Abstract
Carbon-heteroatom bond (especially for C-N bond) formation through nickel catalysis has seen significant development. Well-established Ni(0)/Ni(II) redox cycle and photoinduced Ni(I)/Ni(III) redox cycle have been the dominant mechanisms. We report a thermally driven Ni-catalyzed method for C-N bond formation between haloarenes and B2N4 reagents, yielding N,N-dialkylaniline derivatives in good to excellent yields with broad functional group tolerance under base-free conditions. The catalytic protocol is useful for base-sensitive structures and late-stage modifications of complex molecules. Detailed mechanistic studies and density functional theory (DFT) calculations indicate that a Ni(I)/Ni(III) redox cycle is preferred in the C-N coupling process, and B2N4 reagent serves both as a single electron transfer donor and a N,N-dialkylation source.
Similar content being viewed by others
Introduction
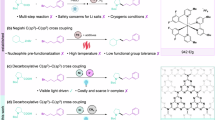
N,N-dialkylanilines are key scaffolds in bioactive compounds1,2,3 and important building blocks for organic synthesis4,5,6,7,8,9. The development of more efficient and sustainable N-alkylation processes has continuously attracted the attention of chemists10,11,12,13. While direct N-alkylation with alkyl halide (e.g., toxic methyl iodide)14 and reductive amination15,16 remains in use, transition-metal (Pd/Ni etc.) promoted C-X amination reactions utilizing dialkylamines or their equivalences through well-established M(0)/M(II) redox cycle17,18,19,20,21,22 have emerged as effective alternatives (Fig. 1a). Productive catalysis through the M(0)/M(II) cycle has been achieved through elegant ligand designs based on phosphorous23,24,25 or N-heterocyclic carbenes (NHC)26,27,28,29. In recent years, Ni(I)/Ni(III)30,31,32,33,34 redox cycle has been confirmed to be more efficient and versatile since high-valent Ni(III) species lead to a faster and energetically downhill C-N reductive elimination (RE)28. Recent developments in photoredox catalysis35,36,37,38,39 (Fig. 1b) have demonstrated that C-N bond formation can be achieved by using simple ligands (even without ligands) under exceptionally mild reaction conditions. MacMillan40,41 and Buchwald42 developed a nickel/photochemical C-N bond formation methodology without complex ligands between haloarenes and amines. Miyake43 also demonstrated a photocatalyst-free C-N cross coupling of electron deficient haloarenes via ultraviolet light photoexcitation of Nickel-amine complexes. In the absence of additional photocatalysts, both Xue44,45 and our group46 have reported the strategies for C-N bonds construction via photo-induced nickel catalysis. Most recently, Ritter47 reported a Ni(I)/Ni(III)-photo-catalyzed C-heteroatom bond formation of pre-functionalized arylthianthrenium salts based on simple Ni(II) salt.
While these approaches have found wide use for C-N bond formation, they are primarily hampered by their inability to efficiently react with electron rich haloarenes and base sensitive substrates, since most of these reactions require the consumption of super-stoichiometric amounts of exogenous bases as sacrificial reagents. Still remains undeveloped, there are limited reports of base-free thermally sustained Ni(I)/Ni(III) coupling strategies, utilizing external single-electron reductants such as manganese and zinc48,49,50,51.
While B2O4 type diboron reagents, such as tetrahydroxydiboron (B2(OH)4), are applied in the Suzuki-Miyaura coupling reaction52,53 for C-C bond construction, bis(pinacolato)diboron (B2pin2) and B2pin2 derivatives (B2cat2, B2nep2 etc.), are often used in C-B bond formation through Miyaura borylation reaction (Fig. 1c)54,55. Diboron compounds are common and useful single electron transfer (SET) reagents56,57 in photoredox catalysis, but they are less used as SET reagents in transition-metal catalysis. While seminal reports58,59,60 in the field of C-C/C-X bonds formation utilizing B2O4 type diboron reagents have been accomplished, in sharp contrast, B2N4-type diboron reagents, such as tetrakis(dimethylamino)diboron (B2(NMe2)4), have not been found to be useful for transition-metal-catalyzed C-N bond construction. Could a B2N4 type diboron reagent be used both as a SET reagent and a “N” source by the Ni(I)/Ni(III) redox cycle without photoexcitation under base-free and mild reaction conditions? Herein, we report such a case, as shown in (Fig. 1d). Remarkably, nickel offers distinct advantages over palladium, such as earth-abundant metal catalyst and a reduced propensity for β-hydride (or β-hydrogen) elimination61 when working with alkyl fragments.
N,N-dimethyl anilines are most valuable intermediates among N,N-dialkylanilines used for the preparation of bioactive molecules, polyester resins and dyes. Direct N,N-dimethylation of haloarenes, especially electron rich haloarenes, with dimethylamine is more challenging because of its low boiling point (~7 °C). Several protocols62,63,64,65 for the dimethylamination of haloarenes using the equivalents of dimethylamine have been reported as well. However, the pre-functionalization of dimethylamine, the requirement for strong base and complex ancillary ligands are necessary, making it nontrivial for high site selectivity. In this work, our photo-free “two-in-one” protocol makes it complementary to other C-N bond formation strategies (especially for syntheses of N,N-dimethyl anilines), whose effectiveness is exemplified through working with base-sensitive partners and late-stage modifications of a series of bioactive complex molecules.
Results and discussion
Reaction design and optimization
Building on this basis, we set out to examine the possibility of base-free, complex ligand-free Ni-catalyzed C-N coupling of haloarenes with commercially available B2(NMe2)4, aiming to establish a protocol that displays an effective N,N-dimethyl aniline synthesis. In our initial study, the coupling reaction of 4-bromophenoxybenzene (1a) with B2(NMe2)4 was selected as a model system for reaction development, as shown in (Table 1). Various transition metal catalysts were systematically evaluated for their efficacy in our study (Table S1). These experiments demonstrated that the N,N-dimethyl aniline product 2a formed with 94% isolated yield in the presence of 10 mol % of Ni(acac)2 catalyst. Copper and palladium catalysts showed no reactivity for this reaction (entries 1-2). The yield of 2a was slightly reduced with NiCl2•6H2O as catalyst (entry 3). In contrast, Ni(0) catalysts, for example Ni(COD)2, did not show activity (entry 4). Thus, Ni(II) is indispensable for optimizing reaction efficiency in this C-N cross coupling reaction. Subsequent studies revealed DMF to be the optimal solvent for the coupling reaction (entries 5-7). Lower reaction temperature and reduced loading of B2(NMe2)4 significantly diminished the yield of 2a (entries 8-9). Control experiments revealed that no reaction occurred without Ni(II) catalyst (entry 10).
Scope of substrates
With the optimal condition in hand, we first investigated the scope and limitations of our Ni-catalyzed C-N coupling reaction with respect to the para-substituted bromoarenes (Fig. 2A). This method showed excellent functional group tolerance, both electron-donating and electron-withdrawing substituents could be well accommodated, including alkyl (2c), alkenyl (2u), alkynyl (2v), thioether (2e), free hydroxyl and amino (2s, 2t), cyclopropyl (2w), (hetero)aryl (2o-2r). Carbonyl functionalities such as esters (2l), amides (2m-2n) and ketones (2w-2x) that might be susceptible to acylation or condensation reaction were not affected when B2(NMe2)4 was used. Nitrogen-, oxygen-, sulfur-, boron-, silicon-containing haloarenes were also prepared in high yields.
A The functional group tolerance of para-substituted haloarenes; B Variation of haloarene coupling partner; C Variation of heterohaloarene coupling partner. Reaction condition: substrates (0.2 mmol, 1.0 equiv.), Ni(acac)2 (0.02 mmol, 10 mol%), B2(NMe2)4 (0.2 mmol, 1.0 equiv.), DMF (0.5 mL), stirring at 80 °C for 24 h. Yields are reported for material obtained following purification and isolation.
We next extended the protocol to the synthesis of poly-substituted aniline derivatives, bearing in mind their importance in medicinal chemistry (Fig. 2B). Bromoarenes bearing electron-donating and electron withdrawing groups at ortho and meta position were all compatible to give the corresponding anilines in good to excellent yields (2y-2ac), bulky ortho-OPh-bromoarenes (2aa), albeit with slightly diminished yields. Di-substituted bromoarenes with electron-donating and electron-withdrawing groups, such as 3,4- 2,4- 2,5 and 3,5-disubstituted bromoarenes could give good to high yields (2ad-2al), showcasing the compatibility of our method with a broad array of functionality. Naphthyl bromides (2am-2ap) were also good partners to afford desired products. Given the critical role of heterocyclic motifs in drug development, we extended this methodology to diverse heteroaryl bromides, spanning benzothiophene, benzofuran, indole, pyridine, coumarin, quinoxaline, (iso)quinoline, pyrazine, and pyrimidine scaffolds. All of these substrates yielded the expected products in good to excellent yields (2aq-2bc, Fig. 2C).
Due to the mild and base-free reaction conditions, we envisioned that our strategy might be applicable to substrates that would readily undergo undesired racemization, elimination and decomposition reactions in the presence of strong bases. Chiral substituents at the α-carbonyl position are highly susceptible to epimerization, while the presence of strong bases can further induce decomposition. Armed with our standard conditions, no ee erosion was observed as exemplified by the enantio-retention experiment utilizing 4-Br-L-phenylalanine as model substrate (3a, Fig. 3A). In contrast, only decomposition product were observed under classic Buchwald-Hartwig C-N coupling conditions (Fig. S2). In addition, elimination-prone coupling partners bearing alkyl chloride (3b) were suitable coupling partners. The alkyl halides would valuable for subsequent synthetic applications. Furthermore, heteroaryl rings (Fig. 3B), such as oxazole (3c), benzothiazole (3d), indazole (3e) and imidazole (metronidazole derivative 3f), which would be decomposed under strong basic conditions66, could be well tolerated in our system. This result inspired us to pursue the three-step synthesis of PET (the positron emission computed tomography) tracer analog 3g for Alzheimer’s disease (AD) in 66% overall yield.
A Enantioretention experiment. B Base-sensitive coupling partners. C Synthesis of base-sensitive AD PET tracer analog. Condition A: 2-amino-6-fluorobenzothiazole (1.0 equiv.), ethylene glycol (4 equiv.), KOH (6.0 M, 30 equiv.), reflux at 140 °C for 24 h; condition B: bis(2-amino-5-fluorophenyl)disulfide (1.0 equiv.), 4-bromobenzaldehyde (1.02 equiv.), sodium metabisulfite (1.02 equiv.), DMSO (0.17 M), reaction at 120 °C for 2 h; condition C: 2-(4-Bromophenyl)−6-fluorobenzothiazole (0.2 mmol), B2(NMe2)4 (0.2 mmol), Ni(acac)2 (10 mol%), DMF (0.5 mL), reaction for 24 h under 80 °C. AD Alzheimer’s disease, PET the positron emission computed tomography. See SI for full experimental details and conditions.
Different B2N4 reagents67,68 were next used to explore the applicability of these reagents in forming diversified amino products (Fig. 4). B2N4 reagents derived from pyrrolidine and piperidine could be utilized to construct corresponding N,N-dialkylanilines (4a-4d). For example, electron-rich and electron-deficient aryl bromides were well tolerated when reacting with tetrakis(pyrrolidino)diborane under standard conditions (4a-4c). Furthermore, more valuable tertiary aniline products (4e-4g) were successfully prepared with good to excellent yields using corresponding B2N4 reagents derived from several secondary amines. Unfortunately, aliphatic primary amine-derived diboron species didn’t work under standard conditions, most likely due to the undesired hydrolysis of diboron species. For example, only benzyl amine was detected when utilizing B2(BnNH)4 as B2N4 reagent. To our delight, pharmaceutical-related diarylamines could be prepared from B2(NHPh)4 with (hetero)aryl bromides (4h-4j). Of note is that more sterically hindered B2N4 reagents were also good partners to afford diarylamine product (4k).
The broad functional group tolerance for this dimethylamination of haloarenes encouraged us to test this strategy for late-stage functionalization of complex molecules, such as natural products and active pharmaceutical ingredients (APIs). As shown in Fig. 5, N,N-dimethyl anilines derived from gallic acid (5a), thymol (5c-5d), estradiol (5e), tyramine (5i), as well as a variety of APIs (5b, 5f-5h, 5j-5l) bearing polar groups, basic heteroatoms, and heterocyclic frameworks were readily synthesized, confirming versatility of our method. Furthermore, a wide variety of powerful synthetic transformations of the resulting N,N-dimethyl complexes were also demonstrated using N,N-dimethyl aniline derivatives (Fig. S5). These valuable transformations revealed the possibility of global derivatization around the core N,N-dimethylaniline structure, and such derivatization can turn N,N-dimethylaniline into a versatile synthetic building block in organic synthesis.
Mechanistic studies
The mechanism of this Ni-catalyzed C-N coupling of haloarenes with B2N4 type reagents was then investigated. A series of probing experiments were conducted to investigate the reaction pathway. No desired products were observed with other potential intermediates such as dimethylamine, N,N-dimethylformamide and tris(dimethylamino)borane (Fig. S7). In the radical trapping experiments (Fig. 6A), when phenyl tert-butyl nitrone (PBN) was introduced into the experiments as a spin-trapping reagent, radical EPR signals were observed (Fig. 6Aa). A g-factor of 2.005 indicates that an organic radical species, whose adduct with PBN was detected by high-resolution mass spectrometry (HRMS [M + K+]: 513.3418), was generated from mixture of Ni(acac)2 and B2N4 reagent after heating. In fact, radical intermediate A1 was identified by oxygen free high-resolution mass spectrometry (HRMS [M + H+]: 298.2716), confirming the single electron transfer progress between Ni(II) and B2N4 reagent. Notably, the C-N coupling reaction was completely suppressed using TEMPO as a radical inhibitor, and the adduct of TEMPO-acac was detected by HRMS (Fig. 6Ab; [M + H+]: 256.1914). We speculated that TEMPO abstracted acac radical69 from key radical cage Ni(acac), releasing non-catalytic nickel(0). Additionally, no obvious inhibition of C-N bond formation was observed using 1,1-diphenylethylene as a neutral radical scavenger, indicating that no radical species was involved in the Ni redox cycle (Fig. 6Ac). In this trapping experiment, a key radical intermediate was captured by 1,1-diphenylethylene (HRMS [M + H+]: 479.3736).
A Radical trapping experiments: (Aa) PBN trapping experiment; (Ab) TEMPO trapping experiment; (Ac) 1,1-diphenylethylene trapping experiment. PBN phenyl tert-butyl nitrone; TEMPO 2,2,6,6-Tetramethyl-1-piperinedinyloxy; EPR electron paramagnetic resonance. B Ni(I)/Ni(III) pathway confirmation. C Ni(I) confirmation through comproportionation reaction (C1) and standard reaction condition (C2) by electron paramagnetic resonance (EPR) experiments in DMF solvent. D Key intermediates detected by HRMS. See SI for full experimental details and conditions.
Next, we performed several experiments to confirm whether the dominant productive pathway involves a Ni(0)/Ni(II) or Ni(I)/Ni(III) redox cycle. As shown in Fig. 6B, when utilizing Ni(COD)2 as the Ni(0) catalyst, instead of C-N coupling product 2a, homo-coupling product 2a’ (room temperature or 80 °C) from 4-bromophenoxybenzene was isolated as the single product through the well-established M(0)/M(II) redox cycle (i.e., Negishi coupling70, Suzuki-Miyaura coupling51,52), revealing that Ni(0) was not involved in the Ni redox cycle. Proceeded at room temperature, no desired C-N coupling product 2a was detected no matter Ni(0) or Ni(II) was used. Well-defined examples of comproportionation reactions71 of Ni(0) and Ni(II) to generate Ni(I) species have been reported since Heimbach’s pioneering work72. Based on these general comproportionation strategies of Ni(0) and Ni(II), after 4 h reaction of Ni(0) and Ni(II) (Ni(COD)2: Ni(acac)2 = 20 mol%: 20 mol%) at room temperature, the Ni mixture was added into the reaction system, the desired C-N coupling product was detected with 5% yield (80% yield at 80 °C), indicating that an active Ni(I) species was formed through comproportionation reaction between Ni(0) and Ni(II). The formation of the Ni(I) species was evidenced by electron paramagnetic resonance (EPR) spectroscopy, which revealed a Ni(I) species with giso = 2.366 from the comproportionation reaction (Fig. 6C, C1). We also conducted EPR measurements on the reaction mixture obtained under standard reaction conditions after 10 h. The unpaired electron in Ni(I) was directly observed by EPR (giso = 2.349 at 100 K Fig. 6C, C2). While the EPR of C1 is clean, that of C2 is likely affected somewhat by the presence of other organic radicals, for example, A1. Perhaps more straightforwardly, radical intermediate A1 and related fragments A2-A4 (m/z of [M + H+]: 298.2716 for A1; m/z of [M+Na+]: 256.0756 for A2; m/z of [M + H+]: 199.1612 for A3; m/z of [M + H+]: 101.1249 for A4; m/z of [M+Na+]: 290.9448 for A5) and key Ni(III) intermediates (m/z of [M-acac] +: 305.9188 for B; m/z of [M + H+]: 371.1028 for C) were also identified by oxygen free high-resolution mass spectrometry (see supporting information for detailed oxygen free HRMS experiments) after 1 h under standard reaction conditions (Fig. 6D), which supports the potential energy surface obtained from the following density functional theory (DFT) calculations, indicating a Ni(I)/Ni(III) redox cycle was involved in the nickel catalyzed C-N coupling of haloarene.
Computational studies were performed to gain an in-depth understanding of the reaction mechanism (Fig. 7, Supplementary Computational Details). Ni(acac)2, B2(NMe2)4, and PhBr were selected as model substrates for this theoretical study. Based on the results of mass spectrometry experiments, we conducted DFT calculations to evaluate the feasibility of forming the B2(NMe2)4(acac) radical species A1 and NiI(acac) via single electron transfer (SET) process between Ni(acac)₂ and B2(NMe2)4. Additionally, we also carried out a comprehensive assessment of the solvents’ role in our reaction (Fig. S26). The DFT results indicate that the Gibbs free energy (ΔG) for this reaction is 6.4 kcal/mol, suggesting that the reaction is thermodynamically feasible. After the SET process, DMF binds to NiI(acac) to form intermediate Int1, and then oxidative addition occurs via transition state TS1, resulting in the Ni(III) intermediate Int2 with an activation barrier of 13.1 kcal/mol. Next, the Ni(III) intermediate Int2 coordinates with B2(NMe2)4 to form Int3. Due to boron’s metalloid property, Int3 undergoes a transmetallation-like four-membered ring transition state TS2 (25.1 kcal/mol) to form Int4. In fact, intermediate C (m/z of [M + H+]: 371.1028 in Fig. 6D), derivative of Int4, has been detected by HRMS. Int4 then produces the Ni(I) intermediate Int5 via the reductive elimination transition state TS3 (20.3 kcal/mol). Finally, another molecule of DMF replaces PhNMe₂ in Int5 to yield the aniline product and regenerate Int1 for the next catalytic cycle.
Based on these findings and previous reports40,41,42,43,44,45,46,47,73,74,75,76,77,78,79,80, we propose the following mechanism (Fig. 8). First, single electron transfer progress between Ni(acac)2 and B2(NMe2)4 (A) generates B2(NMe2)4 radical species A1 and NiI(acac). Next, NiI(acac) undergoes oxidative addition with the aryl bromide 1a, leading to the formation of Ni(III) species B. Subsequent amination of B with B2(NMe2)4 delivers C and A2. Feasible reductive elimination of C enables the formation of product 2a and regenerates Ni(I) catalyst.
Additionally, A2 is also responsible for 2a synthesis through Ni(I)/Ni(III) redox cycle (green line). 85% yield of 2a can be isolated with 0.7 equivalent of B2(NMe2)4, indicating that, in addition to B2(NMe2)4, other dimethylamine sources such as A2 participate in the C-N bond formation reaction. The by-product (A5) of A2 has been captured by HRMS, showcasing the fact that A2 is one of the dimethylamine sources.
In conclusion, we have developed an efficient C-N cross coupling methodology for N,N-dialkylation of haloarenes with B2N4 reagents by complex ligand-free and base-free nickel catalysis without an external reductant. Compared with the traditional N,N-dialkylaniline synthetic strategies, this protocol benefits from high functional group tolerances, reduced synthetic steps and feasible base sensitive aniline derivatives syntheses. The protocol’s excellent functional group tolerance enables the functionalization of a variety of biologically relevant compounds, representing a valuable potential industrial application of the simple nickel catalyst system. A detailed mechanistic investigation and DFT calculations give a reasonable explanation for the Ni(I)/Ni(III) redox pathway.
Methods
General procedure for nickel catalyzed C-N coupling of haloarenes with B2N4 reagents
An oven-dried 4 mL vial was charged with a magnetic stir bar, aryl bromides (0.2 mmol, 1.0 equiv.), Ni(acac)2 (0.02 mmol, 10 mol%), B2N4 (0.2 mmol, 1.0 equiv.), DMF (0.5 mL) in the glove box. The vial was sealed with a plastic cap and then stirring was achieved by placing the assembled reactor at 80 °C on IKA C-MAG HS 7 control magnetic stir bars for 24 h. After reaction completion, the reaction was quenched with H2O and diluted with EtOAc. The resulting mixture was separated and extracted with EtOAc (three times). The combined organic layer was dried over anhydrous Na2SO4, filtered and concentrated in vacuo. The reaction mixture was purified by fresh silica gel chromatography to afford the desired product.
Data availability
The experimental and analytical procedures and full spectral data are available in the Supplementary Information. Cartesian coordinates of the calculated structures are available from Supplementary Data 1. Data supporting the findings of this manuscript are also available from the corresponding author upon request.
References
Kalasz, H. Biological role of formaldehyde, and cycles related to methylation, demethylation, and formaldehyde production. Mini-Rev. Med. Chem. 3, 175–192 (2003).
Roughley, S. D. & Jordan, A. M. The medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 54, 3451–3479 (2011).
Chisholm, T. S. & Hunter, C. A. A closer look at amyloid ligands, and what they tell us about protein aggregates. Chem. Soc. Rev. 53, 1354–1374 (2024).
Hu, X., Martin, D., Melaimi, M. & Bertrand, G. Gold-catalyzed hydroarylation of alkenes with dialkylanilines. J. Am. Chem. Soc. 136, 13594–13597 (2014).
Song, G., Luo, G., Oyamada, J., Luo, Y. & Hou, Z. ortho-Selective C-H addition of N, N-dimethyl anilines to alkenes by a yttrium catalyst. Chem. Sci. 7, 5265–5270 (2016).
Xie, J., Yu, J., Rudolph, M., Rominger, F. & Hashmi, A. S. K. Monofluoroalkenylation of dimethylamino compounds through radical-radical cross-coupling. Angew. Chem. Int. Ed. 55, 9416–9421 (2016).
Cao, Z.-C., Xie, S.-J., Fang, H. & Shi, Z.-J. Ni-catalyzed cross-coupling of dimethyl aryl amines with arylboronic esters under reductive conditions. J. Am. Chem. Soc. 140, 13575–13579 (2018).
Zhang, H.-H., Zhao, J.-J. & Yu, S. Enantioselective α-Allylation of anilines enabled by a combined palladium and photoredox catalytic system. ACS Catal. 10, 4710–4716 (2020).
Zhang, B., Er, F. R., Vasilopoulos, A., Voight, E. A. & Alexanian, E. J. General synthesis of N-Alkylindoles from N,N-Dialkylanilines via [4+1] annulative double C-H functionalization. J. Am. Chem. Soc. 145, 26540–26544 (2023).
Li, Y., Fang, X., Junge, K. & Beller, M. A general catalytic methylation of amines using carbon dioxide. Angew. Chem. Int. Ed. 52, 9568–9571 (2013).
Bobbink, F. D., Das, S. & Dyson, P. J. N-formylation and N-methylation of amines using metal-free N-heterocyclic carbene catalysts and CO2 as carbon source. Nat. Protoc. 12, 417–428 (2017).
Dorel, R., Grugel, C. P. & Haydl, A. M. The buchwald-hartwig amination after 25 years. Angew. Chem. Int. Ed. 58, 17118–17129 (2019).
Lam, R. H. et al. Selective formylation or methylation of amines using carbon dioxide catalysed by a rhodium perimidine-based NHC complex. Green Chem. 21, 538–549 (2019).
Redeker, K. R. et al. Emissions of methyl halides and methane from rice paddies. Science 290, 966–969 (2000).
Afanasyev, O. I., Kuchuk, E., Usanov, D. L. & Chusov, D. Reductive amination in the synthesis of pharmaceuticals. Chem. Rev. 119, 11857–11911 (2019).
Reber, S. et al. Scalable synthesis of C5aR1 antagonist ACT-1014-6470 via N7-selective reductive amination of an unprotected pyrazole starting material and intramolecular urea formation with 1,1′-Carbonyl-di(1,2,4-triazol) (CDT). Org. Proc. Res. Dev. 28, 2269–2283 (2024).
Sambiagio, C., Marsden, S. P., Blacker, A. J. & McGowan, P. C. Copper catalysed Ullmann type chemistry: from mechanistic aspects to modern development. Chem. Soc. Rev. 43, 3525–3550 (2014).
Ruiz-Castillo, P. & Buchwald, S. L. Applications of palladium-catalyzed C-N cross-coupling reactions. Chem. Rev. 116, 12564–12649 (2016).
West, M. J., Fyfe, J. W. B., Vantourout, J. C. & Watson, A. J. B. Mechanistic development and recent applications of the chan-lam amination. Chem. Rev. 119, 12491–12523 (2019).
Ribaucourt, A. & Cossy, J. N-(Hetero)arylations with metalated (Hetero)aryls: recent advances in first-row transition-metal-mediated cross-couplings. ACS Catal. 10, 10127–10148 (2020).
Trowbridge, A., Walton, S. M. & Gaunt, M. J. New strategies for the transition-metal catalyzed synthesis of aliphatic amines. Chem. Rev. 120, 2613–2692 (2020).
Cannalire, R. et al. Visible light photocatalysis in the late-stage functionalization of pharmaceutically relevant compounds. Chem. Soc. Rev. 50, 766–897 (2021).
Tassone, J. P., England, E. V., MacQueen, P. M., Ferguson, M. J. & Stradiotto, M. PhPAd-DalPhos: ligand-enabled, nickel-catalyzed cross-coupling of (hetero)aryl electrophiles with bulky primary alkylamines. Angew. Chem. Int. Ed. 58, 2485–2489 (2019).
Newman-Stonebraker, S. H., Wang, J. Y., Jefrey, P. D. & Doyle, A. G. Structure-reactivity relationships of Buchwald-type phosphines in nickel-catalyzed cross-couplings. J. Am. Chem. Soc. 144, 19635–19648 (2022).
Feng, K. et al. Development of a deactivation-resistant dialkylbiarylphosphine ligand for Pd-catalyzed arylation of secondary amines. J. Am. Chem. Soc. 146, 26609–26615 (2024).
Rull, S. G. et al. Elucidating the mechanism of aryl aminations mediated by NHC-supported nickel complexes: evidence for a nonradical Ni(0)/Ni(II) pathway. ACS Catal. 8, 3733–3742 (2018).
Wang, Z.-C., Xie, P.-P., Xu, Y., Hong, X. & Shi, S.-L. Low-temperature nickel-catalyzed C−N cross-coupling via kinetic resolution enabled by a bulky and flexible chiral N-Heterocyclic carbene ligand. Angew. Chem. Int. Ed. 60, 16077–16084 (2021).
Chernyshev, V. M. et al. Discovery of the N-NHC coupling process under the conditions of Pd/NHC- and Ni/NHC-catalyzed buchwald-hartwig amination. Organometallics 41, 1519–1531 (2022).
Wang, Z.-C., Li, Y.-Y., Zhang, S.-Q., Hong, X. & Shi, S.-L. Unsymmetric N-heterocyclic carbene ligand enabled nickel-catalysed arylation of bulky primary and secondary amines. Chem. Sci. 14, 4390–4396 (2023).
Ting, S. I. et al. 3d-d excited states of Ni(II) complexes relevant to photoredox catalysis: spectroscopic identification and mechanistic implications. J. Am. Chem. Soc 142, 5800–5810 (2020).
Zhu, C., Yue, H., Nikolaienko, P. & Rueping, M. Merging electrolysis and nickel catalysis in redox neutral cross-coupling reactions: experiment and computation for electrochemically induced C-P and C-Se bonds formation. CCS Chem. 2, 179–190 (2020).
Bismuto, A., Finkelstein, P., Müller, P. & Morandi, B. The journey of Ni(I) chemistry. Helv. Chim. Acta. 104, e202100177 (2021).
Cagan, D. A. et al. Elucidating the mechanism of excited-state bond homolysis in nickel-bipyridine photoredox catalysts. J. Am. Chem. Soc. 144, 6516–6531 (2022).
Ting, S. I., Williams, W. L. & Doyle, A. G. Oxidative addition of Aryl Halides to a Ni(I)-Bipyridine complex. J. Am. Chem. Soc. 144, 5575–5582 (2022).
Twilton, J. et al. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 1, 0052 (2017).
Holmberg-Douglas, N. & Nicewicz, D. A. Photoredox-catalyzed C-H functionalization reactions. Chem. Rev. 122, 1925–2016 (2022).
Pitre, S. P. & Overman, L. E. Strategic use of visible-light photoredox catalysis in natural product synthesis. Chem. Rev. 122, 1717–1751 (2022).
Beil, S. B., Chen, T. Q., Intermaggio, N. E. & MacMillan, D. W. C. Carboxylic acids as adaptive functional groups in metallaphotoredox catalysis. Acc. Chem. Res. 55, 3481–3494 (2022).
Zhang, J. et al. Recent advances in C(sp3)-N bond formation via metallaphotoredox catalysis. Chem. Commun. 60, 6340–6361 (2024).
Terrett, J. A., Cuthbertson, J. D., Shurtlef, V. W. & MacMillan, D. W. C. Switching on elusive organometallic mechanisms with photoredox catalysis. Nature 524, 330–334 (2015).
Till, N. A., Tian, L., Dong, Z., Scholes, G. D. & MacMillan, D. W. C. Mechanistic analysis of metallaphotoredox C-N coupling: photocatalysis initiates and perpetuates Ni(I)/Ni(III) coupling activity. J. Am. Chem. Soc. 142, 15830–15841 (2020).
Park, B. Y., Pirnot, M. T. & Buchwald, S. L. Visible light-mediated (Hetero)aryl amination using Ni(II) salts and photoredox catalysis in flow: a synthesis of tetracaine. J. Org. Chem. 85, 3234–3244 (2020).
Lim, C.-H., Kudisch, M., Liu, B. & Miyake, G. M. C-N cross-coupling via photoexcitation of nickel-amine complexes. J. Am. Chem. Soc. 140, 7667–7673 (2018).
Song, G. et al. Chiral arylated amines via C-N coupling of chiral amines with aryl bromides promoted by light. Angew. Chem. Int. Ed. 60, 21536–21542 (2021).
Song, G. et al. Adaptive photochemical amination via Co(II) Catalysis. J. Am. Chem. Soc. 146, 26936–26946 (2024).
Bao, H. & Wang, L. Photoinduced reduction of nitroarenes and tandem C-N cross-coupling with haloarenes. Org. Lett. 25, 8872–8876 (2023).
Ni, S. et al. C-heteroatom coupling with electron-rich aryls enabled by nickel catalysis and light. Nat. Catal. 7, 733–741 (2024).
Sun, R., Qin, Y. & Nocera, D. G. General paradigm in photoredox nickel-catalyzed cross-coupling allows for light-free access to reactivity. Angew. Chem. Int. Ed. 59, 9527–9533 (2020).
Ni, S. et al. Nickel meets aryl thianthrenium salts: Ni(I)-catalyzed halogenation of arenes. J. Am. Chem. Soc. 145, 9988–9993 (2023).
Li, H., Tian, X., Dang, Q.-Q., Zhang, J. & Wen, Z.-K. Overcoming electron bias in hydroarylation of phenylpropiolic acid derivatives to enable highly reversed regio- and stereoselectivity via steric and transient post addition coordination. ACS Catal. 14, 9985–9992 (2024).
Huang, S. & Zhou, J. S. Nickel-catalyzed enantioselective reductive arylation of common ketones. J. Am. Chem. Soc. 146, 12895–12900 (2024).
Beletskaya, I. P., Alonso, F. & Tyurin, V. The Suzuki-Miyaura reaction after the Nobel prize. Coord. Chem. Rev. 385, 137–173 (2019).
Niwa, T. et al. Lewis acid-mediated Suzuki-Miyaura cross-coupling reaction. Nat. Catal. 4, 1080–1088 (2021).
Tian, Y.-M., Guo, X.-N., Braunschweig, H., Radius, U. & Marder, T. B. Photoinduced borylation for the synthesis of organoboron compounds. Chem. Rev. 121, 3561–3597 (2021).
Tse, M. H., Zhong, R.-L. & Kwong, F. Y. Palladium-catalyzed miyaura borylation of overly crowded aryl chlorides enabled by a complementary localized/remote steric bulk of ligand chassis. ACS Catal. 12, 3507–3515 (2022).
Corcé, V., Ollivier, C. & Fensterbank, L. Boron, silicon, nitrogen and sulfur-based contemporary precursors for the generation of alkyl radicals by single electron transfer and their synthetic utilization. Chem. Soc. Rev. 51, 1470–1510 (2022).
Liu, T.-T. et al. Neutral boryl radicals in mixed-valent B(III)Br-B(II) adducts. Chem. Eur. J. 29, e202202634 (2023).
Lennox, A. J. J. & Lloyd-Jones, G. C. Selection of boron reagents for Suzuki-Miyaura coupling. Chem. Soc. Rev. 43, 412–443 (2014).
Fan, Y., Huang, Z., Lu, Y., Zhu, S. & Chu, L. Defluorinative alkylboration of alkenes enabled by dual photoredox and copper catalysis. Angew. Chem. Int. Ed. 63, e202315974 (2024).
Lu, L. et al. Enantioselective synthesis of β-aminoboronic acids via borylalkylation of enamides. J. Am. Chem. Soc. 146, 16639–16647 (2024).
Choi, J. & Fu, G. C. Transition metal-catalyzed alkyl-alkyl bond formation: another dimension in cross-coupling chemistry. Science 356, eaaf7230 (2017).
Wang, D., Kuang, D., Zhang, F., Yang, C. & Zhu, X. Room-temperature copper-catalyzed arylation of dimethylamine and methylamine in neat water. Adv. Synth. Catal. 357, 714–718 (2015).
Li, J. et al. Nickel-catalyzed amination of aryl chlorides with amides. Org. Lett. 23, 687–691 (2021).
Taeufer, T. & Pospech, J. Palladium-catalyzed synthesis of N,N-dimethylanilines via buchwald-hartwig amination of (Hetero)aryl triflates. J. Org. Chem. 85, 7097–7111 (2020).
Beletskaya, I. P. & Averin, A. D. Metal-catalyzed reactions for the C(sp2)-N bond formation: achievements of recent years. Russ. Chem. Rev. 90, 1359–1396 (2021).
Reichert, E. C., Feng, K., Sather, A. & Buchwald, S. L. Pd-catalyzed amination of base-sensitive five-membered heteroaryl halides with aliphatic amines. J. Am. Chem. Soc. 145, 3323–3329 (2023).
Baber, R. A. et al. Primary amido substituted diborane(4) compounds and imidodiborate(4) anions. Dalton Trans. 34, 3137–3139 (2005).
Loderer, D., Nöth, H., Pommerening, H., Rattay, W. & Schick, H. Chemistry of diborane(4) derivatives: mixed tetraaminodiboranes(4) and additions of diborane(4) derivatives to an amino-imino-borane. Chem. Ber. 127, 1605–1611 (1994).
Choi, Y. S. et al. Metal acetylacetonate as a radical initiator and catalyst for polyurethane in dual-curing reaction at low temperature. Prog. Org. Coat. 151, 105926 (2021).
Haas, D., Hammann, J. M., Greiner, R. & Knochel, P. Recent developments in negishi cross-coupling reactions. ACS Catal. 6, 1540–1552 (2016).
Day, C. S. & Martin, R. Comproportionation and disproportionation in nickel and copper complexes. Chem. Soc. Rev. 52, 6601–6616 (2023).
Heimbach, P. Changes in the coordination numbers of Ni(0) and Ni(I) compounds. Angew. Chem. Int. Ed. Engl. 3, 648 (1964).
Yoo, C. & Lee, Y. A T-shaped nickel(I) metalloradical species. Angew. Chem. Int. Ed. 56, 9502–9506 (2017).
Yue, H., Zhu, C., Kancherla, R., Liu, F. & Rueping, M. Regioselective hydroalkylation and arylalkylation of alkynes by photoredox/nickel dual catalysis: application and mechanism. Angew. Chem. Int. Ed. 59, 5738–5746 (2020).
Chen, Y., Wang, X., He, X., An, Q. & Zuo, Z. Photocatalytic dehydroxymethylative arylation by synergistic cerium and nickel catalysis. J. Am. Chem. Soc. 143, 4896–4902 (2021).
Du, X., Cheng-Sánchez, I. & Nevado, C. Dual nickel/photoredox-catalyzed asymmetric carbosulfonylationof alkenes. J. Am. Chem. Soc. 145, 12532–12540 (2023).
Jeon, J. H. et al. Chemo- and regioselective nickel-catalyzed reductive 1,4-Alkylarylation of 1,3-Enynes through an L2NiAr intermediate. ACS Catal. 14, 8996–9007 (2024).
Mendel, M. et al. Dynamic stereomutation of vinylcyclopropanes with metalloradicals. Nature 631, 80–86 (2024).
Day, C. S. et al. Elucidating electron-transfer events in polypyridine nickel complexes for reductive coupling reactions. Nat. Catal. 6, 244–253 (2023).
Huang, H., Alvarez-Hernandez, J. L., Hazari, N., Mercado, B. Q. & Uehling, M. R. Effect of 6,6′-Substituents on bipyridine-ligated Ni Catalysts for cross-electrophile coupling. ACS Catal. 14, 6897–6914 (2024).
Acknowledgements
We gratefully acknowledge the financial supports from the Shenzhen Key Laboratory of Neural Cell Reprogramming and Drug Research (No. ZDSYS20230626091202006 to L.W.), the National Natural Science Foundation of China (No. 22271316 to L.W.; No. 21933004 to Y.-D.W.; No. 22403067 to T.-Y.S.), the Guangdong Basic and Applied Basic Research Foundation (No. 2022A1515011994 to L.W.) and the Fundamental Research Funds for the Central Universities (No. 23ykbj010 to L.W.). Computational studies were supported by Shenzhen Bay Laboratory Supercomputing Center. We also thank Dr. He Zhiqi for helpful discussion.
Author information
Authors and Affiliations
Contributions
Q.C. and L.W. discovered the reactions. Q.C. and Q.L. performed the reaction optimizations, studied the reaction scope and synthetic utility and studied the reaction mechanisms. Y.-H.D. and T.-Y.S. performed the computational study. L.W. wrote the manuscript with help from T.-Y.S. and Y.-D.W. L.W., T.-Y.S., and Y.-D.W. initiated the project, designed the experiments, and directed the research.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Wei Guan, Xue-Peng Zhang, and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chang, Q., Li, Q., Deng, YH. et al. Nickel catalyzed C-N coupling of haloarenes with B2N4 reagents. Nat Commun 16, 3202 (2025). https://doi.org/10.1038/s41467-025-58438-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-58438-6