Abstract
Real-time monitoring of neurotransmitters is essential in driving basic neuroscience understandings and creating treatments for various brain disorders. However, current neurotransmitter sensing devices are highly limited in their spatiotemporal resolution and ability to integrate with neuronal recording. Here, we introduce a unique carbon coating approach to achieve high-performance voltammetry electrodes with extraordinary scalability and interoperability. Surprisingly, we discovered that mild annealing drastically improves the electrochemical stability of graphene-based carbon coating, enabling the transformation of conventional neuroelectrodes into fast-scan-cyclic-voltammetry-stable carbon sensors. We successfully validated sub-second detection of nanomolar dopamine in vivo using carbon-coated microelectrodes (CCMs) in rodents and demonstrated arrays of one hundred CCMs with high yield and uniformity. Furthermore, we developed a dual-modal neural probe that integrates the CCM with electrophysiological recording sites, allowing us to demonstrate that dopamine fluctuation in the ventral striatum of awake rats strongly correlates with the high gamma power in the brain with sub-second-level precision. Together, these advances pave the way for spatiotemporally scalable and multiplexed brain interfacing, with also broad applicability in electrochemical-related diagnostic and interventional approaches.
Similar content being viewed by others
Introduction
Neurotransmitters are chemical messengers that heavily regulate basic physiological processes, stabilize neural circuits, and participate in various cognitive processes, including motivation, mood, and learning1,2. Real-time monitoring of their rapid dynamics is essential in both normal and pathologic conditions to drive basic neuroscience understandings and create treatments for various brain disorders, such as Parkinson’s disease, drug addiction, depression, and attention deficit hyperactivity disorder3,4,5,6,7. However, despite tremendous progress in tools for biochemical sensing, precisely measuring the rapid fluctuations of neurotransmitters in vivo remains challenging8. Furthermore, there is a pressing need to simultaneously monitor electrical activity and neurotransmitter dynamics to decode the intricate relationships between complex signal patterns and functional connectivity within the brain. Among various approaches for neurotransmitter measurement, electrochemical sensing is uniquely advantageous due to its sub-second temporal resolution9,10, human translatability11,12, and scalability13. Despite decades of development, electrochemical neurotransmitter sensing still largely relies on silica-encapsulated carbon fiber electrodes (CFEs), which are brittle and challenging to scale up for high spatial resolution or integrate with microelectrode-array-(MEA)-based neural interfaces commonly used for electrophysiological recording14,15. Great strides have been made to create alternative carbon electrodes from carbonization strategies such as pyrolysis of polymers16,17,18,19, and laser-induced graphitization20, but they are generally limited by prohibitively high temperatures or low spatial resolution (Supplementary Table 1).
We introduce a low-temperature carbon coating and engineering approach to transform standard microelectrodes into carbon microelectrodes for high-performance electrochemical sensing of neurotransmitters (Fig. 1a). Based on gold microelectrodes made from standard micro/nanofabrication, the creation of stable carbon-coated microelectrode (CCM) requires only two steps: (i) electroplating of graphene-based carbon coating at room temperature, followed by (ii) a mild annealing process at 250 °C, without the need for additional protection, etching, transfer, or lamination processes. We discovered that the mild annealing dramatically enhanced the electrochemical stability of the CCMs. As such, the CCMs achieved reliable, fast-scan cyclic voltammetry (FSCV) sensing of multiple monoamines with high sensitivity and nanomolar detection limits. Their ability to detect sub-second dopamine (DA) transients in vivo was validated in rats and mice with electrical stimulation and reward delivery. We achieved scalable fabrication of CCM arrays with up to 100 channels, demonstrating high yield and uniformity. Additionally, we effectively integrated the CCM with electrophysiological recording sites in a monolithic deep neural probe, which further unveiled fine timescale correlations between DA and high-gamma oscillations in response to addictive drugs in awake rats. We envision that the carbon coating and stabilization approach will enable spatiotemporally resolved and multiplexed brain activity mapping, directly impacting both neuroscience studies and clinical applications.
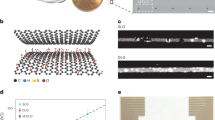
a Schematic of the surface type CCM array and penetration probe integrating CCM with electrophysiological recording sites (indicated by green). The presence of the oxide groups, sp2 carbon, and defects in the carbon coating contributes to achieving high-performance DA sensing. The compatibility of CCMs with the FSCV technique enables sub-second neurochemical dynamics measurement. The yellow trace represents phasic DA transients measured by FSCV using a CCM in awake animals. b Charge transfer resistance (Rct) and double-layer capacitance (Cdl) extracted from a simplified Randles equivalent circuit model as a function of the soak time (n = 4 CCMs, mean ± SD) in PBS (pH 7.4) at 37 °C. CFE: carbon fiber electrode. c Transmission Electron Microscopy images of cross-sectional CCM: as deposited (left) and 250 °C annealed (right). Inset: magnified view of horizontally stacked carbon flakes in the coating. d Grazing incidence X-ray diffraction pattern of the carbon coating, revealing the characteristic peaks corresponding to the parallel stacking of the carbon flakes. e Atomic ratio of oxygen obtained from Energy-dispersive X-ray spectroscopy (n = 4 CCMs, mean ± SD). f Schematic of the interface of the electrolyte and carbon coating to show the effect of annealing on the infiltration of water and ions into the carbon coating. Inset: equivalent circuit model for CCMs in PBS. Rs: solution resistance. The annealed carbon coating can resist the infiltration of water and ions because of the decrease of interlayer spacing and the removal of the oxide groups.
Results
Carbon coating and stabilization
We realized electrochemically stable carbon coating through first electroplating via potentiostatic deposition onto gold microelectrodes using electrochemical reduction of graphene oxide (GO) in aqueous dispersion, followed by mild annealing at 250 °C for 1 h in an N2 environment. GO micro-flakes were chosen as the precursor due to their dispersibility to water, large specific area, and tunable properties21,22. The detailed electroplating process is described in “Methods” and Supplementary Figs. 1 and 2. The resulting carbon coating, ~100 nm thick, possessed an intimate contact with the gold surface (Supplementary Fig. 3). We observed that the mild annealing dramatically improved the stability of the electrochemical interface of the CCM. As deposited, the CCM exhibited a significant impedance drop within 1-day soaking in phosphate-buffered saline (PBS) (Supplementary Fig. 4). Detailed equivalent circuit (EC) modeling (Supplementary Fig. 5) revealed a similar instability of charge transfer resistance (Rct) of the CCM/electrolyte interface with more than two orders of magnitude drop (Fig. 1b), indicating severe water/ion infiltration into the coating. Surprisingly, the annealing, although mild, significantly stabilized the Rct (Fig. 1b), with only a minor reduction comparable to commercial CFEs. The double-layer capacitance (Cdl) of the annealed CCM-electrolyte interface also exhibited negligible change with minimal sample-to-sample variation (Fig. 1b). Note that the entire process, including the room temperature deposition and low-temperature annealing, is fully compatible with the essential materials used in neural interfaces.
To shed light on why mild annealing dramatically improved the electrochemical stability, we further characterized the fundamental structure of the carbon coating. Transmission electron microscopy (TEM) image showed that it was constructed by horizontally stacked graphene layers, similar to graphite (Fig. 1c). Grazing incidence X-ray diffraction demonstrated that the stacking distance decreased from 4.0 to 3.7 Å after mild annealing, and the carbon micro-flakes exhibited better ordering (Fig. 1d and Supplementary Fig. 6). Additionally, the decrease in interlayer spacing was accompanied by a reduction in oxygen content in the carbon coating from 15.9% to 8.7% (Fig. 1e and Supplementary Fig. 7), which may arise from the removal of the intercalated water23 and/or hydroxy and carbon-oxygen single bonds (Supplementary Fig. 8). This dual reduction approach results in a lower oxygen content (8.7%) and reduced interlayer spacing (3.7 Å) compared to previous hydrothermal reduction methods (15% oxygen, 3.9 Å spacing)22. Additionally, the oxygen content in CCMs annealed at 250 °C is significantly lower than that of previous GO thermally reduced at 250 °C to 350 °C (~15% oxygen)24,25,26. Fundamentally, how annealing will affect the material structure of GO-based coating and its electrochemical sensing performance is largely unknown and nontrivial.
These structural changes support the potential degradation mechanism from water/ion infiltration (Fig. 1f). In ideal graphite, the interlayer space is impermeable to ions and molecules, including protons27. However, in the as-deposited carbon coating, larger interlayer distance and the presence of more hydrophilic oxide groups facilitate water and ions to infiltrate through the edges of the graphene micro-flakes and their interlayer space, which then increases the effective area of charge transfer at the CCM-electrolyte interface and reduces Rct. Quantitatively, a 100-nm carbon consists of ~250 graphene layers, and a complete coating infiltration would align with the observed two orders of magnitude Rct reduction. Conversely, the Cdl exhibited less notable change as the interlayer spacing was insufficient to form the electrical double layer. In contrast, denser packing of the layers and a more hydrophobic surface resulting from annealing-induced reduction in oxide groups make the carbon coating highly resistant to water/ion infiltration, thus ensuring high electrochemical stability. We note the reduction of interlayer spacing is significant, considering that the value in ideal graphite is 3.35 Å. This water/ion infiltration mechanism also synergizes with the previous studies of intercalating aqueous KCl ions in single-crystalline graphite28 and the interlayer spacing of the porous hydrothermally reduced GO films29. In addition to the major changes in interlayer spacing and oxygen content, we observed a decreased intensity ratio of disorder-induced (D) and graphite (G) bands from the Raman spectra post-annealing (Supplementary Fig. 9), suggesting a restoration of sp2 carbon.
CCM scalability and neurotransmitter sensing performance
Leveraging this versatile material platform, we have successfully fabricated monolithic large-scale CCM arrays. Figure 2a displays representative 100-channel CCM arrays in the form of 10 × 10 grids with an electrode pitch of 530 μm on the two most commonly used substrates in neural interfaces: silicon (rigid) and Kapton (flexible). The resulting CCMs have a site area of 60 × 60 μm2 (inset, Fig. 2a) and up to 100% yield from visual inspection (Supplementary Fig. 10a). Cyclic voltammetry (CV) characterization validates that all the CCMs in the 100-channel array offer a wide electrochemical window from −0.6 V to 1.5 V; in contrast, the gold microelectrodes exhibit a significant peak at 1.2 V, indicating gold oxidation (Fig. 2b). The CV plots and impedance distribution (Supplementary Fig. 10b) of the CCM array demonstrate the uniformity of carbon coating and its electrochemical interface across all CCMs.
a 100-channel CCM arrays on silicon (left) and 25.4-μm-thick Kapton (flexible, right) substrate. Inset: Corresponding magnified view of two CCMs. b Mean and individual cyclic voltammograms (100 mV/s) of CCMs in a 100-channel CCM array in PBS (pH 7.4). 99 out of the 100 channels are included. Inset: Cyclic voltammogram of a representative Au microelectrode. c Concentration-dependent response of a CCM to DA. Left: background-subtracted color plot (bottom) and current-time trace at 0.6 V (top). Right: Background-subtracted cyclic voltammograms for the indicated DA concentrations. d Oxidation peak current at 0.6 V vs. DA concentration over 50–1000 nM at CCMs (n = 4 CCMs, mean ± SD) and commercial CFEs. The sensitivity is 125.5 vs. 15.5 nA/μM. e Cyclic voltammogram in the presence of 5 nM DA to show the low LOD of a CCM. f Schematic of the 100-channel CCM array testing in PBS (left) and the heatmap of background current value at 0.6 V with respect to actual channel position (right). g Histogram of the peak current to background current ratio (PC/BC, sensitivity) of the 100-channel CCM array. Inset: Voltammograms illustrate the background current at 0.6 V (BC) and the peak current of the background-subtracted cyclic voltammograms in the presence of 1 μM DA (PC). h Histogram of the peak-to-peak potential distance (Vp-p) of 100-channel CCM array. The voltammogram in the inset illustrates the Vp-p: potential difference between the oxidation peak and reduction peak of 1 μM DA. FSCVs were conducted with a scan range of −0.4 to 1.1 V, a scan rate of 400 V/s, and at 10 Hz. Ninety-eight out of the 100 channels function for DA sensing, exhibiting consistent DA sensing results.
Encouraged by the wide electrochemical window and the stable baseline current achieved through annealing (Supplementary Fig. 11), we then examined the neurotransmitter sensing performance of CCMs fabricated on 1-mil Kapton using FSCV (Supplementary Fig. 12), known for sub-second sensing capability. To ensure compatibility of the annealing processing with broader neural interface materials, we consistently use 250 °C-annealed CCM for the FSCV measurements unless otherwise specified. We use a photoresist (PR) confinement method that restricts carbon deposition within the PR window after PR lift-off (Supplementary Fig. 12) to preserve the high spatial resolution of our electrochemical deposition method. Using DA as a model analyte, we observed that CCMs exhibited a linear response to DA across physiologically relevant concentrations from 50 nM to 1 μM (Fig. 2c), with sensitivity significantly outperformed that of commercial CFEs (125.5 vs. 15.5 nA/μM, Fig. 2d), and demonstrating a low limit of detection (LOD) of 5 nM (three times the standard deviation of baseline signal, Fig. 2e). This high sensitivity can be attributed to large specific surface area (Supplementary Fig. 13), as well as residual oxide groups and edge states of our carbon coating30,31. Non-annealed CCMs, with the presence of more oxide groups, exhibit even higher sensitivity. We were able to demonstrate 100-channel CCM arrays with a near unity yield of FSCV capability with a narrow background current distribution at the DA oxidation potential (0.6 V) (Fig. 2f and Supplementary Fig. 14a) and well-behaved DA sensing current (Supplementary Fig. 14b). Their sensitivity, characterized by peak current/background current, had a median value of 5% (Fig. 2g), surpassing that of typical commercial CFEs (2.4%). The peak-peak distance (Vp-p) exhibited minimal deviation (<0.02 V), suggesting that precise oxidation peak potential can be obtained (Fig. 2h). These results demonstrate the scalability of neurotransmitter sensing with precisely controlled locations and predictable, homogeneous sensitivity.
We also evaluated the neurotransmitter sensing specificity of the CCMs. With the same triangle waveforms for DA sensing, distinct background-subtracted cyclic voltammograms were obtained for serotonin (5-HT) and epinephrine (EP), allowing for their identification (Fig. 3a, b). The relationships between oxidation peak current and 5-HT/EP concentrations are also linear across the physiological range (Supplementary Fig. 15a). By extending the switching potential to 1.5 V, CCMs allow adenosine (ADE) sensing with good linear dependence as well as simultaneous ADE and DA sensing (Fig. 3c, d and Supplementary Fig. 15b). The background-subtracted cyclic voltammograms for ADE and 5-HT, as well as ADE and EP mixtures, also show all main characteristic peaks of the individual neurotransmitters, allowing their simultaneous detection (Supplementary Fig. 16). Although achieving simultaneous detection of all these neurotransmitters directly with the traditional triangular waveform is challenging, machine learning32 and modified sensing waveforms offer promising solutions to address these difficulties. When using a Jackson waveform, the CCM exhibits an ultra-low 5-HT detection limit, with 1 nM producing a clear oxidation peak (Fig. 3e), meanwhile, the CCM demonstrates highly selective sensing of 5-HT over DA (Fig. 3e and Supplementary Fig. 17). Even with a high scan rate of 1000 V/s applied to the CCM using the Jackson waveform, the background current remains within 2 μA (Supplementary Fig. 18), which is low enough to prevent any tissue damage33,34. Besides FSCV, the CCMs are also compatible with differential pulse voltammetry, another selective and sensitive technique for chemical analysis (Supplementary Fig. 19). The versatile sensing capabilities of CCMs make them promising for applications in various electrochemical-sensing scenarios.
Concentration-dependent response of CCMs to (a) serotonin (5-HT), (b) epinephrine (EP), and (c) adenosine (ADE). The ADE was detected with an extended 1.5 V switching potential. The main oxidation peak potentials are, 5-HT: 0.40 and 0.64 V; EP: 0.62 V; ADE: 1.10 and 1.37 V. d Simultaneous detection of DA and ADE. Top: Background-subtracted voltammogram collected at the time indicated by the vertical dash line; Bottom: A color plot serially displaying all background-subtracted voltammograms. 100 μL PBS containing 2 μM DA and 10 μM ADE was introduced to the surface of a CCM. e Jackson waveform for 5-HT detection. Top: Jackson waveform with a scan rate of 1000 V/s; Left: Background-subtracted voltammogram in the presence of 1 nM 5-HT to demonstrate a low limit of detection; Right: A comparison of the background-subtracted voltammograms in the presence of 500 nM DA and 100 nM 5-HT to show its selective sensing of 5-HT over DA. The highlighted portions with corresponding colors in the molecular structure in (a–c) indicate the electrochemical active groups. Except for the Jackson waveform, a triangle waveform with a scan rate of 400 V/s was applied to CCMs. All waveforms were repeated at 10 Hz. All tests were performed in PBS (pH 7.4).
CCM reliability and in vivo validation
To evaluate the performance of our CCMs under physiological-like conditions, we performed FSCV detection of DA using CCMs in a dynamic flow cell (Fig. 4a). The extracted current-time trace at the oxidation potential of DA reveals a fast time response, with a half-peak response time (t50%) of 0.30 ± 0.07 s (Fig. 4b and Supplementary Fig. 20). Importantly, the CCM demonstrates quick self-regeneration during continuous FSCV measurement (Fig. 4b). After 100 repetitions of DA sensing, the extracted waveforms remain nearly identical (Fig. 4c). Furthermore, the CCMs exhibit 2-week stable DA sensing performance (Supplementary Fig. 21), demonstrating superior reliability compared to commercial glass-sealed CFEs (Supplementary Fig. 22). The enhanced reliability of the CCMs is attributed to reduced infiltration of water and ions achieved through annealing, which results in a stable background current. When annealed at 300 °C, CCMs maintain stable performance for DA sensing even after 10 weeks of soaking at 37 °C in PBS (Supplementary Fig. 23).
a Schematic for dynamic DA sensing in a flow cell. b Color plot (bottom) and current-time trace at 0.6 V (indicated by the white dashed line in the color plot, top) measured over nine consecutive injections of DA. 100 μL PBS containing 5 μM DA was injected into the flow cell approximately every 30 s. The color plot is generated from continuous background-subtracted voltammograms. c Background-subtracted voltammograms extracted from 100 DA sensing events using the same CCM in a flow cell. The waveforms were taken at the peak current times identified in the current-time traces at 0.6 V. d Schematic of DA sensing in the NAc by a CCM while electrically stimulating the MFB in rats. Representative trace of phasic DA release and corresponding background-subtracted color plot from electrical stimulation (60 Hz, 60 pulses at 300 µA; indicated by the red bar) of the MFB. NAc, nucleus accumbens; MFB, medial forebrain bundle. e Schematic for measurements of DA release in the NAc induced by 10% sucrose feeding in head-fixed mice (left) and representative voltammetric signal in response to reward delivery (right). The red triangle marker indicates the time point when the mouse started eating. Representative (f) current-time traces of, and (g) half-peak response time (t50%) calculated from spontaneous phasic DA transients in freely behaving rats (n = 10, 8, 6, 6 DA transients). Data are presented as mean ± SEM. The mean value (center line), lower and upper quartile (box edges) are indicated in the box plot.
Next, we assessed the ability of the CCM to detect DA in vivo. We first implanted the CCMs in the nucleus accumbens (NAc) of anesthetized rats, which is one of the main regions receiving projections from DA neurons in the ventral tegmental area through the medial forebrain bundle (MFB). Using the CCM, we detected immediate voltammetric responses correlated with electrical stimulation of the MFB, with the background-subtracted cyclic voltammogram resembling that of in vitro analyzed DA (Fig. 4d and Supplementary Fig. 24). Histological verification confirmed the placement of the CCM within the NAc (Supplementary Fig. 25). Furthermore, administration of amphetamine (AMPH), a psychostimulant known to augment evoked DA response35, resulted in a pronounced increase in release magnitude post-injection (Supplementary Fig. 26), confirming that the signals evoked by MFB stimulation were indeed originated from DA release. Subsequently, to detect behaviorally evoked signals, we implanted the CCM in the NAc of awake mice and monitored the DA release elicited by natural rewards. The CCM effectively detected phasic DA release in response to sugar feeding (Fig. 4e). Additionally, we have established successful empirical calibration protocols to determine absolute DA concentrations in vivo. The sensitivity of CCMs is expected to be lower in vivo than in PBS due to biofouling, leading to higher LOD. Post-in vivo calibration of the explanted CCMs was conducted to obtain calibration results that more accurately reflect in vivo conditions (Supplementary Fig. 27). In freely behaving rats, the spontaneous phasic DA transients detected at CCM ranged from 50–200 nM (Fig. 4f and Supplementary Fig. 28), consistent with that measured by CFEs36,37. The variations in signal magnitude can be attributed to the specific location of the probes within the brain and the unique behavior that evoked the DA transients in each rat. The consistent phasic DA transients and sub-second response time demonstrate the reproducibility and stability of our CCM neural probes. Additionally, the electrical stimulation evoked and sugar reward elicited DA concentrations are around 45 and 80 nM, respectively, which are in the normal range compared to those recorded by CFEs14,38,39. Temporal analysis of the representative phasic DA transients revealed sub-second temporal resolution of CCMs with a t50% of 0.15 ± 0.06 s (averaged from 30 DA transients from four rats, Fig. 4g). The duration of the phasic DA transients, defined as the width at half-height of the current peak, is ~200 ms (Supplementary Fig. 29) and aligns with values reported in the literature40,41,42. Benefiting from the stable baseline current of CCM, tonic DA releases were also detected in the NAc of behaving rats following AMPH administration (Supplementary Fig. 30).
Monolithic neural probe integrating CCM and electrical recording sites
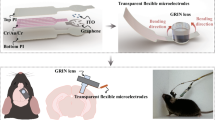
Our simple coating approach facilitates the development of multifunctional devices for neuroscience studies. We monolithically integrated the CCM with electrophysiological recording sites to create a dual-modal neural probe. Specifically, probes with 16-channel gold microelectrodes were fabricated on Kapton with a minimum feature size of 4 μm. While one channel at the probe tip received carbon coating, the remaining 15 channels were electroplated with Pt-Ir to ensure a low-impedance surface (Fig. 5a and Supplementary Fig. 31). The mild annealing process, conducted following the electrochemical deposition of carbon coating, was fully compatible with the probe fabrication, and the CCM maintained high sensing performance through subsequent processing steps. Constructed entirely from lithography, the integrated probe is highly miniaturized, measuring 110 μm (thickness) ×150 μm (width) × 8.3 mm (length) (Fig. 5a and Supplementary Fig. 31). We connected the integrated probe to a custom printed-circuit-board-(PCB)-based connector and implanted it into the rat brain with the CCM positioned at the NAc and the electrophysiological recording sites distributed along NAc, dorsal striatum (CPu), and primary motor cortex (M1) (Fig. 5b). The polymer nature of the integrated probe ensured a 100% probe survival rate throughout the implantation process.
a Dual-modal neural probe integrating neurochemical sensor (CCM) and electrophysiological (E-phys) recording sites. Explosive view (top), photograph (middle), and SEM image (bottom). b Photograph of the neural probe connected to the PCB connector and head stage (left); Schematic of AMPH administration and device implantation in a rat (right). Inset: Distribution of recording sites across multiple brain regions. c In vivo monitoring of DA and electrophysiological activity. Top: DA events between 15 s before and 185 s after AMPH injection. Bottom: Representative spectrogram of LFP power; 1, 2, and 3 indicate times from which the 1-s episode of raw LFP traces were taken. AMPH was injected at time 0, as indicated by the dashed line. d Single-unit spikes. Top: Raster plot of the recorded spikes. From top to bottom, the spikes correspond to single unit i-v; all channels were in the striatum. Bottom: Firing rates and sorted waveforms. The mean waveform (thick line) with error band (SD) is overlaid on the individual waveforms (thin lines). e Representative DA and high gamma power (60–100 Hz) traces (top); Spatial distribution of the correlation coefficient between DA concentration and high gamma power change (bottom). The bottom right panel displays the recording sites. These data correspond to a DA event in Rat 3 shown in (f). f Statistical analysis of the correlation between DA concentration and high gamma power change over 0-65 min post-AMPH injection (n = 9, 9, 11, 11 DA events). The coefficient value was selected from the electrophysiological channel showing the highest correlation. p values are determined by a one-sample Wilcoxon signed-rank test compared to 0.3, which is a threshold that has been commonly used to indicate significant correlations in brain function studies65,66. The median value (center line), lower and upper quartile (box edges), and maximum and minimum value whiskers are indicated in the box plot. The resulting p values with a confidence interval of 0.95 were 0.0039, 0.0020, 0.021, and 0.00098, respectively. Statistical significance: * (p < 0.05), ** (p < 0.01), *** (p < 0.001).
Utilizing the dual-modal neural probe, we successfully recorded DA transients at the NAc, spatial distribution of local field potential (LFP) oscillations, and single-unit spikes across the cortex and striatum following AMPH administration (Fig. 5b–d). The DA sensing was synchronized with the electrophysiological recording by sending a transistor-transistor logic (TTL) pulse train from the FSCV instrument to the digital input of the Intan electrophysiological recording system. Leveraging the sub-second temporal resolution of FSCV, we identified rapid DA transients 2 s post-AMPH injection and the subsequent slower DA event 1 min post-injection. Coinciding with both DA events, an increase in broad gamma-band power (40–100 Hz) was observed in the NAc and M1, whereas a gamma band with a different frequency composition (45–85 Hz peaked at 60 Hz), specifically occurred in the CPu associated with the 1-min post-AMPH DA event (Fig. 5c and Supplementary Fig. 32a). Spatial analysis revealed stronger high gamma-band power (60–100 Hz) in the NAc and M1 compared to the CPu (Supplementary Fig. 32b). Additionally, the gamma band exhibited a more prominent and prolonged power increase post-AMPH than saline injection (Supplementary Figs. 32b and 33). The DA transient occurring 2 s post-AMPH injection is attributed to an injection-evoked response, as supported by previous studies43 and the presence of DA events induced by saline injection (Supplementary Fig. 34); in contrast, the enhanced DA event and persistent gamma rhythms44 observed 1 min post-AMPH administration are compatible with previous literature reports on AMPH’s effects (Fig. 5c). We also demonstrated the high yield and reliability of the dual-modal neural probe in recording single-unit spikes, which were identified in 12 out of 15 channels across the 6 mm deep brain (Supplementary Fig. 35).
Dopamine dynamics and gamma oscillations are of clinical interest as they control connectivity between brain regions, and their abnormalities are linked to central nervous diseases (CNS), including Parkinson’s45, Alzheimer’s46,47, and schizophrenia48. AMPH is widely used to create animal models of schizophrenia based on manipulation of the dopaminergic system49,50. While it is speculated that AMPH-evoked DA release in NAc is related to gamma oscillations51, direct evidence is scarce due to a lack of appropriate tools. Our dual-modal neural probe enables quantitative analysis of the correlation between changes in DA concentrations and gamma power. The sub-second resolution of the CCM and precise spatial definition of the recording sites allow us to relate the DA concentrations in NAc with the high gamma power of known brain depth with high spatiotemporal precision. We observed that tonic DA releases were highly and positively correlated to high gamma power change in the ventral striatum close to CCM and cortex (Fig. 5e and Supplementary Fig. 36). Analysis of multiple tonic DA events lasting seconds to minutes from 4 rats post-AMPH injection further confirmed a robust positive correlation between the changes of DA concentrations and high gamma power, with an average correlation coefficient of 0.58 ± 0.21 (Fig. 5f), which is considerably higher than the correlation to any other LFP bands (Supplementary Fig. 37). The positive correlations between high gamma power and DA signal in both cortex and striatum observed in this study are consistent with the functional connectivity between these regions52 and align with findings from previous research53. Correlation coefficients were consistently derived at lag = 0 s from cross-correlation analysis (Supplementary Fig. 38), as detailed in the Methods section. Thus, we conclude that DA concentration changes are strongly correlated with high gamma power changes in the NAc and M1 at fine timescales. Unlike gamma-band power, the spiking frequencies didn’t show consistent positive or negative correlations with DA transients across different units and animals (Fig. 5d and Supplementary Figs. 35 and 39). We also observed more frequent DA events of higher concentrations post-AMPH injection (Supplementary Fig. 40) than the saline group (Fig. 5f and Supplementary Fig. 41). Notably, the injection-evoked DA (Supplementary Fig. 42), and DA events detected in the saline group, without any AMPH effect, also indicate a potential positive correlation between DA and high gamma power (Supplementary Fig. 41), suggesting that the correlations may potentially extend to normal states of cortical-striatal circuitry rather than a pure pharmacological effect44.
The in vivo findings from this study demonstrate the effectiveness of our dual-modal neural probe in capturing complex interactions between neurochemical dynamics and spatially organized electrical signals at sub-second precision. Previous efforts of neurochemical sensing have utilized platinum-based materials54,55,56, PEDOT57 or also carbon-based nano-materials58,59,60,61 as electrochemical sensors, however, these studies employed amperometry or differential pulse voltammetry, which either lack chemical specificity, particularly in the unknown and complex in vivo conditions, or sub-second temporal resolution. GO-modified CFEs have been developed to enhance the sensing performance such as sensitivity, but such modification is still based on traditional CFEs62,63. In comparison to sensors in the existing dual-modal neural probes for simultaneous electrophysiology recording and neurochemical sensing54,55,56, our carbon coating offers a broader water window and ensures a stable baseline when using the FSCV method. This advance enables chemical specificity while achieving sub-second temporal resolution. Our analyses demonstrate the strong potential of this dual-modal neural probe for quantitatively studying the correlation between DA and electrophysiological signals. We envision that a higher-density probe with an increased number of electrodes could yield further insights in future work, such as correlations between DA and single-unit activities, as well as depth-resolved relationships between DA and electrophysiological signals.
Discussion
We discovered that mild annealing dramatically stabilized electroplated carbon coatings and the resulting carbon microelectrodes. This simple approach allows the transformation of conventional MEAs into carbon MEAs with the capability of high-performance neurochemical sensing, an excellent yield, and remarkable uniformity. The scalability and high sensing performance of the CCMs makes them highly promising for multiplexed neurochemical dynamics monitoring. Moreover, the compatibility with micro/nanofabrication techniques and a broad range of substrates—including silicon and polymers—provides an avenue for developing various high spatial resolution hybrid devices. Notably, our work showcases the application enabled by the CCM using a dual-modal integrated neural probe, which reveals the correlations between sub-second DA dynamics and electrical activities in the brain. Compared to the state-of-the-art carbon microelectrodes that are compatible with FSCV (Supplementary Table 1), the CCM platform stands out in terms of its low-temperature compatibility, high sensitivity, high scalability, and the capability of multifunctional integration.
On a broader level, the scalability of CCMs facilitates high throughput neurotransmitter sensing with precisely controlled locations across various brain depths and regions, offering remarkable opportunities for a deeper understanding of the complex spatiotemporal chemical dynamics in biological systems. The interoperability of CCMs, together with their scalability, will enable arbitrary combinations of electrochemical sensors and electrophysiological recording sites. This versatility makes our technology platform adaptable for studying various neural communication networks, encompassing both chemical and electrical signals. Further studies should also pursue chronic stability in vivo and address the artifacts caused by FSCV to electrical recording. Given the translational advantages of carbon sensors for human use, we also envision our approach can be further extended to clinical applications for the diagnosis and treatment of a wide range of neurological disorders and mental health conditions. Future directions for the clinical translation of CCMs should focus on developing robust anti-fouling strategies and integrating on-probe reference electrodes, aiming at enhancing sensing stability and minimizing neural tissue damage.
Methods
Fabrication of lithographic metal microelectrodes and MEAs
Overall, the flexible metal microelectrodes and MEAs were constructed by five layers: a Kapton/Polyimide (PI) bilayer as the substrate, a Cr/Au bilayer as the conductor, and SU-8 as an encapsulation layer. We firstly laminated 1 mil Kapton film (Fralock) onto a glass slide pre-spin-coated with polydimethylsiloxane (base/crosslinker 10:1, Sylgard 184, Dow). Then a 4 μm PI (PI-2545, HD Microsystems) was spin-coated onto the Kapton substrate to improve the surface smoothness, and the PI-coated substrate was cured in ambient air at 250 °C for 70 min. Subsequently, we deposited Cr (10 nm)/Au (70 nm) films on the Kapton/PI substrates using an electron-beam (e-beam) evaporator, with Cr serving as an adhesion layer between the PI and Au. Photolithography steps were then undertaken to pattern the metallic traces using a positive photoresist (PR, S1805, Dow). The un-patterned Cr/Au was removed with Cr and Au etchants (Transene Co., Inc.), and the photoresist layer was removed by swabbing and rinsing with acetone. Finally, an SU-8 encapsulation layer (1.5 μm; SU-8 2, Kayaku Advanced Materials) was spin-coated on the device to define the microelectrodes and the SU-8 was cured in ambient air at 180 °C for 30 min. Depending on the different mask designs, single-channel gold microelectrodes, 100-channel, and 16-channel gold MEAs were fabricated on flexible substrates through these processes.
All the single microelectrodes and 100-channel MEAs had 30 × 30 µm2 openings. For the 16-channel MEAs, a 30 × 30 µm2 opening was defined at the tip while the other 15 channels had a 20 × 20 µm2 window. The single-channel microelectrodes and 16-channel MEAs were used for carbon deposition and Pt-Ir deposition directly. For the 100-channel MEAs, following SU-8 encapsulation, Ni was used as a temporary shorting layer for all contact pad matrices to facilitate simultaneous carbon deposition on all the 100 channels. To confine the area of the CCMs, another layer of 1.5 µm PR with 60 × 60 µm2 openings was added and aligned with the 30 × 30 μm2 gold microelectrodes. This layer restricts the lateral growth of the deposited carbon, resulting in 60 × 60 µm2 CCMs after the PR lift-off (Supplementary Fig. 43).
The single-channel microelectrode used for TEM characterization was fabricated on Si substrates to avoid the charging effect, and 100-channel MEAs were also fabricated on Si substrate to show that our carbon coating approach is compatible with essential substrate materials used in neural interfaces. Except for the substrates used, all the fabrication processes were the same as described above.
Fabrication of carbon-coated microelectrodes (CCMs)
Single CCMs and 100-channel CCM arrays
Single CCMs and 100-channel CCM arrays were prepared by potentiostatic deposition of carbon coating on the gold microelectrodes fabricated as described above. The electrodeposition was performed in 1 mg/mL GO dispersion with Ag/AgCl (3 M NaCl) as the reference electrode, Pt wire as the counter electrode and 30 × 30 μm2 gold microelectrode (array) as the working electrode. The 1.0 mg/mL GO dispersion was obtained by diluting the as-purchased 4 mg/mL GO water dispersion (Graphenea, Spain) with PBS (1×, pH = 7.4) in a 1:3 ratio. The deposition potential was optimized as −1.0 V for 30 × 30 μm2 gold microelectrodes. Prior to the electrodeposition, the gold microelectrodes were treated with 10 min UVO (UV/Ozone ProCleanerTM, Bioforce Nanosciences) to clean the surface and improve its hydrophilicity. Using a potentiostat (Gamry Instruments, Warminster, PA), −0.8 V was applied to the working electrode for 5 min to remove the oxygen in GO dispersion, and −1.0 V was adopted to reduce the GO and achieve electrodeposition of carbon coating. The electrodeposition took 3–5 min, during which mechanical stirring was conducted at ~70 rpm. The CCMs were left at room temperature overnight followed by mild annealing in a furnace tube (Eurotherm, Thermo Scientific) for 1 h at 250 °C under an N2 atmosphere.
For the PR-confined CCMs (arrays), the PR was removed with acetone before annealing. The temporary shorting Ni layer on the contact pad area of the 100-channel CCM array was removed with nickel etchant after carbon deposition.
Dual-modal integrated neural probe
The 30 × 30 μm2 gold microelectrode located at the tip of the neural probe was electroplated with carbon coating and annealed at 250 °C as described above. The remaining 15-channel 20 × 20 μm2 gold microelectrodes along the probe were electroplated with Pt-Ir using cyclic voltammetry method (−0.1 to 0.1 V, 500 mV·s−1, 700 cycles) in freshly made Pt-Ir solution at 55 °C to 60 °C. The Pt-Ir solution was made by adding 640 μL HNO3 (ACS grade, 68–70%), 0.02 g Na3IrCl6·xH2O (Sigma-Aldrich) and 0.0186 g Na2PtCl6·6H2O (Sigma-Aldrich) into 100 mL DI water. After boiling for 3 min, the solution was cooled down to 55 °C to 60 °C for electroplating. The MEAs of CCM and Pt-Ir fabricated on 1 mil Kapton were then stacked onto 3 mil Kapton using epoxy (Gorilla) as the adhesive to stiffen the probe for deep brain insertion. After overnight curing of the epoxy, the probe profile was cut out with the laser cutting machine (ProtoLaser U4, LPKF). The laser parameters used were as follows: frequency: 200 kHz, power: 1.1 W, pulse energy: 6.6 μJ, and mark speed: 1000 mm/s. The laser spot size was ~20 μm. The probe was cut with a tip angle of 27° to facilitate insertion and minimize tissue damage.
To avoid any interference of the ascorbic acid and biofouling from the biological fluids, a Nafion (D2020, Ion Power) coating was adopted by drop-casting 0.2 μL 0.1% w/v Nafion solution (solvent, 1:1 ethanol and water) on the CCMs with an estimated coating thickness of ~0.31 μm. Prior to the implantation, the neural probe was carefully released from the PDMS-coated glass slide and bonded to a custom-designed PCB.
Characterization of carbon coating and CCMs
Electrochemical impedance spectroscopy (EIS) analysis of CCMs
The EIS test (Reference 600+, Gamry) was conducted for as-deposited and 250 °C-annealed CCMs before soaking and after specified soaking time over 2 weeks in PBS (pH 7.4) at 37 °C. For comparison, CFEs with a tip diameter of 10 μm and length of 100 μm (CF10-100, World Precision Instruments) were tested under the same conditions as a control. The EIS test was performed at open circuit potential with an alternating current amplitude of 10 mV and applied frequencies from 10 MHz to 0.01 Hz. The electrochemical impedance spectra were fitted with the R(CR) EC model in ZSimpWin software. The values of charge transfer resistance (Rct) and double-layer capacitance (Cdl) of 4 CCMs for each group were extracted from EC modeling.
Transmission electron microscopy (TEM) characterization
The TEM samples were made by cutting out 15 μm length × 10 μm width and ~100 nm thickness from 60 × 60 μm2 250 °C-annealed CCM on silicon substrate with focused ion beam scanning electron microscope (FIB-SEM, Helios 5CX DualBeam, Thermo Fisher Scientific). The TEM images of the cross-section of carbon coating on gold were taken by Tecnai F 20 (FEI Corporation) with an accelerating voltage of 200 kV.
Grazing incidence X-ray diffraction (GIXRD)
The interlayer spacing of carbon flakes in the carbon coating was determined using GIXRD. The samples were prepared by electrodeposition of carbon coating on gold film on a Si substrate. Four as-deposited and 250 °C-annealed samples were characterized using a Rigaku D/Max 2000 rotating anode XRD system with a Cu target. Conditions for GIXRD were the following: tube: Cu Kα = 0.154 06 nm, filter: Ni, scan rate: 0.06°/min, sampling interval: 0.02°, accelerating voltage: 40 kV, current: 300 mA and two theta scan range: 15°–36°. The incident angle was fixed at 0.5°.
Energy-dispersive X-ray spectroscopy (EDS)
The effect of annealing on oxygen content in the carbon coating was characterized by EDS using an Ultim Extreme detector from Oxford Instruments equipped with the FIB-SEM. Single-channel CCMs were prepared as the tested samples and the 30 × 30 μm2 center area of the CCMs was analyzed by EDS. The atomic ratio of O versus (C + O) was calculated and averaged from 4 CCMs for each group.
Cyclic voltammetry (CV) characterization of 100-channel CCM array
The 250 °C-annealed 100-channel CCM (60 × 60 μm2) array fabricated on 1 mil Kapton was scanned with CV (Reference 600+, Gamry) from −0.6 to 1.5 V with a scan rate of 100 mV/s in PBS. The tested CCM array had a 100% yield while visually observed under microscope (Supplementary Fig. 10a). After connecting to the custom PCB-based connector and CV scanning, 99 out of the 100 channels showed a stable cyclic voltammogram window except that one channel was open circuit. Bare gold microelectrode (30 × 30 μm2) was used as a control and scanned with the same parameters.
Neurochemical sensing performance in PBS
CCMs for multiple neurotransmitter sensing
The CCMs were used for neurotransmitter sensing with FSCV. Prior to the neurotransmitter sensing, all electrodes, unless otherwise specified, were electrochemically conditioned in PBS to stabilize the background current using a triangular waveform. Specifically, the electrode potential was held at −0.4 V, ramped to 1.1 V, and then ramped back to the holding potential, with a scan rate of 400 V/s at 100 ms intervals (10 Hz). The FSCV was performed using High-Definition Cyclic Voltammetry software (HDCV, Department of Chemistry, University of North Carolina at Chapel Hill) in conjunction with data acquisition cards (National Instruments, Austin TX) to control and record data from a WaveNeuro potentiostat (Pine Research, Durham, NC). Once the background current became stable (current drift <0.5‰ over 100 scans), for DA (≥99%, Sigma-Aldrich), serotonin (≥98%, Fisher Scientific), and epinephrine (≥98%, TCI) sensing, the same triangular waveform was applied to a CCM and 50–1000 nM of each neurotransmitter were detected in PBS. CFEs were used for DA sensing with the same waveform as a comparison. A triangular waveform with a wider scan range (−0.4 to 1.5 V) but the same scan rate and frequency was applied for adenosine (≥99%, Sigma-Aldrich) sensing (0.5–10 μM). Simultaneous DA and adenosine sensing was tested by promptly injecting 100 μL PBS containing 2 μM DA and 10 μM adenosine into the flow cell, during which PBS was continuously flowed at 3 mL/min using a syringe pump (GenieTouchTM, Kent Scientific).
Jackson waveform scanning from 0.2 V to a switching potential of 1.0 V to −0.1 V back to the holding potential of 0.2 V at 1000 V/s and 10 Hz was used for serotonin and DA (25 nM to 500 nM) sensing. 5 nM DA and 1 nM serotonin were tested in PBS with triangular waveform and Jackson waveform, respectively, to show the low LOD of the CCMs. The LOD for DA was calculated by \({{{\rm{LOD}}}}=\frac{3\times {{{\rm{\sigma }}}}}{{{{\rm{sensitivty}}}}}\), where σ is the standard deviation of the background current at 0.6 V, and sensitivity is the slope of the calibration curve. Six CCMs were used for the LOD calculation. All bench testing was performed with a sintered Ag/AgCl counter/reference electrode (2.0 × 4 mm, World Precision Instrument). The background-subtracted cyclic voltammograms were obtained by subtracting the background current (in the absence of neurotransmitters) from the cyclic voltammograms in the presence of the corresponding concentrations of the neurotransmitters.
Performance of 100-channel CCM array
For the 100-channel CCM array, we achieved 100% yield carbon deposition with careful step-by-step processing. All the 100-channel CCM were tested one by one with the custom PCB connector since the FSCV instrument for simultaneous 100-channel testing is not commercially available. For each channel, the CCM was conditioned by FSCV scanning (−0.4 V to 1.1 V, 400 V/s) at 60 Hz for 5 min followed by 10 Hz for another 5 min to achieve a relatively stable baseline current. The background current at 0.6 V (corresponding to the DA oxidation peak potential) of all the 100 channels was statistically analyzed and shown in the heatmap. 1 μM DA was measured by each channel. The potential distance between the reduction peak and oxidation peak of 1 μM DA was characterized as peak-peak distance (Vp-p). The sensitivity was characterized by the ratio of the oxidation peak current of 1 μM DA to the background current at 0.6 V. All the 100-channel CCMs work for DA sensing except that two channels showed the same sensing performance and background current, indicating a shorting issue.
Stability tested in flow cell
The single-channel CCM probe on 1 mil Kapton was positioned at the output of a flow injection instrument (Pine Research Instrumentation, NC, USA) where a pulse of DA could be introduced and removed from the electrode surface as illustrated in Fig. 4a. PBS buffer was flowed at 3 mL/min using a syringe pump. One hundred microliter PBS containing 5 μM DA was promptly injected into the flow cell about every 30 s and 100 times were repeated. A typical triangle waveform (−0.4 V to 1.1 V, 400 V/s, 10 Hz) was applied at the electrode. Voltammograms taken before DA exposure were subtracted from those obtained during the presence of the maximum concentration of DA to collect the background-subtracted cyclic voltammograms. Current-vs-time traces were obtained at oxidation peak potential (0.6 V) for each cyclic voltammogram. The variation in the peak height is primarily due to the differences in manual injection speed.
Animal studies
Male and Female Sprague Dawley rats (250–350 g, 8–12 weeks, Charles River Laboratories) were used for electrical stimulation under anesthetized and amphetamine administration while awake. These rats were housed with a 12-h light/dark cycle and provided ad libitum access to food and tap water. All animal procedures involving rats were performed in accordance with the Animal Care and Use Committee of Dartmouth College.
Electrical stimulation
Rats were anesthetized with urethane (1.5 mg/kg, i.p.; Millipore Sigma) and, were immobilized in a stereotaxic frame (51500UD, Stoelting, WI, USA) once reaching the surgical plane of anesthesia. A heating pad was used to maintain their body temperature. The sensing probe with a CCM at the tip was precisely lowered 7.1 mm below the cortical surface at a speed of 50 μm/s to reach the NAc core (AP: +1.2 mm, ML: +2.0 mm) using a precision-controlled inserter (NeuralGlider, Actuated Medical Inc., PA, USA). A bipolar electrical stimulation probe (125 μm diameter MS303S/3-A/SP, Plastic One) with ~1 mm tip separation was positioned in MFB (AP: −4.0 mm, ML: −1.3 mm) employing standard stereotaxic procedures, starting at a depth of 7.0 mm from the dura. The Ag/AgCl reference electrode was lowered 2–3 mm into the cerebral cortex contralaterally to the sensing probe. After a waiting period of 30 min, CCM was conditioned at 10 Hz for 30 min with a triangular waveform (−0.4 V to 1.2 V, 400 V/s). Constant current, biphasic stimulus pulses (60 Hz, 60 pulses, 300 μA, 2 ms each phase) were then delivered to MFB from a 128ch Intan recording/stimulation system (Intan Technology) using charge-balanced biphasic stimuli (cathodic first). The stimuli were triggered by the analog output of WaveNeuro to avoid artifacts induced by stimulation to voltammetric recordings. The timestamps of the triggering signals were sent to the digital input of the Intan recorder for synchronization purposes. The positioning of the stimulating electrode was optimized by electrically stimulating the MFB and advancing the electrode 100–200 μm each step until a maximal evoked response was detected in NAc, where its depth was around 8.3 mm. To allow the recovery from previous trials, 5 min were given between consecutive stimulations. To confirm the chemical specificity of our measurements, rats were administered with 10 mg/kg D-amphetamine (AMPH, i.p., in 0.9% saline, Sigma-Aldrich). Electrical stimulation was conducted 5 min after AMPH injection.
The voltammetric recording was conducted with the same parameters as conditioning. The cyclic voltammograms collected before electrical stimulation were set as the baseline and subtracted from those obtained after stimulation. The resulting subtracted voltammograms provided confirmation that DA is the substance observed. We extracted the current vs. time trace at the oxidation peak potential of DA.
AMPH administration in behaving rats
On the day of surgery, rats were induced with 2–3% isoflurane, then maintained at 1.5%–2.5%. Three cranial windows were made: one above the NAc core for a dual-modal neural probe, one above the contralateral cortex for an Ag/AgCl reference electrode, and one window contralateral to NAc for a ground screw. These exposed windows were covered with Kwik-Sil (World Precision Instrument), and a temporary head chamber was secured on the head with dental cement to protect the surgery area. Following surgery, rats received buprenorphine (0.65 mg/kg, Ethiqa XR) for analgesic purposes and were allowed to recover for 3–5 days.
On the day of experiments, rats were anesthetized with the same isoflurane conditions as during the surgery day and positioned on a stereotaxic frame. Following the removal of the temporary head chamber and Kwik-Sil, an Ag/AgCl reference electrode was inserted 2–3 mm in the brain and the dual-modal neural probe was lowered down 7.1 mm to access the NAc core (AP: +1.2 mm ML: +2.0 mm). After positioning the neural probe, the craniotomy windows were filled with Kwik-Sil, and the entire skull was covered with dental cement to secure the connector in place. Once the dental cement was fully cured, isoflurane was discontinued. The neural probe was connected to a custom PCB connector to interface with data acquisition systems. Specifically, the CCM was connected to the WaveNeuro potentiostat via a female connector pin on the PCB, while the 15-channel Pt-Ir microelectrodes were connected to the electrophysiology recording system (Intan Technology) through an RHD 16-channel recording head stage (Intan Technology), also interfaced with the PCB, for data acquisition. The amplifier for electrophysiology recording was connected to the ground screw, providing a low-impedance reference for electrical noise. The rats were then placed in a 60 × 65 × 45 cm chamber to allow them freely moving. After the rats fully awoke from anesthesia, CCMs were conditioned at 10 Hz with a triangular waveform (–0.4 V to 1.1 or 1.2 V, 400 V/s) for 30 min to stabilize the baseline current, and naturally occurring DA transients were recorded over the subsequent 30 min. Subsequently, we did a 0.9% saline injection and a 10-min recording followed by amphetamine (2.5 mg/kg base weight) administration and another 65-min recording. The volume of saline and amphetamine were controlled as the same and both were injected intraperitoneally. Voltametric and electrophysiological recordings were conducted simultaneously post-injection. A DA event in this work is defined as a period marked by continuous fluctuations in DA concentration, identified based on the following criteria: (i) initial characteristics: A DA event begins with a recognizable DA signature in the background-subtracted cyclic voltammograms, showing a peak oxidation potential around 0.6 V and a negative reduction potential near −0.25 V. (ii) consistency of DA background-subtracted cyclic voltammogram: The DA characteristic cyclic voltammogram must be continuous and consistent over the event period, with the DA event concluding at the end of this cyclic voltammogram. (iii) amplitude threshold: The peak oxidation current at 0.6 V must exceed a threshold of 4 nA, corresponding to an approximate DA concentration of 50 nM. This threshold prevents the inclusion of weak signals that could result from noise.
Sugar reward
Female C57BL/6J mice (30–35 g, 6–12 months, Jackson Laboratory, ME) were used for sugar reward in a head-fixed setup. These mice were housed with a 12-h light/dark cycle and provided ad libitum access to food and water. The ambient temperature was controlled at 21.5 ± 1 °C, with relative humidity maintained between 30% and 70%. All animal procedures involving mice were performed in accordance with the Institutional Animal Care & Use Committee of Weill Cornell Medical College.
Cranial windows were made above the NAc core (AP + 1.2 mm; ML 1.4 mm) for the CCM probe and the contralateral cortex for an Ag/AgCl reference electrode. Animals recovered 2–3 weeks before recording.
On recording day, the CCM probe was inserted into the NAc core using the NeuralGlider at a speed of 50 μm/s until reaching a depth of 4.2–4.3 mm and remained tethered to the arm for the entire duration of the recording. Ag/AgCl reference electrode was inserted 2–3 mm in depth contralaterally. Triangular waveforms sweeping from −0.4 to 1.3 V were applied at 400 V/s and 10 Hz. The unexpected reward of 0.3 mL of 10% sucrose solution was delivered to the mice. The animal behavior was captured by a camera with 40 frames/s to correlate the timing of the observed behaviors with the DA signals.
For all the animal studies, the Ag/AgCl reference electrode was prepared by soaking insulated silver wire (Dia. 254 μm, A-M systems, WA) with an exposed tip of 0.5–1 mm in bleach (Clorox, Oakland, CA) overnight. The CCMs were calibrated in a PBS solution of known DA concentration pre- and post-in vivo to derive a calibration factor to convert measured changes in current to differential DA concentrations. Background subtraction and digital filtering were done with HDCV software.
Electrophysiological recording and data processing
Signal acquisition
The custom PCB connector for dual-modal neural probe was designed with two terminals to separate FSCV and electrophysiological recording with minimal crosstalk. The probe/connector bundle was then connected to a 512-channel recording system (Intan Technology) for electrophysiological recording. After implantation, electrophysiological data was sampled at 20 kHz. To synchronize electrophysiology signals with FSCV measurements, we generated a TTL pulse train from WaveNeuro to the digital input of the Intan recorder.
Artifact removal
FSCV-induced artifacts were removed offline using a signal processing approach15. Specifically, electrophysiological signals were divided into 5-s snippets with 2.5-s step and converted to the frequency domain by discrete fast Fourier transform (FFT). Then, individual peaks at 10 Hz and its harmonics were interpolated using a piecewise cubic Hermite interpolation polynomial at the full width of each peak at 75% of the maximum. The data was then converted back to time domain using inverse FFT.
Local field potential (LFP) analysis
The raw data was bandpass filtered to 1–300 Hz for the LFP analysis. We performed baseline power subtraction to the LFP spectrogram for the better visualization of power change before and after AMPH/Saline injection. The baseline power used for subtraction is the average value calculated from 10 to 20 s prior to the AMPH/Saline injection. For gamma-band analysis, data was bandpass filtered to 60–100 Hz. All the LFP analyses, including spectrogram, power spectra density (PSD), and power calculations were conducted in custom Python scripts.
Correlation analysis
To study the correlation between DA and electrophysiological signals, the total power in the high gamma band (60–100 Hz) as a function of time was calculated using custom Python scripts. To match the temporal resolution of FSCV recordings, we calculated the high gamma power within each 0.1-s snippet. Then, the calculated power data as a function of time was convolved with a window from 2 to 40 based on the length of data to eliminate the noise peaks caused by the rat’s head hitting the sides of the cage while moving. We aligned the prominent DA events with their synchronized high gamma-band power from electrophysiological recording. The cross-correlations between the 2 signals were then derived for all the recording channels over a lag of ±15 s. The cross-correlation curve was calculated with a resolution of 0.1 s. We observed that the peak value of correlation coefficients could vary within a lag of ±500 ms. For consistency, we used data at lag = 0 s throughout this study.
Spike sorting
The raw data was filtered to the 300–6000 Hz band for spike analysis. The spike sorting was performed using MountainSort4&564. Common median referencing and whitening were applied to the filtered data before sorting. The threshold was set to 5-5.5 times the standard deviation. The adjacency radius was set to be 100 μm. The sorted clusters were manually curated to satisfy the following criteria simultaneously as well-isolated single-unit clusters: Firing rate > 0.1 Hz; Inter-spike-interval violation (<2 ms) <1%; Full-width half maximum of the mean waveform between 0.15 and 0.75 ms; Peak-to-valley time of the mean waveform between 0.15 and 0.85 ms; L-ratio < 0.1; Median spike SNR > 4. All the remaining clusters were treated as multi-units. All single-unit analyses used curated spike clusters that meet the criteria above.
Statistical analysis
For statistical analyses, Origin and Excel were used. All replicate numbers are indicated in the figure legends. Given the data is not normally distributed due to the sample size (Shapiro-Wilk test, P = 0.00014), a one-sample Wilcoxon signed-rank test was used for correlation coefficients analysis.
Ethics
Every experiment involving animals has been carried out following a protocol approved by an ethical commission.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting the findings of this study are available within the article and its supplementary files. Any additional requests for information can be directed to, and will be fulfilled by, the corresponding authors. Source data are provided with this paper. The electrophysiological data have been deposited in Figshare https://doi.org/10.6084/m9.figshare.28446950. Source data are provided with this paper.
Code availability
Code for spike sorting and other neural data analysis is deposited in GitHub: https://github.com/qiangy0819/dual-modal-sensing.git.
References
Wise, R. A. Dopamine, learning and motivation. Nat. Rev. Neurosci. 5, 483–494 (2004).
Marder, E. Neuromodulation of neuronal circuits: back to the future. Neuron 76, 1–11 (2012).
Phillips, P. E. M., Stuber, G. D., Heien, M. L. A. V., Wightman, R. M. & Carelli, R. M. Subsecond dopamine release promotes cocaine seeking. Nature 422, 614–618 (2003).
Poewe, W. et al. Parkinson disease. Nat. Rev. Dis. Prim. 3, 17013 (2017).
Patriarchi, T. et al. Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science 360, eaat4422 (2018).
Bang, D. et al. Sub-second dopamine and serotonin signaling in human striatum during perceptual decision-making. Neuron 108, 999–1010 (2020).
Bang, D. et al. Noradrenaline tracks emotional modulation of attention in human amygdala. Curr. Biol. 33, 5003–5010 (2023).
Wu, Z., Lin, D. & Li, Y. Pushing the frontiers: tools for monitoring neurotransmitters and neuromodulators. Nat. Rev. Neurosci. 23, 257–274 (2022).
Wightman, R. M. Probing cellular chemistry in biological systems with microelectrodes. Science 311, 1570–1574 (2006).
Venton, B. J. & Cao, Q. Fundamentals of fast-scan cyclic voltammetry for dopamine detection. Analyst 145, 1158–1168 (2020).
Kishida, K. T. et al. Subsecond dopamine fluctuations in human striatum encode superposed error signals about actual and counterfactual reward. Proc. Natl. Acad. Sci. USA 113, 200–205 (2016).
Batten, S. R. et al. Dopamine and serotonin in human substantia nigra track social context and value signals during economic exchange. Nat. Hum. Behav. 8, 718–728 (2024).
Xia, M. et al. Scalable, flexible carbon fiber electrode thread arrays for three-dimensional probing of neurochemical activity in deep brain structures of rodents. Biosens. Bioelectron. 241, 115625 (2023).
Clark, J. J. et al. Chronic microsensors for longitudinal, subsecond dopamine detection in behaving animals. Nat. Methods 7, 126–129 (2010).
Schwerdt, H. N. et al. Dopamine and beta-band oscillations differentially link to striatal value and motor control. Sci. Adv. 6, eabb9226 (2020).
Zachek, P. T. M. K., Moody, B., Wightman, R. M. & McCarty, G. S. Simultaneous decoupled detection of dopamine and oxygen using pyrolyzed carbon microarrays and fast-scan cyclic voltammetry. Anal. Chem. 81, 6258–6265 (2009).
Wu, T., Alharbi, A., Kiani, R. & Shahrjerdi, D. Quantitative principles for precise engineering of sensitivity in graphene electrochemical sensors. Adv. Mater. 31, e1805752 (2019).
Nasri, B. et al. Hybrid CMOS-graphene sensor array for subsecond dopamine detection. IEEE Trans. Biomed. Circuits Syst. 11, 1192–1203 (2017).
Castagnola, E. et al. Flexible glassy carbon multielectrode array for in vivo multisite detection of tonic and phasic dopamine concentrations. Biosensors 12, 540 (2022).
Li, J. et al. A tissue-like neurotransmitter sensor for the brain and gut. Nature 606, 94–101 (2022).
Liu, Y., Dong, X. & Chen, P. Biological and chemical sensors based on graphene materials. Chem. Soc. Rev. 41, 2283–2307 (2012).
Viana, D. et al. Nanoporous graphene-based thin-film microelectrodes for in vivo high-resolution neural recording and stimulation. Nat. Nanotechnol. 19, 514–523 (2024).
Acik, M. et al. The role of intercalated water in multilayered graphene oxide. Acs Nano 4, 5861–5868 (2010).
Acik, M. et al. The role of oxygen during thermal reduction of graphene oxide studied by infrared absorption spectroscopy. J. Phys. Chem. C 115, 19761–19781 (2011).
Sengupta, I., Chakraborty, S., Talukdar, M., Pal, S. K. & Chakraborty, S. Thermal reduction of graphene oxide: How temperature influences purity. J. Mater. Res. 33, 4113–4122 (2018).
Valentini, C. et al. Tuning the electrical properties of graphene oxide through low-temperature thermal annealing. Nanoscale 15, 5743–5755 (2023).
Hu, S. et al. Transport of hydrogen isotopes through interlayer spacing in van der Waals crystals. Nat. Nanotechnol. 13, 468–472 (2018).
Saini, L., Nemala, S. S., Rathi, A., Kaushik, S. & Kalon, G. Selective transport of water molecules through interlayer spaces in graphite. Nat. Commun. 13, 498 (2022).
Bernicola, Md. P. et al. On the electrochemical activation of nanoporous reduced graphene oxide electrodes studied by in situ/operando electrochemical techniques. Adv. Funct. Mater. 34, 2408441 (2024).
Heien, M. L., Phillips, P. E., Stuber, G. D., Seipel, A. T. & Wightman, R. M. Overoxidation of carbon-fiber microelectrodes enhances dopamine adsorption and increases sensitivity. Analyst 128, 1413–1419 (2003).
McCreery, R. L. Advanced carbon electrode materials for molecular electrochemistry. Chem. Rev. 108, 2646–2687 (2008).
Batten, S. R. et al. Emotional words evoke region- and valence-specific patterns of concurrent neuromodulator release in human thalamus and cortex. Cell Rep. 44, 115162 (2025).
Keithley, R. B. et al. Higher sensitivity dopamine measurements with faster-scan cyclic voltammetry. Anal. Chem. 83, 3563–3571 (2011).
Cogan, S. F., Ludwig, K. A., Welle, C. G. & Takmakov, P. Tissue damage thresholds during therapeutic electrical stimulation. J. Neural Eng. 13, 021001 (2016).
Daberkow, D. P. et al. Amphetamine paradoxically augments exocytotic dopamine release and phasic dopamine signals. J. Neurosci. 33, 452–463 (2013).
Robinson, D. L., Heien, M. L. A. V. & Wightman, R. M. Frequency of dopamine concentration transients increases in dorsal and ventral striatum of male rats during introduction of conspecifics. J. Neurosci. 22, 10477–10486 (2002).
Wightman, R. M. et al. Dopamine release is heterogeneous within microenvironments of the rat nucleus accumbens. Eur. J. Neurosci. 26, 2046–2054 (2007).
Day, J. J., Roitman, M. F., Wightman, R. M. & Carelli, R. M. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat. Neurosci. 10, 1020–1028 (2007).
Gonzalez, I. L. et al. Sex differences in dopamine release in nucleus accumbens and dorsal striatum determined by chronic fast-scan cyclic voltammetry: effects of social housing and repeated stimulation. J. Neurosci. 44, e1527232024 (2024).
Robinson, D. L. & Wightman, R. M. Nomifensine amplifies subsecond dopamine signals in the ventral striatum of freely-moving rats. J. Neurochem. 90, 894–903 (2004).
Redgrave, P., Gurney, K. & Reynolds, J. What is reinforced by phasic dopamine signals? Brain Res. Rev. 58, 322–339 (2008).
Liu, C., Goel, P. & Kaeser, P. S. Spatial and temporal scales of dopamine transmission. Nat. Rev. Neurosci. 22, 345–358 (2021).
Berridge, C. W. & Stalnaker, T. A. Relationship between low-dose amphetamine-induced arousal and extracellular norepinephrine and dopamine levels within prefrontal cortex. Synapse 46, 140–149 (2002).
Berke, J. D. Fast oscillations in cortical-striatal networks switch frequency following rewarding events and stimulant drugs. Eur. J. Neurosci. 30, 848–859 (2009).
Brown, P. et al. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson’s disease. J. Neurosci. 21, 1033–1038 (2001).
Nobili, A. et al. Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer’s disease. Nat. Commun. 8, 14727 (2017).
Palop, J. J. & Mucke, L. Network abnormalities and interneuron dysfunction in Alzheimer’s disease. Nat. Rev. Neurosci. 17, 777–792 (2016).
Uhlhaas, P. J. & Singer, W. Abnormal neural oscillations and synchrony in schizophrenia. Nat. Rev. Neurosci. 11, 100–113 (2010).
Jones, C. A., Watson, D. J. & Fone, K. C. Animal models of schizophrenia. Br. J. Pharmacol. 164, 1162–1194 (2011).
Ahnaou, A., Huysmans, H., Van de Casteele, T. & Drinkenburg, W. Cortical high gamma network oscillations and connectivity: a translational index for antipsychotics to normalize aberrant neurophysiological activity. Transl. Psychiatry 7, 1285 (2017).
Ma, J. & Leung, L. W. S. Relation between hippocampal g waves and behavioral disturbances induced by phencyclidine and methamphetamine. Behav. Brain Res. 111, 1–11 (2000).
Bolam, J. P., Hanley, J. J., Booth, P. A. & Bevan, M. D. Synaptic organisation of the basal ganglia. J. Anat. 196, 527–542 (2000).
von Nicolai, C. et al. Corticostriatal coordination through coherent phase-amplitude coupling. J. Neurosci. 34, 5938–5948 (2014).
Johnson, M. D., Franklin, R. K., Gibson, M. D., Brown, R. B. & Kipke, D. R. Implantable microelectrode arrays for simultaneous electrophysiological and neurochemical recordings. J. Neurosci. Methods 174, 62–70 (2008).
Zhang, S. et al. An implantable microelectrode array for dopamine and electrophysiological recordings in response to L-dopa therapy for Parkinson’s disease. In Proc. 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 1922–1925 (IEEE, 2016).
Zhang, S. et al. Real-time simultaneous recording of electrophysiological activities and dopamine overflow in the deep brain nuclei of a non-human primate with Parkinson’s disease using nano-based microelectrode arrays. Microsyst. Nanoeng. 4, 17070 (2018).
Fan, P. et al. Flexible microelectrode array probe for simultaneous detection of neural discharge and dopamine in striatum of mice aversion system. Sens. Actuators B Chem. 390, 133990 (2023).
Tandel, R. D., Pawar, S. K. & Seetharamappa, J. Synthesis and characterization of bentonite-reduced graphene oxide composite: application as sensor for a neurotransmitter, dopamine. J. Electrochem. Soc. 163, H705–H713 (2016).
Kim, M. K., Leong, J. C., Jo, Y., Kook, G. & Lee, H. J. Multimodal neural probes with small form factor based on dual‐side fabrication. Adv. Mater. Technol. 8, 2200692 (2022).
Ko, S. H., Kim, S. W., Lee, S. H. & Lee, Y. J. Electrodeposited reduced graphene oxide-PEDOT:PSS/Nafion hybrid interface for the simultaneous determination of dopamine and serotonin. Sci. Rep. 13, 20274 (2023).
Pitiphattharabun, S. et al. Reduced graphene oxide/zinc oxide composite as an electrochemical sensor for acetylcholine detection. Sci. Rep. 14, 14224 (2024).
Taylor, I. M. et al. Enhanced dopamine detection sensitivity by PEDOT/graphene oxide coating on in vivo carbon fiber electrodes. Biosens. Bioelectron. 89, 400–410 (2017).
Chang, Y. & Venton, B. J. Optimization of graphene oxide-modified carbon-fiber microelectrode for dopamine detection. Anal. Methods 12, 2893–2902 (2020).
Chung, J. E. et al. A fully automated approach to spike sorting. Neuron 95, 1381–1394 (2017).
Cohen, J. Statistical Power Analysis for the Behavioral Sciences 2nd ed. (Lawrence Erlbaum Associates, 2013).
Smyser, C. D. et al. Longitudinal analysis of neural network development in preterm infants. Cereb. Cortex 20, 2852–2862 (2010).
Acknowledgements
We acknowledge support from NIH R01MH139342 (to H.F.), the NSF CAREER award ECCS-2140392 (to H.F.), Dartmouth College institutional fund (to H.F.), the foundation for Anesthesia Education & Research 230444-01 (to J.L.), and the Van Poznak Anesthesiology Research Scholarship (to J.L.). We thank Kyle Smith for the helpful discussion on the original probe design.
Author information
Authors and Affiliations
Contributions
Y. Qi, Y. Qiang, and H.F. conceived the ideas and designed the research. Y. Qi, J.R., Y. Qiang, and H.F. designed the devices. Y. Qi characterized the materials and tested the devices. Y. Qi, J.R., T.B., Y. Qiang, Y.S., W.G., and G.L. fabricated the devices. T.B. and Y. Qiang designed and prototyped the electronics boards. Y. Qi, Y. Qiang, D.J., J.L., H.M., A.I., M.v.d.M., and H.F. designed and performed in vivo experiments and signal processing. Y. Qi, Y. Qiang, and H.F. prepared the figures. Y. Qi, Y. Qiang, and H.F. co-wrote the manuscript while all the authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The carbon coating technique is covered in the patent with H.F. and Y. Qi listed as the applicants: TRUSTEES OF DARTMOUTH COLLEGE, H.F., Y. Qi, WO Patent, Pub. No. WO/2024/145690, Published. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Luis Jacinto and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qi, Y., Jang, D., Ryu, J. et al. Stabilized carbon coating on microelectrodes for scalable and interoperable neurotransmitter sensing. Nat Commun 16, 3300 (2025). https://doi.org/10.1038/s41467-025-58388-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-58388-z