Abstract
To thrive in extreme conditions, organisms have evolved a diverse arsenal of adaptations that confer resilience. These species, their traits, and the mechanisms underlying them comprise a valuable resource that can be mined for numerous conceptual insights and applied objectives. One of the most dramatic adaptations to water limitation is desiccation tolerance. Understanding the mechanisms underlying desiccation tolerance has important potential implications for medicine, biotechnology, agriculture, and conservation. However, progress has been hindered by a lack of standardization across sub-disciplines, complicating the integration of data and slowing the translation of basic discoveries into practical applications. Here, we synthesize current knowledge on desiccation tolerance across evolutionary, ecological, physiological, and cellular scales to provide a roadmap for advancing desiccation tolerance research. We also address critical gaps and technical roadblocks, highlighting the need for standardized experimental practices, improved taxonomic sampling, and the development of new tools for studying biology in a dry state. We hope that this perspective can serve as a roadmap to accelerating research breakthroughs and unlocking the potential of desiccation tolerance to address global challenges related to climate change, food security, and health.
Similar content being viewed by others
Introduction
Desiccation tolerance is one of nature’s most extraordinary phenomena. Macromolecules, cells, and organisms typically require high internal hydration to function1 and most of our understanding of biology occurs within a narrow moisture window. However, there are some organisms, tissues, and cells that can survive the near complete loss of internal water without dying. Desiccation-tolerant cells and organisms survive drying so extreme that there is insufficient liquid water to form even a single layer of hydration around cellular structures and molecules2,3,4. Understanding the adaptive mechanisms that preserve life in a desiccated state holds promise for various practical applications, including the production, storage, and utilization of agricultural, medicinal, and material products. For example, insight into this phenomenon could drive innovations in optimizing dry storage of germplasms and labile macromolecules, facilitating the long-term preservation of natural diversity, and has potential to accelerate the bioengineering of more resilient crops. Advancing these objectives is critical–now more than ever–given the unprecedented rates of species and genetic diversity loss and the increasing frequency and magnitude of natural disasters5.
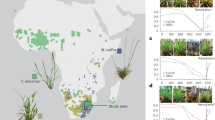
The phenomenon of desiccation tolerance has long captivated and perplexed scientists (Fig. 1)6,7,8,9,10,11,12,13,14,15,16,17, but unraveling the evolutionary, physiological, and genetic basis of this trait has proven a formidable challenge. The inherent complexity of desiccation tolerance and diversity of desiccation-tolerant organisms, coupled with the technical limitations in experimental assays that require an aqueous environment, has hindered progress. Still, research on desiccation tolerance has accelerated in recent years18,19, and this growing interest, combined with technological advances in omics and other high-throughput methodologies, has led to a wealth of data across numerous modalities. This growth offers exciting opportunities to accelerate breakthroughs and potential translational applications, but integration across diverse study systems, scales of biological organization, and individual labs remains a roadblock. Historically, desiccation tolerance research has been conducted in siloed sub-disciplines defined primarily by study organisms, with limited integration of findings across kingdoms18. Currently, there is still considerable variation in the organisms and questions being investigated, as well as the methodologies employed and outcomes tracked. While this diversity is an asset for data and hypothesis generation, standardization across sub-disciplines is necessary to facilitate integration and unlock the full potential of desiccation tolerance research.
Summaries of a evolutionary, b ecological, c morphological, d cellular, and e molecular aspects of desiccation tolerance. f timeline of major natural and research milestones in desiccation tolerance. Colors represent the biological scale of each discovery: purple for discoveries in evolution, blue for discoveries in geography, green for organismal-scale discoveries, yellow for cellular-scale discoveries, and red for discoveries on the molecular scale. Orange represents a historical biological event. For a brief history of the modern discovery of desiccation tolerance, see Alpert (2000). Illustrations in (c–e) by Rachel Torrez.
Here, we provide an overview of the current understanding of desiccation tolerance, summarize gaps and technical roadblocks that should be addressed to accelerate desiccation tolerance research, and highlight its potential translational applications. We outline core conceptual frameworks and methodologies central to desiccation tolerance research, and present working definitions of key terms. We suggest generalized best practices that span organisms and sub-disciplines, which we hope will facilitate knowledge integration. As the field becomes more unified through the adoption of these shared practices, we anticipate significant advances in xeropreservation technology, synthetic biology, and other futuristic applications.
Definition of desiccation tolerance
What is desiccation tolerance? And what does it mean to survive or maintain viability in a dry state? Developing language and consensus around these concepts is critical for integration across the field. We propose a set of working definitions (Box 1), built on historical frameworks, which could be readily adopted by diverse sub-disciplines to facilitate collaboration. To contextualize these definitions, it is important to note that desiccation tolerance and the related term ‘anhydrobiosis’ are fundamentally different from drought avoidance and resistance. Desiccation-tolerant cells dehydrate so completely that essentially all measurable cellular activity ceases, yet somehow they resume healthy cellular function within minutes to hours of rehydration. Desiccation-tolerant cells possess adaptations, either constitutive or induced during dehydration, which preserve cellular integrity in a dry state and support repair upon rehydration. Decades ago, desiccation tolerance was defined as the ability to revive from equilibration with the water potential of the air, which is predominantly low2. In practice, this often equated to surviving equilibration to 50% relative humidity at 20 °C, which corresponds roughly to a water potential of −100 MPa and the point at which the monolayer of water molecules around cellular organelles breaks down4,20. Similarly, anhydrobiosis was defined as the process by which an organism can maintain viability in the dried state where there is insufficient water to support the monolayered sphere of hydration around macromolecules and membranes essential for enzyme activity3,21,22. These definitions, though useful, lack flexibility regarding intermediate water potentials where biological activity is severely inhibited, despite residual water, and do not fully account for the multiple physiological, ecological, and anatomical factors that influence desiccation tolerance. Therefore, we suggest updated definitions (Box 1) that acknowledge the spectrum of dehydration, placing desiccation at one extreme and hydration at the other. While we still find it useful to define thresholds and cutoff points highlighting the extremity of desiccation tolerance, we suggest that tolerance should be described on a continuum, allowing for a more quantitative assessment. Massive shifts in cellular dynamics and material properties occur between −5 MPa and −100 MPa20,23, and tolerance of these intermediate dehydration states is notably variable across organisms and tissues. Dehydration tolerance should therefore be described in relation to minimum recorded water content of the sample, with desiccation tolerance being reserved only for those samples with a minimum recorded water potential < −100 MPa (Box 1).
Evolution of desiccation tolerance
Understanding the evolution of desiccation tolerance is critical for identifying the genetic, physiological, and ecological conditions that enable life at the extreme. The evolutionary processes that gave rise to desiccation tolerance also provide a blueprint for potential translation into applied contexts. Desiccation tolerance has evolved recurrently and convergently across the tree of life24,25,26,27 and is widely distributed across taxa, spanning diverse prokaryotic and eukaryotic lineages (Fig. 1a). Desiccation tolerance is an ancestral adaptation to periodically dry conditions that played a critical role in enabling the transition of early life from aquatic to terrestrial environments. Desiccation tolerance likely arose in ancestral bacteria, archaea, and algal populations as a response to periodic drying28. The rise and diversification of terrestrial organisms was enabled by ancestral traits present in these lineages29,30,31,32. As some lineages diversified and adapted to terrestrial life, they evolved other mechanisms to cope with water scarcity, and many lineages lost their ability to tolerate desiccation as they evolved alternative ways to escape or resist drought33. Interestingly, a few animals maintained desiccation tolerance in their eggs or larvae, as did many vascular plants in their seeds or spores, indicating that the genetic potential for desiccation tolerance was widely retained across hundreds of millions of years of evolution, but its expression was often developmentally restricted to specific tissue types and life stages. In vascular plants, the occurrence of desiccation tolerance in vegetative tissues is thought to be secondary, as compared to the ancestral vegetative desiccation tolerance present in extant streptophytes and bryophytes. This secondary evolution is thought to have occurred through the rewiring of ancestral pathways that were maintained in spores and seeds34,35,36.
There have been multiple evolutionary losses of desiccation tolerance, suggesting that there is a cost associated with maintaining desiccation tolerance when it is not required for survival29,33,37. Indeed, tradeoffs do exist between desiccation tolerance and other traits, such as growth and productivity33, and these tradeoffs partially explain the restricted distribution of many desiccation-tolerant organisms, although there are exceptions38. The evolution of desiccation tolerance is often associated with expansion into marginal habitats, such as bare rock outcrops in the tropics39, tidal zones40, and hyper-arid microclimates. Desiccation tolerance has evolved, been lost, and sometimes subsequently re-gained at different times than the genes that influence desiccation tolerance phenotypes, complicating the identification of evolutionary homologies. For example, genes that underlie non-homologous desiccation tolerance phenotypes (e.g., due to convergent evolution) might well be homologous (e.g., due to shared ancestry), highlighting that some lineages could be genetically predisposed to evolve desiccation tolerance.
The complex evolutionary history of desiccation tolerance has given rise to a wide diversity of desiccation-tolerant organisms and tissues. Not surprisingly, there is substantial variability in the combination of traits expressed in different lineages, and this remains poorly understood, especially on a broad phylogenetic scale41. However, comparisons within and across taxa have been leveraged to identify phenotypic and genomic homologies underlying desiccation tolerance27,42,43,44,45,46. For example, a growing number of sister-species comparisons in vascular plants within families such as Selaginellaceae, Linderniaceae, and Poaceae have shown that the convergent expansion of Early Light Inducible Proteins (ELIPs) distinguish the genomes of desiccation-tolerant plants47,48,49,50,51,52,53. Desiccation tolerance also appears to build on the deeply conserved genetic architecture of water deficit responses27,42,46 and similar genetic features are evident across diverse taxa43. Comparative studies at increasingly shallow evolutionary time scales (e.g., within single species) have highlighted the role of gene duplication and polyploidy in enhancing desiccation tolerance45. Phenotypic plasticity in desiccation tolerance also exists, perhaps best studied in bryophytes54,55 and is itself a trait that can itself evolve. Phenotypic plasticity is a matter of degree, so rather than using categorical terms, a more quantitative framework such as the norm of reaction approach, which compares the phenotypes of each genotype across an environmental gradient56 is recommended. Untangling the complex evolutionary dynamics of desiccation tolerance requires integration across scales and study systems.
Technological and computational advancements have accelerated the use of omics approaches for untangling the evolutionary history of desiccation tolerance across kingdoms57,58. Currently, over a dozen genome assemblies of desiccation-tolerant or so-called “resurrection” plants have been published including for Boea hygrometrica16, Craterostigma plantagineum59, Eragrostis nindensis52, Haberlea rhodopensis60, Lindernia brevidens49, Microchloa afra27, Oropetium thomaeum15,61, O. capense27, Selaginella tamariscina, S. lepidophylla50, Sporobolus stapfianus53, Syntrichia caninervis17, S. ruralis, Tripogon minimus27, and Xerophyta schlechteri, many of which are hosted at the Drying Without Dying database (http://desiccation.novogene.com/home)62 and are coupled with vast RNAseq and multi-omics datasets. Comparatively, fewer genomic resources are available for desiccation-tolerant animals, but genome assemblies exist for the midge Polypedilum vanderplanki63, tardigrades Ramazzottius varieornatus64 Hypsibius dujardini65 and Paramacrobiotus sp66, brine shrimp Artemia franciscana67, rotifers Adineta vaga68, Rotaria macrura, and R. magnacalcarata69, and nematodes Aphelenchus avenae70, Anguina tritici71, and Caenorhabditis elegans72. Genome assemblies are also available for numerous desiccation-tolerant algae73, bacteria, and fungi. Comparative studies that leverage these phylogenetically diverse datasets are a powerful way to understand the evolutionary history and mechanisms of desiccation tolerance43. However, widespread gaps in taxonomic sampling and genomic resources hinder our ability to reconstruct the deep evolutionary history of desiccation tolerance, and additional data are needed to understand contemporary evolution and local adaptation.
Ecology of desiccation tolerance
Understanding the ecological dynamics of desiccation-tolerant species is critical for identifying processes that sustain ecosystems and communities in extreme environments. This knowledge will provide a foundation for translating natural processes into conservation efforts, sustainable management practices, and ecosystem engineering. In many cases, desiccation-tolerant organisms can tolerate a variety of extreme conditions beyond water limitation, including temperature extremes above 100 °C and below 0 °C, high salinity, nearly complete vacuum, intense radiation, and toxins2,74,75. This remarkable cross-tolerance is typically only observed in the desiccated state76,77, but many species also have mechanisms for accelerating their life cycle78,79,80 and avoiding carbon starvation81,82 that facilitate survival in extreme environments.
It might seem intuitive that desiccation-tolerant organisms would be linked to arid regions, but this is common only in some taxonomic groups24,83. Many desiccation-tolerant organisms are found in wetter climates but in microhabitats where water availability is sometimes or always very low, including hypersaline lakes, ephemeral pools, rock outcrops, or tree trunks and canopies76,84,85. So while desiccation-tolerant organisms can be found almost anywhere on Earth, taxa are not evenly distributed (Fig. 1b). Some groups have a cosmopolitan distribution such as desiccation-tolerant tardigrades, nematodes, lichens, bryophytes, and seeds, which are all found from the tropics to Antarctica83,86, but other groups show more geographical and ecological specificity. For example, the diversity of angiosperms with vegetative desiccation tolerance increases towards the tropics and in areas with moderate seasonal conditions41,84. More subtle patterns of spatial distribution and habitat specification are evident within narrower taxonomic groups. For example, desiccation-tolerant eudicots are nearly absent from the Americas41. It is likely that inter- and intra-specific differences in desiccation tolerance phenotypes partially explain ecological and biogeographical patterns. Species native to drier environments tend to tolerate more rapid, complete, or prolonged desiccation81 than species from more mesic environments.
There is a need to link species responses to community dynamics and ecosystem functions, particularly in heterogeneous environments where multiple selective pressures are at play. While data in this area remain limited, assessing community-level dynamics of desiccation tolerance has been explored in some systems. For example, soil crusts that combine desiccation-tolerant bacteria, fungi, algae, lichens, and bryophytes decrease erosion and fix nitrogen in arid systems worldwide and are strongly affected by both climate change and human disturbance87,88. Investigating the interaction of soil crusts (and the desiccation-tolerant organisms that comprise them) with other ecological processes will enhance our understanding of the roles of desiccation tolerance in maintaining biodiversity, ecosystem resilience, and stability.
Physiology and cell biology of desiccation tolerance
Understanding the precise physiological and cellular mechanisms that enable desiccation tolerance is essential for nearly all potential applications. Desiccation tolerance is a complex, emergent phenotype that requires the coordination of numerous cellular processes and biochemical pathways. While many of the central cellular processes are known, how they are regulated and coordinated remains unclear. Despite the diversity of desiccation-tolerant organisms, certain responses are widely shared across distantly related taxa20,43. For example, leaf curling is commonly observed in desiccating plants (Fig. 1c)53,89,90,91,92,93. Similarly, the dauer larvae of midges and nematodes coil up, and tardigrades contract their bodies and retract their limbs during desiccation (Fig. 1c)25,86,94. These structural changes could simply be a consequence of water loss but are also thought to help mitigate mechanical damage due to volume loss, protect brittle appendages, and reduce exposure to photooxidative damage. The pre-emptive cessation of metabolism, including photosynthesis in photoautotrophs, also occurs during desiccation. However the cessation of metabolic activity is a consequence of dehydration95 that does not predict survival, but is only reversible in tolerant cells upon rehydration.
The impacts of dehydration and desiccation on cellular macromolecules and compartments are multifacteted: from intracellular molecular crowding and concomitant higher local concentrations of damaging reactive oxygen species (ROS), to the loss of the molecular hydration layer94,96. ROS-associated DNA damage accrues during drying and in the dry state97,98,99 but chromatin condensation observed in desiccation-tolerant tissues may minimize damage (Fig. 1d)98,100,101,102,103,104,105. Increased molecular concentration leads to partial protein unfolding, misfolding, and aggregation106, which in turn affects the function of enzymes and macromolecular complexes, with the electron transport chains of chloroplasts and mitochondria being major sites of damage in sensitive organisms107,108. In desiccation-tolerant plants and some bacteria, gross changes in cell wall shape are observed, and these dynamics require cellular and tissue remodeling (Fig. 1d)109,110,111. Cellular integrity is maintained under these conditions by complex cell wall remodeling and folding to alleviate mechanical stress112, or by increased vacuolation to maintain volume and shape111. The cytoskeleton also undergoes significant changes as microtubules depolymerize during drying and reassemble during rehydration (Fig. 1d)113. Lipid bilayers exhibit increased fusion events and lipid phase transitions (Fig. 1d), which desiccation-tolerant organisms counter by altering lipid composition early in the drying response114.
Desiccation-tolerant organisms exhibit a dynamic accumulation of protective proteins during the early phase of drying, including various DNA-binding proteins, late embryogenesis abundant (LEA) proteins, heat shock proteins (HSPs), lipocalins, and, in plants, ELIPs (Fig. 1e)106,115,116,117,118,119. Many of these proteins are intrinsically disordered (IDPs) and likely help to prevent protein aggregation, unfolding, and membrane disruption, thereby preserving cellular organization106,120. Some IDPs have now convincingly been shown to confer protection via percolation or gel transitions that maintain cellular organization and prevent drying-induced damage95,121,122,123. The formation of membrane-less compartments via biomolecular condensation or gelation of proteins could also play a role in the desiccation tolerance phenotype124,125,126,127,128.
In desiccation-tolerant organisms, drying also leads to major shifts in carbohydrate metabolism and the accumulation of protective metabolites129,130. Non-reducing sugars such as trehalose, raffinose, and sucrose play central roles in stabilizing desiccated cells and tissues across various life forms, including bacteria, yeast, nematodes, invertebrates, desiccation-tolerant (orthodox) seeds, and vegetatively desiccation-tolerant plants130,131,132,133. For example, animals like brine shrimp, nematodes, and tardigrades accumulate trehalose25,130,134, and the accumulation of raffinose or sucrose appears to play an analogous role in many desiccation-tolerant plants135. DNA damage is mitigated by DNA-binding proteins that enhance desiccation protection136 but there is also evidence of upregulated DNA repair machinery during drying98, suggesting that both DNA protection and repair contribute to establishing the maintenance of DNA integrity when the cells reach the desiccated state. Similarly, sugars and IDPs appear to function synergistically to help maintain membrane fluidity114,130 during the drying process.
At sufficiently low water content, the cytosol will undergo vitrification to adopt a non-crystalline or “glassy” solid state (Box 1; Fig. 1d, e)137. This state reduces molecular motions that would otherwise allow for the unfolding and aggregation of proteins, the fusion of membranes, and the general loss of cellular organization and integrity115,138. While cytosol vitrification is associated with desiccation tolerance, it is not sufficient to confer tolerance, as any sufficiently heterogeneous system (e.g., a cell) will form a vitrified, non-crystalline state upon drying139. The ‘vitrification hypothesis’ has been a longstanding theory which posits that desiccation-tolerant organisms survive drying by vitrifying or forming non-crystalline glasses. The theory suggests that by forming intracellular glasses, an organism induces a super-viscous state in which molecular motions, such as protein unfolding, are slowed to the point that they no longer take place on biological time scales–thus preserving biological form and function. Current work is focused on identifying the properties that distinguish a protective vitrified system from a non-protective one. While there is clearly much more to learn about the material properties and biophysics underlying protective vitrification, an emerging picture makes it clear that glass transition temperature is not the only property contributing to protection by glasses in the dry state139,140,141. Other properties, such as glass former fragility, a measure of how a system’s viscosity increases as it approaches its glass transition temperature and/or dries, also often trend positively with survival in the dry state25,139,140,142.
Roadblocks and key questions in desiccation tolerance research
Despite significant progress in understanding the mechanisms underlying desiccation tolerance, many questions remain (Box 2), and persistent roadblocks hinder comprehensive insights and translational applications. A critical challenge is the lack of standardization in experimental practices, including different methods of assessing water status, methods of drying and rehydrating specimens, the timing of sampling, set of traits measured, and selection of metadata reported. This variability complicates comparisons and synthesis across different studies, organisms, and scales of biological organization. Technical limitations to studying biology in a dry state, including the reliance on water in traditional cell biology techniques, taxonomic sampling gaps, and sparse ecological data, pose added challenges.
To advance research on desiccation tolerance, answer key questions (Box 2), and accelerate potential translational applications, it is essential to address these gaps through the adoption of standardized practices and by promoting the open and equitable sharing of resources to strengthen collaborations. Below, we present a brief guide to central concepts, approaches, and best practices for data generation, standardization, curation, and synthesis in the context of desiccation tolerance. We outline classic dehydration and rehydration experiments, discuss key measurements, and propose essential metadata that can be used to answer key questions in the field.
How do you know if a specimen is desiccation-tolerant?
In order to standardize and integrate across disciplines, we need to agree on how to test if an organism, tissue, or cell is desiccation-tolerant. To determine if a specimen is desiccation-tolerant, we must first observe it in a desiccated state, and subsequently observe it recover when rehydrated. However, this is not as simple as it sounds and many factors impact drying and subsequent recovery. Thus, it is important to test a selection of drying and rehydration scenarios before drawing a conclusion (Fig. 2). Different species tolerate different rates and intensities of drying, durations in a dry state, and rates of rehydration54,92,143. Each species likely has an optimal drying scenario that maximizes desiccation tolerance and this can be established empirically by implementing sequential drying experiments (Box 3). Considering the habitat and environment where the organism naturally occurs can inform the design of ecologically relevant drying scenarios and rehydration methods. Additional factors such as the temperature, light, and starting condition of the specimen can also have a substantial impact on survival and these should always be reported. For example, exposure to previous dry or wet conditions can influence recovery from desiccation in some species144,145 so the experimental and growth conditions of materials should always be reported. Rehydration methods also matter, and while specimens are commonly rehydrated by the addition of liquid water, in some cases, exposure to high humidity prior to the addition of water improves recovery146.
a Shows organismal water content as a function of environmental water availability and the drying parameters that can be varied (rate of drying, intensity of drying, time in the dry state and rate of rehydration). Boxes in b show examples of measurements that can be performed in organismal biology, physiology, cell biology, and multi-omics. Pre-measurement conditions and data integration are important additional considerations. Illustrations in (a) by Rachel Torrez.
Our working definition of desiccation tolerance hinges on measuring the water status of a specimen in its most dehydrated state, and then detecting viability of the sample after rehydration (Box 1). While measuring water potential directly is ideal147, for practical reasons, measuring relative water content (RWC), water content (WC as g H2O g dwt-1), or drying to equilibration with a known relative humidity (RH), may be more feasible (Box 3). Most methods for the direct measurement of water potentials, such as thermocouple psychrometers, dew point sensors, and pressure bombs have practical or technical limitations148,149,150. It is for these reasons that gravimetry-based methods for assessing water status, including RWC and WC, although less comparable across species151, have been adopted (Box 3). Physiological markers associated with water status, such as the cessation of respiration, photosynthesis and other metabolic processes are useful indicators for the early stages of dehydration (between −2 to −15 MPa20), and the extent of vitrification may be used to help validate the more extreme changes in water status. We suggest that measures of both water status and physiology be coupled to improve robustness.
Drying to equilibrium with known relative humidities (Box 3) is the most precise method for varying the rate and intensity of desiccation. This approach works well in many organisms such as bacteria, yeasts, algae, bryophytes, and seeds, but is less useful for vascular plants as it does not mimic their natural processes. Desiccation-tolerant animals are typically desiccated by extended exposure to low humidity environments, but it is unclear if drying continues to equilibrium as water status is typically reported as WC25. Desiccation-tolerant seeds are routinely dried to equilibrium with air at 10 to 20% RH152. However, simply drying an organism to equilibrium with a particular RH to establish if the organism is desiccation-tolerant does not consider the impact of drying rate and rehydration on the tolerance, so again, multiple drying and rehydration scenarios should be assayed.
Our definition of desiccation tolerance also relies on detecting viability after rehydration. Viability is determined by evidence of cellular function after rehydration including metabolism, growth, and reproduction. In practice, this can take multiple forms. Some studies measure growth and development following rehydration, while others measure physiological markers of metabolism and photosynthesis, and others measure water status. Water status alone is not sufficient to determine recovery, and we recommend that a combination of these metrics be reported, with the caveat that researchers measuring growth must distinguish between recovery of existing cells vs. new growth, as only the former demonstrates tolerance. Viability after desiccation is also influenced by the rehydration process, which can impose additional stresses on organisms, so again multiple scenarios should be assayed.
How do you measure physiological and cellular responses to dehydration in desiccation-tolerant organisms?
A suite of physiological processes linked to desiccation tolerance can be measured to gain insight into the degree of stress and mechanisms of tolerance. Most of these manifest in the early stages of desiccation, while water still remains in the tissues. The majority of cellular processes cease at water potentials below −15 MPa20,153 and the components required for desiccation tolerance likely need to be assembled prior to this. Cellular processes vary in their need for water: protein and nucleic acid synthesis ceases when water potentials reach approximately −5 MPa, respiration ceases at ~ −15 MPa, and enzyme activity ceases at ~ −25 MPa153. Biochemical changes at water potentials below this are likely determined by chemical activity and physical forces and are not biologically driven. The specific water requirements for these processes vary across life forms and tissues, highlighting the need for careful characterization of each desiccation-tolerant organism under consideration. Measuring these parameters across a drying timecourse can help to determine the water content at which various cellular processes cease.
Many properties and pathways involved in desiccation tolerance can be surveyed across drying time courses, including oxidant and antioxidant activity, changes in pigment concentration, and in the case of plants, chlorophyll fluorescence parameters such as the maximum (FV/FM) or effective (ΦPSII) quantum yield of photosystem II. To complement these traditional physiological measures, changes in the amount and combination of various sugars, such as trehalose, sucrose, raffinose family oligosaccharides, and other metabolites can be tested via metabolomics135, and proteins and transcripts can be quantified via proteomics and transcriptomics. For desiccation-tolerant organisms that undergo dry-wet-dry cycles in nature, the carbon balance metric82,154,155, employing infrared gas analysis, is especially illustrative of physiological tolerance. While this technique was originally developed for use with photosynthetic organisms, any organism that exchanges gas with a headspace (e.g., biocrust communities156) can be measured with respect to carbon balance, providing insights into the relative level of metabolism during dry-wet-dry cycles. Carbon balance measurements can be applied to communities to assess combined and emergent phenotypes or on individual community members (excised or grown separately) to examine individual roles of organisms82. Similarly, oxygen consumption can be measured in non-photosynthetic anhydrobiotes (e.g., brine shrimp, nematodes, tardigrades) during desiccation157. Biophysical and material properties of dried organisms, like changes in cytoplasmic vitrification, stability of sugar glasses, molecular mobility, H-bonding patterns, and molecular packing are informative and can be assayed by various forms of spectroscopy158,159. Traditionally, glass transition temperature has been considered a key protective property distinguishing desiccation-tolerant from sensitive vitrified systems. However, recent work suggests that properties such as glass fragility can be just as important in conferring desiccation tolerance and therefore warrant further research attention139.
Viewing organisms and cells in hydrated, dehydrated, and rehydrating states is useful and relies on microscopy and, in some cases, spectroscopy. Changes to the structure of cells during desiccation and rehydration can be observed using electron and light microscopy160 if appropriate fixation techniques are utilized to prevent rehydration of dried samples. To observe changes in protein localization and organelle morphology during desiccation and recovery, proteins can be tagged with fluorescent markers and visualized by fluorescent microscopy122,125,161. Changes in cellular rehydration can be measured using genetically encoded multimeric nanoparticles162, which are fluorescently labeled particles within cells that allow monitoring of changes in sub-cellular diffusion and cytoplasmic viscosity163. Similarly, changes to intracellular molecular crowding during osmotic stress can further be assayed using the Förster Resonance Energy Transfer based sensor SED1164. Cell viability and division can be measured either by using appropriate stains, microscopy, or growth assays such as colony forming units in microbes165, or tissue growth in plants90. In addition, cell membrane phase behavior can be quantified using Fourier transform infrared spectroscopy166, and the viscosity of tissue can be probed by electron paramagnetic resonance158.
How do you integrate omics data to understand desiccation tolerance at the systems level?
Systems-level analyses coupling genomics, transcriptomics, proteomics, and metabolomics with traditional physiology and cell biology offer powerful tools to elucidate the complex mechanisms underlying desiccation tolerance. Desiccating cells undergo massive shifts in transcriptomic, proteomic, and metabolomic profiles, and capturing these changes has been central to dissecting the mechanisms of desiccation tolerance. However, these high-dimensional omics studies often suffer from inconsistent methodologies and incomplete metadata reporting, similar to those observed more broadly in the plant drought response literature167. It is also important to recognize that the presence of a compound (e.g., mRNA, protein, metabolite) does not provide information about the balance between synthesis and degradation. Indeed, there is often very little correlation between transcript abundance and protein synthesis168, and this also extends to the relationship between protein abundance and metabolite levels169. As cells dry, their ability to synthesize transcripts, proteins, and metabolites becomes compromised20. Thus, increases in transcripts, proteins, and metabolites late in the drying process are likely the result of changes in turnover rates, stability, or sequestration, as seen in dehydrating Syntrichia ruralis170. This is particularly relevant to studies that are focused on the longevity of desiccated samples where alterations in cellular components (transcripts, metabolites, etc.) are likely to result from instability, chemical activity, or physical forces rather than biological activity since cellular processes are non-operative in the desiccated state. Such samples may also contain changing fractions of potentially viable and nonviable components (cells, tissues), which can be difficult to decipher in pooled samples. This begs the question as to whether or not components that accumulate late in the drying process are important during drying, are necessary for recovery upon rehydration, or are needed to replenish depleted pools.
Paired multi-omic datasets with complete metadata are needed to decipher these dynamics (Fig. 2). Recent technological advances that enable measurements of genome accessibility and ribosome occupancy can help resolve these uncertainties and many types of measurements can be performed simultaneously171. Experiments that are designed to integrate physiological, cellular, and -omic level processes will allow for critical connections between form and function in desiccation tolerance research. Expanding these studies further to investigate community level responses is another important area for future research. Such experiments also provide an obvious opportunity to collaborate across labs and institutions to bring diverse expertises together.
How do you manage, curate, and share your desiccation tolerance research data?
Desiccation tolerance research has generated large volumes of data across all omics modalities as well as established and emerging laboratory and in silico techniques. Such data can be expensive to generate and, in the absence of harmonized metadata referring to common technical standards, difficult to integrate between experiments, let alone between laboratories167 and sub-disciplines.
To make efficient use of research funds and embrace productivity gains from emerging computational techniques, data must be shared in a FAIR (Findable, Accessible, Interoperable and Reusable) manner. An attempt to achieve this has been initiated for desiccation-tolerant plants in the Drying without Dying database62, but community buy-in and extension to additional study organisms are needed. Shared practices should not only apply to the laboratory, but be documented and annotated with appropriate machine and human-readable metadata (Supplementary Table 1), and deposited in reputable repositories that can guarantee longevity (Supplementary Table 2). Appropriate metadata standards should capture and report sample histories and status, and relevant environmental parameters such as the RH, light, temperature, and water status. Standardized physiological data (e.g., water status, respiration, photosynthesis, etc.) should accompany all omics studies to enable comparative analyses, and sampling times should account for circadian regulation. Relevant standards and guidelines for reporting data and metadata are listed in Supplementary Table 3.
Prospects and future vision
Research on desiccation tolerance has immense potential (Fig. 3). The adoption of standardized methods and FAIR data practices will streamline the development of novel medical drugs and biofluids, enhance techniques for the dry storage of cells, and stable biologicals such as therapeutic antibodies and mRNA vaccines, and provide genetic tools for engineering biostasis. This standardization could also accelerate the engineering of crops that are more resilient to water limitation, improve seed priming and conservation efforts, and target pathogen dormancy, while inspiring the creation of new environmentally responsive materials that mimic these biological processes for industrial, agricultural, and medical applications.
a De-siloing sub-disciplines of desiccation tolerance research. b Future prospects and possible applications of desiccation tolerance research. In (a), colors represent research in desiccation tolerance at different biological scales (evolution, geography, organism, cell, and molecule). In (b), colors represent broad categories for aspirational goals in desiccation tolerance research: purple for social and community growth and development, blue for geographical advancement, green for organismal-scale applications, yellow for cellular-scale applications, and red for applications on the molecular scale. Orange represents broad, general advancements in desiccation tolerance research.
The study of desiccation tolerance has long promised to improve human health and medical technology. For example, understanding how pathogens or disease vectors tolerate desiccation will help combat the spread of disease and could yield new drug targets172,173,174. Metabolites such as trehalose, phenolic compounds, and essential oils derived from desiccation-tolerant organisms have been recognized for their antioxidant, anti-obesity, anti-inflammatory, antimicrobial, osmotic stress reducing, and chemoprotective properties, and thus carry potential for cosmetics, nutrition, and food storage175. Extracts from several desiccation-tolerant plants are already used in anti-aging, skin protective, and moisturizing lotions. Dietary intake of these compounds has been associated with lower rates of cancer, diabetes, and cardiovascular diseases and might improve meat quality, growth, and gut function when used in animal nutrition176,177,178,179,180,181,182. However there is a lack of information on possible side effects or contaminations in these extracts and low biomass production challenges the sustainable harvesting of desiccation-tolerant organisms. These challenges should be tackled by the development of effective production systems, regulations to ensure sustainable use, and comprehensive toxicological studies, guidelines, and regulatory frameworks to facilitate safe consumer products178,182,183,184.
Promising applications also exist in “xero-” or dry-preservation of biological materials that would mitigate the dependence on costly and logistically difficult cryo-preservation and cold chain logistics. Currently, the state of the art lies in xeropreservation of simple biologics such as vaccines and other molecular therapeutics185,186 as well as cell-based biologics including platelets and stem cells187,188,189,190, but xeropreservation of tissues and increasingly complex systems could be on the horizon. Advancing these techniques will require refinement of preservation techniques, possibly leveraging novel excipients, formulations, and loading methods. Future advances could someday move beyond molecules and cells to tissues, organs, or even whole-organism stabilization. Of course, immunogenicity and toxicity studies are needed to assess the safety of novel excipients and biologics stored under new conditions, but this area holds promise.
Desiccation-tolerant plants and seeds also hold a wealth of information relevant to agriculture, biotechnology, and material science. Through direct genetic manipulation of key pathways and their regulators, desiccation-sensitive seeds and crops could be made more tolerant of water deprivation. Seeds of many tropical plants, including high-value crops such as coffee, cocoa, and mango are desiccation sensitive. Understanding desiccation sensitivity and tolerance in these seeds is crucial for safeguarding the germplasm of this important biodiversity. Even for traditional crops which are unlikely to face vegetative desiccation, research on desiccation tolerance mechanisms could help improve performance under periodic water deficits. For instance, desiccation inspired traits related to osmotic adjustment, protective pigments, and oxidative stress management could inform breeding strategies that enhance recovery under drought stress. We acknowledge that extreme water deficit experienced by desiccation-tolerant organisms differs substantially from milder, sub-lethal drought stresses that agricultural crops are faced with. However, metabolic and protective pathways activated in desiccation-tolerant plants could be used to enhance survival across a spectrum of water-limited conditions, especially in subsistence agriculture, not just for engineering crops that survive desiccation outright. As modern crop breeding shifts focus from purely maximizing productivity to enhancing resilience and ecological functionality, desiccation tolerance research provides an opportunity for developing crops that balance productivity with resilience under marginal conditions. Studies have already demonstrated that overexpression of LEA, HSPs, and other key desiccation tolerance genes can improve photosynthetic rates and recovery under moderate drought stress191,192,193,194. While industrialized, high-input agriculture seeks to optimize yield, in regions where drought-related crop failures can lead to total food insecurity (e.g., smallholder or subsistence farming systems), having a crop that can survive a period of water deficit—enabling partial yield after rain returns—would be transformative. Indirect approaches, which circumvent the negative public perception of genetically modified organisms and the plant transformation bottleneck, should also be exploited. For example, desiccation-tolerant plants harbor valuable microbial diversity that can be leveraged to enhance microbe-mediated drought tolerance195,196,197. The root-associated microbiota of multiple desiccation-tolerant plants are currently being tested as biostimulants and stress-tolerance enhancers with promising results198,199.
Maximizing potential through interdisciplinary collaboration and community building
Tremendous progress in understanding desiccation tolerance has been driven by diverse research communities around the world. These communities have pushed our understanding of how organisms across the domains of life survive desiccation, providing insight into the biophysical, molecular, cellular, organismal, ecological, and evolutionary mechanisms of tolerance. With these advances, our hope is that we are now on the verge of unifying the field to translate these results to practical uses.
In order to achieve these lofty goals, global partnerships and interdisciplinary collaborations are needed. Collaborations that span the scientific disciplines of biology, engineering, computer science, and beyond should be coupled with partnerships across governmental agencies, entrepreneurs, and local communities. Integrating expertise and principles from material engineering, computer science, and biophysics will accelerate the development of new tools for studying biology in a dry state and analytical approaches for integrating multidimensional datasets. Of course, partnerships with governmental agencies, non-profit organizations, and entrepreneurs are critical for bringing advances to consumers while ensuring legal and ethical compliance.
Breaking down the silos within desiccation tolerance research will require investing in collaborations across diverse disciplines and global regions. Given that desiccation-tolerant organisms are widely distributed with many important diversity hotspots in the Global South, collaborations should respect the sovereignty and intellectual property rights of local and indigenous communities, with equitable benefit-sharing agreements established at their inception to ensure compensation for germplasm or traditional knowledge. Partnerships should also ensure that technologies for studying desiccation tolerance are both accessible and affordable. Open access to research tools, data, and sharing platforms can democratize science, allowing a broader range of researchers, including those from less affluent regions, to participate. We advocate for developing methods and approaches that are cost-effective and easily accessible across different geographical and economic landscapes. By continuing to encourage and push for an inclusive, equitable, and interdisciplinary global research network, we can help support a new standard for desiccation tolerance research.
References
Walters, C., Hill, L. M. & Wheeler, L. J. Dying while dry: kinetics and mechanisms of deterioration in desiccated Organisms1. Integr. Comp. Biol. 45, 751–758 (2005).
Bewley, J. D. Physiological aspects of desiccation tolerance. Annu. Rev. Plant Physiol. 30, 195–238 (1979).
Crowe, J. H., Hoekstra, F. A. & Crowe, L. M. Anhydrobiosis. Annu. Rev. Physiol. 54, 579–599 (1992).
Alpert, P. & Oliver, M. J. Drying without dying. in Desiccation and survival in plants: drying without dying (CABI Publishing, 2002).
IPCC, Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (eds. Core Writing Team, H. Lee and J. Romero), pp. 35–115, (IPCC, Geneva, Switzerland, 2023).
Wellman, C. H., Osterloff, P. L. & Mohiuddin, U. Fragments of the earliest land plants. Nature 425, 282–285 (2003).
Hayes, E. H. et al. 65,000-years of continuous grinding stone use at Madjedbebe. North. Aust. Sci. Rep. 12, 1–17 (2022).
Snir, A. et al. The origin of cultivation and proto-weeds, long before neolithic farming. PLoS ONE 10, e0131422 (2015).
Theophrastus. Enquiry into Plants and Minor Works on Odours and Weather Signs. (W. Heinemann, London, 1916).
Hamarneh, S. Measuring the Invisible World. The life and works of Antoni van Leeuwenhoek. (Abelard-Schuman, New York, 1959).
Keilin, D. The Leeuwenhoek Lecture - The problem of anabiosis or latent life: history and current concept. Proc. R. Soc. Lond. Ser. B - Biol. Sci. 150, 149–191 (1997).
Heckly, R. J. & Dimmick, R. L. Correlations between free radical production and viability of lyophilized bacteria. Appl. Microbiol. 16, 1081–1085 (1968).
Koster, K. L. & Leopold, A. C. Sugars and desiccation tolerance in seeds. Plant Physiol. 88, 829–832 (1988).
Bartels, D., Schneider, K., Terstappen, G., Piatkowski, D. & Salamini, F. Molecular cloning of abscisic acid-modulated genes which are induced during desiccation of the resurrection plant Craterostigma plantagineum. Planta 181, 27–34 (1990).
VanBuren, R. et al. Single-molecule sequencing of the desiccation-tolerant grass Oropetium thomaeum. Nature 527, 508–511 (2015).
Xiao, L. et al. The resurrection genome of Boea hygrometrica: 112, 5833–5837 (2015).
Silva, A. T. et al. To dry perchance to live: insights from the genome of the desiccation-tolerant biocrust moss Syntrichia caninervis. Plant J. 105, 1339–1356 (2021).
Lotreck, S. G., Ghassemi, M. & VanBuren, R. T. Unifying the research landscape of desiccation tolerance to identify trends, gaps, and opportunities. bioRxiv https://doi.org/10.1101/2024.06.06.597802 (2024).
Tebele, S. M., Marks, R. A. & Farrant, J. M. Two decades of desiccation biology: a systematic review of the best studied angiosperm resurrection plants. Plants 10, 2784 (2021).
Oliver, M. J. et al. Desiccation Tolerance: avoiding cellular damage during drying and rehydration. Annu. Rev. Plant Biol. https://doi.org/10.1146/annurev-arplant-071219-105542 (2020).
Womersley, C. Biochemical and physiological aspects of anhydrobiosis. Comp. Biochem. Physiol. Part B: Comp. Biochem. 70, 669–678 (1981).
Billi, D. & Potts, M. Life and death of dried prokaryotes. Res. Microbiol. 153, 7–12 (2002).
Walters, C., Farrant, J. M., Pammenter, N. & Berjak, P. Desiccation stress and damage. in Desiccation and Survival in Plants: Drying Without Dying (eds. Black, M. & Pritchard, H. W.) 263–291 (CABI, 2002).
Oliver, M. J., Velten, J. & Mishler, B. D. Desiccation tolerance in bryophytes: a reflection of the primitive strategy for plant survival in dehydrating habitats? Integr. Comp. Biol. 45, 788–799 (2005).
Hibshman, J. D., Clegg, J. S. & Goldstein, B. Mechanisms of desiccation tolerance: themes and variations in brine shrimp, roundworms, and tardigrades. Front. Physiol. 11, 592016 (2020).
Burki, F., Sandin, M. M. & Jamy, M. Diversity and ecology of protists revealed by metabarcoding. Curr. Biol. 31, R1267–R1280 (2021).
Marks, R. A., Van Der Pas, L., Schuster, J., Gilman, I. S. & VanBuren, R. Convergent evolution of desiccation tolerance in grasses. Nat. Plants 10, 1112–1115 (2024).
Jaffe, A. L., Castelle, C. J. & Banfield, J. F. Habitat transition in the evolution of bacteria and Archaea. Annu. Rev. Microbiol. 77, 193–212 (2023).
Oliver, M. J., Tuba, Z. & Mishler, B. D. The evolution of vegetative desiccation tolerance in land plants. Plant Ecol. 151, 85–100 (2000).
Herburger, K. & Holzinger, A. Localization and quantification of callose in the streptophyte green Algae Zygnema and Klebsormidium: 56, cv139 (2015).
Fürst-Jansen, J. M. R., de Vries, S. & de Vries, J. Evo-physio: on stress responses and the earliest land plants. J. Exp. Bot. 71, 3254–3269 (2020).
Bierenbroodspot, M. J. et al. Phylogenomic insights into the first multicellular streptophyte. Curr. Biol. 34, 670–681.e7 (2024).
Alpert, P. Constraints of tolerance: why are desiccation-tolerant organisms so small or rare? J. Exp. Biol. 209, 1575–1584 (2006).
Costa, M.-C. D. et al. A footprint of desiccation tolerance in the genome of Xerophyta viscosa. Nat. Plants 3, 17038 (2017).
VanBuren, R. Desiccation tolerance: seedy origins of resurrection. Nat. Plants 3, 17046 (2017).
VanBuren, R. et al. Seed desiccation mechanisms co-opted for vegetative desiccation in the resurrection grass Oropetium thomaeum. Plant Cell Environ. 40, 2292–2306 (2017).
Gusev, O. et al. Comparative genome sequencing reveals genomic signature of extreme desiccation tolerance in the anhydrobiotic midge. Nat. Commun. 5, 4784 (2014).
Smrithy, V., Kulkarni, A., Shigwan, B. K., Porembski, S. & Datar, M. N. Desiccation‐tolerant vascular plants from Western Ghats, India: review, updated checklist, future prospects and new insights. Nord. J. Bot. https://doi.org/10.1111/njb.03939 (2023).
Porembski, S. et al. An overview on desiccation-tolerant mat-forming monocotyledons on tropical inselbergs. Flora 285, 151953 (2021).
Peredo, E. L. & Cardon, Z. G. Shared up-regulation and contrasting down-regulation of gene expression distinguish desiccation-tolerant from intolerant green algae. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.1906904117 (2020).
Bondi, L. The desiccation-tolerant vascular plants’ paradox: the role of environmental constraints on the diversity and distribution of plants able to resurrect from dryness. (Universität Rostock). https://doi.org/10.18453/rosdok_id00004404 (2023).
Alejo-Jacuinde, G., González-Morales, S. I., Oropeza-Aburto, A., Simpson, J. & Herrera-Estrella, L. Comparative transcriptome analysis suggests convergent evolution of desiccation tolerance in Selaginella species. BMC Plant Biol. 20, 468 (2020).
Browne, J., Tunnacliffe, A. & Burnell, A. Anhydrobiosis: plant desiccation gene found in a nematode. Nature 416, 38 (2002).
Marks, R. A. et al. Variability in functional traits along an environmental gradient in the South African Resurrection Plant Myrothamnus flabellifolia. Plants 11, 1332 (2022).
Marks, R. A. et al. Higher order polyploids exhibit enhanced desiccation tolerance in the grass Microchloa afra. J. Exp. Bot. https://doi.org/10.1093/jxb/erae126 (2024).
Zhang, X. et al. Syntrichia ruralis: emerging model moss genome reveals a conserved and previously unknown regulator of desiccation in flowering plants. N. Phytol. https://doi.org/10.1111/nph.19620 (2024).
Oliver, M. J. et al. A sister group contrast using untargeted global metabolomic analysis delineates the biochemical regulation underlying desiccation tolerance in Sporobolus stapfianus. Plant Cell 23, 1231–1248 (2011).
Yobi, A., Wone, B. W., Xu, W., Alexander, D. C. & Guo, L. Comparative metabolic profiling between desiccation‐sensitive and desiccation‐tolerant species of Selaginella reveals insights into the resurrection trait. Plant 6, 983–999 (2012).
VanBuren, R. et al. Desiccation tolerance evolved through gene duplication and network rewiring in Lindernia. Plant Cell 30, 2943–2958 (2018).
VanBuren, R. et al. Extreme haplotype variation in the desiccation-tolerant clubmoss Selaginella lepidophylla. Nat. Commun. 9, 13 (2018).
Silva Artur, M. A., Costa, M.-C. D., Farrant, J. M. & Hilhorst, H. W. M. Genome-level responses to the environment: plant desiccation tolerance. Emerg. Top. Life Sci. 3, 153–163 (2019).
Pardo, J. et al. Intertwined signatures of desiccation and drought tolerance in grasses. Proc. Natl Acad. Sci. USA 117, 10079–10088 (2020).
Montes, R. A. C. et al. A comparative genomics examination of desiccation tolerance and sensitivity in two sister grass species. Proc. Natl Acad. Sci. USA 119, e2118886119 (2022).
Stark, L. R., Greenwood, J. L., Slate, M. L. & Brinda, J. C. Syntrichia norvegica shoots exhibit a complex inducible response to desiccation: separating the effects of rate of drying and water content. Botany 95, 481–491 (2017).
Xiao, L., Yobi, A., Koster, K. L., He, Y. & Oliver, M. J. Desiccation tolerance in Physcomitrella patens: Rate of dehydration and the involvement of endogenous abscisic acid (ABA). Plant Cell Environ. 41, 275–284 (2018).
Sultan, S. E. Phenotypic plasticity for plant development, function and life history. Trends Plant Sci. 5, 537–542 (2000).
Dinakar, C. & Bartels, D. Desiccation tolerance in resurrection plants: new insights from transcriptome, proteome and metabolome analysis. Front. Plant Sci. 4, 482 (2013).
Gechev, T., Lyall, R., Petrov, V. & Bartels, D. Systems biology of resurrection plants. Cell. Mol. Life Sci. https://doi.org/10.1007/s00018-021-03913-8 (2021).
VanBuren, R. et al. Core cellular and tissue-specific mechanisms enable desiccation tolerance in Craterostigma. Plant J. 114, 231–245 (2023).
Gupta, S. et al. The genome of Haberlea rhodopensis provides insights into the mechanisms for tolerance to multiple extreme environments. Cell. Mol. Life Sci. 81, 117 (2024).
VanBuren, R., Wai, C. M., Keilwagen, J. & Pardo, J. A chromosome‐scale assembly of the model desiccation tolerant grass Oropetium thomaeum. Plant Direct 2, e00096 (2018).
Gao, B. et al. Drying without Dying: a genome database for desiccation-tolerant plants and evolution of desiccation tolerance. Plant Physiol. https://doi.org/10.1093/plphys/kiad672 (2023).
Yoshida, Y. et al. High quality genome assembly of the anhydrobiotic midge provides insights on a single chromosome-based emergence of extreme desiccation tolerance. NAR Genom. Bioinform. 4, lqac029 (2022).
Hashimoto, T. et al. Extremotolerant tardigrade genome and improved radiotolerance of human cultured cells by tardigrade-unique protein. Nat. Commun. 7, 12808 (2016).
Arakawa, K., Yoshida, Y. & Tomita, M. Genome sequencing of a single tardigrade Hypsibius dujardini individual. Sci. Data 3, 160063 (2016).
Hara, Y., Shibahara, R., Kondo, K., Abe, W. & Kunieda, T. Parallel evolution of trehalose production machinery in anhydrobiotic animals via recurrent gene loss and horizontal transfer. Open Biol. 11, 200413 (2021).
De Vos, S. et al. The genome of the extremophile Artemia provides insight into strategies to cope with extreme environments. BMC Genom. 22, 635 (2021).
Flot, J.-F. et al. Genomic evidence for ameiotic evolution in the bdelloid rotifer Adineta vaga. Nature 500, 453–457 (2013).
Nowell, R. W. et al. Comparative genomics of bdelloid rotifers: insights from desiccating and nondesiccating species. PLoS Biol. 16, e2004830 (2018).
Wan, X. et al. The Aphelenchus avenae genome highlights evolutionary adaptation to desiccation. Commun. Biol. 4, 1232 (2021).
Singh, A. K. et al. Unveiling the draft genome of the seed gall nematode, Anguina tritici: insights and analysis. Physiol. Mol. Plant Pathol. 133, 102330 (2024).
C. elegans Sequencing Consortium. Genome sequence of the nematode C. elegans: a platform for investigating biology. Science 282, 2012–2018 (1998).
Feng, X. et al. Genomes of multicellular algal sisters to land plants illuminate signaling network evolution. Nat. Genet. 56, 1018–1031 (2024).
Hinton, H. E. Cryptobiosis in the larva of Polypedilum vanderplanki Hint. (Chironomidae). J. Insect Physiol. 5, 286–300 (1960).
Worland, M. R. & Block, W. Desiccation stress at sub-zero temperatures in polar terrestrial arthropods. J. Insect Physiol. 49, 193–203 (2003).
Vanhaecke, P., Tackaert, W. & Sorgeloos, P. The biogeography of Artemia: an updated review. 1, 129–155 (1987).
Browne, R. A. & Wanigasekera, G. Combined effects of salinity and temperature on survival and reproduction of five species of Artemia. J. Exp. Mar. Bio. Ecol. 244, 29–44 (2000).
Su, Z. et al. Flower development under drought stress: morphological and transcriptomic analyses reveal acute responses and long-term acclimation in Arabidopsis. Plant Cell 25, 3785–3807 (2013).
Centeno, D. C., Hell, A. F., Braga, M. R., Del Campo, E. M. & Casano, L. M. Contrasting strategies used by lichen microalgae to cope with desiccation-rehydration stress revealed by metabolite profiling and cell wall analysis. Environ. Microbiol. 18, 1546–1560 (2016).
Hell, A. F. et al. Metabolic changes on the acquisition of desiccation tolerance in seeds of the Brazilian native tree Erythrina speciosa. Front. Plant Sci. 10, 1356 (2019).
Alpert, P. & Oechel, W. C. Comparative patterns of net photosynthesis in an assemblage of mosses with contrasting microdistribution. Am. J. Bot. 74, 1787–1796 (1987).
Coe, K. K., Belnap, J. & Sparks, J. P. Precipitation-driven carbon balance controls survivorship of desert biocrust mosses. Ecology 93, 1626–1636 (2012).
Kranner, I., Beckett, R., Hochman, A. & Nash, T. H. III Desiccation-tolerance in lichens: a review. Bryologist 111, 576–593 (2008).
Porembski, S. & Barthlott, W. Granitic and Gneissic Outcrops (inselbergs) as Centers of Diversity for Desiccation-Tolerant Vascular Plants. 151 19–28 https://link.springer.com/content/pdf/10.1023%2FA%3A1026565817218.pdf (2000).
Ngarari, M. M. et al. Salinity tolerance, growth and survival of three Artemia franciscana (Kellogg, 1906) populations under laboratory conditions. Aquac. Fish Fish. 4, e166 (2024).
Wełnicz, W., Grohme, M. A., Kaczmarek, L., Schill, R. O. & Frohme, M. Anhydrobiosis in tardigrades-the last decade. J. Insect Physiol. 57, 577–583 (2011).
Elbert, W. et al. Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nat. Geosci. 5, 459–462 (2012).
Ferrenberg, S., Reed, S. C. & Belnap, J. Climate change and physical disturbance cause similar community shifts in biological soil crusts. Proc. Natl. Acad. Sci. USA 112, 12116–12121 (2015).
Suguiyama, V. F., Silva, E. A., Meirelles, S. T., Centeno, D. C. & Braga, M. R. Leaf metabolite profile of the Brazilian resurrection plant Barbacenia purpurea Hook. (Velloziaceae) shows two time-dependent responses during desiccation and recovering. Front. Plant Sci. 5, 96 (2014).
Leprince, O. & Buitink, J. Introduction to desiccation biology: from old borders to new frontiers. Planta 242, 369–378 (2015).
Neeragunda Shivaraj, Y. et al. Perspectives on structural, physiological, cellular, and molecular responses to desiccation in resurrection plants. Scientifica 2018, 9464592 (2018).
Coe, K. K. et al. Strategies of desiccation tolerance vary across life phases in the moss Syntrichia caninervis. Am. J. Bot. 108, 249–262 (2021).
Prats, K. A. & Brodersen, C. R. Desiccation and rehydration dynamics in the epiphytic resurrection fern Pleopeltis polypodioides. Plant Physiol. 187, 1501–1518 (2021).
Giovannini, I. et al. Production of reactive oxygen species and involvement of bioprotectants during anhydrobiosis in the tardigrade Paramacrobiotus spatialis. Sci. Rep. 12, 1938 (2022).
Sanchez-Martinez, S. et al. Labile assembly of a tardigrade protein induces biostasis. Protein Sci. 33, e4941 (2024).
Ren, Q., Brenner, R., Boothby, T. C. & Zhang, Z. Membrane and lipid metabolism plays an important role in desiccation resistance in the yeast Saccharomyces cerevisiae. BMC Microbiol. 20, 338 (2020).
Neumann, S., Reuner, A., Brümmer, F. & Schill, R. O. DNA damage in storage cells of anhydrobiotic tardigrades. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 153, 425–429 (2009).
Gusev, O. et al. Anhydrobiosis-associated nuclear DNA damage and repair in the sleeping chironomid: linkage with radioresistance. PLoS ONE 5, e14008 (2010).
Horikawa, D. D. et al. Analysis of DNA repair and protection in the Tardigrade Ramazzottius varieornatus and Hypsibius dujardini after exposure to UVC radiation. PLoS ONE 8, e64793 (2013).
Hickernell, L. M. A study of desiccation in the rotifer, Philodina roseola, with special reference to cytological changes accompanying desiccation. Biol. Bull. 32, 343–406 (1917).
Kater, J. M. A cytological study of dormancy in the seed of phaseolus vulgaris. Ann. Bot. os-41, 629–642 (1927).
Hallam, N. D. & Luff, S. E. Fine structural changes in the leaves of the desiccation-tolerant plant talbotia elegans during extreme water stress. Bot. Gaz. 141, 180–187 (1980).
Proctor, M. C. F., Ligrone, R. & Duckett, J. G. Desiccation tolerance in the moss Polytrichum formosum: physiological and fine-structural changes during desiccation and recovery. Ann. Bot. 99, 75–93 (2007).
Czerneková, M., Janelt, K., Student, S., Jönsson, K. I. & Poprawa, I. A comparative ultrastructure study of storage cells in the eutardigrade Richtersius coronifer in the hydrated state and after desiccation and heating stress. PLoS ONE 13, e0201430 (2018).
Crèvecoeur, M., Deltour, R. & Bronchart, R. Cytological study on water stress during germination of Zea mays. Planta 132, 31–41 (1976).
Romero-Perez, P. S., Dorone, Y., Flores, E., Sukenik, S. & Boeynaems, S. When phased without water: biophysics of cellular desiccation, from biomolecules to condensates. Chem. Rev. 123, 9010–9035 (2023).
Challabathula, D., Zhang, Q. & Bartels, D. Protection of photosynthesis in desiccation-tolerant resurrection plants. J. Plant Physiol. 227, 84–92 (2018).
Chen, C.-L. et al. A crucial role of mitochondrial dynamics in dehydration resistance in Saccharomyces cerevisiae. Int. J. Mol. Sci. 22, 4607 (2021).
Moore, J. P. et al. Response of the leaf cell wall to desiccation in the resurrection plant Myrothamnus flabellifolius. Plant Physiol. 141, 651–662 (2006).
Moore, J. P., Vicré-Gibouin, M., Farrant, J. M. & Driouich, A. Adaptations of higher plant cell walls to water loss: drought vs desiccation. Physiol. Plant. 134, 237–245 (2008).
Chen, P., Jung, N. U., Giarola, V. & Bartels, D. The dynamic responses of cell walls in resurrection plants during dehydration and rehydration. Front. Plant Sci. 10, 1698 (2019).
Ilias, I. A., Wagiran, A., Azizan, K. A., Ismail, I. & Samad, A. F. A. Irreversibility of the cell wall modification acts as a limiting factor in desiccation tolerance of Oryza sativa ssp. Indica cv MR303. Plant Stress 12, 100463 (2024).
Faria, J. M. R., Buitink, J., van Lammeren, A. A. M. & Hilhorst, H. W. M. Changes in DNA and microtubules during loss and re-establishment of desiccation tolerance in germinating Medicago truncatula seeds. J. Exp. Bot. 56, 2119–2130 (2005).
Hoekstra, F. A., Golovina, E. A. & Buitink, J. Mechanisms of plant desiccation tolerance. Trends Plant Sci. 6, 431–438 (2001).
Boothby, T. C. et al. Tardigrades use intrinsically disordered proteins to survive desiccation. Mol. Cell 65, 975–984.e5 (2017).
Vanburen, R., Pardo, J., Wai, C. M., Evans, S. & Bartels, D. Massive tandem proliferation of ELIPs supports convergent evolution of desiccation 3 tolerance across land plants 4 5 Short title: Desiccation tolerance arose via ELIP duplication. Plant Physiol. Preview https://doi.org/10.1104/pp.18.01420 (2019).
Hernández-Sánchez, I. E. et al. LEAfing through literature: late embryogenesis abundant proteins coming of age-achievements and perspectives. J. Exp. Bot. 73, 6525–6546 (2022).
Kc, S. et al. Disordered proteins interact with the chemical environment to tune their protective function during drying. eLife https://doi.org/10.7554/eLife.97231.3 (2024).
Biswas, S. et al. Helicity of a tardigrade disordered protein contributes to its protective function during desiccation. Protein Sci. 33, e4872 (2024).
Boothby, T. C. & Pielak, G. J. Intrinsically disordered proteins and desiccation tolerance: elucidating functional and mechanistic underpinnings of anhydrobiosis. Bioessays 39, 1700119 (2017).
Hesgrove, C. S. et al. Molecular Swiss Army Knives: Tardigrade CAHS proteins mediate desiccation tolerance through multiple mechanisms. bioRxiv https://doi.org/10.1101/2021.08.16.456555 (2021).
Tanaka, A. et al. Stress-dependent cell stiffening by tardigrade tolerance proteins that reversibly form a filamentous network and gel. PLoS Biol. 20, e3001780 (2022).
Sanchez-Martinez, S., Ramirez, J. F., Meese, E. K., Childs, C. A. & Boothby, T. C. The tardigrade protein CAHS D interacts with, but does not retain, water in hydrated and desiccated systems. Sci. Rep. 13, 10449 (2023).
Belott, C., Janis, B. & Menze, M. A. Liquid-liquid phase separation promotes animal desiccation tolerance. Proc. Natl Acad. Sci. USA 117, 27676–27684 (2020).
Dorone, Y. et al. A prion-like protein regulator of seed germination undergoes hydration-dependent phase separation. Cell 184, 4284–4298.e27 (2021).
Ginsawaeng, O. et al. Subcellular Localization of Seed-Expressed LEA_4 Proteins Reveals Liquid-Liquid Phase Separation for LEA9 and for LEA48 Homo- and LEA42-LEA48 Heterodimers. Biomolecules 11, (2021).
Boothby, T. C. & Wolniak, S. M. Masked mRNA is stored with aggregated nuclear speckles and its asymmetric redistribution requires a homolog of Mago nashi. BMC Cell Biol. 12, 45 (2011).
Fleming, M. B., Richards, C. M. & Walters, C. Decline in RNA integrity of dry-stored soybean seeds correlates with loss of germination potential. J. Exp. Bot. 68, 2219–2230 (2017).
Koshland, D. & Tapia, H. Desiccation tolerance: an unusual window into stress biology. Mol. Biol. Cell 30, 737–741 (2019).
Nguyen, K., Kc, S., Gonzalez, T., Tapia, H. & Boothby, T. C. Trehalose and tardigrade CAHS proteins work synergistically to promote desiccation tolerance. Commun. Biol. 5, 1046 (2022).
Ma, X. et al. A small stress protein acts synergistically with trehalose to confer desiccation tolerance on mammalian cells. Cryobiology 51, 15–28 (2005).
Reina-Bueno, M. et al. Role of trehalose in heat and desiccation tolerance in the soil bacterium Rhizobium etli. BMC Microbiol. 12, 207 (2012).
Tapia, H., Young, L., Fox, D., Bertozzi, C. R. & Koshland, D. Increasing intracellular trehalose is sufficient to confer desiccation tolerance to Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 112, 6122–6127 (2015).
Erkut, C. & Kurzchalia, T. V. The C. elegans dauer larva as a paradigm to study metabolic suppression and desiccation tolerance. Planta 242, 389–396 (2015).
Dace, H. J., Adetunji, A. E., Moore, J. P., Farrant, J. M. & Hilhorst, H. W. A review of the role of metabolites in vegetative desiccation tolerance of angiosperms. Curr. Opin. Plant Biol. 75, 102410 (2023).
Hibshman, J. D., Carra, S. & Goldstein, B. Tardigrade small heat shock proteins can limit desiccation-induced protein aggregation. Commun. Biol. 6, 1–10 (2023).
Crowe, J. H., Carpenter, J. F. & Crowe, L. M. The role of vitrification in anhydrobiosis. Annu. Rev. Physiol. 60, 73–103 (1998).
Boothby, T. C. Water content influences the vitrified properties of CAHS proteins. Mol. Cell 81, 411–413 (2021).
Ramirez, J. F., Arulsamy, N. & Boothby, T. C. The fragility of vitrified glasses correlates with their protective capacity during desiccation. Biophys. J. 122, 292a (2023).
Kumara, U. G. V. S. S., Ramirez, J. F. & Boothby, T. C. The effect of sucrose polymer-size on glass transition temperature, glass former fragility, and water retention during drying. Front. Mater. 11, 1351671 (2024).
Ramirez, J. F., Kumara, U. G. V. S. S., Arulsamy, N. & Boothby, T. C. Water content, transition temperature and fragility influence protection and anhydrobiotic capacity. BBA Adv. 5, 100115 (2024).
Ballesteros, D. & Walters, C. Solid-state biology and seed longevity: a mechanical analysis of glasses in pea and soybean embryonic axes. Front. Plant Sci. 10, 920 (2019).
Csintalan, Z. Chlorophyll Fluorescence during drying and rehydration in the Mosses Rhytidiadelphus loreus (Hedw.) Warnst.,Anomodon viticulosus (Hedw.) Hook. & Tayl. and Grimmia pulvinata (Hedw.) Sm. Ann. Bot. 84, 235–244 (1999).
Zhu, Y. et al. Global transcriptome analysis reveals acclimation-primed processes involved in the acquisition of desiccation tolerance in Boea hygrometrica. Plant Cell Physiol. 56, 1429–1441 (2015).
Sun, R.-Z., Liu, J., Wang, Y.-Y. & Deng, X. DNA methylation-mediated modulation of rapid desiccation tolerance acquisition and dehydration stress memory in the resurrection plant Boea hygrometrica. PLoS Genet. 17, e1009549 (2021).
Slate, M. L., Brinda, J. C., Coe, K. K., Greenwood, J. L. & Stark, L. R. Prehydration mitigates damage accrued from prolonged periods of desiccation in cultured shoot apices of Syntrichia ruralis. J. Bryol. 43, 138–149 (2021).
Juenger, T. E. & Verslues, P. E. Time for a drought experiment: do you know your plants’ water status? Plant Cell 35, 10–23 (2023).
Wiebe, H. H. Measuring water potential (activity) from free water to oven dryness. Plant Physiol. 68, 1218–1221 (1981).
Campbell, G. S., Smith, D. M. & Teare, B. L. Application of a dew point method to obtain the soil water characteristic. in Experimental Unsaturated Soil Mechanics 71–77 (Springer Berlin Heidelberg, 2007).
Bittelli, M. Measuring soil water potential for water management in agriculture: a review. Sustain. Sci. Pract. Policy 2, 1–26 (2010).
Sun, W. Q. Methods for the study of water relations under desiccation stress. in Drying without Dying (ed. Black M. Pritchard H.) 47–91 (2002).
Solberg, S. Ø. et al. Long-term storage and longevity of orthodox seeds: a systematic review. Front. Plant Sci. 11, 1007 (2020).
Bewley, J. D. & Black, M. Seeds: Physiology of Development and Germination (Springer, 2013).
Mishler, B. D. & Oliver, M. J. PuttingPhyscomitrella patens on the tree of life: the evolution and ecology of mosses. in The MossPhyscomitrella patens 1–15 (Wiley-Blackwell, 2009).
Coe, K. K., Neumeister, N., Gomez, M. I. & Janke, N. C. Carbon balance: a technique to assess comparative photosynthetic physiology in poikilohydric plants. Appl. Plant Sci. https://doi.org/10.1002/aps3.11585 (2024).
Grote, E. E., Belnap, J., Housman, D. C. & Sparks, J. P. Carbon exchange in biological soil crust communities under differential temperatures and soil water contents: Implications for global change. Glob. Chang. Biol. 16, 2763–2774 (2010).
Pigon, A. & Weglarska, B. Rate of metabolism in tardigrades during active life and anabiosis. Nature 176, 121–122 (1955).
Buitink, J., Hemminga, M. A. & Hoekstra, F. A. Characterization of molecular mobility in seed tissues: an electron paramagnetic resonance spin probe study. Biophys. J. 76, 3315–3322 (1999).
Buitink, J., Leprince, O., Hemminga, M. A. & Hoekstra, F. A. The effects of moisture and temperature on the ageing kinetics of pollen: interpretation based on cytoplasmic mobility. Plant Cell Environ. 23, 967–974 (2000).
Erkut, C. et al. Trehalose renders the Dauer Larva of Caenorhabditis elegans resistant to extreme desiccation. Curr. Biol. 21, 1331–1336 (2011).
Harry, C. J. et al. Protocol for fluorescent live-cell staining of tardigrades. STAR Protoc. 5, 103232 (2024).
Delarue, M. et al. mTORC1 controls phase separation and the biophysical properties of the cytoplasm by tuning crowding. Cell 174, 338–349.e20 (2018).
Meneses-Reyes, G. I., Rodriguez-Bustos, D. L. & Cuevas-Velazquez, C. L. Macromolecular crowding sensing during osmotic stress in plants. Trends Biochem. Sci. 49, 480–493 (2024).
Cuevas-Velazquez, C. L. et al. Intrinsically disordered protein biosensor tracks the physical-chemical effects of osmotic stress on cells. Nat. Commun. 12, 5438 (2021).
Salazar, J. K. et al. Desiccation survival of Salmonella enterica,Escherichia coli, and Enterococcus faecium related to initial cell level and cellular components. J. Food Prot. 85, 398–405 (2022).
Scherber, C. M., Schottel, J. L. & Aksan, A. Membrane phase behavior of Escherichia coli during desiccation, rehydration, and growth recovery. Biochim. Biophys. Acta 1788, 2427–2435 (2009).
VanBuren, R. et al. Variability in drought gene expression datasets highlight the need for community standardization. bioRxiv, https://doi.org/10.1101/2024.02.04.578814 (2024).
Stitt, M. Systems-integration of plant metabolism: means, motive and opportunity. Curr. Opin. Plant Biol. 16, 381–388 (2013).
Fernie, A. R. & Stitt, M. On the discordance of metabolomics with proteomics and transcriptomics: coping with increasing complexity in logic, chemistry, and network interactions scientific correspondence. Plant Physiol. 158, 1139–1145 (2012).
Wood, A. J. & Oliver, M. J. Translational control in plant stress: the formation of messenger ribonucleoprotein particles (mRNPs) in response to desiccation of Tortula ruralis gametophytes. Plant J. 18, 359–370 (1999).
Xu, X. et al. Molecular insights into plant desiccation tolerance: transcriptomics, proteomics and targeted metabolite profiling in Craterostigma plantagineum. Plant J. https://doi.org/10.1111/tpj.15294 (2021).
Russo, T. A. et al. The response regulator BfmR is a potential drug target for Acinetobacter baumannii. mSphere 1, 10–1128 (2016).
Loftus, R. W., Dexter, F., Robinson, A. D. M. & Horswill, A. R. Desiccation tolerance is associated with Staphylococcus aureus hypertransmissibility, resistance and infection development in the operating room. J. Hosp. Infect. 100, 299–308 (2018).
Green, E. R. et al. Bacterial hydrophilins promote pathogen desiccation tolerance. Cell Host Microbe 30, 975–987.e7 (2022).
Esbelin, J., Santos, T. & Hébraud, M. Desiccation: an environmental and food industry stress that bacteria commonly face. Food Microbiol. 69, 82–88 (2018).
Moore, J. P. et al. The predominant polyphenol in the leaves of the resurrection plant Myrothamnus flabellifolius, 3,4,5 tri-O-galloylquinic acid, protects membranes against desiccation and free radical-induced oxidation. Biochem. J. 385, 301–308 (2005).
Nicoletti, M. et al. In vitro biological activities of the essential oil from the ‘resurrection plant’ Myrothamnus moschatus (Baillon) Niedenzu endemic to Madagascar. Nat. Prod. Res. 26, 2291–2300 (2012).
Gechev, T. S. et al. Natural products from resurrection plants: potential for medical applications. Biotechnol. Adv. 32, 1091–1101 (2014).
Cassano, R. & Trombino, S. Trehalose‐based hydrogel potentially useful for the skin burn treatment. J. Appl. Polym. Sci. 44755. https://doi.org/10.1002/app.44755 (2017).
Bentley, J., Moore, J. P. & Farrant, J. M. Metabolomic profiling of the desiccation-tolerant medicinal Shrub Myrothamnus flabellifolia indicates phenolic variability across its natural habitat: implications for tea and cosmetics production. Molecules 24, 1240 (2019).
Biscaro, R. C. et al. Modulation of autophagy by an innovative phytocosmetic preparation (Myrothamnus flabelifolia and Coffea arabica) in human fibroblasts and its effects in a clinical randomized placebo-controlled trial. J. Cosmet. Dermatol. 21, 4901–4912 (2022).
Nantapo, C. W. T. & Marume, U. Exploring the potential of Myrothamnus flabellifolius Welw. (Resurrection Tree) as a Phytogenic Feed Additive in Animal Nutrition. Animals 12, 1973 (2022).
Pinto, L., Tapia-Rodríguez, M. R., Baruzzi, F. & Ayala-Zavala, J. F. Plant antimicrobials for food quality and safety: recent views and future challenges. Foods 12, 2315 (2023).
Djilianov, D. et al. Resurrection Plants-A valuable source of natural bioactive compounds: from word-of-mouth to scientifically proven sustainable use. Metabolites 14, 113 (2024).
Leung, V. et al. Thermal stabilization of viral vaccines in low-cost sugar films. Sci. Rep. 9, 1–11 (2019).
Packebush, M. H. et al. Natural and engineered mediators of desiccation tolerance stabilize Human Blood Clotting Factor VIII in a dry state. Sci. Rep. 13, 4542 (2023).
Wolkers, W. F., Walker, N. J., Tablin, F. & Crowe, J. H. Human platelets loaded with trehalose survive freeze-drying. Cryobiology 42, 79–87 (2001).
Li, S. et al. Late embryogenesis abundant proteins protect human hepatoma cells during acute desiccation. Proc. Natl. Acad. Sci.USA 109, 20859–20864 (2012).
Kikuta, S. et al. Towards water-free biobanks: long-term dry-preservation at room temperature of desiccation-sensitive enzyme luciferase in air-dried insect cells. Sci. Rep. 7, 1–10 (2017).
Rolsma, J. L., Darch, W., Higgins, N. C. & Morgan, J. T. The tardigrade-derived mitochondrial abundant heat soluble protein improves adipose-derived stem cell survival against representative stressors. Sci. Rep. 14, 11834 (2024).
Xiao, B., Huang, Y., Tang, N. & Xiong, L. Over-expression of a LEA gene in rice improves drought resistance under the field conditions. Z.üchter Genet. Breed. Res. 115, 35–46 (2007).
Sato, Y. & Yokoya, S. Enhanced tolerance to drought stress in transgenic rice plants overexpressing a small heat-shock protein, sHSP17.7. Plant Cell Rep. 27, 329–334 (2008).
Amara, I., Capellades, M., Ludevid, M. D., Pagès, M. & Goday, A. Enhanced water stress tolerance of transgenic maize plants over-expressing LEA Rab28 gene. J. Plant Physiol. 170, 864–873 (2013).
Xiang, J. et al. Overexpressing heat-shock protein OsHSP50.2 improves drought tolerance in rice. Plant Cell Rep. 37, 1585–1595 (2018).
Rousk, K., Jones, D. L. & DeLuca, T. H. The resilience of nitrogen fixation in feather moss (Pleurozium schreberi)-cyanobacteria associations after a drying and rewetting cycle. Plant Soil 377, 159–167 (2014).
Tebele, S. M., Marks, R. A. & Farrant, J. M. Exploring the root-associated microbiome of the resurrection plant Myrothamnus flabellifolia. Plant Soil 500, 53–68 (2023).
Sun, R.-Z., Wang, Y.-Y., Liu, X.-Q., Yang, Z.-L. & Deng, X. Structure and dynamics of microbial communities associated with the resurrection plant Boea hygrometrica in response to drought stress. Planta 260, 24 (2024).
Fernandes-Júnior, P. I. et al. The resurrection plant Tripogon spicatus (Poaceae) harbors a diversity of plant growth promoting bacteria in northeastern Brazilian Caatinga. Rev. Bras. Cienc. Solo 39, 993–1002 (2015).
Lozo, J. et al. Rhizosphere microbiomes of resurrection plants Ramonda serbica and R. nathaliae: comparative analysis and search for bacteria mitigating drought stress in wheat (Triticum aestivum L.). World J. Microbiol. Biotechnol. 39, 256 (2023).
Alpert, P. The limits and frontiers of desiccation-tolerant life. Integr. Comp. Biol. 45, 685–695 (2005).
Barrs, H. D. & Weatherley, P. E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. Jnl. Bio. Sci. 15, 413–428 (1962).
Hsiao, T. C. Plant responses to water stress. Annu. Rev. Plant Physiol. 24, 519–570 (1973).
Stark, L. R., Greenwood, J. L. & Brinda, J. C. How to dry a bryophyte: A review and experimental test of four methods to induce desiccation tolerance. bryo 125, 1–22 (2022).
Roszkowska, M. et al. How long can tardigrades survive in the anhydrobiotic state? A search for tardigrade anhydrobiosis patterns. PLoS ONE 18, e0270386 (2023).
Fernández-Marín, B. et al. Non-invasive diagnosis of viability in seeds and lichens by infrared thermography under controlled environmental conditions. Plant Methods 15, 147 (2019).
Acknowledgements
This work was supported by the Water and Life Interface Institute (WALII: US National Science Foundation DBI-2213983). We thank Rachel Torrez for assistance in drawing original artwork used in figures. We thank Kent Bradford for conversations and feedback that improved the clarity and direction of the paper.
Author information
Authors and Affiliations
Contributions
Conceptualization of this project was led by R.A.M., L.V.D.P., M.J.O., S.Y.R., and O.M.S.C. with contributions from MASA, L.B., T.C.B., D.C., K.C., H.J.W.D., A.H., S.P., A.T., R.V., B.D.M., and D.B. among others. Figures were drawn by JTBE with support from R.A.M., O.M.S.C., and K.C. All authors contributed to writing the original draft, editing and reviewing later drafts, and approving the final draft.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marks, R.A., Ekwealor, J.T.B., Artur, M.A.S. et al. Life on the dry side: a roadmap to understanding desiccation tolerance and accelerating translational applications. Nat Commun 16, 3284 (2025). https://doi.org/10.1038/s41467-025-58656-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-58656-y
This article is cited by
-
The phenomenon of anhydrobiosis—structural and functional changes in yeast cells
Applied Microbiology and Biotechnology (2025)