Abstract
Organic semiconductor crystals (OSCs) offer mechanical flexibility, high carrier mobility, and tunable electronic structures, making them promising for optoelectronic and photonic applications. However, traditional lithographic techniques damage OSCs due to high-energy beams or solvents, leading to high defect densities, poor uniformity, and significant device-to-device variation. Existing methods also struggle to eliminate residual layers while forming independent, complex two-dimensional patterns. A chemical-free nanoimprint crystallography (NICL) method is introduced to overcome these challenges by balancing residual-layer-free nanoimprinting with the fabrication of independent, complex 2D patterns. In situ control of crystallization kinetics via temperature gradient adjustment yields OSC nanostructures with low defect densities and good uniformity. Patterning of various OSCs over a range of feature sizes is demonstrated. The patterned OSCs exhibit good lasing performance and low device-to-device variation (as low as 2%), indicating that NICL is a promising approach for fabricating high-performance, uniform OSC-based devices.
Similar content being viewed by others
Introduction
Organic semiconductor crystals (OSCs) are the packing of small organic molecules into well-defined structures with long-range order and lattice symmetry. Compared with their amorphous counterparts, the long-range ordered arrangement of molecules in crystalline materials is a prerequisite to achieve interacting molecular π-orbitals for efficient charge transport1,2. In the past decades, a bundle of research has focused on the design and synthesis of new molecules, as well as to explore and improve the physical properties of OSCs. The enriched molecular library provides material candidates to develop low-cost, flexible, and low-power consumption molecular devices, such as field-effect transistors3, solar cells4, light-emitting diodes5,6, photodetectors7,8, lasers9,10, and so on. However, there remain several challenges that must be addressed towards practical device applications, notably achieving low defect density and high uniformity in OSC patterns. Specifically, the fabrication of OSC devices requires precise patterning of OSCs on different substrates with consistent quality and minimal device-to-device variation (DDV). The stochastic nucleation and growth of OSCs often lead to random distribution of crystals with uncontrollable morphology and high defect densities, which impedes their device fabrication and integration11. Traditional lithographic techniques normally use ultraviolet light or electron beam for region-selective modification of the polymer resist for mask preparation, which is followed by the mask-assisted etching or growth of materials12. However, as organic molecules are normally vulnerable to high temperatures, ultraviolet light, electron beams, and organic solvents, such lithographic techniques can inevitably damage the organic crystals, introduce defects, and lead to deterioration of their optoelectronic properties1.
The good solubility of small organic molecules has driven the widespread development and significant progress of patterned methodologies based on solution techniques. However, these methods also come with a series of challenges. Solution-based processes require strict control over numerous parameters, including solution concentration, solvent selection, temperature control, and evaporation rate, subtle changes in which can significantly affect the orientation and uniformity of crystals. The uneven diffusion of solutes in solution and solvent evaporation can lead to an increase in internal defects and heterogeneous structures in crystals, leading to significant device-to-device variation (DDV) and compromising the performance consistency across large-area devices.
Solution methods face limitations in achieving high precision and complex patterning, as their patterning capabilities are constrained by the capillary filling and solvent evaporation mechanisms of the solution, which may make filling complex patterns difficult, thereby limiting the freedom of pattern design. Currently, most patterning techniques are limited to the preparation of one-dimensional linear structures, and the realization of two-dimensional complex pattern arrays remains challenging. Solution methods may accompany higher material waste, as not all solutes in the solvent can be effectively converted into crystals, such as in spin-coating processes. In addition, existing methods often cannot simultaneously achieve residual-layer-free patterning and independent, complex two-dimensional structures. The residual layers between patterns can cause crosstalk and stress-induced cracks, adversely affecting device performance and reliability. In the preparation of complex patterns, the gas phase method often relies on masking and etching techniques, which may be detrimental to sensitive organic crystal materials. Therefore, developing a method that can achieve high-resolution, residual-layer-free, high-crystallinity, and high-uniformity patterning of OSCs with low DDV remains an urgent need in the field.
Here, we propose a technique termed nanoimprint crystalithography (NICL) to address the above-mentioned technical impediments. Using OSC powders as the feedstock, NICL is primarily a chemical-free technique which overcomes the harmful influences from organic solvents or other chemicals. NICL effectively addresses the trade-off between residual-layer-free nanoimprinting and the fabrication of independent arrays of two-dimensional complex patterns. The adsorption effect of PDMS during crystal growth ensures that the residual layer between micro-patterned crystals is gradually absorbed, preventing crosstalk and reducing stress-induced cracks. In addition, the rational management of temperature gradient during molding provides an efficient strategy for in-situ control of the crystallization kinetics, enabling the growth of high-quality OSC micro/nanopatterns with low defect densities and good uniformity across large areas. With its general applicability, high patterning resolution, and tunable crystallinity, ultra-low defect density, and low device-to-device variation, NICL paves a way towards fabrication of high-performance OSC devices that meet the stringent requirements for practical optoelectronic and photonic applications.
Results
Concept and working principle
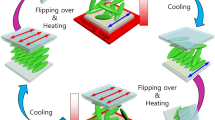
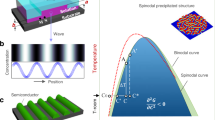
The basic concept of NICL is illustrated in Fig. 1. The organic powder is employed as the feedstock and loaded onto the target substrate. The temperature of the substrate is controlled by an electric wire heater. The powders are heated to the liquid phase, and a patterned polydimethylsiloxane (PDMS) mold is applied to the organic feedstock to shape the molecules under external pressure and capillary force. Finally, the template is cooled down, and the crystallite OSC microstructures are obtained after the liquid-solid phase transition and demolding. In order to improve the crystallinity of OSCs and the imprint resolution, the processing temperature should be carefully controlled. Figure 1b shows the temperature trajectories of both substrate (hot end, TH) and PDMS mold (cold end, TC) during NICL. At the heating stage, both TH and TC are set above the melting point (TM) of the organic powders to ensure that the melt is in high fluidity and could fully fills the mold (A-B). The system is then cooled down. It is noted that the crystallization cannot occur immediately, the organic molecules are still disordered melts even when the temperature of the feedstock is below TM (B-C-D). Such undercooling behavior is attributed to the free energy of the liquid-solid phase transitions ΔG, which can be described by the melting latent heat ΔHM at a relatively low undercooling temperature ΔTU13 (1)
a Schematic showing the NICL technique. b The temperature trajectories during the NICL process. The red line, green dashed line, and blue line represent the temperature of the hot end, the phase frontier, and the cold, respectively. The yellow and purple dashed line marks the melting temperature and the crystallization temperature, respectively. The inner schematics illustrate the evolution of molecular structure at different stages.
The crystallization of organic molecules is initialized when the cold end reaches the critical temperature Tcry (Tcry = TM - ΔTU), where ΔG is sufficient to trigger the nucleation. The nucleus grows rapidly from the top of the mold and forms patterned OSCs (D-E-F). In the crystallization stage, the temperature at the growth forefront (TF) is raised due to the release of crystallization latent heat (D-E) and then maintained at a constant above Tcry (E-F) until crystallization is completed. After crystallization, the temperature of OSCs continues to decrease with the environmental temperature (F-G). It is known that during the rapid undercooling crystallization, defects such as vacancies, dislocations, and grain boundary are easily formed in the crystals as the solidification process is too fast for the molecules to form perfectly ordered lattices14. Thus, the temperature of the system is maintained around TM for post-crystallization annealing, where the defect density is reduced for the growth of high-quality crystals (G-H). Finally, the system is naturally cooled down to room temperature to release the thermal stress, and the PDMS template is removed from the substrate to obtain high-quality OSC patterns. It is noted that no chemical is used during the NICL process, and the chemical damage is essentially eliminated.
To verify the above working mechanism, we took 1,4-bis[2-(2-methylphenyl)ethenyl]-Benzene (OMSB, see Supplementary Fig. 1 the thermal properties) as an example and carried out a variety of control experiments. Figure 2a shows the trajectories of both TH and TC during the NICL process. Since the top surface of the mold is not atomically smooth, heterogeneous nucleation occurs, and thus, the undercooling required to activate the crystallization is low15. Since energy exchange occurs during NICL, it can be treated as a non-isothermal crystallization process, where the crystallization temperature Tcry relies on the cooling rate16. As shown in the inset of Fig. 2a, the undercooling temperature is elevated from 24 to 34 K when the cooling rate varies from ~ 0.5 to ~ 1800 K s−1. The bottom substrate was in direct contact with a heating stage, delivering a precise and uniform heating environment. To simplify the experimental apparatus and procedure, the PDMS mold was maintained at a different temperature by harnessing the cooling effect of controlled room-temperature air flowing across its surface. During the cooling process, an exponential decay function was applied to fit the temperature data according to Newton’s Law of Cooling. Although direct observation of the crystallization process within PDMS microcavities is not feasible, we conducted proxy experiments to elucidate its kinetics and thermal parameters (see Supplementary Note 1, Movie 1, and Supplementary Fig. 2). Under the experimental conditions outlined in Fig. 2a, the crystallization temperature was determined to be 438 K, and the average crystallization rate was measured at 30.4 μm/s, as demonstrated in Supplementary Movie 1. For verification, we took Raman spectra of the OSC samples, with the Raman intensity as a function of the cooling rate summarized in the inset of Fig. 2a. We can see that the Raman intensity is reduced as the cooling rate increases, indicating the deterioration of crystallinity17.
a Temperature trajectory of the hot end and cold end during NICL. The initial cooling process and post-crystallization thermal treatment process are marked with green and pink, respectively. The inset shows the undercooling temperature (ΔTU) and normalized Raman intensity of the OMSB crystals (IR) as functions of the cooling rate in the first cooling process. b The evolution of Raman spectra during the post-crystallization thermal treatment process. c One-dimensional diffraction peak profiles of the OMSB crystals grown at different cooling rates and temperature holding times derived from two-dimensional XRD data. d–g Two-dimensional XRD patterns of the OMSB crystals grown at different cooling rates and temperature holding time. h the summarized FWHM of (003) diffraction peak of the OMSB crystals grown at different cooling rates and temperature holding time. i Time-resolved photoluminescence of the micro-patterned OMSB arrays with different NICL parameters. The photoluminescence lifetime τ of each sample is derived with a single exponential fitting and marked in the legend. j The phase diagram summarizes the crystallinity of OSCs grown at different temperature holding times and undercooling temperatures. Different colors mark parameters that lead to different crystallinity.
In order to reveal the key role of post-crystallization annealing on the improvement of crystallinity, we held the hot end of the OMSB sample at Tset = 433 K for time tset and recorded the Raman spectra at different tset. As shown in Fig. 2b, we can see the enhancement of the Raman intensity and narrowing of the full width at half maximum (FWHM) when tset is increased. This can be ascribed to the lattice healing of the organic crystals during the post-crystallization annealing18. The molecules own high mobility at the temperature of 433 K, and the migration and re-organization of organic molecules repair the molecular lattices through defect passivation, grain boundaries migration, and crystal re-orientation. The defect density is significantly reduced19, and small grains merge into larger ones, with the formation of high-quality and highly orientated OSCs20. Moreover, the latent heat of crystallization is released during this process to avoid stress and crack generation in the following cooling step21.
The OMSB structure was characterized using X-ray diffraction (XRD, see Fig. 2c, d). A fast-cooling rate results in significantly broader XRD peaks, while post-crystallization annealing (20 K/min, tset = 10 min) produces narrower peaks. In addition, some diffraction peaks observed in the non-annealed sample (20 K/min, tset = 0 min) disappear after annealing, indicating improved crystal quality and more uniform orientation22. The two-dimensional XRD patterns (Fig. 2d–g) show that an appropriate cooling rate and annealing process are critical for obtaining high-quality single crystals. Compared to the circular diffraction pattern of the powder sample (Fig. 2d), the sample cooled at 10800 K/min exhibits clearer spots and a more circular pattern (Fig. 2e), indicating improved crystal orientation. Further reduction of the cooling rate to 20 K/min results in a more spot-like diffraction pattern (Fig. 2f), reflecting enhanced crystal orientation and suppression of misaligned crystals. The annealing process further refines this by promoting single-crystal growth with a single orientation (Supplementary Fig. 3), producing distinct and sharp diffraction spots (Fig. 2g). Grazing Incidence XRD (GIXRD) was applied to further investigate the crystal quality and preferred orientation of the prepared micro/nanopatterned crystals under different substrates and growth conditions (Supplementary Fig. 4 and Note 2).
The time-resolved photoluminescence spectra in Fig. 2i show a reduced fluorescence lifetime from 1.42 ns to 1.07 ns as the crystallinity of OMSB is promoted at optimized NICL parameters, which can be ascribed to the enhanced crystallinity and diminished molecular distance23. It is noted that both Tset and tset are critically important for the lattice healing of OSCs and should be carefully controlled. As summarized in Fig. 2j, when Tset is too high (region I), the organic molecules are highly active and could be easily adsorbed by the PDMS film (see Supplementary Fig. 5). The demolding becomes challenging, and the impairment to the patterned crystals is inevitable. When Tset is too low (region V), the mobility of the organic molecules is insufficient for lattice repair, and the crystallinity is low for the patterned OSCs. For OMSB molecules, a Tset value 10–30 K lower than TM is preferred. Similarly, when tset is too short, the migration of the organic molecules is insufficient for lattice optimization (region II). While an overlong tset leads to unpreferred adsorption of organic molecules by PDMS and defect generation (region IV, see Supplementary Fig. 6). It is also noted that the optimized values of both Tset and tset (region III) varies with the type of target organic molecules. The boundaries between regions II and III, as well as III and IV, reflect the delicate balance between the thermodynamic factors driving crystallization and the kinetic barriers impeding it during the crystallization of organic semiconductor crystals via NICL (Supplementary Note 3 and Supplementary Fig. 7).
Morphology control
Similar to the traditional nanoimprint technique, the size and thickness of the OSC micropatterns can be precisely controlled through the geometric design of the molds used in NICL. As shown in Fig. 3a–d and Supplementary Fig. 8, the microdisk arrays of OMSB molecules with diameter ranging from 163.7 nm to 60 μm were fabricated. Figure 3e–h summarize the side-view SEM images of the OMSB microdisks with a diameter of ~ 20 μm and height ranging from 436 nm to 15 μm. Besides the microdisk arrays, we also demonstrate more complicated structures such as triangular, quadrilateral, pentagonal, hexagonal, and star-shaped micropatterns, as characterized by white light interferometry (WLI) in Fig. 3i. Atomic force microscopy (AFM) characterization under varying crystallization conditions reveals disparate surface roughness distributions, suggesting that the post-crystallization annealing process can facilitate the formation of smoother crystal surfaces (see Supplementary Fig. 9). The statistical analysis from SEM images indicate that crystal sizes are generally slightly smaller than template sizes, as shown in Supplementary Fig. 10. To more clearly illustrate the changes in size uniformity, the ratio of the standard deviation to the mean value of crystal diameters is shown in Supplementary Fig. 11. As the template size decreases, the uniformity of crystal size also decreases, indicating that a smaller template size reduces the constraining effect on the crystal growth process. Under the excitation of UV light, all these micropattern arrays emit bright blue light (see Supplementary Fig. 12). The dark regimes around the micropatterns indicate that the patterns are well isolated from each other and that there is no residual layer between the patterns. Through nanoimprint lithography, it has been possible to fabricate two-dimensional micro and nano-patterned arrays, particularly for some organic materials such as polymers. However, these two-dimensional patterns are usually not completely independent, and often there is a residual layer between them, which is detrimental to the functional performance of certain optoelectronic devices. Directly fabricating a patterned crystal array without any residual layer is typically challenging and often requires additional processing steps to remove the residual layer between the patterned micropillars. Here, by utilizing the absorption capacity of PDMS for the residual layer (see Supplementary Fig. 13), we successfully directly constructed a micro and nano-patterned array with no residual layer. Three-dimensional laser scanning confocal microscopy was also used to characterize the height of the OMSB micropatterns over a large area. The uniform color in Fig. 3i reveals that the patterned OSCs have good surface quality and uniform height distribution.
a–d Top-view SEM images of OMSB micro-disks with diameter of 0.16, 30, 60, and 160 μm, respectively. e–h Side-view SEM images of OMSB micro-disks with heights of 0.44, 2.4, 4.1, and 15 μm, respectively. i Tilt-view WLI images of the OMSB micro-structures of different shapes showing uniform height and smooth surfaces. j Tset and (k) tset as a function of the diameter of the micropatterns, respectively. The error bars represent the variability in crystallization outcomes across multiple experiments conducted under varying temperature and time conditions. They are derived from the statistical analysis of crystallinity (≥ 90%), circularity (≥ 80%, calculated as P²/4πA, where P is the perimeter and A is the area), and intact morphology without residual layers.
It is noted that the crystal growth kinetics during NICL is strongly influenced by the mold size. Thus, the parameters of NICL should be adjusted according to the feature sizes of the target patterns. Taking a mold with a diameter of D for consideration, the crystallization temperature of the melt in the mold Tcry can be written as ref. 24
where Tb is the crystallization temperature of the bulk melt. γws and γwl are the surface tensions at the PDMS-solid and PDMS-melt interface, respectively. ν is the molar volume of the melt, while λsb is the latent heat of the bulk phase. For PDMS, γws is larger than γwl, and we can infer that the crystallization temperature of the OSC micropatterns decreases with pattern size. As the pattern size decreases, the undercooling temperature required to initialize the crystallization increases, while the required Tset is reduced (see Fig. 3j). In contrast, for the micropatterns with larger sizes, there are high-density defects and grain boundaries inside the patterns while the specific surface area to release the latent heat of crystallization is smaller. Thus, a sufficiently long tset is expected to improve the crystallinity (see Fig. 3k). The error bars indicate the range of growth parameters where high-quality patterned organic crystals consistently form, demonstrating the robustness and reproducibility of the process. The dark I − V curve also reveals the low defect density and high carrier mobility of the micro-patterned OMSB structure fabricated by NICL (see Supplementary Note 4 and Supplementary Fig. 14).
Diversity of NICL
NICL is a general approach which can be extended to many other organic crystals besides OMSB. For demonstration, we printed different micropatterns of 2 - (4-tert-butylphenyl) - 5 - (4-biphenyl) - 1,3,4-oxadiazole (Butyl PBD), 1,4-bis [2 - [4 - [n, n-di (p-tolyl) amino] phenyl] vinyl] benzene (DPAVB), and Tris (dibenzoylmethane) mono (phenantholine) europium (lll) (EU (dbm) 3 (phen)) (EU (DBM)3(phen)), which emit light at 477, 520, and 615 nm, respectively (Fig. 4a–f, also see Supplementary Table 1 the corresponding NICL parameters). It should be noted that all these organic molecules have different molecular configurations, thermal properties, and crystallization kinetics. Consequently, The NICL parameter should be optimized according to the target materials. Normally, organic molecules with large molecular weight or complex structures has a lower diffusion coefficient25, therefore requiring a longer time for defect passivation and grain boundary migration (see Supplementary Fig. 15). The general applicability of NICL also allows the patterning of OSCs composed of various kinds of organic molecules in individual micropatterns. when dealing with multi-component systems, solution-based methods often encounter challenges due to the narrow range of orthogonal solvents, making it difficult to achieve micro-nano fabrication of complex compositions. The NICL technique, conversely, effortlessly circumvents this issue. As shown in Supplementary Fig. 16, the micropatterns composed of two or three kinds of organic materials are fabricated. The NICL parameters are shown in Supplementary Table 2.
OSC microlasers
The molecular crystals patterned by NICL with precise geometric control and high crystallinity provide an ideal platform for microlasers. As shown in Fig. 5a, the patterned OMSB microdisk acts as both the gain medium and the resonant micro-cavity for self-coupling. When pumped by a 400 nm laser (see Supplementary Fig. 17 for the experiment setup), room-temperature lasing at 450 nm was observed in a single microdisk. Figure 5b shows the emission spectra of a single OMSB microdisk of 20 μm in diameter pumped at different laser intensities. At a pump of 5.98 μJ/cm2, the emission band with a broad linewidth of more than 20 nm was observed, which is attributed to the amplified spontaneous emission of the OMSB crystal. When the pump laser intensity exceeds 19 μJ/cm2, the emission intensity increases rapidly, and the broad spontaneous emission peak splits into several narrow and sharp peaks, which implies lasing emission. A high-quality factor of 1667 further reveals the high crystallinity and good surface quality of the OSC micropatterns (compared with Q ~ 1500 in single crystals26). Figure 5c summarizes the FWHM and emission intensities of the OMSB microdisk under different pumping intensities. When the pump light intensity exceeds the threshold of 17.48 μJ/cm2, the emission intensity dramatically increases while the FWHM rapidly decreases, implying the transition from amplified spontaneous radiation to laser emission. The observed emission characteristics are attributed solely to stimulated emission from the active medium, with amplified spontaneous emission (ASE), directional scattering, and cavity-induced modifications to the fluorescence spectrum ruled out based on spectral width, experimental setup, and stability of spectral positions. The laser mode can be tuned readily by adjusting the size (see Supplementary Fig. 18). As the diameter increases, the number of laser resonance modes increases, and the mode spacing Δλm becomes smaller. For resonant cavities with whispering gallery modes, the mode spacing can typically be calculated by:
where D is the diameter of the micro-disk crystal and n is the refractive index of the crystal. According to Eq. 3, there should be a linear relationship between the mode spacing and the reciprocal of the circumference L = πD. The laser spectra of OMSB micro-disk crystals with diameters ranging from 10 µm to 30 µm is shown in Supplementary Fig. 18a. The relationship between the mode spacing and 1/L is plotted in Supplementary Fig. 18b, presenting a good linear correlation. The laser mode can also be tuned readily by adjusting the shape (see Supplementary Figs. 19–22) of the microdisks. These microlasers show good uniformity, adaptability in different environments, and long-time stability (see Supplementary Note 5, 6, and Supplementary Figs. 23–26), suggesting that NICL is a promising platform for the fabrication of high-performance OSC photonic devices.
a Schematic of laser emission of the OMSB microdisk arrays fabricated by NICL. b The PL spectra of the OMSB microdisk at different pumping powers of 5.98, 19.02, 19.42, 22.07 μJ/cm2. c The emission intensity and FWHM as functions of the pumping power. d Schematic of OFET based on patterned OSCs fabricated by NICL. e Output curves of the OFET. f The transfer curve of the OFET. g Transfer curves of 6 × 6 OFET array based on patterned OSCs fabricated by NICL. h Mobility distribution in the 6 × 6 OFET array. i Corresponding histogram of saturation mobility. The average value is 3.15 cm2 V−1 s−1, and the standard deviation is 0.067 cm2 V−1 s−1.
Organic Field-Effect Transistors
To further demonstrate the applicability of the NICL methodology in electronic devices, Organic Field-Effect Transistors (OFETs) with a top-contact/bottom-gate architecture were fabricated (Fig. 5d). The ultra-low defect density of patterned 2,7-dioctyl[1]benzothieno[3,2-b][1]benzothiophene (C8-BTBT) crystals has been confirmed through the space charge limited current (SCLC) method, which shows a trap density of 1.0 × 1010 cm−3 (Supplementary Fig. 27). Patterned C8-BTBT were first synthesized on a Si/SiO2 substrate using the NICL process, followed by the deposition of gold electrodes through thermal evaporation to form the top contacts. Figures 5e, f show the characteristic transfer and output curves of the device, which display typical p-type conductive behavior. The carrier mobility of these patterned crystalline devices was measured at 4.25 cm² V−1 s−1, comparable to the performance of C8-BTBT single-crystal OFETs reported in previous studies. This mobility represents a 36-fold improvement over non-patterned thin-film devices fabricated under similar conditions (see Supplementary Fig. 28), highlighting the efficacy of NICL in enhancing carrier transport in organic crystals.
In addition, devices based on annealed crystals (see Supplementary Fig. 29) exhibited 1.5 times enhancement of carrier mobility compared to their non-annealed counterparts, emphasizing the crucial role of post-crystallization annealing in improving crystal quality. Notably, carrier mobility is an intrinsic property, independent of crystal size, suggesting that the improvement primarily results from lattice self-healing rather than extended crystal growth. Furthermore, these OFETs demonstrated long-term stability (see Supplementary Fig. 30).
Most importantly, the OFET array fabricated using NICL exhibited good uniformity across devices. The Device-to-Device Variation (DDV), defined as the ratio of standard deviation to mean value, was only 2.12% (Fig. 5g–i), significantly lower than the variability seen in organic semiconductor thin films produced via alternative solution-printing methods (which typically range between 12.3% and 28.1%, Supplementary Table 3). This low DDV highlights the ability of NICL to mitigate mobility fluctuations caused by anisotropic charge transport in organic crystals. Such uniformity is critical for addressing challenges in large-scale device integration, including panel luminance inconsistencies, errors in digital circuits, and current gain mismatches in analog circuits.
Discussion
NICL presents a compelling alternative to both traditional and emerging methods for organic crystal patterning (as supported by Supplementary Table 4–6). Unlike conventional techniques such as photolithography, which involve multiple steps like photoresist coating, exposure, and development, NICL employs template-guided melt-crystal growth, streamlining the process and significantly reducing fabrication time. This method also avoids the need for high-cost equipment like electron beam lithography and nanoimprint lithography, and eliminates the specialized inks required for techniques such as laser direct writing or inkjet printing, thus reducing production costs while maintaining high precision and large-area fabrication capability (Supplementary Note 7 and Fig. 31). In comparison to emerging solution-based and vapor-phase patterning techniques, NICL excels in its ability to produce high-resolution, complex two-dimensional patterns without residual layers. The PDMS mold’s adsorption of excess melt effectively prevents residual layers between crystals, which are often a limitation in other methods, ensuring higher crystal quality and eliminating issues like stress-induced cracks and crosstalk between devices. This feature is particularly important for improving the reliability of optoelectronic and OFET applications.
Furthermore, NICL achieves ultra-low defect densities, as demonstrated with patterned C8-BTBT crystals showing a defect density of 1.0 × 1010 cm−3, which rivals the highest-quality crystals produced by other techniques27. This reduction in defects leads to enhanced carrier mobility and overall device performance. In addition, NICL ensures good uniformity across large areas, with OFET arrays exhibiting a device-to-device variation of just 2.12%, which is significantly lower than the variation seen in organic semiconductor films fabricated using solution-based methods27. Its ability to create complex, residue-free patterns with high uniformity and low defect density positions NICL as a good method for next-generation organic electronics and photonics, offering a precise, cost-effective, and scalable solution that addresses the limitations of existing technologies.
In summary, we develop a lithographic technique termed NICL for precise and non-invasive patterning of OSCs. Taking organic powders as the feedstock, NICL harnesses good morphology control in nanoimprint lithography and in-situ crystallization kinetics control during molding to improve the patterning resolution and crystallinity. As a chemical-free approach, it essentially eliminates the chemical damage from solvent or developer in traditional lithographic techniques. The good surface quality, tunable crystallinity, and low trap density of the fabricated OSC patterns reveal that NICL is a promising platform to fabricate high-quality OSC devices for various applications in microelectronics, optoelectronics, and nanophotonics.
Methods
Materials
PDMS prepolymer and curing agent were purchased from Dow Corning company. Organic crystals OMSB and Butyl PBD were purchased from Alfa Aesar. DPAVB and EU (DBM)3(phen) were purchased from TCI (Shanghai). All the materials were used as purchased without further purification.
Preparation of PDMS templates
The preparation procedure was as shown in Supplementary Fig. 32. The SU8 microstructures were first prepared on a silicon substrate by conventional lithography, and then a mixture of PDMS prepolymer and curing agent with a mass ratio of 10:1 was spin-coated on top. After heating at 95 °C for 90 min, the PDMS template with micropatterned cavity array structures was obtained. The SEM images of the PDMS template are shown in Supplementary Fig. 33.
Preparation of organic micro-patterned crystal arrays
The crystal powder was uniformly spread on the substrate and heated to a melted liquid state. The PDMS template was heated to the same temperature and covered on the melt. The temperature was hold for 1 minute to allow the melt to fully fill the micro-cavities on the template. The temperature is lowered to the melting point and held for several minutes to ensure that the residual layer of melt is fully absorbed and then gradually cooled down. When the cooling process and the crystal growth is complete, the PDMS template is peeled off to obtain micro-patterned crystal arrays consistent with the template pattern. The bottom substrate was in direct contact with a thermally controlled hot stage, delivering a precise and uniform heating environment. To simplify the experimental apparatus and procedure, the PDMS mold was maintained at a different temperature by harnessing the cooling effect of controlled room-temperature air flowing across its surface. Throughout the crystallization process, the infrared camera continuously captured the thermal distribution on both the heated substrate and the PDMS mold. During the cooling process, an exponential decay function was applied to fit the temperature data.
Characterization
Thermogravimetry (TG), derivative thermogravimetry (DTG), and differential scanning calorimetry (DSC) curves were obtained by TA Instruments (America) at a heating rate of 10 K / min. SEM images were taken with JEOL JSM-7500f at an accelerating voltage of 1 kV. The fluorescence and Raman signals were obtained by using the Nikon A1RMP system under 405 nm excitation. XRD was obtained by Rigaku X-ray. The height distribution over a large area was obtained by Confocal Laser Scanning Microscopy (Nikon). The I-V curve of the organic crystal wire array was tested using a Keithley 2600 SourceMeter with Ti/Au (5 nm/50 nm) electrodes fabricated using electron beam lithography and electron beam evaporation method.
Characterization of laser emission
An infrared femtosecond laser (wavelength 800 nm, repetition frequency 2500 Hz) is converted into 400 nm by a BBO crystal. The 400 nm pump light is focused on the micro-patterned crystal through an objective lens. The emission of the micro-patterned crystal is focused by a lens and collected by a spectrometer with a resolution of 0.01 nm.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available from the corresponding author upon request. Source data are provided in this paper.
Change history
28 April 2025
The original version of this Article omitted from the author list the equal contribution statement of Shun-Xin Li and Guan-Yao Huang. Consequently, the following was added to the author list: “These authors contributed equally: Shun-Xin Li, Guan-Yao Huang.” This has now been corrected in both the PDF and HTML versions of the Article.
References
Zhang, X., Deng, W., Jia, R., Zhang, X. & Jie, J. Precise patterning of organic semiconductor crystals for integrated device applications. Small 15, 1900332 (2019).
Rigas, G.-P. et al. Spray printing of organic semiconducting single crystals. Nat. Commun. 7, 13531 (2016).
Kim, Y.-H., Yoo, B., Anthony, J. E. & Park, S. K. Controlled deposition of a high-performance small-molecule organic single-crystal transistor array by direct ink-jet printing. Adv. Mater. 24, 497–502 (2012).
Li, H., Fan, C., Fu, W., Xin, H. L. & Chen, H. Solution-grown organic single-crystalline donor-acceptor heterojunctions for photovoltaics. Angew. Chem. Int. Ed. 54, 956–960 (2015).
Yang, X., Feng, X., Xin, J., Wang, H. & Yan, D. High-performance crystalline organic light-emitting diodes based on multi-layer high-quality crystals. Org. Electron. 64, 236–240 (2019).
Nakanotani, H. & Adachi, C. Organic light-emitting diodes containing multilayers of organic single crystals. Appl. Phys. Lett. 96, 053301 (2010).
Cao, M. et al. Enhanced photoelectrical response of thermodynamically epitaxial organic crystals at the two-dimensional limit. Nat. Commun. 10, 756 (2019).
Deng, W. et al. Aligned single-crystalline perovskite microwire arrays for high-performance flexible image sensors with long-term stability. Adv. Mater. 28, 2201–2208 (2016).
Wei, C. et al. Controlled self-assembly of organic composite microdisks for efficient output coupling of whispering-gallery-mode lasers. J. Am. Chem. Soc. 137, 62–65 (2014).
Chen, S. et al. Single-crystal organic heterostructure for single-mode unidirectional whispering-gallery-mode laser. Adv. Opt. Mater. 10, 2101931 (2022).
Yu, L. et al. Programmable and coherent crystallization of semiconductors. Sci. Adv. 3, e1602462 (2017).
Galatsis, K. et al. Patterning and templating for nanoelectronics. Adv. Mater. 22, 769–778 (2010).
Dhanaraj, G., Byrappa, K., Prasad, V. & Dudley, M. Springer Handbook of Crystal Growth. Springer Berlin, Heidelberg (2010).
Li, M. et al. Growth defects of organic crystals: A review. Chem. Eng. J. 429, 132450 (2022).
Holbrough, J. L., Campbell, J. M., Meldrum, F. C. & Christenson, H. K. Topographical control of crystal nucleation. Cryst. Growth Des. 12, 750–755 (2012).
Hu, Y. et al. Influence of cooling rate on crystallization behavior of semi-crystalline polypropylene: experiments and mathematical modeling. Polymers 14, 3646 (2022).
Sugiyama, T., Furukawa, Y. & Fujimura, H. Crystalline/amorphous Raman markers of hole-transport material NPD in organic light-emitting diodes. Chem. Phys. Lett. 405, 330–333 (2005).
Khalfin, S. et al. Self-healing of crystal voids in double perovskite nanocrystals is related to surface passivation. Adv. Func. Mater. 32, 2110421 (2022).
Li, P. et al. The effect of thermal annealing on defect states, light intensity and photocurrent of organic solar cells. Optik 265, 169488 (2022).
Dheepika, R., Shaji, A., Imran, P. M. & Nagarajan, S. Improving device performance of p-type organic field-effect transistor using butterfly like triarylamines. Org. Electron 81, 105568 (2020).
Wang, L. et al. Annealing engineering in the growth of perovskite grains. Crystals 12, 894 (2022).
Bharti, D. & Tiwari, S. P. Crystallinity and performance improvement in solution processed organic field-effect transistors due to structural dissimilarity of the additive solvent. Synth. Met. 215, 1–6 (2016).
Sun, P., Liu, D., Zhu, F. & Yan, D. An efficient solid-solution crystalline organic light-emitting diode with deep-blue emission. Nat. Photonics 17, 264–272 (2023).
Lev, D. G., Gubbins, K. E., Radhakrishnan, R. & Sliwinska-Bartkowiak, M. Phase separation in confined systems. Rep. Prog. Phys. 62, 1573 (1999).
Perkins, W. G., Capiati, N. J. & Porter, R. S. The effect of molecular weight on the physical and mechanical properties of ultra-drawn high density polyethylene. Polym. Eng. Sci. 16, 200–203 (1976).
Wang, X., Li, H., Wu, Y., Xu, Z. & Fu, H. Tunable morphology of the self-assembled organic microcrystals for the efficient laser optical resonator by molecular modulation. J. Am. Chem. Soc. 136, 16602–16608 (2014).
Deng, W. et al. Scalable growth of organic single-crystal films via an orientation filter funnel for high-performance transistors with excellent uniformity. Adv. Mater. 34, 10 (2022).
Acknowledgements
The authors acknowledge the financial support of the National Key Research and Development Program of China (2020YFA0715000, L.L.), the National Natural Science Foundation of China (62075111, L.L., 61960206003, 21903035, 61825502, 61827826, H.B.S., 62075081, H.X. and 62304089, S.X.L.), the Natural Science Foundation of Jilin Province (20220101105JC, H.X.), the Tsinghua-Foshan Innovation Special Fund (2021THFS0102, H.B.S.) and Tsinghua University Initiative Scientific Research Program (L.L.).
Author information
Authors and Affiliations
Contributions
H.X., L.L., and H.B.S. conceived and supervised the project. S.X.L. and G.Y.H. performed the experiments and contributed equally to this work. S.X.L. prepared the patterned PDMS molds and performed the thermal properties characterization (TG, DTG, and DSC) of the OSCs. S.X.L. and G.Y.H. fabricated the patterned OSCs by NICL and performed multiple characterizations of the OSCs (XRD, photoluminescence, Raman, SEM, current, and so on). X.J.W., X.Z., X.L., Q.D.C., and Y.H.Y. conducted the laser emission characterization of patterned organic crystal arrays. S.X.L, G.Y.H., T.F., and L.L. wrote the manuscript with the contribution from all the authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Chan Paddy Kwok Leung, Tomoya Oshikiri, and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, SX., Huang, GY., Xia, H. et al. Nanoimprint crystalithography for organic semiconductors. Nat Commun 16, 3636 (2025). https://doi.org/10.1038/s41467-025-58934-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-58934-9
This article is cited by
-
Electric-Field-Driven Generative Nanoimprinting for Tilted Metasurface Nanostructures
Nano-Micro Letters (2026)