Abstract
Nucleoporin 98 (NUP98) fusion oncoproteins are strong drivers of pediatric acute myeloid leukemia (AML) with poor prognosis. Here we show that NUP98 fusion-expressing AML harbors an epigenetic signature that is characterized by increased accessibility of hematopoietic stem cell genes and enrichment of activating histone marks. We employ an AML model for ligand-induced degradation of the NUP98::KDM5A fusion oncoprotein to identify epigenetic programs and transcriptional targets that are directly regulated by NUP98::KDM5A through CUT&Tag and nascent RNA-seq. Orthogonal genome-wide CRISPR/Cas9 screening identifies 12 direct NUP98::KDM5A target genes, which are essential for AML cell growth. Among these, we validate cyclin-dependent kinase 12 (CDK12) as a druggable vulnerability in NUP98::KDM5A-expressing AML. In line with its role in the transcription of DNA damage repair genes, small-molecule-mediated CDK12 inactivation causes increased DNA damage, leading to AML cell death. Altogether, we show that NUP98::KDM5A directly regulates a core set of essential target genes and reveal CDK12 as an actionable vulnerability in AML with oncogenic NUP98 fusions.
Similar content being viewed by others
Introduction
Acute myeloid leukemia (AML) is a heterogeneous malignant disease of the hematopoietic system that is characterized by the accumulation of immature myeloid blasts in the bone marrow (BM). The landscape of somatic mutations in pediatric AML differs substantially from adult AML and is characterized by lower mutational burden1. Hence, targeted therapies against recurrent mutations approved for the treatment of adult AML are often unsuitable therapeutic options for pediatric patients.
Fusion oncoproteins arise from structural chromosomal rearrangements and frequently act as oncogenic drivers, with a particularly high prevalence in pediatric patients1. The family of Nucleoporin 98 (NUP98) oncofusions features more than 30 distinct partner genes which often comprise factors that harbor DNA-binding homeodomains, plant homeodomain (PHD) fingers and Su(var)3-9, Enhancer-of-zeste, and Trithorax (SET) domains2,3,4.
Fusion of the first 14 exons of the NUP98 gene to the last exons of the KDM5A (also JARID1A) gene results in a cryptic translocation giving rise to the expression of the chimeric NUP98::KDM5A protein5,6,7. Besides its enrichment in pediatric acute megakaryoblastic leukemia (AMKL)6, NUP98::KDM5A is the most common NUP98 fusion found in infant AML and is associated with an aggressive form of the disease and particular poor prognosis7,8. The catalytic Jumonji C (JmjC) domain of the H3K4 demethylase KDM5A is not present in the chimeric fusion protein. However, the C-terminal PHD chromatin recognition domain of KDM5A is preserved in the NUP98::KDM5A fusion protein and is necessary for its oncogenic potential9.
We and others have shown that NUP98 fusion proteins are associated with chromatin and induce global transcriptional dysregulation leading to AML10,11,12. Chromatin association of many NUP98 fusion oncoproteins is mediated by the chromatin-binding domains of the fusion partners and often occurs at genomic regions rich in H3K27ac and H3K4me312,13,14. NUP98 fusion oncoproteins are able to recruit the transcriptional co-activators CBP/p300 and epigenetic regulators, such as histone deacetylase 1 (HDAC1), Non-Specific Lethal, and MLL1/MENIN complexes12,15,16. The formation of transcriptional hubs via the aberrant recruitment of transcription factors and chromatin regulators has been explained by the ability of NUP98 fusion proteins to form biomolecular condensates through liquid-liquid phase separation11,13,17,18. This is mediated by the FG repeats in the intrinsically disordered NUP98 N-terminus and is crucial for the oncogenic potential of NUP98 fusion proteins13,17. Furthermore, expression of NUP98::KDM5A and other NUP98 oncofusions leads to sustained upregulation of HOXA and HOXB genes and other transcription factors that induce a stem/progenitor-like phenotype, such as MEIS19. However, it is unclear how NUP98 fusion oncoproteins actively induce and maintain these transcriptional programs and which effector proteins are required to orchestrate the observed patterns.
In this work we reason that a better understanding of how NUP98 fusion proteins deregulate gene expression on the epigenetic and transcriptional level will provide a rational basis for the development of tailored therapies. By comparing global chromatin accessibility of AML patient samples featuring different disease subtypes with healthy blood cells, we identify epigenetic patterns that are specific to NUP98 fusion-expressing AML. Using a model for ligand-induced degradation of the NUP98::KDM5A fusion oncoprotein we find that NUP98::KDM5A maintains activating H3K27ac and H3K4me3 histone marks on target genes. Furthermore, nascent RNA sequencing upon induced fusion oncoprotein degradation reveals direct transcriptional target genes of NUP98::KDM5A. Integration of these data with results from a genome-wide CRISPR/Cas9 screen in NUP98::KDM5A-driven AML cells identifies a core set of 12 essential direct target genes which are critical for the survival of NUP98::KDM5A-driven leukemia cells. Among them, we validate the kinase CDK12 as an actionable target. NUP98::KDM5A-driven AML expresses high levels of CDK12 and is highly sensitive to genetic and pharmacological CDK12 perturbation. In line with the role of CDK12 in regulating the transcription of DNA damage repair genes, CDK12-inactivating small molecules cause increased DNA damage in NUP98::KDM5A AML cells. These results represent the basis for further investigations of CDK12-targeting compounds and the development of tailored treatments for patients with NUP98 rearrangements.
Results
Epigenomic analysis of pediatric AML reveals NUP98 fusion-specific patterns
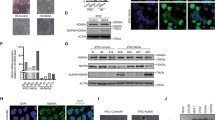
To gain a better understanding of the epigenetic landscape of NUP98 oncofusion-driven leukemia, we performed ATAC-seq on primary AML patient samples to determine global patterns of chromatin accessibility. In addition to samples expressing NUP98::KDM5A and NUP98::NSD1 (n = 4, each), we included samples from other pediatric AML patients (n = 7) with different oncogenic driver mutations (KMT2A::MLLT3, CBFB::MYH11, CBFA2T3::GLIS2, and normal karyotype) (Supplementary Data 1). We also included publicly available ATAC-seq data from adult AML samples and additional pediatric AML samples (of which two expressed NUP98::NSD1)19, as well as data from healthy hematopoietic stem and progenitor cells (HSPC) and mature myeloid blood cells (Supplementary Data 2). Principal component analysis revealed that chromatin accessibility patterns of AML patient samples were broadly distributed across healthy blood cells, with most of them ranging within different stages of hematopoietic progenitors (Fig. 1A and Supplementary Fig. 1A, B). This is in line with the concept of AML arising from progenitor cells with a block of differentiation that are skewed to particular stages of hematopoietic differentiation20,21. To derive epigenetic signatures that are specific for NUP98 fusion-driven AML we next aimed to identify the healthy progenitor subtype that is closest to the NUP98 fusion-expressing AML cells. Inter-sample distance visualized by minimum spanning tree analysis revealed that granulocyte-monocyte progenitor (GMP) cells are the closest normal counterpart of NUP98 fusion-expressing AML cells based on chromatin accessibility (Fig. 1B). Comparison of these two cell populations identified 1921 and 6054 regions close to gene promoters that were significantly more or less accessible in NUP98 fusion-driven AML cells compared to GMP cells, respectively (Supplementary Data 3). Accessibility of the 1921 more open regions was highest in NUP98::KDM5A AML cells over hematopoietic stem cells (HSC), while the same regions were not accessible in GMPs and mature myeloid cells (Fig. 1C). The 6054 regions enriched in GMPs are also highly accessible in HSCs and associated with reduced accessibility in mature myeloid cells (Supplementary Fig. 1C). Overall, these analyses show that NUP98::KDM5A-expressing AML exhibits a global reduction in chromatin accessibility when compared to healthy progenitors, but a gain in the accessibility of HSC-specific regions.
A Principal component (PC) analysis of ATAC-seq data from pediatric and adult AML patients and samples from healthy blood cells, including HSCs, progenitor cells, and mature myeloid cells. B Minimum spanning tree of distances between ATAC-seq signals of NUP98 fusion-expressing and other pediatric AML samples and samples of healthy blood cells. C ATAC-seq profile plots showing 1921 more accessible regions in NUP98 fusion-expressing AML vs. GMPs. D Heatmap and profile plots of ATAC-seq, H3K27ac, and H3K4me3 CUT&Tag data showing 1921 genomic regions that are more accessible in NUP98 fusion-expressing AML vs. GMPs. E Heatmap showing ATAC-seq unsupervised clustering of 893 differentially accessible regions in NUP98 fusion-expressing AML patient samples compared to NUP98 wild-type pediatric AML patient samples (HSC hematopoietic stem cell, MPP multipotent progenitor, CMP common myeloid progenitor, MEP Megakaryocyte-erythrocyte progenitor, GMP granulocyte-monocyte progenitor). Source data are provided as a Source Data file.
Genomic regions with high accessibility are often enriched for activating histone marks22. Indeed, by performing CUT&Tag (Cleavage Under Targets and Tagmentation) for H3K27ac and H3K4me3 marks in blasts from a NUP98::KDM5A patient-derived xenograft (PDX) AML model, we found that the majority of the 1921 genomic regions that are specifically accessible in NUP98 fusion-driven AML also showed overlapping H3K27ac and H3K4me3 marks (Fig. 1D). Importantly, genes associated with these genomic regions were highly expressed in NUP98::KDM5A-positive AML cells compared to other subtypes of pediatric AML23 (Supplementary Fig. 1D). Analysis of ChIP-seq data from a murine HA-tagged NUP98::KDM5A model10 (Table 1) showed that genes associated with these 1921 more accessible regions featured particularly high levels of fusion oncoprotein binding (Supplementary Fig. 1E). Finally, transcription factor motif analysis revealed that consensus motifs of GATA- and AP1-families were enriched in genomic regions with high accessibility in NUP98::KDM5A AML, while binding sites for ETS and IRF transcription factors were enriched in regions that were less accessible in NUP98::KDM5A-expressing cells (Supplementary Fig. 1F).
Unsupervised clustering of ten NUP98 fusion-expressing versus ten NUP98 wild-type AML samples revealed a cluster of regions which were more accessible in AML samples expressing NUP98::NSD1 and NUP98::KDM5A (Fig. 1E). In addition, we also found regions with NUP98::KDM5A-specific accessibility. These regions might reflect lineage-specific characteristics associated with acute megakaryocytic leukemia (M7) diagnosed in these NUP98::KDM5A-expressing samples.
Taken together, our analysis of chromatin accessibility across a multitude of AML patient samples and healthy hematopoietic cell types showed that NUP98 fusion-driven AML harbors an epigenetic signature that is characterized by increased accessibility of HSC-associated regions and enrichment of activating histone marks.
Induced NUP98::KDM5A degradation causes terminal differentiation of AML blasts
Given the specific effects of NUP98::KDM5A on the epigenome of AML cells we aimed to develop a model that allows the direct investigation of NUP98::KDM5A-dependent molecular effects. We used the degradation tag (dTAG) system for ligand-induced protein degradation24,25 to establish a dTAG-NUP98::KDM5A cell line (Fig. 2A and Supplementary Fig. 2A). The dTAG-NUP98::KDM5A construct was introduced into murine fetal liver-derived HSPCs alongside the clinically observed activated NrasG12D co-mutation3,7,26 and cells were transplanted into sublethally irradiated recipient mice (Supplementary Fig. 2B). In this model, recipient animals developed an aggressive, transplantable AML-like disease (Supplementary Fig. 2C, D)10. The immuno-phenotype of leukemic blasts from the BM, spleen and blood of recipient mice was similar to previously established murine NUP98::KDM5A models10, showing moderate levels of c-Kit and high levels of Mac-1 and Gr-1 surface markers, confirming the myeloid origin of the leukemia (Supplementary Fig. 2E, F). The BM of AML-engrafted mice was collected and cultured in vitro until a stably growing dTAG-NUP98::KDM5A-expressing cell line was established (Table 1). ATAC-seq and RNA-seq analysis of dTAG-NUP98::KDM5A cells revealed a strong correlation of chromatin accessibility and gene expression with NUP98::KDM5A AML patient data (Supplementary Fig. 2G), further substantiating the clinical relevance of our mouse model for the analysis of the human disease.
A Schematic overview of the constructs used for the generation of the dTAG-NUP98::KDM5A cell line. B Representative plots of flow cytometric analysis of intracellular NUP98::KDM5A protein levels upon treatment with dTAG13 or DMSO. (Blue, dTAG-NUP98::KDM5A cell line; grey, untagged NUP98::KDM5A control cell line (Table 1)). C Intracellular flow cytometric analysis of NUP98::KDM5A protein levels after treatment of dTAG-NUP98::KDM5A cells with indicated concentrations of dTAG13 for 18 h (n = 3 biological replicates, mean ± SD, two-sided, unpaired t-test), D and after treatment with 35 nM dTAG13 for indicated time points (n = 3 biological replicates, mean ± SD, two-sided, unpaired t-test). E Flow cytometric analysis of cell cycle distribution of dTAG-NUP98::KDM5A cells after treatment with dTAG13 (35 nM) and DMSO for 3 days (n = 3 biological replicates, mean ± SD, two-sided, unpaired t-test), including representative histogram plots (right). F Flow cytometric analysis of apoptosis after treatment of dTAG-NUP98::KDM5A cells with dTAG13 (35 nM) and DMSO for 3, 7, and 10 days (n = 3 biological replicates, mean ± SD, two-sided, unpaired t-test). G Representative cytospin images showing the morphology of dTAG-NUP98::KDM5A cells after treatment with dTAG13 (35 nM) or DMSO for 3 and 5 days. Scale bar = 20 μm. Representative images of n = 2 samples. H Flow cytometric analysis of surface expression of c-Kit, (I) Gr-1 and (J) Mac-1 of dTAG-NUP98::KDM5A cells after treatment with dTAG13 (35 nM) and DMSO for 7 days (n = 3 biological replicates, mean ± SD, two-sided, unpaired t-test), including representative histogram plots (bottom). Parts of the figure were created in BioRender. Grebien, F. (2025) https://BioRender.com/d32l249. Source data are provided as a Source Data file.
Degradation of the dTAG-NUP98::KDM5A fusion oncoprotein was induced by addition of the dTAG13 ligand molecule24. Flow cytometric quantification of dTAG-NUP98::KDM5A levels revealed that dTAG13 treatment induces near-complete degradation of the dTAG-NUP98::KDM5A protein without affecting NrasG12D levels (Fig. 2B and Supplementary Fig. 2H). 35 nM of the dTAG13 molecule caused efficient NUP98::KDM5A degradation within 15 min of treatment and complete fusion oncoprotein degradation was achieved after 1 h (Fig. 2C, D). Degradation of the NUP98::KDM5A fusion protein leads to a G1-phase cell cycle arrest followed by apoptosis at later stages (Fig. 2E, F). Induced NUP98::KDM5A degradation caused significant morphological changes in AML cells that are characteristic of terminal myeloid differentiation (Fig. 2G), including loss of the progenitor marker c-Kit and an increase of the myeloid differentiation markers Mac-1 and Gr-1 (Fig. 2H–J).
Altogether, these data show that the dTAG-NUP98::KDM5A AML cell line model allows fast and complete dTAG13-induced degradation of the fusion protein. This NUP98::KDM5A-driven model is fully dependent on sustained expression of the oncogenic driver, as its degradation leads to cell cycle arrest, differentiation, and apoptosis of leukemia cells (Supplementary Fig. 2I).
NUP98::KDM5A actively maintains H3K27ac marks on target genes
To obtain further insights into how NUP98::KDM5A shapes epigenetic patterns to induce malignant transformation of hematopoietic progenitor cells, we investigated changes in the epigenetic landscape upon acute NUP98::KDM5A degradation. Chromatin immunoprecipitation (ChIP)-qPCR showed that the H3K27ac mark was strongly decreased on the promoters of the NUP98::KDM5A target genes Hoxa9 and Meis1 already after 3 h of fusion protein degradation (Fig. 3A). While loss of the H3K4me3 mark was also detected in the same regions, its decrease occurred at a slower rate, peaking at 6 h of dTAG13 treatment (Supplementary Fig. 3A). Western blot analysis showed that global levels of H3K27ac and H3K4me3 did not change upon dTAG13 treatment (Supplementary Fig. 3B).
A H3K27ac ChIP-qPCR in dTAG-NUP98::KDM5A cells for Hoxa9 and Meis1 after treatment with dTAG13 for indicated time points compared to DMSO and a negative control genomic region (n = 2 technical replicates, mean ± SD). B Heatmaps and profile-plots of H3K27ac CUT&Tag data of dTAG13 (35 nM) and DMSO-treated dTAG-NUP98::KDM5A cells showing 161 significantly downregulated sites compared to 1000 random sites with no significant changes (n = 3, FDR < 0.05). C Representative tracks of H3K27ac CUT&Tag data (dTAG13 and DMSO). D Time-series plots showing cluster analysis of up and downregulated transcripts from RNA-seq data of dTAG-NUP98::KDM5A cells treated with dTAG13 (35 nM) for 8 h or 24 h compared to DMSO (0 h) (n = 4 biological replicates per time point). The upper and lower ends of the bars represent the minimum and maximum expression values for the respective group. The lines represent the mean expression profile across replicates for each group. E Heatmap of RNA-seq data in dTAG-NUP98::KDM5A cells after 8 h and 24 h of dTAG13 treatment compared to DMSO, showing 249 significantly differentially expressed genes at 24 h dTAG13 treatment (n = 4, log2FC > 1 or < −1, padj < 0.05, statistical analysis and p-value calculations were performed using DESeq2). F Scatter plot showing log2FC values in gene expression (QUANT-seq) and H3K27ac levels (CUT&Tag) upon dTAG13 treatment (35 nM, 8 h) compared to DMSO. G Hockey stick plot of H3K27ac CUT&Tag data in dTAG-NUP98::KDM5A cells (left). Genes are ranked according to normalized read signal, including a quantitative box plot representation (right, target genes n = 38, other genes n = 24,415, two-sided Wilcoxon rank sum test with continuity correction, p-value < 2.2e-16). Data are presented as boxplots where the center line represents the median, the bounds of the box indicate the first (25th percentile) and third (75th percentile) quartiles and the whiskers extend to 1.5 × inter-quartile range from the hinges. Data points beyond this range are shown as individual dots and represent outliers. Source data are provided as a Source Data file.
Next, we performed CUT&Tag after 8 h of dTAG13 treatment to investigate global changes of H3K27ac and H3K4me3 distribution on chromatin. 161 genomic regions showed a significant reduction of H3K27ac levels while only 37 regions were associated with reduced H3K4me3 levels upon NUP98::KDM5A degradation. No regions showed an increase in the levels of these histone marks upon degradation of the fusion protein (Fig. 3B and Supplementary Fig. 3C, D). The genomic regions with altered H3K27ac and H3K4me3 levels strongly overlapped and corresponded to 84 and 29 genes, respectively (Supplementary Fig. 3E). The accessibility of chromatin associated with these genes was highly conserved between mouse and human NUP98::KDM5A AML cells, further indicating that the NUP98::KDM5A fusion oncoprotein controls similar regulatory circuits in mouse and human AML (Supplementary Fig. 3F). Among the genes associated with reduced H3K27ac levels upon dTAG13 treatment we found known NUP98::KDM5A target genes like Meis1 and genes of the Hoxa cluster, but also several genes that had not been linked to AML before (Fig. 3C and Supplementary Data 4). Gene Ontology (GO) analysis confirmed that genes with differential H3K27ac signals were significantly enriched for regulators of transcription (Supplementary Fig. 3G).
To determine if the observed epigenetic changes upon loss of NUP98::KDM5A are also reflected at the transcriptional level we performed mRNA sequencing (RNA-seq) after 8 h and 24 h of NUP98::KDM5A degradation. We identified four major clusters of genes that depict time-resolved changes in gene expression with different kinetics (Fig. 3D). Differential expression analysis revealed that 153 genes were significantly downregulated and 96 genes were upregulated after 24 h of fusion protein loss (Fig. 3E and Supplementary Data 5). The expression of genes from the Hoxa gene cluster, as well as other transcription factors like Nkx2-3 and Bcl11a was already downregulated 8 h after NUP98::KDM5A degradation. However, Meis1 downregulation appeared to be a later transcriptional event, since the expression of this gene was only significantly downregulated after 24 h. GO analysis confirmed the concomitant loss of stem cell-associated gene expression and the activation of transcriptional programs characteristic of myeloid differentiation, including Camp, Irf8 and Cebpe (Fig. 3E and Supplementary Fig. 4A). Chromatin accessibility was significantly higher at genes that were downregulated upon NUP98::KDM5A degradation in mouse and human AML samples (Supplementary Fig. 4B). The intersection of H3K27ac CUT&Tag data with RNA-seq data identified 38 NUP98::KDM5A target genes that exhibited a significant loss of H3K27ac marks and were downregulated after fusion protein degradation (Fig. 3F, Supplementary Fig. 4C and Supplementary Data 5). These 38 NUP98::KDM5A target genes were characterized by significantly higher H3K27ac signals when compared to all other genes, and chromatin accessibility at these genes was highest in samples of NUP98::KDM5A AML patients (Fig. 3G and Supplementary Fig. 4D). Additionally, analysis of ChIP-seq data10 showed that NUP98::KDM5A fusion oncoprotein binding was exceptionally high in the promoters of these 38 target genes (Supplementary Fig. 4E).
Taken together, these data show that NUP98::KDM5A drives transcriptional programs of hematopoietic progenitors while repressing programs associated with myeloid differentiation via the maintenance of a specific epigenetic signature.
Identification of direct transcriptional target genes of NUP98::KDM5A
Although our RNA-seq analysis revealed genes that were differentially expressed after NUP98::KDM5A degradation, it is not possible to discern whether these genes are under direct control of the NUP98::KDM5A fusion oncoprotein or if their expression is indirectly affected by changes in NUP98::KDM5A levels. The SLAM-seq (Thiol [SH]-linked alkylation for the metabolic sequencing of RNA) approach enables highly sensitive identification of global changes in nascent mRNA transcripts27,28. We induced complete degradation of NUP98::KDM5A by dTAG13 treatment and labeled newly synthesized mRNA with 4-thiouridine, which allowed the identification of changes in the synthesis of mRNA transcripts that happen within 1 h after NUP98::KDM5A degradation (Fig. 4A). Differential expression analysis revealed 45 genes whose expression was significantly downregulated upon NUP98::KDM5A loss, while the expression of no gene was significantly induced upon fusion oncoprotein degradation (Fig. 4B and Supplementary Data 6). The expression of all 45 direct NUP98::KDM5A target genes was also found downregulated upon prolonged depletion of the fusion protein in the RNA-seq datasets (8 h and/or 24 h), highlighting the necessity of the driver oncoprotein to maintain high expression of these genes (Fig. 4C). Several of the direct NUP98::KDM5A target genes have been described to play roles in leukemia, such as CDKN2C29, CDKN1B30, NKX2-331, IGF2BP332,33, and MN134. Other genes in this list are still unexplored or have been implicated in other cancer types, such as CDK1235,36,37,38,39,40. Analysis of NUP98::KDM5A ChIP-seq data10 revealed that the majority of the direct target genes featured high levels of NUP98::KDM5A chromatin binding (Fig. 4D, E). Furthermore, H3K27ac and H3K4me3 marks were strongly enriched at the transcriptional start sites and throughout the gene bodies of these genes (Fig. 4F). dTAG13 treatment caused a strong reduction of the H3K27ac mark on the promoters of the 45 direct NUP98::KDM5A target genes (Fig. 4G). Finally, the direct NUP98::KDM5A target genes were significantly overexpressed in NUP98::KDM5A-expressing AML cells compared to control cells that only express the NUP98 N-terminus and NrasG12D10 and were highly expressed in samples of NUP98::KDM5A AML patients (Fig. 4H, I).
A Schematic representation of the experimental setup for nascent RNA-seq (SLAM-seq) (4SU, 4-thiouridine). B Volcano plot of SLAM-seq data in dTAG-NUP98::KDM5A cells showing log2FC and –log10(p-value) values after dTAG13 (35 nM, 2 h) vs. DMSO treatment (statistical analysis and p-value calculations were performed using DESeq2). C Heatmap showing gene expression changes of the 45 direct target genes 2 h (SLAM-seq), 8 h and 24 h (RNA-seq) after dTAG13 treatment compared to DMSO treatment. D Hockey stick plot of ChIP-seq data of NUP98::KDM5A chromatin binding. All genes were ranked according to their normalized read counts and 45 direct targets are highlighted in color. E HA-NUP98::KDM5A ChIP-seq data showing normalized read signal of the 45 direct target genes compared to all other genes. F H3K27ac and H3K4me3 CUT&Tag data from dTAG-NUP98::KDM5A cells showing normalized read signal of the 45 direct target genes compared to all other genes. G H3K27ac CUT&Tag data from dTAG-NUP98::KDM5A cells showing normalized read signal for the 45 direct target genes after 8 h dTAG13 treatment compared to DMSO. H RNA-seq data showing gene expression levels of the 45 direct targets compared to all other genes in murine NUP98::KDM5A AML cells compared to control cells expressing a NUP98 N-terminal fragment (all genes n = 18,899, direct targets n = 45, two-sided Mann–Whitney U test, p-value < 0.0001). Horizontal bars represent the mean, error bars +/− SD. I Expression of 42 human homologs of direct NUP98::KDM5A target genes in pediatric AML patients from the St. Jude Cloud data repository. Data are presented as boxplots where the center line represents the median, the bounds of the box indicate the first (25th percentile) and third (75th percentile) quartiles and the whiskers extend to 1.5 × inter-quartile range from the hinges. Data points beyond this range are shown as individual dots and represent outliers. Parts of the figure were created in BioRender. Grebien, F. (2025) https://BioRender.com/w52j392. Source data are provided as a Source Data file.
In summary, we identified 45 immediate downstream target genes of the NUP98::KDM5A fusion oncoprotein. These direct target genes are bound by NUP98::KDM5A at their promoters, feature high levels of activating histone marks, and are highly expressed.
Genome-wide CRISPR/Cas9 screening identifies 12 essential direct target genes of NUP98::KDM5A
To identify genes whose mutational inactivation causes a fitness defect in NUP98::KDM5A cells, we performed a genome-wide CRISPR/Cas9 loss-of-function screen in a NUP98::KDM5A AML cell line (Supplementary Fig. 5A). We generated a single-cell-derived Cas9-expressing variant of murine NUP98::KDM5A AML cells (Table 1) and introduced a genome-wide single guide RNA (sgRNA) library to determine the representation of sgRNAs after 14 population doublings. High technical quality of screening results was confirmed by clear separation of core essential and non-essential genes (Supplementary Fig. 5B)41. Mutational disruption of 4105 genes caused a fitness defect in NUP98::KDM5A-driven cells (Fig. 5A). These genes were enriched for basic cellular functions, but also contained common proto-oncogenes (e.g., Myc, Brd4) and hematopoietic progenitor cell-specific essential genes like Myb42,43. Knockout of 645 genes caused a proliferative advantage, including the tumor suppressor genes Trp53 and Kdm5c44. Twelve of the 45 direct NUP98::KDM5A target genes were essential for NUP98::KDM5A-driven AML cell growth (Fig. 5B). Interestingly, more than half of the essential direct NUP98::KDM5A target genes encode transcription factors, including several members of the Hoxa cluster (Hoxa3, Hoxa7, Hoxa9, Hoxa10). Additionally, we found the homeobox transcription factors SIX1 and NKX2-3, and the transcription factor BCL11A. While the RNA binding protein IGF2BP3 has previously been shown to be important for AML physiology32,33,45, the roles of VCPIP1, SLMAP, and EXOC8 in malignant hematopoiesis are unknown. The cyclin-dependent kinase CDK12 is involved in transcriptional elongation and has been mainly studied in association with solid tumors. Among the 45 direct NUP98::KDM5A target genes, knockout of Mxi1 and Tnrc18 led to a proliferative advantage.
A Volcano plot of genome-wide CRISPR/Cas9 screen data from NUP98::KDM5A-expressing cells showing the log2FC and –log10(p-value) values of mean sgRNA abundance at the screen endpoint vs. the sgRNA pool (log2FC > 2.25 and <−2.25, statistical analysis and p-value calculations were performed using MAGeCK). B Intersection of SLAM-seq and genome-wide CRISPR screen data. C Scheme of competition-based proliferation assay (Dox doxycycline, AUC area under curve). D Heatmap representation of proliferation assay data after shRNA-mediated target knockdown (2× shRNAs/gene) showing mean percent of iRFP670-positive cells over 21 days, with positive controls shown at the top and the negative control shown at the bottom (n = 2, biological replicates). E Scatter plot of SLAM-seq and H3K27ac CUT&Tag data showing log2FC values upon dTAG13 (35 nM) treatment compared to DMSO and essential direct target genes are highlighted in color. F Hockey stick plot of H3K27ac CUT&Tag data from primary material of a NUP98::KDM5A PDX model (left). Genes are ranked according to normalized read signal and essential direct target genes are highlighted in color including a quantitative box plot representation (right, essential direct targets n = 12, all genes n = 26,917, two-sided Wilcoxon rank sum test with continuity correction, p-value = 0.0001632). Data are presented as boxplots where the center line represents the median, the bounds of the box indicate the first (25th percentile) and third (75th percentile) quartiles and the whiskers extend to 1.5 × inter-quartile range from the hinges. Data points beyond this range are shown as individual dots and represent outliers. Parts of the figure were created in BioRender. Grebien, F. (2025) https://BioRender.com/n44f632. Source data are provided as a Source Data file.
To validate the essentiality of the 12 target gene candidates using an orthogonal approach, we performed an arrayed competition-based growth proliferation assay using two doxycycline-inducible small hairpin RNAs (shRNA) targeting each transcript in NUP98::KDM5A AML cells stably expressing the reverse Tet-transactivator (rtTA3)10 (Fig. 5C and Table 1). We observed a strong and fast decrease in cell fitness upon knockdown (KD) of the transcription factors Bcl11a and Nkx2-3 that was comparable to the effects of downregulation of the essential gene controls Myc and Myb and the KD of the NUP98::KDM5A fusion transcript itself (Fig. 5D). A similarly strong but slightly slower effect was observed upon Cdk12 knockdown. Interestingly, we observed a more subtle reduction in cellular fitness upon KD of single Hoxa genes, which could be due to redundant functions of genes in this cluster. However, we confirmed that knockdown of all 12 essential direct target genes caused impaired AML cell growth, thus validating the results from the genome-wide CRISPR/Cas9 screen.
dTAG13-mediated NUP98::KDM5A degradation caused downregulated expression and a reduction in H3K27ac marks in the promoters of these 12 direct essential target genes, indicating that the fusion protein maintains high levels of activating histone marks and active transcription of these genes (Fig. 5E). The majority of the 12 essential direct target genes also featured significantly higher H3K27ac marks in human NUP98::KDM5A leukemia cells from a PDX model compared to the mean signal of all other genes in this dataset (Fig. 5F).
Collectively, we identified a core set of 12 essential direct target genes of the NUP98::KDM5A fusion oncoprotein. These comprise master transcription factors of the leukemogenic program of NUP98::KDM5A-driven AML, as well as other genes which have not been associated with hematologic malignancies.
CDK12 is an essential direct target gene of the NUP98::KDM5A fusion oncoprotein
Among the essential direct targets of NUP98::KDM5A, the cyclin-dependent kinase 12 (CDK12) stood out, as it is currently the only directly druggable protein in this list. CDK12 plays a critical role in the regulation of transcriptional elongation, as its canonical function is to phosphorylate the C-terminal domain of RNA polymerase II upon its interaction with Cyclin K46. Several small molecules to perturb CDK12 have been described46,47,48,49,50, including kinase inhibitors and the heterobifunctional CDK12-specific degrader BSJ-4-11651. CDK12 has been proposed as a functionally relevant, potential biomarker in solid cancers35,37,52,53, but its role in hematologic malignancies has not been extensively studied.
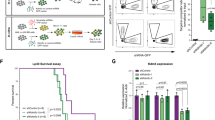
In a large dataset comprising gene expression data from 2487 pediatric cancer patients23, CDK12 expression was slightly but significantly higher in AML patients (n = 320) compared to all other pediatric cancer entities (Fig. 6A). Among AML patients, NUP98 fusion-driven leukemia patients (n = 11) showed even higher CDK12 expression, with highest levels detected in NUP98::KDM5A AML (n = 4). In line with this, CDK12 was the gene with the highest H3K27ac signal in our NUP98::KDM5A PDX CUT&Tag data among the 12 essential direct target genes of the fusion oncoprotein (Figs. 5F and 6B). The CDK12 promoter also showed high H3K4me3 levels in the same PDX sample and was accessible in NUP98::KDM5A patient samples (Fig. 6B and Supplementary Fig. 6A). The NUP98::KDM5A fusion oncoprotein binds to the promoter region and the gene body of Cdk12, and dTAG13-induced loss of NUP98::KDM5A caused a reduction in H3K27ac levels at the Cdk12 locus and decreased Cdk12 expression (Fig. 6B). shRNA-mediated knockdown of Cdk12 using three individual shRNAs caused a strong proliferative disadvantage in NUP98::KDM5A-driven AML cells, highlighting the dependency of these cells on CDK12 (Fig. 6C). Proliferation of murine AML cell lines driven by the KMT2A::MLLT3 fusion (RN2)54 and N-terminal Cebpa mutations (Cebpap30/p30)55 was also dependent on CDK12, suggesting CDK12 is a broad AML dependency, in line with a previous report56 (Supplementary Fig. 6B, C). The kinase activity of CDK12 was required for the proliferation of NUP98::KDM5A AML cells, as exogenous expression of wild-type CDK12, but not a kinase-dead mutant of CDK12 (CDK12D873N)57 restored proliferation in cells harboring Cdk12-targeting shRNAs (Fig. 6D). Finally, knockdown of Cdk12 caused a mild but significant impairment of growth of NUP98::KDM5A-driven AML in vivo (Fig. 6E, F), and immunophenotyping of leukemic blasts showed that Cdk12-deficient, iRFP670/CD45.2-positive AML cells displayed reduced levels of the progenitor marker c-Kit, which is in line with their reduced leukemogenic potential (Fig. 6G and Supplementary Fig. 6D, E).
A Gene expression data for CDK12 of 2487 pediatric cancer patients, AML patients (n = 320), AML with NUP98 rearrangements (n = 11) and AML with NUP98::KDM5A (n = 4) from the St. Jude Cloud data repository (two-sided, unpaired t-test). Data are presented as boxplots, where the center line represents the median, and the bounds of the box indicate the first (25th percentile) and third (75th percentile) quartiles. The whiskers extend down to the 10th percentile and up to the 90th percentile. Data points beyond this range are shown as individual dots and represent outliers. B Representative IGV tracks of H3K27ac CUT&Tag data and SLAM-seq data from dTAG-NUP98::KDM5A cells, and HA-NUP98::KDM5A ChIP-seq data showing the murine Cdk12 locus (top). H3K27ac and H3K4me3 CUT&Tag data in NUP98::KDM5A PDX cells showing the human CDK12 locus (bottom) (PDX patient-derived xenograft). C Competition-based proliferation assay upon doxycycline-inducible shRNA-mediated knockdown of Cdk12 in a murine NUP98::KDM5A (rtTA3) AML cell line, showing percentage of iRFP670-positive cells over 24 days (n = 3 biological replicates). The upper and lower ends of the bars represent the minimum and maximum percent of iRFP670+ cells (normalized to shRen and day 3) for the respective point. D Competition-based proliferation assay upon doxycycline-inducible shRNA-mediated knockdown of Cdk12 in dTAG-NUP98::KDM5A cells stably expressing exogenous CDK12 wild type and CDK12D873N, showing percentage of iRFP670-positive cells on days 2, 7, and 11 on doxycycline (n = 3 biological replicates, mean ± SD, two-sided, unpaired t-test). E Schematic representation of the experimental setup for in vivo shRNA-induced knockdown of Cdk12. F Kaplan–Meier curve of sublethally irradiated recipient mice transplanted with doxycycline-inducible shCdk12-expressing NUP98::KDM5A (rtTA3) AML cells (n = 5 biological replicates, Log-rank (Mantel-Cox) test). G Flow cytometric analysis of CD45.2/iRFP670+ AML blasts from bone marrow of moribund mice showing the percentage of c-Kit surface marker expression (n = 5, mean ± SD, two-sided, unpaired t-test). Source data are provided as a Source Data file.
CDK12 is a druggable vulnerability in NUP98::KDM5A-driven leukemia
As the kinase activity of CDK12 was required for the proliferation of AML cells with NUP98 fusion oncoproteins, we used the CDK12/13 inhibitor THZ53158 to pharmacologically target CDK12. In line with an AML-specific vulnerability, primary human AML cells expressing NUP98::KDM5A and NUP98::NSD1 were 10 times more sensitive to THZ531-mediated CDK12 inhibition than BM mononuclear cells (MNCs) and CD34+ progenitor cells from healthy donors (Fig. 7A). In contrast, the sensitivity of healthy cells and AML cells to the CDK12 degrader BSJ-4-11651 was comparably low (Supplementary Fig. 7A), which is likely attributed to the very high potency of BSJ-4-116 that precluded the faithful evaluation of a potential therapeutic window. Murine and human AML cells expressing different driver mutations were also sensitive to THZ531 and BSJ-4-116, in line with reported data56 (Fig. 7B and Supplementary Fig. 7B).
A Cell viability assay of primary human NUP98-rearranged AML cells, healthy donor BM MNC and CD34+ progenitors treated with indicated concentrations of THZ531 for 3 days (n = 3 technical replicates). B GI50 values from cell viability assays of murine AML cells treated with THZ531 for 3 days. C RT-qPCR analysis of dTAG-NUP98::KDM5A cells treated with THZ531 (2 µM, 24 h) showing log2FC values (n = 3 technical replicates). D Gene set enrichment analysis of RNA-seq data from a doxycycline-controlled NUP98::KDM5A cell line (Tet-Off) after 5 days of doxycycline-induced NUP98::KDM5A downregulation compared to DMSO. E Time series plot of Tet-Off RNA-seq data showing gene expression levels of DNA double strand break repair genes at day 3 and day 5 after NUP98::KDM5A downregulation normalized to NUP98::KDM5A-expressing cells, highlighting selected genes (left), with a density plot of normalized expression of all genes (right). F Proliferation assay of dTAG-NUP98::KDM5A cells stably expressing exogenous CDK12 wild type and CDK12D873N, showing the cumulative cell number on days 2, 7, and 11 of dTAG13 treatment (initial dTAG13 concentration, 35 nM, maintained at 3,5 nM after day 2) (n = 3 biological replicates, mean ± SD, two-sided, unpaired t-test). G Representative images of γH2A.X immunofluorescence staining of dTAG-GFP-NUP98::KDM5A cells treated with THZ531 (2 µM, 24 h) (left) and quantification of γH2A.x signal (right). Midline represents the median, bounds of the box represent the inter-quartile range (Q1 to Q3), and whiskers represent minimum and maximum values (DMSO: n = 125 nuclei (technical replicates), THZ531: n = 131 nuclei (technical replicates) two-sided, unpaired t-test, right). Representative images of n = 8 samples. Source data are provided as a Source Data file.
CDK12 has been proposed to control the expression of DNA repair genes47,51,53. RT-qPCR and RNA-seq analyses of dTAG-NUP98::KDM5A cells confirmed that treatment with THZ531 and BSJ-4-116 induced the downregulation of genes in the DNA damage repair pathway, including the key factors Brca2, Rad50, and Mdc1 (Fig. 7C and Supplementary 7C, D). As Cdk12 is a direct target gene of NUP98::KDM5A, we reasoned that loss of the fusion oncoprotein driver itself would result in similar gene expression changes. Indeed, the expression of DNA repair genes was downregulated after NUP98::KDM5A repression in a doxycycline-controlled Tet-Off model (Table 1)10 (Fig. 7D, E). Additionally, many genes with functions in DNA damage repair are essential for the proliferation of NUP98::KDM5A AML cells, as their knockout caused cell depletion in the genome-wide CRISPR screen (Supplementary Fig. 7E). In fact, exogenous expression of wild-type but not kinase-dead CDK12 was able to partially overcome the proliferation arrest that is associated with dTAG13-mediated NUP98::KDM5A degradation (Fig. 7F). To strengthen our hypothesis that NUP98::KDM5A-expressing AML cells depend on intact DNA damage repair to ensure protection against the accumulation of excessive DNA damage, we measured the levels of the DNA damage marker phosphorylated H2A.X59 in a dTAG-GFP-NUP98::KDM5A cell line (Table 1). Indeed, both CDK12 perturbation as well as dTAG13-induced loss of the NUP98::KDM5A fusion protein caused a significant increase in γH2A.X foci in these cells (Fig. 7G and Supplementary Fig. 7F, G).
Altogether, we show that NUP98::KDM5A-driven AML is hypersensitive to CDK12 perturbation. Therefore, our observations suggest that CDK12 inhibition should be investigated further as a potential therapeutic opportunity for AML patients with NUP98::KDM5A rearrangements.
Discussion
Targeted treatment options for pediatric AML are very limited and the approval of novel therapies is lagging behind recent developments for the treatment of adult leukemia60. In that regard, AML with NUP98 rearrangements is particularly problematic, as affected patients are often refractory to therapy and frequently suffer from early disease relapse7,61. Here, we studied the direct epigenetic and transcriptional effects of the NUP98::KDM5A fusion oncoprotein with a focus on identifying its immediate downstream target genes (Supplementary Fig. 8A). We identify 12 direct transcriptional target genes of the NUP98::KDM5A fusion oncoprotein that are essential for AML cell growth. Mechanistically, we find that the strong dependency of NUP98::KDM5A-driven cells on CDK12 involves efficient DNA damage repair for the survival of AML cells with NUP98 fusions.
Even though global patterns of chromatin accessibility are similar in adult versus childhood leukemia we found genomic regions that were selectively accessible in NUP98-rearranged AML but not in healthy hematopoietic progenitors and other subtypes of pediatric AML. These regions were enriched for activating H3K27ac and H3K4me3 histone marks, which is in line with previous results showing that actively transcribed target genes of NUP98::HOXA9 or NUP98::NSD1 fusion oncoproteins are marked by H3K4me3 and H3K27ac12,13,14.
The establishment of a ligand-inducible model for fast and efficient NUP98::KDM5A degradation enabled the investigation of immediate cellular effects upon complete loss of the oncogenic driver protein. NUP98::KDM5A degradation induced the loss of H3K27ac marks at a defined set of genomic regions, but also the loss of H3K4me3, albeit to a lesser extent. Thus, NUP98::KDM5A chromatin binding appears to actively maintain the specific epigenetic signature associated with these genes. As NUP98::KDM5A degradation also caused the transcriptional downregulation of many of these genes, these data suggest that the NUP98::KDM5A-mediated active maintenance of H3K27ac and H3K4me3 marks at their target genes is involved in the regulation of their expression. Furthermore, the rapid loss of these histone marks upon NUP98::KDM5A degradation suggests that the fusion oncoprotein actively restricts access of histone demethylases and deacetylases to its target genes. As NUP98 fusion proteins were shown to form biomolecular condensates, these higher-order structures likely provide versatile platforms enabling the fast recruitment and/or exclusion of various epigenetic modifiers at target gene loci11. While the content and function of NUP98::KDM5A condensates are incompletely understood, these structures may affect additional epigenetic mechanisms that involve the deposition of other histone marks.
The fast degradation kinetics of the dTAG-NUP98::KDM5A model enabled us to determine immediate transcriptional changes upon loss of the fusion protein. Intersection of this dataset with results from a genome-wide CRISPR/Cas9 screen identified 12 direct target genes of the NUP98::KDM5A fusion oncoprotein that are essential for AML cell growth. This set of genes is enriched for known master regulators of NUP98::KDM5A-driven AML but also features novel candidates that have not been implicated in AML pathogenesis before. It is well known that NUP98 fusion oncoproteins cause overexpression of HOXA cluster genes, which are important regulators of development and self-renewal2,62. Furthermore, deregulated expression of NKX2-3 has been described in a variety of other hematologic malignancies31,63,64. Interestingly, NKX2-3 plays a role in megakaryoblastic development and is important in AMKL, which is the AML subtype with a particular high prevalence of the NUP98::KDM5A fusion31. The SIX1 homeobox transcription factor has also been linked to the maintenance of leukemia stem cells in AML and is important for oncogenic transformation in KMT2A fusion-driven leukemia65,66,67. The BCL11A transcription factor represses the expression of fetal hemoglobin and represents an attractive target in sickle cell anemia and β-thalassemia68. In AML, BCL11A was shown to repress PU.1 target genes, which is in line with our observation that PU.1 motifs are less accessible in NUP98::KDM5A AML cells, and high expression of BCL11A is linked to worse prognosis in AML patients69,70. The oncogenic properties of the RNA-binding protein IGF2BP3 have been studied across many different cancer types, including AML71,72. IGF2BP3 targets mRNAs encoding important factors in leukemia, like MYC, CDK6, HOXA genes, and EPOR, causing their stabilization and overexpression in leukemia cells32,33,45. The direct regulation of Igf2bp3 by NUP98 fusion oncoproteins could therefore represent a mechanism that contributes to the overexpression of Cdk6 that we have previously reported10. The deubiquitinating enzyme VCPIP1, the exocyst complex member EXOC8 and the Sarcolemma associated protein SLMAP were not known to play roles in malignant hematopoiesis and will have to be investigated in future studies.
The direct NUP98::KDM5A target CDK12 is an important regulator of transcriptional elongation via phosphorylating Serine 2 at the C-terminal domain of RNA polymerase II46. Additionally, CDK12 is involved in transcription termination, pre-mRNA splicing, RNA turnover and suppression of intronic polyadenylation47,48. CDK12 is important for the transcription of DNA damage repair genes, in particular those related to homologous recombination (HR)-mediated repair, as these transcripts are long and harbor multiple intronic polyadenylation sites47. In solid cancers, CDK12 has been associated with both tumor suppressive and oncogenic functions73. Our data show that CDK12 is crucial for the growth of NUP98::KDM5A-driven leukemia in vitro and in vivo and that small-molecule-mediated perturbation of CDK12 leads to a profound downregulation of genes involved in DNA repair, as reported in previous studies47,48,53,74. In line with CDK12 being a direct target of NUP98::KDM5A, downregulation of the fusion oncoprotein itself phenocopied the effects of CDK12 loss. We propose that expression of NUP98 fusion proteins and other AML oncogenes cause oncogene-induced replication stress, which has been described as a source of genomic instability in cancer75,76. In line with this, CDK12 has been shown to protect genome integrity by preventing transcription-replication conflicts77,78. However, its role in preventing excessive accumulation of DNA damage via the efficient induction of repair mechanisms likely represents one aspect amongst the broader role of CDK12 in mediating leukemia cell survival (Supplementary Fig. 8B).
Preclinical studies have demonstrated that CDK12 is amenable to pharmacological targeting73. Our data as well as a recent study using several human cell lines show high sensitivity of AML cells towards dual CDK12/13 inhibition, suggesting that CDK12 might be a more broadly relevant target for hematologic malignancies56. While THZ531 has not been optimized for in vivo use, animal studies using the precursor compound THZ1 and related agents showed effective cancer killing without reports of hematotoxicity38,49,50. Further preclinical studies will be required to investigate the potential of CDK12-targeting compounds in NUP98-oncofusion-driven pediatric AML and related diseases.
Methods
All research complies with relevant ethical regulations of the University of Veterinary Medicine Vienna. Fresh-frozen samples of primary bone-marrow mononuclear cells (MNCs) were obtained from the biobank of the St. Anna Children’s Hospital Vienna, Austria. Collection of samples and their use in research was performed with clearance of the appropriate Ethics committee (ethics vote No.1500/2014 of the Ethics Committee of the Medical University Vienna, Vienna, Austria). Samples were collected at diagnosis from patients enrolled in the AML-BFM studies. All patients or their respective legal guardians gave written informed consent prior to the study. The use of human material was approved by the institutional review boards in accordance with the Declaration of Helsinki.
Expression vectors and viral vectors
The dTAG-NUP98::KDM5A construct was generated from a plasmid for constitutive expression of NUP98::KDM5A as previously described10. The FKBP12F36V degradation tag (dTAG) and a triple-V5 affinity tag were fused to the NUP98 N-terminus of the of NUP98::KDM5A fusion protein, as we have previously shown that the addition of exogenous tag sequences does not affect the leukemogenic potential of NUP98::KDM5A10. The FKBP12F36V-tag24 and 3× V5-tag were ordered as one double-stranded DNA fragment and introduced in frame with the N-terminus of the NUP98 sequence via Gibson assembly. The sequence of the 3× V5-tag was kindly provided by the J. Zuber lab. The dTAG-eGFP-NUP98::KDM5A construct was generated from the dTAG-NUP98::KDM5A construct by restriction enzyme-guided removal of the eGFP-IRES-FKBP12F36V-3 × V5 sequence and introduction of an in frame FKBP12F36V-3 × V5-eGFP double-stranded DNA fragment via Gibson assembly. MSCV-rtTA3-IRES-NrasG12D-EF1as-Luc2 has been described previously41. For the generation of the CDK12 wild type overexpression construct pCR8/GW_TOPO_Cdk12_Nterm_FlagHa (Addgene plasmid #127177) expressing the mouse Cdk12 transgene (NM_001109626.1 isoform) was cloned into pLX303-Gw (Addgene plasmid #25897) using Gateway LR Clonase II Enzyme Mix (Invitrogen, USA) according the manufacturer’s protocol. The CDK12 kinase-dead variant CDK12D873N was designed as the murine equivalent of the previously published human CDK12D877N kinase-dead mutant57. The CDK12D873N overexpression construct was generated by inserting the corresponding point mutation into the CDK12 wild type expression construct using the Q5 Site-Directed Mutagenesis Kit (NEB, USA) and the NEBase Changer tool (primers in Supplementary Data 7) according to the manufacturer’s protocol. Plasmid lentiCas9-Blast was a gift from Feng Zhang (Addgene plasmid #52962)79. Lentiviral plasmid psPAX2 (Addgene plasmid #12260) and pMD2.G (Addgene plasmid #12259) were gifts from Didier Trono. Retroviral plasmid pCMV-gag/pol was acquired from Cell Biolabs, USA. The mouse genome-wide Vienna sgRNA library was used as previously described80. shRNA sequences were cloned into pRRL-TRE3G-iRFP670-miR-PGK-NeoR (Supplementary Data 7)81.
Cell culture
Murine fetal liver cells were cultivated in DMEM/IMDM (50:50% vol/vol, Gibco, USA), supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Merck, Germany), 100 U/ml penicillin, 100 µg/ml streptomycin, 4 mM l-Glutamine and 50 µM 2-Mercaptoethanol (all Gibco, USA) in the presence of 100 ng/ml mouse stem cell factor (mSCF), 10 ng/ml mIL-3 nd 10 ng/ml mIL-6 (all from PeproTech, Vienna, Austria). Ex vivo-isolated leukemia cells were cultivated in RPMI 1640 (Gibco, USA), supplemented with 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, 4 mM l-Glutamine, 100 ng/ml mSCF, and 10 ng/ml mIL-3 (all from PeproTech, Austria). After one week, media of the ex vivo-isolated cells was switched to RPMI 1640 supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin, 4 mM l-Glutamine, 1 mM Sodium pyruvate (Merck, Germany), 50 µM 2-Mercaptoethanol (Gibco, USA) and 20 mM 4-(2-Hydroxyethyl)−1-piperazineethanesulfonic acid (HEPES) (Merck, Germany). Stable cell lines were established by continuous culture for over 4 weeks and all murine NUP98::KDM5A cell lines (Table 1) were maintained as described above. Murine AML cell lines driven by NUP98::NSD110, KMT2A::MLLT3 (RN2)54, RUNX1::MECOM41, N-terminal CEBPA mutant (Cebpap30/p30)55, and N/C-terminal CEBPA mutants (CNC)82 were maintained as previously described. All human cell lines, Nomo-1 (ACC 542), HL-60 (ACC 3), MOLM-13 (ACC 554), Kasumi-1 (ACC 220), OCI-AML3 (ACC 582) and K562 (ACC 10) were maintained using RPMI 1640 supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin and 4 mM l-Glutamine and were obtained from DSMZ. Platinum-E (Cell Biolabs, USA) and Lenti-X 293 T cells (Takara, France) were cultivated in DMEM (Gibco, USA) supplemented with 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin and 2 or 4 mM l-Glutamine, respectively. Primary patient-derived leukemia cells, human BM CD34+ Progenitor Cells (#2M-101C) and Human BM MNC (#2M-125C) (Lonza, Switzerland) and PDX cells were cultured in IMDM (Gibco, USA) containing 15% BIT 9500 Serum Substitute (STEMCELL Technologies, Canada), 100 ng/ml SCF, 50 ng/ml Flt3-Ligand, 20 ng/ml IL-3, 20 ng/ml G-CSF (all from PeproTech, Austria), 100 µM 2-Mercaptoethanol, 50 µg/ml gentamicin (Gibco, USA), and 10 µg/ml ciprofloxacin (Merck, Germany) plus 500 nM SR1 (APExBIO, USA) and 1 µm UM729 (APExBIO, USA) as previously described83. All cells were cultured at 37 °C, 5% CO2, and 95% humidity. Human cell lines were validated using STR profiling. Primary cell lines were not authenticated, since testing is not applicable for primary samples and primary cell lines. All cell lines were routinely tested for mycoplasma contamination and confirmed negative.
Virus production and infections
For retrovirus production, Platinum-E cells were co-transfected with 20 µg transfer vector and 5 µg pCMV-gag/pol using Polyethyleneimine (branched, MW 25.000, Sigma-Aldrich, Austria). Virus supernatant was harvested 48 h and 56 h post transfection, filtered (0.45 µm) and supplemented with recombinant mIL-3 (10 ng/ml), mIL-6 (10 ng/ml) (both from PeproTech, Austria) and mSCF (100 ng/ml). Murine progenitor cells were spinoculated with viral supernatants (1:2 diluted) for 45 min at 37 °C at 500 × g in the presence of polybrene (4 µg/ml) (Merck, Germany). For lentivirus production Lenti-X 293 T cells were transfected with 4 µg transfer vector, 2 µg psPAX2 and 1 µg pMD2.G using PEI. Lentivirus was harvested 48 h and 72 h post transfection and filtered (0.45 µm). Target cells were spinoculated after addition of virus supernatant (1:5 diluted) followed by centrifugation for 90 min at 37 °C at 1000 × g in the presence of polybrene (5 µg/ml).
Transplantation-based models and cell line generation
NUP98::KDM5A (control) and dTAG-NUP98::KDM5A cell lines were generated by retroviral co-transduction of either MSCV-eGFP-IRES-NUP98::KDM5A or MSCV-eGFP-IRES-dTAG-3 × V5-NUP98::KDM5A together with MSCV-rtTA3-IRES-NrasG12D-EF1a-Luc2 of murine fetal liver cells (C57BL/6, Ly5.2), followed by continuous culture for 12 weeks. 4 × 106 cells (96% GFP+) cells were transplanted into sublethally irradiated (4,5 Gy) recipient mice (C57BL/6, Ly5.1) via tail vain injection. Disease progression was monitored by whole-body luminescence imaging as previously described84. Mice were euthanized upon disease onset and BM and spleen cells were harvested. Stable cell lines were established by continuous culture of BM cells for 4 weeks without supplemented cytokines. For the secondary transplant of the dTAG-NUP98::KDM5A model, 2 × 106 GFP+ spleen cells (93% GFP+) of primary transplant mice were transplanted into recipient mice (C57BL/6, Ly5.1) via tail vain injection. The dTAG-GFP-NUP98::KDM5A cell line was generated by retroviral co-transduction of MSCV-dTAG-3 × V5-eGFP-NUP98::KDM5A and MSCV-rtTA3-IRES-NrasG12D-EF1a-Luc2 of murine fetal liver cells (C57BL/6, Ly5.2). 4 × 106 cells (6% GFP+) were transplanted into sublethally irradiated (4.5 Gy) recipient mice (C57BL/6, Ly5.1) via tail vain injection. Stable cell lines were established by continuous culture of harvested BM cells of leukemia-bearing mice for 4 weeks without supplemented cytokines. For in vivo shRNA-mediated knock down of Cdk12, the NUP98::KDM5A (rtTA3, Ly5.2) cell line was lentivirally transduced with indicated doxycycline-inducible shRNA constructs and selected with G418 (1 mg/ml, InvivoGen, France). 1 × 106 cells were transplanted into sublethally irradiated (4.5 Gy) recipient mice (C57BL/6, Ly5.1) via tail vain injection. Disease progression was monitored by whole-body luminescence imaging. Upon detectable engraftment at day 3 doxycycline hyclate (4 mg/ml) (Merck, Germany) and Sucrose (20 mg/ml) (Merck, Germany) was dissolved in water and administration was started and exchanged every 2 and 3 days. Mice were euthanized upon disease onset and BM cells were harvested and analyzed. In all cases leukemia development was regularly monitored and animals were euthanized before maximal disease burden was reached. All animal studies were approved by the Ethics and Animal Welfare Committee of the University of Veterinary Medicine, Vienna in accordance with the University’s guidelines for Good Scientific Practice and authorized by the Austrian Federal Ministry of Education, Science and Research (ref BMBWF 68.205/0199-V/3b/2018, 2022-0.874.042) in accordance with current legislation. Animals were maintained on a 12-h light-dark cycle with a room temperature of 21 ± 1 °C and a relative humidity of 40–55%. Animals were housed in specific pathogen-free conditions, fed a standard chow (V1534; Ssniff, Germany), and had access to acidified drinking water ad libitum. Qualified personnel executed all animal experiments and monitored animal welfare daily. Upon the first signs of illness (loss of escape reflex, white paws), the mice were humanely and painlessly euthanized by cervical dislocation. The PDX model of a NUP98::KDM5A AML sample was established as previously described under approval of the legal authorities (131/19)85. Sex was not considered in the study design because no sex bias for NUP98::KDM5A-driven AML has been reported.
Intracellular flow cytometry
NUP98::KDM5A (control) and dTAG-NUP98::KDM5A cells were treated with indicated concentrations of dTAG13 (Merck, Germany) or DMSO for indicated durations. 1 × 106 cells were harvested, washed with PBS and stained with Zombie Aqua Fixable Viability Dye (1:1000, BioLegend, USA) for 10 minutes. After PBS wash, cells were fixed with 2% phosphate-buffered formaldehyde solution (Roti-Histofix 4,5%, Carl Roth, Germany) for 15 min. After washing with PBS, cells were permeabilized with 0.2% TritonX-100 (PanReac AppliChem, Germany) in PBS supplemented with 10% FBS for 15 min, followed by incubation in 0.1% TritonX-100 in PBS supplemented with 10% FBS for 30 min. Next, cells were incubated with anti-mouse CD16/CD32 antibody (1:200, Mouse BD Fc Block, clone 2.4G2, BD Biosiences, Germany) for 10 min, followed by the direct addition of the 2× primary antibody staining solution (1:400 final dilution) in 0.1% TritonX-100 buffer (V5-Tag, clone D3H8Q, Cell signaling, USA) and incubation for 45 min. After a wash with 0.1% TritonX-100 buffer, cells were incubated in secondary antibody staining solution (1:200, Anti-rabbit IgG AF647, #A-21246, Thermo Fisher Scientific, USA) for 45 min. All incubation steps were performed at room temperature with light protection. Stained cells were washed twice with 0.1% TritonX-100 buffer and samples were analyzed on a BD FACSCanto II (BD Biosciences, Germany). Data analysis was done using the FlowJo software package (FlowJo LLC, Ashland, USA).
Flow cytometry
For characterization of ex-vivo-isolated leukemia blasts, cells from BM and spleen were washed with PBS and resuspended in PBS with 0.5% FCS, followed by staining for 30 min with dilutions (1:200) of the following antibodies (all from Biolegend, USA): anti-mouse CD45.2 PE-Cy7 (clone 104), anti-mouse CD11b/Mac-1 PerCP-Cy5.5 (clone M1/70), anti-mouse Gr-1/Ly-6C BV421 (clone RB6-8C5), anti-mouse CD117/c-Kit APC (clone 2B8) and anti-mouse CD117/c-Kit biotin (clone 2B8) together with anti-streptavidin BV421. Engraftment of murine leukemia was assessed by measurement of GFP fluorescence marker expression or staining with anti-CD45.2 antibody (1:200). Analysis of surface marker expression of leukemia cells was performed after gating on GFP-positive or CD45.2/iRFP670-positive cells. Cellular effects after NUP98::KDM5A degradation were assessed after treatment with 35 µM dTAG13 for indicated durations. Myeloid differentiation was monitored by measurement of surface marker expression as described above using the following antibodies (1:200) (all from Biolegend, USA): anti-mouse CD11b/Mac-1 PerCP-Cy5.5 (clone M1/70), anti-mouse Gr-1/Ly-6C PE-Cy7 (clone RB6-8C5) and anti-mouse CD117/c-Kit APC (clone 2B8). Apoptosis analysis was performed using the Annexin V AF647 Conjugate (#A23204, Invitrogen, USA) according to the manufacturer’s instructions. For cell cycle analysis, cells were fixed in 70% Ethanol for 30 min before staining with propidium iodide solution for 30 min. Samples were analyzed on a BD FACSCanto II or on an Intellicyt IQue Screener (Sartorius, Germany). Data analysis was done using the FlowJo or ForeCyte (Essen Bioscience, USA) software packages.
Cytospins
Cells were cytocentrifuged onto glass slides and stained with Rapid-Chrome Kwik-Diff Staining Kit (Thermo Fisher Scientific, USA) followed by microscopic analysis. Photographs were taken on a Zeiss Imager Z.1 microscope and images were processed using Zeiss ZEN software (Zeiss, Germany).
Competitive cell proliferation assay with shRNA knockdown and shCdk12 rescue experiment
To investigate the effect of the gene knockdown, competition-based proliferation assays were performed using rtTA3-expressing AML cells driven by NUP98::KDM5A (with or without stable expression of exogenous CDK12 wild type or CDK12D873N), KMT2A::MLLT3 and N-terminal CEBPA mutations (Cebpap30/p30) that were transfected with doxycycline-inducible shRNAs at an infection rate of approximately 50%10. 5 × 105 cells were seeded in 24-well plates in triplicates. Doxycycline (Merck, Germany) (1 µg/ml) was added to the media to induce knockdown and fluorescence expression of iRFP670 (coupled to shRNAs) was measured every 2 and 3 days using the IntelliCyt iQue Screener Plus (Sartorius, Germany). To compare the effects of candidate gene knockdown on cell growth we calculated the mean area under the curve (AUC) of the iRFP670-positive population, which accounts for the dynamics of the shRNA-induced growth inhibitory effect over time. Data analysis was done using the ForeCyte (Essen Bioscience, USA) software packages and values were normalized to day 3 or 2 after doxycycline induction and the shRenilla negative control.
Proliferation assay for dTAG13 rescue experiment
dTAG-NUP98::KDM5A cells (with or without stable expression of exogenous CDK12 wild type or CDK12D873N) were seeded in triplicates and treated with 35 nM dTAG13 and maintained on 3,5 nM dTAG13 after day 2. Cells were split and counted at regular intervals. Growth rates and cumulative cell numbers were calculated using Microsoft Excel. Growth rate was determined according to Eq. (1).
Cell viability assay
Cells were seeded in white 96-well plates in triplicates and treated with BSJ-4-116 or THZ531 (both MedChemExpress, USA) in a dilution series at indicated concentrations, followed by incubation for 3 days. Cell viability was determined using the CellTiter-Glo® Luminescent Cell Viability Assay (Promega, USA), on a Spark multimode microplate reader (TECAN, Switzerland). Dose–response curves were calculated using the Prism software v6.0.1 (GraphPad, USA).
Immunofluorescence staining and microscopy
dTAG-GFP-NUP98::KDM5A cells were treated with dTAG13, THZ531, BSJ-4-116 or DMSO with indicated concentrations samples were taken after 4 h or 72 h. Cells were cytocentrifuged onto glass slides and spots were then fixed with 4% formaldehyde (Histofix, P087.6, Carl Roth, Germany) for 10 min and permeabilized with 0.2% Triton X-100 in PBS for 15 min at room temperature. For detection of DNA damage, samples were incubated overnight with a polyclonal anti-gamma H2A.X antibody (ab2893, Abcam, UK) (1:1500) followed by 1 h of incubation with a secondary Alexa Fluor-647-conjugated antibody (#A-21246, Invitrogen, USA) (1:333). Slides were counterstained with DAPI and mounted using a water-based fluorescence mounting medium (Agilent, S302380-2). Images were acquired using an inverted confocal microscope with Airyscan super-resolution capability Zeiss LSM 880 Airyscan (Zeiss, Germany) and a 63× oil objective (Zeiss 63×/1.40 Plan-Apochr., Oil, DIC III) in Airyscan mode and Zen Black software. Laser intensities and microscope detectors were carefully set to avoid pixel saturation. The same settings were used to acquire all the samples. Raw.czi files were imported into Arivis Vision 4D (Arivis AG, Germany) and an automated segmentation pipeline was designed manually. The segmented objects were manually proofread and adjusted and the mean pixel intensity was calculated for every individual nucleus.
Western blotting
Western blotting was performed according to standard protocols. Briefly, dTAG-NUP98::KDM5A cells were treated with 35 nM dTAG13 or DMSO for indicated durations, harvested by centrifugation, washed with PBS, snap frozen in liquid nitrogen. Whole-cell lysates were prepared by incubating samples at 95 °C for 10 min in Laemmli buffer, followed by 30 min of sonication. Proteins were separated on a 15% sodium dodecyl sulfate polyacrylamide gel and transferred to nitrocellulose membranes. The following primary antibodies were used for immunoblotting: Histone marks: anti-H3 (ab1791) (1:1000), anti-H3K4me3 (ab8580) (1:1000) and anti-H3K27ac (ab4729) (Abcam, UK) (1:1000), anti-HSC70 (sc-7298, Santa Cruz, USA) (1:10000) and anti-NRas (sc-31, Santa Cruz, USA) (1:1000). The membrane was stripped for 20 min in between antibody incubations.
Real-time quantitative PCR
dTAG-NUP98::KDM5A cells were treated with THZ531 or DMSO for 24 h, harvested by centrifugation, washed with PBS and snap frozen in liquid N2. Total RNA was extracted using the Monarch Total RNA Miniprep Kit (NEB, USA). Reverse transcription was done using RevertAid RT Reverse Transcription Kit (Thermo Fisher Scientific, USA) and quantitative PCR was performed using the SsoAdvanced Universal SYBR Green Supermix (Bio-Rad, USA) on a Bio-Rad CFX-Connect Real-Time PCR Detection System. Results were normalized to Gapdh and analyzed using the 2−ddC(t) method. Primers are listed in Supplementary Data 7.
Chromatin immunoprecipitation (ChIP)-qPCR
ChIP was performed as previously described86. In brief, dTAG-NUP98::KDM5A cells were treated with 35 nM dTAG13 (Sigma-Aldrich, USA) or DMSO for indicated durations. Cells were harvested and washed with PBS, followed by crosslinking with 11% formaldehyde (Thermo Fisher Scientific, USA). The reaction was quenched with 2.5 M glycine (Sigma-Aldrich, USA), and cells were lysed in 1% SDS-buffer. After sonication, cells were incubated overnight with 1 µg/µl anti-H3K4me3 (ab8580, Abcam, UK) or anti-H3K27ac (ab4729, Abcam, UK) antibodies. Antibody-bound DNA was isolated using G-protein coupled magnetic beads (Dynabeads Protein G, Invitrogen, USA) and decrosslinked. Enrichment of selected genomic regions was tested by qRT-PCR and compared to a negative region control. SsoAdvanced Universal SYBR Green Supermix (Bio-Rad, USA) was used for qRT-PCR, which was performed on a Bio-Rad CFX96 TouchTM Real-Time PCR Detection System (Bio-Rad, USA). Data of technical triplicates were analyzed as percent of input. qRT-PCR primer sequences can be found in Supplementary Data 7.
Cleavage under targets and tagmentation (CUT&Tag)
For measurement of H3K27ac and H3K4me histone mark distribution the iDeal CUT&Tag kit for histones compatible with histones and some non-histone proteins (Cat# C01070020, Diagenode, Belgium) was used according to the manufacturer’s protocol. dTAG-NUP98::KDM5A cells were treated with 35 nM dTAG13 (Merck, Germany) or DMSO for 8 h and harvested for processing with the iDeal CUT&Tag kit. Three individual cryopreserved BM samples from a NUP98::KDM5A PDX model were thawed and stained for human CD45 (all approx. 97% hCD45+) and immediately processed with the iDeal CUT&Tag kit. After wash with complete CT Wash Buffer, ConA beads were added and samples were incubated on a rotator. Cells were permeabilized with Complete CT Antibody Buffer and samples were incubated with 1 µg of either anti-H3K4me3 (ab8580), anti-H3K27ac (ab4729) (Abcam, UK) or rabbit IgG (Antibody Package for CUT&Tag anti-rabbit, Diagenode, Belgium) antibodies overnight at 4 °C, while rotating. After removing supernatant, anti-rabbit secondary antibody (1:100) (Antibody Package for CUT&Tag anti-rabbit, Diagenode, Belgium) was added and samples were incubated for 45 min at RT, followed by washing with complete CT Wash Buffer 2. pA-Tn5 transposase diluted in complete CT Wash Buffer 3 (1:250) was added and samples were incubated for 1 h at RT. After washing with complete CT Wash Buffer 3, CT Tagmentation Buffer was added for activation of tagmentation and samples were incubated at 37 °C for 1 h at 800 rpm. To stop tagmentation, CT Buffer E, CT Buffer S, and proteinase K were added and samples were incubated at 55 °C for 1 h at 800 rpm. Column-based DNA purification was performed by the addition of ChIP DNA Binding Buffer. For library amplification, purified DNA was mixed with primer index pairs (24 UDI for Tagmented libraries Set I & II, Diagenode, Belgium) and 2× High Fidelity Mastermix using the manufacturer’s PCR protocol. Libraries were purified using AMPure XP beads (Beckman Coulter, Germany) and analyzed with an Agilent TapeStation instrument (Agilent Technologies, USA) to assess the library quality, size, and concentration. Purified libraries were sequenced on an Illumina NextSeq2000 instrument (Illumina, USA) using paired-end 50 bp chemistry.
RNA sequencing
For RNA-seq analysis, dTAG-NUP98::KDM5A cells were treated with 35 nM dTAG13 (Merck, Germany) or 100 nM BSJ-4-116 (MedChemExpress, USA) for indicated durations. RNA was isolated according to the standard protocol using the NEB Monarch Total RNA Miniprep Kit (NEB, USA). Sequencing libraries were generated using the QuantSeq 3′ mRNA-Seq Library Prep Kit (FWD) for Illumina (Lexogen, Austria) according to manufacturer’s protocol. Purified libraries were sequenced on an Illumina NextSeq2000 instrument (Illumina, USA) using single-read 100 bp chemistry. SLAM-seq was performed as previously described28. dTAG-NUP98::KDM5A cells were treated with 35 nM of dTAG13 (Merck, Germany) for 1 h, followed by the addition of 100 μM 4-thiouridine (4sU, Merck, Germany) for 1 h. RNA was isolated according to the standard protocol using the RNeasy Plus Mini Kit (Qiagen, Germany). Total RNA was alkylated using 10 mM iodoacetamide (Merck, Germany) and RNA was purified by ethanol precipitation. Sequencing libraries were generated using the QuantSeq 3′ mRNA-Seq Library Prep Kit (FWD) for Illumina (Lexogen, Austria) according to manufacturer’s protocol. Purified libraries were sequenced on an Illumina NovaSeq instrument (Illumina, USA) using single-read 100 bp chemistry.
ATAC-seq
Fresh-frozen samples of primary bone-marrow mononuclear cells were rapidly thawed, resuspended in Iscove’s Modified Dulbecco’s Medium (IMDM, Gibco, USA) and washed with PBS (Gibco, USA). Blast populations were identified with a routine diagnostics panel of monoclonal antibodies and sorted via flow cytometry (FACSCalibur, BD Biosciences, USA). dTAG-NUP98::KDM5A cells were harvested by centrifugation and washed with PBS. Subsequent DNA-isolation and library preparation for ATAC-seq was performed on 50,000 sorted blasts using Tagment DNA TDE1 Enzyme and Buffer Kits (Illumina, USA) as previously described87.
Genome-wide CRISPR/Cas9 screen
The design and construction of the murine genome-wide Vienna sgRNA library, as well as the experimental workflow for loss-of-function screening have been previously described80,88. Briefly, a single-cell-derived Cas9-expressing clone of a NUP98::KDM5A-driven AML cell line was infected with a lentivirally-packaged sgRNA library at a low multiplicity of infection (MOI) (≤10%). Infected cells were selected using G418 (1 mg/ml, InvivoGen, France), which was kept in the medium until the end of the screen. Screen endpoint samples were harvested after 14 cell population doublings, and cell pellets corresponding to at least 500-fold library representation were harvested. Next generation sequencing libraries of screen endpoint samples and sgRNA plasmid pools were prepared and sequenced on a HiSeq 2500 instrument (Illumina, USA) using single-read 50 bp chemistry.
Bioinformatics analysis
Genome-wide CRISPR screen
Raw.bam files underwent processing using the crispr-process-nf Nextflow89 pipeline, available at https://github.com/ZuberLab/crispr-process-nf, which included barcode trimming, filtering, and alignment using Bowtie290. The crispr-mageck-nf Nextflow pipeline, available at https://github.com/ZuberLab/crispr-mageck-nf incorporating MAGeCK91, was employed for comprehensive analysis of the CRISPR screen data.
RNA-seq (QUANT-seq)
Raw files underwent preprocessing using prinseq-lite92 (version 0.20.4). Subsequently, alignment against the mouse reference genome (mm10) was performed using STAR93 (version 2.7.9a). Post-processing and sorting of aligned reads was conducted using samtools94 (version 1.4). Counts per gene were obtained utilizing featureCounts95 (version 2.0.3) from the subread package. Normalized expression levels and identification of differentially expressed genes were calculated using the DESeq296 R package. Heatmaps depicting expression patterns were created using the heatmap.2 function from the gplots97 R package. Time-series plots illustrating dynamic expression changes over time were generated using the maSigPro98 R library. Gene set enrichment analysis was performed with GSEA software99 (version 4.3.2). GO-enrichment was performed with EnrichR100 and g:Profiler101 (g:GOst) and plots were generated with SR-plot102.
Nascent RNA-seq (SLAM-seq)
Raw.bam files underwent processing using the slamseq Nextflow pipeline, accessible at https://github.com/nf-core/slamseq. This pipeline integrates multiple tools including fastqc, trim_galore103 and slamdunk104 available at: https://github.com/t-neumann/slamdunk, for quality assessment, adapter trimming, and read mapping. DESeq2 was employed for comparative analysis, facilitating the identification of newly transcribed genes. Heatmaps depicting expression patterns were created using the heatmap.2 function from the gplots R package.
CUT&Tag
Raw files underwent preprocessing using prinseq-lite for quality control and filtering. Alignment against either the mouse (mm10) or human (hg38) reference genomes was conducted using BWA105 (version 0.7.17-r1188). Post-processing and sorting of aligned reads were performed using samtools (version 1.13). The function bamCoverage from Deeptools106 (version 3.5.1) was utilized to generate BigWig files with a binSize of 10, employing Counts Per Million for normalization. Peaks were called using macs2107 (version 2.1.0) with the –broad option. Differential CUT&Tag regions were identified using the R package DiffBind108, applying DESeq2. Tornado and profile plots were created using the generateEnrichedHeatmap from the profileplyr109 R package or Deeptools. Read counts per genes were obtained using featureCounts from the subread package. All hockey-stick and scatter plots were generated using ggplot2110 in R. Coverage plots were generated with IGV111.
ATAC-seq
Publicly available AML patient ATAC-seq datasets were obtained from GEO accessions listed in Supplementary Data 2. Pediatric AML patient data were obtained from the St. Anna Children’s Cancer Research Institute (CCRI) and Yokohama City University Hospital19.
A modified version of the ATAC-seq Data Processing Pipeline112,113 was applied to the raw Bam files, accessible at: https://github.com/epigen/atacseq_pipeline. The pipeline utilized fastp114 for adapter removal and Bowtie2 for read alignment to the GRCh38 (hg38) human reference genome. Duplicate marking was performed with samblaster115 and aligned BAM files were sorted, indexed, and filtered for ENCODE blacklisted regions using samtools. Peaks were called with macs2 (version 2.1.0) applying the –broad option. Differential ATAC-seq regions were identified with DiffBind and region-to-gene annotation was conducted with ChIPseeker116. Counts over exons were obtained using featureCounts and normalization was performed with DESeq2. Principal Component Analysis (PCA) plots were generated using the ggplot2110 package in R. For the minimum spanning tree visualization batch correction was done with ComBat117 and we utilized vegdist from the VEGAN118,119 package and ggraph120. Tornado and profile plots were created with Deeptools and the differential ATAC-seq regions showcased via heatmaps were generated with DiffBind.
Motif enrichment analysis
Motif enrichment analysis was conducted using the findMotifsGenome.pl function from HOMER121 (version 4.1). This analysis was performed on 1921 regions with increased accessibility and 6054 regions with decreased accessibility in the hg38 genome, to identify enriched motifs.
Analysis of AML patient gene expression data
Gene expression data from AML patients were retrieved using the St. Jude Cloud PeCan tool (https://pecan.stjude.cloud/), which provides access to the St. Jude Cloud data repository23. Raw count data were normalized using DESeq2, followed by further data processing in R. Visualizations were generated using the ggplot2110 package.
Statistical analysis
For Cleavage Under Targets and Tagmentation (CUT&Tag), RNA sequencing (RNA-seq), genome-wide CRISPR screen, Gene Ontology (GO) term analyses, assay for transposase-accessible chromatin using sequencing (ATAC-seq), chromatin immunoprecipitation DNA sequencing (ChIP-seq) and gene set enrichment analyses published statistical packages were used as referenced in the respective methods sections. Otherwise, the Prism software (version 6.0.1, GraphPad, USA) was used for statistical analyses, and data are shown as mean ± SD. The unpaired Student’s t test or the Mann–Whitney test were used for P value determination. Results were considered significant when P < 0.05. P values are indicated in each figure. The individual sample size is reported in figure legends.
Schematic figures were generated with Adobe Illustrator CS6 (Adobe, USA) or BioRender.com.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The publicly available Tet-Off RNA-seq data used in this study are available in the Gene Expression Omnibus (GEO) database under accession code GSE13478410. The raw ATAC-seq data from primary pediatric AML samples obtained from the Yokohama City University (YCU)19 are not available due to data privacy laws. The processed ATAC-seq count data are available on Zenodo under https://doi.org/10.5281/zenodo.14943880. The publicly available ATAC-seq data from 15 primary pediatric AML samples obtained from the St. Anna Children’s Cancer Research Institute (CCRI) used in this study are available in the GEO database under accession code GSE282258. The mapping of sample names to GEO accessions is provided in Supplementary Data 1. The genome-wide CRISPR-screen, RNA (QUANT)-seq, nascent (SLAM)-seq, Cut&Tag and ATAC-seq cell line data generated in this study have been deposited in the GEO database under accession code GSE255808. The remaining data are available within the Article, Supplementary Information or Source Data file. Source data are provided with this paper.
References
Bolouri, H. et al. The molecular landscape of pediatric acute myeloid leukemia reveals recurrent structural alterations and age-specific mutational interactions. Nat. Med. 24, 103–112 (2018).
Michmerhuizen, N. L., Klco, J. M. & Mullighan, C. G. Mechanistic insights and potential therapeutic approaches for NUP98-rearranged hematologic malignancies. Blood 136, 2275–2289 (2020).
Gough, S. M., Slape, C. I. & Aplan, P. D. NUP98 gene fusions and hematopoietic malignancies: common themes and new biologic insights. Blood 118, 6247–6257 (2011).
Rasouli, M. et al. NUP98 oncofusions in myeloid malignancies: an update on molecular mechanisms and therapeutic opportunities. HemaSphere 8, e70013 (2024).
Van Zutven, L. J. C. M. et al. Identification of NUP98 abnormalities in acute leukemia: JARIDIA (12p13) as a new partner gene. Genes Chromosom. Cancer 45, 437–446 (2006).
De Rooij, J. et al. NUP98/JARID1A is a novel recurrent abnormality in pediatric acute megakaryoblastic leukemia with a distinct HOX gene expression pattern. Leukemia 27, 2280–2288 (2013).
Bertrums, E. J. M. et al. Comprehensive molecular and clinical characterization of NUP98 fusions in pediatric acute myeloid leukemia. Haematologica 108, 0390–6078 (2023).
Noort, S. et al. The clinical and biological characteristics of NUP98-KDM5A in pediatric acute myeloid leukemia. Haematologica 106, 0390–6078 (2020).
Wang, G. G. et al. Haematopoietic malignancies caused by dysregulation of a chromatin-binding PHD finger. Nature 459, 847–851 (2009).
Schmoellerl, J. et al. CDK6 is an essential direct target of NUP98 fusion proteins in acute myeloid leukemia. Blood 136, 387–400 (2020).
Terlecki-Zaniewicz, S. et al. Biomolecular condensation of NUP98 fusion proteins drives leukemogenic gene expression. Nat. Struct. Mol. Biol. 28, 190–201 (2021).
Xu, H. et al. NUP98 fusion proteins interact with the NSL and MLL1 complexes to drive leukemogenesis. Cancer Cell 30, 863–878 (2016).
Ahn, J. H. et al. Phase separation drives aberrant chromatin looping and cancer development. Nature 595, 591–595 (2021).
Heikamp, E. B. et al. The menin-MLL1 interaction is a molecular dependency in NUP98-rearranged AML. Blood 139, 894–906 (2022).
Kasper, L. H. et al. CREB binding protein interacts with nucleoporin-specific FG repeats that activate transcription and mediate NUP98-HOXA9 oncogenicity. Mol. Cell. Biol. 19, 764–776 (1999).
Bai, X. T. et al. Trans-repressive effect of NUP98-PMX1 on PMX1-regulated c-FOS gene through recruitment of histone deacetylase 1 by FG repeats. Cancer Res. 66, 4584–4590 (2006).
Chandra, B. et al. Phase separation mediates NUP98 fusion oncoprotein leukemic transformation. Cancer Discov. 12, 1152–1169 (2022).
Jevtic, Z. et al. SMARCA5 interacts with NUP98-NSD1 oncofusion protein and sustains hematopoietic cells transformation. J. Exp. Clin. Cancer Res. 41, 34 (2022).
Yamato, G. et al. Genome-wide DNA methylation analysis in pediatric acute myeloid leukemia. Blood Adv. 6, 3207–3219 (2022).
Olsson, I., Bergh, G., Ehinger, M. & Gullberg, U. Cell differentiation in acute myeloid leukemia. Eur. J. Haematol. 57, 1–16 (1996).
Agarwal, A. et al. Differentiation of leukemic blasts is not completely blocked in acute myeloid leukemia. Proc. Natl. Acad. Sci. USA 116, 24593–24599 (2019).
Mansisidor, A. R. & Risca, V. I. Chromatin accessibility: methods, mechanisms, and biological insights. Nucleus 13, 236 (2022).
McLeod, C. et al. St. Jude Cloud—a pediatric cancer genomic data sharing ecosystem. Cancer Discov. 11, 1082 (2021).
Nabet, B. et al. The dTAG system for immediate and target-specific protein degradation. Nat. Chem. Biol. 14, 431–441 (2018).
Winter, G. E. et al. Selective target protein degradation via phthalimide conjugation. Science 348, 1376 (2015).
Lavallée, V. P. et al. Identification of MYC mutations in acute myeloid leukemias with NUP98-NSD1 translocations. Leukemia 30, 1621–1624 (2016).
Herzog, V. A. et al. Thiol-linked alkylation of RNA to assess expression dynamics. Nat. Methods 14, 1198–1204 (2017).
Muhar, M. et al. SLAM-seq defines direct gene-regulatory functions of the BRD4-MYC axis. Science 360, 800–805 (2018).
Olsen, S. N. et al. MLL::AF9 degradation induces rapid changes in transcriptional elongation and subsequent loss of an active chromatin landscape. Mol. Cell 82, 1140–1155 (2022).
Xia, Z. B. et al. The MLL fusion gene, MLL-AF4, regulates cyclin-dependent kinase inhibitor CDKN1B (p27kip1) expression. Proc. Natl. Acad. Sci. USA 102, 14028–14033 (2005).
Nagel, S., Pommerenke, C., Meyer, C. & Macleod, R. A. F. Nkl homeobox genes nkx2-3 and nkx2-4 deregulate megakaryocytic-erythroid cell differentiation in aml. Int. J. Mol. Sci. 22, 11434 (2021).
Tran, T. M. et al. The RNA-binding protein IGF2BP3 is critical for MLL-AF4-mediated leukemogenesis. Leukemia 36, 68–79 (2021).
Fan, J., Zhuang, M., Fan, W. & Hou, M. RNA N6-methyladenosine reader IGF2BP3 promotes acute myeloid leukemia progression by controlling stabilization of EPOR mRNA. PeerJ 11, e15706 (2023).
Heuser, M. et al. MN1 overexpression induces acute myeloid leukemia in mice and predicts ATRA resistance in patients with AML. Blood 110, 1639–1647 (2007).
Lei, H. et al. CRISPR screening identifies CDK12 as a conservative vulnerability of prostate cancer. Cell Death Dis. 12, 740 (2021).
Houles, T. et al. CDK12 is hyperactivated and a synthetic-lethal target in BRAF-mutated melanoma. Nat. Commun. 13, 1–16 (2022).
Filippone, M. G. et al. CDK12 promotes tumorigenesis but induces vulnerability to therapies inhibiting folate one-carbon metabolism in breast cancer. Nat. Commun. 13, 2642 (2022).
Iniguez, A. B. et al. EWS/FLI confers tumor cell synthetic lethality to CDK12 inhibition in Ewing sarcoma. Cancer Cell 33, 202–216.e6 (2018).
Valerio, D. G. et al. Histone acetyltransferase activity of MOF is required for MLL-AF9 leukemogenesis. Cancer Res. 77, 1753 (2017).
Hoshii, T. et al. A non-catalytic function of SETD1A regulates cyclin K and the DNA DAMAGE Response. Cell 172, 1007–1021.e17 (2018).
Schmoellerl, J. et al. EVI1 drives leukemogenesis through aberrant ERG activation. Blood 141, 453–466 (2023).
Mucenski, M. L. et al. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell 65, 677–689 (1991).
Lieu, Y. K. & Reddy, E. P. Conditional c-myb knockout in adult hematopoietic stem cells leads to loss of self-renewal due to impaired proliferation and accelerated differentiation. Proc. Natl. Acad. Sci. USA 106, 21689–21694 (2009).
Trempenau, M. L. et al. The histone demethylase KDM5C functions as a tumor suppressor in AML by repression of bivalently marked immature genes. Leukemia 37, 593 (2023).
Palanichamy, J. K. et al. RNA-binding protein IGF2BP3 targeting of oncogenic transcripts promotes hematopoietic progenitor proliferation. J. Clin. Invest. 126, 1495–1511 (2016).
Fan, Z. et al. CDK13 cooperates with CDK12 to control global RNA polymerase II processivity. Sci. Adv. 6, eaaz5041 (2020).
Dubbury, S. J., Boutz, P. L. & Sharp, P. A. CDK12 regulates DNA repair genes by suppressing intronic polyadenylation. Nat 564, 141–145 (2018).
Magnuson, B. et al. CDK12 regulates co-transcriptional splicing and RNA turnover in human cells. iScience 25, 105030 (2022).
Jiang, B. et al. Structure-activity relationship study of THZ531 derivatives enables the discovery of BSJ-01-175 as a dual CDK12/13 covalent inhibitor with efficacy in Ewing sarcoma. Eur. J. Med. Chem. 221, 113481 (2021).
Chang, Y. et al. Development of an orally bioavailable CDK12/13 degrader and induction of synthetic lethality with AKT pathway inhibition. Cell Rep. Med. 5, 101752 (2024).
Jiang, B. et al. Discovery and resistance mechanism of a selective CDK12 degrader. Nat. Chem. Biol. 17, 675–683 (2021).
Quereda, V. et al. Therapeutic targeting of CDK12/CDK13 in triple-negative breast cancer. Cancer Cell 36, 545–558.e7 (2019).
Joshi, P. M., Sutor, S. L., Huntoon, C. J. & Karnitz, L. M. Ovarian cancer-associated mutations disable catalytic activity of CDK12, a kinase that promotes homologous recombination repair and resistance to cisplatin and poly(ADP-ribose) polymerase inhibitors. J. Biol. Chem. 289, 9247–9253 (2014).
Zuber, J. et al. Toolkit for evaluating genes required for proliferation and survival using tetracycline-regulated RNAi. Nat. Biotechnol. 29, 79 (2011).
Kirstetter, P. et al. Modeling of C/EBPalpha mutant acute myeloid leukemia reveals a common expression signature of committed myeloid leukemia-initiating cells. Cancer Cell 13, 299–310 (2008).
Savoy, L. et al. CDK12/13 dual inhibitors are potential therapeutics for acute myeloid leukemia. Br. J. Haematol. 202, 195–198 (2023).
Bösken, C. A. et al. The structure and substrate specificity of human Cdk12/Cyclin K. Nat. Commun. 5, 3505 (2014).
Zhang, T. et al. Covalent targeting of remote cysteine residues to develop CDK12 and 13 inhibitors. Nat. Chem. Biol. 12, 876 (2016).
Paull, T. T. et al. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 10, 886–895 (2000).
Bazinet, A. & Assouline, S. A review of FDA-approved acute myeloid leukemia therapies beyond ‘7 + 3’. Expert Rev. Hematol. 14, 185–197 (2021).
McNeer, N. A. et al. Genetic mechanisms of primary chemotherapy resistance in pediatric acute myeloid leukemia. Leukemia 33, 1934–1943 (2019).
Wang, G. G., Cai, L., Pasillas, M. P. & Kamps, M. P. NUP98–NSD1 links H3K36 methylation to Hox-A gene activation and leukaemogenesis. Nat. Cell Biol. 9, 804–812 (2007).
Nagel, S. & Drexler, H. G. Deregulated NKL homeobox genes in B-cell lymphoma. Cancers (Basel) 11, 1–15 (2019).
Villarese, P. et al. TCRα rearrangements identify a subgroup of NKL-deregulated adult T-ALLs associated with favorable outcome. Leukemia 32, 61–71 (2017).
Wang, Q. F. et al. MLL fusion proteins preferentially regulate a subset of wild-type MLL target genes in the leukemic genome. Blood 117, 6895–6905 (2011).
Chu, Y. et al. Six1 regulates leukemia stem cell maintenance in acute myeloid leukemia. Cancer Sci. 110, 2200 (2019).
Zhang, L. S. et al. Installation of a cancer promoting WNT/SIX1 signaling axis by the oncofusion protein MLL-AF9. EBioMedicine 39, 145 (2019).
Liu, N. et al. Direct promoter repression by BCL11A controls the fetal to adult hemoglobin switch. Cell 173, 430 (2018).
Sunami, Y. et al. BCL11A promotes myeloid leukemogenesis by repressing PU.1 target genes. Blood Adv. 6, 1827–1843 (2022).
Xutao, G. et al. BCL11A and MDR1 expressions have prognostic impact in patients with acute myeloid leukemia treated with chemotherapy. Pharmacogenomics 19, 343–348 (2018).
Mancarella, C. & Scotlandi, K. IGF2BP3 from physiology to cancer: novel discoveries, unsolved issues, and future perspectives. Front. Cell Dev. Biol. 7, 363 (2020).
Liu, X. et al. Targeting IGF2BP3 in Cancer. Int. J. Mol. Sci. 24, 9423 (2023).
Wu, W., Yu, S. & Yu, X. Transcription-associated cyclin-dependent kinase 12 (CDK12) as a potential target for cancer therapy. Biochim. Biophys. Acta - Rev. Cancer 1878, 188842 (2023).
Blazek, D. et al. The Cyclin K/Cdk12 complex maintains genomic stability via regulation of expression of DNA damage response genes. Genes Dev. 25, 2158 (2011).
Kotsantis, P., Petermann, E. & Boulton, S. J. Mechanisms of oncogene-induced replication stress: jigsaw falling into place. Cancer Discov. 8, 537 (2018).
Bowry, A., Kelly, R. D. W. & Petermann, E. Hypertranscription and replication stress in cancer. Trends Cancer 7, 863–877 (2021).
Curti, L. et al. CDK12 controls transcription at damaged genes and prevents MYC-induced transcription-replication conflicts. Nat. Commun. 15, 1–18 (2024).
Yang, Y. et al. Transcription and DNA replication collisions lead to large tandem duplications and expose targetable therapeutic vulnerabilities in cancer. Nat. Cancer 5, 1885–1901 (2024).
Sanjana, N. E., Shalem, O. & Zhang, F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 11, 783 (2014).
Michlits, G. et al. Multilayered VBC score predicts sgRNAs that efficiently generate loss-of-function alleles. Nat. Methods 17, 708–716 (2020).
Fellmann, C. et al. An optimized microRNA backbone for effective single-copy RNAi. Cell Rep. 5, 1704–1713 (2013).
Kodali, S. et al. RNA sequestration in P-bodies sustains myeloid leukaemia. Nat. Cell Biol. 26, 1745–1758 (2024).
Skucha, A. et al. MLL-fusion-driven leukemia requires SETD2 to safeguard genomic integrity. Nat. Commun. 9, 1983 (2018).
Zuber, J. et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nat 478, 524–528 (2011).
Schmitz, M. et al. Xenografts of highly resistant leukemia recapitulate the clonal composition of the leukemogenic compartment. Blood 118, 1854–1864 (2011).
Schmidt, L. et al. CEBPA-mutated leukemia is sensitive to genetic and pharmacological targeting of the MLL1 complex. Leukemia 33, 1608–1619 (2019).
Shashikant, T. & Ettensohn, C. A. Genome-wide analysis of chromatin accessibility using ATAC-seq. Methods Cell Biol. 151, 219–235 (2019).
de Almeida, M. et al. AKIRIN2 controls the nuclear import of proteasomes in vertebrates. Nature 599, 491–496 (2021).
Di Tommaso, P. et al. Nextflow enables reproducible computational workflows. Nat. Biotechnol. 35, 316–319 (2017).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Li, W. et al. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biol. 15, 554 (2014).
Schmieder, R. & Edwards, R. Quality control and preprocessing of metagenomic datasets. Bioinformatics 27, 863–864 (2011).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Li, H. et al. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Warnes, G. et al. gplots: Various R Programming Tools for Plotting Data. CRAN. R package version 3.2.0, https://CRAN.R-project.org/package=gplots (2024).
Conesa, A., Nueda, M. J., Ferrer, A. & Talón, M. maSigPro: a method to identify significantly differential expression profiles in time-course microarray experiments. Bioinformatics 22, 1096–1102 (2006).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550 (2005).
Xie, Z. et al. Gene set knowledge discovery with enrichr. Curr. Protoc. 1, e90 (2021).
Kolberg, L. et al. g:Profiler—interoperable web service for functional enrichment analysis and gene identifier mapping (2023 update). Nucleic Acids Res. 51, W207–W212 (2023).
Tang, D. et al. SRplot: A free online platform for data visualization and graphing. PLoS ONE 18, e0294236 (2023).
Krueger, F. et al. FelixKrueger/TrimGalore: v0.6.10, Zenodo. https://doi.org/10.5281/zenodo.7598955 (2023).
Neumann, T. et al. Quantification of experimentally induced nucleotide conversions in high-throughput sequencing datasets. BMC Bioinformatics. 20, 258 (2019).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Ramirez, F., Dundar, F., Diehl, S., Gruning, B. A. & Manke, T. deepTools: a flexible platform for exploring deep-sequencing data. Nucleic Acids Res. 42, W187–W191 (2014).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Ross-Innes, C. et al. Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature 481, 389–393 (2012).
Carroll, T. & Barrows, D. profileplyr https://doi.org/10.18129/B9.BIOC.PROFILEPLYR.
Wickham, H. Ggplot2: Elegant Graphics for Data Analysis. Use R! CN - QA90.W53 2009 (Springer, 2009).
Robinson, J. T. et al. Integrative genomics viewer. Nat. Biotechnol. 29, 24–26 (2011).
Reichl, S. et al. Ultimate ATAC-seq Data Processing & Analysis Pipeline: v0.1.1, Zenodo. https://doi.org/10.5281/ZENODO.6346319 (2022).
Casteels, T. et al. SMNDC1 links chromatin remodeling and splicing to regulate pancreatic hormone expression. Cell Rep. 40, 111288 (2022).
Chen, S., Zhou, Y., Chen, Y. & Gu, J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890 (2018).
Faust, G. G. & Hall, I. M. SAMBLASTER: fast duplicate marking and structural variant read extraction. Bioinformatics 30, 2503–2505 (2014).
Yu, G., Wang, L.-G. & He, Q.-Y. ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics 31, 2382–2383 (2015).
Leek, J. T., Johnson, W. E., Parker, H. S., Jaffe, A. E. & Storey, J. D. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 28, 882–883 (2012).
Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 14, 927–930 (2003).
Oksanen, J. et al. vegan: Community Ecology Package. CRAN. R package version 2.6-8, https://CRAN.R-project.org/package=vegan (2024).
Pedersen, T. ggraph: An Implementation of Grammar of Graphics for Graphs and Networks. CRAN. R package version 2.2.1, https://CRAN.R-project.org/package=ggraph (2024).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576 (2010).
Acknowledgements
All members of the Grebien group took part in scientific discussions, Georg Winter advised on the establishment of the dTAG model, Michaela Fellner provided experimental support with the genome-wide CRISPR screen, Raúl Jiménez Heredia and Anna Hurt provided experimental support with patient material and ATAC-seq library preparations and Gabriele Stefanzl gave scientific advice on healthy donor primary cells. Elizabeth Heyes assisted in the finalization of the manuscript. The team of the Vienna BioCenter Core Facilities (https://www.vbcf.ac.at) performed next generation sequencing services. Norio Shiba (Yokohama City University; Japan) provided ATAC-seq raw data and additional information on pediatric patient samples19. This work was supported by a starting grant from the European Research Council to F.G. (636855) (F.G.), the Austrian Science Fund (Grants TAI490, P35298 (F.G.) and KLI 1056-B (K.B.)) and a Heidi Ras Grant of the FZK University Children’s Hospital Zurich (N.S.). S.T., J.S., and M.A. are recipients of a Doctoral Fellowship (DOC) of the Austrian Academy of Sciences at the Institute for Medical Biochemistry at the University of Veterinary Medicine Vienna. J.S. is supported by the Fellinger Cancer Research Fund.
Author information
Authors and Affiliations
Contributions
F.G. and S.T. conceived the work and designed the study; T.E. performed the bioinformatics analyses; S.T., N.W., M.P., P.F.P., J.S., B.H., G.M., M.A., and M.M.G. performed experiments; B.H., K.N., M.N.D., and K.B. provided ATAC-seq data and scientific input on the patient data; N.S. and B.B. established the PDX model; R.M. provided patient material; P.V. provided healthy donor primary material; S.T., F.G., and T.E. wrote the manuscript; J.Z., K.B., and G.S.F. were involved in experimental design and scientific discussions; F.G. supervised the study and all authors revised and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.Z. is a founder, shareholder, and scientific advisor of Quantro Therapeutics GmbH. J.Z. and the Zuber laboratory receive research support and funding from Boehringer Ingelheim. P.V. received consultancy honoraria from Pfizer, AOP Orphan, Delbert, Novartis, and Blueprint. The other authors declare no conflict of interest.
Peer review
Peer review information
Nature Communications thanks Alexandre Orthwein, Jay Sarthy, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Troester, S., Eder, T., Wukowits, N. et al. Transcriptional and epigenetic rewiring by the NUP98::KDM5A fusion oncoprotein directly activates CDK12. Nat Commun 16, 4656 (2025). https://doi.org/10.1038/s41467-025-59930-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-59930-9