Abstract
Personalized synergistic antibacterial agents against diverse bacterial strains are receiving increasing attention in combating antimicrobial resistance. However, the current research has been struggling to strike a balance between strain specificity and broad-spectrum bactericidal activity. Here, we propose a bacterial cell wall-specific antibacterial strategy based on an in situ engineered nanocomposite consisting of carbon substrate and decorated TiOx dots, termed TiOx@C. The fiber-like carbon substrate of TiOx@C is able to penetrate the bacterial membrane of Pseudomonas aeruginosa (P. aeruginosa), but not that of Staphylococcus aureus (S. aureus) due to its thicker bacterial wall, thus achieving bacterial wall specificity. Furthermore, a series of experiments demonstrate the specific electro-mechanical co-sterilization effect of TiOx@C. On the one hand, TiOx@C can disrupt the electron transport chain and block the energy supply of S. aureus. On the other hand, TiOx@C capable of destroying the membrane structure of P. aeruginosa could cause severe mechanical damage to P. aeruginosa as well as inducing oxidative stress and protein leakage. In vivo experiments demonstrate the efficacy of TiOx@C in eliminating 97% of bacteria in wounds and promoting wound healing in wound-infected female mice. Overall, such a bacterial cell wall-specific nanomedicine presents a promising strategy for non-antibiotic treatments for bacterial diseases.
Similar content being viewed by others
Introduction
The emergence of antimicrobial resistance (AMR) in bacteria has posed a challenge to intractable infections1. Antibiotics and microorganisms have combatted each other for nearly a century, in which bacteria have developed adaptive ability to evade the activity of many conventional antibiotics2. AMR bacteria bring a global crisis, increasing the morbidity and mortality of infected individuals and negatively impacting clinical outcomes in diverse populations3. In 2022, the WHO Global Report on Infection Prevention and Control identified 4.95 million deaths related to bacterial AMR worldwide in 20194. There is an urgent need to tackle the progression of bacterial AMR, which requires global comprehensive initiatives, including the rational use of antibiotics, stronger regulation, and the development of innovative ‘outside of the box’ therapeutics.
Therapeutics based on nanomaterials are promising tools for combating hard-to-treat bacterial infections, with the characteristic advantage of being able to evade existing mechanisms associated with acquired drug resistance5,6. In recent years, a broadening range of nanomaterials has been developed to efficiently fight infections, which can kill bacteria through several mechanisms including membrane damage through physical/chemical interactions, disruption of electron transport homeostasis and the respiratory chain, and inducing bacterial oxidative stress through direct generation of reactive oxygen species (ROS) through catalytic processes7,8. Nevertheless, the development of drug resistance is a complicated process, and different bacterial strains exhibit varying degrees of resistance9. For example, compared to Gram-positive bacteria, Gram-negative bacteria usually have more specific protein pumps that pump antibiotics out of the cell10. So far, the antibacterial nanomaterials have shown a significant increase in bactericidal efficacy, whereas the recognition of specific bacterial strains is unsatisfactory. Therefore, specific sterilization of different strains of bacteria via multi-targeting bactericidal mechanisms is of great significance for the development of elaborated antibacterial nanomedicine11,12. Prevailing strategies have proven successful in targeting Gram-positive (G + ) or Gram-negative (G-) bacteria in a strain-selective manner based on differences in cell membranes, such as membrane charge and sugar composition13,14,15. Another approach to achieving selective antimicrobial effect is to modify the chemical structure of the antimicrobial nanoagent, such as charge, hydrophobicity and functionality, which is also determined by the bacterial outer membrane, the primary interface between the material and the bacteria14,16,17,18,19. Considering that Gram-negative and positive bacteria have completely different membrane structures, we hypothesize that the binding interactions of the same antimicrobial agent with different bacterial membranes will be differing. Tang et al. have reported a series of phospholipid mimetic aggregation-induced emission luminogens (AIEgens) that selectively kill bacteria through regulating the lengths of the substituted alkyl chains of the AIEgens, which were designed based on the different structures of the two types of bacterial membranes20. Unfortunately, such a model of selective elimination of bacteria would deactivate the antibacterial effect on another bacterium, which is not conducive to the development of broad-spectrum nano-antimicrobials.
Thanks to their component-tunable and easily modifiable features, nanomaterials have the prospect of achieving strain-specific, multi-target, broad-spectrum bactericidal effects, which greatly facilitate the handling of antibiotic resistance21,22,23. Titanium dioxide (TiO2)-based photocatalysts have shown promising potential in the field of antimicrobial agents thanks to their high efficiency, low cost, and biocompatibility24,25,26. In recent years, a growing number of researchers have developed many emerging titanium oxide-based antibacterial strategies. Yang et al. prepared TiO2/TiO2-x metasurfaces with potent NIR-responsive antimicrobial activity on titanium alloy implants by an alkaline–acid bidirectional hydrothermal (aaBH) method, which showed great antibacterial effect under low-power NIR irradiation27. However, to enhance the ability to treat infections in vivo, nanomaterials with antimicrobial capacity in dark condition are required. Li et al. reported that black titania nanotube arrays (B-TNT) on a titanium substrate had significant bactericidal capacity in dark condition by generating superoxide anions, hydroxyl radicals and singlet oxygen28. Therefore, it is imperative to develop new titanium oxide-based nanomaterials which possess broad-spectrum bactericidal activity without the need for external stimuli, and have a synergistic antibacterial effect with multiple bactericidal mechanisms.
Here, we present an antibacterial nanoagent (TiOx@C) with both bacterial cell wall specificity and broad-spectrum bactericidal properties. The TiOx@C nanocomposite, which is composed of a carbon substrate and decorated multivalent TiOx quantum dots, was obtained from the MXene nanomaterial (Ti3C2) through its self-structural evolution via facile, gentle oxidation. In such a two-in-one nanomaterial, the carbon substrate endows it with cell wall selectivity, and the TiOx quantum dots on the surface enable an enhanced electron-donor effect. A series of in vitro experiments have revealed that TiOx@C, which is unable to penetrate the bacterial membrane of Staphylococcus aureus (S. aureus) due to size effects, can selectively localize on its peptidoglycan layer and further disrupt the electron transport chain through excess electron inflow. Simultaneously, the bacterial membrane of Pseudomonas aeruginosa (P. aeruginosa) was significantly destroyed after TiOx@C treatment and resulting in severe contents leakage. It is noteworthy that the synergistic electro-mechanical bacterial inhibition strategy does not cause the evolution of drug resistance. In animal models of wound infection, both the bactericidal activity and the wound-healing promoting effect of TiOx@C have been demonstrated. The as-presented bacterial cell wall-specific, multi-target nanomedicine provides a distinct electron-mechanical co-intervention strategy in the personalized and effective treatment of infections, which has paved the way for emerging antibacterial nanoagents in combating drug resistant bacteria (Fig. 1).
The fiber-like carbon substrate of TiOx@C is capable of selectively entangling with the peptidoglycan layer of S. aureus, further disrupting the electron transport chain and killing the bacteria by delivering excess electrons to key enzymes of the electron transport chain via TiOx dots. Meanwhile, the carbon substrate of TiOx@C could directly penetrate the cell wall of P. aeruginosa, disrupt the bacterial membrane structure and penetrate into the bacteria, and trigger oxidative stress and protein leakage, thus leading to the bacterial death.
Results and discussion
Synthesis and characterization of TiOx@C
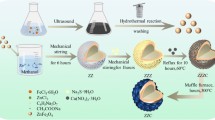
First, Ti3C2 nanosheets were synthesized by a chemical exfoliation method according to the previous literature29. In general, Ti3C2 nanosheets are considered to be highly sensitive to strong oxidants due to their large specific surface area, making active Ti atoms easily to be oxidized to titanium oxide30. Here, we treated the Ti3C2 aqueous dispersion with H2O2 to obtain the oxidation product (Fig. 2a). Transmission electron microscopy (TEM) images show the microstructure transformation of Ti3C2 before and after H2O2 oxidation (Figs. S1, Supplementary information). The final product, referred to as TiOx@C, displayed a carbon layer with a silk-like structure in which TiOx dots of 2 to 5 nm were uniformly distributed, thus forming the TiOx@C nanocomposites (Fig. 2b). The stability of TiOx@C in aqueous solution was further investigated. Digital photographs, TEM images and UV-vis spectra results show that the morphology and composition of TiOx@C in water remained unchanged for up to 30 days (Figs. S2 and S3, Supplementary information). Furthermore, TiOx@C exhibits excellent dispersion in aqueous solution, saline, and LB Broth, thereby making it a promising nanomedicine for antimicrobial therapy (Figs. S4, Supplementary information).
a Schematics for the synthesis of TiOx@C nanocomposite. b TEM images of TiOx@C nanocomposites scale bar, 50 nm. c HRTEM image of TiOx@C, scale bar, 2 nm. The white arrows indicate the lattice distortion. n = 3 samples with similar results. XRD pattern (d) and Raman spectra (e) of Ti3C2 and TiOx@C. (f–i) EPR spectra (f) and Ti 2p (g), O1s (h), C 1s (i) XPS profiles of TiOx@C.
The corresponding lattice planes of TiOx quantum dots are observed by high-resolution TEM (HRTEM), indicating that the lattice distortion defects are conspicuous (Fig. 2c). Energy-dispersive X-ray spectroscopy (EDS) mappings by TEM and SEM demonstrate that the Ti, O, and C elements are uniformly distributed throughout the nanohybrids, revealing that the TiOx are evenly decorated on the carbon layer without significant aggregation (Figs. S5–6, Supplementary information). The X-ray diffraction (XRD) patterns of the precursor Ti3C2 and TiOx@C show that the disappearance of 2θ peak at 9.5° after the formation of TiOx@C, indicating that the layered structure no longer existed (Fig. 2d)29. While TiOx@C shows no obvious indication of crystallinity, due to the amorphous structure of carbon layer. Raman results reveal that the characteristic peaks of Ti3C2 completely disappeared in the Raman spectra of TiOx@C, while the different peaks at 153.9 and 623.5 cm−1 can be observed, which correspond to the symmetric stretching and bending vibration of O-Ti-O in Ti3O5 respectively (Fig. 2e)31. Electron paramagnetic resonance (EPR) was used to confirm the presence of oxygen vacancies (OVs). The EPR spectrum of TiOx@C displays a g value of about 2.004, confirming the presence of OVs in TiOx (Fig. 2f)32. As evidence for the presence of multivalent titanium grew, X-ray photoelectron spectroscopy (XPS) was used to verify the chemical state of TiOx@C. In the Ti 2p spectrum (Fig. 2g), the characteristic peaks of Ti 2p1/2 at 463.8 eV and Ti 2p3/2 at 458.2 eV indicate the Ti3+ in TiOx, and the characteristic peaks of Ti 2p1/2 at 464.8 eV and Ti 2p3/2 at 458.8 eV correspond to Ti4+, confirming the multivalent state of Ti in TiOx. The O1s spectrum shows four characteristic peaks centered at 530.0, 530.7, 531.7 and 532.6 eV, corresponding to the Ti-O bonds, hydroxylated surface, the presence of OVs and adsorbed moisture respectively, which is consistent with the EPR results (Fig. 2h). The C 1 s spectrum exhibits two peaks at 284.8 and 287.5 eV, corresponding to the C-C and C-O bonds in the carbon layer, respectively (Fig. 2i)33. The high intensity of the C-C peak indicates the enhanced electrical conductivity of TiOx@C.
To gain deeper insight into the effect of structural evolution on the performance of Ti3C2, first-principles DFT calculations were performed, and the optimized models were provided (Figs. S7, Supplementary information). According to the partial density of states (PDOS) calculation results, the pristine oxygen-capped Ti3C2 shows metalloid properties; above the Fermi level (Ef), the dominant contribution to the DOS comes from the d orbitals of Ti atoms, while below the Fermi level, the prevailing contribution to the DOS comes from the p orbitals of the O atom orbitals. Besides, the PDOS of Ti 3 d of TiOx exhibits a stronger intensity around the Fermi level, thus improving conductivity and promoting the electron transfer of TiOx (Figs. S8, Supplementary information). It is typically believed that the arc radius in electrochemical impedance spectroscopy (EIS) is proportional to the resistance of the material; a smaller arc radius indicates a lower electron transport resistance in the material34. As shown in Figure S9, the lower electrochemical impedance of TiOx@C indicates a stronger electron transport capability than Ti3C2.
Antibacterial performance of TiOx@C
Inspired by the unique structure and improved electron-donating ability of TiOx@C, the potential of TiOx@C as a bactericidal agent was further investigated. Staphylococcus aureus (S. aureus), which is a major bacterial human pathogen representative of gram-positive bacteria (G + )35, and Pseudomonas aeruginosa (P. aeruginosa) that is one of the top-listed pathogens causing hospital-acquired infections36, representing for gram-negative bacteria (G−), were used as model bacteria. As shown in Fig. 3a–c, TiOx@C exhibited broad-spectrum and concentration-dependent antibacterial activity, while the titanium-based nano-antibacterial agent Ti3C2 nanosheets and TiO2 nanoparticles showed negligible intrinsic antibacterial performance, which depend on additional external light irradiation to generate photothermal/photodynamic therapy as previously reported37,38. The results suggest that the specific electronical property of TiOx@C with abundant oxygen vacancies and multivalent states contributes to its antimicrobial activity. Notably, P. aeruginosa was more easily eradicated than S. aureus when the concentration of TiOx@C was increased to 30 μg mL−1, which highlights the potential strain-specific bactericidal mechanism of TiOx@C. Confocal laser scanning microscope (CLSM) was used to observe the viability and cytotoxicity of bacteria stained by NucGreen and EthD-III. EthD-III is a cell membrane-impermeable nucleic acid dye that can determine bacteria with membrane damage. The CLSM images demonstrated the concentration-dependent antibacterial performance of TiOx@C (Fig. 3d). Then, the bacterial growth inhibition effect of TiOx@C was determined. The results demonstrate that TiOx@C inhibited the growth of S. aureus by 57.3% and P. aeruginosa by 64.9% at a concentration of 100 μg/mL for 24 h. Therefore, the minimum inhibitory concentration that inhibits 50% of the bacteria (MIC50) of TiOx@C for S. aureus and P. aeruginosa is 100 μg/mL (Figs. S10, Supplementary information). It can be found that TiOx@C inhibited the growth of S. aureus by 94.2% and P. aeruginosa by 91.6% at a concentration of 200 μg/mL for 24 h. Therefore, the minimum inhibitory concentration that inhibits 90% of the bacteria (MIC90) of TiOx@C for S. aureus and P. aeruginosa was 200 μg/mL.
a Representative optical images of S. aureus and P. aeruginosa colonies formed on LB agar plates after various treatments and (b, c) the corresponding colony counting results. n = 3, biological replicates. Data are presented as mean ± SD. One-way ANOVA with Bonferroni post test was used to analyze multiple groups. NS, not significant. d CLSM images of S. aureus and P. aeruginosa stained with NucGreen (EX488) and EthD-III /PI (EX532) after different treatments, scale bar, 40 μm. Green color indicates live bacteria, and red color indicates membrane-damaged bacteria. n = 3, biological replicates with similar results. e Representative optical images of biofilms stained by crystal violet after the treatment by TiOx@C at varied concentrations. (f, g) The corresponding quantitative results of S. aureus biofilm (f) and P. aeruginosa (g) biofilm. n = 4, biological replicates. Data are presented as mean ± SD.
Considering the accelerated evolution of bacterial AMR against antibiotics, we further evaluated whether prolonged exposure to TiOx@C nanocomposites would similarly lead to the acquisition of bacterial resistance. The antibacterial efficacy of TiOx@C was evaluated by screening S. aureus and P. aeruginosa strains for a period of 21 days at sub-MIC90 values39. The results demonstrate that TiOx@C exhibited a prolonged antibacterial effect without the induction of resistance (Figs. S11 and S12, Supplementary information). A growing number of studies have revealed that nanomaterials can induce bacterial resistance through a range of mechanisms, such as Ag NPs40, TiO2 NPs41, and CNTs42. It is notable that TiOx@C has two components which have parallel correspondences to independent bacterial targets i.e., the bacterial cell wall, the electron transport system and redox homeostasis, which can achieve the synergy of the multiple independent bactericidal mechanisms in circumventing bacterial drug resistance8.
Biofilms exhibit a higher prevalence of multidrug resistance to most clinically available drugs, which is a crucial issue needing to be tackled43. Unfortunately, extracellular polymeric substances (EPSs) bound to the cell surfaces of the biofilm can hinder the interaction of nanomaterials or drugs with the biofilm, thus reducing their germicidal efficacy44. Excitedly, it has been reported that boosted electron transfer between nanomaterials and bacteria can destroy the EPSs and eliminate biofilms without developing drug resistance45. The crystal violet method was performed to qualitatively and quantitatively evaluate the ability of TiOx@C to eliminate biofilms. The results indicate that the amount of crystal violet adhered to the biofilm treated by TiOx@C was significantly reduced, confirming that such material could effectively eliminate both S. aureus and P. aeruginosa biofilms (Fig. 3e). As shown in Fig. 3f, g, the quantitative results demonstrate that the anti-biofilm activity of TiOx@C exhibits a concentration-dependent manner, and the elimination rate of P. aeruginosa biofilm could reach up to 92% at a concentration of 400 μg mL−1.
Bacterial cell wall-specific performance of TiOx@C
The morphological changes of the bacteria before and after co-incubation with TiOx@C were observed by scanning electron microscopy (SEM). The SEM images exhibit that the initial cell walls of S. aureus are roughened after TiOx@C treatment, indicating that S. aureus were wrapped on the surface by the fibrous carbon layers (marked by orange labels) (Fig. 4a). As shown in the SEM images of P. aeruginosa, the original smooth cell walls become shrunk and the morphology exhibit shriveled after co-incubation with TiOx@C, indicating that TiOx@C has led to the loss of bacterial cytoplasm, shrinkage and/or deformation of bacterial membranes of P. aeruginosa. The corresponding elemental mapping demonstrates that the TiOx quantum dots were distributed uniformly on the bacterial surfaces to trap and disarm bacteria (Fig. 4b). The differences between the cell wall of S. aureus and P. aeruginosa after TiOx@C treatment inspire us to investigate its potential bacterial cell wall -specific antibacterial mechanism.
a, b SEM images and elemental mapping of S. aureus (a) and P. aeruginosa (b) after various treatments, scale bar, 1 μm. n = 3, biological replicates with similar results. c, d Bio-TEM images and elemental mapping of S. aureus (c) and P. aeruginosa (d) before and after TiOx@C treatment, scale bar, 500 nm. n = 3, biological replicates with similar results. e, f Permeability of bacterial membrane determined by ONPG assay and protein leakage analysis of S. aureus (e) and P. aeruginosa (f) after various treatments. n = 4, biological replicates. Data are presented as mean ± SD. Two-way ANOVA with Bonferroni post test was used to analyze multiple groups. NS, not significant.
Given the above hypotheses of the potential bacterial cell wall-specific antibacterial activity of TiOx@C, the intrinsic antibacterial mechanisms were further investigated. Atomic force microscopy (AFM) is a powerful tool for obtaining in-depth understanding of microbial morphology and structural details46. The AFM results show that the smooth surface of S. aureus became rough after TiOx@C treatment, and the corresponding root mean square roughness (Rq) increased significantly, which was consistent with the SEM results, implying that TiOx@C nanomaterials were extensively attached to the surface of the S. aureus bacterium (Figs. S13a, Supplementary information). In contrast, the surface roughness of P. aeruginosa did not change significantly, instead, the thickness of the bacterium is markedly reduced (Figure S13b, Supplementary information). The three-dimensional morphology-reconstructed images intuitively reflect the deflation and indentation of the bacteria after TiOx@C treatment, due to the leakage of bacterial contents (Figs. S14, Supplementary information). Bio-TEM observation was performed to further visualize the details of the internal structure of microbial cells after exposure to TiOx@C, and localize the position of TiOx@C within different bacteria. It can be observed that the TiOx@C adhere uniformly and tightly attached to the surface of individual S. aureus bacteria, showing minimal ultrastructural and morphological changes. The elemental mapping images more intuitively exhibit that the carbon networks of TiOx@C were entangled with the peptidoglycan (the major component of G+ cell wall) and close to the surface of the S. aureus inner membrane due to the outer thick peptidoglycan layer of G+ bacterium (approximately 20 ~ 40 nm), thus the carbon networks were blocked to get into the phospholipid bilayer of the bacterial inner membrane due to the size effect (Fig. 4c)47,48.
In contrast, P. aeruginosa with a much thinner peptidoglycan layer (~3 nm) treated with TiOx@C displayed significant morphological changes, including disruption of the cell wall, areas of clear cytoplasm, leakage of cytoplasm, and plasmolysis. In P. aeruginosa, the hydrophobicity and polarity of lipopolysaccharides (LPS) of its cell envelope drastically reduce the membrane permeability49. It has been widely reported that nanomaterials with unique morphology such as nanowire or nanorods can cause mechanical damage by disrupting the bacterial cell membrane or cell wall, which in turn kills the bacteria50,51,52. However, such mechanical damage largely depends on the angle of interaction of material with bacteria, which greatly limits in vivo applications. In our work, The elemental mapping results show a uniform distribution of Ti element on the bacteria, suggesting the carbon network of TiOx@C enables to penetrate the bacterial envelope and further enter the bacterium due to the strong hydrophobic interactions between the large number of C-C bonds, finally disrupting the bacterial membrane significantly (Fig. 4d)53. The interaction between TiOx@C and bacterial membrane can be further confirmed by detecting the permeability of the bacterial membrane using o-nitrophenyl-β-D-galactopyranoside (ONPG). As a result, the permeability of the bacterial membrane of P. aeruginosa elevated significantly with increasing concentration and prolonging incubation time after exposure to TiOx@C (Fig. 4f), while those of S. aureus showed negligible change (Fig. 4e).
Next, the detailed changes after TiOx@C treatment of the cell envelope were further elucidated at the micro/nanoscale by small angle X-ray scattering (SAXS). The exhibited SAXS profile can be divided into three regions: Region I represents the overall size of the bacterial cell in the system, including the core-shell structure. Region II contains information about the cell wall and its thickness, while region III reveals the structural arrangement of groups of objects (e.g., DNA, ribosomes, and proteins) in the cytoplasm54. As a result, the scattering curves of TiOx@C-treated S. aureus shifted to higher q values in region I and to lower values in region II and III, suggesting that the overall structure of the bacteria has become larger and looser, while the thinning of the bacterial wall may be accompanied by denaturation and loss of contents of the bacterial cytoplasm (Fig. 5a). On the contrary, the scattering curves of treated P. aeruginosa shifted toward lower q values in regions II and III, which implies that the membrane structure and cytoplasmic contents of the bacteria were significantly disrupted (Fig. 5b). Meanwhile, the protein leakage of bacteria was determined by BCA Protein Assay Kit. It could be found that the P. aeruginosa individuals suffered membrane damage with a decrease in total protein amount, which indicates the significant protein degradation (Fig. 5c, d).
a, b SAXS scattering profile for the dispersions of S. aureus (a) and P. aeruginosa (b) before and after TiOx@C treatment. c, d Protein leakage analysis of S. aureus (c) and P. aeruginosa (d) after various treatments. e The corresponding schematic illustrations of interactions of TiOx@C with Gram-positive or Gram-negative bacterial membrane. n = 4, biological replicates. Data are presented as mean ± SD. One-way ANOVA with Bonferroni post test was used to analyze multiple groups. NS not significant.
In summary, the same antimicrobial agent would have varied binding interactions with different bacterial cell wall due to the size effect, which may lead to various antibacterial mechanisms against different bacteria. In the case of S. aureus, TiOx@C can merely be enriched in its much thicker peptidoglycan layer owing to the large sizes of carbon substrate. Thus, few of them could disrupt the inner membrane. For P. aeruginosa, TiOx@C is able to penetrate its lipopolysaccharide layer and the outer membrane due to hydrophobic interaction and further disrupts the thinner peptidoglycan layer to enter the bacteria, which is directly and strongly toxic to the bacteria (Fig. 5e).
Self-driven electron transfer in bacteria-TiOx@C interface
Compared to previous bacterial wall-specific antimicrobials15,16,17, TiOx@C was unable to enter S. aureus but still showed great bactericidal activity. As is known in literature, the electron transport chain (ETC) on the bacterial inner membrane plays a key role in physiological processes of bacteria such as metabolism and energy supply55. In recent years, interference/disruption of the bacterial electron transport chain has been applied as an emerging antibacterial strategy in a number of studies6,8. However, most of these are based on piezoelectric materials to achieve electron transport which require additional ultrasonic stimulation. Considering the unique electronic structure of TiOx, we further explored the potential self-driven electron transport in bacteria-TiOx interface. To investigate the electrochemical activity of the TiOx@C-bacteria systems, the cyclic voltammetry (CV) experiment was performed. As a result, the CV curves of S. aureus- TiOx@C exhibit a greatly increased current signal compared to untreated bacteria, while the CV curves of P. aeruginosa show negligible change before and after TiOx@C treatment (Fig. 6a). Subsequently, electrochemical impedance spectroscopy (EIS) was used to evaluate the ability of TiOx@C to boost electron transport capacity. As shown in Fig. 6b, the semicircle diameter (Rct) of the S. aureus- TiOx@C was significantly smaller than that of untreated S. aureus, indicating that the TiOx@C facilitates electron transfer, thus increasing the electrical conductivity of the S. aureus-TiOx@C systems. Similarly, no significant difference was found in the EIS profiles of P. aeruginosa before and after TiOx@C treatment.
a Cyclic voltammetry (CV) curves of suspended S. aureus and P. aeruginosa after various treatments. b EIS spectrum of suspended S. aureus and P. aeruginosa after various treatments. c, d The corresponding INT-ETS assay outcomes containing UV-vis spectrum and quantitative ETS results of S. aureus (c) and P. aeruginosa (d) after various treatments. n = 3, biological replicates. Data are presented as mean ± SD. One-way ANOVA with Bonferroni post test was used to analyze multiple groups. (e, f) The ATPase concentration of S. aureus (e) and P. aeruginosa (f) treated with TiOx@C. n = 3, biological replicates. Data are presented as mean ± SD. One-way ANOVA with Bonferroni post test was used to analyze multiple groups. NS, not significant. (g, h) The ATP level of S. aureus (g) and P. aeruginosa (h) treated with TiOx@C. n = 3, biological replicates. Data are presented as mean ± SD. One-way ANOVA with Bonferroni post test was used to analyze multiple groups. NS, not significant. (i) Structural optimization of heme c adsorption on TiOx@C and electron density difference plots at the heme c and TiOx@C interface from top view and side view.
It has been reported that nanocatalysts with similar TiOx structure exhibited efficient Fenton-like catalytic performance attributed to the abundance of surface defects and the presence of multivalent titanium56. The TiOx@C-mediated Fenton-like reaction was evaluated using a colorimetric method based on the degradation of methylene blue (MB) after selective ·OH trapping. It can be found that the MB absorption showed a significant decay after TiOx@C + H2O2 treatment, suggesting the production of ·OH radicals (Figs. S15, Supplementary information). Thus, we further measured the ROS levels of bacteria after TiOx@C treatment. The results indicate that the bacteria in the control group emitted negligible green fluorescence, while a concentration-dependent enhancement of fluorescence emission was observed in the TiOx@C-treated bacteria, possibly due to the excess ROS attack via a Fenton-like catalytic reaction57. (Figs. S16, Supplementary information).
Next, 2-para-(iodophenyl)-3(nitrophenyl)-5(phenyl)tetrazolium chloride (INT) was applied to detect the activity of the electron transport system (ETS). INT is a readily reducible reagent with its yellow color, which can accept hydrogen and electrons and be reduced to iodonitrotetrazolium formazan (INTF) turning purple; the rate of H+/e− transfer in the ETS can be quantified by the change in absorbance. The corresponding results demonstrate an increase of absorbance at 485 nm of S. aureus-TiOx@C system after addition of the INT detector. However, after prolonged co-incubation with TiOx@C, the absorbance decreased significantly. It can be found that TiOx@C is able to temporarily enhance the electron transport activity of S. aureus, which is attributed to the electronic property of TiOx and the superior electrical conductivity of carbon substrate to boost electron transport capacity in a short period of time, while the electronic activity was strongly inhibited upon prolonged incubation due to abnormal electron stacking (Fig. 6c). As to P. aeruginosa, a marked enhancement of ETS activity was observed over a short period, which could be attributed to two aspects. On the one hand, the ETS activity was improved by TiOx@C via boosted electron transport performance, and on the other hand, it may be related to the cell membrane disruption of P. aeruginosa, allowing more reductase enzymes in the bacterium to reduce INT. Notably, the decrease of U-values within 20 min of P. aeruginosa was greater than that of S. aureus, which indicates that P. aeruginosa suffers a mass death more quickly, corresponding to the results of the above experiments (Fig. 6d).
We then determined the stability of TiOx@C before and after exerting antimicrobial effects using XPS and MB degradation tests. The results show that the valence states of Ti in TiOx@C before and after co-incubation with S. aureus and P. aeruginosa has no significant changes, as the presence of carbon substrate is of great importance for maintaining the titanium in a relatively stable valence state or metastable state (Fig. S17, Supplementary information). And TiOx@C still exhibited significant Fenton-like activity after co-incubation with bacteria (Fig. S18, Supplementary information), suggesting that TiOx@C is able to inhibit bacterial growth efficiently over a long period of time. Moreover, the microbial ATP synthase concentration of the bacteria after various treatments was determined by enzyme linked immunosorbent assay (ELISA). The results indicate that the ATPase level of both S. aureus and P. aeruginosa decreased significantly after co-incubation with TiOx@C (Fig. 6e, f). The ATPase activity of S. aureus decreased more rapidly in a short period of time due to the direct damage to the ATPase by the TiOx@C anchored on the bacterial membrane. Simultaneously, the adenosine triphosphate (ATP) levels of bacteria show the same trend as the decreased ATP synthase activity, indicating that the inhibition of the bacterial electron transport chain leads to insufficient motive force of the proton pump to enable ATP synthesis, resulting in a decrease in ATP levels (Fig. 6g, h).
Cytochrome c (cyt c) consisting of numerous heme c groups, an iron-containing biomolecule, plays a crucial role in the electron transport chain of bacteria, which facilitates the ordered transfer of electrons within the cell through its structural domains with iron-copper centers58. Therefore, we simulated the electron flow between heme c and TiOx@C. The electron accumulation and depletion regions are presented as yellow and blue areas respectively. Notably, the redistribution of electrons occurs at the interface between heme c and TiOx@C. The electron flow (e−) values of TiOx@C to heme c were further elucidated by Bader charge analysis (Fig. S19, Supplementary information). Consequently, a large accumulation of electrons occurred in the heme (iron center) structural domain, which is critical for heme c (Fig. 6i). The results demonstrate that TiOx@C is capable of overloading heme c with electrons and further inactivating cyt c activity, disrupting the bacterial electron transport chain and inducing bacterial death.
In summary, TiOx@C exhibits a synergistic electronic-mechanical bactericidal effect. For P. aeruginosa, TiOx@C can directly penetrate the bacterial membrane and cause severe mechanical damage. For S. aureus, TiOx@C is unable to directly damage the bacterial membrane, but can adhere to the bacterial membrane and disrupt the bacterial electron transport chain.
Specialized antibacterial mechanisms of TiOx@C against S. aureus and P. aeruginosa
Further, we performed a transcriptomic analysis on S. aureus and P. aeruginosa samples to investigate the effects of the membrane interaction-guided electron-mechanical intervention of TiOx@C against bacteria on gene expression and regulation. Preliminary identification of differentially expressed genes (DEGs) between the normal bacteria group and the TiOx@C-treated group was performed. The volcano plot shows that the TiOx@C-treated group had 968 DEGs compared to the untreated normal S. aureus (Fig. 7a).
a, f Volcano plots depicting fold changes and p-value per gene of S. aureus (a) and P. aeruginosa (f) comparing after TiOx@C treatment. b, g Differential gene GO enrichment column diagrams of enriched pathways of S. aureus (b) and P. aeruginosa (g). BP indicates biological process; CC indicates cellular component and MF indicates molecular function. c, h Heatmap depicting representative DEGs of different comparison groups of S. aureus (c) and P. aeruginosa (h); orange, upregulation; blue, downregulation; log2 fold change ≥ 1, Q values < 0.05. Key DEGs are marked on the right, n = 3, biological replicates. d, i Chordal graph presenting enriched GO pathway of the differentially represented genes in S. aureus (d) and P. aeruginosa (i) comparing after TiOx@C treatment. e, j Schematic mechanism of strain-specific antibacterial activity of TiOx@C against S. aureus (e) and P. aeruginosa (j).
Gene Ontology (GO) enrichment analysis reveals that DEGs in the S. aureus groups were involved in biological processes, cellular components and molecular functions especially in structural constitution of ribosome, extracellular region and translation, indicating that TiOx@C induced a stress response (Fig. 7b). It is noted that DEGs involved in electron carrier activity and ATP synthase activity were significantly down-regulated in S. aureus after TiOx@C treatment, which is consistent with the experimental results. Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis shows that photosynthesis pathway involving proton motive and reducing forces and ATP synthase, was the most differentially expressed in S. aureus treated with TiOx@C (Figs. S20a, Supplementary information). And the corresponding eight genes involved in this pathway which are genetically associated with the composition and activity of the FoF1-ATPase were significantly down-regulated (Fig. S21, Supplementary information), demonstrating that the antibacterial mechanism of TiOx@C against S. aureus is to disrupt ATP synthase activity and interfere with the energy metabolism of the bacteria. Expression heat maps disclose that the expression of succinate dehydrogenase (SDH)-related genes such as sdhA (representing flavoprotein subunit) and sdhB (representing iron-sulfur protein subunit) was downregulated, demonstrating the efficacy of TiOx@C in disrupting the electron transport chain of S. aureus, thus suppressing its respiratory chain (Fig. 7c)59. Moreover, the expression of the atpC gene, which encodes the γ subunit of the ATP synthase F1 complex that plays a pivotal role in cellular respiration in bacteria and energy supply system was significantly downregulated in the TiOx@C-treated group60. The chordal diagram depicting the enriched GO pathways of the DEGs exhibits that downregulation of key genes implies the disruption of bacterial ETCs due to blocked ATP synthesis and energy depletion. leading to S. aureus death (Fig. 7d). Cluster of homologous genes (COG) analysis is a common technique for microbial genome annotation and comparative genomics. The COG results indicated that genes related to translation, ribosomal structure and cell wall/membrane/envelope biogenesis were significantly downregulated, reflecting those physiological metabolic processes, such as cell wall structure, RNA transcription and protein synthesis were significantly suppressed in the bacteria after TiOx@C treatment (Figure S22a, Supplementary information).
Taken together, the antibacterial mechanisms of TiOx@C against gram-positive bacteria could be summarized as follows: Owing to the size effect, the fiber-like carbon substrate of TiOx@C entangles with peptidoglycan layer of S. aureus, which allows direct interface with the electron transport chain complexes on the cell membrane. Then, TiOx quantum dots with enhanced electron-donating ability exert electron flow into crucial enzymes of the ETC leading to electron stacking which disrupts the bacterial electron homeostasis. As a result, the damaged electron transport chain is unable to form the proton gradient for ATP synthesis. This disrupts bacterial metabolism and ultimately induces bacterial death (Fig. 7e).
The DEGs of P. aeruginosa differed significantly from those of S. aureus. In terms of P. aeruginosa, the treated group showed only 89 DEGs compared to the normal group (Fig. 7f). Specifically, GO enrichment analysis demonstrates that the oxidative stress response is of most differential expression in TiOx@C-treated bacteria, suggesting that TiOx@C induces oxidative stress in P. aeruginosa, which is consistent with the flow cytometry results (Fig. 7g). KEGG analysis results show that important pathways such as glyoxylate and dicarboxylate metabolism output and lipopolysaccharide biosynthesis were enriched in TiOx@C-treated P. aeruginosa, revealing that TiOx@C induces a stress response in bacteria to generate adaptation to environmental changes (Figs. S20b, Supplementary information). Moreover, several genes related to oxidative stress and bacterial redox homeostasis were significantly upregulated (Fig. 7h). The chordal diagram depicting enriched GO pathways of the 6 representative DEGs exhibits that the upregulation of ahpC (known as alkyl hydroperoxide reductase subunit C), ahpF (known as alkyl hydroperoxide reductase subunit F), katA and katB (encode for the enzyme catalase). In detail, the katB indicates that the bacteria have been damaged by oxidative stress, resulting in the upregulation of important enzymes involved in the detoxification of reactive oxygen species (Fig. 7i). The COG results indicated that genes related to the inorganic ion transport and metabolism which is largely related to the synthesis of oxidative stress-related enzyme were upregulated, verifying that P. aeruginosa suffered from oxidative stress after treatment with TiOx@C (Figure S22b, Supplementary information).
To sum up, the antibacterial mechanism of TiOx@C against Gram-negative bacteria can be summarized as follows: Due to the hydrophobic effect, the fibrous carbon substrate of TiOx@C can penetrate the outer and inner phospholipid bilayers of Gram-negative bacteria, further disrupting the bacterial membrane structure and altering membrane permeability. Then, TiOx quantum dots with unique electronic property introduce electron flow to macromolecules, proteins, metabolic enzymes in the bacterial cytoplasm to disrupt redox homeostasis, inducing bacterial oxidative stress. Finally, the membrane of Gram-negative bacteria is destroyed, resulting in severe oxidative stress damage and significant protein degradation and loss, which ends up in bacterial death (Fig. 7j).
Treatment of wound infection in vivo. Biocompatibility is of great significance for the development and application of biomedical nanomaterials. Thus, we investigated the cytotoxicity of TiOx@C using the Cell Counting Kit-8 (CCK-8) assay. The results demonstrate that TiOx@C shows negligible cytotoxicity to PC12 and L929 cells even when the concentration was up to 300 μg mL−1 (Figs. S23, Supplementary information). In addition, for further biological applications, we verified the biosafety of TiOx@C to human cells. CCK-8 assay demonstrate the biocompatibility of TiOx@C to HacaT cells (Human Keratinocytes cells) (Figs. S24, Supplementary information). Considering the ability of TiOx@C to disrupt bacterial membranes, we further investigated the membrane integrity of the cells after co-incubation with the material. By staining the cells with PI dye, which is able to penetrate damaged cell membranes, it can be found that the cell membrane of HacaT cells incubated with TiOx@C was not disrupted (Figure S25, Supplementary information). The results suggest that TiOx@C is unable to penetrate the cell membrane of eukaryotic cells, due to differences in the composition of bacterial and eukaryotic cell membranes. It is well known that cholesterol (Chol), which regulates the bending rigidity of cell membranes is uniquely linked to cell evolution—it is universally absent in prokaryotic membranes and is present in differing amounts in eukaryotic membranes. The differential nature of the cell membrane serves as a barrier to prevent the carbon substrate from disrupting the integrity of the mammalian cell membrane, thus enabling microbial selectivity.
Inspired by the effective, multi-target antibacterial mechanism of TiOx@C, we constructed a S. aureus-infected wound infection model to assess the in vivo therapeutic efficacy. The in vivo experimental protocol is shown in Fig. 8a. As shown in Fig. 8b, c, the number of bacteria remaining in the infected lesions decreased significantly after treatments with TiOx@C. The quantitative results of plate counting demonstrated that the TiOx@C nanocomposites effectively eliminated 97% of the bacteria in the wounds, indicating that TiOx@C is promising as a potent antimicrobial agent for the treatment of wound infections. Also, the amounts of inflammatory cells in the peripheral blood of treated mice in response to infection were mostly close to normal levels, which demonstrates that TiOx@C can effectively alleviate infection-induced inflammation in vivo (Fig. 8d). Furthermore, no significant suppuration, edema and lesion expansion could be observed in TiOx@C-treated group, and the wounds were able to form scabs and heal faster within two weeks in comparison to the infected (untreated) group (Fig. 8e). The schematic morphological changes of the wounds in different groups over the 14-day course display the rapid wound healing in the TiOx@C -treated group, and such a rate of healing was comparable to that of the uninfected (Control) group (Fig. 8f), as evidenced by the time-dependent curves of wound areas (Figure S26a, Supplementary information). After wound modeling, all groups of mice exhibited a transient weight loss followed by an increase in body weight during the 14-day recovery monitoring period, during which the TiOx@C-treated and control groups showed a comparable weight gaining rate and the infected group grew at the slowest rate (Figs. S26b, Supplementary information).
(a) Scheme of the wound infection treatments. (b) Photographs of S. aureus colonies from infected tissues in 1 day post treatment. (c) Bacterial counting results corresponding to (b). n = 3, biological replicates. Data are presented as mean ± SD. One-way ANOVA was used to analyze multiple groups. (d) Inflammatory cell numbers in the blood of mice in different groups. n = 3, biological replicates. Data are presented as mean ± SD. One-way ANOVA with Bonferroni post test was used to analyze multiple groups. NS, not significant. e Representative images of S. aureus-infected skin wound of mice after treatments for different days. Scale bar, 5 mm. n = 3, biological replicates with similar results. f Schematic diagrams of the wound size changes corresponding to (e). g Giemsa staining images on day 7, scale bar, 100 μm. Orange arrows indicate the S. aureus-infected area. H&E, Masson and IHC, Collagen I and III (Col I and Col III) staining images of wound tissues after various treatments on day 14, scale bar, 200 μm. Scale bar for smaller magnifications of H&E images is 1 mm. n = 3, biological replicates with similar results. The muscle fibers are presented as red, and the collagen fibers are presented as blue in Masson staining images. Brown color indicates the Col I and III-positive.
Accordingly, the remaining bacteria in the infected wound tissue could be further observed by Giemsa staining, which are negligible in the tissues on day 7 after TiOx@C treatment. Pathomorphological analysis of the wound tissues on day 14 was conducted (Fig. 8g). The hematoxylin and eosin (H&E) staining results indicate that the healing wound skin tissue in the TiOx@C-treated group had an intact tissue composition and follicle regeneration, with a distinct stratum corneum on the surface. However, in the untreated group, the incomplete skin structure could be observed accompanied by a noticeable scab attached to the surface of the wound. The Masson’s trichrome staining images suggest that more complete wound epithelium and the deposition of regenerated collagen fiber (stained blue) were observed in the infected wound tissue treated by TiOx@C. Additionally, immunohistochemical (IHC) staining outcome shows markedly large Collagen type I and type III (Col I and Col III)-positive area in the healing wound skin after TiOx@C treatment. Collagen type I is the major component of the ECM in skin and plays crucial role in the early inflammatory and proliferative phases of wound healing, which provides tensile strength to the wound and promotes tissue reconstruction. Col III, on the other hand, gives the wound elasticity and flexibility61. Col I has a higher tensile strength and will form more resilient scar tissue, while Col III allows for better wound recovery and is less likely to form scar. It can be found that Col III is more positive in the treatment group than in the control group, which indicates that TiOx@C with promoting healing effect could achieve optimal tissue repair and minimize scar formation, possibly attributed to its porous fiber-like carbon substrate with good electrical conductivity.
Encouraged by the great antimicrobial and pro-wound healing properties of TiOx@C in wound-infected mice, we then further evaluated the long-term toxicity of TiOx@C in healthy mice to assess its potential application as an antibiotic alternative. The assessment of hematological and blood biochemical markers for mice on day 14 showed no significant abnormalities or deleterious effects in TiOx@C-treated mice, suggesting that TiOx@C has insignificant toxicity and can be used for further in vivo experiments (Figs. S27a, b, Supplementary information). Furthermore, histological analyzes of major organs (heart, liver, spleen, lungs and kidneys) were performed. H&E staining images show negligible histological abnormalities or pathological changes in TiOx@C -treated mice compared to normal healthy mice (Figs. S27c, Supplementary information). In conclusion, TiOx@C exhibits favorable biosafety and possesses the potential to be applied as an effective nanoagent for the synergistic treatment of bacterial infections and wound healing.
Discussion
In this study, a concept of bacterial cell wall-specific nanomedicine has been introduced, which can achieve multi-targeting and highly efficient elimination of both S. aureus and P. aeruginosa via electron-mechanical bactericidal mechanisms. In vitro experiments and further transcriptomic analyses reveal that TiOx@C can be localized into the inner membrane and donate electrons to the significant enzymes of S. aureus electron transport chain, which disrupts the respiratory and metabolic pathways of the bacteria. Meanwhile, P. aeruginosa treated by TiOx@C would suffer from cell membrane damage, oxidative stress, and protein leakage, which would eventually lead to bacterial death. In vivo experiments have demonstrated that TiOx@C possesses greatly enhanced bactericidal activity and wound healing-promoting capacities simultaneously, which promotes fibroblast proliferation, and damaged skin rehabilitation and collagen regeneration. Therefore, such a safe and effective nano-antimicrobial agent can achieve efficient elimination of S. aureus and P. aeruginosa through synergistic electron-mechanical intervention without inducing drug resistance, which is expected to serve as a promising nanomedicine alternative to conventional antibiotics to combat difficult-to-treat bacterial infections by circumventing current mechanisms of drug resistance of bacteria.
Methods
Ethical approval
All animal experiments were under the context of the animal protocols approved by the Institutional Animal Care and Use Committee guidelines in Shanghai Tenth Peoples’ Hospital, Tongji University School of Medicine (approval number: SHDSYY-2018-Z0026). All mice were kept in accordance with the policies on animal research of the National Ministry of Health. All mice used for animal experiments are female with a gentle character, and the sex of the mice does not affect the results of the experiments.
Materials
Ti3AlC2 powder (≥95 wt%) was purchased from XFNANO Materials Tech Co., Ltd. (Nanjing, China). Hydrofluoric acid aqueous solution (HF, ≥40 wt%), N-methylpyrrolidone (NMP, ≥99.7 wt%), ethanol (≥99.9 wt%) and hydrogen peroxide (H2O2, ≥30 wt%) were obtained from Adamas Reagent Chemical Co., Ltd. (Shanghai, China). Iodonitrotetrazolium chloride (INT, ≥98%) and Methylene blue (MB, ≥70%) were bought from Aladdin Scientific Co., Ltd. (Shanghai, China). Bacterial live/dead staining kits was bought from US Everbright Inc. (Suzhou, China). DCFH-DA, CCK-8, Calcein-AM/PI assay were bought from Keygen Biotech Co., Ltd. (Nanjing, China). ATP Assay Kit and Crystal Violet Staining Solution was obtained from Beyotime Biotech Co., Ltd. (Shanghai, China). Microbial ATPase ELISA kit was purchased from Hengyuan Biotechnology (Shanghai, China). Dulbecco’s modified eagle’s medium (DMEM, high glucose) and Roswell Park Memorial Institute (RPMI) 1640 medium were purchased from iCell Bioscience Co., Ltd. (Shanghai, China). Fetal bovine serum (FBS), PBS, and penicillin/streptomycin were obtained from Gibco (Thermo Fisher Scientific Inc., USA). All chemicals were used directly without further purification.
Synthesis of TiOx@C
-
a.
Synthesis of Ti3C2. The Ti3AlC2 ceramic bulk was ground to a fine powder. About 10 g of the powder was immersed in 80 ml of 40% HF aqueous solution and left at room temperature for 3 days. Then collected by centrifugation (4601 g, 10 min) and washed several times with water and ethanol. The obtained Ti3C2 was dispersed in NMP (20 mg/mL) and sonicated in a water bath (200 W, 8 h). Afterwards, the precipitate was obtained by centrifugation (6010 g, 15 min) and freeze-dried to powder.
-
b.
Synthesis of TiOx@C. 1 mL of 3.0 wt% H2O2 was dropwise added into 10 mL of Ti3C2 suspension (1 mg/mL) under vigorous stirring at 4 °C and reacted for 12 h. The precipitated bulk Ti3C2 was removed by centrifugation (9390 g, 20 min) to obtain a stably dispersed TiOx@C solution. Then TiOx@C aqueous dispersion was obtained and powdered products can be obtained by freeze-drying.
Cells and Bacteria
Mouse Fibroblast cell line (L929 cells) (SCSP-5039), rat PC12 cells (SCSP-517) and human HacaT cells (SCSP-5091) were obtained from Cell Bank/Stem Cell Bank, Chinese Academy of Science. L929 cells were cultured in in RPMI-1640 medium containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. PC12 cells were cultured in DMEM high glucose medium added with 10% FBS and 1% penicillin/streptomycin. Both cells were cultured in a humidified atmosphere of 5% CO2 at 37 °C. Staphylococcus aureus (S. aureus, ATCC6538), and Pseudomonas aeruginosa (P. aeruginosa, ATCC9027) were ordered from Guangdong Microbial Culture Collection Center. (Guangzhou, China). Bacteria were grown in Luria-Broth (LB) broth and LB agar (Beyotime Biotechnology). Biofilms were grown in 1/10 LB broth. The bacteria were cultured at 37 °C and passaged every day
Antibacterial experiments
Before the experiment, bacteria (S. aureus, P. aeruginosa) were incubated overnight in LB broth at 37 °C (240 rpm shaking). The bacteria in logarithmic growth period (OD600 = 0.5, 108 Colony Forming Units (CFU) mL−1) were centrifuged (3280 g, 5 min) and resuspended in 100 μL saline. At the same time, 900 μL TiOx@C saline dispersion at different concentration (20, 30, 40 μg/mL) was added at 37 °C for 1 h. To explore the antibacterial performance of other Ti-based nanomaterials, the bacteria were treated with Ti3C2 and TiO2 with the Ti concentration of 40 ppm under the same conditions. After incubation, the bacteria were diluted and plated on LB agar overnight at 37 °C. To quantify bacteriostasis, the number of colonies were counted.
Animals
Six-to-eight-week-old healthy female BALB/c mice were provided by SPF biotechnology co. (Beijing, China). All animals were housed under the standard conditions for seven days prior to treatment (temperature 20–25 °C, 12 h light/dark cycle, humidity of 60–70%). Animals were assigned to experimental groups randomly.
Biosafety evaluation
Ten female BALB/C mice were equally divided into two groups, then the mice of TiOx@C group treated with TiOx@C suspension through external application. The control group was fed normally without any treatment. For blood analysis, the blood samples were obtained from mice on day 14. Then, the mice were sacrificed after blood collection, and the major organs samples were taken for H&E staining and pathological evaluation.
S. aureus-infected wound model
30 female BALB/c mice were equally divided into three groups (Control, Untreated, TiOx@C treatment). To establish wound model, all the mice were removed hair and excised a square cut (10 mm*10 mm appropriately) on the back. For bacterial infection, S. aureus suspension (OD600 = 1.0, 50 μL) was injected into the wound and dressed with plaster (Hynaut, China). After infection for 24 h, the TiOx@C materials (50 μg/mL, 50 μL) were sprayed on the wounds. The lesions were recorded by taking photographs and measured by vernier. Some mice were killed on day 2 and the infected tissue were collected and homogenized to count the number of bacteria. For histopathological analysis, the lesioned skins were collected after sacrifice on day 14. The BALB/c mice with uninfected wound were used as negative control (group Control), and the infected mice without any treatments were regarded as positive control (group Untreated).
Statistical analysis
All the statistical data were presented as the mean ± standard deviation (SD) from at least three independent experiments. One-way/two-way analysis of variance (ANOVA) with Bonferron’s significant difference post-hoc test was conducted for multiple comparisons, in which a P value of less than 0.05 was considered as significant difference.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data underlying this study are available from the corresponding author upon request. The authors declare that all data needed to support the findings of this study are provided within the article, Supplementary information, and Source data file. The raw data of RNA-sequencing has been deposited in the China National Center for Bioinformation under the BioProject accession number PRJCA034742 (https://www.cncb.ac.cn/). Source data are provided as a Source Data file. Source data are provided with this paper.
References
Murray, C. J. L. et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399, 629–655 (2022).
Smith, W. P. J., Wucher, B. R., Nadell, C. D. & Foster, K. R. Bacterial defences: mechanisms, evolution and antimicrobial resistance. Nat. Rev. Microbiol. 21, 519–534 (2023).
Willyard, C. The drug-resistant bacteria that pose the greatest health threats. Nature 543, 15–15 (2017).
Balakrishnan, V. S. WHO’s first global infection prevention and control report. Lancet Infect. Dis. 22, 1122 (2022).
Gupta, A., Mumtaz, S., Li, C.-H., Hussain, I. & Rotello, V. M. Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev. 48, 415–427 (2019).
Makabenta, J. M. V. et al. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 19, 23–36 (2021).
Huh, A. J. & Kwon, Y. J. Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Controlled Release 156, 128–145 (2011).
Xie, M. et al. Antibacterial nanomaterials: mechanisms, impacts on antimicrobial resistance and design principles. Angew. Chem. Int. Ed. 62, e202217345 (2023).
Van Acker, H., Van Dijck, P. & Coenye, T. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol. 22, 326–333 (2014).
Darby, E. M. et al. Molecular mechanisms of antibiotic resistance revisited. Nat. Rev. Microbiol. 21, 280–295 (2023).
Pillai, P. P., Kowalczyk, B., Kandere-Grzybowska, K., Borkowska, M. & Grzybowski, B. A. Engineering gram selectivity of mixed-charge gold nanoparticles by tuning the balance of surface charges. Angew. Chem. Int. Ed. 55, 8610–8614 (2016).
Fang, G. et al. Differential Pd-nanocrystal facets demonstrate distinct antibacterial activity against gram-positive and gram-negative bacteria. Nat. Commun. 9, 129 (2018).
Wang, L. et al. The density of surface coating can contribute to different antibacterial activities of gold nanoparticles. Nano Lett. 20, 5036–5042 (2020).
Niu, J. et al. Antibody mimics as bio-orthogonal catalysts for highly selective bacterial recognition and antimicrobial therapy. ACS Nano 15, 15841–15849 (2021).
Yuan, H. et al. Cationic conjugated polymers for discrimination of microbial pathogens. Adv. Mater. 26, 4333–4338 (2014).
Liu, K. et al. Supramolecular photosensitizers with enhanced antibacterial efficiency. Angew. Chem. Int. Ed. 52, 8285–8289 (2013).
Jia, H. R., Zhu, Y. X., Duan, Q. Y. & Wu, F. G. Cell surface-localized imaging and sensing. Chem. Soc. Rev. 50, 6240–6277 (2021).
Zhu, C. et al. Multifunctional cationic poly(p-phenylene vinylene) polyelectrolytes for selective recognition, imaging, and killing of bacteria over mammalian cells. Adv. Mater. 23, 4805–4810 (2011).
Wu, X. et al. Reactivity differences enable ROS for selective ablation of bacteria. Angew. Chem. Int. Ed. 61, e202200808 (2022).
Wang, J., Li, J., Shen, Z., Wang, D. & Tang, B. Z. Phospholipid-mimetic aggregation-induced emission luminogens for specific elimination of gram-positive and gram-negative bacteria. ACS Nano 17, 4239–4249 (2023).
Miller, K. P., Wang, L., Benicewicz, B. C. & Decho, A. W. Inorganic nanoparticles engineered to attack bacteria. Chem. Soc. Rev. 44, 7787–7807 (2015).
Koo, H., Allan, R. N., Howlin, R. P., Stoodley, P. & Hall-Stoodley, L. Targeting microbial biofilms: current and prospective therapeutic strategies. Nat. Rev. Microbiol. 15, 740–755 (2017).
Caster, J. M., Patel, A. N., Zhang, T. & Wang, A. Investigational nanomedicines in 2016: a review of nanotherapeutics currently undergoing clinical trials. WIREs Nanomed. Nanobiotechnology 9, e1416 (2017).
Arun, J. et al. Synthesis and application of titanium dioxide photocatalysis for energy, decontamination and viral disinfection: a review. Environ. Chem. Lett. 21, 339–362 (2023).
Kumaravel, V. et al. Antimicrobial TiO2 nanocomposite coatings for surfaces, dental and orthopaedic implants. Chem. Eng. J. 416, 129071 (2021).
Chen, X. & Mao, S. S. Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem. Rev. 107, 2891–2959 (2007).
Yang, M. et al. NIR-responsive TiO2 biometasurfaces: toward in situ photodynamic antibacterial therapy for biomedical implants. Adv. Mater. 34, 2106314 (2022).
Li, Z. et al. Antibacterial ability of black titania in dark: Via oxygen vacancies mediated electron transfer. Nano Today 50, 101826 (2023).
Naguib, M. et al. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 23, 4248–4253 (2011).
Ahmed, B., Anjum, D. H., Hedhili, M. N., Gogotsi, Y. & Alshareef, H. N. H2O2 assisted room temperature oxidation of Ti2C MXene for Li-ion battery anodes. Nanoscale 8, 7580–7587 (2016).
Chen, Y. & Mao, J. Sol–gel preparation and characterization of black titanium oxides Ti2O3 and Ti3O5. J. Mater. Sci.: Mater. Electron. 25, 1284–1288 (2014).
Luo, D. et al. Revealing the rapid electrocatalytic behavior of ultrafine amorphous defective Nb(2)O(5-x) nanocluster toward superior Li-S performance. ACS Nano 14, 4849–4860 (2020).
Zhang, H. et al. MXene-derived TinO2n−1 quantum dots distributed on porous carbon nanosheets for stable and long-life Li–S batteries: enhanced polysulfide mediation via defect engineering. Adv. Mater. 33, 2008447 (2021).
Wang, S. et al. Electrochemical impedance spectroscopy. Nat. Rev. Methods Prim. 1, 41 (2021).
Lowy, F. D. Staphylococcus aureus infections. N. Engl. J. Med. 339, 520–532 (1998).
Rossi, E. et al. Pseudomonas aeruginosa adaptation and evolution in patients with cystic fibrosis. Nat. Rev. Microbiol. 19, 331–342 (2021).
Wang, J. et al. NIR-dependent photothermal-photodynamic synergistic antibacterial mechanism for titanium carbide nanosheets intercalated and delaminated by tetramethylammonium hydroxide. Biomater. Adv. 152, 213492 (2023).
Wang, R. et al. Graphdiyne-modified TiO2 nanofibers with osteoinductive and enhanced photocatalytic antibacterial activities to prevent implant infection. Nat. Commun. 11, 4465 (2020).
Ling, L. L. et al. A new antibiotic kills pathogens without detectable resistance. Nature 517, 455–459 (2015).
Stabryla, L. M. et al. Role of bacterial motility in differential resistance mechanisms of silver nanoparticles and silver ions. Nat. Nanotechnol. 16, 996–1003 (2021).
Qiu, Z. et al. Effects of nano-TiO2 on antibiotic resistance transfer mediated by RP4 plasmid. Nanotoxicology 9, 895–904 (2015).
Anh Le, T. T., Thuptimdang, P., McEvoy, J. & Khan, E. Phage shock protein and gene responses of Escherichia coli exposed to carbon nanotubes. Chemosphere 224, 461–469 (2019).
Ciofu, O., Moser, C., Jensen, P. Ø. & Høiby, N. Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol. 20, 621–635 (2022).
Flemming, H.-C. & Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 8, 623–633 (2010).
Shi, T. et al. Nanohole-boosted electron transport between nanomaterials and bacteria as a concept for nano–bio interactions. Nat. Commun. 12, 493 (2021).
Dufrêne, Y. F. Towards nanomicrobiology using atomic force microscopy. Nat. Rev. Microbiol. 6, 674–680 (2008).
Linklater, D. P. et al. Antibacterial action of nanoparticles by lethal stretching of bacterial cell membranes. Adv. Mater. 32, 2005679 (2020).
Hayden, S. C. et al. Aggregation and interaction of cationic nanoparticles on bacterial surfaces. J. Am. Chem. Soc. 134, 6920–6923 (2012).
Nikaido, H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev.: MMBR 67, 593–656 (2003).
Wang, G. et al. Quantifiable relationship between antibacterial efficacy and electro-mechanical intervention on nanowire arrays. Adv. Mater. 35, e2212315 (2023).
Ivanova, E. P. et al. Bactericidal activity of black silicon. Nat. Commun. 4, 2838 (2013).
Liu, Z. et al. Bio-inspired self-adaptive nanocomposite array: from non-antibiotic antibacterial actions to cell proliferation. ACS Nano 16, 16549–16562 (2022).
Karunakaran, S., Pandit, S., Basu, B. & De, M. Simultaneous exfoliation and functionalization of 2H-MoS2 by thiolated surfactants: applications in enhanced antibacterial activity. J. Am. Chem. Soc. 140, 12634–12644 (2018).
Duarte, H., Gummel, J., Robles, E., Berti, D. & Fratini, E. Ultra-/small angle x-ray scattering (USAXS/SAXS) and static light scattering (SLS) modeling as a tool to determine structural changes and effect on growth in s. epidermidis. ACS Appl. Bio Mater. 5, 3703–3712 (2022).
Vercellino, I. & Sazanov, L. A. The assembly, regulation and function of the mitochondrial respiratory chain. Nat. Rev. Mol. Cell Biol. 23, 141–161 (2022).
Jiang, Y. et al. In situ turning defects of exfoliated Ti(3)C(2) MXene into fenton-like catalytic active sites. Proc. Natl Acad. Sci. USA 120, e2210211120 (2023).
Mitchell, A. M. & Silhavy, T. J. Envelope stress responses: balancing damage repair and toxicity. Nat. Rev. Microbiol. 17, 417–428 (2019).
Brausemann, A., Zhang, L., Ilcu, L. & Einsle, O. Architecture of the membrane-bound cytochrome c heme lyase CcmF. Nat. Chem. Biol. 17, 800–805 (2021).
Moosavi, B., Berry, E. A., Zhu, X.-L., Yang, W.-C. & Yang, G.-F. The assembly of succinate dehydrogenase: a key enzyme in bioenergetics. Cell. Mol. Life Sci. 76, 4023–4042 (2019).
Fenoll, A., Muñoz, R., García, E. & de la Campa, A. G. Molecular basis of the optochin-sensitive phenotype of pneumococcus: characterization of the genes encoding the F0 complex of the Streptococcus pneumoniae and Streptococcus oralis H(+)-ATPases. Mol. Microbiol. 12, 587–598 (1994).
Singh, D., Rai, V. & Agrawal, D. K. Regulation of collagen i and collagen iii in tissue injury and regeneration. Cardiol. Cardiovascular Med. 7, 5–16 (2023).
Acknowledgements
We would like to express our sincere gratitude to Shanghai Synchrotron Radiation Facility (SSRF) for their support to the research. The authors acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 22422510, to H. L., 52372276, to H. L., 22335006, to J. S.), National Key R&D Program of China (Grant No. 2022YFB3804500, to J.S.), Youth Innovation Promotion Association CAS (Grant No. 2023262, to H. L.), Young Elite Scientists Sponsorship Program by CAST (Grant No. YESS20210149, to H. L.), Shanghai Science and Technology Committee Rising-Star Program (Grant No. 22QA1410200, to H. L.), Natural Science Foundation of Shanghai (Grant No. 23ZR1472300, to H. L.), and CAMS Innovation Fund for Medical Sciences (Grant No. 2021-I2M-5-012, to J. S.).
Author information
Authors and Affiliations
Contributions
Conceptualization: Y.Y. and H.L. Methodology: Y.Y., X.Y., J.J., Z.C., Y.X. Z., Y.C. Investigation: Y.Y. Supervision: H.L. and J.S. Writing, original draft: Y.Y. Writing—review & editing: H.L. and J.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Peer review
Peer review information
Nature Communications thanks Xiangang Hu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
You, Y., Yu, X., Jiang, J. et al. Bacterial cell wall-specific nanomedicine for the elimination of Staphylococcus aureus and Pseudomonas aeruginosa through electron-mechanical intervention. Nat Commun 16, 2836 (2025). https://doi.org/10.1038/s41467-025-58061-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-58061-5
This article is cited by
-
Identification and antibacterial activity of a novel antimicrobial peptide attacin from Conogethes punctiferalis
Archives of Microbiology (2025)