Abstract
Label-free optical imaging and sensing of single nanoparticles are vital for fundamental research, disease diagnosis, and nanomaterial studies. Surface plasmon resonance microscopy (SPRM) is a label-free detection technology which is widely used in the detection of single nanoparticles. However, conventional SPRM suffers from poor spatial resolution, a limited field-of-view, system complexity, and high operating costs. In this study, we introduce a compact, low-cost, and large field-of-view chip-based plasmonic scattering microscopy (Chip-PSM). Compared with SPRM, Chip-PSM retains high detection sensitivity and in situ label-free analysis capability, while offering a larger field-of-view, an isotropic point-spread-function and higher spatial resolution. With these advantages, Chip-PSM enables detecting and imaging dielectric nanoparticles, gold nanoparticles, and biological samples. Additionally, the hygroscopic growth dynamics of aerosol nanoparticles and the chemical reactions occurring on nanocrystals are successfully characterized via Chip-PSM. We anticipate that the proposed Chip-PSM will have broad applications across many scientific fields, including physics, chemistry, and atmospheric sciences.
Similar content being viewed by others
Introduction
Imaging and detecting single nanoparticles via optical label-free methods is critical for environmental monitoring, understanding chemical reaction mechanisms, and studying biological processes1,2,3,4,5,6,7. Surface plasmon resonance microscopy (SPRM) is a popular label-free detection technique because of its extraordinary sensitivity to tiny signals. Surface plasmons are one type of evanescent field and can largely enhance light‒material interactions. Therefore, surface plasmons can be used to develop highly sensitive label-free imaging methods such as SPRM. Recently, SPRM has been employed for imaging single cells8,9,10, subcellular organelles11, viruses12, nanoparticles13,14,15,16, exosomes17, and chemical reactions18,19 owing to its extreme sensitivity. However, SPRM has two significant disadvantages that limit its broader application. First, SPRM suffers from poor spatial resolution because of interference between the reflective wave and the leakage radiation scattered by nanoparticles, resulting in a point-spread-function with a parabolic tail17,20. Although using azimuthal rotation illumination can improve spatial resolution21,22, it requires a pair of high-speed scanning galvanometers and complex optical path adjustments. Second, SPRM images are formed by collecting the reflected light from the gold surface. The reflection light creates a strong background noise on the image, reducing image contrast and making it difficult to image small nanoparticles. Recently, a research group proposed plasmonic scattering microscopy (PSM) to overcome these two disadvantages23,24,25. Unlike SPRM, PSM collects plasmonic waves scattered by nanoparticles with an air objective placed on top of the sample. PSM retains the advantages of SPRM because surface plasmons still function as the illumination field. At the same time, the scattering light is directly imaged to form PSM images without collecting the reflection light, which results in high image contrast and high spatial resolution.
However, PSM require bulky, complicated, and expensive optical setups, such as a high numerical aperture objective lens used to excite surface plasmons, which are cumbersome to operate, especially for untrained users. Additionally, the angle of incident light at the metal‒dielectric interface must be meticulously adjusted to achieve momentum matching for surface plasmons’ excitation26. Furthermore, the use of a high numerical aperture (NA) oil-immersion objective to excite the surface plasmons in the PSM and SPRM limits the field-of-view of the imaging system. This makes simultaneous imaging and sensing of large quantities of nanoparticles difficult or impossible. In recent years, interest in developing chip-based imaging instruments to simplify the optical setup and expand the field-of-view has increased27,28,29.
In this work, we propose a compact, low-cost, and large field-of-view chip-based plasmonic scattering microscopy (Chip-PSM) for the label-free imaging and sensing of nanoparticles with isotropic point-spread-function. Chip-PSM greatly simplifies the PSM setup by using a compact optical illumination device to excite surface plasmons. This compact optical illumination device is a combination of a gold-coated glass slide, a well-designed one-dimensional photonic crystal (1DPC), a polymer layer and an LED light source. All these components are of planar shape and easily integrated. This compact optical illumination device is much simpler than the devices used in the previous works on chip-based label-free optical microscopy28,29, thus it enables significant miniaturization and simple operation of the microscope setup. This compact optical illumination device can be installed in a conventional optical microscopy to realize plasmonic imaging. Omnidirectional surface plasmons illumination can be excited via this compact optical illumination device; thus, the Chip-PSM will provide an isotropic point-spread-function, which cannot be realized via conventional PSM or SPRM. Like PSM or evanescent scattering microscopy30, Chip-PSM collects the scattering light with an air objective placed above the sample, and there is no need for any high NA oil-immersion objective. Owing to the expanded surface plasmons illumination zone (exceeding 1 × 1 cm2), Chip-PSM can realize a large field-of-view to simultaneously detect numerous nanoparticles. Chip-PSM also enables real-time imaging of biological cells or nanoparticles in liquid solution. Additionally, the label-free sensing of hygroscopic growth in single aerosol nanoparticles and chemical reactions in individual perovskite nanocrystals are achieved via the Chip-PSM. In comparison to SPRM and PSM, the Chip-PSM offers a simple, low-cost solution for high-quality and high-throughput imaging of single nanoparticles due to its isotropic point-spread-function and large field-of-view.
Results
Principles of chip-based plasmonic scattering microscopy (Chip-PSM)
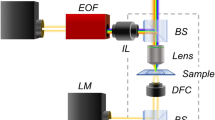
As shown in Fig. 1a, Chip-PSM integrates a standard upright microscope with a compact optical illumination device. A real photo of the fabricated compact optical illumination device is shown in Fig. S1a. The optical device consists of an LED source, a polymer layer doped with TiO2 nanoparticles, an all-dielectric 1DPC, and a gold-coated glass slide from the bottom to the top. The LED source (center wavelength at 660 nm) with a wavelength bandwidth of ~20 nm (Fig. S1b) was used as the light source for illumination. The light from LEDs has low spatial coherence and does not induce speckles on the microscopy images as those induced by the laser beams used in conventional SPRM or PSM9,13,23,24. The polymer layer, doped with dispersed TiO2 nanoparticles (with a diameter of ~60 nm) and a thickness of 2 μm, is placed above the LED source. The high refractive index and low imaginary characteristic of TiO2 particles increase the scattering efficiency. The well-dispersed TiO2 nanoparticles can uniformly scatter light from the LED source in all directions. An all-dielectric 1DPC is placed on the polymer layer, where its angular-dependent transmission is precisely tailored to function as a momentum-space filter31. The light scattered from the polymer layer can be transmitted through this 1DPC at a designed angular range that is matched with the surface plasmon resonant angle. The thin gold film was coated on a glass substrate that was then placed on the 1DPC. A drop of refractive index matching oil was placed between the 1DPC and the gold film-coated glass substrate. By using this configuration, omnidirectional surface plasmons can be excited at the gold/air interface, which can be used as the near-field illumination field for single nanoparticles, as shown in Fig. 1a and Fig. 1b. There is no need for a high-NA oil-immersion objective or other bulky optical element for the excitation of the surface plasmons, as used in conventional PSM23,24.
a Experimental setup of Chip-PSM based on an upright microscope. A compact optical illumination device consists of an LED source, a polymer layer, a 1DPC, and a gold-coated glass slide. The plasmonic waves scattered by single nanoparticles and the rough surface are collected by the above air objective to form a Chip-PSM image. b single nanoparticle is illuminated by omnidirectional Surface plasmons. c Angular-dependent transmittance of the 1DPC (left Y-axis) and angular-dependent reflectance of the Gold-coated glass slide (right Y-axis), in the case of the Transverse-Magnetic (left part), and Transverse-Electric polarized (right part) incident light. d PSM images of single nanoparticles (diameter of 150 nm, n = 1.51) simulated via finite-difference time-domain methods under single-direction illuminations and omnidirectional illuminations. The white arrowhead line represents the propagation direction of the single-direction propagating surface plasmons. Scale bar, 100 nm.
To enable the 1DPC to function as a momentum‒space filter, we need to find the best suitable structural parameters of the 1DPC. Using the transfer matrix method32, we determined the suitable parameters for the 1DPC. All dielectric 1DPC is made of an altering layer of SiNX (thickness d = 75 nm, refractive index n = 2.45) and SiO2 (thickness d = 110 nm, refractive index n = 1.46), with 15 pairs in total. The angular-dependent transmittance map of the 1DPC is calculated and shown in Fig. S2. Both the transmittance intensity profile of the 1DPC at 660 nm and the angular-dependent reflectance of the gold film-coated glass slide is shown in Fig. 1c. The two calculated curves can be seen as the transmittance and the reflectance spectra in momentum space. The horizontal axis is defined as k/k0 = n sin(θ), where n is the refractive index of the glass substrate (~1.5), θ is the incident angle, and \({k}_{0}\) is the wavenumber of the light in vacuum. Notably, the momentum space filter made of the 1DPC can be tuned by altering the thickness and refractive index of the dielectric layers, allowing the design of a filter working at various excitation wavelengths.
On the reflectance curve, there is a dip (at k/k0 = n sin(θ) = 1.04, \(\theta\) = 43.9°) when the incident light is transverse magnetic polarized, which is the typical surface plasmon resonant curve26. The horizontal position of this dip represents the excitation angle of the surface plasmons at the gold/air interface. This dip is clearly within the transmittance peak of the 1DPC. The transverse magnetic polarized light is transmitted through the 1DPC and then excites the surface plasmons to illuminate the single nanoparticles. Since only the transverse magnetic polarized beam can excite surface plasmons at the surface plasmon resonant angle, the transverse electric polarized light is then blocked by using the designed 1DPC (see the right part of Fig. 1c). Furthermore, surface plasmons can be excited only at surface plasmon resonant angles that are larger than the critical angle; thus, the 1DPC was also designed to block or filter the transmitted light at small incident angles, as shown in Fig. 1c. Thus, the momentum-space filtering functions of the designed 1DPC reduce the background and enhance the imaging contrast of the PSM.
Unlike SPRM, which records reflected light, PSM or Chip-PSM images of surface plasmons are scattered by single nanoparticles with an air objective placed on top of the samples. As expected, the flat gold film surface also scatters surface plasmons, creating a background (\({E}_{b}\)). Consequently, the Chip-PSM image of single nanoparticles is described by
where \(\phi\) is the phase difference between light scattered by single nanoparticles and light scattered by a rough surface. Owing to the short distance between the nanoparticles and the rough surface, the phase difference is close to zero, resulting in positive interference contrast, as in PSM or evanescent scattering microscopy23,30. To enhance image contrast, we can subtract the background intensity \({\left|{E}_{b}\right|}^{2}\), which is obtained from areas without nanoparticles. From Eq. (1), when the diameter of the nanoparticles decreases, the interference term becomes dominant. This technique is useful for detecting the tiny signals of single nanoparticles.
For conventional PSMs, single nanoparticles are illuminated by surface plasmons propagating along one direction, and the PSM image of single nanoparticles (diameter at 150 nm, \(n\) = 1.51) is simulated via finite-difference time-domain methods33, as shown in Fig. 1d (the white arrowhead indicates the propagation direction of the excited surface plasmons). The simulated pattern represents the point-spread-function of the PSM, which is noncircular and nonuniform; thus, the image of a single nanoparticle on the PSM image is distorted (the first three images in Fig. 1d). This distortion will be more obvious when the imaged object has complicated shapes, which will be demonstrated in the following experiments on the imaging of curved nanowires.
In contrast, in the case of Chip-PSM, the excited surface plasmons can propagate in all directions along the gold/air interface (omnidirectional surface plasmons), as shown in Fig. 1b; thus, single nanoparticles can be more uniformly illuminated. As a result, the Chip-PSM image of single nanoparticles is circularly symmetric and uniform, as shown in the last image in Fig. 1d, meaning that the Chip-PSM has an isotropic point-spread-function and thus better imaging quality. This simulation result is explained by the discrete dipole approximation (Supplementary Section 1)34.
Isotropic point-spread-function and large field-of-view of the Chip-PSM
To verify the above principle, the 1DPC was fabricated via PECVD (Plasma-Enhanced Chemical Vapor Deposition) of SiO2 and SiNX on a coverslip. The process of PECVD depends on the chemical reaction of SiH4 with N2O and NH3 at high temperature. The refractive index of SiNX can be precisely adjusted by changing the ratio of SiH4 to NH329. The compact optical illumination device consists of a gold-coated glass slide, the 1DPC, a polymer layer, and an LED source. The fabrication details of the compact optical illumination device are provided in Supplementary Section 2. The 1DPC and the polymer layer were characterized by scanning electron microscopy, as shown in Fig. S3. To experimentally demonstrate the functions of the fabricated 1DPC and gold film, we measured the angular-dependent transmittance of the 1DPC and the angular-dependent reflectance of the gold film-coated coverslip (Fig. S4) via a home-built back focal plane imaging setup, as shown in Fig. S5. The back focal plane imaging setup is now widely used for momentum space optical measurements, such as dispersion relation curves, wavevectors of guided modes, and angular distributions of light from various emitters35,36. The experimentally measured transmittance curve of the 1DPC at a wavelength of 660 nm is nearly consistent with the theoretical results, as shown in Fig. S6. Then, the fabricated gold film-coated coverslip, the 1DPC, and the nanoparticle-doped polymer film are placed onto a commercial LED source one by one. A drop of index-matching oil (\(n\) = 1.51) was placed between the 1DPC and the gold film-coated coverslip. The gold film is placed on the top layer to sustain the propagation of surface plasmons at the gold/air interface. The coupling efficiency of the compact optical illumination device is discussed in Supplementary Section 3.
To demonstrate the advantages of the Chip-PSM, we also established the setup of typical PSM, azimuthally-rotation-illumination-PSM, typical SPRM, and azimuthally-rotation-illumination-SPRM, as shown in Supplementary Section 4. In azimuthally-rotation-illumination-PSM and azimuthally-rotation-illumination-SPRM, a pair of galvanometers were used to achieve rotational illumination (Fig. S7). First, single polymer nanoparticles with a diameter of 150 nm (\(n\) = 1.51) were imaged using these configurations as well as Chip-PSM. Figure 2a–c shows the experimental results for single polystyrene nanoparticles obtained via conventional PSM, azimuthally-rotation-illumination-PSM, and Chip-PSM, respectively. For all three systems, an air objective (40×, NA = 0.6) was used, limiting the resolution to ~550 nm (\(\frac{\lambda }{2{{{\rm{NA}}}}}\)). Therefore, images of single 150 nm nanoparticles can be taken as the experimental point-spread-function of the imaging system.
a–c Experimental images of single polystyrene nanoparticles (diameter of 150 nm, n = 1.51) obtained via PSM, Azimuthally-Rotation-Illumination-PSM, and Chip-PSM. Scale bar, 3.3 μm. d–f Experimental images of silver nanowires obtained via PSM, Azimuthally-Rotation-Illumination-PSM, and Chip-PSM. Scale bar, 33 μm. An air objective (40×, NA = 0.6) is used for capturing images on (a–f). The white arrowhead in Fig. 2a and d represents the propagation direction of the excited surface plasmons. g–h Chip-PSM images of silver nanowires. Air objectives of 10× (NA = 0.3) and 4× (NA = 0.2) were used for image on (g) and (h) respectively. The zone marked with a white box on (g–h) represents the same imaging areas as shown in Fig. 2d–f. The scale bars represent 132 μm in Fig. 2g and 330 μm in Fig. 2h.
Figure 2a shows the point-spread-function of the conventional PSM with single-direction illumination, which is noncircular or distortional, which is consistent with the finite-difference time-domain simulation results (Fig. 1d). In contrast, the point-spread-function for the azimuthally-rotation-illumination-PSM (Fig. 2b) and Chip-PSM (Fig. 2c) are more symmetric and isotropic. To make a more comprehensive comparison, we also measured the point-spread-function of SPRM and azimuthally-rotation-illumination-SPRM, which exhibited a parabolic tail shape and two closely spaced bright spots, respectively. As shown in Fig. S8a and S8b, the shape of a single nanoparticle in the image is obviously distorted. The comparisons among the images obtained via SPRM (Fig. S8a), azimuthally-rotation-illumination-SPRM (Fig. S8b), PSM (Fig. 2a), azimuthally-rotation-illumination-PSM (Fig. 2b), and Chip-PSM (Fig. 2c) verify that image distortion is much greater for SPRM and azimuthally-rotation-illumination-SPRM and that the Chip-PSM image is distortion-free.
As shown in Fig. S7c, d, for the PSM and azimuthally-rotation-illumination-PSM, the surface plasmons are excited by the high-NA oil-immersion objective lens and work as the illumination field for microscopic imaging. Owing to the limit field-of-view of this oil-immersion objective (100×, NA = 1.49), surface plasmons can be excited in a limited area that is smaller than the field-of-view of the imaging objective (air objective, 40×, NA = 0.6). Thus, the field-of-view of the PSM and azimuthally-rotation-illumination-PSM is determined by the area of the excited surface plasmons, meaning that the field-of-view cannot be increased even when an air objective is used for imaging. In contrast, surface plasmons can be excited over a large area by using the proposed compact optical illumination device, as demonstrated in Fig. 1a, b; thus, the field-of-view of Chip-PSM can be increased by using an air objective with a low magnification factor. To demonstrate the difference in the field-of-views of the three imaging systems, silver nanowires with a line width of 50 nm were placed on the gold film and used as the imaging objects. As shown in Fig. 2d, e, the PSM and azimuthally-rotation-illumination-PSM have a field-of-view of ~220 μm in diameter; a 100× oil-immersion objective is used to excite the surface plasmons, and a 40× air objective is used to image the nanoparticles (Fig. S7c, d). In contrast, the Chip-PSM has a large illumination zone (larger than 1 × 1 cm2). When the Chip-PSM is equipped with 40× (NA = 0.6), 10 × (NA = 0.3), and 4 × (NA = 0.2) air objectives, its field-of-view reaches 330 × 330 μm2, 1.32 × 1.32 mm2, and 3.3 × 3.3 mm2, respectively (Fig. 2f–h). Owing to the large field-of-view of the Chip-PSM, many more silver nanowires can be recorded on a single microscopy image, as shown in Fig. 2h. The large field-of-view of the Chip-PSM is favorable for high-throughput optical measurements. It is worth noting that prism-based37 PSM can also achieve a millimeter-scale imaging field of view. However, this approach requires precise control of light coupling into surface plasmons and relies on a bulky prism, which poses challenges for system integration. Additionally, the prism-based system typically excites SPs in a single direction, which can lead to a noncircular or distortional point-spread-function (PSF). ln contrast, the proposed Chip-PSM integrates the light source with micro-nano structures designed for omnidirectional surface plasmons excitation, significantly reducing the system’s footprint and simplifying operational complexity.
In addition to the large field-of-view, the Chip-PSM achieves superior imaging quality compared with the PSM and azimuthally-rotation-illumination-PSM. As indicated by the green arrow-1 in Fig. 2d–f, an alpha-shaped nanowire is fully resolved in the C-PSM image (Fig. 2f), whereas it is partially invisible in the PSM and azimuthally-rotation-illumination-PSM images (Fig. 2d, e). The straight line indicated by the green arrow-2 is nearly invisible in Fig. 2d and can be detected only on the Chip-PSM and azimuthally-rotation-illumination-PSM images (Fig. 2e, f), which directly demonstrates the advantages of azimuthally rotated illuminations and omnidirectional illuminations. The mechanism of this advantage can be briefly explained as follows. When the orientation of the nanowire is along the propagation path of the surface plasmons, its scattering light will be weak, and the light scattered from each point on this nanowire will experience destructive interference; thus, it becomes nearly invisible in the image. When the orientation of the nanowire is perpendicular to the propagation direction of the surface plasmons, the scattering light is stronger, as demonstrated in Fig. 2d; thus, the nanowire is more visible in the image. As a result, when the surface plasmons’ propagation directions are rotating or omnidirectional, the orientation of the nanowire is always perpendicular to the propagation direction of the surface plasmons, which is the reason why the nanowires or various orientations are all visible on the azimuthally-rotation-illumination-PSM and Chip-PSM images (Fig. 2e, f). The advantage of the Chip-PSM over the azimuthally-rotation-illumination-PSM is that there is no need for mechanical scanning or bulky optical setups, resulting in higher temporal resolution, lower cost, and simpler experimental configuration.
In our experiments, we also imaged the nanowires with SPRM and azimuthally-rotation-illumination-SPRM (Fig. S8c, d), where the nanowires were surrounded by considerable background noise and nearly unresolved, indicating that the imaging quality of SPRM is worse than that of PSM. For comparison, we also replaced the gold film-coated coverslip with a bare coverslip for Chip-PSM, and the two corresponding images are shown in Fig. S9, which demonstrates that the imaging contrast for single nanoparticles is enhanced if the gold film is used (Supplementary Section 5).
Label-free imaging of single polymer nanoparticles
The advantages of Chip-PSM have been demonstrated, and we verify the detection ability of Chip-PSM by imaging polystyrene nanoparticles with diameters varying from 40–200 nm. Polystyrene nanoparticles (\(n\) = 1.51) were placed on the gold film and illuminated by the surface plasmons at the gold/air interface via the setup shown in Fig. 1a. To obtain a more accurate scattering intensity of polystyrene nanoparticles, we average a sequence of images to suppress random noise and subtract the background (\({\left|{E}_{b}\right|}^{2}\)) from the image area without nanoparticles; the image processing is shown in Supplementary Section 6. The Chip-PSM images of polystyrene nanoparticles with diameters of 40, 60, 80, 120, 150, and 200 nm are shown in Fig. S10. We determine the scattering intensity of the polystyrene nanoparticles by integrating the pixel intensity within a square area (7 × 7 pixels; the nanoparticle is located at the center of this area). Image intensity histograms of polystyrene nanoparticles with diameters of 40, 60, 80, 120, 150, and 200 nm are constructed (Fig. 3a), which approximately fit with Gaussian distributions12, as indicated by the red lines in Fig. 3a. The deviation of the experimental results from the ideal Gaussian distribution can be attributed to slight variations in nanoparticle sizes, which affect the scattering intensity distribution, as well as the limited number of nanoparticles measured in the experiment.
a Image intensity histograms of polystyrene nanospheres with diameters of ~200, 150, 120, 80, 60 and 40 nm, where the red solid lines are Gaussian fits. The incident light intensity remains unchanged for different nanospheres, ~1 W cm−2. The exposure times are 5 ms, 10 ms, 15 ms, 30 ms and 100 ms for 200 nm, 150 nm, 120 nm, 80 nm, 60 nm, and 40 nm, respectively. The image intensity was normalized with an exposure time of 50 ms. b image intensity versus nanosphere’s diameter. The error bars indicate the standard deviation obtained from the corresponding histograms. The experiments were repeated three times, yielding similar results for the relationship between scattering intensity and nanoparticle size.
Curves of the image intensity at the peak of each histogram versus the nanoparticle diameter on a logarithmic scale are plotted in Fig. 3b, and the error bars represent the standard deviations of the Gaussian fits of the corresponding histograms (Fig. 3a). Considering the exponential decay of surface plasmons with increasing distance from the surface, the diameters of these nanoparticles are replaced by the effective diameters (Supplementary Section 7). In the large nanoparticle regime (diameters > 90 nm), the image intensity follows a power law of \({d}^{5.8}\), where the exponent is close to six. This is expected since the light scattered from nanoparticles dominates as the diameter increases, so the measured image intensity is proportional to \({\left|{E}_{s}\right|}^{2}\) according to Eq. (1). However, in the small nanoparticle regime, the image intensity is proportional to \({d}^{3}\), which is predicted by the interference term \(2\left|{E}_{b}\right|\left|{E}_{s}\right|\cos \left(\phi \right)\) in Eq. (1). The interference effect makes Chip-PSM possible for detecting and imaging smaller nanoparticles. Chip-PSM is also suitable for imaging nanoparticles and biological cells immersed in water (Supplementary Section 8). Two videos show the dynamic motion of the nanoparticles and the biological cells (Movie S1 and Movie S2).
Label-free sensing of the hygroscopic growth of single aerosol nanoparticles
In the following experiments, we verify the sensing ability of Chip-PSM by detecting tiny changes in single aerosol nanoparticles with diameters as small as ~50 nm. Atmospheric aerosols can pose significant threats to human health38, as smaller particles such as PM2.5 (particles with a diameter of 2.5 micrometers or less) can penetrate deep into the lungs and even enter the bloodstream. In our previous work, SPRM39 and Bloch surface wave microscopy40 were proposed to detect the hygroscopic growth of single aerosol nanoparticles. However, these methods require scanning galvanometers, precise optical path alignment, and high-NA oil-immersion objectives to excite surface waves for probing aerosol nanoparticles with diameters of ~90 nm. Therefore, developing an imaging system with a simpler configuration for detecting smaller aerosol nanoparticles is necessary and important.
Here, we use Chip-PSM to image and detect the hygroscopic growth of single aerosol nanoparticles with a diameter of ~50 nm. The component of aerosol nanoparticles is NaCl, which is the primary chemical component involved in breaking ocean droplets and has a well-established deliquescence relative humidity41. The corresponding optical setup is shown in Fig. S11a, where the aerosol nanoparticles are deposited on the substrate (gold-coated glass slide). A sample chamber was placed on the substrate, as shown in Fig. S11b, and the relative-humidity in the chamber was controlled dynamically from 60 to 87% with an uncertainty of 1% relative-humidity via a custom-made humidification system (Fig. S11c). An air objective is placed on top of the sample chamber to collect the scattered light from the single aerosol nanoparticles, as shown in Fig. 1a. Deposition onto the substrate was discussed in our previous work39,40,42, and a differential mobility analyzer was used to screen the desired deposited nanoparticle size (50 nm). The scanning electron microscopy image of the deposited NaCl nanoparticles is presented in Fig. 4a, with the size distribution histogram primarily concentrated at 50–60 nm (Fig. 4b), which is consistent with the differential mobility analyzer screening size (50 nm).
a scanning electron microscopy image of the deposited NaCl nanoparticles. Scale bar, 500 nm. b The diameter distribution histogram of the NaCl nanoparticles. c Chip-PSM images of a single aerosol nanoparticle at four Relative-Humidity (RH). Scale bar, 1.375 μm. d Experimental relative intensity of the light scattering from single aerosol nanoparticles at various relative-humidity (left Y-axis). Theoretical hygroscopic curve (right Y-axis) predicted by the extended aerosol inorganic model. The error bars represent the standard deviation of the three selected nanoparticle intensities.
At each relative-humidity value, the residence time is ~15 min to allow sufficient reaction of the aerosol nanoparticles. We averaged a sequence of images and determined the scattering intensity of the aerosol nanoparticles by integrating the pixel intensity within a square area (7 × 7 pixels). Image processing is like that used for polystyrene nanoparticles. The Chip-PSM image revealed many nanoparticles. Three aerosol nanoparticles with smaller and similar brightness values were selected at a low relative humidity (20%), each with an approximate diameter of 50 nm. We averaged the three aerosol nanoparticle intensities as the final value (\({I}_{{RH}}\)) at the selected environmental relative-humidity. As shown in Fig. 4c, Chip-PSM images for single aerosol nanoparticles at four relative-humidity setpoints are presented, which reveal that the intensity of the aerosol nanoparticles increases with increasing relative-humidity due to the absorption of water vapor. This demonstrates that the size of aerosol nanoparticles increases during the hygroscopic growth process. The curve of this relative intensity (defined as \({I}_{{RH}}/{I}_{{RH}=60\%}\)) versus the relative-humidity value is plotted in Fig. 4d. The error bars represent the standard deviation of the three selected nanoparticle intensities. When the relative-humidity increases from 60% to 75%, the relative intensity increases slightly, indicating that the NaCl aerosol nanoparticles do not absorb much water vapor from the air space. However, when the relative-humidity reaches 77%, the scattering intensity increases significantly, which can be explained by the deliquescence of NaCl aerosol nanoparticles. The threshold relative-humidity value is called deliquescence relative humidity, at which time aerosol nanoparticles undergo a solid‒aqueous phase transition. In our experiment, the measured deliquescence relative humidity of pure NaCl aerosol nanoparticles was 77%, which agreed with previous work42,43. To evaluate the accuracy of Chip-PSM in sensing the hygroscopic growth of aerosol nanoparticles, an extended aerosol inorganic model (Fig. 4d, solid red curve) [http://www.aim.env.uea.ac.uk/aim/aim.php] is used to predict hygroscopic growth, which is consistent with the experimental results. The extended aerosol inorganic model prediction results verify that Chip-PSM can image and sense the hygroscopic growth of single NaCl aerosol nanoparticles with diameters of ~50 nm.
Enhancing the detection limit of Chip-PSM
Finally, we demonstrate that the detection limit of the proposed Chip-PSM can be further enhanced through optimization of the chip structures. We added a layer of silicon (thickness: 8 nm) on top of the gold film. The red lines in Fig. 5a show the calculated reflectivity spectra of conventional surface plasmon resonant sensors (the gold film) and silicon-enhanced surface plasmon resonant sensors as a function of k/k0 = n sin(θ). The solid red line indicates that the conventional surface plasmon resonant sensor has a full width at half maximum of ~0.03 (\(\Delta \theta\) = 1.6°). Moreover, the dashed red line shows that the reflectivity spectrum of the silicon-enhanced surface plasmon resonant sensors has a broader full width at a half maximum of ~0.15 (\(\Delta \theta\) = 9.4°). The calculated transmittance spectrum of the designed 1DPC is also plotted in Fig. 5a with a dark line. These results indicate that, compared with conventional surface plasmon resonant sensors, surface plasmons in silicon-enhanced surface plasmon resonant sensors can be excited over a wider incident angle, improving the energy efficiency of the light transmitted from the 1DPC and the detection limit for single nanoparticles. The calculation results can be attributed to the change in the effective refractive index on the air side after the silicon film is added to the gold film44,45. The experimental reflectivity spectra of the silicon-enhanced surface plasmon resonant sensors are shown in Fig. S4f and are nearly consistent with the calculated results.
a Angular-dependent transmittance of the 1DPC (left Y-axis), and angular-dependent reflectance of the gold film and silicon-coated gold film (right Y-axis). The incident beam is Transverse-Magnetic (TM) polarized. b Chip-PSM image of gold nanoparticles with diameter at about 10 nm. The exposure time is 300 ms. Scale bar, 27.5 μm. c Enlarged images of the zones marked with three green boxes (i, ii, and iii) in Fig. 5b. Scale bar, 3.575 μm. d–f Chip-PSM images of CSPbI3 nanoparticles before reaction with HCl vapor (d), after reaction with HCl (e) and after reaction with HI vapor (f). The exposure time was 200 ms. Scale bar, 2.75 μm.
Owing to the broad angular range of surface plasmons’ excitation, enhanced Chip-PSM enables the detection and imaging of smaller nanoparticle signals over a large field-of-view. Moreover, the silicon-enhanced surface plasmon resonant sensors have a higher excitation angle (at \(k/{k}_{0}\) = 1.19, \(\theta\) = 52.5°) than conventional surface plasmon resonant sensors do, allowing PSM imaging with a higher NA air objective. As shown in Fig. 5b, single gold nanoparticles with a diameter of ~10 nm are imaged via Chip-PSM with a 40× (NA = 0.6) air objective, achieving a field-of-view of 275 × 275 μm2. The very bright spots in Fig. 5b are signals from large particles originally present on the substrate. The enlarged images of the 10 nm gold nanoparticles (marked with green boxes) are shown in Fig. 5c and can be clearly detected and individually imaged. Fig. 5b, c demonstrate that Chip-PSM can detect and image many nanoparticles in real time without image postprocessing, which is challenging for traditional PSM. Increasing the exposure time allows the detection and imaging of weaker signals, such as the scattering light from single 30 nm polystyrene nanoparticles and 5 nm gold nanoparticles, as shown in Fig. S12.
Finally, the Chip-PSM was used to detect and image anion exchange reactions in single cesium halide perovskite nanocrystals. These materials are known for their outstanding photoelectric conversion properties, making them highly relevant for applications in solar cells and photodetectors46,47,48. Anion exchange in cesium lead halide perovskites enables tuning of photoluminescence across the visible spectrum while preserving high quantum yields and narrow emission linewidths49. In the experiment, cesium lead halide perovskite (CSPbX3, X = Cl, I, Br) nanoparticles are spin-coated on the substrate (silicon-coated gold film). The transmission electron microscopy image, shown in Fig. S13e, revealed an edge length of ~10 nm. Fig. 5d shows a PSM image of CSPbI3 nanoparticles without image postprocessing. After HCl vapor is introduced to the substrate that is spin-coated with CSPbI3 nanoparticles, the intensity of the single CSPbX3 nanoparticles significantly decreases due to the anion exchange reaction, which converts the CSPbI3 nanoparticles to CSPbCl3, as shown in Fig. 5e. In the next step, HI vapor is added, and another anion-exchange reaction occurs, in which the nanoparticles are recovered from CSPbCl3 to CSPbI3. The intensity of the single nanoparticles increases again, as shown in Fig. 5f. The experimental results are consistent with our previous work50, demonstrating that Chip-PSMs can detect and image the chemical reactions of single nanoparticles with high sensitivity. Notably, the perovskite nanoparticles on the substrate were exposed to HCl or HI for ~30 min prior to Chip-PSM imaging under the assumption that the ion exchange reaction had fully occurred. Direct introduction of acidic gas onto the perovskite surface will damage the air objective used in Chip-PSM. However, the use of an acid-resistant sample chamber in combination with Chip-PSM could enable real-time observation of the ion exchange process.
In fact, the existing imaging system can be entirely replaced by compact imaging devices such as smartphones, microlenses, or metalenses, enabling the full integration and portability of the PSM system. To verify the compatibility of compact imaging devices with the compact optical illumination device, we have demonstrated the use of a smartphone and a small lens as the imaging system for the Chip-PSM and successfully imaged silver nanowires with a diameter of 70 nm. The results are showed in supplementary section 9.
Discussion
In summary, we developed a Chip-PSM for large-view, label-free, highly sensitive imaging of single nanoparticles with an isotropic point-spread-function. The Chip-PSM combines a standard upright microscope with a compact optical illumination device. Compared with conventional PSM or SPRM, which use oil-immersion objectives and bulky optical components, the proposed Chip-PSM is more compact, cost-effective, and user-friendly. Taking advantage of the filtering function of a well-designed 1DPC in momentum space, omnidirectional surface plasmons can be excited at the gold/dielectric interface, providing omnidirectional illumination and resulting in an isotropic point-spread-function; thus, the imaging quality of the Chip-PSM is much better than that of the SPRM or PSM. Taking advantage of its high sensitivity, label-free nature, better image quality, and wide-field imaging ability, we successfully adopt Chip-PSM to image and sense the hygroscopic growth of single aerosol nanoparticles and the chemical reactions of single perovskite nanocrystals. Unlike traditional PSM23 or evanescent scattering microscopy30, which are typically used to detect biomolecules, Chip-PSM expands the application to detect and image single-particle changes in atmospheric environments and chemical reactions. Additionally, Chip-PSM can also image the dynamic behaviors of metal nanoparticles and biological cells in liquid solutions. The versatility of Chip-PSM makes it applicable to a wide range of disciplines, including biological, physical, environmental, and chemical sciences5,18,23,30,51,52,53.
However, the main shortcoming of Chip-PSM is that the incident light intensity of the LED source is ~1 W cm−2, which is much smaller than the incident light intensity of the conventional PSM (~3 kW cm−2). This results in Chip-PSM being less effective at detecting small nanoparticles23. However, compared with PSM or SPRM, Chip-PSM offers superior imaging quality, a larger field-of-view, and a simpler configuration. Chip-PSM can be used as a beneficial auxiliary to PSM to broaden its application scope. In the future, we can overcome the limitations of Chip-PSM by using a stronger LED source or focusing the LED sources with microlens arrays.
Methods
Optical experimental setup
All Chip-PSM images were taken on a modified optical microscope (Nikon Ni-U) using an air objective (CFI Super Plan Fluor ELWD and 40×, NA = 0.6, WD = 3.6–2.8 mm), except for Fig. 2g (CFI Plan Fluor DL and 10×, NA = 0.3, WD = 15.2 mm) and Fig. 2h (CFI Plan Apochromat Lambda D 4×, NA = 0.2, WD = 20.0 mm), for which additional air objectives were used. All Chip-PSM images were recorded by Prime 95B sCMOS (TELEDYNE PHOTOMETRICS). The LED light source was purchased from Shanghai Xilong Optoelectronics Technology Co., Ltd., with a model specification of LED09-660-1W. The total optical power is ~1 W, and the optical power density incident on the polymer layer is ~1 W cm−2.
All the BFP measurements were performed on a modified optical microscope (Nikon Ti2-U). An oil-immersion objective (CFI Apochromat TIRF 100×, NA = 1.49, WD = 0.12 mm) from Nikon Japan was used to measure the angular distribution on the BFP fully. A broadband white light source with a bandpass filter was used to measure the reflectivity BFP (Fig. S5b). Three filters were used to measure the transmission BFP of the 1DPC, where the source was the LED source used in the experiment. The Neo sCMOS detector for recording the BFP images was from Andor Oxford Instruments (UK).
Preparation of the nanoparticles and nanowires
The polystyrene nanoparticles were purchased from Thermo Fisher Scientific (USA). The certified mean diameters of the nanoparticles supplied were ~30, 40, 60, 80, 120, 150, and 200 nm. Gold nanoparticles with diameters of 5 nm and 10 nm, as well as silver nanowires with a diameter of 50 nm and 70 nm, were purchased from XFNANO Materials Tech. Co., Ltd. Theses gold nanoparticles, polystyrene nanoparticles and sliver nanowires were diluted in anhydrous ethanol and dropped on the substrate to dry. To image gold nanoparticles in water, the nanoparticles are first dissolved in deionized water, after which a drop of the solution is placed on the surface of the gold film and covered with a clean coverslip. Transmission electron microscopy images of Au nanoparticles with diameters of 5 nm, 10 nm, 20 nm, and 50 nm, along with scanning electron microscopy images of polystyrene nanoparticles with sizes of 30 nm, 40 nm, 60 nm, 80 nm, 120 nm, 150 nm, and 200 nm, as well as silver nanowires (m) with a linewidth of 50 nm, are presented in Fig. S13. The fabrication details of the CSPbI3 nanoparticles were presented in our previous work50.
Preparation of neutrophil samples
A commercial neutrophil extraction kit was used to isolate neutrophils from 1 mL of whole blood, followed by dilution to 1 × 106 cells/mL in culture medium containing 5% fetal bovine serum. The neutrophils were then incubated at 37 °C in a 5% CO2 incubator for 30 min. Then, the cell suspension was quickly dropped onto the Au film surface, and another piece of glass slide was used as the coverslip, which was placed on the suspension and flattened.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data that support the plots within this paper and other finding of this study are available from the corresponding author upon request. Source data for Figs. 1–5 are available at https://doi.org/10.6084/m9.figshare.28645445.
Code availability
The codes that support the findings of this study are available from the corresponding authors upon request.
References
Shaked, N. T., Boppart, S. A., Wang, L. V. & Popp, J. Label-free biomedical optical imaging. Nat. Photonics 17, 1031–1041 (2023).
Stollmann, A. et al. Molecular fingerprinting of biological nanoparticles with a label-free optofluidic platform. Nat. Commun. 15, 4109 (2024).
Miao, X., Yan, L., Wu, Y. & Liu, P. Q. High-sensitivity nanophotonic sensors with passive trapping of analyte molecules in hot spots. Light. Sci. Appl. 10, 5 (2021).
Ohannesian, N., Misbah, I., Lin, S. H. & Shih, W.-C. Plasmonic nano-aperture label-free imaging (PANORAMA). Nat. Commun. 11, 5805 (2020).
Lee, S. F. & Klenerman, D. Weighing one protein with light. Science 360, 378–379 (2018).
Chocarro-Ruiz, B., Fernández-Gavela, A., Herranz, S. & Lechuga, L. M. Nanophotonic label-free biosensors for environmental monitoring. Curr. Opin. Biotechnol. 45, 175–183 (2017).
Jiang, B., Li, H., Wang, W. & Wang, H. Optical in situ deciphering of the surface reconstruction–assistant multielectron transfer event of single Co3O4 nanoparticles. Proc. Natl. Acad. Sci. USA 121, e2407146121 (2024).
Wang, W. et al. Single cells and intracellular processes studied by a plasmonic-based electrochemical impedance microscopy. Nat. Chem. 3, 249–255 (2011).
Wang, W. et al. Label-free measuring and mapping of binding kinetics of membrane proteins in single living cells. Nat. Chem. 4, 846–853 (2012).
Yang, Y. et al. Imaging action potential in single mammalian neurons by tracking the accompanying sub-nanometer mechanical motion. ACS Nano 12, 4186–4193 (2018).
Yang, Y. et al. Label-free tracking of single organelle transportation in cells with nanometer precision using a plasmonic imaging technique. Small 11, 2878–2884 (2015).
Wang, S. et al. Label-free imaging, detection, and mass measurement of single viruses by surface plasmon resonance. Proc. Natl. Acad. Sci. USA 107, 16028–16032 (2010).
Shan, X. et al. Imaging the electrocatalytic activity of single nanoparticles. Nat. Nanotechnol. 7, 668–672 (2012).
Wang, Y. et al. Single-molecule calorimeter and free energy landscape. Proc. Natl. Acad. Sci. USA 118, e2104598118 (2021).
Wang, H. et al. Phase imaging of transition from classical to quantum plasmonic couplings between a metal nanoparticle and a metal surface. Proc. Natl. Acad. Sci. USA 117, 17564–17570 (2020).
Fang, Y. et al. Intermittent photocatalytic activity of single CdS nanoparticles. Proc. Natl. Acad. Sci. USA 114, 10566–10571 (2017).
Yang, Y. et al. Interferometric plasmonic imaging and detection of single exosomes. Proc. Natl. Acad. Sci. USA 115, 10275–10280 (2018).
Shan, X., Patel, U., Wang, S., Iglesias, R. & Tao, N. Imaging local electrochemical current via surface plasmon resonance. Science 327, 1363–1366 (2010).
Wang, Y. et al. Fast electrochemical and plasmonic detection reveals multitime scale conformational gating of electron transfer in cytochrome c. J. Am. Chem. Soc. 139, 7244–7249 (2017).
Yang, Y., Zhai, C., Zeng, Q., Khan, A. L. & Yu, H. Quantitative amplitude and phase imaging with interferometric plasmonic microscopy. ACS Nano 13, 13595–13601 (2019).
Kuai, Y. et al. Label-free surface-sensitive photonic microscopy with high spatial resolution using azimuthal rotation illumination. Sci. Adv. 5, eaav5335 (2019).
Wu, G., Qian, C., Lv, W.-L., Zhao, X. & Liu, X.-W. Dynamic imaging of interfacial electrochemistry on single Ag nanowires by azimuth-modulated plasmonic scattering interferometry. Nat. Commun. 14, 4194 (2023).
Zhang, P. et al. Plasmonic scattering imaging of single proteins and binding kinetics. Nat. Methods 17, 1010–1017 (2020).
Zhang, P., Zhou, X. & Wang, S. Plasmonic scattering microscopy for label-free imaging of molecular binding kinetics: from single molecules to single cells. Chemistry–Methods 3, e202200066 (2023).
Zhou, X., Chieng, A. & Wang, S. Label-free optical imaging of nanoscale single entities. ACS Sens. 9, 543–554 (2024).
Maier S. A. Plasmonics: Fundamentals and Applications 1st edn, Vol. 224 (Springer, 2007).
Diekmann, R. et al. Chip-based wide field-of-view nanoscopy. Nat. Photonics 11, 322–328 (2017).
Chazot, C. A. C. et al. Luminescent surfaces with tailored angular emission for compact dark-field imaging devices. Nat. Photonics 14, 310–315 (2020).
Kuai, Y. et al. Planar photonic chips with tailored angular transmission for high-contrast-imaging devices. Nat. Commun. 12, 6835 (2021).
Zhang P. et al. Evanescent scattering imaging of single protein binding kinetics and DNA conformation changes. Nat. Commun. 13, 2298 (2022).
Liu, Y., Huang, M., Chen, Q. & Zhang, D. Single planar photonic chip with tailored angular transmission for multiple-order analog spatial differentiator. Nat. Commun. 13, 7944 (2022).
Anemogiannis, E., Glytsis, E. N. & Gaylord, T. K. Determination of guided and leaky modes in lossless and lossy planar multilayer optical waveguides: reflection pole method and wavevector density method. J. Lightwave Technol. 17, 929 (1999).
Taflove A. Computational Electrodynamics: The Finite-difference Time-domain Method 3rd edn, Vol. 1038 (Artech House, 1995).
Evlyukhin, A. B. & Bozhevolnyi, S. I. Point-dipole approximation for surface plasmon polariton scattering: Implications and limitations. Phys. Rev. B 71, 134304 (2005).
Descrovi, E. et al. Leakage radiation interference microscopy. Opt. Lett. 38, 3374–3376 (2013).
Drezet, A. et al. Leakage radiation microscopy of surface plasmon polaritons. Mater. Sci. Eng.: B 149, 220–229 (2008).
Zhang, P. et al. In situ analysis of membrane-protein binding kinetics and cell–surface adhesion using plasmonic scattering microscopy. Angew. Chem. Int. Ed. 61, e202209469 (2022).
Pöschl, U. Atmospheric aerosols: composition, transformation, climate and health effects. Angew. Chem. Int. Ed. 44, 7520–7540 (2005).
Xie, Z. et al. In situ quantitative observation of hygroscopic growth of single nanoparticle aerosol by surface plasmon resonance microscopy. Anal. Chem. 92, 11062–11071 (2020).
Kuai, Y. et al. Real-time measurement of the hygroscopic growth dynamics of single aerosol nanoparticles with bloch surface wave microscopy. ACS Nano 14, 9136–9144 (2020).
Castarède, D. & Thomson, E. S. A thermodynamic description for the hygroscopic growth of atmospheric aerosol particles. Atmos. Chem. Phys. 18, 14939–14948 (2018).
Fan Z. et al. Chip-Based Dark-filed Microscopy for Sensing the Dynamical Changes of Single Aerosol Nanoparticles. IEEE Journal of Selected Topics in Quantum Electronics, 1-7 (IEEE, 2023).
Peng, C., Chen, L. & Tang, M. A database for deliquescence and efflorescence relative humidities of compounds with atmospheric relevance. Fundamental Res. 2, 578–587 (2022).
Hu, W. P. et al. A novel ultrahigh-resolution surface plasmon resonance biosensor with an Au nanocluster-embedded dielectric film. Biosens. Bioelectron. 19, 1465–1471 (2004).
Hohenau, A. et al. Dielectric optical elements for surface plasmons. Opt. Lett. 30, 893–895 (2005).
Protesescu, L. et al. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 15, 3692–3696 (2015).
Huang, H. et al. Colloidal lead halide perovskite nanocrystals: synthesis, optical properties and applications. NPG Asia Mater. 8, e328 (2016).
Jena, A. K., Kulkarni, A. & Miyasaka, T. Halide perovskite photovoltaics: background, status, and future prospects. Chem. Rev. 119, 3036–3103 (2019).
Nedelcu, G. et al. Fast anion-exchange in highly luminescent nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 15, 5635–5640 (2015).
Liu, Y. et al. Cascaded momentum-space polarization filters enabled label-free black-field microscopy for single nanoparticles analysis. Proc. Natl. Acad. Sci. USA 121, e2321825121 (2024).
Williamson, C. J. et al. A large source of cloud condensation nuclei from new particle formation in the tropics. Nature 574, 399–403 (2019).
Yu, X.-C. et al. Optically sizing single atmospheric particulates with a 10-nm resolution using a strong evanescent field. Light.: Sci. Appl. 7, 18003–18003 (2018).
Zheng, T. et al. A chemical timer approach to delayed chemiluminescence. Proc. Natl. Acad. Sci. USA 119, e2207693119 (2022).
Acknowledgements
This work was supported by the National Nature Science Foundation of China (grant nos. 12134013, 42375124, 62127818, 52373122), the National Key Research and Development Program of China (2021YFA1400700), the Fundamental Research Funds for the Central Universities (WK2340000109), and the Innovation Program for Quantum Science and Technology (No. 2021ZD0303301). The work was partially performed at the University of Science and Technology of China’s Center for Micro- and Nanoscale Research and Fabrication and at the Instruments Center for Physical Science, University of Science and Technology of China. The authors thank Professor Ling Zhu and Mr. Xiao Yang (Anhui Institute of Optics and Fine Mechanics. CAS) for providing the biological cells.
Author information
Authors and Affiliations
Contributions
X.X.Y., Z.T.F. and D.G.Z. initiated the studies. X.X.Y., Z.T.F. and C.E.L. conducted the optical experiments and obtained the theoretical and simulated results. X.L. and Q.W.Z. contributed to the design of the compact optical illumination device and the experimental setup. H.L.Z. and G.Z. contributed to the design and analysis of the anion exchange reactions in single cesium halide perovskite nanocrystals. Z.B.X., H.Q.G., and J.G.L. contributed to the design and analysis of the hygroscopic growth of single aerosol nanoparticles. X.X.Y., Z.T.F. and D.G.Z. wrote the manuscript. D.G.Z., X.L., J.G.L., Q.W.Z., G.Z. and H.Q.G. supervised the research. All authors contributed to the interpretation of the results.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Shaopeng Wang, Xinyu Zhou and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
You, X., Fan, Z., Zhang, H. et al. Large field-of-view plasmonic scattering imaging and sensing of nanoparticles with isotropic point-spread-function. Nat Commun 16, 5104 (2025). https://doi.org/10.1038/s41467-025-60460-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-60460-7
This article is cited by
-
Sensitivity enhancement of surface plasmon resonance biosensors based on versatile nanostructures: principle, fabrication, and illustrative applications
Microsystems & Nanoengineering (2026)