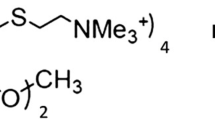
Abstract
Cancer remains a global health challenge necessitating innovative therapies. We introduce a strategy to disrupt cancer cell redox balance using gold nanoparticles (Au NPs) as electron sinks combined with electroactive membranes. Utilizing Shewanella oneidensis MR-1 membrane proteins, we develop liposomes enriched with c-type cytochromes. These, coupled with Au NPs, facilitate autonomous electron transfer from cancer cells, disrupting redox processes and inducing cell death. Effective across various cancer types, larger Au NPs show enhanced efficacy, especially under hypoxic conditions. Oxidative stress from Au@MIL (MIL: membrane-integrated liposome) treatments, including mitochondrial and endoplasmic reticulum lipid oxidation and mitochondrial membrane potential changes, triggers apoptosis, bypassing iron-mediated pathways. Surface plasmon band and X-ray absorption near-edge structure (XANES) analyses confirm electron transfer. A SiO2 insulator coating on Au NPs blocks this transfer, suppressing cancer cell damage. This approach highlights the potential of modulated electron transfer pathways in targeted cancer therapy, offering refined and effective treatments.
Similar content being viewed by others
Introduction
In today’s world, cancer continues to be a pervasive and pressing health concern, impacting millions of lives worldwide1. With its ability to affect virtually any part of the human body, cancer poses a significant threat to individuals of all ages and backgrounds. Despite advancements in medical research and treatment options, the global population of people living with cancer remains alarmingly high, underscoring the urgent need for continued efforts in prevention and innovative therapies to combat this formidable disease.
Surgery is the most direct method for tumor removal, but its drawback lies in its invasive nature. Chemotherapy is the most common method used in cancer treatments, but its challenges often involve the need for specific drugs tailored to target certain cancer cells2. When combined with nanoparticles (NPs), the NPs typically serve as targeted carriers3. Radiation therapy is an effective cancer treatment method, but it requires expensive radiation facilities and consideration of the impact on healthy tissues. If integrated with NPs, heavy element particles, which raise concerns regarding toxicity, are necessary4. Photodynamic therapy is another cancer treatment strategy that requires the combination of photosensitizers and external light. Therefore, whether integrated with NPs or not, the water solubility of photosensitizers and the penetration of light into tissues need to be considered5,6. With the advancement of nanotechnology, some nanomaterials have been further developed to possess photothermal capabilities to kill cancer cells7. However, like the photodynamic strategy, they face the challenge of tissue penetration of external light. Recently, the development of nanozymes NPs has received attention for their catalytic properties, generating free radicals to kill cancer cells through delicate design8. Overall, these nanomedicinal particles all have their inherent limitations such as the need for specific drug design, NPs toxicity, light penetration issues, and delicate design and preparation of nanomaterials.
Shewanella oneidensis MR-19, renowned for its remarkable extracellular electron transfer prowess, efficiently channels metabolic electrons to external acceptors9,10. The outer membrane of this microorganism hosts pivotal proteins like MtrA, MtrB, and MtrC, instrumental in ferrying electrons to its surface. MtrC and OmcA proteins on the bacterial surface further aid in electron transfer to surrounding metal ions11,12,13,14. Our innovative method, liposome fusion-induced membrane exchange (LIME), allows for the extraction of electroactive membranes from these bacteria, yielding membrane-integrated liposomes (MIL) abundant in c-type cytochromes but free of intact bacteria15. Recent our investigations revealed that these MIL structures, when coupled with TiO2 NPs, generate electrons upon X-ray exposure, initiating superoxide production via the electroactive membrane15. This underscores the vital role of membranes in triggering outward electron transport, underscoring their significance in orchestrating electron transport mechanisms and signaling their importance in cellular electron dynamics. Moreover, these electroactive membranes exhibit bidirectional electron transfer capabilities, as evidenced by various studies elucidating the inward movement of electrons across biological membranes16,17,18. The interplay between inward and outward electron movements across biological membranes adds complexity to electron transfer processes, showcasing the versatility of membranes in cellular electron transport.
Au NPs have demonstrated promise as electron sinks19,20,21,22,23. In the electroactive membrane of Shewanella oneidensis MR-1, cytochrome, a protein with a redox potential of −0.3 eV24, plays a pivotal role. This potential is more negative than the Fermi level of Au, typically ranging from +0.35 to +0.45 eV compared to the Normal Hydrogen Electrode (NHE)25. Consequently, facilitated by cytochrome, we propose that extracellular electrons can potentially flow to Au, indicating Au’s potential as an electron sink within specific electron transfer pathways. Ten heme groups construct an electron channel within cytochromes that allow efficient electron hopping and transferring through the redox cycle of Fe2+ and Fe3+ between these hemes, thus endowing an idiographic electroactive feature.
In this work, we provide an additional platform to address the limitations in cancer therapy by using common gold (Au) NPs as electron sinkers, combined with electroactive membrane. This enables the autonomous transfer of electrons from cancer cells to Au NPs, thereby disrupting the redox processes within cancer cells and causing their death. We found that this simple design and strategy can be applied experimentally to induce the death of various types of cancer cells. Our goal is to disrupt the redox equilibrium within cancer cells, capitalizing on their highly active redox systems. By utilizing Au@MIL NPs, we aim to redirect electron transfer pathways within these cancer cells (i.e. directing electron transfer from cancer cells to Au NPs), disrupting their redox balance and undermining their survival mechanisms (Fig. 1a). Notably, a size effect is observed in Au NPs in this study, with larger size demonstrating greater efficacy in eradicating cancer cells. Significant observation shows that Au@MIL effectively induces cellular damage and death in hypoxic condition, which typically confers treatment resistance. Oxidative stress induced by Au@MIL treatments, including lipid oxidation in mitochondrial and endoplasmic reticulum, mitochondrial membrane potential changes, and ROS generation, has been evaluated. Lipid peroxidation, indicative of the oxidative breakdown of lipids, serves as a hallmark of ferroptosis. Rather than relying on iron, disrupting the redox balance of cancer cells with Au@MIL induces cell death, accompanied by notable lipid peroxidation. The alteration in mitochondrial membrane potential, suggestive of mitochondrial depolarization, indicates the initiation of apoptosis in the cells. Interestingly, no ROS is detected during apoptosis. Notably, the introduction of a SiO2 insulator between Au NPs and MIL directly blocks electron transfer from cancer cells, preventing the death of cancer cells. The manipulation of electron transfer pathways, particularly within cancer cells boasting hyperactive redox systems, presents a promising avenue for targeted therapies. Disrupting this delicate redox equilibrium holds potential for impeding the adaptive and growth mechanisms of cancer cells, offering a pathway for refined and effective therapeutic strategies against cancer.
a The schematic illustration demonstrates how Au@MIL induces cell damage by redirecting electron transfer to Au NPs, thereby disrupting the redox equilibrium within cancer cells. b High-resolution TEM images of Au NPs, MILs, and Au@MIL NPs (with the red arrow indicating the MIL membrane area) were captured. The lattice spacing of the (200) plane is shown in Au@MIL NPs, and the electron diffraction pattern confirms a crystalline structure of Au@MIL. c Energy-dispersive X-ray spectroscopy (EDS) line scanning and d EDS mapping along the Au@MIL NPs reveal P Kα1 signal from the phosphorus element in MIL distributed on Au@MIL NPs. e The Fourier-transform infrared (FTIR) spectra of Au NPs, MILs, and Au@MIL NPs were analyzed. f SDS-PAGE results of S. oneidensis MR-1, MILs, Au@MIL NPs, Au NPs, and Au@PEG NPs display protein bands stained with Coomassie Brilliant Blue (CBB) for total protein detection and TMBZ for haem-based protein detection. g The fluorescence spectra of the membrane-specific dye FM 4-64 with maximum fluorescence at 680 nm confirm the presence of the lipid membrane on the surface of the Au NPs. b, d, f Each experiment was repeated three times with similar results. Source data are provided as a Source Data file.
Results
Synthesis and Characterization of Au@MIL NPs
Au NPs with an average size of 70 nm were synthesized using a modified Turkevich method26 Fig. 1b. Following our developed technique termed LIME, vesicular structures known as membrane-integrated-liposomes (MILs) were efficiently produced from Shewanella oneidensis MR-1 Fig. 1b. During the sonication-cooling process, the MILs deconstructed and subsequently self-assembled onto the surface of the Au NPs, forming MIL-coated Au NPs Au@MIL NPs; Fig. 1b. High-resolution TEM (HRTEM) images clearly show the MIL membrane adhering to the surface of the Au NPs, with a thickness of approximately 5 nm, closely correlating with that of a single-layer cell membrane27. The lattice spacing of the (200) plane in the face-centered cubic (FCC) structure of Au was observed to be 2 Å (ICDD PDF card no. 00-004-0784). Additionally, the electron diffraction pattern indicates a crystalline structure of Au@MIL, corresponding to the FCC structure of Au with (111) and (200) planes, suggesting no structural change after membrane coating.
To optimize the MIL coating on the Au NPs, different ratios of MIL to Au were used during the sonication-cooling process. The resulting Au@MIL NPs were then analyzed for hydrodynamic diameter and zeta potential. The hydrodynamic diameter increases gradually with higher MIL/Au ratios, reaching a maximum value at a ratio of 10 Supplementary Fig. 1. Conversely, the zeta potential values decrease as the MIL/Au ratio increased, reaching −13.1 mV, which is close to the value of MIL alone. This indicates complete coverage of the MIL on the Au NPs at a ratio of 10 Supplementary Fig. 2. Based on both hydrodynamic diameter and zeta potential analyses, a MIL/Au ratio of 10 is determined to be optimal for fabricating Au@MIL NPs.
Figure 1c, d presents the energy dispersive spectroscopy (EDS) signal of the phosphorus element around the Au NPs, attributed to the phosphate lipid component in MIL, confirming the presence of the Au core and MIL shell. Furthermore, FT-IR measurements provide additional evidence of MIL on the Au NPs Fig. 1e. For MILs, characteristic peaks were detected in the regions of 690–1200 cm−1, 1500–1700 cm−1, and 2850–3300 cm−1, corresponding to the phospholipid components, amino acids from membrane proteins, and the hydrocarbon skeleton and hydroxyl groups in lipids, respectively28,29. These findings indicate the successful integration of MIL onto the Au NPs.
Characterization of membrane on the Au@MIL NPs
Characterizing the membrane proteins on the Au@MIL NPs is crucial to ensure the functionality of cytochromes in facilitating electron transfer from cancer cells to Au NPs. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was used to separate proteins by molecular weight, followed by staining to identify the proteins on the gel, providing evidence of proteins on the Au@MIL NPs. As expected, both MILs and Au@MIL NPs stained with Coomassie Brilliant Blue (CBB) reveal the presence of membrane proteins extracted from S. oneidensis MR-1 Fig. 1f. Heme staining further identified representative heme-based cytochromes, including MtrA, MtrC, and OmcA, from S. oneidensis MR-1, MILs, and Au@MIL NPs Fig. 1f. This analysis confirms the successful coating of MIL on Au NPs without protein loss. No proteins were detected in the Au NPs alone or in Au@PEG NPs. Polyethylene glycol thiol (mPEG-SH, MW = 3400) was used to modify the Au NPs as a negative control for comparison.
The concentration of total proteins on Au@MIL NPs was further quantified using a standard protein assay Supplementary Fig. 3. The protein amounts on Au@MIL NPs increase gradually as a function of the MIL/Au ratio, with an optimal ratio of 10 achieving the maximum cytochrome content. The presence of cytochromes in MILs, constructed from heme proteins, allows the concentration of heme to serve as an index for determining the cytochrome content. Due to the specific structure of heme, which contains a porphyrin ring chelating an Fe center, heme concentration can be determined by measuring Fe concentration. Fe concentration in Au@MIL NPs was measured using ICP-OES (iCAP™ 7400). In a 3.5 mL solution containing 274 ppm Au in Au@MIL NPs, the Fe concentration was found to be 0.065 ppm. Consequently, the mass of Fe, the moles of Fe, and the number of Fe atoms were calculated to be 2.27 × 10−7 g, 4.07 × 10−9 moles, and 2.45 × 1015 atoms, respectively. Given that MtrC, MtrA, and OmcA are part of the decaheme cytochromes, the maximum amount of decaheme cytochrome was determined to be 2.55 × 1014 per mg of NPs. The density of Au NPs was estimated to be approximately 19.32 g/cm330, assuming the volume of Au NPs is close to that of a sphere with a 70 nm diameter. The mass and number of Au NPs were calculated to be 3.47 × 10−15 g and 2.88 × 1011 NPs per mL, respectively, corresponding to 887 decaheme cytochromes per Au NP. To provide additional evidence for the presence of the membrane on the NPs, a membrane-specific fluorescent dye, FM 4-64, which emits at 680 nm, was utilized Fig. 1g. Fluorescence signals were detected in both MILs and Au@MIL NPs, indicating the presence of the membrane. No fluorescence emission was observed in the bare Au NPs.
In vitro cytotoxicity evaluation using 70 nm sized Au in Au@MIL NPs
To investigate the anti-cancer efficacy of Au@MIL, thirteen different cell lines were selected for testing. The study includes two normal cell lines and eleven cancer cell lines, utilizing the LIVE/DEAD cell imaging kit assay to determine the cytotoxic effects of the 70 nm Au@MIL NPs Supplementary Fig. 4. Live and dead cells were stained with calcein AM and ethidium homodimer-1 (EthD-1), respectively, followed by treatment with 70 nm Au@MIL NPs for imaging evaluation. After 72 h of incubation, no cell death signal was observed in the normal cell lines (NeHepLxHT, M10). In contrast, substantial damage was observed in the cancer cell lines post-treatment with Au@MIL NPs after the same incubation period. These results indicate a significant reduction in the viability of cancer cells incubated with 70 nm Au@MIL NPs, suggesting that Au@MIL can be broadly applied across different types of cancer.
Having demonstrated the cytotoxic effect of 70 nm Au@MIL NPs on various cancerous cells, we selected five cell lines for more detailed investigation: two normal cell lines (NeHepLxHT and M10) and three cancerous cell lines (Hep G2, HA22T, and MDA-MB-231) Fig. 2a. To clarify the role of MIL in targeting cancerous cells, we prepared Au NPs with different coatings: Au@PEG, Au@Lipo (liposome), and Au@E. coli (Escherichia coli membrane) for comparison with Au@MIL regarding therapeutic efficacy. The membrane of E. coli lacks the necessary proteins to facilitate electron transfer, making it a relevant control. We followed the standardized LIME method to extract the membrane from E. coli, forming Au@E. coli Supplementary Figs. 5–8. Protein-free liposome-coated Au NPs (Au@Lipo) were also prepared for evaluation Supplementary Figs. 9–11. Notably, no cytotoxic effect was observed in either cancerous or normal cells treated with Au@PEG, Au@Lipo, or Au@E. coli.
a A variety of cancer cell lines, including Hep G2 hepatoma cells, HA22T hepatoma cells, and MDA-MB-231 triple-negative breast cancer cells, along with the normal cell lines NeHepLxHT hepatocytes and M10 breast epithelial cells, were chosen for detailed cytotoxicity studies. Representative images illustrate the distribution of live and dead cells in NeHepLxHT, M10, Hep G2, HA22T, and MDA-MB-231 cells following a 72-h treatment with 70 nm Au@PEG, Au@Lipo, Au@E. coli, and Au@MIL NPs at a concentration of 300 ppm Au. Nuclei, live cells, and dead cells were stained with Hoechst 33342 (blue), calcein AM (green), and EthD-1 (red), respectively. These images are representative of three independent experiments. Scale bars, 100 μm. b Colony assay of 70 nm Au-based NPs in NeHepLxHT, M10, Hep G2, HA22T, and MDA-MB-231 cells (n = 3, a repeated experiment). Cells were incubated with 300 ppm of Au-based NPs for 72 h and maintained without them for another 14 days. The formation of clones was fixed and stained with 0.5% crystal violet, and the results were measured using ImageJ software. Data were presented as the mean ± SD, and p-values were calculated using one-way ANOVA. Source data are provided as a Source Data file.
The colony assay quantified the cytotoxicity of 70 nm Au@MIL NPs across the five selected cell lines. Specifically, there is approximately 74% inhibition in Hep G2 cells, around 29% inhibition in HA22T cells, and about 70% inhibition in MDA-MB-231 cells. In contrast, no cytotoxicity was observed in the normal cell lines NeHepLxHT and M10 Fig. 2b. Control groups containing Au@PEG, Au@Lipo, and Au@E. coli showed no toxicity to either cancerous or normal cell lines. These findings underscore the critical role of electron transfer facilitated by MILs, which is essential for inducing cytotoxic effects, specifically in cancer cells.
Size-dependent efficacy of Au@MIL NPs in cancer cell eradication
The discovery that 70 nm sized Au NPs can kill cancer cells has sparked interest from a nanomaterials perspective in understanding whether this effect is size-dependent. To explore this, smaller (15 nm) and larger (100 nm) Au NPs were used to study their efficacy in eradicating cancer cells compared to 70 nm size in forming Au@MIL NPs. Initially, the effect of NP size was examined using Hep G2 cells to evaluate their electron attraction capacity. Live and dead cell observations illustrated in Fig. 3a show Hep G2 cell viability when treated with 70 nm and 100 nm sizes at 300 ppm for 24, 48, and 72 h. A size-dependent behavior in cell viability was observed, with 100 nm Au@MIL NPs inducing greater Hep G2 cell death compared to 70 nm NPs at the same concentration. Thus, further studies involve treating two normal cell lines (NeHepLxHT, M10) and three cancer cell lines (Hep G2, HA22T, and MDA-MB-231) with 100 nm Au@MIL NPs at 300 ppm for 48 h, as depicted in Fig. 3b. Once again, no cell death was observed in the normal cell lines, while significant cell damage was seen in the cancer cell lines post-treatment. The colony assay further demonstrated significant anti-cancer cytotoxicity of 100 nm Au@MIL NPs across cancer cell lines, with approximately 96% inhibition in Hep G2 cells, 68% inhibition in HA22T cells, and 85% inhibition in MDA-MB-231 cells Fig. 3c. Notably, the above-mentioned anti-cancer cytotoxicity was weakened in the 15 nm-sized groups, as no cell death was observed Supplementary Figs. 12, 13. These results highlight a significant size-dependent anti-cancer effect of Au@MIL NPs.
a The size and incubation-dependent curve of Au@MIL NPs illustrates the impact of NP size on enhancing the cytotoxic effect against Hep G2 cells over 24, 48, and 72-h intervals (n = 3 replicates). Data were presented as the mean ± SEM, and the p-values were calculated using the two-tailed student’s t-test. b Additional cytotoxicity studies were performed by treating two normal cell lines (NeHepLxHT, M10) and three cancer cell lines (Hep G2, HA22T, and MDA-MB-231) with 100 nm Au@MIL NPs at 300 ppm for 48 h. Nuclei, live cells, and dead cells were stained with Hoechst 33342 (blue), calcein AM (green), and EthD-1 (red), respectively. These images are representative of three independent experiments. Scale bars, 100 μm. c Colony assay of 100 nm Au@MIL in NeHepLxHT, M10, Hep G2, HA22T, and MDA-MB-231 cells (n = 3, a repeated experiment). Cells were incubated with 300 ppm of Au@MIL for 72 h and maintained without them for another 14 days. The formation of clones was fixed and stained with 0.5% crystal violet, and the results were measured using ImageJ software. Data were presented as the mean ± SD, and the p-values were calculated using the two-tailed student’s t-test. d The Venn diagram of the intersection of differentially expressed genes (Benjamini-Hochberg correction, padj ≦0.05) and GO enrichment analysis in Hep G2 and MDA-MB-231 cells. Cells were treated with 300 ppm of 100 nm Au@MIL for 24 h (N) or left untreated (C). mRNA samples were then isolated and analyzed by Genomics BioSci & Tech. Co. Ltd. e Heatmap of the top 10 differentially expressed genes involved in the mitochondrial transition process in HepG2 and MDA-MB-231 cells. The * symbol denoted intersecting genes. f Heatmap of the top 10 differentially expressed genes involved in the cellular response to oxidative stress process in HepG2 and MDA-MB-231 cells. The * symbol denoted intersecting genes. Source data are provided as a Source Data file.
Prior to further in vitro examination, we conducted RNA sequencing analysis on Hep G2 and MDA-MB-231 cells treated with 100 nm Au@MIL NPs (N), chosen based on greater anti-cancer efficacy, for 24 h, comparing them to untreated control groups (C) to comprehensively explore regulatory mechanisms. As depicted in Fig. 3d, we identified 8230 significantly differentially expressed genes in the Hep G2 groups (H_C vs. H_N) and 6100 in the MDA-MB-231 groups (M_C vs. M_N), with an overlap of 5032 genes. Gene Ontology (GO) enrichment analysis reveals that these intersected genes are involved in various biological processes, including DNA repair, mitochondrial transition, cell cycle regulation, oxidative stress response, and apoptotic processes. They are associated with cellular components such as the cytosol, mitochondrion, nucleus, cytoplasm, and membrane, and are linked to molecular functions such as RNA, ATP, DNA, protein, and metal ion binding. Given the importance of electron transfer from cancer cells to Au NPs, as indicated by the enrichment of the Gene Set Enrichment Analysis (GSEA) term of electron transfer activity in the Au@MIL-treated groups Supplementary Fig. 14, this process appears to influence the signal transduction pathways in cancer cells comprehensively. Here, we particularly focus on mitochondrial function and oxidative stress, as we have identified several regulatory genes involved in these processes, including MRPS18A, MRPL28, MRPL37, MRPL42, GPX1, SLC7A11, and SLC25A24 Fig. 3e, f, which have been recently implicated in tumor progression and cell death regulation31,32,33,34.
Au@MIL NPs-induced mitochondrial dysfunction
Mitochondria are crucial organelles that regulate cellular energy balance, oxidative stress responses, and cell survival. To investigate the mechanism of cell death induced by Au@MIL NPs, we used the dual-emissive fluorescent probe JC-1 to assess mitochondrial membrane potential. High membrane potential leads to red fluorescence due to mitochondrial J-aggregates formation, while low potential results in green fluorescence from cytosolic J-monomers. Mitochondrial dysfunction, indicative of decreased membrane potential, was assessed by a decreased ratio of red-to-green fluorescence intensity. In both Hep G2 and MDA-MB-231 cancer cells Fig. 4a, b, the Au@MIL-treated groups exhibited a lower red-to-green fluorescence ratio compared to the control, indicating increased mitochondrial depolarization. Furthermore, mitochondrial depolarization intensified with longer exposure times (24, 48, and 72 h), underscoring a time-dependent effect of Au@MIL NPs. Additionally, ATP-Red 1, a mitochondrial ATP fluorescence probe, was used to investigate ATP depletion induced by Au@MIL NPs. Our results demonstrate a significant reduction in ATP synthesis in the Au@MIL-treated groups compared to controls Fig. 4c. Moreover, the XF Cell Mito Stress Test indicates defects in mitochondrial function in Hep G2 cells treated with Au@MIL for 24 h. These defects include impairments in basal respiration, ATP production, and maximal respiration Fig. 4d, e. Therefore, our findings highlight that Au@MIL NPs lead to diminished ATP production and loss of mitochondrial membrane potential.
a, b Analysis of mitochondrial membrane potential in Hep G2 and MDA-MB-231 cells (average of three random fields) treated with 100 nm Au@MIL NPs for 24, 48, and 72 h using JC-1 staining. Enlarged cells (white arrows) show JC-1 fluorescence localized in the cytoplasm. Mitochondrial depolarization increased over time, as indicated by the rising green signal. c Fluorescence imaging of mitochondrial ATP fluctuations with 100 nm Au@MIL NPs. Mitochondrial ATP production was detected using ATP Red-1 staining. d, e Oxygen consumption rate (OCR) analysis in Hep G2 cells using the Seahorse XFe24 Extracellular Flux Analyzer (n = 3). Cells were treated with 100 nm Au@MIL NPs (300 ppm) for 24 h, followed by the XF Cell Mito Stress Test with oligomycin, FCCP, rotenone, and antimycin A (2 µM each). Basal respiration, ATP production, and maximal respiration were measured. f Lipid peroxidation analysis in Hep G2 cells treated with 100 nm Au@MIL NPs for 24 and 48 h using C11-BODIPY 581/591 staining. Ferroptocide was used as a positive control. Confocal imaging was performed for analysis, and quantification of lipid peroxidation is provided (n = 40 cells). g Distribution of lipid peroxidation in Hep G2 cells treated with 100 nm Au@MIL NPs for 24 h. Cells expressing mitochondrial Cox8-mRFP or endoplasmic reticulum ERp57-RFP were stained with C11-BODIPY 581/591 before imaging with a confocal microscope. Scale bars: 5 μm. h Evaluation of intracellular ROS generation by Au@MIL NPs. Fluorescence images of Hep G2 cells show intracellular ROS levels following treatment with TBHP, Au@MIL, and Au@E. coli at Au concentration of 300 ppm for 48 h. a, b, c, h Scale bars: 50 μm. f Scale bar: 20 μm. c, g are representative of three independent experiments, while a, b, d, e, f were repeated three times with similar results. a, b, d, e Data are presented as mean ± SD, while c, f are shown as mean ± SEM. Statistical analysis was conducted using a two-tailed Student’s t-test. Source data are provided as a Source Data file.
Au@MIL NPs-induced lipid peroxidation
Lipid peroxidation, a critical marker of ferroptosis, involves the oxidative degradation of lipids due to disruption in cellular redox balance. To detect lipid peroxidation, the lipophilic fluorescent probe BODIPY™ 581/591 C11 was utilized, which shifts its fluorescence emission from red (590 nm) to green (510 nm) upon oxidation. As a positive control for inducing rapid ferroptotic cell death, Ferroptocide was employed. Confocal microscopy images of Hep G2 cells reveals an increase in green fluorescence following treatment with Au@MIL NPs, indicating heightened lipid peroxidation compared to untreated cells Fig. 4f. To further elucidate the localization of lipid peroxidation, mitochondrial Cox8-mRFP and endoplasmic reticulum ERp57-RFP markers were employed in conjunction with BODIPY™ 581/591 C11 staining. Our findings confirm that Au@MIL NPs induced lipid peroxidation in both mitochondria and the endoplasmic reticulum Fig. 4g.
Absence of intracellular ROS by Au@MIL NPs
Intracellular ROS production induced by Au@MIL NPs was assessed using the ROS probe 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA), which emits green fluorescence upon oxidation. Tert-butyl hydroperoxide (tBHP) served as a positive control for inducing oxidative stress. However, as shown in Fig. 4h, no ROS production was observed in the control, Au@MIL, and Au@E. coli groups. This indicates that Au@MIL NPs do not induce the production of intracellular ROS, specifically hydrogen peroxide (H2O2), hydroxyl radicals (•OH), peroxyl radicals (ROO•), or peroxynitrite (ONOO–). Overall, the cytotoxicity of Au@MIL NPs in cancer cells is primarily attributed to lipid peroxidation rather than ROS production.
Ferroptosis is a type of programmed cell death characterized by the accumulation of lipid peroxides. It occurs when reactive oxygen species (ROS), such as hydroxyl radicals (•OH), extract electrons from lipid molecules in the cell membrane. This process, known as lipid peroxidation, leads to the disruption of membrane integrity and, ultimately cell death. Unlike other forms of cell death, ferroptosis is specifically driven by the iron-dependent accumulation of lipid ROS, making it distinct and significant in various physiological and pathological contexts. In our study, Au@MIL NPs appear to induce lipid peroxidation through a mechanism that does not involve the production of traditional ROS intermediates. Instead, Au@MIL NPs directly extract electrons from lipid molecules. This suggests that Au@MIL NPs facilitate lipid oxidation and potentially ferroptosis through an alternative pathway that bypasses the generation of conventional ROS. The unique behavior of Au@MIL NPs in directly extracting electrons from lipids highlights its distinct mode of action, which could offer advantages in controlling oxidative stress and cell death in specific applications where ROS production is undesirable or needs to be minimized.
Examination of stability for Au@MIL NPs
The hemolytic property of Au@MIL NPs in defibrinated sheep blood was evaluated to ensure their safe circulation in blood vessels Supplementary Fig. 15. Au@PEG NPs served as a control reference. No hemolysis of erythrocytes was observed, indicating the feasibility and safety of administering Au@MIL NPs in mice. Next, the stability of Au@MIL NPs was examined. It was found that Au@MIL NPs can be safely stored in water before further in vitro and in vivo investigations. No apparent aggregation-induced precipitation or morphological changes were observed after incubation at 4 °C or 25 °C for 7 days, indicating satisfactory stability Supplementary Fig. 16a. Supplementary Fig. 16b shows intact Au@MIL NPs at 4 °C and 25 °C before and after 7 days (red arrows indicating the presence of MILs). Furthermore, the anti-cancer efficacy of Au@MIL remained evident at 4 °C and 25 °C after 7 days of storage, demonstrating sustained performance and potential for further applications Supplementary Fig. 16c.
Tumor regression in orthotopic xenograft mice
To demonstrate the efficacy of Au@MIL for in vivo anti-tumor, we prepared Hep G2-Red-FLuc orthotopic hepatocellular carcinoma (HCC) xenograft mice and administered a single dose of Au@MIL, Au@PEG, and sterilized PBS via intravenous injection. Biodistribution analysis reveals apparent accumulation of Au@MIL in the spleen, liver, and feces, indicating typical metabolic processes through hepatobiliary clearance Fig. 5a. Notably, substantial NPs accumulation within the orthotopic HCC tumor site was observed in a time-dependent manner, peaking at 24 h post-treatment, likely due to the enhanced permeability and retention effect. In anti-tumor experiments, the Au@MIL group showed significant tumor regression compared to PBS and Au@PEG treatments, as evidenced by an IVIS luminance signal ratio of less than 1 (compared to Day 0, with no therapy) over the 14-day post-treatment period Fig. 5b, c. No significant changes in body weight were observed among the groups Supplementary Fig. 17. After the therapeutic mice were sacrificed on Day 14 post-treatment, ex vivo IVIS detection and histological analysis confirmed the lowest luminance signal and the smallest tumor area of HCC in the Au@MIL group Fig. 5d, e. As expected, we observed abundant expression of cell death and lipid peroxidation markers within the cytoplasm of Au@MIL-treated HCC cells in tumor sections Fig. 5f. Importantly, a single dose of Au@MIL significantly prolonged the survival rate of Hep G2-Red-FLuc HCC xenograft mice, with no deaths observed up to Day 50 post-treatment Fig. 5g. Finally, we validated the in vivo biosafety of Au@MIL in immunocompetent C57BL/6 mice, finding no impact on murine body weight, serum/urine biochemical indices, or histological features of normal organs Supplementary Fig. 18a–d. These data suggest that Au@MIL has potential as a therapeutic strategy for HCC, leveraging electron transfer from cancer cells to Au NPs to combat tumors.
HepG2-Red-FLuc orthotopically injected xenograft mice (8–10 weeks, male) were administered with a single dose of sterilized PBS (100 µL), 3000 ppm of 100 nm Au@PEG (100 µL sterilized PBS), and 3000 ppm of 100 nm Au@MIL (100 µL sterilized PBS) via intravenous injection. a ICP-MS assessed the biodistribution of Au@MIL at different post-treatment time points (1, 4, 8, 24, and 72 h) in the following samples: heart, lung, spleen, kidney, liver, HCC tumor, and feces collected from treated mice (n = 3 replicates). b, c Orthotopic tumor growth of HepG2-Red-FLuc cells in xenograft mice treated with PBS, Au@PEG, and Au@MIL monitored by IVIS system (n = 5). The luminance signal was measured as the total flux ratio on post-treatment Day 0, 4, 7, 11, and 14 compared to Day 0. d Ex vivo luminance signals (total flux) of HepG2-Red-FLuc cells in the liver, collected from mice treated with PBS, Au@PEG, and Au@MIL after 14 days, were monitored using the IVIS system (n = 5). e The appearance of livers with HCC from mice treated with PBS, Au@PEG, and Au@MIL was assessed at Day 14 post-treatment (n = 5). The yellow dashed circle indicates the visible tumor area. f Morphology of HCC in liver sections collected from mice treated with PBS, Au@PEG, and Au@MIL after 14 days was assessed by hematoxylin and eosin staining (Scale bar, 1 mm). The yellow dashed circle indicated the tumor areas, and the expression of cleaved caspase-3 (Asp175) and 4-Hydroxynonenal within them was detected by IHC staining (Scale bar, 200 µm). The results were repeated at least three times independently with similar tendencies, and one representative experiment is shown. g The survival rate of HepG2-Red-FLuc HCC xenograft mice treated with PBS, Au@PEG, and Au@MIL (n = 5) was assessed, and p-values were calculated using the log-rank test. a Data are presented as mean ± SEM, with p-values calculated using a two-tailed Student’s t-test. b, c, d Data are presented as mean ± SD, with p-values calculated using One-way ANOVA. Source data are provided as a Source Data file.
Discussion
The presentation of cellular uptake results at 100 nm indicates a lack of clear trend regarding the efficacy of Au@MIL NPs in cancer cell eradication Fig. 6a. While some cancer cells display higher uptake levels, others exhibit lower uptake. For example, Au@MIL NPs are taken up 2.5 times more by Hep G2 cells compared to MDA-MB-231 cells and the colony assay survival rates are at 4% for Hep G2 cells and 15% for MDA-MB-231 cells. In contrast, while the cellular uptake by HA22T cells is 1.6 times that of MDA-MB-231 cells, the survival rates are at 32% for HA22T cells and 15% for MDA-MB-231 cells. This discrepancy implies an inconsistency between the uptake of Au@MIL NPs and their efficacy in killing cancer cells.
a The cellular uptake of Au@MIL NPs was assessed in two normal cell lines and three cancer cell lines treated with 100 nm Au@MIL NPs for 48 h. (n = 3 replicates). Data were presented as the mean ± SEM. Statistical analysis was conducted using a two-tailed Student’s t-test. b A schematic illustration depicting the transfer of electrons to Au NPs through the MIL. The redox potentials of Au NPs and MIL versus NHE are also indicated. c To assess the role of oxidative stress in cell death, fluorescence microscopy was used to evaluate live and dead staining in two normal and three cancer cell lines following antioxidant (Vitamin E, Vit E) treatment. Cells were treated with 100 nm Au@MIL NPs (300 ppm Au) for 48 h, with 2.5 μM Vit E added every 24 h in the Vit E and Au@MIL + Vit E groups. d The UV-visible spectra of Au NPs, Au@MIL NPs, and Au@MIL NPs after 72 h of incubation with cells were analyzed. The corresponding SPR and full width at half maximum (FWHM) bandwidth values are provided in the table below. e The XANES spectra at the Au L3-edge were obtained for Au NPs, Au@MIL NPs, Au@MIL NPs after 72 h of cell incubation, and Au foil. f TEM images of 100 nm Au@SiO2 NPs and Au@SiO2@MIL NPs. Dark spheres indicate Au NPs, the gray layer represents SiO2, and the outer transparent layer is MIL. Fluorescence images show live/dead cell distribution in Hep G2 cells after 48 h treatment with 70 nm and 100 nm Au@SiO2@MIL (300 ppm Au). g Cytotoxicity of 100 nm Au@MIL NPs under hypoxic conditions (1% O2). Fluorescence images of Hep G2 cells after 48 h of incubation with 100 nm Au@MIL NPs show the distribution of live and dead cells. (n = 3 replicates). c, f The images are representative of three independent experiments. c, f, g Scale bars: 100 μm. Source data are provided as a Source Data file.
The enhanced effectiveness of 100 nm Au NPs in killing cancer cells compared to 70 and 15 nm NPs may be attributed to their surface area and the number of cytochromes on their surface. While larger Au NPs have a smaller surface area per particle, their total surface area can be relatively large, especially at higher concentrations. Consequently, larger particles can accommodate more charge when combined with MIL. For the additional evidence, the number of cytochromes in MIL coating the surface of 100 nm Au NPs is 3.4 times that of 70 nm particles, allowing for more efficient electron transfer. Cytochromes embedded in the outer membrane of Shewanella oneidensis MR-1 play the most crucial role in facilitating the extracellular electron transfer process. These factors likely result in more electrons being transferred to the larger 100 nm NPs, thereby more effectively disrupting the redox balance of cancer cells.
For the argument for the transfer of electrons from cancer cells to Au NPs through the membrane, cytochrome with a redox potential of −0.3 eV24 plays a crucial role. This potential is more negative than the Fermi level of gold (Au), ranging from +0.35 to +0.45 eV compared to the Normal Hydrogen Electrode (NHE)25 Fig. 6b. Consequently, extracellular electrons can potentially flow to Au through cytochrome. Therefore, we conducted the following experiments to demonstrate that the death of cancer cells involves the electron transfer from cancer cells to Au NPs.
The oxidation-reduction (redox) process is essential for cellular activities, but insufficient antioxidant capacity can lead to oxidative stress and damage. Au@MIL NPs act as electron shuttles, extracting electrons from lipids and causing lipid peroxidation. Antioxidants like vitamin E counteract this by donating electrons35, stabilizing the redox environment and preventing lipid oxidation. In our study, vitamin E effectively reduced the redox damage caused by 100 nm Au@MIL NPs in cancer cells Fig. 6c, highlighting its role in mitigating oxidative stress and influencing the therapeutic efficacy of Au@MIL NPs.
Additional evidence is the observed a notable phenomenon wherein there is blue shift and narrowing of the bandwidth of the Au surface plasmon band (SPR) for Au@MIL after incubation with cancer cells. The SPR of noble metallic NPs is highly sensitive to the charge density accumulated on the nanostructures. Theoretical calculations and experimental observation36,37,38 suggest that the plasmonic frequency is directly correlated with the electron density within the NP. An increase in the concentration of free electrons leads to a blue shift in the plasmon resonance wavelength. Excess electrons on the surface of an Au NP can modify the collective oscillation of electrons (plasmons), impacting the absorption or scattering of light and causing the resonance wavelength to shift towards the blue end of the spectrum. Specifically, in the case of 100 nm Au, Au@MIL incubated with cells for 72 h clearly displayed a blue shift to 559 nm in the SPR, whereas both Au and Au@MIL without cell incubation exhibited identical surface plasmon wavelength (566 nm) towards the red end of the spectrum (Fig. 6d). Additionally, we found that the bandwidth of the SPR is inversely proportional to the electron density38. As electron concentration increases, the bandwidth decreases. Indeed, we observed the bandwidth narrow down from 86 nm to 52 nm in Au@MIL.
The XANES technique is very sensitive to small changes in Au 5 d counts, especially the first spike at the beginning of the edge jump (also known as the white line). Figure 6e shows the normalized Au L3-edge XANES of the three NPs (Au NPs, Au@MIL, and Au@MIL after cancer cells incubation) and Au metal (bulk). The intensity systematic of the resonance at the threshold (white line) associated with a 2p3/2 to 5 d5/2;3/2 dipole transition probing the unoccupied densities of d states at the Fermi level. In principle, the Au 5 d orbital is fully filled, but due to the s-d hybridization, there will still be some vacancies, resulting in the intensity of the white line of Au metal. As Au is nanosized, the intensity of the white line will also increase39. When Au NPs are coated with an organic membrane, charge redistribution will also occur, thereby affecting the intensity of the white line40,41. When Au@MIL interacts with cells, we observe a significant decrease in white line intensity, which means that the electron orbitals of Au 5d are filled with more electrons. In other words, more electrons are transferred from the cells to the Au NPs.
Finally, to directly demonstrate the electron flow into Au NPs, an insulating SiO2 layer (6 nm) was introduced between Au NPs and MIL. Remarkably, neither in vitro cancer cell death nor in vivo tumor inhibition was observed when using SiO2 as an insulator Fig. 6f and Supplementary Fig. 19, strongly indicating that the electron transfer from cancer cells to Au NPs is a critical mechanism underlying their anti-cancer activity. Previous studies have suggested a thickness for blocking electron tunneling effect around 2–3 nm42,43.
The last but not least, in cancer treatment, it is known that tumor hypoxia can lead to increased resistance of cancer cells, reducing the effectiveness of treatments. Hypoxic conditions induce genetic and metabolic changes in tumor cells, making them more adaptable to harsh environments, thereby increasing the complexity and failure rate of treatments. Therefore, effective strategies targeting tumor hypoxia are crucial for improving the success rate of cancer therapies. Thus, we tested cancer cells in hypoxic condition because the entire research hinges on the electron transfer from cancer cells to Au NPs, disrupting the cancer cell redox balance. As expected, the significant results show that Au@MIL still effectively caused damage to cancer cells under hypoxic condition Fig. 6g. The fact that Au@MIL can still effectively target this imbalance under hypoxia underscores its robustness and specificity in targeting cancer cell vulnerabilities. However, it is worth mentioning that the overall lethality of cancer cells under hypoxic condition is not as high as under non-hypoxic conditions, with a lethality ratio of 1:2 based on live & dead observation. The lower lethality of hypoxia cancer cells when treated with Au@MIL NPs is likely due to the differences in their redox balance, metabolic adaptations, activation of survival pathways, and microenvironmental factors. These differences enable hypoxia cancer cells to better withstand the oxidative stress induced by the Au@MIL NPs, resulting in a lower death rate. We have further observed that mitochondrial depolarization and the reduction of ATP production are less pronounced under hypoxic conditions compared to normoxic conditions Supplementary Figs. 20, 21, highlighting a phenomenon worthy of further investigation.
Our study introduces a therapeutic approach using Au@MIL NPs to disrupt the redox equilibrium within cancer cells, effectively inducing their death. By harnessing the electron transfer capabilities of Shewanella oneidensis MR-1 derived membranes, these NPs induce oxidative stress and apoptosis without generating reactive oxygen species. Ultimately, the Au@MIL NPs offer a promising and versatile platform for developing refined and effective cancer therapies.
Methods
Materials
All reagents were of analytical grade and used without additional purification. 3,3,5,5-tetramethylbenzidine (TMBZ, C16H20N2, ≥99.0 %), Brilliant Blue R (C45H44N3NaO7S2), sodium phosphate dibasic (Na2HPO4, 99%), sodium phosphate monobasic (NaH2PO4, 99%), thiol-PEG-Amine (HS-PEG3500-NH2), ATP Red-1 and bovine serum albumin (BSA) were purchased from Sigma-Aldrich (USA). Sodium citrate dihydrate (Na3C6H5O7•2H2O, 100.5%) was bought from Mallinckrodt. Hydroquinone (C6H4(OH)2, ≥99.5%) was purchased from Riedel-de Haën. Hydrogen tetrachloroaurate (III) trihydrate (HAuCl4•3H2O, 99.99% metal biasis, Au ≥ 49.0%) and Lithium dodecyl sulfate (4X LDX sample buffer) were purchased from Thermo Scientific. Methoxypolyethylene glycol thiol (mSH-PEG) was obtained from Biopharma PEG Scientific Inc. Ethanol (C2H5OH, Anhydrous, ≥99.5%) was bought from Jingming Chemical Co., Ltd. Tetraethyl orthocilicate (TEOS, C8H20O4Si, 98%) was purchased from Acros Organics. Sodium hydroxide (NaOH) was purchased from Katayama Chemical. Ammonium chloride (NH4Cl, 99.5%), calcium chloride dihydrate (CaCl2·2H2O, 95%), sodium bicarbonate (NaHCO3, 99.5%), sodium chloride (NaCl, 99.5%), and magnesium chloride hexahydrate (MgCl2·6H2O, 98%) were sourced from Wako. 2-[4-(2-hydroxyethyl)-1-piperazinyl] ethanesulfonic acid (HEPES, C8H18N2O4S, 99%) and Cell Counting Kit-8 (CCK-8) were purchased from Dojindo. LB broth (Luria-Bertani, Miller) and yeast extract were bought from BD. Sodium acetate (CH3COONa·3H2O, 99%) and hydrogen peroxide (H2O2, 30%) were obtained from SHOWA. Isopropanol (95%) was obtained from Fisher Chemical. 6X Laemmli SDS sample buffer was purchased from Alfa Aesar. Commercial page gel (10%) and tris-glycine-SDS (TGS) buffer were purchased from GenScript. High-glucose Dulbecco’s Modified Eagle’s Medium (DMEM), Minimum Essential Medium (MEM), Dulbecco’s Modified Eagle’s Medium/Ham’s F-12 Mixture (DMEM/F12), and fetal bovine serum (FBS) were obtained from Gibco. Phosphate-buffered saline (PBS) and penicillin–streptomycin was bought from Caisson (Smithfield, VA, USA). Hoechst 33342, calcein AM and ethidium homodimer-1 (EthD-1) were sourced from Invitrogen (Carlsbad, CA, USA). Chloroform (CHCl3, ≥99.8%) was purchased from Merck. 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC, C44H84NO8P, >99.0%) and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE, C41H78NO8P, >99.0%) were acquired from Avanti Polar Lipids. 2′,7′-dichlorofluorescein diacetate (DCFH-DA, C24H16Cl2O7, 20 mM) was purchased from Abcam (Cambridge, UK). Water purified with a Milli-Q Synergy system was used throughout the studies.
Synthesis of Au NPs
Spherical Au NPs ranging from 15 to 100 nm were synthesized using different methods. Specifically, 15 nm Au NPs were synthesized using a modified Turkevich method, which involves the reduction of hydrogen tetrachloroaurate (III) trihydrate (HAuCl4) with sodium citrate bihydrate at 120 °C. Prior to conducting the syntheses, all glassware was thoroughly cleaned using aqua regia. Aqua regia was prepared by mixing one-part nitric acid with three parts hydrochloric acid. Initially, 1.8 mL of 5 mM HAuCl4 was dissolved in 28.5 mL of ultrapure water and heated to 120 °C in an oil bath. Meanwhile, 1.22 mL of 38.8 mM sodium citrate bihydrate was freshly prepared in cold ultrapure water. Once the HAuCl4 solution reached 120 °C, the sodium citrate bihydrate solution was added dropwise with continuous stirring. Following the addition of sodium citrate bihydrate, the solution was stirred for an additional 10 min and then allowed to cool to room temperature while avoiding light exposure. The color of the mixture transitioned from pale yellow to wine red, indicating successful NP synthesis. The resulting 15 nm Au NPs, serving as the Au NP seeds, were harvested and stored at 4 °C in the dark until further use.
To synthesize larger Au NPs, ranging from 70 to 100 nm in diameter, a seeded growth method was employed. This method utilized 15 nm Au seeds as nucleation sites, and the final NP size was controlled by varying the amount of these seeds. The 15 nm Au seeds were prepared as previously described. For the synthesis of larger Au NPs, 0.5 mL of 50 mM HAuCl4 was added to 100.5 mL of deionized water at room temperature. While rapidly stirring, the appropriate volume of 15 nm Au seeds (750 μL for 70 nm, 300 μL for 100 nm) was added to the solution to achieve the desired sizes. Notably, greater volumes of 15 nm seeds produced smaller NPs. To initiate the NP growth reaction, 220 μL of 34 mM sodium citrate bihydrate was added to the solution, followed immediately by the addition of 1 mL of 0.03 M hydroquinone. The solution was allowed to stir at room temperature for three hours. Distinct color transitions were observed during the synthesis: the solution changed from black to wine-red for 70 nm Au NPs, and from black to red-brown for 100 nm Au NPs. Finally, the synthesized Au NPs (70 nm and 100 nm) were obtained by centrifugation for 10 min and washed twice with deionized water to remove residual reactants. The resulting Au NPs were re-dispersed in deionized water and stored at 4 °C in the dark until further use.
Surface PEGylation of Au NPs
Au NPs were modified via PEGylation. Specifically, 5 mL of Au NP solution (Au concentration 400 ppm) was mixed with 1 mL of mSH-PEG (2 mg/mL) in double-distilled water (DDW) in glass vials and stirred at room temperature for 2 h in the dark to modify the Au NP surface with mSH-PEG. After the reaction, the Au@PEG NPs were collected by centrifugation for 10 min at 15 °C and washed with DDW. This washing process was repeated at least twice. The resulting Au@PEG NPs were resuspended in DDW and stored at 4 °C in the dark until further use.
Preparation of silica-insulated Au NPs
The synthesis of silica-insulated Au NPs was carried out using a modified standard sol-gel method. Initially, 2 mL of Au NPs at a concentration of 1,500 ppm were mixed with 1 mL of NH2-PEG-SH at 5 mg/mL. This mixture was then sonicated for 5 min in an ice bath. Following this, 6 mL of double-distilled water (DDW), 1 mL of ethanol (99.5%), 10 µL of NaOH (1 M), and 50 µL of tetraethyl orthosilicate (TEOS) were added sequentially, with sonication performed for 3, 3, 1, and 1 min, respectively, after each addition. The solution was then stirred vigorously for 18 h in an ice bath to form SiO2 coatings on the Au NPs. The product was collected by centrifugation for 3 min and washed at least three times with ethanol. Finally, the insulated Au NPs were dispersed in DDW for subsequent MIL encapsulation, resulting in the formation of Au@SiO2@MIL NPs.
Preparation of defined medium (DM)
The DM buffer solution was prepared by dissolving 1.25 g of NaHCO3, 0.04 g of CaCl2, 0.5 g of NH4Cl, 0.1 g of MgCl2, 5 g of NaCl, 3.6 g of HEPES, and 0.25 g of yeast extract in 0.5 L of deionized water. The mixture was autoclaved at 122 °C for 25 min to ensure sterility.
Preparation of liposomes
The individual stock solutions of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) in chloroform were prepared in sealed glass vials at a concentration of 10 mg/mL and stored at 4 °C. A lipid mixture was then generated by combining DOPC and DOPE in a volume ratio of 0.504 mL:0.118 mL. Subsequently, this mixture underwent nitrogen purging to achieve a multi-layered lipid film at the vial’s bottom. The dried lipid film was rehydrated in 0.8 mL phosphate-buffered saline (PBS) to form liposomes with a composition of 70% DOPC and 30% DOPE. These liposomes were homogenized by extrusion through a polycarbonate filter with 100-nm pores. The resulting dispersed liposomes were stored at 4 °C for subsequent experiments.
Bacterial culture and MILs preparation
Shewanella oneidensis MR-1 (obtained from ATCC) underwent cultivation on Luria broth (LB) agar at 30 °C, yielding bacterial colonies. A single colony was subsequently inoculated into 80 mL fresh LB medium and incubated under slow shaking at 30 °C for 24 h. Following this, the bacterial medium was substituted with defined medium (DM), adjusting the S. oneidensis MR-1 concentration to 4 optical density (O.D.). A 100 μL pre-prepared liposome solution was added to the 1 mL culture for further aerobic cultivation at 30 °C for 24 h, during which MILs were predominantly produced through the LIME process15,44. The resulting solution underwent centrifugation for 5 min at 4 °C to separate bacteria and MILs. Supernatants containing MILs and residual S. oneidensis MR-1 were filtered using a 0.45 μm syringe filter to remove remaining bacteria. The MIL suspensions were then ultracentrifuged at 4 °C for 4 h. The resulting MIL pellet was resuspended in DM and stored at −20 °C for subsequent experiments.
Wild-type Escherichia coli (E. coli) was obtained from ATCC. For E. coli culture, LB medium containing 50 mg/L kanamycin was used, and the culture was maintained at 30 °C. The process for preparing MILs was identical to that used for S. oneidensis MR-1, resulting in the production of E. coli-derived MIL.
Protein quantification
The protein concentration was determined in accordance with the manufacturer’s instructions using a Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Bovine serum albumin (BSA) solutions were employed to establish the standard calibration curve. Initially, samples were combined with radioimmunoprecipitation assay buffer (RIPA) and incubated for 10 min. Following this, the supernatants containing proteins were obtained through centrifugation for 5 min. Each sample (4 μL) was then mixed with 50 μL of Bio-Rad protein assay reagent (reagent A/reagent S 50:1), followed by the addition of 400 μL of reagent B (as labeled by the manufacturer) for 5 min. Later, 200 μL of supernatants or standard solutions were transferred to a 96-well plate, and the optical density (O.D.) at 690 nm for each well was measured using a microplate reader (800 TS, BioTek, USA).
Preparation of membrane-coated Au NPs
The combination of Au NPs (100 ppm) and MILs (1 mg/mL) underwent sonication (DELTA-DC80, 40 kHz, 80 W, and 10 min) to disintegrate MILs. Subsequently, the mixture was rapidly frozen in an ice bath for 5 min to facilitate the integration of MIL components onto the surface of Au NPs, resulting in the formation of Au@MIL NPs. This sonication-cooling process was iterated three times to optimize membrane coverage on Au NPs. The resulting Au@MIL NPs were then collected through centrifugation for 5 min and subjected to three washes with PBS. The same procedure was followed to obtain the Au@Lipo NPs and Au@E. coli NPs.
Protein electrophoresis
The protein electrophoresis system (Hoefer SE260) was set up with TGS running buffer in the tank and a commercial PAGE gel installed. Samples were prepared by mixing 20 µL of bacterial extract, MILs, Au@PEG, or Au@MIL with 4 µL of 6X Laemmli SDS sample buffer and incubating at 95 °C for 5 min. A 15 µL sample was loaded into each well of the PAGE gel, and electrophoresis was run at 120 V for approximately 40 min. Gels were stained with Brilliant Blue R for whole protein visualization.
For heme-staining, gels were prepared similarly but using a 2-mercaptoethanol-free 4X LDX sample buffer to replace the 6X Laemmli SDS sample buffer. Staining was done in a 50 mL solution containing 6.3 mM TMBZ and 0.25 M sodium acetate (pH 5.0) in the dark for 2 h, followed by the addition of 47.5 µL of 30% hydrogen peroxide and incubation for 30 min. The gel background was destained using a solution of 30% isopropanol and 70% sodium acetate.
Cultivation and treatment of cancer and normal cell lines
A diverse array of cancer cell lines, including Huh-7, Hep G2, SK HEP-1, HA22T, A549, Hep 3B, AsPC-1, PANC-1, MDA-MB-231, HeLa, and T24, along with the normal cell lines NeHepLxHT and M10, were propagated in media formulations comprising high-glucose DMEM, MEM, and RPMI. These media were supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin (P/S), and 1% non-essential amino acids (NEAA), all sourced from Caisson (Smithfield, VA, USA). Additionally, human neonatal hepatocyte cells (NeHepLxHT)45,46, genetically modified to express the hTERT gene, were cultured in high-glucose DMEM/F-12 enriched with 10% FBS, 1% P/S, 100 nM dexamethasone, 0.1% ITS premix, 20 ng/mL human epidermal growth factor (EGF), and 5 mg/mL ciprofloxacin. All cell cultures were incubated at 37 °C within a humidified atmosphere containing 5% CO2.
To simulate hypoxic conditions, a specialized hypoxia chamber was utilized, maintaining an atmospheric composition of 1% O2, 5% CO2, and 94% N2 within the incubator. After 48 h of hypoxic exposure, cellular specimens were harvested for further examination.
Visualization of cellular cytotoxic and oxidative stress responses
To visualize cytotoxicity in cancer treatments, cancerous and normal cells (30,000 cells per well) were seeded into 24-well plates and cultured for 24 h. The cells were then treated with Au@PEG, Au@Lipo, Au@E. coli, or Au@MIL NPs (Au concentration of 300 ppm) and incubated for an additional 48 h for 15 nm and 100 nm NPs, or 72 h for 70 nm NPs. After NPs internalization, the medium was carefully aspirated, and the cells were washed three times with PBS to remove non-internalizing NPs. The cells were subsequently stained with 2 μg/mL Hoechst 33342 for nuclei, 1 μM calcein AM for live cells, and 1 μM EthD-1 for dead cells. Fluorescence images were captured using a 20× objective lens on an Olympus IX71 fluorescence microscope.
To examine intracellular oxidative stress, Hep G2 cells were seeded in 24-well plates and cultured overnight. The positive control group was treated with 500 μM tert-butyl hydroperoxide (tBHP) for 3 h. The other groups were treated with Au@MIL or Au@E. coli NPs (Au concentration of 300 ppm) and incubated for an additional 48 h. Following the incubation period, all cells were washed three times with PBS and stained with 10 μM DCFH-DA fluorescent dye to determine intracellular ROS levels.
Detection of Intracellular ATP Production
Hep G2 cells (30,000 per well) were seeded into 24-well plates and cultured for 24 h. The cells were then treated with Au@MIL medium solution (100 nm Au of 300 ppm), with untreated cells serving as the control group. After 48 h of incubation, the cells were incubated with the fluorescent probe ATP Red-1 (10 μM, Sigma-Aldrich) at 37 °C for 15 min to detect mitochondrial ATP production. Fluorescence images were captured using a 20X objective lens on a fluorescence microscope (IX71, Olympus, Tokyo, Japan) and fluorescence intensity was measured using ImageJ software (National Institutes of Health, MD).
Hemolysis analysis
A 2% suspension of red blood cells was introduced into various solutions, including deionized water (positive control group), PBS (negative control group), PBS with Au@PEG NPs, or PBS with Au@MIL NPs, with an Au concentration of 50 ppm. The sample solutions were incubated at room temperature in the dark for 1 hour. Subsequently, hemoglobin quantification in the supernatants of different treatments was determined by measuring the absorbance of hemoglobin at 450 nm using a microplate reader. After completing the assay, the percentage of hemolysis was calculated using the following equation:
Clonogenic assay
One thousand cells were seeded in a 6-well culture plate overnight, then treated with 300 ppm Au-based NPs, including Au@MIL (15 nm, 70 nm and 100 nm), Au@PEG (70 nm), Au@Lipo (70 nm), and Au@E. coli (70 nm), for 72 h. After refreshing the cells with medium without NPs, they were further incubated for another 14 days. The formation of colonies was fixed with methanol for 30 min, stained with 0.5% crystal violet for another 30 min, and then measured using ImageJ software (version 1.53r).
RNA sequencing and bioinformatics analysis
Total ribonucleic acid (RNA) was extracted from cancer cells treated with PBS and 300 ppm Au@MIL (100 nm) using Trizol Reagent (#15596026, Thermo Fisher Scientific). For RNA library preparation, the refined RNA underwent processing with the TruSeq Stranded mRNA Library Prep Kit from Illumina, San Diego, CA, USA, following the manufacturer’s guidelines.
In summary, mRNA (1 µg) was isolated from total RNA using oligo(dT)-coupled magnetic beads and subsequently fragmented at an elevated temperature. The first-strand cDNA was synthesized using reverse transcriptase and random primers. After the generation of double-strand cDNA and adenylation at the 3’ ends of DNA fragments, adaptors were ligated and purified using the AMPure XP system from Beckman Coulter, Beverly, USA. The library quality was assessed using the Agilent Bioanalyzer 2100 and Real-Time PCR systems. Subsequently, the qualified libraries were sequenced on an Illumina Novaseq X plus platform, generating 150 bp paired-end reads, by Genomics, BioSci & Tech Co., New Taipei City, Taiwan.
The fastp program (version 0.20.0) was employed to eliminate low-quality bases and sequences originating from adapters in the raw data. The processed reads were aligned to the reference genomes using HISAT2 (version 2.1.0). The FeatureCounts software (v2.0.1) within the Subread package was utilized for gene abundance quantification. Differentially expressed genes (DEGs) were identified using either DESeq2 (version 1.28.0) or EdgeR (version 3.36.0), depending on the presence or absence of biological replicates. The R package clusterProfiler (version 4.0.0) was employed to conduct functional enrichment analysis of gene ontology (GO) and Gene Set Enrichment Analysis (GSEA) within gene clusters.
JC-1 staining
1 × 105 cancer cells were seeded in 6-well overnight and treated with PBS, and 300 ppm Au@MIL (100 nm). After 24, 48, and 72 h post-treatment, the cancer cells were stained with a JC-1 mitochondrial membrane potential assay kit (HY-K0601, MedChemExpress) according to the manufacturer’s guidelines. The images were observed using an inverted fluorescence microscope (CKX53, Olympus), excited at 510 nm and emitted at 527 nm (green) and 590 nm (red). Three different fields were taken for each group and analyzed using ImageJ software (version 1.53r).
Measurement of lipid ROS in Hep G2 cells
Lipid peroxidation in Hep G2 cells was assessed using the C11-BODIPY 581/591 fluorescence probe (ThermoFisher, Massachusetts, USA) according to the manufacturer’s instructions. Hep G2 cells were seeded at a density of 4000 cells per well in 96-well glass-bottom microplates (Greiner, Kremsmünster, Austria). The cells were treated with Au@MIL (100 nm Au of 300 ppm) for 24 and 48 h to induce oxidation. Additionally, cells treated with Ferroptocide (50 μM) for 1 hour served as a positive control, while untreated cells were used as the control group. All groups were gently washed twice with PBS to remove non-internalizing NPs and then treated with 10 μM C11-BODIPY 581/591 solution and 4 μg/mL Hoechst 33258 (Merck, USA) at 37 °C for 1 h. Finally, the cells were washed with PBS and imaged using an ImageXpressMicro (IXM) high-content imaging system operated by MetaXpress software version 6.7.0.211.
Subcellular Localization of Lipid Peroxidation
To determine the subcellular localization of lipid peroxidation, Hep G2 cells were transfected with mitochondrial Cox8-mRFP or endoplasmic reticulum ERp57-RFP plasmids using Lipofectamine 3000 (Invitrogen, USA). After 24 h, the transfected cells were treated with 100 nm Au@MIL NPs (300 ppm) for an additional 24 h. Following treatment, the cells were washed twice with phenol red-free medium to remove residual nanoparticles. Lipid peroxidation was visualized by incubating the cells with 10 μM C11-BODIPY 581/591 for 1 h in the dark at 37 °C. After staining, the cells were washed with PBS and imaged using a confocal laser scanning microscope (FV3000, Olympus) equipped with a 100X oil immersion objective.
Trafficking of lipid peroxidation by Au@MIL NPs
To study the lipid peroxidation induced by Au@MIL NPs, fluorescent protein RFP-tagged proteins (mitochondrial Cox8-mRFP and the endoplasmic reticulum ERp57-RFP) were used to label mitochondria and the endoplasmic reticulum, respectively. Hep G2 hepatoma cells were seeded at a density of 5 × 105 cells per dish in 3.5 cm glass-bottom dishes and incubated for 24 h. The cells were then transfected with Cox8-mRFP and ERp57-RFP plasmids using Lipofectamine 3000 (Thermo Fisher Scientific) and incubated for an additional 24 h. Subsequently, cells were treated with Au@MIL NPs for 24 h. After another 24 h of incubation, the cells were rinsed with fresh phenol red-free medium. Images were obtained using a confocal microscope (FV3000, Olympus) equipped with a 100X oil lens.
Mice
Animal care adhered to the Laboratory Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals, receiving approval from the Institutional Animal Care and Use Committee of National Cheng Kung University (NCKU). All animal treatments and surgical procedures followed the protocols outlined by the NCKU Laboratory Animal Center (IACUC No.113084). The experimental mice, including NOD-SCID and C57BL/6 strains, were housed in cages with three to five mice per cage. They were maintained at 22–23 °C with 55 ± 10% humidity, following a 13 h/11 h light/dark cycle.
Establishment of the Hep G2-Red-FLuc orthotopic HCC xenograft mice
NOD-SCID mice (male, 6–8 weeks old) were anesthetized with Zoletil 100 (Virbac), administered intraperitoneally, and placed supine. Subsequently, 2 × 106 Hep G2-Red-Fluc cells in a solution comprising 10 μL of PBS and 10 μL of Basement Membrane Matrix (BD) were surgically implanted into either the right or left lobe of the liver using BD Insulin Syringes 30 G 3/10cc (BD). The incision was closed using CT204 Chromic Catgut (20 mm, 75 cm, UNIK SURGICAL SUTURES MFG. CO.) and NC193 Monofilament Nylon (19 mm, 45 cm, UNIK SURGICAL SUTURES MFG. CO.). Subsequently, the mice were allowed to recover fully.
Evaluation of anti-tumor efficacy and biodistribution of Au@MIL in vivo
Hep G2-Red-FLuc orthotopic HCC xenograft mice were treated with 100 μL of sterilized PBS, 100 μL of 3000 ppm Au@PEG (100 nm), 100 μL of 3000 ppm Au@MIL (100 nm), and 100 μL of 3000 ppm Au@SiO2@MIL (100 nm) through intravenous administration for a single dose. The body weight of each group was recorded twice a week, and tumor growth in Hep G2-Red-FLuc hepatocellular carcinoma cells was monitored using the IVIS imaging system (Caliper Life Sciences). For sample collection, all mice were sacrificed on Day 14 post-treatment. For survival analysis, the Institutional Animal Care and Use Committee of NCKU stipulated that the maximal tumor burden should not exceed 10% of the body weight, and ascites formation should be absent. All experimental mice were sacrificed before reaching these specified limits.
For biodistribution analysis, samples of the heart, lung, spleen, kidney, liver, HCC tumor, and feces were collected from Au@MIL-treated HCC mice at 1, 4, 8, 24, and 72 h post-treatment. The samples were disrupted into powder and acid-digested in aqua regia for one week. The concentration of Au in the sample solution was determined by iCAP™ 7400 ICP-OES (Thermo Fisher Scientific).
IVIS imaging system and quantification
The mice were anesthetized using a combination of oxygen and isoflurane and subsequently intraperitoneally administered 100 μL of D-luciferin (catalog # 122796, Caliper Life Sciences). Ten minutes later, the mice were imaged using the Xenogen IVISR Spectrum Noninvasive Quantitative Molecular Imaging System (Caliper Life Sciences) and analyzed with Living Image 4.7.3 (PerkinElmer, USA). The liver was collected and subjected to ex vivo IVIS detection after the mice’s sacrifice.
Biosafety of Au@MIL in vivo
C57BL/6 mice (male, 6–8 weeks old) were treated with 100 μL of sterilized PBS and 100 μL of 3000 ppm Au@MIL (100 nm) through a single intravenous administration. The body weight of each group was recorded daily, and all mice were sacrificed on Day 7 post-treatment.
Hematoxylin and eosin (H&E) staining
Tumor and standard organ samples, including the heart, lung, spleen, liver, and kidney, were embedded in paraffin and sliced into 5 μM thickness. The sections underwent deparaffinization, rehydration, and PBS washing, then staining with hematoxylin solution for 3 min. After washing in tap water, eosin solution was applied for 1 min. Subsequently, the sections were immersed in ethanol and xylene before being mounted for evaluation. The sections were examined under a microscope (CX31, Olympus), and three fields were captured for each group.
Immunohistochemistry (IHC) staining
Tumor samples were embedded in paraffin and sliced into 5 μm thickness. The sections underwent deparaffinization, rehydration, and incubation with cleaved caspase-3 (Asp175) antibody (1:400 dilution, #9661, Cell Signaling Technology) and 4-Hydroxynonenal (1:200 dilution, #bs-6313R, Bioss). Staining was carried out using an ABC peroxidase standard staining kit (Thermo Fisher Scientific) containing biotinylated affinity-purified goat anti-rabbit IgG (1:1000 dilution, #32054, Thermo Fisher Scientific) and a DAB peroxidase (HRP) substrate kit (Vector Laboratories) as per the manufacturer’s protocol. Finally, the sections were examined under a microscope (CX31, Olympus), with three different fields captured for each group.
Biochemical analysis
Blood was extracted from the mice’s hearts, and heparin sodium was promptly added. The collected blood samples underwent centrifugation for 10 min to obtain the serum. Subsequently, the serum samples were utilized for blood biochemistry analysis, including alanine aminotransferase (ALT), alkaline phosphatase (ALP), aspartate aminotransferase (AST), total bilirubin (T-Bil), blood urea nitrogen (BUN), creatine (CREA), and uric acid (UA) expression. The analysis was performed using a FUJI DRI-CHEM 4000i (FUJIFILM). For urine analysis, the collected samples were analyzed using the Thinka Urine Test Strip 10UB (Arkray) with the RT-4010 analyzer (Arkray).
Cellular uptake of Au@MIL NPs
Two normal cell lines (NeHepLxHT, M10) and three cancer cell lines (Hep G2, HA22T, and MDA-MB-231) were seeded into 60-mm culture dishes at a density of 2 × 105 cells per dish. After 48 h of culturing, the cells were incubated with either control medium or 100 nm Au@MIL NPs (100 ppm). Following 48 h of incubation, the medium was removed, and the cells were washed three times with PBS. Subsequently, trypsin was added to collect the cells, which were then immersed in aqua regia for 1 day. Finally, the Au ion concentrations in the aqua regia were determined using ICP-AES (iCAP 7400 Duo, Thermo Scientific, US) measurements.
XANES measurement
The XANES spectra were measured at TLS 01C1 beamline in the National Synchrotron Radiation Research Center. XANES measurements at the Au L3-edge were conducted in fluorescence mode using a Lytle detector. The spectra of Au foil were collected in transmission mode for comparison and monochromatic energy calibration. The acquired XANES data were processed according to the standard procedures using the Athena program47.
Statistical Analysis
Statistical analysis was performed using Microsoft Excel 2016 or Origin software version 8.1 for Windows. Student’s t-test or one-way ANOVA was employed to determine statistical significance, with a p-value less than 0.05 considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and its supplementary files. The RNA sequencing data from this study is available in the Gene Expression Omnibus (GEO) database under the accession code GSE285028. Source data are provided with this paper. Source data is available for Figs. 1f, g; 2b; 3a, c, d, e, f; 4a, b, c, d,e, f; 5a, c, d, g; 6a and Supplementary Figs. 1–3, 5, 7, 8, 10, 11, 13, 15, 17–21 in the associated source data file. Source data are provided with this paper.
References
Shi, J. J., Kantoff, P. W., Wooster, R. & Farokhzad, O. C. Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer 17, 20–37 (2017).
Anand, U. et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 10, 1367–1401 (2023).
Jin, K. T. et al. Recent trends in nanocarrier-based targeted chemotherapy: Selective delivery of anticancer drugs for effective lung, colon, cervical, and breast cancer treatment. J. Nanomater. 2020, 9184284 (2020).
Chong, L. M., Tng, D. J. H., Tan, L. L. Y., Chua, M. L. K. & Zhang, Y. Recent advances in radiation therapy and photodynamic therapy. Appl. Phys. Rev. 8, 041322 (2021).
Correia, J. H., Rodrigues, J. A., Pimenta, S., Dong, T. & Yang, Z. C. Photodynamic therapy review: principles, photosensitizers, applications, and future directions. Pharmaceutics 13, 1332 (2021).
Lucky, S. S., Soo, K. C. & Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 115, 1990–2042 (2015).
Shanmugam, V., Selvakumar, S. & Yeh, C. S. Near-infrared light-responsive nanomaterials in cancer therapeutics. Chem. Soc. Rev. 43, 6254–6287 (2014).
Manoharan, D., Wang, L.C., Chen, Y.C., Li, W.P. & Yeh, C.S. Catalytic Nanoparticles in Biomedical Applications: Exploiting Advanced Nanozymes for Therapeutics and Diagnostics. Adv. Healthc. Mater. e2400746 (2024).
Shi, L. et al. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 14, 651–662 (2016).
Castelle, C. et al. A new iron-oxidizing/O-reducing supercomplex spanning both inner and outer membranes, isolated from the extreme acidophile. J. Biol. Chem. 283, 25803–25811 (2008).
Beliaev, A. S., Saffarini, D. A., McLaughlin, J. L. & Hunnicutt, D. MtrC, an outer membrane decahaem cytochrome required for metal reduction in MR-1. Mol. Microbiol. 39, 722–730 (2001).
Myers, C. R. & Myers, J. M. MtrB is required for proper incorporation of the cytochromes OmcA and OmcB into the outer membrane of MR-1. Appl. Environ. Microb. 68, 5585–5594 (2002).
Bretschger, O. et al. Current production and metal oxide reduction by MR-1 wild type and mutants. Appl. Environ. Microb. 73, 7003–7012 (2007).
Edwards, M. J., White, G. F., Butt, J. N., Richardson, D. J. & Clarke, T. A. The crystal structure of a biological insulated transmembrane molecular wire. Cell 181, 665–673 (2020).
Chen, Y. C. et al. Electroactive membrane fusion-liposome for increased electron transfer to enhance radiodynamic therapy. Nat. Nanotechnol. 18, 1492–1501 (2023).
Rowe, A. R. et al. Tracking electron uptake from a cathode into shewanella cells: implications for energy acquisition from solid-substrate electron donors. Mbio 9, e02203–e02217 (2018).
Rowe, A. R. et al. Identification of a pathway for electron uptake in shewanella oneidensis. Commun. Biol. 4, 957 (2021).
Abuyen, K. & El-Naggar, M. Y. Soluble iron enhances extracellular electron uptake by MR-1. Chemelectrochem 10, e202200965 (2023).
Chen, S. W. et al. Gold nanoelectrodes of varied size: Transition to molecule-like charging. Science 280, 2098–2101 (1998).
Chen, S. W. & Murray, R. W. Electrochemical quantized capacitance charging of surface ensembles of gold nanoparticles. J. Phys. Chem. B 103, 9996–10000 (1999).
Ansar, S. M. & Kitchens, C. L. Impact of gold nanoparticle stabilizing ligands on the colloidal catalytic reduction of 4-nitrophenol. Acs. Catal. 6, 5553–5560 (2016).
Feng, W. H. et al. Near-infrared-activated NaYF4: Yb3+, Er3+/Au/CdS for H2 production via photoreforming of bio-ethanol: plasmonic Au as light nanoantenna, energy relay, electron sink and co-catalyst. J. Mater. Chem. A 5, 10311–10320 (2017).
Bano, A. et al. Enhancing catalytic activity of gold nanoparticles in a standard redox reaction by investigating the impact of AuNPs size, temperature and reductant concentrations. Sci. Rep. 13, 12359 (2023).
Qian, F. et al. Photoenhanced electrochemical interaction between and a hematite nanowire photoanode. Nano Lett. 14, 3688–3693 (2014).
Subramanian, V., Wolf, E. E. & Kamat, P. V. Catalysis with TiO/gold nanocomposites.: Effect of metal particle size on the fermi level equilibration. J. Am. Chem. Soc. 126, 4943–4950 (2004).
Dong, Y. C. et al. Effect of gold nanoparticle size on their properties as contrast agents for computed tomography. Sci. Rep. 9, 14912 (2019).
Liu, L. Z. et al. Cell membrane coating integrity affects the internalization mechanism of biomimetic nanoparticles. Nat. Commun. 12, 5726 (2021).
Mihály, J. et al. Characterization of extracellular vesicles by IR spectroscopy: Fast and simple classification based on amide and C-H stretching vibrations. Bba-Biomembranes 1859, 459–466 (2017).
Bonechi, C. et al. Physicochemical characterization of hyaluronic acid and chitosan liposome coatings. Appl. Sci. (Basel) 11, 12071 (2021).
Varo, E. S. et al. Gold Nanoparticles for CO Electroreduction: An optimum defined by size and shape. J. Am. Chem. Soc. 146, 2015–2023 (2024).
Wang, S. H., Lee, D.-S., Kim, T.-H., Kim, J.-E. & Kang, T.-C. Reciprocal regulation of oxidative stress and mitochondrial fission augments parvalbumin downregulation through CDK5-DRP1- and GPx1-NF-κB signaling pathways. Cell Death Dis. 15, 707 (2024).
Zhao, J.-W. et al. The role of the mitochondrial ribosomal protein family in detecting hepatocellular carcinoma and predicting prognosis, immune features, and drug sensitivity. Clin. Transl. Oncol. 26, 496–514 (2024).
Ning, N. et al. A novel microtubule inhibitor promotes tumor ferroptosis by attenuating SLC7A11/GPX4 signaling. Cell Death Discov. 9, 453 (2023).
Gao, L.-F. et al. Tumor bud-derived CCL5 recruits fibroblasts and promotes colorectal cancer progression via CCR5-SLC25A24 signaling. J. Exp. Clin. Cancer Res. 41, 81 (2022).
Lobo, V., Patil, A., Phatak, A. & Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 4, 118–126 (2010).
Sheldon, M. T., van de Groep, J., Brown, A. M., Polman, A. & Atwater, H. A. Plasmoelectric potentials in metal nanostructures. Science 346, 828–831 (2014).
Navarrete, J. et al. Merely measuring the UV-visible spectrum of gold nanoparticles can change their charge state. Nano Lett. 18, 669–674 (2018).
Ershov, V., Tarasova, N. & Ershov, B. Evolution of electronic state and properties of silver nanoparticles during their formation in aqueous solution. Int. J. Mol. Sci. 22, 10673 (2021).
Zhang, P. & Sham, T. K. X-ray studies of the structure and electronic behavior of alkanethiolate-capped gold nanoparticles: The interplay of size and surface effects. Phys. Rev. Lett. 90, 245502 (2003).
Zhang, P. & Sham, T. K. Tuning the electronic behavior of Au nanoparticles with capping molecules. Appl. Phys. Lett. 81, 736–738 (2002).
Benfield, R. E. et al. Structure and bonding of gold metal clusters, colloids, and nanowires studied by EXAFS, XANES, and WAXS. J. Phys. Chem. B 105, 1961–1970 (2001).
Lin, Y. X. et al. Connecting the irreversible capacity loss in Li-ion batteries with the electronic insulating properties of solid electrolyte interphase (SEI) components. J. Power Sources 309, 221–230 (2016).
Brown, M. D. et al. Plasmonic dye-sensitized solar cells using core-shell metal-insulator nanoparticles. Nano Lett. 11, 438–445 (2011).
Long, X. et al. Scalable liposomes functionalization via membrane lipid exchange mechanisms. Nano Today. 11, 102630 (2025).
Reid, Y., Gaddipati, J. P., Yadav, D. & Kantor, J. Establishment of a human neonatal hepatocyte cell line. Vitr. Cell Dev. Biol. Anim. 45, 535–542 (2009).
Li, T. N. et al. Intrahepatic hepatitis B virus large surface antigen induces hepatocyte hyperploidy via failure of cytokinesis. J. Pathol. 245, 502–513 (2018).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using. J. Synchrotron Radiat. 12, 537–541 (2005).
Acknowledgements
C.-S.Y. acknowledges the financial support from the National Science and Technology Council (NSTC), Taiwan (NSTC 113-2113-M-006-015). This research was also partially supported by the Higher Education Sprout Project, Ministry of Education, to the Headquarters of University Advancement at National Cheng Kung University. Additional financial support was provided by the Center of Applied Nanomedicine, National Cheng Kung University, under the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project of the Ministry of Education (MOE) in Taiwan. W.-T.C. acknowledges the financial support from NSTC, Taiwan (NSTC 113-2740-B-006-002). W.-P.L. acknowledges the financial support provided by NSTC, Taiwan (NSTC 112-2113-M-037-014-MY2 and 113-2320-B-037-007) and the Yushan Fellow Program by the MOE, Taiwan (MOE-109-YSFMS-1019-001-P1). W.-P.S. thanks the financial support by the NSTC, Taiwan (NSTC 113-2314-B-006-038-MY3; NSTC 113-2314-B-006-014; NSTC 113-2321-B-006-010) and National Cheng Kung University Hospital (NCKUH-T11310005). We are grateful for the support from the Laboratory Animal Center, College of Medicine, National Cheng Kung University, and the Core Facility of Taiwan Mouse Clinic and Animal Consortium. We thank the staff of the Clinical Medicine Research Center, National Cheng Kung University Hospital, for the technical support. We thank Genomics BioSci & Tech Co., Ltd for the technical support in RNA-seq and bioinformatics analysis. We appreciate the technical services provided by the Bioimaging Core Facility of the National Core Facility for Biopharmaceuticals, NSTC, Taiwan. We also thank the Core Research Laboratory, College of Medicine, National Cheng Kung University, Taiwan, for their equipment support. The authors gratefully acknowledge the use of equipment from the Core Facility Center of National Cheng Kung University. Additionally, we thank the staff of TLS 01C1 and TLS 01C2 of NSRRC for their assistance with XAS and PXRD experimental measurements and data analysis.
Author information
Authors and Affiliations
Contributions
C.-S.Y. conceived the research idea and proposed mechanism. C.-S.Y., W.-T.C., W.-P.L., W.-P.S., and H.-S.S. designed the experiments and supervised the entire project. Y.-C.C., Y.-L.L., and M.-C.C. synthesized and characterized the Au NPs. W.-P.L. provided the LIME technique and all bacterial strains. Y.-C.C., Y.-L.L., M.-C.C., W.-J.W., and J.-S.C. fabricated the liposomes. Y.-C.C., Y.-L.L., and M.-C.C. fabricated the MILs. W.-J.W. and J.-S.C. fabricated the E. coli-derived MILs and contributed to the protein characterization analysis. Y.-C.C. and Y.-L.L. performed the in vitro studies. Y.-C.C. and H.-C.W. conducted the lipid ROS measurements. W.-P.S. designed the animal disease model experiments. L.-C.C., Y.-F.L., and W.-P.S. assisted with the colony assay and in vivo studies. Y.-C.C., Y.-L.L., and M.-C.C. measured the ICP values. P.-Y.C. and H.-S.S. conducted the XANES measurements. D.M. was involved in the discussion. C.-S.Y., Y.-C.C., L.-C.C., W.-T.C., W.-P.L., H.-S.S., and W.-P.S. wrote the paper. All authors discussed the experimental data, reviewed, and commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Stefanie Klein and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, YC., Chang, LC., Liu, YL. et al. Redox disruption using electroactive liposome coated gold nanoparticles for cancer therapy. Nat Commun 16, 3253 (2025). https://doi.org/10.1038/s41467-025-58636-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-58636-2
This article is cited by
-
Multifunctional gold nanoparticles: bridging detection, diagnosis, and targeted therapy in cancer
Molecular Cancer (2025)
-
Tumor microenvironment-activated ROS enhancers for effective inhibition of osteosarcoma
BMC Biotechnology (2025)