Abstract
Disease tolerance mitigates organ damage from non-resolving inflammation during persistent infections, yet its underlying mechanisms remain unclear. Here we show, in a Pseudomonas aeruginosa pneumonia mouse model, that disease tolerance depends on the mitochondrial metabolite itaconate, which mediates cooperative host-pathogen interactions. In P. aeruginosa, itaconate modifies key cysteine residues in TCA cycle enzymes critical for succinate metabolism, inducing bioenergetic stress and promoting the formation biofilms that are less immunostimulatory and allow the bacteria to integrate into the local microbiome. Itaconate incorporates into the central metabolism of the biofilm, driving exopolysaccharide production—particularly alginate—which amplifies airway itaconate signaling. This itaconate-alginate interplay limits host immunopathology by enabling pulmonary glutamine assimilation, activating glutaminolysis, and thereby restrain detrimental inflammation caused by the inflammasome. Clinical sample analysis reveals that P. aeruginosa adapts to this metabolic environment through compensatory mutations in the anti-sigma-factor mucA, which restore the succinate-driven bioenergetics and disrupt the metabolic synchrony essential for sustaining disease tolerance.
Similar content being viewed by others
Introduction
To confront infection, the host activates metabolic rewiring1,2,3,4,5. This process, known as “resistance to infection”, involves various adjustments that amplify phagocyte effector activity, including the release of reactive oxygen species and pro-inflammatory cytokines2,6,7. While these factors reduce pathogen burden, they also inflict considerable collateral damage on the surrounding tissue1,2,3,4,5,8. Despite significant progress in understanding how metabolism supports resistance to infection, its role in alternative defense mechanisms against persistent pathogens, such as disease tolerance, remains poorly understood.
Disease tolerance, also known as “tolerance to infection”, is an essential defense strategy against the immunopathology caused by difficult-to-eradicate pathogens9,10. This platform minimizes tissue damage by curbing protractive inflammation, preserving the function of infected organs11,12. To establish disease tolerance, the host orchestrates a complex metabolic program that fosters reciprocal interactions with the pathogen. The tolerant host selects for microbial communities with reduced immunostimulatory traits, suppressing inflammatory pathways that disrupt the mucosa, like the cytokine IL-1β. This dynamic promotes a host-pathogen “détente” that limits organ damage and facilitates coexistence9,10,11,13. However, the precise mechanisms by which host and pathogen co-transition into disease tolerance remain unclear.
Itaconate is a major immunoregulatory metabolite that preserves inflamed tissues by reducing IL-1β signaling, particularly by limiting inflammasome function1,3,14,15. Yet, its role on disease tolerance remains poorly understood3,14. Macrophages exposed to inert bacterial surrogates in vitro, such as lipopolysaccharide (LPS), promote the synthesis of itaconate via immunoresponsive gene 1 (Irg1)1,3,14,15. Itaconate suppresses IL-1β by inhibiting complex II (i.e., succinate dehydrogenase) in the tricarboxylic acid (TCA) cycle. This process abrogates succinate oxidation and the electron transport chain (ETC), decreasing the generation of ATP that fuels IL-1β production16. Furthermore, itaconate dampens IL-1β release by modifying key cysteine residues of inflammasome proteins, a process termed “S-itaconation”15,17,18. Despite its clear role in IL-1β modulation, how itaconate regulates disease tolerance remains underexplored, especially during infection by organisms like Pseudomonas aeruginosa that must adapt their metabolism to tolerant tissues to thrive.
P. aeruginosa, a prominent Gram-negative opportunist and ESKAPE pathogen, triggers acute inflammation in the respiratory tract19, mediated by succinate and IL-1β in alveolar macrophages (AM)20. Instead of resolving the infection, succinate-driven IL-1β signaling exacerbates tissue damage and promotes bacterial growth, as P. aeruginosa metabolizes succinate19,20,21. Succinate is the preferred P. aeruginosa carbon source, supporting its TCA cycle bioenergetics and generation of virulence factors, like the type IV pili22 and the iron-scavenging siderophore pyoverdine23. However, most P. aeruginosa infected hosts transition to a state of disease tolerance, where IL-1β and immunopathology subside to preserve pulmonary integrity. This immune shift enables host-pathogen coexistence, fostering bilateral interactions conducive of respiratory maintenance. The mechanisms underlying the evolution from harmful inflammation to P. aeruginosa tolerance remain unclear.
This study reveals how itaconate orchestrates disease tolerance by inducing bidirectional host-pathogen metabolic interactions. By modifying P. aeruginosa TCA cycle enzymes via S-itaconation, itaconate couples pathogen bioenergetics with the manufacture of immunosuppressive exopolysaccharides (EPS) like alginate. This EPS reprogramming favors a range of host-pathogen adaptations, such as reduced P. aeruginosa glutamine consumption, augmented host glutamine assimilation, and activation of AMs glutaminolysis. This metabolically synchronized, bilateral environment suppresses IL-1β. To maintain this tolerant milieu, itaconate integrates into the pathogen central metabolism, fueling alginate synthesis and further itaconate signaling. These findings not only reveal how itaconate facilitates the host-pathogen metabolic dialog that is integral for disease tolerance in the lung but also provide with a distinctive conceptual framework for the development of efficient therapies against intractable infections.
Results
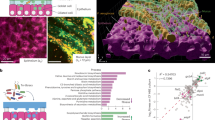
Disease tolerance P. aeruginosa strains couple EPS synthesis with impaired succinate bioenergetics
To understand the basis of tolerance to P. aeruginosa lung infection, we examined the immunometabolic features of strains associated with this state. When transitioning to a tolerant program, hosts favor P. aeruginosa strains that are metabolically primed to manufacture the EPS alginate, driven by algD expression (Supplementary Fig. 1a). These alginate-producing communities help maintain respiratory integrity by suppressing IL-1β, while sparing cytokines like IL-6 or tumor necrosis factor α (TNFα), as shown by the laboratory strain of P. aeruginosa PAO1 (WT PAO1), its ΔalgD PAO1 mutant (Fig. 1a, Supplementary Fig. 1b, c), and alginate-programmed isolates from tolerant individuals (Supplementary Fig. 1d). These alginate-specialized strains do not produce type III secretion system toxins like pcrV, exoS and exoT, which trigger inflammasome activation and alveolar destruction24,25,26 (Supplementary Fig. 1e). This milieu limits alveolar permeabilization (Fig. 1b, Supplementary Fig. 1f) and host mortality (Fig. 1c, Supplementary Fig. 1g), hallmarks of disease tolerance11,12,20. The tolerogenic program enabled by alginate-producing pathogens does not rely on other major immunomodulatory mechanisms, such as recruitment of regulatory T cells (Treg)27 or enrichment of the anti-inflammatory cytokine IL-1028 (Supplementary Fig. 1h, i).
Mice were exposed to either PBS, WT PAO1, or ΔalgD PAO1 (n = 3, total of 9–10 mice per group). Measures outcomes included: a BAL cytokines; b BAL albumin; c host survival. d Bacterial energy production (Biolog Technology) (n = 3). e, f Bacterial oxygen consumption rate (Seahorse) (e) and total oxygen consumption (AUC: arbitrary unit count) (f) (n = 5). g Caloric expenditure (μW/OD600). Right graph: total heat along growth (n = 4). h Growth curves (OD600) (n = 3). i Biofilm quantification (n = 12). j BAL succinate (n = 3, total of 6–10 mice per group); k bacterial burden (n = 3, total of 12 mice per group); l BAL itaconate (n = 3, total of 6–9 mice per group). WT and Irg1−/− mice were exposed to either PBS or WT PAO1 (n = 3, total of 8–10 mice per group). The following were measured: m BAL cytokines; n bacterial burden. Data are shown as average +/− SEM. d, f–g, i: t-Student test. a, b, j–n One-Way ANOVA (Tukey multiple comparison test). e, g, h Two-Ways ANOVA; C: Kaplan-Meier test. All statistical tests are two-sided. Source data are provided as a Source Data file.
Alginate-specialized P. aeruginosa strains also exhibit distinct bioenergetic profiles. Producing alginate through algD constrains TCA cycle activity, limiting succinate and citrate bioenergetics (Fig. 1d). This correlates with lower aerobic metabolic rates (Fig. 1e, f). Alginate synthesis demands substantial calories at the initial phase of proliferation, as illustrated by integrating isothermal calorimetric curves with growth assays29 (μM/OD600 - energy utilization per biomass unit) (Fig. 1g, gray and red zones). This energy expenditure limits growth, as shown by lower WT PAO1 biomass versus its ΔalgD PAO1 mutant in stationary phase (Fig. 1h, blue zone), similar to alginate-programmed isolates from tolerant hosts (Supplementary Fig. 1j–l). To balance this energy trade-off, P. aeruginosa prioritizes biofilm formation (Fig. 1I), a hallmark of limited bioenergetic resources30. In the respiratory tract, these alginate-rich biofilms reduce succinate levels (Fig. 1j) and pathogen burden (Fig. 1k, Supplementary Fig. 1m, n), leading to decreased IL-1β signaling (Fig. 1a, Supplementary Fig. 1d). However, pre-nourishing these strains with succinate increases biomass and IL-1β but not IL-6 or TNFα (Supplementary Fig. 2). Overall, these findings illustrate how alginate-rich P. aeruginosa strains adapt bioenergetically to support lung health through disease tolerance by prioritizing alginate manufacture over succinate metabolism.
Alginate enhances host itaconate signaling during P. aeruginosa pneumonia
Alginate-rich P. aeruginosa strains promote airway itaconate enrichment (Fig. 1l). In vitro assays using bone-marrow derived macrophages (BMDM) showed that alginate specifically induced itaconate signaling (Supplementary Fig. 3a, b). Soluble alginate dampens BMDMs inflammatory responses to LPS, particularly reducing the release of IL-1β and TNFα (Supplementary Fig. 3c, d), but not IL-6 (Supplementary Fig. 3e). Using Irg1−/− animals, which cannot produce itaconate14 (Supplementary Fig. 3f), we demonstrated that the immunometabolite during P. aeruginosa pneumonia restricted IL-1β signaling without affecting IL-6 and TNFα (Fig. 1m). This IL-1β inhibition linked with decreased NLPR3 inflammasome activity, particularly expression of both pro-Caspase 1 and its cleaved p20 form15 (Supplementary Fig. 3g). Itaconate promoted downstream cytoprotective routes that abrogate IL-1β production and favor mucosal integrity17, like Hemoxygenase 1 (HO-1) (Supplementary Fig. 3h). This environment reduced P. aeruginosa burden in the respiratory tract (Fig. 1n). These results show that P. aeruginosa alginate manufacture aligns with increased pulmonary itaconate levels, creating an immunometabolic state that promotes disease tolerance.
Itaconate blocks P. aeruginosa succinate bioenergetics and drives alginate-mediated biofilms
Itaconate promoted P. aeruginosa alginate-based biofilms, especially when combined with carbohydrates like glucose (Fig. 2a). Adding succinate, the preferred P. aeruginosa nutrient31,32,33, dispersed these biofilms (Fig. 2a), showing that itaconate enables this lifestyle by altering succinate bioenergetics in the TCA cycle.
a Biofilm specialization by WT and ΔalgD PAO1 (OD540/OD600) (n = 3, 3-5 replicates per assay). WT PAO1 was exposed or not to itaconate in nutrient-rich media (LB). The following were measured: b intracellular TCA cycle metabolite abundance (n = 3); c Global chemoproteomic profiling of S-itaconation of the WT PAO1 proteome (n = 3). d Succinate oxidation (generation of anion superoxide (O2*−)) in WT PAO1 and Δict PAO1, which cannot degrade itaconate (n = 4). e oxygen consumption rates (OCR) by Seahorse technology (n = 4); f total oxygen consumed along time (n = 4); g growth (OD600) (n = 3). Data are shown as average +/− SEM. a: One-Way ANOVA (Tukey multiple comparison test). g Two-Way ANOVA. b–d, f t-Student test. All statistical tests are two-sided. Source data are provided as a Source Data file.
In PAO1, itaconate provoked a ~ 15,000-fold accumulation of the succinate precursor succinyl-CoA (Fig. 2b). This succinyl-CoA enrichment was accompanied by accrual of all its predecessors, such as αketoglutarate (αKG), aconitate, and citrate (Fig. 2b), but not the succinyl-CoA byproducts fumarate, malate, and oxaloacetate (Fig. 2b). This indicates a bottleneck in the TCA cycle, impairing succinyl-CoA conversion to succinate.
In P. aeruginosa, the TCA cycle enzyme that converts succinate into succinyl-coA is succinyl-CoA ligase (sucCD). Chemoproteomic profiling using the bioorthogonal probe of itaconate, C3A, which retained the α,β-unsaturated carboxylic acid groups of the immunometabolite, revealed that itaconate modifies key cysteine residues of succinyl-CoA ligase through “S-itaconation”18,34, inactivating its function. Among all proteins exhibiting C3A-modified cysteines, we found sucC and sucD, subunits β and α of succinyl-CoA ligase, respectively, clustering among the most significantly S-itaconated proteins (Fig. 2c). Modifications in sucC mapped to Cys101 (Log2FC ~ 1.08) and Cys211 (Log2FC ~ 3.58), and sucD PTM to Cys13 (Log2FC ~ 2.45) and Cys124 (Log2FC ~ 3.79) (Fig. 2c). Of note, in Gram-negative organisms, sucD Cys124 is highly conserved (Supplementary Fig. 4a), as it coordinates the removal of the CoA group from succinyl-CoA to form succinate35. Transcriptomic data confirmed sucD inhibition by itaconate (Supplementary Fig. 4b).
Itaconate also modified sdhB at Cys209 and Cys213 (Log2FC ~ 1.56), a succinate dehydrogenase subunit, the enzyme that oxidizes succinate in the P. aeruginosa TCA cycle-ETC interface (Fig. 2c). Functional assays using a P. aeruginosa strain unable to breakdown itaconate (Δict PAO1) indicated that this metabolite limited the ability of the organism to oxidize succinate and produce anion superoxide (O2*−) at the ETC (Fig. 2d). This bioenergetic impairment provoked compensatory increases in oxygen consumption to maintain ATP homeostasis (Fig. 2e, f). Consistently, the growth of Δict PAO1 in itaconate was rescued when succinate was added in increasing concentrations (Fig. 2g). Glucose failed to restore Δict PAO1 growth, showing itaconate’s effects are succinate-specific (Supplementary Fig. 4c–e). Thus, itaconate blocks P. aeruginosa succinate bioenergetics.
Itaconate promotes P. aeruginosa EPS reprogramming
We found itaconate supports alginate-based P. aeruginosa biofilms via bacterial metabolic remodeling. The specialization of P. aeruginosa into less immunostimulatory communities - alginate-rich biofilms - requires the concerted participation of many anabolic platforms, such as gluconeogenesis, the pentose phosphate pathway (PPP), and the de novo pathway of pyrimidine synthesis (DNPPS)36,37,38,39 (Fig. 3a). These circuits generate different EPS precursors, like carbohydrates, cycled sugars, and nucleotides36,37,38,39, which further stimulate algD function for alginate synthesis. We found that itaconate stimulated PAO1 enrichment of many gluconeogenic intermediates that facilitate alginate generation, like phosphoenolpyruvate (PEP), fructose-6-phosphate, glucose-6-phosphate and glucose (Fig. 3b). Furthermore, itaconate provoked PAO1 concentration of diverse sugars that emerge from gluconeogenesis, such as glucuronate, glucosamine (Glc), and N-Acetyl-Glc-1-phosphate (GlcNAc-1-P) (Fig. 3c). Itaconate also augmented in P. aeruginosa different cycled carbohydrates formed in the PPP, including D-Ribose, D-Sedoheptulose-7-phosphate, and D-Erythrose-4-phosphate (Fig. 3d). This metabolic reconfiguration was consistent with the accumulation of both orotate, the rate-limiting step of the DNPPS, and uracil, which is in direct equilibrium with orotate via uridine monophosphate (UMP) (Fig. 3e). The priming by itaconate of all P. aeruginosa platforms involved in EPS was confirmed by the increase of both UDP-glucuronate and GDP-glucose, nucleotide-activated carbohydrates that act as building blocks for many of these biopolymers (Fig. 3f). Of note, other EPS precursors like ADP-glucose and UDP-glucose were depleted by itaconate (Fig. 3f), indicating their rapid redirection to the extracellular milieu to strengthen the biofilm shield.
a P. aeruginosa central metabolism; b–f Intracellular metabolite abundance in WT PAO1 exposed or not to itaconate (n = 3); g–k Isotope carbon tracing in WT PAO1 exposed or not to 13C-itaconate. Different isotopologues per metabolite are coded with numbers-colors (n = 3). Data are shown as average +/− SEM. b–f: t-Student test. All statistical tests are two-sided. Source data are provided as a Source Data file.
Itaconate fuels P. aeruginosa EPS remodeling
We wondered how itaconate promoted P. aeruginosa EPS reprograming. We hypothesized that this process occurs as part of the pathogen’s strategy to withstand the immunometabolite toxicity40. Itaconate is considered a major bactericidal agent40. P. aeruginosa, unlike other opportunists, can endure itaconate burden by breaking it down into acetyl-CoA and pyruvate, two sources of gluconeogenesis40 (Supplementary Fig. 5a and b). We postulated that itaconate drives P. aeruginosa anabolic reprograming by incorporating carbon flux into gluconeogenesis.
We tracked the integration of 13C-itaconate into the P. aeruginosa PAO1 central metabolism through isotope carbon labeling (Fig. 3g). These assays showed that itaconate highly assimilated into the backbone of both acetyl-CoA and pyruvate, as ~70% and ~60% of all isotopologues of these molecules, respectively, showed 13C signature (Fig. 3h). This incorporation enabled the avid circulation of 13C-itaconate carbon atoms into gluconeogenesis; while ~60% of all isotopologues from both PEP and glyceraldehyde-3-phosphate (G3P) presented the 13C signature, ~80–90% of isotopologues from fructose-1,6-biphosphate, fructose-6-phosphate, and glucose-6-phosphate, end products of gluconeogenesis, manifested this labeling (Fig. 3h).
Carbon atoms of 13C-itaconate reached upstream networks that employ byproducts of gluconeogenesis to facilitate EPS synthesis, like the PPP and DNPPS. This was illustrated by a prominent 13C signature in substantial isotopologue fractions of D-Ribose-5-P ( ~ 80%), orotate ( ~ 40%), uracil ( ~ 80%), uridine ( ~ 90%) and nucleotides like UMP ( ~ 90%), UDP ( ~ 90%) and UTP ( ~ 90%) (Fig. 3i–k). Ultimately, carbon atoms of itaconate dominated the architecture of many EPS precursors like UDP-glucoronate ( ~ 95%), UDP-GlcNAc ( ~ 95%), and UDP-glucose ( ~ 95%) (Supplementary Fig. 5c), confirming how this mitochondrial factor effectively assumes control over the P. aeruginosa production of pro-biofilm determinants.
Itaconate suppresses harmful inflammation by facilitating pulmonary glutaminolysis
Given that disease tolerance is coordinated by bilateral host-pathogen adaptations, we anticipated that itaconate, in addition to reprogram P. aeruginosa metabolism, would also impact pulmonary cells. Itaconate’s inhibition of host mitochondrial complex II14 would initiate an array of compensatory mechanisms to replenish the TCA cycle and restore cell bioenergetics, such as glutamine catabolism via glutaminolysis41. Indeed, in neurons, complex II blockade by itaconate prompts glutaminolysis and accumulation of the glutamine byproduct αKG42. Of interest, in macrophages, αKG represses IL-1β signaling via epigenetic changes, particularly through the H3K27 demethylase Kdm6b41. We anticipated that during P. aeruginosa pneumonia itaconate would suppress IL-1β in AMs via glutaminolysis and Kdm6b stimulation.
To promote glutaminolysis, AMs assimilate glutamine through transporters like Slc38a2, Slc38a1, Slc1a5, and Slc3a241. Then, glutamine is converted into glutamate via Gls (rate-limiting step) and subsequently to αKG by Glud1 (Fig. 4a, blue arrows)41. Transcriptomic analyzes of AMs through single-cell RNA sequencing (scRNA-Seq) in lungs of infected Irg1+/+ and Irg1−/− mice confirmed that itaconate upregulates glutaminolysis during P. aeruginosa pneumonia, particularly by promoting expression of Slc38a1, Slc38a2, Slc3a2, Gls and Kdm6b (Fig. 4b–d). This setting was supported by reduced levels of Glul, which antagonizes Gls (Fig. 4c), and lower mRNA abundance of the inflammasome constituents nlpr3 and Il1b (Fig. 4c). Consistently, itaconate provoked glutamine depletion from the infected airway (Fig. 4e), its enrichment within the pulmonary tissue (Fig. 4f, left panel), and its conversion into glutamate (Fig. 4f, right panel). Inhibition of glutaminolysis with the Gls inhibitor BPTES in infected mice exacerbated IL-1β levels and bacterial burden (Fig. 4g, h), affirming its role in controlling inflammation without affecting IL-6 or TNFα (Fig. 4g). Itaconate did not affect other routes involved in AMs glutamine assimilation and stimulation of IL-1β signaling, such as the GABA shunt (Gad1, Abat, Aldh5a1, hif1a)6 (Fig. 4a, red arrows; Supplementary Fig. 6a).
a Glutamine metabolism in AMs. Lungs from Irg1+/+ and Irg1−/− mice exposed to either PBS or WT PAO1 were studied by scRNA-Seq: b cell subsets; c glutamine metabolism genes in AMs; d Glutaminolysis score in AMs (n = 1, pool of two mice per group, total of 12-48 cells); e BAL metabolite enrichment (n = 3, total of 6-9 mice per group); f lung tissue metabolite levels (DESI-2D). Mice were exposed or not to WT PAO1 and administered or not with BPTES (n = 3, total of 3–5 mice per group). The following were measured: g BAL cytokines; h bacterial burden. i Bacterial energy production with glutamine (Biolog Technology) (n = 3). j Bacterial growth in glutamine (n = 3). k BAL metabolite enrichment (n = 3, total of 6–9 mice per group). l Bacterial energy production with glutamate (Biolog Technology) (n = 3). m Bacteria growth in glutamate (n = 3). Data are shown as average +/− SEM. e, i, k, l t-Student test. d, g, h One-Way ANOVA (Tukey multiple comparison test); j, m Two-Way ANOVA. All statistical tests are two-sided. Source data are provided as a Source Data file.
Together, these findings indicate that, during P. aeruginosa infection, respiratory cells employ glutamine to mitigate pathological inflammation through glutaminolysis, a process facilitated by itaconate.
Itaconate stimulates AMs glutaminolysis by interfering with ETC
Since glutaminolysis is typically activated to compensate for mitochondrial bioenergetic insufficiency, particularly by fueling the TCA cycle43, we hypothesized that itaconate promotes this response during P. aeruginosa pneumonia by disrupting ETC integrity. ETC activity, driven by complexes I, II, III and IV in the inner membrane of the mitochondrion, is coordinated by a mega molecular structure known as the “respirasome” (I2II2III2IV2)44. The respirasome supports ATP synthesis by complex V, which energizes the inflammasome activity to produce IL-1β 16. ScRNA-Seq studies and pathway enrichment analyzes of lungs from infected Irg1+/+ and Irg1-/- mice revealed that itaconate alters the expression of key ETC subunits involved in respirasome integrity in AMs (Supplementary Data 1-2). The immunometabolite increased mRNA levels of mt-Nd1234, mt-Cytb, mt-Co123, and mt-Atp6, which, respectively, form the core membrane embedded subunits of complexes I, III, IV and V44 (Supplementary Fig. 6b, c). However, itaconate reduced expression of essential supernumerary subunits required for the proper assembly of these complexes, such as Ndufa4-Ndufa11-Ndufb2-Ndufb4 (complex I), Uqcrb-Uqcrh (complex III), and Cox7a2-Cox4i1-Cox5b-Cox7c (complex IV)45 (Supplementary Fig. 6b, c). While reducing Cox7a2, itaconate conserved its isoform Cox7a2l (P > 0.05) (Supplementary Fig. 6b, c, red quadrant), which forms less efficient respirasomes45. These effects are specific to AMs, with no significant changes in other pulmonary cell subsets (P > 0.05 for all genes) (Supplementary Fig. 7). Together, these findings confirm that itaconate facilitates glutaminolysis and tolerance to P. aeruginosa infection through bioenergetic impairment of specific respiratory cells.
Alginate-producing P. aeruginosa strains facilitate airway glutaminolysis precursors
Unlike the ΔalgD PAO1 strain, its WT PAO1 counterpart, which produces alginate, relies less on environmental glutamine for energy generation and growth (Fig. 4i, j). This metabolic configuration enabled more airway glutamine availability during infection (Fig. 4k). Additionally, alginate did not affect P. aeruginosa’s ability to employ glutamate as energy source to proliferate (Fig. 4l, m), facilitating its depletion from the airway by the host (Fig. 4k). Collectively, these findings confirm how P. aeruginosa tailored for disease tolerance facilitates immunoregulatory pathways that rely on glutamine accessibility.
P. aeruginosa reprograms its bioenergetics in response to itaconate
The bioenergetic adjustment imposed by itaconate on the host prompted us to evaluate whether this might also occur in P. aeruginosa, leading to pathogen adaptation to survive. Integrating RNA-Seq with unbiased pathway analysis showed that P. aeruginosa PAO1 responded to itaconate by activating catabolic networks producing the ketone body acetoacetate (AcAc), including metabolism of the nutrient lipoic acid, degradation of the branched amino acids valine, leucine, and isoleucine, catabolism of terpenes like pinene, camphor, and geraniol, oxidation of propanoate, and metabolism of 2-oxocarboxylic acids (Supplementary Fig. 8a, Supplementary Data 3). Adding exogenous AcAc restored growth in strains unable to cope with itaconate stress, like Δict PAO1 (Supplementary Fig. 8b). P. aeruginosa also downregulated energy-intensive processes that incur substantial ATP expenditure, such as repressors of biofilm formation, phenazine biosynthesis, quorum sensing, the type VI secretion system (T6SS), sulfur assimilation routes and ABC transporters (Supplementary Fig. 8a). These changes conserved the pathogen ATP-GTP homeostasis (Supplementary Fig. 8c, d). However, the preserved energy was redirected to early alginate production, limiting biomass growth (Supplementary Fig. 8e–g). This indicates that P. aeruginosa adapts to itaconate but remains constrained by the metabolic demands of alginate synthesis.
P. aeruginosa inactivation of the anti-sigma factor mucA is a hallmark of adaptation to disease tolerance
The metabolic constrain found in the tolerant lung leads P. aeruginosa to evolve compensatory mutations46,47,48. The adaptation of P. aeruginosa to this milieu, particularly the interaction between itaconate and alginate synthesis, attenuates the anti-sigma factor mucA (Fig. 5a). MucA inactivation is common in the lung of long-term infected hosts, such as people with cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD), and bronchiectasis (BC), which associates with tolerance disruption and severe pulmonary deterioration49,50. Among 51 types of inactivating mucA mutations found in 1014 genomes of P. aeruginosa isolates from these tolerant subjects (Supplementary Data 4), mucA22 emerged as the most frequent adaptation (Fig. 5b, c, Supplementary Fig. 9a). This mutation is not observed in strains from individuals at intensive care unit (ICU), which have not transitioned into disease tolerance yet (Fig. 5b, c, Supplementary Fig. 9a).
a mucA mRNA levels (n = 3, 8-12 technical replicates). Number of P. aeruginosa genomes studied: ICU: 182; CF: 532; COPD: 90; COPD: 210. In these genomes, the following was evaluated: b Frequency of mucA mutations in P. aeruginosa isolates genomes. c Frequency of different mucA mutations in isolates; d, e Unbiased pathway enrichment analyzes between WT and mucA22 PAO1 (n = 3). Top 5 significantly changed pathways are shown. f, g Bacterial oxygen consumption rate (Seahorse) (f) and total oxygen consumption (area under the curve) (g) (AUC: arbitrary unit count) (n = 4). h Bacterial energy production (Biolog Technology) (n = 3). i growth curves (OD600) (n = 3). Data are shown as average +/− SEM. d, e t-Student test; a, g, h One-Way ANOVA (Tukey multiple comparison test); f, i Two-Ways ANOVA. All statistical tests are two-sided. Source data are provided as a Source Data file.
MucA22 is a stop-codon mutation that fully abrogates mucA function50,51,52. Despite bolstering P. aeruginosa alginate production53 (Supplementary Fig. 1b, c), mucA22 causes severe pulmonary exacerbations linked with secretion of cytokines that are not suppressed by itaconate, such as TNFα and TNFα-dependent chemoattractants like IL-8, MIP1α and MIP254,55,56, and massive infiltration of phagocytes that hinder the airway57. MucA22 also boosts P. aeruginosa succinyl-coA ligase (sucCD) activity48, potentially mitigating the TCA cycle impairment induced by itaconate (Fig. 2). We anticipated that, by offsetting the bacterial bioenergetic stress, this mutation would disrupts the host-pathogen synchrony that maintains disease tolerance.
MucA22 enhances P. aeruginosa TCA cycle bioenergetics
Analysis of publicly available transcriptomic datasets for WT PAO1 and its isogenic mucA22 PAO1 mutant58,59 revealed that inactivation of the anti-sigma factor boosts P. aeruginosa TCA cycle function, making the pathogen resilient to the bioenergetic stress imposed by itaconate. By integrating unbiased pathway enrichment analyzes with the Kyoto Encyclopedia of Genes and Genomes (KEGG) and the Gene Ontology for Biological Processes (GOTERM BP) libraries, we identified the top five P. aeruginosa networks with the highest frequency of significantly altered (P < 0.05) genes by mucA22 (Fig. 5d–e, Supplementary Data 5). Within the “metabolic pathways” category, notable increased genes included aconitate hydratase (acnA), which converts citrate to aconitate, a αKG’s precursor that stimulates the TCA cycle60 (Fig. 5d, Supplementary Data 5), as well as adenylosuccinate lyase (PA3517), fumarate hydratase (fumC2), and cytochrome C (PA2266), which collectively facilitate production of the succinate byproduct fumarate61, enable its oxidation to malate62, and transport electrons from this reaction across the ETC to synthesize ATP63 (Fig. 5d, Supplementary Data 5). Consistently, mucA22 PAO1 demonstrated enhanced aerobic metabolism with succinate compared to WT PAO1, a process independent of alginate, as shown by an isogenic mucA22ΔalgD PAO1 mutant (Fig. 5f–g, Supplementary Fig. 1b, c). This heightened oxygen consumption linked to a 2-fold increase in energy generation with succinate, rising to 4-fold when alginate synthesis occurred (Fig. 5h). Such bioenergetic reprogramming likely provides P. aeruginosa with an energy surplus under itaconate stress. Indeed, despite reduced proliferation during EPS production, alginate-generating mucA22 PAO1 exhibited resilience to itaconate-induced growth inhibition, both in vitro (Fig. 5i) and in vivo (Supplementary Fig. 9b). Furthermore, this setting dispersed surface-attached P. aeruginosa biofilms by itaconate (Supplementary Fig. 9c–d). These findings demonstrate how mucA22 equips P. aeruginosa to overcome metabolic stress imposed by the itaconate-alginate axis, enhancing its bioenergetic capacity and adaptive fitness.
MucA22 P. aeruginosa conserves alginate-mediated tolerance but exacerbates TNFα signaling
Transcriptomic analyzes revealed that mucA22 enhances P. aeruginosa’s resilience to environmental threats while activating inflammatory pathways independent of itaconate, such as TNFα signaling. MucA22 elicited loci involved in the breakdown of complex carbohydrates, resistance to osmotic pressure, and tolerance to desiccation stress49,64,65, such as trehalase (treA), glycogen synthase (PA2165), glycogen phosphorylase (glgP), UDP-glucose 6-dehydrogenase (PA2022), ABC transporter permeases PA3888-PA3891 and glutamine amidotransferase (PA3459) (Supplementary Data 5). This stress-resistance program aligned with activation of the “alginate biosynthetic process” (Fig. 5d), utilizing sugar byproducts to sustain EPS production through algD and auxiliary algABEFGJKLU8 genes49,64,65.
Interestingly, mucA22 downregulated pyoverdine synthesis genes but paradoxically boosted pyoverdine release, a siderophore that exacerbates airway destruction (Fig. 5e, Supplementary Fig. 9e, Supplementary Data 5). This equilibrium might be part of a negative feedback loop triggered by the siderophore itself. Additionally, mucA22 induced pro-inflammatory adjustments linked to TNFα signaling, such as pilA and pilB, components of type IV pili, and the algZ (amrZ)-algR system, which disperses biofilms into planktonic forms by repressing PelA and Psl EPS genes (Fig. 5d–e, Supplementary Data 5). These adaptations escalate airway immunopathology, undermining disease tolerance through alginate and itaconate-driven mechanisms.
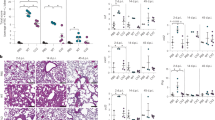
MucA22 promotes airway disruption through TNFα signaling
In mice infected with mucA22 P. aeruginosa, TNFα and related chemoattractants - e.g., MIP1α (Ccl3), MIP2 (Cxcl2), and the murine IL-8 homolog KC (Cxcl1) – increased, leading to higher pathogen loads and airway inflammation (Fig. 6a, b). As indicated by the mucA22ΔalgD PAO1 mutant, alginate partially attenuated bacterial burden (Fig. 6b), but not TNFα signaling (Fig. 6a). Although alginate production by mucA22 PAO1 favored many parameters of disease tolerance, such as airway itaconate enrichment (Supplementary Fig. 10a), less generation of T3SS necrotizing toxins (Supplementary Fig. 10B), decreased IL-1β and IL-6 (Fig. 6c), reduced alveolar permeabilization (Fig. 6d), and limited host mortality (Fig. 6e), these pathogens exacerbated the pulmonary recruitment of myeloid cells, such as monocytes (Supplementary Figs. 10c–e; recruited monocytes 2 by scRNA-Seq, Fig. 4b) and neutrophils (Supplementary Figs. 10c–e; recruited neutrophils 1 by scRNA-Seq, Fig. 4b).
a Mice were exposed to either PBS, WT PAO1, mucA22 PAO1 or mucA22ΔalgD PAO1 (n = 3, total of 8-11 mice per group). The following were analyzed: a, c BAL cytokines; b bacterial burden; d BAL albumin; e host survival. Mice were exposed to either PBS, WT PAO1, or mucA22 PAO1 (n = 2, total of 4–9 mice per group). The following were analyzed: f, g numbers and viability of type 1 pneumocyte in BAL; h, i, k lung cell numbers; j BAL VEGF. Data are shown as average +/− SEM. a–d, f–k: One-Way ANOVA (Tukey multiple comparison test). e: Kaplan-Meier test. All statistical tests are two-sided. Source data are provided as a Source Data file.
As indicated by scRNA-Seq and unbiased pathway enrichment analyzes, the shared signature of recruited monocytes 2 and recruited neutrophils 1 is expression of Tnf, its receptor Tnfrsf1b, and the regulatory protein Tnfaip3, as well as different inflammatory circuits elicited by TNFα, such as toll-like receptors 2 and 4 (Tlr2, Tlr4), cytoplasmic kinases (Map3k8, Ikbke), NFkΒ signaling (Nfkb1, Nfkb2, Nfkbia), and the chemoattracting factors Ccl3 (MIP1α), Cxcl2 (MIP2) and Cxcl1 (KC) (Supplementary Figs. 10f, g, Supplementary Data 6). Separately, recruited monocytes 2 downregulate pathways critical for pathogen clearance, including the respirasome (mt-Nd1234, Sdha, mt-Cytb, mt-Co123, mt-Atp6, Ndufa11-Ndufb247, Uqcrb, and Coxi1-Cox7c-Cox7a2-Cox7a2l)44,45 and phagocytosis promoters (Pi3kr1, Sirpb1bc, Sirpa, Mapk14, c-Fos, Calm2, Calm3)66,67,68 (Supplementary Fig. 10f). Analogously, recruited neutrophils 1 abrogate expression of essential genes for effector function (Csf1r, Csf3r)69,70, phagocytosis (c-Fos)66, oxidative burst (Rock2, Ncf2)71,72, bacterial degradation (Lyz2)73, and priming of naïve T cells against foreign antigens (H2-Ab1, H2-Q10, Cd74, Itga4, Itgal, Ptgs2, Il6ra, Cxcr4, Sell, Cst3, and Hsp90ab1)74 (Supplementary Fig. 10g). Together, these findings reveal how mucA22 hinders airway integrity by facilitating the accretion of TNFα-producing phagocytes incapable of eliminating P. aeruginosa.
TNFα-driven inflammation damages type 1 pneumocytes, vital for alveolar function75,76,77, leading to their detachment into the airway space (Fig. 6f), subsequent death (Fig. 6g), and compensatory type 2 pneumocyte hyperplasia to replenish them in the lung tissue78,79 (Fig. 6h, i). This repair response increases VEGF levels80 (Fig. 6j) and ciliated cell proliferation (Fig. 6k), attempting to clear airway debris but ultimately disrupting respiratory-vascular homeostasis. Thus, mucA22 exacerbates airway pathology by driving ineffective, necrotic immune responses that destabilize respiratory equilibrium.
Evidence of P. aeruginosa adaptation to alginate-driven disease tolerance in the human lung
To gain clinical insights in the mechanistic described, we studied FRD1, a widely used, multidrug resistant (MDR) isolate of P. aeruginosa from the lung of a chronically infected CF patient81,82. Although this strain produces copious alginate, its mucA22 mutation drives severe immunopathology83,84. Our analyzes confirmed FRD1 harbors vestiges of progressive adaptation to disease tolerance; 1) by preserving many traits linked with itaconate immunoregulation, such increased alginate synthesis and less production of inflammatory toxins; and 2) by heightening succinate bioenergetics via mucA22.
Compared with WT PAO1, FRD1 harbors 9,603 NSM, including 82 that significantly impact gene function (frameshift, stop gained, start lost, etc), 5,635 with moderate effects on gene activity (missense variant, conservative in frame deletion, conservative in frame insertion, etc), and 3,886 affecting intergenic regions (Supplementary Data 7). While few NSM affected alginate-related genes, including algC, algI, algK, alg8, and alg44 (Fig. 7a), and the integrity of the algD locus was fully preserved (Fig. 7a), clusters for alternative EPS like Pel and Psl accumulated numerous inactivating mutations (Fig. 7a). This mutation pattern correlated with reduced mRNA levels (Fig. 7a). In vivo, FRD1’s alginate-centered metabolism reduces bacterial burden (Fig. 7b) and dampens immunopathology, lowering markers like pro-inflammatory cytokines (IL-1β, IL-6, TNFα, MIP1α, MIP2, and KC) (Fig. 7c), airway permeability (Fig. 7d), and loss of body temperature regulation (Fig. 7e), as shown by an isogenic ΔalgD FRD1 strain. However, alginate has no effect on FRD1 lethality (Fig. 7f), suggesting complementary adaptations that mitigate bacterial pathology in the absence of the EPS.
a Number of non-synonymous mutations (# NSM) and gene expression level (logFC) for loci involved in EPS synthesis in FRD; control: WT PAO1. Mice were exposed to either PBS, WT PAO1, FRD1 or ΔalgD FRD1 (n = 2, total of 11-12 mice per group). The following were analyzed: b pathogen burden; c BAL cytokines; d BAL albumin; e body temperature; f Animal survival. Data are shown as average +/− SEM. B-E: One-Way ANOVA (Tukey multiple comparison test); f Kaplan-Meier. All statistical tests are two-sided. Source data are provided as a Source Data file.
FRD1 accrued substantial NSM in genes related to immunopathology, such as the involved in LPS O-antigen modification (e.g., WbpMZYW, wzt, PA5455-PA5459), LPS lipid A synthesis and modification (e.g., wzz2, waaGPL, eptC, lpxBD), and LPS surface exposure (e.g., lptD, lptG) (Supplementary Fig. 11a). LptD inactivation in FRD1 is sufficient to preserve host survival, as confirmed by a PAO1 strain lacking this locus (ΔlptD PAO1) (Supplementary Fig. 11b). Thus, FRD1 exhibits vestiges of adaptation to disease tolerance, balancing EPS and LPS integrity to mitigate disease.
P. aeruginosa FRD1 enhances succinate bioenergetics
As result of its mucA mutation mucA22, FRD1 reprograms its TCA cycle to prioritize succinate metabolism. Compared with WT PAO1, key genes for succinate generation and oxidation (sucCD, sdhABCD) are preserved and upregulated, whereas earlier TCA cycle steps (icd, idh, sucB, lpdG, lpdV, lpd3) are less conserved (Fig. 8a). FRD1 compensates for a mutation in fumC1, which utilizes the succinate byproduct fumarate, by maintaining the sequence integrity of fumC2, mqoA, mqoB, and gltA, as well as promoting their expression (Fig. 8a). This setting ensures efficient succinate bioenergetics and increases aerobic metabolism, independent of alginate (Fig. 8b, c). Like mucA22 PAO1 (Fig. 5h), FRD1 couples alginate synthesis with enhanced energy generation from succinate and fumarate, overcoming the metabolic burden of EPS production (Fig. 8d). Indeed, despite synthesizing alginate, FRD1 still generated higher biomass than WT PAO1 (Fig. 8e). Thus, by optimizing succinate-fumarate bioenergetics, FRD1 outcompetes the bioenergetic encumbrance of alginate production, enabling increased bacterial loads.
a Number of non-synonymous mutations (# NSM) and gene expression level (logFC) in FRD1 for TCA cycle clusters; control: WT PAO1. b, c oxygen consumption rates (OCR) by Seahorse technology (b) and total oxygen consumed along time (c) (AUC: arbitrary unit count) (n = 4). d bacterial energy production (Biolog Technology) (n = 3). e growth curves (OD600) (n = 3). Data are shown as average +/− SEM. c, d: One-Way ANOVA (Tukey multiple comparison test); e Two-Ways ANOVA. All statistical tests are two-sided. Source data are provided as a Source Data file.
Discussion
This study reveals how metabolism shapes bidirectional host-pathogen interactions that drive disease tolerance. Itaconate, a key mitochondrial carboxylate, plays a central role by synchronizing the metabolic programs of both host and pathogen to reduce harmful lung inflammation. In the pathogen, itaconate limits IL-1β-driven immunopathology by disrupting planktonic growth, primarily through inhibition of succinate nutrition. By impairing succinate bioenergetics, itaconate induces the formation of small bacterial communities with reduced immunostimulatory properties. These communities form biofilms that integrate into the local microbiome to coexist with the host. Itaconate supports this environment by redirecting carbon flux towards EPS synthesis, such as alginate, which consolidates biofilm lifestyle and stimulates further itaconate production. In the host, the itaconate-alginate axis disrupts pathways like ETC, reducing energy production and suppressing IL-1β synthesis in AMs. Furthermore, itaconate promotes AMs glutaminolysis, enhancing IL-1β suppression via epigenetic regulation41. Thus, itaconate, in synergy with alginate, shape metabolic reprogramming in both host and pathogen, preventing IL-1β-mediated immunopathology and establishing disease tolerance.
Our work illustrates how P. aeruginosa progressively circumvents the bioenergetic pressure linked to disease tolerance – i.e., itaconate-alginate axis and TCA cycle protein S-itaconation - through compensatory mutations like mucA22. This mutation enhances the pathogen’s capability to generate energy via succinate oxidation, while leveraging the alginate-itaconate axis to further disable mucA function. Despite producing alginate to control inflammation, P. aeruginosa strains harboring mucA22 skew the tolerant microenvironment towards TNFα signaling, driving the infiltration of harmful phagocytes incapable of resolving the infection. This immune dysfunction leads to airway obstruction, epithelial damage, altered mucosal remodeling, and the progressive buildup of cellular debris. This setting results in deleterious consequences for host health, as observed in people with CF, where P. aeruginosa isolates with mucA disruption accelerate pulmonary decline and demise83,84.
Our work challenges the long-standing belief that alginate is a major virulence factor produced by P. aeruginosa in the human lung36,83,85. This assumption originated from clinical observations correlating pulmonary decline in chronically infected patients with the isolation of strains producing high levels of the EPS, such as those harboring mucA mutations52,83,84. However, contrary to this view, our findings suggest that alginate is not responsible for airway damage, but, instead, plays a key protective role by limiting immunopathology and supporting alveolar integrity. This interpretation aligns with the well-documented biocompatibility of alginate. As an FDA-approved biopolymer, alginate has been widely utilized in food preservation86,87, regenerative medicine88,89,90,91,92, and biodegradable materials93,94. Our study highlights its synergistic relationship with itaconate, a mitochondrial metabolite that maintains pulmonary stability by fueling its synthesis.
By assessing metabolically active pathogens instead of inert bacterial surrogates like LPS, our study redefines how itaconate preserves integrity of the infected airway via disease tolerance. Contrary to traditional views, itaconate orchestrates this defense strategy by aligning host-pathogen metabolism, fostering an immunosuppressed microenvironment that limits immunopathology. These findings underscore the crucial role of mitochondrial metabolism in interkingdom interactions during infection, particularly during pneumonia caused by multi-drug resistant ESKAPE opportunists like P. aeruginosa. By offering a conceptual framework for the forces driving bacterial persistence and evolution in the human lung, this work encourages the scientific community to integrate host and pathogen metabolism in studying host-pathogen interactions. This comprehensive approach could unveil new defense mechanisms beyond resistance to infection and disease tolerance, paving the way for more effective therapies against hard-to-eradicate pathogens.
Methods
Reagents used in this study
For flow cytometry of mouse samples, antibodies used included: anti-mouse CD45 AF700 (Biolegend, Cat 103128), anti-mouse CD11c Bv605 (Biolegend, Cat 117333), anti-mouse SiglecF (BD Bioscience, Cat 562680), anti-mouse CD11b AF594 (Biolegend, Cat 101254), anti-mouse MHC II APC/Cy7 (Biolegend, Cat 107628), anti-mouse Ly6C Bv421 (Biolegend, Cat 128032), anti-mouse Ly6G PerCP/Cy5.5 (Biolegend, Cat 127616), anti-mouse Epcam FITC (Biolegend, Cat 118208), and anti-mouse F4/80 (Biolegend, Cat 123114). Live/Dead DAPI dye used was acquired from Invitrogen (Cat L34962). For FACS assays, dilution for all antibodies was 1/200.
Mouse experiments
All animal assays were performed following institutional guidelines at Columbia University Irving Medical Center. Mice experiments were approved by protocol IACUC AABE8600. 8–10-week-old C57BL/6 J (000664) and C57BL/6NJ (005304) WT mice (20-25grs) were obtained from The Jackson Laboratories. Irg1−/− (Acod1−/−) (Jax 029340) and il1r−/− (Jax 003245) C57BLl/6 mice were also obtained from The Jackson Laboratories and bred in our facilities at Columbia University Irving Medical Center. WT and Irg1−/− mice are immunocompetent animals, and they received neither medical nor drug treatments prior to infection. Each in vivo experiment was performed using 50% female and 50% male animals, and results were not expected to be influenced by sex. Animals were randomly assigned to cages. Experiments were performed in our animal facilities under barrier isolation. Animals were maintained in standard rodent light-dark cycles, 18-23 degrees Celcius, ~30–50% humidity, as well as fed with regular irradiated chow diet (Purina Cat 5053, distributed by Fisher).
P. aeruginosa strains
WT PAO1, ΔalgD PAO1, mucA22 PAO1, mucA22ΔalgD PAO1, FRD1, and ΔalgD FRD1 were grown in LB overnight and sub-cultured until exponential phase for studies. P. aeruginosa strains from a tolerant subject (PA 605 and PA 686) were obtained from sputum of a 10-year chronically infected CF patient. These studies were performed under our approved IRB protocols AAAR1395 and AAAS6553. All these strains were plated in LB agar and their phenotypes were characterized regarding small colony variants or mucoid morphology. Infection experiments with either of these pathogens were performed from LB subcultures on exponential phase of growth, which derived from overnight grown stocks. Overnight cultures and subcultures were grown at 37 °C under shacking.
Infection of mice
Animals used were WT, Irg1−/−, and il1r−/− mice. Animals were exposed to either PBS, WT PAO1, ΔalgD PAO1, mucA22 PAO1, mucA22ΔalgD PAO1, the P. aeruginosa isolates PA 605 or PA 686, FRD1 or ΔalgD FRD1. When indicated, the bacterial dose varied accordingly with the experiment performed. We used different inoculum: ~105, or ~106 CFUs or PBS alone (non-infected). When indicated, 24 h prior and during the moment of the infection, animals were treated or not with 10 mg/Kg of BPTES. Survival of mice was monitored for 5 days. When indicated, animal temperature was tracked with an infrared thermometer that measured surface and internal temperature. To evaluate the host immune response to infection, mice were euthanized 24 h (Euthasol) after exposure to the pathogens, and BAL and lungs were collected. CFU amounts were measured through LB agar plating. CFUs below limit of detection (102 CFU per tissue) were represented in graphs as “non-detected” (ND). Immune cells (flow cytometry) and cytokines (ELIZA) were quantified in BAL and in lungs. Flow cytometry: alveolar macrophages were identified as DAPI−CD45+Epcam−CD11blow/−SiglecFhighCD11c+F4/80−Ly6G−Ly6C−, recruited neutrophils 1 as DAPI−CD45+Epcam−CD11bhighSiglecF−CD11c−MHCII- F4/80−Ly6G+Ly6C+, recruited monocytes 1 as DAPI−CD45+Epcam−CD11bhighSiglecF−CD11c−MHCII− F4/80−Ly6G−Ly6Clow, recruited monocytes 2 as DAPI−CD45+Epcam−CD11bhighSiglecF−CD11c−MHCII− F4/80−Ly6G−Ly6Chigh, resident neutrophils as DAPI−CD45+Epcam−CD11bhighSiglecF−CD11c−MHCII− F4/80−Ly6G+Ly6Clow, recruited monocytes 2 as DAPI−CD45+Epcam−CD11blowSiglecF−CD11c−MHCII− F4/80−Ly6G−Ly6Chigh, regulatory myeloid cells as DAPI−CD45+Epcam−CD11blowSiglecF−CD11c−MHCII− F4/80−Ly6G−Ly6C−, type 1 pneumocytes DAPI−CD45−EpcamlowCD11b−SiglecF−CD11c−MHCII−F4/80−Ly6G−Ly6C−, type 2 pneumocytes DAPI−CD45−EpcammiddleCD11b−SiglecF−CD11c−MHCII+F4/80−Ly6G−Ly6C−, and ciliated cells as DAPI−CD45−EpcamhighCD11b−SiglecF−CD11c−MHCII−F4/80−Ly6G−Ly6C−. Samples were analyzed with FlowJo, vX. Cell viability was determined by using live/dead DAPI staining (Thermo Fisher Scientific, L34962). When indicated, BAL were analyzed by Mass-Spec for metabolomics studies.
Airway metabolomics
BAL from either PBS-exposed or 24h-infected mice were collected with 3 ml of sterile PBS. Samples were immediately placed on ice. Then, samples were diluted with 100% methanol in a 1:1 proportion, mixed and stored at −80 °C for future metabolomics analysis. Just prior to mass spectrometry, samples were thawed and dried under a stream of N2 and were resuspended in HPLC-grade water at a 4:1 dilution (relative to the original BAL volume). High-resolution mass spectrometry data were acquired on a Thermo Fisher Exactive Mass spectrometer in negative mode using 25 min reverse phase gradients and ion-pairing chromatography. Metabolites were identified using the known chromatographic retention times of standards, and metabolite signals were quantified using MAVEN. Metabolite signal intensities were used to quantify difference between treatments in BAL and respect to PBS-treated animals.
Mouse lung processing for single-cell RNA-sequencing analysis
WT or Irg1−/− C57bl/6 mice (8-10-week-old) were treated with PBS or infected with WT PAO1 ( ~ 106 total CFU per mouse in 50uL). 24 h after infection, mice were euthanized, their lungs were harvested, and single cell suspensions of the lungs were prepared as described. Briefly, the lungs were placed in an Eppendorf tube containing an enzymatic digestion solution of collagenase I (2 mg/mL), dispase (20 mg/mL), elastase (1 mg/mL), and DNAse (1 uL/mL) in PBS. The lungs were minced within the tube and then incubated with shaking at 37 °C for 30 minutes. Subsequently, 4 volumes of PBS supplemented with 10% FBS were added to quench the digestion, and the digestion solution was strained over a 70-micron filter. The cell suspension was spun down at 4 °C and 1400 rpm for 7 min. Red blood cell lysis was performed using the Invitrogen RBC lysis buffer. The resulting cell pellet was resuspended in PBS supplemented with 0.04% BSA before being loaded onto the 10X Genomics Chromium Single Cell Controller (Accession numbers GEO: GSE203352). Cell viability analysis was performed before loading the samples, and was above 95% per sample. A total of ~8000 cells were analyzed per sample. FASTQ file generation, alignment, filtering, barcode counting, and UMI counting were done using the 10X Cell Ranger software. Scores for glutaminolysis in alveolar macrophages were calculated using the AddModuleScore function in Seurat. Briefly, glutaminolysis scores included the Slc38a1, Slc38a2, Slc1a5, Slc3a2, Gls, and Kdm6b.
Single cell RNA-sequencing analysis
Matrix data generated by scRNA-Seq were analyzed using the Seurat library (https://satijalab.org) in R Studio (https://www.rstudio.com). Data were cleaned for mitochondrial RNA (less than 5% per sample), and unbiased cell clustering was performed. The Seurat algorithm identified 31 cell clusters with unique transcriptomic signatures. To determine the biological nature of each one of these clusters, we did comparative analyzes of the transcriptomic profile of each cluster with available data bases using the SingleR algorithm (using ImmGen as the reference data set). In addition, this cluster characterization was manually cross-checked with cell markers from publicly available datasets using the top 10 markers for each cluster. To identify genes affected by Irg1, we contrasted the expression of each cluster in the same cell population from either WT or Irg1−/− mice. Genes significantly (P < 0.05) modified by itaconate were identified by MAST statistical test. We submitted all significantly changed genes for unbiased pathway enrichment analysis to Database for Annotation, Visualization and Integrated Discovery (DAVID; https://david.ncifcrf.gov).
DESI-MS of lung tissue
DESI-MS, an ambient ionization imaging technique, was performed to detect metabolites and their spatial distributions in situ. Prior to data acquisition, murine lungs from infected mice were embedded in 2% carboxymethylcellulose (CMC, w/v, Sigma, 419273) (Nguyen et al., Stoeckli et al.,), sectioned to 10 mm thickness using a Leica CM3050 S cryostat (Columbia University Molecular Pathology Shared Resource (MPSR) histology service) and stored at −80 C. The frozen 10 mm lung tissues were dried for 20 min at 300 torr using a vacuum desiccator (Bel-Art). Briefly, DESI-MS was performed in the negative ion mode (0.6 kV) from m/z 50–1000 using the Waters Synapt G2-Si QTOF coupled to a Waters DESI source with a motorized stage. The lung tissues were scanned under charged droplets generated from the electrospray nebulization of a histologically compatible solvent, methanol:acetonitrile 1:1 (v/v) containing 40 pg/ml of the internal standard leucine enkephalin (Leu-Enk) (Waters Corp.), that is used as a lock mass to correct any mass drifts during imaging runs (flow rate 1.5 ml/min). Raw MS files are processed using the HDI software (version 1.5, Waters Corp.) or exported to imzML (Schramm et al.,) to be used in SCiLS Lab MVS Pro (2020 version).
qRT-PCR for genes in P. aeruginosa
P. aeruginosa strains were grown in LB overnight and then subcultured until exponential phase in LB media supplemented with or without itaconate (30 mM each metabolite). Total RNA was extracted using either the E.Z.N.A. Total RNA Kit1 or RNeasy Mini Kit. The RNA was then processed with DNase. The RNA concentration was measured using NanoDrop One. cDNA was synthesized using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems), and qPCR was performed with a StepOnePlus Real-Time PCR System (Applied Biosystems) using POWER SYBR Green PCR Master Mix (Applied Biosystems). Relative gene expression was calculated by the 2−ΔΔCT method. The rpsL transcript was used as a reference housekeeping gene, and the WT PAO1 strain grown in glucose was used as a calibrator. All primers used in this work are provided in Supplementary Data 8.
Caloric expenditure of biofilm
WT PAO1, ΔalgD PAO1, mucA22 PAO1, and mucA22ΔalgD PAO1 mutants were grown in LB overnight, then inoculated (1/100) into 200 μl of LB with or without 30 mM of Itaconate (Sigma-Aldrich #I29204) in the 48-well cell culture inserts (calWellTM #1901004). Each insert was then transferred to a titanium cup (calVialTM) and locked airtight with a screwdriver. The titanium cups were anchored to the 48-well cup holder (calPlateTM) and inserted into the microcalorimetry instrument (calScreenerTM) in the front zone for 10 minutes, followed by the mid-zone for 20 minutes for calibration and temperature stabilization. Once the reading was fully calibrated and stabilized, the holder was then inserted into the inner zone. The reading was recorded using the microcalorimetry software calViewTM 2.0.
Biofilm quantification
Plates were prepared by inoculating (1/100) of the overnight bacterial culture of WT PAO1, ΔalgD PAO1, mucA22 PAO1, and mucA22ΔalgD PAO1 mutants into 200 μl of LB with or without 30 mM of itaconate in a 96-well clear, flat-bottom plate (Falcon #353072). The plate was then sealed off with a clear optical adhesive film (Applied BiosystemsTM #4311971) and placed inside the Varioskan Lux plate reader (Thermo Scientific #3020-82355) at 37 oC for 30 minutes before running the plate at static for 48 hr. Bacterial concentration was captured with optical density at 600 nm (OD600) using Skanlt Software 7.0 RE.
Bacterial growth in glutamine
WT PAO1 and ΔalgD PAO1 mutants were grown in LB overnight, then washed twice with filtered PBS before inoculating (1/100) into 200 μl of 1X of M9 minimal media (Gibco #A1374401) supplemented with 2 mM of MgSO4 and 0.1 mM of CaCl2, and 7.5 mM of Glutamine (Fisher #O2956-100).The plate was then sealed off with clear optical adhesive film and placed inside the Varioskan Lux at 37 oC for 30 minutes before shaking the plate at 240 rpm for 48 hr. Bacterial concentration was captured with optical density at 600 nm (OD600) using Skanlt Software 7.0 RE. OD400 was also tracked for pyoverdine production.
Bacterial respiration
WT PAO1, ΔalgD PAO1, mucA22 PAO1, and mucA22ΔalgD PAO1 mutants were grown in LB overnight, then washed twice with filtered PBS before inoculating (1 × 107) into 450 μl of 1X of M9 minimal media supplemented with 2 mM of MgSO4 and 0.1 mM of CaCl2 in Seahorse XFe24 cell culture plate (Agilent #102334-000B). 50 μl of 75 mM Succinate in M9 media was loaded onto the Seahorse XFe24 cartridge, which was previously hydrated in Seahorse calibrant (Agilent #100840-000) overnight at 37 oC. After 60 minutes of stabilization at the basal state, succinate was acutely injected into the plate to achieve the final concentration of 7.5 mM. Bacterial oxygen consumption rate was captured with Seahorse XFe24 Analyzer (Agilent #1002238-100) using Seahorse Wave Desktop v2.6.0.
Targeted polar metabolomics profiling
WT PAO1 was grown in LB overnight and inoculated (1/100) into LB with or without 30 mM itaconate, and grown at 37 oC until late exponential phase in triplicate. The cultures were standardized to OD600 of 7.5. For metabolite extraction, each culture was diluted in filtered PBS in 1:3 and centrifuged at 2000 × g for 10 minutes at 1oC. The supernatant was discarded, and the pellet was washed twice with PBS. The pellet (about 30 μl in volume) was then resuspended in 80% methanol-water extraction solution and lysed with 10 freeze-thaw cycles. This was done by alternating emersion of samples in liquid nitrogen and a dry-ice/100% ethanol bath. The debris was removed by centrifugation at high speed (14,000 × g) for 5 minutes at 1oC and the supernatant was then transferred to a 0.65 ml snap-cap tube and stored at −80 oC until analysis. Targeted LC/MS analysis was performed using a Q Exactive Orbitrap mass spectrometer coupled Vanquish UPLC system (Thermo Scientific). The Q Exactive was operated in polarity-switching mode, and a Sequant ZIC-HILIC column (2.1 mm i.d. × 150 mm, Merck) was used for separation of metabolites with flow rate set at 150 μL/min. Buffers consisted of 100% acetonitrile for mobile A, and 0.1% NH4OH and 20 mM CH3COONH4 in water for mobile B. Gradient ran from 85 to 30% mobile A for 20 minutes followed by a wash with 30% mobile A and re-equilibration at 85% mobile A. Metabolites were identified on the basis of exact mass within 5 ppm and standard retention times. All data analysis was done using MAVEN software 2011.6.17.
Stable isotope tracing with 13C-itaconate
WT PAO1 was grown in LB overnight and inoculated (1/100) into LB with or without 7.5 mM 13C5-itaconate (LGC #TRC-1931004) and grown at 37 oC until late exponential phase in triplicate. The cultures were then standard to OD600 of 2.4. Using the metabolite extraction method and target LC/MS analysis as described previously, relative quantitation was performed based on peak area for each isotopologue. All data analysis was done using MAVEN software 2011.6.17.
Carbon source utilization assay
For the Carbon Source Phenotype MicroarrayTM (Biolog), a stock solution of 2 × 107 bacteria/mL of overnight bacteria grown in LB was washed and prepared in 1X IF-Oa buffer (Biolog #72268) supplemented with 1X Redox Dye Mix A (Biolog #74221). Subsequently, 100 μL of this stock solution (delivering 2 × 106 bacteria) was added to each well of a PM1 MicroplateTM (Biolog #12111) and the plate was incubated at 37 °C for 24 hr. Absorbance was read at 590 nm on the Varioskan Lux plate reader.
Single-nucleotide polymorphism (SNP) calling
Comparison of SNPs between P. aeruginosa isolates was done by means of short-read alignment to the genome of PAO1 as reference (GenBank: AE004091), using the Bacterial and Viral Bioinformatics Resource Center (BV-BRC) tool (available at: https://www.bv-brc.org). SNP calls were made using the Variation Analysis Tool (available at: https://www.bv-brc.org/app/Variation).
Chemoproteomic profiling of S-itaconated cysteines in P. aeruginosa
We used the protocol described by Zhang et al, 34,41. For identification of S-itaconated cysteines by C3A, 800 μL of PAO1 suspension was incubated with 80 μL of either PBS or itaconate (pH adjusted) on the ThermoMixer (950 rpm, 30 min, 37 °C). Both groups were incubated with 8 μL of the C3A probe on the ThermoMixer (950 rpm, 37 °C, 1 h). Bacteria were then centrifuged, washed, resuspended in 1 mL of 0.1% PBST and lysed. Lysates were spun down and the supernatant was passed into a new 1.5 mL tube. Click reaction was carried out in the ThermoMixer (1200 rpm, 1 h, 29 °C) with 106 μL of Click reagent mix (60 μL of TBTA ligand, 20 μL of 50 mM CuSO4, 20 μL of freshly prepared 50 mM TCEP and 6 μL of 20 mM acid-cleavable azide-biotin tag (Confluore). Then, click-labeled lysates were precipitated with 5 mL of MeOH/chloroform (4:1) and 3 mL of ddH2O. Precipitated fractions were washed twice with pre-chilled MeOH, resuspended in 1 mL of 1.2% SDS in PBS, and sonicated. The solutions were then heated for 10 min at 90 °C and centrifuged (20,000 g, 10 min, RT) to remove excessive copper. Afterwards, the copper-free solutions were mixed with the washed streptavidin beads (Thermo Fisher Scientific) and the resulting solutions were incubated for 4 hours at 29 °C, followed by washing with PBS and ddH2O. The resulting beads were resuspended in 500 μL of 8 M urea (Sigma)/100 mM TEAB (Sigma). For reduction, 25 μL of 200 mM DTT (Shanghai Yuanye Bio-Technology Co., Ltd) was added, and tubes were incubated under agitation on the ThermoMixer (1200 rpm, 30 min, 37 °C). For alkylation, 25 μL of 400 mM 2-iodoacetamide (Sigma) was added, and the tubes were incubated on the ThermoMixer (1200 rpm, 30 min, 35 °C). The beads were then collected by centrifugation and resuspended in 200 μL of 2 M urea/100 mM TEAB containing 1 mM CaCl2 and 10 ng μL − 1 trypsin (Promega). After trypsin digestion was performed on the ThermoMixer (1350 rpm, 18 h, 37 °C), the beads were centrifuged (1400 g, 3 min, RT) and supernatants were reassigned into a new 1.5 mL Protein LoBind® Tubes (Eppendorf). Beads were washed and all the supernatants were combined into the former tubes. These samples were analyzed by mass spectrometry analysis.
Statistical analysis
We modeled the number of independent experiments required to reach significance between groups by using JMP, a computer program dedicated for statistical analysis. We based this simulation on preliminary data, experimental design, and past experience. These analyzes were done assuming a 20% standard deviation (SD), and equivalent variances within groups. Significance (P < 0.05) with power 0.8 was used. Experiments in this study were not performed in a blinded fashion. All analyzes and graphs were performed using the GraphPad Prism 9 software. Data in graphs are shown as average ± SEM and data were assumed to fit normal distribution. For comparison between average values for more than 2 groups, we performed One-Way ANOVA with a multiple posteriori comparison. When studying two or more group along time, data was analyzed using Two-Way ANOVA with a multiple posteriori comparison. Differences between two groups in samples’ average values were analyzed using analysis of variance (parametric) or Student’s t test for normally distributed data or Mann-Whitney or Kruskal-Wallis test otherwise. Differences were considered significant when P value (two-sides) was under 0.05 (P < 0.05). P values and amounts of independent experiments and replicates are indicated in each Figure Legend.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data are available in the manuscript or the supplementary materials. To ensure access to data and materials associated to this study, readers can contact Dr. Sebastián A. Riquelme (sr3302@cumc.columbia.edu). Access to reagents not found at commercial suppliers will be facilitated by the corresponding author. Transcriptomic data have been deposited in GEO under accession number GSE203352. Chemoproteomic profiling data has been deposited in iProx under accession number PXD050510. Source data are provided with this paper.
Code availability
This work does not include new code.
References
Auger, J. P. et al. Metabolic rewiring promotes anti-inflammatory effects of glucocorticoids. Nature 629, 184–192 (2024).
Mills, E. L. et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 167, 457–470.e413 (2016).
O’Neill, L. A. J. & Artyomov, M. N. Itaconate: the poster child of metabolic reprogramming in macrophage function. Nat. Rev. Immunol. 19, 273–281 (2019).
Kelly, B. & O’Neill, L. A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res 25, 771–784 (2015).
Ogger, P. P. & Byrne, A. J. Macrophage metabolic reprogramming during chronic lung disease. Mucosal Immunol. 14, 282–295 (2021).
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature 496, 238–242 (2013).
Mills, E. L., Kelly, B. & O’Neill, L. A. J. Mitochondria are the powerhouses of immunity. Nat. Immunol. 18, 488–498 (2017).
Schneider, D. S. & Ayres, J. S. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat. Rev. Immunol. 8, 889–895 (2008).
McCarville, J. L. & Ayres, J. S. Disease tolerance: concept and mechanisms. Curr. Opin. Immunol. 50, 88–93 (2018).
Soares, M. P., Teixeira, L. & Moita, L. F. Disease tolerance and immunity in host protection against infection. Nat. Rev. Immunol. 17, 83–96 (2017).
Tomlinson, K. L. et al. Ketogenesis promotes tolerance to Pseudomonas aeruginosa pulmonary infection. Cell Metab. 35, 1767–1781.e1766 (2023).
dos Santos, G., Kutuzov, M. A. & Ridge, K. M. The inflammasome in lung diseases. Am. J. Physiol. Lung Cell Mol. Physiol. 303, L627–L633 (2012).
Martins, R. et al. Disease tolerance as an inherent component of immunity. Annu Rev. Immunol. 37, 405–437 (2019).
Lampropoulou, V. et al. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab. 24, 158–166 (2016).
Hooftman, A. et al. The immunomodulatory metabolite itaconate modifies NLRP3 and inhibits inflammasome activation. Cell Metab. 32, 468–478.e467 (2020).
Billingham, L. K. et al. Mitochondrial electron transport chain is necessary for NLRP3 inflammasome activation. Nat. Immunol. 23, 692–704 (2022).
Mills, E. L. et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 556, 113–117 (2018).
Qin, W. et al. Chemoproteomic profiling of itaconation by bioorthogonal probes in inflammatory macrophages. J. Am. Chem. Soc. 142, 10894–10898 (2020).
Torres, A. et al. Pneumonia. Nat. Rev. Dis. Prim. 7, 25 (2021).
Cohen, T. S. & Prince, A. S. Activation of inflammasome signaling mediates pathology of acute P. aeruginosa pneumonia. J. Clin. Invest 123, 1630–1637 (2013).
Riquelme S. A., et al. CFTR-PTEN-dependent mitochondrial metabolic dysfunction promotes Pseudomonas aeruginosa airway infection. Sci Transl Med 11, eaav4634 (2019).
Comolli, J. C. et al. Pseudomonas aeruginosa gene products PilT and PilU are required for cytotoxicity in vitro and virulence in a mouse model of acute pneumonia. Infect. Immun. 67, 3625–3630 (1999).
Kang, D., Xu, Q. & Kirienko, N. V. In vitro lung epithelial cell model reveals novel roles for Pseudomonas aeruginosa siderophores. Microbiol Spectr. 12, e0369323 (2024).
Soong, G., Parker, D., Magargee, M. & Prince, A. S. The type III toxins of Pseudomonas aeruginosa disrupt epithelial barrier function. J. Bacteriol. 190, 2814–2821 (2008).
Shaver, C. M. & Hauser, A. R. Relative contributions of Pseudomonas aeruginosa ExoU, ExoS, and ExoT to virulence in the lung. Infect. Immun. 72, 6969–6977 (2004).
Arlehamn, C. S. & Evans, T. J. Pseudomonas aeruginosa pilin activates the inflammasome. Cell Microbiol 13, 388–401 (2011).
Sakaguchi, S., Yamaguchi, T., Nomura, T. & Ono, M. Regulatory T cells and immune tolerance. Cell 133, 775–787 (2008).
Hara, M. et al. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J. Immunol. 166, 3789–3796 (2001).
Lichtenberg, M. et al. Cyclic-di-GMP signaling controls metabolic activity in Pseudomonas aeruginosa. Cell Rep. 41, 111515 (2022).
Riquelme, S. A. et al. Pseudomonas aeruginosa utilizes host-derived itaconate to redirect its metabolism to promote biofilm formation. Cell Metab. 31, 1091–1106.e1096 (2020).
Gorke, B. & Stulke, J. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat. Rev. Microbiol 6, 613–624 (2008).
Rojo, F. Carbon catabolite repression in Pseudomonas: optimizing metabolic versatility and interactions with the environment. FEMS Microbiol Rev. 34, 658–684 (2010).
Valentini, M. & Lapouge, K. Catabolite repression in Pseudomonas aeruginosa PAO1 regulates the uptake of C4 -dicarboxylates depending on succinate concentration. Environ. Microbiol 15, 1707–1716 (2013).
Zhang, Y., Qin, W., Liu, D., Liu, Y. & Wang, C. Chemoproteomic profiling of itaconations in Salmonella. Chem. Sci. 12, 6059–6063 (2021).
Hidber, E., Brownie, E. R., Hayakawa, K. & Fraser, M. E. Participation of Cys123alpha of Escherichia coli succinyl-CoA synthetase in catalysis. Acta Crystallogr D. Biol. Crystallogr 63, 876–884 (2007).
Franklin, M. J., Nivens, D. E., Weadge, J. T. & Howell, P. L. Biosynthesis of the Pseudomonas aeruginosa extracellular polysaccharides, Alginate, Pel, and Psl. Front Microbiol 2, 167 (2011).
Banerjee, P. C., Vanags, R. I., Chakrabarty, A. M. & Maitra, P. K. Alginic acid synthesis in Pseudomonas aeruginosa mutants defective in carbohydrate metabolism. J. Bacteriol. 155, 238–245 (1983).
Ye, R. W., Zielinski, N. A. & Chakrabarty, A. M. Purification and characterization of phosphomannomutase/phosphoglucomutase from Pseudomonas aeruginosa involved in biosynthesis of both alginate and lipopolysaccharide. J. Bacteriol. 176, 4851–4857 (1994).
Al Ahmar R., Kirby B. D., Yu H. D. Pyrimidine biosynthesis regulates the small-colony variant and mucoidy in Pseudomonas aeruginosa through sigma factor competition. J. Bacteriol. 201, e00575 (2019).
Sasikaran, J., Ziemski, M., Zadora, P. K., Fleig, A. & Berg, I. A. Bacterial itaconate degradation promotes pathogenicity. Nat. Chem. Biol. 10, 371–377 (2014).
Liu, P. S. et al. alpha-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat. Immunol. 18, 985–994 (2017).
Cordes, T. et al. Itaconate modulates tricarboxylic acid and redox metabolism to mitigate reperfusion injury. Mol. Metab. 32, 122–135 (2020).
Yoo, H. C., Yu, Y. C., Sung, Y. & Han, J. M. Glutamine reliance in cell metabolism. Exp. Mol. Med 52, 1496–1516 (2020).
Guo, R., Zong, S., Wu, M., Gu, J. & Yang, M. Architecture of human mitochondrial respiratory megacomplex I(2)III(2)IV(2). Cell 170, 1247–1257.e1212 (2017).
Fernandez-Vizarra, E. et al. Two independent respiratory chains adapt OXPHOS performance to glycolytic switch. Cell Metab. 34, 1792–1808.e1796 (2022).
Winstanley, C., O’Brien, S. & Brockhurst, M. A. Pseudomonas aeruginosa evolutionary adaptation and diversification in cystic fibrosis chronic lung infections. Trends Microbiol 24, 327–337 (2016).
La Rosa R., Johansen H. K., Molin S. Convergent metabolic specialization through distinct evolutionary paths in Pseudomonas aeruginosa. mBio 9, e00269 (2018).
Schlictman, D., Kavanaugh-Black, A., Shankar, S. & Chakrabarty, A. M. Energy metabolism and alginate biosynthesis in Pseudomonas aeruginosa: role of the tricarboxylic acid cycle. J. Bacteriol. 176, 6023–6029 (1994).
Behrends, V., Ryall, B., Wang, X., Bundy, J. G. & Williams, H. D. Metabolic profiling of Pseudomonas aeruginosa demonstrates that the anti-sigma factor MucA modulates osmotic stress tolerance. Mol. Biosyst. 6, 562–569 (2010).
Jung, I. Y. et al. Risk factors for mortality in patients with Pseudomonas aeruginosa pneumonia: Clinical impact of mucA gene mutation. Respir. Med 140, 27–31 (2018).
Ciofu, O. et al. Investigation of the algT operon sequence in mucoid and non-mucoid Pseudomonas aeruginosa isolates from 115 Scandinavian patients with cystic fibrosis and in 88 in vitro non-mucoid revertants. Microbiol. (Read.) 154, 103–113 (2008).
Boucher, J. C., Yu, H., Mudd, M. H. & Deretic, V. Mucoid Pseudomonas aeruginosa in cystic fibrosis: characterization of muc mutations in clinical isolates and analysis of clearance in a mouse model of respiratory infection. Infect. Immun. 65, 3838–3846 (1997).
Bragonzi, A. et al. Sequence diversity of the mucABD locus in Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Microbiol. (Read.) 152, 3261–3269 (2006).
Yu, H., Hanes, M., Chrisp, C. E., Boucher, J. C. & Deretic, V. Microbial pathogenesis in cystic fibrosis: pulmonary clearance of mucoid Pseudomonas aeruginosa and inflammation in a mouse model of repeated respiratory challenge. Infect. Immun. 66, 280–288 (1998).
Malhotra, S., Hayes, D. Jr. & Wozniak, D. J. Mucoid Pseudomonas aeruginosa and regional inflammation in the cystic fibrosis lung. J. Cyst. Fibros. 18, 796–803 (2019).
Chroneos, Z. C., Wert, S. E., Livingston, J. L., Hassett, D. J. & Whitsett, J. A. Role of cystic fibrosis transmembrane conductance regulator in pulmonary clearance of Pseudomonas aeruginosa in vivo. J. Immunol. 165, 3941–3950 (2000).
Cohen, T. S. & Prince, A. Cystic fibrosis: a mucosal immunodeficiency syndrome. Nat. Med 18, 509–519 (2012).
Cross, A. R. et al. The histone-like protein AlgP regulon is distinct in mucoid and nonmucoid Pseudomonas aeruginosa and does not include alginate biosynthesis genes. Microbiol. (Read.) 166, 861–866 (2020).
Limoli D. H., et al. Pseudomonas aeruginosa Alginate Overproduction Promotes Coexistence with Staphylococcus aureus in a Model of Cystic Fibrosis Respiratory Infection. mBio 8, e00186 (2017).
Somerville, G., Mikoryak, C. A. & Reitzer, L. Physiological characterization of Pseudomonas aeruginosa during exotoxin A synthesis: glutamate, iron limitation, and aconitase activity. J. Bacteriol. 181, 1072–1078 (1999).
Toth, E. A. & Yeates, T. O. The structure of adenylosuccinate lyase, an enzyme with dual activity in the de novo purine biosynthetic pathway. Structure 8, 163–174 (2000).
Hassett, D. J. et al. An operon containing fumC and sodA encoding fumarase C and manganese superoxide dismutase is controlled by the ferric uptake regulator in Pseudomonas aeruginosa: fur mutants produce elevated alginate levels. J. Bacteriol. 179, 1452–1459 (1997).
Cutruzzola, F. et al. Pseudomonas aeruginosa cytochrome C(551): probing the role of the hydrophobic patch in electron transfer. J. Inorg. Biochem 88, 353–361 (2002).
Woodcock, S. D. et al. Trehalose and alpha-glucan mediate distinct abiotic stress responses in Pseudomonas aeruginosa. PLoS Genet 17, e1009524 (2021).
Chen, C. & Beattie, G. A. Characterization of the osmoprotectant transporter OpuC from Pseudomonas syringae and demonstration that cystathionine-beta-synthase domains are required for its osmoregulatory function. J. Bacteriol. 189, 6901–6912 (2007).
Collart, M. A. et al. Receptor-mediated phagocytosis by macrophages induces a calcium-dependent transient increase in c-fos transcription. Oncogene 4, 237–241 (1989).
Schlam, D. et al. Phosphoinositide 3-kinase enables phagocytosis of large particles by terminating actin assembly through Rac/Cdc42 GTPase-activating proteins. Nat. Commun. 6, 8623 (2015).
Hayashi, A. et al. Positive regulation of phagocytosis by SIRPbeta and its signaling mechanism in macrophages. J. Biol. Chem. 279, 29450–29460 (2004).
Panopoulos, A. D. & Watowich, S. S. Granulocyte colony-stimulating factor: molecular mechanisms of action during steady state and ‘emergency’ hematopoiesis. Cytokine 42, 277–288 (2008).
MacDonald, K. P. et al. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood 116, 3955–3963 (2010).
Jacob, C. O. et al. Lupus-associated causal mutation in neutrophil cytosolic factor 2 (NCF2) brings unique insights to the structure and function of NADPH oxidase. Proc. Natl Acad. Sci. USA 109, E59–E67 (2012).
Tlili, A. et al. ROCK2 interacts with p22phox to phosphorylate p47phox and to control NADPH oxidase activation in human monocytes. Proc. Natl Acad. Sci. USA 120, e2209184120 (2023).
Nordenfelt, P. & Tapper, H. Phagosome dynamics during phagocytosis by neutrophils. J. Leukoc. Biol. 90, 271–284 (2011).
Roche P. A., Cresswell P. Antigen processing and presentation mechanisms in myeloid cells. Microbiol. Spectr. https://doi.org/10.1128/microbiolspec.mchd-0008-2015 (2016).
Kasper M., Barth K. Potential contribution of alveolar epithelial type I cells to pulmonary fibrosis. Biosci. Rep. 37, BSR20171301 (2017).
Mukhopadhyay, S., Hoidal, J. R. & Mukherjee, T. K. Role of TNFalpha in pulmonary pathophysiology. Respir. Res 7, 125 (2006).
Mazzon, E. & Cuzzocrea, S. Role of TNF-alpha in lung tight junction alteration in mouse model of acute lung inflammation. Respir. Res 8, 75 (2007).
Barkauskas, C. E. et al. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Invest 123, 3025–3036 (2013).
Desai, T. J., Brownfield, D. G. & Krasnow, M. A. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 507, 190–194 (2014).
Zepp J. A., et al. Genomic, epigenomic, and biophysical cues controlling the emergence of the lung alveolus. Science 371, eabc3172 (2021).
Silo-Suh L. A., Suh S. J., Ohman D. E., Wozniak D. J., Pridgeon J. W. Complete genome sequence of pseudomonas aeruginosa mucoid strain FRD1, isolated from a cystic fibrosis patient. Genome Announc 3, e00153–15 (2015).
Ohman, D. E. & Chakrabarty, A. M. Genetic mapping of chromosomal determinants for the production of the exopolysaccharide alginate in a Pseudomonas aeruginosa cystic fibrosis isolate. Infect. Immun. 33, 142–148 (1981).
Pedersen, S. S., Hoiby, N., Espersen, F. & Koch, C. Role of alginate in infection with mucoid Pseudomonas aeruginosa in cystic fibrosis. Thorax 47, 6–13 (1992).
Henry, R. L., Mellis, C. M. & Petrovic, L. Mucoid Pseudomonas aeruginosa is a marker of poor survival in cystic fibrosis. Pediatr. Pulmonol. 12, 158–161 (1992).
May, T. B. et al. Alginate synthesis by Pseudomonas aeruginosa: a key pathogenic factor in chronic pulmonary infections of cystic fibrosis patients. Clin. Microbiol Rev. 4, 191–206 (1991).
Senturk Parreidt T., Muller K., Schmid M. Alginate-based edible films and coatings for food packaging applications. Foods 7, 170 (2018).
Gheorghita Puscaselu R., Lobiuc A., Dimian M., Covasa M. Alginate: from food industry to biomedical applications and management of metabolic disorders. Polymers (Basel) 12, 2417 (2020).
Cattelan, G. et al. Alginate formulations: current developments in the race for hydrogel-based cardiac regeneration. Front Bioeng. Biotechnol. 8, 414 (2020).
Tsujimoto, T. et al. An acellular bioresorbable ultra-purified alginate gel promotes intervertebral disc repair: a preclinical proof-of-concept study. EBioMedicine 37, 521–534 (2018).
Hernandez-Gonzalez, A. C., Tellez-Jurado, L. & Rodriguez-Lorenzo, L. M. Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: A review. Carbohydr. Polym. 229, 115514 (2020).
Pourali, P. & Yahyaei, B. Wound healing property of a gel prepared by the combination of Pseudomonas aeruginosa alginate and Alhagi maurorum aqueous extract in rats. Dermatol Ther. 32, e12779 (2019).
Froelich A., et al. Alginate-based materials loaded with nanoparticles in wound healing. Pharmaceutics 15, 1142 (2023).
Cui, X., Ng, K. R., Chai, K. F. & Chen, W. N. Clinically relevant materials & applications inspired by food technologies. EBioMedicine 75, 103792 (2022).
Sun, J. & Tan, H. Alginate-based biomaterials for regenerative medicine applications. Mater. (Basel) 6, 1285–1309 (2013).
Acknowledgements
This study was funded by grants R35GM146776 (NIH, SR), 1R21AI181780 (NIH, SR), RIQUEL21I0 (CFF), and GT007268 (Innovation Nucleation Fund, SR). We thank Dr. Joanna B. Goldberg for providing us with the WT PAO1, ∆algD PAO1, mucA22 PAO1 and mucA22∆algD PAO1 strains. We thank Dr. Peter Jorth, Dr. Catherine Armbruster, and Dr. Jennifer M. Bomberger for providing us the FRD1 and ∆algD FRD1 strains. We appreciate the help provided by Dr. Ian Lewis and Marija Drikic at University of Calgary, Alberta, Canada, on metabolomic studies. We highly appreciate the help of Dr. Alice Prince reading and criticizing the rigorous preparation of this work. We thank Dr. Guoan Zhang, Director of the Proteomics and Metabolomics Core Facility at Weill Cornell Medicine for this help with bacterial metabolomics assays.
Author information
Authors and Affiliations
Contributions
Conceptualization: Y.T.C., G.K.L., S.R. Methodology: Y.T.C., G.K.L., S.C., Z.L., T.W.F.L., C.W., S.R. Investigation: Y.T.C., G.K.L., S.C., Z.L., T.W.F.L., C.W., S.R. Visualization: Y.T.C., G.K.L., S.R. Funding Acquisition: S.R. Project Administration: C.W., S.R. Supervision: S.R. Writing—original draft: Y.T.C., G.K.L., S.R. Writing—review editing: T.W.F.L., C.W., S.R. Y.T.C. and G.K.L. authors contributed equally.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Luke O’Neill, Teresa Zelante and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, YT., Lohia, G.K., Chen, S. et al. A host-pathogen metabolic synchrony that facilitates disease tolerance. Nat Commun 16, 3729 (2025). https://doi.org/10.1038/s41467-025-59134-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-59134-1
This article is cited by
-
Development and validation of a sandwich ELISA for equine IL-1β
Animal Diseases (2025)
-
Fecal microbiota transplantation in pigs: current status and future perspective
Animal Microbiome (2025)