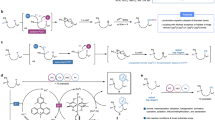
Abstract
In recent years, amination of alkylboronates through ionic copper catalysis or boron-ate complex 1,2-metalation has been well established, but complementary radical processes remain less studied before. Herein, based on rational design, we develop several imine-type N-centered radical scavengers and apply them to the radical amination of alkylboronates. The reaction proceeds under mild photoredox-catalyzed transition-metal-free conditions and features excellent functional group tolerance. It also enables the preparation of a range of medicinally valuable amine derivatives from complex natural products. Further application of this reagent in C-H amination, deoxygenative amination, decarboxylative amination and three component trifluoromethylative/sulfonylative aminations are also realized. Further mechanistic studies and DFT calculations are conducted to provide detailed evidence for the mechanism.
Similar content being viewed by others
Introduction
Organoboron compounds are some of the most useful compounds in synthetic chemistry because of the versatile reactivity of carbon–boron bonds1,2,3,4. Among them, boronic acid pinacol esters have received a great deal of attention from the synthetic community due to their stability, which facilitates their handling and allows them to be used for a broad range of reactions. Amines and other nitrogen-containing functional groups are highly important and can be found in many bioactive alkaloids, small molecule pharmaceuticals, and agrochemicals5. Thus, transformations of pinacol boronates to corresponding amines are highly valuable, especially for medicinal chemistry.
Since 1964, amination of organoboranes (such as trialkylboranes, dichloroboranes, and dialkylborinates) has been well studied6. But until recent decades, the amination of bench-stable boronic acids or boronic esters has been reported. Among them, since 1998, the copper-promoted C-N bond formation of arylboronic acids with amines and amides (Chan–Lam coupling) is an attractive method7,8,9,10,11. Later, the substrate scope has been expanded to arylboronates, alkyl boronic acids, alkylboronates and potassium organotrifluoroborates12,13,14,15,16,17. Besides copper catalysis, an alternative strategy is the conversion of a boron reagent to a boron ‘ate’ complex, mediated by the aminating reagent, followed by a 1,2-metalate rearrangement that establishes a C-N bond and yields the amination product.18,19,20,21,22,23,24. Those aminations are generally transition-metal-free and stereospecific. From 2012, the groups of Morken19,21,23, Kürti20, and Liu22,24 have made great contributions to this area. Very recently, an enzymatic process has also been reported25.
Ionic amination of alkylboronates usually requires high temperature for copper catalysis or a strong base (e.g. n-BuLi, t-BuOK etc.) for boron-ate complex formation, which has resulted in limited functional group compatibility. A radical protocol through photoredox catalysis may provide an alternative milder pathway. However, the development of such a protocol is not straightforward due to the challenges in achieving single-electron oxidation of alkylboronates, so further activation is necessary. In 2017, the Ley group reported that a weak Lewis base could act as an activator to promote this process (Fig. 1a), but this protocol is limited to benzylic boronates and α-heteroatom-substituted primary alkyl boronates26. Soon after, the Aggarwal group realized the activation of general alkyl boronic esters with PhLi as the activator27,28,29,30, and recently the Liu group reported single-electron oxidation of alkylboronates with NaOMe or NaOH as the activator, however strong bases were required31. In contrast, in 2022, Maier reported a milder amino radical transfer (ART) activation strategy, where alkylboronates could be activated by an in situ generated amino radical32. Recently, the Studer group also applied this strategy to generate alkyl radicals, and further pointed out that this process proceeded through a nucleohomolytic substitution mechanism (Fig. 1a)33. Since no further activation step was required, and the boronic esters could be activated by the amino radical generated from a weak organic base34,35,36, we decide to apply this strategy into our transformations.
In recent years, radical amination has attracted much attention. Several different strategies for radical C-N couplings have been reported37,38,39,40, for example, the reaction of N-centered radical addition to olefins and aromatics has been well established, whereas the development of transition-metal-catalyzed strategies and radical-radical coupling strategies have been fast growing38,39,40. However, the research field of N-centered radical scavengers has suffered from slow development41. Although different reagents (Fig. 1b), such as nitric oxide41, azodicarboxylates42,43,44,45,46, diazonium salts47,48,49, diazirines50, nitrosoarenes51 and sulfonyl azides52,53, have been used as N-centered radical scavengers for many years, there are only few reagents developed in the recent 20 years, such as α-diazoacetates54,55,56 and sulfonyl triazoles57,58,59. After carefully analysis, we note that most of these reagents contain N–N or N–O multiple bonds, so that subsequent reduction is required to obtain the free amine, which reduces the redox economy of the process. Until 2010, the Studer group applied stannylimine as the scavenger to give an imine as the final product, which only requires hydrolysis to give the corresponding amine60. This synthetic approach is redox-economical, but due to the high toxicity of stannylimine, further application is limited. Very recently, the research groups of Cho61,62, Glorius63,64,65,66,67,68,69, Prieto70 and others71,72,73,74 have developed an oxime ester derivative as the amination reagent. For this reagent, through photoinduced triplet energy transfer (EnT) pathway, a persistent iminyl radical was generated as the N-centered radical scavenger.
In this study, inspired by the works of Studer60,75, Cho61,62 and Glorius63,64,65,66,67,68,69, we decide to develop an imine-type N-centered radical scavenger that benefits from stability, convenient scale-up preparation, and properties that traditional reagents lack, such as non-explosivity (compared to azide reagents) and low toxicity (compared to stannylimines). We also want the resulting imines to be easily hydrolyzed to access free amines while the by-product ketone could be recovered and recycled (Fig. 1c). Based on the development of this reagent, we would then investigate the visible-light photoredox-catalyzed radical amination of alkyl pinacol boronates, and application of this method in the synthesis of several functional molecules. Once this is successfully achieved, we aim to show the generality of the developed reagent in more challenging C-H aminations, deoxygenative aminations and some other transformations.
Results
Reaction development
We began our studies by rational design and synthesis of imine derivatives as potential N-centered radical scavengers. Firstly, we synthesized 2a and 2b61. Our hypothesis is that if the photo-oxidation step to generate an alkyl radical is quicker than triplet energy transfer (EnT), the newly formed radical will add to 2a/2b to generate a more thermodynamically stabilized radical intermediate INTa/INTb, which will promote the progression of the reaction. Then, considering the polarity of the C=N bond in imines, N is more electronegative than C, so any (partial) negative charge would localize on the N atom. As most alkyl radicals are nucleophilic radicals, which prefer addition to electropositive sites, thus umpolung natural polarity of the imines is required to revert its reactivity (Fig. 2a). Inspired by the creative work from Kurti76, oxime tosylate 2c was synthesized. We then synthesized 2d and 2e with two aromatic rings to help stabilize the anticipated radical center and a ketone to induce umpolung. Similarly, oxime derivatives with a sulfone group 2f, 2g and 2h were synthesized (Fig. 2b). It should be noted that, putting an electron-withdrawing group on the carbon side of the imines would not only invert the polarity of the C=N bond, but also make the molecules redox-active. As traditional oxime derivatives (like 2a/2b) are well known for EnT activation to generate a persistent iminyl radical for radical-radical coupling reactions; our newly developed reagent 2d to 2h would be much easier for single-electron transfer (SET) activation to generate corresponding persistent iminyl radicals77,78,79,80.
a Rational design of the imine-type N-centered radical scavengers. b Different N-centered radical scavengers been designed and synthesized. c Optimization of the reactions, standard reaction conditions: 1a (0.2 mmol), 2 (0.4 mmol), morpholine (0.6 mmol), 4-CzIPN (5 mol%), MeCN (0.1 M), blue LEDs, 16 h. Yields were determined by 1H NMR analysis with CH2Br2 as an internal standard. amorpholine (0.4 mmol). bPhotocatalyst = Ir[(dFCF3ppy)2(dtbbpy)]PF6 (1 mol%). cWithout photocatalyst. dIsolated yield. eMeCN as solvent. fWith 390 nm LEDs.
With these potential scavengers in hand, we proceeded to investigate the reactivity of aminating reagents 2 with alkyl boronic ester 1a. Unsurprisingly, 2a and 2b failed to give any desired product (Fig. 2c, entry 1). Scavenger 2c also showed no reactivity (entry 2). Delightfully, employing 2d gave the desired product in 27% yield (entry 3), whereas 2e resulted in a slight improved yield (entry 4). Much to our surprise, 2f showed no reactivity (entry 5), but when we switched to 2g, the desired product was obtained in excellent yield (entry 6), whereas reagent 2h gave a much lower yield (entry 7). When morpholine was reduced to 2 equivalents, the yield decreased significantly (entry 8). Using 1 mol% Ir[(dFCF3ppy)2(dtbbpy)]PF6 instead of 4-CzIPN as the photocatalyst resulted in a significantly lower yield (entry 9). Switching the solvent to DMF resulted in a decreased yield (entry 10). Interestingly, irradiation without a photocatalyst in DMF also afforded the desired product in 12% yield, but in contrast, when MeCN was used as the solvent under photocatalyst-free conditions, no desired product was obtained, which could be due to poor solubility (entry 11). Further optimization results showed that irradiation with 390 nm LEDs in DMF without a photocatalyst could give the desired product in 28% yield (entry 12). However, we could not obtain any better results under photocatalyst-free conditions.
Substrate scope
With the optimized conditions in hand, we investigated the scope of the deboronative amination (Fig. 3). Cyclic secondary alkyl-Bpin (1a-1f) were converted effectively and delivered the corresponding imines in moderate to good yields. Interestingly, we found that substrates with a non-strained 6-membered or 5-membered ring (1a-1d) could give the desired product in good yield, however much lower yields were obtained from substrates with a 4-membered or 12-membered ring (1e, 1f). Acyclic substrates (1g-1m) were also efficiently transformed to the desired products. It should be noted, substrates with a cyclic ether (1c), Boc-protected amine (1d), acetate (1i), ester (1j), alkyne (1l) or nitrile (1m) all gave the desired products in good yields, demonstrating excellent functional group tolerance. However, the substrate with an amide group (1k) resulted in a lower yield. Primary alkyl-Bpin (1n-1v) were smoothly transformed into the desired alkyl imines (3n-3t) in moderate yields. A range of functional groups were tolerated, including bromide (1o), silyl ether (1p), ketal (1q), indole (1t), carbazole (1u) and sulfonamide (1v). Much to our surprise, under the standard conditions, tertiary substrates (1w-1y) could not give the corresponding imine products but instead afforded saturated amines in moderate yields. We believed these saturated amines were produced from further reduction of the corresponding imines by the excess morpholine under the photoredox-catalyzed conditions81. Notably, derivatives of drug molecules and natural products, such as gemfibrozil (3y), cedrol (3z), dehydroabietic acid (3aa), santonin (3ab), diosgenin (3ac), vespertilin (3ad), and oleanolic acid (3ae) could be obtained in moderate to good yields, demonstrating that this method can be used for late-stage installation of C-N bonds from corresponding boronic esters in complex molecules.
Mechanistic studies and DFT calculations
Mechanistic studies were conducted to provide evidence for the proposed radical pathway. Radical clock experiments with substrate 1af provided the corresponding ring-opening product 3af in 43% yield, indicating that a cyclopropylmethyl radical intermediate was involved in the reaction (Supplementary Fig. S1a). Then, a series experiments of 2g with different photocatalysts were conducted to investigate how much starting material would remain (Supplementary Fig. S1b). We chose four photocatalysts which differs in both triplet energies and reduction potentials82,83,84,85,86,87. DFT calculations indicated that the triplet energy of 2g was 43.6 kcal/mol, while the excited energy of 4-CzIPN is 59.7 kcal/mol, which means triplet 4-CzIPN is strong enough to consume 2g in the absence of a reductant. However, our experiments showed that most of the starting material remained in each case for all four photocatalysts. Although through these experiments we cannot completely rule out the triplet EnT pathway, however, it does indicate that an EnT pathway is not likely to be the major pathway. Another strong evidence is that, in DMF, 12% yield of desired product was still obtained without a photocatalyst (Fig. 2c, entry 11). In this case, as blue light could not excite 2g directly, a triplet EnT pathway could be ruled out, and we believe that, in the absence of a photocatalyst, there may be an EDA complex that initiates the reaction. This was further confirmed by UV-Vis experiments (Supplementary Fig. S1c). Besides, the quantum yield of our deboronative amination reaction under the standard conditions with 1a was determined to be 0.674, indicating that radical chain processes are not supported (see Supplementary Information for details).
To gain further understanding of the mechanism, DFT calculations for the deboronative imination reaction were performed (Fig. 4). Firstly, morpholine INT1 could be oxidized by the excited photocatalyst through a SET process to give a radical cation species INT2 and the reduced PC.- species. Next, INT2 could be converted to an N-centered radical INT3 via proton transfer with another morpholine molecule. Then, the yielded N-centered radical INT3 could attack the B-site of 1a via TS1 to generate a cyclohexyl radical INT4. The predicted activation barrier for this step is 8.5 kcal/mol relative to 1a + INT3. Subsequently, the yielded cyclohexyl radical INT4 could attack the C=N moiety of 2g. Computational results show that it is more likely for INT4 to attack the N-site of 2g via TS2a, than the C-site via TS2b, to afford the radical adduct INT5. For the following transformations, there are two possible pathways. In pathway a (black in Fig. 4), INT5 is further reduced by the reduced photocatalyst to form the anionic intermediate, which undergoes β-elimination through transition state TS3a to give desired product 3a. Alternatively, in pathway b (red in Fig. 2), INT5 could undergo β-scission directly through transition state TS3b to give 3a and release the radical intermediate INT6b, which could be further reduced by the reduced photocatalyst to give a tosylate anion. The transition state energy barrier for the β-elimination step (TS3a) is 1.3 kcal/mol lower than that for the β-scission step (TS3b), indicating that pathway a is more favorable. On the other hand, the reduced PC.- species might undergo SET with 2g to give the corresponding radical anion. The predicted energy barrier for this SET process is 6.9 kcal/mol (Supplementary Fig. S2). Next, a β-elimination step could follow via TS-S1 to give an iminyl radical and tosylate anion. The calculated activation barrier for this step is only 5.8 kcal/mol. The cross-coupling between the iminyl radical and the cyclohexyl radical could also give the desired product 3a, so may not be ruled out.
One may wonder whether an EnT pathway is feasible for this reaction. The predicted singlet-triplet energy gap of 2g is 43.6 kcal/mol. Nevertheless, the excited 2g* needs to overcome a barrier of 10.1 kcal/mol to undergo homolytic cleavage to give the iminyl radical (Supplementary Fig. S2), which is less favorable than the SET process.
Based on the mechanistic studies and DFT calculations, a possible mechanism was proposed (Fig. 5). Firstly, morpholine is oxidized by an excited photocatalyst (PC*) and undergoes deprotonation by another morpholine molecule to generate radical A. Radical A then activates substrate 1a through nucleohomolytic substitution to generate cyclohexyl radical B, which in turn adds to aminating reagent 2g and give intermediate C. Then C is reduced by the reduced photocatalyst to give anionic intermediate D, which undergoes β-elimination to give the desired product 3a. Also, there is another possible mechanism (Fig. 5, pathway II), where after cyclohexyl radical B is generated, 2g could be further reduced by the reduced photocatalyst to give intermediate E. This in turn generates iminyl radical F after β-elimination of a tosylate anion. Finally, cyclohexyl radical B undergoes cross coupling with the iminyl radical F to give desired product 3a. Since reagent 2g is present in large excess, whereas iminyl radical F is generated in low concentration in the reaction system, we still believe that pathway I is more probable than pathway II, but we cannot rule out the latter.
Application in functional molecules syntheses and other transformations
The excellent functional group tolerance of the mild reaction conditions further encouraged us to apply our method in the synthesis of functional molecules. In recent years, the cedrol derivatized amine 8 was found to have interesting anti influenza virus activity88. But starting from cedrene (4), the traditional synthetic route required 4 steps (Fig. 6a), including hydroboration-oxidation to give alcohol 5, then oxidation to give ketone 6, followed by further transformation into oxime 7, and finally reduction to give amine 8. It is worth noting that, unfortunately, this route affords product 8 as an inseparable mixture. Recently, the Shu group reported an improved synthesis of 8, through direct radical hydroamination of cedrene (4), where the desired product 8 could be obtained in a single step with 95% yield89. Unfortunately, with his method, 8 was still obtained as an inseparable mixture (d.r. = 1.6:1). Now, using our method, we have demonstrated that it only requires 2 steps (hydroboration and deboronative amination) to synthesise the desired product 3z as a single diastereomer. After hydrolysis, the desired free amine 8 was obtained as a single diastereomer with a yield of 68%. We then applied our method into the synthesis of (S)-phenibut, starting from boronic ester 9. Previously, the Yun group transformed the pinacol boronate into dichloroborane 1090, and then reacted it with benzyl azide to obtain protected phenibut 11. Through our method, pinacol boronate 9 could be transformed into protected phenibut 12 in a single step, with 100% es. Our method was further applied to the synthesis of a chiral amino alcohol91. Starting from boronic ester 13, desired product 14 was obtained with good es. Interestingly, in this reaction we also obtained side-product 15, possibly through a radical 1,2-aryl migration process.
To further demonstrate the generality of this developed reagent, more challenging transformations (such as, deoxygenative amination, C-H amination and three-component radical relay aminations) and other radical precursors (such as carboxylic acid, boronic acid, and potassium organotrifluoroborate) were tested (Fig. 7). Delightfully, under slightly optimized conditions (see Supplementary Information for details), the use of an NHC activation strategy developed by the MacMillan group92,93,94,95 enabled the conversion of alcohol 16 into imine 3d in 15% yield (Fig. 7a). Additionally, cyclohexane 17 could be transformed into imine 3a in 19% yield (Fig. 7b). Starting from carboxylic acid 18, the corresponding decarboxylative amination product 3a was obtained in 44% yield (Fig. 7c). With our standard conditions, boronic acid 19 could be converted into imine 3ag in 89% yield (Fig. 7d). With slightly optimized conditions, potassium trifluoroborate 20 could be transformed to imine 3r in 33% yield (Fig. 7e). Also, three-component trifluoromethylative amination96 of unactivated olefin 21 could deliver the desired difunctionalized product 22 in 45% yield (Fig. 7f), and three-component sulfonylative amination of styrene 23 successfully afforded desired product 24 in 50% yield (Fig. 7g).
In order to demonstrate the accessibility of the aminating reagent, we performed a large scale synthesis of 2g in single sequence and obtained 19.4 g of reagent 2g (Fig. 8a). To demonstrate the practical use of our deboronative amination reaction, we also carried out a scale up experiment, starting from 841 mg (4 mmol) boronate 1a, where desired product 3a was obtained in 53% yield (Fig. 8b). It should be highlighted that our imine products can be treated as protected amines, which can be easily converted into the corresponding amines through simple hydrolysis. In order to demonstrate the advantage of our method (Fig. 8c), we hydrolyzed product 3b with HCl to provide amine-HCl salt 25 in 55% yield, meanwhile recycling ketone 26 in 79% yield. After neutralizing 25 with NaOH, free amine 27 was obtained in 75% yield. The recycled ketone 26 could be further transformed into aminating reagent 2g.
Discussion
In conclusion, based on rational design, we have developed several imine-type N-centered radical scavengers and successfully applied them in visible-light photoredox-catalyzed transition-metal-free radical amination of alkyl pinacol boronates. The reaction proceeds under mild conditions, features excellent functional group tolerance, and enables the preparation of medicinally valuable imine derivatives of a range of complex natural products. Detailed mechanistic studies and DFT calculations has shown that a photoinduced EnT process is unlikely to be the major pathway; this reaction most likely proceeds through a photoredox-catalyzed SET process. Furthermore, DFT calculations support a radical addition, reduction, then anionic β-elimination pathway rather than a radical addition, β-scission pathway. To demonstrate the generality of these developed reagents, further application in C-H amination, deoxygenative amination, decarboxylative amination and three component trifluoromethylative/sulfonylative aminations were also realized. Given the importance of amination of organoboron compounds, we believe our radical process will be an important addition to this research area.
Methods
General procedure for deboronative amination reactions
An oven-dried vial (12 mL) equipped with a magnetic stirrer bar was sequentially charged with alkyl pinacol boronate (0.2 mmol, 1.0 equiv.), 2 (0.4 mmol, 2.0 equiv.), 4-CzIPN (4.0 mg, 0.01 mmol, 0.05 equiv.). The vial was evacuated and back-filled with nitrogen three times before CH3CN (1.0 mL, 0.2 M) and morpholine (52.3 mg, 0.6 mmol, 3.0 equiv.) were added. Then, the vial was irradiated under blue LEDs for 16 h. After the reaction was completed, the solution was concentrated under reduced pressure. The residues were directly purified by column chromatography to give the desired product (notably, the silica gel used here was pre-neutralized with 5% triethylamine in petroleum ether solution prior to the usage, in order to minimize the product loss).
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Should any raw data files be needed in another format they are available from the corresponding author upon request. Source data are provided with this paper.
References
Ollivier, C. & Renaud, P. Organoboranes as a Source of Radicals. Chem. Rev. 101, 3415–3434 (2001).
Xu, L., Zhang, S. & Li, P. Boron-selective reactions as powerful tools for modular synthesis of diverse complex molecules. Chem. Soc. Rev. 44, 8848–8858 (2015).
Diner, C. & Szabó, K. J. Recent Advances in the Preparation and Application of Allylboron Species in Organic Synthesis. J. Am. Chem. Soc. 139, 2–14 (2017).
Corpas, J., Mauleón, P., Arrayás, R. G. & Carretero, J. C. Transition-Metal-Catalyzed Functionalization of Alkynes with Organoboron Reagents: New Trends, Mechanistic Insights, and Applications. ACS Catal. 11, 7513–7551 (2021).
Bräse, S. Amino Group Chemistry: From Synthesis to the Life Sciences. Edited by Alfredo Ricci. ChemBioChem 9, 1509–1509 (2008).
Brown, H. C., Heydkamp, W. R., Breuer, E. & Murphy, W. S. The Reaction of Organoboranes with Chloramine and with Hydroxylamine-O-sulfonic Acid. A Convenient Synthesis of Amines from Olefins via Hydroboration. J. Am. Chem. Soc. 86, 3565–3566 (1964).
Chan, D. M. T., Monaco, K. L., Wang, R.-P. & Winters, M. P. New N- and O-arylations with phenylboronic acids and cupric acetate. Tetrahedron Lett. 39, 2933–2936 (1998).
Lam, P. Y. S. et al. New aryl/heteroaryl C-N bond cross-coupling reactions via arylboronic acid/cupric acetate arylation. Tetrahedron Lett. 39, 2941–2944 (1998).
Evans, D. A., Katz, J. L. & West, T. R. Synthesis of diaryl ethers through the copper-promoted arylation of phenols with arylboronic acids. An expedient synthesis of thyroxine. Tetrahedron Lett. 39, 2937–2940 (1998).
Peacock, D. M., Roos, C. B. & Hartwig, J. F. Palladium-Catalyzed Cross Coupling of Secondary and Tertiary Alkyl Bromides with a Nitrogen Nucleophile. ACS Cent. Sci. 2, 647–652 (2016).
Górski, B., Barthelemy, A.-L., Douglas, J. J., Juliá, F. & Leonori, D. Copper-catalysed amination of alkyl iodides enabled by halogen-atom transfer. Nat. Catal. 4, 623–630 (2021).
Cazorla, C., Métay, E., Andrioletti, B. & Lemaire, M. Ritter-type amidation of alkylboron derivatives with nitriles. Tetrahedron Lett. 50, 6855–6857 (2009).
González, I., Mosquera, J., Guerrero, C., Rodríguez, R. & Cruces, J. Selective Monomethylation of Anilines by Cu(OAc)2-Promoted Cross-Coupling with MeB(OH)2. Org. Lett. 11, 1677–1680 (2009).
Larrosa, M., Guerrero, C., Rodríguez, R. & Cruces, J. Selective Copper-Promoted Cross-Coupling of Aromatic Amines with Alkyl Boronic Acids. Synlett 2010, 2101–2105 (2009).
Matsuda, N., Hirano, K., Satoh, T. & Miura, M. Copper-Catalyzed Amination of Arylboronates with N,N-Dialkylhydroxylamines. Angew. Chem. Int. Ed. 51, 3642–3645 (2012).
Rucker, R. P., Whittaker, A. M., Dang, H. & Lalic, G. Synthesis of Tertiary Alkyl Amines from Terminal Alkenes: Copper-Catalyzed Amination of Alkyl Boranes. J. Am. Chem. Soc. 134, 6571–6574 (2012).
Sueki, S. & Kuninobu, Y. Copper-Catalyzed N- and O-Alkylation of Amines and Phenols using Alkylborane Reagents. Org. Lett. 15, 1544–1547 (2013).
Larouche-Gauthier, R., Elford, T. G. & Aggarwal, V. K. Ate Complexes of Secondary Boronic Esters as Chiral Organometallic-Type Nucleophiles for Asymmetric Synthesis. J. Am. Chem. Soc. 133, 16794–16797 (2011).
Mlynarski, S. N., Karns, A. S. & Morken, J. P. Direct Stereospecific Amination of Alkyl and Aryl Pinacol Boronates. J. Am. Chem. Soc. 134, 16449–16451 (2012).
Zhu, C., Li, G., Ess, D. H., Falck, J. R. & Kürti, L. Elusive Metal-Free Primary Amination of Arylboronic Acids: Synthetic Studies and Mechanism by Density Functional Theory. J. Am. Chem. Soc. 134, 18253–18256 (2012).
Edelstein, E. K., Grote, A. C., Palkowitz, M. D. & Morken, J. P. A Protocol for Direct Stereospecific Amination of Primary, Secondary, and Tertiary Alkylboronic Esters. Synlett 29, 1749–1752 (2018).
Liu, X. et al. Aminoazanium of DABCO: An Amination Reagent for Alkyl and Aryl Pinacol Boronates. Angew. Chem. Int. Ed. 59, 2745–2749 (2020).
Xu, P. et al. Construction of Azacycles by Intramolecular Amination of Organoboronates and Organobis(boronates). Org. Lett. 23, 3379–3383 (2021).
Xu, J., Qin, Y. & Liu, C. Direct Amination of Benzylic Pinacol Boronates by an Aminoazanium. Synlett 34, 2244–2248 (2023).
Hanley, D. et al. Stereospecific Enzymatic Conversion of Boronic Acids to Amines. J. Am. Chem. Soc. 146, 19160–19167 (2024).
Lima, F. et al. A Lewis Base Catalysis Approach for the Photoredox Activation of Boronic Acids and Esters. Angew. Chem. Int. Ed. 56, 15136–15140 (2017).
Kaiser, D., Noble, A., Fasano, V. & Aggarwal, V. K. 1,2-Boron Shifts of β-Boryl Radicals Generated from Bis-boronic Esters Using Photoredox Catalysis. J. Am. Chem. Soc. 141, 14104–14109 (2019).
Shu, C., Noble, A. & Aggarwal, V. K. Photoredox-Catalyzed Cyclobutane Synthesis by a Deboronative Radical Addition–Polar Cyclization Cascade. Angew. Chem. Int. Ed. 58, 3870–3874 (2019).
Wang, H., Han, W., Noble, A. & Aggarwal, V. K. Dual Nickel/Photoredox-Catalyzed Site-Selective Cross-Coupling of 1,2-Bis-Boronic Esters Enabled by 1,2-Boron Shifts. Angew. Chem. Int. Ed. 61, e202207988 (2022).
Wang, H., Wu, J., Noble, A. & Aggarwal, V. K. Selective Coupling of 1,2-Bis-Boronic Esters at the more Substituted Site through Visible-Light Activation of Electron Donor–Acceptor Complexes. Angew. Chem. Int. Ed. 61, e202202061 (2022).
Shi, D., Xia, C. & Liu, C. Photoinduced Transition-Metal-Free Alkynylation of Alkyl Pinacol Boronates. CCS Chem. 3, 1718–1728 (2021).
Speckmeier, E. & Maier, T. C. ART─An Amino Radical Transfer Strategy for C(sp2)–C(sp3) Coupling Reactions, Enabled by Dual Photo/Nickel Catalysis. J. Am. Chem. Soc. 144, 9997–10005 (2022).
Wang, Z., Wierich, N., Zhang, J., Daniliuc, C. G. & Studer, A. Alkyl Radical Generation from Alkylboronic Pinacol Esters through Substitution with Aminyl Radicals. J. Am. Chem. Soc. 145, 8770–8775 (2023).
Cauley, A. N. et al. Ni/Photoredox-Catalyzed C(sp2)–C(sp3) Cross-Coupling of Alkyl Pinacolboronates and (Hetero)Aryl Bromides. Org. Lett. 24, 5663–5668 (2022).
Lan, H., Huo, X., Jia, Y. & Wang, D. Silyl Radical Generation from Silylboronic Pinacol Esters through Substitution with Aminyl Radicals. Org. Lett. 26, 1011–1016 (2024).
Yue, F. et al. Deboronative functionalization of alkylboron species via a radical-transfer strategy. Chem. Sci. 15, 14241–14247 (2024).
Xiong, T. & Zhang, Q. New amination strategies based on nitrogen-centered radical chemistry. Chem. Soc. Rev. 45, 3069–3087 (2016).
Luo, J. & Wei, W.-T. Recent Advances in the Construction of C–N Bonds Through Coupling Reactions between Carbon Radicals and Nitrogen Radicals. Adv. Synth. Catal. 360, 2076–2086 (2018).
Zhao, Y. & Xia, W. Recent advances in radical-based C–N bond formation via photo-/electrochemistry. Chem. Soc. Rev. 47, 2591–2608 (2018).
Chan, C. M., Chow, Y. C. & Yu, W. Z. Recent Advances in Photocatalytic C-N Bond Coupling Reactions. Synthesis 52, 2899–2921 (2020).
Höfling, F. S. B. & Heinrich, M. R. Nitrogen-Centered Radical Scavengers. Synthesis 52, 2899–2921 (2020).
Guo, J.-J. et al. Photocatalytic C−C Bond Cleavage and Amination of Cycloalkanols by Cerium(III) Chloride Complex. Angew. Chem. Int. Ed. 55, 15319–15322 (2016).
Lang, S. B., Cartwright, K. C., Welter, R. S., Locascio, T. M. & Tunge, J. A. Photocatalytic Aminodecarboxylation of Carboxylic Acids. Eur. J. Org. Chem. 2016, 3331–3334 (2016).
Zhang, M.-J., Schroeder, G. M., He, Y.-H. & Guan, Z. Visible light-mediated decarboxylative amination of indoline-2-carboxylic acids catalyzed by Rose Bengal. RSC Adv. 6, 96693–96699 (2016).
Hu, A. et al. δ-Selective Functionalization of Alkanols Enabled by Visible-Light-Induced Ligand-to-Metal Charge Transfer. J. Am. Chem. Soc. 140, 1612–1616 (2018).
An, Q. et al. Cerium-Catalyzed C–H Functionalizations of Alkanes Utilizing Alcohols as Hydrogen Atom Transfer Agents. J. Am. Chem. Soc. 142, 6216–6226 (2020).
Lu, Z., Hennis, O., Gentry, J., Xu, B. & Hammond, G. B. Base-Promoted Radical Azofluoromethylation of Unactivated Alkenes. Org. Lett. 22, 4383–4388 (2020).
Zhang, J. et al. Transition-metal free C–N bond formation from alkyl iodides and diazonium salts via halogen-atom transfer. Nat. Commun. 13, 7961 (2022).
Ke, S. et al. Radical N2-Retention Cyclizations of Aryl Diazoniums: Access to 7/8/9-Membered Heterocycles. Org. Lett. 26, 3622–3627 (2024).
Shu, X., Xu, R. & Liao, S. Photocatalytic divergent decarboxylative amination: a metal-free access to aliphatic amines and hydrazines. Sci. China Chem. 64, 1756–1762 (2021).
Gao, Y., Yang, S., Xiao, W., Nie, J. & Hu, X.-Q. Radical chemistry of nitrosoarenes: concepts, synthetic applications and directions. Chem. Commun. 56, 13719–13730 (2020).
Liu, C., Wang, X., Li, Z., Cui, L. & Li, C. Silver-Catalyzed Decarboxylative Radical Azidation of Aliphatic Carboxylic Acids in Aqueous Solution. J. Am. Chem. Soc. 137, 9820–9823 (2015).
Marcote, D. C. et al. Photoinduced decarboxylative azidation of cyclic amino acids. Org. Biomol. Chem. 17, 1839–1842 (2019).
Zheng, J., Qi, J. & Cui, S. Fe-Catalyzed Olefin Hydroamination with Diazo Compounds for Hydrazone Synthesis. Org. Lett. 18, 128–131 (2016).
Chan, C.-M., Xing, Q., Chow, Y.-C., Hung, S.-F. & Yu, W.-Y. Photoredox Decarboxylative C(sp3)–N Coupling of α-Diazoacetates with Alkyl N-Hydroxyphthalimide Esters for Diversified Synthesis of Functionalized N-Alkyl Hydrazones. Org. Lett. 21, 8037–8043 (2019).
Essayan, D. E., Schubach, M. J., Smoot, J. M., Puri, T. & Pronin, S. V. Directed Hydrogen Atom Transfer for Selective Reactions of Polyenols. J. Am. Chem. Soc. 146, 18224–18229 (2024).
Ji, J. et al. Copper-Catalyzed Intermolecular 1,2-Carbotriazolization of Alkenes with N-Sulfonyl-1,2,3-Triazoles and Aldehydes. ACS Sustain. Chem. Eng. 11, 16240–16248 (2023).
Deng, Y. et al. Visible-Light-Promoted α-C(sp3)–H Amination of Ethers with Azoles and Amides. Org. Lett. 26, 933–938 (2024).
Ji, J. et al. Iodine-catalyzed intermolecular 1,2-thio (seleno)amination of alkenes with 1,2,3-triazoles and disulfides (diselenides) in air. Org. Chem. Front. 11, 3066–3071 (2024).
Lamas, M.-C., Vaillard, S. E., Wibbeling, B. & Studer, A. Radical Amination with Trimethylstannylated Benzophenone Imine. Org. Lett. 12, 2072–2075 (2010).
Soni, V. K. et al. Reactivity Tuning for Radical–Radical Cross-Coupling via Selective Photocatalytic Energy Transfer: Access to Amine Building Blocks. ACS Catal. 9, 10454–10463 (2019).
Kang, J., Hwang, H. S., Soni, V. K. & Cho, E. J. Direct C(sp3)–N Radical Coupling: Photocatalytic C–H Functionalization by Unconventional Intermolecular Hydrogen Atom Transfer to Aryl Radical. Org. Lett. 22, 6112–6116 (2020).
Patra, T., Bellotti, P., Strieth-Kalthoff, F. & Glorius, F. Photosensitized Intermolecular Carboimination of Alkenes through the Persistent Radical Effect. Angew. Chem. Int. Ed. 59, 3172–3177 (2020).
Patra, T., Das, M., Daniliuc, C. G. & Glorius, F. Metal-free photosensitized oxyimination of unactivated alkenes with bifunctional oxime carbonates. Nat. Catal. 4, 54–61 (2021).
Tan, G. et al. Photochemical single-step synthesis of β-amino acid derivatives from alkenes and (hetero)arenes. Nat. Chem. 14, 1174–1184 (2022).
Tan, G. et al. Highly Selective Radical Relay 1,4-Oxyimination of Two Electronically Differentiated Olefins. J. Am. Chem. Soc. 144, 21664–21673 (2022).
Tan, G. et al. Energy transfer-enabled unsymmetrical diamination using bifunctional nitrogen-radical precursors. Nat. Catal. 5, 1120–1130 (2022).
Erchinger, J. E. et al. EnT-Mediated N–S Bond Homolysis of a Bifunctional Reagent Leading to Aliphatic Sulfonyl Fluorides. J. Am. Chem. Soc. 145, 2364–2374 (2023).
Tan, G. et al. Metal-free photosensitized radical relay 1,4-carboimination across two distinct olefins. Chem. Sci. 14, 2447–2454 (2023).
Geniller, L., Taillefer, M., Jaroschik, F. & Prieto, A. Photocatalyzed Amination of Alkyl Halides to Access Primary Amines. J. Org. Chem. 89, 656–664 (2024).
Zheng, Y. et al. Regioselective Access to Vicinal Diamines by Metal-Free Photosensitized Amidylimination of Alkenes with Oxime Esters. Angew. Chem. Int. Ed. 61, e202212292 (2022).
Qi, X.-K. et al. Metal-Free Amino(hetero)arylation and Aminosulfonylation of Alkenes Enabled by Photoinduced Energy Transfer. J. Am. Chem. Soc. 145, 16630–16641 (2023).
Li, S.-S. et al. The Merger of Halogen Atom Transfer (XAT) and Energy Transfer Catalysis (EnT) for the Modular 1,2-Iminylalkylation of Diazenes. Org. Lett. 25, 7009–7013 (2023).
Zhuang, Z. et al. Visible-Light-Induced Decarboxylative Aminosulfonylation of (Hetero)aryl Carboxylic Oxime Esters. Org. Lett. 26, 713–718 (2024).
Kran, E., Mück-Lichtenfeld, C., Daniliuc, C. G. & Studer, A. Synthesis of Stannylated Aryl Imines and Amines via Aryne Insertion Reactions into Sn−N Bonds. Chem. Eur. J. 27, 9281–9285 (2021).
Kattamuri, P. V. et al. Practical Singly and Doubly Electrophilic Aminating Agents: A New, More Sustainable Platform for Carbon–Nitrogen Bond Formation. J. Am. Chem. Soc. 139, 11184–11196 (2017).
Zhang, H., Wei, Z., Zhang, A. H. & Yu, S. Access to Cyanoimines Enabled by Dual Photoredox/Copper-Catalyzed Cyanation of O-Acyl Oximes. Org. Lett. 22, 7315–7320 (2020).
Davies, J., Sheikh, N. S. & Leonori, D. Photoredox Imino Functionalizations of Olefins. Angew. Chem. Int. Ed. 56, 13361–13365 (2017).
Jiang, H. & Studer, A. Iminyl-Radicals by Oxidation of α-Imino-oxy Acids: Photoredox-Neutral Alkene Carboimination for the Synthesis of Pyrrolines. Angew. Chem. Int. Ed. 56, 12273–12276 (2017).
Davies, J., Morcillo, S. P., Douglas, J. J. & Leonori, D. Hydroxylamine Derivatives as Nitrogen-Radical Precursors in Visible-Light Photochemistry. Chem. Eur. J. 24, 12154–12163 (2018).
Wang, R. et al. Visible-Light-Mediated Umpolung Reactivity of Imines: Ketimine Reductions with Cy2NMe and Water. Org. Lett. 20, 2433–2436 (2018).
Romero, N. A. & Nicewicz, D. A. Organic Photoredox Catalysis. Chem. Rev. 116, 10075–10166 (2016).
Strieth-Kalthoff, F., James, M. J., Teders, M., Pitzer, L. & Glorius, F. Energy transfer catalysis mediated by visible light: principles, applications, directions. Chem. Soc. Rev. 47, 7190–7202 (2018).
Zhou, Q.-Q., Zou, Y.-Q., Lu, L.-Q. & Xiao, W.-J. Visible-Light-Induced Organic Photochemical Reactions through Energy-Transfer Pathways. Angew. Chem. Int. Ed. 58, 1586–1604 (2019).
Nicastri, M. C., Lehnherr, D., Lam, Y.-h, DiRocco, D. A. & Rovis, T. Synthesis of Sterically Hindered Primary Amines by Concurrent Tandem Photoredox Catalysis. J. Am. Chem. Soc. 142, 987–998 (2020).
Sakakibara, Y. & Murakami, K. Switchable Divergent Synthesis Using Photocatalysis. ACS Catal. 12, 1857–1878 (2022).
Corpas, J., Mauleón, P., Gómez Arrayás, R. & Carretero, J. C. E/Z Photoisomerization of Olefins as an Emergent Strategy for the Control of Stereodivergence in Catalysis. Adv. Synth. Catal. 364, 1348–1370 (2022).
Liu, J. et al. Cedrol derivative, preparation method and application thereof. CN Patent.CN112592252A (2021).
Du, Y.-D., Chen, B.-H. & Shu, W. Direct Access to Primary Amines from Alkenes by Selective Metal-Free Hydroamination. Angew. Chem. Int. Ed. 60, 9875–9880 (2021).
Jang, W. J. & Yun, J. Catalytic Asymmetric Conjugate Addition of a Borylalkyl Copper Complex for Chiral Organoboronate Synthesis. Angew. Chem. Int. Ed. 58, 18131–18135 (2019).
Dong, W., Ye, Z. & Zhao, W. Enantioselective Cobalt-Catalyzed Hydroboration of Ketone-Derived Silyl Enol Ethers. Angew. Chem. Int. Ed. 61, e202117413 (2022).
Dong, Z. & MacMillan, D. W. C. Metallaphotoredox-enabled deoxygenative arylation of alcohols. Nature 598, 451–456 (2021).
Sakai, H. A. & MacMillan, D. W. C. Nontraditional Fragment Couplings of Alcohols and Carboxylic Acids: C(sp3)–C(sp3) Cross-Coupling via Radical Sorting. J. Am. Chem. Soc. 144, 6185–6192 (2022).
Wang, J. Z., Lyon, W. L. & MacMillan, D. W. C. Alkene dialkylation by triple radical sorting. Nature 628, 104–109 (2024).
Chen, R. et al. Alcohol-alcohol cross-coupling enabled by SH2 radical sorting. Science 383, 1350–1357 (2024).
Majhi, J. et al. Metal-Free Photochemical Imino-Alkylation of Alkenes with Bifunctional Oxime Esters. J. Am. Chem. Soc. 144, 15871–15878 (2022).
Acknowledgements
The authors gratefully acknowledge financial support from National Natural Science Foundation of China (NSFC), Youth program (Grant No: 22101173), Fundamental Research Funds for the Central Universities (23X010301599, 24X010301678), “Thousand Talents Plan, Youth project” and startup funding from Shanghai Jiao Tong University (SJTU). We thank Prof. Zhaoguo Zhang from Shanghai Jiao Tong University for sharing the laboratory.
Author information
Authors and Affiliations
Contributions
J.W. conceived and directed the project. C.Z. performed the experiments and collected the data. X.B., J.L. conducted the DFT calculations. J.W., X.B., C.Z., and J.L. discussed the results. J.W. wrote the manuscript with contributions from all authors. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, C., Lin, J., Bao, X. et al. Development of N-centered radical scavengers that enables photoredox-catalyzed transition-metal-free radical amination of alkyl pinacol boronates. Nat Commun 16, 3225 (2025). https://doi.org/10.1038/s41467-025-58347-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-58347-8