Abstract
Increasing attention has been paid to silacyclobutanes because of their wide application in ring opening and ring extension reactions. However, the synthesis of functionalized silacyclobutanes remains an unmet challenge because of the limited functional group tolerance of the reactions with organometallic reagents and chlorosilacyclobutanes. Herein, we report a conceptually different solution to this end through a visible-light-induced metal-free hydrosilylation of unactivated alkenes with hydrosilacyclobutanes. A wide range of unactivated alkenes with diverse functional groups including the base-sensitive acid, alcohol and ketones participated in this reaction smoothly. In particular, the first hydrosilylation reaction of alkenes with dihydrosilacyclobutane provides a facile access to various functionalized alkyl monohydrosilacyclobutanes. Unsymmetrical dialkyl silacyclobutanes have also been synthesized through consecutive hydrosilylation with dihydrosilacyclobutane in one pot. The mechanism study reveals that the Lewis basic solvent could promote the generation of strained silyl radicals by direct light irradiation without a redox-active photocatalyst and the thiol catalyst plays an important role in accelerating the reaction.
Similar content being viewed by others
Introduction
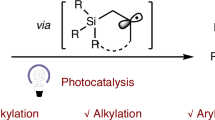
Organosilicon compounds are of great value in organic synthesis, medicinal chemistry and material science1,2,3. As a kind of important organosilicon reagents, silacyclobutanes (SCBs) have attracted much attention in recent years4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33. Taking advantage of the high ring tension (strain energy: 24.5 kcal/mol)8, diverse ring opening and ring extension reactions of SCBs have been developed to synthesize a wide range of important organosilicon compounds (Fig. 1a)4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32. Although SCBs have emerged as lynchpins in the synthesis of organosilicon compounds, the synthesis of functionalized SCBs is challenging. Traditional synthesis of 1,1-disubstituted SCBs relied on the nucleophilic substitution reaction of 1-chlorine-substituted silacyclobutanes with organometallic reagents (Fig. 1b)4,5,6,7,8,9,10,11. Most of the substituents introduced by this method are simple aryl groups and non-functionalized alkyl groups, such as ethyl and n-butyl, because of the limited functional group tolerance of the basic and nucleophilic organometallic reagents. Recently, Zhao and coworkers reported a seminal method for the synthesis of SCBs through a Ni-catalyzed reductive coupling reaction between 1-chloro-substituted silacyclobutanes and aryl/vinyl halides/pseudo-halides, which bypassed the pre-synthesis of organometallic reagents (Fig. 1b)33. However, the corresponding reactions with alkyl halides/pseudo-halides were not successful, and the 1-chloro-substituted silacyclobutanes used in these reactions still need to be synthesized from organometallic reagents. To the best of our knowledge, there is no general method for the synthesis of alkyl SCBs with broad functional group diversities. As a step- and atom-economical approach to synthesizing organosilicon compounds, hydrosilylation of alkenes has been extensively studied34,35,36,37,38,39,40,41,42,43,44,45,46. The most widely studied olefin hydrosilylation reactions are based on transition-metal catalysis1,34,35, but recent visible-light-induced photocatalytic reactions through the generation of silyl radicals provided a conceptually different approach for organosilicon compounds synthesis (Fig. 1c)36,37,38,39,40,41,42,43,44,45,46. In particular, the employment of organophotocatalyst to induce the generation of silyl radicals bypassed the intermediacy of transition-metals37,38,39,40,41,42. Despite these advances, photocatalytic hydrosilylation reactions are still limited to non-cyclic hydrosilanes (Fig. 1c). The radical hydrosilylation with HSCBs is more challenging because the strained rings could lead to problematic C–Si bond cleavage side reactions.
a Silacyclobutanes are important lynchpin intermediates for the synthesis of value-added organosilicon compounds through ring-opening and ring-expansion reactions. b Previous methods for the synthesis of silacyclobutanes relied on the nucleophilic substitution reactions with organometallic reactions and transition-metal catalyzed reductive coupling reactions, but they are not applicable in the synthesis of functionalized alkyl SCBs. c Previous photocatalytic radical hydrosilylation of alkenes was limited to linear hydrosilanes, and the radical hydrosilylation with hydrosilacyclobutanes is more challenging because of the labile C–Si bonds of the strained four-membered ring compounds. d This study: photo-induced metal-free radical hydrosilylation of alkenes with broad substrate scope and excellent functional group tolerance, in the absence of any redox-active photocatalyst.
In this work, we report a unique photo-induced metal-free radical hydrosilylation of unactivated alkenes without redox active photocatalyst, which avoids the photocatalyst-induced decomposition of SCBs (Fig. 1d). The practical method significantly broadens the diversity of SCBs, enhancing the research of using SCBs as lynchpins in the synthesis of organosilicon compounds. In particular, the first successful application of dihydrosilacyclobutane (DHSCB) in hydrosilylation reactions not only provides a general synthesis of functionalized alkyl monohydrosilacyclobutanes (MHSCBs) but also provides a facile synthesis of unsymmetrical dialkyl SCBs through sequential hydrosilylation in one pot.
Results
Reaction development
Since the ketone-containing 1,1-disubstituted SCB 3a cannot be prepared by previous methods employing organometallic reagents or through the transition-metal-catalyzed reactions, MHSCB 1a and alkene 2a were chosen as model substrates to test the idea of hydrosilylation of alkenes with MHSCBs to prepare SCBs (Table 1). Inspired by Wu’s seminal work37, we first tested the employment of 5 mol% of eosin Y as the photocatalyst in the hydrosilylation reaction between 1a (1.0 equiv.) and 2a (1.0 equiv.), in the presence of 10 mol% of i-Pr3SiSH as the co-catalyst, under 460 nm in 1,4-dioxane, but only 36% yield of 3a was observed by 1H NMR, and significant decomposition of MHSCB 1a was observed (Table 1, entry 1). Replacement of neutral eosin Y with eosin Y-Na as the photocatalyst did not improve the reaction (100% conversion of 1a, 38% yield of 3a, Table 1, entry 2). Fortunately, when the catalyst was changed to TBADT or benzophenone and the light was changed to 390 from 460 nm, the yield of 3a was significantly improved (64%, Table 1, entry 3; 85%, Table 1, entry 4). However, the control experiment showed that 85% yield of 3a could also be obtained under 390 nm without a photocatalyst, albeit the corresponding reactions at 420 and 460 nm led to significantly decreased yield (Table 1, entries 5–7). The reaction did not proceed without light irradiation (Table 1, entry 8). Further investigation of the reaction conditions without adding a photocatalyst by changing the solvent revealed that THF was the best solvent (Table 1, entries 9–13). The less sterically hindered THF is better than the more sterically hindered Et2O and MeOtBu (Table 1, entries 9–11). The use of less coordinating PhMe as the solvent led to only a 2% conversion of 1a. It is known that the Lewis acidity of SCBs (Denmark’s strain release Lewis acidity) is higher than that of linear silanes27,47,48,49,50. The above results indicate that the coordination of solvent to the Lewis acidic SCBs could promote the hydrosilylation reaction. However, the reaction in CH2Cl2 resulted in a 54% conversion of 1a, but only a 2% yield of 3a was obtained because of the competing chlorine atom abstraction reaction by the silyl radical from CH2Cl2 (Table 1, entry 13). The chloro derivative of 1a was observed by Liu et al.29 Si NMR and quenched by MeMgBr, affording 1-methyl-1-(p-tolyl)siletane 1a-1 in 63% yield (see Supplementary Fig. 3). With THF as the solvent, slightly increasing the amount of 1a to 1.2 equivalents and extending the reaction time to 36 h result in the optimal conditions for the preparation of 3a (95% yield, Table 1, entry 14).
Substrate scope exploration
With the optimized conditions, we examined the scope of unactivated alkenes 2. As shown in Fig. 2, mono-substituted alkenes bearing various functional groups, including ketone, free alcohol, carboxylic acid, carboxylic ester, aldehyde, nitrile, chloride, SiMe3, B(Pin), ether and epoxide, were all well tolerated, giving the corresponding SCBs 3a−3m in 61−96% yields. Selective hydrosilylation of the terminal alkene in the presence of an internal alkene was achieved without altering the reaction conditions, and compound 3n was isolated in 57% yield. If a diene containing two terminal alkenes was employed, decreasing the amount of MHSCB 1a to 1.0 equivalent was needed to suppress the bis-silylation side reaction, and compound 3o was isolated in 64% yield. When the amount of 1a was increased to 2.4 equivalents, a successful bis-silylation was achieved, affording compound 3p in 74% yield. Moreover, electron-rich vinyl ethers, vinyl ester, and vinyl sulfide could also participate in the reactions to produce products 3q–3t in 61–95% yields. Vinyl amides and vinyl carbazole also worked well in the reactions with MHSCB 1a, affording the corresponding hydrosilylation products 3u–3x in 77–92% yields. In addition, aryl hydrosilacyclobutanes possessing different substituents (F, t-Bu, NMe2) on the aromatic rings also participated in the reaction smoothly to generate 3y–3ab in 77–93% yields. It is worth noting that the 1,1-disubstituted alkene was well accommodated and compound 3ac was isolated in 77% yield with excellent anti-Markovnikov regioselectivity. Mono-substituted alkenes derived from menthol and DHEA could also undergo the hydrosilylation smoothly to afford products 3ad and 3ae in 74% yield and 75% yield, respectively.
After achieving the efficient synthesis of 1,1-disubstituted SCBs with MHSCBs, we subsequently studied the selective synthesis of alkyl MHSCBs through radical hydrosilylation reaction with DHSCB (1f), because alkyl MHSCBs that contain functional groups are also difficult to synthesize by previous methods (Fig. 3). Compound 1f is a gas that has not been explored in synthetic chemistry, probably because of its difficulty to access51,52,53,54,55. We first developed an efficient synthesis of 1f with 77% yield that can be stored as a stock solution in Et2O through the reduction of commercially available 1,1-dichlorosiletane with LiAlH4 (for details, see Supporting Information). With compound 1f as the reaction partner, aliphatic alkenes bearing various functionalized groups, including Ph, CO2H, Cl, OH, OAc, OCOPh, and Bpin, worked well, giving the corresponding MHSCBs 3af−3al in 72−91% yields. Vinyl carbazole was also well tolerated in this reaction, giving the product 3am in 75% yield. The alkene derived from l-menthol also reacted well with dihydrosilacyclobutane 1f, affording product 3an an 86% yield. Diene 2g, which contains two terminal double bonds participated in this reaction smoothly, and bis-silylation product 3ao was isolated in 77% yield. The reactions with internal alkene such as (Z)-cyclooctene and 1,1-disubstituted alkene such as (3-methylbut-3-en-1-yl)benzene worked well, giving corresponding hydrosilylation products 3ap and 3aq in 50% and 67% yield, respectively.
a1f (0.76 mmol, 0.30–0.4 M in Et2O), 2 (0.5 mmol), i-Pr3SiSH (10 mol%), THF (1 mL), 390 nm (6 W), 36–48 h. b1f (1.52 mmol, 0.30–0.4 M in Et2O), 2o (0.5 mmol), i-Pr3SiSH (10 mol%), THF (1 mL), 390 nm (6 W), 60 h. c1f (1.14 mmol, 0.30–0.4 M in Et2O), 2 (0.5 mmol), i-Pr3SiSH (10 mol%), THF (1 mL), 390 nm (6 W), 48 h. d1f (1.14 mmol, 0.30–0.4 M in Et2O), 2 (0.75 mmol), i-Pr3SiSH (10 mol%), THF (1 mL), 390 nm (6 W), 48 h; then the mixture was concentrated under vacuum and 2’ (0.5 mmol) and THF (2 mL) was added and the resulting mixture was stirred under 390 nm (6 W) for 48 h. e1f (1.52 mmol, 0.30–0.4 M in Et2O), 2 (1 mmol), i-Pr3SiSH (10 mol%), THF (1 mL), 390 nm (6 W), 48 h; then the mixture was concentrated under vacuo and 2’ (0.5 mmol), i-Pr3SiSH (5 mol%) and THF (2 mL) was added and the resulting mixture was stirred under 390 nm (6 W) for 48 h.
Encouraged by the above results, we then studied the synthesis of unsymmetrical dialkyl SCBs through sequential hydrosilylation of alkenes in one pot (Fig. 3). Reagent 1f is volatile, and the excess amount of 1f can be easily removed from the reaction mixture, thus no column purification of the first hydrosilylation product is needed, and the reaction mixture can be directly used in the next hydrosilylation process after removal of 1f under vacuum. Under the simple procedures, various unsymmetrical dialkyl SCBs have been prepared. Alkenes bearing various functional groups, including carboxylic ester, Cl, OH, Bpin, CN, and carbazole were all well tolerated, giving the corresponding products 4aa−4ag in 68−86% yields. Moreover, the one-pot consecutive hydrosilylation reaction could also be applied in the late-stage functionalization of complex alkenes, affording the derivatives from l-menthol, Citronellol, Cholesterol, and DHEA (Dehydroepiandrosterone) in synthetically useful yields (4ah–4ak, 43–75% yields).
Synthetic applications
To show the synthetic potential of this method, we scaled up the reaction to 5 mmol scale, and SCB 3s was isolated in 83% yield (1.087 g, Fig. 4a). Thanks to the ring tension, silacyclobutanes could undergo diverse ring opening or ring expansion reactions to access a wide variety of functionalized organosilicon compounds. For example, 3s could undergo a ring-opening reaction with styrene to give E-alkenylsilane 5 in 86% yield through the Ni-catalyzed reaction12. In the presence of a Pd-catalyst, ethyl buta-2,3-dienoate underwent [4 + 2] cycloaddition with SCB 3s, affording 2-(E)-enoate-substituted silacyclohexene 6 in 85% yield23. The Rh-catalyzed intermolecular C(sp)–H bond silylation reaction of a terminal alkyne with compound 3s also performed well, giving alkynylsilane 7 in 80% yield56. Treatment of 3s with diphenyl acetylene under Ni-catalyzed conditions afforded silacene 8 in 73% yield57. Moreover, the reaction of compound 3af with MeOH in the presence of an N-heterocyclic carbene catalyst afforded siloxane 9 in 73% yield58. In addition, the hydrosilylation of activated alkenes with MHSCBs was also achieved, affording unsymmetrical dialkyl silacyclobutanes 10a–10c in 55–81% yields, in the presence of 4CzIPN (2,4,5,6-Tetra(9H-carbazol-9-yl)isophthalonitrile) as the photocatalyst.
Mechanistic studies
In order to shine some light on the mechanism of this reaction, several control experiments have been conducted. Under standard conditions, non-strained silacyclopentane (1g) and acyclic Et2SiH2 have been tried, both the conversion of 2h and the yield of product decreased compared with the reaction of 1f. Moreover, the reaction with Et3SiH was much slower, affording compound 13 in only 5% yield. These results indicate that the existence of ring leads to higher reactivity of HSCB (Fig. 5a) Under the standard conditions, we found that the product 3h could be detected without the addition of thiol catalyst, albeit the reaction was slow and only 11% yield was obtained after 120h, indicating that silyl radical could be generated by direct light irradiation of 1a (Fig. 5b). Alkene 2ah was then used to conduct a radical clock experiment. When the reaction was performed under the standard conditions, compound 14 was isolated in 41% yield, and compound 15 was isolated in 81% yield (Fig. 5c), supporting the generation of a silyl radical and a thiyl radical in the reaction. Without i-PrSiSH, compound 14 was generated in 5% yield. The reaction of i-PrSiSH and 2ah, in the absence of 1a, afforded compound 15 an 85% yield. Furthermore, EPR experiments were performed to investigate the generation of silyl radical intermediate by light irradiation of 1a. With the addition of radical spin trapping reagent DMPO (5,5-dimethyl-1-pyrroline-N-oxide), a mixture signal of possible silyl radicals (g = 2.0076, AN = 14.30 G, AH = 20.60 G), carbon radicals (g = 2.0076, AN = 13.90 G, AH = 18.0 G) and hydrogenated DMPO (g = 2.0076, AN = 14.50 G, AH1 = 18.80 G, AH2 = 18.80 G) were identified (Fig. 5d)59. EPR studies of i-Pr3SiSH and the reaction system were also conducted (for details, see Supplementary Fig. 4). These results provided strong evidence for the radical process of this reaction. To investigate the possible coordination between THF and HSCB, we conducted the NMR experiments, and the obvious different chemical shift of the Si–H in THF-d8 and toluene-d8 support the coordination of 1a with THF (for details, see Supplementary Figs. 5 and 6). However, we cannot rule out the stabilization of radical intermediate by polar solvent THF in facilitating the reaction. Subsequently, we employed density functional theory (DFT) to calculate the bond dissociation energies (BDEs) of the X–H bonds in compounds 1b, 1f, and the thiol. Figure 5e shows the BDEs with/without explicit THF. For compound 1b, the Si–H BDE is 85.1 kcal/mol with explicit THF and 86.4 kcal/mol with implicit THF. For compound 1f, the BDEs are 87.0 and 87.3 kcal/mol, respectively. For the S–H bond, the BDEs are 87.0 and 86.4 kcal/mol, respectively. Therefore, the presence of THF might affect the BDEs, notably for the Si–H bond in compound 1b (1.3 kcal/mol difference), albeit this energy difference might be in the error of the calculation method. Based on the above experimental results and literature reports37,38,39,60, a plausible reaction mechanism is proposed for the hydrosilylation of unactivated alkenes with HSCBs, which is also supported by DFT calculations (Fig. 5f). HSCBs are excited by light to form silyl radicals I, which then selectively add to the less sterically hindered site of the unactivated alkenes via the transition state TS-1 with an energy barrier of 10.2 kcal/mol, leading to carbon radicals II. The nucleophilic radicals II then undergo a polarity-matched hydrogen atom transfer (HAT) process via the transition state TS-2 to afford the hydrosilylation product, along with the formation of thiyl radical III, with a total activation free energy of 9.7 kcal/mol. Thiyl radical III could abstract the hydrogen atom from HSCBs to form silyl radicals I via transition state TS-3, with an activation free energy of 7.8 kcal/mol, while regenerating i-Pr3SiSH. We also considered the HAT process of nucleophilic radical II with the HSCB through transition state TS-4, which could also produce the hydrosilylation product. However, the activation free energy for this step is 17.3 kcal/mol, which allows us to rule out this process in the reaction in the presence of a thiol catalyst. However, for the initiation of the reaction, we cannot rule out the possibility of first generating thiyl radical III from the direct irradiation of i-Pr3SiSH followed by the HAT process to generate key intermediate I.
a Control experiments: non-strained cyclic and acyclic silanes under the standard conditions. “c.”: conversion; “y.”: yield. b Control experiment without i-Pr3SiSH. c Radical clock experiment. d EPR study of 1a. e The bond dissociation energies (ΔH) of Si/S–H bonds. f Proposed mechanism. The strained silyl radicals could be generated by direct irradiation and added to the alkene; the presence of the catalytic amount of i-Pr3SiSH could accelerate the reaction through polarity-matched hydrogen atom transfer. Calculations were performed at the M06-2X/6-311G (d, p)/SMD (THF) level of theory with (data shown outside the parentheses) and without (data shown inside the parentheses) explicit THF. “TS”: transition state.
In conclusion, we have developed a visible-light-induced Lewis basic solvent-promoted metal-free silylation of unactivated alkenes with hydrosilacyclobutanes. The strained-ring silicon-centered radicals could be directly generated under visible-light irradiation without a redox-active photocatalyst. The thiol catalyst plays an important role in accelerating the reaction. A wide range of unactivated alkenes with diverse functional groups participate in this reaction smoothly, which significantly broadened the diversity and scope of silacyclobutanes. This unique metal-free single-electron process offers distinct advantages to the previous metal-based two-electron processes for the synthesis of SCBs. We believe the successful applications of hydrosilacyclobutanes in radical reactions and the ability to prepare various previously unreached highly functionalized SCBs will significantly promote the development of organosilicon chemistry.
Methods
General procedure for hydrosilylation of unactivated alkenes with hydrosilacyclobutanes (HCBs)
In an argon-filled glovebox, to a flame-dried screw-cap reaction tube equipped with a magnetic stir bar were added i-Pr3SiSH (9.5 mg, 0.05 mmol, 10 mol%), hydrosilacyclobutanes (0.6 mmol, 1.2 equiv.), alkenes (0.5 mmol, 1 equiv.) and THF (2.0 mL) sequentially. The tube was sealed with a screw cap equipped with a septum, and removed from the glovebox. The reaction mixture was stirred at rt for 36 h under 6 W 390 nm LED lamps. The reaction mixture was concentrated under reduced pressure. The crude product was purified with column chromatography on silica gel to obtain the pure product.
Data availability
All data needed to support the conclusions of this manuscript are included in the main text or supplementary information. Source Data are provided with this paper. All data are available from the corresponding author upon request. Source data are provided with this paper.
References
Hiyama, T. & Oestreich, M. Organosilicon Chemistry: Novel Approaches and Reactions (Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2020).
Franz, A. K. & Wilson, S. O. Organosilicon molecules with medicinal applications. J. Med. Chem. 56, 388–405 (2013).
Ji, P., Park, J., Gu, Y., Clark, D. S. & Hartwig, J. F. Abiotic reduction of ketones with silanes catalysed by carbonic anhydrase through an enzymatic zinc hydride. Nat. Chem. 13, 312–318 (2021).
Hirano, K., Yorimitsu, H. & Oshima, K. Nickel-catalysed reactions with trialkylboranes and silacyclobutanes. Chem. Commun. 3234, 3241 (2008).
Liu, J., Zhang, W. & Xi, Z. Synthesis and reactivity of four-membered silacycles. Chin. J. Org. Chem. 29, 491–503 (2009).
Li, L., Zhang, Y., Gao, L. & Song, Z. Recent advances in C–Si bond activation via a direct transition metal insertion. Tetrahedron Lett. 56, 1466–1473 (2015).
Mu, Q.-C., Chen, J., Xia, C.-G. & Xu, L.-W. Synthesis of silacyclobutanes and their catalytic transformations enabled by transition-metal complexes. Coord. Chem. Rev. 374, 93–113 (2018).
Huang, J., Liu, F., Wu, X., Chen, J.-Q. & Wu, J. Recent advances in the reactions of silacyclobutanes and their applications. Org. Chem. Front. 9, 2840–2855 (2022).
Huang, W.-S., Wang, Q., Yang, H. & Xu, L.-W. State-of-the-art advances in enantioselective transition-metal-mediated reactions of silacyclobutanes. Synthesis 54, 5400–5408 (2022).
Liu, M., Qi, L. & Zhao, D. Recent advances in transition metal-catalyzed C–Si bond cleavage of silacyclobutanes. Chin. J. Org. Chem. 43, 3508 (2023).
Chen, F., Liu, L. & Zeng, W. Synthetic strategies to access silacycles. Front. Chem. 11, 1200494 (2023).
Hirano, K., Yorimitsu, H. & Oshima, K. Nickel-catalyzed regio- and stereoselective silylation of terminal alkenes with silacyclobutanes: facile access to vinylsilanes from alkenes. J. Am. Chem. Soc. 129, 6094–6095 (2007).
Shintani, R., Moriya, K. & Hayashi, T. Palladium-catalyzed enantioselective desymmetrization of silacyclobutanes: construction of silacycles possessing a tetraorganosilicon stereocenter. J. Am. Chem. Soc. 133, 16440–16443 (2011).
Ishida, N., Ikemoto, W. & Murakami, M. Cleavage of C–C and C–Si σ-bonds and their intramolecular exchange. J. Am. Chem. Soc. 136, 5912–5915 (2014).
Zhang, Q.-W. et al. Rhodium-catalyzed intramolecular C–H silylation by silacyclobutanes. Angew. Chem. Int. Ed. 55, 6319–6323 (2016).
Wang, X. B. et al. Controllable Si–C bond activation enables stereocontrol in the palladium-catalyzed [4 + 2] annulation of cyclopropenes with benzosilacyclobutanes. Angew. Chem. Int. Ed. 59, 790–797 (2020).
Qin, Y., Han, J. L., Ju, C. W. & Zhao, D. Ring expansion to 6-, 7-, and 8-membered benzosilacycles through strain-release silicon-based cross-coupling. Angew. Chem. Int. Ed. 59, 8481–8485 (2020).
Zhang, L. et al. A combined computational and experimental study of Rh-catalyzed C–H silylation with silacyclobutanes: insights leading to a more efficient catalyst system. J. Am. Chem. Soc. 143, 3571–3582 (2021).
Huo, J. et al. Palladium-catalyzed enantioselective carbene insertion into carbon−silicon bonds of silacyclobutanes. J. Am. Chem. Soc. 143, 12968–12973 (2021).
Chen, H. et al. Enantioselective synthesis of spirosilabicyclohexenes by asymmetric dual ring expansion of spirosilabicyclobutane with alkynes. Angew. Chem. Int. Ed. 61, e202212889 (2022).
Chen, S. et al. Nickel-catalyzed cross-redistribution between hydrosilanes and silacyclobutanes. Angew. Chem. Int. Ed. 61, e202213431 (2022).
An, K. et al. Rhodium hydride enabled enantioselective intermolecular C–H silylation to access acyclic stereogenic Si–H. Nat. Commun. 13, 847 (2022).
Tang, X. et al. Ring expansion of silacyclobutanes with allenoates to selectively construct 2- or 3-(E)-enoate-substituted silacyclohexenes. ACS Catal 12, 5185–5196 (2022).
Chen, Y. et al. Copper-catalyzed synthesis of S–S bond-containing silanols from SCBs and trisulfide-1,1-dioxides. J. Org. Chem. 88, 7953–7961 (2023).
Zhu, W.-K. et al. Rhodium-catalyzed hydrolytic cleavage of the silicon-carbon bond of silacyclobutanes to access silanols. Org. Lett. 25, 7186–7191 (2023).
Zhang, J. et al. Reversing site-selectivity in formal cross-dimerization of benzocyclobutenones and silacyclobutanes. CCS Chem. 5, 1753–1762 (2023).
Sun, Z.-H., Wang, Q. & Xu, L.-P. Mechanism and origins of enantioselectivity in the nickel-catalyzed asymmetric synthesis of silicon-stereogenic benzosiloles. J. Org. Chem. 89, 5675–5682 (2024).
Liu, M. et al. Nickel(0)-catalyzed ring-opening reaction of silacyclobutanes with 1,3-dienes to access allylsilane. Org. Chem. Front. 11, 3821–3826 (2024).
Liu, S., Chen, Y.-S., Wu, Y. & Wang, P. Pd-catalyzed intermolecular Si–O formation via Si–C activation. Sci. China Chem. 67, 2661–2669 (2024).
Wang, Y. et al. Tunable regiodivergent reactivity of N-allenamides with silacyclobutanes via palladium catalysis in the synthesis of silacyclic β-aminosilanes. ACS Catal 14, 10882–10892 (2024).
Sun, Y., Zhou, K., Ma, C., Li, Z. & Zhang, J. Rhodium/Ming-Phos-catalyzed asymmetric annulation reaction of silacyclobutanes with terminal alkynes. Green Synth. Catal. 5, 205–210 (2024).
Liu, M., Yan, N., Tian, H., Li, B. & Zhao, D. Ring expansion toward disila‐carbocycles via highly selective C−Si/C−Si bond cross‐exchange. Angew. Chem. Int. Ed. 63, e202319187 (2024).
Yang, L.-Y., Qin, Y., Zhao, Z. & Zhao, D. Nickel-catalyzed reductive protocol to access silacyclobutanes with unprecedented functional group tolerance. Angew. Chem. Int. Ed. 63, e202407773 (2024).
Lewis, K. M. & Couderc, S. 1946 and the early history of hydrosilylation. Molecules 27, 4341 (2022).
Troegel, D. & Stohrer, J. Recent advances and actual challenges in late transition metal catalyzed hydrosilylation of olefins from an industrial point of view. Coord. Chem. Rev. 255, 1440–1459 (2011).
Radchenko, A. V. & Ganachaud, F. Photocatalyzed hydrosilylation in silicone chemistry. Ind. Eng. Chem. Res. 61, 7679–7698 (2022).
Fan, X. et al. Stepwise on-demand functionalization of multihydrosilanes enabled by a hydrogen-atom-transfer photocatalyst based on eosin Y. Nat. Chem. 15, 666–676 (2023).
Kong, D. et al. Photocatalyzed regioselective hydrosilylation for the divergent synthesis of geminal and vicinal borosilanes. Nat. Commun. 14, 2525 (2023).
Zhou, R. et al. Visible-light-mediated metal-free hydrosilylation of alkenes through selective hydrogen atom transfer for Si−H activation. Angew. Chem. Int. Ed. 56, 16621–16625 (2017).
Zhu, J., Cui, W.-C., Wang, S. & Yao, Z.-J. Radical hydrosilylation of alkynes catalyzed by Eosin Y and thiol under visible light irradiation. Org. Lett. 20, 3174–3178 (2018).
Zhu, J., Cui, W.-C., Wang, S. & Yao, Z.-J. Visible light-driven radical trans-hydrosilylation of electron-neutral and -rich alkenes with tertiary and secondary hydrosilanes. J. Org. Chem. 83, 14600–14609 (2018).
Huang, Z. et al. Photocatalytic metal-free radical hydrosilylation for polymer functionalization. Chin. J. Chem. 41, 2275–2281 (2023).
Liang, H., Ji, Y.-X., Wang, R.-H., Zhang, Z.-H. & Zhang, B. Visible-light-initiated manganese-catalyzed E-Selective hydrosilylation and hydrogermylation of alkynes. Org. Lett. 21, 2750–2754 (2019).
Zhong, M., Pannecoucke, X., Jubault, P. & Poisson, T. Copperphoto catalyzed hydrosilylation of alkynes and alkenes under continuous flow. Chem. Eur. J. 27, 11818–11822 (2021).
Oezkar, S., Akhmedov, V. M. & Kayran, C. Photocatalytic hydrosilylation of conjugated dienes with triethylsilane in the presence of Cr(CO)5L (L = CO, P(CH3)3, P(OCH3)3). J. Organomet. Chem. 553, 103–108 (1997).
Abdelqader, W. et al. Photocatalytic hydrosilylation of conjugated dienes with triethylsilane in the presence of Cr(CO)6. Organometallics 15, 604–614 (1996).
Denmark, S. E., Jacobs, R. T., Dai-Ho, G. & Wilson, S. Synthesis, structure, and reactivity of an organogermanium lewis acid. Organometallics 9, 3015–3019 (1990).
Denmark, S. E., Griedel, B. D., Coe, D. M. & Schnute, M. E. Chemistry of enoxysilacyclobutanes: highly selective uncatalyzed aldol additions. J. Am. Chem. Soc. 116, 7026–7043 (1994).
Kinnaird, J. W. A., Ng, P. Y., Kubota, K., Wang, X. & Leighton, J. L. Strained silacycles in organic synthesis: a new reagent for the enantioselective allylation of aldehyde. J. Am. Chem. Soc. 124, 7920–7921 (2002).
Zhang, X., Houk, K. N. & Leighton, J. L. Origins of stereoselectivity in strain-release allylations. Angew. Chem. Int. Ed. 44, 938–941 (2005).
Auner, N., Grobe, J., Mueller, T. & Rathmann, H. W. Transient silenes and their dimethyl-d6 ether donor complexes from the gas-phase pyrolysis of siletanes. Organometallics 19, 3476–3485 (2000).
Ushakov, N. V. & Pritula, N. A. Synthesis of unsymmetrically substituted dialkenyl derivatives of silacyclobutane and silacyclopent-3-ene. Zh. Obshch. Khim. 62, 1318–1324 (1992).
Jackson, R. A. & Zarkadis, A. K. Strained-ring silicon-centered radicals: silacyclobut-1-yl and related radicals. J. Organomet. Chem. 341, 273–279 (1988).
Barton, T. J. & Tillman, N. Mechanism of the decomposition of silacyclobutane to silylene and propene. J. Am. Chem. Soc. 109, 6711–6716 (1987).
Auner, N. & Grobe, J. Silaethenes: I. Preparation and characterization of monosilacyclobutanes. J. Organomet. Chem. 188, 25–52 (1980).
He, T., Li, B., Liu, L., Ma, W. & He, W. Rhodium-catalyzed intermolecular silylation of Csp−H by silacyclobutanes. Chem. Eur. J. 27, 5648–5652 (2021).
Wang, X. C., Li, B., Ju, C. W. & Zhao, D. Nickel(0)-catalyzed divergent reactions of silacyclobutanes with internal alkynes. Nat. Commun. 13, 3392 (2022).
Chen, S., He, X., Jin, Y., Lan, Y. & Shen, X. Copper-catalyzed regio- and stereo-selective hydrosilylation of terminal allenes to access (E)-allylsilanes. Nat. Commun. 13, 3691 (2022).
Buettner, G. R. Spin trapping: ESR parameters of spin adducts. Free Radic. Biol. Med. 3, 259–303 (1987).
Alektiar, S. N. et al. Radical hydrocarboxylation of unactivated alkenes via photocatalytic formate activation. J. Am. Chem. Soc. 145, 10991–10997 (2023).
Acknowledgements
We are grateful to the National Key R&D Program of China (2022YFA1506100, X.S.), the National Natural Science Foundation of China (22471201, 21901191, X.S.), the China Postdoctoral Science Foundation (2023TQ0252, 2023M742687, S.C.), the Postdoctoral Foundation of Hubei Province (211000032, S.C.) and the Postdoctoral Fellowship Program of CPSF (GZC20231960, S.C.) for financial support. The theoretical calculations were performed on the supercomputing system in the Supercomputing Center of Wuhan University.
Author information
Authors and Affiliations
Contributions
X.S. conceived the idea, guided the project and wrote the manuscript with revisions by all the other authors; S.C. developed the catalytic methods, performed the mechanistic studies and the synthetic applications. S.C. and M.G. prepared the substrates and studied the scope. X.H. performed the calculations. All the authors are involved in the discussion and analysis of the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Jian Cao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, S., Gao, M., He, X. et al. Photo-induced ring-maintaining hydrosilylation of unactivated alkenes with hydrosilacyclobutanes. Nat Commun 16, 2468 (2025). https://doi.org/10.1038/s41467-025-57705-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-57705-w