Abstract
The large-scale implementation of proton-exchange membrane water electrolyzers relies on high-performance membrane-electrode assemblies that use minimal iridium (Ir). In this study, we present a support-free Ir catalyst developed through a metal-oxide-based molecular self-assembly strategy. The unique self-assembly of densely isolated single IrO6H8 octahedra leads to the formation of μm-sized hierarchically porous Ir hydroxide particles. The support-free Ir catalyst exhibits a high turnover frequency of 5.31 s⁻¹ at 1.52 V in the membrane-electrode assembly. In the corresponding proton-exchange membrane water electrolyzer, notable performance with a cell voltage of less than 1.75 V at 4.0 A cm⁻² (Ir loading of 0.375 mg cm⁻²) is achieved. This metal-oxide-based molecular self-assembly strategy may provide a general approach for the development of advanced support-free catalysts for high-performance membrane-electrode assemblies.
Similar content being viewed by others
Introduction
Electrochemical technologies based on renewable energy, such as water electrolysis1,2, CO2 upgrading3, nitrate reduction to NH34, and oxygen reduction to H2O25, are expected to play a critical role in achieving a sustainable future. In these technologies, the oxygen evolution reaction (OER) is often the reaction at the counter electrode and provides electrons and protons. The high kinetic barrier of OER significantly affects the efficiency of these electrochemical processes6.
The advancement of perfluorosulfonic acid-based proton-exchange-membrane (PEM)7 has made PEM-based electrochemical techniques highly promising. For instance, PEM water electrolyzers (PEMWEs), with high dynamic range, are suitable for coupling with fluctuating renewable energy sources1. In PEM CO2 reduction reactors, the acidic environment can increase carbon efficiency3. However, the harsh acidic and oxidizing conditions at the anode limit the choice of OER catalysts to Ir (or Ir-based ones)2,8. The activity and/or stability of state-of-the-art Ir-free OER catalysts remains far inferior to Ir-based ones9. For potential large-scale implementation of PEM-based devices, the Ir loading at the anode should be low enough (e.g., Ir loading of ~0.4 mgIr cm−2 in PEMWEs2) to address concerns related to cost and sustainability.
After decades of research and screening, two Ir-based catalysts, rutile IrO23,5 and amorphous IrOxHy10, have been widely used as OER catalysts in state-of-the-art PEMWEs. Notably, amorphous IrOxHy was initially observed on the surface of Ir metal undergoing consecutive potential cycling11. This IrOxHy was later referred to as the anodic iridium oxide film (AIROF)12,13. The Ir metal can be considered as a precatalyst. For IrO2, a high loading (e.g., >1 mgIr cm−2 in PEMWEs2) is generally required to compensate for its very low intrinsic activity. By contrast, AIROF demonstrates considerably higher intrinsic activity than rutile IrO214, highlighting its potential to reduce Ir loading in devices.
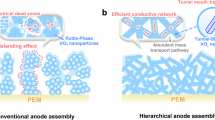
However, the application of IrOxHy in MEAs faces significant challenges. Specifically, to ensure a fine dispersion of nano IrOxHy, the use of support material is often mandatary15,16. Despite considerable progress17, a highly conductive and stable support material is yet to be found18. In the case of support-free IrOxHy, precatalysts of Ir metal or alloy, with structures of nanowire19 and nanosheet20 are generally used. However, stability could be a significant concern as the formation of AIROF from Ir metal is accompanied by massive dissolution/loss of Ir21.
Designing precatalysts using Ir-based complex oxides, coupled with reconstruction, is a strategy for developing advanced IrOxHy catalysts22,23,24. This strategy is intriguing as these complex oxides can be easily synthesized using scalable and environmentally benign methods, such as solid-state synthesis22,23. In addition to the formation of highly active IrOxHy, our recent research has revealed that the high activity of Sr(Co/Sc)1-xIrxO3-derived IrOxHy is partially attributed to an increased active surface area (approximately tenfold increment)22. This increase is a result of leaching soluble cations during the reconstruction process25. Coincidentally, it is found that the surface area of a Sr2IrO4, with a higher Sr content, increases by ~13 times after the leaching of Sr26. This cation-leaching effect provides a simple way to improve the utilization of Ir. However, due to the unclear origin of the cation-leaching-induced increase in active surface area, limited efforts have been dedicated to take advantage of this reconstruction-induced promoting effect.
Herein, we present a support-free IrOxHy featuring a hierarchically porous (HP) structure. With such support-free IrOxHy as the anode, the MEA exhibits a notable performance of 1.75 V @ 4.0 A cm−2. The key to this HP-IrOxHy is the introduction of an advanced Sr4IrO6 precatalyst. A straightforward acid leaching of Sr2+ from Sr4IrO6 results in the formation of HP-IrOxHy, which brings about a significant surface area increase of ~400-fold. The formation of this HP structure is due to a unique self-assembly of densely isolated IrO6H8 octahedra. This HP structure significantly enhances mass transport in the anode, reducing the related loss to a mere ~24 mV @ 4 A cm−2. Our findings show that the MEA-based turnover frequency of Ir from HP-IrOxHy (~5.31 s−1 at 1.52 V) exceeds previously reported values.
Results and discussion
Hierarchically porous structure
In Sr4IrO6 (Fig. 1a), the arrangement of Ir and Sr alternates along the c-axis, forming chains that are separated by chains composed solely of Sr atoms. Consequently, all Ir atoms in Sr4IrO6 are fully coordinated, with six oxygen atoms, and each IrO6 octahedron is strictly isolated by Sr atoms. The synthesis of highly pure Sr4IrO6 (Fig. S1) is achieved through the solid-state method. The tetravalence state of Ir and the structural characteristic of isolated IrO6 octahedra are confirmed by X-ray adsorption analysis (Fig. S2). As depicted in the scanning electron microscope (SEM) images (Figs. 1b and S3), the as-synthesized Sr4IrO6 exhibits large solid particles, with sizes mostly exceeding 10 μm.
a Arrangement of IrO6 octahedra (isolated by Sr) in Sr4IrO6. b SEM image of the as-prepared Sr4IrO6 particles. c SEM images of acid-treated Sr4IrO6 particles and a surface slit. d XPS of Sr_3d from the as-prepared and acid-treated Sr4IrO6. e, f SEM images and corresponding sketch maps of the cross-sectional specimen (acid-treated Sr4IrO6) prepared by FIB milling. g TEM images of local structure of pores/channels within the bulk of acid-treated Sr4IrO6. h The surface areas for the as-prepared and acid-treated Sr4IrO6. The upper limit of the surface area for the as-prepared Sr4IrO6 is estimated using a spherical model, with a diameter >10 μm (marked in the green circle). The orange circle represents the equivalent dense IrO2 sphere for the acid-treated Sr4IrO6, with a calculated diameter of ~12.8 nm based on the BET surface area of the acid-treated Sr4IrO6.
The leaching of Sr2+ ions from Sr4IrO6 is achieved by immersing the material in a 0.1 mol HClO4 solution (details can be found in the methods). After the acid treatment, the particles remain μm-sized (Fig. S3), but additional slits with a typical width of 100 nm can be observed on the surface (Fig. 1c). The disappearance of the X-ray photoelectron spectroscopy (XPS) signal from Sr_3d (Fig. 1d) indicates the successful leaching of Sr2+ ions. Furthermore, by subjecting the acid-treated Sr4IrO6 to additional calcination at 1000 °C, a pure rutile IrO2 phase is obtained (Fig. S4), confirming the complete removal of Sr2+ ions. Note that, in addition to Sr²⁺ leaching, approximately 19% of the initial Ir from the as-prepared Sr4IrO6 will also leach out (Table S1). Based on the proposed molecular assembly mechanism in this study (please see the discussions below), one likely reason for this Ir loss is that the dissolution of Sr interrupts the formation of Ir–O(H)–Ir bonds, leading to the loss of some IrO6 molecules.
The microstructure and surface area changes, induced by Sr2+ leaching, are further analyzed with focused ion beam (FIB)-SEM and transmission electron microscopy (TEM). As shown in Fig. 1e, f are the cross-sectional specimens prepared by FIB milling. Remarkably, a highly porous bulk structure, with apparent macropores (diameter >100 nm), can be observed. The local structure is analyzed with TEM (Fig. 1g), where a hierarchically porous structure is observed, with pore/channel widths varying from ~10 nm to over 100 nm.
Consequently, the acid-treated Sr4IrO6 exhibits a high BET surface area of 40.3 m2 g−1 (Figs. 1h and S5). This surface area is equivalent to the formation of nano IrO2 particles with a diameter of ~12.8 nm. Note that due to the large particle size of the as-prepared Sr4IrO6, analyzing the corresponding surface area and pore size distribution was unsuccessful. Instead, an estimated surface area of <0.092 m2 g−1 was calculated, assuming a spherical particle with a diameter of >10 μm. The presence of surface slits and bulk pores/channels (Fig. 1c, e–g) indicates that a simple Sr2+ leaching process can stimulate the development of a hierarchically porous structure within the μm-sized particles (i.e., a support-free structure). Intriguingly, such morphology changes result in a notable ~400-fold increase in surface area. The increment is substantially higher than the cases observed in other Ir-based complex oxides (Table S2), highlighting the promoting effect of the Sr2+ leaching process in Sr4IrO6.
Sr2+-leaching-triggered IrO6H8 self-assembly
To better understand the origin of the surface area increment induced by Sr2+ leaching, the properties of the acid-treated Sr4IrO6 are analyzed. The powder X-ray diffraction pattern (XRD) in Fig. 2a shows no diffraction peaks, confirming the amorphous nature of the acid-treated Sr4IrO6. In addition, the position of Ir–LIII absorption edge from the acid-treated Sr4IrO6 is similar to that of rutile IrO2 (Fig. S6), confirming that the acid treatment does not induce the redox of the initial Ir4+ state. This is reasonable as protons from the electrolyte are expected to enter the lattice, maintaining charge neutrality. Consequently, the acid-treated Sr4IrO6 can be described as HP-IrOxHy, representing an amorphous Ir hydroxide with a hierarchically porous structure.
a Powder XRD pattern of the acid-treated Sr4IrO6. b Ir LIII-edge extended X-ray absorption fine structure spectra from HP-IrOxHy and rutile IrO2. c CV curves from HP-IrOxHy and AIROF. d Thermogravimetry profile of HP-IrOxHy. e Kinetic barriers for the first and second dehydration processes of two Ir(OH)4(OH2)2 octahedra units in case 1. f Kinetic barriers for the first dehydration process of two Ir(OH)4(OH2)2 octahedra units in case 2. g Energy of the coupled Ir(OH)4(OH2)2 octahedra, with different initial Ir–Ir distance, after optimization. h IrO6 octahedra arrangements in Sr4IrO6-type H8IrO6 and rutile-type H8IrO6. The H atoms are omitted for simplification. i Schematic of the formation of μm-sized HP-IrOxHy from Sr4IrO6.
Interestingly, the analysis of the Ir LIII-edge extended X-ray absorption fine structure (EXAFS) spectra from HP-IrOxHy and rutile IrO2 (Fig. 2b) reveals the presence of local structures corresponding to edge- and corner-sharing IrO6 octahedra in HP-IrOxHy. This suggests that Sr2+ leaching triggers the self-assembly of initially isolated IrO6 octahedra, leading to the formation of Ir–O(H)-Ir bonds. In the Ir LIII-edge EXAFS spectra from rutile IrO2, two marked scattering peaks (~1.63 Å and ~2.85 Å) correspond to the Ir–O path in the first Ir–O shell and the Ir-Ir1 path in the edge-sharing IrO6 octahedra, respectively. The broad scattering peak above 3 Å is mainly attributed to the Ir-Ir2 path in corner-sharing IrO6 octahedra. In the acid-treated Sr4IrO6, three scattering peaks are observed at positions of ~1.60 Å, ~2.85 Å, and ~3.32 Å. By comparing these spectra with those of rutile IrO2 as a reference, the three scattering peaks in HP-IrOxHy can be attributed to the first Ir–O shell, the edge-sharing IrO6 octahedra, and the corner-sharing IrO6 octahedra, respectively.
Meanwhile, the formation of a rutile IrO2-like structure has been proposed and identified in several amorphous IrOxHy phases, such as AIROF27 and electrodeposited iridium oxide film (EIROF)28. The electrochemical behaviors of various IrOxHy materials have also been extensively studied13,22,26,29,30,31,32. The cyclic voltammetric (CV) curve, for instance, is one of the most reported data. The profiles of CV curves, reflecting the electrochemical redox behavior, can be considered as a “fingerprint” for material identification. Inspired by this, we conducted a thorough comparison of the CV curves obtained from HP-IrOxHy and previously reported IrOxHy catalysts (for detailed information, refer to Fig. S7). The comparison reveals that the formed HP-IrOxHy, derived from Sr4IrO6, exhibits similarities to the well-known AIROF. Representative CV curves from HP-IrOxHy and AIROF (prepared by electrochemical oxidation of Ir nanoparticles, Fig. S8) are shown in Fig. 2c. In both CV curves, a couple of redox peaks between ~0.60 V and ~1.05 V can be attributed to the redox process of Ir3+/Ir4+. Another pair of redox peaks, including a less pronounced oxidation peak (started at ~1.3 V but masked by the OER current) and a distinctive reduction peak (~1.4 V), can be associated with the redox process of Ir4+/Ir5.x+ (x≥0).
Ideally, considering the ion exchange between proton and Sr2+ during the leaching process, the theoretical stoichiometry of HP-IrOxHy should be IrO6H8 (disregarding the possible presence of adsorbed or crystal water). With this stoichiometry, the IrO6 octahedron can be represented as Ir(OH)4(OH2)2. However, based on the thermogravimetry profile of HP-IrOxHy (Fig. 2d), the calculated values for x and y are 3.76 and 3.52, respectively. It is important to note that these values include the contribution from adsorbed or crystal water. Consequently, the content of OH/H2O in HP-IrOxHy is significantly lower than the theoretical values, suggesting that the self-assembly of Ir(OH)4(OH2)2 octahedra occurs through a dehydration process, which can be expressed by the following equation:
The occurrence of dehydration is further supported by the instability of H8IrO6, which is expected with a structure resembling Sr4IrO6 and can be the most likely intermediate after the Sr2+ leaching from Sr4IrO6. From the calculated phonon spectra from the optimized H8IrO6 (Fig. S9), a large imaginary phonon mode around Γ point can be observed, indicating that such a H8IrO6 structure is dynamically unstable33. Then, in the following discussion, we employ a model system, consisting of two isolated Ir(OH)4(OH2)2 octahedra, to investigate the dehydration process with DFT simulation. Initially, three possibilities were considered: dehydration between two –OH groups (case 1), between –OH2 and –OH (case 2), and between two –OH2 groups (case 3). The Ir–Ir distance is fixed at 6 Å to be consistent with the practical Ir–Ir distance of 5.932 Å in Sr4IrO6 (based on the XRD refinement in Fig. S1). It is found that case 3 is unlikely, as the two Ir(OH)4(OH2)2 octahedra, with two –OH2 groups in a “head-to-head” arrangement, prefer to rotate to the arrangement of “head-to-head” –OH2 and –OH, i.e., case 2 (Fig. S10). Thus, the dehydration processes in case 1 (Fig. 2e) and case 2 (Figs. 2f and S11) are explored, and the details of the optimized structures are provided in Supplementary Data 1. In both cases, the first dehydration processes (loss of the first H2O molecule) lead to the formation of corner-sharing IrO6 octahedra and are kinetically favorable (Fig. 2e, f). Specifically, the corresponding kinetic barriers are 0.257 and 0.187 eV for case 1 and case 2 (Fig. 2e, f), respectively, both of which are easily conquered at room temperature. Interestingly, additional calculations show that further dehydration (loss of the second H2O molecule) is favorable in case 1 with a kinetic barrier of 0.270 eV (Fig. 2e) but unlikely to occur in case 2 with a high kinetic barrier of 0.412 eV (Fig. S11). As a result, the formation of edge-sharing IrO6 octahedra is expected to be the final state in case 1 (Fig. 2e). This theoretical prediction aligns with the experimental findings of the formation of both corner- and edge-sharing IrO6 octahedra in HP-IrOxHy.
It is worth noting that HP-IrOxHy particles maintained their μm-size after the dehydration process. Specifically, the hydrolysis method, which also involves the dehydration process of free monomeric [IrOxHy]z- complexes, has long been used for the preparation of IrOxHy nanoparticles or colloids34,35. Similarly, after the leaching of Sr2+ from Sr4IrO6, the resulting IrO6H8 octahedra are initially isolated (before dehydration). Then, if following the previous findings, the self-assembly of these IrO6H8 octahedra should lead to the formation of IrOxHy nanoparticles or clusters.
Apart from the dehydration reaction, another crucial requirement for the self-assembly of Ir4+OxHy octahedra is that the two octahedra should be in close proximity, with an intermolecular distance (IMD) below a critical value (the IMD is defined as Ir–Ir distance between the two Ir(OH)4(OH2)2 octahedra units). In comparison to the random distribution of free monomeric [IrOxHy]z- complexes in solution, the isolated IrOxHy octahedra derived from Sr4IrO6 exhibit a distinctive feature, namely, a confined IMD of ~6 Å. This confined IMD is inherited from the IMD of IrO6 octahedra in the initial Sr4IrO6 lattice.
Next, we employ two isolated Ir(OH)4(OH2)2 octahedra as a model system to investigate the critical IMD. The initial IMD between the two octahedra varies from 6.0 Å to 9.5 Å, with an increment of 0.5 Å. Subsequently, the two octahedra were allowed to move freely, and the optimized structures were examined. It was found that a critical IMD of 8.5 Å exists. Specifically, when the IMD is ≤8.0 Å (Fig. S12), the two isolated octahedra spontaneously approach each other. The corresponding reduced system energies (Fig. 2g) indicate the thermodynamic feasibility of this approach. In contrast, when the IMD is ≥8.5 Å, such approaching behavior does not occur (Fig. S12), and the system energies remain high (Fig. 2g), suggesting that the driving force, such as intermolecular forces, between the two octahedra is relatively weak. The confined IMD (~6 Å) of IrO6 octahedra in Sr4IrO6 is lower than the critical IMD. This implies that when Sr2+ leaches out from the lattice of Sr4IrO6, the IrO6H8 octahedra prefer to approach each other (leading to the dehydration and formation of -Ir–O(H)-Ir- framework) rather than dispersing into the solution.
Simultaneously, the initial loose arrangement of IrO6 octahedra in Sr4IrO6 likely plays a crucial role in the formation of a highly porous structure. Figure 2h illustrates the arrangement of IrO6 octahedra in Sr4IrO6-type and rutile-type H8IrO6, with the latter being a possible motif in HP-IrOxHy. In a single unit of Sr4IrO6-type H8IrO6, there are six isolated Ir(OH)4(OH2)2 octahedra occupying a space of approximately 976 Å3. On the other hand, in a single unit of rutile-type H8IrO6, the occupied space is around 192 Å3 when there are six equivalent Ir(OH)4(OH2)2 octahedra. By considering the conservation of Ir atoms/octahedra, it can be found that the Sr2+ leaching and Ir(OH)4(OH2)2 self-assembly in a single H8IrO6 unit result in a significant volume change of ~784 Å3 (an approximately 80% reduction), leading to densification. Evidently, such densification accompanied by a distinctive volume change, if does not cause particle shrinkage, will induce the formation of a porous structure within the particles.
With the aforementioned findings, the process of the formation of HP-IrOxHy from Sr4IrO6 can be summarized as follows (as depicted in Fig. 2i): First, the leaching of Sr2+ enables the generation of free and isolated IrOxHy octahedra, such as Ir(OH)4(OH2)2 octahedra. Secondly, with an initial IMD of ~6 Å (which is below the critical value of 8.5 Å), the formation of a framework comprising corner- and edge-sharing IrO6 octahedra through dehydration within μm-sized particles is facilitated. Thirdly, the densification of the lattice ensures the high porosity of the resulting framework, which is observed to be hierarchically porous in this study.
Activity evaluation
Encouraged by the promising results of HP-IrOxHy, we further employ ball milling (BM) to reduce the particle size. The BET surface area of the ball-milled HP-IrOxHy (HP-IrOxHy-BM) increased to 80.4 m2 g−1 (Fig. S13). Furthermore, a comprehensive analysis is conducted to assess the impact of the BM process on the formation of the AIROF-like phase. XPS analysis (Fig. S14), CV profile comparison (Fig. S15), and activity evaluation (Fig. S16) all confirm that the BM process does not affect the formation of the AIROF-like active phase.
Rotating disk electrode (RDE)-based three-electrode system is then applied to study the catalytic activity of selected electrocatalysts, including IrOxHy nanoparticle from Alfa-Aesar (IrOxHy-AA), 20 wt. % Ir nanoparticle/C from Fuel Cell store (Ir/C-FC), electrochemically activated Sr4IrO6-BM (E-Sr4IrO6-BM), and the HP-IrOxHy-BM (Fig. 3). In addition, in Fig. S18, the mass activities of state-of-the-art nano IrO2, nano Ir, and AIROF-like nano IrOxHy are summarized to assess the advancement of the HP-IrOxHy-BM. As expected, the mass activities of nano IrO2 catalysts are significantly lower compared to the AIROF-like catalysts, emphasizing the high intrinsic activity of AIROF. Finally, HP-IrOxHy-BM demonstrates a notable mass activity, supporting its cost competitiveness as a water oxidation catalyst in practical MEAs.
Mass activity of state-of-the-art nano-sized IrO2, nano-sized Ir metal, nano-sized IrOxHy, E-Sr4IrO6-BM, and μm-sized HP-IrOxHy-BM catalysts. E-Sr4IrO6-BM is derived from Sr4IrO6-BM, which is electrochemically activated with CV scanning (Fig. S17). The error bars represent the standard deviation derived from three independent measurements.
Performance in MEAs
Activity
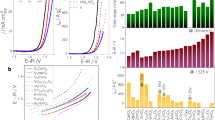
Figure 4a illustrates the polarization curves of MEAs with the HP-IrOxHy-BM anode. The state-of-the-art Ir-based MEAs with anode catalysts of IrVI-ado36, IrOx@TiO215, IrO2@TiO215, NSTF37, Sr2CaIrO638, and IrOxHy-hybrid39 are also included. The MEA with the Ir/C-FC anode catalyst exhibits much inferior performance, despite its high mass activity over RDE measurements (Fig. 3). This difference can be attributed to the oxidation-induced degradation of the carbon support. Due to the high intrinsic activity of AIROF-like catalysts, the two MEAs with IrOxHy-AA and HP-IrOxHy-BM outperform the MEA with the rutile IrO2 from Sigma-Aldrich (IrO2-SA). For example, at a current density of 1 A cm−2, the corresponding cell voltage decreases from 1.81 V (IrO2-SA) to 1.57 V (IrOxHy-AA) and 1.54 V (HP-IrOxHy-BM). Notably, even lower cell voltages are achieved with the MEA utilizing HP-IrOxHy-BM, especially at high current densities. For example, an additional drop in cell overpotential (\({\Delta \eta }_{{overall}}\)) of approximately 190 mV is observed at a working current density of 4 A cm−2. As a result, the performance of the MEA with HP-IrOxHy-BM anode catalyst surpasses many previous reports and is comparable to that reported by Bernt et al.40.
a Polarization curves of MEAs with the HP-IrOxHy-BM anode and the state-of-the-art Ir-based MEAs in PEMWEs. Both supported and support-free electrocatalysts are considered. Four MEAs, with anode catalysts of Ir/C-FC (0.375 mgIr cm−2), IrOxHy-AA (0.375 mgIr cm−2), IrO2-SA (0.375 mgIr cm−2), and HP-IrOxHy-BM (0.375 mgIr cm−2), are measured in this work, while the rest MEAs, with anode catalysts of IrVI-ado (0.08 mgIr cm−2), IrOx@TiO2 (Heraeus, 0.3 mgIr cm−2), IrO2@TiO2 (Umicore, 2.5 mgIr cm−2), NSTF (3 M, 0.375 mgIr cm−2), Sr2CaIrO6 (0.4 mgIr cm−2), and IrOxHy-hybrid (0.2 mgIr cm−2) are from references. For a fair comparison, only those subjected to similar testing conditions (membrane thickness of ~50 μm, 80 °C, 1 atm) are selected. b Tafel plots of the HFR-free cell voltage in cells with IrOxHy-AA and HP-IrOxHy-BM anodes. Inset is the Tafel plots between 10 and 100 mA cm−2, with the Tafel slopes obtained through linear fitting. c \({\eta }_{m}\) from PEM water electrolyzers with engineered Ir-based anodes. d MEA-based TOF from HP-IrOxHy-BM. TOFs from state-of-the-art anode catalysts of IrO2@TiO2 (Elyst Ir75 0480, Umicore) and IrVI-ado are also included. The error bars (shaded areas) represent the standard deviation calculated from three independent measurements using the same MEA for each sample.
In PEMWEs, the overall cell overpotential can be primarily attributed to three factors: cell Ohmic loss, electrode kinetic loss, and mass-transport-related loss. Therefore, the \({\Delta \eta }_{{overall}}\) can be expressed as follows:
where \({\Delta \eta }_{o}\), \({\Delta \eta }_{k}\), and \({\Delta \eta }_{m}\) are anode-difference-induced change of Ohmic loss, electrode kinetic loss, and mass-transport related loss, respectively.
where \(i\) is the current density and \(\Delta R\) is anode-difference-induced change of cell ohmic resistance (can be estimated from the measured high-frequency resistances (HFRs). It can be found that, for both anodes, the HFRs (Fig. S19) are almost unchanged as the current density increase, and a reduction (∆R) of ≈20 mΩ cm2 can be observed when changing the anode from IrOxHy-AA to HP-IrOxHy-BM. Thus, the lower cell voltages can be partially related to a reduced HFRs.
The analysis of electrode kinetic and mass-transport-related losses is conducted by subtracting the total Ohmic loss, referred to as HFR-free cell voltage. Figure 4b illustrates the Tafel plots of the iR-corrected cell voltages, and the Tafel slopes are determined by fitting the data from 0.01 A cm−2 to 0.1 A cm−2 (inset of Fig. 4c), where the mass-transport related loss is considered negligible. At low current densities (≤0.1 A cm−2), the HFR-free cell voltages with the two anodes exhibit close proximity to each other. Tafel slopes of 41.5 ± 1.4 mV dec−1 and 39.6 ± 0.8 mV dec−1 are obtained from HP-IrOxHy-BM and IrOxHy-AA, respectively. It is then reasonable to conclude that the kinetic losses for the two anodes are nearly identical, i.e., \({\Delta \eta }_{k}\approx 0\). Then, the observed \({\Delta \eta }_{{overall}}\) can be predominantly attributed to the mass-transport-related loss (\({\Delta \eta }_{m}\)) within the anode catalyst layer.
Similar to the venation pathway in a tree, a hierarchical transport structure is deemed as an optimal solution for mass transport41. Compared to the catalyst layer with non-structured IrOxHy particles (Fig. S20a, b), it can be asserted that the key role is played by the anode layer composed of HP-IrOxHy-BM particles (Fig. S20c, d). As shown in Fig. 4c, the advancement of such hierarchically porous structure is further supported by the estimated mass-transport overpotentials (\({\eta }_{m}\)), which are substantially lower than those from the electrolyzers with engineered anodes, such as ionomer-free porous transport electrodes (Ir PTE)42, nanoporous Iridium nanosheets (Ir nanosheets)20, IrO2 from Alfa-Aesar (IrO2-AA)43, IrO2@TiO2 (Elyst Ir75 0480, Umicore)44, IrO2 anode with patterned grooves43, IrOxHy-AA39, and IrOxHy-hybrid39. Note that the turnover frequency (TOF) obtained from a three-electrode system is often used to evaluate the intrinsic activity of an active site. In this study, we apply MEA-based TOF, which includes MEA-related factors such as mass transport, proton transport, and Ir utilization rate to express the activity of Ir in practical electrolyzers. As shown in Figs. 4d and S21, the HP-IrOxHy-BM shows high MEA-based turnover frequencies (TOFs), which outperform those from state-of-the-art anode catalysts, such as IrO2@TiO2 and IrVI-ado.
Stability/durability
The stability of HP-IrOxHy-BM is evaluated using a PEMWE, and chronopotentiometric measurements are employed. The PEMWE (Fig. 5a) with HP-IrOxHy-BM anode exhibits stable performance. This is reasonable considering the short working hours and the intrinsic high stability of Ir. The activity versus stability of HP-IrOxHy-BM during operation is then assessed using the S-number metric, which considers Ir dissolution45. In the PEMWE measurement (Fig. 5a), the S-number increases from \(1.5\times {10}^{8}\) (~41 h) to \(0.94\times {10}^{9}\) (~354 h) to a relatively stable value of \(1.1\times {10}^{9}\) (~380 h). This monotonic increment can be related to the effect of operation time46. Intriguingly, the final S-number of HP-IrOxHy-BM in the MEA-based electrolyzer is substantially higher than a benchmark S-number of \(1.5\times {10}^{8}\)36, highlighting the practical application of HP-IrOxHy-BM in PEMWEs.
a Top is chronopotentiometric measurement (at 1 A cm−2) for the MEA with the HP-IrOxHy-BM anode and bottom is the estimated S-number at different time slots. The benchmark S-number is from IrVI-ado. b Extracted power output profile from practical solar panels in the daytime (green solid line). The orange dots are the applied power input profile (~10.5 h) for the MEA. c The recorded cell voltage and current profiles. The tests are repeated/cycled seven times. d Estimated Sd-numbers vs. cycle number. The stability/durability measurements were performed once, and the standard deviation of S-/Sd-numbers is estimated from ICP-MS results.
A major advantage of PEMWEs is their rapid response to load changes. Therefore, a qualified catalyst for PEMWEs should exhibit high durability under significantly fluctuating input power, particularly from renewable energy sources. Here, to better simulate the practical operating conditions of PEMWE, we apply fluctuating power instead of programmed potential or current profiles to study the durability of our PEMWE. In Fig. 5b (solid line), a typical power output profile obtained from practical solar panels (Fig. S22) during the daytime is presented. The durability of the PMEWE with the HP-IrOxHy-BM anode is then assessed using a simulated power input profile (open dots in Fig. 5b), which is cycled seven times. The recorded voltage and current profiles are shown in Fig. 5c. In seven cycles, the voltage/current profiles exhibit a high degree of overlap, indicating the good durability of the PEMWE under investigation. Furthermore, the stable HFR-free cell voltages (Fig. S23) also suggest the robustness of the anode during the cycling process.
To evaluate the activity versus durability of HP-IrOxHy-BM, the total Ir dissolution is measured in each cycle. To differentiate it from the commonly used S-number, we use Sd-number to emphasize the applied renewable energy-based power input profile. As shown in Fig. 5d, over the course of 7 cycles, the corresponding Sd-numbers can range from \(2.3\times {10}^{8}\) to \(\sim1.0\times {10}^{9}\). Intriguingly, the evolution of the Sd-number differs from that of the S-number (Fig. 5a) from chronopotentiometric measurements (Fig. 5a). On one hand, the Sd-number reaches a relatively stable value of \(\sim1.0\times {10}^{9}\) in ~30 h (initial 3 cycles), which is much shorter than the time required for the S-number (>200 h). On the other hand, the increase in the Sd-number is not consistently monotonic. From cycles 3 to 7, the Sd-numbers exhibit slight variation in the vicinity of \(1.0\times {10}^{9}\). Considering the observed disparity, gaining a deeper understanding of catalyst durability under renewable energy-based power input profiles would be highly valuable for catalyst optimization. Furthermore, considering the benchmark stability of crystalline rutile IrO29, more effort is needed to enhance the stability/durability of amorphous IrOxHy.
In this work, we demonstrate a metal-oxide-based molecular self-assembly strategy to construct support-free electrocatalysts with hierarchically porous structure, perfectly matching the requirements of anode catalysts for practical MEAs. In this strategy, the key is designing a proper precatalyst that facilitates the cation-leaching-triggered molecular self-assembly. The designed Sr4IrO6 precatalyst provides densely isolated single IrO6 octahedra. The distance between the neighboring IrO6 octahedra, ~6 Å, is neither too short nor too long, ensuring the formation of a robust -Ir–O(H)-Ir- framework through dehydration and the development of a hierarchically porous structure through densification. The μm-sized hierarchically porous structure enhances anode mass transport. In the MEA-based measurements, the HP-IrOxHy-BM catalyst exhibits notable electrochemical performance (e.g., a TOF of 5.31 s−1 @ 1.52 V and a S-number of \( > 1\times {10}^{9}\)), outperforming several other Ir-based catalysts (e.g., a TOF of 3.36 s−1 @ 1.55 V and a S-number of \(1.5\times {10}^{8}\) from IrVI-ado). Such a metal-oxide-based molecular self-assembly strategy, in conjunction with well-designed precatalysts, presents an easily accessible approach to develop advanced electrocatalysts for high-performance MEA devices.
Methods
Sample preparation
The Sr4IrO6 was synthesized with the solid-state reaction. Stoichiometric amounts of SrCO3 (Sigma-Aldrich, 99.9%) and IrO2 (Sigma-Aldrich, 99.9%), were thoroughly ground and calcined at 1200 °C for 0.5 h under ambient air. The chemical leaching was performed by immersing ~0.1 g sample in 50 mL 0.1 M HClO4 solution at room temperature, the Sr2+ leaching would proceed spontaneously. During the leaching process, the solution needed to be refreshed 1–2 times. The final product will be rinsed with pure water to remove the residual acid and Sr2+. For the preparation of ball-milled samples, the as-prepared Sr4IrO6 is ball-milled with a planetary ball mill (FRITSCH PULVERISETTE 7 premium line). ZrO2 grinding bowls (80 mL) and balls (3 mm) are applied and the rotational speed is 400 rpm.
Electrode preparation and RDE-based electrochemical characterization
The electrodes were prepared by drop-casting as-prepared catalyst ink (10 μL) on a glassy carbon RDE with a surface area of 0.196 cm2. The ink was prepared by mixing 2 mg catalyst powder with 1 mg acetylene black carbon, which was ultrasonically dispersed in the solution containing 750 μL H2O, 245 μL isopropanol, and 5 μL Nafion solution (5 wt.%, Sigma-Aldrich). The electrochemical tests were conducted in Ar-saturated 0.1 M HClO4 (pH = 1.08), and the temperature is set to be 25 °C (controlled with circulating water bath). OER measurements are performed with a biologic SP-150 potentiostat coupled with the modulated speed rotator (Pine Research Instrumentation). In total, 100% iR-compensation was applied, and the uncompensated resistance (R value of ~30 ohm) was measured by electrochemical impedance spectroscopy (EIS). A Pt plate was used as the counter electrode and a saturated calomel electrode (SCE) was used as the reference electrode. Scaling to RHE is performed using Evs.RHE = Evs. SCE + 0.241 V + 0.059 V× pH. A high rotation speed of 2500 rpm was applied to ensure a fast O2 bubble removal. Non-Faradaic currents are excluded with capacitance correction. Specifically, CV scanning is performed at a rate of 10 mV s−¹, and the OER currents are obtained by averaging the forward and backward CV currents. At least three measurements were performed when evaluating the OER activities of different catalysts.
Characterization
XRD measurements were performed with a BRUKER D8 Advance diffractometer in Bragg-Brentano geometry with Cu Kα radiation. A GSAS program and EXPGUI interface were used for the Rietveld refinement47. SEM was performed with JEOL JSM-7600F. FIB-SEM and TEM were performed on FEI Quata 3D FEG and JEOL 2100 F, respectively. The surface composite was analyzed with X-ray photoelectron spectroscopy (Kratos AXIS Supra). The BET surface areas were measured with nitrogen adsorption-desorption tests (ASAP Tri-Star II 3020). X-ray adsorption spectroscopy experiments were performed at the Singapore Synchrotron Light Source, XAFCA beamline. The Athena software packages were used for the data analysis. Inductively coupled plasma mass spectrometry (ICP-MS) is performed on PerkinElmer ELAN DRC-e. Inductively coupled plasma-optical emission spectroscopy (ICP-OES) is performed on a Thermo Fisher iCAP6000 ICP emission spectrometer.
MEA preparation and cell assembly
The MEA was prepared by a catalyst-coated membrane (CCM) method. As received Nafion 212 membrane was air sprayed with 0.375 mgIr cm−2 IrOxHy (IrO2-SA, Ir/C-FC, IrOxHy-AA, and HP-IrOxHy-BM) and 0.5 mgPt cm−2 40 wt. % Pt/C on each side as anode and cathode catalysts, respectively. The catalyst ink was prepared by mixing 50 mg of IrOxHy or 40 wt. % Pt/C catalyst with 7.41 mL isopropanol, 2.47 mL deionized water and 0.12 mL 5 wt. % Nafion D-520 solution, then ultrasonicating it for at least 1 h. The CCM was then sandwiched between the Pt-coated Ti felt (anode porous transport layer) and Toray 090 carbon paper (cathode gas diffusion layer). Two Pt-coated Ti blocks with a single serpentine channel were used as bipolar plates and current collectors. Viton gaskets with suitable thicknesses were also placed to prevent liquid/gas leakage. The torque applied to assemble the cell was 8.5 Nm, and the active area of the MEA was 5 cm2.
Electrochemical tests of MEAs
The electrochemical tests were performed with an Autolab potentiostat or a biologic SP-150 with a 20 A booster. The cell temperature was maintained at 80 °C by two electric heating plates and measured by a thermocouple placed beside the two bipolar plates. Deionized water was pumped into the cell at 2 ml min-1 by peristaltic pump and preheated in a heating coil before flowing into the cell. The PEMWE was first pretreated/activated with chronoamperometry at 1.8 V for 30 min. The polarization curve was obtained by chronopotentiometry of current densities from 10 mA cm−2 to 4 A cm−2 at each current density for 60 s. EIS measurements, in a range of 10 Hz–20 kHz with current perturbations of ± 200 mA, were carried out at each current step to determine the high-frequency resistance, which was obtained from the high-frequency intercept of the Nyquist plot with the real axis. The fixed current stability tests were conducted by applying the current density of 1 A cm−2 for ~400 h. The outlet anolytes were collected at different time points, and the dissolved Ir in the collected electrolyte was measured by ICP-MS. A model solar profile, extracted from a practical solar profile was also used to mimic the durability of PEMWE with solar power supply. The applied power input was controlled under 35 W. The same profile pattern was applied for seven cycles, and the outlet anolyte (after each cycle) was collected, and the dissolved Ir was measured by ICP-MS.
DFT computations
Spin-polarized density functional theory (DFT) is completed within the Vienna ab initio simulation package (VASP)48. The ion-electron interaction is described using the projector-augmented plane-wave49, and the exchange-correlation interaction is described using the Perdew–Burke–Ernzerhof functional of the generalized gradient approximation50. A cut-off energy of 500 eV with 3 × 3 × 3 Monkhorst−Pack k-point grids are used for the calculation of two Ir(OH)4(OH2)2 octahedra units. All the systems are optimized until energy and force were less than 10−5 eV and 0.01 eV/Å. The vacuum layer larger than 15 Å is used to prevent the interaction between periodical slabs. Grimme’s D3 method is adopted to consider the van der Waals (vdW) interactions51. The climbing-image nudged elastic band (CINEB) method52 is used to identify transition states and kinetic barriers. Phonon bands are obtained employing density functional perturbation theory as implemented in Phonopy code53.
Data availability
All data supporting the findings of this study are available within the article and its Supplementary Information/Source Date file, or from the authors upon reasonable request. Source data are provided with this paper.
Change history
26 June 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41467-025-61210-5
References
Chatenet, M. et al. Water electrolysis: from textbook knowledge to the latest scientific strategies and industrial developments. Chem. Soc. Rev. 51, 4583–4762 (2022).
Ayers, K. et al. Perspectives on low-temperature electrolysis and potential for renewable hydrogen at scale. Annu. Rev. Chem. Biomol. Eng. 10, 219–239 (2019).
Huang, J. E. et al. CO2 electrolysis to multicarbon products in strong acid. Science 372, 1074–1078 (2021).
van Langevelde, P. H., Katsounaros, I. & Koper, M. T. M. Electrocatalytic nitrate reduction for sustainable ammonia production. Joule 5, 290–294 (2021).
Zhang, X. et al. Electrochemical oxygen reduction to hydrogen peroxide at practical rates in strong acidic media. Nat. Commun. 13, 2880 (2022).
Hong, W. T. et al. Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis. Energy Environ. Sci. 8, 1404–1427 (2015).
Mauritz, K. A. & Moore, R. B. State of understanding of Nafion. Chem. Rev. 104, 4535–4586 (2004).
Kibsgaard, J. & Chorkendorff, I. Considerations for the scaling-up of water splitting catalysts. Nat. Energy 4, 430–433 (2019).
Wei, C. et al. Benchmarking electrocatalyst stability for acidic oxygen evolution reaction: the crucial role of dissolved ion concentration. ACS Catal. 13, 14058–14069 (2023).
Alia, S. M. et al. Activity and durability of iridium nanoparticles in the oxygen evolution reaction. J. Electrochem. Soc. 163, F3105–F3112 (2016).
Rand, D. A. J. & Woods, R. Cyclic voltammetric studies on iridium electrodes in sulphuric acid solutions: nature of oxygen layer and metal dissolution. J. Electroanal. Chem. Interfacial Electrochem. 55, 375–381 (1974).
Beni, G., Rice, C. E. & Shay, J. L. Electrochromism of anodic iridium oxide films: III. anion mechanism. J. Electrochem. Soc. 127, 1342 (1980).
Gottesfeld, S. & Srinivasan, S. Electrochemical and optical studies of thick oxide layers on iridium and their electrocatalytic activities for the oxygen evolution reaction. J. Electroanal. Chem. Interfacial Electrochem. 86, 89–104 (1978).
Geiger, S. et al. Activity and stability of electrochemically and thermally treated iridium for the oxygen evolution reaction. J. Electrochem. Soc. 163, F3132 (2016).
Bernt, M. et al. Current challenges in catalyst development for PEM water electrolyzers. Chem. Ing. Tech. 92, 31–39 (2019).
Böhm, D. et al. Highly conductive titania supported iridium oxide nanoparticles with low overall iridium density as OER catalyst for large-scale PEM electrolysis. Appl. Mater. Today 24, 101134 (2021).
Wang, Y. et al. Nano-metal diborides-supported anode catalyst with strongly coupled TaOx/IrO2 catalytic layer for low-iridium-loading proton exchange membrane electrolyzer. Nat. Commun. 14, 5119 (2023).
Moriau, L., Smiljanić, M., Lončar, A. & Hodnik, N. Supported iridium-based oxygen evolution reaction electrocatalysts—recent developments. ChemCatChem. 14, e202200586 (2022).
Alia, S. M., Shulda, S., Ngo, C., Pylypenko, S. & Pivovar, B. S. Iridium-based nanowires as highly active, oxygen evolution reaction electrocatalysts. ACS Catal. 8, 2111–2120 (2018).
Chatterjee, S. et al. Nanoporous iridium nanosheets for polymer electrolyte membrane electrolysis. Adv. Energy Mater. 11, 2101438 (2021).
Cherevko, S., Geiger, S., Kasian, O., Mingers, A. & Mayrhofer, K. J. J. Oxygen evolution activity and stability of iridium in acidic media. Part 2.—Electrochemically grown hydrous iridium oxide. J. Electroanal. Chem. 774, 102–110 (2016).
Chen, Y. et al. Lattice site–dependent metal leaching in perovskites toward a honeycomb-like water oxidation catalyst. Sci. Adv. 7, eabk1788 (2021).
Chen, Y. et al. Exceptionally active iridium evolved from a pseudo-cubic perovskite for oxygen evolution in acid. Nat. Commun. 10, 572 (2019).
Seitz, L. C. et al. A highly active and stable IrOx/SrIrO3 catalyst for the oxygen evolution reaction. Science 353, 1011–1014 (2016).
Seow, J. Z. Y., Chen, Y., Ge, J., Fisher, A. C. & Xu, Z. J. Deciphering the poisoning effect of sulfate on a perovskite-derived IrOxHy catalyst for water oxidation in acid. J. Electrochem. Soc. 170, 044507 (2023).
Zhang, R. et al. First example of protonation of ruddlesden–popper Sr2IrO4: a route to enhanced water oxidation catalysts. Chem. Mater. 32, 3499–3509 (2020).
Pavlovic, Z., Ranjan, C., Gao, Q., van Gastel, M. & Schlögl, R. Probing the structure of a water-oxidizing anodic iridium oxide catalyst using Raman spectroscopy. ACS Catal. 6, 8098–8105 (2016).
Huang, J. et al. Domain structure for an amorphous iridium-oxide water-oxidation catalyst characterized by X-ray pair distribution function analysis. Phys. Chem. Chem. Phys. 16, 1814–1819 (2014).
Nong, H. N. et al. A unique oxygen ligand environment facilitates water oxidation in hole-doped IrNiOx core–shell electrocatalysts. Nat. Catal. 1, 841–851 (2018).
Bozal-Ginesta, C. et al. Redox-state kinetics in water-oxidation IrOx electrocatalysts measured by operando spectroelectrochemistry. ACS Catal. 11, 15013–15025 (2021).
Kang, K. S. & Shay, J. L. Blue sputtered iridium oxide films (blue SIROF’s). J. Electrochem. Soc. 130, 766 (1983).
Pearce, P. E. et al. Revealing the reactivity of the iridium trioxide intermediate for the oxygen evolution reaction in acidic media. Chem. Mater. 31, 5845–5855 (2019).
Wang, L. et al. Intercalated architecture of MA2Z4 family layered van der Waals materials with emerging topological, magnetic and superconducting properties. Nat. Commun. 12, 2361 (2021).
Zhao, Y., Hernandez-Pagan, E. A., Vargas-Barbosa, N. M., Dysart, J. L. & Mallouk, T. E. A high yield synthesis of ligand-free iridium oxide nanoparticles with high electrocatalytic activity. J. Phys. Chem. Lett. 2, 402–406 (2011).
Wöhler, L. & Witzmann, W. Die Oxyde des Iridiums. Z. f. ür. Anorganische Chem. 57, 323–352 (1908).
Li, A. et al. Atomically dispersed hexavalent iridium oxide from MnO2 reduction for oxygen evolution catalysis. Science 384, 666–670 (2024).
Lewinski, K. A., van der Vliet, D. & Luopa, S. M. NSTF advances for PEM electrolysis—the effect of alloying on activity of NSTF electrolyzer catalysts and performance of NSTF based PEM electrolyzers. ECS Trans. 69, 893 (2015).
Retuerto, M. et al. Highly active and stable OER electrocatalysts derived from Sr2MIrO6 for proton exchange membrane water electrolyzers. Nat. Commun. 13, 7935 (2022).
Hegge, F. et al. Efficient and stable low iridium loaded anodes for PEM water electrolysis made possible by nanofiber interlayers. ACS Appl. Energy Mater. 3, 8276–8284 (2020).
Bernt, M. et al. Effect of the IrOx conductivity on the anode electrode/porous transport layer interfacial resistance in PEM water electrolyzers. J. Electrochem. Soc. 168, 084513 (2021).
Pham, C. V., Escalera-López, D., Mayrhofer, K., Cherevko, S. & Thiele, S. Essentials of high performance water electrolyzers—from catalyst layer materials to electrode engineering. Adv. Energy Mater. 11, 2101998 (2021).
Lee, J. K. et al. Ionomer-free and recyclable porous-transport electrode for high-performing proton-exchange-membrane water electrolysis. Nat. Commun. 14, 4592 (2023).
Yuan, S. et al. Bubble management in PEM water electrolysis via imprinting patterned grooves on catalyst layer. Int. J. Heat. Mass Transf. 212, 124249 (2023).
Bernt, M., Siebel, A. & Gasteiger, H. A. Analysis of voltage losses in PEM water electrolyzers with low platinum group metal loadings. J. Electrochem. Soc. 165, F305 (2018).
Geiger, S. et al. The stability number as a metric for electrocatalyst stability benchmarking. Nat. Catal. 1, 508–515 (2018).
Knöppel, J. et al. On the limitations in assessing stability of oxygen evolution catalysts using aqueous model electrochemical cells. Nat. Commun. 12, 2231 (2021).
Toby, B. H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 34, 210–213 (2001).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 27, 1787–1799 (2006).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Togo, A. & Tanaka, I. First principles phonon calculations in materials science. Scr. Mater. 108, 1–5 (2015).
Acknowledgements
The authors thank the Facility for Analysis, Characterization, Testing, and Simulation (FACTS) at Nanyang Technological University for materials characterization. This work is supported by the National Natural Science Foundation of China (No. 22409174), The National Key Research and Development Program of China (No. 2024YFA1509200, No. 2024YFA1509203, No. 2024YFA1509204), and Startup Foundation for Hundred-Talent Program of Zhejiang University to Y.C. This work is also supported by the Agency for Science, Technology and Research (A*STAR) MTC Individual Research Grants (IRG) M22K2c0078 and Singapore National Research Foundation under its Campus for Research Excellence and Technological Enterprise (CREATE) programme to Z.J.X.
Author information
Authors and Affiliations
Contributions
Supervision: Z.J.X. Conceptualization: Y.C. Methodology: Y.C., C.D., Q.W., and Z.J.X. Investigation: Y.C., C.D., Q.W., H.L., S.X., J.Z.Y.S., S.L., Y.B., Y.X., and Y.J. Visualization: Y.C. and F.M. Funding acquisition: Z.J.X., A.C.F., and Y.C. Project administration: Y.C. and Z.J.X. Writing—original draft: Y.C., C.D., and Q.W. Writing—review and editing: Z.J.X. and Y.C.
Corresponding authors
Ethics declarations
Competing interests
Y.C., Z.J.X., and A.C.F. have filed a provisional PCT patent application (PCT/SG2024/050309) regarding the preparation of support-free iridium hydroxide for oxygen evolution reaction. The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Min-Rui Gao, Huabin Zhang and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, Y., Dai, C., Wu, Q. et al. Support-free iridium hydroxide for high-efficiency proton-exchange membrane water electrolysis. Nat Commun 16, 2730 (2025). https://doi.org/10.1038/s41467-025-58019-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-58019-7