Abstract
All organisms face a certain risk of dying before reproducing, putting strong pressure on individuals to reproduce as early as possible. Despite this, some organisms delay maturity, defer reproduction, and age slowly. The evolution of such slow-paced life is classically attributed to allometric effects and reduced extrinsic mortality, but might also result from the invasion of challenging environments requiring adaptations that boost adult survival yet impose substantial energetic and developmental costs. Here, we reveal that the invasion of marine environments by endotherms may have triggered adaptive shifts towards slow life histories, particularly in pelagic lineages. Such life history convergences may have been facilitated by the slow-paced nature of their non-marine ancestors, and were associated with adaptations for enhanced energy acquisition and storage, enabling a long reproductive lifespan at the expense of extended development. Ancestral traits and lifestyle changes might thus have been important in shaping the evolution of slow life histories.
Similar content being viewed by others
Introduction
The life history of organisms —expressed through the differential allocation of time and resources to growth, survival, and reproduction— represents the resolution of conflicts among competing adaptations to maximise fitness1. As such, it is a fundamental way through which organisms adapt to their environment1,2,3. The adaptive relevance of life history is evident in environments where adults face significant extrinsic mortality. Under such conditions, natural selection favours adaptations for early reproduction and high fecundity to increase the likelihood of leaving descendants before death3,4,5. Along with extrinsic mortality, allometric constraints are also believed to play a crucial role in shaping life history evolution1. Body size limits the rate at which biomass can be produced, with smaller animals typically having higher metabolic rates than larger animals. While a higher metabolism allows animals to reproduce more quickly6, it can also lead to increased oxidative stress and accelerated cellular wear, ultimately reducing longevity7. Thus, smaller animals, like mice, tend to have much shorter lives than larger animals, like elephants—a pattern known as the ‘mouse-to-elephant curve'8,9.
While life history theory has achieved considerable success in explaining why some organisms live fast and die young10,11, the evolutionary reasons why others mature late, defer reproduction and age slowly remain less well-understood. Both the “mouse-to-elephant curve” and “extrinsic mortality” theories offer insights into the factors shaping such slow-paced life histories3,4,5,11, though neither fully accounts for their evolution8. When adult mortality risk decreases, for example, natural selection is expected to favour longer lifespans by removing deleterious genes and directing greater investment in building and maintaining a durable soma1,12. However, merely relaxing extrinsic mortality seems insufficient to explain the evolution of very slow life histories. For such strategies to evolve, the age-specific contribution to fitness should further shift from juveniles to adults13,14, which requires ‘buffer’ adaptations that ensure a high probability of adult survival regardless of extrinsic mortality risks. If developing these adaptations implies diverting important resources and time, increasing the fitness value of adults should lead to reduced reproductive effort and delayed maturity1. Such evolutionary reconfigurations are more likely when organisms invade ecological niches that not only present reduced threats to adults but also expose them to novel adaptive challenges.
Although it has long been appreciated that life history diversification has been shaped by major niche changes that altered the selective pressures on traits affecting age-specific fecundity and/or mortality15,16, the limited exploration of such scenarios has hindered a better understanding of the evolution of slow-lived strategies8. For terrestrial vertebrates, a particularly relevant scenario is the invasion of the marine environment. Some of the most fascinating and enigmatic examples of very long longevities are found in endotherms that have invaded marine environments17,18,19. Whales boast longer lifespans than most contemporary mammals, with the potential to reach up to 200 years. Among birds, albatrosses hold the record for maximum lifespan, with some living over 70 years. The remarkable longevity of these marine endotherms is associated with delayed reproduction, longer development periods and reduced fecundity, raising the intriguing hypothesis that the invasion of marine environments has favoured the evolution of a slow pace-of-life18,19,20,21.
The hypothesis that marine environments select for a slow pace of life is grounded in the unique challenges posed by marine environments. Although predation risk is relatively low for endotherms in the sea, they face the significant challenge of exploiting widely dispersed and often clumped prey, which frequently exhibit low spatial and temporal predictability17,18,19,20,21,22,23. This challenge is further accentuated by the necessity to develop foraging strategies drastically distinct from those used by their ancestors on land, and to adapt them to harsh conditions, as marine birds and mammals exhibit their greatest diversity in cold waters24. In environments where resources are scarce, unpredictable or challenging to obtain but threats for adults are not as severe, investing in adaptations to efficiently exploit resources can be advantageous —even if it results in delayed reproduction and reduced fecundity— provided that these adaptations reduce adult mortality and hence facilitate longer lifespans and iterated reproduction1,3.
The intriguing possibility that the invasion of marine environments has selected for slow life histories remains largely untested. Previous research has documented the exceptionally long lifespans of some marine species16,25,26, and explored trade-offs favouring slow-paced lives in particular marine clades19,27. Despite this progress, it remains unclear whether such a slow pace of life is a general feature of marine endotherms that reflects adaptive evolutionary convergences or rather is better explained by alternative factors, such as phylogenetic inertia or specific adaptations tied to particular marine lifestyles. Although the existence of repeated, independent transitions between land and sea provides promising opportunities for macroevolutionary analyses28, contrasting the above alternatives is challenging because life history traits do not leave traces in the fossil record. However, advances in phylogenetic-based comparative analyses29,30,31 now provide opportunities to infer past evolutionary changes based on information about extant species.
In this study, we build on these advances to reconstruct evolutionary transitions of endotherms to marine environments from non-marine ancestors, and to show that these transitions are associated with changes towards slower life histories that are consistent with models of adaptive evolution. Such life history adjustments were associated with adaptations that enhanced energy storage and acquisition, ultimately extending reproductive lifespan. However, this came at the cost of longer developmental periods and delayed sexual maturity. We also find that the ancestors of marine lineages were likely aquatic and tended to occupy the slow extreme of the fast-slow continuum, which likely facilitated their successful colonisation of marine environments while reducing constraints on the evolution of even slower life histories.
Results and discussion
Recolonisation of the sea
Despite oceans covering 70% of the planet’s surface, only a few terrestrial animal clades have successfully colonised the sea32 (Fig. 1a). Using species-level information from 9991 birds and 4408 mammals (Supplementary Data 1), we reconstructed transitions from terrestrial, aquatic freshwater and marine environments in calibrated phylogenies33,34 by means of stochastic character mappings (Supplementary Figs. 1, 2)—a probabilistic approach to mapping characters onto phylogenies that overcomes the limitations of parsimony35. In birds, the average number of independent transitions to marine lifestyles was estimated to be 10.7 (CI95 = 7-15), all within two major radiations (Aequorlithornithes and Anseriformes). In mammals, the number of transitions was 8.6 (CI95 = 5.0-15.5) in three major radiations (Cetartiodactyla, Carnivora and Sirenia). Interestingly, our phylogenetic reconstructions suggest that transitions to marine life generally occurred from aquatic ancestors, but rarely directly from terrestrial ancestors (Supplementary Fig. 3). Likewise, transitions from marine to terrestrial environments are very rare. While these figures are probably underestimations, as suggested by analyses of the fossil record32, both the scarcity of independent invasions from non-marine ancestors and the gradual nature of the transitions highlight the challenges that marine environments pose for colonisation.
a Taxonomic Order containing marine species (blue lines). b–i Density plots describing variation in the fast-slow continuum among marine, aquatic and terrestrial species, described as the first axis of a PCA. Smaller values along the fast-slow axis indicate faster life histories, while higher values correspond to slower life histories. The red line represents the estimated value of the ancestor of the marine species, estimated for 100 phylogenies with the function fastAnc from Phytools. The position of marine species in the fast-slow continuum is shown for all birds (b) and mammals (c), and within major marine radiations: Aequorlithornites, split in non-Charadriformes (d) and Charadriiformes (e), Anseriformes (f), Cetartiodactyla (g), Carnivora (h) and Sirenia (i)). The fast-slow continuum values are not directly comparable between birds and mammals, as they are derived from separate PCAs conducted for each taxon. Sample sizes (species number, birds/mammals) Marine = 339/125, Aquatic = 785/133, Terrestrial = 8865/4150. Abbreviations: ANS Anseriformes, CETAR Cetartiodactyla, CARN Carnivora, Tr Triassic, J Jurassic, K Cretaceous, Pg Paleogene, and Ng Neogene. Animal illustrations created by Daniel Sol.
Marine endotherms in the fast-slow continuum
To investigate whether the invasion of marine environments has led to changes in life history, we described the fast-slow continuum based on seven life history traits: maximum longevity, age at first breeding, gestation/incubation time, weaning/fledging time, litters/broods per year, litter/clutch size and fecundity (Supplementary Data 2). These life history traits were used in a Principal Component Analysis (PCA) to assign the position of each species along the fast-slow continuum. The first axis of the PCA explained 54% and 75% of variation in life history in birds and mammals, respectively, and effectively captured this continuum, with positive loadings for longevity and developmental traits, and negative loadings for fecundity traits (Supplementary Data 3). The axis also captured the fecundity-survival trade-off underpinning the fast-slow continuum1,14, as indicated by its correlation with life expectancy, generation time, and elasticity in fecundity derived from demographic analyses (Supplementary Fig. 4). Along the fast-slow continuum, marine species tend to consistently occupy the slower extreme (Fig. 1b–i), with the only exception of marine Anseriformes (Fig. 1f).
Phylogenetic multivariate analyses on traits describing longevity, fecundity and development time confirmed statistically the above patterns (Supplementary Fig. 5). Marine species show lower fecundity yet longer lifespans and development periods compared with species that are primarily aquatic or terrestrial (Supplementary Fig. 6), clear signatures of a slow pace-of-life. Although life history might vary with trophic niche, migratory tendency and climatic region26, the above pattern held when these alternative explanations were considered in the analyses (Supplementary Fig. 7). Thus, our results confirm and generalise that marine birds and mammals are at the slow extreme of the fast-slow continuum.
Life history characterisation of ancestors
Phylogenetic reconstructions suggest that the ancestors of marine lineages generally had a slow pace-of-life (Fig. 1 and Supplementary Figs. 8, 9). This is true for all clades except Anseriformes. While phylogenetic reconstructions are prone to uncertainties, ancestor estimations were highly consistent across 100 trees from the posterior distributions of phylogenies (Supplementary Figs. 8, 9). Having a slow ancestor is relevant because whether a lineage evolves toward a fast or slow life history can be constrained by the initial position of the ancestor in the fast-slow continuum36. It also suggests that the successful colonisation of marine environments likely demanded a life history co-opted to thrive in these environments.
The adaptive nature of the slow pace-of-life
To investigate the adaptive significance of life history divergences between marine and non-marine lineages, we modelled the fast-slow continuum using evolutionary models based on an Ornstein-Uhlenbeck (OU) process29,30,31. Unlike the commonly used Brownian motion process, which assumes that a trait evolves over time through stochastic changes, the OU process also considers the possibility that the trait changes around one or several adaptive optima dictated by different selective regimes. To test whether transitions from aquatic to marine environments have selected for slower life histories, we fitted multiple optima OU models for life history evolution in a sample of 100 stochastic character mapping reconstructions. These models were then compared with models featuring a single optimum (OU1) or Brownian motion models (BM), which do not assume adaptive processes. Our analyses revealed that in all clades with marine radiations except Anseriformes, an OU model with multiple optima provided a superior fit to the data relative to the other models (Table 1). The estimated optima (parameter θ) of the fast-slow continuum for marine, aquatic and terrestrial selective regimes, derived from the OUMV models, showed certain discrepancies with respect to the actual values of the analysed species (Fig. 2). These discrepancies may reflect incomplete adaptation, the influence of historical and stochastic factors, or potential estimation errors37,38. Nevertheless, the superior fit of the OUMV models remains highly consistent across phylogenies and stochastic character mappings (Table 1). Furthermore, these models consistently identify a slower life history optimum for marine regimes compared to terrestrial and freshwater regimes in analyses within the same phylogeny and stochastic character mapping (Supplementary Figs. 10–13), suggesting a convergent evolutionary trend towards slower life histories in marine environments.
The outer plots are phenograms to visually represent how life history changes across evolutionary time and across taxa for Aequorlithornites (a), Anseriformes (b), Cetartiodactyla (c) and Carnivora (d). The fast-slow continuum corresponds to the first principal component (PC1) derived from a PCA conducted separately for birds and mammals. The inner plots show the kernel probability density of the fast-slow optima (θ) estimated from OUMV models based on a sample of 100 stochastic character mappings (see Supplementary Figs. S11–S15 for estimations of θ for particular phylogenies and stochastic character mappings). Colour codes like in Fig. 1. Abbreviations: TERR Terrestrial, AQ Aquatic and MAR Marine.
Life history divergence in pelagic and coastal environments
The marine environment exhibits significant heterogeneity39. Coastal areas, in particular, boast a higher abundance and diversity of resources in comparison to the vast open ocean, largely due to coastal upwelling40. If the challenging and unpredictable nature of the marine environment is the driving force behind selection for slower life histories, we might expect distinct optima for coastal and oceanic species. Indeed, among marine species, Aequorlithornithes tend to exhibit slower strategies than Anseriformes, while Cetaceans exhibit slower strategies than carnivores, in line with their predominantly pelagic habits. Within Aequorlithornithes, the only clade with a good representation of both pelagic and coastal species (Supplementary Data 1), we also found that an OUMV model with distinct optima for pelagic and coastal selective regimes outperformed a model where both environments are pooled together (average AICc = 897.2 vs 973.1). Although such life history differences may in part reflect selection for higher fecundity driven by the overabundance and diversity of food in coastal upwellings, as predicted by r-selection models of life history evolution3, the striking and consistently much higher optima estimated for pelagic regimes compared to coastal and terrestrial regimes (Supplementary Fig. 14) strongly suggest that much of the life history slow-down occurred in pelagic environments. If this is the case, the relatively limited life history divergence observed in sea ducks is not entirely unexpected; it likely reflects their predominantly coastal habits.
Evolution of the life history components of the fast-slow continuum
The fast-slow continuum reflects trade-offs (positive and negative) between lifespan, fecundity and development. We therefore assessed how exposure to marine environments has selected for each of these particular life history traits. To this end, we applied multivariate multi-regime OU models to jointly analyse longevity, fecundity, and development time. We found a consistent trend towards longer lifespans, lower fecundity, and extended development time in lineages exposed to marine selective pressures (Supplementary Fig. 15). These results further reinforce the hypothesis that the colonisation of marine environments are associated with an adaptive slowing of the pace-of-life in endotherms, especially in lineages that have adopted more pelagic lifestyles.
Body size may partially explain slow-lived strategies
Several adaptive theories might explain how birds and mammals living in the sea have evolved slower life histories, but one that is expected to be particularly relevant is selection for larger body size41,42,43. Although body size is often viewed as a constraint of life history evolution6, a large body may provide adaptive advantages when foraging requires the exploration of large areas, as it allows for reduced metabolic rates and the storage of significant energy reserves, thereby reducing the need for frequent foraging to meet energetic needs44. In addition, a large body offers insulation against cold water temperatures, reduces heat loss, and provides access to prey that smaller animals cannot handle, such as deep-sea organisms that require long dives or small prey that must be amassed in large amounts with a single foraging bout45. Thus, even though a large body demands extended development and a greater food supply for maintenance, these costs may be counterbalanced by a substantial improvement in survival prospects during sea foraging, ultimately enabling longer reproductive spans46.
In a multivariate OU framework, the co-evolution of life history and body size can be analysed by estimating the covariance in the strength of selection (parameter α), which describes the extent to which the evolution of life history is influenced by body size or vice-versa47. Jointly modelling body size and the fast-slow continuum by means of a bi-variate mvOUM, we found that models where α is allowed to co-vary were generally favoured over other models (Supplementary Data 4). The best mvOUM also allowed covariance in the stochastic element sigma (σ). This might reflect a situation where life history and body size respond to similar factors (like secondary adaptive optima or developmental/functional constraints), in addition to being constrained by each other.
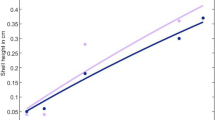
A phylomorphospace representing the fast-slow continuum as a function of body size showed that the slower strategy in marine Anseriformes and Carnivores aligns well with the allometric expectations, suggesting that it is mainly explained by allometry. Instead, in Aequorlithornites and Cetartiodactyla, there is a notable grade shift in the allometric curve, with marine clades showing a more relaxed association between life history and body size (Fig. 3). Marine Aequorlithornites and cetaceans stand out as the clades containing the species with more pelagic habits and the slowest life histories among all endotherms (Supplementary Figs. 16, 17). Consequently, these two clades present valuable opportunities for delving into adaptations other than body size that may explain their slow life histories.
a–d Phylomorphospaces for Aequorlithornites (a), Anseriformes (b), Cetartiodactyla (c) and Carnivora (d). In the plots, the phylogenetic tree is superimposed onto the morphospace by connecting species based on their evolutionary relationships. To more clearly represent the phylomorphospace, the body mass of adults has been log-transformed, and the axis scales have been adjusted across taxa. Colour codes like in Fig. 1.
Further insights into the adaptive basis of slow-lived strategies
The challenges of capitalising on the spatial and temporal aggregation of marine food resources are contingent on the nature of the prey consumed and the adaptations for efficient resource acquisition17,27. In cetaceans, for example, species that mainly feed on plankton (Mysticeti) face abundant but sporadic food availability. This not only requires large bodies to amass prey in large amounts and convert them into fat reserves, but also demands high mobility to efficiently track such temporarily and spatially variable food resources48. Indeed, large whales can undertake long seasonal migrations between the tropics and polar waters, which can involve several months without feeding. Odontocetes, instead, are smaller and primarily prey on fish and squids, resources that are more predictable but more challenging to capture. Exploiting these prey may require accumulating knowledge and socially learning a variety of complex foraging skills, including cooperative hunting and tool use, which may have been facilitated by an encephalized brain and enhanced body manoeuvrability49,50. While high mobility and an encephalized brain may provide significant advantages, they also incur costs, including extended development times, and may also be linked to body size. To explore the intricate ways in which the ecological adaptations of cetaceans interact with life history traits, we employed a phylogenetic path analysis51 (Fig. 4a).
a Adaptations examined in this study. The diagram illustrates variation in the slow pace-of-life as arising from: (1) direct causal relationships between the adaptations and longevity (e.g., an encephalized brain contributing to enhanced longevity), (2) shared causal factors (e.g., increased longevity associated with extended development time, creating spurious associations with adaptations requiring prolonged development), and (3) indirect pathways (e.g., body size influencing longevity indirectly through its impact on locomotory morphology). Since encephalization represents brain size relative to body size (i.e., accounting for allometric effects), no direct arrow connects it to body size. b–e Best causal models based on phylogenetic path analyses for cetaceans (b), cetaceans excluding Mysticeti (c), marine Aequorlithornites (d), and marine Aequorlithornites excluding penguins (e). Purple arrows denote positive effects, while brown arrows represent negative effects. Values represent the standardised path coefficients and associated standard error, with arrow width representing their effect size. Although fecundity is a major component of the fast-slow continuum, it is not included here because most marine species exhibit similarly low fecundities. Abbreviations: LO Maximum longevity; BO Body size; RB Relative brain size; ST Streamlined body type; GE Generation time; IN Incubation time; WI Hand-wing index.
Our phylogenetic path analysis51 supports the importance of encephalization and high mobility in cetaceans’ life history (Fig. 4b). The best supported models highlight the central importance of body size, but also reveals that slow-lived species are characterised by highly encephalized brains and streamlined body types that offer less resistance to move throughout water (Fig. 4b and Supplementary Figs. 18, 19). These correlates may signify the advantages of such adaptations in providing “buffer adaptations” against environmental unpredictability, enhancing survival and enabling longer lives. At the same time, the positive associations of body size and encephalization with development time suggest secondary consequences for life history in terms of extended growth and maturation52. Restricting the analysis to odontocetes generally aligns with these discoveries, but, in line with their dependence on single-prey and their tendency for shorter-distance movements, the path analysis suggests a diminished significance of body size and shape (Fig. 4c and Supplementary Figs. 19–22). Instead, encephalization gains greater importance, especially in social cetaceans.
In marine Aequorlithornites, flight limits body size and the capacity to accumulate reserves. However, being large enough to accumulate fat can still be important in some species, particularly those that have either lost the ability to fly or utilise dynamic soaring to reduce travel costs. In addition, it may be essential for nestlings to endure extended periods without food22. Another factor potentially influencing their life history is the need to travel long distances to meet daily energy requirements and feed the offspring. Albatrosses, shearwaters and petrels can travel vast distances to forage, sometimes covering up to 1000 km in a single day19. This remarkable ability, which is facilitated by their skill in extracting energy from wind currents to soar, not only enables them to efficiently track changes in food abundance but also provides more freedom to choose remote, secure breeding locations that may be far from optimal foraging areas. Besides body size and mobility, cognition can also be relevant for marine birds that rely on resources that vary in time and space, notably to create visual maps of suitable foraging areas in the vast areas of the sea or to develop complex foraging techniques to capture prey in the water. Similar to cetaceans, a phylogenetic path analysis supports the significance of body size, flying efficiency and encephalization for the life history of seabirds. Their remarkable slow life histories are associated with larger bodies, but also with high levels of encephalization and wing morphologies better suited for long-distance movements (Fig. 4d and Supplementary Fig. 23). As in cetaceans, the correlation between these adaptations and life history is likely indicative of both benefits and developmental costs (see also refs. 19,53). The way life history co-varies with body size, encephalization and mobility also changes depending on the foraging strategy, being more pronounced in pelagic species that use surface seizing to access vastly distributed resources that vary in time and space (Fig. 4e and Supplementary Figs. 24, 25).
Overall, our results support the notion that the slow life history of marine endotherms is not merely a consequence of their large body, but also reflects other adaptations to efficiently exploit marine resources. While our analyses focus on general adaptations to understand macroevolutionary convergences across birds and mammals, this does not preclude the relevance of more specific adaptations for taxa exploiting different environments19,22,26,54. Of particular relevance are adaptive divergences that may arise between species that reproduce on the shore vs those that reproduce in the sea, as well as between species with precocial versus altricial offspring, due to their potential to shape how energy and nutrients are allocated to offspring27.
Longevity as a central component of a slow-paced life
Our analyses suggest that the evolution towards very slow life histories partly reflects selection for adaptations that enable the efficient exploitation of challenging food resources. Although these adaptations often result in longer developmental periods, they may offset this cost by enhancing adult survival, thereby supporting an extended reproductive lifespan. The central role of longevity for fitness is supported by growing evidence from long-term population studies55 (Supplementary Fig. 26). In the Kittiwake (Rissa tridactyla), which can live up to 28 years, between 80–83% of variation in lifetime reproductive success can be attributed to longevity56. As the contribution of older age classes to reproductive success increases, longevity can be extended because selection becomes more efficient against the accumulation of deleterious mutations or antagonistic pleiotropic, slowing the aging process and decreasing intrinsic mortality. In bowhead whales, recent analyses of their genome and transcriptome have revealed selection for genes related to cell cycle, DNA repair, cancer, and aging57, substantiating such a possibility.
When fitness largely depends on a long reproductive life, a reduction in fecundity is expected to mitigate the costs of reproduction for adults1,58,59. This cost has been well-documented in marine endotherms, and is exemplified by individuals skipping reproduction when conditions are unfavourable60. Such a strategy is possible because spreading the reproductive effort in many events represents a form of bet-hedging, minimising the fitness impact of any single reproductive attempt1. The severe energy limitations imposed by pelagic ecosystems might also directly select for reduced fecundity by limiting the number of offspring that parents can successfully raise2,19. In seabirds that need to travel long distances to find food, the number of offspring may be constrained by the amount of food that parents can bring in each trip. Similarly, in migratory whales where breeding females spend several weeks without feeding while nursing their calves, commitment to lactation may restrict their capacity to care for a larger number of offspring. When energy limitations are high and development takes a long time, a more successful strategy than producing many offspring is to invest in a few larger offspring that are better prepared to withstand adverse weather conditions and extended periods without food16,21. Such a strategy ensures that at least some offspring will survive to pass the parents’ genes to the next generation, particularly significant considering that a low fecundity implies producing fewer offspring during their lifetime.
Compared to fecundity, the reasons why marine endotherms delay the onset of first reproduction appear less obvious because postponing reproduction should reduce the duration of the reproductive life. However, selection may favour postponing reproduction if breeding earlier imposes mortality costs and is unlikely to produce viable offspring61,62,63. In elephant seals (Mirounga angustirostris), females that bred early have decreased lifespan, low weaning success, and lower lifetime reproductive success than females that postpone first breeding64. Postponing reproduction is often interpreted as reflecting insufficient maturation. Bowhead whales only reach their final body size when 40 or 50 years old, implying that foraging proficiency can be impaired for long periods65. Another factor that may explain why marine endotherms delay the onset of reproduction is the learning time required to acquire sufficient foraging proficiency26, although this explanation is less well supported by evidence66.
Towards a lifestyle perspective of life history evolution
Our study highlights that ancestral legacies and adaptations to exploit scarce, variable or challenging food resources may have favoured convergent evolution towards slow life histories in endotherms that invaded the marine environment. The exact evolutionary pathways remain idiosyncratic, due to differences in ancestors and the new niches occupied27. However, our analyses suggest that evolutionary convergences partially arise because the adaptations necessary to thrive in marine environments are costly to produce but, once developed, offer protection against extrinsic mortality. As a result, the age-specific contribution to fitness has further shifted from juveniles to adults relative to their non-marine ancestors. Thus, our findings support previous claims to broaden life history theory8, highlighting the need to move beyond classic “mouse-to-elephant curve” and “relaxed extrinsic mortality” paradigms to consider the central role of the ecological lifestyle of organisms in favouring slow-lived strategies. Given that the physiology of some long-lived marine ectotherms shares similarities with that of marine endotherms67, verifying whether our findings extend to ectotherms could offer additional insights into the factors shaping the evolution of slow pace-of-life strategies.
Considering the significance of a slow life history strategy and aquatic pre-adaptations to thrive in the sea, and the low prevalence of these features among endotherms, it is unsurprising that only a few lineages have successfully transitioned from terrestrial to marine habitats. Nevertheless, their adaptive radiations in the sea have been truly extraordinary17,22. As apex predators, marine endotherms currently play critical roles in the structure and function of most ocean ecosystems68. Despite their ecological and societal value, many populations of marine endotherms are threatened by human exploitation, habitat loss, pollution, and ocean warming69. This may seem puzzling, as we might expect animals with life histories evolved to thrive in the harsh and dynamic conditions of the sea to also be able to cope successfully with the variety of new challenges posed by human activities. However, the slow-paced life of marine endotherms makes their populations highly vulnerable to threats that impact adult survival—such as ship strikes, acoustic pollution, fishing net entanglements, oil spills, and commercial hunting—threats to which they have had little opportunity to adapt70. In addition, their long generation times limit their potential for population recovery and evolutionary rescue71. Paradoxically, the very slow life history strategy that likely enabled birds and mammals to conquer and diversify in harsh marine environments could leave them poorly prepared to face the rapid and unprecedented threats generated by human activities.
Methods
Habitat assignation
We considered a species marine when a large proportion of the total population foraged in the marine environment for at least part of the year72,73. The rest of the species were classified as either aquatic freshwater or terrestrial. Habitat assignation was based on published information for mammals74 and birds72,73, updated with more recent information from IUCN75. In birds, the above criteria yielded to classify 339 avian species as marine, 785 as aquatic and 8865 as terrestrial. In mammals, we classified 125 species as marine, 133 as aquatic and 4150 as terrestrial—excluding Chiropters (which lack marine representatives and have a life history largely shaped by flight) to speed up analysis. For some analyses, we further subdivided marine species into primarily ‘pelagic’ or ‘coastal’. ‘Pelagic’ species were those that primarily use marine pelagic deep water and/or marine neritic pelagic continental shelf water. ‘Coastal’ species were those that primarily use coastal inshore water (sea along coasts, typically 8 km from the shoreline) throughout the year, excluding species that may occasionally use this habitat, but do not do so typically.
Life history characterisation
We characterised the life history of mammals and birds based on 7 life history traits: maximum longevity, age at first breeding, gestation/incubation time, weaning/fledging time, litters/broods per year, litter/clutch size and fecundity (i.e., the product of the last two traits)(Supplementary Data 1). Data were extracted from76, updated with information from74 for mammals, and from77,78,79,80 and HBW Alive (https://birdsoftheworld.org/bow/home) for birds. For birds, information on maximum longevity was complemented with data from long-term capture-recapture schemes (e.g., USGS, EURING, ABBBS or SAFRING). As the use of maximum longevity data has been criticised because of its dependence on research effort81, we also used this information to test the accuracy of the estimations (see Supplementary Methods and Supplementary Data 6). To explore the co-variation between life history traits, we described the main axes of variation using a principal component analysis, as implemented in the function ‘prcomp’ of the stats package in R82. Following Pigot et al.83, we centred and rescaled each life history trait to unit variance before performing the PCAs. All life history traits were log10-transformed, except broods/litters per year. As evolutionary analyses require highly sampled clades, we also estimated the PCA including species with imputed life history traits. Data imputation was restricted to species with three or more life history traits, with this information integrated with phylogenetic data using the Rphylopars package84. Despite of the potential for imputed data and PCA to introduce undesirable statistical artefacts85, analyses with both raw and imputed data were highly consistent. In the main text, we present the results of the PCA that includes imputed data, along with analyses of individual life history traits based solely on available data.
Confounding variables
Comparative analyses seeking to identify the mechanisms behind the adaptive significance of a phenotypic trait need to consider the possible effects of confounding variables. For example, if species at higher trophic levels have slower life histories and are overrepresented among marine endotherms, this can create a spurious relationship between slow life histories and marine environments. A spurious relationship can also arise in traits that are overrepresented in non-marine species. For example, migratory species are typically characterised by fast-lived life histories. If migration is more common in non-marine species, this may lead to find differences in life history between marine and non-marine species. Finally, trade-offs in life history can be masked by trophic level due to the fact that different species may have different amounts of resources to allocate between survival and reproduction. To address these issues, we gathered published information on trophic level (carnivore, omnivore, herbivore and scavenger), migratory strategy (migratory or resident) and whether the species was tropical, temperate, polar or widespread from the PanTHERIA86 and AVONET87. We classified species as tropical, temperate, polar, or widespread based on their breeding latitude, with the tropics defined as − 23.4° to 23.4° and polar regions as below − 60° or above 60°.
Phylogenetic hypotheses
We used the most comprehensive, updated phylogenies currently available, Lum et al.34 for birds and Upham et al.33 for mammals. Since our evolutionary models require highly sampled clades, we included DNA-missing species randomly assigned to topological positions within taxonomic constraints (genus or family) across the credible set of trees. To deal with this and other sources of phylogenetic uncertainty, we repeated the analyses across a sample of trees (see details below).
Testing for life history differences between marine and non-marine species
We used the function ‘phylolm’ in the R-package phylolm88 to test for differences in the fast-slow continuum among marine and non-marine species, and the function ‘mvgls’ in mvMORPH47 to test for multivariate differences in the underlying life history traits (gestation/incubation, fecundity and maximum longevity). The error term was defined as Brownian motion. To deal with measurement error, we assumed that the variance of measurement errors was the same for all species and estimated it from the data.
Evolutionary transitions between environments
We used the phylogenies to reconstruct evolutionary transitions between marine, aquatic and terrestrial environments. We used a stochastic character mapping approach that applies a Monte Carlo algorithm to sample the posterior probability distribution of ancestral states and timings of transitions on phylogenetic branches under a Markov process of evolution35,89. To accomplish this, we used the ‘make.simmap’ function in the R package phytools90 to generate stochastic character-mapped reconstructions with the ‘ARD’ model, allowing for asymmetrical transitions. In our reconstructions, we considered phylogenetic uncertainty by integrating results from the 100 randomly sampled trees of the posterior distribution of phylogenies, running 5 reconstructions for each phylogenetic tree. Thus, we obtained 500 trees with stochastic character mappings.
Reconstruction of ancestral characters
We used the function ‘fastAnc’ from the phytools package90 to estimate the ancestral values of the fast-slow for each node of the avian and mammalian phylogenies. We then extracted the values for the ancestors of the marine clades. To deal with phylogenetic uncertainty in ancestral estimations, we estimated the values for 100 phylogenies. The function ‘contMap()’ from phytools90 was used to plot changes in the fast-slow continuum onto the phylogeny.
Testing the adaptive significance of the fast-slow: univariate approach
To assess whether life history changed adaptively after the invasion of marine environments, we used the R package OUwie91 to fit several univariate models of phenotypic evolution: (1) single-rate Brownian motion (BM1) model, indicating shared evolutionary history and random change as the best explanation for species similarity; (2) multiple-rate Brownian motion (BMS) model, indicating shared evolutionary history as the best explanation for species similarity, but allowing evolutionary rate to vary across habitats; (3) single-optimum Ornstein-Uhlenbeck (OU1) process, suggesting adaptation to a single selective regime characterised by a specific optimum trait for the entire clade; (4) multiple-optima OU (OUM), with different selective regimes and optima for marine, aquatic and terrestrial species; and 5) a multi-rate multi-optima OU (OUMV), which also allows to the evolutionary rate to also vary across habitats. More complex OU models (e.g., OUMA, OUMVA) led to frequent convergence issues and hence they are not included. All models were run on a random sample of 100 phylogenies, with those used in BMS, OUM and OUMV models sampled from stochastic character mapping trees. Model comparison was based on the second-order Akaike information criterion (AICc). We excluded Sirenia from the analysis due to the insufficient number of species.
Testing the adaptive significance of the fast-slow: a multivariate approach
Because we find support for the hypothesis that marine environments have selected for a slower pace-of-life, we further validated these results by jointly analysing longevity, gestation/incubation, and fecundity using multivariate, multi-regime OU models (mvOU). In addition, we also used a variety of mvOU to explore whether the fast-slow continuum and body size evolved 1) independently (setting the co-variation between alpha and sigma to zero), 2) in a correlated fashion as a response to similar selective pressures (setting the co-variation between alpha to zero), 3) in a correlated fashion because there is a statistically significant interaction between traits toward the optimum (setting the co-variation between sigma to zero; by constraining the alpha matrix but not the sigma matrix this tests for a significant interaction in the “selection” strength); and 4) a correlated fashion because with both sigma and alpha allowed to co-variate. We fitted the mvOU models using the R-package mvMORPH47.
Path analyses for adaptive responses
We used phylogenetic path analyses92,93 to investigate the links between life history and buffer adaptations to thrive in marine environments. We focused on three general buffer adaptations: a large body size41, an encephalized brain94 and a morphology for efficient locomotion46,80. Body size was extracted from PanTHERIA86 and AVONET87. Encephalization, which reflects a higher accumulation of pallial neurons and is correlated with enhanced cognition53, was estimated as the residuals of a log-log phylogenetic generalised least squares model of brain mass against body mass95,96, with brain data extracted from ref. 96 for birds and ref. 97 for Cetaceans. To describe morphology, we used published data on the hand-wing index for birds and streamlining for cetaceans46. The hand-wing index is a morphological metric linked to wing aspect ratio, and associated with avian flight efficiency and dispersal ability80. The streamlining index describes whether a whale species is more or less streamlined based on a log-linear regression of body mass versus body length, with positive residuals indicating ‘less-streamlined’ and negative residuals ‘more-streamlined’46. We estimated the residuals based on a phylogenetic generalised least squares model, with body mass and body length data from ref. 46. We decided to exclude ‘Balaena mysticetus’ from our analysis due to its outlier status and the inability to verify its data, as it originated from a single individual. In mammals, lifespan has also been linked to social organisation98, yet current information on social cohesion is insufficient to test the relevance of this factor. To further delve into foraging links, we used data on the main foraging strategy from ref. 99 for marine Cetartiodactyla (capturing single prey, either primarily squids or other vertebrates and filtering zooplankton) and from the HBW Alive (https://birdsoftheworld.org/bow/home) for marine Aequorlithornites (dipping, generalist, lunge diving, pursuit diving and surface seizing). For Cetartiodactyla, we also used data on fasting strategy (fasting vs non-fasting) and social complexity (mostly social, mostly solitary, and both social and solitary) from ref. 99 (see main text for justification).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All the relevant data are available in Figshare (https://doi.org/10.6084/m9.figshare.28202642). The data include life history traits, ecological confounds and phylogenies for both birds and mammals, as well as character stochastic mappings for 500 phylogenies. All figures in the main and supplementary text may be generated with code and data available in Figshare.
Code availability
The R codes used in the analysis are available in Figshare (https://doi.org/10.6084/m9.figshare.28202642). The code uses R version 4.4.1 (2024-06-14) and allows to prepare the data and phylogenies, generate the fast-slow continuum, estimate ancestral states, and run character stochastic mappings, MANOVAs and univariate and bi-variate evolutionary models. All figures in the main and supplementary text may also be generated with the code.
References
Stearns, S. C. The Evolution of Life Histories (Oxford University Press, 1992).
Lack, D. Ecological Adaptations for Breeding in Birds (Methuen and Co., London, 1968).
Reznick, D., Bryant, M. J. & Bashey, F. r- and K- selection revisited: The role of population regulation in life-history evolution. Ecology 83, 1509–1520 (2002).
Shattuck, M. R. & Williams, S. A. Arboreality has allowed for the evolution of increased longevity in mammals. Proc. Natl. Acad. Sci. USA 107, 4635–4639 (2010).
Healy, K. et al. Ecology and mode-of-life explain lifespan variation in birds and mammals. Proc. R. Soc. B Biol. Sci. 281, 20140298 (2014).
Sibly, R. M. M. & Brown, J. H. H. Effects of body size and lifestyle on evolution of mammal life histories. Proc. Natl. Acad. Sci. USA 104, 17707–17712 (2007).
Brown, J. H., Burger, J. R., Hou, C. & Hall, C. A. S. The pace of life: Metabolic energy, biological time, and life history. Integr. Comp. Biol. 62, 1479–1491 (2022).
Dobson, F. S. A lifestyle view of life-history evolution. Proc. Natl. Acad. Sci. USA 104, 17565–17566 (2007).
Jeschke, J. M. & Kokko, H. The roles of body size and phylogeny in fast and slow life histories. Evol. Ecol. 23, 867–878 (2009).
Reznick, D. N. & Travis, J. Experimental studies of evolution and eco-evo dynamics in guppies (Poecilia reticulata). Annu. Rev. Ecol. Evol. Syst. 50, 335–354 (2019).
Martin, T. E. Age-related mortality explains life history strategies of tropical and temperate songbirds. Science 349, 966–970 (2015).
Kirkwood, T. B. L. & Austad, S. N. Why do we age? Nature 408, 233–238 (2000).
Stearns, S. C. Life history evolution: Successes, limitations, and prospects. Naturwissenschaften 87, 476–486 (2000).
Healy, K., Ezard, T. H. G., Jones, O. R., Salguero-Gómez, R. & Buckley, Y. M. Animal life history is shaped by the pace of life and the distribution of age-specific mortality and reproduction. Nat. Ecol. Evol. 3, 1217–1224 (2019).
Partridge, L. & Harvey, P. H. The ecological context of life history evolution. Science 241, 1449–1455 (1988).
Sibly, R. M. et al. Energetics, lifestyle, and reproduction in birds. Proc. Natl. Acad. Sci. USA 109, 10937–10941 (2012).
Costa, D. P. & Shaffer, S. A. in Metabolic Ecology: A Scaling Approach. (John Wiley and Sons, 2012).
Ricklefs, R. E. Seabird life histories and the marine environment: Some speculations. Colonia. Waterbirds 13, 1–6 (1990).
Dobson, F. S. & Jouventin, P. How slow breeding can be selected in seabirds: Testing Lack’s hypothesis. Proc. R. Soc. B Biol. Sci. 274, 275–279 (2007).
Hamer, K. C., Schreiber, E. A. & Burger, J. in Biology of Marine Birds. (CRC Press, London, 2001).
Ricklefs, R. E. Some considerations on the reproductive energetics of pelagic seabirds. Stud. Avian Biol. 8, 84–94 (1983).
Schreiber, E. A. & Burger, J. Biology of Marine Birds. (CRC Press, Boca Raton, Fla, 2001).
Ashmole, N. P. in Avian Biology (Academic Press, New York, 1971).
Grady, J. M. et al. Biodiversity patterns: Metabolic asymmetry and the global diversity of marine predators. Science 363, eaat4220 (2019).
Okie, J. G. et al. Effects of allometry, productivity and lifestyle on rates and limits of body size evolution. Proc. R. Soc. B Biol. Sci. 280, 20131891 (2013).
Weimerskirch, H. in Biology of Marine Birds. (CRC Press, London, 2001).
Costa, D. P. Reproductive and foraging energetics of high latitude penguins, albatrosses and pinnipeds: Implications for life history patterns. Am. Zool. 31, 111–130 (1991).
Kelley, N. P. & Pyenson, N. D. Evolutionary innovation and ecology in marine tetrapods from the Triassic to the Anthropocene. Science 348, aaa3716 (2015).
Hansen, T. F. in Modern Phylogenetic Comparative Methods and Their Application in Evolutionary Biology. (Springer Berlin Heidelberg, 2014).
Bartoszek, K., Pienaar, J., Mostad, P., Andersson, S. & Hansen, T. F. A phylogenetic comparative method for studying multivariate adaptation. J. Theor. Biol. 314, 204–215 (2012).
Cressler, C. E., Butler, M. A., King, A. A. & Harmon, L. Detecting adaptive evolution in phylogenetic comparative analysis using the Ornstein-Uhlenbeck model. Syst. Biol. 64, 953–968 (2015).
Vermeij, G. J. & Motani, R. Land to sea transitions in vertebrates: The dynamics of colonization. Paleobiology 44, 237–250 (2018).
Upham, N. S., Esselstyn, J. A. & Jetz, W. Inferring the mammal tree: Species-level sets of phylogenies for questions in ecology, evolution, and conservation. PLoS Biol. 17, e3000494 (2019).
Lum, D., Rheindt, F. E. & Chisholm, R. A. Tracking scientific discovery of avian phylogenetic diversity over 250 years. Proc. R. Soc. B 289, 20220088 (2022).
Bollback, J. P. SIMMAP: Stochastic character mapping of discrete traits on phylogenies. BMC Bioinform. 7, 88 (2006).
Schaffer, W. M. Selection for optimal life histories: The effects of age structure. Ecology 55, 291–303 (1974).
Beaulieu, J. M., Jhwueng, D. C., Boettiger, C. & O’Meara, B. C. Modeling stabilizing selection: Expanding the Ornstein-Uhlenbeck model of adaptive evolution. Evolution 66, 2369–2383 (2012).
Cooper, N., Thomas, G. H., Venditti, C., Meade, A. & Freckleton, R. P. A cautionary note on the use of Ornstein-Uhlenbeck models in macroevolutionary studies. Biol. J. Linn. Soc. 118, 64–77 (2016).
Weimerskirch, H. Are seabirds foraging for unpredictable resources? Deep Sea Res. II Top. Stud. Oceanogr. 54, 211–223 (2007).
Sequeira, A. M. M. et al. Convergence of marine megafauna movement patterns in coastal and open oceans. Proc. Natl. Acad. Sci. USA 115, 3072–3077 (2018).
Buddhachat, K. et al. Life expectancy in marine mammals is unrelated to telomere length but is associated with body size. Front. Genet. 12, 675 (2021).
Gearty, W., McClain, C. R. & Payne, J. L. Energetic tradeoffs control the size distribution of aquatic mammals. Proc. Natl. Acad. Sci. USA 115, 4194–4199 (2018).
Koztowski, J. & Weiner, J. Interspecific allometries are by-products of body size optimization. Am. Nat. 149, 352–380 (1997).
Costa, D. in Marine Mammals: Advances in Behavioural and Population Biology. (Academic Press, 1993).
Goldbogen, J. A. et al. Why whales are big but not bigger: Physiological drivers and ecological limits in the age of ocean giants. Science 366, 1367–1372 (2019).
Ferguson, S. H., Higdon, J. W., Schmidt, C., Pomerleau, C. & Matthews, C. J. D. Investigating the relationship between body shape and life history traits in toothed whales: Can body shape predict fast-slow life histories? Evol. Biol. 50, 300–317 (2023).
Clavel, J., Escarguel, G. & Merceron, G. mvMORPH: An R package for fitting multivariate evolutionary models to morphometric data. Methods Ecol. Evol. 6, 1311–1319 (2015).
Costa, D. P., Breed, G. A. & Robinson, P. W. New insights into pelagic migrations: Implications for ecology and conservation. Annu. Rev. Ecol. Evol. Syst. 43, 73–96 (2012).
Brent, L. J. N. et al. Ecological knowledge, leadership, and the evolution of menopause in killer whales. Curr. Biol. 25, 746–750 (2015).
Montgomery, S. H. et al. The evolutionary history of cetacean brain and body size. Evolution 67, 3339–3353 (2013).
Jiménez‐Ortega, D., Kolm, N., Immler, S., Maklakov, A. A. & Gonzalez‐Voyer, A. Long life evolves in large‐brained bird lineages. Evolution 74, 2617–2628 (2020).
Barton, R. A. & Capellini, I. Maternal investment, life histories, and the costs of brain growth in mammals. Proc. Natl. Acad. Sci. USA 108, 6169–6174 (2011).
Sol, D. et al. Neuron numbers link innovativeness with both absolute and relative brain size in birds. Nat. Ecol. Evol. 6, 1381–1389 (2022).
Costa, D. P. & Favilla, A. B. Field physiology in the aquatic realm: ecological energetics and diving behavior provide context for elucidating patterns and deviations. J. Exp. Biol. 226, jeb245832 (2023).
Moreno, J. Lifetime reproductive success in seabirds: interindividual differences and implications for conservation. Sci. Mar. 67, 7–12 (2003).
Coulson, J. C. in Reproductive Success. (University of Chicago Press, 1988).
Keane, M. et al. Insights into the evolution of longevity from the bowhead whale genome. Cell Rep. 10, 112–122 (2015).
Dobson, F. S. & Jouventin, P. The trade-off of reproduction and survival in slow-breeding seabirds. Can. J. Zool. 88, 889–899 (2010).
Williams, G. C. Adaptation and Natural Selection (Princeton University Press, 1966).
Griffen, B. D. Reproductive skipping as an optimal life history strategy in the southern elephant seal, Mirounga leonina. Ecol. Evol. 8, 9158–9170 (2018).
Mourocq, E. et al. Life span and reproductive cost explain interspecific variation in the optimal onset of reproduction. Evolution 70, 296–313 (2016).
Pyie, P., Nur, N., Sydeman, W. J., Emshe, S. D. & Emslie, S. D. Cost of reproduction and the evolution of deferred breeding in the western gull. Behav. Ecol. 8, 140–147 (2011).
Sanz-Aguilar, A., Tavecchia, G., Pradel, R., Mínguez, E. & Oro, D. The cost of reproduction and experience-dependent vital rates in a small petrel. Ecology 89, 3195–3203 (2008).
Boeuf, B. L., Condit, R. & Reiter, J. Lifetime reproductive success of northern elephant seals (Mirounga angustirostris). Can. J. Zool. 97, 1203–1217 (2019).
George, J. C. et al. Severe bone loss as part of the life history strategy of bowhead whales. PLoS ONE 11, e0156753 (2016).
Patterson, E. M., Krzyszczyk, E. & Mann, J. Age-specific foraging performance and reproduction in tool-using wild bottlenose dolphins. Behav. Ecol. 27, 401–410 (2016).
Paladino, F. V., O’Connor, M. P. & Spotila, J. R. Metabolism of leatherback turtles, gigantothermy, and thermoregulation of dinosaurs. Nature 344, 858–860 (1990).
Estes, J. A., Heithaus, M., McCauley, D. J., Rasher, D. B. & Worm, B. Megafaunal impacts on structure and function of ocean ecosystems. Annu. Rev. Environ. Resour. 41, 83–116 (2016).
Pimiento, C. et al. Functional diversity of marine megafauna in the Anthropocene. Sci. Adv. 6, eaay7650 (2020).
Hilde, C. H. et al. The demographic buffering hypothesis: Evidence and challenges. Trends Ecol. Evol. 35, 523–538 (2020).
Bell, G. Evolutionary rescue. Annu. Rev. Ecol. Evol. Syst. 48, 605–627 (2017).
Croxall, J. P. et al. Seabird conservation status, threats and priority actions: A global assessment. Bird. Conserv. Int. 22, 1–34 (2012).
Oppel, S. et al. Spatial scales of marine conservation management for breeding seabirds. Mar. Policy 98, 37–46 (2018).
Soria, C. D., Pacifici, M., Marco, M. D., Stephen, S. M. & Rondinini, C. COMBINE: A coalesced mammal database of intrinsic and extrinsic traits. Ecology 102, e03088 (2021).
IUCN. The IUCN Red List of Threatened Species. Version 2023-1. https://www.iucnredlist.org (2023).
Myhrvold, N. P. et al. An amniote life‐history database to perform comparative analyses with birds, mammals, and reptiles: Ecological Archives E096‐269. Ecology 96, 3109–3109 (2015).
Sol, D., Sayol, F., Ducatez, S. & Lefebvre, L. The life-history basis of behavioural innovations. Philos. Trans. R. Soc. B 371, 20150187 (2016).
Gonzalez‐Voyer, A. et al. Sex roles in birds: Phylogenetic analyses of the influence of climate, life histories and social environment. Ecol. Lett. 25, 647–660 (2022).
Bird, J. P. et al. Generation lengths of the world’s birds and their implications for extinction risk. Conserv. Biol. 34, 1252–1261 (2020).
Sheard, C. et al. Ecological drivers of global gradients in avian dispersal inferred from wing morphology. Nat. Commun. 11, 2463 (2020).
Reinke, B. A. et al. Diverse aging rates in ectothermic tetrapods provide insights for the evolution of aging and longevity. Science 376, 1459–1466 (2022).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ (2024).
Pigot, A. L. et al. Macroevolutionary convergence connects morphological form to ecological function in birds. Nat. Ecol. Evol. 4, 230–239 (2020).
Goolsby, E. W., Bruggeman, J. & Ané, C. Rphylopars: fast multivariate phylogenetic comparative methods for missing data and within-species variation. Methods Ecol. Evol. 8, 22–27 (2017).
Davis, A. M. & Betancur-R, R. Widespread ecomorphological convergence in multiple fish families spanning the marine–freshwater interface. Proc. R. Soc. B 284, 20170565 (2017).
Jones, K. E. et al. PanTHERIA: A species‐level database of life history, ecology, and geography of extant and recently extinct mammals: Ecological Archives E090‐184. Ecology 90, 2648–2648 (2009).
Tobias, J. A. AVONET: Morphological, ecological, and geographical data for all birds. Ecol. Lett. 25, 581–597 (2021).
Ho, L. S. T. & Ane, C. phylolm: Phylogenetic Linear Regression. R package version 2.6.2. https://CRAN.R-project.org/package=phylolm (2023).
Revell, L. J. A comment on the use of stochastic character maps to estimate evolutionary rate variation in a continuously valued trait. Syst. Biol. 62, 339–345 (2013).
Revell, L. J. (2024) phytools 2.0: an updated R ecosystem for phylogenetic comparative methods (and other things). PeerJ 12, e16505 (2012).
Beaulieu J. M. & O’Meara B. OUwie: Analysis of evolutionary rates in an OU framework. R package version 2.10, https://CRAN.R-project.org/package=OUwie (2022).
Hardenberg, Avon & Gonzalez-Voyer, A. Disentangling evolutionary cause-effect relationships with phylogenetic confirmatory path analysis. Evolution 67, 378–387 (2013).
van der Bijl, W. phylopath: Easy phylogenetic path analysis in R. PeerJ 6, e4718 (2018).
Sol, D. Revisiting the cognitive buffer hypothesis for the evolution of large brains. Biol. Lett. 5, 130–133 (2009).
Orme, D. The caper package: Comparative analysis of phylogenetics and evolution in R. R package version 0.5. 2, 1–36 (2013).
Sayol, F., Downing, P. A., Iwaniuk, A. N., Maspons, J. & Sol, D. Predictable evolution towards larger brains in birds colonizing oceanic islands. Nat. Commun. 9, 2820 (2018).
Ridgway, S. H., Carlin, K. P. & Alstyne, K. R. V. Delphinid brain development from neonate to adulthood with comparisons to other cetaceans and artiodactyls. Mar. Mammal. Sci. 34, 420–439 (2018).
Zhu, P. et al. Correlated evolution of social organization and lifespan in mammals. Nat. Commun. 14, 372 (2023).
Albouy, C. et al. Global vulnerability of marine mammals to global warming. Sci. Rep. 10, 548 (2020).
Acknowledgements
We thank Joan Maspons, Kordiyeh Hamidi and Jose Luís Copete for helping us in gathering data, and Jeremy Bellieur and Julien Clavel for advice in the use of their respective R-packages OUwie and mvMorph. This research was part of the project PID2023-152973NB-I00 funded by MICIU AEI /10.13039/501100011033. MG was supported by MINECO PID2021-124731NB-I00. Animal illustrations created with the free and open-source Krita 5.2.3. This work is dedicated to Marc Estiarte.
Author information
Authors and Affiliations
Contributions
Original idea: D.S. and A.H.M. Conceptualisation: D.S. Conceptualisation – refinements: M.G., D.O. and A.H.M. Methodology: D.S. Data collection: A.P., L.O., D.S. Analysis: D.S. Funding acquisition: D.S. Writing – original draft: D.S. Writing – review & editing: A.P., L.O., M.G., D.O. and A.H.M.
Corresponding author
Ethics declarations
Competing interests
Authors declare that they have no competing interests.
Peer review
Peer review information
Nature Communications thanks Cheng-Hsiu Tsai, Daniel Costa, Anna Csergő, who co-reviewed with Nour Elhouda, and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sol, D., Prego, A., Olivé, L. et al. Adaptations to marine environments and the evolution of slow-paced life histories in endotherms. Nat Commun 16, 4265 (2025). https://doi.org/10.1038/s41467-025-59273-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-59273-5
This article is cited by
-
Endangered bowhead whales might buffer climate change with individual variability in movement patterns
Scientific Reports (2026)
-
Aging dynamics in captive sea turtles reflect conserved life-history patterns across the testudine phylogeny
Communications Biology (2025)
-
Evolution under domestication triggers widespread deviation to the coat versus kernel mass allometry in seeds
Vegetation History and Archaeobotany (2025)