Abstract
What is the molecular origin of voltage dependence in skeletal muscle excitation-contraction? Cholinergic transmission to the muscle fiber triggers action potentials, which are sensed by voltage-gated L-type calcium channels (CaV1.1). In turn, the conformational changes in CaV1.1 propagate to and activate intracellular ryanodine receptors (RyR1), causing Ca2+ release and contraction. The CaV1.1 channel has four voltage-sensing domains (VSD-I to -IV) with diverse voltage-sensing properties, so the identity of VSD(s) responsible for conferring voltage dependence to RyR1 opening, is unknown. Using voltage-clamp fluorometry, we show that only VSD-III possesses kinetic, voltage-dependent and pharmacological properties consistent with skeletal-muscle excitability and Ca2+ release. We propose that the earliest voltage-dependent event in the excitation-contraction process is the structural rearrangement of VSD-III that propagates to RyR1 to initiate Ca2+ release and contraction.
Similar content being viewed by others
Introduction
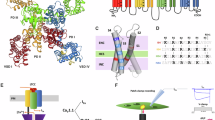
Skeletal muscle contraction is initiated by an electrical excitation, the muscle action potential (AP), which triggers the opening of ryanodine receptors (RyR1) and the release of Ca2+ from the sarcoplasmic reticulum (SR)1. This sequence of events, referred to as excitation-contraction (EC) coupling2, relies on the physical association of RyR1, an intracellular channel that is intrinsically voltage-insensitive, with four voltage-gated Ca2+ channels (CaV1.1, also known as the dihydropyridine receptor, DHPR) located in the sarcolemma and T-tubules (Fig. 1)3,4.
CaV1.1 channel macromolecular complex (α1S pore forming subunit (violet), auxiliary subunits β1a (yellow), α2δ−1 (green), and γ1 (black), and Stac3 adapter protein, orange) is thought to be in physical contact with RyR1 (gray). α1S comprises four concatenated repeats (I-IV), each of which includes a voltage-sensing domain (VSD, blue, red, green, and orange) and a quarter of the pore domain (enlargement). RyR1 is embedded in the SR membrane and cannot sense electrical signals in the sarcolemma directly; instead, it uses CaV1.1 voltage-sensing apparatus to gain voltage dependence. During an action potential, the voltage-dependent conformational rearrangements in one or more CaV1.1 VSD(s) are thought to propagate to RyR1, likely via the repeat II-III loop (red)50, which opens and releases Ca2+ from the sarcoplasmic reticulum (SR). (CaV1.1 Protein Data Bank ID 5GJV53, RyR1 Protein Data Bank ID 3J8H66).
During an action potential, the CaV1.1 channel is thought to transduce the electric potential change across the membrane of muscle fibers by undergoing conformational rearrangements that propagate to and open RyR1, causing contraction (Fig. 1)1,5. Early studies on muscle EC coupling recognized that an intramembrane charge movement originating from CaV1.1 associates with voltage-dependent SR Ca2+ release6,7,8. This measurable current, called “gating current”9, is the electrical manifestation of the concomitant activation of the four voltage-sensing domains (VSDs) of CaV1.1,6,7,10,11 (Fig. 1 and Supplementary Fig. 1a), compelled to by a change in membrane potential. As gating currents are a composite signal generated by the concomitant activation of all four VSDs, they cannot be used to extract information about individual VSDs. Prior studies have attempted to uniquely pinpoint the specific VSD(s) that control EC coupling using different approaches in cultured murine muscle fibers and myotubes, reaching wholly contrasting conclusions11,12: an optical approach reported that all VSDs may be involved except VSD-III11, while a mutagenesis approach concluded that only impairment of VSD-III affects Ca2+ release12.
To solve this recent conundrum and definitively answer the age-old question of how excitation and contraction are coupled, we defined and tested a series of stringent criteria that a CaV1.1 VSD coupled to RyR1 opening should satisfy:
-
1.
VSD activation is kinetically compatible with the time courses of the muscle action potential and Ca2+ release.
-
2.
VSD activation and Ca2+ release have shared voltage dependence, specifically:
-
a.
The VSD is not active at potentials where Ca2+ release does not occur, and
-
b.
The VSD activates over the range of membrane potentials where Ca2+ release occurs.
-
a.
-
3.
A drug that modifies the activation of the VSD(s) assigned to RyR1 should also affect Ca2+ release.
In this work, to test these postulates, we use the Cut-open Oocyte Vaseline Gap Voltage-Clamp Fluorometry (COVG-VCF) approach, which affords exceptional voltage-dependent optical tracking of protein conformational changes under physiologically relevant conditions. In this way, we resolve the activation of the four individual VSDs in the human CaV1.1 channel macromolecular complex (Fig. 1), under diverse and naturalistic voltage protocols and while modified by an antihypertensive drug.
Results
Only VSD-III and -IV display kinetics compatible with muscle Ca2+ release and activate in response to a mammalian muscle action potential
COVG-VCF, a combined electrophysiological-optical approach, is able to detect the voltage and time-dependent conformational rearrangements of individual VSDs10,13 revealing the molecular events that initiate EC coupling in skeletal muscle.
A cysteine (Cys) was introduced at the extracellular flank of S4 helices in each VSD of the CaV1.1 pore-forming subunit (α1S) and labeled with a thiol-reactive fluorophore (Supplementary Fig. 1a) serving as an optical reporter of VSD local conformational changes. The engineered channels retained WT-like functional properties (Supplementary Fig. 1b–d and Supplementary Table 1).
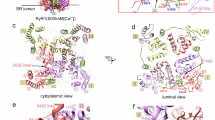
Under physiological conditions (functional channels) and 2 mM external [Ca2+], we simultaneously recorded ionic current and fluorescence changes (reporting transmembrane movements of the individual VSDs) from the entire human CaV1.1 channel expressed in Xenopus oocytes (Fig. 2a).
a Representative Ca2+ currents (ICa) and simultaneously recorded fluorescence signals from human CaV1.1 channels complex (α1S + β1a + α2δ-1 + γ1 + Stac3) reporting local protein structural changes in each VSD. Above the recordings are the step voltage protocol used and the labeling position in the pore-forming α1S subunit of CaV1.1 channels structure (Protein Data Bank ID 5GJV;53, top view). The black lines superimposed to the fluorescence traces at 20 mV are best fits to a single (VSD-I, -III, and -IV) or double exponential functions (VSD-II). b Mean normalized fluorescence representing the VSD activation probabilities during a skeletal muscle AP waveform. For comparison, all the fluorescence traces are presented as positive deflections. An animation combining these AP-clamp data on the CaV1.1 structure is shown in Supplementary Movie 1. c The time constant (τ) of VSD activation to 20 mV. For VSD-II, the relative amplitudes (Amp%) of fast and slow components are reported above the τ values (VSD-I: n = 9, VSD-II: n = 5, VSD-III: n = 9, VSD-IV: n = 6). d VSDs activities at resting membrane potential (Vm). Note the elevated activity of VSD-IV at resting membrane potential (VSD-I: n = 6, VSD-II: n = 4, VSD-III: n = 5, VSD-IV: n = 6). e Maximal VSD activity recorded during AP-clamp from experiments as in (b). Note that only fast-activating VSD-III and -IV respond to an AP stimulus. (VSD-I: n = 6, VSD-II: n = 4, VSD-III: n = 5, VSD-IV: n = 6). Error bars are ± SEM.
Each VSD reacted to depolarizing pulses with distinct kinetics and voltage dependence (Fig. 2a). VSD-I displayed the slowest kinetics of activation (τ activation at 20 mV = 76.9 ± 6.5 ms, n = 9) while VSD-III and -IV activated very rapidly with time constants shorter than 2 ms (τ activation: VSD-III = 1.81 ± 0.2 ms, n = 9, VSD-IV = 1.79 ± 0.2 ms, n = 6, Fig. 2c). On the other hand, VSD-II, whose dominant time constant of activation is slow (τslow = 212.1 ± 31.6 ms, 71% ± 4% of the total amplitude), displayed a minor fast component of 3.2 ± 0.6 ms (n = 5) that accounted for 29% ± 4% of the total amplitude (Fig. 2c). Note that Ca2+ release from the SR is a very fast event that peaks within 3-4 ms11,14: thus, in principle, VSD-II, -III and -IV possess at least one kinetic component of activation fast enough to allow their movement in response to a muscle AP (3–6 ms). This is a necessary condition to act as a voltage sensor for RyR1 activation and fast Ca2+ release.
To determine the sensitivity of each VSD to a physiological stimulus, we optically recorded the activation of each VSD (Fig. 2b and Supplementary Movie 1) using a mammalian skeletal muscle AP waveform as a voltage command. The collected fluorescence signal integrates both voltage dependence and kinetic properties of the individual VSD and informs as to whether a specific sensor is activated at rest and by a physiological AP (duration at half-maximum amplitude = 1.5 ms). First, we found that AP-evoked conformational changes could be detected in all VSDs, similar to optical studies in cultured murine fibers11. Yet, stark differences in the fractional activation of each VSD were revealed when the data were normalized to the limiting maximal and minimal ΔF from the same cell. We found that at the resting membrane potential (Vm = − 93 mV), the activity of VSD-IV is quite high (17% ± 2%), a feature that disqualifies this sensor from having a role in SR Ca2+ release. On the other hand, the other VSDs had no significant activity at resting membrane potential (Fig. 2b and d). We found that only VSD-III and -IV reached a probability of activation of 58% ± 5% (n = 5) and 63% ± 4% (n = 6) of the maximal activation, respectively (Fig. 2b and e). Not surprisingly, the signals from the slowest sensors (VSD-I and -II) were barely detectable as they did not promptly respond to the brief AP, reaching an activation probability of only 6 ± 3%, n = 6 (VSD-I) and 19 ± 4%, n = 4 (VSD-II) (Fig. 2b and e).
Only VSD-III displays both fast kinetics and voltage dependence compatible with skeletal muscle Ca2+ release
VSD-III and -IV are able to activate in response to an AP: however, to act as bona fide RyR1 voltage sensor, a VSD needs to match the voltage dependence of RyR1-mediated Ca2+ release, a measurable parameter in muscle EC coupling. To identify the VSDs with a voltage dependence compatible with that of skeletal muscle Ca2+ release, we constructed their voltage-dependent activation curves. As shown in Fig. 3a, the four VSDs are highly heterogeneous in their voltage sensitivity (z, effective valence) and potential of half-activation (V1/2) (Fig. 3a and Supplementary Table 2):
a Mean voltage dependence of VSD activation from experiments as in Fig. 2a. Lines represent fits to single Boltzmann distributions (data are represented as mean ± SEM, error bars when not visible are within the symbols; fitting parameters are reported in Supplementary Table 2). Note that each VSD exhibited distinct voltage-dependent properties (VSD-I: n = 9, VSD-II: n = 5, VSD-III: n = 10, VSD-IV: n = 6, G(V): n = 6). b Voltage dependence curves from panel (a) superimposed together with the voltage dependence of the Ca2+ release flux curves (black lines) from mammalian adult skeletal muscle fibers for comparison. The reported curves (black lines) were constructed from the Boltzmann distribution fit parameters in (i) Ferreira-Gregorio et al., 2017: V1/2 = − 30 mV, z = 2.85 e018, (ii) Szentesi et al., 1997: V1/2 = − 29.5 mV, z = 2.7 e016, (iii) Hernandez-Ochoa et al., 2014: V1/2 = − 24 mV, z = 1.92 e017, (iv) Struk et al., 1998: V1/2 = − 14 mV, z = 3.25 e015, (v) DiFranco et al., 2022: V1/2 = − 13 mV, z = 3.13 e019. The yellow highlighted area delimits the ranges of membrane potentials where the Ca2+ release flux occurs. Note that only VSD-I and -III activate with a voltage dependence similar to that of SR Ca2+ release.
VSD-IV displayed the most negative half-activation potential and was the least sensitive to changes in membrane potential (V1/2 = − 32 ± 2 mV; z = 0.6 ± 0.02 e0, n = 6). VSD-II moves at the most depolarized membrane potentials with a V1/2 = 8 ± 1 mV, and z = 1.5 ± 0.1 e0 (n = 5). VSD-I and VSD-III exhibited similar activation (VSD-I: V1/2 = − 22 ± 1 mV; z = 2.2 ± 0.1 e0 n = 9; VSD-III: V1/2 = − 24 ± 1 mV; z = 1.6 ± 0.04 e0, n = 10) (Fig. 3a and Supplementary Table 2).
Remarkably, VSD-I and VSD-III display an overall voltage dependence that closely follows that reported for Ca2+ release in muscle fibers measured in 1.8-2.0 mM [Ca2+]ext as in the present study. Figure 3b illustrates this finding, showing the activation curves (F(V)) of the four VSDs superimposed with Ca2+ release curves obtained from human15 and rodent16,17,18,19 studies of adult muscle fibers. Note that only VSD-I and -III voltage dependence falls in the narrow membrane potential range where SR Ca2+ release occurs. Importantly, these results dismiss VSD-II and -IV as sensors for RyR1 activation (and Ca2+ release).
Thus, VSD-III stands out as the single sensor with kinetics (Fig. 2) and voltage dependence (Fig. 3) of the skeletal muscle Ca2+ release process.
Pharmacological evidence reinforces VSD-III role as the voltage sensor of muscle contraction
Dihydropyridines (DHPs) are a class of Ca2+ channel blockers that bind to the pore-forming subunit of L-type CaV channels, including CaV1.1. Nifedipine, a well-known antihypertensive and antianginal drug20, binds to the pore region at the interface of Repeats III and IV21,22,23 (Fig. 4a), preventing Ca2+ entry (Fig. 4b, c). Paradoxically, although Ca2+ influx through the CaV1.1 is not required for skeletal muscle contraction24,25, numerous studies have found that this drug alters, SR Ca2+ release and contraction in mammalian and amphibian muscles7,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39. Remarkably, nifedipine was found to perturb the charge movement of CaV1.17,30. Together, this body of literature provides strong evidence that nifedipine modifies the behavior of the VSD(s) that confer voltage dependence to RyR1 opening and RyR1-mediated SR Ca2+ release. Figure 4 shows representative fluorescence recordings in control (no drug) and in the presence of 10 µM nifedipine. Note that nifedipine binding to the pore perturbed VSD-III activation, such that its voltage dependence shifted towards hyperpolarized potential by ~20 mV (control: V1/2 = −25 ± 1 mV, n = 14; nifedipine V1/2 = −43 ± 2 mV, n = 14, P < 0.001). To a lesser extent, also the activation of VSD-I was affected (control: V1/2 = −30 ± 2 mV, n = 11, nifedipine V1/2 = −45 ± 2 mV, n = 5, P < 0.001). On the other hand, nifedipine did not modify VSD-II and -IV (Fig. 4f and Supplementary Table 3).
a Structure of CaV1.1 α1S interacting with nifedipine. Color code: repeat I in blue; repeat II in red; repeat III in green; repeat IV in yellow; Ca2+ ions in purple and nifedipine in black. The inset shows an enlargement of the nifedipine docking site, which includes parts of the α1S pore domain: S5III, S6III, P-loopIII, and S6IV helices. Side chains of putative residues coordinating nifedipine are shown. Red dashed lines represent potential hydrogen bonds (Protein Data Bank ID 6JP5, adapted from ref. 23). b Representative Ba2+ current traces before (black) and after 10 µM nifedipine (violet). c Normalized current-voltage relationship constructed from traces as in (b) (n = 6). d, e CaV1.1 VSD activations in the absence (“control”, d) and in the presence of 10 µM nifedipine (e). The voltage-clamp protocol is shown above the traces in panel (d). f F(V) curves for the four VSDs in the absence (circles) and presence of nifedipine (10 µM, squares). Note that nifedipine caused a leftward shift of the activation curves of VSD-I and -III, while leaving VSD-II and -IV unaffected. Data were fitted to the Boltzmann distribution. Fitting parameters are reported in Supplementary Table 3. Error bars are ± SEM (VSD-I: control n = 11, nifedipine n = 5; VSD-II: control n = 8, nifedipine n = 5; VSD-III: control n = 14, nifedipine n = 14; VSD-IV: control n = 7, nifedipine n = 7).
In contrast to VSD-I, VSD-III activation is not strongly coupled with the pore-domain open conformation, in the absence of nifedipine10. Moreover, a nifedipine-bound channel structure did not show nifedipine binding on VSD-III23. How does nifedipine facilitate VSD-III activation? One straightforward explanation is that the pore structural configuration induced by nifedipine binding is conformationally coupled to both VSD-I and -III, such that the activations of both domains are allosterically facilitated. In related L-type CaV1.2 channels, VSD-III and the pore are conformationally coupled40, so nifedipine binding to CaV1.1 may not need to induce a very drastic structural change to influence VSD-III.
In conclusion, these results further demonstrate the relevance of VSD-III to skeletal muscle EC-coupling, since this is the sole sensor that: (i) possesses fast kinetics compatible with that of Ca2+ release, (ii) shares the same voltage dependence as of SR Ca2+ release and (iii) is perturbed by nifedipine, a calcium channel blocker that alters Ca2+ release in skeletal muscle (Fig. 4f).
Discussion
VSD-III of CaV1.1 is the voltage sensor of SR Ca2+ release
In 1791, Italian scientist Luigi Galvani made a groundbreaking observation, associating for the first time muscle contraction with electricity41. Since then, great progress has been made in identifying the players of the signaling cascade that, starting from the electrical excitation, leads to contraction11,42,43,44,45. Remarkably, Schneider and Chandler recognized that a movement of charge within the sarcolemma was a key step in skeletal muscle EC coupling6. A few years later, Rios and Brum7 established that the charge movement originated from the CaV1.1 channel, which functions as a voltage sensor of Ca2+ release. We now know that CaV1.1 comprises four distinct and independent VSDs (Fig. 1) with at least one of them functioning as RyR1 voltage sensors.
In our study, we have probed the properties of each VSD of the human CaV1.1 and singled out VSD-III as the only sensor with (i) kinetics, (ii) voltage dependence and (iii) pharmacological properties compatible with the properties of SR Ca2+-release in skeletal muscle fibers (Figs. 2–4 and 5a). During an AP, the activation of VSD-III represents the earliest molecular transition responsible for the mechanical opening of RyR1 (Fig. 5b), thus coupling excitation with contraction.
a Summary of the VSD properties that fit those of skeletal muscle Ca2+ release. b During a skeletal muscle AP, the displacement of the charged VSD-III controls RyR1 activation and muscle contraction. For simplicity, the interaction of one CaV1.1 and one RyR1 monomer is shown. Created in BioRender. Angelini, M. (2025) https://BioRender.com/z78y343.
Two distinct CaV1.1 VSDs control two different ion channels
Our recent work has revealed a stunning heterogeneity in the properties and functional role of the four VSDs of L- and N-type channels including CaV1.1, CaV1.2, CaV2.1, and CaV2.210,40,46,47,48. However, CaV1.1 is unique among the CaV family as its VSDs must control two separate processes with drastically different kinetics and voltage dependence: (i) opening of the CaV1.1 pore and (ii) opening of RyR1. CaV1.1 has assigned these two functions to two distinct VSDs: VSD-I, a slowly activating sensor, drives the opening of CaV1.110. This sensor is the most energetically coupled to the pore, its activation contributing ~80 meV towards the stabilization of the open state10, setting the ionic current kinetics10,49. On the other hand, VSD-III controls RyR1, which inherits its fast kinetics and voltage dependence, distinctive of muscle SR Ca2+ release (Figs. 2, 3). Interestingly, VSD-III has no role in gating CaV1.1 as it was found to have no contribution to CaV1.1 opening10. Thus, CaV1.1 VSD-III’s exclusive role is to be the voltage sensor, in trans, of RyR1.
Prior work, that pioneered the use of voltage-clamp fluorometry in muscle fibers11, could not conclusively assign a voltage-sensing role to a specific VSD(s) but found that the activation of VSD-III was slower than that of Ca2+ release. However, in the present work, COVG-VCF provided the voltage control necessary to correctly assign the activities of all VSDs. Our positive identification of a single VSD consistent with a very stringent set of conditions (and positive exclusion of the other VSDs), including the voltage dependence of Ca2+ release reported in several studies, and a muscle AP clamp, supports the validity of our findings and the major role of VSD-III in the EC coupling. Our finding is also consistent with the finding that mutations in VSD-III alone impaired the voltage dependence of Ca2+ release, reported in a recent study on cultured murine myotubes12.
An important structural element found to be essential for skeletal muscle EC coupling, is CaV1.1 “II-III loop” connecting Repeat-II and -III intracellularly (Fig. 1)50,51. The II-III loop is crucial for the propagation of CaV1.1 conformational rearrangements to RyR1, and perturbations in its amino acid sequence have been found to impair EC coupling51. While available structures of CaV1.1 alone23,52,53 or in complex with RyR14 have not resolved this flexible region, several studies have highlighted a distinct region of interaction with RyR154. Notably, VSD-III is the sensor directly connected with the II-III loop, which is likely to mediate the propagation of VSD-III voltage-dependent movements to RyR1, conferring voltage dependence to contraction. In addition, the β1a subunit42 and Stac3 (which binds II-III loop55,56) have also been found necessary for muscle EC coupling43,44.
Nifedipine perturbs both muscle EC coupling and VSD-III
The role of VSD-III in EC coupling is also supported by its sensitivity to nifedipine, shared only with VSD-I (Fig. 4). Although skeletal muscle EC coupling does not depend on Ca2+ influx24,25, a surprisingly large number of studies found that nifedipine alters skeletal muscle contraction. Most of these studies report an overall reduction in Ca2+ release7, contraction28,29,31,32,33,34,35,37, and force27,38 in fibers and muscles. Other studies have observed the stimulatory effect of nifedipine25,26,36,39. While the endpoints of nifedipine action are still being debated, there is strong consensus on its ability to modify EC coupling, demonstrated in a variety of experimental settings and model species. Based on this body of work, it is not surprising to find that nifedipine altered VSD-III, the only sensor with kinetics and voltage dependence of SR Ca2+ release.
Nifedipine binding to CaV1.1 channel induces a significant change in VSD-III activation by shifting its activation towards more negative membrane potentials (Fig. 4d–f), likely to be the perturbation responsible for altering EC coupling.
Interestingly, we found that nifedipine had also an effect on VSD-I activation (Fig. 4d–f). As nifedipine is known to stabilize an inactivated state of L-type Ca2+ channels20,57,58, the new conformational state of the nifedipine-bound channel is revealed as a leftward shift of the voltage dependency of VSD-I and -III (Fig. 4f). In the nifedipine-bound state, VSD-I and VSD-III appear uncoupled from the CaV1.1 pore and RyR1, as they still respond to depolarizations but are unable to gate CaV1.1 pore and RyR1, respectively, possibly explaining a reduction in EC coupling observed in most studies7,27,28,29,31,32,33,34,35,37,38.
Limitations and advantages of the Xenopus oocyte expression system in this study
A limitation of this study is that the conformational changes of the four VSDs have been captured in the absence of RyR1. We have attempted to reconstitute the EC coupling machinery in oocytes by expressing RyR1 together with the full CaV1.1 complex and Junctophilin, but we could not obtain evidence that functional coupling between the two channels occurred. Thus, CaV1.1 VSD activities are measured in a non-skeletal-muscle environment, independently of Ca2+ release and any retrograde effect of RyR1 on CaV1.1. On the other hand, the Xenopus oocyte expression system, combined with the COVG-VCF technique13,59,60 has provided unprecedented information on the biophysical properties of the individual VSDs of the human CaV1.1 channel, revealing their kinetics, voltage dependence, and sensitivity to nifedipine. The large signal-to-noise ratio of current and fluorescence of the oocyte expression system, combined with the fastest oocyte voltage-clamp (COVG), have revealed significant differences among the four VSDs, and singled out VSD-III as the voltage sensor of muscle contraction.
In summary, we have probed the excitation-driven molecular events within the CaV1.1 channel associated with EC coupling. Among the four VSDs, the activation of VSD-III is likely the earliest voltage-dependent molecular transition in the skeletal muscle EC coupling process. The other sensors do not possess fast kinetics (VSD-I), voltage dependence (VSD-II and -IV), or nifedipine sensitivity (VSD-II and -IV) that characterize skeletal muscle SR Ca2+ release.
We propose that the conformational rearrangements of VSD-III propagate to RyR1, bestowing voltage sensitivity to this receptor: VSD-III activation is the molecular event that makes muscle contraction an electrical phenomenon, as first observed in a frog leg by Luigi Galvani over two centuries ago41.
Methods
Ethical statement
All animals are hosted in UCLA-maintained housing facilities; all animal procedures were approved by the UCLA Institutional Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health. Protocol numbers: ARC-2001-124 (Xenopus laevis) and ARC-2014-081-AM-003 (Mouse).
Molecular biology
Human CACNA1S (α1S, GenBank accession no. BC133671)61 was coexpressed with auxiliary subunits rabbit β1a (UniProt accession no. P19517), rabbit α2δ-1 (UniProt accession no. P13806), human γ1 (UniProt accession no. Q06432) and mouse Stac3 (UniProt accession no. Q8BZ71) in Xenopus laevis oocytes.
A Cys was substituted in α1S at the extracellular flank of the S4 helix of each VSD by site-directed mutagenesis, using the QuikChange Site-Directed Mutagenesis Kit (Agilent Technologies) to create L159C and L164C (VSD-I), M519C (VSD-II), V893C (VSD-II), or S1231C (VSD-IV) as previously described10. The cRNA of the different subunits was transcribed in vitro using mMESSAGE mMACHINE T7 (Ambion) or AmpliCap-Max T7 High Yield Message Maker Kit (CELLSCRIPT) and injected into Xenopus laevis oocytes.
Oocyte preparation
Animals were sourced from Xenopus1, Corp USA. Oocyte lobes were surgically harvested from Xenopus laevis and defolliculated using collagenase type I (207 U/ml), as previously described10. Stage V-VI oocytes were injected at the equator with 50 nl cRNA mix containing the CaV1.1 complex.
Oocytes were incubated for 4–5 days at 18 °C in either SOS (in mM: 100 NaCl, 2 KCl, 1.8 CaCl2, 1 MgC12, and 5 HEPES, pH = 7.0, with 100 U/ml penicillin, 100 µg/ml streptomycin, and 50 µg/ml gentamicin) or in a solution composed by 50% L-15 Leibovitz’s L-15 (Corning cellgro), 47.5% H2O, 10% heat-inactivated horse serum (HyClone), 100 U/ml penicillin, 100 µg/ml streptomycin, and 100 µg/ml amikacin (Cayman Chemical Company).
Cut-open Oocyte Vaseline Gap - Voltage-Clamp Fluorometry (COVG-VCF)
4–5 days after injection, oocytes were incubated on ice with with thiol-reactive fluorophores, sensitive to environmental changes (10 µM tetramethylrhodamine-6-maleimide [TMRM-6′] (AAT Bioquest) for L159C or 10 µM tetramethylrhodamine-5-maleimide [TMRM-5′] (AAT Bioquest) for L164C or 20 µM MTS-5(6)-carboxytetramethylrhodamine [MTS-TAMRA] (Santa Cruz) for VSD-II, -III, or -IV) in a depolarizing solution (in mM: 120 K-methanesulfonate(MES), 2 Ba(MES)2 or 2 Ca(MES)2, and 10 HEPES, pH = 7.0). All stocks were 100 mM in DMSO.
Voltage-clamp fluorometry (VCF)62 was performed at room temperature using the Cut-open oocyte Vaseline gap (COVG), implemented for epifluorescence measurements13,59,60,63. The VCF setup consists of an Olympus BX51WI upright microscope with an LED light source and appropriate filter set. Fluorescence emission was acquired using a 40× water immersion objective (LUMPlanFl, Olympus Optical) and amplified using a Dagan Photomax 200 system (Dagan Corporation).
External solution (mM) contained 120 NaMES, 2 Ca(MES)2, 10 HEPES (pH = 7.0). Internal solution (mM) contained 120 K-Glutamate, 10 HEPES (pH = 7.0). Intracellular micropipette solution (mM) was composed of 2700 NaMES, 10 NaCl, and 10 HEPES (pH = 7.0). Prior to the experiments, oocytes were injected with 100 nl 100 mM BAPTA•4 K (Invitrogen), 10 mM HEPES, pH = 7.0, to prevent activation of native Ca2+- and Ba2+-dependent Cl− channels64. Nifedipine experiments were performed in 2 mM Ba(MES)2 (Fig. 4), and L159C cysteine mutant was used for VSD-I VCF recordings (instead of L164C) since it provides a larger signal-to-noise ratio with a modest change in the voltage-dependent properties (Supplementary Figs. 1,2 and Supplementary Table 1). Nifedipine (Alomone Labs) was dissolved in DMSO to make a 100 mM stock solution.
Fluorescence changes and ionic currents were elicited during a 200 ms depolarizing square pulse or using a skeletal muscle action potential (AP) waveform (see next section) as a voltage command. Holding potential was −90 mV. Ionic current and fluorescence were acquired simultaneously from the same membrane area and signals were filtered at 1/5 of the sampling frequency (typically 1–5 kHz).
Skeletal muscle action potential
Animals were sourced from the Jackson lab, USA. Action potentials (APs) were recorded using the two-electrode voltage-clamp technique from enzymatically dissociated flexor digitorum brevis fibers from C57BL mice as previously described65. When filled with a solution mimicking the intracellular milieu (“intracellular solution”), both electrodes had resistances of 10-12 MΩ. The intracellular solution contained (in mM) 75 aspartate, 5 ATP-Na2, 5 phospho-creatine di-Tris, 5 reduced glutathione, 5 MgCl2, 30 EGTA, 15 Ca(OH)2, and 20 MOPS, pH = 7.4 with KOH. The extracellular solution contained (in mM) 145 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, and 10 MOPS, 10 glucose, pH = 7.4 with NaOH. APs were elicited by supra-maximal 0.5 ms current pulses. AP waveform was digitized and used as a voltage command in VCF experiments.
Data analysis
The voltage dependence of ionic conductance G(V), estimated from the peaks of the tail currents, was fitted to a Boltzmann equation:
where Itail, max is the maximum tail current, z is the valence, V1/2 is the half-activation potential, Vm is the membrane potential, F and R are the Faraday and gas constants, and T the absolute temperature, respectively. G(V) data points and fitting curves were normalized to the fitting parameter Itail, max.
Fluorescence vs voltage curves (F(V)) were constructed by plotting the fluorescence amplitude at the end of 200 ms depolarizing pulses against the membrane potential. Data points were fitted to a Boltzmann equation in this form:
where Fmax is the maximum fluorescence deflection and Fmin is the minimum fluorescence deflection.
Fluorescence recordings under AP-clamp (Fig. 2b, d, e) were normalized as:
where F is the fluorescence recorded during the action potential, Fmin and Fmax are the minimum and maximal fluorescence, respectively, as determined during 200 ms depolarizing square pulse protocol (F(V)) in the same cell.
Fluorescence kinetics in Fig. 2a, c were fitted to one or the sum of two exponential function(s).
where B is the baseline, A is the amplitude, t is time, and τ is the time constant.
Statistical Analysis
Statistical significance was assessed using two-tailed unpaired Student’s t-tests. Data are presented as means ± SEM.
Protein structure depiction
CaV1.1 (Protein Data Bank ID 5GJV53 and 6JP523) and RyR1 (3J8H66) were rendered using PyMOL (Schrödinger). Supplementary Movie 1 was rendered in Blender 4.3 (the Blender Foundation) as previously for CaV2.247. For the pore opening (conductance) in Supplementary Movie 1: first, the current trace recorded during the AP-clamp had the gating current subtracted. The ionic current trace was converted to conductance by dividing by the driving force using the reversal potential value calculated by a current-voltage plot from the same cell. The conductance trace was then normalized by dividing by the maximal conductance. This was derived from the cell’s Itail,max divided by the driving force of the tail current.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data that support this study are available from the corresponding authors upon request. Previously published structures and related PDB codes used in the manuscript are: 5GJV, 3J8H and 6JP5. Source data are provided in this paper.
References
Hernandez-Ochoa, E. O. & Schneider, M. F. Voltage sensing mechanism in skeletal muscle excitation-contraction coupling: coming of age or midlife crisis? Skelet Muscle 8, 22 (2018).
Sandow, A. Excitation-contraction coupling in muscular response. Yale J. Biol. Med. 25, 176–201 (1952).
Block, B. A., Imagawa, T., Campbell, K. P. & Franzini-Armstrong, C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J. Cell Biol. 107, 2587–2600 (1988).
Xu, J. et al. In situ structural insights into the excitation-contraction coupling mechanism of skeletal muscle. Sci. Adv. 10, eadl1126 (2024).
Bannister, R. A. Bridging the myoplasmic gap II: more recent advances in skeletal muscle excitation-contraction coupling. J. Exp. Biol. 219, 175–182 (2016).
Schneider, M. F. & Chandler, W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature 242, 244–246 (1973).
Rios, E. & Brum, G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature 325, 717–720 (1987).
Tanabe, T., Beam, K. G., Powell, J. A. & Numa, S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature 336, 134–139 (1988).
Bezanilla, F. Gating currents. J. Gen. Physiol. 150, 911–932 (2018).
Savalli, N. et al. The distinct role of the four voltage sensors of the skeletal CaV1.1 channel in voltage-dependent activation. J. Gen. Physiol. 153, https://doi.org/10.1085/jgp.202112915 (2021).
Banks, Q. et al. Voltage sensor movements of Ca(V)1.1 during an action potential in skeletal muscle fibers. Proc Natl Acad Sci USA 118, https://doi.org/10.1073/pnas.2026116118 (2021).
Pelizzari, S. et al. Ca(V)1.1 voltage-sensing domain III exclusively controls skeletal muscle excitation-contraction coupling. Nat. Commun. 15, 7440 (2024).
Pantazis, A. & Olcese, R. in Encyclopedia of Biophysics (eds Gordon Roberts & Anthony Watts) 1–9 (Springer Berlin Heidelberg, 2019).
Delbono, O. & Stefani, E. Calcium transients in single mammalian skeletal muscle fibres. J. Physiol. 463, 689–707 (1993).
Struk, A., Lehmann-Horn, F. & Melzer, W. Voltage-dependent calcium release in human malignant hyperthermia muscle fibers. Biophys. J. 75, 2402–2410 (1998).
Szentesi, P., Jacquemond, V., Kovacs, L. & Csernoch, L. Intramembrane charge movement and sarcoplasmic calcium release in enzymatically isolated mammalian skeletal muscle fibres. J. Physiol. 505, 371–384 (1997).
Hernandez-Ochoa, E. O., Olojo, R. O., Rebbeck, R. T., Dulhunty, A. F. & Schneider, M. F. beta1a490-508, a 19-residue peptide from C-terminal tail of Cav1.1 beta1a subunit, potentiates voltage-dependent calcium release in adult skeletal muscle fibers. Biophys. J. 106, 535–547 (2014).
Ferreira Gregorio, J., Pequera, G., Manno, C., Rios, E. & Brum, G. The voltage sensor of excitation-contraction coupling in mammals: Inactivation and interaction with Ca(2). J. Gen. Physiol. 149, 1041–1058 (2017).
DiFranco, M. & Cannon, S. C. Voltage-dependent Ca(2+) release is impaired in hypokalemic periodic paralysis caused by Ca(V)1.1-R528H but not by Na(V)1.4-R669H. Am. J. Physiol. Cell Physiol. 323, C478–C485 (2022).
Zamponi, G. W., Striessnig, J., Koschak, A. & Dolphin, A. C. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol. Rev. 67, 821–870 (2015).
Nakayama, H. et al. Identification of 1,4-dihydropyridine binding regions within the alpha 1 subunit of skeletal muscle Ca2+ channels by photoaffinity labeling with diazipine. Proc. Natl. Acad. Sci. USA 88, 9203–9207 (1991).
Striessnig, J., Murphy, B. J. & Catterall, W. A. Dihydropyridine receptor of L-type Ca2+ channels: identification of binding domains for [3H](+)-PN200-110 and [3H]azidopine within the alpha 1 subunit. Proc. Natl. Acad. Sci. USA 88, 10769–10773 (1991).
Zhao, Y. et al. Molecular basis for ligand modulation of a mammalian voltage-gated Ca(2+) channel. Cell 177, 1495–1506 (2019).
Armstrong, C. M., Bezanilla, F. M. & Horowicz, P. Twitches in the presence of ethylene glycol bis(-aminoethyl ether)-N,N’-tetracetic acid. Biochim. Biophys. Acta 267, 605–608 (1972).
Dayal, A. et al. The Ca(2+) influx through the mammalian skeletal muscle dihydropyridine receptor is irrelevant for muscle performance. Nat. Commun. 8, 475 (2017).
Gallant, E. M. & Goettl, V. M. Effects of calcium antagonists on mechanical responses of mammalian skeletal muscles. Eur. J. Pharmacol. 117, 259–265 (1985).
Ildefonse, M. et al. Excitation contraction coupling in skeletal muscle: evidence for a role of slow Ca2+ channels using Ca2+ channel activators and inhibitors in the dihydropyridine series. Biochem. Biophys. Res. Commun. 129, 904–909 (1985).
Lamb, G. D. Components of charge movement in rabbit skeletal muscle: the effect of tetracaine and nifedipine. J. Physiol. 376, 85–100 (1986).
Caputo, C. & Bolanos, P. Contractile inactivation in frog skeletal muscle fibers. The effects of low calcium, tetracaine, dantrolene, D-600, and nifedipine. J. Gen. Physiol. 89, 421–442 (1987).
Lamb, G. D. & Walsh, T. Calcium currents, charge movement and dihydropyridine binding in fast- and slow-twitch muscles of rat and rabbit. J. Physiol. 393, 595–617 (1987).
Leoty, C. & Noireaud, J. Effects of external cations and calcium-channel blockers on depolarization-contraction coupling in denervated rat twitch skeletal muscles. Pflugers Arch 408, 146–152 (1987).
Dulhunty, A. F. & Gage, P. W. Effects of extracellular calcium concentration and dihydropyridines on contraction in mammalian skeletal muscle. J. Physiol. 399, 63–80 (1988).
Gamboa-Aldeco, R., Huerta, M. & Stefani, E. Effect of Ca2+ channel blockers on K+ contractures in twitch fibres of the frog (Rana pipiens). J. Physiol. 397, 389–399 (1988).
Neuhaus, R., Rosenthal, R. & Luttgau, H. C. The effects of dihydropyridine derivatives on force and Ca2+ current in frog skeletal muscle fibres. J. Physiol. 427, 187–209 (1990).
Cognard, C., Rivet, M. & Raymond, G. The blockade of excitation/contraction coupling by nifedipine in patch-clamped rat skeletal muscle cells in culture. Pflugers Arch. 416, 98–105 (1990).
Kitamura, N., Ohta, T., Ito, S. & Nakazato, Y. Effects of nifedipine and Bay K 8644 on contractile activities in single skeletal muscle fibers of the frog. Eur. J. Pharmacol. 256, 169–176 (1994).
Posterino, G. S. & Lamb, G. D. Effect of nifedipine on depolarization-induced force responses in skinned skeletal muscle fibres of rat and toad. J. Muscle Res. Cell Motil. 19, 53–65 (1998).
Manttari, S. & Jarvilehto, M. Comparative analysis of mouse skeletal muscle fibre type composition and contractile responses to calcium channel blocker. BMC Physiol. 5, 4 (2005).
Menezes-Rodrigues, F. S. et al. Calcium influx through L-type channels attenuates skeletal muscle contraction via inhibition of adenylyl cyclases. Eur. J. Pharmacol. 720, 326–334 (2013).
Pantazis, A., Savalli, N., Sigg, D., Neely, A. & Olcese, R. Functional heterogeneity of the four voltage sensors of a human L-type calcium channel. Proc. Natl. Acad. Sci. USA 111, 18381–18386 (2014).
Galvani, L. De Viribus Electricitatis in Motu Musculari Commentarius (1791).
Gregg, R. G. et al. Absence of the beta subunit (cchb1) of the skeletal muscle dihydropyridine receptor alters expression of the alpha 1 subunit and eliminates excitation-contraction coupling. Proc. Natl. Acad. Sci. USA 93, 13961–13966 (1996).
Nelson, B. R. et al. Skeletal muscle-specific T-tubule protein STAC3 mediates voltage-induced Ca2+ release and contractility. Proc. Natl. Acad. Sci. USA 110, 11881–11886 (2013).
Horstick, E. J. et al. Stac3 is a component of the excitation-contraction coupling machinery and mutated in Native American myopathy. Nat. Commun. 4, 1952 (2013).
Perni, S., Lavorato, M. & Beam, K. G. De novo reconstitution reveals the proteins required for skeletal muscle voltage-induced Ca(2+) release. Proc. Natl. Acad. Sci. USA 114, 13822–13827 (2017).
Savalli, N. et al. The alpha2delta-1 subunit remodels CaV1.2 voltage sensors and allows Ca2+ influx at physiological membrane potentials. J. Gen. Physiol. 148, 147–159 (2016).
Nilsson, M. et al. Voltage-dependent G-protein regulation of Ca(V)2.2 (N-type) channels. Sci. Adv. 10, eadp6665 (2024).
Wang, K. et al. A rich conformational palette underlies human CaV2.1-channel availability. Nat. Commun. 16, 3815 (2025).
Fernandez-Quintero, M. L. et al. Structural determinants of voltage-gating properties in calcium channels. Elife 10, https://doi.org/10.7554/eLife.64087 (2021).
Tanabe, T., Beam, K. G., Adams, B. A., Niidome, T. & Numa, S. Regions of the skeletal muscle dihydropyridine receptor critical for excitation-contraction coupling. Nature 346, 567–569 (1990).
Nakai, J., Tanabe, T., Konno, T., Adams, B. & Beam, K. G. Localization in the II-III loop of the dihydropyridine receptor of a sequence critical for excitation-contraction coupling. J. Biol. Chem. 273, 24983–24986 (1998).
Wu, J. et al. Structure of the voltage-gated calcium channel Cav1.1 complex. Science 350, aad2395 (2015).
Wu, J. et al. Structure of the voltage-gated calcium channel Ca(v)1.1 at 3.6 A resolution. Nature 537, 191–196 (2016).
Ogawa, H., Kurebayashi, N., Yamazawa, T. & Murayama, T. Regulatory mechanisms of ryanodine receptor/Ca(2+) release channel revealed by recent advancements in structural studies. J. Muscle Res. Cell Motil. 42, 291–304 (2021).
Wong King Yuen, S. M., Campiglio, M., Tung, C. C., Flucher, B. E. & Van Petegem, F. Structural insights into binding of STAC proteins to voltage-gated calcium channels. Proc. Natl. Acad. Sci. USA 114, E9520–E9528 (2017).
Polster, A., Nelson, B. R., Papadopoulos, S., Olson, E. N. & Beam, K. G. Stac proteins associate with the critical domain for excitation-contraction coupling in the II-III loop of Ca(V)1.1. J. Gen. Physiol. 150, 613–624 (2018).
Sanguinetti, M. C. & Kass, R. S. Voltage-dependent block of calcium channel current in the calf cardiac Purkinje fiber by dihydropyridine calcium channel antagonists. Circ. Res. 55, 336–348 (1984).
Bean, B. P. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc. Natl. Acad. Sci. USA 81, 6388–6392 (1984).
Stefani, E. & Bezanilla, F. Cut-open oocyte voltage-clamp technique. Methods Enzymol. 293, 300–318 (1998).
Gandhi, C. S. & Olcese, R. The voltage-clamp fluorometry technique. Methods Mol. Biol. 491, 213–231 (2008).
Wu, F., Quinonez, M., DiFranco, M. & Cannon, S. C. Stac3 enhances expression of human Ca(V)1.1 in Xenopus oocytes and reveals gating pore currents in HypoPP mutant channels. J. Gen. Physiol. 150, 475–489 (2018).
Mannuzzu, L. M., Moronne, M. M. & Isacoff, E. Y. Direct physical measure of conformational rearrangement underlying potassium channel gating. Science 271, 213–216 (1996).
Cha, A. & Bezanilla, F. Characterizing voltage-dependent conformational changes in the Shaker K+ channel with fluorescence. Neuron 19, 1127–1140 (1997).
Barish, M. E. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J. Physiol. 342, 309–325 (1983).
DiFranco, M., Kramerova, I., Vergara, J. L. & Spencer, M. J. Attenuated Ca(2+) release in a mouse model of limb girdle muscular dystrophy 2A. Skelet Muscle 6, 11 (2016).
Yan, Z. et al. Structure of the rabbit ryanodine receptor RyR1 at near-atomic resolution. Nature 517, 50–55 (2015).
Acknowledgements
We are thankful to Drs. Michela Ottolia, Alan Neely, and Andreas Schwingshackl, and their laboratory members, for the insightful discussions and comments. This study was supported by grants from the National Institutes of Health/National Institute of General Medical Sciences R35GM131896 (R.O.), National Institute of Arthritis and Musculoskeletal and Skin Diseases R01AR063182 (S.C.), and R21AR080282-02R (M.D). Start-up funds from the Linköping University Wallenberg Center for Molecular Medicine / the Knut and Alice Wallenberg Foundation (A.P.); Hjärnfonden (The Swedish Brain Foundation) grant FO2023-0025 (A.P.); Vetenskapsrådet (The Swedish Research Council) grant 2022-00574 (A.P.).
Author information
Authors and Affiliations
Contributions
Conceptualization: M.A., N.S., M.D., S.C., A.P., and R.O. Investigation: M.A., N.S., F.S., S.M., M.D., S.C., and R.O. Formal analysis M.A., N.S., F.S., S.P., A.P., and R.O. Methodology: M.A., N.S., F.S., A.P., and R.O. Funding acquisition: M.D., S.C., A.P., and R.O. Supervision: M.A. and R.O. Writing – original draft: M.A. and R.O. Writing – review & editing: All.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Angelini, M., Savalli, N., Steccanella, F. et al. The molecular transition that confers voltage dependence to muscle contraction. Nat Commun 16, 4847 (2025). https://doi.org/10.1038/s41467-025-59649-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-59649-7