Abstract
Non-Mendelian transmission of mitochondria has been well established across most eukaryotes, however the genetic mechanism that governs this uniparental inheritance remains unclear. Plants in the genus Cucumis, specifically melon and cucumber, exhibit paternal transmission of the mitochondrial (mt) DNA, making them excellent models for exploring the molecular mechanisms underlying mitochondrial transmission. Here, we develop a toolkit to screen for mutants in mitochondrial inheritance (mti), and use fine mapping to successfully identify a mitochondrially targeted endonuclease gene (MTI1) controlling mitochondrial transmission. Knockout of MTI1 results in a shift from paternal to bi-parental inheritance of the mtDNA, confirming the crucial role of MTI1 in uniparental inheritance of mitochondria. Moreover, we demonstrate that MTI1 exhibits robust endonuclease activity both in vitro and in vivo, specifically expresses in mitochondria of the fertilized ovule within 24 h of pollination. Collectively, this study reveals that a nuclear-encoded but mitochondria-targeted gene plays a causative role in governing the non-Mendelian mitochondrial inheritance, revolutionizing our knowledge about mitochondrial DNA transmission.
Similar content being viewed by others
Introduction
Mitochondrion is the energy-generating powerhouse that plays critical roles in many cellular processes such as respiration, apoptosis, and metabolism1. It is a semi-autonomous organelle possessing its own genome (mtDNA), which governs numerous important cytoplasm-related traits or diseases, including cytoplasmic male sterility in plants and Parkinson’s disease in humans2,3. Unlike nuclear genes that obey Mendel’s laws, non-Mendelian inheritance is regarded as the central dogma for the transmission of mitochondria4. In almost all eukaryotes, mtDNA is maternally inherited. Although sperm-derived paternal mitochondria have been shown to enter the oocyte’s cytoplasm after fertilization, paternal mtDNA is never transmitted to the offsprings5,6. However, how the DNA-containing mitochondria are often transmitted uniparentally, or conversely, is accidentally biparentally transmitted, remains a long-standing and unresolved question.
Recently, the copy number of mtDNA in male gametic cells has been shown to be highly regulated and associated with mitochondrial inheritance in plants7,8. Nuclease activity has been implicated in controlling the quantity of mtDNA, and emerging research has identified nucleases that reduce the copy number of mtDNA during male gametogenesis8,9. Defective in Pollen Organelle DNA Degradation 1 (DPD1) is one such exonuclease, which was found to be directly responsible for the reduction of the copy number of chloroplast DNA (cpDNA) and mtDNA in pollen8. As an exonuclease, DPD1 requires the participation of an endonuclease when degrading circular mtDNA. M20 is this type of endonuclease, involved in mtDNA degradation in pollen by acting with DPD19. Although the proteins encoded by these cloned genes have been proven to have nuclease activity involved in the degradation of mtDNA, none have been shown to regulate the mode of mitochondrial inheritance. That is why when either gene was knocked out, leakage of paternal mtDNA was only found in a few progenies10, suggesting that there must be other mechanisms affecting transmission of mtDNA. Unfortunately, due to the lack of natural mutants and efficient tools to study mitochondrial inheritance with forward genetics, hidden genes that regulate the mode of mitochondrial inheritance remain elusive and unresolved.
Maternal inheritance of mitochondria is considered a general rule, but it is not universal in plants. For instance, paternal inheritance of mtDNA has been observed in the Cucumis species cucumber (Cucumis sativus L.) and melon (C. melo L.)11,12, which are relatively rare exceptions among Angiosperms. Cucumis is the only genus in Cucurbitaceae that exhibits paternal inheritance of mitochondria4, which could serve as a genetic model to explore the important biological question regarding how paternal mitochondria and their mtDNA are selectively transmitted to their zygotes after fertilization. To address this question, we developed a toolkit to screen for key genes regulating mitochondrial inheritance by crossing with the mitochondrial mutant MSC16, which exhibits paternally transmitted mosaic (MSC) phenotypes. Fortunately, we identified an accession whose progeny displayed biparental inheritance of mitochondria after pollination by MSC16, along with a restoration of the wild-type (WT) phenotype. This unique germplasm allowed us to employ forward genetics to clone key genes involved in regulating mitochondrial inheritance. Finally, we identified and validated the causal gene responsible for paternal mitochondrial transmission in cucumber. Our study provides important genetic evidence behind the non-Mendelian inheritance of mitochondria and opens new avenues for further understanding the evolutionary implications of mitochondrial genetics.
Results
Development and validation of a unique mitochondria-specific marker
Paternal transmission of the mtDNA has been well documented in cucumber and melon11. We previously used a highly inbred line (MSC16) of cucumber that possesses a paternally transmitted MSC phenotype on cotyledons and true leaves for genetic analyses of mtDNA transmission13. The MSC phenotype is associated with under-representation of specific regions of the mtDNA, resulting in both reduced transcript numbers and protein abundance of partial mitochondrial genes, including the ribosomal protein S7 (rps7)14. As a result, these gene regions are appropriate for developing molecular markers that minimize competition between paternal and maternal mtDNA during PCR detection and efficiently detect maternal mtDNA leakage in the hybrid progenies15.
When MSC16 is crossed as the paternal parent to WT maternal plants, ~96% of progenies show the MSC phenotype and 4% are WT15. This proportion of WT progenies agrees closely with the frequency of maternal leakage of mtDNA in melon (Supplementary Table 1), when we evaluated the homogeneity of melon seeds with mitochondrial markers. Therefore, we inferred that leakage of maternal mtDNA may occasionally occur in the female gametes of Cucumis plants. Due to the presence of mtDNA sequences in the nucleus (NUMTs), genetic analyses of biparental mtDNA inheritance have been challenging and remain controversial16. To validate our hypothesis of leakage of maternal mtDNA in cucumber, we developed a degenerated cleaved amplified polymorphic sequence (dCAPS) marker, rps7SNP(GA/TC), to determine the origin of mitochondrial polymorphisms in hybrid progenies (Fig. 1a, b). The marker-assisted results from the polymerase chain reaction (PCR) showed paternal transmission of the dCAPS marker in F1 hybrids from reciprocal crosses between Calypso and MSC16 (n = 10) (Fig. 1c), demonstrating the specificity of this marker for differentiating mtDNA from NUMTs. Maternal mtDNA was only detected in WT F1 hybrids (n = 10) from the cross of the WT cucumber cultivar ‘Calypso’ as the maternal parent with MSC16 as the paternal parent. We also used a segregating population for marker and phenotype verification, and the results showed that our newly developed dCAPS marker co-segregated with the MSC phenotype (n = 84, Supplementary Data 1). Therefore, we confirmed that leakage of maternal mtDNA leads to the occurrence of relatively rare WT progenies in the cross with MSC16 as the male parent (Fig. 1d, e).
a The visual mosaic (MSC) phenotype is associated with under-representation in the regions of the mtDNA, and sequence polymorphism is detected in this region near rps7. b The schematic illustration for rps7SNP(GA/TC) dCAPS design for Calypso and MSC16 lines. c Examples of dCAPS analysis detecting the sequence polymorphism. In the WT hybrids from MSC16 × Calypso and the MSC hybrids from Calypso × MSC16, only the band corresponding to the paternal parent was detected. The band corresponding to the maternal parent was detected in all WT hybrids from Calypso × MSC16. The experiment was repeated three times with similar results. d Hybrids from the crossing of MSC16 as the female parent with Calypso as the male produced only WT hybrids, because of paternal inheritance of mtDNA. e Low frequencies of WT hybrids were observed from crosses with MSC16 as the male. For hybrids from individual fruits, frequencies of WT plants varied from 0% to a high level of 4.4%.
However, this observation is not in agreement with previous research proposing that the appearance of rare WT progenies in crosses with MSC16 as the paternal parent was due to the sorting of the WT sublimon that exists at low levels in MSC1617,18. Thus, we reasoned that it should be feasible to screen for mutants that exhibit altered mtDNA transmission by crossing WT cucumber plants from diverse germplasm accessions as maternal parents with MSC16 as the paternal parent and use forward genetics to search for genes affecting mtDNA transmission.
Screening for natural mutants with maternal transmission of the mtDNA
We used plants from a core collection of 78 cucumber accessions as maternal parents in crosses with MSC16 as the male parent, and successfully identified a cucumber line (P3A) that when crossed as the female parent with MSC16 as the male produced high numbers (48/50) of WT progenies and a small number (2/50) of MSC progenies (Fig. 2a and Supplementary Data 2). Based on the rps7SNP(GA/TC) marker, we distinguished the source of mtDNA in the progenies from P3A (♀) × MSC16 (♂) (n = 100). In F1 individuals from P3A × MSC16 that exhibited the MSC phenotype, we detected a weaker signal of WT mtDNA from the maternal parent compared to the WT individuals (Supplementary Fig. 1). Because a specific threshold of mitochondrial numbers seems to affect the progeny phenotype, we speculated that the copy number of maternal mtDNA may not have reached the threshold to alter the phenotype from MSC to WT. To further confirm this, we quantified the mtDNA copy number in hybrid progenies derived from P3A (♀) × MSC16 (♂) using Droplet Digital PCR (ddPCR) and Fast NGS assay for the mitochondrial rps7 gene. The F1 individuals (n = 8) from P3A (♀) × MSC16 (♂) exhibiting MSC phenotype possessed 18.2 ± 1.9 copies of mtDNA per cell, and the average percentage of maternal rps7 was 9.2%. Accordingly, the MSC F1 individuals had 16.5 ± 1.1 and 1.7 ± 0.9 copies of mtDNA per cell coming from the paternal and maternal parents, respectively. In contrast, mtDNA per cell quantified from the F1 individuals (n = 8) of P3A (♀) × MSC16 (♂) exhibiting WT phenotype was 70.4 ± 6.1 copies, and the average percentage of maternal rps7 was 73.4%. As a result, the F1 individuals with WT phenotype had 18.9 ± 3.3 and 51.6 ± 5.5 copies per cell from the paternal and maternal parents, respectively. This result is a 3.9-fold reduction of mtDNA in the cells of MSC phenotype progenies relative to WT phenotype progenies. It was clear that the primary difference between WT and MSC progenies was the difference in the copy number of the mtDNA from the maternal parent. These results suggest that the complete recovery of WT phenotype requires a critical threshold of WT mtDNA from the maternal parent. Our results reveal that cucumber line P3A produces a high proportion of WT phenotype progenies when crossed with MSC16 as the male parent and shows unique biparental transmission of mtDNA compared to other Cucumis plants.
a The phenotypes of P3A (♀), MSC16 (♂) and the F1 progenies. Red arrows indicate the MSC progenies. Scale bars, 10 mm. b Positional cloning of the MTI1 gene in cucumber. Preliminary mapping of the MTI1 using BSA-seq, and fine mapping using KASP markers with 382 F2 plants. 30 genes in the candidate region and the MTI1 were successfully cloned with in silico analysis. c The genomic organization of the MTI1 (CsaV3_3G040940) gene. Blue boxes represent 5′ and 3′ UTRs, yellow boxes and black lines represent exons and introns, respectively. There is 1-bp deletion (Δ909) for P3A line. d Sequence alignment of the MTI1 between the parental lines MSC16 and P3A. The position of the 1-bp deletion (Δ909) in P3A caused frameshift, resulting a premature stop codon. The red asterisk indicates the premature STOP codon in P3A.
Fine mapping, cloning, and gene editing validation of the MTI1 gene-controlling mitochondrial inheritance
To further dissect the genetic basis of biparental transmission of mtDNA in cucumber, we established F1 and F2 families using P3A and MSC16 (Supplementary Fig. 2), and conducted a genetic mapping analysis. The F2 mapping population (CS21) was obtained by the self-pollination of randomly selected WT phenotype F1 progenies from P3A (♀) × MSC16 (♂). The second F2 population (CS22) was generated by crossing WT phenotype F1 progenies as the female parent with MSC phenotype F1 progenies as the male. The phenotype of the CS21 mapping population was obtained from the frequencies of WT versus MSC phenotypes of approximately 100 progenies (F3 individuals) from each 382 F2 individual pollinated with MSC16. The results showed a ratio of 101: 182: 99, fitting a 1: 2: 1 Mendelian ratio (χ2 = 0.87, P = 0.65; Supplementary Data 3). In the second F2 population (CS22), 50 WT and 42 MSC phenotypic segregants were observed, fitting a 1:1 segregation ratio (χ2 = 0.70, P = 0.40). All the results supported the presence of a single nuclear locus controlling mitochondrial inheritance in cucumber P3A. Therefore, we named the locus mitochondrial inheritance 1 (mti1), and it conditions a high frequency of WT progenies in crosses with MSC16 as the male. Our hypothesis is that the WT allele (MTI1) in the female gamete conditions alternative degradation of maternal mtDNA, and that the mti1 allele allows the survival of maternal mtDNA, enabling its transmission to progenies.
To identify the candidate gene for MTI1, we used BSA-seq and fine genetic mapping to place the MTI1 locus within a 326 kb region containing 30 predicted genes (Fig. 2b). In silico analysis revealed that only one gene within this interval harbors a sense mutation leading to a frameshift variant (Supplementary Table 2), providing strong evidence for CsaV3_3G040940 as the candidate gene for MTI1. Sequence analysis of the candidate MTI1 gene revealed a single-nucleotide deletion (Δ909) within exon 8 of CsaV3_3G040940, leading to the premature termination of translation and production of a truncated protein (MTI1truc) in P3A (Fig. 2c, d). CsaV3_3G040940 is predicted to encode a DNA- (apurinic or apyrimidinic site) lyase belonging to a large gene family that includes magnesium-dependent endonucleases and phosphatases involved in intracellular signaling19.
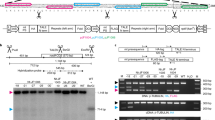
To confirm whether the mutation in CsaV3_3G040940 is associated with the change in mtDNA transmission, we employed CRISPR-Cas9 technology to construct knock-outs. Cucumber transformation is extremely genotype-dependent, limiting its efficiency. According to the previous study, the cucumber line CU2 has been shown to have very high transformation efficiency20. In addition, we observed that all the hybrids produced by crossing CU2 (♀) with MSC16 (♂) exhibited the MSC phenotype, indicating CU2 contains the WT genotype for the MTI1 locus. Thus, we selected CU2 for knockout constructs. We finally obtained two null mutants (mti1-1 and mti1-2) (Fig. 3a, b). Through our whole genome sequencing and comparative analysis, off-target sequences of mti1 mutants were identical to those of wild type, ruling out off-target effects (Supplementary Table 3). We observed that the cross of mti1-1 as the female parent with MSC16 as the male produced only progenies with the WT phenotype (Fig. 3c, d and Supplementary Table 4). When crossing heterozygotes at the MTI1 locus as females with MSC16 as the male parent, the segregations of WT to MSC progenies fit a 1:1 ratio (Fig. 3c, d and Supplementary Table 4). Similar results were obtained with the mti1-2 line. These results clearly support the hypothesis that the fate of maternal mtDNA is determined by the allele at the MTI1 locus in the female gamete. All together, we concluded that the MTI1 gene (CsaV3_3G040940) controls mitochondrial inheritance in cucumber. In addition, we observed high variability of growth rates in progenies of the mti1 knockout lines crossing with MSC16 as the male, particularly in the WT progenies (Fig. 3c). Given that all these WT progenies had identical nuclear genotypes, the primary difference between these progenies might be attributed to the variation in the copy number of the mtDNA from the maternal parent. This variation may potentially alter mitochondrial genome stability and result in differences in mitochondrial activity, but further experimental evidence is required.
a Creation of null mutants of MTI1 using CRISPR/Cas9 carrying a 1 bp deletion (mti1-1) and a 239 bp deletion (mti1-2) in exons. The sequences of the sgRNA target sites are underlined, and the protospacer-adjacent motif (PAM) sites are highlighted in red. b Isolation of gene-edited progeny without Cas9 element by GFP marker within the self-pollinated progenies of T0-1 and T0-2. Yellow arrows indicate the plants without the CRISPR/Cas9 vector. Scale bars, 1 cm. c The phenotype of the hybrids from the mti1-1, mit1-2, (mti1-1 × CU2) and (mti1-2 × CU2) lines as the female parent crossing with MSC16 as the male. Red arrows indicate the MSC progenies. Scale bars, 1 cm. d The segregation results of hybrids from the crossing of edited female plants with MSC16 as the male parent. The number represents the proportion of each phenotype in the hybrid offspring.
MTI1 possesses magnesium-dependent endonuclease activity
Our results have shown that the mutation (Δ909) in CsaV3_3G040940 rendered the protein nonfunctional and allowed maternal transmission of mtDNA in cucumber. Because MTI1 was annotated as a DNA- (apurinic or apyrimidinic site) lyase, we generated a recombinant MTI1-His protein (6× histidine-tagged at C terminus) in Escherichia coli followed by affinity purification, in order to determine if MTI1 has endonuclease activity in vitro. In addition to the WT MTI1-His gene, we also expressed a mutant MTI1truc-His from the P3A line. To avoid His tag missing for purification, we used the truncated sequence to construct the vector for the mutant MTI1 (Supplementary Fig. 3a). The two recombinant proteins were purified, and endonuclease activity was assayed in a standard buffer with the magnesium bivalent cation as a cofactor (Supplementary Fig. 3b, c). The results showed that plasmid DNA was degraded by MTI1-His during the first 10 min of incubation (Fig. 4a). In contrast, only a small amount of degradation was detected in the mutant MTI1truc-His protein. Therefore, we postulated that the mutation of MTI1 in P3A occurs in the middle of the coding region, and the protein may still retain some nuclease activity. Heat-denatured MTI1 did not degrade plasmid DNA even after 60 min of incubation. The addition of EDTA inhibited the nuclease activity of MTI1-His, suggesting that Mg2+ is an essential cofactor. When Zn2+ or Mn2+ was added as the cation source, the endonuclease showed much lower activities than Mg2+ (Fig. 4a). In conclusion, these in vitro enzymatic studies indicate that MTI1-His possesses a magnesium-dependent endonuclease activity.
a Endonuclease assay of recombinant MTI1-His proteins. Effects of heat treatment and EDTA were tested simultaneously as negative controls. Requirements of bivalent cations: magnesium (Mg2+), manganese (Mn2+), and zinc (Zn2+) were tested. The experiment was repeated three times with similar results. M, DNA marker; OC, open-circular DNA; L, linear DNA; SC, supercoiled DNA. b Quantitative analysis of mtDNA copy number with the hairy-root materials overexpressing the transcript of MTI1. Mock, the hairy roots overexpressing RUBY. MTI1, the hairy-root materials overexpressing MTI1 and RUBY. Data are means ± SD. Statistical analysis was performed using two-sided student’s t test. **P < 0.01 and n = 6 biological replicates. c Subcellular localization of MTI1-GFP fusion protein in cucumber hairy-root cells. Red arrows indicate the RUBY-positive hairy roots. Yellow arrows indicate the mitochondria with GFP and Mitotracker signals. GFP indicates the fusion protein, MitoTracker fluorescence is the mitochondrial marker, and the yellow color is the merged image. Scale bars, 40 μm. d–h The development stages of the cucumber female flower. −2, −1, 0 days after anthesis (DAA) and 1, 2 days after pollination (DAP). Scale bars, 5 mm. i–m Cross sections of female flowers hybridized with digoxigenin-labeled antisense probes (red color) to MTI1 transcript. Scale bars, 0.5 mm.
To further test whether MTI1 is associated with degradation of mtDNA in vivo, we constructed 35S promoter-driven overexpression vectors with the MTI1 transcript fused with GFP and used Agrobacterium rhizogenes strain K599 for cucumber transformation. Quantification analysis revealed a 3-fold degradation of mtDNA in hairy-root cells transformed with MTI1 compared with the control (Fig. 4b), while there was no significant difference in plastid copy number (Supplementary Fig. 4). Moreover, the GFP signals of the MTI1 transcript were co-localized with the red fluorescence of Mitotracker Orange-labeled mitochondria (Fig. 4c) in cucumber hairy-root cells. Collectively, we concluded that MTI1 is a nuclear-encoded and mitochondrially targeted endonuclease that determines the transmission of mtDNA by affecting levels of maternal mtDNA in progenies.
MTI1 degrades maternal mtDNA after fertilization, potentially by ubiquitination labeling
The mechanism for non-Mendelian transmission of the mtDNA is a long-standing and unsolved question21, and different mechanisms have been proposed to explain the mode of mitochondrial inheritance in plants22,23. Two prevalent hypotheses are the ‘simple dilution model’ and ‘active degradation model’24. The selective degradation of mtDNA from one parent has gradually been recognized as the consensus model5,25, although it is still controversial about the timing of mitochondrial degradation prior to or upon fertilization26,27.
Identification of the MTI1 gene provides a unique opportunity to leverage our knowledge about mitochondrial inheritance in cucumber. We designed a specific RNA probe for MTI1 and conducted in situ hybridization analysis within the ovule and ovary. Hybridization results demonstrated that MTI1 was primarily expressed within 24 h after pollination (Fig. 4d–m). Almost no MTI1 signals were observed in the ovary or ovule after 24 h of pollination. These results were consistent with the expression analysis with qRT-PCR in the ovary (Supplementary Fig. 5). Therefore, we concluded that MTI1-mediated degradation of the maternal mtDNA specifically occurs shortly after fertilization. This phenomenon has also been observed in Caenorhabditis elegans and Drosophila melanogaster, in which a mitochondrial endonuclease G (EndoG) regulates the degradation of paternal mitochondria after fertilization28,29. However, the mutation of EndoG only delayed the degradation of mtDNA from the paternal parent and did not change the fundamental fate of mtDNA from the parents. In this study, we identified a mutation in the MTI1 gene that altered the fate of mtDNA from the parents, supporting its key role in regulating mitochondrial transmission. Our finding that MTI1 is expressed in ovules after fertilization, which is consistent with the ‘digestion of the organelle after fertilization’ hypothesis for mitochondrial inheritance.
In most living organisms, the paternal mitochondria and their mtDNA are eliminated from zygotes. But why maternal mitochondrial inheritance predominates in eukaryotes remains an unresolved question4,30. A reasonable hypothesis is that paternal mitochondria could be severely damaged by reactive oxygen species during spermatogenesis, resulting in higher mutational loads after fertilization31. However, this hypothesis does not explain the occurrence of biparental or paternal mitochondrial transmission in some plants, such as in the genus Cucumis. In addition, how organisms selectively degrade the mitochondria coming from one of the parents after biparental mtDNA coexistence in the zygote is another standing question32. To address this question and reveal the molecular mechanism associated with MTI1, we performed RNA-seq to identify gene expression changes between MTI1 and mti1 gene-edited plants. A total of 93 differentially expressed genes (DEGs) (|fold change| > 2 and p < 0.05) were detected, of which 76 genes including MTI1 were upregulated (higher expression in MTI1 plants) and 17 genes were downregulated (Supplementary Data 4 and Supplementary Fig. 6a). KEGG pathway enrichment analysis was conducted, and protein ubiquitylation was the most significantly enriched pathways for DEGs. All genes associated with protein ubiquitylation were significantly upregulated, indicating that the ubiquitination process may participate in the recognition of target mitochondria (Supplementary Fig. 6b). Western blot analysis of the ovaries one day after pollination revealed that the ubiquitination level of total protein in the knockout line (mit1-1) was significantly higher than the wild type (CU2) (Supplementary Fig. 6c). Ubiquitination has been shown to cause degradation of sperm mitochondria and mtDNA in mammalian zygotes and embryos, ensuring that stringent maternal inheritance of mtDNA arises from the selective destruction of sperm mitochondria33,34. Similar mechanisms may also exist in plants and provide for the selective destruction of the mitochondria.
MTI1 exhibits selection footprints in Cucumis during domestication
MTI1 encodes a DNA- (apurinic or apyrimidinic site) lyase belonging to the apurinic/apyrimidinic sites endonucleases (APEs). Plants typically contain three APE homologous35, as do cucumber and melon. Phylogenetic analysis of APE family proteins revealed that plant APEs belong to three different clades, namely the ARP, APE1L, and APE2 clades, respectively, and MTI1 clustered with Arabidopsis ARP and human hsAPE1 clades (Supplementary Fig. 7). However, we did not observe any clear differentiation of this gene in plants.
Cucurbitaceae is a large plant family that includes 95 genera. To date, Cucumis is the only Cucurbit genus reported to show paternal inheritance of mitochondria, which makes the Cucurbit family an ideal system to explore the evolution of mitochondrial inheritance11. Maternal mtDNA signals were also detected in hybrids with the CmMTI1 knockout line (the MTI1 homolog in melon) as the maternal parent (Supplementary Fig. 8a–c), demonstrating that the CmMTI1 plays a similar role to CsMTI1 in cucumber. Therefore, we analyzed the MTI1 sequences of 115 cucumber accessions and 171 melon accessions from different geographic origins (Fig. 5a) using published resequencing datasets36. We identified only two accessions with a non-synonymous mutation (Supplementary Table 5). Both of these accessions were collected from Zambia, where a high diversity of wild Cucumis species exists and may be the center of origin for cucumber and melon. Based on these findings, we questioned whether the genetic diversity of MTI1 conferred any adaptive advantages and whether MTI1 experienced selection during domestication and improvement. Notably, we found that the nucleotide diversity (π) of the genomic region around MTI1 was much lower in the domesticated cucumber groups from East Asian, Eurasian, and Xishuangbanna in comparison to the wild group from India, suggesting that MTI1 underwent selection during domestication (Fig. 5b). And we discovered similar domesticated footprints at the region around the CmMTI1 locus in melon populations (Fig. 5c). These results suggest that MTI1 has undergone positive selection during the domestication of Cucumis plants. As a result, only cucumber and melon have evolved to retain paternal inheritance of mtDNA11, a trait seemingly linked to the selection of MTI1. Taken together, our results provide strong evidence that paternal inheritance of mtDNA in Cucumis is the derived state, and biparental inheritance of mtDNA is suppressed during the domestication of Cucumis. However, the biological significance of paternal transmission in Cucumis needs further study.
a The geographical distribution of 286 Cucumis accessions used in the domestication analysis. A total 115 cucumber accessions were used, consisting of wild populations (Indian accessions) and cultivated populations (Eurasian, East Asian, and Xishuangbanna accessions). A total of 171 melon accessions were used, including wild and cultivated populations. Distributions of the Cucumis plants across geographic areas are shown as pie charts, colored in green, yellow, and blue, respectively, with the numbers indicated. The colored lines represent the four cucumber populations: Indian (green), Xishuangbanna (purple), Eurasian (yellow), and East Asian (blue). b Genomic region around the CsMTI1 locus shows evidence of a domestication sweep. The distribution of π is shown for the Indian (green), Xishuangbanna (purple), Eurasian (orange), and East Asian (blue) populations. The CsMTI1 is located in the gray region. c Genomic region around the CmMTI1 locus shows evidence of a domestication sweep. The distribution of π is shown for the wild (light blue) and cultivated (light orange) populations. The CmMTI1 is located in the gray region.
Discussion
Non-Mendelian mitochondrial inheritance is a high research priority for mitochondrial biologists and clinical geneticists37,38. It is widely recognized that the mitochondria in male gametic cells participate in the fertilization process even in species with known maternal inheritance of mitochondria39,40. The degradation of mtDNA within the male gametic cells of plants and animals occurs during male gametophyte development or after fertilization41,42. Increasing studies have found that nucleases might be the key factors in mitochondrial inheritance. In Drosophila, EndoG was thought to be the main effector of degradation of paternal mitochondria through autophagy soon after fertilization29. In Arabidopsis, DPD1 was the first nuclease to be relevant to the degradation of mtDNA after mitosis during pollen cell development, and DPD1 expression is associated with the gene Autophagy 7 (ATG7) that plays a critical role in the autophagy pathway8,43. Endonuclease M20 was identified based on in-gel detection of endonuclease activity from pollen, which helps to understand the molecular mechanism underlying mtDNA degradation, as the exonuclease DPD1 requires the participation of endonucleases to degrade the circular mtDNA9. Recently, Chung and colleagues demonstrated that loss of dpd1 and mild chilling stress during male gametogenesis could increase the frequency of paternal leakage of chloroplasts tobacco (Nicotiana tabacum), which suggested that both genetic and environmental factors could affect the organelle inheritance10. However, mutants of the above-mentioned genes had no effect on the inheritance mode of mitochondria, although the paternal mitochondria remained in the zygote transiently in the EndoG mutant.
Limited research and progress on mitochondrial inheritance are partially due to a lack of natural mutants and efficient approaches to detect mitochondrial transmission. The previous reports about relevant genes could only be identified by the degradation of mtDNA within the male gametic cells. As a result, these genes are supposed to be primarily involved in the degradation process, rather than determining the mode of mitochondrial inheritance. Researchers have demonstrated that mitochondrial degradation in pollen may be widespread for nutrient recycling and unrelated to mitochondrial inheritance44. In our study, we used the paternally transmitted MSC mutants of cucumber to identify a natural mutant among 115 cucumber accessions, which enabled us to pinpoint the key gene determining the fate of mitochondrial DNA through forward genetics. Remarkably, paternal inheritance of cucumber mitochondria is controlled by a single nuclear gene, MTI1, providing direct evidence for the participation of a nuclear-encoded and mitochondrially targeted endonuclease in mitochondrial inheritance of plants. As an endonuclease involved in mtDNA degradation, various factors can influence its activity, potentially leading to leakage of mtDNA. In our in vitro nuclease activity analysis, the truncated MTI1 exhibited partial endonuclease activity, which helps explain the presence of a few MSC individuals among hybrids from P3A × MSC16. Our data also showed that MTI1 activity is enhanced by Mg2+, whereas in the presence of Mn2+ or Zn2+ the activity was lower. This finding is consistent with numerous earlier nuclease studies8,45. Our current study provides valuable insights into screening mutants relevant to mtDNA inheritance. It enables further identification of additional genes participating in mitochondrial inheritance, thereby deepening our global understanding of mitochondrial genetic regulation. Currently, organelle transgenic and mitochondrial gene editing technologies are undergoing breakthroughs46,47, which is promising to create mitochondrial mutants.
Another standing question regarding mitochondrial inheritance is the timing of organelle degradation, that is, before or after fertilization. Our research revealed that mitochondrial inheritance is affected by the MTI1 genotype of the female gametophyte in cucumber. Similar phenomena have been observed in studies of plastid inheritance48. Moreover, we found that the expression of the MTI1 occurs after fertilization rather than before. This raises the question of how MTI1 distinguishes maternal and paternal mitochondria. Our RNA-seq data indicated that the expression of MTI1 is primarily associated with the ubiquitination process. Ubiquitin is a highly conserved peptide that is covalently linked to protein lysine residues, influencing protein sorting, degradation, or signal transmission depending on the ubiquitination pattern49. In Caenorhabditis elegans, ubiquitination is the primary mechanism responsible for the removal of paternal mitochondria through autophagy after fertilization50,51. Therefore, we assume that a similar mechanism may exist in plants, where mitochondria from one parent may be ubiquitinated and selectively destroyed after fertilization, resulting in uniparental inheritance of mitochondria.
While our study established that MTI1 governs paternal mitochondrial transmission, we are still puzzled by why Cucumis plants exhibit paternal inheritance rather than the more common maternal inheritance as seen in most eukaryotes. For maternal inheritance, a reasonable hypothesis is that paternal mitochondria could be severely damaged by reactive oxygen species created during spermatogenesis and higher mutational loads after fertilization28,52. This hypothesis does not explain the occurrence of biparental or paternal mitochondrial transmission in non-canonical examples such as plants in the genus Cucumis. Although Cucurbitaceae is a big plant family including 95 genera, Cucumis is the only genus found to show paternal mitochondrial inheritance. We analyzed the MTI1 sequences of 115 cucumber accessions and identified only two accessions with a non-synonymous mutation. Both of these accessions were collected from Zambia, which has a high diversity of wild Cucumis species and may be the center of origin for cucumber and melon53. Presumably, understanding why Cucumis plants developed the paternal transmission of mitochondria will shed light on the elucidation of evolutionary trade-offs about the non-Mendelian inheritance. Previous studies have shown that mitochondrial inheritance might be associated with sex determination54,55. For example, mitochondrial inheritance can be directly controlled by sex-determining genes in the fungus Phycomyces blakesleeanus, and mtDNA itself could be a key factor in sex determination. The non-canonical genetic systems of mtDNA transmission are often found in organisms with unusual sexual determination, which is consistent with Cucumis species that show paternal mitochondrial transmission and complex genetic control of sex determination56,57,58. Interestingly, MTI1 is homologous to animal and human APEX1. The variants of APEX1 in animals and humans are strongly associated with embryonic development and female reproductive organ disease, like breast, cervical, and ovarian cancers59. Genes that control mitochondrial inheritance might be associated with sex determination or reproductive organ development, and it may be worthwhile to combine mitochondrial inheritance with sex determination or reproductive organ development.
In summary, our study reveals a nuclear-encoded but mitochondria-targeted gene that acts as a switch-on/off in controlling the non-Mendelian mitochondrial inheritance. Accordingly, we proposed a model for mitochondrial inheritance in Cucumis plants (Fig. 6). When the egg cell carries the WT MTI1 allele, maternal mtDNA is degraded after fertilization, and paternal mtDNA predominates in the zygote and eventual embryo. In egg cells carrying the mutant mti1 allele, maternal mtDNA remains after fertilization, and biparental mtDNAs co-exist in the zygote. Our studies revealed the transcript diversity and functional differentiation of MTI1 gene in Cucumis. As endonucleases are ubiquitously present and highly conserved in living organisms, these findings will contribute to our understanding of how non-Mendelian inheritance of mitochondria was shaped during evolution. Besides, we observed selection footprints of MTI1 during Cucumis domestication, but the evolutionary implications as to why this gene is under selection and why Cucumis plants favor non-canonical paternal transmission of the mtDNA require further investigation.
Methods
Plant materials and growth conditions
The melon experiments were carried out using the inbred line DHL92 (Cucumis melo L. subsp. melo), Trigonus (TRI, C. melo L. subsp. agrestis), TMR (Cucumis melo L. subsp. melo), Mapao (C. melo L. subsp. agrestis). DHL92 and TRI were reciprocally crossed to obtain F1 hybrids for detecting maternal leakage of mtDNA. TMR was used to do gene editing for melon. TMR and Mapao were reciprocally crossed to obtain F1 hybrids for analyzing the transmission of mtDNA. The cucumber experiments were carried out using the inbred lines Hardwickii (a wild cucumber), P3A (inbred line from a single plant from USDA PI 401734)18, MSC16 (inbred line, selected after passage of the nonmosaic, highly inbred line B through cell culture)13, and CU2 (inbred line)20 and the cultivar Calypso60. The segregated populations were grown and phenotyped in the greenhouse (Zhejiang Academy of Agriculture Sciences, Hangzhou, China).
Mitochondrial DNA marker development
The mitochondrial resequencing data of MSC16 were downloaded from the NCBI (SRR1744691)14. The reads were aligned to the mtDNA reference sequence of ‘Calypso’60. SNPs were filtered using the criteria for high quality. All SNPs in the rps7 region were delimited by 195,676 and 283,618 in mitochondrial chromosome 1 and were considered to be designed for mitochondrial markers. The dCAPS primers design was performed with the dCAPS Finder 2.0 program, introducing a single nucleotide mismatch adjacent to the SNP position (SNP position: 263660–263661) in the reverse primer that created a HindIII restriction site in the amplified PCR product of Calypso but not in MSC1661. The forward primer was designed with Primer Premier 6.0. Following PCR reactions, the HindIII restriction enzyme was added to the PCR reaction and incubated for 30 min at 37 °C according to the manufacturer’s instructions (New England Biolabs, United States). Two microliters of digested PCR products were then mixed with 1 µL of 10× loading buffer, and subjected to electrophoresis in an 8% polyacrylamide gel.
A phenotypic segregation population was created through hybridization (P3A × Hardwickii) × MSC16, including 84 individual plants. After sowing, collect MSC or wild phenotypes of each individual plant. Extract the DNA from the first true leaf and use the dCAPS program to identify the mitochondrial genotypes of each individual plant.
Quantitative RT-PCR analysis
Total RNA was isolated using a Tiangen RNA extraction kit (Tiangen, China) and then reverse transcribed using Reverse Transcription Kit with gDNA Eraser (Tiangen, China) following the manufacturer’s protocol. The RT-qPCR was performed with the StepOne PCR System (Thermo Fisher Scientific, United States), and amplification was conducted using ArtiCanATM SYBR qPCR Mix (Tsingke, China) with 40 cycles of denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s, and extension at 72 °C for 30 s. The amplification specificity was tested by a dissociation curve (65–90 °C) to compare the results from different reactions and samples. At least three independent biological and technical replicates were tested per sample.
Positional cloning of MTI1
To map the causal gene in the mti1 mutant, the F2 mapping population (CS21) was obtained by crossing P3A and MSC16 and then by self-pollination of the subsequent WT progenies. A total of 382 F2 plants were grown in the greenhouse, and testcrossed with MSC16 as the male parent, and the frequencies of WT versus MSC testcross progenies were scored to determine the genotype of the F2 individual. The homozygous genotype with the allele (MTI1/MTI1) produced >90% MSC progenies, while homozygotes with the unique allele (mti1/mti1) from P3A produced more than 90% WT progenies. The heterozygous genotype produced approximately equal numbers of WT and MSC progenies. And a second F2 population (CS22) was generated by crossing WT F1 progenies as the female parent with MSC F1 progenies as the male.
BSA-seq (Bulked Segregation Analysis Sequencing) was performed to map the mti1 locus with parents and bulked DNA samples of MTI1/MTI1 and mti1/mti1 F2 plants. Equal amounts of DNA from 30 MTI1/MTI1 and 30 mti1/mti1 F2 plants were prepared for the bulked pools. The genomic DNA libraries were re-sequenced with an Illumina HiSeq 2500 (500 bp) in Novogene (Beijing, China). The clean data obtained from the DNA pools were aligned to the ‘Chinese Long’ cucumber reference genome (v3.0)19. Variant callings, including SNPs and indels, were filtered to identify the candidate interval. To further determine the location of mti1, we developed the Kompetitive Allele-Specific PCR (KASP) markers based on the genome sequence of the two parents and determined the genotypes of the recombinants with these markers62.
To further find the candidate gene, an in silico analysis was performed with re-sequenced cucumber accessions that had the MTI1 genotype63. All the resequencing data of cucumber accessions were downloaded from the NCBI (SRR2096458, SRR2096471, SRR2096523, SRR9914340, SRR543284, SRR543239, SRR543255, SRR543211, SRR543208, SRR543209) and separately mapped on the candidate region. By comparing the P3A sequence within the localization interval, we successfully identified a candidate gene.
Plasmid construction and plant transformation
To generate the mti1 CRISPR-Cas9-edited plants, the binary vector pBSE402 containing a CRISPR cassette with a functional Cas9 under a constitutive promoter (CaMV 35S) plus a 35S-GFP expression cassette was used for plasmid construction (gift from Xueyong Yang, Chinese Academy of Agricultural Sciences). The single guide RNA (sgRNA) target site from the N terminus of MTI1 was selected to construct the pBSE402-sgRNA-MTI1 vector. Then, Agrobacterium cells harboring the target vector were used to transform the cotyledon explant of cucumber and melon with a syringe under negative pressure. Currently, the genotype of the material still limits the genetic transformation of cucumber and melon, with cucumber CU2 and melon TMR lines being the most efficient materials employed for transformation20. In brief, cucumber or melon seeds were sterilized and plated on 1/2 Murashige and Skoog medium (Phytotech, United States) supplemented with 2 mg/L of 6-benzylaminopurine (Sigma-Aldrich, United States) and 1 mg/L of ABA (Phytotech, United States) and incubated for 2 d at 28 °C. Shoot regeneration, elongation, and rooting were performed. Genomic DNA was extracted from callus and plants using the Hi-Pure Plant Genomic DNA Kit (Tsingke, China). PCR was performed using KOD-FX (Toyobo, Japan) and the gene-specific primers listed in Supplementary Data 5. PCR products were cloned into pEASY-Blunt Zero (Transgen Biotech, China), and the MTI1 alleles were identified by sequencing.
Off-target analysis based on whole-genome resequencing
The genomic DNA was extracted from fresh leaves of T1 plants using CTAB method. DNA sequencing was completed by the biological company (Novogene, China), with at least 25× coverage depth. The CRISPR-P 2.0 website was used to seek potential off-target sites by comparing the sgRNA target sites to the cucumber reference genome64. The IGV program (V2.8.0) was used to analyze the sequencing data65.
A. rhizogenes-mediated root transformation
For the construction of the GFP plasmid, we amplified the CDS sequence of MTI1 except for the stop codon using KOD plus polymerase (Toyobo, Japan). The amplified fragment was digested with KpnI and XbaI, and subcloned into the pMDC32-GFP-RUBY vector, which contains a visible RUBY reporter to the naked eye66. The plasmid was used for the A. rhizogenes-mediated root transformation, which was performed as the following steps67. Cucumber seedlings with cotyledons just unfolded were cut at the hypocotyl 1 cm below the cotyledons with a sterile scalpel in the liquid of A. rhizogenes. Then, the cut of residual hypocotyl was scraped on the plate-grown A. rhizogenes. The hairy roots were induced in a growth medium with high humidity by a plastic covering. After two weeks of culture, the transgene-positive hairy roots (with RUBY) were harvested and used for the subsequent analysis.
Quantification of copy numbers of mtDNA
Droplet digital PCR (Bio-Rad, United States) was performed to estimate the copy numbers of mtDNA in the WT and MSC progenies. For mtDNA or cpDNA quantification, two sets of primers and probes were designed targeting the mitochondrial and nuclear genomes, respectively. The probe targeting mtDNA or cpDNA was labeled with the FAM fluorophore, whereas the probe targeting the nuclear sequence was labeled with HEX. All probes had the BHQ1 quencher. Detailed information on the probes and primers for ddPCR is provided in Supplementary Data 5. The ddPCR method was performed according to the manufacturer’s instructions (QX200 Droplet Digital PCR) modified by the use of cucumber genomic DNA15. Data were analyzed using the QuantaSoft software v1.4 (Bio-Rad), which determines the numbers of droplets that were positive and negative for each fluorophore in each sample. The fraction of positive droplets was then fit to a Poisson distribution in the QuantaSoft software to determine the absolute copy number per μL. By calculating the ratio of the copy numbers of the mitochondrial gene and nuclear gene, the copy number of mitochondrial DNA in a single cell can be obtained. During the calculation process, our previous studies have confirmed that CsDPD1 is a single copy in the nuclear genome15. Considering the little impact of cell cycle on the copy number of nuclear genes68, we determined the copy number of the nuclear genome in a single cell to be 2. To distinguish the proportion of mtDNA from paternal or maternal parents, we amplified the mitochondrial sequence of rps7 from the whole genome using KOD plus polymerase (Toyobo, Japan). The amplified fragments were sent for Fast NGS sequencing with ≥100× depths (Tsingke, China) to obtain the number of reads from each parent.
Subcellular localization experimentation
The transgene-positive hairy roots (with RUBY) were used for the subcellular localization analysis. The confocal microscope (Olympus, Japan) was used to observe green and red fluorescence, and the fluorescence signals were captured with Olympus Fluoview (version 2.0). To stain mitochondria, hairy root cells were incubated with 0.5 mM MitoTracker Orange CM-H2TMRos (Invitrogen, United States) for 30 min before observation.
RNA-Seq analysis
The shoot apex of WT (MTI1) and knockout cucumber mutants (mti1) created by the CRISPR-Cas9 strategy were sampled. Total RNA was extracted from frozen tip meristem samples using TRIzol reagent (Invitrogen, United States) according to the manufacturer’s protocol. The cDNA library was prepared for sequencing according to the Illumina TruSeqTM RNA Sample Kit protocol. Sequencing was performed using an Illumina HiSeq 2500 system (Genepioneer Biotechnologies). RNA-seq reads were generated and processed to calculate expression levels, which were averaged over four biological replicates. Differential expression analysis was performed using DESeq2 with Galaxy69, and an absolute log2(FC) value > 1 and a corrected p value < 0.05 were set as the thresholds for DEGs. All DEGs were mapped to the KEGG database and searched for significantly enriched KEGG pathways.
Western blot analysis
The total protein was extracted from the ovaries of knockout cucumber mti1-1 and WT (CU2) lines one day after pollination. And the samples were separated by 12.5% SDS polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes and blotted with monoclonal antibodies against Ubiquitin (Servicebio, China) and polyclonal antibodies against Actin (Abbkine, China), respectively.
Nuclease activity assay
To construct pET-32a for expressing recombinant MTI1-His protein in Escherichia coli, an MTI1 cDNA containing the entire reading frame was amplified by PCR using the cDNA library. And the mutagenesis of pET-32a to express MTI1truc-His was conducted using the cDNA of P3A. The His-tag MTI1 proteins were induced by isopropyl β-D-1-thiogalactopyranoside (IPTG) and purified using a HiLoad Superdex column (GE Healthcare, United States) according to the manufacturer’s instructions. Nuclease activity assay was performed as previously described9. Briefly, the nuclease assay reaction (40 μL) consisted of 40 mM Tris-HCl, pH 7.5, 10 mM MgCl2, and 1 μg pUC18 plasmid DNA substrate, and the purified MTI1-His or MTI1truc-His (3 μg protein) was finally added to initiate the reaction. For inhibition of the activity of nuclease, MTI1-His was incubated at 85 °C for 5 min prior to nuclease assay, or the reactions were mixed with 10 mM EDTA. Reactions were completed by immediately adding the stopping buffer (1% SDS, 50% glycerol, and 0.05% bromophenol blue) and were subjected to 1% (w/v) agarose gel electrophoresis. After staining with ethidium bromide, the remaining undigested DNA was image-captured and quantified using AlphaVIEW SA software (v3.2.4).
In situ hybridization analysis
The ovaries (−48h, −24h, 0 h, 24 h, and 48 h after pollination) were collected and fixed in 3.7% formol-acetic-alcohol and stored at 4 °C until use. The probe of MTI1 was designed according to unique gene fragments. Sample sectioning and in situ hybridization were performed as the following steps. Briefly, ten micrometer-thick sliced sections were prepared, which were deparaffinized in 100% Histo-clear, followed by dehydration in an ethanol gradient. Proteinase K working solution was added for incubation and followed by adding pre-hybridization solution and incubating. Then, the digoxin-labeled probe was added for incubation. After incubating in the sections were incubated with anti-DIG-HRP. Freshly prepared NBT/BCIP chromogenic reagent was used to mark the tissue.
The sections were naturally air-dried and mounted with a glycerol jelly mounting medium. Images were taken with a Nikon DS-Ri1 microscope (Nikon, Japan).
Nucleotide diversity analysis
Resequencing data from 115 published cucumber accessions (37 EastAsian, 29 Eurasian, 19 Xishuangbanna, and 30 Indian) and 171 melon accessions from different geographic origins were used for the analysis36. Nucleotide diversity was estimated as a π value for each population using VCFtools. The SNPs near the MTI1 locus in the cucumber genome were obtained (corresponding to Chr3: 32.0–33.0 Mb, 9930 genome v2). We measured the level of π using a 100-kb window with a step size of 10 kb.
Phylogenetic analysis of APE family proteins
The cucumber MTI1 endonuclease protein sequence was used as a query to search against the genome database of each species with BLASTP. The obtained protein sequences were aligned using the ClustalW program in MEGA X. The neighbor-joining method in MEGA X was used to construct the phylogenetic tree, and 500 bootstrap replicates were conducted.
Statistical analysis
All experiments were carried out with at least three replicates. Student’s t test and one-way ANOVA (with Tukey’s post hoc test) were performed to assess the statistical significance of the experiment results using the GraphPad Prism 9 software (GraphPad Software, United States) and Excel 2019 (Microsoft, United States). Wilcoxon rank-sum tests were used to compare the mtDNA levels for the transgenic samples.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The transcriptomic data generated in this study have been deposited in the NCBI database under accession code PRJNA997008. All sequencing data for the off-target mutations generated in this study have been deposited in the NCBI database under accession code PRJNA996879. Source data are provided with this paper.
References
Zhao, Y. N. et al. Malate transported from chloroplast to mitochondrion triggers production of ROS and PCD in Arabidopsis thaliana. Cell Res. 28, 448–461 (2018).
Takatsuka, A., Kazama, T., Arimura, S. & Toriyama, K. TALEN-mediated depletion of the mitochondrial gene proves that it is a Tadukan-type cytoplasmic male sterility-causative gene in rice. Plant J. 110, 994–1004 (2022).
Müller-Nedebock, A. C. et al. Mitochondrial DNA variation in Parkinson’s disease: analysis of “out-of-place” population variants as a risk factor. Front. Aging Neurosci. 14, 921412 (2022).
Munasinghe, M. & Ågren, J. A. When and why are mitochondria paternally inherited? Curr. Opin. Genet. Dev. 80, 102053 (2023).
Sato, M., Sato, K., Tomura, K., Kosako, H. & Sato, K. The autophagy receptor ALLO-1 and the IKKE-1 kinase control clearance of paternal mitochondria in Caenorhabditis elegans. Nat. Cell Biol. 20, 81–91 (2018).
Matsushima, R. et al. Mitochondrial dynamics in plant male gametophyte visualized by fluorescent live imaging. Plant Cell Physiol. 49, 1074–1083 (2008).
Wang, D. Y. et al. The levels of male gametic mitochondrial DNA are highly regulated in angiosperms with regard to mitochondrial inheritance. Plant Cell 22, 2402–2416 (2010).
Matsushima, R. et al. A conserved, Mg2+-dependent exonuclease degrades organelle DNA during Arabidopsis pollen development. Plant Cell 23, 1608–1624 (2011).
Ma, F. et al. The mitochondrial endonuclease M20 participates in the down-regulation of mitochondrial DNA in pollen cells. Plant Physiol. 178, 1537–1550 (2018).
Chung, K. P., Gonzalez-Duran, E., Ruf, S., Endries, P. & Bock, R. Control of plastid inheritance by environmental and genetic factors. Nat. Plants 9, 68–80 (2023).
Havey, M., McCreight, J., Rhodes, B. & Taurick, G. Differential transmission of the Cucumis organellar genomes. Theor. Appl. Genet. 97, 122–128 (1998).
Havey, M. Predominant paternal transmission of the mitochondrial genome in cucumber. J. Heredity 88, 232–235 (1997).
Bartoszewski, G., Malepszy, S. & Havey, M. J. Mosaic (MSC) cucumbers regenerated from independent cell cultures possess different mitochondrial rearrangements. Curr. Genet. 45, 45–53 (2004).
Del Valle-Echevarria, A. R., Kiełkowska, A., Bartoszewski, G. & Havey, M. J. The mosaic mutants of cucumber: a method to produce knock-downs of mitochondrial transcripts. G3: Genes Genomes Genet. 5, 1211–1221 (2015).
Shen, J. et al. Rare maternal and biparental transmission of the cucumber mitochondrial DNA reveals sorting of polymorphisms among progenies. Theor. Appl. Genet. 132, 1223–1233 (2019).
Wei, W. et al. Nuclear-embedded mitochondrial DNA sequences in 66,083 human genomes. Nature 611, 105–114 (2022).
Havey, M. J., Park, Y. H. & Bartoszewski, G. The Psm locus controls paternal sorting of the cucumber mitochondrial genome. J. Heredity 95, 492–497 (2004).
Del Valle-Echevarria, A., Sanseverino, W., Garcia-Mas, J. & Havey, M. Pentatricopeptide repeat 336 as the candidate gene for paternal sorting of mitochondria (Psm) in cucumber. Theor. Appl. Genet. 129, 1951–1959 (2016).
Li, Q. et al. A chromosome-scale genome assembly of cucumber (Cucumis sativus L.). GigaScience 8, giz072 (2019).
Xin, T. et al. Targeted creation of new mutants with compact plant architecture using CRISPR/Cas9 genome editing by an optimized genetic transformation procedure in cucurbit plants. Hortic. Res. 9, uhab086 (2022).
Zlotorynski, E. Mechanism of sperm mtDNA elimination. Nat. Rev. Mol. Cell Biol. 24, 855–855 (2023).
Zhang, Q., Zhang, Y., Sakamoto, W. & Kuroiwa, T. Reduction in amounts of mitochondrial DNA in the sperm cells as a mechanism for maternal inheritance in Hordeum vulgare. Planta 216, 235–244 (2002).
Kuroiwa, T. Review of cytological studies on cellular and molecular mechanisms of uniparental (maternal or paternal) inheritance of plastid and mitochondrial genomes induced by active digestion of organelle nuclei (nucleoids). J. Plant Res. 123, 207–230 (2010).
Nagata, N. Mechanisms for independent cytoplasmic inheritance of mitochondria and plastids in angiosperms. J. Plant Res. 123, 193–199 (2010).
Sato, M. & Sato, K. Degradation of paternal mitochondria by fertilization-triggered autophagy in C. elegans embryos. Science 334, 1141–1144 (2011).
Kimata, Y. et al. Mitochondrial dynamics and segregation during the asymmetric division of Arabidopsis zygotes. Quant. Plant Biol. 1, e3 (2020).
Onraet, T. & Zuryn, S. C. elegans as a model to study mitochondrial biology and disease. Semin. Cell. Dev. Biol. 154, 48–58 (2023).
Wang, W. J. et al. Endonuclease G promotes autophagy by suppressing mTOR signaling and activating the DNA damage response. Nat. Commun. 12, 476 (2021).
Zhou, Q. H. et al. Mitochondrial endonuclease G mediates breakdown of paternal mitochondria upon fertilization. Science 353, 394–399 (2016).
Zuidema, D., Jones, A., Song, W. H., Zigo, M. & Sutovsky, P. Identification of candidate mitochondrial inheritance determinants using the mammalian cell-free system. Elife 12, RP85596 (2023).
Durairajanayagam, D., Singh, D., Agarwal, A. & Henkel, R. Causes and consequences of sperm mitochondrial dysfunction. Andrologia 53, e13666 (2021).
Ross, L., Mongue, A. J., Hodson, C. N. & Schwander, T. Asymmetric inheritance: the diversity and evolution of non-Mendelian reproductive strategies. Annu. Rev. Ecol. Evolution Syst. 53, 1–23 (2022).
Song, W. H., Yi, Y. J., Sutovsky, M., Meyers, S. & Sutovsky, P. Autophagy and ubiquitin-proteasome system contribute to sperm mitophagy after mammalian fertilization. Proc. Natl Acad. Sci. USA 113, E5261–E5270 (2016).
Sutovsky, P. et al. Ubiquitin tag for sperm mitochondria. Nature 402, 371–372 (1999).
Li, J. C. et al. Functional importance and divergence of plant apurinic/apyrimidinic endonucleases in somatic and meiotic DNA repair. Plant Cell 35, 2316–2331 (2023).
Qi, J. et al. A genomic variation map provides insights into the genetic basis of cucumber domestication and diversity. Nat. Genet. 45, 1510–1515 (2013).
Strome, S., Bhalla, N., Kamakaka, R., Sharma, U. & Sullivan, W. Clarifying Mendelian vs non-Mendelian inheritance. Genetics 227, iyae078 (2024).
Zschocke, J., Byers, P. H. & Wilkie, A. O. M. Mendelian inheritance revisited: dominance and recessiveness in medical genetics. Nat. Rev. Genet. 24, 442–463 (2023).
Ben-Hur, S. et al. Egg multivesicular bodies elicit an LC3-associated phagocytosis-like pathway to degrade paternal mitochondria after fertilization. Nat. Commun. 15, 1–25 (2024).
Togashi, T., Parker, G. A. & Horinouchi, Y. Mitochondrial uniparental inheritance achieved after fertilization challenges the nuclear-cytoplasmic conflict hypothesis for anisogamy evolution. Biol. Lett. 19, 20230352 (2023).
Lee, W. et al. Molecular basis for maternal inheritance of human mitochondrial DNA. Nat. Genet. 55, 1632–1639 (2023).
Sato, M. & Sato, K. Maternal inheritance of mitochondrial DNA by diverse mechanisms to eliminate paternal mitochondrial DNA. Biochim. Biophys. Acta Mol. Cell Res. 1833, 1979–1984 (2013).
Tang, L. Y. & Sakamoto, W. Tissue-specific organelle DNA degradation mediated by DPD1 exonuclease. Plant Signal. Behav. 6, 1391–1393 (2011).
Sakamoto, W. & Takami, T. Nucleases in higher plants and their possible involvement in DNA degradation during leaf senescence. J. Exp. Bot. 65, 3835–3843 (2014).
Li, Y. et al. An AP endonuclease functions in active DNA demethylation and gene imprinting in Arabidopsis. PLoS Genet. 11, e1004905 (2015).
Fuentes, I., Stegemann, S., Golczyk, H., Karcher, D. & Bock, R. Horizontal genome transfer as an asexual path to the formation of new species. Nature 511, 232–235 (2014).
Kazama, T. et al. Curing cytoplasmic male sterility via TALEN-mediated mitochondrial genome editing. Nat. Plants 5, 722–730 (2019).
Rusche, M. L., Mogensen, H. L., Zhu, T. & Smith, S. E. The zygote and proembryo of alfalfa: quantitative, three-dimensional analysis and implications for biparental plastid inheritance. Protoplasma 189, 88–100 (1995).
Punzi, E., Milani, L., Ghiselli, F. & Passamonti, M. Lose it or keep it: (how bivalves can provide) insights into mitochondrial inheritance mechanisms. J. Exp. Zool. Part B-Mol. Dev. Evol. 330, 41–51 (2018).
Sasaki, T. & Sato, M. Degradation of paternal mitochondria via mitophagy. Biochim. Biophys. Acta Gen. Subj. 1865, 129886 (2021).
de Melo, K. P. & Camargo, M. Mechanisms for sperm mitochondrial removal in embryos. Biochim. Biophys. Acta Mol. Cell Res. 1868, 118916 (2021).
Liao, S., Chen, L., Song, Z. & He, H. The fate of damaged mitochondrial DNA in the cell. Biochim. Biophys. Acta Mol. Cell Res. 1869, 119233 (2022).
Zhao, G. et al. A comprehensive genome variation map of melon identifies multiple domestication events and loci influencing agronomic traits. Nat. Genet. 51, 1607–1615 (2019).
Flanagan, B. A., Li, N. & Edmands, S. Mitonuclear interactions alter sex-specific longevity in a species without sex chromosomes. Proc. R. Soc. B 288, 20211813 (2021).
Allison, T. M., Radzvilavicius, A. L. & Dowling, D. K. Selection for biparental inheritance of mitochondria under hybridization and mitonuclear fitness interactions. Proc. R. Soc. B 288, 20211600 (2021).
Martin, A. et al. A transposon-induced epigenetic change leads to sex determination in melon. Nature 461, 1135–1138 (2009).
Boualem, A. et al. A cucurbit androecy gene reveals how unisexual flowers develop and dioecy emerges. Science 350, 688–691 (2015).
Zhang, S. et al. The control of carpel determinacy pathway leads to sex determination in cucurbits. Science 378, 543–549 (2022).
Peng, L. et al. APEX1 regulates alternative splicing of key tumorigenesis genes in non-small-cell lung cancer. BMC Med. Genomics 15, 147 (2022).
Alverson, A. J., Rice, D. W., Dickinson, S., Barry, K. & Palmer, J. D. Origins and recombination of the bacterial-sized multichromosomal mitochondrial genome of cucumber. Plant Cell 23, 2499–2513 (2011).
Neff, M. M., Turk, E. & Kalishman, M. Web-based primer design for single nucleotide polymorphism analysis. Trends Genet. 18, 613–615 (2002).
Shen, J., Xu, X., Zhang, Y., Niu, X. & Shou, W. Genetic mapping and identification of the candidate genes for mottled rind in Cucumis melo L. Front. Plant Sci. 12, 769989 (2021).
Wang, D. et al. Fine mapping a ClGS gene controlling dark-green stripe rind in watermelon. Sci. Hortic. 291, 110583 (2022).
Liu, H. et al. CRISPR-P 2.0: an Improved CRISPR-Cas9 tool for genome editing in plants. Mol. Plant 10, 530–532 (2017).
Thorvaldsdóttir, H., Robinson, J. T. & Mesirov, J. P. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform. 14, 178–192 (2013).
He, Y. B., Zhang, T., Sun, H., Zhan, H. D. & Zhao, Y. D. A reporter for noninvasively monitoring gene expression and plant transformation. Hortic. Res. 7, 152 (2020).
Fan, Y. L. et al. A fast, simple, high efficient and one-step generation of composite cucumber plants with transgenic roots by Agrobacterium rhizogenes-mediated transformation. Plant Cell Tissue Organ Cult. 141, 207–216 (2020).
Shen, J., Zhang, Y., Havey, M. J. & Shou, W. Copy numbers of mitochondrial genes change during melon leaf development and are lower than the numbers of mitochondria. Hortic. Res. 6, 95 (2019).
Jalili, V. et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2020 update. Nucleic Acids Res. 48, W395–W402 (2020).
Acknowledgements
We gratefully acknowledge the gift of MSC16 from Drs. Grzegorz Bartoszewski and Stefan Malepszy of the Warsaw University of Life Sciences. We also thank Xueyong Yang (Chinese Academy of Agricultural Sciences) for technical help with the transformation of cucumber, and Yiqun Weng (USDA and University of Wisconsin-Madison) and Xingfang Gu (Chinese Academy of Agricultural Sciences) for providing the germplasm materials. This study was funded by the Department of Science and Technology of Zhejiang Province (Grant 2022C02032 to J.S.), the National Natural Science Foundation of China (Grant 32372692 to J.S.), the Natural Science Foundation of Zhejiang Province (Grant LY20C150005 to X.X.), the Major Science and Technology Project of Plant Breeding in Zhejiang Province (Grant 2021C02065-3 to W.S.), Special Support Plan for high-level talents of Zhejiang Province (Grant 2021R51007 to M.Z.) and ZAAS Program of Transdisciplinary Research.
Author information
Authors and Affiliations
Contributions
M.J.H., W.S., M.Z., J.S., and X.L. conceived and designed the project; J.S. and X.L. conducted most of the experiments and analysis; X.X., E.D.M., C.W., Y.Z., and Z.W. performed some field and some data analysis work; J.S. and X.L. wrote the manuscript; M.Z., E.D.M., and M.J.H. revised it.
Corresponding authors
Ethics declarations
Competing interests
Authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Sodmergen Sodmergen and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shen, J., Lyu, X., Xu, X. et al. A nuclear-encoded endonuclease governs the paternal transmission of mitochondria in Cucumis plants. Nat Commun 16, 4266 (2025). https://doi.org/10.1038/s41467-025-59568-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-59568-7