Abstract
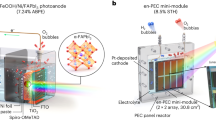
An artificial leaf mimicking the function of a natural leaf has recently attracted significant attention due to its minimal space requirement and low cost compared to wired photoelectrochemical and photovoltaic-electrochemical systems for solar hydrogen production. However, it remains a challenge to achieve a practical-size solar water-splitting device that can fulfill the criteria of a solar-to-hydrogen conversion efficiency above 10%, long-term durability, and scalability. Here, we develop 1 cm2 perovskite-based photoelectrodes using a defect-less, chlorine-doped formamidinium lead triiodide as photo-absorber and ultraviolet-insensitive tin oxide as an electron transport layers. This device is encapsulated using electrocatalyst-deposited nickel foils, which demonstrates high photocurrent density and high stability for 140 h. Ultimately, we fabricate a scalable mini-module-sized artificial leaf (16 cm2) consisting of a side-by-side/parallel configuration of photoanode and photocathode architecture integrated with a 4 × 4 array of 1 cm2 photoelectrodes, which maintains a stable ‘module-level’ solar-to-hydrogen efficiency of 11.2% in an unbiased solar water-splitting under 1-sun illumination.
Similar content being viewed by others
Introduction
Photovoltaic-electrochemical (PV − EC) and photoelectrochemical (PEC) systems are two representative techniques for solar hydrogen (H2) production with high efficiency. The PV − EC system consists of separate PV and EC components, which require much space and high installation costs for both components. The PEC system integrates these two components into a single device, but it still requires external wiring, leading to increased resistivity and decreased efficiency, especially in scaled-up systems1,2,3,4. In contrast, the natural leaf has a simple and integrated structure that performs its complex photosynthesis processes without external wires. Inspired by this, the artificial leaf is designed to mimic the natural leaf by integrating the light or photo-absorbing semiconductors and EC without external wiring5,6. As a result, this system requires less space and incurs lower costs compared with PV − EC or PEC − PV systems7. Moreover, since no external wiring is needed, low resistivity and high efficiency can be maintained even in the scaled-up artificial leaf systems8.
To commercialize this artificial leaf technology for solar H2 production9,10,11, three criteria must be satisfied: (i) high solar-to-hydrogen (STH) conversion efficiency exceeding 10%, (ii) long-term durability, and (iii) facile scalability. Particularly, the most significant challenge is maintaining the high STH efficiency with stability and scalability in a practical-size, large-area device8. Despite extensive research for more than a decade, there is still no report of artificial leaf technology that meets all three requirements stated above because metal oxide-grade semiconductors, such as TiO2, Fe2O3, BiVO4 and WO3, offer durability but lack the high efficiency, while PV-grade semiconductors, such as III-V compounds, silicon (Si), and perovskites (PSK) provide a high efficiency but are typically less durable8,12,13. Fabricating large-area photoelectrodes while maintaining high efficiency and durability is an especially critical issue because uniform thickness of semiconductor and electrocatalyst, resistivity of the substrate, and surface passivation - all of which are easier to maintain in small-area photoelectrodes. Fundamentally, the reduced PEC performance in large-area photoelectrodes is due to the solution resistance and electrolyte’s pH gradient between cathodic and anodic sides as a function of the catalyst electrode’s geometrical area, and these issues are more significant than substrate resistivity and catalyst inhomogeneity5,7,13,14. Nocera et al. introduced an artificial leaf made from a triple-junction Si solar cell integrated with catalysts on both sides without any external wire5,6,8. However, the STH efficiency of their devices was rather low (2.5–3.4%) because the low photovoltage of Si solar cell (<0.6 eV) forced them to use a triple-junction Si solar cell, and the co-catalyst deposited on the Si surface blocked the incident light5,15,16. Metal oxide-based artificial leaf demonstrated even lower STH efficiencies due to their wide bandgaps and low charge-transfer efficiencies17,18,19,20. For example, small-area devices of BiVO4-PSK wireless tandem PEC cells achieved only ~3% STH efficiency due to the low performance of BiVO417. Moreover, STH efficiency was dramatically reduced to 0.58% upon scaling-up to 100 cm2 due to uneven deposition of BiVO4 and electrocatalyst layers, as well as increased substrate’s resistance in the large-scale photoelectrode18,19,20. A PV − EC system achieved an STH efficiency of 30% using an InGaP/GaAs/GaInAs triple-junction solar cells under concentrated light21, but such efficient systems remain expensive, complicated, and yet to be scalable. A scalable particulate photocatalytic farm showed an STH efficiency of 0.76%22 under normal light and required a concentrated light to reach 9%23. In this regard, PEC systems, especially of artificial leaf-type configurations, are efficient20, promising, and favorable for scale-up without much loss in efficiency24.
To achieve the high STH efficiency in artificial leaf based PEC devices, organic-inorganic hybrid metal-halide perovskites (MHP-PSK)25,26 are promising photoelectrode materials due to their ideal and optimal bandgap range of 1.23–2.0 eV for achieving a high photovoltage of 1.0–1.2 V27,28 and a theoretical STH efficiency limit of 27.9%29,30,31,32. These materials offer a high charge transfer properties, and easily tunable band positions to locate the conduction band more negative than the potential of 0 V for H2 evolution, while the valence band is more positive than the potential of 1.23 V for O2 evolution by changing the composition of A, B, and X sites in ABX3-based MHP-PSK materials18,20,33,34,35. However, the intrinsic instability problem of MHP-PSK is critical in water and under light illumination, and scaling up of these devices while maintaining the high STH efficiency still remains challenging36,37. Many PSK-based PEC systems have been reported recently24,36,38,39,40, including individual photoelectrodes37,39,40,41,42,43,44 and unbiased PEC devices36,37,40, yet very few are artificial leaves36,40,45,46, rarely focused on stability41,47 as well as scalability24,37,39. For example, an all-PSK PEC system achieved a STH efficiency of 13.4%, yet without considering further scale-up38. Another PSK-based stand-alone scalable PEC devices (4 cm2), using a 4 × 4 array of 0.25 cm2-sized total 16 small-cells, showed an STH efficiency of 3.54%37. A large-area unbiased PSK-based PEC system39 achieved a STH efficiency of >9% at 64 cm2 but showed an STH efficiency of 3.09% when scaled-up to 100 cm2. Although it was not an artificial leaf, our recent work24 demonstrated a good scalability of FAPbI3 photoelectrode area from 0.25 to 123.2 cm2 with minimal efficiency loss, but the STH efficiency still remained just below 10% threshold, and the device also presented an intrinsic stability problem due to titanium oxide (TiO2) as an electron transfer layer (ETL) degrading the PSK layer under UV irradiation.
Here, we present an MHP-PSK-based artificial leaf that satisfies all three requirements outlined above. Our artificial leaf design incorporates the photoelectrodes made from high-efficiency, durable, and large-area PSC featuring a chlorine (Cl)-doping in tin oxide (Cl:SnO2) ETL and Cl-doping in formamidinium lead triiodide (Cl:FAPbI3) as PSK photo-absorber, as per our reported work48. The synergistic interaction between Cl:SnO2 and Cl:FAPbI3 enhances both the phase stability and efficiency through passivating defect sites at the ETL/PSK interface, with acetylcholine chloride (ACCl) serving as a stabilizer (Cl-dopant) for α-FAPbI3 to improve long-term stability48. Furthermore, Cl:SnO2 as ETL is expected to improve electron transport and enhance FAPbI3 PSK’s stability under UV irradiation compared to TiO2 as ETL. We protect the Cl:FAPbI3 thin-film from water permeation by using an encapsulation layer of Ni foil and Ag-coated poly-methyl methacrylate (PMMA) particles as an ohmic or electrical contact between Cl:FAPbI3 layer and Ni foil. We apply nickel-iron-cobalt (NiFeCo) oxyhydroxide as an oxygen evolution catalyst (OEC) to improve the stability of PEC water-splitting reaction performance of the photoanode while measuring in an O-ring type PEC reactor, where only NiFeCo/Ni anode keeps exposed to the electrolyte while epoxy bond and PSK layers are isolated from direct contact with the electrolyte. Such O-ring PEC reactors have been employed previously to stabilize the MHP-PSK photoelectrodes24,26,49. Notably, this photoelectrode with an active illumination area of 1 cm2 demonstrates a durable performance for 140 h, maintaining 99% of its initial performance until that point. In addition, FAPbI3 is not degraded, and no lead (Pb) leaching occurs after the long-term durability test. The result demonstrates that a durable photoelectrode could be fabricated by improving the intrinsic stability of PSK phase and robust encapsulation of the material to prevent electrolyte permeation and subsequent degradation. A Cl:FAPbI3-based photocathode is also fabricated similarly with a Co9S8–MoS2-Pt based hydrogen evolution catalyst (HEC). Note that the OEC in our artificial leaf does not use expensive Pt-group metals like Ir or Ru, and HEC uses only a minimal amount of Pt (~0.1 wt%) but still show a high STH efficiency >10%. Finally, a scalable mini-module-sized artificial leaf is demonstrated by confined integration of optimized photoanode and photocathode sub-cells in a 4 × 4 array (16 cm2) and achieved a ‘module-level’ STH efficiency of 11.2%.
Device engineering of FAPbI3 photoanodes
We selected intrinsically stable FAPbI3 as the PSK photo-absorbing layer, a crystalline semiconductor, which has recently gained wide usage for photovoltaics (PV) and can be fabricated using scalable and low-cost synthetic methods48,50. FAPbI3 exhibits an intrinsic stability and efficiency due to its higher crystallinity with fewer lattice defects, relatively smaller bandgap, higher thermal stability, and longer carrier diffusion length than to the more frequently used MAPbI3 PSK51,52,53. Accordingly, Cl-doped and undoped FAPbI3 devices were prepared using an n-i-p structured FAPbI3 thin-film with either Cl:SnO2 or TiO2 as ETLs (Fig. 1a; See Methods)24. The Cl-doping in FAPbI3 PSK layer and SnO2 ETL was optimized individually, and their optoelectronic, surface, and solar properties were analyzed (Fig. S1–S3, Supplementary Note S1). Figure 1b and Fig. S4a show the cross-sectional scanning electron micrographs (SEM) of Cl:FAPbI3 thin-films fabricated on top of the Cl:SnO2 and TiO2 as an ETLs, respectively.
a Device structure of n-i-p configured FAPbI3 thin-film photoanode with functional layers including Au layer as top electrode, Spiro-OMeTAD as HTL, Cl:FAPbI3 as a photo-absorbing PSK layer with Cl:SnO2 as ETL and NiFeCo OEC layer. The structure of photoanode is copper wire/FTO/Cl:SnO2/Cl:FAPbI3/Spiro-OMeTAD/gold/Ni foil/NiFeCo. White arrows indicate the hole transport from the HTL to NiFeCo/Ni foil via gold layer. b High-resolution (HR) cross-sectional SEM image of n-i-p structured thin-film device with functional layers including Au layer as top electrode, Spiro-OMeTAD as HTL, Cl:FAPbI3 as PSK layer, and Cl:SnO2 as ETL. c HR- TEM image of NiFeCo/Ni foil surface. d Jph-E curves of Ni/Cl:FAPbI3(TiO2), NiFeCo/Ni/Cl:FAPbI3(TiO2) (green color), Ni/Cl:FAPbI3(Cl:SnO2), and NiFeCo/Ni/Cl:FAPbI3(Cl:SnO2) (blue color) photoanodes measured in 1 M KOH electrolyte at a scan rate of 10 mV s−1. e Jsc-E curves of Cl:FAPbI3 PV cells with Cl:SnO2 (blue color) and TiO2 (green color) as ETLs. f Charge transport, photophysical properties and charge transfer kinetics of FAPbI3 photovoltaics using Cl:SnO2 (blue color) and TiO2 (green color) as ETLs. Photoluminescence (PL) measurement of FAPbI3 films with Cl:SnO2 (blue color) and TiO2 (green color) as ETLs. Here ETL/Cl:FAPbI3 films were prepared on FTO glass. g–j Statistics solar performances represented in box-plot and overlap graph showing JSC, VOC, FF, and PCE of Cl:FAPbI3 PV cells using TiO2 and Cl:SnO2 as ETLs. Here blue box, Cl:SnO2 as ETL, green box, TiO2 as ETL; for both ETLs, the box represents 25–75% standard deviation; bottom line, median; dark green full circle, mean; whiskers, outliers with 1.5 as coefficient. In the statistical error bars, more than 15 devices were used for measurements of each ETL.
The Cl:FAPbI3 thin-films using both ETLs (TiO2 vs. Cl:SnO2) exhibit a well-compacted interface between each layer without altering the absorbance, bandgap, and external quantum efficiency (Fig. S4b–d, Supplementary Note S2). The NiFeCo OEC was synthesized in situ on a Ni foil using an all-solution-based deposition method involving metal-nitrate precipitation-PMND (See Methods)54. Here, NiFeCo was chosen as OEC due to its superior OER performance compared to unary and binary catalysts of these elements. The PMND method54 was selected for its cost-effectiveness, simplicity, and ability to synthesize amorphous metal oxides in a single-step process under ambient conditions, offering precise compositional control without the need for complex deposition techniques. High-resolution transmission electron micrographs (HR − TEM) (Fig. 1c, Fig. S5, Supplementary Note S3) verify the formation of lattice fringes (inset image) in multi-directions showing d-spacings of 0.2435 nm and 0.2135 nm corresponding to (111) and (103) planes of NiFeCo catalyst surface. Uniform elemental distribution of Ni, Fe, and Co is observed in the HR-Energy-Dispersive X-ray Spectroscopy (EDS) images (Fig. S5a). As expected, X-ray photoelectron spectroscopy (XPS) reveals that the NiFeCo oxyhydroxide catalyst contains the oxidation states of Ni2+, Fe3+, and Co2+ (Fig. S6, Supplementary Note S4). The NiFeCo/Ni foil electrode shows the higher performance, and superior charge transport than the those of Ni foil itself, and NiFeCo/Ni foil fabricated using a conventional hydrothermal method in 1 M KOH55,56,57 (Fig. S7, Supplementary Note S5, Table S1). To fabricate the photoelectrode, this NiFeCo as OEC deposited on Ni foil was integrated with Cl:FAPbI3 thin-film using Ag-coated poly-methyl methacrylate (PMMA) particles, which served as a transparent conductive adhesive layer and formed an ohmic junction (denoted as NiFeCo/Ni/Cl:FAPbI3(Cl:SnO2 or TiO2) photoanodes; See Methods; Fig. S8).
To highlight the advanced features of our artificial leaf device, we conducted some comparative experiments with specially designed control devices. First, the PEC performance comparison of NiFeCo/Ni/FAPbI3(Cl:SnO2) and NiFeCo/Ni/Cl:FAPbI3(Cl:SnO2) photoanodes were investigated the effect of Cl-doping into the crystal lattice of FAPbI3. As shown in Fig. S9, the fill factor (FF) was dramatically enhanced upon Cl-doping, along with an increase in photocurrent density. This improvement in PEC performance is attributed to the bulk and surface passivation of the FAPbI3 photoanode via Cl-doping, wherein ACCl was introduced in the solution precursor to stabilize α−FAPbI3 phase and passivate the defect sites located at grain boundaries. Subsequent surface passivation using ACCl and 4-methoxy-phenethylammonium iodide (MeO-PEAI) further improved the performance by suppressing undesired recombination losses48. We found that Cl-doping into FAPbI3 layer is also effective in enhancing the performance of PEC water-splitting cells, similar to its impact on solar PV cells. Next, we compared the PEC performance of Cl:FAPbI3-based photoanodes with different ETL layers of Cl:SnO2 and TiO2. The NiFeCo/Ni/Cl:FAPbI3(Cl:SnO2) photoanode demonstrated a plateau photocurrent of 24.3 mA cm−2 at 1.23 VRHE in water photooxidation and achieved an applied bias photon-to-current conversion efficiency (%ABPE) of 12.7%, which is approximately 1.7 times higher than that of NiFeCo/Ni/Cl:FAPbI3(TiO2) (Fig. 1d, Fig. S9a, and Table S2). The incident-photon-to-electron conversion efficiency (%IPCE) and integrated photocurrent of both photoanodes (Fig. S9b) reveal an absorption edge at 827.6 nm, aligning the consistency with that of Cl:FAPbI3 PV thin-films (Fig. S4b). The NiFeCo/Ni/Cl:FAPbI3(Cl:SnO2) photoanode also shows better PEC performance than NiFeCo/Ni/Cl:FAPbI3(TiO2) in the wavelength range of 450–800 nm. Improvements in Jsc, Voc, FF, and PCE (%) (Fig. 1e) can be attributed to the relatively faster quenching of electrons from the PSK layer to the ETL when utilizing Cl:SnO2 ETL, as observed in photoluminescence (PL) measurements of Cl:FAPbI3(Cl:SnO2) films (Fig. 1f)58, which enables a more efficient charge transportation compared to that of Cl:FAPbI3(TiO2) films. Since the figures of merit (FoM) parameters (Jph, and Eon) of PEC photoanode are related to the corresponding solar cell performance metrics (Fig. 1g–j, Fig. S10, Supplementary Note S6) of PV cells59, the formation of a coherent interlayer between the Cl:FAPbI3 layer and Cl:SnO2 ETL60 greatly enhances the electron extraction and plays a significant role in efficient PEC charge transport (Fig. S11) compared to the TiO2 ETL (Fig. 1d, e) of the similar devices.
Stabilization of FAPbI3 photoanodes
The intrinsic instability of MHP-PSK materials under highly humid conditions with continuous light illumination poses a critical issue in PV applications, which becomes more serious in PEC water-splitting applications, where the device has to be operated in an electrolyte. The bare FAPbI3 photoanode without Ni encapsulation lost its activity within a few minutes (red curve; Fig. S12), whereas Ni/Cl:FAPbI3(TiO2) and Ni/Cl:FAPbI3(Cl:SnO2) photoanodes exhibited stability for 40 h (until t90: assigned as the time until the device retains 90% of the initial performance) under sulfite oxidation conditions (Fig. S12). The slightly lower photocurrents and stability of Ni/Cl:FAPbI3(TiO2) could be attributed to the photoactivity of UV-sensitive TiO2 ETL, which creates deep charge trapping and recombination sites and makes the device less stable compared to SnO2 ETL under sunlight conditions60,61,62. Furthermore, as reported in previous studies63,64, Cl:SnO2 ETL can more easily form a stable interface with Cl:FAPbI3 due to the presence of Sn2+, halides, and organic cations60. This emphasizes the impact of proper ETL selection in determining the performance and stability of the photoanodes. We further dig deeply into why the stability of NiFeCo/Ni/Cl:FAPbI3(Cl:SnO2) is dependent upon the choice of Cl:SnO2 ETL in 1 M KOH without any sacrificial agent (such as sulfite). There are three major degradation mechanisms for destabilizing the PSK-based photoelectrodes24,65: (i) chemical instability due to water that can permeate into the encapsulated PSK layer, (ii) photocorrosion caused by the accumulation of the surface-reaching holes, and (iii) a photo-instability of PSK layer by UV irradiation. Accordingly, we performed the chemical, photocorrosion, and photostability tests under simulated sunlight.
In all three stability tests, the NiFeCo/Ni/Cl:FAPbI3(Cl:SnO2) photoanode exhibited higher photocurrent density ( Jph) than the NiFeCo/Ni/Cl:FAPbI3(TiO2) photoanode (Fig. 2a–c, Supplementary Note S7). Both photoanodes remained stable in chemical stability tests, attributed to effective encapsulation with Ni foil (Fig. 2a). During photocorrosion stability test under water oxidation conditions, the NiFeCo/Ni/Cl:FAPbI3(TiO2) photoanode was significantly less stable than NiFeCo/Ni/Cl:FAPbI3(Cl:SnO2), as accumulated holes corroded the PSK layer (Fig. 2b). However, the surface-reaching holes are readily consumed in sulfite oxidation on any surface, and thus both photoanodes demonstrated good stability in the photocorrosion test conducted with a sacrificial agent (0.2 M Na2SO3). Finally, the NiFeCo/Ni/Cl:FAPbI3(Cl:SnO2) photoanode exhibited significantly better photo-stability, as it showed a negligible loss during the 10-h photochemical stability test under UV light (Fig. 2c). Thus, the three stability tests unanimously demonstrate the superior photo-activity as well as stability of Cl:SnO2 ETL, confirming its efficacy for PEC water-splitting applications.
a Chemical stability (J–E) curves showing LSV plots of NiFeCo/Ni/Cl:FAPbI3(TiO2) (green color) and NiFeCo/Ni/Cl:FAPbI3(Cl:SnO2) (blue color) photoanodes measured in 1 M KOH (pH 14) electrolyte after immersing them in the electrolyte for continuous 5 days in dark and recording the J–E curves in light once per day. b Photocorrosion was measured with and without a sacrificial agent (0.2 M Na2SO3) to see if there was charge-accumulation at the surface without being consumed. Photocorrosion tests were conducted at applied voltage with highest %ABPE, i.e., 0.78 VRHE for NiFeCo/Ni/Cl:FAPbI3(TiO2) (green color) and 0.64 VRHE for NiFeCo/Ni/Cl:FAPbI3(Cl:SnO2) (blue color) photoanodes. c Photostability under continuous illumination with and without a UV cut-off filter. These three stability tests were conducted in a PEC reactor without O-ring. d Extended long-term stability measurements of NiFeCo/Ni/Cl:FAPbI3(TiO2) (green color) and NiFeCo/Ni/Cl:FAPbI3(Cl:SnO2) (blue color) photoanodes at 1.23 VRHE for 140 h. The measurement was performed in an O-ring type PEC reactor (inset photographs) where only NiFeCo/Ni anode is exposed to electrolyte while epoxy bond and PSK layers do not directly contact with electrolyte. Light was illuminated from the backside of the O-ring type PEC reactor. e Absorbance spectra of the NiFeCo/Ni/Cl:FAPbI3(Cl:SnO2) photoanode measured before and after the stability test. The absorbance measurement was conducted through diffusive reflectance spectroscopy (DRS) from back side (PSK side) in wavelengths of 550–875 nm, because the front side is integrated with catalyst. f Stability and photocurrent density of our NiFeCo/Ni/Cl:FAPbI3(Cl:SnO2) photoanode compared with reported state-of-the-art PSK photoanodes (FeNi/Ni/FAMACsPbIBr346, NiFe/Ni/CCP/FAMAPbI376, NiFe/Ni/carbon/FAMAPbI359, Ni/Graphite/FACsPbBrI377, Carbon/Ag/MAPbI378, Ni/Ag/MAPbI379). The “tx” in graph indicates the time till x% of the photocurrent generation is retained (See Table S4 for detail). All PEC measurements were conducted using a 3-electrode set-up at a scan rate of 10 mV s−1 in 1 M KOH (pH 14) unless specified otherwise under the 1-sun (AM 1.5 G) illumination.
The advantage of Cl:SnO2 over TiO2 as ETL was much more pronounced in a 140-h long-term stability test comparing NiFeCo/Ni/Cl:FAPbI3(Cl:SnO2) and NiFeCo/Ni/Cl:FAPbI3(TiO2) photoanodes (Fig. 2d). To prevent the encapsulant dissolution during prolonged sunlight exposure (AM 1.5 G, 1-sun condition), this long-term stability test was conducted in an O-ring type PEC reactor, which was designed in a such a way that only electrocatalyst is exposed to electrolyte while PSK and epoxy are prevented from direct contact with electrolyte (inset photographs of Fig. 2d). After 140 h of continuous water oxidation operation, NiFeCo/Ni/Cl:FAPbI3(Cl:SnO2) photoanode retained 99%, while the NiFeCo/Ni/Cl:FAPbI3(TiO2) photoanode retained only 60% of its original performance. This result indicates that the stability of Cl:FAPbI3 photoanode can be improved greatly by using the Cl:SnO2 ETL layer. This improvement can be attributed to trap sites, which might be created in the TiO2 ETL-based Cl:FAPbI3 photoanodes during prolonged illumination, thus hindering the charge transport towards the NiFeCo/Ni surface due to enhanced recombination. Gas productivity of 169.8 μmol cm−2 of O2 was quantified with gas-chromatography (GC) analyses and its corresponding faradaic efficiency (%FE) of >90% was measured during a 1-h operation (Fig. S13).
After the PEC-stability test, the material states of the PSK and catalyst layers were analyzed (Figs. S14, 15 and Supplementary Note S8). The cross-sectional FE-SEM images of FAPbI3 thin films (Fig. S14b, c) confirmed that use of the O-ring type PEC reactor can prevent direct interaction of organic components of epoxy with permeated electrolyte during long periods of reaction. However, after 140 h, the slight performance loss was mainly observed in fill factor (FF) of the photoanode, as shown in LSV curves data (Fig. S14a). A minor shift near the absorption edge was also observed in the absorbance spectra before and after the PEC-stability test for the NiFeCo/Ni/Cl:FAPbI3(Cl:SnO2) photoanode (Fig. 2e), which is mainly attributed to the ion migration originating in the PSK films66,67. After 140 h of PEC stability test, no significant morphological changes were observed in the FE-SEM analyses of the catalyst layer (Fig. S15a–d), indicating that the NiFeCo is a potential robust OEC candidate that avoids the accumulation of the holes and withstands possible photocorrosion during the long period of reactions (Fig. 2d). This also co-relates with inductively coupled plasma-optical emission spectroscopy (ICP-OES) analyses, which revealed negligible leaching of catalyst metals (Table S3) as well as minimal lead (Pb) leaching from the NiFeCo/Ni/Cl:FAPbI3(Cl:SnO2) photoanode. Overall, the NiFeCo/Ni/Cl:FAPbI3(Cl:SnO2) photoanode exhibits long-term durability, retaining 99% of the initial activity, and demonstrates good PEC performances in terms of Jph, Eon and %ABPE, as summarized in Fig. 2f and Table S4. Notably, this performance and durability achieved with relatively large-area photoelectrodes (>1 cm2) are comparable to those of reported conventional MHP-based small photoelectrodes («1 cm2).
Scalable mini-module-sized artificial leaf for solar H2 generation
Fabricating a scalable mini-module-sized artificial leaf device that maintains both high STH efficiency and high stability is critical for realizing the practical application of artificial leaves as discussed above. Here we demonstrate a scalable mini-module-sized artificial leaf by interconnecting a 4 × 4 array of 1 cm2 sub-cells (total area; 16 cm2) made of 8 photoanodes and 8 photocathodes (Fig. 3a). A photocathode was fabricated in a similar manner to the photoanode demonstration, except that Co9S8–MoS2 (CoSMoS) or Pt-doped CoSMoS (CoSMoS–Pt, ~0.1 wt% Pt) was used as an HER catalyst instead of OER catalyst. The performance of HER catalyst was benchmarked after optimizing the CoSMoS–Pt catalyst (Fig. S16-S17, Supplementary Notes S9, S10) and conducting electrochemical analyses (Fig. S18, Supplementary Note S11, Table S5)68,69,70. Detailed fabrication and PEC characterization of CoSMoS−Pt loaded Cl:FAPbI3(Cl:SnO2) photocathode are described in Methods section (Fig. S19, Supplementary Note S12), and their benchmarked PEC performance (Fig. 3b, Table S6) are discussed in Supplementary Information (Fig. S20, Supplementary Note S13).
a Schematic of a large mini-module-sized artificial leaf (16 cm2) prepared by 4 × 4 array design using sub-cells (each size of 1 cm2) containing 8 photoanodes and 8 photocathodes. White arrows indicate the hole-transport from HTL to NiFeCo/Ni foil via gold layer for photoanode, while electron-transport from FTO to Ni Foil/CoSMoS–Pt via In-Sn alloy for photocathode. b J–E plots for Cl:FAPbI3(Cl:SnO2)/Ni/CoSMoS−Pt (orange color), Cl:FAPbI3(Cl:SnO2)/Ni/Pt (black color) and Cl:FAPbI3/Ni (cyan color) photocathodes. c Maximum possible STH efficiency (operating point) from J–E curves of photoanode vs. photocathode connected in series as wired PEC device (1 cm2 each device) measured in 3-electrode set-up. d Photographs of our large-area mini-module. e Gas evolution from the mini-module-sized artificial leaf (16 cm2). f State-of-art STH efficiency of reported artificial leaves with different illumination areas of photoelectrodes, including all-perovskite based (12.5%40, 11.5%36, 6.7%80), all metal-oxide based (0.21%19, 0.8%81, 1.1%82, 3%17, 3.7%83), all-silicon based (4.7 and 6.2%5), all III-V class based (6%84, 11.2%85). For details of each point, see Table S8.
Before scaling-up the artificial leaf (“wireless” configuration), we tested the feasibility of unassisted solar H2 production using “wired” configurations, where each 1 cm2 sub-cell of the PSK photoanode (NiFeCo/Ni/Cl:FAPbI3(Cl:SnO2)) and photocathode (Cl:FAPbI3(Cl:SnO2)/Ni/CoSMoS–Pt) was connected in series using external wires (Fig. S21, Supplementary Note S14). We used the champion device of Cl:FAPbI3(Cl:SnO2)/Ni/CoSMoS–Pt photocathode, which had a positive Eon of ~1.1 VRHE and sufficient high Jph of −24.5 mA cm−2 at 0 VRHE, which is capable of efficient proton reduction during water-splitting (Fig. 3b, Fig. S20b–d, Table S7).
The optimized “wired” photoanode–photocathode configuration for unassisted PEC-OWS systems (comparing undoped vs. doped FAPbI3, small- vs. large-cells, and binary vs. ternary electrocatalysts, Fig. S22a–c) achieved a maximum STH efficiency of 12.67% in 1 M KOH in a 3-electrode set-up (Fig. 3c, Fig. S22d, Supplementary Note S15), and 12.17% STH efficiency in a 2-electrode set-up in >14 h of continuous operation (Figs. S22e, f, S23a,b). The H2-O2 gas productivity of 354.1 ± 30.1 and 169.2 ± 10.8 μmol cm−2 was measured during stable operation of 1 h, which corresponded to an STH efficiency of 12.1% (Fig. S23c, Supplementary Note S16). To scale-up the sub-cell size PEC-OWS system into a panel-type artificial leaf architecture (to a mini-module-sized device >10 cm2), we strategically increased the FAPbI3 photoanode size from 1 cm2 up to 16 cm2 by applying step-wise fabrication using (i) metal grid line design (Fig. S24a) and (ii) multi-cell array of 1 cm2 sub-cells (Fig. S24b–e). We also developed large photoanodes and photocathodes (2 and 4 cm2) composed of two units of sub-cells (1 × 2 and 2 × 2 cm2), which had to be connected with each other in series to generate sufficient photovoltage (>2 V) for unassisted PEC-OWS (Figs. S24–S26, Supplementary Note S17).
In the experimental scale-up study (Figs. S24–S27), the J–E curves show Jph of 22.9 mA cm−2 for the sub-cell photoanode (1 cm2) and 20.25 mA cm−2 for the large photoanode (8 cm2) at 1.23 VRHE (Fig. S27d), which is very same as that of the small sub-cell (0.25 cm2, 24.3 mA cm−2 at 1.23 VRHE; Fig. 2d; Fig. S27d). This indicates only ~6% of efficiency loss upon upscaling the unit cell size, demonstrating a good scalability. This is because the large-device made by arraying the small sub-cells possesses a low surface resistance compared to the devices fabricated on a single large-area substrate with high surface resistivity.
Multi-cells (8 sub-cells) required parallel connections to enlarge the photoanodes and photocathodes (Figs. S24, S25), and series connections between them to obtain sufficient photovoltage (~2 V). This ensures that efficient charge transport can be realized from each other in both photoelectrodes (Fig. S26). Therefore, a combination of series and parallel connections is necessary to optimize photovoltage and efficient charge transport in large-area photoelectrode devices. Our mini-module-sized artificial leaf (8 photoanodes and 8 photocathodes; total 16 cm2, Fig. 3d) exhibited >10% of module-level STH efficiency, along with H2 and O2 gas production in a 2:1 molar ratio (Fig. 3e, Supplementary Movie S1). As shown in Fig. 3f, this performance places our artificial leaf among those reported devices with STH efficiency of >10% at sizes of >10 cm2).
Discussion
We demonstrated a scalable and durable mini-module-sized artificial leaf device (16 cm2) of a STH efficiency exceeding 10%, using defect-less Cl-doped FAPbI3, UV-insensitive Cl-doped SnO2 ETL, and multi-component cocatalyst layers comprising of NiFeCo OEC and CoSMoS−Pt HEC for OER and HER, respectively. This benchmark study demonstrates a successful scale-up of photoelectrodes (more than 10 times increase in illumination area) with a minimal efficiency loss of <10%, which is indeed essential requirement for the commercialization of artificial leaf technology. Because our scaled-up mini-module-sized artificial leaf is based on a modular design, the STH efficiency would be almost the same when it is further scaled-up to meter-scale devices, which is equivalent size of commercial solar panels. In addition to achieving a high STH conversion efficiency of >10% target, the most remarkable feature of our artificial leaf is its high durability, which is one of the critical requirements for a commercial device. This durability was achieved through Cl-doping of FAPbI3 and adoption of Cl:SnO2 ETL, both synergistically enhanced an intrinsic stability of the photo-absorbing PSK layer. Additionally, the potential device degradation failure from encapsulant dissolution was avoided by using an O-ring type PEC reactor, which exposes only the electrocatalyst to the electrolyte while PSK and epoxy are not in direct contact with electrolyte. From device engineering perspective, the side-by-side (parallel-configured photoanode–photocathode) setup offers the advantage of using the same electrolyte, reducing resistance due to pH gradient. To optimize this configuration, we tailored both OER and HER catalysts for PEC-OWS operation in alkaline media (pH 14) and carefully selected individual PSK sub-cells with similar performance, resulting in enhanced PEC efficiency. However, some challenges remain, such as (i) establishing an efficient conductive pathway for proton transport between the anode and cathode, and (ii) separating difficulty in the generated hydrogen and oxygen on the same side, requiring a membrane or external gas separator. A potential solution is to adopt a tandem PEC structure or membrane-integrated design facilitating easier gas separation even in the side-by-side setup.
Methods
Chemicals and materials
The chemicals and materials received from suppliers were as per follows: lead(II) iodide (PbI2, 99.99%, TCI Chemicals), 4-methoxy-phenethylammonium iodide (MeO-PEAI, 99%, Greatcell Solar Materials), urea (CO(NH2)2, 99%, ACS), tin(II) chloride dihydrate (SnCl2·2H2O, 98%, Acros), deionized (DI) water (Alfa Aesar), hydrochloric acid (HCl, 37 wt% in H2O, Sigma Aldrich), thioglycolic acid (C2H4O2S, 99%, Sigma Aldrich), acetylcholine chloride (ACCl, 98%, TCI Chemicals), formamidinium iodide (FAI, 99.99%, Greatcell Solar Materials), N,N-dimethylformamide (DMF, >99.8%, Sigma Aldrich), dimethyl sulfoxide (DMSO, 99.9%, Sigma Aldrich), 2-propanol (IPA; 99.5%, Sigma Aldrich), chlorobenzene (CB, 99.8%, Sigma Aldrich), 2-methoxyethanol (2-ME, 99.8%, Sigma Aldrich), ether (99%, Samchun) and ethyl alcohol (EtOH, 99.9%, Samchun), conductive glass substrate of fluorine doped tin oxide (FTO), (Asahi Glass; 12–13 Ω cm−2). Main electrolytes (1 M KOH with pH 14, 0.5 M H2SO4 with pH 1) were purchased from Samchun. Additionally, 1 M KOH + 0.2 M sodium sulfite (Na2SO3) as a sacrificial electrolyte, 1 M potassium borate (KBi with pH 9.1) and 0.1 M potassium phosphate (KPi with pH 7) were prepared to adjust the desired level of pH.
Synthesis of FAPbI3 perovskite inks
Undoped FAPbI3 powder and its corresponding inks, TiO2 inks and HTL inks were prepared according to our previous reports including PV cell works by Min et al.71,72, Yoo et al.48,50 and a PEC study by Hansora et al.24.
Undoped FAPbI3 powder
Briefly, 20 mg of formamidine acetate (99%, Alfa Aesar) was reacted in 30 mL of hydroiodic acid (57 wt%, Sigma Aldrich) at 60 °C at 1 mbar for 1 h with continuous stirring. After the reaction, the solvent was evaporated for 1 h at 60 °C, yielding a white powder of FAI. Then product was dissolved first in ethanol and then recrystallized using diethyl ether followed by drying for 24 h in a vacuum oven at room temperature. The resulting FAI powder (19.5 mmol) was mixed with PbI2 (99.99%, TCI Chemicals) and 11 mL of 2-ME with 1:1 molar ratio and stirred continuously at 120 °C. A black precipitate of formamidinium lead triiodide (FAPbI3) was obtained via a retrograde method. The black powder was then filtered and annealed at 150 °C for 30 min.
Undoped FAPbI3 ink
FAPbI3 ink was formulated using freshly obtained black powder with a concentration of 889 mg mL−1 in 8:1 v/v ratio of DMF:DMSO.
HTL ink
For preparing HTL ink, 39.5 µL 4-tert-butylpyridine (t-BP, Sigma Aldrich), 23 µL bis(trifluoromethane)sulfonimide lithium salt (LiTFSI, 99.95%, Sigma Aldrich) with a concentration of 520 mg mL−1 in acetonitrile (CAN, 99.8%, Sigma Aldrich), and 10 µL Co(III)-TFSI (FK209, Lumtec Corp.) with a concentration of 375 mg mL−1 in ACN and 1 mL Spiro-OMeTAD (Lumtec Corp.) with a concentration of 90 mg mL−1 in anhydrous chlorobenzene were all mixed together in a vial.
TiO2 paste
The TiO2 paste (SC-HT040, ShareChem) was prepared by mixing 2-ME with terpineol in 3.5:1 w/w ratio with 0.2 M of titanium diisopropoxide bis(acetylacetonate) [Ti(acac)2, 75 wt% in IPA, Sigma Aldrich].
Chlorine-doped FAPbI3 and SnO2 inks
Cl:FAPbI3 precursor ink was prepared by mixing 1.4 M FAPbI3 in 40 mol% of methylammonium chloride (MACl) with adding 0.3 mol% of acetylcholine chloride (ACCl, 98%, TCI Chemicals) in a DMF:DMSO (v/v ratio of 8:1) solution as per our previous works48,71. To prepare Cl-doped SnO2 ink by chemical bath deposition (CBD) method, as per our work60, 14.2 mM (0.108 g) of SnCl2·2H2O (98%, Acros) and 500 mg of urea (CO(NH2)2; 99%, ACS) were dissolved into 40 mL of DI water, 10 μL of thioglycolic acid (C2H4O2S; 99%, Sigma Aldrich), and 500 μL of HCl (37 wt% in H2O, Sigma Aldrich). For the undoped SnO2 layer, a commercial solution (15% SnO2 in H2O colloidal dispersion, Alfa Aesar) of tin(iv) oxide was diluted further in H2O with w/w ratio of 1:5 before its further use for ETL deposition.
Fabrication of FAPbI3 thin-films
Undoped FAPbI3 thin-films
As per PV cell works by Min et al.71,72, Yoo et al.48,50 and a PEC work by Hansora et al.24, we fabricated the undoped FAPbI3 thin-films without using any chlorine precursor. A compact blocking layer of TiO2 (bl-TiO2) was deposited on FTO glass of 2.5 cm × 2.5 cm or 6.25 cm2 (TEC 8, 10–15 Ωcm−1, Pilkington) by depositing a 0.02 M Ti(acac)2 (75 wt% in IPA, Sigma Aldrich) ink by using a spray-pyrolysis method at 450 °C to avoid contact between HTL and FTO. A 200 nm mesoporous TiO2 (50 nm anatase particles) layer was deposited by spin-coating at 1500 rpm for 40 s using SC-HT040 paste (ShareChem) in a solvent mixture of 2-ME:terpineol (w/w ratio of 3.5:1), followed by calcination at 500 °C for 1 h. The FAPbI3 layer was deposited on the TiO2 thin-film and annealed at 150 °C for 10 min, and then at 100 °C for 10 min. The surface passivation was done by spin-coating the 0.015 M of PEAI (98%, Greatcell Solar) solution in 75% IPA at 5000 rpm, followed by vacuum drying for 24 h or annealing at 100 °C for 5 min. The HTL (Spiro-OMeTAD) was deposited by spin-coating at 3000 rpm for duration of 30 s. Finally, the gold layer was deposited as a top electrode by using the thermal evaporation, while the indium layer was soldered as a bottom electrode onto FTO for proper ohmic contact.
Cl-doped FAPbI3 thin-films
The FAPbI3-based PV solar cell with a device structure of FTO/TiO2/Spiro-OMeTAD/Au and Cl:SnO2/Cl:FAPbI3/Spiro-OMeTAD/Au were fabricated as follows. First, the electron transport layers (ETLs) were deposited on the etched and UV-ozone-treated FTO glass substrate. TiO2 and Cl:SnO2 inks were used for fabricating ETLs. For the compact bl-TiO2 layer, a TiO2 ink (diluted Ti(acac)2 in EtOH) was first coated by spray pyrolysis deposition at 450 °C. The mp-TiO2 layer was next deposited on layers of bl-TiO2/FTO substrate. Mesoporous TiO2 solution (TiO2 paste dissolved in 2-ME:terpineol with 3.5:1 w/w ratio) was deposited by spin-coating at 1500 rpm and then calcined at 500 °C for 1 h.
The CBD method60 with SnCl2-containing inks as discussed earlier was utilized for the deposition of the Cl-doped SnO2 layer. The FTO substrate was kept inclined while immersing in the SnO2 solution at 70 °C for a 6-h reaction. After successful deposition of SnO2 layer, these films were annealed at 150 °C for 1 h, and an additional layer of 10 mM of KCl dissolved in DI water was deposited by spin-coating at 3000 rpm for 30 s and annealed at 100 °C for 10 min. The Cl:FAPbI3 layer was deposited onto the ETL by spin-coating the Zwitterionic acetylcholine cation (AC+) and Cl− anions or AC+Cl− stabilized α-FAPbI3 (ACCl-FAPbI3 or Cl:FAPbI3) ink48,71 on Cl-doped SnO2 layer at 1000 rpm for 10 s and 5000 rpm for 15 s, while dropping the 1 mL ink of ethyl ether during the spin coating. These PSK layers were then annealed at 120 °C for 1 h. A 4.5 mM of ACCl and 0.016 M of MeO-PEAI in IPA solutions was sequentially deposited by spin-coating at 5000 rpm, followed by annealing at 100 °C for 5 min48.
The non-passivated pure FAPbI3 was deposited in the same way without additive ACCl in PSK precursor, and without any PSK surface passivation by ACCl or sequential treatment by MeO-PEAI. A mixture of Spiro-OMeTAD solution (90 mg mL−1 dissolved in CB) containing 39.5 µL of t-BP, 23 µL of Li-TFSI salt (520 mg mL−1 dissolved in ACN), and 10 µL of Co-TFSI salt (375 mg mL−1 dissolved in ACN) was deposited by spin-coating at 3000 rpm for 30 s as HTL onto the PSK films, followed by thermal evaporation of the gold (Au) layer of 70 nm thickness as a counter electrode (top-electrode) for small cells (0.096 cm2) and large cells (1 cm2).
Fabrication of OEC/Ni electrodes
A required size of Ni foil of 25 μm thickness (99% metal, Alfa Aesar) was washed for 10 min under bath sonication in a sequence of DI water, EtOH, and then DI water again.
NiFeOOH/Ni anodes
The NiFeOOH cocatalyst was prepared by precipitating metal nitrate deposition (PMND) method as per our previous works24,54. Briefly, 0.0290 g of Ni(NO3)2·6H2O (99.999%, Sigma Aldrich) and 0.0404 g of Fe(NO3)3·9H2O (>98.5%, Kanto Chemicals) were dissolved in each of a 5 mL 2-ME solvent to get individual precursor solutions of 0.02 M of Ni and Fe. These catalyst solutions were mixed properly in a 1:1 molar ratio and stirred, followed by sonication for 10 min and further stirring for 60 min at room temperature to obtain the required NiFeOOH inks.
NiFeCo/Ni anodes
By applying the same PMND method54, NiFeCo cocatalyst ink was also prepared by adding 0.02 M (0.0290 g) of Co(NO3)2·6H2O (98%, Sigma Aldrich) as Co precursor in 5 mL of 2-ME to make 0.02 M of solution with 1:1:1 equimolar ratio.
Following this, electrodes were prepared using Ni:Fe (1:1) and Ni:Fe:Co (1:1:1) catalyst ink solutions by dropping 10 μL cm−2 on the pre-treated Ni foils. These catalyst electrodes were dried at a low temperature (LT) of 80 °C in an oven for 10 min. We also prepared NiFeCo(HT)/Ni electrodes by annealing the same electrodes at a high temperature (HT) of 400 °C for 20 min in a box furnace.
RuOx/Ni and IrOx/Ni anodes
For comparison, we also prepared the benchmark catalysts including RuOx and IrOx electrodes as per our previous works24,54. Following this, 0.02 M of Ru- or Ir-based cocatalyst inks were prepared by utilizing RuCl3·xH2O (99.9%, Sigma Aldrich) or Na2IrCl6·6H2O (99.9%, Sigma Aldrich) as the precursors and added to 10 mL of acetylacetone. To obtain metal oxide films, respective inks were deposited by drop casting onto treated Ni substrate (10 μL cm−2) and annealed in two steps: at 80 °C for 20 min and 500 °C for 1 h.
Fabrication of Ni/HEC electrodes
Ni/CoSMoS and Ni/CoSMoS−Pt cathodes
The Co9S8-MoS2 (CoSMoS) cocatalyst was prepared by two-step synthesis as per our previous work73. Briefly, 150 mg of Co(OAc)2·4 H2O (Sigma Aldrich) was dissolved in 50 mL of ethanol, and an aqueous solution of pyrazole (C3H3N2H, 173.1 mg) was gradually poured. The resulting mixture was then stirred for 2 h at 60 °C and kept overnight as it is without stirring to obtain the precipitates of Co-pyrazole. This Co-based complex was dispersed in an aqueous solution of (NH3)2·MoS4 (200.1 mg, Sigma Aldrich) and sonicated for duration of 1 h. The resulting mixture was treated in a hydrothermal reactor at 120 °C for 12 h. The Co3O4-(am-MoSx) precursor was collected by vacuum filtration. The CoSMoS co-catalyst was finally acquired by reductive annealing the Co3O4-(am-MoSx) precursor in the tubular furnace at 400 °C for 2 h. Approximately, 10 mg of as-synthesized CoSMoS powder was sonicated for 1 h after adding it into a 1 mL mixture of DI water, IPA, and 10 µL of 5% Nafion solution. For CoSMoS−Pt ink, 10 µL of H2PtCl6 (8 wt%, Sigma Aldrich) solution and 5 µL mL−1 of IPA was mixed by sonication for 1 h before preparing the electrodes. The 10 µL of CoSMoS or CoSMoS−Pt ink was drop-casted onto a Ni foil and dried at 80 °C for 10 min to form a working cathode for electrochemical testing as well as attachment to a FAPbI3 thin-films.
Ni foil/Pt cathodes
A Pt layer of 20 nm thickness was deposited by e-beam evaporation on Ni foil before electrochemical testing as well as attachment to FAPbI3 thin-films.
Ni foil/Pt@C cathodes
Pt@C ink was prepared in a 1 mL mixture of DI water, IPA, and 10 µL of 5% Nafion solution, which was drop-casted onto a Ni foil and dried at 80 °C for 10 min.
Fabrication of FAPbI3 photoelectrodes
Fabricated PSC thin-films and OEC/Ni and HEC/Ni electrodes were used to develop both photoanodes (as a regular n-i-p structure) and photocathodes (as a flipped n-i-p structure), respectively.
Cocatalyst/Ni foil-encapsulated photoanodes
Undoped and doped-FAPbI3 thin-films were used to develop photoanodes (refer to fabrication steps in Fig. S8a–c) as we reported previously24. An OEC/Ni foil electrode was physically attached on the Au layer (towards hole transporting side) using Ag-coated polymethyl-methacrylate (PMMA) particles. To provide an efficient charge transfer for OER, an electrical contact between PSK device with the underlying charge extracting layer and OEC/Ni foil was developed using acrylic (Loctite 401, Henkel) based adhesive mixed with conductive Ag-coated PMMA particles (0.5 wt% of PMPMS-AG-1.53 (Cospheric), 45–53 μm size of PMMA particles coated with 250 nm-thick Ag layer)74 uniformly pasted the Au layer. The bottom electrode (electron transporting side from the FTO) was connected using copper wire before further PEC testing. The entire OEC/Ni/FAPbI3 photoanode was encapsulated using an epoxy-adhesive containing 2-component (J-B Weld) and dried overnight at room temperature. The actual illumination area of device was defined by proper encapsulation applying epoxy with the pre-determined geometrical area (0.096 to 1 cm2) for small sub-cell photoanodes, which were measured by the vernier caliper scale.
Integrated photocathodes
Cl-doped FAPbI3/Ni/HEC integrated photocathode was also prepared similarly but by flipping n-i-p configuration of FAPbI3 thin-films and detailed fabrication steps are depicted with relevant photographs in Fig. S8d–f and Fig. S19a–h. Ni foil/HEC cathodes were connected using Ag paste on the InSn alloy bottom electrode (on electron transporting FTO side), while the top electrode (on hole transporting gold side) was connected using copper wire. The entire photocathode was encapsulated using solid epoxy adhesive and dried overnight at room temperature. The structure of photoanode is copper wire/FTO/Cl:SnO2/Cl:FAPbI3/Spiro-OMeTAD/Gold/Ni foil/NiFeCo and the OER catalyst NiFeCo/Ni foil is integrated to hole transportation side at gold top electrode, where Ni foil is also used to encapsulate the FAPbI3 films to avoid the penetration of electrolyte. The structure of photocathode is CoSMoS–Pt/Ni foil/In-Sn alloy/FTO/Cl:SnO2/Cl:FAPbI3/Spiro-OMeTAD/Gold/copper wire, in which HER catalyst is integrated towards the electron transportation side at FTO bottom electrode using In-Sn alloy and Ag paste.
Fabrication of large photoelectrode array
The large OEC/Ni-encapsulated FAPbI3 photoanodes were developed using an array design by arranging the 1 cm2 sub-cells. Additionally, we also prepared a FAPbI3 large photocathode while attaching 1 cm2 HEC/Ni on the InSn alloy bottom electrode via Ag paste and then encapsulated the device with epoxy. Detailed fabrication steps with schematics and photographs are shown in Fig. S24–S26.
Fabrication of wireless PEC mini-module or stand-alone mini-module-sized artificial leaf
The small-area wireless device was prepared by connecting an OEC/Ni/Cl:FAPbI3 photoanode to a Cl:FAPbI3/Ni/HEC integrated photocathode (Fig. S24–S26) and encapsulating it using 2-component epoxy adhesive (J-B weld, USA), which was dried overnight at room temperature. For Ni foil–encapsulated, large-area mini-module, OEC/Ni foil anode was attached to the Au layer by bar press coating of Ag-coated PMMA-acrylic adhesive, while Ni foil/HEC was attached to a bottom electrode (exposed electron transporting FTO side).
Design and development of PEC reactor panel
The PEC reactor panel (small and large size) was designed and fabricated using transparent acrylic plastic in the MakerLab factory at Ulsan National Institute of Science and Technology (UNIST). The PEC performance of all individual photoelectrodes were optimized in two compartments of PEC reactor panel for overall water-splitting reaction like in our reported work24.
Material characterizations
The cross-sectional morphology of the FAPbI3 thin-films and energy-dispersive X-ray spectroscopy (EDS) of OEC/Ni and Ni/HEC electrodes were measured using a scanning electron microscope (Cold FE-SEM, SU-8220, Hitachi). The optical properties of FAPbI3 thin-films, OEC/Ni/FAPbI3 photoanodes, and FAPbI3/Ni/HEC photocathodes were measured before and after their stability tests using a UV-Vis spectrophotometer (UV-2600, Shimadzu) and UV-Vis-NIR spectrophotometer (UV-3600, Shimadzu). X-ray diffraction (XRD) measurements were performed using a high-resolution XRD instrument (D8 Discovery, Bruker). The steady-state photoluminescence (PL) spectra and the time-correlated single-photon counting for time-resolved photoluminescence (TRPL) spectra of PSK films deposited on FTO glass substrate and PSK films with and without ETLs deposited on slide glass substrate were measured using a FluoTime 300 (PicoQuant) equipment connected with a PMA-C-192-M detector, high-resolution excitation monochromator and assembly of hybrid photomultiplier detector (PMA Hybrid 40, PicoQuant). The fitting of PL and TRPL spectra were done using an Origin software (Academic OriginPro version 2020). The XPS spectra were measured using a high-transmission instrument (K-alpha, ESCALAB 250 Xi, ThermoFisher) equipped with a high flux, monochromatic Al-Kα as an X-ray energy source in the range 200–3 keV. The XPS spectrum analysis was performed using the Thermo Scientific Advantage software, and XPS spectra were fitted by Gaussian method in the Origin software. A high-resolution transmission electron microscopy (HR-TEM, JEM-2100F, Jeol) was used to investigate structural, elemental mapping, and compositions of OER and HER cocatalysts. Fringes and lattices of catalyst nanoparticles were identified using Gatan Microscopy Suit Software (Gatan). ICP-OES (700-ES, Varian) was used to measure the leaching of Fe, Ni, Co, and Pb in 1 M KOH electrolyte while measuring the long-term stability tests.
Photovoltaic measurements
The PV performance of FAPbI3 PV cells was measured using a solar simulator (Oriel Sol3A class AAA, Newport) and calibrated using a silicon reference cell certificated by NREL, USA. The J-E characteristics of the FAPbI3 PV cells were measured using the Keithley source meter (Keithley 2420) under the one sun illumination of simulated AM 1.5 G conditions at an intensity of 100 mW cm−2. The J-E characteristics were measured in a potential range of 0–1.5 V with a 10-mV step voltage and a delay time of 40 ms. The active area of FAPbI3 PV cells was controlled by a metal mask (with an aperture size of 0.096 and 1 cm2) placed in front of the device to avoid an overestimation of the photocurrent density measurement. For measurement of the high-efficiency devices, antireflective coating layer was applied onto the FAPbI3 PV cell’s surface. The EQE measurement was performed with an internal quantum efficiency system instrument (IQ200B, Oriel) in dark conditions.
Electrochemical measurements
All electrocatalytic measurements of OEC/Ni anodes and Ni/HEC cathodes were carried out using 3-electrode or 2-electrode set-ups. Various electrolytes, such as acidic (0.5 M H2SO4 with pH 0.3), neutral (0.5 M KPi with pH 7), near-neutral alkaline (1 M KBi with pH 9.1), and alkaline (1 M KOH with pH 14), were used for linear sweep voltammetry (LSV, i-E curves), chronopotentiometry (E-t curves), and chronoamperometry (i-t curves) measurements at an electrochemical set-up (Iviumstat, Ivium). All LSV (i-E) curves were measured from a potential range of 1 to 2 VRHE under dark for OER and 0.2 to −1 VRHE for HER with 10 mV s-1 scan rate, and the 10th scan of each measurement was analyzed without -iR compensation. Electrochemical impedance spectroscopy (EIS) measurement was performed using the electrochemical set up (Iviumstat, Ivium) at different frequencies and applied voltages depending on OER and HER catalyst. The Nyquist plots (EIS curves) of OEC/Ni foil and HEC/Ni foil electrodes were measured at applied voltages of 1.43 and 0 VRHE under dark mode and in applied frequency range of 0.01–10 kHz and 0.1–100 KHz, respectively. The fitting of EIS curves was done by using a modified Randles circuit model.
Photoelectrochemical activity measurements
All PEC measurements were performed using 3- or 2-electrode set-ups in a PEC water-splitting cell or a PEC reactor panel. Electrolytes of various conditions such as acidic (0.5 M H2SO4 with pH 0.5), alkaline (1 M KOH with pH 14), and neutral (0.5 M KPi with pH 7, 1 M KBi with pH 9) solutions were tested to select an electrolyte for optimized photoelectrodes during overall PEC reactions. The working areas of OEC/Ni/FAPbI3 and FAPbI3/Ni/HEC photoelectrodes were kept at 0.096 to 0.25, 1, 4, and 16 cm2. A solar light simulator (Oriel Sol3A, Newport) provided 1-sun irradiation to measure the PEC performance, while an electrochemical set-up (Iviumstat, Ivium) was used to measure LSV curves or J–E curves and chronoamperometry (J-t) curves. All LSV curves were measured from a potential range of 0 to 2 VRHE under dark for OER and 0.2 to −1 VRHE for the HER with a scan rate of 10 mV s-1 and the 10th scan of each measurement was analyzed. All PEC stability tests were conducted at the applied voltage of the highest %ABPE and %HC-STH efficiency of photoanodes and photocathodes. These stability tests were conducted without an O-ring PEC reactor except Fig. 2d. The light intensity of 100 mW cm−2 was calibrated using a thermopile detector with a spectrum response range of 0.19–10.6 µm (818P-020-12, Newport). Detailed procedures of various PEC measurements and calculations including PEC water-splitting activity are provided as below.
3-electrode set-up measurements
The PEC performance of OEC/Ni/FAPbI3 photoanodes and FAPbI3/Ni/HEC integrated photocathodes were tested using a 3-electrode set-up in a two-compartment PEC water-splitting reactor, i.e., photoanode or photocathode as working electrode (WE), Pt wire as counter electrode (CE) and Hg/HgO (1 M NaOH) as reference electrode (RE). The PEC measurements in 3-electrode set-up were conducted with an optimized scan rate of 10 mV s−1 to obtain the LSV curves or J-E curves of photoelectrodes. The applied potential of all photoelectrodes was calculated by conversion equation, ERHE = E(Hg/HgO) + 0.118 + 0.0591 × pH. In this equation, E(Hg/HgO) indicates applied bias potential in VHg/HgO from electrochemical set-up and 0.118 indicates a conversion factor from Hg/HgO reference electrode to reversible hydrogen electrode (RHE) scale. For 3-electrode set-up, applied bias photon-to-current efficiency (%ABPE) of OEC/Ni/FAPbI3 photoanode and half-cell solar-to-hydrogen (%HC-STH efficiency) of FAPbI3/Ni/HEC integrated photocathode were calculated using the following equations.
In this equation, Jph indicates photocurrent density of photoelectrode (mA cm−2); Pin, an incident power density of solar light (100 mW cm−2); Eapp, indicates applied bias in RHE scale; 1.23 V, thermodynamic potential for water oxidation (EO2/H2O); 0 V, thermodynamic potential for water-reduction (EH+/H2); ηF, faradaic efficiency (%FE).
The solar-to-hydrogen (%STH) efficiency can be calculated using the Eq. 3, which is generally used to obtain maximum predictable %STH efficiency of small- and large sub-cells (0.25 and 1 cm2) from data obtained in 3-electrode set-up.
2-electrode set-up measurements
The 2-electrode PEC measurements of OEC/Ni/FAPbI3 photoanode or FAPbI3/Ni/HEC integrated photocathode (as WE) were conducted with photocathode or photoanode (as CE), respectively, but without using RE in the same physical distance in a two-compartment PEC cell reactor. Both photoelectrodes were connected with each other with a “wired” configuration using Cu-wire attached with the crocodile pins. The J-t curves were measured under different and specific applied biases between the photoanode and photocathode. We used the operating photocurrent density Jop at the intersecting point from J-E curves measured using a 2-electrode set-up to calculate %STH efficiency using the following Eq. 4.
In this equation, Jop indicates the operating photocurrent density (mA cm−2) obtained during the J-t measurements at an applied potential 0 V in 2-electrode set-up, Pin indicates power density (100 mW cm−2) of incident solar light, 1.23 V indicates thermodynamic potential for overall water-splitting.
Photonic charge-transfer efficiency measurements
Incident photon-to-current efficiency (%IPCE)
A solar light source (TLS, Driel Instruments from Newport) with monochromator tunability was used for IPCE measurements. The %IPCE of the OEC/Ni/FAPbI3 photoanode was measured at an applied potential of 1.23 VRHE and the potential corresponding to maximum %ABPE, while that of the HEC/Ni/FAPbI3 integrated photocathode was measured at an applied potential of 0 VRHE and the potential corresponding to maximum %HC-STH efficiency under the monochromatic light (with a wavelength range of 300–900 nm) and a chopping frequency of 200 Hz. The electrochemical set-up was used for Jph measurements with time-dependency. The illumination area of each photoelectrode device was kept at 1 cm2. The %IPCE of photoelectrodes was calculated using Eq. 5:
In this equation, Jph and λ indicate the photocurrent density (mA cm−2) as a function of wavelength (nm) and wavelength of incident light, respectively. The Pin indicates the calibrated power (mW cm−2) of diode used at individual wavelengths. This calibration was done before actual PV measurements and we used the calibrated value of λ using a certified diode.
Open-circuit potential (OCP)
For photovoltage measurements of photoelectrodes, we measured open-circuit potential (OCP), E (VHg/HgO) when the current of 0 mA flowing through the potentiostat in dark and 1-sun illumination. The measured voltage E (VHg/HgO) was converted to a potential, E (VRHE) scale. Both photoanodes Ni/FAPbI3 and OEC/Ni/FAPbI3 were measured to check their photovoltage.
Fill factor
The FF values of photoelectrodes were determined from Eq. 6, in which the numerator indicates the maximum power obtained from the current density, Jmax, indicates an applied potential of Emax (VRHE) for water oxidation75.
Gas productivity measurements
Faradaic efficiency (%FE)
The %FE of evolved gases (H2 and O2) was determined from a product analysis by gas chromatography equipment (GC, Agilent 7890 A) with a molecular sieve (5-Å size) packed column connected to a thermal conductivity detector (TCD). To conduct an online gas analysis, Ar flow at a constant flow rate of 5 mL min−1 was used as a carrier gas into a gas-tight PEC water-splitting reactor filled with the electrolyte, and the evolving gas outlet was directly connected to the GC to analyze evolved H2-O2 gases, which were auto-samples every 10 min. The 1-sun solar light condition and the alkaline electrolyte were used the same as the above PEC measurements. Controlled chronoamperometry (J-t) tests were performed at an applied voltage depending on 3- or 2-electrode set-ups. The %FE of photoelectrodes was measured at the applied potential of maximum %ABPE. Theoretical rates of gas generation were calculated from measured photocurrents, while real production rates were measured by GC analyses.
In this equation, nH2 and nO2 indicate the moles of H2 and O2, respectively, which were quantified by GC during the stability tests in 2- or 3- electrodes set-up, Faraday constant (F) is 96485 C mol−1, and Q/2 and Q/4 are the charges passed in Coulomb (C) for H2 and O2, respectively.
Solar-to-hydrogen efficiency of wireless mini-module-sized artificial leaf device
Based on gas productivity measurements from GC analyses, we calculated H2 evolved from the wireless mini-module-sized artificial leaf device kept in PEC reactor, and these data were used to calculate %STH efficiency using following Eq. 9.
In this equation, nH2 indicates the mmol of H2 gas per second measured by GC, A indicates the illumination area of small-cell, large-cell photoanodes and mini-module-sized artificial leaf device, Pin indicates the power density (100 mW cm–2) of incident solar light, ΔG indicates the Gibb’s free energy (237,000 J mol–1) for overall water-splitting. Moreover, the geometrical areas of the catalyst and photoanode were kept the same. We used a division factor ‘2’ to calculate %STH efficiency due to parallel or side-by-side connections of two photoelectrodes in the PEC system, where the illumination areas of the photoanodes and photocathodes as well as geometrical area of cocatalysts on each electrode were kept the same.
Data availability
All data generated or analyzed during this study are included in Supplementary Information (Supplementary Notes S1 to S17, Figures S1 to S27, Tables S1 to S8, References, Supplementary Video 1 as a separate movie, and Source Data files). The data for all the graphs within the main article Figures and Supplementary Figs. are provided as Source Data. Source data are provided with this paper.
References
Ciamician, G. The photochemistry of the future. Science 36, 385–394 (1912).
Bard, A. J. & Fox, M. A. Artificial photosynthesis: solar splitting of water to hydrogen and oxygen. Acc. Chem. Res. 28, 141–145 (1995).
Tachibana, Y., Vayssieres, L. & Durrant, J. R. Artificial photosynthesis for solar water-splitting. Nat. Photonics 6, 511–518 (2012).
Ye, S. et al. Mimicking the key functions of photosystem II in artificial photosynthesis for photoelectrocatalytic water splitting. J. Am. Chem. Soc. 140, 3250–3256 (2018).
Reece, S. Y. et al. Wireless solar water splitting using silicon-based semiconductors and earth-abundant catalysts. Science 334, 645–648 (2011).
Nocera, D. G. The artificial leaf. Acc. Chem. Res. 45, 767–776 (2012).
Surendranath, Y., Bediako, D. K. & Nocera, D. G. Interplay of oxygen-evolution kinetics and photovoltaic power curves on the construction of artificial leaves. Proc. Natl. Acad. Sci. USA 109, 15617–15621 (2012).
Kim, J. H., Hansora, D., Sharma, P., Jang, J. W. & Lee, J. S. Toward practical solar hydrogen production - an artificial photosynthetic leaf-to-farm challenge. Chem. Soc. Rev. 48, 1908–1971 (2019).
Lewis, N. S. Developing a scalable artificial photosynthesis technology through nanomaterials by design. Nat. Nanotechnol. 11, 1010–1019 (2016).
Guan, X. et al. Making of an industry-friendly artificial photosynthesis device. ACS Energy Lett 3, 2230–2231 (2018).
Bonke, S. A., Wiechen, M., MacFarlane, D. R. & Spiccia, L. Renewable fuels from concentrated solar power: towards practical artificial photosynthesis. Energy Environ. Sci. 8, 2791–2796 (2015).
Vilanova, A., Dias, P., Lopes, T. & Mendes, A. The route for commercial photoelectrochemical water splitting: a review of large-area devices and key upscaling challenges. Chem. Soc. Rev. https://doi.org/10.1039/d1cs01069g (2024).
Spitler, M. T. et al. Practical challenges in the development of photoelectrochemical solar fuels production. Sustain. Energy Fuels 4, 985–995 (2020).
Dogutan, D. K. & Nocera, D. G. Artificial photosynthesis at efficiencies greatly exceeding that of natural photosynthesis. Acc. Chem. Res. 52, 3143–3148 (2019).
Jin, J. et al. An experimental and modeling/simulation-based evaluation of the efficiency and operational performance characteristics of an integrated, membrane-free, neutral pH solar-driven water-splitting system. Energy Environ. Sci. 7, 3371–3380 (2014).
Bogdanoff, P. et al. Artificial Leaf for water splitting based on a triple‐junction thin‐film silicon solar cell and a PEDOT:PSS/catalyst blend. Energy Technol 4, 230–241 (2016).
Kim, J. H. et al. Wireless solar water splitting device with robust cobalt-catalyzed, dual-doped BiVO4 photoanode and perovskite solar cell in tandem: a dual absorber artificial leaf. ACS Nano 9, 11820–11829 (2015).
Andrei, V. et al. Scalable triple cation mixed halide perovskite-BiVO4 tandems for bias-free water splitting. Adv. Energy Mater. 8, 1801403 (2018).
Andrei, V., Reuillard, B. & Reisner, E. Bias-free solar syngas production by integrating a molecular cobalt catalyst with perovskite-BiVO4 tandems. Nat. Mater. 19, 189–194 (2020).
Andrei, V. et al. Floating perovskite-BiVO(4) devices for scalable solar fuel production. Nature 608, 518–522 (2022).
Jia, J. et al. Solar water splitting by photovoltaic-electrolysis with a solar-to-hydrogen efficiency over 30. Nat. Commun. 7, 13237 (2016).
Nishiyama, H. et al. Photocatalytic solar hydrogen production from water on a 100-m(2) scale. Nature 598, 304–307 (2021).
Zhou, P. et al. Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting. Nature 613, 66–70 (2023).
Hansora, D. et al. All-perovskite-based unassisted photoelectrochemical water splitting system for efficient, stable and scalable solar hydrogen production. Nat. Energy 9, 272–284 (2024).
Hamann, T. Perovskites take lead in solar hydrogen race. Science 345, 1566–1567 (2014).
Khamgaonkar, S. S., Taka, A. L. & Maheshwari, V. Engineering and design of halide perovskite photoelectrochemical cells for solar‐driven water splitting. Adv. Func. Mater. 34, 2405414 (2024).
Zhou, Y., Poli, I., Meggiolaro, D., De Angelis, F. & Petrozza, A. Defect activity in metal halide perovskites with wide and narrow bandgap. Nat. Rev. Mater. 6, 986–1002 (2021).
Isikgor, F. H. et al. Molecular engineering of contact interfaces for high-performance perovskite solar cells. Nat. Rev. Mater. 8, 89–108 (2022).
Pinaud, B. A. et al. Technical and economic feasibility of centralized facilities for solar hydrogen production via photocatalysis and photoelectrochemistry. Energy Environ. Sci. 6, 1983–2002 (2013).
Fountaine, K. T., Lewerenz, H. J. & Atwater, H. A. Efficiency limits for photoelectrochemical water-splitting. Nat. Commun. 7, 13706 (2016).
Hu, S., Xiang, C., Haussener, S., Berger, A. D. & Lewis, N. S. An analysis of the optimal band gaps of light absorbers in integrated tandem photoelectrochemical water-splitting systems. Energy Environ. Sci. 6, 2984–2993 (2013).
Shaner, M. R., Atwater, H. A., Lewis, N. S. & McFarland, E. W. A comparative technoeconomic analysis of renewable hydrogen production using solar energy. Energy Environ. Sci. 9, 2354–2371 (2016).
Pan, S. et al. Halide perovskite materials for photo(electro)chemical applications: dimensionality, heterojunction, and performance. Adv. Energy Mater. 12, 2004002 (2021).
Singh, S. et al. Hybrid organic-inorganic materials and composites for photoelectrochemical water splitting. ACS Energy Lett 5, 1487–1497 (2020).
Chen, J., Dong, C., Idriss, H., Mohammed, O. F. & Bakr, O. M. Metal halide perovskites for solar‐to‐chemical fuel conversion. Adv. Energy Mater. 10, 1902433 (2019).
Park, J. et al. Hybrid perovskite-based wireless integrated device exceeding a solar to hydrogen conversion efficiency of 11%. Small 19, e2300174 (2023).
Choi, H. et al. Organometal halide perovskite-based photoelectrochemical module systems for scalable unassisted solar water splitting. Adv. Sci. 10, e2303106 (2023).
Fehr, A. M. K. et al. Integrated halide perovskite photoelectrochemical cells with solar-driven water-splitting efficiency of 20.8%. Nat. Commun. 14, 3797 (2023).
Jeong, W. et al. Large-area all-perovskite-based coplanar photoelectrodes for scaled-up solar hydrogen production. Energy Environ. Sci. 17, 3604–3617 (2024).
Lee, H. et al. A dual spin-controlled chiral two-/three-dimensional perovskite artificial leaf for efficient overall photoelectrochemical water splitting. Nat. Commun. 15, 4672 (2024).
Daboczi, M., Cui, J., Temerov, F. & Eslava, S. Scalable all-inorganic halide perovskite photoanodes with >100 h operational stability containing earth-abundant materials. Adv. Mater. 35, e2304350 (2023).
Poli, I. et al. Graphite-protected CsPbBr3 perovskite photoanodes functionalised with water oxidation catalyst for oxygen evolution in water. Nat. Commun. 10, 2097 (2019).
Choi, H. J. et al. Suppression of undesired losses in organometal halide perovskite‐based photoanodes for efficient photoelectrochemical water splitting. Adv. Energy Mater. 13, 202300951 (2023).
Yun, J. et al. Conductive passivator and dipole layer mixture enabling high‐performance stable perovskite photoelectrode‐based solar water splitting. Adv. Energy Mater. 202301693, https://doi.org/10.1002/aenm.202301693 (2023).
Sharifi, T. et al. Toward a low‐cost artificial leaf: driving carbon‐based and bifunctional catalyst electrodes with solution‐processed perovskite photovoltaics. Adv. Energy Mater. 6, 1600738 (2016).
Chen, H. et al. Integrating low‐cost earth‐abundant co‐catalysts with encapsulated perovskite solar cells for efficient and stable overall solar water splitting. Adv. Func. Mater. 31, 2008245 (2020).
Zhu, Z. et al. Ultrastable halide perovskite CsPbBr(3) photoanodes achieved with electrocatalytic glassy-carbon and boron-doped diamond sheets. Nat. Commun. 15, 2791 (2024).
Yoo, J. W. et al. R4N+ and Cl− stabilized α-formamidinium lead triiodide and efficient bar-coated mini-modules. Joule 7, 797–809 (2023).
Khamgaonkar, S. S., Chen, Q., Musselman, K. & Maheshwari, V. Stable perovskite photocathodes for efficient hydrogen evolution in acidic and basic conditions. J. Mater. Chem. A 11, 20079–20088 (2023).
Yoo, J. W. et al. Efficient perovskite solar mini-modules fabricated via bar-coating using 2-methoxyethanol-based formamidinium lead tri-iodide precursor solution. Joule 5, 2420–2436 (2021).
Park, B. W. et al. Stabilization of formamidinium lead triiodide α-phase with isopropylammonium chloride for perovskite solar cells. Nat. Energy 6, 419–428 (2021).
Jeong, J. et al. Pseudo-halide anion engineering for alpha-FAPbI3 perovskite solar cells. Nature 592, 381–385 (2021).
Lu, H. et al. Vapor-assisted deposition of highly efficient, stable black-phase FAPbI3 perovskite solar cells. Science 370, eabb8985 (2020).
Kim, Y. K., Kim, J. H., Jo, Y. H. & Lee, J. S. Precipitating metal nitrate deposition of amorphous metal oxyhydroxide electrodes containing Ni, Fe, and Co for electrocatalytic water oxidation. ACS Catal 9, 9650–9662 (2019).
Smith, R. D. et al. Photochemical route for accessing amorphous metal oxide materials for water oxidation catalysis. Science 340, 60–63 (2013).
Morales-Guio, C. G., Liardet, L. & Hu, X. Oxidatively electrodeposited thin-film transition metal (oxy)hydroxides as oxygen evolution catalysts. J. Am. Chem. Soc. 138, 8946–8957 (2016).
Morales-Guio, C. G. et al. An optically transparent iron nickel oxide catalyst for solar water splitting. J. Am. Chem. Soc. 137, 9927–9936 (2015).
Jiang, Q. et al. Enhanced electron extraction using SnO2 for high-efficiency planar-structure HC(NH2)2PbI3-based perovskite solar cells. Nat. Energy 2, 16177 (2016).
Kim, T. G. et al. Monolithic lead halide perovskite photoelectrochemical cell with 9.16% applied bias photon-to-current efficiency. ACS Energy Lett 7, 320–327 (2021).
Min, H. et al. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 598, 444–450 (2021).
Leijtens, T. et al. Overcoming ultraviolet light instability of sensitized TiO(2) with meso-superstructured organometal tri-halide perovskite solar cells. Nat. Commun. 4, 2885 (2013).
Yoo, J. J. et al. Efficient perovskite solar cells via improved carrier management. Nature 590, 587–593 (2021).
Kamat, P. V., Bisquert, J. & Buriak, J. Lead-free perovskite solar cells. ACS Energy Lett 2, 904–905 (2017).
Hao, F., Stoumpos, C. C., Cao, D. H., Chang, R. P. H. & Kanatzidis, M. G. Lead-free solid-state organic–inorganic halide perovskite solar cells. Nat. Photonics 8, 489–494 (2014).
Yu, J. M. et al. High-performance and stable photoelectrochemical water splitting cell with organic-photoactive-layer-based photoanode. Nat. Commun. 11, 5509 (2020).
Xia, J. X. et al. Ionic selective contact controls the charge accumulation for efficient and intrinsic stable planar homo-junction perovskite solar cells. Nano Energy 66, 104098 (2019).
Ahn, N. et al. Trapped charge-driven degradation of perovskite solar cells. Nat. Commun. 7, 13422 (2016).
Xu, J. et al. Frenkel-defected monolayer MoS2 catalysts for efficient hydrogen evolution. Nat. Commun. 13, 2193 (2022).
Sun, Y. et al. Plasma-induced large-area N,Pt-doping and phase engineering of MoS2 nanosheets for alkaline hydrogen evolution. Energy Environ. Sci. 15, 1201–1210 (2022).
Han, X. et al. Ultrafine Pt nanoparticle‐decorated pyrite‐type CoS2 nanosheet arrays coated on carbon cloth as a bifunctional electrode for overall water splitting. Adv. Energy Mater. 8, 1800935 (2018).
Min, H. et al. Efficient, stable solar cells by using inherent bandgap of alpha-phase formamidinium lead iodide. Science 366, 749–753 (2019).
Kim, G. et al. Impact of strain relaxation on performance of α-formamidinium lead iodide perovskite solar cells. Science 370, 108–112 (2020).
Kim, M. K. et al. Covalent 0D–2D heterostructuring of Co9S8–MoS2 for enhanced hydrogen evolution in all pH electrolytes. Adv. Func. Mater. 30, 2002536 (2020).
Choi, I. Y. et al. Two-terminal mechanical perovskite/silicon tandem solar cells with transparent conductive adhesives. Nano Energy 65, 104044 (2019).
Pihosh, Y. et al. Ta3N5-Nanorods enabling highly efficient water oxidation via advantageous light harvesting and charge collection. Energy Environ. Sci. 13, 1519–1530 (2020).
Rhee, R. et al. Unassisted overall water splitting with a solar‐to‐hydrogen efficiency of over 10% by coupled lead halide perovskite photoelectrodes. Carbon Energy 5, e232 (2022).
Wang, M. et al. High-performance and stable perovskite-based photoanode encapsulated by blanket-cover method. ACS Appl. Energy Mater 4, 7526–7534 (2021).
Tao, R., Sun, Z., Li, F., Fang, W. & Xu, L. Achieving organic metal halide perovskite into a conventional photoelectrode: outstanding stability in aqueous solution and high-efficient photoelectrochemical water splitting. ACS Appl. Energy Mater 2, 1969–1976 (2019).
Nam, S., Mai, C. T. K. & Oh, I. Ultrastable photoelectrodes for solar water splitting based on organic metal halide perovskite fabricated by lift-off process. ACS Appl. Mater. Interfaces 10, 14659–14664 (2018).
Liang, J. et al. A low-cost and high-efficiency integrated device towards solar-driven water splitting. ACS Nano 14, 5426–5434 (2020).
Edwardes Moore, E. et al. Semi-artificial photoelectrochemical tandem leaf with a CO2-to-formate efficiency approaching 1%. Angew. Chem. Int. Ed. 60, 26303–26307 (2021).
Moore, E. E., Andrei, V., Zacarias, S., Pereira, I. A. C. & Reisner, E. Integration of a hydrogenase in a lead halide perovskite photoelectrode for tandem solar water splitting. ACS Energy Lett 5, 232–237 (2019).
Liu, B. et al. Double‐side Si photoelectrode enabled by chemical passivation for photoelectrochemical hydrogen and oxygen evolution reactions. Adv. Func. Mater. 31, 2007222 (2020).
Varadhan, P., Fu, H. C., Kao, Y. C., Horng, R. H. & He, J. H. An efficient and stable photoelectrochemical system with 9% solar-to-hydrogen conversion efficiency via InGaP/GaAs double junction. Nat. Commun. 10, 5282 (2019).
Okamoto, S., Deguchi, M. & Yotsuhashi, S. Modulated III–V triple-junction solar cell wireless device for efficient water splitting. J. Phys. Chem. C 121, 1393–1398 (2017).
Acknowledgements
This work was supported by Climate Change Response Project (NRF-2019M1A2A2065612), the Brainlink Project (NRF-2022H1D3A3A01081140, NRF-2021R1A4A3027878) (awarded to J.S.L.), the NRF grant (RS-2023-00222006) (awarded to J.-W.J.), and the Basic Science Research Program (NRF-2018R1A3B1052820) (awarded to S.I.S.) funded by Ministry of Science and the ICT of Korea via National Research Foundation (NRF), as well as by research funds from UNIST (1.190013.01) (awarded to J.S.L. and J.-W.J.) and the Institute for Basic Science (IBS-R019-D1). The authors are thankful for material charaterization and instrumentation provision of the UNIST Central Research Facility (UCRF).
Author information
Authors and Affiliations
Contributions
D.H., R.M., N.E., J.W.Y., J.-W.J., S.I.S., and J.S.L. designed and directed the research. D.H. and R.M. conceived the concept of stabilizing FAPbI3 PSK-based photoanodes and photocathodes using a metal encapsulation strategy and optimized the improvements in the PEC system. N.E. and J.W.Y. prepared the FAPbI3 thin-films and measured their performance, while D.H. and R.M. designed FAPbI3 mini-module photoelectrodes using multi-cell array and metal grid designs. D.H. prepared NiFeCo and CoSMoS−Pt and optimized them for OER and HER experiments and their integration to develop both FAPbI3 photoelectrodes. M.K.K. prepared CoSMoS catalysts. W.J.B. conducted gas-chromatography measurements and gas productivity analysis. D.H. and W.J.B. designed and fabricated the small- and large-PEC water-splitting reactors using acrylic components at Makelab at UNIST. During the revision of this work, J.P. prepared the doped and undoped ETL films. D.H., R.M., N.E., J.W.Y., J.-W.J., S.I.S., and J.S.L. co-wrote the paper. All authors read and commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
D.H., R.M., W.J.B., J.-W.J., and J.S.L. prepared a Korean patent application (UTP24079KR-00 and 10-2024-0063224 dated 2024-04-10) concurrently with this paper. The other authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Munkhbayar Batmunkh and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hansora, D., Mehrotra, R., Noh, E. et al. Scalable and durable module-sized artificial leaf with a solar-to-hydrogen efficiency over 10%. Nat Commun 16, 4186 (2025). https://doi.org/10.1038/s41467-025-59597-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-59597-2