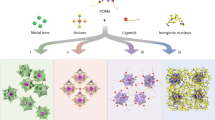
Abstract
Two-dimensional (2D) materials with intrinsic pores have attracted attention for catalytic and electronic applications. However, a significant gap exists between all-inorganic 2D networks with inorganic connectors and those with organic connectors due to the greater complexity of functionalizing inorganic molecules. Addressing this gap, we present a new class of 2D all-inorganic porous networks: single-layer cluster ionic-chain networks (CINs), constructed by using PW10M2 (M = Mn, Co) polyoxometalate (POM) clusters as nodes and end-capping agents for ionic chains. The integration of POM clusters into these networks significantly alters the electronic and band structures. Notably, the Mn-based CIN exhibits extremely high catalytic activity, achieving a toluene oxidation conversion rate of over 1.45 mmol g−1 h−1. Calculations suggest that POM clusters act as an ‘electron buffer’, stabilizing electron density at Mn sites and lowering the activation energy for toluene oxidation. This development showcases POM clusters as ‘superatom’ capping agents, establishing a pathway for all-inorganic 2D networks that could advance new catalytic materials with unique electronic properties.
Similar content being viewed by others
Introduction
Due to quantum confinement effects, atomically thin two-dimensional (2D) materials have unique physical and chemical characteristics that set them apart from their bulk counterparts1,2,3,4,5,6,7,8. Although 2D materials such as graphene9, transition metal oxides10, chalcogenides2,7, and Mxene11 derived from layered crystals have been extensively researched, the exploration of single-layer materials with intrinsic pores or cavity structures that characterize periodic network patterns is still in its infancy. Currently, constructing 2D network structures relies mainly on organic molecule linkers12,13,14,15, as seen in 2D metal–organic frameworks (MOFs)14 and 2D covalent organic frameworks (COFs)15. In comparison with 2D MOFs and COFs, all-inorganic 2D networks are significantly less common and are typically assembled from inorganic linkers. Fundamentally, this disparity arises from the greater difficulty in functionalizing inorganic molecules compared to organic ones. Among all-inorganic 2D network structures, layered zeolites have received the most attention16,17,18. However, because layered zeolites often form three-dimensional (3D) frameworks and synthesizing 2D zeolite nanosheets frequently requires template agents, the accurate manufacture of monolayer zeolite materials is difficult and complex17,19. Fortunately, chain-like fragments of specific ionic crystals have potential for performing the linkers of network structures, but synthesizing ionic chains with precisely controlled lengths and subsequently constructing all-inorganic 2D networks remains a significant challenge.
Clusters with atomic-scale precision and nano- or sub-nanometer dimensions offer diverse coordination modes and directional chemical bonding interactions, making them ideal end-capping agents for inorganic ionic species20,21. By precisely controlling the coordination modes of the cluster nodes and orienting the assembly of these nodes with ionic chains, a novel all-inorganic porous network can be obtained. Specifically, polyoxometalates (POMs), a distinct class of metal–oxygen molecular clusters, exhibit various structural types and exceptional catalytic properties, allowing them to act as multidentate ligands that link both metal and non-metal species22,23,24. Structural modifications or substituent variations in POMs enable precise control over their coordination abilities, thereby facilitating fine-tuned assembly of resulting complexes25. For example, Su et al. synthesized a POM cluster with an inorganic vacancy that aligns precisely with the coordination environment of hexavalent americium, resulting in a water-soluble nanoscale composite through robust interactions with americium ions20. Similarly, Wang’s group demonstrated a structural transformation of POM cluster assemblies from nanowire to nanoring by adjusting solution acidity, subsequently arranging these into 3D superstructures26. Moreover, the co-assembly of POM cluster and various inorganic compounds (such as NiSx-NiOy, CuO, ZnO, etc.) has proven the compatibility between them, and a series of nanosheets with sub-1 nm thick and dense structures were obtained27,28,29. Accordingly, it is feasible to construct a single-layer porous network through the precise co-assembly of POM clusters and ion chains. Additionally, POM clusters can influence the electronic and band structures of materials by acting as “electron buffers,” enhancing catalytic performance and informing industrial applications30,31,32,33,34. On the other hand, the interactions between clusters and substrates are typically surface-limited30,31. Therefore, preparing cluster-based materials at the single cluster level is expected to modify their electronic structures and confer significantly enhanced catalytic properties.
In this study, we design a new class of all-inorganic 2D network structure known as the cluster ionic-chain network (CIN). These 2D networks are composed of {PW10M2} (M = Mn, Co) clusters capped with ionic chains {MH2PO4}3, derived from the fragments of non-layered inorganic crystal M(H2PO4)2·2H2O. Characterization reveals that the 2D network features square pores measuring 1.7 × 1.7 nm with an angle of approximately 87°. The accurate molecular model of the CIN is further established. Notably, the integration of POM clusters into inorganic crystal networks forming sub-nanometric structures effectively alters their electronic and band structures. Therefore, the Mn-CIN exhibits remarkable catalytic activity, achieving a conversion rate exceeding 1.45 mmol g⁻¹ h⁻¹ for toluene oxidation, along with notable cycle stability. Theoretical calculations indicate that spatial constraints significantly influence the interaction between the clusters and ionic chains. Moreover, the electron buffering properties of the POM clusters help balance the electron density of the Mn sites in the ionic chains, thereby lowering the activation energy required for toluene oxidation. This research presents a pioneering approach for the seamless integration of molecular clusters with inorganic crystal lattices at a sub-nanometer scale. It highlights the potential of utilizing these clusters as “superatom” capping agents on ionic chains to create all-inorganic 2D network catalysts with superior activities and unique electronic properties.
Results
Preparation and structure of CIN built by PW10Mn2 cluster and ionic chains
Nodes and organic linkers generally possess distinct functional groups for reported network structures, which induce regular skeletons through coordination or chemical bonds12 (Fig. S1). Creatively, when di-Mn-substituted POM (PW10Mn2) clusters are used as nodes, chain fragments of Mn(H2PO4)2·2H2O crystals can be used as linkers to construct single-layer all-inorganic networks. The formation mechanism of CIN may be as follows. POM clusters with special symmetry and metal substitution sites have the potential to serve as nodes for constructing network structures35. The di-M-substituted POM cluster exhibits Cs symmetry (a mirror plane perpendicular to the M-M direction). The two M sites are symmetrically equivalent and oriented to allow bidirectional coordination with ionic chains (Fig. 1a). Unlike isotropic clusters, the directional bonding preference of di-substituted clusters minimizes off-plane interactions, thereby promoting monolayer formation. In addition, carbonates and phosphates have been demonstrated to yield ionic chains through crosslinking strategies, resulting in a series of inorganic mineral materials with unique physical properties36,37,38. Manganese phosphate with chain-like segments has the potential to form ionic chains and further serve as linkers in the network structure (Fig. 1a). As a representative, the loss of two WO6 octahedra from the Keggin ion (PW12) via base hydrolysis results in di-Mn-substituted POM (PW10Mn2) cluster39, marked as PW10Mn2 cluster (Fig. 1a). Subsequently, chain-like fragments ({MH2PO4}3) of Mn(H2PO4)2·2H2O crystals acting as linkers combines PW10Mn2 cluster node to generate 2D monoclinic network structure with γ ≈ 87°. Figure 1b clearly illustrates the structure of single-layer Mn-CIN, where the PW10Mn2 cluster nodes and {MH2PO4}3 linkers are connected via Mn-O bonds. As shown in Figs. S2 and S3, high-purity Mn-CIN nanosheets were obtained and can be seen under transmission electron microscopy (TEM) observation, and the lattice structure can be seen in TEM images taken at higher magnification. The high-angle annular dark-field scanning TEM (HAADF-STEM) images clearly show the pure Mn-CIN nanosheets (Fig. S2a) and their distinct pore structures within the networks (Fig. S2b). Interestingly, as shown in Fig. S4, the spherical aberration corrected HAADF-STEM (AC-HAADF-STEM) image shows the side view of Mn-CIN, indicating that Mn-CIN is only a single cluster thick. Additionally, the atomic force microscope (AFM) results show that the thickness of Mn-CIN sheets is approximately 1.0 nm (Fig. 1c). Further, the fast Fourier transformation (FFT) diffraction pattern of high-resolution TEM (HRTEM) image displays a tetragonal arrangement of PW10Mn2 clusters (Fig. S5). Notably, under cryogenic conditions with liquid nitrogen, cryogenic transmission electron microscopy (cryo-TEM) can observe a more regular network structure within the nanosheets (Fig. 1d). The nearly spherical cluster nodes exhibit higher contrast due to the presence of heavier elements, while the linkers between clusters show low contrast. The AC-HAADF-STEM image in Fig. 1e shows a large-scale tetragonal network structure with approximately 1.0 nm spherical cluster particles at the nodes. Moreover, the molecular structure is supported by the higher-magnification AC-HAADF-STEM image in Fig. 1f, which shows a 4*4 network made up of 16 clusters. The node-forming POM cluster and the linear linkers connecting them are both easily discernible. The grid spacing of Mn-CIN is strictly uniform and shows high crystallinity, as seen by the clear tetragonal diffraction spots produced by the further FFT (Fig. 1g). Inter-cluster spacings (~2.7 nm) in the FFT results are consistent with those found in the molecular model and small-angle X-ray diffractometry (SXRD) data. Furthermore, the energy-dispersive spectroscopy (EDS) analysis reveals the uniform distribution of Mn, P, and W elements in the network (Fig. 1h). Similarly, di-Co-substituted phosphotungstate (PW10Co2) and cobalt phosphate ionic chains also serve as building blocks for the network structure, enabling the formation of Co-CIN. The successful synthesis and characterization of Co-CIN further validate the generality and robustness of the CIN design strategy. Structural analyses confirm that Co-CIN adopts a monolayer 2D architecture analogous to Mn-CIN, with square pores of ~1.7 × 1.7 nm (HAADF-STEM, Fig. S6) and a uniform thickness of ~1.0 nm (AFM, Fig. S7). As shown in Fig. S8, the cryo-TEM image reveals the network structure of Co-CIN and its good crystallinity. The EDS mapping spectrum (Fig. S9) shows the uniform distribution of Co, P, and W elements in Co-CIN. Raman (A₁ mode, Fig. S10) and FTIR spectra (Fig. S11) confirm the retention of Keggin-type POM cluster signatures. The successful integration of Co into the CIN framework demonstrates the platform’s versatility for tailoring materials with diverse transition metals. This result validates the general applicability of the strategy for constructing 2D CINs.
a The schematic of the CIN construction strategy through combining the chain-like fragment of inorganic crystals with disubstituted clusters. b The structure model of the single-layer CIN fragment optimized by density functional theory (DFT). c AFM result of a monolayer Mn-CIN sheet with single-cluster thickness. Inset: porous network structure shown by STEM image (top right), molecular model of a PW10Mn2 cluster (bottom left). d Cryo-TEM image of Mn-CIN. Inset: zoomed-in view of Cryo-TEM image, wherein the nearly spherical cluster nodes exhibit higher contrast due to the presence of heavier elements, while the linkers between clusters show low contrast. e AC-HAADF-STEM image of Mn-CIN with regular, approximately square-shaped pores. f The local structure of CIN with a 4*4 cluster arrangement in the AC-HAADF-STEM image. Inset: the molecular model of a 2*2 cluster net. g FFT pattern of Mn-CIN. h STEM image of Mn-CIN sheets and corresponding EDS elemental mapping analysis of Mn, P, and W.
Whether electron transfer can occur directly depends on the linking modes between cluster nodes and ionic chain linkers. Therefore, the composition of ionic chains and clusters is analyzed, and the coordination environment of manganese is further determined (Fig. 2a, b). According to the differential scanning calorimetry analysis, the thermal breakdown of Mn(H2PO4)2·2H2O and Mn-CIN both entails the dehydration of coordinated water molecules, which is followed by the intramolecular dehydration and condensation of the protonated phosphate ions (Fig. 2a). Differently, in the one-dimensional ionic chains of CIN, the coordination environment of Mn demonstrates a greater degree of freedom for coordinated water (II in Fig. 2c). This increased flexibility contrasts with the more constrained environment found in the Mn(H2PO4)2·2H2O crystal (I in Fig. 2c). Consequently, this difference in coordination leads to a shift from the endothermic peak 1, observed at approximately 400 K, to peak 1* (Fig. 2a), suggesting an earlier release of coordinated water. In addition, the decomposition of protonated phosphate groups is observed in both the CIN and the crystal at about 450 K, indicating that their phosphate groups are similar. As shown in the extended X-ray absorption fine structure (EXAFS) analysis (Fig. 2b), oxygen atoms surround Mn atom with an average coordination number of 4.6 (Mn−O: 2.14 Å) in Mn-CIN and 5.5 (Mn−O: 2.13 Å) in cluster precursor (Table S1), indicating the coexistence of low-coordination Mn atoms of linkers and high-coordination Mn atoms of nodes within Mn-CIN. The structural integrity of the PW10Mn2 cluster could be distinguished through infrared and Raman spectroscopy (Fig. 2d, e). As shown in Fig. 2d, the P-O bond splitting stretching vibration signal around 1050 nm is one of the characteristics of the asymmetric Keggin-type cluster structure. Additionally, Fig. 2e shows the characteristic vibration mode (A1) of the cluster structures40. The shift (from 975 cm−1 for PW10Mn2 clusters to 938 cm−1 for Mn-CIN) is possibly caused by changing the symmetry of the clusters by interacting with the ionic chains.
a DSC images of Mn-CIN sheets. b R-space EXAFS spectra of intrinsic PW10Mn2 clusters and Mn-CIN. c Typical simulated coordination mode of Mn atom in Mn(H2PO4)2·2H2O crystals (I) and in CIN (II). d Infrared spectroscopy of PW10Mn2 clusters and Mn-CIN. e Raman spectroscopy of PW10Mn2 clusters and Mn-CIN.
Moreover, interlayer stacking between Mn-CIN sheets induces peaks at 2θ ≈ 2.46° and its secondary peak 2θ ≈ 4.95° in the SXRD (Fig. 3a). The weak signal at 2θ ≈ 3.19° belongs to the edge of the square pore in CIN, originating from clusters connected by linker. The XRD shows no significant signal peaks (Fig. 3b), indicating that neither the POM nor the phosphate forms a separate crystalline phase. The Mn-CIN material has a layered structure where layers stack periodically, but within each layer, the arrangement is not perfectly ordered over long distances. The linkers (ionic chains) exhibit rotational and conformational freedom, leading to a lack of long-range atomic order. Furthermore, the X-ray absorption near-edge spectroscopy (XANES) and EXAFS analysis are used to confirm the chemical state and local environment of the Mn atom in Mn-CIN. As shown in Figs. 3c and S12, the XANES spectra of Mn-CIN are shifted to higher energies compared to the intrinsic PW10Mn2 clusters and MnO, reflecting that the valence state of the Mn atoms in Mn-CIN is beyond +2 and the electron transfer from the Mn atoms of the linkers to the cluster. Wavelet transform (WT) analysis further characterizes the Mn state in CIN, revealing the fingerprints of Mn-O bonds at R = 1.6 Å (Fig. 3d). Additionally, there are also features of P and W in the outer shell around the Mn atom, which is the average chemical environment information of Mn in both the clusters and the linkers (Table S1).
a Small-angle XRD results of Mn-CIN. The a and c peaks are attributed to interlayer stacking of the monolayer network, while the b peak arises from the ordered arrangement of adjacent clusters within the monolayer network. b XRD results of Mn(H2PO4)2·2H2O crystals and Mn-CIN. c XANES spectra of Mn-CIN. d WT representation of the EXAFS signal. e The XPS data of Mn 3 s in Mn(H2PO4)2·2H2O and Mn-CIN. f The XPS data of W 4 f in PW10Mn2 clusters and Mn-CIN. g The band gaps of Mn(H2PO4)2·2H2O crystals, PW10Mn2 clusters, and Mn-CIN obtained from UV-vis diffuse reflectance spectra.
Further, the structure of Mn-CIN is analyzed by using X-ray photoelectron spectroscopy (XPS). The splitting amplitude ΔE of the two split peaks of Mn 3s reflects the valence state of manganese41,42,43. The result of ΔE{CIN} < ΔE{Mn(H2PO4)2·2H2O} indicates that the valence state of manganese in Mn-CIN increases (Fig. 3e). Correspondingly, the W 4f signals in Mn-CIN shift to lower energy regions compared to the cluster monomer, with W 4f 5/2 and W 4f 7/2 shifting to lower energies, indicating that the tungsten in the node gains electrons and its valence state decreases (Fig. 3f). The signals of Mn 3s and W 4f indicate the electron transfer from the linker to the cluster node in Mn-CIN. This electron transfer may endow the novel 2D all-inorganic network material with unique catalytic activity, which will be discussed in subsequent sections. Interestingly, clusters and ionic chains form a continuous structure at the sub-nanometer scale, and the interaction between them could alter the band structure of the material23,44. The band gaps of Mn(H2PO4)2·2H2O crystals, PW10Mn2 cluster monomers, and Mn-CIN are measured by ultraviolet–visible diffuse reflectance spectroscopy (Fig. 3g). Results show that the band gap of Mn-CIN is narrower than that of the former two.
Catalytic performance of Mn-CIN for toluene oxidation
The conversion of toluene into value-added products such as benzaldehyde, benzoic acid, and benzyl alcohol is of significant importance in the chemical industry (Fig. 4a). The activation of the C(sp3)-H bond of toluene under mild conditions (particularly at ambient temperature and pressure) is generally preferred but remains challenging45,46,47. Besides, industrial production of benzaldehyde and benzoic acid typically involves the chlorination of toluene and catalytic toluene oxidation, respectively48. It has been reported that manganese-based catalysts can achieve the conversion of toluene46,47,48; however, they require higher temperatures (above 190 °C) and exhibit lower conversion efficiency49. Interestingly, the effect of electron buffer within the Mn-CIN on the high-density Mn atoms renders it highly promising for catalytic oxidation of toluene. As illustrated in Fig. 4a, the Mn-CIN catalyst combines environmentally friendly peroxide oxidants to accelerate the transformation of toluene and produce aldehydes with great selectivity at mild temperatures (50 °C). Compared to both manganese salts and cluster monomers, the 2D network formed by their combination at the sub-nanometer scale exhibits superior catalytic activity and stability (Fig. 4b). The Mn-CIN nanosheets show a high conversion rate (1452 μmol g−1 h−1) at 50 °C for 6 h, which is 5.9-fold and 5.2-fold higher than that of the manganese salts catalyst (246 μmol g−1 h−1) and the cluster catalyst (279 μmol g−1 h−1), respectively. The toluene conversion increases over time, and the selectivity for benzaldehyde remains above 85% after 18 h (Fig. 4c). The six-cycle durability tests and the well-retained catalyst morphologies both reflect the excellent durability of Mn-CIN (Figs. 4d and S13). To evaluate the stability of the pores and structural integrity after catalysis (under conditions of 50 °C for 3 h), we conducted post-catalysis characterization using EDS elemental mapping, FFT analysis, and HAADF-STEM imaging. EDS mapping of the used Mn-CIN catalyst (Fig. S14) confirmed that Mn, P, and W elements remained uniformly distributed across the network, with no significant aggregation or elemental migration observed, demonstrating robust structural retention. The FFT pattern of high-resolution TEM images (Fig. S15) retained sharp tetragonal diffraction spots after catalysis, consistent with that of the pristine material (Fig. 1g), confirming the preservation of the long-range periodic arrangement of clusters and ionic chains within the network. HAADF-STEM images (Fig. S16) revealed that the square pore structure (1.7 × 1.7 nm) and node–linker connectivity were preserved post-catalysis. These results collectively validate the exceptional stability of Mn-CIN’s pore architecture and crystallinity under mild catalytic conditions. The selectivity for benzaldehyde remained at 100%. Additionally, the catalysis by Mn-CIN was conducted at temperatures ranging from 50 °C to 80 °C (Fig. 4e). The conversion rate of toluene reached 6981 μmol g−1 h−1 at 80 °C. Compared with most reported thermal catalysts or photocatalysts, the Mn-CIN catalyst exhibited higher selectivity for benzaldehyde and greater toluene conversion under mild reaction conditions (Fig. 4f and Table S2). Additionally, we tested the catalytic performance of Co-CIN. Under identical conditions, Co-CIN achieved a toluene conversion rate of 568 µmol g−1 h−1 over 6 h and exhibited 94% selectivity for benzaldehyde (Fig. S17).
a Photographs of the product mixture after oxidation. The toluene conversion was catalyzed by Mn(H2PO4)2·2H2O crystals (left) and Mn-CIN (right) for 3 h at 50 °C. b The conversion rates of toluene catalyzed by Mn(H2PO4)2·2H2O crystals ( ), PW10Mn2 clusters (
), PW10Mn2 clusters ( ), and Mn-CIN (
), and Mn-CIN ( ). Reaction conditions: 50 °C, 6 h. c The toluene conversion catalyzed by Mn-CIN at 50°C over 18 h. d Catalytic durability for Mn-CIN. Reaction conditions: 50 °C, 3 h. e The conversion rate of toluene oxidation for Mn-CIN at different temperatures for 3 h. f The toluene conversion rate of Mn-CIN compared with other reported systems for toluene oxidation.
). Reaction conditions: 50 °C, 6 h. c The toluene conversion catalyzed by Mn-CIN at 50°C over 18 h. d Catalytic durability for Mn-CIN. Reaction conditions: 50 °C, 3 h. e The conversion rate of toluene oxidation for Mn-CIN at different temperatures for 3 h. f The toluene conversion rate of Mn-CIN compared with other reported systems for toluene oxidation.
To elucidate the reaction mechanism, the reaction system was supplemented with scavengers for holes, electrons, and radicals (Fig. S18). It is discovered that the critical step involves the activation of the C(sp3)-H bond, resulting in carbon-centered radicals (R·). This indicates that the catalytic oxidation process involves hydrogen abstraction to generate carbon radicals50,51,52. The electron transfer process involving manganese atoms plays a decisive role in hydrogen abstraction. The in situ electron paramagnetic resonance data provide direct evidence that Mn atoms in Mn-CIN undergo reduction during hydrogen abstraction, generating stronger paramagnetic reductive Mn species (Fig. S19). This unambiguously establishes Mn as the active site for C(sp³)-H bond activation. Therefore, the superior catalytic performance of the 2D network is presumably due to the combination of cluster and ionic chain and the unique sub-nanometric structure. The calculations for the dangling O-W groups of the POM cluster revealed a high activation energy (1.49 eV) for hydrogen abstraction (Fig. S20), confirming that the reaction predominantly occurs at Mn sites in the ionic chains, as further supported by in situ Raman spectroscopy (Fig. S21). The catalytic activity of Mn-CIN is governed by the interplay between Mn site proximity to the POM cluster and the cluster’s ability to delocalize charge changes. Based on these results, the Mn site directly connected to the cluster via Mn-O bonds was selected, and a formation mechanism for benzyl radicals was proposed to elucidate how the cluster enhances the catalytic activity of the Mn sites (Fig. S22). According to earlier research30,31, clusters can balance the charge density of active sites through electron buffer, lowering system energy. This is supported by the fact that clusters lower the activation energy for hydrogen abstraction at the manganese sites. The Bader charge calculations for the CIN–toluene interaction systems during benzyl radical generation are summarized in Fig. S23. The change in Bader charges primarily occurs across the entire cluster (rather than at one certain metal atom), which may be a crucial factor in the enhanced catalytic activity of the sub-nanometer structure of Mn-CIN.
Sub-nanometric orbital overlap in CIN mediated by the cluster
Clusters can be employed as electron buffers to balance the electronic density of active catalytic species, thereby enhancing catalytic oxidation efficiency by reducing the free energy of intermediates30,31. Usually, the influence of the electronic buffer is limited, possibly only significant in the transmission of a few chemical bonds around the cluster (limited to sub-nanometric distance). Therefore, the integration strategy of clusters and ionic chains at the sub-nanometer scale will maximize the influence of clusters on the electronic properties of the material. The cluster in Mn-CIN acts as a dynamic electron buffer, enabling reversible charge exchange between Mn sites and the POM cluster during catalysis (Fig. S24). This bidirectional electron transfer mechanism stabilizes the Mn active centers and sustains high catalytic efficiency without irreversible changes to the valence state of both Mn and W. According to density functional theory (DFT) studies on the interaction between clusters and the ionic chains in Mn-CIN, clusters have a stronger electronic “transport” effect on nearby manganese atoms, balancing their charges and improving their capacity to transfer electrons, which speeds up the peroxide-induced oxidation of toluene. The DFT differential charge density analysis simulates the dynamic assembly process between POM clusters and ionic chains, capturing the transient charge redistribution. These negatively charged POM clusters electrostatically attract positively charged {Mn(H2PO4)2}3 chain fragments. This interaction leads to electron accumulation in the ionic chains (linkers) and concomitant depletion in the clusters (Fig. 5a). Although the electrostatic interactions induce electron accumulation throughout the linkers, localized depletion at specific Mn sites (e.g., Mn3 and Mn6 in Fig. 5a) demonstrates the electron transfer from Mn to the cluster, consistent with the elevated Mn oxidation state evidenced by both XANES (Fig. 3c) and XPS (Fig. 3e, f). This demonstrates enhanced interaction between the clusters and ionic chains through charge transfer. The Bader charge analysis and energy barrier calculations (Figs. S25 and S26) collectively demonstrate that the POM cluster’s electron-buffering capability is distance-dependent, and the catalytic activity is optimized at cluster-proximal sites. By redistributing charge across the network, the cluster stabilizes reactive intermediates and lowers activation energy at Mn sites. This mechanism rationalizes the superior catalytic performance of Mn-CIN compared to isolated clusters or ionic chains. To further explore the interaction between Mn atoms and clusters at different spatial distributions, Bader charge analysis was conducted on the structural units in Mn-CIN before and after losing one or even two electrons (Figs. 5b and S27). Mn3 and Mn6 atoms directly connected to the terminal oxygen of the cluster show minimum charge changes. Mn1 and Mn8 atoms, connected to the cluster via phosphate groups, also exhibit slight Bader charge changes after losing electrons. In contrast, Mn2 and Mn7 atoms, located more than 1.2 nm from the cluster center, showed significant Bader charge changes after the structural unit lost electrons. Notably, the Bader charge changes in the substituted Mn atoms (4 and 5) within the cluster are also notable due to the spatially asymmetrical molecular orbitals of the substituted cluster53. This indicates that the electronic buffering effect of clusters on neighboring chemical groups is distance-dependent, and restricting the distance to within the sub-nanometric scale can most efficiently leverage the electronic buffering effect of clusters. Meanwhile, the electron localization function (ELF) results (Fig. S28) and ELF slice (Fig. 5c) illustrate electron delocalization both in the cluster and ionic chain. Compared to isolated Mn(H2PO4)2·2H2O crystals (~3.86 eV) and PW₁₀Mn₂ cluster monomers (~3.32 eV), the Mn-CIN exhibits a narrower bandgap (~3.0 eV) (Fig. 3g). This reduction originates from the sub-nanoscale integration of POM clusters with ionic chains, which induces significant electronic restructuring. The sub-nanometric architecture of Mn-CIN promotes orbital overlap between adjacent clusters and ionic chains. Projected density of states calculations (Fig. S29) reveal that covalent Mn-O bonds between the POM clusters and ionic chains facilitate the hybridization of O 2s, O 2p, and Mn 4s orbitals. This creates new energy bands, which bring the conduction band minimum closer to the Fermi level, thereby reducing the bandgap. Moreover, the similar proximity of W 5d and Mn 3d orbitals to the Fermi level in Mn-CIN provides a possibility for electron transfer between the clusters and ionic chains (Fig. 5d). The compatibility between clusters and sub-nanometer inorganic crystal fragments plays a crucial role in the development of novel network materials. Additionally, the introduction of these clusters can directly modify the electronic structure of the inorganic crystals and lead to the creation of materials with enhanced catalytic properties.
a The differential charge density of the Mn-CIN. The regions with electron loss are shown in blue, and the isosurface is 0.0003 e Bohr−3. b The Bader charge variation of Mn atoms at corresponding positions in (a) after losing one (green dot) and two electrons (blue dot) in the Mn-CIN. c ELF results from one fragment of Mn-CIN. The slice is perpendicular (a) to the paper surface. d The PDOS of Mn(H2PO4)2·2H2O crystals (up) and Mn-CIN (down); the Fermi level was set as 0 eV as shown by dashed lines.
Discussion
This study has synthesized a new class of all-inorganic 2D materials: CINs built with PW10M2 (M = Mn, Co) POM clusters as capping agents for ionic chains. Unlike traditional catalysts where cluster–substrate interactions are surface-limited, CIN integrates POM clusters (e.g., PW10Mn2) with ionic chains ({MH2PO4}3) at a sub-nanometer scale. This intimate coupling enables electronic interactions across the entire network rather than localized surface effects. The preparation of CINs allowed for the modification of the electronic and band structures of traditional inorganic crystals through cluster engineering, thereby achieving superior catalytic activity compared to both clusters and the original inorganic crystals. The Mn-based CIN exhibited exceptional catalytic activity, converting toluene at a rate exceeding 1.45 mmol g−1 h−1. Moreover, a precise molecular model of CIN is proposed to reveal how the electron buffer effect of clusters on metal sites is limited by distance. Theoretical calculations indicate that the POM clusters function as an “electron buffer,” stabilizing electron density at the Mn sites. Leveraging the electronic buffering effect of clusters reduces the activation energy of catalytic reactions. These findings underscore the potential of POM clusters as “superatom” capping agents for ionic chains, establishing a viable approach for constructing all-inorganic 2D networks and paving the way for designing new catalytic materials with advanced electronic properties. Future studies could extend this strategy to other clusters (e.g., transition metal oxides, chalcogenides) and ionic systems, enabling the design of advanced catalysts. Integrating a wider variety of clusters and inorganic substances within the sub-nanometer scale may be one of the future directions for creating novel 2D network materials.
Methods
General synthesis of CIN
For the synthesis of PW10Mn2 clusters, 2.88 g of H3PW12O40·nH2O was dissolved in 10 mL of water, and the pH of the solution was adjusted to 6.4 using saturated NaHCO3 solution. The solution was heated to 100 °C with stirring. To this hot solution, 0.4 g of MnCl2·4H2O dissolved in a minimal amount of water was added. The solution was heated at 100 °C with stirring for 2 h and filtered hot, then 4 mmol tetrabutylammonium bromide was immediately added. The resulting orange-yellow precipitates were filtered, dried at 50 °C, and designated as (TBA)4H3[PW10Mn2O38(H2O)2]. For the synthesis of PW10Co2 clusters, the same procedure was followed except that 0.5 g of CoCl2·6H2O was used instead of MnCl2·4H2O.
For the synthesis of Mn-CIN, 0.020 mmol (TBA)4H3[PW10Mn2O38(H2O)2] clusters39,54 were dispersed in 5.0 mL ethanol. Subsequently, a mixed solution consisting of 0.5 mL water, 0.014 mL concentrated HCl, and 0.15 mmol Mn(H2PO4)2·2H2O were introduced into the mixture. After adding 2.0 mL oleylamine and 3 min of stirring, the mixture was sealed within a 10 mL Teflon-lined autoclave and heated at 180 °C for 12 h. The resultant products were then collected via differential centrifugation, washed multiple times with cyclohexane, and dried in a vacuum oven at 60 °C for 5 h. In the synthesis of Co-CIN, 0.020 mmol (TBA)4H3[PW10Co2O38(H2O)2] clusters and 0.12 mmol CoCl2·6H2O were dispersed in 5.0 mL ethanol. To this dispersion, a mixed solution of 0.75 mL water and 30 mg H3PO4 (85 wt.%) was added. After adding 1.0 mL oleylamine and 0.75 mL oleic acid, the subsequent steps, including stirring, autoclave heating, product collection, washing, and drying, were conducted in the same manner as described for the Mn-CIN synthesis.
Catalytic measurement for toluene oxidation
For the typical toluene oxidation, 14 mg of catalyst and 0.2 ml of tert-butyl hydroperoxide were added into 1.0 g toluene. The reaction was performed under continuous magnetic stirring. Finally, the solution was centrifuged, and the supernatant was studied using chromatography. In the recycle experiment, the precipitate was reused after washing by cyclohexane to remove the residual substrates.
Computational details for DFT calculations
Spin-polarized DFT simulations were conducted via Vienna Ab initio Simulation Package (VASP). The cut-off energy was set to 450 eV. The Generalized Gradient Approximation with Perdew–Burke–Ernzerhof (PBE) functional was used for describing the exchange–correlation of electrons. The core–valence interaction was performed by the Projector-Augmented Wave (PAW) with PBE as the energy functional, and the PAW potentials represent the valence electrons of W as 5 d46s2, Mn as 3p63 d54s2, H as 1s1, P as 3s23p3, C as 2s22p2, and those of O as 2s22p4. The DFT + U method with Becke–Johnson damping was added for the highly localized 5d orbitals of W and 3d orbitals of Mn. The Brillouin zone was sampled by the gamma-centered Monkhorst–Pack Grid with k-points of 1 × 1 × 1 for the structural optimization. The atomic positions were relaxed until the force on each atom was less than 0.1 eV/Å and electronic energies were converged within 10-4 eV. To model the monolayer CIN, the cell included a vacuum of 30 Å, which was large enough to avoid interaction between two layers along the c-axis. The crystal structure graphics and isosurfaces were produced using VESTA.
A Hubbard U value of 2.6 eV was applied to the Mn 3d orbitals using the DFT + U method with LDAUU = 3 and LDAUJ = 0.4. To validate this parameter, we conducted a systematic U-dependent bandgap analysis for Mn(H2PO4)2·2H2O crystals. As shown in Fig. S30, U = 2.6 eV yielded a theoretical bandgap (3.87 eV) in agreement with the experimental value (3.86 eV; Fig. 3g). The same U value was adopted for Mn-CIN, ensuring consistency between its calculated (2.99 eV) and experimental (3.00 eV) bandgaps (Fig. S31). No U correction was applied to W 5d orbitals, as their extended spatial distribution and high delocalization result in negligible electron correlation effects.
The complete crystallographic data, including fractional atomic coordinates, are provided in Table S3 (Mn-CIN) and Table S4 (Co-CIN) in the Supplementary Information. All coordinates correspond to the fully relaxed configurations employed for both catalytic activity evaluation and electronic structure analysis.
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
References
Tan, C. et al. Recent advances in ultrathin two-dimensional nanomaterials. Chem. Rev. 117, 6225–6331 (2017).
Zhao, X. et al. Engineering covalently bonded 2D layered materials by self-intercalation. Nature 581, 171–177 (2020).
Leng, K. et al. Molecularly thin two-dimensional hybrid perovskites with tunable optoelectronic properties due to reversible surface relaxation. Nat. Mater. 17, 908–914 (2018).
Chen, T.-A. et al. Wafer-scale single-crystal hexagonal boron nitride monolayers on Cu (111). Nature 579, 219–223 (2020).
Hou, L. et al. Synthesis of a monolayer fullerene network. Nature 606, 507–510 (2022).
Wang, M. et al. Exceptionally high charge mobility in phthalocyanine-based poly(benzimidazobenzophenanthroline)-ladder-type two-dimensional conjugated polymers. Nat. Mater. 22, 880–887 (2023).
Li, J. et al. Towards the scalable synthesis of two-dimensional heterostructures and superlattices beyond exfoliation and restacking. Nat. Mater. 23, 1326–1338 (2024).
Xiao, D. et al. Single crystals of purely organic free-standing two-dimensional woven polymer networks. Nat. Chem. 16, 1906–1914 (2024).
Pumera, M. & Sofer, Z. Towards stoichiometric analogues of graphene: graphane, fluorographene, graphol, graphene acid and others. Chem. Soc. Rev. 46, 4450–4463 (2017).
Tan, J. et al. Metal-lattice-heredity synthesis of single-crystalline 2D transition metal oxides. Matter 8, 101873 (2024).
Wang, D. et al. Direct synthesis and chemical vapor deposition of 2D carbide and nitride MXenes. Science 379, 1242–1247 (2023).
Chakraborty, G., Park, I.-H., Medishetty, R. & Vittal, J. J. Two-dimensional metal-organic framework materials: synthesis, structures, properties and applications. Chem. Rev. 121, 3751–3891 (2021).
Jiang, Y. et al. Porous two-dimensional monolayer metal-organic framework material and its use for the size-selective separation of nanoparticles. ACS Appl. Mater. Interfaces 9, 28107–28116 (2017).
Luo, L. et al. Self-condensation-assisted chemical vapour deposition growth of atomically two-dimensional MOF single-crystals. Nat. Commun. 15, 3618 (2024).
Yang, J. et al. Advancing osmotic power generation by covalent organic framework monolayer. Nat. Nanotechnol. 17, 622–628 (2022).
Roth, W. J., Nachtigall, P., Morris, R. E. & Čejka, J. Two-dimensional zeolites: current status and perspectives. Chem. Rev. 114, 4807–4837 (2014).
Varoon Agrawal, K. et al. Dispersible exfoliated zeolite nanosheets and their application as a selective membrane. Science 334, 72–75 (2011).
Chlubná, P. et al. 3D to 2D routes to ultrathin and expanded zeolitic materials. Chem. Mater. 25, 542–547 (2013).
Korde, A. et al. Single-walled zeolitic nanotubes. Science 375, 62–66 (2022).
Zhang, H. et al. Ultrafiltration separation of Am(VI)-polyoxometalate from lanthanides. Nature 616, 482–487 (2023).
Li, Z., Liu, Q. & Wang, X. Two-dimensional cluster-assembled materials with properties beyond their individualities and bulks. Matter 6, 3747–3762 (2023).
Zhang, S., Shi, W. & Wang, X. Locking volatile organic molecules by subnanometer inorganic nanowire-based organogels. Science 377, 100–104 (2022).
Li, Z., Zhang, Z., Hu, H., Liu, Q. & Wang, X. Synthesis of two-dimensional polyoxoniobate-based clusterphenes with in-plane electron delocalization. Nat. Synth. 2, 989–997 (2023).
Li, H., Zheng, L., Lu, Q., Li, Z. & Wang, X. A monolayer crystalline covalent network of polyoxometalate clusters. Sci. Adv. 9, eadi6595 (2023).
Zhang, F., Li, H., Li, Z., Liu, Q. & Wang, X. Phase engineering of polyoxometalate assembled superstructures. Nat. Synth. 3, 1039–1048 (2024).
Liu, Q. et al. Single molecule-mediated assembly of polyoxometalate single-cluster rings and their three-dimensional superstructures. Sci. Adv. 5, eaax1081 (2019).
Liu, J. et al. Incorporation of clusters within inorganic materials through their addition during nucleation steps. Nat. Chem. 11, 839–845 (2019).
Liu, J., Shi, W. & Wang, X. Cluster-nuclei coassembled into two-dimensional hybrid CuO-PMA sub-1 nm nanosheets. J. Am. Chem. Soc. 141, 18754–18758 (2019).
Liu, J., Shi, W. & Wang, X. ZnO-POM cluster sub-1 nm nanosheets as robust catalysts for the oxidation of thioethers at room temperature. J. Am. Chem. Soc. 143, 16217–16225 (2021).
Zhang, Y. et al. Fullerene on non-iron cluster-matrix co-catalysts promotes collaborative H2 and N2 activation for ammonia synthesis. Nat. Chem. 16, 1781–1787 (2024).
Zheng, J. et al. Ambient-pressure synthesis of ethylene glycol catalyzed by C60-buffered Cu/SiO2. Science 376, 288–292 (2022).
Zhao, W. et al. Bismuth telluride supported sub-1 nm polyoxometalate cluster for high-efficiency thermoelectric energy conversion. Nano Lett. 24, 5361–5370 (2024).
Yang, Y. et al. Polyoxometalate clusters confined in reduced graphene oxide membranes for effective ion sieving and desalination. Adv. Sci. 11, 2402018 (2024).
Cao, X. et al. Cluster-level heterostructure of PMo12/Cu for efficient and selective electrocatalytic hydrogenation of high-concentration 5-hydroxymethylfurfural. J. Am. Chem. Soc. 146, 25125–25136 (2024).
Xiao, Y., Chen, Y., Wang, W., Bu, X. & Feng, P. Advancing pore-space-partitioned metal-organic frameworks with isoreticular cluster concept. Angew. Chem. Int. Ed. 63, e202403698 (2024).
Liu, Z. et al. Crosslinking ionic oligomers as conformable precursors to calcium carbonate. Nature 574, 394–398 (2019).
Ma, Z. et al. High mechanical strength alloy-like minerals prepared by inorganic ionic co-cross-linking. Adv. Mater. 36, e2308017 (2023).
Mu, Z. et al. Pressure-driven fusion of amorphous particles into integrated monoliths. Science 372, 1466–1470 (2021).
Patel, A. & Patel, K. Cs salt of di-manganese(II) substituted phosphotungstate: one pot synthesis, structural, spectroscopic characterization and solvent free liquid phase oxidation of styrene using different oxidants. Polyhedron 69, 110–118 (2014).
Nakka, L., Molinari, J. E. & Wachs, I. E. Surface and bulk aspects of mixed oxide catalytic nanoparticles: oxidation and dehydration of CH3OH by polyoxometallates. J. Am. Chem. Soc. 131, 15544–15554 (2009).
Santos, V. P. et al. Structural and chemical disorder of cryptomelane promoted by alkali doping: Influence on catalytic properties. J. Catal. 293, 165–174 (2012).
Hou, J., Li, Y., Mao, M., Ren, L. & Zhao, X. Tremendous effect of the morphology of birnessite-type manganese oxide nanostructures on catalytic activity. ACS Appl. Mater. Interfaces 6, 14981–14987 (2014).
Yang, Y. et al. Novel photoactivation and solar-light-driven thermocatalysis on ε-MnO2 nanosheets lead to highly efficient catalytic abatement of ethyl acetate without acetaldehyde as unfavorable by-product. J. Mater. Chem. A 6, 14195–14206 (2018).
Mancuso, J. L., Mroz, A. M., Le, K. N. & Hendon, C. H. Electronic structure modeling of metal-organic frameworks. Chem. Rev. 120, 8641–8715 (2020).
Kesavan, L. et al. Solvent-free oxidation of primary carbon-hydrogen bonds in toluene using Au-Pd alloy nanoparticles. Science 331, 195–199 (2011).
Zhang, W. et al. Selective aerobic oxidation reactions using a combination of photocatalytic water oxidation and enzymatic oxyfunctionalizations. Nat. Catal. 1, 55–62 (2018).
Cao, X. et al. A photochromic composite with enhanced carrier separation for the photocatalytic activation of benzylic C-H bonds in toluene. Nat. Catal. 1, 704–710 (2018).
Partenheimer, W. Methodology and scope of metal/bromide autoxidation of hydrocarbons. Catal. Today 23, 69–158 (1995).
Gao, J., Tong, X., Li, X., Miao, H. & Xu, J. The efficient liquid-phase oxidation of aromatic hydrocarbons by molecular oxygen in the presence of MnCO3. J. Chem. Technol. Biotechnol. 82, 620–625 (2007).
Mir, B. A., Rajamanickam, S., Begum, P. & Patel, B. K. Copper(I) catalyzed differential peroxidation of terminal and internal alkenes using TBHP. Eur. J. Org. Chem. 2020, 252–261 (2020).
Barman, P. et al. Deformylation reaction by a nonheme manganese(III)-peroxo complex via initial hydrogen-atom abstraction. Angew. Chem. Int. Ed. 55, 11091–11095 (2016).
Rerek, M. E., Weil, I. & Hill, M. Kinetics and mechanism of the Mn(III)gluconate catalyzed decomposition of hydrogen peroxide. Coord. Chem. Rev. 105, 251–268 (1990).
Li, Z. et al. Single-walled cluster nanotubes for single-atom catalysts with precise structures. J. Am. Chem. Soc. 146, 450–459 (2024).
Liu, G. et al. Indirect electrocatalysis S-N/S-S bond construction by robust polyoxometalate based foams. Adv. Mater. 35, 2304716 (2023).
Acknowledgements
This work was supported by NSFC (92461314, 22035004, 22241502, 22405047) (to X.W. and Z.L.) and the XPLORER PRIZE (to X.W.). The calculations were supported by the Center of High Performance Computing, Tsinghua University. We sincerely thank the Tsinghua University Analysis Center for TEM testing. We thank Tao Liu for technical support during cryo-EM image acquisition, and we acknowledge the Technology Center for Protein Sciences at Tsinghua University for providing the cryo-EM facility support.
Author information
Authors and Affiliations
Contributions
X.W. proposed and guided the project. H.L. and Q. Lu carried out the experiments and wrote the manuscript. F.Z., Q. Liu, and J.Z. helped with the data analysis. Z.L. and Q. Lu performed the calculations and analyzed the results. X.W. and Z.L. helped with article revisions. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Ye Zhang and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, H., Lu, Q., Zhang, F. et al. Single-layer cluster ionic-chain networks with tetragonal pores. Nat Commun 16, 5778 (2025). https://doi.org/10.1038/s41467-025-60879-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-60879-y