Abstract
Neonatal bacterial meningitis is associated with substantial mortality and morbidity worldwide. Neonatal meningitis-causing Escherichia coli (NMEC) is the most common gram-negative bacteria responsible for this disease. However, the interactions of NMEC with its environment within the host are poorly understood. Here, we showed that a low level of leucine, a niche-specific signal in the blood, promotes NMEC pathogenicity by enhancing bacterial survival and replication in the blood. A low leucine level downregulates the expression of NsrP, a small RNA (sRNA) identified in this study, in NMEC in an Lrp-dependent manner. NsrP destabilizes the mRNA of the purine biosynthesis-related gene purD by direct base pairing. Decreased NsrP expression in response to low leucine levels in the blood, which is a purine-limiting environment, activates the bacterial de novo purine biosynthesis pathway, thereby enhancing bacterial pathogenicity in the host. Deletion of NsrP or purD significantly increases or decreases the development of E. coli bacteremia and meningitis in animal models, respectively. Furthermore, we showed that intravenous administration of leucine effectively reduces the development of bacteremia and meningitis caused by NMEC by blocking the Lrp-NsrP-PurD signal transduction pathway. This study provides a potential strategy for the prevention and treatment of E. coli-induced meningitis.
Similar content being viewed by others
Introduction
Meningitis is a state of inflammation of the meninges and subarachnoid space that can also involve the cortex and parenchyma of the brain1. Bacterial meningitis is considered the most severe form of this disease. Despite the availability of antibiotic therapy, the mortality of bacterial meningitis has remained at 20–25% for several decades2, and ~50% of survivors sustain permanent/lifelong neurological sequelae, such as hearing loss, developmental delay, and cognitive impairment3,4,5. Escherichia coli is the most common Gram-negative bacteria that causes meningitis. Among the plethora of serotypes, E. coli strains possessing the K1 capsular polysaccharide are more frequently neonatal meningitis-causing Escherichia coli (NMEC)4. With the increasing emergence of drug resistance, innovative strategies for the treatment of bacterial meningitis are becoming increasingly needed.
Bacterial meningitis is typically initiated by NMEC through a series of bacterial-host interactions. These interactions encompass vertical transmission of the causative agent from mother to infant and colonization of mucosal surfaces, typically the upper respiratory or gastrointestinal tract6,7. Subsequently, the bacteria invade the intravascular space, surviving and multiplying there, ultimately resulting in bacteremia. NMEC subsequently enters the central nervous system by translocating across the blood-brain barrier (BBB) and induces inflammation in the meninges6,8. Among these steps, a high level of bacteremia is indispensable for NMEC invasion of the BBB, which is a prerequisite for meningitis development9. Thus, inhibiting NMEC multiplication in the bloodstream is considered a means of preventing E. coli meningitis. However, the bacterial determinants and mechanisms that are essential for inducing a high level of bacteremia remain largely unclear.
The ability to switch between different lifestyles allows bacterial pathogens to thrive in diverse ecological niches10. Small noncoding RNAs (sRNAs) are robust posttranscriptional regulators of gene expression, typically by base pairing to target mRNAs, and play an important role in the niche adaptation of pathogens11,12. Over the past few years, a growing number of sRNAs that influence bacterial pathogenicity have been identified in E. coli13,14,15. For example, a previous study demonstrated that the sRNA EsrF responds to high ammonium concentrations in the colon and enhances the pathogenicity of enterohemorrhagic E. coli (EHEC) O157 by promoting bacterial motility and adhesion to host cells14. Under oxygen-limited conditions, the oxygen-responsive small RNA DicF enhances the expression of the EHEC type three secretion system to promote host colonization13. MavR, a sRNA that is conserved among pathogenic Enterobacteriaceae, regulates the expression of genes encoding proteins involved in nutrient acquisition, motility, oxidative stress responses, and attaching and effacing (AE) lesion formation16. In addition, a previous study identified an mEp460 phage-encoded sRNA, sRNA-17, in NMEC. The expression of sRNA-17 was found to be inhibited in the bloodstream, leading to increased survival of NMEC and enhanced penetration of the BBB17. Another example in NMEC is the sRNA Nsr69, which suppresses flagellin expression and thereby enhances NMEC survival in cerebrospinal fluid (CSF)18. However, whether sRNAs influence NMEC adaptation to the host remains poorly studied.
During the evolution of NMEC RS218 (serotype O18:K1, a reference strain isolated from the CSF of a neonate with meningitis), 22 genomic islands were acquired, distributed across different regions of the genome, with a total length of ~0.45 Mb19. These genomic islands are referred to as E. coli RS218-derived genomic islands (RDIs)19. Nearly half of the RDIs have been shown to play a role in meningitis development19. It is likely that some of these RDIs harbor sRNAs that can regulate NMEC pathogenicity.
Due to their central role in the metabolism of bacteria, nucleotide biosynthesis pathways are strongly linked to the virulence of bacterial pathogens20,21,22,23,24. De novo purine biosynthesis is a major pillar of nucleotide metabolism. The purine biosynthesis pathway leads to the conversion of 5-phosphoribosyl-1-pyrophosphate (PRPP) to inosine 5-monophosphate (IMP) to generate adenine and guanine, which serve as biosynthetic precursors for polymerizing reactions that generate DNA and RNA and as potential energy carriers20,25. The survival and proliferation of bacterial pathogens in the host are influenced by the ability of the pathogens to adjust their metabolism in response to the changing availability of purines within the host environment, especially in the bloodstream, which is characterized by purine-limiting conditions23,24.
Here, we examined how NMEC employs niche-specific strategies to counteract host nutritional immunity. NMEC senses low levels of leucine, a niche-specific signal in the blood, to downregulate the expression of the NMEC-specific sRNA NsrP in an Lrp-dependent manner. NsrP destabilizes the mRNA of the purine biosynthesis-related gene purD. A decrease in NsrP expression in response to low leucine levels activates the de novo purine biosynthesis pathway, resulting in enhanced bacterial pathogenicity in the host. In addition, we showed that the survival and replication of NMEC decreased when the Lrp-NsrP-PurD regulatory pathway was blocked by increasing the level of leucine in the blood via intravenous administration. This study reveals a potential strategy for using leucine as a potential drug candidate for the prevention and treatment of E. coli bacteremia and meningitis.
Results
NsrP is an sRNA that negatively regulates NMEC virulence
By analyzing our previous RNA sequencing (RNA-seq) data of RS218 derived from the blood of mice that were injected with NMEC RS218 via the tail vein18, we identified a potential NMEC-specific sRNA, which we named NsrP. NsrP is encoded by RDI 13, which is reportedly involved in the invasion of human brain microvascular endothelial cells (HBMECs), an in vitro BBB model19,26. This finding suggests that NsrP may play a role in NMEC virulence. Utilizing an NsrP-specific probe, we performed Northern blotting to determine whether NsrP is an sRNA transcribed in RS218. The findings showed an RNA band for the RS218 wild-type strain (WT) and the ΔNsrP complemented strain (cNsrP, which was generated by introducing a plasmid containing NsrP with its native promoter into ΔNsrP), but not for the ΔNsrP strain, indicating that NsrP is an sRNA transcribed from the reverse strand of the NMEC RS218 genome (Fig. 1a).
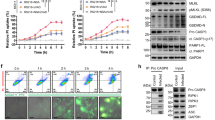
a Northern blotting was performed with a specific probe directed against NsrP sRNA in the NMEC WT, ΔNsrP, and ΔNsrP complemented (cNsrP) strains. 5S rRNA was used as a loading control. b The genomic position of the NsrP sequence in the NMEC RS218 genome. c Northern blotting for NsrP in RNA preparations from NMEC WT, and Δhfq. d, e Bacterial counts in the blood (CFU/mL, d) and meningitis development (e) were determined 4 h after intravenous injection of 1 × 106 CFU of the WT, ΔNsrP, or cNsrP strain (n = 8 for each group in d). CSF culture positivity was defined as meningitis (e). f H&E staining of brain sections was performed 4 h after intravenous injection of 1 × 106 CFU of the WT or ΔNsrP strain. The right panels are higher-magnification images of the boxed regions, showing meningeal thickening and neutrophil infiltration (arrows). Scale bar, 100 μm. The images shown are representative of three independent experiments. g Survival curves of 2- to 5-day-old rats subcutaneously injected with 1 × 105 CFU of the WT or ΔNsrP strain (n = 16 for each group). The number of living animals was recorded every 8 h. ns, nonsignificant. The two-tailed Mann–Whitney U-test (d), and the log-rank (Mantel–Cox) test (g) were applied.
The genomic location of the NsrP is shown in Fig. 1b. The 5′ and 3′ rapid amplification of cDNA ends (RACE) technique was used to determine the transcription start and termination sites of NsrP. The results showed that NsrP was exactly 244 nucleotides in length, spanning coordinates 2,920,694 to 2,920,937 in the genome (Supplementary Fig. 1a). Since Hfq is an indispensable chaperone for both sRNAs and their target mRNAs to facilitate base pairing and to protect sRNAs from degradation by cellular nucleosidases14, we therefore examined whether NsrP is hfq dependent. Northern blotting showed that the expression of NsrP exhibited no significant difference in the Δhfq strain compared with that in the WT strain (Fig. 1c). Moreover, when bacterial transcription was blocked by rifampicin treatment, the half-life of NsrP showed no significant difference in the Δhfq strain compared with that in the WT strain, indicating that NsrP is an Hfq-independent sRNA (Supplementary Fig. 1b).
To investigate the potential influence of NsrP on the pathogenicity of NMEC, we initially performed HBMEC invasion assays. Our findings indicated that the deletion of NsrP did not have any discernible impact on the capacity of the bacteria to invade the HBMECs (Supplementary Fig. 1c). To determine the potential role of NsrP in the development of bacteremia and meningitis in vivo, we used a mouse model of experimental hematogenous meningitis18. The results showed that the quantity of ΔNsrP in the blood was 7.26 ± 0.30 log CFU/ml, which was 10.42-fold higher than that of the WT strain (6.29 ± 0.22 log CFU/ml) and 11.10-fold higher than that of the cNsrP strain (6.25 ± 0.24 log CFU/ml), suggesting that NsrP hinders the survival and replication of NMEC within the murine bloodstream (Fig. 1d). In addition, CSF was collected from the mice to assess bacterial penetration across the BBB. The results showed a significant increase in the incidence of E. coli meningitis (defined on the basis of positive CSF cultures) in ΔNsrP-infected mice compared with that in mice infected with the WT or cNsrP strain (Fig. 1e). This finding is consistent with the fact that achieving a high level of bacteremia is a prerequisite for meningitis development for NMEC6. Since NsrP overlaps with a hypothetical open reading frame (orf14550), we mutated the putative start codon of orf14550 in the chromosome of WT (from ATG to CTG), generating strain orf14550mut, and found that this site mutation did not influence the virulence of NMEC (Fig. 1b and Supplementary Fig. 1d, e), suggesting the influence of NsrP on the virulence of NMEC is not related with orf14550.
Additionally, brain sections of the infected mice were examined by hematoxylin and eosin (H&E) staining. Compared with those in WT strain-infected mice, thickened meninges with neutrophil infiltration were observed in the mice infected with ΔNsrP (Fig. 1f), demonstrating that NsrP deletion leads to aggravated inflammation in the cerebral meninges. We next investigated the influence of NsrP on NMEC virulence by monitoring the survival of 2- to 5-day-old rats infected subcutaneously with WT or ΔNsrP NMEC27. Survival curves showed that all the neonatal rats infected with the ΔNsrP strain died at 48 h post-infection, while only 50% of the neonatal rats infected with the WT strain died at 48 h post-infection (Fig. 1g), suggesting that the mortality induced by the ΔNsrP strain was accelerated compared with that induced by the WT strain. These results demonstrated that NsrP inhibits bacterial survival in the blood and penetration of the BBB, resulting in a decrease in bacterial virulence.
NsrP inhibits NMEC virulence by repressing purD expression
In total, 387 putative target genes of NsrP were predicted in the genome of RS218 using TargetRNA3 (Supplementary Data 1). Among these potential target genes, purD may be a potential target of NsrP because the predicted base-pairing domain was included in the purD mRNA near the initiation codon (Fig. 2a). The purD gene encodes a phosphoribosylamine-glycine ligase that has been identified by transposon insertion sequencing as an NMEC gene required for survival in human serum20,28. qRT-PCR assays revealed that purD expression was significantly greater in the ΔNsrP strain than in the WT and cNsrP strains, suggesting that NsrP negatively regulates purD expression (Fig. 2b). To determine the expression of PurD at the protein level, a chromosomally 3×FLAG C-terminal fusion construct of purD was generated. Western blotting was performed to quantify the levels of FLAG-tagged PurD in LB medium for the WT, ΔNsrP and cNsrP strains. The results showed that, compared with the WT and cNsrP strains, the ΔNsrP strain exhibited increased PurD-FLAG production (Fig. 2c). These results demonstrate that NsrP inhibited PurD production.
a The regions of base pairing between NsrP and purD as predicted by the TargetRNA3 with the probability threshold of 0.25 and p-value of 0.05. Point mutations to generate the disrupted alleles. b qRT-PCR analysis of purD in the NMEC WT, ΔNsrP, and cNsrP. c Western blot analysis and quantification of PurD-FLAG in the NMEC WT, ΔNsrP, and cNsrP. DnaK was used as the loading control. d, e Bacterial counts in the blood (CFU/mL, d) and meningitis development (e) were determined 4 h after intravenous injection of 1 × 106 CFU of the WT, ΔNsrP, ΔpurD, ΔNsrPΔpurD or ΔpurD complemented (cpurD) strain (n = 8 for each group in d). CSF culture positivity was defined as meningitis. f H&E staining of the brain sections was performed 4 h after intravenous injection of 1 × 106 CFU of the WT or ΔpurD. The right panels are higher-magnification images of the boxed regions, showing meningeal thickening and neutrophil infiltration (arrows). Scale bar, 100 μm. The images shown are representative of three independent experiments. g Survival curves of 2- to 5-day-old rats subcutaneously injected with 1 × 105 CFU of the WT or ΔpurD (n = 16 for each group). The number of living animals was recorded every 8 h. h Growth curves of the WT and ΔpurD in M9 medium with or without 40 mM IMP. i, j Replication of the WT and ΔpurD in 60% NMS with (j) or without (i) 40 mM IMP. k, l Bacterial counts in the blood (CFU/mL, k) and meningitis development (l) were determined 4 h after intravenous injection of 1 × 106 CFU of the WT, ΔpurD, or cpurD with or without IMP (n = 8 for each group in k). CSF culture positivity was defined as meningitis (l). ns nonsignificant. In b, c, h–j, data were presented as the means ± SDs (n = 3 independent experiments). One-way ANOVA (b, c, i, j), two-tailed Mann–Whitney U-test (d, k), log-rank (Mantel–Cox) test (g), and two-way ANOVA (h) were applied.
To determine the effect of purD on NMEC pathogenicity, we performed HBMEC invasion assays and found that there was no significant difference in the bacterial capacity to invade HBMECs among the WT, ΔpurD and ΔpurD complemented (cpurD) strains (Supplementary Fig. 2a). A previous investigation established the limited availability of nucleotide precursors in blood23,29. In addition, the inactivation of nucleotide biosynthesis genes in Salmonella enterica and Bacillus anthracis has been demonstrated to impede their growth in human serum24. Therefore, we examined the impact of purD on the capacity of NMEC to elicit bacteremia and meningitis in a mouse model of experimental hematogenous meningitis. Compared with the WT strain, the ΔpurD strain elicited a 12.4-fold lower level of bacteremia in mice, with bacterial loads of 6.46 ± 0.32 log CFU/ml for the WT strain and 5.38 ± 0.28 log CFU/ml of blood for the ΔpurD strain) (Fig. 2d). Similarly, the development of E. coli meningitis was markedly suppressed in ΔpurD-infected mice compared to that in mice infected with the WT or cpurD strain (Fig. 2e). H&E staining of brain sections revealed thickened meninges with neutrophil infiltration in mice infected with WT, whereas no discernible histopathology was observed in the meninges of the animals infected with ΔpurD (Fig. 2f). Survival curves showed that 50% of the neonatal rats infected with the WT strain died 48 h post-infection, whereas those infected with the ΔpurD strain remained viable at the end of the observation period (96 h post-infection, Fig. 2g). Collectively, these results provide evidence that purD facilitates the survival and replication of NMEC within the murine bloodstream, which enhances bacterial penetration across the BBB and meningitis development.
Furthermore, we investigated whether NsrP regulates the virulence of NMEC via purD. Animal experiments showed that the double-mutant ΔNsrPΔpurD and ΔpurD strains induced comparable levels of bacteremia in mice, with no significant difference in their capacity to cause meningitis development (Fig. 2d, e). Both strains exhibited significantly reduced virulence compared to the WT and ΔNsrP strains in infected mice (Fig. 2d, e). Additionally, the ΔNsrP strain induced significantly higher levels of bacteremia and an increased incidence of meningitis in mice compared to the WT strain (Fig. 2d, e), consistent with the results presented above (Fig. 1d, e). These findings suggest that the deletion of NsrP had no effect on NMEC virulence in the ΔpurD background and that the regulatory effect of NsrP on the virulence of NMEC is mediated by purD.
Bacterial growth curves demonstrated that, compared with the WT strain, the ΔpurD strain exhibited a growth deficiency when cultured in the M9 medium (Fig. 2h), which is a minimal medium devoid of purines and their derivatives. However, when M9 medium was supplemented with 40 mM IMP, the growth of the ΔpurD strain was similar to that of the WT strain (Fig. 2h). In addition, we showed that compared with the WT strain, the ΔpurD strain exhibited a reduced capacity for growth in both normal mouse serum (NMS) and heat-inactivated normal mouse serum (HI-NMS) after 2 or 4 h of incubation in vitro (Fig. 2i and Supplementary Fig. 2b). Conversely, the WT and ΔpurD strains exhibited comparable growth rates in NMS and HI-NMS when supplemented with IMP (Fig. 2j and Supplementary Fig. 2c). Furthermore, animal experiments demonstrated that the intravenous injection of IMP enhanced the ability of the ΔpurD but not the WT and cpurD strains to induce bacteremia and meningitis in mice (Fig. 2k, l). These findings suggest that the survival impairment observed for the ΔpurD strain is attributable to the deficiency of purines in the bloodstream and the impairment of purine synthesis.
NsrP directly binds to purD mRNA
To test for a direct interaction between NsrP and purD mRNA in vitro, we performed an RNA electrophoretic mobility shift assay (REMSA) using in vitro–transcribed and purified NsrP and purD mRNA or the NsrP complementary strand as a positive control (NsrP+) (Supplementary Fig. 3a, b). NsrP-purD mRNA complexes were observed, and the band intensity increased as the NsrP concentration increased (Supplementary Fig. 3a), confirming the direct interaction between NsrP and purD mRNA in vitro.
IntaRNA analysis showed that NsrP may interact with the purD mRNA through nine-nucleotide base (CUAAUUACU) pairing beginning at nucleotide +12 in the purD mRNA (based on the ATG site) (Fig. 2a). The prediction of the secondary structure of NsrP sRNA via RNAfold revealed that this binding motif of NsrP is located in a loop structure (Supplementary Fig. 3c). To confirm that this motif is the key sequence of NsrP required for binding to purD mRNA, we performed REMSA using a mutated NsrP (NsrPmut) (the motif AAUUACU was complementarily mutated to UUAAUGA, which did not affect the secondary structure of NsrP), and a mutated purDmut mRNA (the sequence AGUGAUU was complementarily mutated to UCACUAA) (Fig. 2a). No interaction was observed between the NsrPmut sRNA and purD mRNA or between the NsrP sRNA and purDmut mRNA (Supplementary Fig. 3d, e). However, REMSA showed a direct interaction between the NsrPmut sRNA and purDmut mRNA (Supplementary Fig. 3f). Moreover, we constructed a plasmid containing purDH-lux fusion (purD and purH are located in an operon30) (Fig. 3a and Supplementary Fig. 3g), and introduced this plasmid into ΔNsrP, generating strain ΔNsrP-purDH-lux. We found a significant reduction in the luminescence intensity of the ΔNsrP-purDH-lux strain when NsrP was overexpressed (Fig. 3b). In contrast, overexpressing NsrPmut with mutated binding motif did not influence the luminescence intensity (Figs. 2a, 3b). Furthermore, there was no significant difference in the luminescence intensity of the ΔNsrP-purDmutH-lux strain (the binding motif AGUGAUU of purDH-lux on the plasmid was complementarily mutated to UCACUAA) when NsrP was overexpressed, whereas a significant reduction in the luminescence intensity was observed when NsrPmut was overexpressed in ΔNsrP-purDmutH-lux strain (Fig. 3a, c). In addition, northern blotting showed that there is no significant difference between the expression of NsrP and NsrPmut (Supplementary Fig. 3h, i). Collectively, these data indicate that NsrP binds directly and specifically to the purD mRNA through the identified motif (AAUUACU).
a Schematic of the structure of purDH-lux. The region of the purDH promoter and the expected full-length purDH without termination codon were cloned and inserted into pMS402 to construct a purDH-lux fusion. Point mutations to generate the disrupted alleles in the purDmutH transcript are indicated. b, c Quantification of the PurD (b) or PurDmut (c) protein expression level in the ΔNsrP-purDH-lux strain (b) or ΔNsrP-purDmutH-lux strain (c) by measurement of luminescence. The ΔNsrP-purDH-lux and ΔNsrP-purDmutH-lux strains were transformed with the expression construct pNM12 (Control), or pNM12 encoding NsrP or NsrPmut. d qRT-PCR analysis of purD expression in rifampicin-treated the WT and ΔNsrP strains. e Graphical presentation of proposed interaction of NsrP sRNA with the purD mRNA, and of base-pair changes to generate NsrPbind-mut and PurDsyn-mut. f–i Bacterial counts in the blood (CFU/mL, f, h) and meningitis development (g, i) were determined 4 h after intravenous injection of 1 × 106 CFU of the WT, NsrPbind-mut, and ΔNsrP strains (f, g) or WT, PurDsyn-mut, ΔNsrP, and PurDsyn-mutΔNsrP strains (h, i) (n = 8 for each group in f, h). CSF culture positivity was defined as meningitis. In b–d, data were presented as the means ± SDs (n = 3 independent experiments). ns nonsignificant. One-way ANOVA (b–d), and two-tailed Mann–Whitney U-test (f, h) were applied.
To investigate whether NsrP influences the stability of purD mRNA, we performed a rifampicin transcription inhibition assay. The results showed that the purD mRNA was more stable in the ΔNsrP strain than in the WT strain (Fig. 3d), indicating that NsrP was detrimental to purD mRNA stability. Furthermore, we found that the expression of purD in the Δhfq strain exhibited no significant difference compared with that in the WT strain in rifampicin transcription inhibition assay (Supplementary Fig. 3j). In contrast, the expression of purD was significantly increased in the ΔhfqΔNsrP strain compared with that in the Δhfq strain (Supplementary Fig. 3k). These results suggest that NsrP-purD base pairing promotes purD mRNA degradation in an Hfq-independent way and thus decreases PurD expression.
To further identify the contribution of the NsrP binding motif to NMEC virulence in vivo, we mutated the NsrP binding motif (AAUUACU to UUAAUGA) on the chromosome of WT, generating strain NsrPbind-mut. We also mutated the binding motif on purD by synonymous mutation (AGUGAUU to GGUCAUA) on the chromosome of WT, generating strain PurDsyn-mut (Fig. 3e). We found that the levels of bacteremia and the incidence of meningitis elicited by the NsrPbind-mut and PurDsyn-mut strains in mice were significantly higher than that in WT-infected mice, which exhibited no significant difference from that in ΔNsrP-infected mice (Fig. 3f–i). Moreover, we further deleted NsrP in strain PurDsyn-mut (generating strain PurDsyn-mutΔNsrP), and found that the levels of bacteremia and the incidence of meningitis in mice infected by PurDsyn-mutΔNsrP exhibited no significant difference from that in mice infected by PurDsyn-mut (Fig. 3h, i). This finding suggests that the regulatory effect of NsrP on the virulence of NMEC is mediated by its binding to purD mRNA.
NMEC senses low leucine levels in the blood to suppress NsrP expression
qRT-PCR analysis of WT NMEC recovered from the blood of mice infected via tail vein injection showed that NsrP expression decreased compared with that of WT NMEC in LB medium (Fig. 4a). To investigate the potential factors regulating NsrP expression, an analysis of the NsrP promoter region was conducted using the online promoter prediction tool BProm. This analysis confirmed the presence of an Lrp-binding site within the NsrP promoter region (Fig. 4b). qRT-PCR analysis revealed a significant decrease in NsrP expression in the Δlrp strain compared with that in the WT strain when cultured in LB medium (Fig. 4c), indicating that Lrp positively regulates NsrP expression.
a qRT-PCR analysis of NsrP expression in the NMEC WT strain in LB medium and mouse blood. b The NsrP promoter region and the potential binding site of Lrp were predicted by the BProm program (SoftBerry). c qRT-PCR analysis of the NsrP expression in WT and Δlrp. d Biacore SPR kinetic analyses of Lrp binding to the promoter of NsrP. e Fold enrichment of the NsrP promoter in the Lrp-ChIP samples compared with that in the mock-ChIP samples. f qRT-PCR analysis of the NsrP expression in WT NMEC in M9 medium supplemented with 0.1 mM leucine (Leu-L) or 5 mM leucine (Leu-H). g Northern blotting of NsrP sRNA in WT NMEC in M9 medium supplemented with 0.1 or 5 mM leucine. 5S rRNA was used as the loading control. h, i qRT-PCR analysis of the NsrP expression in the Δlrp (h) and NsrPpro-mut (i) strain in M9 medium supplemented with 0.1 or 5 mM leucine. In a, c, e–i, data were presented as the means ± SDs (n = 3 independent experiments). ns nonsignificant. Two-tailed unpaired Student’s t-test (a, c, f–i) and two-way ANOVA (e) were applied.
To investigate whether Lrp directly binds to the NsrP promoter, we performed a surface plasmon resonance (SPR) assay to monitor the real-time binding of Lrp to the NsrP promoter. The result showed that the purified Lrp (but not BSA) binds to the promoter of NsrP in a dose-dependent manner (Fig. 4d and Supplementary Fig. 4a). Consistently, chromatin immunoprecipitation-qPCR (ChIP-qPCR) analysis revealed 9.51-fold greater enrichment of the NsrP promoter region in Lrp-ChIP samples than in the mock-ChIP samples (Fig. 4e). In contrast, the fold enrichment of rpoS did not significantly differ between these two samples (Fig. 4e). These results indicate that Lrp specifically binds to the NsrP promoter region both in vitro and in vivo.
E. coli Lrp was initially identified as a leucine-responsive regulatory protein, but subsequent research has also indicated its responsiveness to various other small effector molecules, including alanine, methionine, threonine, lysine, histidine, and isoleucine31. Thus, we carried out qRT-PCR to analyze NsrP expression in response to each of these amino acids. Mid-logarithmic phase WT strain incubated in LB medium was collected and reincubated in M9 minimal medium supplemented with different concentrations of these amino acids for 30 min. The qRT-PCR results revealed that NsrP expression decreased at a low leucine concentration (0.1 mM), which mimics conditions found in human blood32, compared to its expression at a high leucine concentration (5 mM). Conversely, the expression of NsrP did not significantly change in response to the other amino acids (Fig. 4f and Supplementary Fig. 4b). Similarly, northern blot analysis also revealed a decrease in NsrP expression in the WT strain under conditions of low leucine levels, suggesting that NsrP expression is repressed by low leucine levels (Fig. 4g). Furthermore, we showed that leucine levels no longer regulate NsrP expression in the strains Δlrp and NsrPpro-mut (with Lrp binding site on the promoter of NsrP was deleted from the genome) (Fig. 4b, h, i). These findings provide evidence that NMEC senses low leucine levels through Lrp to suppress NsrP expression by binding to its promoter directly.
NMEC promotes purD expression in mouse blood by sensing low leucine levels in an NsrP-dependent manner
As NsrP expression was repressed at a low level of leucine and NsrP served as a negative regulator of purD expression, our subsequent investigation focused on the impact of leucine on purD expression. qRT-PCR was performed on mid-logarithmic phase WT strains reincubated in M9 minimal medium supplemented with different concentrations of leucine (0.1 or 5 mM) for 30 min. The results revealed a significant increase in purD expression under low-leucine conditions (Fig. 5a). This finding is consistent with the observed upregulation of purD expression in mouse blood, which is a low-leucine environment compared with LB medium (Fig. 5b). To determine the impact of leucine on PurD expression at the protein level, a 3×FLAG-PurD WT strain was incubated in the same conditions. Western blotting results showed that PurD-FLAG production was significantly induced by low leucine levels (Fig. 5c). In contrast, in the Δlrp, ΔNsrP, and NsrPpro-mut strains, the regulation of purD expression by leucine was no longer observed (Fig. 5d–f). These results demonstrated that NMEC senses low leucine levels in the blood to increase purD expression in an Lrp- and NsrP-dependent manner.
a qRT-PCR analysis of the purD expression in the NMEC WT strain in M9 medium supplemented with 0.1 or 5 mM leucine. b qRT-PCR analysis of the purD expression in WT NMEC in LB medium or mouse blood. c Western blot analysis and quantification of PurD-FLAG in WT NMEC in M9 medium supplemented with 0.1 or 5 mM leucine. DnaK was used as the loading control. d–f qRT-PCR analysis of the purD expression in the Δlrp (d), ΔNsrP (e), and NsrPpro-mut (f) strain in M9 medium supplemented with 0.1 or 5 mM leucine. In a–f, data were presented as the means ± SDs (n = 3 independent experiments). ns, nonsignificant. Two-tailed unpaired Student’s t-test was applied.
In summary, our study demonstrated that NMEC can sense low leucine levels in the bloodstream, leading to a decrease in NsrP expression, which in turn increases purD expression in an Lrp-dependent manner. This phenomenon increases NMEC survival in the host bloodstream. These findings suggest that a low leucine level is beneficial for NMEC survival in the bloodstream, which is consistent with the finding that NsrP mutation promotes NMEC pathogenicity.
purD did not affect the intestinal colonization of NMEC
NMEC infections arise due to the colonization of the neonatal gastrointestinal tract by maternally derived NMEC at or soon after birth, followed by transcytosis through enterocytes into the bloodstream and ultimately the development of meningitis6,7. In addition, the gut can serve as a reservoir for the recurrence of NMEC infection33. To investigate the impact of purD on the colonization of NMEC in the intestine, we performed qRT-PCR to determine the expression of NsrP and purD in the rat intestine. The results showed that NsrP and purD expression in the WT strain that colonized the small intestine did not significantly differ from that in the LB medium (Supplementary Fig. 5a). Moreover, bacterial colonization in the small intestine also did not significantly differ among the NMEC WT, ΔpurD, and cpurD strains (Supplementary Fig. 5b), suggesting that purD was not necessary for NMEC intestinal colonization. Previous research has demonstrated that the small intestine of infants is abundant in purines and amino acids, including leucine34,35,36,37. Therefore, the small intestine serves as a niche for NMEC infection that does not require upregulation of the de novo purine synthesis pathway.
Intravenous administration of leucine reduced NMEC virulence in mice by blocking the Lrp-NsrP-PurD regulatory pathway
The above results showed that NMEC senses low levels of leucine in the blood to increase the expression of purD by repressing NsrP expression, which results in significantly increased NMEC pathogenicity. Therefore, the leucine level may be a potential factor for the treatment of NMEC infection. Thus, mice infected by NMEC WT were treated with leucine through tail vein injection at 0.5 h post-infection to determine the effect of leucine on NMEC pathogenicity. The results showed that leucine treatment resulted in significantly increased or decreased bacterial expression of NsrP and purD in vivo, respectively, comparted to control mice (WT-infected mice receiving PBS) (Fig. 6a, b). Moreover, leucine-treated mice exhibited significantly decreased levels of bacteremia and inhibited development of meningitis compared to control mice (Fig. 6c, d). Additionally, the leucine-treated mice exhibited less thickened meninges with neutrophil infiltration than did the control mice (Fig. 6e). Conversely, there was no significant difference in bacteremia levels or the development of meningitis between leucine-treated mice and control mice when they are infected with strain ΔNsrP, ΔpurD, NsrPpro-mut, NsrPbind-mut- or PurDsyn-mut (Fig. 6f–k). Collectively, these data indicate leucine is able to reduce the pathogenicity of NMEC by blocking the Lrp-NsrP-PurD regulatory pathway.
a, b qRT-PCR analysis of the NsrP (a) and purD (b) expression in mouse blood were determined 4 h after intravenous injection of 1 × 106 CFU of the NMEC with the administration of leucine or PBS. (c, d, f–k) Bacterial counts in the blood (CFU/mL, c, f–j) and meningitis development (d, k) were determined 4 h after intravenous injection of 1 × 106 CFU of the WT (c), ΔpurD (f), ΔNsrP (g), NsrPpro-mut (h), NsrPbind-mut (i), and PurDsyn-mut (j) strains with the administration of leucine or PBS at 0.5 h post-infection (n = 8 for each group). CSF culture positivity was defined as meningitis (d, k). e H&E staining of the brain sections was performed 4 h after intravenous injection of 1 × 106 CFU of the WT strain and of leucine or PBS at 0.5 h post-infection. The right panels are higher-magnification images of the boxed regions, showing meningeal thickening and neutrophil infiltration (arrows). Scale bar, 100 μm. The images shown are representative of three independent experiments. l The distribution of NsrP in 930 publicly available complete E. coli genomes. In a, b, data were presented as the means ± SDs (n = 3 independent experiments). ns nonsignificant. Two-tailed unpaired Student’s t-test (a, b) and two-tailed Mann–Whitney U-test (c, f–j) were applied.
To investigate whether the administration of leucine, which targets the Lrp-NsrP-PurD regulatory pathway, could be a potential strategy for the treatment of E. coli bacteremia and meningitis, we analyzed the distribution of NsrP in E. coli by performing a comparative genomics analysis using 930 publicly available complete E. coli genomes. The genomes of the strains included 46 NMEC strains, 77 uropathogenic E. coli (UPEC) strains, 85 enteropathogenic E. coli (EPEC) strains, 366 EHEC strains, and 356 nonpathogenic E. coli strains. The results showed that NsrP was present in the majority of the NMEC strains (36/46, 78.3%) and in a few UPEC strains (12/77, 15.6%) whose serotypes were O1 and O18 (Fig. 6l, Supplementary Data 2). In contrast, NsrP was absent in the EHEC, EPEC, and nonpathogenic E. coli strains (Fig. 6l). Furthermore, among the 34 NMEC strains that possessed a K1 capsule (34/46, 73.9%, defined as E. coli K1+), 29 harbored NsrP (29/34, 85.3% in E. coli K1+), suggesting that NsrP is widely present in E. coli K1+ strains. These results indicate that NsrP is widely distributed in NMEC but not in nonpathogenic E. coli or other pathotypes of E. coli. Thus, the administration of leucine to target the Lrp-NsrP-PurD regulatory pathway is a potential strategy for the prevention and treatment of E. coli bacteremia and meningitis.
Discussion
To survive and thrive in an often hostile environment, bacteria must monitor their surroundings and adjust their gene expression and physiology accordingly. This is especially important for pathogenic bacteria, which continuously interact with the host during an infection38. In this study, we elucidated an NsrP-mediated regulatory signal transduction pathway employed by NMEC to sense low leucine levels in the host bloodstream. This perception leads to the augmentation of bacterial purine biosynthesis, thereby facilitating the progression of bacteremia and meningitis. Specifically, NsrP destabilizes the mRNA of the purine biosynthesis-related gene purD, resulting in the downregulation of purD expression. A low leucine level in the blood decreases NsrP expression via Lrp, which ultimately increases purD expression, resulting in enhanced purine biosynthesis. This mechanism significantly contributes to NMEC proliferation within the bloodstream, consequently inducing bacteremia and bacterial meningitis (Fig. 7).
In bacterial pathogens, sRNAs can directly influence bacterial pathogenicity by regulating the expression of virulence factors. For example, in EHEC under microaerobic conditions, the sRNA DicF activates translation of pchA, which subsequently activates the transcription of ler resulting in the enhanced expression of genes within the LEE pathogenicity island13. Additionally, the sRNAs GlmY and GlmZ of EHEC repress the expression of espADB encoded by LEE4 and the transcripts from LEE539. In hypervirulent Klebsiella pneumonia, the sRNA ArcZ regulates many hypermucoviscosity-related genes, causing the overproduction of the hypermucoviscous capsule, which is crucial for the hyper-virulence40. This direct regulation of virulence genes by sRNAs enables pathogens to efficiently thrive within the host, offering a rapid response mechanism with minimal energy expenditure. However, sRNAs can also indirectly influence bacterial pathogenicity by modulating physiological activities such as adaptation to environmental stresses. For example, iron scarcity and oxidative stress are common challenges pathogens face within the host. In Pseudomonas aeruginosa, the sRNA PrrH, regulates genes involved in pyochelin biosynthesis, enabling the pathogen to scavenge iron from human proteins like lactoferrin and transferrin in the form of Fe3+ 41. In Salmonella Typhimurium, the sRNAs CyaR, MicA, and OxyS have been identified as regulators of ompX, a gene involved in responses to oxidative stress42. Additionally, in P. aeruginosa, the sRNA SicX regulates anaerobic ubiquinone biosynthesis, facilitating the transition between chronic and acute infections43. Whether through direct regulation of virulence factors or modulation of bacterial metabolic responses to environmental stresses, these mechanisms are crucial for pathogen colonization and survival within the host. In our study, we identified an sRNA-mediated adaptive mechanism that helps NMEC respond to purine-limited environments in the host bloodstream, highlighting the essential role of sRNAs in facilitating bacterial survival under nutrient-restricted conditions.
The horizontal acquisition of certain genes can drastically alter the fitness of a bacterium, allowing it to utilize additional substrates and thrive in otherwise toxic environments44,45,46. Numerous key E. coli pathogenicity genes have been identified by horizontal gene transfer, such as the locus of enterocyte effacement (LEE) and genomic O island 122 in EHEC47. NsrP is a horizontally acquired sRNA that mediates the repression of purD expression in response to the leucine level. The scarcity of purines in blood, a nutrient-limiting condition, plays an important role in suppressing bacterial growth in blood24. NMEC increases its purine synthesis in response to low leucine levels in the blood via the Lrp-NsrP-PurD signal transduction pathway, which is beneficial for NMEC survival and replication. In contrast, the small intestine of infants is abundant in purines since human milk contains considerable amounts of nucleotides that are digested in the small intestine36. Moreover, the majority of dietary proteins are fully degraded in the small intestine, which makes this portion of the gastrointestinal tract abundant in amino acids, including leucine33,37. Thus, we speculate that in the intestinal environment, NMEC does not need to upregulate the expression of purine synthesis genes (Supplementary Fig. 5a, b), enabling it to precisely regulate its energy consumption during infection (Fig. 7).
E. coli Lrp is a nucleoid-associated protein involved in the transcriptional regulation of numerous genes. Leucine is recognized as the primary effector of Lrp, significantly modulating its regulatory activity by either enhancing or inhibiting its effects48. Additionally, recent studies have shown that Lrp also responds to other amino acids, including alanine, methionine, isoleucine, lysine, histidine, and threonine31. For example, in addition to the canonical Lrp-leucine regulon in E. coli, the next most well-characterized group is the Lrp-alanine regulon, which includes genes involved in alanine export and metabolism49. However, alanine regulates only a small subset of the Lrp regulon compared to leucine50. This highlights leucine’s unique ability to broadly influence Lrp-regulated gene networks compared to other effectors, although the underlying mechanism remains unclear. Previous studies have shown that leucine promotes the dissociation of the Lrp hexadecamer into leucine-bound octamers with altered DNA-binding affinity51. Based on this, we speculate that Lrp’s ability to activate the expression of NsrP in response to leucine, rather than other amino acids, may be due to the specific oligomeric state induced by leucine. This hypothesis warrants further investigation in future studies.
Among clinical isolates, E. coli K1+ is predominant among isolates from neonatal E. coli meningitis and possesses a limited diversity of O-serotypes, dominated by O18, O1, O7, O16, and O454. We found that NsrP is predominant in O18:K1 and O1:K1 strains, which are the dominant serotypes that cause neonatal meningitis in many countries52,53. The strategy for treating NMEC infection usually involves the use of antibiotics54. However, antibiotic treatment changes the composition of the gut microbiota, resulting in a negative impact on host health55. Moreover, the widespread use of antibiotics has increased the antibiotic resistance of many pathogenic bacteria, making treatment more challenging56. Leucine is an essential amino acid that cannot be synthesized within the body and has been found to be, along with isoleucine and valine (the three components of branched-chain amino acids, BCAA), an effective pharmaconutrient in several diseases57. For instance, BCAA administration has been applied in septic patients to improve their nutritional status and outcomes58. BCAA administration is also recommended for the treatment of liver failure due to their effect on the detoxification of ammonia to glutamine and their decreased concentration in patients with liver cirrhosis59. Moreover, leucine supplementation has been proven as a nutritional treatment for sarcopenia due to its anabolic effects on cell signaling and protein synthesis in muscle in clinical trials60,61,62. Therefore, leucine administration targeting the Lrp-NsrP-PurD signal transduction pathway is a potential strategy for the prevention and treatment of E. coli bacteremia and meningitis. In addition, NsrP is absent in other E. coli strains, suggesting that intravenous administration of leucine may have a minor effect compared to that of antibiotic treatment on the gut microbial composition and diversity.
Methods
Ethics statement
All animal experiments were conducted in compliance with the guidelines outlined in the Guide for the Care and Use of Laboratory Animals. All animal studies were conducted according to protocols approved by the Institutional Animal Care Committee of Nankai University (Tianjin, China) and were performed under protocol no. IACUC 2016030502.
Bacterial strains, plasmids, and growth conditions
All the E. coli K1 strains used in this study were derived from a clinical isolate RS218 (serotype O18:K1:H7), which was isolated from the CSF of a newborn infant with meningitis63. Plasmids, strains, and primers are listed in Supplementary Tables 1, 2. The mutants were generated using the λ-red recombination system to substitute the target genes with the chloramphenicol or kanamycin resistance genes in plasmid pKD3 or pKD464. The complemented strains were constructed by cloning the open reading frame and promoter region of corresponding genes into a plasmid, pACYC184. For purification of Lrp-6×His protein, lrp was cloned into the pET-28a expression vector and transferred into E. coli BL21 (DE3). For ChIP-qPCR, lrp was tagged with 3×FLAG, cloned into pTRC99A and transferred into Δlrp. All the resulting clones were verified by PCR amplification and DNA sequencing. The test strains were grown in LB broth at 37 °C with required antibiotics (ampicillin, 100 μg/mL; chloramphenicol, 25 μg/mL; kanamycin, and 50 μg/mL).
Small RNA candidate prediction
RNA sequencing data (SRA: PRJNA680436) were initially mapped to the genome RS218 (GenBank accession number: CP007149.1) using Bowtie2. Rockhopper software was then employed to identify intergenic and Cis-natural antisense transcripts65. A BlastX search against the non-redundant NCBI database was performed to annotate these transcripts. Transcripts that remained unannotated were considered potential candidates for noncoding sRNA66.
5′ and 3′ RACE assay
5′ and 3′ RACE assay were carried out using 5′ and 3′ RACE System for Rapid Amplification of cDNA Ends kits (Invitrogen, CA, USA) according to the manufacturer’s instructions. In brief, for 5′ RACE, the first strand of cDNA is synthesized from total RNA using a gene-specific primer. Following purification, cDNA was Oligo (dC)-tailed, and PCR was performed using an Abridged Anchor Primer and a nested gene-specific primer. The products were cloned into pEASY-T1 (TransGen, Beijing, China; CT111) and sequenced. For 3′ RACE, the total RNA was applied for 3′-poly (A) tailing before reverse transcription using the adapter primer. The gene-specific primer and the universal amplification primer that targets the adapter region were used for amplification. The products were cloned into pEASY-T1 and sequenced. The oligonucleotides used in these assays are listed in Supplementary Table 2.
RNA extraction and quantitative PCR (qPCR)
For NMEC collected from the blood of NMEC-infected mice, the specimens were collected as follows. 18-day-old BALB/c mice received 1 × 106 CFU of NMEC WT or mutant strains in the logarithmic phase of growth via the tail vein. Four hours later, blood specimens were collected. For NMEC collected from the small intestine, 2- to 5-day-old Sprague-Dawley rat pups were orally infected by pipette feeding of 20 μL PBS containing 2 × 106 CFU of NMEC WT or mutant strains in the logarithmic phase of growth. Small intestine specimens were collected following the contents were removed after 24 h infection. For the effect of leucine levels on NMEC, mid-logarithmic phase NMEC WT or mutant strains incubated in LB medium were collected and reincubated in M9 minimal medium (1×M9 salts, 2 mM MgSO4, 0.1 mM CaCl2, 0.4% glucose) supplemented with 0.1 or 5 mM leucine for 30 min. For the effect of Hfq protein on the stability of NsrP and degradation of purD mRNA, LB-cultured NMEC and Δhfq strains in the logarithmic phase of growth were incubated with 100 μg/mL of rifampicin for 0, 2, 4, 8, 16, 32 min. The specimens above were collected by centrifugation at 5000×g for 5 min.
The total RNA was extracted using a TRIzol reagent (Invitrogen, USA). RNA was treated with DNase I at 37 °C for 30 min, and the successful removal of DNA contamination was verified by PCR. The quality and quantity of RNA were determined by NanoDrop spectrophotometer (Thermo Fisher, USA). cDNA synthesis was performed by PrimeScript RT reagent Kit (Takara, USA, RR037A). qRT-PCR was performed using SYBR green PCR master mix (Applied Biosystems, 4367659) on QuantStudio 5 (Applied Biosystems, CA, USA). The relative mRNA expression level was analyzed using the ΔΔCt method after normalizing to the Ct of the rpoA13. Each experiment was performed in triplicate.
In vitro transcription and RNA-RNA EMSA (REMSA)
The DNA templates were transcribed into NsrP, NsrP+ (positive control), NsrPmut sRNA, purD, and purDmut mRNA using the T7 High Efficiency Transcription Kit (TransGen, Beijing, China, JT101). RNA was purified by MagicPure® RNA Beads (TransGen, Beijing, China, EC501) and then checked in an 8% Tris-Acr-urea gel. REMSA was performed with NsrP, NsrPmut sRNA (0, 10, 20, and 30 μM) and purD, purDmut mRNA or NsrP+ (positive control, 10 μM) in REMSA binding buffer containing 10 mM HEPES [pH 7.3], 20 mM KCl, 2.4 mM MgCl2, and 2.4 mM DTT. Reactions were incubated for 2–3 min at 85 °C and then at 37 °C for 45 min. The samples were separated by 8% native polyacrylamide gels and stained with SYBR Gold Nucleic Acid Gel Stain (Invitrogen, CA, USA, S11494) for 10 min, then imaged using SuperSignal west pico chemiluminescent substrate (Thermo). ImageJ software was used to measure band intensities.
Purification of Lrp protein and surface plasmon resonance (SPR)
The lrp sequence from NMEC RS218 genomic DNA was cloned into the pET-28a to obtain the pET-Lrp plasmid. Lrp-6×His protein was expressed in E. coli BL21 (DE3) containing pET-Lrp and purified using HiTrap Ni2+-chelating column. The concentration of Lrp was determined by the Bradford method67. The PCR fragment of the NsrP promoter (NsrP-pro) were amplified with the 5′-biotinylated primers and purified using a SPARKeasy Gel DNA Extraction Kit (Sparkjade, Shandong, China, AE0101). The interactions between 5′-biotinylated NsrP promotor DNA and Lrp (DNA-binding protein, ~20 KDa) or BSA (fatty acid-free bovine serum albumin, the negative control, ~66.5 KDa) were examined using a Biacore X100 (BR110073) equipped with research-grade streptavidin (SA)-coated sensor chips (Cityva, England) at 25 °C. Following the manufacturer’s recommendations, the SA-coated sensor chips were preconditioned with 1 M NaCl in 50 mM NaOH and then washed with 50% dimethyl carbinol, 1 M NaCl in 50 mM NaOH to ensure a clean surface.
The biotinylated NsrP promoter DNA was immobilized at a flow rate of 30 μL/min using 100 μg/mL DNA oligonucleotides diluted in 1× HBS-EP+ running buffer, generating low-density surfaces with approximately 1000 response units (RU). Lrp or BSA at concentrations ranging from 20 nM to 1.25 μM was injected over the active surface containing NsrP promoter DNA using the Kinetics/Affinity mode at a flow rate of 30 μL/min. The association phase lasted for 180 s, followed by a 300-s dissociation phase.
Between sample injections, surfaces were regenerated at a flow rate of 30 μL/min using two 30 s pulses of 0.5% [vol/vol] SDS solution, followed by Extra-clean and Rinse procedures as specified by the instrument protocol. The Biacore X100 evaluation software was used to fit the affinity curves using a steady-state affinity model. The equilibrium dissociation constant (KD) was calculated.
Bacterial growth curve assays
Overnight cultures were used to seed in M9 minimal medium with or without 40 mM IMP at an optical density at 600 nm (OD600) of 0.1 as previously described22. A 200 μL aliquot was added to a 96-well microplate and incubated at 37 °C with shaking at 180 rpm for 24 h. The OD600 was recorded every 20 min. Experiments were independently performed three times.
Lux-based light production measurements
The DNA fragment containing purDH promoter and full length of purDH or purDmutH were amplified, purified, and digested with BamHI/HindIII. The products were ligated to pMS402 plasmid, respectively, to construct lux fusion plasmids. The DNA fragments of NsrP and NsrPmut (AAUUACU to UUAAUGA) were amplified, purified, digested with MscI/EcoRI and ligated to pNM12 plasmid, respectively, to construct sRNA overexpression plasmids. The empty pNM12 vector was labeled as control. The plasmids and insertions were verified by colony PCR and Sanger sequencing. Single colonies of ΔNsrP strains harboring lux fusions and sRNA overexpression plasmids were grown to logarithmic phase in LB medium at 37 °C, and then incubated with 0.1% of arabinose for 30 min to induce the expression of NsrP or NsrPmut. The luminescence was measured using the Spark multimode microplate reader (Tecan).
Northern blotting
Northern blotting was performed using the DIG Northern Starter kit (Roche). About 15 μg total RNA were electrophoresed in a 1.2% agarose gel containing 37% formaldehyde. RNA was transferred to nitrocellulose membranes and immobilized at 120 °C for 30 min. Prehybridization was performed in digoxigenin (DIG) Easy Hyb buffer for 30 min. The DIG-labeled RNA probe was added and incubated overnight. The membrane was washed twice in 2× SSC/0.1% SDS for 5 min at room temperature and followed by 1× SSC/0.1% SDS buffers for 15 min at 68 °C. Signals were visualized using an Amersham Imager 680, 5S rRNA being used as an internal control.
Western blotting
LB-cultured NMEC, ΔNsrP, and cNsrP strains grown to the logarithmic phase were pelleted and lysed to obtain total protein. The proteins (100 μg) were separated by 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes. The membranes were blocked with Tris-buffered saline with 0.1% (w/v) Tween 20 (TBST) containing 5% skim milk for 1 h at room temperature. The membranes were then incubated with the primary antibody anti-FLAG (1:1000 dilution, Sigma, F1804) and DnaK (1:1000 dilution, Abcam, ab69617) overnight at 4 °C followed by the secondary antibody Goat Anti-Mouse IgG H&L-HRP (1:5000 dilution, Abcam, ab6789) for 1 h at room temperature. Samples were visualized with chemiluminescence and the protein levels were analyzed using ImageJ which normalized to DnaK from three independent experiments.
ChIP and ChIP-qPCR
The pTRC99A-Lrp-3×FLAG recombinant plasmid was electroporated into Δlrp. The LB-cultured Δlrp containing pTRC99A-Lrp-3×FLAG grown up to late-logarithmic phase was treated with 1% formaldehyde for 25 min at room temperature. The reaction was quenched by the addition of 0.5 M glycine and sonicated to generate DNA fragments of 200–500 bp. The sample was pelleted, and the supernatant was used for immunoprecipitation with an anti-3×FLAG antibody (Sigma, F1804) and protein A magnetic beads (Invitrogen, 10002D) according to the manufacturer’s instructions. An aliquot without antibody served as negative control (Mock). The sample then was incubated with RNaseA for 2 h at 37 °C, and proteinase K for 2 h at 55 °C. DNA fragments were purified using a PCR purification kit (Roche, 11732668001). The relative enrichment of the promotor of NsrP and rpoS (the negative control) was examined with qRT-PCR as mentioned above and rpoA gene was used as reference control.
Animal experiments
Two- to 5-day-old Sprague-Dawley in both sexes and 18-day-old BALB/c male mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (licensed by Charles River), and housed in a temperature-controlled room in a specific pathogen-free facility with a 12 h light/12 h dark cycle. 18-day-old BALB/c mice were used for the experimental hematogenous meningitis model, in which animals develop a high level of bacteremia followed by bacterial traversal of the BBB mimicking the pathogenesis of human E. coli meningitis18. Two- to 5-day-old Sprague-Dawley rats were used for intestine colonization assays and mouse survival assays as previously described27,28. For the experimental hematogenous meningitis model, each mouse received 1 × 106 CFU of NMEC WT or mutant strains in the logarithmic phase of growth via the tail vein. Four hours later, blood and CSF specimens were collected for bacterial cultures (CFU). For the leucine administration assay, l-leucine (10 mg/mL) or PBS was administered via the tail vein injection at a dose of 1 mg/30 g body weight at 0.5 h post-infection of NMEC68. For intestine colonization assays, 2- to 5-day-old Sprague-Dawley rats were orally infected by pipette feeding of 20 μL PBS containing 2 × 106 CFU of NMEC WT or mutant strains in the logarithmic phase of growth. Small intestine specimens were collected following the contents were removed after 24 h infection, then weighed, washed with PBS, and homogenized for bacterial cultures. The in vivo colonization efficiency was determined by counting the number of CFU per g of tissues28. For mouse survival assays, 2- to 5-day-old Sprague-Dawley rats were subcutaneously injected with 1 × 105 CFU of NMEC WT or mutant strains in the logarithmic phase of growth27. The number of animals alive was recorded every 8 h.
E. coli invasion assay in HBMEC
Immortalized cell line HBMEC were a generous gift from Dr. K.S. Kim (Johns Hopkins University, Baltimore, MD). The immortalized HBMECs have been verified for their function and morphological structure, which are similar to primary cells69. E. coli invasion into HBMECs were assessed as described17,70. HBMECs were cultured in Dulbecco’s Modified Eagle Medium (DMEM, Gibco) supplemented with 10% fetal bovine serum and 10% Nu-Serum (BD Biosciences) at 37 °C in 5% CO2. The NMEC WT or mutant strains in the logarithmic phase of growth were collected and resuspended in an experimental medium (M199-HamF12 [1:1], containing 5% heat-inactivated fetal bovine serum, 2 mM glutamine, and 1 mM pyruvate). HBMEC grown in collagen-coated 24-well plates were infected with the suspension in a multiplicity of infection of 100: 1 and incubated at 37 °C in 5% CO2 for 90 min. HBMEC were washed with PBS three times and reincubated with an experimental medium containing gentamicin (100 mg/ml) for 1 h at 37 °C in 5% CO2 to kill extracellular bacteria before the cells were lysed. The lysates were plated on LB agar plates and cultured overnight to count the bacterial CFU for quantifications3. The results were expressed as percent relative invasion compared to the percent invasion of NMEC WT.
Analysis of the distribution of NsrP in E. coli
930 E. coli complete genomes containing 46 NMEC strains, 77 Uropathogenic E.coli (UPEC) strains, 85 Enteropathogenic E.coli (EPEC) strains, 366 EHEC strains, and 356 nonpathogenic E. coli strains were downloaded from the GenBank database at the National Center for Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov/genbank/). NsrP was identified using blastn (minimum sequence coverage/identify 80/85%).
Quantification and statistical analysis
Data are shown as bar graphs or dot plots (means ± SDs) of three independent experiments. Statistical significance was analyzed with GraphPad Prism 9.5.0 software (GraphPad Inc) using the two-tailed unpaired Student’s t-test, one-way ANOVA, two-way ANOVA, log-rank (Mantel–Cox) test, or Mann–Whitney U-test according to the test requirements, as stated in the figure legends.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All study data were available in the main text and/or Supplementary information. Source data are provided with this paper.
References
van de Beek, D., Brouwer, M. C., Koedel, U. & Wall, E. C. Community-acquired bacterial meningitis. Lancet 398, 1171–1183 (2021).
van de Beek, D., de Gans, J., Tunkel, A. R. & Wijdicks, E. F. Community-acquired bacterial meningitis in adults. N. Engl. J. Med. 354, 44–53 (2006).
Zhao, W. D. et al. Caspr1 is a host receptor for meningitis-causing Escherichia coli. Nat. Commun. 9, 2296 (2018).
Kim, K. S. Human meningitis-associated Escherichia coli. EcoSal Plus 7, ESP-0015-2015 (2016).
Kim, K. S. Acute bacterial meningitis in infants and children. Lancet Infect. Dis. 10, 32–42 (2010).
Kim, K. S. Pathogenesis of bacterial meningitis: from bacteraemia to neuronal injury. Nat. Rev. Neurosci. 4, 376–385 (2003).
Croxen, M. A. & Finlay, B. B. Molecular mechanisms of Escherichia coli pathogenicity. Nat. Rev. Microbiol. 8, 26–38 (2010).
Doran, K. S. et al. Host-pathogen interactions in bacterial meningitis. Acta Neuropathol 131, 185–209 (2016).
Xie, Y., Kim, K. J. & Kim, K. S. Current concepts on Escherichia coli K1 translocation of the blood-brain barrier. FEMS Immunol. Med. Microbiol. 42, 271–279 (2004).
Rossi, E. & La Rosa, R. Pseudomonas aeruginosa adaptation and evolution in patients with cystic fibrosis. Nat. Rev. Microbiol. 19, 331–342 (2021).
Storz, G., Vogel, J. & Wassarman, K. M. Regulation by small RNAs in bacteria: expanding frontiers. Mol. Cell 43, 880–891 (2011).
Sauder, A. B. & Kendall, M. M. After the fact(or): posttranscriptional gene regulation in enterohemorrhagic Escherichia coli O157:H7. J. Bacteriol. 200, e00228–18 (2018).
Melson, E. M. & Kendall, M. M. The sRNA DicF integrates oxygen sensing to enhance enterohemorrhagic Escherichia coli virulence via distinctive RNA control mechanisms. Proc. Natl Acad. Sci. USA 116, 14210–14215 (2019).
Jia, T. et al. A novel small RNA promotes motility and virulence of enterohemorrhagic Escherichia coli O157:H7 in response to ammonium. mBio 12, e03605–e03620 (2021).
Jia, T. & Wu, P. The phosphate-induced small RNA EsrL promotes E. coli virulence, biofilm formation, and intestinal colonization. Sci. Signal. 16, eabm0488 (2023).
Sauder, A. B. & Kendall, M. M. A pathogen-specific sRNA influences enterohemorrhagic Escherichia coli fitness and virulence in part by direct interaction with the transcript encoding the ethanolamine utilization regulatory factor EutR. Nucleic Acids Res. 49, 10988–11004 (2021).
Sun, H. et al. An ArcA-modulated small RNA in pathogenic Escherichia coli K1. Front. Microbiol. 11, 574833 (2020).
Sun, H. et al. Bacteria reduce flagellin synthesis to evade microglia-astrocyte-driven immunity in the brain. Cell Rep. 40, 111033 (2022).
Xie, Y. et al. Identification and characterization of Escherichia coli RS218-derived islands in the pathogenesis of E. coli meningitis. J. Infect. Dis. 194, 358–364 (2006).
Goncheva, M. I., Chin, D. & Heinrichs, D. E. Nucleotide biosynthesis: the base of bacterial pathogenesis. Trends Microbiol. 30, 793–804 (2022).
Kim, G. L. et al. Growth and stress tolerance comprise independent metabolic strategies critical for Staphylococcus aureus infection. mBio 12, e0081421 (2021).
Shaffer, C. L. et al. Purine biosynthesis metabolically constrains intracellular survival of uropathogenic Escherichia coli. Infect. Immun. 85, e00471–16 (2017).
Connolly, J. et al. Identification of Staphylococcus aureus factors required for pathogenicity and growth in human blood. Infect. Immun. 85, e00337–17 (2017).
Samant, S. et al. Nucleotide biosynthesis is critical for growth of bacteria in human blood. PLoS Pathog. 4, e37 (2008).
Zhang, Y., Morar, M. & Ealick, S. E. Structural biology of the purine biosynthetic pathway. Cell. Mol. Life Sci. 65, 3699–3724 (2008).
Teng, C. H. et al. NlpI contributes to Escherichia coli K1 strain RS218 interaction with human brain microvascular endothelial cells. Infect. Immun. 78, 3090–3096 (2010).
Zhang, X. W. et al. Lpp of Escherichia coli K1 inhibits host ROS production to counteract neutrophil-mediated elimination. Redox Biol. 59, 102588 (2023).
McCarthy, A. J. & Stabler, R. A. Genome-wide identification by transposon insertion sequencing of Escherichia coli K1 genes essential for in vitro growth, gastrointestinal colonizing capacity, and survival in serum. J. Bacteriol. 200, e00698–17 (2018).
Mei, J. M., Nourbakhsh, F., Ford, C. W. & Holden, D. W. Identification of Staphylococcus aureus virulence genes in a murine model of bacteraemia using signature-tagged mutagenesis. Mol. Microbiol. 26, 399–407 (1997).
Aiba, A. & Mizobuchi, K. Nucleotide sequence analysis of genes purH and purD involved in the de novo purine nucleotide biosynthesis of Escherichia coli. J. Biol. Chem. 264, 21239–21246 (1989).
Hart, B. R. & Blumenthal, R. M. Unexpected coregulator range for the global regulator Lrp of Escherichia coli and Proteus mirabilis. J. Bacteriol. 193, 1054–1064 (2011).
Yokoyama, K. et al. Feast/famine regulation by transcription factor FL11 for the survival of the hyperthermophilic archaeon Pyrococcus OT3. Structure 15, 1542–1554 (2007).
Nhu, N. T. K. & Phan, M. D. High-risk Escherichia coli clones that cause neonatal meningitis and association with recrudescent infection. eLife 12, RP91853 (2024).
Dallas, D. C. et al. Personalizing protein nourishment. Crit. Rev. Food Sci. Nutr. 57, 3313–3331 (2017).
Solmonson, A. & Faubert, B. Compartmentalized metabolism supports midgestation mammalian development. Nature 604, 349–353 (2022).
Thorell, L., Sjöberg, L. B. & Hernell, O. Nucleotides in human milk: sources and metabolism by the newborn infant. Pediatr. Res. 40, 845–852 (1996).
Malo, C. Free amino acid levels in serum and small intestine during the post-natal development of normal and sparse-fur mutant mice. Comp. Biochem. Physiol. A Physiol. 109, 1049–1057 (1994).
Gripenland, J. et al. RNAs: regulators of bacterial virulence. Nat. Rev. Microbiol. 8, 857–866 (2010).
Gruber, C. C. & Sperandio, V. Global analysis of posttranscriptional regulation by GlmY and GlmZ in enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 83, 1286–1295 (2015).
Wu, K. et al. RNA interactome of hypervirulent Klebsiella pneumoniae reveals a small RNA inhibitor of capsular mucoviscosity and virulence. Nat. Commun. 15, 6946 (2024).
Hoang, T. M., Huang, W., Gans, J. & Weiner, J. The heme-responsive PrrH sRNA regulates Pseudomonas aeruginosa pyochelin gene expression. mSphere 8, e0039223 (2023).
Briones, A. C. et al. Genetic regulation of the ompX porin of Salmonella Typhimurium in response to hydrogen peroxide stress. Biol. Res. 55, 8 (2022).
Cao, P., Fleming, D. & Moustafa, D. A. A Pseudomonas aeruginosa small RNA regulates chronic and acute infection. Nature 618, 358–364 (2023).
Juhas, M. Horizontal gene transfer in human pathogens. Crit. Rev. Microbiol. 41, 101–108 (2015).
Frost, L. S., Leplae, R., Summers, A. O. & Toussaint, A. Mobile genetic elements: the agents of open source evolution. Nat. Rev. Microbiol. 3, 722–732 (2005).
Koonin, E. V. & Wolf, Y. I. Genomics of bacteria and archaea: the emerging dynamic view of the prokaryotic world. Nucleic Acids Res. 36, 6688–6719 (2008).
Konczy, P. et al. Genomic O island 122, locus for enterocyte effacement, and the evolution of virulent verocytotoxin-producing Escherichia coli. J. Bacteriol. 190, 5832–5840 (2008).
Ziegler, C. A. & Freddolino, P. L. The leucine-responsive regulatory proteins/feast-famine regulatory proteins: an ancient and complex class of transcriptional regulators in bacteria and archaea. Crit. Rev. Biochem. Mol. Biol. 56, 373–400 (2021).
Ihara, K. et al. Expression of the alaE gene is positively regulated by the global regulator Lrp in response to intracellular accumulation of l-alanine in Escherichia coli. J. Biosci. Bioeng. 123, 444–450 (2017).
Trouillon, J., Doubleday, P. F. & Sauer, U. Genomic footprinting uncovers global transcription factor responses to amino acids in Escherichia coli. Cell Syst 14, 860–871 (2023).
Chen, S., Iannolo, M. & Calvo, J. M. Cooperative binding of the leucine-responsive regulatory protein (Lrp) to DNA. J. Mol. Biol. 345, 251–264 (2005).
Geslain, G. et al. Genome sequencing of strains of the most prevalent clonal group of O1:K1:H7 Escherichia coli that causes neonatal meningitis in France. BMC Microbiol. 19, 17 (2019).
Sáez-López, E. et al. Outbreak caused by Escherichia coli O18: K1: H7 sequence type 95 in a neonatal intensive care unit in Barcelona, Spain. Pediatr. Infect. Dis. J. 36, 1079–1086 (2017).
Barichello, T. et al. Bacterial meningitis in Africa. Front. Neurol. 14, 822575 (2023).
Zhang, Q., Cheng, L., Wang, J., Hao, M. & Che, H. Antibiotic-induced gut microbiota dysbiosis damages the intestinal barrier, increasing food allergy in adult mice. Nutrients 13, 3315 (2021).
Duan, Y. et al. Antibiotic resistance and virulence of extraintestinal pathogenic Escherichia coli (ExPEC) vary according to molecular types. Front. Microbiol. 11, 598305 (2020).
Leenders, M. & van Loon, L. J. Leucine as a pharmaconutrient to prevent and treat sarcopenia and type 2 diabetes. Nutr. Rev. 69, 675–689 (2011).
De Bandt, J. P. & Cynober, L. Therapeutic use of branched-chain amino acids in burn, trauma, and sepsis. J. Nutr. 136, 308s–313s (2006).
Holecek, M. Branched-chain amino acids and ammonia metabolism in liver disease: therapeutic implications. Nutrition 29, 1186–1191 (2013).
Borack, M. S. & Volpi, E. Efficacy and safety of leucine supplementation in the elderly. J. Nutr. 146, 2625s–2629s (2016).
Bauer, J. M. et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: a randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 16, 740–747 (2015).
Lo, J. H., U, K. P., Yiu, T., Ong, M. T. & Lee, W. Y. Sarcopenia: current treatments and new regenerative therapeutic approaches. J. Orthop. Translat. 23, 38–52 (2020).
Huang, S. H. et al. Identification and characterization of an Escherichia coli invasion gene locus, ibeB, required for penetration of brain microvascular endothelial cells. Infect. Immun. 67, 2103–2109 (1999).
Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl Acad. Sci. USA 97, 6640–6645 (2000).
McClure, R. et al. Computational analysis of bacterial RNA-Seq data. Nucleic Acids Res. 41, e140 (2013).
Mann, B. et al. Control of virulence by small RNAs in Streptococcus pneumoniae. PLoS Pathog. 8, e1002788 (2012).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976).
Ma, C. et al. L-leucine promotes axonal outgrowth and regeneration via mTOR activation. FASEB J. 35, e21526 (2021).
Stins, M. F., Badger, J. & Sik Kim, K. Bacterial invasion and transcytosis in transfected human brain microvascular endothelial cells. Microb. Pathog. 30, 19–28 (2001).
Zhu, L. et al. Arachidonic acid metabolism regulates Escherichia coli penetration of the blood-brain barrier. Infect. Immun. 78, 4302–4310 (2010).
Acknowledgements
This work was supported by the National Natural Science Foundation of China Program under Grant No. 82402643 (to H.S.), 32070130 (to B.L.), 82372267 (to B.L.), 32070133 (to L.F.), 32470111 (to L.F.), 32130003 (to L.W.), 32370194 (to L.W.); the Natural Science Foundation of Shenzhen under Grant No. JCYJ20220530164604010 (to B.L.), JCYJ20230807151559009 (to B.L.); Guangdong Basic and Applied Basic Research Foundation Grant 2024A1515010588 (to B.L.); Shenzhen Science and Technology Program Grant JCYJ20210324135007019 (to L.F.); Key Laboratory Major Project of 2024 (Tianjin) Grant 24ZXZSSS00140 (to B.L.), and Fundamental Research Funds for Central Universities under Grant No. 63233172 (to B.L.).
Author information
Authors and Affiliations
Contributions
Conceptualization, B.L., H.S., and X.L.; methodology, H.S., X.L., X.Y., J.Q., Y.L., Y.Z., H.S., Q.W., R.L., X.C., Q.Z., T.J., and X.W.; visualization, H.S., X.L., and X.Y.; supervision, B.L.; writing—original draft, B.L., H.S., and X.L.; writing—review and editing, B.L., H.S., X.L., L.F., and L.W.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Sahar Melamed, Andrew Roe and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sun, H., Li, X., Yang, X. et al. Low leucine levels in the blood enhance the pathogenicity of neonatal meningitis-causing Escherichia coli. Nat Commun 16, 2466 (2025). https://doi.org/10.1038/s41467-025-57850-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-57850-2