Abstract
The self-powered photoelectrochemical components themselves featured advancements in operating independently without external supply. Ultimately, due to lack of assistance from the external bias, the photoelectrochemical response is commonly restricted by the deficient photo-quantum efficiency for the absence of carrier multiplication. This work demonstrates a self-powered photoelectrochemical photodetector based on CuOx/AlGaN nanowires with staggered band structure and enhanced built-in potential for efficient exciton extraction. The generated multiple excitons within reach-through CuOx layer could be speedily separated before Auger recombination. This yields a 131.5% external quantum efficiency and 270.6 mA W−1 responsivity at 255 nm. The work confirms the role of multiple exciton generation in photoelectrochemical systems, offering a solution on paving path of advance for self-powered optoelectronics and weak-light UV imaging applications.
Similar content being viewed by others
Introduction
Self-powered electronics based on photic driving can operate independently and sustainably, which are considered as core components in the low-carbon industrial system. Self-powered photodetectors (PDs), for instance, are indispensable modules for constructing energy-saving optoelectronic systems1,2. Among the existing self-powered PDs, the developing photoelectrochemical (PEC) PDs stand out due to their low cost, non-lithographic fabrication, robust water-resistance and multifunctional adjustable light response, making it receive dramatic increasing attentions in recent years3,4. However, for these conventional self-powered PEC PDs, the photoelectric response cannot benefit from the internal photoelectric gain aroused by the assistance of a power supply and thus the corresponding responsivity is suppressed by the deficient quantum efficiency5 (normally not exceeding 100%).
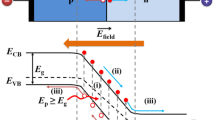
Generally, acquiring access to the photoelectric gain effects in solid-state photodetectors, such as avalanche photodiodes6, photomultipliers7, and photodetectors combined with low-noise trans-resistance amplifiers8, is an effective method to achieve the ultra-high quantum efficiency. However, all the cases above are inevitable to require for the external supplied voltage. In fact, introducing the multiple exciton generation (MEG) effect into the self-powered PEC PDs is a feasible approach to beat the gain-voltage tradeoff. In 2001, Nozik predicted that the highly efficient multiple exciton generation effect in nano-semiconductors9,10. Due to quantum size effects, the bandgap (Eg) of nano-semiconductors can be effectively tuned by adjusting their size11, leading to the formation of discrete intra-band energy levels12. The discreteness of energy reduces the probability of phonon-mediated relaxation, slowing down the electron relaxation process and effectively suppressing the Auger recombination effect. In addition, the reduction in nano-semiconductor size increases the surface-to-volume ratio, resulting in a higher density of surface electrons, which intensifies the Coulomb interaction11. Upon absorbing a high-energy photon, a hot electron is created in the nano-semiconductor. Due to the effective suppression over Auger recombination and the enhancement on Coulomb interaction, the hot electron with sufficient kinetic energy could avoid cooling via radiating phonons but instead excites other ground-state electrons to higher energy states through collisions13. In this process, if the photon energy (E) is above the energy threshold (Eth > 2Eg), a high-energy photon can generate two or more electron-hole pairs in the nano-semiconductor14,15,16, resulting in internal photoelectric gain and breaking through the restriction of the quantum efficiency.
To achieve the effect of photoelectric gain without bias, aside from the MEG by high-energy photon excitation, an appropriate band alignment and the internal electric field intensity enhancement should be accounted in, which facilitates exciton extraction and carrier transfer over the thermal exciton relaxation process17,18,19. Herein, a PEC PD based on the p-type CuO-Cu2O (CuOx) nanocomposite/n-type AlGaN nanowire heterostructure is developed to achieve high photoelectric conversion efficiency. Under 255 nm UV light illumination and 0 V bias, the high-energy electrons in the CuOx auxiliary light-absorbing layers on AlGaN NWs are excited from the valence band to an energy level above the minimum of the conduction band, resulting in collision ionization and an efficient MEG process. The strong built-in electric field at the AlGaN/CuOx type-II heterojunction interface promotes the separation of multiple carriers without cooling, significantly enhancing the photocurrent. The AlGaN/CuOx UV PD exhibits a high responsivity of 270.6 mA W−1 and an external quantum efficiency (EQE) of 131.5% at the light intensity of 5 μW cm−2, achieving one of the highest results among the reported self-powered PEC PDs to date. Due to excellent photoresponse, the efficient AlGaN/CuOx PEC PD can be applied for high-quality UV imaging techniques. This work provides a method for developing the next generation of high-performance PEC UV detectors.
Results
Reach-through band bending at the solid–electrolyte interface
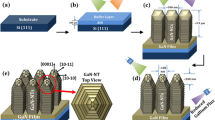
The photoelectrode, adopted for this work, is composed of Si-doped n-Al0.3Ga0.7N nanowires (~400 nm long), vertically grown on n-Si (111) substrate, as shown in Fig. 1a and Supplementary Fig. 1. The AlGaN-based PEC PDs were fabricated using an encapsulation technique to access the PEC response of AlGaN-based photoelectrodes (see “Methods”). A typical three-electrode quartz window cell was employed (Fig. 1a), consisting of a working electrode (PEC PD), a counter electrode (Pt), a reference electrode (Ag/AgCl), and the electrolyte solution (Na2SO4). When bare n-type AlGaN contacts the electrolyte, upward band bending occurs on the surface of AlGaN, resulting in positive photocurrent under deep-UV light irradiation (detailed mechanism of carrier transport can be seen in Supplementary Fig. 2a).
a Construction and working principles of n-AlGaN nanowires-based light-detection electrochemical cell. b–d Wavelength-dependent operation model of the cell with low-level, high-level and moderate-level amount of decorated CuOx, respectively. The energy band diagrams, CuOx decorated AlGaN nanowires and photocurrent signals are schematically shown. e Spectral response of AlGaN/CuOx-nanowire-based PEC PDs at three different deposition times.
In order to modify the surface physicochemical properties and control the behavior of carriers at the surface, the rational metal oxides decoration on nanostructural semiconductor is an effective strategy for the pursuit of PEC devices with superior performance. Herein, the n-AlGaN nanowires were treated with surface modulation by depositing CuOx composites, composed of CuO and Cu2O nano-mixture. Regarding to conduction type of CuOx, the AlGaN/CuOx heterostructure, indeed, could blur the direct evidence of the surface band bending. In order to get rid of the interfering from the heterojunction, the specific photoelectrode, with depositing CuOx layer on FTO substrate, was prepared for I–t test to observe the polarity of the photocurrent generated by the bare CuOx. When the bare p-type semiconductor contacts the electrolyte, the energy band at the semiconductor/electrolyte interface bends downward (Supplementary Fig. 2b), resulting in a negative photocurrent under UV light illumination. As shown in Supplementary Fig. 2c, the photocurrent with the negative polarity means the CuOx as the p-type semiconductor. According to our best knowledge, the operating mechanisms on the manipulation of carrier dynamics for the n-type III nitrides decorated by the CuOx could be analyzed through two types of role definition of the CuOx in the PEC process: as a surface co-catalyst to improve surface chemical dynamics2 or as the p-type light-absorbing layer to construct the pn junction for inner structure optimazation3,4.
When the n-AlGaN nanowires are decorated by a small amount of nano-structural CuOx, the deposited oxides play the role of co-catalyst to promote the oxygen evolution reaction (OER). Therefore, the resulting positive-polarity photocurrent under deep UV light with λ1 (defined as E(λ1) > Eg(AlGaN), where E(λ1) is the photon energy of λ1 wavelength light and Eg(AlGaN) is the bandgap of AlGaN) are improved, comparing to the bare AlGaN nanowires with a lower positive photocurrent, which is schematically shown in Fig. 1b. While under longer wavelength λ2 light irradiation (Eg(CuOx) < E(λ2) < Eg(AlGaN), where λ2 is the wavelength of lower-energy light and Eg(CuOx) is the bandgap energy of CuOx composites), due to the excessively low amount of loaded CuOx, there is no obvious photocurrent detected by electrochemical working station (Fig. 1b).
When the AlGaN is covered with a sufficient amount of CuOx nanocrystals (Fig. 1c), a compact thin capping layer was formed on the top of the nanowires. As an intrinsic p-type semiconductor for oxides of copper, the constructed pn junction on the basis of CuOx/AlGaN contact should not be ignored in this case. When this p-CuOx/n-AlGaN junction contacts the electrolytes, it is typically assumed that the energy band at CuOx/electrolytes interface is commonly deemed to bend downwards by default, achieving electrochemical equilibrium at the p-type semiconductor/electrolyte interface. In this case, the direction of the built-in electric fields at the interfaces of CuOx/AlGaN and CuOx/electrolytes are opposite and, thus, there is competition on carrier transport at the two interfaces (Fig. 1c). When p-CuOx/n-AlGaN nanowires are irradiated by high-energy λ1 light, exhibiting the positive photocurrent for p-CuOx/n-AlGaN nanowires. While irradiated by lower-energy λ2 light, a negative photocurrent signal is generated under λ2 light illumination. The detailed analysis on the transport of photogenerated carriers can be referred to in Supplementary Note 1.
In fact, besides of the described cases in Fig. 1b, c, there is another energy band structure for p-CuOx/n-AlGaN nanowires in electrolyte, which is rarely mentioned before. Since the significant difference of work function between the CuOx and heavily n-doped AlGaN, the cladding layer of moderate amount of p-type CuOx could be completely depleted by n-type AlGaN. More than that, to further achieve the electrochemical equilibrium, the energy band would bend upward at the CuOx/electrolyte interface to prevent the the electrons constantly transferring from the semiconductor to the electrolyte, which is shown in Fig. 1d. Although the CuOx is a p-type semiconductor, the downwards band bending is absent at semiconductor/electrolyte interface, which is replaced by abnormal upwards bending. From the viewpoint of the energy band characteristics, the upwards band bending of the reach-through CuOx layer perfectly matches with the band bending of p-CuOx/n-AlGaN junction space charge region, significantly accelerating carrier transport and efficiently facilitating charge separation in the entire CuOx segment. Therefore, a large photocurrent with positive polarity is detected by the illumination of λ1 light. Similarly, when the p-CuOx/n-AlGaN junction is exposed under λ2 light, a small magititude of positive photocurrent generates in the circuit loop for this case, which is opposite to the direction of photocurrent for λ1 light in case of Fig. 1b. In the other word, detecting the polarity switching of photocurrent between high- and lower- energy light is an effective method to confirm the truth of direction change of band bending at p-CuOx/electrolyte interface, as the amount of deposited CuOx increases on the AlGaN nanowire. It is worth noting that the photoelectrode with the configuration of CuOx/AlGaN/Si multi-heterojunction was designed for optimizing band alignment and carrier transfer. Generally speaking, as the results shown by the FDTD simulation (see below), most of UV light could be absorbed by the AlGaN/CuOx-nanowire, which determines that a great many of the carriers are generated within the nanowires. Thus, for the upper CuOx/AlGaN interface, it was assigned to photogenerated carrier regulation. For the lower AlGaN/Si interface, the heavily n-type doped Si substrates were adopted for plasma-enhanced molecular beam epitaxy (MBE) growth to achieve good electroconductivity between the n-AlGaN and Si. As shown in Supplementary Fig. 2c, due to the characteristics of the n-AlGaN/n-Si band alignment, either the electrons or holes separated from CuOx/AlGaN interface can easily migrate across the AlGaN/Si interface and into Si substrate. It means that the AlGaN/Si interface plays the role as the linker for electric conduction.
In this work, the PEC light-detection devices based on the pristine AlGaN nanowires and the modified AlGaN/CuOx samples by chemical bath deposition (CBD) method for 10, 20, 30, 40 and 50 min are denoted as CBD-0, CBD-10, CBD-20, CBD-30, CBD-40 and CBD-50, respectively. Among these, the thickness of CuOx on AlGaN for CBD-10, CBD-30 and CBD-50 are ~3, ~20 and ~50 nm, respectively, which is supported by transmission electron microscopy (TEM) images in Supplementary Fig. 3. From this, the simulated energy band of AlGaN/CuOx heterojunction were obtained by the Silvaco TCAD (see “Methods”) and are shown in Supplementary Fig. 4. It is not difficult to see that the tilt energy band of CuOx suggests the cladding oxides layer are totally depleted by the n-AlGaN nanowire for CBD-10 and CBD-30. Nevertheless, when the thick of CuOx layer increase to 50 nm, the energy band of CuOx become flattened at the far end of the junction and the the downward band bending could be established at the interface of un-depleted CuOx/electrolyte. More importantly, the photocurrent on the spectral response of CBD-0, CBD-30 and CBD-50 in Fig. 1e shows the polarity-switching occurrence, which implies that the direction changes of band bending at p-CuOx/electrolyte interface, from upwards for CBD-30 to downwards for CBD-50 as the increase of the CuOx thickness.
Structural characterization of CuOx/AlGaN nanowires
Si-doped n-type AlGaN nanowires were grown vertically on n-type silicon substrates by high-frequency MBE (see “Methods”). In this work, in order to enhance the photoresponse of the AlGaN nanowire-based PEC devices, surface decoration of CuOx nanostructures was involved on n-type AlGaN nanowires by CBD20,21. To elucidate the chemical properties and electronic states of the decorated AlGaN NWs, X-ray photoelectron spectroscopy (XPS) measurements were performed. In Fig. 2a, the XPS spectra of the CBD-modified AlGaN NWs shows typical peaks of Cu2p3/2 and Cu2p1/2, representing features of Cu2O and CuO, respectively22,23. Two binding energy peaks in Fig. 2a at 933.2 and 953.1 eV are associated with Cu2O, while the other two smaller binding energy peaks at 934.6 and 954.5 eV are attributed to CuO. Additionally, the satellite peaks of Cu2p3/2 and Cu2p1/2 also confirm the existence of CuO on AlGaN. Since the peak area shows proportionality to the number of copper atoms in their corresponding oxidation states, by calculating the ratio of peak areas, it is evident that the combined atomic ratio of Cu2+ to Cu+ are nearly one to one (1:1). On the one hand, the peak at 529.5 eV corresponds to the metal-oxygen bonding; on the other hand, the peak locating at 531.6 eV could be attributed to surface hydroxide in the XPS spectrum of O1s (see Supplementary Fig. 5)22. All of these XPS results demonstrate that the CuOx are well-structured on AlGaN nanowires by CBD method.
As shown in Fig. 2b and Supplementary Fig. 6, scanning electron microscopy (SEM) was conducted to characterize the changes on the morphology of AlGaN NWs before and after CBD deposition. In the top half of Fig. 2b, the morphology of the pristine AlGaN specimen shows that the as-grown AlGaN nanowires by MBE are vertically arranged on the substrate and have a uniform morphology in size. After CBD modification, the AlGaN nanowires are coated by a copper-oxide thin layer and the CuOx layer exhibiting the concavo-convex morphology, which is shown in the bottom half of Fig. 2b. Supplementary Fig. 6 provides the cross-sectional SEM images, where it is obvious that the CuOx layer resides atop the AlGaN NWs (~400 nm long). Furthermore, TEM image, shown in Fig. 2c, indicates that the more deposited CuOx mainly resides on the top of the nanowires and the side walls of the nanowires are covered with thin CuOx layer, forming a core-shell structure, which corresponds to the distribution properties of CBD deposition. In Fig. 2d, the delicate distribution of chemical composition is further characterized by scanning transmission electron microscopy (STEM) images and the corresponding energy spectrum element mapping. Figure 2e displays the high-resolution microstructure of CuOx, in which the lattice fringes of Cu2O and CuO are clearly visible. The stripes with a pitch of 2.46 Å correspond to the (111) lattice planes of cuprous oxide (JCPDS 005-0667), while the stripes with a pitch of 2.53 Å correspond to the (002) lattice plane of copper oxide (JCPDS 01-080-1268). In summary, these results confirm that CuOx are primarily loaded on the top as well as the side walls of AlGaN nanowires, forming AlGaN/CuOx heterostructures. This heterostructure enables efficient charge transfer at the interface and optimizes surface chemical reactivity, leading to the significant enhancement of photoresponse.
Photoelectrochemical response enhanced by CuOx decoration
Surface decoration on n-type III-nitrides with metal oxides is an effective strategy to promote surface dynamics of target reaction and protect the III-nitrides from surface degradation3,4. Supplementary Fig. 7 depicts photoelectrochemical measurements of the original AlGaN nanowires and AlGaN/CuOx samples with different impregnation times under UV irradiation at a light intensity of 100 μW cm−2. The photocurrent density results show that the current density first increased and then decreased with increasing CuOx modification time, showing that the 30-min CuOx deposition corresponds to the highest photoresponse. The photocurrent density of sample CBD-30 was recorded as 17.39 μA cm−2 and is 6.1 times higher than that of the original sample (CBD-0). With increased deposition time, the amount of CuOx on the AlGaN nanowire surface increases, completing the construction of AlGaN/CuOx heterostructure. The photogenerated carrier separation is facilitated by the strong built-in electric field created by the reach-through band bending and the staggered-gap (type-II) band structure, as shown in Fig. 1d. However, when the CBD deposition time is prolonged to 40 min, the overloaded CuOx leads to downward band bending at CuOx/electrolyte, causing severe carrier transport competition between the two interfaces and the decrease of current density (Fig. 1c).
Figure 3a shows the current–time (I–t) curves of PEC-PDs with sample AlGaN/CuOx nanowires (CBD-30) and the pristine AlGaN nanowires (CBD-0) at different light intensity illumination. It is not hard to identify that the AlGaN sample modified with CuOx nanostructures shows significantly improved PEC photoresponse compared with the bare AlGaN nanowires. Figure 3a, b shows that the photocurrent density (Ip) of the two samples are improved by the increasing light intensity, which implies the PDs can sensitively make response on the light power changing.
a Repeated on-off I–t characteristics of bare AlGaN and AlGaN/CuOx PEC PDs at different light power without bias. For making comparison more obvious, the I–t curve of the bare AlGaN PEC PD was enlarged by 2.5 times (scale bar, 20 μA cm−2). b Photoresponse characteristics (current density and responsivity) and c EQE of bare AlGaN and AlGaN/CuOx PEC PDs under 255 nm illumination at different light power. d EIS analysis of bare AlGaN and AlGaN/CuOx PEC PDs. The inset in (d) is the equivalent circuit model used to fit the Nyquist plots.
To more comprehensively evaluate the optical response of the two AlGaN-based PEC PDs, the calculated responsivity (R) and EQE are shown in Fig. 3b, c. In the first instance, the responsivity was adopted to characterize the sensitivity of the PEC PDs, which can be calculated through the equation:
where P is the incident light power intensity. The responsivity of the two PEC-PDs at 0 V bias and various light intensities are shown in Fig. 3b. The responsivity of the AlGaN/CuOx samples varied between 90.5 mA W−1 and 270.6 mA W−1, demonstrating excellent light sensitivity across a wide range of light intensity. In contrast, the maximum responsivity of the bare AlGaN PEC PD was only 29.04 mA W−1 at 200 μW cm−2. It is noted that, as shown in Fig. 3b, the responsivity of AlGaN/CuOx PD monotonously decrease with the light power intensity increase. The maximum responsivity of the AlGaN/CuOx PEC PD is obtained at the weakest light power intensity (5 μW cm−2), indicating that the AlGaN-CuOx co-catalyst has great potential for weak-light detection. Herein, it is important to introduce EQE to assess the photoelectric conversion efficiency:
where c is the speed of light, h is Planck’s constant, q is the electronic charge and λ is the wavelength of incident light, respectively. As shown in Fig. 3c, the EQE of AlGaN/CuOx PD increases as the optical power intensity decreases, which is keeping with the results of responsivity. When the light intensity is 5 μW cm−2, AlGaN/CuOx PD achieved an impressively high EQE of 131.5%, which was 11.1 times higher than the original PD (11.8%). Notably, due to the MEG effect triggered by the absorption of high-energy photons by CuOx, the EQE of AlGaN/CuOx PD exceeds 100% without external bias. Within the range of light intensity from 5 to 1000 μW cm−2, there is a significant change in the EQE of the AlGaN/CuOx PEC PD, dropping from 131.7% to 44%. In contrast, the EQE of the AlGaN sample shows no significant change and remains at a consistently low level. The main reason for this is that, compared with the bare AlGaN, the AlGaN/CuOx heterojunction sample, under the aid of MEG, generated many more photogenerated carriers at higher light power. These excessive carriers cannot be timely transferred away, thus lowering down the EQE at high light power. Besides of the responsivity and EQE, specific detectivity (D*) is another crucial parameter for assessing the ability of PD to detect weak signals. When the noise is mainly dominated by the shot noise of the dark current (Idark), the equation of the specific detectivity can be simplified as:
Where R is the responsivity of PD, S is the area, and q is the electron charge. The calculated D* values for different light intensities are shown in Supplementary Fig. 8. Impressively, the AlGaN and AlGaN/CuOx PEC PDs exhibit remarkable D* value of 5.56 × 1011 and 6.17 × 1012 Jones at 5 μW cm−2, respectively, revealing the ability of AlGaN/CuOx PEC-type PD to detect weak light.
To evaluate the contribution of CuOx nanocomposites on PEC process, electrochemical impedance spectroscopy (EIS) measurement was employed under illumination of 255 nm light. EIS diagram could demonstrate the transport properties at the semiconductor/electrolyte interface. The diameter of EIS curve reveals the interfacial charge transfer resistance (Rct), providing information about the charge transfer kinetics at the interface. The EIS plots of the bare AlGaN and the CuOx decorated AlGaN samples are demonstrated in Fig. 3d. In order to quantitatively study the change of the Rct, we employed an equivalent circuit that consists of a series resistance (Rs), a bulk resistance (R1), a constant-phase element (CPE), and Rct, which is shown in the inset of Fig. 3d. The value of Rct, which decreased by more than an order of magnitude, dropped from 3.77 × 106 Ω cm2 for AlGaN to 2.44 × 105 Ω cm2 for AlGaN/CuOx. The AlGaN/CuOx sample shows the smaller Rct, which implies that the CuOx deposition can facilitate ultra-fast charge transfer at the interface, benefiting the exciton extraction from CuOx layer.
Photoresponse enhancement mechanisms
To elucidate the underlying mechanism on the enhanced PEC performance of AlGaN/CuOx nanowires assisted by the MEG effect, the behavior of charge generation and recombination were investigated using femtosecond transient absorption (TA) spectroscopy24,25, in which low flux pulse excitation is designed to ensure that the number of excitons in the multiple photon excitation (MPE) state (i.e., absorbing multiple photons to create multiple excitons) can be neglected15,26,27. In order to prevent the Si substrate from interfering with absorption spectra, TA characterization were carried out on CuOx samples deposited on quartz substrates (see “Methods” and Supplementary Fig. 9). Supplementary Fig. 10 shows the time-resolved transient absorption spectra measured at a pump energy of 4.13 eV, a ground-state bleaching (GSB) peak appears near 520 nm under the excitation pulse, corresponding to the steady-state absorption peak of CuOx, which is consistent with the result of UV-visible absorption spectrum (Supplementary Fig. 11a). In the meantime, the bandgap of CuOx composites is 1.9 eV, which is also confirmed by UV–visible absorption spectrum shown in Supplementary Fig. 11b. When a photon (with photon energy ≥3.8 eV) is absorbed by the CuOx nanostructure, a high-energy exciton is generated. In a very short time (~0.1 ps), the exciton decays via impact ionization, creating two electron-hole pairs (process I). The two hot electrons can then relax non-radiatively, e.g., via phonon emission (hot electron cooling), to form a ground-state biexciton (process II). Ultimately, the biexciton decays into a single exciton via Auger recombination13 (process III). The entire evolution process of carrier dynamics is shown in Fig. 4a. The time-lapse spectral (Fig. 4b) shows strong peaks observed within the 1 ns, which gradually decrease over time, representing the thermal relaxation of multiple excitons. After 5 ns, the further decrease in peak value corresponds to the radiative recombination of the single exciton28.
a The process of MEG effect. b The TA spectra showing the first exciton bleach of CuOx at different delay time. The pump is at 300 nm with a pulse fluence of 10 nJ cm−2. The A represents the absorption for TA measurement. c Bleaching dynamics with and without MEG in CuOx (Eg = 1.9 eV). The lines are a two-exponential fit that indicates the presence of the short-lived bi-excitonic component. Inset: MEG QE in CuOx as a function of pump photon energy normalized by Eg. d Normalized responsivity as a function of wavelength for the bare AlGaN and AlGaN/CuOx PEC PD at 0 V bias. Simulated electric-field intensity distribution of AlGaN/CuOx NWs at 255 nm (e), 275 nm (f), 295 nm (g), 310 nm (h), 365 nm (i) and 385 nm (j) wavelength. The white dotted line represents the interface between AlGaN and CuOx.
To determine the existence of MEG process and its quantum yield, TA spectroscopy probing the dynamic of ground-state bleaching is performed on spin-coated CuOx composite layer on quartz substrates. Since the TA instrumental response time (35 fs) is much shorter than the carrier lifetime (~1 ps), the influence of the instrument response is negligible, and it is feasible to the fit carrier dynamics curves using a biexponential function. Under small pump energy of hv < 2Eg at low pump intensity, the GSB decay of CuOx only single exponential decay with a lifetime of >10 nm, indicating the neutral single-exciton recombination. The existence of MEG at higher photoexcitation energy (>2Eg) is evidenced by the emergence of fast decay with increased amplitudes. The lifetime of fast decay of 430 ps can be attributed to the biexciton Auger recombination. As shown in Fig. 4c, the quantum yield of CuOx at 4.13 eV (2.12Eg) high-energy photons is about 1.8, whereas it is only 0.98 at 3.4 eV (1.74Eg) low-energy photons, strongly evidencing the existence of over 100% quantum yield efficiency in CuOx nanostructure under high-energy ultraviolet light illumination. In the meanwhile, the efficiency of the MEG effect can be quantitatively characterized by carrier lifetime spectra, where the signal intensity is proportional to the number of excitons excited by the pump pulse in CuOx24,25,28. In general, the calculated quantum efficiency is applied to quantitatively measure the MEG in a nano-semiconductor structure. As shown in Supplementary Fig. 12, the TA signal rapidly decays from the initial peak to a steady value. The ratio of the peak signal strength A to the platform signal strength B is proportional to the average number of excitons generated in each stimulated nanosemiconductor. Therefore, at low flux incidence, the quantum efficiency of multiple exciton generation is expressed as15,29:
Where A and B are the absolute signal intensity of before and after Auger recombination. Here, as shown in Supplementary Fig. 13, it is worth noting that the multiphoton absorption events are excluded to ascertain the behavior of the photon absorption and further support the MEG effects.
Actually, besides the effective multiple exciton generation, efficient multiple exciton extraction and charge transfer promoted by the built-in electric field between the hetero-interface is crucial, which could effectively suppress the Auger recombination and take full advantage of the MEG effect to photoelectric gain in PEC process17,19. It is notable that the formed reach-through CuOx layer combined with the staggered-gap band structure of AlGaN/CuOx heterojunction, shown in Fig. 1d, is perfectly fit for extracting the multiplicative electrons from CuOx nanostructure into AlGaN. Once the multiple excitons were generated by high-energy photons in the CuOx layers, an appropriate method of extracting charges is very crucial for suppressing thermal relaxation, due to the short lifetime of the multiple excitons. The electric field in the reach-through CuOx layer can impel the charge separation effectively. The significance of reach-through CuOx layer is that photo-induced carriers can be impelled to drift across the whole depleted layer by the built-in electric fields between AlGaN and electrolyte, yielding much higher efficiency of charge extraction than diffusion movement. Since then, the following issue should be emphatically considered is that the characteristics of spatial distribution on optical field and the photogenerated multiple excitons. In Fig. 4d, when the pristine AlGaN NWs are irradiated by the light with longer wavelength (>300 nm), there are no obvious photoresponse to be detected. Only under the photoexcitation by the short-wavelength light (<300 nm), an observable photocurrent occurred, which is attributed to the intrinsic band-to-band carrier transition in AlGaN. By contrast, within the region of 255–365 nm, the more prominent photoresponse of AlGaN/CuOx PD could be attributed to the excited CuOx coating layer. The most fascinating enhancement of photoresponse for the AlGaN/CuOx PD appears under <300 nm UV light irradiation, which is ascribed to the participation of MEG. Here, it needs to be mentioned that the AlGaN/CuOx PD, there is no photocurrent can be observed under longer than 400 nm light irradiation, though the photon energy is exceeding the bandgap of CuOx. The EQE of bare AlGaN and AlGaN/CuOx PEC PDs under different light wavelengths (255–620 nm) was also charted in Supplementary Fig. 14. It is clearly indicated that EQE of AlGaN/CuOx PD exceeds 100% irradiated by UV light below 300 nm. Specifically, the photon energy dependence of the distribution of electric field in the nanowires should be deeply considered, which totally determines the spatial distribution of photogenerated carriers. According to the results of the finite-difference time-domain (FDTD) simulation (the numerical model can be referred to Supplementary Fig. 15), it can be explicitly recognized that, under short-wavelength light illumination, the distribution center of the optical field as well as its induced photogenerated carriers locate at within CuOx layer as well as the vicinity of AlGaN/CuOx interface. However, as the light wavelength increases from 255 to 385 nm, the strongest absorption region shifts downwards, from AlGaN/CuOx interface into the bulk of AlGaN nanowires, as shown in Fig. 4e–j. In other words, in the short-wavelength light irradiation, the electron-hole pairs generated could be immediately swept into built-in electric field of the AlGaN/CuOx interface, expediting the carrier separation and then resulting in detectable photocurrent. On the contrary, under long-wavelength light illumination, the generated carriers occur in the bulk of the AlGaN nanowires, keeping away from the top of the nanowires and failing to make a contribution to the photocurrent. Moreover, the optical absorption efficiency of CuOx at 255 nm is 2 times higher than at 400 nm through comparison. In General, under long-wavelength light, such fast relaxation process impedes the application of MEG theory to PEC photodetection for enhancing external quantum efficiency28. By manipulating the spatial distribution of photogenerated carrier and combining with the effects of the strong built-in electric field in AlGaN/CuOx interface, the multiplied carriers in the CuOx layer can be effectively separated and transferred without cooling, enabling an efficient MEG process14,17,19,30 and achieving 131.5% external quantum efficiency.
High-resolution deep-ultraviolet imaging
Because the PEC-based device involves both physical and chemical processes, it offers diverse methods for adjusting light-responsive behavior. Figure 5a–c demonstrates the sensitivity of the light response to the bias potential and electrolyte concentration. When the bias potential was adjusted between −0.2 and 0.8 V, the photoresponse is enhanced from 16.5 to 48.3 μA cm−2, which is attributed to the acceleration on carrier separation and transport in the photoelectrode by bias potential3,4. In addition to the external bias, the photocurrent of AlGaN/CuOx PD also depends on the electrolyte concentration. As the electrolyte concentration increases, the photocurrent density increases at first and then decreases. When the electrolyte concentration is 0.5 M, the Ip reaches the maximum value. However, at higher ion concentrations than 0.5 M, redox at the interface is hindered, resulting in a decrease in photocurrent4. This phenomenon can be attributed to the adsorption of high-concentration SO42− ions onto the photoanode surface, which occupies active sites and impedes hole transfer to reactants. In addition, the localized accumulation of oxidation products (O2 and H+) induces proton overconcentration, together suppressing oxidation kinetics.
a–c I–t curve and photocurrent density of AlGaN/CuOx PEC PD under different electrolyte concentration (0.1 M, 0.3 M, 0.5 M and 1 M; a scale bar, 20 μA cm−2) and bias voltage (−0.2 V, 0 V, 0.2 V,0.5 V and 0.8 V; b scale bar, 20 μA cm−2). d The rise and decay time for AlGaN/CuOx PEC PD. e The frequency response curve with −3 dB cut-off frequency. f Comparison of responsivity and response time between AlGaN and AlGaN/CuOx PEC PD. g In all, 50,000-s stability test of AlGaN/CuOx PEC PD.
To display the light response speed of the PD, Fig. 5d (AlGaN/CuOx) and Supplementary Fig. 16 (AlGaN) show the response time (tr) and decay time (td) intervals of the photocurrent curve at a bias potential of 0 V under 255 nm illumination. The tr and td are defined as intervals from 10 to 90% of the maximum photoelectric values and from 90% to 10%, respectively. The corresponding tr and td of AlGaN/CuOx are 183.3 and 154.6 μs, respectively, while the response speeds of the bare AlGaN nanowires were 360.8 and 241.3 μs, respectively. It is not difficult to see that the improved response speed of AlGaN/CuOx PD is most likely due to the accelerated charge transfer process and redox rate at the interface. To thoroughly study the frequency characteristic of the AlGaN/CuOx PEC PD in electrolyte, frequency-based tests were conducted, with presenting the time response of the PEC PD in the 1 Hz–20 kHz frequency range in Supplementary Fig. 17. It can be observed that the output waveform remains stable as a square wave with clear rising/falling edges up to 1 kHz, yet when the frequency increases to 10 kHz, the output waveform transitions from a square wave to a triangular wave, probably because the PD reaches its response-speed limit and lacks sufficient time to stabilize at high frequencies. The frequency-response curve (Fig. 5e) was obtained by measuring the amplitudes of output signals at different frequencies, enabling to estimate that the −3 dB cut-off frequency of the AlGaN/CuOx PEC PD in sodium sulfate solution is higher than 10 kHz. In our opinion, although achieving ultra-high-frequency response for PEC devices is still a challenge, a few promising strategies were proposed, such as interface engineering to eliminate recombination, nanostructure design to reduce the carrier diffusion path, circuit optimization for impedance matching. Figure 5f shows the comparison on the performance of the prepared AlGaN/CuOx PD with the recently reported PEC photodetectors1,3,4,5,31,32,33,34,35,36. The AlGaN/CuOx PD, in our work, shows more superior photoresponse performance than the other listed works in Fig. 5f, with the responsivity of 270.6 mA W−1 and EQE of 131.5%.
Generally speaking, the durability and long-term stability of multi-cycle photodetectors are the crucial characteristics for further application. Figure 5g shows the 50,000-s long-term on/off response of AlGaN/CuOx samples under illumination of 200 μW cm−2 without bias. The photocurrent of AlGaN/CuOx samples only exhibits slight attenuation after 50,000 s of long-term operation, highlighting the remarkable stability of AlGaN/CuOx samples in multiple cycles. Actually, because of the surface passivation by deposited CuOx, the AlGaN nanowires were substantially mitigated from the photo-induced corrosion, resulting in excellent durability and stability for AlGaN/CuOx PD. And more notably, the main reason for selecting Na2SO4 electrolyte is that it could create a relatively stable ionic environment, suppressing interfering with the photoelectrochemical (PEC) process by the side reactions. In addition, though the alkaline electrolyte could provide higher OH− ion concentration and might improve the photoresponse, the original Na2SO4 is not suitable to be replaced with the alkaline electrolyte, in consideration of the chemical stability of PEC PD. Alkaline electrolytes might corrode the surface of nanowires during PEC testing, thereby reducing the long-term stability of PEC PDs. As shown in Supplementary Fig. 18, the photoresponse performance drops by over 50% within the initial 12,000 s.
Finally, the excellent photon response of the AlGaN/CuOx PEC PD to specific wavelengths was further substantiated through monochromatic light imaging. Figure 6a illustrates the component schematic, where the light source is provided by a light-emitting diode, and a “butterfly” pattern mask (Fig. 6b) is positioned between the light source and PD. The mask, having a subtle pattern with a fine outline, is capable of scanning in both the X and Y axes via a displacement platform. As shown in Fig. 6c, owing to the superior responsivity of the AlGaN/CuOx PEC PD at 255 nm compared to the AlGaN PEC PD, a distinct “butterfly” pattern is discernible with the AlGaN/CuOx PEC PD at a wavelength of 255 nm. More details about the imaging tests can be found in Supplementary Fig. 19. In contrast, a significantly more blurred pattern is observed with the original AlGaN PEC PD (Fig. 6d). As previously mentioned, the remarkable contrast in image clarity between the two devices further corroborates the ultra-high responsivity of the AlGaN/CuOx PEC PD under weak UV illumination. The high-responsivity PDs facilitate high-resolution image acquisition in dynamic environments.
Because of the high photoresponse performance, it is feasible to discuss the proposed PEC PD for further application and even for commercialization in the future. Totally, there are still several issues to be solved, such as device packing and system integration. At present, from the perspective of the authors, two technical issues need to be addressed. Because of no external supply, the photoresponse of the self-powered PEC PD should be improved further. Certainly, the MEG involved PEC PD in this paper provides one effective solution. For another thing, the liquid electrolyte as well as the bulky vessel impedes the commercial progress. In fact, the traditional water and electrolytic vessel for the PEC PD could be replaced by the solid electrolyte-based capsule, which significantly reduces the volume of PEC PD, less than several hundredths of the original PEC cells. It greatly improves the practicality in the field of portable and wearable electronics37,38.
Discussion
In summary, up to now, the quantum efficiency of MEG is still inefficient in optoelectronics because the incident light energy must be much greater than the 2Eg of the excited nano-semiconductors to generate sufficient excess carriers. Recently, optimizing energy band structure and enhancing the internal electric field intensity has been proved to be a promising strategy to extract excitons generated by the MEG effect and improve the photoelectric conversion efficiency17,19.
We report a PEC device with high responsivity and external quantum efficiency of over 100% after appropriate CuOx composite modification, providing the reach-through band bending on the AlGaN nanowire surface by CBD method. By manipulating spatial distribution of optical fields, the MEG occurs within the whole depleted CuOx layer with tilt energy band, the generated multiple excitons can be instantaneously separated by the built-in electric field against the exciton Auger recombination. The separated electrons migrate to the interface of AlGaN/CuOx and are swept into the AlGaN by the strong built-in electric field of the type-II heterojunction; the holes, at the same time, transfer to the CuOx/electrolyte interface to participate in the OER at surface. From this, comparing to the bare AlGaN PEC PD, the EQE of the device based on AlGaN/CuOx heterojunction significantly increased from 11.8 to 131.5%, with an 11.1-fold increase in responsivity of 270.6 mA W−1 and a fast response speed under 255 nm light illumination. Notably, both the photoresponsivity and quantum efficiency achieve high-level results among self-powered PEC PD reported to date. This work proves the necessity of precise energy band designing for realizing the carrier multiplication from MEG, opening up the insight into constructing self-powered PEC-based optoelectronics in the future.
Methods
Growth of nanowires and CuOx nanocomposite
AlGaN nanowires were grown on Si substrates using plasma-assisted MBE under nitrogen-rich conditions. To remove organic contaminants and surface oxides from the silicon substrate, it is thoroughly cleaned sequentially with methanol, acetone, and hydrofluoric acid. After cleaning, the substrate was installed in the MBE chamber. Before the growth process, the Si substrate was first annealed at 860 °C for 20 min to remove the remaining oxides and restore the growth front. The flows of Al, Ga, Si, and Mg are controlled by a melt pool, with nitrogen ions provided by a radio frequency plasma source. The AlGaN nanowires were grown at 780 °C for 0.5 h.
Subsequently, CuOx was grown using chemical bath deposition. 0.5 M CuSO4 was dissolved in 10 mL of deionized water to form a solution. Then, 40 mL of 0.5 M Na2SO3 aqueous solution was slowly added to create mixed solution I, containing SO42− and Cu2+ ions. The prepared AlGaN samples were immersed in solution I and stirred for 15 min. Then, 0.25 M NaOH aqueous solution was added to the mixed solution at 70 °C for 30 min. The sample was then removed, washed three times with deionized water, and dried at 70 °C in a vacuum oven. Finally, AlGaN samples with CuOx nanocomposite structures were obtained. Similarly, immerse the cleaned quartz substrate into Solution I. Under the same conditions, a sample of the CuOx nanocomposite structure supported on the quartz substrate will be obtained.
Characterization of nano-structure
The morphology and microstructure of AlGaN and AlGaN/CuOx nanowires were characterized using a field emission scanning electron microscope (FESEM, Hitachi Regulus 8220) and a transmission electron microscope (TEM, Talos F200s). The chemical states of the elements in the samples were evaluated using an X-ray photoelectron spectrometer (XPS, Thermo Scientific, Escalab 250xi) with a monochromatic Al kα source (15 kV, 150 W). The position of the binding energy peak was calibrated by the C1s peak (284.6 eV). The carrier dynamics curves of CuOx nanostructures with an excitation wavelength of 300 nm were obtained using CEL-TAS3000 femtosecond transient absorption spectroscopy. Transient absorption spectroscopy was carried out by using an optical instrument combined a frequency-doubled mode-locked Ti/sapphire femtosecond laser (coherent) and an optical parametric amplifier system. The amplified Ti/sapphire femtosecond laser generates seed pulses with a 35 fs pulse width and a repetition rate of 1 kHz. The seed pulses are divided into two distinct beams. The strong beam is sent to the OPA system and provided the 300 nm pump laser pulse, and the other one is focused onto a nonlinear crystal to generate a white light continuum, providing broadband of 400–800 nm UV–vis probe light. The excitation beam has a low energy of 10 nJ cm−2 per pulse to avoid the exciton–exciton and exciton–charge annihilation effects.
Fabrication and measurements of photoelectrodes
AlGaN and AlGaN/CuOx samples were prepared as photoelectrodes for PEC testing. The natural oxides on the back of the silicon are first removed and then the substrate is coated with a Ga-In alloy to ensure ohmic contact. The sample is then attached to a copper strip using conductive silver slurry. Then, in addition to the nanowires required for light detection, the photoelectrodes are coated with insulating epoxy resin. Finally, the photoelectrodes were dried for 24 h before measurements.
To record the photoresponse properties of the photoelectrode, a three-electrode system was used, consisting of the photoelectrode (working electrode), Pt (counter electrode), and Ag/AgCl (reference electrode). Four different concentrations of Na2SO4 (0.1 M, 0.3 M, 0.5 M and 1 M) were used as the electrolyte in a quartz cell with high UV transmittance. Apart from taking into account the convenience and biosecurity of experimental operation, the long lifespan of LED light source is conducive to long-term stability characterization. The photoelectrodes were exposed to 255 nm LED light with varying light power intensities, and current–time, and electrochemical impedance spectroscopy (EIS) were analyzed on an electrochemical workstation (CHI 760E, Shanghai Chenhua). The incident light was provided by an array of light-emitting diodes with wavelengths of 255–620 nm. The intensity of the light involved in the experiment were calibrated in real time by an UV radiometer (LS-125) and a solar power meter (LH-122). During the light calibration, a real-time and mature protocol was implemented.
Finite-difference time-domain simulations
FDTD calculations with commercial software (FDTD solution, Lumerical Inc., Vancouver, Canada) were used to simulate the optical properties of AlGaN/CuOx nanostructures. To ensure the accuracy of electric field calculations, the sizes of the AlGaN and CuOx in the simulation models are based on the SEM and TEM results. To simplify the simulations, the shape of AlGaN and CuOx is set to be a rectangle with 50 × 400 nm and 20 × 50 nm, and the substrate is set as Si. The light source is a plane wave. The refractive index of the AlGaN and CuOx nanostructure was taken from the reported values39,40.
Simulation of semiconductor energy band
Silvaco TCAD, based on the ATLAS device simulator, was adopted to analyze the energy band structure of AlGaN/CuOx heterojunction NWs. Poisson’s equation and the continuity equation were used in the numerical procedures. For the III-nitride semiconductors, the spontaneous and piezoelectric polarization effects were both taken into account for the polarization physical model.
Data availability
The data that support the findings of this study are available in the article, supplementary information file, source data file or from the corresponding authors upon request. The refractive indices of the AlGaN and CuOx nanostructure used for simulations were taken from the reported values39,40 and are included in the source data file. Source data are provided with this paper.
References
Wang, D. et al. Bidirectional photocurrent in p–n heterojunction nanowires. Nat. Electron. 4, 645 (2021).
Fang, S. et al. Light-induced bipolar photoresponse with amplified photocurrents in an electrolyte-assisted bipolar p–n junction. Adv. Mater. 35, 2300911 (2023).
Kang, Y. et al. Achieving record-high photoelectrochemical photoresponse characteristics by employing Co3O4 nanoclusters as hole charging layer for underwater optical communication. ACS Nano 17, 3901 (2023).
Wang, D. et al. Observation of polarity-switchable photoconductivity in III-nitride/MoSx core-shell nanowires. Light Sci. Appl. 11, 227 (2022).
Tang, Q. et al. Enhanced photoresponse of self-powered ZnO-based photoelectrochemical-type UV photodetectors via Ga-doping for optical communication application. J. Alloy Compd. 972, 172864 (2024).
Jung, H. et al. Low excess noise and high quantum efficiency avalanche photodiodes for beyond 2 µm wavelength detection. Commun. Mater. 5, 219 (2024).
Kublitski, J. et al. Enhancing sub-bandgap external quantum efficiency by photomultiplication for narrowband organic near-infrared photodetectors. Nat. Commun. 12, 4259 (2021).
Kamrani, E., Lesage, F. & Sawan, M. Low-noise, high-gain transimpedance amplifier integrated with SiAPD for low-intensity near-infrared light detection. IEEE Sens. J. 14, 258 (2014).
Nozik, A. J. Quantum dot solar cells. Phys. E: Low. -Dimens. Syst. Nanostruct. 14, 115 (2002).
Nozik, A. J. Spectroscopy and hot electron relaxation dynamics in semiconductor quantum wells and quantum dots. Annu. Rev. Phys. Chem. 52, 193 (2001).
Brus, L. Size, dimensionality, and strong electron correlation in nanoscience. Acc. Chem. Res. 47, 2951 (2014).
Klimov, V. I. Spectral and dynamical properties of multiexcitons in semiconductor nanocrystals. Annu. Rev. Phys. Chem. 58, 635 (2007).
Franceschetti, A., An, J. M. & Zunger, A. Impact ionization can explain carrier multiplication in PbSe quantum dots. Nano Lett. 6, 2191 (2006).
Chen, Y. et al. Multiple exciton generation in tin–lead halide perovskite nanocrystals for photocurrent quantum efficiency enhancement. Nat. Photon. 16, 485 (2022).
Li, M. et al. Low threshold and efficient multiple exciton generation in halide perovskite nanocrystals. Nat. Commun. 9, 4197 (2018).
Yan, Y. et al. Multiple exciton generation for photoelectrochemical hydrogen evolution reactions with quantum yields exceeding 100. Nat. Energy 2, 17052 (2017).
Zhang, Y. et al. Internal quantum efficiency higher than 100% achieved by combining doping and quantum effects for photocatalytic overall water splitting. Nat. Energy 8, 504 (2023).
Cirloganu, C. M. et al. Enhanced carrier multiplication in engineered quasi-type-II quantum dots. Nat. Commun. 5, 4148 (2014).
Li, Y. et al. Efficient carrier multiplication in self-powered near-ultraviolet γ-InSe/graphene heterostructure photodetector with external quantum efficiency exceeding 161. Nano Lett. 24, 7252 (2024).
Han, P. et al. Cu2O quantum dots modified α-Ga2O3 nanorod arrays as a heterojunction for improved sensitivity of self-powered photoelectrochemical detectors. J. Alloy Compd. 952, 170063 (2023).
Raheman Ar, S., Momin, B. M., Wilson, H. M., Annapure, U. S. & Jha, N. Optimal fabrication of 0D/1D Cu2O quantum dots sensitized CdS nanorods heterojunction: efficient photoredox catalyst for H2 generation under visible light irradiation. J. Alloy. Compd. 835, 155262 (2020).
Urbain, F. et al. Multijunction Si photocathodes with tunable photovoltages from 2.0 V to 2.8 V for light induced water splitting. Energy Environ. Sci. 9, 145 (2016).
Huang, Q., Kang, F., Liu, H., Li, Q. & Xiao, X. Highly aligned Cu2O/CuO/TiO2 core/shell nanowire arrays as photocathodes for water photoelectrolysis. J. Mater. Chem. A 1, 2418 (2013).
Karki, K. J. et al. Multiple exciton generation in nano-crystals revisited: consistent calculation of the yield based on pump-probe spectroscopy. Sci. Rep. 3, 2287 (2013).
Schaller, R. D., Agranovich, V. M. & Klimov, V. I. High-efficiency carrier multiplication through direct photogeneration of multi-excitons via virtual single-exciton states. Nat. Phys. 1, 189 (2005).
Manzi, A. et al. Resonantly enhanced multiple exciton generation through below-band-gap multi-photon absorption in perovskite nanocrystals. Nat. Commun. 9, 1518 (2018).
Stubbs, S. K. et al. Efficient carrier multiplication in InP nanoparticles. Phys. Rev. B 81, 081303 (2010).
Huang, Z. & Beard, M. C. Dye-sensitized multiple exciton generation in lead sulfide quantum dots. J. Am. Chem. Soc. 144, 15855 (2022).
Choi, Y., Sim, S., Lim, S. C., Lee, Y. H. & Choi, H. Ultrafast biexciton spectroscopy in semiconductor quantum dots: evidence for early emergence of multiple-exciton generation. Sci. Rep. 3, 3206 (2013).
Davis, N. J. L. K. et al. Multiple-exciton generation in lead selenide nanorod solar cells with external quantum efficiencies exceeding 120%. Nat. Commun. 6, 8259 (2015).
Fang, S. et al. Breaking the responsivity-bandwidth trade-off limit in GaN photoelectrodes for high-response and fast-speed optical communication application. Adv. Funct. Mater. 33, 2214408 (2023).
Huang, L. et al. A simple, repeatable and highly stable self-powered solar-blind photoelectrochemical-type photodetector using amorphous Ga2O3 films grown on 3D carbon fiber paper. J. Mater. Chem. C. 9, 10354 (2021).
Feng, Y. et al. Catalyst-Free β-Ga2O3@a-Ga2O3 core−shell nanorod arrays grown on Si substrate for High-performance self-powered solar-blind photoelectrochemical photodetection. Appl. Surf. Sci. 624, 157149 (2023).
Yang, X. et al. High-performance broadband photoelectrochemical photodetectors based on ultrathin Bi2O2S nanosheets. ACS Appl. Mater. Interfaces 14, 7175 (2022).
Yu, H. et al. One stone, three birds: SnO2 nanosheet arrays toward self-powered visible-blind UV photodetection with high responsivity and rejection ratio. Adv. Opt. Mater. 12, 2301460 (2024).
Zhang, M. et al. Ultrathin In2O3 nanosheets toward high responsivity and rejection ratio visible-blind UV photodetection. Small 19, 2205623 (2023).
Wang, B. et al. Photoelectrochemical self-powered photodetector based on 2D liquid-exfoliated bismuth nanosheets: with novel structures for portability and flexibility. Mater. Today Nano 14, 100109 (2021).
Ren, X. et al. Flexible self-powered photoelectrochemical-type photodetector based on 2D WS2-graphene heterojunction. FlatChem 25, 100215 (2021).
Antoine-Vincent, N., Natali, F., Mihailovic, M. & Vasson, A. Determination of the refractive indices of AlN, GaN, and AlxGa1−xN grown on (111) Si substrates. J. Appl. Phys. 93, 5222 (2003).
Kneer, J. et al. Alterations in the complex refractive index of copper oxide thin films as sensing effect for hydrogen sulfide monitoring. Microsyst. Technol. 20, 607 (2014).
Acknowledgements
This work was funded by the National Key Research and Development Program of China (2022YFB3605400); National Natural Science Foundation of China (62374094, 62104110, and 62174016); Project funded by China Postdoctoral Science Foundation (2023T160332); Training Plan for Principals of Key Projects in Nanjing University of Posts and Telecommunications (NY224084); Basic Research Pilot. Project of Suzhou (SJC2022004).
Author information
Authors and Affiliations
Contributions
J.X., J.W., R.Z., G.Y. and D.C. developed the idea and designed the experiments. P.S., X.W., G.X., X.T., T.P., B.L. and K.W. performed the MBE growth and materials characterization. X.W., G.X., X.T., T.P., Z.C., Q.C. and Z.H. measured the photodetection experiments and analyzed the data. J.X., T.Z. and X.W. co-wrote and revised the paper. All the authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xue, J., Wang, X., Xu, G. et al. Multiple exciton generation boosting over 100% quantum efficiency photoelectrochemical photodetection. Nat Commun 16, 5275 (2025). https://doi.org/10.1038/s41467-025-60420-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-60420-1