Abstract
Reactivating the human epicardium post-cardiac injury holds promise for cardiac tissue regeneration. Despite successful differentiation protocols yielding pure, self-renewing epicardial cells from induced pluripotent stem cells (iPSCs), these cells maintain an embryonic, proliferative state, impeding adult epicardial reactivation investigation. We introduce an optimized method that employs mammalian target of rapamycin (mTOR) signaling inhibition in embryonic epicardium, inducing a quiescent state that enhances multi-step epicardial maturation. This yields functionally mature epicardium, valuable for modeling adult epicardial reactivation. Furthermore, we assess cardiac organoids with cardiomyocytes and mature epicardium, probing molecular mechanisms governing epicardial quiescence during cardiac maturation. Our results highlight iPSC-derived mature epicardium’s potential in investigating adult epicardial reactivation, pivotal for effective cardiac regeneration. Additionally, the cardiac organoid model offers insight into intricate cardiomyocyte-epicardium interactions in cardiac development and regeneration.
Similar content being viewed by others
Introduction
The human epicardium is the outermost layer of the heart, and it plays an important role in the development and regeneration of the heart1. During embryonic development, the epicardium gives rise to a population of cell-fate-oriented cardiac progenitor cells that can differentiate into various cell types2,3,4. Recent studies in mice have indicated that in the initial days following birth, as well as during embryonic development, epicardial cells possess the capacity to regenerate following myocardial injury5. This property is progressively lost during postnatal life. While most studies identify both commonalities and differences in the transcriptomic characteristics defining the mature epicardium, it is widely accepted that the adult epicardium, in a healthy state, is a dormant tissue with low migratory and regenerative capacities, and remains quiescent6.
Despite being non-proliferative, quiescent epicardial cells remain metabolically and transcriptionally active7. Interestingly, the period of epicardial quiescence is concurrent with the increased maturation of the heart8.
Current differentiation protocols to produce epicardial cells from human-induced pluripotent stem cells (hiPSCs) can generate highly pure, long-term self-renewing epicardial cell populations retained in an embryonic, proliferative state as determined by the expression of the Wilms tumor protein, encoded by the WT1 gene9,10,11,12,13,14,15,16,17,18. However, the immaturity of iPSC-derived epicardial cells presents a significant barrier to creating cellular platforms for modeling mature epicardium, which is crucial for elucidating the fundamental biology of epicardial reactivation in adulthood. Understanding this reactivation is essential for effective cardiac regeneration19. Recent studies have succeeded in isolating primary epicardial tissue in adult individuals and have studied its transcriptional profile in contrast to that of fetal counterparts6,20,21,22.
Cellular quiescence, a reversible state of cell cycle arrest23, is a critical mechanism for maintaining tissue homeostasis in adult tissues24,25,26. The mTOR signaling pathway plays a crucial role in regulating cellular quiescence in mature tissues by sensing and responding to changes in nutrient availability, growth factors, and other environmental cues27,28,29.
mTOR inhibition has been shown to assist the maturation phenotypes of other cell types, including pancreatic β cells30,31, cardiomyocytes32, natural killer cells33,34,35, and erythroid cells36,37. During in vivo heart development, mTOR signaling generally declines, suggesting that mTOR inhibition is associated with the induction of functional maturation in various cell types within the cardiovascular system. However, the extent to which mTOR signaling regulates the activation and deactivation of the human epicardium, the outermost layer of the heart wall, remains largely unexplored. Our study focused on identifying molecular mechanisms that regulate epicardial quiescence and the loss of regenerative capacity in adulthood. Understanding these mechanisms may provide new targets for the development of therapies to promote cell-specific cardiac regeneration following injury.
In this study, we designed a robust method to model mature epicardium from hiPSCs and to understand its developmental dynamics during the transition from the embryonic to neonatal/postnatal stages. This differentiation approach utilizes methods that involve inhibiting mTOR signaling in embryonic epicardial cells to induce a quiescent state, thereby mimicking the functional maturation of the tissue. We observed that the maturation of the epicardium relies on a mechanism dependent on mTOR complex 2 (C2). This is evidenced by the fact that rapamycin, an inhibitor of mTOR complex 1 (C1) kinase, did not promote complete mature phenotypes. The MAFF gene, which encodes a basic region-leucine zipper (bZIP) transcription factor with crucial functions in maintaining physiological homeostasis38, was identified as a transcription factor responsible for regulating cell cycle withdrawal during epicardial maturation. The YBX3 gene, an RNA-binding protein coding gene39, is instrumental in regulating specific subsets of mRNAs. Moreover, it modulates its own abundance via distinct mechanisms39. Crucially, YBX3 is vital for inhibiting the embryonic epicardial program during maturation. The results of this study demonstrate that our platform of mature hiPSC-derived epicardium is applicable for high-throughput screening approaches and has identified GSK3α/β inhibitors as active compounds that reactivate epicardial proliferation. The inhibition of mTOR signaling to induce cellular quiescence can facilitate the differentiation of functional and mature human heart organoids, which comprise a mature epicardium and cardiomyocytes and exhibit a higher proportion of endothelial cells at single-cell resolution under mTOR-inhibiting conditions. These organoids represent an invaluable resource in basic cell biology with clinical applications and help to investigate the molecular mechanisms underlying epicardial reactivation in the context of cardiac regeneration during adulthood and aging. Thus, this study highlights the potential utility of targeting cellular quiescence through mTOR inhibition as a means to induce advanced cardiac models for studying development and regeneration.
Results
Reduced activity of the mTOR signaling pathway accompanies physiological heart maturation
mTOR is a central regulator of cell growth and metabolism, playing a key role in tissue homeostasis. mTOR signaling is particularly intertwined with the insulin-like growth factor 1 (IGF-1) pathway40,41,42. IGF-1 promotes the activation of the mTOR pathway, which in turn fosters cell proliferation and inhibits cell apoptosis during development and regeneration, thus controlling the overall cellularity in tissues. By regulating the balance between cell proliferation and death, mTOR maintains tissue integrity and function, particularly through the IGF-1 pathway. Therefore, mTOR signaling switches may be associated with the initiation cues of tissue maturation, regulating the balance between proliferation and functional maturity. First, to evaluate the mTOR signaling dynamics during murine cardiogenesis, we retrospectively analyzed publicly available transcriptomic data with time intervals to monitor changes in gene expression related to maturation of the heart43, and we confirmed a transcriptomic switch progressively deactivating mTOR signaling starting from E12.5 onwards (Fig. 1a). We conducted experiments to investigate the downregulation of genes involved in the mTOR pathway, specifically those responsible for assembling the two major mTOR complexes, C1 and C2 (Fig. 1b), namely MTOR, RPTOR, and RICTOR. These complexes play a crucial role in phosphorylating downstream targets when the pathway gets active. mTORC1 primarily regulates protein synthesis, cell growth, and autophagy through ribosomal protein S6 kinase (S6K) and the eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1)27. In contrast, mTORC2 is involved in cell survival and cytoskeleton organization, primarily through the phosphorylation of protein kinase B (also known as AKT) in conjunction with complex 144 (Fig. 1b).
a Dataset analysis of mouse heart development reveals multi-step mTOR signaling inactivation. b Schematic of heart’s mTOR network. c Mouse heart Western blot characterizes developmental mTOR targets (pAKT, pS6K, p4E-BP1, p27; β-actin normalization). d Dataset retroanalysis shows heart development’s cell cycle dynamics in GSE1479. a, b, d Partly created in BioRender. Lucena-Cacace, A. (2025) https://BioRender.com/2uhoset. e Sample confocal images of ZO-1 and WT1 co-immunostaining of E12, E14, E18, and adult mouse left ventricle (scale bar: 100 µm). f Phase contrast image of epicardial outgrowth from E12 heart explant. g Epicardial explant Western blot depicts mTOR target suppression over time (pAKT, pS6K, p4E-BP1, WT1; β-actin normalization). h Epicardiogenesis relative gene expression (to E12) of Wt1, Tbx18, Cdh18 and Nbl1 (n = 3 biological replicates). Data are presented as means ± s.e.m.; two-tailed unpaired Student’s t-test. * p < 0.05, ** p < 0.01, *** p < 0.001. Source data is provided as a Source Data file.
To determine the developmental switch towards heart maturation, we examined the phosphorylation targets of mTORC1/2 in protein lysates obtained from embryonic hearts at different time intervals. At E18, we observed a significant decrease in pAKT, pS6K, and p4E-BP1 levels compared to their levels at the younger E12 stage. This indicates a more proliferative state at E12 and confirms the progressive reduction of mTOR signaling over time. Additionally, at one week after birth (P7), we confirmed the upregulation of p27 (Fig. 1c) a key cell cycle inhibitor associated with cellular quiescence45,46,47. Increased levels of p27 arrest cell cycle progression, maintaining cells in the non-dividing, yet metabolically active, G0 phase and preventing excessive cell proliferation, allowing cells to acquire adult functionality and specialization. Furthermore, we observed a progressive upregulation of essential cyclin-dependent kinase inhibitors over time (Fig. 1d), indicating a correlation with cellular quiescence as part of the heart’s developmental trigger towards maturation.
Based on the notion that epicardial maturation likely involves a similar pattern of quiescence as an integral mechanism of heart maturation, at least one shared with cardiomyocytes, we investigated this tissue in isolation. Initially, this study confirmed the cortical distribution of the tight junction protein ZO-1 and observed the epicardium-specific transcription factor WT1 expression on E12 (Fig. 1e and Supplementary Fig. 1a). From E14 onwards, there was a decrease in WT1 protein expression, suggesting the onset of a transcriptional departure from the fetal epicardial network (Fig. 1e). In accordance with recent studies profiling mature epicardium, an increase in mesothelin (MSLN) protein expression was observed6,48 (Supplementary Fig. 1b). Intriguingly, while recent studies have suggested that transcriptomic elevation of C3 is associated with mature epicardium20, our in vivo observations showed a reduction in epicardial C3 protein in adult hearts compared to fetal ones (Supplementary Fig. 1c).
To gain a deeper understanding of maturation in an epicardial-specific context, we conducted experiments involving outgrown epicardial explants from various developmental stages (Fig. 1f). We observed that the acquisition of epicardial maturation was correlated with a decline in mTOR signaling (Fig. 1g), suggesting a shift towards quiescence and tissue homeostasis in adulthood. Additionally, we monitored the expression and protein dynamics over time until the neonatal stage. Our findings confirmed a gradual decrease in fetal markers over time, accompanied by a gradual increase in Nbl1—a maturation marker known for countering the Wt1-mediated Bmp4 signaling pathway during murine epicardial maturation49 (Fig. 1h).
Chemical inhibition of mTOR during embryonic development assists epicardial maturation ex vivo
To elucidate the physiological implications of our observations, we investigated the response of E12 hearts when exposed to an ectopic maturation stimulus. To fully inhibit mTOR signaling, we examined the influence of Torin1, a mTOR kinase inhibitor (mTORKi) able to interrupt a broad spectrum of mTOR-regulated cellular activities. Torin1 interacts with the ATP-binding pockets of both mTOR complexes50, hence its potential to inhibit the functionalities of mTORC1 and mTORC2.
We obtained freshly isolated hearts from E12 embryos and cultivated them under anchorage-independent conditions, using chemical conditions that ensured cellular viability and sustained heart contractions over time. To determine an optimal dose that balances efficacy with cellular safety, we gauged the inhibitory capacity of the inhibitor on mouse epicardial cells. We defined the IC50 of Torin1 using monolayers of an established mouse epicardial cell line (MEC1). The IC50 elucidated that Torin1 can inhibit 50% of cellular growth at a concentration of 60 nM in a span of 96 h (Supplementary Fig. 2a). In MEC1 cells, Torin1 exposure reduced the expression of both proliferative and fetal epicardial markers (Supplementary Fig. 2b) while also impairing EMT-triggering capacity in response to TGF-β stimulation, both morphologically and transcriptomically (Supplementary Fig. 2c, d). Subsequently, we chose to analyze the mTOR inhibition via a brief acute exposure to Torin1 at a concentration of 200 nM for 24 h. This condition had previously been proven to enhance the maturation attributes of hiPSC-derived cardiomyocytes. Our results confirmed that a single administration of Torin1 significantly reduced WT1 protein expression in the mouse epicardium, similar to what is observed in adult hearts. This reduction was accompanied by an elevation of the pro-apoptotic Caspase-3 in the myocardium of E12-treated hearts (Supplementary Fig. 1d) but did not cause observable cell death in the epicardium (Fig. 2a and Supplementary Fig. 1d). This reinforces that mTOR pathway inhibition can expedite the developmental transition essential for improving epicardial maturation and turning off its embryonic scheme.
a WT1 expression in E12 heart after 24-hour of 200 nM Torin1 treatment (scale bar: 500 µm). b Western blot of E12 and E14 whole heart: mTOR suppression during embryonic development of heart and maturation with Torin1 (cTnI (TNNI3), pS6K, pAKT, WT1, SNAI1; β-actin normalization). Partly created in BioRender. Lucena-Cacace, A. (2025) https://BioRender.com/a73fm2y. c Western blot of E12 epicardial explant after Torin1 treatment (pS6K, p4E-BP1, pAKT, WT1 and TNNI1; β-actin normalization). b, c Experiments were repeated with similar results 2 times. d Size of epicardial outgrowth from heart explant over time (n = 6 biological replicates; scale bar: 2 mm). Data are presented as means ± s.e.m. e PCA of Torin1-treated vs untreated E12 epicardial explants (black circles, control, n = 2; pink circles, Torin1, n = 2; red circles, adult, n = 2). f Hierarchical clustering dendrogram depicting the relationship between identified clusters based on Manhattan distance. The vertical axis represents the degree of similarity between clusters, with shorter branch lengths indicating closer transcriptional relationships. Clusters with shared gene expression profiles are grouped together, revealing the hierarchical structure of cellular populations within the dataset. This analysis provides insight into the relative transcriptional proximity of different cell types and their potential lineage relationships. g Bar graph displaying the top 10 most upregulated and h downregulated pathways identified from differentially expressed genes (DEGs). Pathway enrichment significance is represented as −log10 (p-value) on the x-axis, with higher values indicating stronger statistical significance. The y-axis lists the names of the enriched pathways, each represented by a bar. This analysis highlights key biological processes and signaling pathways that are significantly upregulated in the dataset, providing insights into the functional impact of the transcriptional changes observed. The statistical method used is over-representation analysis (ORA) using Fisher’s exact test, with significant pathways represented by p < 0.05. i Three heatmaps displaying the z-score normalized expression of gene signatures related to cell cycle (proliferation), epithelial-to-mesenchymal transition (EMT, migration), and mTOR signaling in fetal and adult samples. The color scale represents the z-score of gene expression, with positive values indicating upregulation and negative values indicating downregulation relative to the mean expression of each gene. The cell cycle heatmap highlights differences in proliferative activity, the EMT heatmap reveals changes in migratory and mesenchymal properties, and the mTOR signaling heatmap demonstrates variations in pathway activation between fetal and adult states. These comparative analyses provide insights into the transcriptional dynamics underlying developmental transitions and pathway-specific regulation. j Heatmap displaying the z-score normalized expression of genes associated with epicardial maturation across different sample conditions, including Torin1-treated samples. The color scale represents the z-score of gene expression, where positive values indicate upregulation and negative values indicate downregulation relative to the mean expression of each gene. This visualization highlights transcriptional differences in epicardial maturation signatures between untreated and Torin1-treated conditions, providing insights into the regulatory effects of Torin1 on epicardial cell state and development. Clustering of samples and genes reveals distinct expression patterns that may reflect developmental transitions and pathway-specific modulations in response to treatment. k Volcano plot visualizing differentially expressed genes (DEGs) based on statistical significance and fold-change. The x-axis represents the log₂ fold-change (log₂FC), indicating the magnitude of gene expression differences, while the y-axis shows the −log₁₀(p-value), representing statistical significance. DESeq2 was used as the statistical method to identify significant differentially expressed genes (DEGs). Upregulated genes are displayed on the right, and downregulated genes on the left, with significantly differentially expressed genes highlighted. This analysis provides an overview of transcriptional changes, distinguishing key genes that exhibit strong differential expression in the dataset. Source data is provided as a Source Data file.
To corroborate the maturation extent induced by Torin1 in embryonic mouse hearts, we systematically assessed and contrasted its effects on mouse hearts from both E12 and E14 stages. Initial results in untreated hearts verified that markers WT1, phospho-AKT, and phospho-S6K exhibit greater expression in E12 compared to E14 (Fig. 2b). Following Torin1 treatment, WT1 levels markedly decreased. At the same time, the adult isoform of cardiac troponin I, TNNI3, increased significantly, suggesting an accelerated synced onset of mature indicators in both the epicardium and myocardium (Fig. 2b).
Considering the potential underrepresentation of epicardial markers in bulk heart tissue, we expanded again the epicardial explant to focus on epicardial maturation in primary cultures. After a seven-day treatment period, there was a consistent reduction in WT1 and other mTOR downstream targets, further confirming the specificity of mTOR downregulation in contributing to cell and tissue maturation (Fig. 2c and Supplementary Fig. 1e). Epicardial explants treated with Torin1 exhibited an absence of cardiac TNNI1 indicating residual absence of cardiomyocytes (Fig. 2c).
We next extended the epicardial explant culture of WT1-expressing cells (Supplementary Fig. 2e, h) for a week in the presence of SB431542. This ALK5 inhibitor prevents the spontaneous activation of the TGF-β signaling pathway and subsequent epithelial-to-mesenchymal transition (EMT)-driven migratory capacity (Supplementary Fig. 2f). The results revealed that samples treated with Torin1 displayed a decrease in proliferation of epicardial outgrowth (Fig. 2d). Notably, no significant morphological changes were observed (Supplementary Fig. 2h). This suggests an impaired migratory ability of the cells, a characteristic feature of mature epicardium (Supplementary Fig. 2f, g).
To understand the role of mTOR suppression in epicardial maturation at the transcriptomic level, we subjected Torin1-treated epicardial explants to RNA sequencing, benchmarking them against the transcriptomic profile of freshly isolated postnatal epicardium from an adult heart (Supplementary Fig. 2i).
Principal component analysis revealed that the first two principal components (PC1 and PC2) accounted for 54.60% of the total variation, with Torin1-treated epicardium plotting spatially closer to adult samples. This suggests a whole-transcriptomic shift from a fetal state toward further maturation (Fig. 2e). This proximity was further visualized in the cluster dendrogram, which showed that Torin1-treated explants clustered more closely with their postnatal counterparts, highlighting that mTOR inhibition contributes to the transcriptomic acquisition of profiles resembling those of mature epicardium (Fig. 2f).
Next, to achieve a more accurate characterization of adult murine epicardium, we performed a pathway enrichment analysis of differentially expressed genes between fetal and adult epicardium. This analysis revealed that the p53 pathway is among the top 10 upregulated pathways (Fig. 2g), whereas the mTOR signaling, glycolysis, and insulin pathways are significantly downregulated among the top 10 downregulated pathways (Fig. 2h).
We then compared gene sets related to cell cycle (proliferation), EMT (migration), and the mTOR signaling pathway to confirm that postnatal epicardium is a quiescent, non-migratory, mTOR-inactive cardiac mesothelium, properties shared by Torin1-treated tissues (Fig. 2i).
Finally, we conducted a direct comparison of genes related to epicardial identity and maturation and confirmed that Torin1-treated tissues exhibit greater transcriptomic similarity to epicardial maturation gene signatures than their fetal, untreated counterparts (Fig. 2j). Although the treatment does not result in a perfect match, our data indicate that mTOR inhibition is an important factor in the maturation of fetal epicardium. This is further supported by the low number of differentially expressed genes (both upregulated and downregulated) between Torin1-treated and adult epicardium (Fig. 2k).
Dual inhibition of mTORC1/2 enhances epicardial quiescence and maturation in hiPSC-derived epicardium
To fully understand the contribution of cellular quiescence in the maturation of the human embryonic epicardium, we used hiPSC-derived epicardium to engineer them and study systematically the effects of the cell cycle withdrawal via the complete inhibition of the mTOR pathway. We first differentiated epicardial-like cells (EPI) from hiPSCs18 (Supplementary Fig. 3a) to form cardiac mesoderm (d1 ~ 3/4) using Activin A and BMP4, and then specified epicardial fate by activating WNT signaling (d3/4 ~ 8). We inhibited TGF-β signaling (SB431542) from d3/4 onwards to maintain epicardial hallmarks and enable long-term EPI cell culture without spontaneous differentiation. We consistently derived EPI cells from hiPSC line 409 B2, which showed typical cobblestone-like morphology at day 24 (Supplementary Fig. 3b) and upregulated WT1, TBX18, and ALDH1A2 during differentiation (Supplementary Fig. 3c). On day 24, EPI cells expressed nuclear WT1, which was confirmed by flow cytometry and immunofluorescence co-staining with ZO1 (Supplementary Fig. 3d, e). Next, to trigger epicardial quiescence, we evaluated the effects of Torin1 and its capacity to dually inhibit mTORC1 and mTORC2 (Fig. 3a) to promote a complete cell cycle withdrawal. First, we assessed the cytotoxic effects of Torin1 at various dosage levels over a 96-h period in vitro using an MTT assay (Supplementary Fig. 3f). Following the outcomes from these procedural refinement steps evaluating cytotoxicity (Supplementary Fig. 3g), we treated the cells to 200 nM Torin1 for 7 days. This treatment commenced post-verification of peak WT1 protein levels on the 24th day of differentiation.
a Workflow to monitor Torin1 effects on hiPSC-derived epicardial monolayers. Created in BioRender. Lucena-Cacace, A. (2025) https://BioRender.com/qbjorue. b Western blot of Torin1-treated hiPSC-epicardial cells assesses mTOR target inhibition and quiescence markers (pAKT, pS6K, p53, p16, and p21; β-actin normalization). c Phase contrast microscopy shows day 31 Torin1-treated hiPSC-derived epicardium retains cobblestone morphology (scale bar: 200 µm). d Growth curve of Torin1-treated epicardium for 7 days (n = 3 biological replicates). e Immunocytochemical analysis of Torin1-treated hiPSC-epicardial cells for WT1, TBX18, and ZO-1 expression (scale bar: 200 µm). f, g Immunocytochemical analysis of Torin1-treated hiPSC-epicardial cells for MSLN and ZO-1 expression (scale bar: 200 µm), and immunofluorescence intensity quantification (n = 3 biological replicates). h MKI67 expression (left) and quantification (right) via Immunocytochemistry (scale bar: 200 µm; n = 3 biological replicates). i Immunocytochemistry for phosphor-histone 3 (pH3) expression (left) and quantification (right) (scale bar: 200 µm; n = 6 biological replicates). j Invasion assay on Torin1-treated hiPSC-epicardial cells. Representative images show invaded cells stained by crystal violet. The total number of celling invading through membrane are quantified. (scale bar: 500 µm; n = 3 biological replicates). k FACS analysis based on Pyrorin Y and Hoechst 33342 staining discriminates G0 cells. l Quantification and statistical analysis of quiescent hiPSC-epicardial cells after Torin1 treatment (Torin1, purple bars; n = 3 biological replicates). Data are presented as means ± s.e.m.; two-way ANOVA with Sidak’s multiple comparison test (d); two-tailed unpaired Student’s t-test (g–j, l). *p < 0.05, **p < 0.01, ***p < 0.001. Source data is provided as a Source Data file.
Upon administration to differentiated epicardial cells, Torin1 treatment (Fig. 3a) resulted in a notable reduction in phosphorylation levels of both S6K and AKT (Fig. 3b). This highlights its effect on both mTORC1 (S6K) and mTORC2 (AKT), while preserving the epicardial structure (Fig. 3c). We noted that AKT regained its phosphorylated form within two days following a one-time Torin1 application (Supplementary Fig. 3h), implying that ongoing treatment might be required to ensure continued mTORC2 suppression. Torin1-treated cells rapidly underwent proliferative arrest (Fig. 3d), a key characteristic of the human adult epicardium. However, proliferative arrest alone was not sufficient, as confirmed by targeting CDK4-driven proliferation with 1 µM Palbociclib (Supplementary Fig. 5a–d), which did not fully mimic a mature epicardial state as Torin1 does, as observed in the murine counterpart.
Next, we verified that Torin1 treatment effectively reduced nuclear WT1 and TBX18 levels while preserving epicardial morphology and maintaining tight junction integrity, as indicated by ZO-1 expression (Fig. 3e and Supplementary Fig. 3i). Additionally, we confirmed a robust elevation of mesothelin (Fig. 3f, g and Supplementary Fig. 5d) in Torin1-treated cells but not under Palbociclib treatment (Supplementary Fig. 4a), along with a significant reduction in C3 protein levels (Supplementary Fig. 4a), consistent with our previous in vivo observations.
Additionally, we observed a noteworthy decrease in the proliferative protein Ki67 (Fig. 3h), along with retained inactivation of the EMT marker SNAI1 and SNAI2 (Supplementary Fig. 4b, c), confirmed by a loss of migratory capacity (Fig. 3j). Furthermore, phosphohistone H3 (pH3)—which stains condensed chromosomes in the mitotic (M) phase—was significantly reduced in Torin1-treated cells (Fig. 3i). To discriminate between senescent and quiescent phenotypes, we performed a beta-galactosidase (β-gal) assay, known for its widely accepted specificity to senescent cells. Torin1-treated epicardium significantly reduced the β-gal+ cells (Supplementary Fig. 4d, e), suggesting that dual inhibition of mTORC1 and C2 may redirect epicardium away from a senescent phenotype, also confirmed by decreased expression of SASP-related genes CXCL8 and SPP1 (Supplementary Fig. 4f). We verified a significant decrease in the transcription of markers associated with cell proliferation (MKI67, CCNA1, CCNB1 and E2F1), while observing a trend of increased expression of markers associated with cell quiescence, including p53 (Supplementary Fig. 4g). To eliminate the possibility of variations arising from donor-specific or protocol-specific differences in the origin and culture conditions of the iPS cells, we validated our primary findings using epicardial monolayers obtained from feeder-free colonies of the 1390C1 line, following the established epicardial GiWiGi protocol16 (Supplementary Fig. 7a). Furthermore, we confirmed our initial observations by demonstrating that Torin1 effectively reduced the expression of the WT1 protein, corroborating our earlier protein analysis (Supplementary Fig. 7b). To further investigate the level of quiescence, we utilized Pyronin Y staining, which has proven to be a reliable method for detecting cells in the G0 phase due to its ability to selectively stain RNA content in cells51. As cells in the G0 phase exhibit reduced RNA synthesis compared to actively cycling cells, we coupled this technique with Hoechst 33342 staining to simultaneously label DNA content, allowing us to identify distinct cell populations in different phases of the cell cycle. Torin1 treatment resulted in a substantial withdrawal from the cell cycle, as demonstrated in FACS dynamics (Fig. 3k). This withdrawal was evidenced by a significant increase in the number of quiescent (G0) cells from 14.2% to 82.5%, along with a decrease in G1 cells from 75.9% to 1.83% (Fig. 3l). To investigate the transcriptional impact of Torin1 on iPS-derived epicardium, we performed a detailed RNA-sequencing analysis52. Consistent with our earlier observations regarding mTOR inhibition in mouse epicardium, cells treated with Torin1 profoundly shifted the transcriptomic landscape of hiPSC-derived epicardium, with principal components 1 and 2 accounting for 64.54% of the total variance in the PCA (Fig. 4a). Our analysis detected 379 differentially expressed genes specifically attributable to Torin1’s treatment (Fig. 4b). Although TJP1 expression—the gene responsible for ZO-1 protein—remained unaffected post-treatment, we confirmed a significant decrease in embryonic epicardial genes WT1, TBX18, ALDH1A2 and CDH18, a cadherin linked to the development of the epicardium (Fig. 4c). Furthermore, genes that drive EMT showed significant downregulation (Fig. 4d). This downregulation coincided with a marked decrease in MKI67 mRNA levels, aligning with our protein analyses (Fig. 4e). Elevated transcription of RB1 and HES1 reaffirmed the transcriptomic hallmark of a quiescent state53,54,55 (Fig. 4e). Finally, several transcripts associated with the metabolic shift toward OXPHOS and FAO, such as PPARGC1A, PDK4, and SIRT1, were substantially elevated in Torin1-treated cells (Fig. 4f). On the other hand, FASN and SREBF1, proteins involved in lipogenesis, were downregulated, which can indicate a shift toward FAO over lipid synthesis (Fig. 4f). In a broader context, we juxtaposed our RNA-seq with GSE840859, a dataset containing human adult epicardium primary samples. Comparative curated heatmaps underscored that Torin1-treated iPS-epicardium (Fig. 4g, h) and the adult epicardium (Fig. 4i, j) exhibit overarching characteristics consistent with a systemically quiescent and matured human epicardium, relative to their respective fetal counterparts (Fig. 4g–j).
a PCA of Torin1-treated and untreated d31 hiPSC-epicardial monolayers (black circles, control, n = 3; purple circles Torin1, n = 3). b Differentially enriched gene plot (DE Plot) highlighting up- and down-regulated genes upon Torin1 treatment. mRNA expression analysis for (c) genes related to fetal epicardial program (TJP1, WT1, TBX18, ALDH1A2, CDH18), (d) genes related to EMT (SNAI1, SNAI2, TFEB, CDH2, CDH1), (e) genes related to quiescence (RB1, HES1) and proliferation (MKI67, PCNA, MCM2), (f), genes related to fatty acid oxidation (PDK4, PPARGC1A, SIRT1, FASN, SREBF1) (control, black bars; Torin1, purple bars; n = 3). Heatmap showing up- and down-regulated gene signatures required for the activation of epicardial quiescence and maturation enriched in Torin1-treated hiPSC-epicardium (g, h) compared to primary tissue of human adult epicardium (GSE84085) (i, j). Data are presented as means ± s.e.m.; two-tailed unpaired Student’s t-test. *p < 0.05, **p < 0.01, ***p < 0.001. Source data is provided as a Source Data file.
High-Throughput Screening (HTS) for Reactivation of the hiPSC-Derived Mature Epicardium
To evaluate the applicability of our hiPSC-derived mature epicardial platform in vitro, we conducted a high-throughput screen (HTS) of ~1600 bioactive compounds for their ability to induce MKI67-expressing cells, both in number and expression level (see the “Methods” section and Supplementary Fig. 6g).
As a positive control, we used thymosin beta-4 (TB4), a small molecule that has been reported to contribute to epicardial reactivation in a limited yet supportive manner56,57,58. We first assessed the effects of TB4 in our cellular platform by treating mature epicardial monolayers with 100 nM TB4 for one day (Supplementary Fig. 6a), a concentration that mildly increased MKI67 expression (Supplementary Fig. 6b), modestly reactivated migratory capacity (Supplementary Fig. 6c, d), and increased detectable WT1 protein expression in the whole cell lysate (Supplementary Fig. 6e).
For the HTS experiment, cells were treated with Torin1 for 7 days to induce epicardial quiescence and functional maturation before further screening (Supplementary Fig. 6f). To ensure standardization, all bioactive compounds were tested at 1 µM, resulting in 29 successful hits (1.8% HIT rate) (Supplementary Fig. 6g), based on a threshold defined as the median value (MED) plus four times their standard deviation (SD) in total MKI67-expressing cell number (Supplementary Fig. 6h, j) and intensity (Supplementary Fig. 6i, j).
Pathway enrichment analysis of the identified compounds revealed GSK3α/β inhibitors as the primary active class (kinase inhibitors). Our findings support the preclinical applicability of our mature hiPSC-derived epicardium and establish a scalable cellular platform for high-throughput drug discovery in clinical applications for regenerative purposes.
YBX3 and MAFF are essential mTORC2-dependant transcription factors that facilitate the quiescence and maturation of epicardium derived from hiPSCs
mTOR functions as the central kinase of two unique multi-protein complexes: mTORC1 and mTORC2. These complexes differ in their constituent proteins and in their sensitivity to the drug rapamycin. Specifically, mTORC1 incorporates Raptor as a primary subunit, whereas Rictor is integral to mTORC2. While mTORC1’s activity is quickly suppressed by rapamycin, mTORC2 remains largely unaffected during short-term rapamycin exposure59. To determine the role of these mTOR complexes in epicardial maturation, hiPSC-derived epicardial monolayers were exposed to either Rapamycin or Torin1, which inhibits both mTOR complexes. Results showed that Rapamycin did not arrest proliferation in terms of MKI67 expression in epicardial cells, suggesting a failure in the acquisition of a quiescent state (Fig. 5a and Supplementary Fig. 5f, g), likely due to residual phospho-AKT activity. This suggests that mTORC2 plays a pivotal role, in conjunction with mTORC1, in epicardial maturation and quiescence, as the use of JR-AB2-011, a selective mTORC2 inhibitor, fails to reduce WT1 levels (Supplementary Fig. 5f).
a Immunocytochemical analysis of MKI67 expression in untreated, Torin1- and Rapamycin-treated hiPSC-epicardial cells at d24 + 7 (white arrows indicate positive signals; scale bar: 200 µm). Experiments were repeated with similar results 3 times. b Venn diagrams illustrating mTORC2-dependent elements in adult epicardium. Venn1 (top left) shows Torin1-specific transcription factors (TFs) not shared with Rapamycin. Venn2 (bottom left) presents TFs from differentially expressed genes (DEGs) significant in adult epicardium. Venn3 (right) displays the unique elements between Torin1-TFs (from Venn1) and adult-TFs (from Venn2). c mRNA of the top 5 upregulated TFs with Torin1 treatment. d Workflow for siRNA transfection against YBX3 and MAFF. Created in BioRender. Lucena-Cacace, A. (2025) https://BioRender.com/r4acodl. e mRNA expression of specific genes in Torin1-treated epicardial monolayers with siRNAs against YBX3 and MAFF (n = 4 biological replicates). Data are presented as means ± s.e.m.; two-tailed unpaired Student’s t-test. f Immunocytochemistry of siYBX3 Torin1-treated cells for WT1 and ZO-1 expression (scale bar: 100 µm). Experiments were repeated with similar results 3 times. g, h Western blot analyses of Torin1-treated hiPSC-epicardium with siRNAs against YBX3 and MAFF, examining proteins of the fetal program and cellular proliferation (WT1, TBX18, MKI67; β-actin normalization). Western blots were repeated with similar results 2 times. Source data is provided as a Source Data file.
Tacrolimus, commonly known as FK50660, is primarily used as an immunosuppressive agent for solid organ transplants61. Although its primary mechanism of action is as a calcineurin inhibitor, it is notable that both tacrolimus and rapamycin bind to FKBP12, which may influence in the maturation phenotype of the epicardium. However, tacrolimus treatment also failed to reduce the epicardial proliferation (Supplementary Fig. 8e), suggesting that inhibition of calcineurin or mTORC1 alone is insufficient to promote epicardial maturation of hiPSC-derived epicardial cells.
To examine how each treatment shaped the epicardial transcriptomic landscape, we performed RNA-sequencing to establish one-to-one comparisons. We pinpointed 66 transcription factors (TFs) that were uniquely altered by Torin1 treatment, denoting them as mTORC2-responsive transcription factors (mTORC2-TFs). In contrast, when comparing the effects of Rapamycin, these were classified as mTORC1-responsive TFs (mTORC1-TFs). A comparative transcriptomic analysis between fetal and adult epicardium derived from pluripotent stem cells (PSCs) uncovered 104 differentially expressed TFs, which we labeled as mature epicardium TFs. Notably, out of these, 24 TFs were present within the Torin1-responsive elements (Fig. 5b).
Next, we refined our list to include only those transcription factors with a fold change expression that is at least twice as high in mature epicardium compared to embryonic epicardium (GSE84085). This narrowed our list to the following five transcription factors: MAFF, RELB, MAFG, HIVEP2, and YBX3 (Fig. 5c).
Out of these five factors, MAFF62 and YBX339 have described functions in the regulatory signaling of PI3K/Akt/mTOR axis, and we have used them in the subsequent experiments. To demonstrate a causal relationship and biological relevance of the individual activation of MAFF and YBX3 in the process of epicardial maturation, we systematically explored the effects of the Torin1 treatment in epicardial cells, ectopically downregulating MAFF and YBX3 achieved by the expression of small-interference RNAs (siRNA; siMAFF and siYBX3, respectively) (Fig. 5d).
Upon confirming the selective downregulation of each gene individually, we verified that Torin1 treatment did not promote their expression (Fig. 5e). We then proceeded to evaluate the expression of two embryonic epicardial markers, WT1 and TBX18; a proliferative marker, MKI67; and the expression of C3, in order to validate our in vivo observations related to this complement component (Fig. 5e). Intriguingly, our results confirmed that YBX3 and MAFF might play roles in epicardial maturation in different ways. While the downregulation of MAFF did not decrease the proliferative levels of epicardial cells (as evidenced by MKI67 mRNA expression), cells with downregulated YBX3 failed to terminate the fetal epicardial program following Torin1 treatment. Indeed, cells with downregulated YBX3 showed a significant increase in C3, a result suggesting that this gene is possibly more related to a fetal state rather than epicardial adulthood (Fig. 5e). This aligns with recent studies based on single-cell RNA-sequencing analyses20. Notably, WT1 protein levels remained consistent in immunocytochemistry (ICC), indicating that YBX3 is pivotal in regulating WT1 and, consequently, the embryonic program (Fig. 5f). Finally, we also verified TBX18 (Fig. 5g) and MKI67 (Fig. 5h) protein levels in the western blot, further validating the distinct roles of YBX3 and MAFF in the maturation of the human epicardium.
Mature hiPSC-epicardium expressing IGF2 and FN1 promotes paracrine-driven transcriptional maturation of sarcomere-related genes in hiPSC-derived cardiomyocytes
IGF2/IGF1R signaling plays a pivotal role in human cardiogenesis, with IGF2 serum levels being more abundant postnatally and thus potentially contributing to cardiac biology. In rodent models, epicardial IGF2’s interaction with myocytic IGF1R has been pinpointed as an important catalyst for myocardial compaction63. Moreover, a recent study identified epicardial fibronectin (FN1) secretion driving cardiac maturation in 3D-engineered tissues64. Another study with epicardioids examines the interactions between myocardium and epicardium, elucidated that this myocardial compaction process in humans’ mirrors that in rodents63. We wondered if the mature epicardium could potentially amplify the paracrine effects of IGF2 and FN1 expression, thereby promoting the transcriptional maturation of hiPSC-derived cardiomyocytes. To explore this, we initiated the differentiation of ventricular cardiomyocytes and epicardial cells independently. For cardiac differentiation, we employed a reporter iPS cell line65 to oversee the transitional switch from the fetal to the adult isoform of the cardiac troponin I—a pivotal developmental switch capable of distinguishing between fetal, neonatal, or adult myocardium66 (Fig. 6a). Post differentiation and maturation of epicardial cells as monolayers, we proceeded to co-culture them with beating embryoid bodies (EBs) composed of ventricular cardiomyocytes (TNNT2+) (Supplementary Fig. 7e) to monitor the expression of TNNI3 in the presence of matured epicardium. Prior studies have outlined various interactions between hiPSC-derived epicardial cells and cardiomyocytes, primarily emphasizing their role in regenerative capabilities and cardiomyocyte cell cycle activation1. Following treatment with Torin1, epicardial cells showed a marked overexpression of IGF2 and FN1 (Fig. 6b). To confirm the paracrine involvement of IGF2 in this observation, we measured secreted IGF2 by ELISA, further confirming its differential secretion into the media under Torin1-treated conditions (Fig. 6c). Notably, a ten-day co-culture period revealed an increased expression of TNNI3 and a corresponding reduction in TNNI1, suggesting that the EBs have started adopting specific characteristics of structural maturation while in paracrine contact with mature epicardial cells (Fig. 6d). Finally, we treated hiPSC-CMs with an IGF2 antibody (Supplementary Fig. 7f) to block its biological activity in Torin1-derived epicardial supernatants and confirmed the impaired cardiomyocyte TNNI3 increase under IGF2-blocking conditions (Supplementary Fig. 7g). Additionally, mRNA expression analysis demonstrated that EBs co-cultured with mature epicardial cells exhibited enhanced expression of genes essential for left ventricular compaction, such as TBX5, HEY2, and PRDM16 (Fig. 6f). A comprehensive secretome protein analysis (Supplementary Fig. 7c) further confirmed the presence of IGF2 and several candidate proteins that could, either alone or in combination with IGF2 and FN1, contribute to the mRNA increments of these genes (Supplementary Fig. 7d). Interestingly, we found haptoglobin (HP) to be significantly upregulated among secreted proteins (Supplementary Fig. 7d). This secreted glycoprotein was identified as the most predictive coding gene exclusively in the aging-associated module of the fetal and adult human epicardium comparison6, suggesting that its secretion may serve as an additional surrogate marker of maturation, given that HP expression is absent in fetal tissues.
a Schematic representation of the co-culture experiment to assess paracrine signaling of mature hiPSC-epicardium over hiPSC-derived ventricular cardiomyocytes. b mRNA expression levels of epicardial IGF2 and FN1 expression after one week of Torin1-driven mTOR inhibition (purple bars, Torin1; n = 3 biological replicates). c IGF2 expression measured in the culture supernatant of Torin1-treated hiPSC-derived epicardial cells (n = 4 biological replicates). d IF detection of adult isoform of cardiac troponin I (red, mCherry + , TNNI3) and fetal cardiac troponin (green, eGFP + , TNNI1) in beating EBs composed of hiPSC-derived ventricular cardiomyocytes in co-culture for 10 days with Torin1-treated mature hiPSC-epicardium (yellow arrows) (scale bar: 500 µm). The experiment was repeated three times with similar results. e mRNA expression levels in hiPSC-derived EBs of cardiac TNNI3 expression after ten days of co-culture with Torin1-treated hiPSC-epicardium (red bars, mature epi) (n = 3 biological replicates). f mRNA expression levels in hiPSC-derived EBs of genes related to left ventricle compaction (TBX5, HEY2, and PRDM16) after ten days of co-culture with Torin1-treated hiPSC-epicardium (red bars, mature epi) (n = 3 biological replicates). Data are presented as means ± s.e.m.; two-tailed unpaired Student’s t-test. *p < 0.05, **p < 0.01, ***p < 0.001. Source data is provided as a Source Data file.
Altogether, our findings validate that mTOR inhibition can promote, at least partially, the upregulation of transcripts related to sarcomere maturation in a non-cell-autonomous manner when culturing untreated EBs but supported through paracrine IGF2 and FN1 signaling mediated by the mature (Torin1-treated) epicardium.
Generation of mature iPSC-derived heart organoids with quiescent epicardium
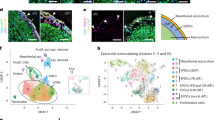
To evaluate the efficacy of mTOR inhibition within a context that more closely mirrors cardiac development, we differentiated self-assembling human heart organoids (hHOs) derived from hiPSC lines67,68. Our aim was to investigate the effect of Torin1 treatment on the epicardial and myocardial maturation of this human cardiac organoid. Specifically, we sought to discern the maturation process of the human epicardium in a complex multicellular environment, where multiple non-autonomous signaling pathways are active. Utilizing a three-step Wnt signaling modulation, we differentiated the feeder-free human iPS cell line 1390C1 into hHOs, as described previously68 (Fig. 7a). We observed minimal variance in the cellular composition of epicardium and myocardium ratios across different differentiation batches—the target populations of the study (Supplementary Figs. 8g and 9a,c)—with an average of 10% for WT1-expressing cells and 18–20% for TNNT2-expressing cardiomyocytes. Subsequently, we characterized the cardio-cytotoxicity profiles associated with varying Torin1 dosages over a 96-hour in vitro period using an MTT assay. The hHOs exhibited a tolerance to Torin1 comparable to that of human epicardial cells in a monolayer, with an IC50 value of 2.51 µM (Fig. 7b). Consequently, we decided to continue using a concentration of 200 nM for further studies, a dosage below the IC50, and proved to have an optimal effect in promoting epicardial quiescence and maturation in hiPSC-derived epicardial monolayers. After administering Torin1 to day-15 growing hHOs for seven days, we observed a notable effect on the overall organoid cell density at day 22 (Supplementary Fig. 8l). While the untreated organoids consistently expanded in size, those treated with Torin1 exhibited halted growth (Fig. 7c, d).
a Protocol for self-assembling mature human heart organoids (hHOs) by mTOR downregulation. Created in BioRender. Lucena-Cacace, A. (2025) https://BioRender.com/9j18jf6. b MTT assay determines hHO viability and IC50 value after Torin1 treatment (96 hours, descending concentrations, 1:2 dilutions; n = 2 technical replicates). Data are presented as means ±s.d. c Human heart organoid size (mm2) before (● black, untreated) and after one-week Torin1 treatment ( purple, Torin1) (untreated at day 15, n = 5; untreated at day 15 + 7, n = 19; Torin1-treated at day 15 + 7, n = 20). Data are presented as means ± s.e.m.; two-tailed unpaired Student’s t-test. d Phase contrast microscopy of untreated and Torin1-treated hHO (scale bar: 500 µm). e Immunofluorescence images of WT1 and TNNI3 expression in hHOs treated with Torin1 (200 nM), Tacrolimus (1 µM), or Rapamycin (200 nM) (scale bar: 500 µm). f Immunofluorescence images of SNAI1 and ZO-1 expression in hHOs treated with Torin1 (200 nM), Tacrolimus (1 µM), or Rapamycin (200 nM) (scale bar: 500 µm). g schematic representation of the effects of partial (Rapamycin, Tacrolimus) and total (Torin1) mTOR inhibition in the hHO maturation h, UMAP visualization of single-cell RNA sequencing (scRNA-seq) data depicting 11 transcriptionally distinct clusters (0–10) identified based on differential gene expression profiles. Cluster 0 corresponds to epicardial cells, characterized by the expression of WT1, TCF21, and ALDH1A2. Clusters 1 and 9 represent cardiomyocytes, marked by the expression of TNNT2, TTN, MYH6, and MYH7, with cluster 1 denoted as Cardiomyocytes I and cluster 9 as Cardiomyocytes II. Clusters 2, 3, 5, 7, and 10 correspond to resident fibroblasts, expressing COL1A1, COL3A1, VIM, and THY1, with distinct subtypes labeled as Resident Fibroblasts I-IV. Cluster 6 represents activated fibroblasts/myofibroblasts, identified by high expression of ACTA2, TAGLN, MYH11, and PDGFRB. Clusters 4 and 8 correspond to endocardial cells, marked by the expression of NPR3, PROX1, NFATC1, and ESAM, with cluster 4 labeled as Endocardial Cells I and cluster 8 as Endocardial Cells II. The clustering highlights the cellular heterogeneity within the heart organoids and the presence of distinct fibroblast, cardiomyocyte, epicardial, and endocardial populations. i UMAP visualization of single-cell RNA sequencing (scRNA-seq) data displaying 13 transcriptionally distinct clusters (0–12) based on differential gene expression profiles. Clusters 0 and 12 represent cardiomyocytes, marked by the expression of MYH6, MYL7, NPPA, and NR2F2, with cluster 0 designated as Cardiomyocytes I and cluster 12 as Cardiomyocytes II. Clusters 1, 3, 5, and 8 correspond to resident fibroblasts, expressing COL1A1, COL3A1, THY1, VIM, DCN, and LUM, with subtype labels Resident Fibroblasts I-IV. Cluster 6 represents epicardial cells, characterized by the expression of WT1, TCF21, and ALDH1A2. Cluster 4 consists of endothelial cells, identified by the expression of VWF, PECAM1, CDH5, KDR, ESAM, and TIE1. Clusters 2, 7, 9, 10, and 11 represent endocardial cells, marked by NPR3, PROX1, NFATC1, ESAM, and SOX17, and are further subdivided into Endocardial Cells I-V. This clustering underscores the cellular heterogeneity within the heart organoids, revealing distinct populations of cardiomyocytes, fibroblasts, endothelial, epicardial, and endocardial cells. j Dot plot for control and k Torin1-treated hHOs illustrating the expression patterns of key marker genes across identified clusters, enabling cell type annotation based on characteristic gene signatures. The size of each dot represents the proportion of cells within a cluster expressing the given gene, while the color intensity reflects the average expression level. l Heatmap displaying the log fold-change (logFC) of differentially expressed genes (DEGs) related to cardiomyocyte maturation across identified clusters. The color scale represents the relative expression levels m Heatmap displaying the log fold-change (logFC) of differentially expressed genes (DEGs) related to epicardial maturation across identified clusters. The color scale represents the relative expression levels n Bar graph illustrating the expression levels of key endothelial marker genes in Cluster 4 following Torin1 treatment in human heart organoids (hHOs). Expression values are normalized and presented as mean expression per cell, highlighting the relative abundance of endothelial-specific genes. The analysis reveals differential regulation of endothelial markers in response to Torin1 treatment. Source data is provided as a Source Data file.
purple, Torin1) (untreated at day 15, n = 5; untreated at day 15 + 7, n = 19; Torin1-treated at day 15 + 7, n = 20). Data are presented as means ± s.e.m.; two-tailed unpaired Student’s t-test. d Phase contrast microscopy of untreated and Torin1-treated hHO (scale bar: 500 µm). e Immunofluorescence images of WT1 and TNNI3 expression in hHOs treated with Torin1 (200 nM), Tacrolimus (1 µM), or Rapamycin (200 nM) (scale bar: 500 µm). f Immunofluorescence images of SNAI1 and ZO-1 expression in hHOs treated with Torin1 (200 nM), Tacrolimus (1 µM), or Rapamycin (200 nM) (scale bar: 500 µm). g schematic representation of the effects of partial (Rapamycin, Tacrolimus) and total (Torin1) mTOR inhibition in the hHO maturation h, UMAP visualization of single-cell RNA sequencing (scRNA-seq) data depicting 11 transcriptionally distinct clusters (0–10) identified based on differential gene expression profiles. Cluster 0 corresponds to epicardial cells, characterized by the expression of WT1, TCF21, and ALDH1A2. Clusters 1 and 9 represent cardiomyocytes, marked by the expression of TNNT2, TTN, MYH6, and MYH7, with cluster 1 denoted as Cardiomyocytes I and cluster 9 as Cardiomyocytes II. Clusters 2, 3, 5, 7, and 10 correspond to resident fibroblasts, expressing COL1A1, COL3A1, VIM, and THY1, with distinct subtypes labeled as Resident Fibroblasts I-IV. Cluster 6 represents activated fibroblasts/myofibroblasts, identified by high expression of ACTA2, TAGLN, MYH11, and PDGFRB. Clusters 4 and 8 correspond to endocardial cells, marked by the expression of NPR3, PROX1, NFATC1, and ESAM, with cluster 4 labeled as Endocardial Cells I and cluster 8 as Endocardial Cells II. The clustering highlights the cellular heterogeneity within the heart organoids and the presence of distinct fibroblast, cardiomyocyte, epicardial, and endocardial populations. i UMAP visualization of single-cell RNA sequencing (scRNA-seq) data displaying 13 transcriptionally distinct clusters (0–12) based on differential gene expression profiles. Clusters 0 and 12 represent cardiomyocytes, marked by the expression of MYH6, MYL7, NPPA, and NR2F2, with cluster 0 designated as Cardiomyocytes I and cluster 12 as Cardiomyocytes II. Clusters 1, 3, 5, and 8 correspond to resident fibroblasts, expressing COL1A1, COL3A1, THY1, VIM, DCN, and LUM, with subtype labels Resident Fibroblasts I-IV. Cluster 6 represents epicardial cells, characterized by the expression of WT1, TCF21, and ALDH1A2. Cluster 4 consists of endothelial cells, identified by the expression of VWF, PECAM1, CDH5, KDR, ESAM, and TIE1. Clusters 2, 7, 9, 10, and 11 represent endocardial cells, marked by NPR3, PROX1, NFATC1, ESAM, and SOX17, and are further subdivided into Endocardial Cells I-V. This clustering underscores the cellular heterogeneity within the heart organoids, revealing distinct populations of cardiomyocytes, fibroblasts, endothelial, epicardial, and endocardial cells. j Dot plot for control and k Torin1-treated hHOs illustrating the expression patterns of key marker genes across identified clusters, enabling cell type annotation based on characteristic gene signatures. The size of each dot represents the proportion of cells within a cluster expressing the given gene, while the color intensity reflects the average expression level. l Heatmap displaying the log fold-change (logFC) of differentially expressed genes (DEGs) related to cardiomyocyte maturation across identified clusters. The color scale represents the relative expression levels m Heatmap displaying the log fold-change (logFC) of differentially expressed genes (DEGs) related to epicardial maturation across identified clusters. The color scale represents the relative expression levels n Bar graph illustrating the expression levels of key endothelial marker genes in Cluster 4 following Torin1 treatment in human heart organoids (hHOs). Expression values are normalized and presented as mean expression per cell, highlighting the relative abundance of endothelial-specific genes. The analysis reveals differential regulation of endothelial markers in response to Torin1 treatment. Source data is provided as a Source Data file.
To understand the effects of mTOR inhibition more comprehensively on human cardiac organoids, we first confirmed two conditions prior to Torin1 treatment: (1) WT1-positive cells are proximate to TNNT2-positive cells, maintaining distinct cellular identities independently (Supplementary Fig. 8a), and (2) EMT is actively operational through SNAI1 due to the inherent growth dynamics of a developing organoid (Supplementary Fig. 8b).
To rigorously validate the mTORC2-dependent phenotype associated with organoid maturation, we systematically assessed the effects of Tacrolimus and Rapamycin on growing heart organoids. Notably, our results demonstrated that Torin1 remains the only compound capable of decreasing epicardial WT1 protein levels (Fig. 7e and Supplementary Figs. 8c, 9b), confirming that mTORC1 inhibition alone is insufficient to trigger maturation phenotypes.
Additionally, we verified that only Torin1 significantly reduced epicardial SNAI1 (Fig. 7f and Supplementary Figs. 8d, 9e) and augmented the expression of the adult cardiac troponin protein TNNI3 (Fig. 7e and Supplementary Figs. 8c, 9d), as previously demonstrated in hiPSC-derived cardiomyocytes32. These findings confirm that a pulse of mTOR inhibition may play a pivotal role in cardiac maturation, affecting both epicardial and myocardial cell types during organ development (Fig. 7g).
In order to study cellular heterogeneity and mTOR-dependent maturation dynamics at single-cell resolution, we performed single-cell (sc)RNA-seq on pooled untreated and Torin1-treated hHOs to provide a statistically robust representation of organoid cellular composition and maturation dynamics. Our UMAP-based cluster identification revealed 11 distinct clusters (0–10) for untreated hHOs (Fig. 7h) and 13 distinct clusters for Torin1-treated hHOs (Fig. 7i), using the same cluster resolution for each condition.
Cluster identification revealed a clear epicardial cluster (cluster 0 in control and cluster 6 in Torin1-treated hHOs) (Fig. 7j, k) based on WT1, TBX18, and TCF21 expression, while two distinct cardiomyocyte clusters were identified (clusters 1 and 9 in control and clusters 4 and 12 in Torin1-treated hHOs) (Fig. 7j, k), reflecting ventricular and atrial cardiomyocyte types, as indicated by GJA5 and KCNA5 expression.
To assess the transcriptomic maturation of these populations in a cluster-specific manner, we performed a differentially expressed gene analysis, which revealed that Torin1-treated hHOs exhibited a strong maturation gene signature, including MYL7, RYR2, MYH7, CORIN, HOPX, TCAP, and TNNI3, in both cardiomyocyte populations (Fig. 7l). The epicardial cluster also displayed a consistent maturation profile, as evidenced by increased expression of NBL1, BNC1, and EFEMP (Fig. 7m), along with a marked increase in IGF2 and FN1, which contribute to cardiomyocyte maturation, as demonstrated in the previous section.
Remarkably, Torin1-treated hHOs exhibited the emergence of a new cluster (cluster 4), representing 4% of the total cells, which corresponded to endothelial cells with strong expression of FLT1 (VEGFR1), KDR (VEGFR2), and EMCN (Endomucin) (Fig. 7n). Altogether, these findings indicate that mTOR-inhibiting conditions facilitated endothelial cell differentiation, suggesting a more vascularized organoid, being another hallmark of maturation. The epicardial cluster in untreated condition is the solely cluster indicating EMT activity (SNAI1/2 positive cells), suggesting that epicardial cells are in the process of active differentiation into epicardial-derived cells (EPDCs), contributing to the enhanced cellular population of the heart organoid. Following the Torin1 treatment, we observed a diminished expression of WT1, while the TNNT2 population remained unaffected (Fig. 7g, Supplementary Fig. 7b and Supplementary Fig. 8h–k). Followed by a significant reduction of MKI67-expressing organoids (Supplementary Fig. 8e), we functionally confirmed that Torin1-treated hHOs had a significant lower beats per minute, another hallmark of a more mature heart organoid, as it reflects a more synchronous systolic function (Supplementary Fig. 8f). This suggests that the epicardium can effectively undergo maturation upon mTOR-inhibiting conditions, a finding consistent with our in vivo observations in mouse heart, and our in vitro maturation of hiPSC-derived epicardial monolayers.
We finally confirmed the mTORC2-reliance of the EMT program suppression through the observed reduction of SNAI1 levels (Supplementary Fig. 8d), confirming the loss of EMT-initiation capacity and endorsing this mature-like heart organoid to resemble a more adult counterpart. In summary, our pharmacological approach aimed at maturing the hiPSC-epicardium has demonstrated efficacy in producing mature human iPS-cardiac organoids expressing markers of adult cardiomyocytes and epicardial cells. This lays the foundation for establishing a platform to investigate the reactivation of adult epicardium, providing insights into its fundamental role in regenerative responses.
Discussion
The human epicardium plays a crucial role in cardiogenesis and heart regeneration. Still, it loses its regenerative functionality as the heart acquires functional maturity, entering into a cellular quiescence state unknown to reactivate in adulthood. In this study, we modeled human epicardiogenesis in vitro utilizing hiPSCs and engineered cell behavior through the pharmacological inhibition of mTOR to assist the entry of quiescence ectopically and enhance the maturation of the epicardial tissue. The maturation of the epicardium could be partially explained by an initial reduction of its proliferative state as a primary trigger of unknown physiological cue, but representing a cell cycle state rapidly decreasing the expression of WT1 protein, an essential protein marking the embryonic, active, and regenerative epicardium49,69,70. Recent studies have claimed that mRNA expression of WT1 is present in the mature epicardium6,20. Nonetheless, our study consistently revealed that WT1 is reduced during maturation and may be completely lost as the epicardium develops, particularly in the postnatal heart. We hypothesize that this could represent a discrepancy between mRNA expression and protein levels71, thus highlighting the necessity of relying on protein studies for further epicardial characterization. Additionally, our study found that the C3 protein—another transcript whose elevation is associated with epicardial maturation in recent studies—is also reduced in protein levels as the epicardium matures, exemplifying a potential RNA-protein mismatch. In all cases, Torin1-treated hiPSC-derived epicardium faithfully mimicked our in vivo observations of mature epicardium.
Despite the rapid decline in WT1 expression upon mTOR inhibition, the switch of the entire fetal program to functional maturation unfolds dynamically and sequentially in time, as evidenced by a late decrease of TBX18 expression. Our study demonstrated that Torin1, an mTOR kinase inhibitor capable of targeting a broad range of mTOR-dependent effectors, effectively orchestrates the onset and consolidation of the mature epicardial program, and the proof of concept of this phenotype is achieved by seven days of treatment, when we observe a significant suppression of TBX18. We suggest that this phenotype critically relies on the comprehensive reduction of phospho-AKT and is consequently dependent on mTORC2, since phospho-AKT levels are dually controlled by the axis governed by mTORC1 and C272, as suggested by the experiments performed with Rapamycin and Tacrolimus.
It is essential to mention that tissue ontogenesis differentiates functional development and aging. A functional mature epicardium does not involve a concept of aged epicardium from the fetal stage. This hypothesis is demonstrated here, where we characterized that dual inhibition of mTORC1/C2 enhances functional maturation but shifts cells away from senescence, as reduced p21 levels, which expression and accumulation are associated with biological aging73 in some tissues, indicate this. Reduction in p21 levels is a recognized phenotype resulting from the hypophosphorylation of 4E-BP1. This process directs p21 towards degradation by the proteasome, which is a direct outcome of mTOR inactivation74. While this study did not primarily concentrate on the characterization of p53 signaling (which has shown synergistic effects on the maturation of hiPSC-CMs when combined with Torin132), our engineered hiPSC-epicardium does enhance p53 expression, a pathway shown to be upregulated in postnatal murine epicardium as well. This cell-autonomous enhancement represents sufficiently what we believe to be a functionally mature epicardium without resorting to additional ectopic p53 overexpression. Using Torin1, we were able to discern a broad range of transcription factors that play roles in this maturation process. Specifically, we examined two transcription factors, YBX3 and MAFF, both of which have distinct contributions to tissue maturation. While MAFF interferes with epicardial proliferation, YBX3’s mechanistic impact appears more noteworthy. YBX3 directly influences WT1 and TBX18, both of which are critical components of the fetal program. Suppressing these factors is crucial for promoting functional maturation. YBX3 regulates SLC7A5 mRNA stability39, a transporter responsible for amino acid intake and intracellular homeostasis. This suggests that the metabolic changes induced by mTOR reduction could also significantly affect epicardial maturation.
Complete suppression of mTOR has been found to enhance the functional maturation of hiPSC-derived epicardium effectively. This is evident through (1) suppression of the epicardial program while retaining epicardial identity, (2) induction of cellular quiescence, (3) loss of EMT-initiation capability, and (4) functional augmentation in IGF2 and FN1 signaling. Such capabilities enable the epicardium to partially promote the transcriptional maturation of hiPSC-derived cardiomyocytes. The adult mouse epicardium appears to upregulate OXPHOS and fatty acid oxidation (FAO) while reducing glycolytic dependence. We observed that although mTOR inhibition decreases glycolysis and elevates FAO-related transcripts, the metabolic maturation of the epicardium remains an area for improvement and a potential gap in creating a more complete postnatal counterpart.
Creating cellular models that emphasize intercellular interactions is of utmost interest in these areas. Engineered cardiac organoids serve as essential tools in this context. These organoids, an expanding domain in biomedical engineering, operate multifunctionally and are pivotal in understanding cardiac health, disease modeling, and promoting generative responses.
Presently, heart organoids are categorized as (1) those where researchers modulate cellular composition to emphasize interactions between specific cells or (2) those designed to mimic the intrauterine environment, promoting self-assembly and autonomous regulation75, thereby better reflecting multicellular developmental and regenerative responses—like the organoid in our study. While recent studies on epicardioids have illuminated the close connection and signaling between the epicardium and myocardium63,76, we contend that the fetal conditions of these organoids may limit their potential for exploring advanced cardiogenesis stages. Our study offers pivotal insights by: (1) elucidating how epicardial cells achieve quiescence following mTOR pathway inhibition, (2) detailing the sequential suppression of the epicardial program and the associated decline in migration and EPDC production, resembling adult epicardium, (3) understanding the functional acquisition by mature epicardial cells in IGF2 and FN1 production—a crucial paracrine signal in cardiac maturation, and (4) shedding light on how cardiac maturation may be bolstered by stabilizing signals from the epicardium to the myocardium and further layers of the heart wall. Part of our discoveries align with those described in pivotal studies on multilineage epicardium–myocardium organoids, such as the one developed by Branco et al. 76. However, each model, including ours, exploits unique organoid features and advantages.
The in vitro platform of hiPSC-derived mature epicardium described in this study enables preclinical applications for epicardial regeneration, including approaches such as high-throughput screening (HTS) of bioactive compounds. In this study, HTS successfully identified compound hits capable of reactivating WT1 and KI67, thereby restoring epicardial regenerative potential. Notably, we identified GSK-3α/β inhibitors, which we disclose in this study as kinase inhibitors previously shown to promote cardiomyocyte proliferation77 and now exhibiting extended potential in reactivating quiescent epicardial cells.
Finally, our pharmacologically engineered organoid contributed to the emergence of new cell types at single-cell resolution, with a distinct presence of endothelial cells, indicating a higher degree of vascularization—another hallmark of hHO maturation78. This mature organoid provides a robust model to characterize and mimic the in vivo multi-step maturation of the postnatal epicardium. It serves as a powerful tool for testing strategies aimed at reactivating the human epicardium, potentially driving natural regenerative responses in clinical settings. Moreover, it represents an invaluable cellular model for studying heart regeneration, cardiac aging, and the complex mechanisms underlying late-onset cardiovascular diseases (CVDs).
This study has not investigated the potential roles of three other factors: RELB, MAFG, and HIVEP2. However, given their mTORC2-dependent regulation and elevated expression in the primary adult epicardium, they might also contribute to epicardial maturation through mechanisms not explored here. Moreover, suppression of mTOR does not fully recapitulate the phenotype of a mature heart; thus, its inhibition supports only partially the multi-step process required for achieving a comprehensive mature phenotype.
Additionally, since the primary focus of this study is the fundamental biology of epicardial maturation derived from hiPSCs in both 2D and 3D contexts (epicardioids), we have not formally characterized the maturation phenotypes of TNNI3-expressing cardiomyocytes resulting from co-culture. Therefore, the observed increase in TNNI3 expression does not necessarily imply enhanced functionality, such as improved calcium handling or electrophysiological properties.
Methods
Cell lines and culture conditions
The hiPSC cell line 409B2, which was reprogrammed using Yamanaka factors via retroviral methods, was grown in Primate ES Cell Medium (ReproCELL) with 4 ng/ml bFGF, supported by MSTO feeder layers. To eliminate the feeder layers, CTK (ReproCELL) was applied to the cells. The 1390C1 and T1/T3 reporter65 cell lines, which don’t require feeders, were maintained in full StemFit® AK02N (ReproCELL) on dishes coated with iMatrix-511 (Nippi). The MEC1 mouse epicardial cell line (Merck, SCC187) was acquired and grown following the guidelines provided by the manufacturer. Regular checks for Mycoplasma were conducted to ensure no contamination.
Epicardial differentiation from hiPSCs
We differentiated epicardial cells from 409B2 cell line following the protocol as described before18, and maintained them in medium supplemented with 10 µM SB431542, with media changes every other day. For epicardial differentiation from 1390C1, cells were dissociated and cultured under the same conditions as cardiomyocyte differentiation. After 24 h, different concentrations of rhBMP4 (3 ng/mL) and Activin A (4 ng/mL) were used from day 1 to day 4, followed by rhBMP4 (10 ng/mL), SB431542 (6 μM), CHIR (0.5 μM), and Retinol (2 μM, Sigma) until day 6. From day 6, cells were cultured Stempro-34 medium with SB431542 (6 μM). Cells were replated on day 10 and moved to normoxia conditions one day after.
Cardiomyocyte differentiation from hiPSCs
We differentiated ventricular cardiomyocytes as follows: hiPSCs were separated into individual cells using a half-strength solution of TrypLE select (Gibco), which was combined with 0.5 mM EDTA. On day 0, these cells were then added to 1.5 mL/well of StemPro-34 medium (Gibco) containing L-glutamine (2 mM; Gibco), MTG (57.6 μM; Sigma-Aldrich), Ascorbic Acid (AA, 50 μg/mL; Sigma-Aldrich), Transferrin (150 μg/ml; Wako), ROCK inhibitor (Y-27632, 10 μM; Nacalai), rhBMP4 (2 ng/mL; R&D Systems), and Matrigel Matrix (0.5%; Corning), targeting to produce embryoid bodies (EBs) at a concentration of 2 × 106 cells/well. After 24 h, the well received an extra 1.5 mL of StemPro-34 medium containing L-glutamine (2 mM), MTG (57.6 μM), AA (50 μg/mL), Transferrin (150 μg/mL), bFGF (10 ng/mL; Wako), Activin A (12 ng/mL; R&D Systems), and rhBMP4 (18 ng/mL). On day 3, EBs were cleansed with IMDM (Gibco) and then cultured with 3 mL of StemPro-34 medium containing L-glutamine (2 mM), MTG (57.6 μM), AA (50 μg/mL), Transferrin (150 μg/mL), VEGF (10 ng/mL; R&D Systems), IWP-3 (1 μM; ReproCELL), SB431542 (5.4 μM; Nacalai), and Dorsomorphin (0.6 μM; Sigma). On day 6, medium was changed to StemPro-34 medium containing L-glutamine (2 mM), MTG (57.6 μM), AA (50 μg/mL), Transferrin (150 μg/mL), VEGF (5 ng/mL). Thereafter, this medium (CM culture medium) was renewed every 2 to 3 days. Initially, the plate was kept in a reduced oxygen setting (5% O2). From day 10 onwards, it was shifted to a regular oxygen atmosphere.
For direct co-culture of EPIs and CMs, day 21 CMs were transferred into wells containing a monolayer of untreated/Torin1-treated epicardial cells. The medium was refreshed with CM culture medium every 2–3 days.
For indirect co-culture, conditioned medium from EPIs were collected and transferred to wells containing CMs every 3 days. IGF2 neutralizing antibody (2 μg/mL, R&D, MAB292) was added to the medium during each medium change.
Heart organoid induction from hiPSCs
The process to develop human cardiac organoids from 1390C1 followed an established procedure67.
Proliferation assay
To assess cell proliferation, day 24 hiPSC-EPIs were seeded at 4 × 104 cells/well in a 6-well plate. The following day, the medium was replaced with fresh medium, and cell number was counted, designating this time point as day 0. Cell growth was monitored daily by counting cell numbers, and a growth curve was plotted based on these values. At the endpoint, crystal violet staining was performed to compare cell density between groups.
Cell transfection
In the gene suppression tests, Silencer® Select siRNAs (ThermoFisher) were prepared as a 10 µM concentrate, specifically siYBX3 (ID: s16222) and siMAFF (ID: s24372). To perform the gene silencing, a mixture was made by mixing 10 µl of the siRNA solution in 500 µl Opti-MEM, and then 10 µl of RNAiMax (ThermoFisher) was introduced. We transfected hiPSC-cells monolayers of 50,000 cells following the manufacturer instructions.
Isolation of mouse fetal hearts and whole-heart lysate
Embryos at E12, E14, and E18 were gathered into PBS, and any surrounding extraneous tissue was carefully removed using tweezers. The heads of the embryos were detached, and to access the heart, the front side of the chest cavity was torn open. The fetal hearts were then mechanically separated and placed into another dish filled with PBS, ready for further culture or analysis. To prepare the heart protein lysate, the hearts were first chopped into tiny bits and then exposed to trypsin for 20 min at 37 °C. The cell mixture was stirred again, processed for another 20 min at 37 °C, and after spinning it at 300 g for 5 min, the clear liquid (supernatant) was collected.
Mouse heart cryosection and immunohistochemical staining
Mouse hearts were fixed in 4% PFA and then transferred to 30% sucrose in PBS overnight at 4 °C for dehydration. After it, hearts were embedded in optimal cutting temperature (OCT) compound and frozen in liquid nitrogen. Cryosections (8–10 µm) were cut using a cryostat at −20 °C and mounted onto glass slides. Sections were washed in PBS to remove residual OCT, then blocked with 0.2% Triton X-100 (Nacalai Tesque), 0.05% bvovine serum albumin (BSA, Nacalai Tesque), and 0.5% normal goat serum in PBS (blocking buffer) for 20 min at room temperature. Sections were incubated overnight at 4 °C with primary antibodies diluted in blocking buffer. Primary antibodies: WT1 (1:200, ab89901, abcam), ZO-1 (1:200, 33-9100, Invitrogen), MSLN (1:200, ab93620, abcam), C3 (1:200, ab97462, abcam) and Caspase-3 (1:500, AF835, R&D). The next day, sections were incubated with secondary antibodies in blocking buffer for 1 h at room temperature and Hoechst (1:10,000) before imaging. Sections were visualized using Keyence BZ-X810 or Olympus FV3000.
Expansion of epicardial explants ex vivo
Harvested hearts were transferred onto 0.1% gelatin-coated plates with a minimal volume of culture medium (DMEM (Low Glucose) + 15% FBS) to facilitate adhesion. After overnight incubation, additional culture medium was added to promote epicardial cell outgrowth. Explants were maintained at 37 °C with 5% CO₂, and cells migrating from the heart tissue were expanded. TGF-β inhibitor (SB431542) was added to the culture medium two days after isolation to maintain the epicardial phenotype. The same protocol was applied across different developmental stages.
Cytotoxic assay
For every experiment, Torin1 was freshly prepared in DMSO. Cells were seeded individually into 96-well plates at a density of 5000 cells per well. To assess treatment efficacy, diminishing concentrations of Torin1 were applied to rapidly dividing cells in a consistent 1:3 ratio. A day after seeding, cells were treated with the designated Torin1 dilutions. After 96 h, cell viability was gauged using crystal violet. To measure cytotoxic effects, absorbance was recorded at 595 nm with a microplate reader. Based on these results, IC50 values were calculated using the GraphPad Prism 7 software. The cytotoxicity assay on heart organoids was performed following the same protocol, with one organoid placed per well before treatment.
Invasion assay
Cell invasive property was assessed using BioCoat Matrigel Invasion Chambers (Corning, #354480) following manufacturer’s protocol.
Senescence β-Galactosidase activity detection
β-Galactosidase activity was measured using the Senescence β-Galactosidase Staining Kit (Cell Signaling TECHNOLOGY, #9860) following the manufacturer’s protocol.
Immunocytochemistry (ICC)
Cells fixed with 4% PFA for 15 min, preserved in PBS at 4 °C. Blocked for 30–45 min in 1% BSA, 0.5% Triton X-100, 0.1 M glycine in PBS. Primary antibodies: anti-TBX18 (1:200, ab15262, Abcam); anti-WT1 (1:50, ab89901, Abcam); anti-ZO-1 (1:200, 33-9100, Invitrogen); anti-pH3 (1:200, C36B11, CST); anti-Ki67 (1:200, ab16667, Abcam); anti-SNAI (1:200, 3879, CST); anti-SNAI2 (1:200, 9585, CST); anti-MSLN (1:100, ab93620, Abcam); anti-C3 (1:200, ab97462, Abcam) in staining buffer (1% BSA, 0.5% Triton X-100 in PBS), incubated overnight at 4 °C. The next day, after three PBS rinses, secondary antibodies, Goat anti-Mouse 488 (1:1000, A11001, Invitrogen), Goat anti-Rabbit 647 (1:1000, A21245, Invitrogen), were introduced in staining buffer for 2 h at room temperature. Post three PBS washes, counterstained with Hoechst 33342 (1:10,000). Imaging using Keyence.
Immunofluorescence staining for hHOs: hHOs fixed in 4% PFA for 45–60 min, overnight incubation at 4 °C in 10% normal donkey serum (Abcam), 0.5% Triton X-100, 0.5% BSA. Primary and secondary antibodies in PBS mixture containing 1% normal donkey serum, 0.5% Triton X-100, 0.5% BSA. Nuclear staining with Hoechst 33342 (1:1000) before mounting. Fluorescence was observed using an Olympus FV3000 confocal microscope. Primary antibodies: anti-Cardiac Troponin T (1:200, ab8295, Abcam); anti-TNNI3 (1:200, ab10231, Abcam). Secondary antibodies: Donkey anti-Mouse 488 (1:200, A21202, Invitrogen); Donkey anti Rabbit 594 (1:200, A21207, Invitrogen).
Flow cytometry analysis (FACS)
For epicardial cells, cells were separated into individual cells using Accumax, rinsed twice, and then fixed with 4% PFA for 15 min. To identify quiescent cells in G0, we employed Hoechst 33342 and Pyronin Y Staining, following the method previously described.
For cardiomyocyte, cells were dissociated into single cells by incubating with Collagenase I overnight at 37 °C, followed by Accumax for 5 min at 37 °C. Heart organoids were dissociated into single cells using 0.25% Trypsin-EDTA for 10 min at 37 °C. Cells were fixed with 4% PFA for 15 min and (for cytoplasmic and nuclear target protein) permeabilized with ice-cold 90% methanol for 15 min. Cells were stained with primary antibodies in FACS buffer (5% FBS in PBS) for 30 min or overnight at 4 °C: WT1 (1:2000, ab89901, abcam); TNNT2 (1:1000, 565744, BD); CD31 (1:500,303116, BioLegend); CD90 (1:1000, 559869, BD). Secondary antibody for unconjugated antibody: Goat anti-Rabbit 647 (1:1000).
The analysis was done with BD FACSDiva v6 or v8 and FlowJo v10. We recognized the cell group by FSC/SSC gating and eliminated any double cells. We used unstained samples or isotype controls to set up negative gates and ensured that positive gates had no signals from the negatives.
Immunoblotting
Cells were broken down using the M-PER Mammalian Protein Extraction Reagent buffer (78501, Thermo Fisher Scientific), while hHOs were lysed with T-PER Tissue Protein Extraction Reagent (78510, Thermo Fisher Scientific). The Bradford assay, with BSA as a reference, was used to measure protein content. The primary antibodies were sourced commercially, including: anti-phospho-S6 Ribosomal Protein (5364, CST), anti-phospho-4E-BP1 (2855, CST), anti-4E-BP1 (9644, CST), anti-phospho-AKT (9271, CST), anti-β-actin (A5441, Sigma), anti-WT1 (ab89901), anti-cTnI TNNI3 (ab10231), anti-SNAI1 (ab63371), anti-NBL1 (ab174843), anti-p53 (12790S, D963E, CST), anti-p16 (12D1, CST), anti-p21 (D7C1M, CST), anti-TBX18 (ab15262), anti-KI67 (Biolegend; 350502), anti-p27 (D69C12, 3686, CST) and TNNI1 (ab203515), all at a dilution of 1:1000. The secondary antibodies applied: goat anti-rabbit IgG with HRP (sc-2054, Santa Cruz) and anti-mouse IgG with HRP (7076, CST), both diluted at 1:5000.
ELISA
Conditioned media were harvested from 1390C1-derived EPI after 48 h of incubation at 37 °C and kept at −80 °C until analysis. Cell numbers were counted at the time of sample harvest. IGF2 was quantified using Human IGF-II/IGF2 ELISA Kit (R&D, DG200) and normalized by cell number.
Proteomic analysis
Conditioned media from 1390C1-derived human epicardial cells treated with or without Torin1 (n = 4 biological replicates per group) were collected after a 48-h incubation and subjected to proteomic analysis. Samples were fragmented by digestive enzymes following SP3 Method. The prepared samples were measured under the following nanoLC-MS/MS analysis conditions. Peptides were analyzed using a nanoElute 2 LC system coupled to a Bruker timsTOF Pro mass spectrometer in diaPASEF mode. Peptides were separated on a C18 column (IonOpticks, 250 mm × 75 µm, 1.6 µm) using a 100-minute gradient from 2% to 80% acetonitrile. MS1 scans ranged from m/z 340–1105 with ion mobility range 0.75–1.32 1/K₀, and fragmentation was performed via CID. The cycle time was 1.03 s with 11 isolation windows of 15 Th each.
Raw data were analyzed using DIA-NN (v1.9) (identification software) in library-free mode against the UniProt/Swiss-Prot human database (2024_02) (protein sequence database). Trypsin/P was used as the protease, allowing one missed cleavage (protease cleavage sites and number of missed cleavages). Cysteine Carbamidomethylation was set as a fixed modification, while methionine oxidation and N-terminal acetylation were variable. The precursor and fragment ion mass tolerances were set to 10 ppm and 10 ppm, respectively. Peptides were filtered by a minimum length of 7 amino acids. Identifications were accepted with a false discovery rate (FDR) ≤ 1% at both the peptide and protein levels, and proteins were retained if supported by at least one unique peptide. Statistical analysis was performed using unpaired two-tailed t-tests.
Image acquisition, processing, and analysis
Images from the microscope were captured using the BZ-X710 and then processed with the BZ-X Analyzer software by Keyence. The color representations were as specified in the figure captions. A select portion of the image was selected, trimmed, and enlarged to showcase both phase contrast and fluorescence details. The data for Western blot was gathered using the ImageQuant 800 from Cytiva. Broad image evaluations were conducted using Microsoft PowerPoint and ImageJ version 1.52.
Quantitative RT-PCR
As instructed in the manufacturer’s guide, QIAzol® Lysis Reagent (Qiagen) was used to extract total RNA. Using the ReverTra Ace system (Toyobo BIOTECH), 1 μg of RNA was converted to cDNA. The StepOnePlus Real-Time PCR system was employed to conduct quantitative RT-PCR (qPCR) using the Next SYBR® qPCR Mix (Thunderbird). GAPDH served as the internal reference control. The sequences for the primers can be found in Supplementary Table 1.
Bioinformatics and RNA-Seq
Retrospective bioinformatical analysis performed in this study was conducted using R2: Genomics analysis and visualization platform (http://r2.amc.nl/). The publicly available datasets used in this study for the retrospective analysis are GSE1479 and GSE84085. We used DESeq2-based normalization. Both the processed and raw fastq files created during this research were stored on the Gene Expression Omnibus (GEO) under the accession code GSE252590. Using RStudio, we analyzed the raw data for deeper insights into gene expression. For reading, exploring, and pre-processing gene expression data, we used the NOISeq pipeline from the Bioconductor package as described before52.
Single-cell RNA sequencing (scRNAseq)
Heart organoids (20 for control and 30 for Torin1-treated) were dissociated into single cells using 0.25% Trypsin-EDTA, and the cells were filtered through a 70-µm strainer. After centrifugation, the cells were resuspended in 0.04% BSA-PBS and counted using trypan blue. Only samples with at least 90% viability were used for library preparation. After dissociation, samples were processed for scRNA-seq with a targeted cell recovery of 10,000.
Libraries were prepared using the Chromium GEM-X Single Cell 3’ Kit v4 (10x Genomics), following the manufacturer’s standard protocol. The pooled libraries were sequenced on an Illumina NovaSeq 6000 (Illumina) using the 10x Genomics recommended 28-10-10-90 cycle configuration (Standard Workflow). bcl2fastq (v2.20.0.422) and Cell Ranger (v7.1.0) were used to generate FASTQ files. The cellranger mkfastq pipeline was executed with default parameters.
scRNA-seq data from control and treated conditions were processed using standard Python libraries. After normalization and logarithmic transformation of the original data, we performed principal component analysis and used the Leiden algorithm implementation available in scanpy to derive clusters of similar cells. Dot plots of traditional markers were used to identify target epicardial and cardiomyocyte clusters. Differential expression levels between clusters of the same type in treated and control conditions were evaluated using the Wilcoxon test implementation available in scanpy. Finally, expression levels of individual clusters were assessed by obtaining the mean expression after normalizing counts to account for variability within cells.
High throughput screening
Epicardial cells derived from 409 B2 cell line on day 23 were seeded in PhenoPlate 384-well microplates (PerkinElmer) coated with 0.1% gelatin at a density of 1500 cells/well by using automated dispenser (Thermo Fisher Scientific). The next day, medium was replaced with fresh medium containing 200 nM Torin1, and the cells were treated for 7 days to induce maturation. After the treatment, a library of 1600 chemical compounds was added using a multistage workstation automatically from a 10 mM stock solution that was prediluted by medium. The final concentration of compound was 1 μM. Cells were then incubated for 2 days to assess the effect on proliferation. At the end of the treatment, ICC for MKI67 was conducted, and fluorescent images were captured automatically. The percentage of MKI67-positive cells and mean intensity of MKI67 fluorescence were analyzed. The standard threshold was defined as the mean signal of negative controls plus four times their standard deviation. Compounds exhibiting signals above this threshold were selected and considered potential regenerative candidates.
Statistics
Statistical analysis was performed with GraphPad Prism 7. Differences between experimental groups were analyzed by unpaired two-tailed t-test or two-way ANOVA. *p < 0.05, **p < 0.01, ***p < 0.001.
Animal experiments
Kyoto University approved all experiments involving animals Animal Experimentation Committee. They were carried out following the Guidelines for Animal Experiments and the Guide to the Care and Use of Laboratory Animals.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting this study are available within this publication as source data. Sequencing data has been deposited under the accession code GSE252590. Mass spectrometry proteomics data has been deposited to the jPOST repository79 with the dataset identifier JPST003846 and ProteomeXchange ID PXD064589. Source data are provided with this paper.
References
Bargehr, J. et al. Epicardial cells derived from human embryonic stem cells augment cardiomyocyte-driven heart regeneration. Nat. Biotechnol. 37, 895–906 (2019).
Pérez-Pomares, J. M. & de la Pompa, J. L. Signaling during epicardium and coronary vessel development. Circ. Res. 109, 1429–1442 (2011).
van Wijk, B. & van den Hoff, M. Epicardium and myocardium originate from a common cardiogenic precursor pool. Trends Cardiovasc. Med. 20, 1–7. https://doi.org/10.1016/j.tcm.2010.02.011. (2010).
Zhou, B. et al. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J. Clin. Investig. 121, 1894–1904 (2011).
Cai, W. et al. Limited regeneration potential with minimal epicardial progenitor conversions in the neonatal mouse heart after injury. Cell Rep. 28, 190–201.e3 (2019).
Knight-Schrijver, V. R. et al. A single-cell comparison of adult and fetal human epicardium defines the age-associated changes in epicardial activity. Nat. Cardiovasc. Res. 1, 1215–1229 (2022).
Hesse, J. et al. Single-cell transcriptomics defines heterogeneity of epicardial cells and fibroblasts within the infarcted murine heart. eLife 10, e65921 (2021).
Quijada, P., Trembley, M. A. & Small, E. M. The role of the epicardium during heart development and repair. Circ. Res. 126, 377–394 (2020).
Bao, X. et al. Long-term self-renewing human epicardial cells generated from pluripotent stem cells under defined xeno-free conditions. Nat. Biomed. Eng. 1, 0003 (2016).
Bax, N. A. M. et al. In vitro epithelial-to-mesenchymal transformation in human adult epicardial cells is regulated by TGFβ-signaling and WT1. Basic Res. Cardiol. 106, 829–847 (2011).
Burridge, P. W., Keller, G., Gold, J. D. & Wu, J. C. Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell 10, 16–28 (2012).
Compton, L. A., Potash, D. A., Mundell, N. A. & Barnett, J. V. Transforming growth factor-beta induces loss of epithelial character and smooth muscle cell differentiation in epicardial cells. Dev. Dyn. Publ. Am. Assoc. Anat. 235, 82–93 (2006).
Iyer, D. et al. Robust derivation of epicardium and its differentiated smooth muscle cell progeny from human pluripotent stem cells. Dev. Camb. Engl. 142, 1528–1541 (2015).
Mummery, C. L. et al. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ. Res. 111, 344–358 (2012).
van Tuyn, J. et al. Epicardial cells of human adults can undergo an epithelial-to-mesenchymal transition and obtain characteristics of smooth muscle cells in vitro. Stem Cells Dayt. Ohio 25, 271–278 (2007).
Witty, A. D. et al. Generation of the epicardial lineage from human pluripotent stem cells. Nat. Biotechnol. 32, 1026–1035 (2014).
Zhao, J. et al. Efficient differentiation of TBX18+/WT1+ epicardial-like cells from human pluripotent stem cells using small molecular compounds. Stem Cells Dev. 26, 528–540 (2017).
Junghof, J. et al. CDH18 is a fetal epicardial biomarker regulating differentiation towards vascular smooth muscle cells. Npj Regen. Med. 7, 1–14. https://doi.org/10.1038/s41536-022-00207-w (2022)
Cao, J. & Poss, K. D. The epicardium as a hub for heart regeneration. Nat. Rev. Cardiol. 15, 631–647 (2018).
Du, J. et al. Single-cell and spatial heterogeneity landscapes of mature epicardial cells. J. Pharm. Anal. 13, 894–907 (2023).
Litviňuková, M. et al. Cells of the adult human heart. Nature 588, 466–472 (2020).
Wang, Z. et al. Cell-type-specific gene regulatory networks underlying murine neonatal heart regeneration at single-cell resolution. Cell Rep. 33, 108472 (2020).
Marescal, O. & Cheeseman, I. M. Cellular mechanisms and regulation of quiescence. Dev. Cell 55, 259–271 (2020).
Yao, G. Modelling mammalian cellular quiescence. Interface Focus 4, 20130074 (2014).
Cho, I. J. et al. Mechanisms, hallmarks, and implications of stem cell quiescence. Stem Cell Rep. 12, 1190–1200 (2019).
van Velthoven, C. T. J. & Rando, T. A. Stem cell quiescence: dynamism, restraint, and cellular idling. Cell Stem Cell 24, 213–225 (2019).
Liu, G. Y. & Sabatini, D. M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 21, 183–203 (2020).
Saxton, R. A. & Sabatini, D. M. mTOR signaling in growth, metabolism, and disease. Cell 168, 960–976 (2017).
Weichhart, T. mTOR as regulator of lifespan, aging, and cellular senescence: a mini-review. Gerontology 64, 127–134 (2018).
Asahara, S., Inoue, H., Watanabe, H. & Kido, Y. Roles of mTOR in the regulation of pancreatic β-cell mass and insulin secretion. Biomolecules 12, 614 (2022).
Blandino-Rosano, M. et al. mTORC1 signaling and regulation of pancreatic β-cell mass. Cell Cycle 11, 1892–1902 (2012).
Garbern, J. C. et al. Inhibition of mTOR signaling enhances maturation of cardiomyocytes derived from human-induced pluripotent stem cells via p53-induced quiescence. Circulation 141, 285–300 (2020).
Yang, C. et al. mTORC1 and mTORC2 differentially promote natural killer cell development. eLife 7, e35619. https://doi.org/10.7554/eLife.35619 (2018).
Yang, C. & Malarkannan, S. Transcriptional regulation of NK cell development by mTOR complexes. Front. Cell Dev. Biol. 8, 566090 (2020).
Li, D., Wang, Y., Yang, M. & Dong, Z. mTORC1 and mTORC2 coordinate early NK cell development by differentially inducing E4BP4 and T-bet. Cell Death Differ. 28, 1900–1909 (2021).
Zhang, X. et al. FOXO3-mTOR metabolic cooperation in the regulation of erythroid cell maturation and homeostasis. Am. J. Hematol. 89, 954–963 (2014).
Malik, N. et al. mTORC1 activity is essential for erythropoiesis and B cell lineage commitment. Sci. Rep. 9, 16917 (2019).
Mass, E., Nimmerjahn, F., Kierdorf, K. & Schlitzer, A. Tissue-specific macrophages: how they develop and choreograph tissue biology. Nat. Rev. Immunol. 23, 563–579 (2023).
Cooke, A. et al. The RNA-binding protein YBX3 controls amino acid levels by regulating SLC mRNA abundance. Cell Rep. 27, 3097–3106.e5 (2019).
Feng, Z. & Levine, A. J. The regulation of energy metabolism and the IGF-1/mTOR pathways by the p53 protein. Trends Cell Biol. 20, 427–434 (2010).
Floyd, S. et al. The insulin-like growth factor-I–mTOR signaling pathway induces the mitochondrial pyrimidine nucleotide carrier to promote cell growth. Mol. Biol. Cell 18, 3545–3555 (2007).
Schiaffino, S. & Mammucari, C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet. Muscle 1, 4 (2011).
Li, X. et al. Transcriptional atlas of cardiogenesis maps congenital heart disease interactome. Physiol. Genomics 46, 482–495 (2014).
Sen, B. et al. mTORC2 regulates mechanically induced cytoskeletal reorganization and lineage selection in marrow derived mesenchymal stem cells. J. Bone Miner. Res. 29, 78–89 (2014). 10.1002/jbmr.2031.
Lemons, J. M. S. et al. Quiescent fibroblasts exhibit high metabolic activity. PLOS Biol. 8, e1000514 (2010).
Ladha, M. H., Lee, K. Y., Upton, T. M., Reed, M. F. & Ewen, M. E. Regulation of exit from quiescence by p27 and cyclin D1-CDK4. Mol. Cell. Biol. 18, 6605–6615 (1998).
Oki, T. et al. A novel cell-cycle-indicator, mVenus-p27K-, identifies quiescent cells and visualizes G0-G1 transition. Sci. Rep. 4, 4012 (2014).
Maselli, D. et al. Epicardial slices: an innovative 3D organotypic model to study epicardial cell physiology and activation. Npj Regen. Med. 7, 1–16. https://doi.org/10.1038/s41536-021-00202-7 (2022).
Velecela, V. et al. Epicardial cell shape and maturation are regulated by Wt1 via transcriptional control of Bmp4. Dev. Camb. Engl. 146, dev178723 (2019).
Liu, Q. et al. Discovery of 1-(4-(4-propionylpiperazin-1-yl)−3-(trifluoromethyl)phenyl)−9-(quinolin-3-yl)benzo[h][1,6]naphthyridin-2(1H)-one as a highly potent, selective mammalian target of rapamycin (mTOR) inhibitor for the treatment of cancer. J Med. Chem. 53, 7146–7155 (2010).
Eddaoudi, A., Canning, S. L. & Kato, I. Flow cytometric detection of G0 in live cells by Hoechst 33342 and pyronin Y staining. Methods Mol. Biol. Clifton NJ 1686, 49–57 (2018).
Lucena-Cacace, A. & Yoshida, Y. Analysis of transcriptional profiling of chamber-specific human cardiac myocytes derived from pluripotent stem cells. Methods Mol. Biol. Clifton NJ 2320, 219–232 (2021).
Sang, L., Coller, H. A. & Roberts, J. M. Control of the reversibility of cellular quiescence by the transcriptional repressor HES1. Science 321, 1095–1100 (2008).
Harris, L. & Guillemot, F. HES1, two programs: promoting the quiescence and proliferation of adult neural stem cells. Genes Dev. 33, 479–481 (2019).
Sueda, R. & Kageyama, R. Regulation of active and quiescent somatic stem cells by Notch signaling. Dev. Growth Differ. 62, 59–66 (2020).
Smart, N. et al. Thymosin β4 induces adult epicardial progenitor mobilization and neovascularization. Nature 445, 177–182 (2007).
Bock-Marquette, I. et al. Thymosin beta-4 denotes new directions towards developing prosperous anti-aging regenerative therapies. Int. Immunopharmacol. 116, 109741 (2023).
Gladka, M. M. et al. Thymosin β4 and prothymosin α promote cardiac regeneration post-ischaemic injury in mice. Cardiovasc. Res. 119, 802–812 (2023).
Sarbassov, D. D. et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. CB 14, 1296–1302 (2004).
Dumont, F. J. FK506, an immunosuppressant targeting calcineurin function. Curr. Med. Chem. 7, 731–748 (2000).
Wallemacq, P. E. & Reding, R. FK506 (tacrolimus), a novel immunosuppressant in organ transplantation: clinical, biomedical, and analytical aspects. Clin. Chem. 39, 2219–2228 (1993).
Brundage, M. E. et al. MAF mediates crosstalk between Ras-MAPK and mTOR signaling in NF1. Oncogene 33, 5626–5636 (2014).
Meier, A. B. et al. Epicardioid single-cell genomics uncovers principles of human epicardium biology in heart development and disease. Nat. Biotechnol. 1–14. https://doi.org/10.1038/s41587-023-01718-7 (2023).
Ong, L. P. et al. Epicardially secreted fibronectin drives cardiomyocyte maturation in 3D-engineered heart tissues. Stem Cell Rep. 18, 936–951 (2023).
Miki, K. et al. ERRγ enhances cardiac maturation with T-tubule formation in human iPSC-derived cardiomyocytes. Nat. Commun. 12, 3596 (2021).
Bedada, F. B. et al. Acquisition of a quantitative, stoichiometrically conserved ratiometric marker of maturation status in stem cell-derived cardiac myocytes. Stem Cell Rep. 3, 594–605 (2014).
Lewis-Israeli, Y. R. et al. Self-assembling human heart organoids for the modeling of cardiac development and congenital heart disease. Nat. Commun. 12, 5142 (2021).
Tian, Y. et al. Immunosuppressants Tacrolimus and Sirolimus revert the cardiac antifibrotic properties of p38-MAPK inhibition in 3D-multicellular human iPSC-heart organoids. Front. Cell Dev. Biol. 10, 1001453 (2022).
Duim, S. N., Goumans, M.-J., & Kruithof, B. P. T. WT1 in Cardiac Development and Disease. In Wilms T., van den Heuvel-Eibrink, M. M. ed. (Codon Publications, 2026).
Marques, I. J. et al. Wt1 transcription factor impairs cardiomyocyte specification and drives a phenotypic switch from myocardium to epicardium. Dev. Camb. Engl. 149, dev200375 (2022).
Schwanhäusser, B. et al. Global quantification of mammalian gene expression control. Nature 473, 337–342 (2011).
Jhanwar-Uniyal, M. et al. Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship. Adv. Biol. Regul. 72, 51–62 (2019).
López-Domínguez, J. A. et al. Cdkn1a transcript variant 2 is a marker of aging and cellular senescence. Aging 13, 13380–13392 (2021).
Llanos, S., García-Pedrero, J. M., Morgado-Palacin, L., Rodrigo, J. P. & Serrano, M. Stabilization of p21 by mTORC1/4E-BP1 predicts clinical outcome of head and neck cancers. Nat. Commun. 7, 10438 (2016).
Kim, H., Kamm, R. D., Vunjak-Novakovic, G. & Wu, J. C. Progress in multicellular human cardiac organoids for clinical applications. Cell Stem Cell 29, 503–514 (2022).
Branco, M. A., Dias, T. P., Cabral, J. M. S., Pinto-do-Ó, P. & Diogo, M. M. Human multilineage pro-epicardium/foregut organoids support the development of an epicardium/myocardium organoid. Nat. Commun. 13, 6981 (2022).
Singh, A. P. et al. Inhibition of GSK-3 to induce cardiomyocyte proliferation: a recipe for in situ cardiac regeneration. Cardiovasc. Res. 115, 20–30 (2019).
Voges, H. K. et al. Vascular cells improve functionality of human cardiac organoids. Cell Rep. 42, 112322 (2023).
Okuda, S. et al. jPOSTrepo: an international standard data repository for proteomes. Nucleic Acids Res. 45, D1107–D1111 (2017).
Acknowledgements
We thank Mio Iwasaki for her work on the proteome analysis, Akira Ohta and Hiromitsu Fuse for their assistance with high-throughput screening, and all members of the Yoshida laboratory for their constructive feedback on this study. Y.T. thanks the Japanese Government (MEXT) scholarship for its financial support. This work was supported by a grant from the Leducq Foundation (18CVD05), JSPS KAKENHI Grants (22K16137 (A.L-C.), 24K11267 (A.L-C.), and 21H02912 (Y.Y.)), the Research Center Network for Realization of Regenerative Medicine, Japan Agency of Medical Research and Development (AMED) (JP19bm0104001 and JP20bm0804022) (Y.Y.), the Acceleration Program of R&D and implementation for Regenerative Medicine and Cell and Gene Therapy, AMED (JP23bm1423011, JP23bm1323001, and JP24bm1123054) (Y.Y.), the Research on Regulatory Science of Pharmaceuticals and Medical Devices, AMED (JP22mk0101241, JP 24mk0121280) (Y.Y.), Program for Creating Deep Tech Startups with Global Reach, Japan Science and Technology Agency (JPMJSF2323) (Y.Y.), and the iPS Cell Research Fund (A.L-C and Y.Y.). We want to express our gratitude to Mikako Marx-Mori, Rumi Fujihara, Tomomi Gibson, and Hiroko Sata for their administrative support. Figures 1a, b, d, 2b, 5d, 6a, and 7a were generated by BioRender.
Author information
Authors and Affiliations
Contributions
A.L-C. and Y.Y. conceived the study and designed experiments. Y.T, A.P.E., K.T., I.C.P., Y.M., A.I. and A.L-C. performed experiments. R.S.A.O. assisted in the analysis of scRNA-Seq. M.N. assisted in RNA-sequencing and secretome. J.J. improved epicardial differentiation methods. Y.T and A.L-C. analyzed and interpreted the data. Y.Y. and A.L-C provided funding and supervision. A.L-C., Y.T. and Y.Y. wrote the manuscript. All authors discussed the results.
Corresponding authors
Ethics declarations
Competing interests
Y.Y. is a scientific advisor of Orizuru Therapeutics and receives grants from Takeda Pharmaceutical Company and Altos Labs, Inc outside the submitted work. All other authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tian, Y., Lucena-Cacace, A., Tani, K. et al. Generation of mature epicardium derived from human-induced pluripotent stem cells via inhibition of mTOR signaling. Nat Commun 16, 5902 (2025). https://doi.org/10.1038/s41467-025-60934-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-60934-8