Abstract
The effect of Levosimendan on postoperative natriuretic peptides in noncardiac surgical patients remains unknown. Thus, this study evaluates the effect of a perioperative levosimendan administration on postoperative N-terminal brain pro natriuretic peptide (NT-proBNP) concentrations. In this prospective, double-blinded, parallel group, placebo-controlled, phase III trial 115 patients were assigned to perioperative single-dose of 12.5 mg of levosimendan and 115 to placebo between October 2020 through November 2023 (clinicaltrials.gov: NCT04329624). The primary outcome was postoperative maximum NT-proBNP concentration within the first 3 postoperative days. 228 patients completed the trial. Postoperative maximum NT-proBNP concentrations did not differ significantly between the groups (effect estimate: −64.51 ng.L-1; 95% CI −332.66 to 195.56; p = 0.61). Here, we show that perioperative levosimendan administration did not lead to a significantly lower release in postoperative NT-proBNP after noncardiac surgery.
Similar content being viewed by others
Introduction
Cardiovascular complications after noncardiac surgery are the most common cause of postoperative morbidity and mortality and still affect 1 of every 33 patients over 45 years1. N-terminal brain pro natriuretic peptide (NT-proBNP), a marker for increased myocardial wall strain, commonly increases up to 4- to 5-fold after noncardiac surgery and is independently associated with cardiac complications and mortality2,3,4,5,6,7. The underlying reason for this steep increase after noncardiac surgery is still unclear but was interpreted as postoperative subclinical heart failure affecting outcome2,3.
Levosimendan is an intravenous inotropic drug, that binds at troponin C and increases the sensitivity of myofilaments to calcium8,9. The more facilitated actin-myosin complexes increase contractility, without increasing myocardial oxygen demand. The active metabolite of levosimendan acts more than 7 days after a single infusion10,11. Thus, levosimendan might be an optimal drug to improve perioperative myocardial function, possibly leading to a lower release of postoperative NT-proBNP.
Levosimendan significantly increased cardiac output and improved myocardial function11,12. In the SURVIVE (Levosimendan vs. Dobutamine for Patients With Acute Decompensated Heart Failure) and the REVIVE I & II (Effect of Levosimendan on the Short-Term Clinical Course of Patients With Acutely Decompensated Heart Failure) trials, levosimendan led to a significant decrease in BNP concentrations up to 1 month after treatment in patients with decompensated heart failure13,14. Based on the previously observed steep increase in postoperative NT-proBNP concentrations, we expected similar effects of levosimendan in patients undergoing major noncardiac surgery, in whom in fact studies are still lacking.
In this work, we tested the hypothesis that a single infusion of levosimendan will lead to a significantly lower release of postoperative NT-proBNP in cardiovascular high-risk patients undergoing moderate- to high-risk major noncardiac surgery as compared to placebo. As our secondary and exploratory aims we evaluated the effect of levosimendan on the incidence of myocardial injury after noncardiac surgery (MINS), postoperative troponin T release, and postoperative cardiovascular complications.
Results
Patient population
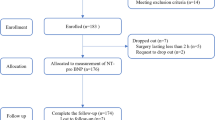
A total of 230 patients were randomized between October 2020 and November 2023. We ceased enrollment when our target sample size of 230 patients was obtained. A total of 228 patients completed the trial, two patients were excluded from the analysis (Fig. 1).
Patient characteristics and morphometric data, ASA physical status, comorbidities, long-term medication, type of surgery and preoperative laboratory parameters are shown in Table 1. Patients receiving levosimendan infusion had a significantly lower mean arterial blood pressure and required a significantly higher noradrenaline support. Otherwise, intraoperative characteristics including duration of anesthesia and surgery, fluid management, anesthesia management, blood pressure and hemodynamic parameters, and intraoperative arterial blood gas analyses were similar between the groups (Table 2 and Supplementary Table 1 in the Supplementary).
Primary endpoint
Postoperative maximum NT-proBNP concentrations were not significantly different between the placebo and the levosimendan group (p = 0.902) (Table 3 and Fig. 2a, b). There was also no significant difference in the median regression model between groups (effect estimate: −64.51 ng L−1; 95% CI −332.66 to 195.56; p = 0.61). Detailed results of the median regression model are presented in Supplementary Table 3 in the Supplementary. Descriptive statistics of NT-proBNP and troponin T are shown in Supplementary Table 2 in the Supplementary.
Definition of boxplots: the median is shown as black bar, the box gives 25% and 75% percentiles, the Whiskers are chosen to extend up to 1.5 times the interquartile range from the box. Values outside this range are shown as dots. a Bloxplots are showing the perioperative time course of NT-proBNP within the first 3 days separately for the groups. b The violin plots show the distribution of maximum NT-proBNP concentrations within the first three postoperative days separately for the groups. The p value of the two-sided Wilcoxon-Rank-Sum test to compare treatment with placebo was 0.902.
In contrast to the times points (2 h after surgery, 1st and 2nd postoperative day) we found a significantly larger increase in NT-proBNP concentrations for the placebo group as compared to the levosimendan group for the times points 3rd postoperative day (p = 0.007) and 5th postoperative days (p = 0.002) after surgery. Detailed results of the regression model evaluating the time course of NT-proBNP are shown in Supplementary Table 4 in the Supplementary.
Secondary endpoint
In our secondary outcome, we did not find a statistically significant difference in the incidence of MINS between the levosimendan group (74/115; 64%) and the placebo group (58/112; 51%) (p = 0.063) (Table 3 and Fig. 3a, b). We also did not find a statistically significantly treatment effect in the logistic regression model for MINS (OR 1.883, 95% CI 1.001 to 3.395; p = 0.051). Detailed results of the regression model for MINS are presented in Supplementary Table 5 in the Supplementary.
Definition of boxplots: the median is shown as black bar, the box gives 25% and 75% percentiles, the Whiskers are chosen to extend up to 1.5 times the interquartile range from the box. Values outside this range are shown as dots. a Bloxplots are showing the perioperative time course of high-sensitive troponin T within the first 3 days separately for the groups. b The violin plots show the distribution of maximum high-sensitive troponin T concentrations within the first three postoperative days separately for the groups. The p value of the two-sided Wilcoxon-Rank-Sum test to compare treatment with placebo was 0.085.
We found no significant difference in postoperative maximum troponin T concentrations between the groups (p = 0.085) (Table 3). Detailed results of the median regression model are presented in Supplementary Table 6 and median regression model of the time course of troponin T between the groups are in Supplementary Table 7 in the Supplementary.
Exploratory endpoint
We found no statistically significant difference in the incidence of major cardiovascular adverse events (MACE) within 30 days after surgery between the groups (p = 0.245) (Table 3). No significant treatment effect was observed in the logistic regression model (OR 0.352, 95% CI 0.044 to 1.826; p = 0.245). Detailed description of the logistic regression model is shown in Supplementary Table 8 in the Supplementary.
Due to the high incidence of MINS we post-hoc analyzed the association between MINS and the composite outcome. We added MINS as a confounder in the median regression analysis for the composite outcome. We did not find a significant association between MINS and the occurrence of MACE (p = 0.919). All other complications, side effects and arrhythmias are shown in Table 4.
We observed no significant difference in the number of administered catecholamines and the dose of administered catecholamines between patients, who with preoperative betablocker therapy and patients without preoperative betablocker therapy. Detailed description of the post-hoc analyses are shown in Supplementary Tables 9 and 10 in the Supplementary.
Only 42 patients required catecholamines during the postoperative stay, whereas in 29 patients the cumulative dose was lower than 1 mg (19 patients in the levosimendan group and 10 patients in the placebo group, p = 0.074). Six patients in the levosimendan group received more than 1 mg of noradrenaline within 24 h (1.6 mg ± 0.4) as compared to 7 patients in the placebo group received more than 1 mg of noradrenaline within 24 h (1.8 mg ± 0.5).
Duration of study drug administration and dose changes are presented in Supplementary Table 11 in the Supplementary.
Discussion
In this randomized, double-blind, placebo-controlled, phase III, trial in cardiovascular risk patients undergoing moderate- to high-risk major noncardiac surgery, the administration of levosimendan did not lead to a significant lower release in postoperative NT-proBNP compared to placebo. There was also no statistically significant difference in the incidence of MINS, postoperative troponin T concentrations, and the incidence of postoperative cardiovascular complications.
Natriuretic peptides are independent and strong predictors for postoperative cardiovascular complications and identifiers for asymptomatic pancardiac organ damage3,15. Several studies showed that postoperative NT-proBNP concentrations are commonly elevated after noncardiac surgery, irrespectively of the patient population, indicating increased myocardial strain in the immediate postoperative period2,3,6,16. Therefore, we assumed that pre-emptive levosimendan increases myocardial function and finally counteracts acute myocardial strain occurring immediately after surgery. Nevertheless, in contrast to the SURVIVE and the REVIVE I & II trials, levosimendan did not lead to a significant lower maximum postoperative NT-proBNP release13,14. However, we observed significantly lower NT-proBNP concentrations on the third and fifth postoperative day in the levosimendan group. Besides hemodynamic perturbations or general anesthetics, postoperative inflammation is also a trigger factor for BNP release17,18,19,20. The association between inflammation and BNP release was revealed in a study by Fish-Trotter et al., who analyzed data from two large retrospective studies and one observational study in healthy volunteers, who were exposed to lipopolysaccharide to induce inflammation17. The authors have shown that increased inflammation is associated with higher natriuretic peptide concentrations17. In this context, it might be possible that increased postoperative inflammation triggers postoperative subclinical heart failure, which is asymptomatic but can be detected by BNP measurements. Thus, our hypothesis is that preemptive perioperative levosimendan might mitigate inflammation-induced subclinical heart failure and prevent further deterioration reflected by lower NT-proBNP concentration on the third and fifth postoperative day. Nevertheless, the clinical meaningful impact of preemptive levosimendan on postoperative long-term cardiovascular outcome has to be tested in large-scale studies.
In the CHEETAH trial (Levosimendan for hemodynamic support after cardiac surgery) and the LICORN trial (Effect of Levosimendan on low cardiac output syndrome in patients with low ejection fraction undergoing coronary artery bypass grafting with cardiopulmonary bypass) perioperative Troponin concentration was not affected by perioperative levosimendan administration21,22. Although we also did not observe any statistical significant differences in our study, Troponin T concentrations and MINS were noticeably higher in the levosimendan group. An explanation for these results could be the dosage of levosimendan in our trial. In contrast to the CHEETAH trial, in which an infusion dose of 0.05 mcg kg−1 min−1 was used, and the LICORN trial, in which an infusion dose of 0.1 mcg kg−1 min−1 was used, we administered levosimendan in the maximum dose of 0.2 mcg kg−1 min−121,22. Thus, it seems likely that the administration of the maximum infusion rate of levosimendan resulted in a more marked myocardial oxygen-perfusion mismatch due to enhanced inotropy, which finally led to a higher Troponin T release.
Since the number of MINS was higher in the levosimendan group, we assumed a higher incidence of major cardiac complications. In contrast to previous studies, MINS was not significantly associated with 30-day cardiovascular complications in our post-hoc analysis23,24. A possible explanation might be that although the criteria for MINS were fulfilled, troponin T increase was relatively low, and thus not clinically meaningful. Interestingly, the incidence of major cardiovascular complications was 6% lower in the levosimendan group, but did not reach statistical significance. In this context, we also observed significantly lower NT-proBNP concentrations on the third and fifth postoperative day. This can be interpreted that pre-emptive levosimendan possibly improves myocardial function helping to compensate increased postoperative myocardial strain, which finally reduces the risk for major cardiovascular complications. Again, this was not statistically significant and must thus be interpreted as preliminary. To confirm this observation, a large, randomized trial is still necessary.
Our trial has some limitations. Firstly, we defined postoperative maximum NT-proBNP as our primary endpoint. Since this was a phase III trial, we used NT-proBNP to assess postoperative myocardial function in noncardiac surgical patients. Of course, the effect on major cardiovascular complications is regarded to be more clinically relevant, but based on our observed incidence, a sample size of more than 1600 patients would have been needed, which is unfeasible for a single-center.
Secondly, we excluded patients with a preoperative ejection fraction of <30% since the incidence of patients with severely reduced ejection fraction undergoing noncardiac surgery is very low and those patients receive levosimendan a day before surgery for myocardial optimization as a standard of care in our institution. As indicated in the SURVIVE I and REVIVE I & II, it might very well be, that those patients benefit most from a perioperative treatment with levosimendan, but this must be tested seperately13,14.
Third, a possible limitation is the timepoint of starting the study drug infusion, which was at skin incision and not as previously described, administered a day before surgery25. We decided on this approach for two reasons. Firstly, for feasibility because of the limited capacity for preoperative monitoring positions to administer the study infusion prior to surgery. Also, in daily practice, restrictions of time and space might constitute a major barrier for systematically implementing preoperative levosimendan treatment in high-risk patients. Secondly, highest free plasma concentrations of the active metabolites of levosimendan are reached approximately 72 h after starting the infusion, which corresponds well to the previously observed postoperative peak in cardiac biomarkers26. Therefore, we assumed that levosimendan infusion would have a beneficial effect at this point in time as well.
Finally, there was an imbalance of baseline comorbidities between our groups. Incidence of coronary artery disease was 9%, myocardial infarction was 6%, and previous revascularizations were 9% higher in the placebo group. However, we included those factors in the regression analysis and did not observe a significant effect on our outcomes of interest, which, however, could be explained by the relatively small sample size and the low number of incidences.
In conclusion, in cardiovascular risk patients undergoing noncardiac surgery a perioperative treatment with levosimendan did not lead to a lower release in postoperative maximum NT-proBNP as compared to placebo. We found also neither a significant nor a clinically meaningful effect on the postoperative incidence of MINS or Troponin T release. In fact, we observed no statistically significant increase in side effects and clinically meaningful complications. In contrast, we observed a slight, but not significant, lower incidence of cardiovascular complications within 30 days after surgery. Therefore, perioperative levosimendan might be a promising prophylactic treatment strategy in cardiovascular high-risk patients undergoing major noncardiac surgery. To clarify if the preemptive administration of levosimendan leads to a reduction of postoperative major cardiovascular complications after noncardiac surgery large-scale trials are needed.
Methods
The trial was approved by the Institutional Review Board of the Medical University of Vienna on 14th July 2020 and was registered at ClinicalTrials.gov (NCT04329624) and the European Clinical Trial Database (EudraCT 2019-04464-22). The trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice and the authors have followed the applicable CONSORT guidelines. The protocol was published previously and presented in the Supplementary Note 17.
Study drug was provided partly by the manufacturer (Orion Corporation, Finland). Orion Corporation had no role in the trial design, data collection and analysis, writing the manuscript, or decisions regarding manuscript submissions. There were no payments from Orion Corporation.
Patients
Patients undergoing major noncardiac surgery planned for longer than 2 h and overnight stay, who were ≥65 and ≤85 years of age, provided written informed consent, and met at least two inclusion criteria (Appendix B in the Supplementary), were eligible for participation. Our exclusion criteria are also listed in Appendix B. Patients were screened a day before surgery according to the surgery schedule. Patients were enrolled after giving written informed consent. Definitions of surgeries are listed in Appendix C in the Supplementary.
Conduct of trial
This prospective double-blinded, parallel-arm, phase III, randomized trial was conducted at the Medical University of Vienna. Patients, investigators, clinicians, and outcome assessors were blinded. Patients were randomly assigned to receive a continuous infusion of a total dose of 12.5 mg (solved in 500 mL 5% glucose solution) of levosimendan or placebo in a 1:1 ratio without stratification. Study drug infusion was started at skin incision with 0.48 mL kg−1 min−1, which is equivalent to 0.2 mcg kg−1 h−1 without a loading dose and was administered until the end of the 500 mL. In cases of clinical meaningful hypotension, defined as requiring more than 0.1 mcg kg−1 min−1 noradrenaline to maintain a mean arterial pressure of at least 65 mmHg without signs of hypovolemia, bleeding, or too deep anesthesia, the infusion rate was decreased by 0.05 mcg kg−1 h−1. When blood pressure stabilized, the infusion rate of study drug was increased by 0.05 mcg kg−1 h−1 up to a maximum rate of 0.2 mcg kg−1 h−1. This was repeated if necessary.
Randomization and study drug preparation were performed by an unblinded trained study nurse, who had no further involvement in the trial. Study drugs were stored between 2 °C and 8 °C in locked refrigerators until use according to the investigator brochure. Vials containing 12.5 mg levosimendan in 5 mL or riboflavin sodium phosphate 0.4 mg (to receive identical the color to levosimendan) for placebo, were diluted in 500 mL 5% glucose solution by a trained study nurse and provided to the attending anesthesiologist.
We used the online randomization program “Randomizer” (Randomizer, Medical University of Graz, Graz, Austria) provided by the Medical University of Vienna and used permuted blocks randomization. Randomization was performed within 1 h before surgery7.
Primary and secondary endpoints
The primary endpoint was postoperative maximum NT-proBNP concentrations within the first three postoperative days. The secondary endpoints were the incidence of MINS and postoperative maximum troponin T concentrations. MINS was defined as follows: a high-sensitivity troponin T of 20 to <65 ng L−1 with an absolute change of at least 5 ng L−1 or high-sensitivity troponin T ≥65 ng L−1 23.
Our exploratory endpoint was major cardiovascular complications (MACE) including myocardial infarction, stroke, and death within 30 days after surgery. Detailed definitions of perioperative complications and outcomes are listed in Appendix D in the Supplementary. The 12-item WHODAS 2.0 and the risk stratification of maximum NT-proBNP concentrations will be published within the 1-year follow-up analysis.
Measurements
Intraoperative hemodynamic parameters and blood gas analysis (measured hourly during surgery) were recorded. All patients received continuous arterial blood pressure monitoring until the end of the study drug infusion for approximately 24 h in post-anesthesia care unit and intensive care unit, respectively. Plasma NT-proBNP and troponin T concentrations were measured preoperatively, within 2 h after surgery and on the first, second, third and fifth postoperative day or discharge occurred first. Measurements on the fifth postoperative day were performed for safety reasons (required by our IRB) were presented but not part of the outcome. We measured heart rate, and blood pressure, and performed an ECG on the first, second, third and fifth postoperative day.
Any adverse events and cardiovascular complications were evaluated during study visits in person. A phone follow-up and a chart review were performed on the 30th day after surgery. Any complication during hospital stay or the first 30 postoperative days were evaluated and recorded.
Sample size estimation
For sample size estimation, we used data from a previous study, in which NT-proBNP concentrations were measured within 1 h after surgery and on the first and third postoperative day6. The maximum of these values was calculated. We assumed, based on a previous study in cardiac patients, that levosimendan would reduce postoperative median maximum NT-proBNP concentration by 37% 14. To estimate the power, the distribution of the z-approximations of the Wilcoxon test statistics was estimated using the bootstrap method. The data for the control group was sampled from the previous data available. The data for the treatment group was sampled from the same data set, where values were reduced by 37%. We considered a drop-out rate of 5%. With a total sample size of 230 (115 per group, with a planned interim analysis after 38 patients per group) the overall estimated power (i.e. the power to reject either at interim or at the final analysis) is 80%.
Statistical analysis
For description of the data, descriptive statistics were used: mean, standard deviations (SD), median 1st quartile (Q1), 3rd quartile (Q3) as well as minimum, maximum, and sample size (N) were calculated for continuous variables; number and percentages were calculated for categorical variables.
The primary endpoint, maximum NT-proBNP concentration within the first three postoperative days, was first compared between the two groups using Wilcoxon rank-sum test. As sensitivity analyses, a median regression model (due to possibly skew distribution of maximum NT-proBNP concentration) was performed for the primary endpoint, accounting for treatment (placebo or levosimendan), age, sex, baseline NT-proBNP concentration, baseline troponin T, BMI, history of coronary artery disease (CAD; Yes vs. No), peripheral artery disease (PAD; Yes vs. No), cerebrovascular disease (CVD; Yes vs. No), arterial fibrillation (AF; Yes vs. No), nephrectomy (Yes vs. No), preoperative use of betablockers (Yes vs. No), and angiotensin-converting-enzyme inhibitors (ACE-I)/angiotensin receptor blockers (ARB) (Yes vs. No). To investigate the time course of NT-proBNP in more detail, a median regression model with random factor subject for NT-proBNP was calculated accounting for the factors treatment, visit and the interaction between treatment and visit as well as including age, sex, baseline troponin T, CAD, PAD, CAD, AF, nephrectomy, preoperative use of betablockers (Yes vs. No), and angiotensin-converting-enzyme inhibitors/angiotensin receptor blockers (Yes vs. No) as potential confounding factors in the model.
The secondary endpoint maximum troponin T was analyzed similarly to the primary endpoint. The secondary endpoint MINS within the first three postoperative days (binary endpoint) was first investigated using chi-square test. Furthermore, a logistic regression model for MINS was performed accounting for treatment, age, sex, baseline NT-proBNP concentration, baseline troponin T concentration, BMI, CAD, PAD, CAD and AF. The exploratory endpoint the composite of complications (binary endpoint) was investigated similar to MINS.
We further post hoc analyzed the effect of preoperative beta-blocker treatment on the effect on intraoperative catecholamine use. We also analyzed the number and dose of catecholamines used during the first 24 h after surgery.
The interim analysis was planned and performed after enrollment of 75 patients (1 drop out). To control for the overall type 1 error rate of 0.05 due to the planned interim analysis, O´Brien- Flemming boundaries for group sequential designs were used; hence, for the interim analysis, the two-sided significance level was 0.0007 and for the group comparisons, the two-sided significance level was 0.0497. A p value < 0.05 was considered as statistically significant for the secondary and explorative outcomes. Due to the lack of meeting the pre-specified boundaries and lack of a significant difference in adverse events between the groups, the data safety and monitoring board led to a decision to continue the study.
All analyses were performed using R release 4.2.2.
Data management
Blinded research personnel obtained all data. All data were recorded and stored in the data management system ‘Clincase‘ Version 2.7.0.12 hosted by IT Systems & Communications, Medical University of Vienna, Vienna, Austria.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The study protocol is available within the Supplementary Information. Data supporting the findings of this study are available in the article, its Supplementary Information, and the source data file. The raw clinical data are available under restricted access for academic use, within the limitations of the provided informed consent and under the General Data Protection Regulation law, access can be obtained by request to the corresponding author. All data requests will be reviewed by the Ethics Committee of the Medical University of Vienna and the Data Clearing House of the Medical University of Vienna and must be supported by the Principal Investigator of the study. The researcher will need to sign a data access agreement. Data will be available until publication of two pre-planned sub-studies (at least 6 months after publication of the main trial) for at least 5 years. We aim to provide an initial response to data access requests within 3 weeks. Source data are provided with this paper.
References
Smilowitz, N. R. et al. Perioperative major adverse cardiovascular and cerebrovascular events associated with noncardiac surgery. JAMA Cardiol. 2, 181–187 (2017).
Rodseth, R. N. et al. Postoperative B-type natriuretic peptide for prediction of major cardiac events in patients undergoing noncardiac surgery: systematic review and individual patient meta-analysis. Anesthesiology 119, 271–283 (2013).
Rodseth, R. N. et al. The prognostic value of pre-operative and post-operative B-type natriuretic peptides in patients undergoing noncardiac surgery: B-type natriuretic peptide and N-terminal fragment of pro-B-type natriuretic peptide: a systematic review and individual patient data meta-analysis. J. Am. Coll. Cardiol. 63, 170–180 (2014).
Payne, C. J. et al. B-type natriuretic peptide predicts long-term survival after major non-cardiac surgery. Br. J. Anaesth. 107, 144–149 (2011).
Álvarez Zurro, C. et al. High levels of preoperative and postoperative N terminal B-type natriuretic propeptide influence mortality and cardiovascular complications after noncardiac surgery. Eur. J. Anaesthesiol. 33, 444–449 (2016).
Reiterer, C. et al. Perioperative supplemental oxygen and NT-proBNP concentrations after major abdominal surgery—a prospective randomized clinical trial. J. Clin. Anesth. 73, 110379 (2021).
Reiterer, C. et al. Effect of perioperative levosimendan administration on postoperative N-terminal pro-B-type natriuretic peptide concentration in patients with increased cardiovascular risk factors undergoing non-cardiac surgery: protocol for the double-blind, randomised. BMJ Open 12, 1–11 (2022).
Papp, Z., Csapó, K., Pollesello, P., Haikala, H. & Édes, I. Pharmacological mechanisms contributing to the clinical efficacy of levosimendan. Cardiovasc. Drug Rev. 23, 71–98 (2005).
Haikala, H. et al. Cardiac troponin C as a target protein for a novel calcium sensitizing drug, Levosimendan. J. Mol. Cell Cardiol. 27, 1859–1866 (1995).
Ukkonen, H. et al. Myocardial efficiency during levosimendan infusion in congestive heart failure. Clin. Pharm. Ther. 68, 522–531 (2000).
Lilleberg, J. et al. Duration of the haemodynamic action of a 24-h infusion of levosimendan in patients with congestive heart failure. Eur. J. Heart Fail. 9, 75–82 (2007).
Lilleberg, J. M., Sundberg, S., Leikola-Pelho, T. & Nieminen, M. S. Hemodynamic effects of the novel cardiotonic drug simendan: echocardiographic assessment in healthy volunteers. Cardiovasc Drugs Ther. 8, 263–269 (1994).
Packer, M. et al. Effect of levosimendan on the short-term clinical course of patients with acutely decompensated heart failure. JACC Heart Fail. 1, 103–111 (2013).
Mebazaa, A. et al. Levosimendan vs dobutamine for patients. J. Am. Med. Assoc. 297, 1883–1891 (2007).
Struthers, A. & Lang, C. The potential to improve primary prevention in the future by using BNP/N-BNP as an indicator of silent “pancardiac” target organ damage: BNP/N-BNP could become for the heart what microalbuminuria is for the kidney. Eur. Heart J. 28, 1678–1682 (2007).
Reiterer, C. et al. A comparison of intraoperative goal-directed intravenous administration of crystalloid versus colloid solutions on the postoperative maximum N-terminal pro brain natriuretic peptide in patients undergoing moderate-to high-risk noncardiac surgery. BMC Anesthesiol. 20, 1–9 (2020).
Fish-Trotter, H. et al. Inflammation and circulating natriuretic peptide levels. Circ. Heart Fail. 13, 135–144 (2020).
Devereaux, P. J. & Sessler, D. I. Cardiac complications in patients undergoing major noncardiac surgery. N. Engl. J. Med. 373, 2258–2269 (2015).
Devereaux, P. J. et al. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ 173, 627–634 (2005).
Hannon, J. D., Cody, M. J., Sun, D. X. & Housmans, P. R. Effects of isoflurane and sevoflurane on intracellular calcium and contractility in pressure-overload hypertrophy. http://pubs.asahq.org/anesthesiology/article-pdf/101/3/675/356184/0000542-200409000-00016.pdf (2004).
Landoni, G. et al. Levosimendan for hemodynamic support after cardiac surgery. N. Engl. J. Med. 376, 2021–2031 (2017).
Cholley, B. et al. Effect of levosimendan on low cardiac output syndrome in patients with low ejection fraction undergoing coronary artery bypass grafting with cardiopulmonary bypass. JAMA 318, 548 (2017).
Devereaux, P. J. et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. J. Am. Med. Assoc. 317, 1642–1651 (2017).
Devereaux, P. J. et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. J. Am. Med. Assoc. 307, 2295–2304 (2012).
Putzu, A., Clivio, S., Belletti, A. & Cassina, T. Perioperative levosimendan in cardiac surgery: a systematic review with meta-analysis and trial sequential analysis. Int. J. Cardiol. 251, 22–31 (2018).
Kivikko, M., Antila, S., Eha, J., Lehtonen, L. & Pentikäinen, P. J. Pharmacokinetics of levosimendan and its metabolites during and after a 24-hour continuous infusion in patients with severe heart failure. Int. J. Clin. Pharmacol. Ther. 40, 465–471 (2002).
Acknowledgements
We thank all the patients for participating in this study. We thank our study team members S. Schallmeiner, M. Morgenroth, V. Xu, T. Marr and G. Vitousek for the support in data collection. We thank our sponsor, the Medical University of Vienna. We thank Orion Pharma for partly funding the study medication. Orion Pharma had no role in data collection, data analysis, data interpretation, or writing of the report.
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, funding acquisition, data interpretation, writing—original draft, writing—review & editing of the final draft: C.R. Conceptualization, methodology, supervision, visualization, data interpretation, writing—original draft, writing—review & editing of the final draft: B.K. and E.F. Formal analysis, final analysis, visualization, writing—review & editing of the final draft: A.G. Data acquisition, investigation, writing—review & editing of the final draft: A.T., D.E., O.Z., N.H., B.H., N.A., and K.H. Data acquisition, software, data management, writing—review & editing of the final draft: M.F. Investigation, data management, writing—review & editing of the final draft: T.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Alessandro Belletti and the other, anonymous, reviewer for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Reiterer, C., Kabon, B., Taschner, A. et al. Levosimendan for postoperative subclinical heart failure after noncardiac surgery: a randomized, double-blinded, phase III trial. Nat Commun 16, 5847 (2025). https://doi.org/10.1038/s41467-025-60601-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-60601-y