Abstract
The many successes of AlphaFold2 (AF2) have inspired methods to predict multiple protein conformations, many of which have biological significance. These methods often assume that AF2 relies on evolutionary couplings to predict alternative protein conformations, but they perform poorly on fold-switching proteins, which remodel their secondary structures and modulate their functions in response to cellular stimuli. Here we present a method designed to leverage AF2’s learning of protein structure more than evolutionary couplings. This method–called CF-random–outperforms other methods for predicting alternative conformations of not only fold switchers but also dozens of other proteins that undergo rigid body motions and local conformational rearrangements. It also enables predictions of fold-switched assemblies unpredicted by AlphaFold3. Several lines of evidence suggest that CF-random sometimes works by sequence association: relating patterns from homologous sequences to a learned structural landscape. Through a blind search of thousands of Escherichia coli proteins, CF-random suggests that up to 5% switch folds.
Similar content being viewed by others
Introduction
Alternative protein conformations can play critical roles in protein function and regulation1,2,3. These alternative conformations can be accessed by rigid body reorientations, local fluctuations, or remodeling secondary and/or tertiary structure (fold switching)4. Physics-based methods, such as molecular dynamics (MD) simulations, have successfully modeled alternative conformations5,6,7,8, but they require too much computational power to predict conformational changes on a large scale. Furthermore, some conformational changes, such as fold switching, occur on a timescale of seconds9,10,11, prohibitively long for MD to reasonably access alternative conformations if they were not known previously. Recently, artificial intelligence (AI)-based protein structure predictors–particularly AlphaFold2 (AF2)12–have offered another way to predict large numbers of alternative protein conformations13,14,15,16,17: modifying or subsampling the inputted multiple sequence alignment (MSA), from which AF2 may infer evolutionarily coupled residue pairs used to predict structure15. These MSA modifications are hypothesized to diminish dominant residue-residue couplings13,18 while sometimes enhancing the couplings of conformational alternatives16,17. However, a recent study found that these methods usually fail on the alternative conformations of 92 experimentally characterized fold-switching proteins likely in AF2’s training set with individual false negative failure rates from 80-93%19.
Here, we present CF-random20, an alternative strategy to predict alternative protein conformations. This strategy leverages ColabFold21 (CF)–an efficient-yet-accurate implementation of AF2–to predict alternative conformations by randomly subsampling input MSAs at depths too shallow for robust coevolutionary inference. While CF-random was shown to perform well on eight fold-switching proteins20, its generalizability was not tested, its inner-workings were not explained, and its implementation was not automated. Here, we address all three of these issues by (1) testing CF-random on 92 fold-switching proteins and 37 other proteins that undergo local conformational fluctuations and rigid body motions, (2) showing that CF-random sometimes works by sequence association: relating patterns from homologous sequences to a learned structural landscape, and (3) providing an automatic implementation. We find that CF-random outperforms all methods reported previously. Compared to the 7−20% success rates of other individual methods for fold-switching proteins, CF-random achieved a 35% success rate while generating 6x fewer structures overall. Further, CF-random captured rigid body reorientations and local conformational fluctuations of the 37 other proteins with a 95% success rate and considerably less sampling than other methods. Encouraged by its success, we develop CF-random to perform blind searches for alternative conformations and run it on >2000 proteins from Escherichia coli. These predictions suggest that up to 5% of E. coli proteins switch folds. We present CF-random as a tool for community use, specifying its strengths and limitations.
Results
CF-random outperforms other AF-based predictors of fold switching
CF-random is a ColabFold-based pipeline that generates putative conformational ensembles by combining predictions from deep and shallow MSA sampling (Fig. 1, Methods). AF2-based methods, such as ColabFold, are known to generate one dominant conformation of fold-switching proteins from deep MSAs22. Thus, the challenge is to sample the alternative conformation. CF-random aims to overcome this challenge by sampling very shallow random input MSAs with as few as 3 sequences. Shallow sampling directs the AF2 network to predict structures from sparse sequence information insufficient for robust coevolutionary inference, setting it apart from previously proposed methods that used a minimum of 24 sequences14,23. Because some predictions are not well folded at shallow MSA sampling depths, CF-random also explores deeper depths. Typically, shallow sampling occurs at seven depths including between 3 and 192 sequences. Template modeling (TM)-scores24 of the fold-switching regions of each prediction are compared to their experimentally determined structures, since this has been shown to discriminate between fold switchers better than overall TM-score19, though the overall score is considered also. We report sampling depth with the following notation: x:y, where x is the value assigned to ColabFold’s –max-seq argument (the number of sequences randomly selected as cluster centers) and y is the value assigned to –max-extra-seq, the number of extra sequences randomly sampled from the clusters is defined by –max-seq. The total number of sequences inputted into ColabFold at each recycling step is x + y.
CF-random (ColabFold-based random MSA sampling) generates dominant and alternative protein structures by combining ColabFold predictions generated from a deep multiple sequence alignment (MSA) and shallow random MSAs, respectively. To run default mode, a full-length MSA is required. ColabFold samples the MSA at default depth (512:5120) to produce the dominant conformation and then randomly subsamples at shallower depths to predict putative alternative conformations. It was benchmarked against known fold-switching proteins by calculating the TM-scores of all predicted structures against two reference structures. Success was considered correct predictions of both conformations from a single target sequence. Source data are provided as a Source Data file.
We tested CF-random on 92 fold-switching proteins likely in AF2’s training set and found that it predicts both the dominant and alternative conformations of 32 fold switchers successfully. Further, CF-random sampled 89% fewer structures than other AF2-based methods (Fig. 2a), which predicted 25 fold switchers altogether. To explore what AF2 has learned about alternative conformations of fold switchers, all predictions were performed without templates. Very shallow sequence sampling was a key to CF-random’s success: 23 conformations (72%) were successfully predicted at sampling depths of 4:8 sequences or below (Fig. 2b). To our knowledge, random sequence sampling at such shallow depths has not been tested systematically; previous work suggested a minimum sampling depth of 8:1614,23.
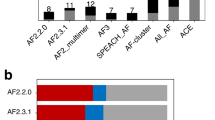
a CF-random performs more successfully and efficiently than SPEACH-AF, AF-cluster, and AF2 with no templates. b Most alternative conformations can be predicted successfully using very shallow sampling depths (those to the left of the dotted line comprise 72% of all predictions). In two cases, sampling at 1:2 produced homogenous well-folded structures similar to the dominant conformation, in which case single sequences were also tested (single). c Successful predictions of three fold-switching proteins not predicted by the other methods: human XCL1, human TRAP1N, and Escherichia coli (E.coli) RepE. Gray represents the reference PDB structures and single-folding regions; blue and pink indicate the fold-switching regions of dominant and alternative structures, respectively. Source data are provided as a Source Data file.
CF-random successfully predicts both global and local fold-switching events, some of which had not been predicted successfully by other methods (Fig. 2c). For instance, CF-random successfully predicts both conformations of human XCL1, which have distinct hydrogen bonding networks and hydrophobic cores25. Though both predictions correspond well with experimentally determined structures, CF-random mispredicts that a disordered C-terminal region of dimeric XCL1 folds into a helix, consistent with other reports that AF2 sometimes incorrectly predicts disordered regions as helices26. CF-random also predicts two conformations of TRAP1-N. This N-terminal domain of a human mitochondrial heat shock protein assumes different conformations in its apo, GTP-, and GDP-bound forms27. The alternative autoinhibitory apo form has a conserved glutamine that binds to the ATP-binding lid region and is proposed to be an on-pathway conformation important for TRAP1N’s function as a protein folding chaperone. The dominant ATP-open form has not been observed to promote protein folding. Finally, RepE, a DNA replication initiator protein from E. coli, has two folds with distinct functions. As a monomer, it functions as a replication initiator whereas the dimeric form functions as a repressor. Its dimeric form is dominant under physiological conditions; the chaperone DnaK mediates its conversion to a monomer28. CF-random predicts its monomeric form from full MSAs and its dimeric form at a very shallow sampling depth (2:4, Fig. 2c).
Combining AF2-multimer with CF-random sampling improves some predictions
Of the 32 successful predictions of fold switchers, six were improved from additional molecular context supplied by the AF2-multimer model. For instance, CF-random predicted a monomer of FraC–a pore-forming toxin from Actinia fragacia–to have its N-terminal helix detached from the rest of the protein, similar to the pore form29 (Supplementary Fig. 1a). Inputting its sequence into AF2-multimer and sampling at the shallowest depth found to produce folded predictions yielded a structure highly consistent with experiment (1.4 Å, Fig. 3a). Interestingly, neither sampling deeper MSAs nor using the AlphaFold330 (AF3) server produced this conformation (Fig. 3a). Furthermore, though AF2-based predictors often struggle to predict amyloid structures31,32, CF-random produced an amyloid-like structure of the human Aβ42 peptide (Supplementary Fig. 1b). Running the multimer model on its sequence at the shallowest depth possible (1:2) yielded a fibril-like conformation consistent with experiment (Fig. 3b). While the morphology of the overall fibril was not completely correct–the experimentally determined structure involved three chains while the prediction involved two in a similar configuration–the prediction suggests that an amyloid-like configuration is possible. Deep-MSA sampling with the AF2 multimer model did not produce amyloid-like fibrils consistent with those in the PDB by a Foldseek33 search, and AF3 predicted a different fibril morphology also loosely consistent with experiment (Fig. 3b). Additionally, CF-random predicted a partially folded conformation of the cell cycle regulatory protein Cks1 from S. cerevisae consistent with its functionally relevant domain-swapped dimer34 (Supplementary Fig. 1c). Running the AF2 multimer model on both deep and shallow MSAs produced an experimentally consistent dimer orientation, while AF3’s was inconsistent (Fig. 3c). The multimer model also enhanced promising predictions of the domain-swapped conformation of human CrkL-SH3C domain (not predicted by AF3) and domain-swapped Escherichia coli rhomboid protease and E. coli FimF (both predicted by AF3). After running CF-random with multimer weights on all 92 fold-switching proteins, only one additional alternative conformation was found: the dimeric form of bone morphogenic protein inhibitor DAN from Mus musculus (PDB ID: 4jph). It was not included among the successes since it was not identified when CF-random was run on a single protein chain.
By contrast, AlphaFold3 (AF3) predicted some assemblies incorrectly but with high confidence. For CF-random, predictions are pink, denoting alternative conformations, while AF3 predictions are colored by plDDT. Experimentally determined predictions are gray with PDB IDs: 4TSY (FraC), 6RHY (Aβ42, above), 2MVX (Aβ42, below), 1QB3 (Cks1). Experimentally determined structures are gray. Source data are provided as a Source Data file.
CF-random predicts rigid body motions and local conformational changes more efficiently than other AF-based methods
CF-random’s performance on alternative conformations of fold switchers raises the question of how well it predicts other conformational changes. To address this question, we tested it on two other datasets used to assess how well AF2-based methods predict alternative conformations. The first was a dataset of 14 proteins, 2 soluble proteins and 12 membrane transporters used to benchmark two previous methods14,16. Like CF-random, one of these two methods (SPEACH-AF) did not use templates to make predictions. SPEACH-AF works by making in silico alanine mutations to columns of multiple sequence alignments (MSAs) corresponding to groups of residues in direct contact. The idea is that these alanine mutations mask dominant evolutionary couplings used for structural inference, allowing AF to detect alternative couplings invisible in the full unmasked MSA16. The other dataset contained 23 proteins with well-defined open and closed conformations (OC23), such as periplasmic binding proteins, used to benchmark another method called AFSample213. Like SPEACH-AF, AFSample2 also masks columns of MSAs but randomly rather than targeting residues in direct contact. It also enables dropout, which set some of AF2’s weights to 0 during inference, making it easier for the network to sample uncertainties and generate more diverse structures.
CF-random sampled alternative conformations more efficiently than both methods while producing accurate predictions. For instance, it successfully captured local conformational fluctuations in the “LID” and “AMP-binding” domains of Escherichia coli Adenylate Kinase (AK) with high accuracy (TM-scores of 0.98 and 0.9 for the dominant and alternative forms, respectively, Fig. 4a,b). Overall, CF-random captured both Fold1 and Fold2 of all SPEACH-AF targets with TM-scores ≥ 0.81 with considerably less sampling than SPEACH-AF: 200 structures/CF-random ensemble compared to 300-811 structures/SPEACH-AF ensemble (Fig. 4c,d, Supplementary Table 2). Similarly, CF-random captured the “cap-open” and “cap-closed” states of β-phosphoglucomutase (βPGM) with TM-scores of 0.97/0.99 for the dominant/alternative conformations (Fig. 4e,f). Overall, CF-random captured both Fold1 and Fold2 of all OC23 targets with TM-scores ≥ 0.7, except for Q9ERE7 (Fig. 4g); AFSample2 also failed to predict its conformations with high accuracy (Supplementary Table 3). However, CF-random successfully captured three other alternative conformations that AFSample2 failed to capture (Supplementary Table 3). Further, CF-random sampled 5x fewer models to predict alternative conformations compared to AFSample2 in 16/23 cases (Fig. 4h). In 6/7 remaining cases, sampling an additional 600 structures yielded high accuracy predictions of both conformations, 20% less sampling than AFSample2 (Fig. 3h).
a, e The open- and closed-conformations of Adenylate Kinase (AK) and β phosphoglucomutase (βPGM) were correctly predicted. Dominant/alternative conformations are blue/pink. b, f CF-random produces ensembles of AK and βPGM accurately and with high confidence, though some inaccurate lower confidence structures are produced also. c, g The highest TM-scores that CF-random produced are reported in these tables and compared with SPEACH-AF and AFSample2, respectively. d, h CF-random sampled substantially fewer structures than either SPEACH-AF or AFSample2. Source data are provided as a Source Data file.
Sequence association drives some predictions of alternative conformations
How does CF-random predict alternative conformations? Since AF2 predictions are often based on evolutionary couplings inferred from multiple sequence alignments (MSAs), it has been proposed that supplying varied MSAs can provide evolutionary restraints unique to alternative conformations15,17. Recent work indicates that this is not the case for rigid body motions and local conformational fluctuations, however18. Instead, conformations consistent with the same set of evolutionary restraints are sampled stochastically. Fold switching differs from rigid body motions and local conformational fluctuations because it involves remodeling of secondary structure, sometimes leading to alternative folds inconsistent with dominant evolutionary couplings20. Since AF2-based predictions of dominant fold-switch conformations are typically consistent with coevolutionary restraints from deep MSAs, we investigated whether successful AF2 predictions of fold switchers are driven by coevolutionary restraints unique to their alternative conformations.
Coevolutionary analysis of the MSAs that successfully produced each alternative fold-switched conformation revealed few unique alternative evolutionary couplings, indicating that predictions of alternative conformations of fold switchers are not generally driven by coevolutionary inference (Fig. 5a). In fact, these MSAs provide more information unique to dominant unpredicted conformations (mean percentage of total couplings, 11%) than alternative predicted conformations (mean percentage of total couplings, 8%). Thus, many of these predictions seem to be driven by AF2’s learning of protein structure rather than couplings unique to alternative conformations. Since previous work suggests that (1) AF2 struggles to predict the large conformational changes that many fold switchers undergo18 and (2) AF2 has memorized some alternative conformations of fold switchers19,20, we hypothesized that AF2 may generate alternative conformations of fold switchers through sequence association: relating features of homologous sequences to a learned structural landscape. This differs from coevolutionary inference, which can be used to infer protein structure without prior knowledge.
a Evolutionary couplings unique to dominant unpredicted structures are generally stronger than evolutionary couplings unique to predicted alternative structures. Each box-and-whisker plot contains 104 data points, with boxes representing interquartile ranges and the middle line representing the median. Whiskers extend to 1.5 times the interquartile range. b CF-random failed to predict the alternative conformation (3-α-helix bundle fold) of Sa1 using the multiple sequence alignment (MSA) with α/β-plait homologs only. c To test for sequence association, three different MSAs were generated containing the Sa1 sequence and a single 3-α-helix homolog over the protein data bank (PDB) search. In all three cases ColabFold (CF) predicted the helical bundle sequence with high confidence, indicating that they are predicted by sequence association. d When the MSA was rebalanced with 3-α-helix (orange and red sequences also represent 3-α homologs) and α/β-plait, CF-random successfully predicts both Sa1 structures. It predicted the α/β-plait from the full MSA and the 3-α-helix bundle with --max-seq = 2, --max-extra-seq = 4. Color bar representing AF2’s confidence metric, per-residue local distance difference test (plDDT) scores, applies to all structures with corresponding colors. Source data are provided as a Source Data file.
We used a recently characterized fold switcher, Sa1 V90T (Sa1 hereafter), to test whether AF2 may produce some alternative conformations through sequence association. Sa1 is a temperature-sensitive fold switcher that assumes a three-α-helix bundle fold at low temperature but switches to an α/β plait as temperature increases13. Some methods for predicting multiple conformations struggle to predict both folds of Sa119. Similarly, with default settings, CF-random predicts the α/β plait fold but not the helical bundle. Investigating, we noticed that although the initial iteration of sequence search for Sa1 yielded sequences unique to both folds, subsequent sequence-search iterations left its final MSA without three-α-helix bundle sequences (Fig. 5b).
We then searched the sequence of Sa1 against the PDB and found it to match 3 three-α-helix folds with sequence identities ranging from 42−70%; these sequences revealed that CF predicts the three-α-helix fold through sequence association. In further detail, we ran CF three times, inputting only one of the three PDB sequences in each run. In all three cases, CF confidently predicted three-α-helix folds from the two-sequence MSAs (Sa1 sequence+homolog, Fig. 5c). Since these two-sequence MSAs are too shallow for robust coevolutionary inference (Supplementary Fig. 2), it seems the best explanation for these predictive successes is sequence association. This approach is much like homology modeling but more powerful because only a single “template” sequence–rather than an input structure–is needed to produce the alternative conformation. Underscoring the explanatory power of this approach, it produces the helical bundle conformation more frequently (40-100% of the time from 20 models, depending on input sequence) than in previous work sampling 1000 structures with dropout and MSA masking (<2% of the time)13. Some of these three-helix-bundle predictions also contained a fourth C-terminal helix experimentally observed to be disordered, again demonstrating that sometimes AF2 sometimes mispredicts disordered regions as helical.
Leveraging these observations, we developed a strategy that enabled CF-random to predict both folds of Sa1 (Fig. 5d). First, Sa1’s MSA was rebalanced to contain hundreds of 3-α-helix bundle homologs but only 3 sequences homologous to the α/β-plait. Interestingly, sampling the modified MSA at full depth produced α/β-plait models with high confidence and RMSDs closer to the experimentally determined structure than the deep MSA with >1000 α/β-plait sequences. Since AF2 predicts the α/β-plait fold of Sa1 from a single sequence with high confidence (Supplementary Fig. 3), sequence association seems to be the best explanation for this prediction. Likewise, sampling at shallow depths such as 2:4 yields high-confidence and accurate predictions of the three-α-helix fold (Fig. 5d). Given the very shallow sampling depth and the observations in Fig. 5c, sequence association seems to be a sensible explanation for this prediction as well.
Blind predictions with CF-random
CF-random was further developed to predict new fold switchers without prior knowledge. This approach extends the algorithm by automatically selecting putative alternative conformations among the predicted models; the remainder of the algorithm is unchanged. In both previous work and this study, we noticed that correctly predicted alternative conformations of fold switchers are not always assigned confident scores by AF219, as measured by plDDT (per-residue local Distance Difference Test). For instance, the alternative fold of XCL1 is predicted with low confidence (plDDT <70) in most cases (Supplementary Fig. 4); low plDDT scores of alternative conformations have been observed in other work as well18,35. To circumvent this problem, we developed an approach to cluster predictions by structural similarity (Fig. 6). Although structural clustering can be straightforward for proteins whose alternative conformations differ substantially from dominant, it is more challenging when structural differences are localized to relatively small protein regions. To overcome this challenge, a Foldseek33 database of all structures predicted for a given target is constructed. This database is used to calculate an all against all similarity matrix for the structures using the Foldseek structural bitscore, which enables both subtle and large conformational differences between predicted structures to be distinguished (Methods). The similarity matrix is then reduced using Principal Component Analysis (PCA) followed by two different density-based scanning algorithms, finally yielding a subset of the full CF-random blind mode ensemble that represents the conformational variance of the predicted structures. Blind mode successfully detected both conformations of fold switchers in 81% of cases identified in default mode (26/32), corresponding to a 28% success rate among all 92 fold switchers. An example of a difficult-to-identify local conformational change is the active-site loop of inositol monophosphatase (IMPase) from Thermatoga maratima shown in Fig. 6. Well-folded predictions of both conformations are present in the yellow and green clusters. Structures from purple and blue clusters are not as well folded (Supplementary Fig. 5). Thus, user discretion is required to identify the most plausible alternative conformations among those suggested by blind mode.
A Foldseek database is created from CF-random generated structures (collectively called the ensemble). This database is used to evaluate each structure’s similarity to the ensemble. Contrasting default mode in Fig. 1, two reference structures are not required, enabling a blind search. Principal component analysis followed by two different clustering algorithms produce the final subset of CF-random conformations that represent the structural variance in the ensemble. From left to right: CF-random blind mode produces 200 predicted structures; all structures are queried against the generated database to create the similarity matrix. Predicted structures from T. maratima IMPase (PDB ID: 2P3V) are depicted within the similarity matrix representation. Principal component analysis and HDBSCAN then produce a reduced space with similar structures clustered near each other (scatter plot top right), and K-medoids selects representative structures from the HDBSCAN clusters (2P3V predicted structures for the green and yellow clusters are shown bottom left, purple and blue structures in Supplementary Fig. 5). Source data are provided as a Source Data file.
Blind predictions of fold-switching E. coli proteins
Finally, we tested CF-random’s blind mode on E. coli proteins–including some from bacteriophage–with 300 residues or fewer (Fig. 7a). This length limit makes them suitable for future experimental testing by nuclear magnetic resonance (NMR) spectroscopy, a method that has successfully identified fold-switching proteins previously36. To ensure maximum plausibility, predictions reported here were also subject to the following quality checks.
a We ran the sequences of 2126 E. coli and phage proteins through CF-random. In total 3237 sequences were attempted, but in 1111 cases, MSAs deep enough for coevolutionary inference could not be generated. Seashell-like image represents these 3237 proteins by length; the inner circle represents 1111 rejected candidates, and outer, the 2126 proteins that were then run through CF-random. If two or more distinct conformations were identified, such as in the case of the successfully identified fold-switching E. coli protein, RfaH, we tested for dual-fold coevolution using ACE. If coevolutionary evidence for both folds was found, the protein was considered a putative fold switcher. Light gray/black contacts on upper/lower diagonals are unique to CF-random predicted dominant/alternative conformations. Teal contacts are from coevolutionary inference. Medium gray contacts are unique to both folds. b Putative fold-switching proteins are involved in diverse functions. C Examples of putative hits. The phage tail tube protein is part of a large assembly that penetrates its host cell envelope. The dominant conformation predicts an extended β-sheet, while in the alternative form, the sheets are incorporated into the larger β-sheet structure. Both conformations were predicted with plDDT scores > 70. NinH is transcription factor protein that may undergo an α-helix-to-β-sheet transition; both conformations were predicted with plDDT scores > 70. Finally, YmcE is a bacterial antitoxin predicted to assume two different folds. Its dominant form was predicted with plDDT > 80, while its alternative was predicted with plDDT > 66 excluding disordered ends. Throughout this figure, dominant folds are blue and alternatives are pink. Source data are provided as a Source Data file.
(1). Coevolutionary evidence for both conformations. Both dominant and alternative protein conformations often have clear coevolutionary signatures37. As shown previously, AF2-based predictions, including those by CF-random, do not necessarily result from coevolutionary inference18,19,20. Predictions of alternative conformations without evolutionary basis can be incorrect20,37. Therefore, we searched for coevolutionary evidence of both conformations using Alternative Contact Enhancement (ACE), a method designed to identify evolutionary couplings unique to multiple protein conformations37. If such evidence was found, a prediction was considered plausible if condition (2) was met. We also note that at shallow depths, CF-random can predict spurious helical conformations. Cross-checking against coevolutionary information often helps to eliminate them. In cases where insufficient evolutionary couplings were present, we used our best judgement to assess the predictions.
(2). Ruling out interchain contact misassignment. Previous work has shown that AF3 can misassign intrachain contacts as interchain19. Here, we observe the opposite problem: sometimes AF2 assigns interchain contacts as intrachain (Supplementary Fig. 6), producing questionable alternative conformations. To circumvent this problem, we performed Foldseek searches on all hits produced by blind mode and examined their oligomeric states. If the target had a close homolog (≥70% sequence identity) with an oligomeric state whose interchain contacts overlapped with contacts unique to the predicted alternative conformation, the prediction was discarded under the assumption that it forms an oligomer and its interchain contacts were misassigned as intrachain, producing a spurious alternative conformation. In cases where a target did not have a close homolog with solved structure, but both of its conformations had coevolutionary support, it was reported. These cases may also conflate intrachain contacts with interchain but lacked evidence to support exclusion.
(3). Confidence ranking. Most confident (Tier 1) predictions satisfied criteria (1) and (2) while also being supported by experiment or having a strong biological basis for the conformational change. Tier 2 predictions satisfied criteria (1) and (2) without further experimental support.
With this approach, we ran CF-random on 2126 E. coli proteins, of which 52 were predicted to switch folds (Supplementary Table 4). Supporting the plausibility of these predictions, the estimated relative Rosetta energy scores38,39 between each pair of putative fold switchers were in the same range as fold switchers determined by experiment (Supplementary Fig. 8). Among the functionally annotated putative switchers, transcription/translation regulators were the most common, followed by toxin-antitoxin proteins, phage proteins, and structural assembly proteins (Fig. 7b). Toxin-antitoxin proteins and proteins of unknown function have not been observed to switch previously, while previous work has established that proteins in the other functional classes sometimes switch, especially transcription factors, which are enriched among fold switchers. Furthermore, the blind search identified some experimentally confirmed fold switchers, including RfaH and MinE, along with homologs of fimbrial proteins known to switch folds.
Three putative fold switchers are presented as examples (Fig. 5c, Supplementary Fig. 7). The phage tail tube protein is part of a large assembly that penetrates its host cell envelope. Its dominant form resembles the conformation of a homolog in its protein assembly, with protruding β-sheets that interact with its partners. Its alternative fold has its β-sheets tucked within the larger body of its structure and stabilized by hydrogen bonds. Though no similar PDB structures could be identified through a Foldseek search, this alternative conformation seems plausible given that similar behaviors in pore forming proteins have been observed40. Both conformations were predicted with plDDT scores ≥ 70. Second, NinH is a transcriptional regulator whose closest FoldSeek matches are Cro repressor proteins. The structures of Cro proteins have evolved over time, and switches have been engineered41,42. This, combined with the fact that several known fold-switching proteins are transcription factors4, supports the prediction. Finally, the bacterial antitoxin YmcE may also switch folds. This is the most speculative of the three predictions since few YmcE homologs were used to construct its MSA, and its evolutionary couplings were noisy. However, the hit is reported because (1) CF-random predicts that several bacterial antitoxins switch folds, and (2) YmcE is predicted to assume two dramatically different conformations.
Based on these predictions, we estimate that up to 5% of E. coli proteins switch folds. Of the 2126 proteins in the set, 52 are predicted to switch folds, a 2.4% hit rate. However, CF-random predicted only 22/47 of known fold-switching proteins with ≤300 residues. If an equal number was missed here, 5.2% would switch folds. Furthermore, 45/92 know fold-switching proteins have > 300 residues. Again, a similar ratio among E. coli proteins would suggest that up to 5% may switch folds.
Discussion
Though AlphaFold2 often predicts single protein conformations with high accuracy, predicting alternative conformations remains a challenge43. Some recently developed methods44,45 generate alternative conformations using new techniques such as the Distributional Graphoformer46 and flow matching46, though these have not been tested on many fold-switching proteins. A diffusion-based model called EigenFold was tested on fold switchers, but its performance was weak47. Several previous methods have relied on coevolutionary information to predict fold switching and other conformational changes, for both AlphaFold215 and ESMFold48,49. Here we show that leveraging AF2’s learning of protein structure–including sequence association–outperforms previous methods for predicting fold-switching proteins. Furthermore, this approach is more efficient than previous methods for predicting rigid body motions and local conformational rearrangements. The generalizability of CF-random suggests that leveraging AF2’s learning of protein structure offers more robust predictions of alternative conformations than coevolutionary inference of input MSAs, and sequence association may be a far-reaching explanation for how AF2 predicts alternative conformations of fold switchers.
CF-random has both advantages and limitations. First, it predicts some fold-switched conformations through AF2’s learning of protein structure rather than coevolutionary inference of input MSAs. To our knowledge, this is the first study showing that AF2 sometimes works by sequence association. Much like homology modeling, sequence association requires prior learning of protein structure. We suspect that this drives many CF-random predictions at very shallow sampling depths (4:8 and below). While shallow random sampling may foster CF-random’s efficiency, it may also impede predictions of alternative conformations very different from those in the training set. CF-random may make new associations between input sequences and structures in its training set, however, as it did with Sa1. Accordingly, it may successfully reveal that certain sequences assumed to be single folders can assume yet-undiscovered alternative conformations in the training set. Previous work indicates that AF2-based methods can incorrectly predict single folders as fold switchers20, however. This is a second notable limitation of CF-random: it may predict alternative conformations of proteins erroneously. For this reason, we suggest using alternative approaches–such as ACE37, AF2-RAVE50, or other MD-based approaches51–to cross-check predictions. Nevertheless, some predictions presented here may be incorrect, particularly those of proteins with unknown function. Thirdly, blind mode sometimes misses alternative conformations that CF-random predicts correctly.
Although CF-random outperforms other methods for predicting known fold switchers, it is a weak predictor (35% success rate), indicating that much work remains to be done in this challenging research area52. CF-random was run without templates and with much less sampling than other methods. More sampling and inclusion of templates may lead to a higher success rate, though we do not expect major improvements since most of the successful predictions reported here were also achieved by combining results from other AF2-based methods with a lot more sampling, and inclusion of templates yielded only a few more unique hits19. The CF-random approach may improve AF3’s performance on fold switchers also, since it was also weak (8% success rate)19.
Nevertheless, CF-random predicts alternative conformations in >50 E. coli proteins, suggesting that up to 5% switch folds. This substantial number supports previous work proposing that fold switching is a widespread natural phenomenon4,53. To demonstrate its potential significance, there are ~20,000 human genes. If 5% of them switched folds, that would be ~1000 human fold-switching proteins. Future work will examine the scope of fold switching in human and other proteomes.
To our knowledge, this is the first study to successfully predict many plausible 3D alternative conformations from thousands of protein sequences. While the focus of this study was on fold switching, CF-random predicted other conformational changes also, suggesting that it can be used to predict alternative conformations in general. Future experimental work will test whether these predictions of fold switchers are correct. We hope that CF-random will help to advance the new frontier of predicting conformational ensembles52.
Methods
Datasets
Fold-switching proteins adopt at least two different conformations (e.g., active/inactive or apo/holo conformations). In the CF-random pipeline, the dominant conformation is typically defined as that which CF predicts most frequently from full MSA sampling, except in a few cases where memorization has been shown or is expected to overrule coevolutionary inference19,35,54. AF2/CF rarely predicts more than one conformation when sampling full MSAs22. By exclusion, the alternative conformation is defined as that which AF2 rarely–if ever–samples from full MSAs and therefore requires an alternative sampling strategy.
PDB IDs corresponding to both conformations of all proteins tested here (fold switchers and other conformational changes) are in Supplementary Tables 1/2/3; regions of proteins that switch folds are also reported, if applicable. These regions are well ordered, as evidenced by having B-factors comparable to regions of solved protein structures that do not switch folds19.
Default mode prediction for predicting alternative conformations and fold-switching proteins
All CF-random predictions were run using ColabFold (CF) version 1.5.5 with monomer-ptm weights and all MSAs were constructed using the CF pipeline unless specified otherwise. Previous work showed that monomer weights can perform better than monomer-ptm20, but E. coli genome proteins were run with monomer-ptm weights before this observation was considered; rerunning with monomer weights was too costly. When run with either set of weights, default mode requires an input MSA and two reference structures as input. It works as follows:
Step 1: Sampling. Multiple Sequence Alignments are sampled at default depth (512:5120) to generate structures using all 5 AF2 models and 5 random seeds. Then 7 shallow depths (1:2, 2:4, 4:8, 8:16, 16:32, 32:64, 64:128) are sampled with 5 random seeds, all 5 models. This leads to 200 predicted structures overall. Importantly, as shown by previous work, the default depth is expected to predict one conformation22; thus, finding the alternative is the challenge. Note that X:2*X refers to the --max-msa:--max-extra-msa ColabFold parameters.
Step 2: Checking against experiment. TM-scores of each prediction are calculated against two distinct experimentally characterized conformations. If predictions with TM-scores ≥ 0.6 for both conformations were generated, and the best TM-score relative to conformation 1 > its TM-score relative to conformation 2 and vice versa, the predictions were considered a success. In some cases, such as human lymphotactin, the TM-score threshold was lowered to account for variability in disordered regions.
Fold-switching predictions
The total number of successes was the number of sequences from which both conformations could be generated; one conformation had a higher TM-score corresponding to Fold1 and the other to Fold2. Models whose accuracies increased by running the Multimer model were included, though their alternative conformations were required to be sampled as monomers also. The total number of structures generated by CF-random was (200)*154 proteins with distinct sequences (many fold switchers have a few mutations to stabilize one conformation or are truncated4) = 30,800 structures. Additionally, models of 100 proteins were generated from single sequences, 25 structures each = 2500 additional structures. In two cases where the alternative conformation was sampled with lower quality from deep MSAs (512:5120), 100 additional structures were sampled each. Finally, as reported in Results, six other proteins were also sampled using the multimer model (alphafold_multimer_v3 weights), 100 conformations each = 600 additional structures, for a total of 34,100 structures. Sampling depths used to produce alternative conformations of fold switchers are reported in Supplementary Table 1. Sampling and success rates for SPEACH-AF, AF-cluster, and AF2 were taken from the supplementary data reported in19.
Multimer predictions
CF was run with the same MSA sampling depths as CF-random using the alphafold2_multimer_v3 weights. The number of chains in predicted homo-oligomers matched those from the biological assemblies in the PDB. For diversity, the AF3 server was sampled with up to 5 seeds (1-5) for each of the oligomeric targets. If the alternative conformation could not be found with those seeds, we reported that AF3 did not predict it.
Other conformational changes
The 14/23 proteins from the SPEACH-AF/OC23 datasets are reported in Supplementary Table 2 and 3. For sampling comparisons, it should be noted that the sampling numbers reported in all cases did not comprise the total number of structures sampled by SPEACH-AF. Rather, the number of good quality structures produced by SPEACH-AF (by MolProbity score55) was reported because the total number of structures generated by SPEACH-AF was not found. MSAs for SPEACH-AF targets were generated using the sequences in their GitHub repository. MSAs for AFsample2 targets were taken directly from their Zenodo repository.
Coevolutionary analysis
With default settings, MSA Transformer56 was used to calculate the evolutionary couplings of each MSA uniformly sampled by CF-random at each sampling depth and each recycling step used to produce the best structure corresponding to each alternative conformation. Sequences from –max-seq and –max-extra-seq were combined to make these MSAs. MSA Transformer contacts were superimposed on residue-residue contacts calculated from AlphaFold2 models of a representative dominant and alternative conformation, except 5 that were better produced by the multimer model and 3l5n/2a73 because their large sizes required too much memory to complete the analysis. Structure-based contacts were calculated by finding pairs of heavy atoms from two different residues within 8 Å of each other. Contact intensities for each protein were normalized by the total number (common+dominant+alternative) for each protein for even comparison.
Sequence association
Protein BLAST57 was used to search the sequence of Sa1 V90T (PDB ID: 8E6Y) against the PDB. Three hits corresponding to the 3-helix-bundle were identified with PDB IDs: 2FS1, 2MH8, 1GJS. Since these 3 sequences were shorter than 8E6Y, pairwise alignments were constructed by adding gaps to the BLAST alignments at both termini as needed. Each MSA was run through ColabFold1.5.5 with the Sa1 V90T sequence as target, 3 recycles for each MSA. To construct the mixed MSA, an MSA for 1GJS was first constructed using ColabFold1.5.5. This MSA was reregistered with gaps at either or both termini to match the pairwise alignment generated previously. Then, the top 3 sequences from the ColabFold1.5.5-generated MSA for 8E6Y were added to the registered 1GJS MSA. Their UniProt IDs were: A0A7C7DMW5, A0A7C6PUQ5, A0A354ECL0. In all cases, CF was run with all 5 models, 4 random seeds/model.
Blind mode predictions
For blind mode of CF-random, only an MSA file is required as input. After CF-random has generated all structures from deep and shallow MSAs, a Foldseek33 database is generated from the predicted ensemble. This database is used to calculate an all against all similarity matrix for the ensemble using the Foldseek structural bitscore. This bitscore is a combination of the Smith–Waterman sequence alignment algorithm, local distance difference test (LDDT), and template modeling (TM)-score. Our approach leverages the combination of LDDT and TM-score within Foldseek’s structural bitscore to discern both subtle and large conformational differences between predicted structures because all predicted structures have the same amino acid sequence, discounting the Smith-Waterman algorithm’s contribution because the sequences of all structures are identical. Unfolded structures can be produced by the CF-random pipeline–particularly at shallow depths. These structures are filtered by calculating the ensemble’s distribution of DSSP assignments and removing outliers from the distribution. The similarity matrix is then reduced using Principal Component Analysis (PCA) to create a lower dimensional space where the distance between points represents how similar/dissimilar the predicted structures are to each other. The HDBSCAN algorithm then clusters similar points together (i.e. points that are both near to each other and of similar density) forming groups of predictions and finally the K-medoids algorithm is used on each group to select three representative structures. The final result is a subset of the full CF-random ensemble that represents the conformational variance of the predicted structures.
Colab notebook prediction with blind mode
Blind mode of CF-random is implemented in a Colab notebook. With job name and full-length MSA file, the user can run blind mode without installing on a local machine. Due to the limited resources of free Colab accounts, a set of shallow MSAs is limited as max-seq = 1, 2, 4, and 8, and max-extra-seq = 2 * max-seq. For running ColabFold with the shallow random MSAs, the user can run ColabFold with selected shallow random MSAs. Running the Colab notebook on proteins >300 residues is not recommended with a free Google account.
E. coli proteome predictions
We ran CF-random on 2126 proteins, representing 65% of proteins ≤300 residues. The remaining 1111 were excluded because we could not generate an MSA deep enough for strong coevolutionary inference by ACE. These proteins were taken from the Refseq58 E. coli genome, consisting of 5386 proteins. Final predictions in Fig. 7 were refined by running CF with 12−24 recycles instead of 3, resulting in higher plDDT scores.
Rosetta energy scores
To estimate the folding energies of E. coli predictions compared to known fold-switching proteins, we calculated the Rosetta Energy Scores (RES) of experimentally characterized fold-switching proteins (Supplementary Table 1) and blind search on the E. coli genome (Supplementary Table 4) using Rosetta38,39. The RES of the crystal structures of fold-switching proteins and the predictions of the E.coli genome were calculated after relaxation with the relax function59. The folding energy difference was obtained following the equation:
Other software
All protein illustrations were generated with PyMOL60. Plots were made with matplotlib61 and Seaborn62. Clustering and PCA were performed with scikit-learn63. The scripts for all data analysis were written in Python 3.10 (https://www.python.org/) with following modules: Biopython 1.79 (https://biopython.org/), NumPy 1.23.5 (https://numpy.org/doc/2.1/index.html), tmtools 0.2.0 (https://pypi.org/project/tmtools/), and pandas 2.2.3 (https://pandas.pydata.org/).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data generated for the analysis and example data to run scripts and to create figures were deposited on GitHub: https://github.com/ncbi/CF-random_software. The supporting data generated in this study are provided in the Supplementary Information and the Source Data file. The structural data used in this study were taken from the Protein Data Bank are listed below with their accession codes –NMR structures of human XCL1: 1J90 [https://www.rcsb.org/structure/1J9O] and 2JP1; crystal structures of human TRAP1N: 5F5R and 5F3K; crystal structures of E. coli RepE: 1REP and 2Z9O; crystal structure of FraC with lipids 4TSY: [https://www.rcsb.org/structure/4TSY]; crystal structure of the cell cycle regulatory protein Cks1 1QB3; NMR structure of amyloid-beta fibrils: the Osaka mutations 2MVX; NMR structure of pore-forming amyloid-beta tetramers 6RHY; crystal structure of Thermotoga maritima IMPase TM1415 2P3V, chains A and D; NMR structure of Sa1_V90T 8E6Y; NMR structure of the Albumin binding domain of Streptococcal Protein G 1GJS; GA-79-MBP CS-rosetta structures 2MH8, and solution NMR structure of PSD-1 2FS1. Unless otherwise stated, all data supporting the results of this study can be found in the article, supplement, and source data files. Source data are provided with this paper.
Code availability
Code can be found at https://github.com/ncbi/CF-random_software and https://doi.org/10.5281/zenodo.15596182.
References
Kim, A. K. & Porter, L. L. Functional and regulatory roles of fold-switching proteins. Structure 29, 6–14 (2021).
Karamanos, T. K., Tugarinov, V. & Clore, G. M. An S/T motif controls reversible oligomerization of the Hsp40 chaperone DNAJB6b through subtle reorganization of a beta sheet backbone. Proc. Natl Acad. Sci. USA 117, 30441–30450 (2020).
Motlagh, H. N., Wrabl, J. O., Li, J. & Hilser, V. J. The ensemble nature of allostery. Nature 508, 331–339 (2014).
Porter, L. L. & Looger, L. L. Extant fold-switching proteins are widespread. Proc. Natl. Acad. Sci. USA 115, 5968–5973 (2018).
Daily, M. D., Phillips, G. N. Jr. & Cui, Q. Many local motions cooperate to produce the adenylate kinase conformational transition. J. Mol. Biol. 400, 618–631 (2010).
Xia, Y. et al. Secondary-structure switch regulates the substrate binding of a YopJ family acetyltransferase. Nat. Commun. 12, 5969 (2021).
Pontiggia, F., Zen, A. & Micheletti, C. Small- and large-scale conformational changes of adenylate kinase: a molecular dynamics study of the subdomain motion and mechanics. Biophys. J. 95, 5901–5912 (2008).
Galaz-Davison, P., Ferreiro, D. U. & Ramirez-Sarmiento, C. A. Coevolution-derived native and non-native contacts determine the emergence of a novel fold in a universally conserved family of transcription factors. Protein Sci. 31, e4337 (2022).
Zuber, P. K. et al. Structural and thermodynamic analyses of the beta-to-alpha transformation in RfaH reveal principles of fold-switching proteins. Elife 11, 76630 (2022).
Tyler, R. C., Murray, N. J., Peterson, F. C. & Volkman, B. F. Native-state interconversion of a metamorphic protein requires global unfolding. Biochemistry 50, 7077–7079 (2011).
Wayment-Steele, H. K. et al. The conformational landscape of fold-switcher KaiB is tuned to the circadian rhythm timescale. Proc. Natl. Acad. Sci. USA 121, e2412293121 (2024).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Kalakoti, Y. & Wallner, B. AFsample2 predicts multiple conformations and ensembles with AlphaFold2. Commun. Biol. 8, 373 (2025).
Del Alamo, D., Sala, D., McHaourab, H. S. & Meiler, J. Sampling alternative conformational states of transporters and receptors with AlphaFold2. Elife 11, 75751 (2022).
Sala, D., Engelberger, F., McHaourab, H. S. & Meiler, J. Modeling conformational states of proteins with AlphaFold. Curr. Opin. Struct. Biol. 81, 102645 (2023).
Stein, R. A. & McHaourab, H. S. SPEACH_AF: Sampling protein ensembles and conformational heterogeneity with Alphafold2. PLoS Comput. Biol. 18, e1010483 (2022).
Wayment-Steele, H. K. et al. Predicting multiple conformations via sequence clustering and AlphaFold2. Nature 625, 832–839 (2024).
Bryant, P. & Noe, F. Structure prediction of alternative protein conformations. Nat. Commun. 15, 7328 (2024).
Chakravarty, D. et al. AlphaFold predictions of fold-switched conformations are driven by structure memorization. Nat. Commun. 15, 7296 (2024).
Schafer, J. W. et al. Sequence clustering confounds AlphaFold2. Nature 638, E8–E12 (2025).
Mirdita, M. et al. ColabFold: making protein folding accessible to all. Nat. Methods 19, 679–682 (2022).
Chakravarty, D. & Porter, L. L. AlphaFold2 fails to predict protein fold switching. Protein Sci. 31, e4353 (2022).
Monteiro da Silva, G., Cui, J. Y., Dalgarno, D. C., Lisi, G. P. & Rubenstein, B. M. High-throughput prediction of protein conformational distributions with subsampled AlphaFold2. Nat. Commun. 15, 2464 (2024).
Zhang, Y. & Skolnick, J. TM-align: a protein structure alignment algorithm based on the TM-score. Nucleic Acids Res. 33, 2302–2309 (2005).
Dishman, A. F. et al. Evolution of fold switching in a metamorphic protein. Science 371, 86–90 (2021).
Pajkos, M., Erdős, G. & Dosztányi, Z. The origin of discrepancies between predictions and annotations in intrinsically disordered proteins. Biomolecules 13, 1442 (2023).
Sung, N. et al. Mitochondrial Hsp90 is a ligand-activated molecular chaperone coupling ATP binding to dimer closure through a coiled-coil intermediate. Proc. Natl. Acad. Sci. USA 113, 2952–2957 (2016).
Nakamura, A., Wada, C. & Miki, K. Structural basis for regulation of bifunctional roles in replication initiator protein. Proc. Natl. Acad. Sci. 104, 18484–18489 (2007).
Tanaka, K., Caaveiro, J. M., Morante, K., González-Mañas, J. M. & Tsumoto, K. Structural basis for self-assembly of a cytolytic pore lined by protein and lipid. Nat. Commun. 6, 6337 (2015).
Abramson, J. et al. Accurate structure prediction of biomolecular interactions with AlphaFold3. Nature 630, 493–500 (2024).
Agarwal, V. & McShan, A. C. The power and pitfalls of AlphaFold2 for structure prediction beyond rigid globular proteins. Nat. Chem. Biol. 20, 950–959 (2024).
Ragonis-Bachar, P. et al. What can AlphaFold do for antimicrobial amyloids?. Proteins Struct. Funct. Bioinforma. 92, 265–281 (2024).
van Kempen, M. et al. Fast and accurate protein structure search with Foldseek. Nat. Biotechnol. 42, 243–246 (2024).
Bourne, Y. et al. Crystal structure and mutational analysis of the Saccharomyces cerevisiae cell cycle regulatory protein Cks1: implications for domain swapping, anion binding and protein interactions. Structure 8, 841–850 (2000).
Schafer, J. W. & Porter, L. L. AlphaFold2’s training set powers its predictions of some fold-switched conformations. Protein Sci. 34, e70105 (2025).
Tuinstra, R. L. et al. Interconversion between two unrelated protein folds in the lymphotactin native state. Proc. Natl. Acad. Sci. USA 105, 5057–5062 (2008).
Schafer, J. W. & Porter, L. Evolutionary selection of proteins with two folds. Nat. Commun. 14, 5478 (2023).
Alford, R. F. et al. The rosetta all-atom energy function for macromolecular modeling and design. J. Chem. Theory Comput 13, 3031–3048 (2017).
Park, H. et al. Simultaneous optimization of biomolecular energy functions on features from small molecules and macromolecules. J. Chem. Theory Comput 12, 6201–6212 (2016).
Iacovache, I. et al. Cryo-EM structure of aerolysin variants reveals a novel protein fold and the pore-formation process. Nat. Commun. 7, 12062 (2016).
Newlove, T., Konieczka, J. H. & Cordes, M. H. Secondary structure switching in Cro protein evolution. Structure 12, 569–581 (2004).
Kumirov, V. K. et al. Multistep mutational transformation of a protein fold through structural intermediates. Protein Sci. 27, 1767–1779 (2018).
Saldano, T. et al. Impact of protein conformational diversity on AlphaFold predictions. Bioinformatics 38, 2742–2748 (2022).
Lewis, S. et al. Scalable emulation of protein equilibrium ensembles with generative deep learning. bioRxiv https://doi.org/10.1101/2024.12.05.626885 (2024).
Jing, B., Berger, B. & Jaakkola, T. AlphaFold meets flow matching for generating protein ensembles. arXiv 2402, 04845 (2024).
Zheng, S. et al. Predicting equilibrium distributions for molecular systems with deep learning. Nat. Mach. Intell. 6, 558–567 (2024).
Jing, B. et al. EigenFold: Generative protein structure prediction with diffusion models. arXiv 2304, 02198 (2023).
del Alamo, D., Jeliazkov, J. R., Truan, D. & Karpiak, J. D. Conformational sampling and interpolation using language-based protein folding neural networks. bioRxiv https://doi.org/10.1101/2023.12.16.571997 (2023).
Swapna, G., Dube, N., Roth, M. J. & Montelione, G. T. Modeling alternative conformational states of pseudo-symmetric solute carrier transporters using methods from machine learning. bioRxiv 16, 2024.07.15.603529 (2024).
Vani, B. P., Aranganathan, A. & Tiwary, P. Exploring kinase Asp-Phe-Gly (DFG) loop conformational stability with AlphaFold2-RAVE. J. Chem. Inf. Model 7, 2789–2797 (2023).
Meller, A., Bhakat, S., Solieva, S. & Bowman, G. R. Accelerating cryptic pocket discovery using AlphaFold. J. Chem. Theory Comput 19, 4355–4363 (2023).
Bowman, G. R. AlphaFold and protein folding: not dead yet! the frontier is conformational ensembles. Annu Rev. Biomed. Data Sci. 7, 51–57 (2024).
Subramanian, V., Appadurai, R., Venkatesh, H., Sekhar, A. & Srivastava, A. Morpheus: A fragment-based algorithm to predict metamorphic behaviour in proteins across proteomes. bioRxiv https://doi.org/10.1101/2025.02.13.637956 (2025).
Chakravarty, D., Lee, M. & Porter, L. L. Proteins with alternative folds reveal blind spots in AlphaFold-based protein structure prediction. Curr. Opin. Struct. Biol. 90, 102973 (2025).
Williams, C. J. et al. MolProbity: More and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018).
Rao, R. M. et al. International Conference on Machine Learning. In Forty-Second International Conference on Machine Learning (PMLR, 2025).
Altschul, S. F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 (1997).
O’Leary, N. A. et al. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res 44, D733–D745 (2016).
Khatib, F. et al. Algorithm discovery by protein folding game players. Proc. Natl. Acad. Sci. USA 108, 18949–18953 (2011).
Lilkova, E. The PyMOL Molecular Graphics System, Version 2.0 Schrödinger, LLC. https://www.scirp.org/reference/referencespapers?referenceid=2403147 (2015).
Hunter, J. D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 9, 90–95 (2007).
Waskom, M. L. seaborn: statistical data visualization. J. Open Source Softw. 6, 3021 (2021).
Pedregosa, F. et al. Scikit-learn: Machine learning in python. J. Mach. Learn. Res. 12, 2825–2830 (2011).
Acknowledgements
L.L.P. thanks Carolyn Ott, Marius Clore, and John Jumper for helpful discussions. This work utilized resources from the NIH HPC Biowulf cluster (http://hpc.nih.gov), and it was supported by the Intramural Research Program of the National Library of Medicine, National Institutes of Health (LM202011, L.L.P.).
Funding
Open access funding provided by the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
Conceptualization: L.L.P., Methodology: M.L., L.L.P., and J.W.S.; Software: M.L, J.W.S., L.L.P., and J.P., Investigation: M.L., J.W.S., J.P., D.C., M.F.C., and L.L.P.,; Data Curation: M.L., J.W.S., D.C., and L.L.P.; Visualization: M.L., J.W.S., D.C., M.F.C., and L.L.P.; Writing – original draft: M.L., L.L.P.; Writing – review & editing: M.L., L.L.P.; Supervision: L.L.P.; Project administration: L.L.P.; Funding acquisition: L.L.P.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Jing Huang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, M., Schafer, J.W., Prabakaran, J. et al. Large-scale predictions of alternative protein conformations by AlphaFold2-based sequence association. Nat Commun 16, 5622 (2025). https://doi.org/10.1038/s41467-025-60759-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-60759-5