Abstract
Alkaline water electrolysis is one of the most prospective technologies for large-scale production of green hydrogen. Nevertheless, current porous membranes face the problem of weak ion transport or poor gas barrier performance. Here, we demonstrate a facile yet massive two-step casting and phase separation strategy to design a thin, asymmetric pore-structure modulated composite membrane for efficient, safe, and industrial-grade alkaline water electrolysis. The prepared composite membrane shows better electrolytic performance (1.71 V at 1 A cm−2) and stability (working for 6352 h). In addition, an industrial-grade electrolyzer equipped with composite membranes exhibits higher hydrogen production efficiency (1.03 Nm3·h−1), H2 purity (99.9%), and faster dynamic response (less than 20 min) compared to mainstream commercial membranes. Ultimately, we propose a semi-empirical model based on the operational characteristics of an electrolyzer equipped with composite membranes and predicting its matching behavior with dynamic renewable energy sources. This work explores the viability of manufacturing high-performance alkaline water electrolysis membranes for green hydrogen production under industrial conditions.
Similar content being viewed by others
Introduction
Hydrogen energy, an efficient and clean secondary energy source, has garnered significant interest from various countries1,2,3. In contrast to conventional hydrogen production methods, green hydrogen technology offers a continuous and eco-friendly energy supply, thereby contributing to the advancement of sustainable energy systems4,5. The water electrolysis hydrogen production technology with scale-up is the key to getting efficient utilization of green hydrogen6,7. Water electrolysis technologies mainly include alkaline water electrolysis (AWE), proton exchange membrane water electrolysis (PEM), and water electrolysis based on solid oxide electrolysis (SOE) cells. Of these, AWE is the most cost-effective technology for industrial production of green hydrogen8,9. However, the current AWE technology still suffers from low efficiency and high cost compared to traditional fossil fuel hydrogen production technologies10,11. Therefore, researchers have developed many methods to improve the efficiency of AWE hydrogen production. For example, exploring efficient and low-cost catalysts12,13,14,15, developing high-performance membranes16,17,18,19,20,21,22, or reducing material cost and increasing operating pressure and temperature23,24,25,26,27. Among these strategies, developing high-performance membranes is considered one of the most effective and easiest approaches to commercialize17,28,29.
The membrane plays an essential role in AWE, as it is placed between the cathode and anode, preventing the mixing of hydrogen and oxygen30. Meanwhile, it allows the hydroxide ions in the solution to travel freely between the cathode and the anode. Therefore, the membrane must provide an effective gas barrier for system safety while ensuring good electrolyte penetration and minimal area resistance. Earlier, asbestos was used as a membrane material for AWE31, but it has been banned due to its poor conductivity and potential health risks. Recently, the polyphenylene sulfide (PPS) fabric membranes have been used in AWE on a large scale32. However, it still faces changes, including high electrolysis energy consumption during electrolysis and low gas barrier due to its hydrophobic properties and large pore size33,34. Thus, an increasing number of researchers have sought to improve the hydrophilicity and gas barrier of PPS mesh by applying functional coatings to its surface. For example, Lee et al. reported the synthesis of a Zirfon-type composite membrane by coating a suspension of hydrophilic nanoparticles (e.g., zirconium oxide35, cerium oxide36, titanium oxide37, or a mixture of nanocellulose nanoparticles38) and polysulfone (PSU) onto a PPS mesh by scraping. The results showed that the fabricated Zirfon-type composite membrane was better than the conventional PPS membrane in terms of electrolytic performance and gas purity. Li et al. designed ZrO2 composite porous membranes containing different polymers, such as poly (oxindole biphenylene)18, polybenzimidazole39. The membranes showed good electrolytic performance to commercial Zirfon membranes under the same electrolytic conditions18.
Currently, there are many studies on AWE composite membranes, but most of them focus on the coating composition on both sides, such as hydrophilic particles36,37,38,40,41,42,43,44, alkaline-resistant polymers18,19,39,45, and so on. However, little attention has been paid to the study of the optimization of the support layer structure, film-forming technology and pore structure modulation. Besides, the current studies on AWE membranes are only run in small single-compartment electrolytic cells (1 cm2 or 4 cm2), resulting in a low squeezing pressure on both sides of the membrane assembly. Meanwhile, both sides of the membrane are at atmospheric pressure, which is a big difference from the actual operating environment of AWE. This cannot reflect the real performance of the membrane, such as mechanical stability, gas-barrier, etc., which undoubtedly has serious safety hazards17,18,29,32,46. Therefore, developing a membrane that meets industrial conditions with better gas barrier properties and ion transport capability is one of the challenges AWE needs to address.
Herein, a facile and large-scale two-step approach is proposed to controllably design an AWE membrane with high efficiency, safety, and fulfillment for industrial applications. The independent modulation of coatings is achieved through stepwise casting and binary phase transition strategies. Besides, we introduce PPS micro-nano-embedded fiber membrane as the porous backbone support layer to produce a thin AWE membrane (PMCM-2) with a top-down differentiated pore structure, achieving a balance between ion transport (0.12 Ω cm2, 30 wt % KOH) and gas barrier (6.44 bar, anhydrous ethanol as the osmotic solution). When applied to AWE, PMCM-2 exhibits better electrolytic performance (1.71 V at 1 A cm−2) and longer durability (operating for about 6352 h). Importantly, we evaluated the suitability of PMCM-2 in an industrial-grade electrolyzer. The results show that the performance of PMCM-2 exhibits good consistency despite 150-fold area expansion, demonstrating the stability of the preparation method. Besides, the industrial electrolyzer fitted with PMCM-2 performs better in terms of hydrogen production efficiency, hydrogen-oxygen barrier, and response time compared to the mainstream commercial AWE membranes, showing great potential in practical applications. Lastly, based on electrochemical and thermodynamic principles23,47, we propose a semi-empirical model for refining the operational characteristics of the PMCM-2-based electrolyzer system and predicting its adaptability to the direct consumption of dynamic renewable energy sources.
Results
Design and preparation of differentially pore-structured composite membrane
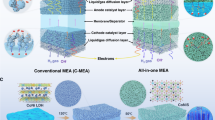
The design of AWE membranes that are efficient, safe, and capable for industrial application should obey the following criteria: i) thin and porous with good electrolyte wettability for fast ion transport; ii) small pore size and high tortuosity to reduce the risk of cross over between H2 and O2; iii) simple preparation method with good bonding between the coating and the support structure to meet the application of industrial hydrogen production system; To satisfy the above three requirements, PMCM is constructed with a differential asymmetry pore structure, consisting of a microporous dense layer, a finger-like pore layer, dense porous skeletal support layer, and a spongy pore layer (Fig. 1a). Briefly, PMCM was composed of PPS micro-nano-embedded fiber membrane as the porous backbone support layer with PSU and ZrO2 as the hydrophilic coatings. The PSU has good film-forming ability and is used as a matrix material to build a compact and highly interconnected organic framework. While ZirO2 possesses hydrophilicity to enhance the ionic conductivity of the membrane and obtain a lower impedance48,49. Specifically, the slurry was cast onto a glass plate using a spatula, and the PPS micro-nano-embedded fiber membrane was immediately laid flat on the casting layer. Subsequently, the slurry was again cast onto the PPS micro-nano-embedded fiber membranes using a spatula to obtain a wet membrane (secondary continuous casting). The wet membrane was exposed to water vapor for a certain period to cause vapor-induced phase separation (VIPS) on its surface50,51. Lastly, the wet membrane was immersed in deionized water for liquid-induced phase separation (LIPS)49,52 to obtain PMCM (binary phase inversion strategy). Figure 1b presents a PMCM measuring 0.8 m × 0.6 m with a flat appearance, and it can be folded in different directions freely, demonstrating better flexibility (Supplementary Fig. 1), which could be used for high-performance AWE in industrial grade. As shown in Fig. 1c, PMCM exhibits an obvious asymmetric structure. Benefiting from the thinner thickness (Supplementary Fig. 2) of the PPS micro-nano-embedded fiber membrane (~45 μm), the total thickness of PMCM is about 190 μm, which is thinner than the current mainstream commercial AWE porous membranes, such as Zirfon UTP-220 (220 μm), Zirfon UTP-500 (500 μm)48. Furthermore, PPS micro-nano-embedded fiber membrane has a rich three-dimensional pore structure (Fig. 1d, and Supplementary Fig. 3, Supplementary Table 1), which effectively enhances the contact area with the coating and results in better coating adhesion. This is beneficial in limiting the generation of internal pores and reducing the risk of coating peeling.
The differential pore structure is modulated mainly by controlling the vapor-induced process, and the prepared membrane is named PMCM-t (t is the vapor-induced time). As shown in Figs. 2a-1, the wet coating was exposed to water vapor at a humidity of 80% RH after secondary casting. At this stage, water vapor continuously penetrated the wet coating and formed polymer-rich and polymer-poor phases at the top of the PMCM, leading to the occurrence of VIPS. With the prolongation of the vapor induction time, the surface of the PMCM from the initial dense structure (Fig. 2b, c) gradually produced pores to obtain a microporous dense layer (Fig. 2d, e). Since the kinetics of gaseous non-solvents are much slower, the membrane morphology near the surface region can be better regulated (Figs. 2a-2, and Supplementary Fig. 4) to obtain a suitable pore structure50,51. Subsequently, the PMCM was immersed in a water bath for LIPS (Figs. 2a-3), and finger-like structures connected by spongy holes were generated below the top layer (Fig. 2f, g). Compared with the conventional finger-like structure, this finger-like structure consisting of sponge-like holes not only preserves the effective path of electrolyte transport, but also reduces the gas interpenetration to a certain extent (Supplementary Fig. 5). However, the process of LIPS was hindered in the region of the porous support layer (Figs. 2a-4). This is the result of the thermodynamic differences between the cast film liquids during the secondary casting process and the staggered arrangement of fibers in the support layer, limiting the exchange of solvent and non-solvent. This led to a further reduction of the exchange flux below the support layer, delaying the process of the liquid-liquid phase separation and eventually forming dense sponge pores. Eventually, as shown in Fig. 2h, with the support structure as the boundary, the upper part of the PMCM is a finger-like macroporous layer and the lower region is a spongy microporous layer. In contrast, under the same formulation, the composite membrane with PPS mesh as the support structure exhibited only a single finger-like pore structure (Supplementary Fig. 6). Moreover, different from the top layer, the bottom layer of PMCM possesses more open pores (Fig. 2i). And with the increase of exposure time, the bottom layer presents a large number of macroporous structures stacked by ZrO2 (Fig. 2j, and Supplementary Fig. 7). This is attributed to the fact that in the vapor-induced process, the cast solution showed a certain concentration gradient difference, while that ZrO2 is easily deposited at the bottom under gravity (Fig. 2k). These ultimately led to a in the dispersibility of the bottom cast solution and weakened the interfacial bonding with the PSF52,53. As a result, pores filled with ZrO2 are formed in the bottom layer of PMCM. Ultimately, the roughness of both the bottom and top of PMCM (Fig. 2l, m, and Supplementary Fig. 8) gradually increased with the prolongation of the vapor induction time. This allows it to be more easily wetted by the electrolyte, thereby improving the ionic conductivity. Meanwhile, the top-down differential pore structure enriches the tortuosity of PMCM to realize the step-by-step interception of gases.
a Schematic diagram of the formation of differential pore structures by binary phase inversion strategy. b Top layer cross-section morphology of PMCM-0. c Top layer morphology of PMCM-0. d Top layer cross-section morphology of PMCM-2 after VIPS. e Top layer morphology of PMCM-2 after VIPS. f Finger-like pore morphology of PMCM-2. g Enlarged morphology of finger-like pores. h Morphology of asymmetric pore structure. i Bottom layer morphology of PMCM-2. j Bottom layer morphology of PMCM-6. k Variation of bottom layer elements of PMCM-2 with different etching time. l AFM image of the top layer of PMCM-2. m AFM image of the bottom layer of PMCM-2.
a Thermal performance. b Contact angle. c Dynamic contact angle. d Porosity. e Area resistance (The average resistance of PMCM-0, PMCM-2, PMCM-4, PMCM-6, UTP-220, and UTP-500 in 30 wt % KOH at 25 °C was 5.178, 5.150, 5.147, 5.145, 5.161, and 5.222 Ω in that order). f Pore size distribution. g Gas flux and permeability coefficient. h BPP (the wetting solution is ethanol). i Variation of area resistance (The average resistance was 5.156, 5.149, 5.154, 5.156, 5.152, and 5.154 Ω at 30 wt % KOH, 25 °C increasing sequentially with immersion time) and BPP (the wetting solution is ethanol) of PMCM-2 for different immersion time. The error bars represent the standard deviation of the measured attributes for three test samples.
a Parameters related to water electrolysis and their schematic diagrams. b Variation of electrolytic performance (without iR compensation, and electrode area of 4 cm2) of PMCM-2 with electrolysis temperature at 30 wt % KOH as electrolyte. c Variation of electrolytic performance (without iR compensation) of membranes (PMCM-2 and UTP-500) with different electrodes (both cathode and anode are Ni foam; cathode is NiCoP and anode is NiFe-LDH; electrode area of 4 cm2) at 80 °C with 30 wt % KOH as electrolyte. d Long-term durability test (without iR compensation) of PMCM-2 under 1.0 A cm−2 (both cathode and anode are Ni foam) and 2.0 A cm−2 (cathode is NiCoP and anode is NiFe-LDH and electrode area of 4 cm2) at 80 °C with 30 wt % KOH as electrolyte. e Surface layer morphology of PMCM-2 after long-term electrolysis. f Variation of C element on the surface of PMCM-2 before and after long-term electrolysis. g BPP (the wetting solution is ethanol) of PMCM-2 before and after long-term electrolysis.
Physical properties of PMCM with different vapor-induced time
Physical properties of PMCM with different vapor-induced time were systematically studied. As seen in Fig. 3a and Supplementary Fig. 9, the thermogravimetric analysis (TG) results indicate that the mass of the PMCM samples with different vapor-induced time are all stable below 450°C, and there is no obvious difference in the thermal decomposition. This suggests that the thermal properties of PMCM are not affected. In addition, as shown in Fig. 3b, the contact angle of the PMCM gradually decreases with the prolongation of vapor induction, and the bottom of the PMCM exhibits a lower contact angle than the top. This is because the bottom surface of the PMCM has more open pores composed of ZrO2 compared to the top surface (shown in Fig. 2e, i), resulting in a higher roughness of the bottom surface (shown in Fig. 2l, m), which makes it easier to be wetted by water droplets. Moreover, with the prolongation of the vapor-induced process, PMCM shows better wettability (Fig. 3c). This is a result of the gradual replacement of the dense structure by the porous structure in the surface layer, which endows the PMCM with a higher porosity (Fig. 3d). As a result, as shown in Fig. 3e and Supplementary Table 2, the area resistance of PMCM decreases rapidly from 0.174 to 0.123 Ω cm2, and then gradually stabilizes at 0.114 Ω cm2, which is lower than commercial membranes. This is a result of the combination of thin thickness and high porosity (Supplementary Fig. 10)2,40. Therefore, the pore size of the PMCM gradually becomes larger as the vapor induction time increased (Fig. 3f). Whereas, the smaller average pore size helps to improve the gas barrier and reduce the gas permeability (Fig. 3g). Eventually, as depicted in Fig. 3h and Supplementary Table 2, the bubble point pressure (BPP) of PMCM-0 was 6.70 bar, while the BPP of PMCM-2 was only reduced to 6.44 bar, but that of PMCM-6 was reduced to 4.43 bar, which was still higher than that of commercial membranes (2.5-3.0 bar). This was attributed to the microporous dense layer and uniform support layer of PMCM, which guaranteed the gas barrier ability (Supplementary Fig. 11). The above results indicate that the design of the differential pore structure endows the PMCM with an effective balance between ion transport and gas barrier properties (Supplementary Table 3). Additionally, the coating must have good adhesion to the support structure to ensure the safety of the system. As indicated in Supplementary Fig. 12, under the same conditions of ultrasonic shock, PMCM-2 exhibits a more stable performance compared to UTP-500. Meanwhile, during 30 days of immersion (80 °C, 30 wt% KOH), the area resistance and BPP of PMCM-2 are stabilized at 0.12–0.14 Ω cm2 and 5.8–6.3 bar (Fig. 3i), respectively, showing notable structural stability.
Electrolytic properties and stability of PMCM-2
To further investigate the electrolytic performance of PMCM in AWE, PMCM-2, which combines ion transport and gas barrier properties (Supplementary Fig. 13), is assembled in a homemade electrolyzer (Fig. 4a, and Supplementary Table 4). An assembly torque of 4 N·m and an alkaline flow rate of 60 mL min−1 are chosen as subsequent electrolysis parameters (Supplementary Fig. 14). As the temperature increased, the electrolytic performance is improved due to the acceleration of ion transfer and catalytic kinetics (Fig. 4b)16. Furthermore, when combined with highly efficient catalyst electrodes (NiCoP for the cathode and NiFe-LDH for the anode), the membranes exhibit better electrolytic performance (Fig. 4c). However, the voltages of PMCM-2 are consistently lower than those of UTP-500 at the same current density, and this difference becomes more obvious with the increase of current density. This is attributed to the better ion transport capability of PMCM-2, which effectively improves the hydrogen production efficiency (Supplementary Fig. 15). Long-term stability is a prerequisite for membranes to be used in AWE. Considering the voltage variability of the electrolysis process, we evaluated the long-term electrolytic reliability of the PMCM-2 with different electrode combinations (as illustrated in Fig. 4d and Supplementary Table 5). During 1776 h of high-voltage operation, there was a partial increase in voltage due to a decrease in the catalytic activity of Ni foam (Supplementary Fig. 16). Then, we used the electrodes (NiFe-LDH/NiCoP) and continued the electrolysis test. PMCM-2 shows better electrolytic stability compared to UTP-500 at the same duration of electrolysis (Supplementary Fig. 17). Ultimately, during the 4576 h of low-voltage testing, there were fewer fluctuations in the electrolyzer voltage, meaning that PMCM-2 demonstrated notable electrolytic stability. The electrolytic performance and durability of PMCM-2 are competitive among the reported AWE porous membranes (Supplementary Table 6)2,17,18,29,32,37,38,39,41,49,53,54, using pure Ni or non-platinum group metal (PGM-free) catalyst as the electrodes. Besides, as seen in Fig. 4e and Supplementary Fig. 18, after long-term electrolysis, the surface pores of PMCM-2 gradually increased and were partly exposed to ZrO2, while the finger pores did not show obvious deformation or collapse. This may be caused by the peeling off of the PSU (Fig. 4f) in the surface layer during the prolonged high-temperature alkaline washing process. In addition, the support structure region and the sponge pores below it still retain a dense pore structure (Supplementary Fig. 19), ensuring the gas barrier capability. Eventually, as illustrated in Fig. 4g and Supplementary Fig. 20, the gas barrier performance of PMCM-2 is partially reduced, while the ion transport property of PMCM-2 is slightly improved, which still offers a better comprehensive performance compared to commercial membranes. In summary, PMCM-2 demonstrates more comprehensive electrolytic performance and stability, which is expected to meet the operating requirements under complex industrial conditions and exhibits potential in practical applications.
Electrolytic properties of PMCM-2 under industrial conditions
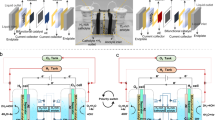
Meeting industrial condition requirements is critical for AWE membrane applications, but is often overlooked. Thus, we tested the PMCM-2 in an AWE system under industrial conditions (Fig. 5a, Supplementary Fig. 21, Supplementary Table 7). The system was an electrolyzer consisting of 10 electrolytic cells connected in series (Fig. 5b). Each cell contained a support grid, sealing gasket, anode, PMCM-2 (with a surface area of 176.7 cm2, Supplementary Fig. 22), cathode (with a surface area of 149.6 cm2), and bipolar plates. After the system was stabilized at 80 °C, 1.6 MPa, with an average current density of 16500 A/m2 for more than 1 h, the voltages of the 10 cells were mainly concentrated at 1.91-2.00 V, whereas the electrolysis consumptions were centered at 4.54–4.77 kW·h·Nm−3 (Fig. 5c), demonstrating a higher efficiency of hydrogen production compared to commercial membranes (Supplementary Fig. 23). Additionally, during the transient operation involving 10 cycles of shutdown and immediate startup (Fig. 5d), the voltage of PMCM-2 reached the pre-shutdown value in less than 20 min after restarting without significant lose. Further, as illustrated in Fig. 5e, PMCM-2 was run intermittently at an average current density of 16500 A m−2 (greatly exceeding the industrial operating current density of 5000-6000 A m−2) for 30 days. As the electrolyzer underwent repeated starts and stops (for security reasons, daily testing was done only during the daytime), the electrode catalytic coatings peeled off and deactivated, resulting in a gradual increase in the average voltage, but the PMCM-2 still maintained good stability (Supplementary Fig. 24). Gas purity is a key factor for the safety of AWE. As shown in Fig. 5f, the content of H2 in O2 (HTO) and O2 in H2 (OTH) gradually increases as the system pressure rises. At a system pressure of 1.0 MPa, the HTO or OTH of the electrolyzer fitted with PMCM-2 are 1.05% and 0.09%, respectively, which are lower than that of commercial membranes of 1.57% and 0.47% (Supplementary Fig. 25). This is attributed to the top-down differential pore structure of PMCM-2, which realizes a step-by-step interception of gases and greatly prevented the permeation of gases. Renewable energy sources have a natural volatility, so safe operation of the electrolyzer at low load is critical55,56. As seen in Fig. 5g, the HTO rises sharply with decreasing load. At a load of 20 % (3300 A m−2), the HTO reaches 1.92%. Further reduction of the load results in a security alarm and the activating of the automatic shutdown procedure. Thus, the minimum load of the electrolyzer system fitted with the PMCM-2 is 20%, which is lower than the load of existing electrolyzers fitted with commercial membranes (30–50%)47,57 and can better match renewable energy sources. Besides, highly responsive start-up characteristics are crucial when dealing with intermittent renewable energy sources. As demonstrated in Fig. 5h, the system reaches a hydrogen production of 1.0 Nm3 h−1 in about 16 and 23 min during hot and cold start-up, respectively, which is faster than the electrolysis system equipped with commercial membranes (Supplementary Fig. 26), suitable for renewable energy. In summary, we compared the overall performance of PMCM-2 and commercial membranes in hydrogen production systems under industrial conditions (Fig. 5i and Supplementary Table 8). Clearly, PMCM-2 shows much better performance in terms of current density, hydrogen production, HTO, purity of H2, and cold-start time, which is expected to be a membrane candidate for high-efficiency AWE.
a Photograph of an industrial alkaline electrolyzer device. b Enlarged view of the industrial electrolyzer and the schematic illustration of the structure of an electrolyzer cell. c Voltage and electrical consumption of different electrolysis cells in 80 °C, 1.6 MPa, and average current density of 16500 A m−2 at 30 wt % KOH as electrolyte. d Effect of number of shutdowns and startup on average cell voltage at 80 °C with 30 wt % KOH as electrolyte, and average current density of 16500 A m−2. e Variation of average cell voltage with time, at an average density of 16500 A m−2 in 80 °C with 30 wt % KOH as electrolyte. f Variation of the HTO and OTH with system pressure at 80 °C with 30 wt % KOH as electrolyte, and average current density of 16500 A m−2. g The HTO at different current densities (loads) at 80 °C with 30 wt % KOH as electrolyte. h Start-up characteristics of electrolyzer fitted with PMCM-2 at 30 wt % KOH as electrolyte, and average current density of 16500 A m−2. i Comparison of the performance of industrial-scale electrolyzers fitted with PMCM-2 and commercial membranes, including current density, hydrogen production, the HTO, purity of H2, and cold-start time at 30 wt % KOH as electrolyte.
Models of dynamic operating behavior of PMCM-2-based electrolyzer
A semi-empirical model was proposed to predict the operational characteristics of PMCM-2-based electrolyzer systems and the matching behavior with dynamic renewable energy sources based on electrochemical and thermodynamic principles23,47,57. Figure 6a, b present the response surface analysis of voltage with current density, temperature, and pressure. The voltage at constant current density decreases with increasing temperature. However, the system pressure has a much smaller effect on the voltage. Thus, regulating the system pressure was not a useful tactic for improving electrolysis efficiency. In addition, the calculated and measured voltages for different parameters display a high degree of agreement (Supplementary Fig. 27a). As shown in Fig. 6c, both experimental and modeled values show similar trends during high temperature (90 °C) electrolysis. The voltage values fluctuated between 1.88 and 1.91 V, confirming the validity of the proposed model. Further, as shown in Fig. 6d, e, the HTO increases exponentially with decreasing current density and increasing pressure. Notably, the increase of temperature also causes an increase in HTO. This is a result of the increase in diffusion phenomena, which enhances hydrogen penetration58. Therefore, it is not feasible to improve the electrolysis efficiency only by increasing the temperature. This may lead to an excess of gas impurities, resulting in a safety alarm and shutdown. Similarly, the measured and simulated values of HTO show good correlation (Supplementary Fig. 27b). Especially, the maximum error in HTO between experimental and modeled values is only 0.07% when electrolyzed at 90 °C (Fig. 6f). The utilization of renewable energy sources to produce green hydrogen is a current trend in AWE technology. Thus, we explore the adaptability of PMCM-2-based electrolyzer under wind-photovoltaic input. As seen in Fig. 6g, the voltage varies rapidly with the input current (which changes with wind energy, Supplementary Fig. 28a), fluctuating between 1.83–1.96 V. Additionally, the variation of HTO matches the input current, operating dynamically within safe limits of 1.14–1.73%. Different from wind power generation, photovoltaic power generation exhibits more pronounced symmetry and greater power fluctuations on sunny days with stable light (Supplementary Fig. 28b). Specifically, the voltage is lower in the morning and evening and highest at noon (Fig. 6h). Whereas, HTO presents an opposite trend to voltage. In particular, the HTO appears to temporarily exceed 2.0 vol% after the morning start-up and before the end of the evening but remains below the lower limit of flammability of HTO (4.0 vol%). In conclusion, effective modeling and analysis of PMCM-based electrolyzer systems can help to better understand the impact of operating strategies on hydrogen production systems and facilitate renewable energy-driven alkaline electrolyzer applications.
a Variation of voltage with current density and temperature. b Variation of voltage with current density and pressure. c Comparison of modeled and experimentally measured values of voltage. d Variation of HTO with current density and temperature. e Variation of HTO with current density and pressure. f Comparison of modeled and experimentally measured values of HTO. g Simulating voltage and HTO versus time for wind power inputs. h Simulating voltage and HTO versus time for photoelectric inputs.
Discussion
In summary, we report a facile, massive two-step approach to controllably fabricate efficient, secure, and industrial-capable AWE membranes. Through a stepwise casting and vapor-induced-immersed phase transition strategy, a thin composite membrane (PMCM-2) with a differentiated pore structure is constructed. Benefiting from the appropriate vapor induction process and the introduction of porous support layer, PMCM-2 forms a special pore structure consisting of microporous dense layer, finger-like pore layer, dense support layer, and spongy pore layer, which endow PMCM-2 with a good gas interception capability (6.44 bar) and ion transport performance (0.12 Ω cm2). Besides, PMCM-2 exhibits notable electrolytic performance (1 A cm−2 @ 2.06 V, Ni foam; 1 A cm−2 @ 1.71 V, NiFe-LDH/NiCoP) and stability (working for 6352 h) when applied to AWE. Furthermore, the industrial electrolyzer equipped with PMCM-2 shows better performance in terms of current density (1.93 V @ 16500 A m−2), hydrogen production (1.03 Nm3 h−1, 10 cells), and hydrogen purity (99.9%, 1.0 MPa) compared with mainstream commercial membrane. Finally, based on the operational characteristics of an industrial electrolyzer equipped with PMCM-2, we present a semi-empirical model to understand the impact of operating strategies on the hydrogen production system and predict its matching behavior with dynamic renewable energy sources. Furthermore, effective modeling and analysis of PMCM-2-based electrolyzer systems can help to better understand the impact of operating strategies on hydrogen production systems and provide an effective predictive tool for the subsequent optimization and design of AWE membranes for green hydrogen production. This work demonstrates the advantages of PMCM-2 in efficient AWE, expecting to drive the practical application of advanced AWE membranes in industrial alkaline electrolyzers.
Methods
Materials
N-methyl-2-pyrrolidone (NMP, 99.5%), isopropanol (99.7%), ethanol (99.5%), ZrO2 (99%), hydrochloric acid (HCl, 37%) and potassium hydroxide (KOH, 95%) were purchased from Aladdin Shanghai Reagent Co. Polysulfone (PSU, P-3500, 99%) was supplied by Solvay Inc. PPS micro-nano-embedded fiber membrane was manufactured by the laboratory. Nickel nitrate (Ni (NO3)2·6H2O, 99%), Ferrous sulfate (FeSO4·7H2O, 99%), cobalt nitrate (Co (NO3)2·6H2O, 99%), urea (CO(NH2)2, 99%), sodium hypophosphite (NaH2PO2, 98%), and ammonium fluoride (NH4F, 98%) were purchased from Shanghai Macklin Biochemical Co. Ltd. All the chemicals were used as received without any other purification. UTP-220 and UTP-500 separators were provided by Agfa. Ni and Raney Ni were purchased from Shandong Hydrogen Boat Green Energy Co. Ltd. The deionized water used in this study was homemade in the laboratory and had a conductivity of less than 10 μS/cm. The electrolyte (1 M KOH, 30 wt % KOH) was configured and used immediately in the laboratory in a procedure that included weighing, initial dissolution, cooling, and volume setting.
Preparation of PMCM
First, a polymer solution was prepared by dissolving 9.5 wt% PSU in 47.5 wt% NMP solvent at 60 °C. Then, 43 wt% ZrO2 was added to the polymer solution to form a homogeneous slurry under mechanical stirring. Details of the formulation optimization process, and their effect on the final membrane properties are given in the Supporting Information (Supplementary Fig. 29). Subsequently, the slurry was cast onto a glass plate using a spatula, and the PPS micro-nano-embedded fiber membrane was immediately laid flat on the casting layer. Subsequently, the slurry was again cast onto the PPS micro-nano-embedded fiber membranes using a spatula and after a certain time of evaporation, the cast membrane was immersed in deionized water for phase conversion to obtain the PMCM.
Pre-treatment of Ni Foam
The pretreatment process of Ni foam (NF) was as follows: firstly, it was immersed in a hydrochloric acid solution with a mass fraction of 30% for 30 min to effectively remove the surface oxidized layer and the attached impurities. Subsequently, the treated NF was placed in water and ethanol in turn, and ultrasonic cleaning was carried out for 40 min respectively, in order to remove the residual acid and other pollutants thoroughly. After cleaning, the NF was transferred to a vacuum oven at 50 °C for use in subsequent experiments.
Preparation of anode electrodes (NiFe-LDH/NF)
3.6 g of Ni (NO3)2·6H2O and 0.49 g FeSO4·7H2O were dissolved in 60 mL isopropanol and 20 mL deionized water, respectively. The above solutions were then mixed under vigorous stirring to form an HN (non-homogeneous nucleation) solution. The pre-washed NF (0.5 mm × 2 cm × 5 cm) was completely immersed in the HN solution at 25 °C for 24 h. It was finally washed with deionized water and dried in a vacuum oven at 25 °C for 12 h to produce NiFe-LDH/NF. The loading mass of NiFe-LDH in NF was 1.4 mg cm−2 obtained by calculating the mass difference between NF and NiFe-LDH/ NF in the dry state.
Preparation of cathode electrodes (NiCoP/NF)
0.87 g Co (NO3)2·6H2O, 0.87 g Ni (NO3)2·6H2O, 0.60 g urea, and 0.22 g NH4F were dissolved in 50 mL deionized water, and then the solution was transferred to an autoclave and the NF (0.5 mm × 2 cm × 5 cm) was immersed in it, which was sealed and then heated at 120 °C for 8 h. Subsequently, the dried precursor and 1.00 g NaPH2O2 were placed downstream and upstream of the tube furnace, respectively. The NiCoP/NF was produced by annealing at 350 °C for 2 h under nitrogen protection with a heating rate of 3 °C·min−1. The loading mass of NiCoP in NF was 1.2 mg cm−2 obtained by calculating the mass difference between NF and NiCoP/NF in the dry state.
Characterizations
The water contact angle of the PMCM was measured with the Dataphysics OCA-25 Contact Angle Meter. The bubble point pressure and pore size distribution of the PMCM (area of 7.07 cm2) were tested using a pore size analyzer (Model PSMA-20, GaoQ Functional Materials Co., Ltd., China). The correlation of pore size and pressure is given in Washburn’s formula.
Where D is the pore diameter, γ is the surface tension of the infiltrating solvent, θ is the angle of contact between the infiltrating solvent (e.g., ethanol) and PMCM, and P is the difference in pressure on both sides of the PMCM. Thus, the smaller the pore diameter, the greater the pressure created by the surface tension. So, this results in a higher pressure needed to push the permeable solvent out of the pore space. This means that the larger the BPP, the smaller the pore diameter of the PMCM. The porosity of the membrane (area of 7.07 cm2) was measured by the weighing method. The morphology of PMCM was observed with a scanning electron microscope (Regulus-8100, HITACHI, Japan) after drying in a freeze dryer for 24 h and under gold plating conditions for 60 s. The surface roughness of PMCM was obtained by atomic force microscopy (Dimension Icon, Bruker, Germany) measurements. The structures of PMCM were analyzed with a Thermo Science Nicolet Is50-FTIR spectrometer in the wave number range of 4000–500 cm−1. The XPS analysis of PMCM was performed with a Thermo Fisher Scientific EscaLab 250Xi electron spectrometer. The thermal stability analysis of PMCM was determined using a Netzsch STA simultaneous thermal analysis (TGA).
Electrochemical characterization
The area resistance of the membrane was measured using electrochemical impedance spectroscopy (EIS). The prepared membranes, with an effective area of 1.767 cm2 were placed between H-cells equipped with Pt electrode plates (as shown in Supplementary Fig. 30), and a 30 wt % KOH solution was injected into both compartments. The impedance was recorded at 25 °C using an electrochemical workstation (GAMRY INTERFACE 1010E) in the frequency range of 0.1 MHz to 10 Hz. The area resistance (AR) of the membrane was calculated by the following formulas:
where R1 and R2 (Ω) represent the resistances of the cell without and with the membrane, respectively. A (cm2) is the effective area of the membrane. Here, the mean value of R1 is 5.08 Ω with a standard deviation of 0.044 (Supplementary Fig. 31) at 25 °C in 30 wt % KOH solution, and A is 1.767 cm2.
The electrochemical performance was estimated by a three-electrode system connected to an electrochemical workstation (GAMRY INTERFACE 1010E). Platinum (1 cm × 1 cm), the as-prepared electrode (1 cm × 1 cm) and a calibrated Hg/HgO of 0.105 V (the value of 0.105 V is the difference between the Hg/HgO reference electrode and standard reversible hydrogen electrode at 25 °C in 1 M KOH) were directly used as the counter electrode, working electrode, and reference electrode. Linear sweep voltammetry measurements at a scan rate of 5 mVs−1 were performed without iR correction. The alkaline OER and HER performance of Ni foam, NiFe-LDH, and NiCoP in 1 M KOH solution were shown in Supplementary Fig. 32. Calibration of the Hg/HgO electrode to the reversible hydrogen electrode (RHE) scale was accomplished, using the formula: ERHE = EHgO/Hg + 0.059 × pH + 0.105 V. Here, the pH for 1 M KOH at 25 °C was 13.78, with a standard deviation of 0.064.
Alkaline water electrolysis
Water electrolysis testing of the membrane (area of 6.25 cm2) was performed in a zero-gap electrolytic cell (Supplementary Fig. 33), consisting of, in order, polar plates, gaskets, cathode (area of 4.0 cm2), membrane, anode, gaskets, and polar plates. Polarization curves (without iR compensation) were obtained by recording the voltage in the current density range of 0.2–3.4 A cm−2 at 20–80 °C in 30 wt % KOH. Long-term stability tests were performed using a potentiostat (CT6002A, Wuhan LAND Electronic Co. Ltd.), with data recorded at 1 min intervals and measurement data collected every 240 h.
Testing of Industrial electrolytic properties
First, electrolytic cells were formed in the order of support grid, anode, membrane (area of 176.71 cm2), gasket, and cathode (area of 149.57 cm2), and 10 electrolytic cells were connected in series to form an electrolyzer. Subsequently, enough electrolyte (30 wt % KOH) was fed into the system to soak the membrane. Prior to each experimental run, the system was purged with nitrogen to avoid mixing the hydrogen produced with residual air. Finally, start the pump and begin circulating the electrolyte, gradually increasing the flow rate to a given flow set point. After the above steps have been completed and the system can be started, the current density was increased and the operating conditions (pressure and temperature) were adjusted to the specified values. The electrolyzer continued to operate in partial load mode to warm up the system. Once the specified temperature was reached, all conditions were kept constant for more than 1 h. After the stabilization phase, performance tests were conducted under different operating conditions.
Mathematical model for alkaline electrolyzer
Electrochemical properties of the electrolyzer can be visualized most intuitively in its polarization curve, known as the U-I curve. Under standard conditions (25 °C and 1 atm), the reverse voltage of the water splitting is 1.229 V. In practice, because of the extra drop in voltage associated with dynamics and resistive effects, the actual voltage of the electrostack will be higher than the theoretical voltage values mentioned above. The voltage drop during AWE includes activation overpotentials, ohmic losses and concentration polarization losses. Among them, with normal working areas of a commercial alkaline electrolyzer, the concentration overpotential can be ignored. Therefore, the voltage of the electrolyzer can be expressed by the following formula:
Where, reversible voltage (Urev) is obtained from the temperature and partial pressures of the reaction products by Nernst equation23,47,59. The activation overpotentials are evaluated by the Butler–Volmer equation. The total ohmic loss is obtained through the law of Ohm. Recently, researchers have developed different models to characterize the electrolyzer. And one of the most extensive models was presented by Ulleberg60, who mathematically described the nonlinear function of the polarization curve with respect to temperature, as depicted below:
On this basis, Sanchez23 presented a mathematical model considering the effects of both temperature and pressure, which could be represented by the below equation:
The above semi-empirical formula defaults to Urev as a constant (1.23 V), which is not entirely accurate. This is because Urev is related to temperature and pressure. For this reason, we introduce the Nernst equation as well as the Antoine equation to obtain the formula for Urev at different temperatures and pressures. Ultimately, the water electrolysis voltage can be expressed as:
The purity of the gas is an essential safety index, especially when the electrolyzer is operating at low loads. At atmospheric pressure and 80 °C, the lower explosion limit (LEL) and upper explosion limit (UEL) for the mixture of H2/O2 are 3.8 and 95.4 mol% H2. Hydrogen crossover from cathode to anode is more critical due to the high dissolution, diffusion, and production rates of H2. To this end, researcher23,61 proposed an empirical model that relates HTO to temperature and pressure, as shown below:
All the statistics obtained from the experimental data (average values obtained after stabilization of the system, Supplementary Table 9) are used as empirical parameters for modeling. The model parameters in Equations are found by fitting the experimental data, using the non-linear least square method (calculated fitting via MATLAB). Eventually, the obtained model parameters are listed in Supplementary Table 10. In addition, the coefficient of determination (R2) is used to determine the goodness of fit, as shown below:
Data availability
All data supporting the findings of this study are available within the article and its Supplementary Information, or from the authors upon request. Source data are provided with this paper.
References
Xie, H. et al. A membrane-based seawater electrolyser for hydrogen generation. Nature 612, 673–678 (2022).
Henkensmeier, D. et al. Separators and membranes for advanced alkaline water electrolysis. Chem. Rev. 124, 6393–6443 (2024).
Wan, L. et al. Key components and design strategy of the membrane electrode assembly for alkaline water electrolysis. Energy Environ. Sci. 16, 1384–1430 (2023).
Boettcher, S. W. Introduction to Green Hydrogen. Chem. Rev. 124, 13095–13098 (2024).
de Kleijne, K. et al. Worldwide greenhouse gas emissions of green hydrogen production and transport. Nat. Energy 9, 1139–1152 (2024).
Ehlers, J. C., Feidenhans’l, A. A., Therkildsen, K. T. & Larrazábal, G. O. Affordable green hydrogen from alkaline water electrolysis: key research needs from an industrial perspective. ACS Energy Lett. 8, 1502–1509 (2023).
Bai, X. et al. Room-temperature, meter-scale synthesis of heazlewoodite-based nanoarray electrodes for alkaline water electrolysis. Adv. Funct. Mater. 34, 2400979 (2024).
Zhang, B. et al. High-valence metals improve oxygen evolution reaction performance by modulating 3d metal oxidation cycle energetics. Nat. Catal. 3, 985–992 (2020).
Tüysüz, H. Alkaline water electrolysis for green hydrogen production. Acc. Chem. Res. 57, 558–567 (2024).
David, M., Ocampo-Martínez, C. & Sánchez-Peña, R. Advances in alkaline water electrolyzers: a review. J. Energy Storage 23, 392–403 (2019).
Zeng, K. & Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 36, 307–326 (2010).
Liu, P. et al. A superhydrophilic NiFe electrode for industrial alkaline water electrolysis. Int. J. Hydrog. Energy 49, 285–294 (2024).
Marquez, R. A. et al. A guide to electrocatalyst stability using lab-scale alkaline water electrolyzers. ACS Energy Lett. 9, 547–555 (2024).
Zhang, J. et al. Tantalum-stabilized ruthenium oxide electrocatalysts for industrial water electrolysis. Science 387, 48–55 (2025).
Xie, L. et al. Flexible tungsten disulfide superstructure engineering for efficient alkaline hydrogen evolution in anion exchange membrane water electrolysers. Nat. Commun. 15, 5702 (2024).
Yin, L. et al. Stable anion exchange membrane bearing quinuclidinium for high-performance water electrolysis. Angew. Chem. Int. Ed. 63, e202400764 (2024).
Liao, Y., Deng, G., Wu, H., Ding, L. & Wang, H. A porous skeleton-supported organic/inorganic composite membrane for high-efficiency alkaline water electrolysis. Adv. Funct. Mater. 34, 2309871 (2023).
Li, H. et al. High chemical stability poly(oxindole biphenylene)/ZrO2 porous separator for alkaline water electrolysis. J. Membr. Sci. 700, 122658 (2024).
Luo, D. et al. Polyphenylene sulfide nonwoven-based composite separator with superior heat-resistance and flame retardancy for high power lithium ion battery. Compos. Sci. Technol. 157, 119–125 (2018).
Abdel et al. Innovative membrane with selective gas permeability for alkaline water electrolysis: Dependable cell performance under industrial conditions. J. Power Sources 587, 233709 (2023).
Tang, T. et al. Bioinspired anion exchange membranes with dual steric cross-linking centers for industrial-scale water electrolysis. Energy Environ. Sci. 17, 7816–7828 (2024).
Wan, L. et al. Oriented intergrowth of the catalyst layer in membrane electrode assembly for alkaline water electrolysis. Nat. Commun. 13, 7956 (2022).
Sánchez, M., Amores, E., Rodríguez, L. & Clemente-Jul, C. Semi-empirical model and experimental validation for the performance evaluation of a 15 kW alkaline water electrolyzer. Int. J. Hydrog. Energy 43, 20332–20345 (2018).
Haug, P., Koj, M. & Turek, T. Influence of process conditions on gas purity in alkaline water electrolysis. Int. J. Hydrog. Energy 42, 9406–9418 (2017).
Denk, K. et al. Mass transfer limitation phenomena across the separator in a zero-gap alkaline water electrolysis stack: Anion-selective polymer electrolyte membrane vs. Zirfon™ Perl UTP 500 case study. Chem. Eng. J. 479, 147354 (2024).
Rocha, F. et al. Proton exchange membrane-like alkaline water electrolysis using flow-engineered three-dimensional electrodes. Nat. Commun. 15, 7444 (2024).
Hodges, A. et al. A high-performance capillary-fed electrolysis cell promises more cost-competitive renewable hydrogen. Nat. Commun. 13, 1304 (2022).
Wan, L., Xu, Z. & Wang, B. Green preparation of highly alkali-resistant PTFE composite membranes for advanced alkaline water electrolysis. Chem. Eng. J. 426, 131340 (2021).
Liao, Y., Deng, G., Ding, L. & Wang, H. A thin and flexible composite membrane with low area resistance and high bubble point pressure for advanced alkaline water electrolysis. J. Membr. Sci. 689, 122182 (2024).
Aili, D. et al. Electrode separators for the next-generation alkaline water electrolyzers. ACS Energy Lett. 8, 1900–1910 (2023).
Wu, Y., Xu, G., Zhou, J. & Cao, D. Research progress of the porous membranes in alkaline water electrolysis for green hydrogen production. Chem. Eng. J. 505, 159291 (2025).
Gao, H. et al. High-performance composite membranes: embedding yttria-stabilized zirconia in polyphenylene sulfide fabric for enhanced alkaline water electrolysis efficiency. Small 21, 2407008 (2025).
Liu, L. et al. Ultrathin reinforced composite separator for alkaline water electrolysis: Comprehensive performance evaluation. Int. J. Hydrog. Energy 48, 23885–23893 (2023).
Li, W., Sun, Z., Ye, X. & He, Y. Ultra-thin PPS mesh-based PSF-ZrO2 composite diaphragm for efficient electrolysis of water for hydrogen production. Int. J. Hydrog. Energy 81, 918–926 (2024).
Lee, H. I. et al. The synthesis of a Zirfon-type porous separator with reduced gas crossover for alkaline electrolyzer. Int. J. Energy Res. 44, 1875–1885 (2020).
Lee, J. W. et al. Cerium oxide–polysulfone composite separator for an advanced alkaline electrolyzer. Polymers 12, 2821 (2020).
Ali, M. F. et al. A study on the effect of TiO2 nanoparticle size on the performance of composite separators in alkaline water electrolysis. J. Membr. Sci. 678, 121671 (2023).
Lee, J. W. et al. Cellulose nanocrystals–blended zirconia/polysulfone composite separator for alkaline electrolyzer at low electrolyte contents. Chem. Eng. J. 428, 131149 (2022).
Li, H. et al. Highly hydrophilic polybenzimidazole/Zirconia composite separator with reduced gas crossover for alkaline water electrolysis. J. Membr. Sci. 683, 121844 (2023).
Burnat, D. et al. Composite membranes for alkaline electrolysis based on polysulfone and mineral fillers. J. Power Sources 291, 163–172 (2015).
Yuan, X., Yan, T., Liu, Z. & Kang, P. Highly Efficient Alkaline Water Electrolysis Using Alkanolamine-Functionalized Zirconia-Blended Separators. ACS Sustain. Chem. Eng. 11, 4269–4278 (2023).
Shiva Kumar, S. et al. Synthesis of titanium (IV) oxide composite membrane for hydrogen production through alkaline water electrolysis. South Afr. J. Chem. Eng. 25, 54–61 (2018).
Song, W. et al. Novel high-safety composite separator: achieving efficient alkaline water electrolysis by compositing microporous polysulfone membrane on one side of complete structure polyphenylene sulfide fabric. Chem. Eng. J. 503, 158688 (2025).
Yu, J. et al. Hydrophilic chitosan-doped composite diaphragm reducing gas permeation for alkaline water electrolysis producing hydrogen. ACS Appl. Mater. Interfaces 16, 1394–1403 (2024).
Du, Y., Gao, H., Zhang, M., Su, K. & Li, Z. Tailoring microstructure of polysulfone composite membranes for alkaline water electrolysis. J. Membr. Sci. 721, 123830 (2025).
Wang, M. et al. A high durable and conductive Mg(OH)2-filled PP/PE composite membrane for alkaline water electrolysis. Int. J. Hydrog. Energy 64, 626–632 (2024).
Ren, Z. et al. Experimental studies and modeling of a 250-kW alkaline water electrolyzer for hydrogen production. J. Power Sources 544, 231886 (2022).
Lee, H. I. et al. Morphological tuning-driven high-performance separator for alkaline water electrolyzer through the surface modification of mechanical support. J. Membr. Sci. 719, 123727 (2025).
Guo, M. et al. Effect of porous irregular ZrO2 nanoparticles on the performance of alkaline water electrolysis composite separator membranes under complex conditions. J. Membr. Sci. 713, 123332 (2025).
Yang, Y.-L. et al. Polyvinylidene difluoride membranes with a broad time window for membrane formation via vapor induced phase separation: Fabricating, modifying, and separation properties. Colloids Surf. A Physicochem. Eng. Asp. 689, 133724 (2024).
Ismail, N. et al. Investigating the potential of membranes formed by the vapor induced phase separation process. J. Membr. Sci. 597, 117601 (2020).
Mohsenpour, S., Leaper, S., Shokri, J., Alberto, M. & Gorgojo, P. Effect of graphene oxide in the formation of polymeric asymmetric membranes via phase inversion. J. Membr. Sci. 641, 119924 (2022).
Hu, B. et al. Porous polybenzimidazole membranes doped with KOH for alkaline water electrolysis. J. Membr. Sci. 694, 122388 (2024).
Lee, H. I. et al. Advanced Zirfon-type porous separator for a high-rate alkaline electrolyser operating in a dynamic mode. J. Membr. Sci. 616, 118541 (2020).
Shimomura, M., Keeley, A. R., Matsumoto, K. I., Tanaka, K. & Managi, S. Beyond the merit order effect: Impact of the rapid expansion of renewable energy on electricity market price. Renew. Sust. Energ. Rev. 189, 114037 (2024).
Wang, J., Song, Y., Liu, F. & Hou, R. Analysis and application of forecasting models in wind power integration: A review of multi-step-ahead wind speed forecasting models. Renew. Sust. Energ. Rev. 60, 960–981 (2016).
Zhang, C., Wang, J., Ren, Z., Yu, Z. & Wang, P. Wind-powered 250 kW electrolyzer for dynamic hydrogen production: A pilot study. Int. J. Hydrog. Energy 46, 34550–34564 (2021).
Oikonomidis, S. et al. Transient modelling of a multi-cell alkaline electrolyzer for gas crossover and safe system operation. Int. J. Hydrog. Energy 48, 34210–34228 (2023).
Amores, E., Rodríguez, J. & Carreras, C. Influence of operation parameters in the modeling of alkaline water electrolyzers for hydrogen production. Int. J. Hydrog. Energy 39, 13063–13078 (2014).
Ulleberg, Ø Modeling of advanced alkaline electrolyzers: a system simulation approach. Int. J. Hydrog. Energy 28, 21–33 (2003).
Hug, W., Bussmann, H. & Brinner, A. Intermittent operation and operation modeling of an alkaline electrolyzer. Int. J. Hydrog. Energy 18, 973–977 (1993).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 22378071, 22308060, 22375047 and 22378068), National Key Research and Development Program of China (No. 2022YFB3804905), Natural Science Foundation of Fujian Province (No. 2022J01244), Orientation Lab Project (No. 00621005), Major Project (No. 00122006), Talent Scientific Research Start-up Project (No. 00524007 and 00525002), and Testing Fund of Precious Apparatus (Nos. QYT2023005 and QYT2023014) of Qingyuan Innovation Laboratory, and Quanzhou Science and Technology Plan Project (No. 2022GZ4).
Author information
Authors and Affiliations
Contributions
J.Y., Y.K.L., and W.L.C. conceived and designed the research. J.Y. carried out the experiments, analyzed data and wrote the manuscript. J.Y.L., C.L.L., and Y.Z.G. assisted in the characterization. Y.Z.L., L.M.L., and X.B.L. discussed the results. M.H.W., W.W., H.Y.C., J.Y.H., and X.J.B. supervised the project and modified the manuscript. All authors discussed the results and reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
You, J., Lu, J., Liu, C. et al. A rationally thin composite membrane with differentiated pore structure for industrial-scale alkaline water electrolysis. Nat Commun 16, 5981 (2025). https://doi.org/10.1038/s41467-025-60985-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-60985-x