Abstract
Frequent SARS-CoV-2 vaccination in vulnerable populations has raised concerns that this may contribute to T cell exhaustion, which could negatively affect the quality of immune protection. Herein, we examined the impact of repeated SARS-CoV-2 vaccination on T cell phenotypic and functional exhaustion in frail older adults in long-term care (n = 23), individuals on immunosuppressive drugs (n = 10), and healthy adults (n = 43), in Canada. Spike-specific CD4+ and CD8+ T cell levels did not decline in any cohort following repeated SARS-CoV-2 vaccination, nor did the expression of exhaustion markers on spike-specific or total T cells increase. T cell production of multiple cytokines (i.e. polyfunctionality) in response to the spike protein of SARS-CoV-2 did not decline in any cohort following repeated vaccination. None of the cohorts displayed elevated levels of terminally differentiated T cells following multiple SARS-CoV-2 vaccinations. Thus, repeated SARS-CoV-2 vaccination was not associated with increased T cell exhaustion in older frail adults, immunosuppressed individuals, or healthy adults.
Similar content being viewed by others
Introduction
One of the unique features of the COVID-19 pandemic was the recommendation for multiple vaccines in a relatively short period of time1,2,3. In Canada, the primary vaccination series typically included 2 mRNA vaccines administered weeks to months apart, and additional doses were recommended for protection against emerging variants1,4,5. As increasingly immune evasive variants continue to emerge, it is expected that vulnerable populations such as older adults and the immunocompromised will continue to be offered multiple updated SARS-CoV-2 vaccinations in relatively short intervals to maintain protective immunity1,2. However, accelerated and frequent boosting in vulnerable patients has raised concerns that this may contribute to detrimental long term effects, such as on the immune system itself6,7. Furthermore, SARS-CoV-2 infection is known to cause changes in the T cell compartment, including differences in expression of receptors associated with exhaustion8,9. While immune responses to infection and vaccination are not equivalent, in the eyes of the public, this concern of immune exhaustion after infection can carry over to vaccination.
Immune exhaustion is a nuanced term, typically used to describe the consequences of chronic stimulation of T cells in the context of infection or malignancy10. Long-term or high levels of exposure to their cognate antigen, and thus repeated stimulation through the T cell receptor, can result in the upregulation of exhaustion markers such as PD-1, LAG-3, TIM-3, and TIGIT10,11,12,13. Sustained co-expression of multiple exhaustion markers produces stronger inhibitory signals which dampen T cell activation and cytokine production, and thus can be used to identify more severely exhausted T cells10,13. The delineation between activation and exhaustion, however, can be unclear. As an example, in convalescent COVID-19 patients, a higher proportion of IFN-γ producing T cells were found in the PD-1+ population than the PD-1− population, which is more consistent with a state of activation rather than exhaustion14. It is therefore critical to consider the co-expression of multiple exhaustion markers, as well as the cytokine-producing functional capacity of T cells when considering cells to be activated or exhausted. While vaccination is not a chronic stimulation condition, the novelty of the mRNA SARS-CoV-2 vaccines, and the frequency of vaccination in vulnerable populations, have prompted consideration of the intricacies of T cell immune exhaustion in the context of mRNA vaccination.
In this study, we investigated the impacts of repeated SARS-CoV-2 vaccination on circulating and spike-specific T cells, including expression of exhaustion markers, and explored their functional capacities after the second, third and fourth doses of mRNA vaccines in vulnerable cohorts of older adults in long-term care facilities and individuals with rheumatoid arthritis on immunosuppressive drugs, and in healthy community-dwelling adults. We show that within each cohort between the second, third, and fourth vaccinations, there were no significant declines in spike-specific T cell levels, their cytokine production capacity, or major changes in exhaustion associated marker expression, indicating that immune exhaustion of T cells does not occur following repeated SARS-CoV-2 vaccination.
Results
Study population
We assessed the impact of repeated SARS-CoV-2 vaccination on T cell phenotypes and function in frail older adults living in long-term care facilities (LTC), people living with rheumatoid arthritis and taking immunosuppressive drugs (RA), and healthy younger adults (HA). To ensure that changes in immune phenotype were not due to SARS-CoV-2 infection, participants were excluded before or during the study period if they had a positive PCR test, rapid antigen test, or seroconverted to become positive for anti-nucleocapsid IgG. The LTC cohort included frail older adults living in long-term care homes (n = 23, median age 84.0 yrs, 60.9% female) (Table 1). The RA cohort included participants with rheumatoid arthritis, who were on immunosuppressive drugs (n = 10, median age 68.0 yrs, 70.0% female) (Table 1). A detailed description of the immunosuppressive drug classes used by participants with RA is available in Supplementary Data 1. The HA cohort was comprised of younger healthy individuals (n = 43, median age 47.0 yrs, 60.5% female) (Table 1). The cohorts did not significantly differ in sex distribution but did significantly differ in age (Table 1). The most common first, second, and third vaccine type was BNT162b2 in the HA and RA cohorts, whereas mRNA-1273 was more commonly used in the LTC cohort (Table 1). mRNA-1273 was the most common fourth dose vaccine type in the LTC and RA cohorts, while the HA cohort had a more even distribution of mRNA-1273 and BNT162b2 (Table 1). The intervals between successive vaccinations significantly differed between the cohorts (Table 1). Blood samples were collected from participants 3 months after their second, third, and fourth SARS-CoV-2 vaccinations.
Expression of markers associated with exhaustion on spike-specific CD4+ and CD8+ T cells
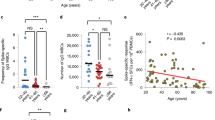
We first examined if repeated SARS-CoV-2 vaccination impacted spike-specific CD4+ and CD8+ T cell levels using activation-induced marker (AIM) assays, and multivariable linear mixed models accounting for age and sex. Compared with post dose 2, older adults (LTC) displayed elevated frequencies of spike-specific CD4+ T cells following the fourth SARS-CoV-2 vaccination (Fig. 1a and Supplementary Data 2). The frequencies of spike-specific CD8+ T cells were also elevated in older adults (LTC) after the third and fourth SARS-CoV-2 vaccinations, compared with the second dose (Fig. 1b and Supplementary Data 2). The immunosuppressed RA and the HA cohorts did not display significant changes in the frequencies of spike-specific CD4+ or spike-specific CD8+ T cells following repeated SARS-CoV-2 vaccination (Fig. 1a, b and Supplementary Data 2).
Spike-specific T cell levels and surface phenotypes were assessed by AIM assay and flow cytometry in the LTC, RA, and HA cohorts. a Frequencies of spike-specific CD4+ T cells. p = 0.0420*. b Frequencies of spike-specific CD8+ T cells. p = 0.0048**, p = 0.0002***. The solid red lines indicate the median of each group. c Frequencies of spike-specific CD4+ T cells expressing each combination of PD-1, TIM-3, and LAG-3, displayed as the mean of each combination as a proportion of all combinations. d Frequencies of spike-specific CD8+ T cells expressing each combination of PD-1, TIM-3, LAG-3, displayed as the mean of each combination as a proportion of all combinations. LTC: 3mo2 n = 14, 3mo3 n = 23, 3mo4 n = 18. RA: 3mo2 n = 7, 3mo3 n = 9, 3mo4 n = 7. HA: 3mo2 n = 42, 3mo3 n = 20, 3mo4 n = 7. For a, b, participant vaccination history is indicated by circles (BNT162b2 only), squares (mRNA-1273 only), triangles (mixed BNT162b2 and mRNA-1273), or diamonds (mRNA only but one vaccine unknown). 3moX denotes 3 months post dose X. Multivariable linear mixed models accounting for age and sex were used to assess changes in spike-specific T cells levels within each cohort, and changes in the frequencies of PD-1, TIM-3, and LAG-3, within each cohort (a–d). FDR adjusted p values were obtained within each cohort for c and d, to account for multiple testing on cells from the same parent population. If p values are not indicated, the result was not significant.
To further elucidate the impact of repeated SARS-CoV-2 vaccinations on the spike-specific CD4+ and CD8+ T cell populations, we investigated their surface co-expression of the exhaustion markers LAG-3, PD-1, and TIM-3. Exhaustion markers can also be markers of activation, and therefore it is expected that there will be some increase in expression on spike-specific T cells after stimulation with the spike peptide14. If the frequencies of cells expressing these markers remained the same after repeated vaccination, this would indicate normal activation; whereas if the frequencies of cells expressing exhaustion markers increased after each dose, this would suggest, but not definitively demonstrate, a progression toward cellular exhaustion. No significant changes in combined exhaustion marker expression on spike-specific CD4+ T cells were observed in older adults (LTC), immunosuppressed individuals with RA, or the HA cohort, following the third or fourth SARS-CoV-2 vaccination, compared with the second vaccination (Fig. 1c and Supplementary Data 3). Compared with post dose 2, older adults living in LTC displayed elevated frequencies of PD-1+LAG-3+TIM-3− spike-specific CD8+ T cells following the third and fourth SARS-CoV-2 vaccinations (Fig. 1d and Supplementary Data 3). No significant changes in expression of exhaustion markers on spike-specific CD8+ T cells were observed in immunosuppressed individuals with RA or the HA cohort after repeated SARS-CoV-2 vaccination (Fig. 1d and Supplementary Data 3).
T cell functionality and polyfunctionality following repeated SARS-CoV-2 vaccination
After observing minimal changes in the surface expression of the exhaustion markers PD-1, TIM-3, and LAG-3 on spike-specific T cells following repeated SARS-CoV-2 vaccination, we investigated whether there were deficits in T cell cytokine production capabilities. In particular, the loss of polyfunctionality—the ability of a T cell to produce multiple different cytokines—is a characteristic of functional exhaustion10. COMPASS employs Bayesian statistics to determine the posterior probabilities of antigen-specific production of various combinations of cytokines, based on examination of flow cytometry data15. The posterior probability of spike-specific CD4+ (Fig. 2a) and CD8+ T cell (Fig. 2b) responses expressing any combination of TNF-α, IL-2, GM-CSF, and IFN-γ was evaluated using COMPASS15. The COMPASS functionality scores (FS), representing the proportion of expressed cytokine combinations out of all the possible combinations in each participant, did not change following repeated SARS-CoV-2 vaccinations, in any cohort, for either CD4+ or CD8+ T cells (Fig. 2c, d and Supplementary Data 4). Therefore, repeated vaccination did not result in a loss of diversity of expressed cytokine combinations10,13. To ensure the lack of change in the FS was not masking movement from higher states of polyfunctionality (i.e., producing 3–4 cytokines) to previously unoccupied states of lower polyfunctionality (i.e., producing 1–2 cytokines), we also employed the COMPASS polyfunctionality score (PFS). The PFS is similar to the FS, but weighs polyfunctional T cell subsets producing 3–4 cytokines more highly than those that produce fewer cytokines15. We found that the PFS for CD4+ or CD8+ T cell cytokine expression did not significantly decline in any cohort between second, third, or fourth vaccinations, indicating that there was no reduction in the most highly polyfunctional T cell subsets following repeated SARS-CoV-2 vaccinations (Fig. 2e, f and Supplementary Data 4).
PBMCs were stimulated for 24 h using a peptide pool derived from the spike protein of SARS-CoV-2, and intracellular cytokine stains were conducted to evaluate cytokine production using flow cytometry. COMPASS heatmap of posterior probabilities of a spike-specific response in a CD4+ T cells b and CD8+ T cells for each cytokine combination. COMPASS functionality score (FS) for c CD4+ T cells and d CD8+ T cells in each cohort following repeated SARS-CoV-2 vaccinations. COMPASS polyfunctionality score (PFS) for e CD4+ T cells and f CD8+ T cells in each cohort following repeated SARS-CoV-2 vaccinations. For a, b, each column represents a different cytokine combination as indicated by the shaded legend beneath the heatmap, while each row is a unique participant. The blue scaling within the heatmaps indicates the posterior probabilities. Colored bars on the left side denote the cohorts associated with the rows, and timepoint within each cohort. For c–f, participant vaccination history is indicated by circles (BNT162b2 only), squares (mRNA-1273 only), triangles (mixed BNT162b2 and mRNA-1273), or diamonds (mRNA only but one vaccine unknown). The solid red lines indicate the median of each group. 2 indicates 3mo2, 3 is 3mo3, and 4 is 3mo4. LTC: 3mo2 n = 14, 3mo3 n = 23, 3mo4 n = 18. RA: 3mo2 n = 7, 3mo3 n = 9, 3mo4 n = 8. HA: 3mo2 n = 41, 3mo3 n = 21, 3mo4 n = 7. Multivariable linear mixed models accounting for age and sex were used to assess changes in the CD4+ and CD8+ T cell FS and PFS within a given cohort following additional SARS-CoV-2 vaccinations. For heatmaps, columns of cytokine combination subsets with posterior probabilities less than 0.005 for all participants are not displayed. If p values are not indicated, the result was not significant.
Immunophenotyping of the circulating T cell compartment following repeated SARS-CoV-2 vaccination
Next, we evaluated whether the circulating T cell compartment, in the absence of ex vivo stimulation, changed in any of the cohorts following repeated SARS-CoV-2 vaccination. Using flow cytometry, we assessed frequencies of CD4+ and CD8+ T cell naïve, effector memory (EM), central memory (CM), and EM re-expressing CD45RA (EMRA) subsets. The older adults in LTC displayed significantly higher frequencies of EM CD4+ T cells following the third and fourth SARS-CoV-2 vaccinations, as compared with post dose 2 (Fig. 3a and Supplementary Data 5). The increase in EM CD4+ T cells in older adults (LTC) was coupled with a decrease in naïve CD4+ T cells following the third and fourth SARS-CoV-2 vaccinations (Fig. 3a and Supplementary Data 5). Interestingly, this change in CD4+ T cell distribution was not observed in the immunosuppressed RA or the HA cohorts (Fig. 3a and Supplementary Data 5).
Flow cytometry was used to assess the frequencies of naïve (N, CCR7+CD45RA+), central memory (CM, CCR7+CD45RA−), effector memory (EM, CCR7−CD45RA−) and EM re-expressing CD45RA (EMRA, CD45RA+CCR7−) CD4+ T cells. a The mean frequency of each T cell subset was determined and plotted in a stacked bar format. b The frequencies of terminally differentiated CD4+ T cells (EMRA+CD57+CD28−), out of all CD4+ T cells, within each cohort following the second, third, and fourth SARS-CoV-2 vaccinations. The solid red lines indicate the median of each group. c The frequency of CD4+ T cells expressing each combination of the exhaustion markers PD-1 and TIGIT, displayed as the mean value within each cohort at each timepoint. For b, participant vaccination history is indicated by circles (BNT162b2 only), squares (mRNA-1273 only), triangles (mixed BNT162b2 and mRNA-1273), or diamonds (mRNA only but one vaccine unknown). 3moX denotes 3 months post dose X. LTC: 3mo2 n = 14, 3mo3 n = 22, 3mo4 n = 15. RA: 3mo2 n = 7, 3mo3 n = 9, 3mo4 n = 8. HA: 3mo2 n = 41, 3mo3 n = 19, 3mo4 n = 5. Multivariable linear mixed models accounting for age and sex were used to assess changes in the frequencies of each T cell subset within a given cohort following repeated SARS-CoV-2 vaccinations. FDR adjusted p values were obtained within each cohort for a and c to account for multiple testing on cells from the same parent population. If p values are not indicated, the result was not significant. p < 0.05 *, p < 0.01 **.
It is well recognized that chronic infections can result in persistent stimulation of T cells, which can push them towards a terminally differentiated phenotype, characterized by expression of CD57 coupled with a loss of CD28 expression, especially within the EMRA compartment16,17,18,19. In contrast, vaccination is an acute stimulus, multiple times, and would not be expected to act as a chronic stimulation20. As such, we evaluated the levels of EMRA+CD28−CD57+ T cells within the CD4+ and CD8+ T cell compartments. There were no significant changes in the frequencies of EMRA+CD28−CD57+ CD4+ T cells in older adults in LTC, immunosuppressed individuals with RA, or the HA cohort following repeated SARS-CoV-2 vaccinations (Fig. 3b and Supplementary Data 5).
We next examined whether repeated SARS-CoV-2 vaccination was associated with changes in overall T cell phenotypic exhaustion, characterized by expression of multiple exhaustion markers (i.e., PD-1, TIM-3, and TIGIT) on unstimulated T cells following the second, third, and fourth SARS-CoV-2 vaccinations. All cohorts displayed minimal levels of TIM-3+ CD4+ T cells, which varied little following additional SARS-CoV-2 vaccinations (Supplementary Fig. 1 and Supplementary Data 6). Older adults in LTC, immunosuppressed individuals with RA, and the HA cohort also did not display any significant changes in the frequencies of PD-1+ or TIGIT+ CD4+ T cells following the third or fourth SARS-CoV-2 vaccinations, compared with post dose 2 (Fig. 3c and Supplementary Data 5).
Older adults in LTC and immunosuppressed individuals with RA did not display significant changes in the CD8+ T cell compartment following repeated SARS-CoV-2 vaccination (Fig. 4a and Supplementary Data 7). The HA cohort had significantly lower frequencies of EMRA CD8+ T cells following the third SARS-CoV-2 vaccination compared with post dose 2, but this observation disappeared following dose 4 (Fig. 4a and Supplementary Data 7). There were no significant changes in the frequencies of EMRA+CD28−CD57+, or PD-1+ and TIGIT+ CD8+ T cells in older adults in LTC, immunosuppressed individuals with RA, or the HA cohort following repeated SARS-CoV-2 vaccinations (Fig. 4b, c and Supplementary Data 7). Similar to what was observed for CD4+ T cells, the CD8+ T cells also expressed only minimal levels of TIM-3 (Supplementary Fig. 1 and Supplementary Data 6).
Flow cytometry was used to assess CD8+ T cell phenotypes. a The mean frequencies of naïve (N), CM, EM, and EMRA CD8+ T cells were determined and plotted in a stacked bar format. b The frequencies of terminally differentiated CD8+ T cells (EMRA+CD57+CD28−), out of all CD8+ T cells, within each cohort following the second, third, and fourth SARS-CoV-2 vaccinations. The solid red lines indicate the median of each group. c The frequency of CD8+ T cells expressing each combination of the exhaustion markers PD-1 and TIGIT, displayed as the mean value within each cohort at each timepoint. For b, participant vaccination history is indicated by circles (BNT162b2 only), squares (mRNA-1273 only), triangles (mixed BNT162b2 and mRNA-1273), or diamonds (mRNA only but one vaccine unknown). 3moX denotes 3 months post dose X. LTC: 3mo2 n = 14, 3mo3 n = 22, 3mo4 n = 15. RA: 3mo2 n = 7, 3mo3 n = 9, 3mo4 n = 8. HA: 3mo2 n = 41, 3mo3 n = 19, 3mo4 n = 5. Multivariable linear mixed models accounting for age and sex were used to assess changes in the frequencies of each T cell subset within a given cohort following repeated SARS-CoV-2 vaccinations. FDR adjusted p values were obtained within each cohort for a and c to account for multiple testing on cells from the same parent population. If p values are not indicated, the result was not significant. p < 0.05 *.
Comparisons of spike-specific T cells, exhausted phenotypes, and functional capacity between the LTC, RA, and HA cohorts
To assess if functional differences in cellular immunity are present between the LTC, RA, and HA cohorts, we examined if the three cohorts differed in their spike-specific T cell responses, T cell functional capacity, and exhausted phenotypes, following the second, third, and fourth SARS-CoV-2 vaccinations. The spike-specific CD4+ and CD8+ T cell levels were similar between the three cohorts after each vaccine dose (Supplementary Data 8). Expression of exhaustion markers on spike-specific CD4+ and CD8+ T cells significantly differed between the cohorts after the second and third vaccinations, but not after the fourth SARS-CoV-2 vaccination (Supplementary Data 8). Changes in exhaustion marker expression between the cohorts varied by vaccine dose (Supplementary Data 8). At all timepoints, the CD4 PFS, CD8 FS, and CD8 PFS were similar between the cohorts (Supplementary Data 8). The CD4 FS of the HA cohort was significantly higher than that of the LTC cohort following the second SARS-CoV-2 vaccination, though this difference was not observed following subsequent vaccinations. Collectively, these data demonstrate that there are subtle differences in T cell responses to vaccination across cohorts.
Consistent with well-documented changes in the T cell compartment that occur with aging, participants in the LTC cohort had higher frequencies of EM, EMRA, and terminally differentiated CD4+ T cells, and lower levels of naïve CD4+ T cells, compared to the HA and/or RA cohorts (Supplementary Data 8). The LTC cohort also had lower levels of naïve CD8+ T cells but higher frequencies of EMRA CD8+ T cells compared to either the HA or RA cohort (Supplementary Data 8). Terminally differentiated CD8+ T cell frequencies were similar between all cohorts after the second vaccination but higher in the LTC cohort than the RA cohort following the third SARS-CoV-2 vaccination (Supplementary Data 8). Similar to what was observed for the spike-specific T cell exhaustion marker expression, the frequencies of overall CD4+ and CD8+ T cells co-expressing PD-1 and TIGIT differed between cohorts following the second and third SARS-CoV-2 vaccinations (Supplementary Data 8). These differences between the cohorts in overall T cell exhaustion marker expression continued following the fourth SARS-CoV-2 vaccination for CD4+ but not CD8+ T cells (Supplementary Data 8). Combined LTC, RA, and HA cohort correlation analyses were also conducted to assess the relationships of the exhaustion parameters (Supplementary Fig. 2).
Impact of dosing interval on T cell exhausted phenotypes and functional capacity
To maximize the number of healthy adults vaccinated with a single dose when SARS-CoV-2 vaccines initially became available, Canadian public health units shifted from using the relatively short manufacturer-recommended dosing interval of 21 days to either a ‘delayed’ interval (35–42 days between dose 1 and 2) or an ‘extended’ interval (> 45 days between dose 1 and 2) for primary series vaccinations2,3,21. We22 and others23 found that a longer dosing interval between first and second vaccinations resulted in higher levels of neutralizing antibodies. To expand those observations to cellular immunity, herein, we investigated whether differences in primary series dosing interval in the HA cohort impacted spike-specific T cell levels, exhaustion, and functional capacity, as well as the overall phenotypes of the T cell compartment at 3 months post dose 2.
The ‘recommended’ (Rec) cohort received the primary series as per the recommended dosing interval (i.e., <35 days between their first and second vaccination), the ‘delayed’ (Del) cohort had 35–42 days between dose 1 and 2, and the ‘extended’ (Ext) cohort had >45 days between the first and second dose22. The Rec, Del, and Ext cohorts did not significantly differ in age, sex, or dose 1 and dose 2 vaccine types (Supplementary Data 9). We found no significant differences in spike-specific CD4+ T cell levels or exhaustion marker expression between any of the HA dosing interval groups (Fig. 5a, b and Supplementary Data 10). There were also no significant differences in spike-specific CD8+ T cell levels or their surface exhaustion marker expression (Fig. 5c, d and Supplementary Data 10). Although CD4+ T cell functionality and polyfunctionality did not differ between dosing intervals (Fig. 5e and Supplementary Data 10), the HA cohort with the shortest dose interval (Rec) had significantly higher CD8+ T cell functionality and PFSs than the longest dosing interval (Ext) (Fig. 5f and Supplementary Data 10). CD4+ and CD8+ CM, EM, EMRA, and naïve T cell frequencies did not differ between the dosing interval groups (Fig. 5g and Supplementary Data 10). Similarly, there were no significant differences in the frequencies of terminally differentiated CD4+ or CD8+ T cells, or the surface expression of the exhaustion markers PD-1 and TIGIT on unstimulated CD4+ T cells as whole, between the dosing intervals (Fig. 5h, I and Supplementary Data 10). Participants in the Rec and Ext dosing intervals had significantly higher frequencies of PD-1+ TIGIT+ CD8+ T cells than participants in the Del cohort (Fig. 5j and Supplementary Data 10).
Flow cytometry was used to assess T cell phenotypes and functional capacity in HA at 3 months post dose 2. The HA ‘recommended’ (Rec) cohort had <35 days between dose 1 and 2, the ‘delayed’ (Del) cohort had 35–42 days between dose 1 and dose 2, and the ‘extended’ (Ext) cohort had >42 days between dose 1 and dose 2. a Frequencies of spike-specific CD4+ T cells in each HA cohort. b Frequencies of spike-specific CD4+ T cells expressing each combination of PD-1, TIM-3, and LAG-3, displayed as mean for each combination. c Frequencies of spike-specific CD8+ T cells in each HA cohort. d Frequencies of spike-specific CD8+ T cells expressing each combination of PD-1, TIM-3, LAG-3, displayed as mean for each combination. COMPASS functionality score (FS) and polyfunctionality score (PFS) for e CD4+ T cells and f CD8+ T cells in response to stimulation with peptides from the spike protein of SARS-CoV-2. g Frequencies of naïve, central memory (CM), effector memory (EM) and EM re-expressing CD45RA (EMRA) CD4+ and CD8+ T cells, displayed as mean of each combination as a proportion of all combinations, in each cohort. h The frequencies of terminally differentiated CD4+ and CD8+ T cells. The frequency of i CD4+ T cells and j CD8+ T cells expressing each combination of PD-1 and TIGIT, displayed as mean of each combination as a proportion of all combinations, in each cohort. For a, c, e, f, h, participant vaccination history is indicated by circles (BNT162b2 only), squares (mRNA-1273 only), triangles (mixed BNT162b2 and mRNA-1273), or diamonds (mRNA only but one vaccine unknown). The solid red lines indicate the median of each group. HA Rec n = 20 (21 for AIMs), HA Del n = 7, HA Ext n = 14. ANOVAs were used to assess differences between dosing interval cohorts. FDR adjusted p values were obtained for b, d, g, i, j to account for multiple testing on cells from the same parent population. If the ANOVA was significant, after FDR correction if applicable, post-hoc tests were used to determine which dosing intervals differed from one another. If p values are not indicated, the result was not significant. p < 0.05 *.
Discussion
Given the concern in the general public that repeated SARS-CoV-2 vaccination may compromise immune protection, we first examined the impact of repeated SARS-CoV-2 vaccination on the spike-specific T cell compartment. Spike-specific CD4+ and CD8+ T cell levels did not decline following repeated vaccination in any cohort. In fact, older adults in LTC displayed elevated spike-specific CD4+ and CD8+ T cell frequencies after additional vaccinations. There are conflicting reports as to whether spike-specific T cell levels increase after additional SARS-CoV-2 vaccinations beyond the primary series, at least in uninfected individuals24,25,26. Others have reported that increases in spike-specific T cell levels following the third SARS-CoV-2 vaccination in healthy donors may be transient, with spike-specific T cell frequencies declining to pre-third dose levels within 60 days post vaccination27. Considering that our samples were collected after this contraction period, when frequencies have returned to pre-boost levels, it was not surprising that we did not observe significant differences in the spike-specific T cell levels of the HA cohort following repeated SARS-CoV-2 vaccination27. The stable spike-specific CD4+ T cell levels we observed in participants with RA following repeated SARS-CoV-2 vaccination aligns with the previous findings of our lab and others28,29. Levels of spike-specific CD8+ T cells were lower than spike-specific CD4+ T cells in all cohorts, which is consistent with prior observations30,31. Overall, evidence suggests that repeated SARS-CoV-2 vaccination does not cause declines in spike-specific T cell levels.
There are previous reports of SARS-CoV-2 infection-associated changes in the expression of the exhaustion markers PD-1, TIM-3, LAG-3, and TIGIT, although it is unclear whether these changes exert functional consequences, or merely indicate a resolving immune response8,9,13,32. In people with a previous SARS-CoV-2 infection, levels of TIM-3+ and PD-1+ CD8+ T cells are elevated after additional SARS-CoV-2 vaccinations24. Although another report demonstrated that repeated exposure to SARS-CoV-2 antigens does not induce a notable CD8+ T cell exhausted phenotype in healthy donors33, concerns have persisted that repeated SARS-CoV-2 vaccination could elicit similar effects to infection on the T cell compartment. While immune responses to SARS-CoV-2 vaccination have some similarity to those after infection, they are not identical, and the distinct conditions of SARS-CoV-2 antigen exposure can result in differences within the SARS-CoV-2-specific T cell compartment34. With this distinction in mind, it was perhaps unsurprising that we did not see elevation of these exhaustion markers in any of the cohorts on total CD4+ T cells, CD8+ T cells, or spike-specific CD4+ T cells following repeated SARS-CoV-2 vaccination. The only change in exhaustion marker expression observed was an increase in PD-1+LAG-3+TIM-3− spike-specific CD8+ T cells in older frail adults, but the functional significance of this change is unclear.
To delineate T cell activation from exhaustion, both exhaustion marker expression and cytokine producing function must be evaluated, as a loss of the ability to produce multiple different cytokines (i.e., polyfunctionality) is associated with T cell exhaustion rather than activation13,14. In healthy adults, the primary SARS-CoV-2 vaccination series has been reported to induce functional and polyfunctional T cell responses, and these T cell cytokine responses have not been reported to decline following the third SARS-CoV-2 vaccination24,27,35,36. Importantly, when we extended the examination of polyfunctional T cell responses to participants from vulnerable populations, and following the fourth SARS-CoV-2 vaccination, we found that repeated SARS-CoV-2 vaccinations were not associated with decreased spike-specific T cell functionality scores or PFSs in any of the cohorts. Thus, despite our observation of an increase in PD-1+LAG-3+TIM-3− spike-specific CD8+ T cells in older frail adults, none of the cohorts displayed decreased cytokine production that would be characteristic of true exhaustion, as opposed to activation.
Severe SARS-CoV-2 infection can also lead to elevated frequencies of terminally differentiated T cells, and decreased frequencies of naïve T cells8,9. To further contrast the immunological impacts of repeated SARS-CoV-2 vaccination from the negative effects observed following SARS-CoV-2 infection, we examined if there were any changes in the composition of the CD4+ or CD8+ T cell compartments after repeated SARS-CoV-2 vaccinations. The RA and HA cohorts did not display changes in their naïve CD4+ or CD8+ T cell levels after repeated vaccination, though the LTC cohort had higher frequencies of EM CD4+ T cells and decreased levels of naïve CD4+ T cells across timepoints. The absence of this change in the other cohorts, and in the CD8+ T cells of the LTC cohort, suggests that this change is not a result of repeated SARS-CoV-2 vaccination, but rather cohort specific to frail older adults living in LTC. Chronic stress, older age, and frailty are all associated with reduced levels of naïve T cells37,38,39. Long-term care residents tend to be frail, and their frailty increases with the length of their stay40,41. Thus, it is possible that the reduction in naïve CD4+ T cells is merely a product of increasing frailty and age over the course of LTC residence rather than repeated vaccinations, but future studies using pre-pandemic samples would be required to disentangle these factors. None of the cohorts displayed elevated levels of terminally differentiated CD4+ or CD8+ T cells following repeated SARS-CoV-2 vaccination, again contrasting repeated SARS-CoV-2 vaccination with the changes observed in SARS-CoV-2 infection and chronic infections8,16,17,18,19.
Considering that, compared to healthy adults, older adults and individuals on immunomodulatory drugs are at risk of poor outcomes associated with SARS-CoV-2 infection42,43,44,45,46, we compared T cell vaccination responses between these three cohorts. We did observe the expected age- and frailty-related changes in the LTC cohort, including lower levels of naïve T cells than their younger counterparts in the HA and RA cohorts38,47. While the spike-specific T cell responses were similar between the cohorts at each timepoint, there were differences in their exhausted phenotype profiles. Only the spike-specific CD4+ T cell FS was significantly higher in the HA cohort than the LTC cohort, while the other FS and PFS did not differ between cohorts, which suggests that the exhaustion marker profile differences do not largely influence the functional capacity of the spike-specific T cells. There are numerous differences in T cell exhaustion marker profiles between the vulnerable cohorts and healthy adults following repeated SARS-CoV-2 vaccination. Whether these influence vaccine effectiveness, or contribute to the severity of SARS-CoV-2 infection, remains a subject for future exploration.
Given that one of the primary concerns regarding immune exhaustion was the frequency of SARS-CoV-2 vaccinations, we explored the impact of primary series dosing interval on overall T cell phenotypes and spike-specific T cell responses in the HA cohort. Dosing interval did not impact spike-specific T cell levels or their expression of exhaustion markers. There is debate regarding the impact of dosing interval on spike-specific T cell functional responses. Some reports indicate that a longer interval between the first and second dose fails to impact polyfunctional spike-specific CD4+ T cells responses48. However, others report that longer dosing intervals did not increase spike stimulation associated IFN-γ production by ELISPOT, but did increase spike-specific CD4+ T cell cytokine production within ELISPOT positive individuals23. This suggests that a longer dosing interval may not elicit higher CD4+ T cell responses in all individuals, but rather may exert the greatest impact among a subset of individuals with strong overall responses. For spike-specific CD8+ T cell responses, previous studies found that the frequencies of polyfunctional spike-specific CD8+ T cells did not differ between dosing intervals23,48. Our study differs from previous publications by including GM-CSF in addition to TNF-α, IFN-γ, and IL-2, which may contribute to our finding that the shortest primary series dosing interval was associated with higher spike-specific CD8+ T cell FS and PFS. While polyfunctionality is implicated in protection against severe disease and disease progression in the context of other pathogens, it remains to be determined if functionality and polyfunctionality are correlates of immune protection against SARS-CoV-2, and if they can explain differences in infection rates or disease severity attributed to dosing interval15,49,50,51. We also found that the total CD8+ T cell compartment of participants in the shortest and longest dosing intervals displayed higher frequencies of PD-1+ TIGIT+ cells than those within the intermediate dosing interval. However, the frequencies of PD-1−TIGIT− CD8+ T cells were not significantly different between the vaccine interval groups, and it is therefore unlikely that this difference in exhaustion marker expressing CD8+ T cells impacts overall immune function.
There are a few limitations to our study including the small size of our RA cohort, and the high number of participants in the HA cohort lost to follow-up, as it became increasingly difficult to obtain samples from individuals without previous SARS-CoV-2 infections after the second dose. Our study included only individuals without previous SARS-CoV-2 infections, to avoid confounding of infection associated changes in T cells with those of vaccination. Consistent with other reports in the literature, we found that repeated SARS-CoV-2 vaccination did not result in enhanced cytokine production in individuals without a previous SARS-CoV-2 infection24. Studies that incorporated individuals with hybrid immunity, or that did not assess history of SARS-CoV-2 infections, could see different trends than those focusing on uninfected individuals. Additionally, previous studies have demonstrated that the majority of double positive cells in the AIM assays are antigen-specific, with little bystander activation52,53,54. However, we cannot eliminate the possibility that bystander activation may contribute to changes in the expression of surface markers. We also did not evaluate the Th1, Th2, Th17, or Treg phenotypes within the population of CD4+ T cells expressing exhaustion markers. The results and conclusions herein can only be definitively applied to the vaccine types and doses included in this study. Future research may seek to extend the vaccine types and doses examined, as well as explore the interplay of exhaustion, T cell receptor sequences, and affinity, as sequencing and specific measures of affinity were not within the scope of the current study.
In conclusion, we have demonstrated that repeated SARS-CoV-2 vaccinations do not increase T cell exhaustion or lead to substantive alterations in the T cell compartment in frail older adults in LTC, immunosuppressed individuals with RA, or healthy adults. Spike-specific T cell levels did not decline upon additional vaccinations, nor did their expression of exhaustion markers increase. T cells also did not display functional deficits in response to stimulation with the spike protein from SARS-CoV-2 following additional SARS-CoV-2 vaccinations. Finally, the overall T cell compartment did not display increases in exhaustion marker expression or terminally differentiated T cells. Repeated SARS-CoV-2 vaccination, as recommended particularly for vulnerable cohorts, is therefore not cause for concern with regards to the T cell compartment, as it does not induce phenotypic or functional characteristics of T cell exhaustion.
Methods
Ethics statement and blood collection
Study recruitment and procedures were reviewed and approved by the Hamilton Integrated Research Ethics Board for protocols #13059 (Long-Term Care, LTC), #13229 (TIMING, HA), and #13307 (SUCCEED, RA). Informed consent for sample and data collection and publication was obtained from all participants or their substitute decision makers. Participants in the LTC cohort were part of the COVID in Long-Term Care Study, a longitudinal observational cohort study of residents of long-term care facilities in Ontario, Canada55. Frailty scores were calculated using the Clinical Frailty Scale, which combines measures of basic activities of daily living, along with health status and comorbidities, as previously described56,57,58. The scoring system ranges from 1 to 9, with 9 indicating terminally ill, very frail, individuals. Participants in the SUCCEED (immunosuppressed RA) cohort were classified as having rheumatoid arthritis based on criteria from the European League Against Rheumatism/American College of Rheumatology and were prescribed immunomodulatory drugs (Supplementary Data 1). The participants in the RA cohort were part of the SUCCEED study and recruited from clinics in Ontario, Canada28,59. The HA cohort included healthcare workers recruited in Ontario22. Previous COVID-19 infection was determined based on a positive PCR or rapid antigen test, or seroconversion for anti-nucleocapsid IgG antibodies60. Only participants without prior SARS-CoV-2 infection, and who received 2, 3, or 4 SARS-CoV-2 mRNA vaccinations were included in the study cohort. Cohort demographic information can be found in Table 1.
Peripheral blood was collected in sodium heparin vacutainers 3 months after the second, third, and fourth SARS-CoV-2 vaccinations. Within the HA cohort, peripheral blood was also collected for a subset of individuals at 3 weeks post dose 2. Peripheral blood mononuclear cells (PBMCs) were isolated using a Ficoll gradient. The PBMCs were cryopreserved in liquid nitrogen before thawing for use in T cell assays. For some participants there were not enough cells to perform all assays at all assessment timepoints.
Increased expression of exhaustion-associated markers can accompany early T cell expansion, but this is likely indicative of activation rather than a state of exhaustion33,61,62. Using matched samples collected from the HA cohort at 3 weeks and 3 months post dose 2, we compared the prevalence of CD4+ and CD8+ T cell subsets (naïve, CM, EM, EMRA) as well as T cell expression of exhaustion markers (Supplementary Fig. 3 and Supplementary Data 11). Although others have reported that peak SARS-CoV-2-specific T cell responses occur within a few weeks of SARS-CoV-2 vaccination and decline in the following 1–3 months, we did not observe a significant decline in the spike-specific T cell frequencies from 3 weeks to 3 months post dose 227,36. As expected, we saw that the prevalence of CD4+ CM T cells increased between 3 weeks and 3 months post dose 2. Frequencies of PD-1+TIGIT- and PD-1+TIGIT+ CD4+ T cells were reduced at 3 months post dose 2, compared with 3 weeks post dose 2 (Supplementary Fig. 3 and Supplementary Data 11). The changes in overall T cell exhaustion marker expression following the second SARS-CoV-2 vaccination suggests that T cells are still activated at 3 weeks post vaccination, so 3 months post vaccination was the appropriate time to measure exhaustion.
Activation induced marker (AIM) assay
Spike-specific memory T cell responses were quantified using an AIM assay63. Briefly, cryopreserved PBMCs were thawed, washed, counted, and resuspended in RPMI supplemented with 10% fetal bovine serum (#12483020, Gibco), 1% Pen/Strep (#15140122, Gibco), 1% HEPES (1 M, #7365-45-9, Sigma), 1% GlutaMAX (#35050061, Gibco), and 0.5% β-mercaptoethanol (#21985023, Gibco) (cRPMI), then rested overnight in a tissue culture incubator at 37 °C/5% CO2. The following day the cells were counted, and the concentration was adjusted so that 100 µL of cRPMI containing 0.5 × 106 cells was added to each well of a 96-well U-bottom plate. Peptides from the spike protein of SARS-CoV-2 (S complete Peptivator, #130-127-951, Miltenyi Biotec) were diluted in cRPMI and used to stimulate PBMCs at a final concentration of 1 µg/mL for 44 h at 37 °C/5% CO2. Cytostim (#130-092-172, Miltenyi Biotec) was used as a positive stimulation control for each sample at 0.25 µL per well, and a media only unstimulated control was also included for each sample.
After 44 h, cells were washed with PBS, then incubated for 30 min at room temperature in the dark with Zombie Near-IR (#423105, BioLegend) to identify live cells. Cells were washed with PBS, then washed with FACS Wash (0.5% (w/v) bovine serum albumin (#A3912, Sigma), 5 mM EDTA (pH 7.4–7.6, #E5134-500G, Sigma) in PBS). The staining cocktail was prepared in PBS and Brilliant Stain Buffer Plus (#566385, BD Biosciences) as detailed in Supplementary Data 12. Antibodies were titrated by lot to determine optimal concentration. Samples were incubated with the antibody cocktail for 30 min at room temperature in the dark, washed with FACS Wash, and resuspended in FACS Wash to run on the cytometer.
AIM+ (spike-specific) T cells were identified by co-expression of CD134 (PE) and CD25 (PE-Cy7) on CD4+ T cells (CD3-BV510, CD4-BB700) and CD137 (APC) and CD69 (BV711) on CD8+ T cells (BB515)52,64,65. Expression of LAG-3 (PE/Dazzle594), TIM-3 (BV605), and PD-1 (BV421) on the AIM + T cells was assessed. The media control well was used to set the gates for co-expression of activation markers (AIM + T cells), and isotype controls were used to set the gates for each exhaustion marker within the AIM + CD4+ or AIM + CD8+ T cells. The gating strategy for the AIM assay is depicted in Supplementary Fig. 4.
Intracellular cytokine stain (ICS)
To evaluate cytokine production, 0.5 × 106 cells were seeded into a 96-well U-bottom plate. Peptides from the spike protein of SARS-CoV-2 (S complete Peptivator, #130-127-951, Miltenyi Biotec) were used to stimulate PBMCs for 19 h. Golgi plug (#555029, BD) was diluted 125× in cRPMI before adding 50 µL to each well. The plate was then incubated at 37 °C/5% CO2 for 5 h. Cells were then washed with PBS, and stained with Zombie Aqua (#423102, BioLegend) for 30 min at room temperature in the dark to detect live cells. Cells were washed with PBS, followed by FACS Wash. The surface stain was prepared in FACS Wash, and included CD4 (Pacific Blue), and CD8 (AF700). Cells were incubated with the surface stain for 30 min in the dark at room temperature, fixed and permeabilized by incubation with Cytofix/Cytoperm (#51-2091KZ, BD Biosciences), and washed twice with 1× Perm/Wash buffer (#51-2091KZ, BD Biosciences).
The intracellular stain antibody cocktail was prepared in 1× Perm/Wash buffer, and included IFN-γ (APC), TNF-α (PE-Cy7), GM-CSF (APCVio770), IL-2 (PE), and CD3 (BV605), as detailed in Supplementary Data 12. Samples were stained with intracellular stain antibody cocktail for 30 min at room temperature in the dark, washed with 1× Perm/Wash buffer, then resuspended in FACS Wash to be run on the cytometer.
Immunophenotyping
CD4+ and CD8+ T cell populations were identified by surface staining and flow cytometry analysis of unstimulated PBMCs as previously described60. Briefly, naïve T cells were classified as CCR7+CD45RA+, CM were CCR7+CD45RA−, EM were CCR7−CD45RA−, and EMRA were CD45RA+CCR7−. Terminally differentiated cells were classified as EMRA+CD57+CD28−. T cells within the CD4+ and CD8+ compartments were also assessed for expression of PD-1 (FITC), TIGIT (BV510), and TIM-3 (BV785). Isotype controls were also used to determine positivity for the exhaustion markers. The gating strategy for the surface immunophenotyping is depicted in Supplementary Fig. 5, details for the antibody staining cocktail can be found in Supplementary Data 12.
All samples assessed by flow cytometry were acquired on a CytoFLEX (Beckman Coulter) using CytExpert software (version 2.4). FCS files were gated using FlowJoTM version 10 software (BD Life Sciences).
Combinatorial polyfunctionality analysis of antigen-specific T cell subsets (COMPASS)
ICS samples were manually gated down to the CD4+ and CD8+ T cell nodes using FlowJoTM version 10 (BD Life Sciences) and then exported from these nodes for analysis using the COMPASS package in R (version 1.42.0)15, as well as the flowCore (v2.8.0), and openCyto (v2.8.4) packages. COMPASS employs Bayesian statistics to determine the posterior probability of antigen-specific responses for each possible cytokine combination (of the explored cytokines GM-CSF, IFN-γ, TNF-α, and IL-2) within the CD4+ or CD8+ T cell population of each participant, based on differences in cytokine production between unstimulated and antigen-stimulated wells. The parameters were set to 40,000 iterations with 8 replications. The COMPASS package also produces two different summary scores to summarize the polyfunctionality of the T cells. The functionality score (FS) uses the posterior probabilities to determine the proportions of different cytokine combinations that are present in each individual for their CD4+ and CD8+ T cells (separately), out of the total possible cytokine combinations. The PFS weighs more heavily the cytokine combinations which are positive for more unique cytokines (i.e., weighs more heavily triple positive, and quadruple cytokine-positive combinations, over those that only produce one or two unique cytokines).
Statistics
Fisher’s exact tests and multivariable linear mixed models were run in R version 4.4.1 (R Core Team) using stats (v4.4.1), usethis (v2.2.3), tidyverse (v2.0.0), tidytext (v0.4.2), devtools (v2.4.5), sjPlot (v2.8.16), lme4 (v 1.1.35.4), and lmerTest (v3.1.3) packages. Data were log2 transformed for analysis using mixed models. Fisher’s exact test was used to compare demographics between the LTC, RA, and HA cohorts for categorical variables. Two-tailed multivariable linear mixed models, accounting for age and sex, were used to assess changes in T cell populations within each cohort after additional SARS-CoV-2 vaccinations. Within each cohort, FDR adjusted p values were calculated for surface stain T cell compartment breakdowns (CM, EM, EMRA, N), surface exhaustion marker expression, and spike-specific CD4+ and CD8+ T cell exhaustion markers, to account for multiple mixed models/repeated testing run on T cells stemming from the same parent population, using the p.adjust function in R. For the three dosing intervals (Rec, Del, Ext) within the HA cohort, and the comparisons between the LTC, RA, and HA cohorts D’Agostino & Pearson tests were used with p < 0.05 indicating a significant departure from normality. Ordinary one-way ANOVAs, or Brown-Forsythe ANOVAs for parameters with unequal standard deviations between cohorts, or Kruskal–Wallis tests for nonparametric measures, were used to assess differences between dosing interval cohorts and the differences between the LTC, RA, and HA cohorts at each post vaccination timepoint. If the ANOVA was significant, after FDR correction if applicable, Tukey’s, Dunnett’s T3, or Dunn’s post-hoc tests were used to determine which dosing intervals differed from one another (for ordinary one-way ANOVAs, Brown-Forsythe ANOVAs, and Kruskal–Wallis tests respectively). COMPASS heatmaps displaying samples clustered by group and timepoint were produced using the cowplot (v1.1.3) package. GraphPad version 8 was used to plot flow cytometry data, and for ANOVAs assessing demographic differences between the cohorts for age and dosing intervals. Correlation coefficients were determined using linear mixed models, accounting for the random effect of cohort, and FDR adjusted p values were obtained to account for multiple testing. The correlation plot was created using the corrplot (v0.92) package. Female sex was entered as 2, male sex was entered as 1. 3mo2 was 2, 3mo3 was 3, 3mo4 was 4. Only complete cases were considered.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data generated in this study for the LTC and RA cohorts are provided in the Source Data File. Data for the HA cohort are available under restricted access, as consent was not obtained for disclosure of individualized participant information. Data from the HA cohort can only be disclosed following the submission and approval of the request to a Population Health Research Institute (PHRI) Hamilton Review Committee22. Requests for access or further information can be sent to the corresponding author, with an anticipated response time of 2 weeks. If access is approved, data would be available to the requesting party for the duration of their study. Measures of central tendency for the HA cohort are provided in the source data file. Source data are provided with this paper.
Code availability
The COMPASS code used in this study is available on Zenodo66.
Change history
19 June 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41467-025-61098-1
References
Canada, P. H. A. of COVID-19 vaccines: Canadian Immunization Guide. https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-26-covid-19-vaccine.html (2021).
Clinical Guidance for COVID-19 Vaccination. CDC https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html (2024).
Kriss, J. L. COVID-19 vaccine second-dose completion and interval between first and second doses among vaccinated persons — United States, December 14, 2020−February 14, 2021. MMWR Morb. Mortal Wkly. Rep. 70, 389–395 (2021).
Canada, P. H. A. of. COVID-19 vaccine doses administered — Canada.ca. aem https://health-infobase.canada.ca/covid-19/vaccine-administration/#a3 (2021).
Canada, P. H. A. of. Guidance on the use of COVID-19 vaccines during the fall of 2024. https://www.canada.ca/en/public-health/services/publications/vaccines-immunization/national-advisory-committee-immunization-guidance-covid-19-vaccines-fall-2024.html (2024).
Manoharan, B. et al. Sociodemographic factors associated with vaccine hesitancy in the South Asian community in Canada. Can. J. Public Health 115, 924–935 (2024).
Hamel, L. et al. KFF COVID-19 Vaccine Monitor: October 2021. KFF https://www.kff.org/coronavirus-covid-19/poll-finding/kff-covid-19-vaccine-monitor-october-2021/ (2021).
De Biasi, S. et al. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat. Commun. 11, 3434 (2020).
Adamo, S. et al. Profound dysregulation of T cell homeostasis and function in patients with severe COVID-19. Allergy 76, 2866–2881 (2021).
Yi, J. S., Cox, M. A. & Zajac, A. J. T-cell exhaustion: characteristics, causes and conversion. Immunology 129, 474–481 (2010).
Blank, C. U. et al. Defining ‘T cell exhaustion. Nat. Rev. Immunol. 19, 665–674 (2019).
Bally, A. P. R., Austin, J. W. & Boss, J. M. Genetic and epigenetic regulation of PD-1 expression. J. I. 196, 2431–2437 (2016).
Rha, M.-S. & Shin, E.-C. Activation or exhaustion of CD8+ T cells in patients with COVID-19. Cell Mol. Immunol. 18, 2325–2333 (2021).
Rha, M.-S. et al. PD-1-expressing SARS-CoV-2-specific CD8+ T cells are not exhausted, but functional in patients with COVID-19. Immunity 54, 44–52.e3 (2021).
Lin, L. et al. COMPASS identifies T-cell subsets correlated with clinical outcomes. Nat. Biotechnol. 33, 610–616 (2015).
Henson, S. M., Riddell, N. E. & Akbar, A. N. Properties of end-stage human T cells defined by CD45RA re-expression. Curr. Opin. Immunol. 24, 476–481 (2012).
Weng, N., Akbar, A. N. & Goronzy, J. CD28− T cells: their role in the age-associated decline of immune function. Trends Immunol. 30, 306–312 (2009).
Brenchley, J. M. et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 101, 2711–2720 (2003).
Strioga, M., Pasukoniene, V. & Characiejus, D. CD8+ CD28- and CD8+ CD57+ T cells and their role in health and disease. Immunology 134, 17–32 (2011).
Röltgen, K. et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell 185, 1025 (2022).
Quach, C. & Deeks, S. COVID-19 vaccination: Why extend the interval between doses? J. Assoc. Med. Microbiol. Infect. Dis. Can. 6, 73–78 (2021).
Leong, D. P. et al. Comparison of three dosing intervals for the primary vaccination of the SARS-CoV-2 mRNA Vaccine (BNT162b2) on magnitude, neutralization capacity and durability of the humoral immune response in health care workers: a prospective cohort study. PLoS ONE 18, e0281673 (2023).
Payne, R. P. et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell 184, 5699–5714.e11 (2021).
Cai, C. et al. SARS-CoV-2 vaccination enhances the effector qualities of spike-specific T cells induced by COVID-19. Sci. Immunol. 8, eadh0687 (2023).
Maringer, Y. et al. Durable spike-specific T cell responses after different COVID-19 vaccination regimens are not further enhanced by booster vaccination. Sci. Immunol. 7, eadd3899 (2022).
Datwani, S. et al. Dynamics of T-cell responses following COVID-19 mRNA vaccination and breakthrough infection in older adults. Pathog. Immun. 8, 117–135 (2023).
Reinscheid, M. et al. COVID-19 mRNA booster vaccine induces transient CD8+ T effector cell responses while conserving the memory pool for subsequent reactivation. Nat. Commun. 13, 4631 (2022).
Benoit, J. M. et al. Immunomodulatory drugs have divergent effects on humoral and cellular immune responses to SARS-CoV-2 vaccination in people living with rheumatoid arthritis. Sci. Rep. 13, 22846 (2023).
Farroni, C. et al. Booster dose of SARS-CoV-2 messenger RNA vaccines strengthens the specific immune response of patients with rheumatoid arthritis: a prospective multicenter longitudinal study. Int. J. Infect. Dis. 125, 195–208 (2022).
Swanson, P. A. et al. AZD1222/ChAdOx1 nCoV-19 vaccination induces a polyfunctional spike protein–specific TH1 response with a diverse TCR repertoire. Sci. Transl. Med. 13, eabj7211 (2021).
Li, L. et al. In-depth analysis of SARS-CoV-2–specific T cells reveals diverse differentiation hierarchies in vaccinated individuals. JCI Insight 7, e156559 (2022).
Loretelli, C. et al. PD-1 blockade counteracts post–COVID-19 immune abnormalities and stimulates the anti–SARS-CoV-2 immune response. JCI Insight 6, e146701 (2021).
Minervina, A. A. et al. SARS-CoV-2 antigen exposure history shapes phenotypes and specificity of memory CD8+ T cells. Nat. Immunol. 23, 781–790 (2022).
Kavazović, I. et al. Vaccination provides superior in vivo recall capacity of SARS-CoV-2-specific memory CD8 T cells. Cell Reports 42, (2023).
Guerrera, G. et al. BNT162b2 vaccination induces durable SARS-CoV-2–specific T cells with a stem cell memory phenotype. Sci. Immunol. 6, eabl5344 (2021).
Özbay Kurt, F. G. et al. Booster dose of mRNA vaccine augments waning T cell and antibody responses against SARS-CoV-2. Front. Immunol. 13, 1012526 (2022).
Klopack, E. T., Crimmins, E. M., Cole, S. W., Seeman, T. E. & Carroll, J. E. Social stressors associated with age-related T lymphocyte percentages in older US adults: evidence from the US Health and Retirement Study. Proc. Natl. Acad. Sci. USA 119, e2202780119 (2022).
Johnstone, J. et al. T-cell phenotypes predictive of frailty and mortality in elderly nursing home residents. J. Am. Geriatrics Soc. 65, 153–159 (2017).
Luo, O. J. et al. Multidimensional single-cell analysis of human peripheral blood reveals characteristic features of the immune system landscape in aging and frailty. Nat. Aging 2, 348–364 (2022).
Castro-Herrera, V. M. et al. Relationships between age, frailty, length of care home residence and biomarkers of immunity and inflammation in older care home residents in the United Kingdom. Front. Aging 2, 599084 (2021).
Milte, R. et al. Prevalence and determinants of physical frailty among people living in residential aged care facilities: a large-scale retrospective audit. BMC Geriatrics 22, 424 (2022).
Wiersinga, W. J., Rhodes, A., Cheng, A. C., Peacock, S. J. & Prescott, H. C. Pathophysiology, transmission, diagnosis, and treatment of Coronavirus Disease 2019 (COVID-19): a review. JAMA 324, 782–793 (2020).
Abul, Y., Leeder, C. & Gravenstein, S. Epidemiology and clinical presentation of COVID-19 in older adults. Infect. Dis. Clin. North Am. 37, 1–26 (2023).
Figueroa-Parra, G. et al. Risk of severe COVID-19 outcomes associated with rheumatoid arthritis and phenotypic subgroups: a retrospective, comparative, multicentre cohort study. Lancet Rheumatol. 4, e765–e774 (2022).
Jin, L. et al. Rheumatoid arthritis and COVID-19 outcomes: a systematic review and Meta-analysis. BMC Rheumatol. 8, 61 (2024).
Grainger, R., Kim, A. H. J., Conway, R., Yazdany, J. & Robinson, P. C. COVID-19 in people with rheumatic diseases: risks, outcomes, treatment considerations. Nat. Rev. Rheumatol. 18, 191–204 (2022).
Leng, S. X. & Pawelec, G. Single-cell immune atlas for human aging and frailty. Life Med. 1, 67–70 (2022).
Hall, V. G. et al. Delayed-interval BNT162b2 mRNA COVID-19 vaccination enhances humoral immunity and induces robust T cell responses. Nat. Immunol. 23, 380–385 (2022).
Seder, R. A., Darrah, P. A. & Roederer, M. T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 8, 247–258 (2008).
Darrah, P. A. et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 13, 843–850 (2007).
Canada, P. H. A. of. Rapid review on protective immunity post infection with SARS-CoV-2: update 3. https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/canadas-reponse/summaries-recent-evidence/rapid-review-protective-immunity-post-infection-sars-cov-2-update-3.html (2022).
Zaunders, J. J. et al. High levels of human antigen-specific CD4+ T cells in peripheral blood revealed by stimulated coexpression of CD25 and CD134 (OX40). J. Immunol. 183, 2827–2836 (2009).
Reiss, S. et al. Comparative analysis of activation induced marker (AIM) assays for sensitive identification of antigen-specific CD4 T cells. PLoS ONE 12, e0186998 (2017).
Nguyen, N. X. et al. Immunogen-specific strengths and limitations of the activation-induced marker assay for assessing murine antigen-specific CD4+ T cell responses. J. Immunol. 210, 916–925 (2023).
Breznik, J. A. et al. Early humoral and cellular responses after bivalent SARS-CoV-2 mRNA-1273.214 vaccination in long-term care and retirement home residents in Ontario, Canada: an observational cohort study. J. Med. Virol. 95, e29170 (2023).
Theou, O. et al. A classification tree to assist with routine scoring of the Clinical Frailty Scale. Age Ageing 50, 1406–1411 (2021).
Pasat, Z. et al. Examining the association between frailty and antibody neutralization of SARS-CoV-2: a multisite retrospective cohort study. J. Am. Med. Dir. Assoc. 25, 647–649.e8 (2024).
Dumitrascu, F., Branje, K. E., Hladkowicz, E. S., Lalu, M. & McIsaac, D. I. Association of frailty with outcomes in individuals with COVID-19: a living review and meta-analysis. J. Am. Geriatrics Soc. 69, 2419–2429 (2021).
Hitchon, C. A. et al. Methotrexate and tumor necrosis factor inhibitors independently decrease neutralizing antibodies after SARS-CoV-2 vaccination: updated results from the SUCCEED study. Vaccines (Basel) 12, 1061 (2024).
Breznik, J. A. et al. Cytomegalovirus seropositivity in older adults changes the T cell repertoire but does not prevent antibody or cellular responses to SARS-CoV-2 vaccination. J. Immunol. 209, 1892–1905 (2022).
Jubel, J. M., Barbati, Z. R., Burger, C., Wirtz, D. C. & Schildberg, F. A. The role of PD-1 in acute and chronic infection. Front. Immunol. 11, 487 (2020).
Hokey, D. A. et al. Activation drives PD-1 expression during vaccine-specific proliferation and following lentiviral infection in macaques. Eur. J. Immunol. 38, 1435–1445 (2008).
Kennedy, A. E. et al. Lasting changes to circulating leukocytes in people with mild SARS-CoV-2 infections. Viruses 13, 2239 (2021).
Sekine, T. et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell 183, 158–168.e14 (2020).
Tarke, A. et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 185, 847–859.e11 (2022).
Verschoor, C. COMPASS analysis to investigate immune exhaustion. https://doi.org/10.5281/zenodo.15329735 (Zenodo, 2025).
Acknowledgements
The authors would like to thank the staff from the Human Immunology Testing Suite (HITS) at McMaster University for their contributions to this project. Thank you to Tara Kajaks for managing the COVID in LTC study, and Hesam Hafezalseheh for his assistance with statistics. We would also like to thank our research participants and coordinators for their contributions to this project. J.A.B. was supported by a Postdoctoral Fellowship from the McMaster Institute for Research on Aging. P.Y.K. was supported by the McMaster University Department of Medicine Mid-Career Award. The RA cohort funding was from the Public Health Agency of Canada (arrangement number 2122-HQ-000070, awarded to M.J.L. and D.M.E.B.). The views herein do not necessarily represent their views. The study involving the HA cohort was funded by the Population Health Research Institute and the Department of Medicine at McMaster University. The study involving the LTC cohort was funded by a grant from the Canadian COVID-19 Immunity Task Force of the Public Health Agency of Canada (021-HQ-000138, awarded to A.P.C., I.N., J.L.B., C.P.V., and D.M.E.B.) and a grant from the Canadian Institutes of Health Research (#192123, awarded to A.P.C., I.N., J.L.B., C.P.V., and D.M.E.B.).
Author information
Authors and Affiliations
Contributions
J.M.B. conducted experiments, analyzed data, and generated figures, wrote manuscript. J.A.B. conducted experiments, contributed to experiment design, data interpretation and manuscript editing. Y.W. conducted experiments, contributed to data interpretation. A.K. conducted experiments, contributed to data interpretation. L.L. conducted experiments. B.C. collected and processed blood samples, collected participant metadata. B.B. clinical coordinator, recruited and contacted patients, collected samples. M.H. contributed to experiment design and project management. C.M.A. collected and organized participant metadata. M.M. clinical coordinator, recruited and contacted patients, collected participant metadata. N.A. Clinical coordinator, recruited and contacted patients, collected participant metadata. J.D.M. contributed to data interpretation and statistics. G.G. Project administration for HA study, sample collection. P.Y.K. Project administration for HA study, sample collection. J.A.D. Designed HA study and obtained funding. A.P.C. Designed LTC study and obtained funding. D.P.L. Designed HA study and obtained funding. I.N. Designed HA and LTC studies and obtained funding. M.D. Designed HA study and obtained funding. J.L.B. Designed LTC study and obtained funding. Contributed to data interpretation and study design. M.J.L. Designed RA study and obtained funding. Contributed to data interpretation. C.P.V. Designed LTC study and obtained funding. Created pipeline for COMPASS analysis, aided in statistical analyses and data interpretation. D.M.E.B. Designed LTC, HA, and RA studies, and obtained funding. Contributed to data interpretation, experimental design, and manuscript writing and revision.
Corresponding authors
Ethics declarations
Competing interests
M.J.L. is the Director of the Canadian Scleroderma Research Group and has received honoraria for consulting or speakers fees from AbbVie, Actelion, Amgen, AstraZeneca, Boehringer-Ingelheim, BMS, Fresenius-Kabi, GSK, Lilly, Mallinckrodt, Novartis, Pfizer, Sanofi, SOBI, UCB, and Scleroderma Society of Ontario/Canada. D.M.E.B. has received honorarium from Pfizer-Global, Pfizer Canada, and AstraZeneca for consulting on the topics of vaccines. D.M.E.B. is on the Board of Directors of the Lung Health Foundation (unpaid, volunteer), and speaks on the topic of adult vaccination and other lung health issues to policymakers, knowledge users, and the general public. D.M.E.B. was an expert witness for the below court cases on the topic of vaccination; (i) Nabil Ben Naoum vs. L’honorable Maxime Bernier, Canada. Provided written affidavit. and was cross examined. June-September, 2022. (ii) David Lavergne-Poitras vs. PMG Technologies Inc., Canada, Montreal, QC. Provided written affidavit. and was cross examined. May-July 2022. (iii) Syndicat des Metallos S.L., 2008, 9599, 2004, 9344, 9554, 1976, 9449, 9519, 5778, 9996 et als. vs. Procureur General du Canada, Canada, Montreal, QC. Provided written affidavit. March-May 2022. (iv) Fisman et al ats Bridle.DM-LSDOCS.FID1093293. Provided written affidavit. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Ching-Tai Huang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Benoit, J.M., Breznik, J.A., Wu, Y. et al. No evidence of immune exhaustion after repeated SARS-CoV-2 vaccination in vulnerable and healthy populations. Nat Commun 16, 5219 (2025). https://doi.org/10.1038/s41467-025-60216-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-60216-3