Abstract
Noninvasive phototherapy with functional preservation is considered to be a promising cancer therapeutic method. However, the clinical application of tumor phototherapy is severely restrained by the lack of appropriate multimodal phototherapy agents exhibiting an ideal tissue penetration depth to maximize the antitumor efficiency as well as to maintain important tissue functions. Herein, an innovative near-infrared ray (NIR)-triggered photodynamic-photocatalytic-photothermal therapy (PDT-PCT-PTT) agent based on an atomically dispersed cobalt single-atom enzyme (Co-SAE) anchored on hollow N-doped carbon sphere (HNCS) has been strategically developed. Reactive oxygen species (ROS) are highly activated and amplified through both the photogenerated electrons and the photothermal conversion induced by NIR irradiation, as systematically demonstrated by the experimental and density functional theory (DFT) calculation results. Mild hyperthermia is eventually achieved through apoptosis and ferroptosis caused by ROS, significantly boosting the interaction of ROS dynamic effects and thermodynamic effects in the tumor microenvironment (TME). More importantly, Co-SAEs/HNCS not only causes multimodal damage through limited TME products but also preserves important organ functions by the induction of mild local hyperthermia. This work expands the biomedical application field of SAEs and presents an innovative all-in-one, multimodal concept for the noninvasive treatment of head and neck cancer.
Similar content being viewed by others
Introduction
Head and neck cancer refers to a group of different malignant tumors originating from the anatomic region of the upper digestive tract1, with 0.6 million new cases diagnosed worldwide each year2, and the 5-year survival rate of these patients is only approximately 60% due to its high malignancy and metastasis rate3. Surgery combined with chemoradiotherapy is the preferred therapy mode4, however, it can lead to undesirable sequelae, for example, lifelong dysfunction of mastication, pronunciation, and respiration, malformation and consequent psychological disturbance owing to defects of nerves, muscles and bones in the head and neck area5. Hence, it is crucial to focus research efforts on the treatment of head and neck cancer.
Recently, phototherapy, including photodynamic therapy (PDT), photocatalytic therapy (PCT) and photothermal therapy (PTT), has emerged as a promising therapeutic modality and provides innovative treatment ideas for cancer treatment because of its unique time-space controllability, noninvasiveness, and reproducible treatment effectiveness6,7,8,9. Currently, porphyrin, zinc phthalocyanine, gold nanoparticles and their derivatives are commonly used in cancer phototherapy, and Photofrin has been approved by the U.S. Food and Drug Administration (FDA)10,11,12. Tumor ablation is achieved through oxidative damage via the generation of reactive oxygen species (ROS) or lethal local temperature via photothermal conversion13,14. However, monomodal phototherapy has its drawbacks: the reaction substrates of PDT or PCT, such as O2 or H2O2, are limited in the tumor microenvironment (TME)15; thermal damage to normal tissues around the tumor often occurs due to thermal diffusion caused by PTT16; and the penetration depth of most visible light is not enough to activate deeper phototherapeutic agents17. Therefore, phototherapy preparations with multiple therapeutic modes to achieve efficient tumor ablation and avoid side effects are urgently demanded.
Although multimodal integrated phototherapy has been widely reported by nanodelivery systems including liposomal18, polymeric micelles19, silica-based materials20, carbon-based materials21, metal-organic frameworks22, etc, however, the complex nanomaterial assembly, premature photosensitizer leakage and high photothermal temperature remain obstacles to higher therapeutic efficacy and improved patient quality of life. As artificial nanoenzymes, single-atom enzyme (SAE) has been widely used in the biomedical field to mimic a variety of natural enzyme functions in organisms, including peroxidase (POD)23, oxidase24, superoxide dismutase25, catalase and so on26,27,28. Owing to their high activity in response to the TME, light or ultrasound, SAE-based materials have been confirmed to exhibit synergistic antitumor effects through multiple reactive pathways29. Nonetheless, the actual efficacy of catalytic therapy is unsatisfactory due to the limited availability of catalytic substrates, restricted visible light penetration depth, and parallel, but not synergistic, reaction modes30. Benefiting from the well-defined structure of the active metal sites of SAE, the ideal model for mechanistic exploration is highly favorable for the activity evaluation, mechanism verification and design optimization of nanocatalysts, thus, a type of SAE that solves the abovementioned problems is expected to be reasonably designed.
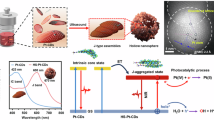
In this work, we prepare a facile strategy to achieve atomically dispersed Co-SAE embedded on the HNCS supports, labeled Co-SAEs/HNCS (Fig. 1). The as-prepared Co-SAEs/HNCS is utilized as a highly efficient photodynamic-photocatalytic-photothermal therapeutic agent to trigger both the interactive ROS dynamic effects and thermodynamic effects by mutually fulfilling multiple pathways in the TME. Experimentally, the burst of ROS and increase of local mild temperature are simultaneously triggered by the photoelectron effect and photothermal effect upon NIR irradiation. Furthermore, density functional theory (DFT) calculations have demonstrated that the superior ROS dynamic activity of Co-SAEs/HNCS in the NIR-I region was remarkably enhanced by the highly atomically dispersed Co-N4 active centers. Encouragingly, apoptosis and ferroptosis are effectively activated and amplified owing to the photothermal effect and glutathione-like (GSHOx-like) activity of Co-SAEs/HNCS, facilitating the downregulation of heat shock protein (HSP) to realize a mild PTT. Thus, our multichannel tumor ablation based on the facile synthesis of SAEs atomically dispersed on highly hierarchical hollow carbon host, as well as the synergistical ROS dynamic effects and mild thermodynamic effects make this all-in-one multimodal concept highly promising for the noninvasive treatment of head and neck cancer.
Results
Synthesis and Characterization of Co-SAEs/HNCS
In this work, the strategy for the synthesis of atomically dispersed and N-coordinated Co-SAE anchored on HNCS support was illustrated in Scheme 1a and experimental section in the supporting information. Briefly, silica suspension with a particle size of ~200 nm was synthesized by the hydrolysis reaction of tetraethyl orthosilicate (TEOS). After magnetic stirring for 24 h, the cobalt salts and dopamine hydrochloride were dissolved in the silica suspension for another stirring for 24 h in air. Since polydopamine (PDA) possesses abundant -OH and N-involved functional groups, Co cations could be efficiently linked with the functional groups via covalent bonds or electrostatic interaction. Hence, the Co cations and dopamine monomers could be simultaneously self-polymerized to form Co-doped PDA around SiO2, marked as Co/PDA@SiO2. Then the Co/PDA@SiO2 was pyrolyzed at 600 °C for 2 h in a N2 atmosphere to obtain the Co-SAE/NCS@SiO2. The SiO2 templates were etched away by the KOH to obtain the final products of Co-SAEs/HNCS.
The morphology of Co-SAEs/HNCS was characterized by scanning electron microscopy (SEM, Fig. 2a) and low-resolution scanning transmission electron microscopy (STEM, Fig. 2b and Supplementary Fig. 1). Obviously, Co-SAEs/HNCS exhibits uniform hollow carbon sphere with a size Co-SAEs/HNCS of ~200 nm. Similar morphology is also observed in HNCS in Supplementary Fig. 2. Additionally, no metal clusters or nanoparticles were found from the low-resolution STEM image shown in Fig. 2b, matching well with the powder X-ray diffraction (PXRD) patterns of Co-SAEs/HNCS presented in Supplementary Fig. 3. The corresponding elemental mappings indicated uniform distributions of Co, C, and N throughout the entire Co-SAEs/HNCS sample, which was further confirmed by X-ray photoelectron spectroscopy (XPS) analysis, as shown in Supplementary Fig. 4. The Co 2p signal was not well detected by XPS analysis due to the low Co concentration in Co-SAEs/HNCS; however, the Co content was confirmed to be ~0.30 wt% via inductively coupled plasma mass spectrometry (ICP–MS). To gain in-depth insight into the structural information of the as-fabricated Co-SAEs/HNCS, spherical aberration correction high-angle annular dark-field STEM (HAADF-STEM) was carried out. As shown in Fig. 2c, isolated Co atoms, marked as yellow dashed circles, were established after proper pyrolysis and chemical leaching. No Co clusters or particles were observed on the highly disordered carbon structures from the HRTEM in Fig. 2d, coinciding with the EDS mapping and XRD results in Fig. 2b and Supplementary Fig. 3, respectively. Co-SAEs/HNCS revealed a negative zeta potential (~50 mV), as shown in Supplementary Fig. 5, which was beneficial for prolonging the lifetime of photogenerated carriers and improving the effect of photogenerated electron-hole pairs, in consistent with the negative zeta potential of those reported N-doped carbon materials31,32,33,34. In addition, the Raman spectrum of Co-SAEs/HNCS shown in Supplementary Fig. 6 contained two main peaks at 1587.0 and 1349.0 cm−1, which correspond to the vibrations of sp2-bonded carbon atoms (G band) and dispersive defect-induced vibrations (D band), respectively, demonstrating plentiful defect sites on the carbon substrates35. The intensity value of ID/IG (1.52) of Co-SAEs/HNCS was evidently higher than that of HNCS (1.46), indicating the more defects were generated with Co-SAEs/HNCS. Additionally, electron paramagnetic resonance (EPR) was further performed to compare the defects in both HCNS and Co-SAEs/HCNS, as the variation of the EPR signal could manifest the unpaired electrons in the carbon substrate. As shown in Supplementary Fig. 7, both HCNS and Co-SAEs/HCNS exhibited resonance signal at 3323.5 G from the EPR spectra, signifying the presence of unpaired electrons that stemming from the defect sites in the samples. Particularly, a prominently higher peak intensity can be seen for Co-SAEs/HCNS compared to HCNS, verifying more intrinsic carbon defects within Co-SAEs/HCNS than that of HCNS36,37.
a SEM image of Co-SAEs/HNCS. b Representative low-resolution STEM image of Co-SAEs/HNCS and the corresponding elemental images showing the distribution of C (green), N (yellow) and Co (red). c High-resolution STEM image of Co-SAEs/HNCS. The isolated Co atoms were highlighted by the yellow dashed circles. d Corresponding HRTEM image in Fig. 2c. e, f Co K-edge XANES spectra and Co K-edge FT-EXAFS spectra of Co-SAEs/HNCS and Co foil. g Experimental and FT-EXAFS fitting curves of Co-SAEs/HNCS at Co K-edge, inset is the corresponding schematic model (Co: blue, N: light blue, and C: brown). h The corresponding EXAFS fitting curves of Co-SAEs/HNCS at k space. i, j Wavelet transforms for the k3-weighted Co K-edge EXAFS signals of Co foil and Co-SAEs. Scale bars: a, 500 nm; b, 100 nm; c-d, 2 nm. a, b, c, d, i, j Each experiment was repeated three times with similar results. Source data are provided as a Source Data file.
X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) were further applied to obtain the chemical state and coordination environments of Co species in HNCS substrates. The structural details are shown in Supplementary Tab. 1. As shown in Fig. 2e, the Co-K-edge XANES profile showed that the edge energy (E0) value of Co-SAEs/HNCS was located below that of Co foil, indicating that the average oxidation state of Co was a positive value38,39,40. The Fourier transform extended X-ray absorption fine structure (FT-EXAFS) spectrum of Co-SAEs/HNCS in Fig. 2f showed a main peak at 1.5 Å (without phase shift correction) in R space, matching well with the dominant Co-N coordination38,40. The detailed Co-N4 coordinate structure was further obtained via the EXAFS experimental spectrum and fitted curve, as observed in Fig. 2g, h. The Co K-edge wavelet transform (WT)-EXAFS was also carried out to confirm the atomic arrangement of Co-SAEs/HNCS as it is very sensitive to identify the atomic configuration of back scatters in both R-space and k-space. The WT contour plots in Co-SAEs/HNCS exhibited only one intensity maximum at ~3.6 Å−1 (Fig. 2j), which is very different from the intensity maximum of Co foil at ~7.0 Å−1 (Fig. 2i), further demonstrating the isolated nature of Co species rather than crystalline structure in Co-SAEs/HNCS. From the comprehensive analysis by EXAFS, the isolated Co-SAEs with the Co-N4 configuration were confirmed on the HNCS substrates.
NIR-Triggered Photothermal and Photodynamic Response of Co-SAEs/HNCS
As light absorption in the NIR wavelength region is essential for NIR-responsive carbon-based materials, the absorption spectra in the UV-vis-NIR region were obtained for HNCS and Co-SAEs/HNCS. Encouragingly, both HNCS and Co-SAEs/HNCS showed profound absorption in the NIR-I region (Supplementary Fig. 8), which inspired us to gain an in-depth understanding of the NIR-responsive photodynamic, photocatalytic and photothermal properties of Co-SAEs/HNSC. To evaluate the photothermal conversion efficiency (PCE) of Co-SAEs/HNSC, photothermal response measurements were performed with an infrared thermal camera by altering the power density of NIR-I (808 nm) and the concentration of Co-SAEs/HNSC aqueous dispersions. The temperatures were gradually elevated with increasing power density of irradiation (Supplementary Fig. 9a) or concentration of Co-SAEs/HNCS aqueous dispersions (Fig. 3a), indicating apparent photothermal conversion phenomena of Co-SAEs/HNCS aqueous dispersions upon NIR-I irradiation. As expected, the HNCS aqueous dispersions showed a photothermal response similar to that of Co-SAEs/HNCS, as depicted in Supplementary Fig. 10 and Supplementary Fig. 11, respectively. Notably, Co-SAEs/HNCS aqueous dispersions showed stable temperature changes after five continuous heating and cooling cycles, indicating excellent stability for Co-SAEs/HNCS as the photothermal agent (Supplementary Fig. 9b). Finally, the PCEs of HNCS and Co-SAEs/HNCS were calculated to be ~33.5% and ~34.2%, respectively (Fig. 3b, c and Supplementary Fig. 10b, c), and these values were consistent with those of other reported carbon-based photothermal agents for efficient PTT41,42,43.
a The Time-dependent temperature changes of Co-SAEs/HNCS receiving irradiation in different concentrations (808 nm, 1 W/cm2, 10 mins). b A random heating-cooling curve of Co-SAEs/HNCS (20 ug/mL) (808 nm, 1 W/cm2, 10 mins). c The linear time data from the cooling curve of Co-SAEs/HNCS. d NIR spectrum for Co-SAEs/HNCS. e Time-dependent irradiated-degradation of DPBF caused by 1O2 generated by Co-SAEs/HNCS upon NIR-I irradiation (808 nm, 1 W/cm2, 10 mins). f Rate constants for DPBF decomposition in the presence of Co-SAEs/HNCS. g Time-dependent absorption changes of TMB in the presence of Co-SAEs/HNCS and H2O2. h The Michaelis-Menten kinetic analysis for Co-SAEs/HNCS in the presence of TMB and H2O2 (n = 3). i ·OH was detected using OPDA in Co-SAEs/HNCS group with and without NIR-I irradiation. (808 nm, 1 W/cm2, 10 mins). Source data are provided as a Source Data file.
To measure the intrinsic photodynamic and photocatalytic properties of Co-SAEs/HNCS, 1,3-diphenylisobenzofuran (DPBF), 3,3,5,5-tetramethylbenzidine (TMB) and o-phenylenediamine (OPDA) were used as the 1O2 and ·OH probe to detect the ROS products. DPBF, which gradually decomposes in the presence of 1O2 generated in the reaction media, was used to quantitatively obtain the 1O2 quantum yield. Please note that indocyanine green (ICG), a commonly used FDA-approved fluorescent dye, was selected as the control sample due to the unequivocal 1O2 quantum yield. First, the absorbance values of each experimental group containing DPBF/ICG (Supplementary Fig. 12a), DPBF/HNCS (Supplementary Fig. 13a) and DPBF/Co-SAEs/HNCS (Fig. 3d) were observed to be quantitatively consistent at 808 nm. In addition, the corresponding characteristic absorption values of DPBF at 415 nm gradually decreased with irradiation time, as shown in Fig. 3e, Supplementary Fig. 12b and Supplementary Fig. 13b. According to Eq. (3), the rate constants for DPBF decomposition were 0.0085 for ICG (Supplementary Fig. 12c), 0.0157 for HNCS (Supplementary Fig. 13c) and 0.0343 for Co-SAEs/HNCS (Fig. 3f). Hence, the final calculated 1O2 quantum yields were 0.0037 for HNCS and 0.0081 for Co-SAEs/HNCS, according to Eq. (4) and the referenced yield of ICG (0.002) in aqueous solution44. In addition, the DPBF absorption spectrum of each experimental group under dark condition, and the change of the absorption peak at 415 nm of each experimental group with or without NIR were shown in Supplementary Fig. 14. Overall, the high 1O2 quantum yield of Co-SAEs/HNCS strongly demonstrated that it could be a promising photodynamic therapeutic agent.
Numerous studies have reported that metal-based SAEs exhibit POD-like activity45,46,47. Hence, the POD-like performance of Co-SAEs/HNCS was identified through the 3,3,5,5-tetramethylbenzidine (TMB) colorimetric reaction experiment by dispersing the Co-SAEs/HNCS sample in acetic acid/sodium acetate buffer solution (pH 6.5). As displayed in Fig. 3g, Co-SAEs/HNCS showed characteristic absorption peaks at 655 nm with increasing time under dark condition. Apparently, the colorimetric reaction of Co-SAEs/HNCS followed typical Michaelis–Menten kinetics48, and the Michaelis–Menten constant (Km) and maximal reaction velocity (Vmax) were 1.906 mM and 0.515·10−7 M s−1 for Co-SAEs/HNCS (Fig. 3h), respectively. Strikingly, ·OH production could be intensified by NIR exposure, as shown in Fig. 3i and Supplementary Fig. 15, the absorbance of the OPDA colorimetric reaction of Co-SAEs/HNCS was significantly enhanced compared to that of HNCS upon NIR-I irradiation, indicating the essential role of Co-SAE, as a photoresponsive catalytically active center, in driving the catalase-like activity. As a result, it was deduced that the greatly improved catalase-like activity of Co-SAEs/HNCS was probably caused by the synergetic effects of photogenerated electrons and photothermal conversion upon NIR-I irradiation.
It is well known that the oxidative damage and lethal effect caused by ROS could be negligible due to antioxidant pathways in tumor cells, in which 1O2 and ·OH would be eliminated by intracellular overexpressed glutathione (GSH)49,50,51. It was expected that Co-SAEs/HNCS could also exhibit excellent glutathione oxidase (GSHOx)-like activity by oxidizing GSH to glutathione oxidized (GSSG). To confirm the GSHOx-like activity of Co-SAEs/HNCS, 5,5’-dithiobis-(2-nitrobenzoic acid) (DTNB) was used to detect GSH remaining after the catalytic reaction, during which TNB could be generated with a characterization absorption peak at 412 nm during the reaction52. Encouragingly, Co-SAEs/HNCS was shown to exhibit Michaelis–Menten kinetics in the catalytic reaction of GSH, with Km and Vmax values were 0.7554 mM and 0.9356·10−6 M s−1, respectively (Supplementary Fig. 16), with Vmax value similar to previously reported SAEs53.
Density functional theory (DFT) simulations
Inspired by the excellent NIR-I response of Co-SAEs/HNCS, the reaction mechanism of ROS generation (1O2 and ·OH) was further investigated by DFT calculations. The electronic spin resonance (ESR) spectra indicated the existence of 1O2 by showing the characteristic intensity ratio of 1:1:1 (Fig. 4a). Based on the experimental quantitative and semiquantitative detection of 1O2 and the ESR spectra, we proceeded to study the spin multiplicity change of O2. The Co-N4 moiety was constructed with four pyridine N atoms surrounding the Co metal center based on previously reported literature35. For comparison, we also studied the impact of the Co-N4 site by removing the Co atom from the N4 site and reran the simulations. As shown in Fig. 4b, the adsorption energies for O2 on the Co-N4 and N4 systems are 0.16 eV and 0.42 eV, respectively, suggesting a more favorable O2 adsorption process on Co-N4 than on the N4 site, which was particularly crucial to determine the generation of 1O2. Next, to determine whether photogenerated electrons could be formed via NIR-I excitation, we applied DFT to examine the impact of infrared emission on Co-N4 and its influence on 1O2 generation. As shown in Supplementary Fig. 17, the computed bandgap of Co-SAEs/HNCS was 0.28 eV, which was relatively narrow and allowed the formation of photogenerated electrons after infrared light (808 nm, 1.5 eV) excitation. Hence, it was proposed that photogenerated electrons could also affect the generation of 1O2 at the Co-N4 site, except for 1O2 generation caused by the common photothermal effect54. A promising pathway ((* + 1e−) + (* + 1 h+) + 3O2 → (*+1 h+) + O2− → 2* + 1O2) was examined for the free energy profile of this photo-driven 1O2 formation as shown in Fig. 4c, where the photogenerated electrons can attack the adsorbed triplet oxygen molecule, forming a superoxide ion (O2-) and a positively charged Co-SAEs/HNCS substrate. This step is highly exothermic with a negative reaction energy of -0.91 eV (blue line). Then, the positive charge transfers back to O2, forming a neutral 1O2 with a negligible energy barrier of 0.16 eV. As a comparison, the pathway (* + 3O2 → (* + 1 h+) + O2- → * + 1O2) of 1O2 formation prior to photo-excitation was much less feasible with a high energy uphill of 0.94 eV (red line). The results indicate that the Co-SAEs could significantly promote the generation of O2- ROS and boost the PDT activity. For comparison, we also studied the impact of the Co site by removing the Co atom from the N4 site and reran the simulations, as shown in Supplementary Fig. 18. The energy required to proceed with the first step was much higher (1.37 eV), further demonstrating the important role of Co-SAEs in the generation of 1O2.
a Signal pattern of 1O2 detected by ESR under different conditions. b O2 adsorption models of Co-SAEs/HCNS (left) and HCNS (right). c Gibbs free energy diagram on Co-N4 site for the formation of 1O2 in the neutral (red) and one e- (blue) condition. d Signal pattern ·OH detected by ESR under different conditions. e The proposed catalytic mechanism of ·OH caused by Co-SAEs/HNCS at pH 6.5 and 25 °C. f Gibbs free energy diagram on Co-N4 site for the formation of ·OH in the neutral (red) and one e- (blue)condition. Source data are provided as a Source Data file.
Furthermore, the ESR spectra indicated the existence of ·OH by showing the characteristic intensity ratio of 1:2:2:1, as evidenced by both the TMB colorimetric reaction experiment and ESR results (Fig. 4d), serving as direct evidence of hydroxyl free radical generation. ·OH was considered the active intermediate in this study. Therefore, the OH-related mechanism of Co-SAEs/HNCS was proposed, as shown in Fig. 4e, which has been validated in many related single-atom catalytic systems1,2. The proposed mechanism is initialized with the adsorption of molecular H2O2 with a very minor uphill free energy of 0.07 eV (red line, Fig. 4f). Then, the adsorbed H2O2 is cleaved homogeneously by the Co-N4 site, forming a reactive ·OH and a hydroxyl group (*OH) adsorbed at the Co-N4 site. The energy barrier was computed to be 0.47 eV, which is easily surmountable under mild conditions. In comparison, the formation of two *OH species is relatively less competitive due to its higher energy of 0.69 eV (Supplementary Fig. 19). Subsequently, the residual *OH can easily react with an approaching proton due to the negligible reaction energy of -0.35 eV, forming an absorbed H2O molecule under the acidic catalytic milieu. The follow-up H2O desorption step is exothermic, enabling regeneration of the catalytic site to accommodate another molecule of H2O2 for OH generation in the next cycle. The overall negative Gibbs free energy (−0.32 eV) and the relatively lower apparent energy barrier (0.47 eV) indicate that the Co-N4-catalyzed Fenton reaction is thermodynamically and kinetically favorable at room temperature, which explains the high experimental Fenton activity. Interestingly, the presence of one photogenerated electron (red line, Fig. 4f), could make the rate-determining step (H2O2 → *OH + OH) even more efficient by significantly reducing the barrier to 0.21 eV.
In addition, Co-SAEs/HNCS was confirmed to catalytically oxidize glutathione in vitro. To evaluate the GSHOx-like activity of Co-SAEs/HNCS, the free energy profiles were also studied using DFT. As shown in Supplementary Fig. 20a, the conformationally optimized GSH molecule can spontaneously adsorb onto the Co-N4 site, enabling its reaction with the oxygen species (O*, OH*) adsorbed at the Co site. With the assistance of O* or OH*, the dissociation of GSH can occur easily to form GS* due to the exothermic nature of this reaction (Supplementary Fig. 20b), generating OH* or H2O, respectively55. The resulting OH* can either continue to dissociate another GSH molecule or react with a proton to generate a H2O molecule. Subsequently, the coupling interaction between the two generated GS* species can occur spontaneously, with a negative reaction free energy of -1.46 eV. The heat released by the exothermic reaction is sufficient to overcome the desorption barrier of the produced GSSG* (1.26 eV). During this GSHOx-like activity, the total Gibbs free energy was drastically reduced by 2.90 eV, exhibiting the remarkable enzymatic activity of Co-SAEs/HNCS in mimicking GSHOx. The mechanism that we proposed has also been validated in other related systems3.
Intracellular oxidative damage induced by Co-SAEs/HNCS
Encouraged by the excellent 1O2 and·OH production ability of Co-SAEs/HNCS, its in vitro antitumor effect was further explored for biomedical applications. The human oral squamous cell carcinoma cell line Cal-33 was selected as the experimental cell line. Whether nanomaterials could be effectively taken up by tumor cells is the primary condition for obtaining therapeutic effects; thus, biological transmission electron microscope (bio-TEM) was first used to evaluate the uptake of Co-SAEs/HNCS by Cal-33 cells. At an incubation concentration of 20 μg/ml, both HNCS and Co-SAEs/HNCS were effectively taken up by Cal-33 cells and dispersed evenly in the cytoplasm. In addition, the complete structural morphology and about ~200 nm diameter were observed before subsequent NIR activation (Fig. 5a). Then, 2’,7’-Dichlorodihydrofluorescein diacetate (DCFH-DA) was used as a fluorescent indicator of 1O2 to investigate the intracellular 1O2 production ability of Co-SAEs/HNCS upon NIR irradiation, and each experimental group, receiving irradiation or not, was observed with confocal laser scanning microscopy (CLSM). Only the HNCS + NIR group and Co-SAEs/HNCS + NIR group showed specific green fluorescence, and the fluorescence intensity of the Co-SAEs/HNCS + NIR group was obviously stronger than that in the HNCS + NIR group, suggesting that there might be more 1O2 production in Cal-33 cells in the Co-SAEs/HNCS + NIR group (Fig. 5b). To further compare the intracellular 1O2 production capacity of the two nanomaterials, flow cytometry (FCM) was used to quantitatively analyze the performance. Similarly, DCFH-DA was used as a fluorescent indicator of 1O2, and Cal-33 cells incubated with HNCS or Co-SAEs/HNCS in each group were irradiated and then immediately detected by FCM. The proportion of 1O2 produced in the Co-SAEs/HNCS (68.4%) group was significantly higher than that in the HNCS (28.2%) group, confirming that Co-SAEs/HNCS had a more significant intracellular NIR-triggered 1O2 production ability than HNCS under the same condition (Supplementary Fig. 21). According to previous studies, the apoptotic pathway represented by mitochondrial damage can be triggered by the burst of a large amount of intracellular 1O256. Therefore, whether Co-SAEs/HNCS also had the ability to damage mitochondria with 1O2 was further explored. The loss of mitochondrial membrane potential is considered to be an important sign of mitochondrial damage, so 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine (JC-1) dye was used to specifically indicate the level of mitochondrial depolarization. CLSM was used to directly observe mitochondrial depolarization in Cal-33 cells. After irradiation, only the HNCS + NIR group and Co-SAEs/HNCS + NIR group had obvious green fluorescence, indicating that the cells in the corresponding experimental group had undergone mitochondrial depolarization, which is the initial manifestation of cell apoptosis (Fig. 5d). Furthermore, FCM was applied to semi-quantitatively analyze the mitochondrial depolarization level. Compared with all the nonirradiated groups and the PBS + NIR group, the Co-SAEs/HNCS + NIR group cells showed a higher proportion of JC1-monomer positive cells, indicating that cells treated with Co-SAEs/HNCS + NIR experienced a certain degree of mitochondrial potential reduction, manifested as mitochondrial depolarization (Fig. 5c).
a The biological TME images of nanoparticle uptake by cells under different magnifications. b The production of 1O2 in cells observed by CLSM under different conditions. c Mitochondrial depolarization in cells semi-quantitative analyzed by FCM (d) and observed by CLSM under different conditions. e The production of ·OH in cells observed by CLSM under different conditions. f The accumulation of LPOs in cells under different conditions. NIR-I irradiation: 808 nm, 1 W/cm2, 10 mins. Scale bars: a, high 3 µm, medium 1 µm, low 250 nm; b, 100 µm; d, 100 µm; e, 50 µm; f, 100 µm. Each experiment was repeated three times with similar results.
At the same time, considering the special high-H2O2 microenvironment in tumor cells, the production of·OH in cells and subsequent cell damage were assessed. Hydroxyphenyl fluorescein (HPF), as a specific fluorescent probe for ·OH, was used to detect the generation of ·OH in cells. After we incubated Cal-33 cells with nanomaterials, each experimental group, receiving irradiation or not, was observed by CLSM. The results showed that only the Co-SAEs/HNCS group, HNCS + NIR group and Co-SAEs/HNCS + NIR group had specific green fluorescence (Fig. 5e). Considering the POD-like catalytic activity of Co-SAEs/HNCS, the intracellular ·OH content of Cal-33 cells under dark condition was also detected fluorescence signal in Co-SAEs/HNCS group, however, the fluorescence intensity of the Co-SAEs/HNCS + NIR group was significantly stronger than others, suggesting that there might be more ·OH production in Cal-33 cells in the Co-SAEs/HNCS + NIR group. This result could be due to the NIR causing an immediate surge of intracellular ·OH levels. The irradiation made the instantaneous intracellular ·OH production far exceed the catalytic generation under dark conditions. To the best of our knowledge, the instantaneous burst of ROS will be more conducive to disrupting the antioxidant environment in tumor cells, and the lower concentration limit also makes biological applications safer57. More importantly, the accumulation of corresponding lipid peroxides (LPOs) is often led to by high intracellular expression of ·OH, which is an important early sign of cell ferroptosis58. Consequently, whether ·OH produced by Co-SAEs/HNCS with irradiation had the ability to cause ferroptosis in Cal-33 cells was further explored. C11-BODIPY581/591 is a fluorescent probe that specifically binds to LPOs in cells and can detect the occurrence of ferroptosis in cells. Excessive intracellular LPO accumulation can specifically bind to the probe to generate obvious green fluorescence, so CLSM was used to directly observe ferroptosis in tumor cells. After similar treatment, only the HNCS + NIR group and Co-SAEs/HNCS + NIR group showed obvious green fluorescence, indicating membrane lipid oxide accumulation at the initial stage of ferroptosis (Fig. 5f). In particular, the fluorescence intensity of the Co-SAEs/HNCS + NIR group was markedly stronger than that of the HNCS + NIR group due to higher intracellular ·OH production, which also led to a higher level of ferroptosis in Cal-33 cells in the Co-SAEs/HNCS + NIR group.
In vitro antitumor ability of Co-SAEs/HNCS
In view of the dual damage mechanism of apoptosis and ferroptosis caused by Co-SAEs/HNCS in Cal-33 cells, the in vitro antitumor ability of Co-SAEs/HNCS and related biomedical safety were further investigated. First, the cell counting kit-8 (CCK-8) method was used to detect the viability of Cal-33 and HGF cells treated with different concentrations of HNCS and Co-SAEs/HNCS. The cells still had great cell viability at the highest culture concentration of 20 μg/ml under dark conditions, suggesting the reliable biosafety of Co-SAEs/HNCS (Supplementary Fig. 22). The CLSM experiment results of HGF cells with Annexin V-FITC/PI dye confirmed the same conclusion (Supplementary Fig. 23). Simultaneously, the result of cell viability of Cal-33 cells treated with different concentrations of HNCS and Co-SAEs/HNCS after irradiation suggested a reasonable concentration-dependent trend (Fig. 6a). More importantly, antitumor effects of thermodynamic effect and ROS dynamic effect were measured under single or interactive conditions, ice bath or vitamin was used as the condition to limit temperature rise or ROS production, respectively. The results showed that the anti-tumor effect of Co-SAEs/HNCS + NIR under normal conditions was better than that of the single-path superposition fitting group, which was a synergistic therapeutic result of the interaction of thermodynamic effect and ROS dynamic effect in cancer cells (Fig. 6b). Correspondingly, there was no significant difference in the treatment effect between the HNCS + NIR group and the single-path superposition fitting group (Supplementary Fig 24). Furthermore, CLSM was used to directly observe cell death and Annexin V-FITC/PI dye was used to stain live and dead cells. Each experimental group without irradiation showed green fluorescence in the field of view, indicating the healthy survival of the cells; in contrast, the HNCS + NIR group or Co-SAEs/HNCS + NIR group showed a certain proportion of red fluorescence in the field of view, respectively, indicating that a certain number of cells had died (Fig. 6c). In the random field of view under the same treatment, compared with the HNCS + NIR group, the Co-SAEs/HNCS + NIR group had a higher red fluorescence ratio and a lower green fluorescence ratio, suggesting stronger cell lethal effects. Moreover, FCM, as a quantitative analysis, obtained a result similar to that CLSM experiment, and it was obvious that the Co-SAEs/HNCS + NIR group had a higher proportion of early/late apoptotic and necrotic cells than the HNCS + NIR group (Fig. 6d).
a The cellular activity of Cal-33 cells under NIR-I condition, the data of each group were analyzed using t-test statistics. Data are shown as mean with SD (n = 3, biological replicates). b The concentration and cell viability curves of the cytotoxicity under different conditions. The data of each group were analyzed using One-way ANOVA statistics. “up” marks Co-SAEs/HNCS + NIR and Co-SAEs/HNCS + NIR + ICE group for statistical analysis, “down” marks Co-SAEs/HNCS + NIR and Co-SAEs/HNCS + NIR+Vc group for statistical analysis. Data are shown as mean with SD (n = 3, biological replicates). c The cell death in cells observed by CLSM under different conditions. d The apoptosis/necrosis detection semi-quantitative analyzed by FCM. e HSP70 expression under different conditions (n = 3, independent experimental replicates). (808 nm, 1 W/cm2, 10 mins). Scale bar: c, 200 µm. c, d, e Each experiment was repeated three times with similar results. Source data are provided as a Source Data file.
In addition, the GSHOx-like activity of Co-SAEs/HNCS played an important role in the apoptosis and ferroptosis pathways (Supplementary Fig. 25). After various experimental treatments, Cal-33 cells were tested for intracellular GSH content using a GSH detection kit, and the results showed a significant decrease in Co-SAEs/HNCS + NIR content (Supplementary Fig. 26). Western blotting was used to verify the changes in the expression of intracellular GPX4. The downregulation of GPX4 confirmed that GSH was converted to GSSG in Cal-33 cells, which amplified the irreversible ferroptosis process (Supplementary Figs. 27–29). Additionally, some researchers have reported that the occurrence of ferroptosis may lead to the downregulation of heat shock proteins (HSPs)55, so the detection of HSP70 expression levels is considered to be an important experiment to assess whether mild photothermal treatment has been realized. Ferrostatin-1 (Fer), a ferroptosis inhibitor, was used to establish a positive control for inhibition of the ferroptosis pathway. As shown in Fig. 6e, Supplementary Figs. 30, 31, the expression of HSP70 was significantly reduced in the Co-SAEs/HNCS + NIR group, while it was significantly upregulated in the Co-SAEs/HNCS + NIR+Fer group, similar to the 43 °C group. This was because the ferroptosis process could inhibit the expression of HSP, thereby aggravating the heat damage of cells at a mild temperature. At the same time, the expression level of ATP in cells was also detected, and it was found that cells treated with HNCS + NIR and Co-SAEs/HNCS + NIR showed a significant decrease in intracellular ATP levels (Supplementary 32). This is due to the accumulation of excessive intracellular reactive oxygen species, which disrupts ATP metabolism in cells. And multiple programmed cell death patterns, including apoptosis and ferroptosis, have effective killing effects on the cells in the experimental group.
In vivo antitumor ability of Co-SAEs/HNCS
Clinically, the occurrence of head and neck cancer often implies loss of function after surgical treatment and a higher tumor recurrence rate. The high fatality rate, dysfunction, psychological trauma and hidden dangers of recurrence have brought great pain to patients with head and neck cancer. Therefore, we hope that there will be a safe, effective and noninvasive way to cure cancer while preserving function. In view of the excellent in vitro antitumor ability of Co-SAEs/HNCS, the antitumor performance of Co-SAEs/HNCS in vivo was explored.
We successfully constructed a nude mouse in situ tongue cancer xenograft model by injecting Cal-33 cell suspension into the anterior one-third lateral edge of the tongue in nude mice. After the tumor grew to approximately 10 mm3, the blood safety of the material was tested by locally injecting PBS or Co-SAEs/HCNS solution into normal nude mice and tumor bearing nude mice. The results of blood routine and biochemistry showed that there were no significant changes in blood indicators in both normal nude mice and tumor bearing nude mice after injection of Co SAEs/HCNS solution compared to those injected locally with PBS solution (Supplementary Figs. 33–34). On the other hand, in experiments of tumor cycle therapy, after the tumor grew to approximately 10 mm3, PBS solution, HNCS or Co-SAEs/HNCS was injected locally into each experimental group. After four hours, each experimental group received irradiation or not. A mild temperature rise that occurred in the irradiation group was confirmed by photothermographic results (Fig. 7a), and the unnecessary thermal damage of adjacent normal tissues was avoided due to timely local temperature not exceeding (Supplementary Fig. 35).
a The photothermal imaging of tumor-bearing mice. b The digital photo of tumor-bearing mice during the treatment cycle. c The digital photo of isolated tumor with tongue. d The tumor volume change curves during the treatment cycle. e The isolated tumor weight, the data of each group were analyzed using One-way ANOVA statistics. Data are shown as mean with SD (n = 5, biological replicates). f The survival curves of tumor-bearing mice. g The tumor growth inhibition rate in tumor-bearing mice, the data of each group were analyzed using One-way ANOVA statistics. Data are shown as mean with SD (n = 5, biological replicates). (808 nm, 1 W/cm2, 10 mins). Source data are provided as a Source Data file.
During the 24-day treatment cycle observation, the local tumors of tumor-bearing nude mice in the nonirradiation experimental groups and PBS + NIR group showed significantly increased growth compared with the obvious growth inhibition in the HNCS + NIR group and Co-SAEs/HNCS + NIR group (Fig. 7d). The digital photograph of the isolated tumor on the 24th day showed that the tumor grew at the anterior one-third of the tongue, and the tumor size trend was the same as that of nude mice bearing tumors in vivo (Fig. 7b, c). Isolated tumor weight measurements also confirmed that the Co-SAEs/HNCS + NIR group achieved the best tumor growth inhibitory effect (Fig. 7e), and the final tumor inhibition rate of Co-SAEs/HNCS + NIR group reached approximately 100% (Fig. 7g). In addition, during observation of the treatment cycle, some tumor-bearing nude mice experienced weight loss (Supplementary Fig. 36), and the survival rate of the nonirradiation experimental groups and PBS + NIR groups decreases over time (Fig. 7f), According to the actual situation and previous research, due to the increased volume of the tumor at the tongue, the tumor-bearing nude mice had difficulty eating and respiratory depression, so they died naturally because of nutritional failure. In contrast, the HNCS + NIR group and Co-SAEs/HNCS + NIR group showed a high survival rate. In the Co-SAEs/HNCS + NIR group, all the treated nude mice were alive after 24 days, suggesting that Co-SAEs/HNCS had an efficient tumor suppression effect while preserving certain tongue function, which will be of great benefit for survival functions such as eating. Furthermore, after the end of the treatment period, the tumors and internal organs of each experimental group were subjected to hematoxylin and eosin (H&E) staining and section observation. The images of normal internal organs showed the well-organized cellular structure of the Co-SAEs/HNCS + NIR group, demonstrating excellent antitumor performance and biological safety (Supplementary Fig. 37). The cancer cells of the Co-SAEs/HNCS + NIR group were the most severely damaged (Supplementary Fig. 38), confirming the strongest tumor suppressor effect. In addition, immunohistochemical (IHC) staining was used to evaluate the expression of Ki67, GPX4 and HSP70 in tumor tissues (Supplementary Fig. 38). Only the HNCS + NIR and Co-SAEs/HNCS + NIR groups showed the downregulation of Ki67, demonstrating the effectiveness of antitumor efficacy. For GPX4, only the Co-SAEs/HNCS and Co-SAEs/HNCS + NIR groups showed significant downregulation, proving that Co-SAEs/HNCS still exerted an effective GSHOx-like effect in vivo. Furthermore, only the Co-SAEs/HNCS + NIR group showed significant downregulation of HSP70, which indicated that the interaction of multimodal phototherapy achieved mild hyperthermia.
Discussion
In summary, we have established an innovative SAE with multimodal phototherapy. Co-SAEs/HNCS agent with Co-N4 configuration as active center exhibited enhanced ROS dynamic effects upon NIR irradiation. The detailed mechanism study with DFT calculations indicated that the efficient generation of 1O2 and ·OH of Co-SAEs/HNCS could significantly triggering both the PDT effect and PCT effect to effectively oxidative damage. GSHOx-like activity strengthened mitochondrial damage and membrane liposome oxidation, and irreversible apoptosis and ferroptosis enabled effective mild photothermal damage. More importantly, this multimodal phototherapy inhibited the growth of xenogeneic head and neck squamous cell carcinoma in situ, preserved head and neck function, and increased the survival rate. This work deeply explored the anti-tumor effect of Co-SAEs/HNCS in response to integrated photodynamic, photocatalytic and photothermal therapy under NIR and triggered the interaction of dual dynamic effects. An orthotopic tumor-bearing model with functional preservation significance verified that Co-SAEs/HNCS had all-in-one multimodal minimally invasive, high-efficiency anti-tumor performance, was a type of phototherapy nano-agent with stable function, credible curative effect and promising application.
Methods
All experimental animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Peking University and approved by the Animal Ethics Committee of Biomedical Ethics Committee of Peking University (the approval number is LA2022168). The maximum diameter of the tumor in animal experiments is prescribed to be less than 17 mm. The experimental animals were maintained in a facility with an ambient temperature ranging from 18 to 29 °C, a relative humidity between 40% and 70%, and a 12:12-hour light: dark cycle.
Synthesis and characterization
Chemicals
Tetraethyl orthosilicate (TEOS, 98%), dopamine hydrochloride (≥98%), ammonium hydroxide (NH3·H2O, 28 wt%), potassium hydroxide (KOH, ≥85%), Cobalt nitrate hexahydrate (Co(NO3)·6H2O, ≥98%) were purchased from Sigma-Aldrich Co. Ltd. 2’, 7’-Dichlorofluorescent yellow diacetate (DCFH-DA, ≥97%), indocyanine green (ICG ≥95%), C11-BODIPY-581/591 ( ≥95%) were purchased from MK bio. Hydroxyphenyl fluorescein (HPF, ≥98%) and ferrostatin-1 (( ≥99%) were purchased from APExBio. 1,3-Diphenylisobenzofuran (DPBF, ≥97%) was purchased from Shanghai yuan ye Bio-Technology Co. Ltd. 3,3,5,5-tetramethylbenzidine (TMB, ≥98%) and o-phenylenediamine (OPDA, ≥98%) were purchased from Aladdin. 2,2,6,6-tetramethylpiperidine (TEMP, ≥98%), 5,5-dimethyl-1-pyrroline N-oxide (DMPO, ≥98%), CCK-8 test kit were purchased from DODJINDO Laboratories. Annexin V-FITC/PI test kit and JC-1 test kit were purchased from Beyotime Biotechnology. Antibodies for western blotting and immunohistonchemistry were purchased from Abcam. Anti-Glutathione Peroxidase 4 (ab125066), Anti-Hsp70 (ab2787), Anti-Ki67 (ab15580). All other reagents and consumables for cell experiments were purchased from Corning. Cells were from the Central Laboratory of Peking University Stomatological Hospital, and animals were purchased from Beijing Vital River Laboratory Animal Technology company.
Characterization
X-ray powder diffractometer (XRD) patterns (Rigaku RU-200b, parameters: Cu Kα radiation, λ = 1.5405 Å) were collected to obtain the crystalline structure and phase purity of SAEs/HNCS. The low-resolution STEM and elemental mappings were obtained on a JEOL JEM-2100F at 200 kV. The high-angle annular dark-field scanning TEM (HAADF-STEM) was recorded on a JEM ARM-200F at 200 kV, which was equipped with a spherical aberration correction. The concentration of Co was determined by inductively coupled plasma mass spectrometry (ICP-MS, iCAP Q, Thermo, Waltham, USA). ESR spectra were collected on a JEOL-FA200 ESR spectrophotometer. The XPS of the N 1 s spectra was acquired using an ESCAlab250XI (Thermal Fischer). The zeta-potential values of the samples were measured using a Malvern Zetasizer (Malvern Nano-ZS). The samples were dispersed in deionized water and sonicated in water for ζ measurements. Raman spectra were conducted on a Horiba HR-800 confocal Raman microscopic system. The X-ray absorption fine structure (XAFS) spectra were obtained at 11B station in Shanghai Synchrotron Radiation Facility. The Co-SAEs/HNCS was collected in the mode of fluorescence excitation mode at room temperature. Co foil was collected in the mode of transmission mode and measured as the reference to calibrate the energy. XAS data were processed by Athena software. The extended X-ray absorption fine structure (EXAFS) data in R-space were acquired by the Artemis software. S02 parameters were calibrated by the Co foil reference sample. EPR spectra were obtained on an X-band Bruker A200 apparatus at 100 K, with the center field, static field, and sweep width of 3320, 3120, and 400 G, respectively.
Synthesis Method
Synthesis of Co/PDA@SiO2 and PDA@SiO2
We prepared through a stepwise polymerization strategy. Firstly, silica suspension with a particle size of ~200 nm was synthesized by the hydrolysis reaction of TEOS. Absolute ethanol (60 ml) and deionized water (20 ml) were poured into a flask under magnetic stirring for 5 mins. Then 2.5 mL TEOS was added into the above solution with stirring for another 5 mins. Later, 2.5 mL NH3·H2O was injected into the mixture for magnetic stirring 24 h at room temperature. The white silica suspension was obtained without any treatment. Then 1000 mg of dopamine hydrochloride solution (dissolved into 20 ml DI water) and 15 mg Co(NO3)·6H2O were quickly poured into the above silica suspension. The color of the solution was rapidly turned into light brown to dark brown, implying the triggering of polymerization of dopamine molecules. The polymerization was kept for another magnetic stirring 24 h at room temperature. The brown products were obtained by centrifugation (13520 x g for 5 mins) and washed with DI water and ethanol, respectively. Finally, the products were dried at 60 °C for overnight in a vacuum oven, which was marked as Co-SAE/PDA@SiO2. PDA@SiO2 was prepared without adding Co precursors.
Synthesis of Co-SAEs/HNCS and HNCS
For the preparation of Co-SAEs/HNCS and HNCS, the Co-SAE/PDA@SiO2. PDA@SiO2 samples were pyrolyzed at 600 °C for 2 h in an N2 atmosphere. After that, the pyrolyzed samples were dispersed into a 50 ml KOH (6 M) solution at 60 °C for 24 h. The black precipitation was collected by centrifugation (13520 x g for 5 mins) and washed with DI water until the solution was neutral. Finally, the as-products were collected after drying at 60 °C for overnight in a vacuum oven.
Biological experiments
The photothermal measurements of Co-SAEs/HNCS
IR thermal camera was used for monitoring all experiments. The HNCS or Co-SAEs/HNCS (0, 20, 50, 75, 100, 150 μg/ml) in PBS aqueous solution was exposed to NIR-I-808 nm (1 W/cm2) for 10 mins, and the Co-SAEs/HNCS (20 μg/ml) in PBS aqueous solution was exposed to NIR-I-808 nm (0.5, 1, 1.5, 2 W/cm2) for 10 mins. The heating-cooling curve was graphed by estimating the Co-SAEs/HNCS (100 μg/ml) in PBS aqueous solution exposed to NIR-I-808 nm (1 W/cm2) for 10 mins and then under dark condition for 15 mins, which was continuously repeated for 5 times. The photothermal conversion effect of HNCS or Co-SAEs/HNCS (20 μg/ml) was exposed to NIR-I-808 nm (1 W/cm2) for 10 mins, then the NIR-I was shut off for 15 mins, thus the photothermal conversion efficiency (PCE) could be estimated through the Eq. (1).
The highest equilibrium temperature and environmental temperature are represented by Tmax and Tsurr, respectively. The heat loss because of the absorbance of the laser by the container is represented by Qdiss, which is estimated to be equal to 0 mW. The NIR power and the absorbance of samples at 808 nm are represented by I and A808, respectively. The heat transfer coefficient and the surface of the container are represented by h and S, respectively. And the hS was calculated through the Eq. (2).
The sample system time constant, heat capacity and the mass of the solvent are represented by τS, cD and mD, respectively. Finally, the η of HNCS and Co-SAEs/HNCS were estimated at ~ 33.5% and ~ 34.2%.
The ROS generation of Co-SAEs/HNCS
The DPBF probe was used as a 1O2 probe to detect the ability of Co-SAEs/HNCS to produce 1O2. At the fixed Co-SAEs/HNCS solution, the decomposition rate (r) of DPBF by 1O2 is proportional to the concentration of DPBF through Eq. (3):
Where the “n” is the order of the reaction with respect to the concentration of DPBF, and equals zero when the DPBF concentration is appropriately controlled, then the “r” is only dependent on 1O2. So that the amount of 1O2 caused by ICG, HNCS and Co-SAEs/HNCS can be calculated through decomposition rate constants of DPBF. And the 1O2 quantum yield of HNCS and Co-SAEs/HNCS can be obtained by comparing the decomposition rate constants of ICG (ΦICG = 0.0020), HNCS and Co-SAEs/HNCS by the Eq. (4).
Where “Φ” is the 1O2 quantum yield, “r” is the photo decomposition rate, “A” is the absorbance of ICG, HNCS and Co-SAEs/HNCS at 808 nm which is controlled to be 0.5. Finally, the 1O2 quantum yield of HNCS and Co-SAEs/HNCS can be estimated to be ~ 0.0037 and ~ 0.0081, respectively.
The TMB was used as a ·OH probe to detect the ability of Co-SAEs/HNCS to produce OH. TMB (1.5 mg/ml) was added to the Co-SAEs/HNCS (75 μg/ml) in acetic acid/sodium acetate buffer solution (pH 6.5) with H2O2 (1 mM) under dark for 0, 2, 4, 6, 10 minutes respectively. The absorbances of color reaction at 655 nm was obtained by a multi-mode microplate inspection system (PerkinElmer). At the meantime, the steady-state kinetic analysis was performed in acetic acid/sodium acetate buffer solution (pH 6.5) with TMB (1.5 mg/ml), H2O2 (1, 2, 3, 4, 5, 20, 50, 100 mM) and Co-SAEs/HNCS (75 μg/ml). The initial catalytic rate was estimated through the absorbance change by Beer-Lambert Law (5) (ε: 39000 M-1 cm-1 for oxTMB).
The rate was graphed by the concentration of H2O2 and fitted with the Michaelis-Menten curves (6). [S] represents the substrate content. Finally, the Michaelis-Menten curves were subjected to nonlinear regression analysis utilizing GraphPad Prism version 8.2.1 to determine the kinetic parameters Km and Vmax.
For ·OH enhancement in NIR response, OPDA was added to the HNCS (20 μg/ml) or Co-SAEs/HNCS (20 μg/ml) in acetic acid/sodium acetate buffer solution (pH 6.5) with H2O2 (1 mM) exposing to irradiation or not (808 nm, 1 W/cm2, 10 mins). Then the absorbances of all samples were immediately recorded.
ESR was used to detect the types of reactive ROS produced by Co-SAEs/HNCS. TEMP, as a 1O2 electron capture agent, was added to the Co-SAEs/HNCS (20 μg/ml) sample with H2O2 (1 mM) after 10 mins of irradiation, and then the sample was analyzed by ESR. DMPO as a ·OH electron capture agent was added to the Co-SAEs/HNCS (20 μg/ml) sample with H2O2 (1 mM) after 10 mins of irradiation, and then the sample was analyzed by ESR.
The GSHOx-like activity of Co-SAEs/HNCS
For the Michaelis-Menten curves analysis, Co-SAEs/HNCS (500 μg/ml) in acetic acid/sodium acetate buffer (pH 6.5) was mixed with GSH (0.06, 0.18, 0.24, 0.6, 1 mM) under dark conditions for 0.5 min, and the DTNB (300 μg/ml) as a probe was added to the mixtures for 0.5 min, then the absorbances of color reaction at 412 nm was obtained by a multi-mode microplate inspection system. The GSH consumed in each sample was obtained by subtracting the remaining GSH from the total GSH. The Michaelis-Menten curves were subjected to nonlinear regression analysis utilizing GraphPad Prism version 8.2.1 to determine the kinetic parameters Km and Vmax through the Eqs. (5, 6) (ε: 13600 M−1 cm−1 for TNB). The expression of intracellular GSH was detected using the GSH detection kit method. Cal-33 cells were inoculated into a 6-well plate (3*105/well), and incubate each experimental group with HNCS, Co-SAEs/HNCS, and H2O2 for 24 hours before receiving or not receiving NIR. After 24 hours, the intracellular GSH expression was tested using a GSH detection kit.
Apoptosis and ferroptosis induced by Co-SAEs/HNCS
Biological transmission electron microscope (Bio-TEM) was used to detect the uptake of nanomaterials in cells. Cal-33 cells were inoculated into a 6-well plate (3*105/well) for 24 h. After changing the medium, each group was added with fresh medium, HNCS (20 μg/ml) or Co-SAEs/HNCS (20 μg/ml). After 24 h, each group was collected for Bio-TEM imaging.
To detect the intracellular 1O2, CLSM and FCM were used as qualitative and semi-quantitative analysis methods, respectively. Cal-33 cells were inoculated into CLSM dishes (2*105/dish) for 24 h. After changing the medium, each group was added with fresh medium, HNCS (20 μg/ml) or Co-SAEs/HNCS (20 μg/ml). A fresh medium containing DCFH-DA (10 μM) was replaced after 24 h. After 0.5 h, each group was replaced with PBS solution for three times and then received irradiation (808 nm, 1 W/cm2, 10 mins) or not. The cells of each group were finally observed by CLSM.
Similarly, Cal-33 cells were inoculated into a 6-well plate (2*105/well) for 24 h. After changing the medium, each group was added with fresh medium, HNCS (20 μg/ml) or Co-SAEs/HNCS (20 μg/ml). After A fresh medium containing DCFH-DA (10 μM) was replaced after 24 h. After 0.5 h, cells in each group were trypsinized and used for FCM immediately after receiving irradiation (808 nm, 1 W/cm2, 10 mins) or not.
CLSM and FCM were used to detect the level of intracellular mitochondrial depolarization. Cal-33 cells were inoculated into CLSM dishes (2*105/dish) for 24 h. After changing the medium, each group was added with fresh medium, HNCS (20 μg/ml) or Co-SAEs/HNCS (20 μg/ml). A fresh medium containing JC-1 (10 μM) was replaced after 24 h. After 0.5 h, each group was replaced with PBS solution for three times and then received irradiation (808 nm, 1 W/cm2, 10 mins) or not. The cells of each group were finally observed by CLSM. Similarly, Cal-33 cells were inoculated into a 6-well plate (2*105/well) for 24 h. After changing the medium, each group was added with fresh medium, HNCS (20 μg/ml) or Co-SAEs/HNCS (20 μg/ml). After 24 h, each group was replaced with PBS solution for three times and then received irradiation (808 nm, 1 W/cm2, 10 mins) or not. Then cells were trypsinized and replaced by PBS containing JC-1 (10 μM). After 0.5 h, cells in each group were used for FCM analysis.
In order to detect the production of OH and LPOs in cells, CLSM was used as a detection method. Cal-33 cells were inoculated into CLSM dishes (2*105/dish) for 24 h. After changing the medium, each group with H2O2 (100 μM) was added with fresh medium, HNCS (20 μg/ml) or Co-SAEs/HNCS (20 μg/ml). A fresh medium containing HPF (5 μM) was replaced after 24 h. After 0.5 h, each group was replaced with PBS solution for three times and then received irradiation (808 nm, 1 W/cm2, 10 mins) or not. The cells of each group were finally observed by CLSM.
Cal-33 cells were inoculated into CLSM dishes (2*105/dish) for 24 h. After changing the medium, each group with H2O2 (100 μM) was added with fresh medium, HNCS (20 μg/ml) or Co-SAEs/HNCS (20 μg/ml). A fresh medium containing C11-BODIPY581/591 (10 μM) was replaced after 24 h. After 0.5 h, each group was replaced with PBS solution for three times and then received irradiation (808 nm, 1 W/cm2, 10 mins) or not. The cells of each group were finally observed by CLSM.
In vitro anti-tumor effect induced by Co-SAEs/HNCS
To detect the apoptosis of Cal-33 cells, CCK-8, CLSM and FCM methods were used respectively. Cal-33 cells were inoculated into 96-well plates (0.5*104/well) for 24 h. After changing the medium, each group was added with fresh medium, HNCS (20 μg/ml) or Co-SAEs/HNCS (20 μg/ml) with H2O2 (100 μM), 24 h later, each group was replaced with PBS solution and then received irradiation (808 nm, 1 W/cm2, 10 mins) or not. Then each group was changed with a fresh medium, the CCK-8 method was used to detect the viability of cells after 24 h. The cytotoxicity detection method for HNCS was the same as above.
For the CLSM, Cal-33 cells were inoculated into CLSM dishes (2*105/dish) for 24 h. After changing the medium, each group was added with medium, HNCS (20 μg/ml) or Co-SAEs/HNCS (20 μg/ml) with H2O2 (100 μM). Each group was replaced with PBS solution after 24 h, and then received with receiving irradiation (808 nm, 1 W/cm2, 10 mins) or not. Then fresh medium containing FITC/PI (10 μM/ 5 μM) was replaced. 0.5 h later, each group was observed by CLSM.
For FCM, Cal-33 cells were inoculated into a 6-well plate (2*105/well) for 24 h. After changing the medium, each group was added with fresh medium, HNCS (20 μg/ml) or Co-SAEs/HNCS (20 μg/ml) with H2O2 (100 μM). After 24 h, each group was replaced with PBS solution and received irradiation (808 nm, 1 W/cm2, 10 mins) or not. Then cells were trypsinized and replaced by PBS containing FITC/PI (10 μM/ 5 μM). After 0.5 h, cells in each group were used for FCM analysis.
Western blot of GPX4 and HSP70
Cal-33 cells were inoculated into a 6-well plate (2*105/well) for 24 h. After changing the medium, each group was added with fresh medium, HNCS (20 μg/ml) or Co-SAEs/HNCS (20 μg/ml) with H2O2 (100 μM). After 24 h, each group was replaced with PBS solution and received irradiation (808 nm, 1 W/cm2, 10 mins) or not. After 24 hours, cells from each experimental group were collected into RIPA solution using a cell scraper. After ultrasonic lysis, they were centrifuged at 4 °C for 30 minutes at 12000 x g. The supernatant was collected and the protein concentration was detected using a BCA protein detection kit. The cells were heated to 100 °C for 5 minutes by adding a loading buffer. Prepare 10% protein gel. After loading the sample, apply 80 V voltage for 30 min, 120 V voltage for 1 h, and transfer the membrane at constant current of 200 mA for 1.5 h. TBST solution containing 5% skimmed milk powder was used to seal for 1 h, and TBST solution containing GPX4 primary antibody was added to incubate at 4 °C overnight, after three times of TBST film washing, TBST solution containing secondary antibody was added to incubate at room temperature for 1 h, then the protein membrane was replaced TBST film washing for three times, incubated with ECL kit for 3 min, and finally exposed in gel imaging system. The steps for detecting HSP70 protein were the same as above.
Detection of intracellular ATP expression levels
Cal-33 cells were inoculated into a 24-well plate (1.5*104/well) for 24 h. After changing the medium, each group was added with fresh medium, HNCS (20 μg/ml) or Co-SAEs/HNCS (20 μg/ml) with H2O2 (100 μM). After 24 h, each group was replaced with PBS solution and received irradiation (808 nm, 1 W/cm2, 10 mins) or not. After 24 hours, cells from each experimental group were collected and detected by ATP detection kit, all test samples are detected by the self luminous mode of the fully automatic multifunctional enzyme-linked immunosorbent assay reader.
Establishment of an animal model of orthotopic xenotransplantation of head and neck cancer
Balb/c nude mice about 6-8 weeks old were received the Cal-33 cells injection in the logarithmic growth phase, and about 1*105 cells were injected into the lateral edge of the anterior one-third of the tongue per mouse. The treatment began when the tumor grew to about 10 mm3.
Blood safety testing
The nude mice were divided into four groups: Normal+ PBS group, Normal+ Co-SAEs/HNCS group, Tumor+ PBS group, and Tumor+ Co-SAEs/HNCS group, with three mice in each group. The nude mice in the Tumor+PBS group and+Tumor+ Co-SAEs/HNCS group were tumor bearing in the aforementioned manner. After approximately 10mm3 of tumor formation, local injection of PBS or Co-SAEs/HNCS solution was performed, and all experimental animals were euthanized seven days later. Whole blood was collected and blood routine indicators were detected using Sysmex XN-1000V fully automated animal blood analyzer. Serum was collected and blood biochemical indicators were detected using Roche diagnostics gmbh fully automated biochemical analyzer cobas 6000 c 501.
In vivo photothermal imaging
Tumor-bearing nude mice were locally injected with PBS, HNCS, Co-SAEs/HNCS (1 mg/kg) respectively, and received irradiation (808 nm, 1 W/cm2, 10 mins) or not after 4 hours. At the same time, infrared thermal imagers were used to monitor the temperature changes of the nude mice’s tumor surface in real time.
In vivo anti-tumor activity of Co-SAEs/HNCS
All balb/c nude mice with tumors growing to about 10 mm3 were randomly divided into 6 groups, each with 5 mice (n = 5). They were locally injected with PBS, HNCS, Co-SAEs/HNCS (1 mg/kg) respectively, and received irradiation (808 nm, 1 W/cm2, 10 mins) or not after 4 hours. Tumor volume and nude mouse body weight were recorded every two days. Images of tumor-bearing mice on day 8, day 14 and day 20 in the treatment cycle were recorded by digital photos. All tumor-bearing nude mice that died naturally during the observation period were collected with tumor and visceral tissues and fixed in formalin for preservation. After 24 days, the rest tumor-bearing nude mice were sacrificed. The tumor and important organ tissues were collected, fixed.
Tissue analysis
The tumor and important organ tissues were dehydrated, sectioned and stained for hematoxylin-eosin staining, then photographed by fluorescence microscope. The tumor tissue sections from different groups were stained with immunohistochemical ki67, GPX4 and HSP70 antibodies, then photographed by fluorescence microscope.
Statistical analysis
All the experimental data were shown as the average value ± SD. The number of duplicate samples in the in vitro experiment section is three (n = 3), and the number of duplicate samples in the in vivo experiment section is five or three (n = 5/ n = 3). The significance of the differences was obtained via t tests or One-way ANOVA statistical method. Data processing and image generation were conducted using Origin 2021 and GraphPad Prism 8 software.
Computation Details
DFT calculations were performed using the Vienna ab initio Simulations Package (VASP.5.4.4.)59 with the frozen-core all-electron projector augmented wave (PAW) model60 and Perdew-Burke-Ernzerhof (PBE) functional61. A kinetic energy cutoff of 400 eV was used for the plane-wave expansion of the electronic wave functions, and the convergence criteria of force and energy were set to 0.01 eV/Å and 10-5 eV respectively. Gaussian smearing of 0.1 eV was applied for optimizations and a k-point grid with a 2 × 2 × 1 Gamma centered mesh was chosen for 6 × 6 × 1 graphene supercell to sample the first Brillouin zone. The GGA + U method was chosen to describe partially filled 3 d orbitals62 with an effective U value of 3.42 eV for Co, which gave reasonable predictions of geometric and electronic structures based on previous reports35,59. The HSE06 functional was utilized to obtain a more accurate prediction of bandgap.60The DFT-D3 correction was used to account for the van der Waals (vdW) interactions63.
For each elementary step of the Fenton reaction, the Gibbs reaction free energy (ΔG) is defined as the difference between free energies of the initial and final states and is given by the expression:
\(\Delta G=\,\Delta E+\Delta {ZPE}-T\Delta S+\Delta {G}_{U}+\,\Delta {G}_{{PH}}\,\)where ΔE is the reaction energy of reactant and product molecules adsorbed on the catalyst surface, which can be obtained from DFT calculations; ΔZPE and ΔS are the change in zero-point energies and entropy at 298 K. The bias effect on the free energy of each initial, intermediate, and final state involving an electron in the electrode is considered by shifting the energy of the state by \(\Delta {G}_{U}=-n{e}_{0}U\), where n is the number of proton-electron pairs transferred. The effect of basic/acidic conditions (pH effect) on thermodynamics is described by \(\Delta {G}_{{pH}}=-{k}_{B}T {ln}\left[{H}^{+}\right]={pH}\times {k}_{B}T{\mathrm{ln}}10\).
The free energy profile of 1O2 formation on Co-N4 site in the neutral condition can be calculated by the following equations:
The free energy profile with the presence of photogenerated electron and hole is computed by:
where *, (*+1 h+), and (*+1e-) denote the neutral substrate, substrate with an extra hole and electron, respectively. We note that while the plane-wave method in VASP cannot give a convincing energy value for O2, the difference in electronic energy between 1O2 and 3O2 (1.07 eV) computed by VASP is in close agreement with the experimental value (0.98 eV)61,62 This indicates that the inaccuracies in the VASP calculations for 1O2 and 3O2 largely cancel each other out when calculating the energy profiles.
where G(H+) + G(e-) = 1/2 G(H2) at U = 0, according to the CHE (computational hydrogen electrode) model63,64,65.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data generated or analyzed during this study are included in this published article and its Supplementary Information file and the Source Data file. Source data are provided with this paper. The full image dataset is available from the corresponding author upon request. Source data are provided with this paper.
References
Mody, M. D., Rocco, J. W., Yom, S. S., Haddad, R. I. & Saba, N. F. Head and neck cancer. Lancet 398, 2289–2299 (2021).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2020. Ca-Cancer J. Clin. 70, 7–30 (2020).
Chinn, S. B. & Myers, J. N. Oral cavity carcinoma: current management, controversies, and future directions. J Clin Oncol 33, 3269–3276 (2015).
Inhestern, J. et al. A two-arm multicenter phase II trial of one cycle chemoselection split-dose docetaxel, cisplatin and 5-fluorouracil (TPF) induction chemotherapy before two cycles of split TPF followed by curative surgery combined with postoperative radiotherapy in patients with locally advanced oral and oropharyngeal squamous cell cancer (TISOC-1). Ann Oncol 28, 1917–1922 (2017).
Su, J. et al. Engineered protein photo-thermal hydrogels for outstanding in situ tongue cancer therapy. Adv. Mater. 33, e2100619 (2021).
Zheng, Q. et al. The recent progress on metal-organic frameworks for phototherapy. Chem. Soc. Rev. 50, 5086–5125 (2021).
Huang, C. et al. In-vitro and in-vivo photocatalytic cancer therapy with biocompatible iridium(iii) photocatalysts. Angew. Chem. Int. Ed. 60, 9474–9479 (2021).
Yu, Y. et al. Biodegradable polymer with effective near-infrared-ii absorption as a photothermal agent for deep tumor therapy. Adv. Mater. 34, e2105976 (2022).
Dai, H. et al. Tumor-targeted biomimetic nanoplatform precisely integrate photodynamic therapy and autophagy inhibiton for collaborative treatment of oral cancer. Biomater. Sci. 10, 1456–1469 (2022).
Ethirajan, M., Chen, Y., Joshi, P. & Pandey, R. K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 40, 340–362 (2011).
Sibuyi, N. R. S. et al. Multifunctional gold nanoparticles for improved diagnostic and therapeutic applications: a review. Nanoscale Res. Lett. 16, 174 (2021).
Lamch, L., Kulbacka, J., Dubinska-Magiera, M., Saczko, J. & Wilk, K. A. Folate-directed zinc (II) phthalocyanine loaded polymeric micelles engineered to generate reactive oxygen species for efficacious photodynamic therapy of cancer. Photodiagn. Photodyn. Ther. 25, 480–491 (2019).
Liang, X. et al. Complementing cancer photodynamic therapy with ferroptosis through iron oxide loaded porphyrin-grafted lipid nanoparticles. ACS Nano 15, 20164–20180 (2021).
Li, J. et al. A facile strategy of boosting photothermal conversion efficiency through state transformation for cancer therapy. Adv. Mater. 33, e2105999 (2021).
Zhou, R., Ohulchanskyy, T. Y., Xu, H., Ziniuk, R. & Qu, J. Catalase nanocrystals loaded with methylene blue as oxygen self-supplied, imaging-guided platform for photodynamic therapy of hypoxic tumors. Small 17, e2103569 (2021).
Yang, Y. et al. 1D Coordination polymer nanofibers for low-temperature photothermal therapy. Adv. Mater. 29, 40 (2017).
Yang, H. et al. A photoresponsive nanozyme for synergistic catalytic therapy and dual phototherapy. Small 17, e2007090 (2021).
Sheng, D. et al. Perfluorooctyl bromide & indocyanine green co-loaded nanoliposomes for enhanced multimodal imaging-guided phototherapy. Biomaterials 165, 1–13 (2018).
Liu, J. et al. Tumor hypoxia-activated combinatorial nanomedicine triggers systemic antitumor immunity to effectively eradicate advanced breast cancer. Biomaterials 273, 120847 (2021).
Cheng, Y. J., Hu, J. J., Qin, S. Y., Zhang, A. Q. & Zhang, X. Z. Recent advances in functional mesoporous silica-based nanoplatforms for combinational photo-chemotherapy of cancer. Biomaterials 232, 119738 (2020).
Bai, Y. et al. Carbon dots with absorption red-shifting for two-photon fluorescence imaging of tumor tissue pH and synergistic phototherapy. ACS Appl. Mater. Interfaces 13, 35365–35375 (2021).
You, Q. et al. Persistent regulation of tumor hypoxia microenvironment via a bioinspired Pt-Based oxygen nanogenerator for multimodal imaging-guided synergistic phototherapy. Adv. Sci. 7, 1903341 (2020).
Su, Y. et al. Dual enzyme-mimic nanozyme based on single-atom construction strategy for photothermal-augmented nanocatalytic therapy in the second near-infrared biowindow. Biomaterials 281, 121325 (2021).
Zhang, J. et al. Cluster nanozymes with optimized reactivity and utilization of active sites for effective peroxidase (and Oxidase) mimicking. Small. 15, e2104844 (2021).
Cao, F. et al. An enzyme-mimicking single-atom catalyst as an efficient multiple reactive oxygen and nitrogen species scavenger for sepsis management. Angew. Chem. Int. Ed. 59, 5108–5115 (2020).
Zhang, Y. et al. Nanozyme decorated metal-organic frameworks for enhanced photodynamic therapy. ACS Nano 12, 651–661 (2018).
Jiao, L. et al. Au@Pt nanodendrites enhanced multimodal enzyme-linked immunosorbent assay. Nanoscale 11, 8798–8802 (2019).
Zhao, Y. et al. A nanozyme- and ambient light-based smartphone platform for simultaneous detection of dual biomarkers from exposure to organophosphorus pesticides. Anal. Chem. 90, 7391–7398 (2018).
Huo, M., Wang, L., Wang, Y., Chen, Y. & Shi, J. Nanocatalytic tumor therapy by single-atom catalysts. ACS Nano 13, 2643–2653 (2019).
Lu, X. et al. Bioinspired copper single-atom catalysts for tumor parallel catalytic therapy. Adv Mater 32, e2002246 (2020).
Xiong, Y. et al. Cobalt single atom site catalysts with ultrahigh metal loading for enhanced aerobic oxidation of ethylbenzene. Nano Res 14, 2418–2423 (2021).
Zhu, P., Xiong, X. & Wang, D. Regulations of active moiety in single atom catalysts for electrochemical hydrogen evolution reaction. Nano Res 15, 5792–5815 (2022).
Zheng, X., Li, B., Wang, Q., Wang, D. & Li, Y. Emerging low-nuclearity supported metal catalysts with atomic level precision for efficient heterogeneous catalysis. Nano Res. 15, 7806–7839 (2022).
Li, R. & Wang, D. Understanding the structure-performance relationship of active sites at atomic scale. Nano Res 15, 6888–6923 (2022).
Han, A. et al. An adjacent atomic platinum site enables single-atom iron with high oxygen reduction reaction performance. Angew. Chem. Int. Ed. 60, 19262–19271 (2021).
Zhang, J., Yin, R., Shao, Q., Zhu, T. & Huang, X. Oxygen vacancies in amorphous InO(x) nanoribbons enhance CO(2) adsorption and activation for CO(2) electroreduction. Angew Chem Int Ed Engl 58, 5609–5613 (2019).
Geng, Z. et al. Oxygen vacancies in ZnO nanosheets enhance CO(2) electrochemical reduction to CO. Angew Chem Int Ed Engl 57, 6054–6059 (2018).
Han, Y. et al. Hollow N-doped carbon spheres with isolated cobalt single atomic sites: superior electrocatalysts for oxygen reduction. J. Am. Chem. Soc. 139, 17269–17272 (2017).
Chen, Y. et al. Atomic-level modulation of electronic density of metal-organic frameworks-derived Co single-atom sites to enhance oxygen reduction performance. Angew Chem. Int. Ed. 60, 3212–3221 (2021).
Han, X. et al. Atomically dispersed binary Co-Ni sites in nitrogen-doped hollow carbon nanocubes for reversible oxygen reduction and evolution. Adv. Mater. 31, 1905622 (2019).
Wang, S. et al. Metal-organic-framework-derived mesoporous carbon nanospheres containing porphyrin-like metal centers for conformal phototherapy. Adv. Mater. 28, 8379–8387 (2016).
Zhang, D. Y. et al. Cobalt carbide-based theranostic agents for in vivo multimodal imaging guided photothermal therapy. Nanoscale 12, 7174–7179 (2020).
Yu, Y. et al. Bortezomib-encapsulated CuS/Carbon dot nanocomposites for enhanced photothermal therapy via stabilization of polyubiquitinated substrates in the proteasomal degradation pathway. ACS Nano 14, 10688–10703 (2020).
Gao, L. et al. Plasmon-mediated generation of reactive oxygen species from near-infrared light excited gold nanocages for photodynamic therapy in vitro. ACS Nano 8, 7260–7271 (2014).
Wang, L. et al. Engineering single-atomic iron-catalyst-integrated 3d-printed bioscaffolds for osteosarcoma destruction with antibacterial and bone defect regeneration bioactivity. Adv. Mater. 33, e2100150 (2021).
Cao, F. et al. Self-adaptive single-atom catalyst boosting selective ferroptosis in tumor cells. ACS Nano 16, 855–868 (2022).
He, H. et al. Bioadhesive injectable hydrogel with phenolic carbon quantum dot supported Pd single atom nanozymes as a localized immunomodulation niche for cancer catalytic immunotherapy. Biomaterials 280, 121272 (2022).
Lin, Y., Wang, F., Yu, J., Zhang, X. & Lu, G. P. Iron single-atom anchored N-doped carbon as a ‘laccase-like’ nanozyme for the degradation and detection of phenolic pollutants and adrenaline. J. Hazard. Mater. 425, 127763 (2022).
Chen, Y. et al. CRISPR screens uncover protective effect of PSTK as a regulator of chemotherapy-induced ferroptosis in hepatocellular carcinoma. Mol. Cancer 21, 11 (2022).
Bai, S. et al. Nanotransferrin-based programmable catalysis mediates three-pronged induction of oxidative stress to enhance cancer immunotherapy. ACS Nano 16, 997–1012 (2021).
Peng, S. Y. et al. Harnessing in situ glutathione for effective ROS generation and tumor suppression via nanohybrid-mediated catabolism dynamic therapy. Biomaterials 281, 121358 (2021).
Su, X. et al. Localized disruption of redox homeostasis boosting ferroptosis of tumor by hydrogel delivery system. Mater. Today Bio 12, 100154 (2021).
Niu, R. et al. Programmed Targeting Pyruvate Metabolism Therapy Amplified Single-Atom Nanozyme-Activated Pyroptosis for Immunotherapy. Adv Mater 36, e2312124 (2024).
Wang, L. et al. Exploiting single atom iron centers in a porphyrin-like MOF for efficient cancer phototherapy. ACS Appl. Mater. Interfaces 11, 35228–35237 (2019).
Chang, M. et al. Single-atom pd nanozyme for ferroptosis-boosted mild-temperature photothermal therapy. Angew. Chem. Int. Ed. 60, 12971–12979 (2021).
He, S., Liu, J., Zhang, C., Wang, J. & Pu, K. Semiconducting polymer nano-regulators with cascading activation for photodynamic cancer immunotherapy. Angew. Chem. Int. Ed. 61, e202116669 (2021).
Dong, P. et al. A mitochondrial oxidative stress amplifier to overcome hypoxia resistance for enhanced photodynamic therapy. Small 5, e2100581 (2021).
Li, Y. et al. Trojan horse-like nano-AIE aggregates based on homologous targeting strategy and their photodynamic therapy in anticancer application. Adv. Sci. 8, e2102561 (2021).
Xu, H., Cheng, D., Cao, D. & Zeng, X. C. A universal principle for a rational design of single-atom electrocatalysts. Nat. Catal. 1, 339–348 (2018).
Krukau, A. V., Vydrov, O. A., Izmaylov, A. F. & Scuseria, G. E. Influence of the exchange screening parameter on the performance of screened hybrid functionals. J. Chem. Phys. 125, 224106 (2006).
Schweitzer, C. & Schmidt, R. Physical mechanisms of generation and deactivation of singlet oxygen. Chem. Rev. 103, 1685–1758 (2003).
ATKINS’PHYSICALCHEMISTRY. Great Britain by Oxford University Press: (Oxford, 2006).
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 355, eaad4998 (2017).
Kulkarni, A., Siahrostami, S., Patel, A. & Nørskov, J. K. Understanding catalytic activity trends in the oxygen reduction reaction. Chem. Rev. 118, 2302–2312 (2018).
Li, X. et al. Exceeding the volcano relationship in oxygen reduction/evolution reactions using single-atom-based catalysts with dual-active-sites. J. Mater. Chem. A 8, 10193–10198 (2020).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22202214-A. H., 22171157-D. W., 51972003-Y.W.) and the Natural Science Foundation of Liaoning Province-Outstanding Youth Foundation (2022-YQ-02-A. H.). We thank the BL11B station in Shanghai Synchrotron Radiation Facility (SSRF).
Author information
Authors and Affiliations
Contributions
These authors contributed equally: H.D., A.H., X.W., H.D. is mainly responsible for the design and completion of in vivo and in vitro biological experiments and article writing. A.H. is mainly responsible for the synthesis and analyzing the structure of the as-prepared materials, and article writing. X.W. is mainly responsible for theoretical calculations and model analysis of material catalysis. P.Z. mainly participated in the analyzing the structure of the as-prepared materials. Y.W. and D.W. are mainly responsible for designing topics and experiments, guiding experimental plans, and writing articles.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Mukund Seshadri, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dai, H., Han, A., Wang, X. et al. NIR-triggering cobalt single-atom enzyme switches off-to-on for boosting the interactive dynamic effects of multimodal phototherapy. Nat Commun 16, 2058 (2025). https://doi.org/10.1038/s41467-025-57188-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-57188-9
This article is cited by
-
Innovative Strategies to Overcome Stability Challenges of Single-Atom Nanozymes
Nano-Micro Letters (2026)