Abstract
Cardiac hypertrophy leads to ventricular dysfunction and heart failure. Deubiquitinating enzymes are responsible for preserving the substrate protein stability and are essential to myocardial hypertrophy. In this study, we aimed to explore the role and regulatory mechanism of a cardiomyocyte-derived deubiquitinating enzyme, USP13, in cardiac hypertrophy. Here we show that USP13 was increased in hypertrophic myocardium and was mainly distributed in cardiomyocytes. Cardiomyocyte-specific Usp13 knockout aggravated TAC or Ang II-induced myocardial hypertrophy and dysfunction in male mice. Correspondingly, USP13 overexpression by AAV9 in hearts exerted a therapeutic impact on cardiac hypertrophy in male mice. Mechanistically, we identified STAT1 as a substrate of USP13 through interactome analysis. USP13 deubiquitinated STAT1, thereby reducing its degradation. Subsequently, USP13 promoted the STAT1-targeted Nppb gene transcription and enhanced mitochondrial function in cardiomyocytes. This study illustrated a beneficial effect of USP13 in hypertrophic cardiomyocytes and identified a cardiomyocyte-specific USP13-STAT1 axis in regulating cardiac hypertrophy.
Similar content being viewed by others
Introduction
Pathological cardiac hypertrophy leads to ventricular dilatation with contractile dysfunction, and frequently results in congestive heart failure1. Myocardial hypertrophy is often accompanied by cardiomyocyte size increase, fetal genes expression and interstitial fibrosis2. Despite the usability of clinical treatments for restraining cardiac hypertrophy, their therapeutic effects seem to be limited so far3. Considering the characteristics of terminal differentiation and poor regeneration of cardiomyocyte4, maintaining the homeostasis of intracellular key proteins might be a vital factor in determining the fate of cardiac hypertrophy. Recently, increasing attentions have been paid to the potential of post-translational modifications (PTMs) of key proteins involved in cardiomyocyte pathophysiology.
Ubiquitination, a dynamic PTM in modulating protein degradation by the 26S proteasome and other non-degradative function, is sequentially catalyzed by a series of ubiquitinating enzymes and reversely regulated by deubiquitinating enzymes (DUBs)5. DUBs are mainly responsible for the cleavage of ubiquitin molecules from substrates and maintain the substrate stability6. Our research team focuses on the regulatory mechanism of DUBs in the pathogenesis of cardiac hypertrophy and has identified two DUBs protecting cardiomyocytes via maintaining the stability of the key Ca2+-handling protein SERCA2a7,8. Therefore, DUBs can be promising as a crucial molecular library for identifying targets to treat cardiac hypertrophy and heart failure.
In the current study, through a bulk transcriptome, a single-cell mRNA sequencing (scRNA-seq) analysis, and serial experimental verification, we identified that cardiomyocyte-derived DUB, ubiquitin-specific peptidase 13 (USP13), might be a crucial factor in cardiac hypertrophy. USP13, as a member of USP family, contains a Zinc finger domain and a USP catalytic domain9. The USP domain of USP13 binds ubiquitin molecules and catalyze the hydrolysis of K27, K48, and K63-linked ubiquitin chains from the substrate proteins9,10,11. In terms of physiological functions, USP13 is mainly associated with autophagy, cell cycle regulation and DNA damage repair. Pathologically, USP13 is involved in the pathogenesis of cancers and neurodegenerative diseases by deubiquitinating specific substrate12,13. However, it remains unclear whether USP13 is associated with cardiac pathophysiology.
This study further explored the effect of cardiomyocyte-derived USP13 on cardiac hypertrophy using cardiomyocyte-specific USP13 knockout mice and recombinant adeno associated virus serotype 9 (AAV9)-mediated overexpression of USP13. Mechanistically, we identified signal transducer and activator of transcription 1 (STAT1) as the crucial substrate of USP13 by interactomes in cardiomyocytes. USP13 promoted the transcriptional function of STAT1 and protected mitochondrial function via deubiquitinating and stabilizing STAT1 in cardiomyocytes. Our study illustrated a beneficial effect of USP13 on hypertrophic cardiomyopathy and identified a cardiomyocyte-specific USP13-STAT1 axis in regulating cardiac hypertrophy.
Results
Identification of cardiomyocyte-derived USP13 as a vital factor in cardiac hypertrophy
We initially analyzed the mRNA profile of DUBs in Ang II-challenged hypertrophic myocardium from our published previously transcriptome dataset (GSE221396)7, where we observed an upregulation of Usp13 gene expression (Fig. 1a and Supplementary Fig. 1a). This finding was corroborated by a series of published transcriptome datasets (Supplementary Fig. 1a). Next, we confirmed the upregulation of Usp13 mRNA levels in mouse hearts induced by Ang II and TAC, as well as in human hypertrophic myocardium, compared to normal controls (Fig. 1b). The protein expression of USP13 was also increased in both Ang II- and TAC- induced mouse heart tissues, compared to their respective control counterparts (Fig. 1c).
a The mRNA profile of DUBs in Ang II-induced mouse hypertrophic myocardium was showed from a published transcriptome data (n = 3; GSE221396; P values were determined by Wald test from DESeq2 software with Benjamini-Hochberg’s correction). b RT-qPCR analysis of Usp13 mRNA level in Ang II- and TAC- induced mouse hypertrophic myocardium (n = 6) as well as human hypertrophic myocardium (n = 4; NCH non-cardiac hypertrophy, CH cardiac hypertrophy) (P values were determined by two-tailed unpaired t test). c Representative western blot of USP13 in Ang II- and TAC- induced mouse heart tissues (upper) and corresponding quantitative analysis (below, n = 6, P values were determined by two-tailed unpaired t test). d–g A single-cell mRNA sequencing was performed in hearts from TAC-treated mice (For each group, single-cell suspensions from 3 to 4 hearts were pooled as 1 sample). d tSNE plot showing 5 main cell types, including cardiomyocytes (CM), fibroblasts (FB), macrophages (MP), endothelial cells (EC) and pericytes (PC). e Biaxial scatter plot showing the expression pattern of Usp13 in these cell types. f UMAP distribution of clustering revealed 3 functional cardiomyocyte clusters. g Dot plot indicated the relative expression of Usp13 in the different functional cardiomyocyte clusters. h The cellular origin of USP13 in Ang II- (left) and TAC- (right) induced heart sections was revealed by immunofluorescence staining (Red: USP13; Green: α-actinin). For b-c, data are presented as mean ± s.e.m.
To identify the cellular source of upregulated USP13 in the heart, we performed scRNA-seq on approximately 17,000 individual heart cells from TAC-induced mice. Based on the specific marker genes expression, 5 main cell types were classified, including cardiomyocytes (CM), macrophages (MP), fibroblasts (FB), pericytes (PC) and endothelial cells (EC) (Fig. 1d). Notably, the Usp13 mRNA expression was mainly distributed in cardiomyocytes during cardiac hypertrophy (Fig. 1e and Supplementary Fig. 1b). Furthermore, using specific molecular markers (Supplementary Fig. 1c), we partitioned the cardiomyocytes into 3 functional clusters14, including normal (CM1), hypertrophic (CM2) and EC- or FB-like (CM3) cardiomyocytes (Fig. 1f and Supplementary Fig. 1d). It is worth noting that Usp13 mRNA expression was mainly distributed in hypertrophic cardiomyocyte (CM2, Fig. 1g) and significantly increased following TAC operation (Supplementary Fig. 1e), suggesting a potential role of USP13 in myocardial hypertrophy. The protein level of USP13 was up-regulated by Ang II challenge in a time-dependent manner in neonatal rat primary cardiomyocytes (NRCMs, Supplementary Fig. 1f). Likewise, we showed that USP13 protein expression in cardiomyocytes was much higher than that in non-cardiomyocytes (Supplementary Fig. 1g). Similarly, double immunofluorescence staining using Ang II- and TAC-induced mouse heart sections showed that up-regulated USP13 was predominantly located in α-actinin+ cardiomyocytes (Fig. 1h). Furthermore, we found that USP13 is mainly localized in the cytoplasm and does not exhibit nuclear or mitochondrial translocation either at baseline or following Ang II stimulation, suggesting that USP13 primarily carries out its deubiquitination function in the cytoplasm (Supplementary Fig. 1h).
Additionally, we endeavored to conduct an investigation on the transcriptional mechanism of increased Usp13 expression. By comparing CHIP atlas data from cardiomyocytes and heart tissues, we identified two cardiac transcription factors targeting the promoter region of Usp13: GATA-4 and Nkx-2.5 (Supplementary Fig. 2a). We then examined the effects of GATA-4 and Nkx-2.5 on USP13 expression. As shown in Supplementary Fig. 2b, the protein levels of USP13 were elevated in HL-1 cells overexpressing GATA-4; however, overexpression of Nkx-2.5 did not enhance USP13 expression (Supplementary Fig. 2c), suggesting that GATA-4 may function as a cardiac transcription regulator inducing Usp13 mRNA transcription in response to hypertrophic challenge.
Cardiomyocyte-specific Usp13 knockout aggravates cardiac hypertrophy challenged by TAC
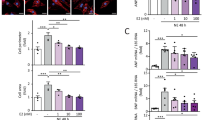
To examine the effect of USP13 on cardiomyocytes, gain- and loss-of-function analysis of USP13 was firstly applied in Ang II-stimulated NRCMs. Ang II-induced increase in cardiomyocyte area was significantly suppressed by USP13 overexpression, while silencing USP13 by siRNA further increased cardiomyocyte hypertrophy (Supplementary Fig. 2d). We then generated a cardiomyocyte-specific Usp13 knockout mice (USP13cKO) by crossing USP13f/f mice and Myh6-Cre mice (Fig. 2a and Supplementary Fig. 3a-b). USP13cKO mice and their littermate USP13f/f mice (serving as the control group) underwent either TAC or sham surgery and were monitored for 4 weeks (Fig. 2a). Non-invasive echocardiography found that USP13 cardiomyocyte deficiency led to more severe heart dysfunction induced by TAC (Fig. 2b–d and Supplementary Table 1). Cardiomyocyte deficiency of USP13 also aggravated the TAC-induced increases in gross heart size (Fig. 2e) and the ratio of heart weight (HW) to tibial length (TL) (Fig. 2f). The cardiomyocyte size, as indicated by H&E staining (Fig. 2g) and WGA staining (Fig. 2h, i), followed similar changing profile. The degree of cardiac fibrosis measured by Masson’s trichrome staining was significantly increased in TAC-induced USP13cKO mice than in USP13f/f mice (Fig. 2j, k). The serum level of ANP, a cardiac injury marker, was further enhanced in TAC-induced USP13cKO mice (Supplementary Fig. 3c). USP13cKO also exacerbated TAC-induced cardiomyocyte death in mice as detected by TUNEL staining (Supplementary Fig. 3d). To examine the potential effect of USP13 on eccentric hypertrophy, we conducted observations at 8 weeks post-TAC modeling. As shown in Supplementary Fig. 3e–g, USP13cKO also exacerbated cardiac dysfunction and structural changes induced by TAC at this time point. Collectively, USP13 derived from cardiomyocytes might serve as a protective regulator against myocardial hypertrophy caused by pressure overload.
a Schematic diagram of the strategy for the generation of cardiomyocyte-specific USP13 knockout mice (USP13cKO) and the experimental timeline of TAC model (USP13cKO mice and USP13f/f mice were subjected to TAC or sham operations for 4 weeks). Myh6-Cre+/- indicates Myh6-Cre heterozygous. b Representative M-mode echocardiographic images of left ventricle were assessed by non-invasive transthoracic echocardiography. c, d M-mode echocardiographic analysis of Ejection fraction (EF) and fractional shortening (FS). e Representative images of gross-heart from each group. f The ratio of heart weight (HW) to tibial length (TL). g Representative images of heart sections stained with H&E. h, i Representative images of heart sections stained with WGA and corresponding quantitative analysis. j, k Representative Masson’s trichrome stained images of heart sections and corresponding quantitative analysis. n = 7 for each group; For (c, d, f, i, k, adjusted P values were determined by one-way ANOVA with Bonferroni’s correction and data are presented as mean ± s.e.m.
USP13cKO exacerbates myocardial hypertrophy stimulated by Ang II
We further demonstrated the role of cardiomyocyte-derived USP13 in Ang II-induced mouse model of cardiac hypertrophy. Mice were subcutaneously implanted with osmotic mini-pumps containing Ang II for a period of 4 weeks. Cardiomyocyte deficiency of USP13 did not affect the increase of serum Ang II (Supplementary Fig. 4a) and systolic blood pressure (SBP) induced by Ang II (Supplementary Fig. 4b). As shown by non-invasive echocardiography, USP13cKO developed more severe cardiac dysfunction in Ang II-induced mice (Supplementary Fig. 4c, d). Serum ANP level followed similar trends (Supplementary Fig. 4e). Moreover, USP13cKO aggravated the hypertrophic response in Ang II- challenged mouse hearts (Supplementary Fig. 4f–h). USP13cKO mice also had an obviously increase in cardiomyocyte size compared with USP13f/f mice challenged by Ang II (Supplementary Fig. 4h–j). In line with this, the extent of interstitial fibrosis in heart tissue was markedly higher in Ang II-induced USP13cKO mice compared to USP13f/f mice (Supplementary Fig. 4k, l). Thus, we confirmed that cardiomyocyte-derived USP13 play a beneficial role in Ang II-induced cardiac hypertrophy.
USP13 directly binds STAT1 and maintains the stability of STAT1 in cardiomyocytes
DUBs perform their specific functions through modifying substrate proteins. Theoretically, the substrates of USP13 interact directly with USP13 protein. Thus, we performed interactomes to identify the potential substrates of USP13. Considering the species consistency with in vivo experiments, we selected mouse cardiomyocyte-like HL-1 cells as the primary target cells for in vitro experiments. Meanwhile, rat-derived NRCMs were also applied to exclude the influence of species difference. HL-1 cells and NRCMs were transfected with Flag-USP13 plasmids, respectively, followed by coimmunoprecipitation combined with LC-MS/MS analysis (interactome workflow shown in Fig. 3a). We identified 81 proteins that physically interact with USP13 in HL-1 cells (dataset I, Fig. 3b) and 204 proteins in NRCMs (dataset II, Fig. 3c). By comparing these two interactomes, we determined 4 potential substrates of USP13, namely STAT1, YWHAZ, ATP1A1, and VAPB (Fig. 3a–c). Among these four candidate substrates, STAT1 has been reported to prevent cardiac hypertrophy caused by pressure overload15. Thus, we hypothesized that USP13 might utilize STAT1 as a substrate in the regulation of cardiac hypertrophy. Subsequently, we verified the endogenous interaction between USP13 and STAT1 in Ang II-stimulated HL-1 cells (Fig. 3d) and heart tissues (Fig. 3e). We also employed NIH/3T3 as a tool cell for studying the mechanism of USP13 binding and deubiquitinating STAT1. The exogenous interaction between USP13 and STAT1 was examined in NIH/3T3 co-transfecting Flag-USP13 and STAT1 plasmids (Fig. 3f). These observations validated that USP13 directly binding STAT1 protein in cardiomyocytes.
a Schematic illustration of the interactomes for USP13 substrate screening. HL-1 (Dataset I) and NRCMs (Dataset II) were transfected with Flag-vector or Flag-USP13 plasmids, followed by Ang II stimulation (1 μM, 24 h). Anti-Flag and protein G-Sepharose beads were added to the cell samples for co-IP. Flag-vector plasmid was used as a negative control to exclude non-specific proteins bound to Flag, anti-Flag and protein G-Sepharose beads. The binding proteins were extracted, digested to peptide, and then subjected to LC-MS/MS analysis. The following table showed the candidate substrates of USP13 screened by interactomes. b, c 2D plots with the log10 signal intensity of the quantified proteins on the y axis (revealing the enrichment in Flag-USP13-IP) and the molecular weight (MW) of proteins on the x axis were identified from Dataset I (b) and II (c). d Co-IP of endogenous USP13 and STAT1 in lysates of Ang II- stimulated HL-1 (1 μM, 24 h). e Co-IP of endogenous USP13 and STAT1 in lysates of Ang II- treated heart tissues (1000 ng/kg/min, 4 weeks). f Co-IP of exogenous Flag-USP13 and STAT1 in lysates from NIH/3T3 expressing Flag-USP13 and STAT1. g Representative western blot of Flag-USP13, P-STAT1 and STAT1 in HL-1 expressing Flag-USP13. h, i Representative western blot of STAT1 and Flag-USP13 in HL-1 expressing Flag-USP13 or Flag-vector with CHX (25 μg/mL) pulse-chase stimulation (h) and the quantitative analysis of STAT1 (i; n = 3 independent experiments, adjusted P values were determined by two-way ANOVA with Bonferroni’s correction). j The mRNA levels of Usp13 and Stat1 in HL-1 expressing Flag-USP13 or Flag-vector (n = 6 independent experiments, P values were determined by two-tailed unpaired t test). For i, j, data are presented as mean ± s.e.m.
We then detected the stability of STAT1 in response to USP13. The protein level of STAT1 was increased in HL-1 cells (Fig. 3g) and NRCMs (Supplementary Fig. 5a) overexpressing Flag-USP13, accompanied with the increased P-STAT1 level. As expected, USP13cKO lead to the decrease of STAT1 protein level in heart tissues with or without TAC (Supplementary Fig. 5b). Next, we traced the stability of STAT1 protein by employing cycloheximide (CHX), an inhibitor of de novo protein synthesis. The degradation rate of endogenous STAT1 protein was decreased in HL-1 cells (Fig. 3h, i), NRCMs (Supplementary Fig. 5c, d) and NIH/3T3 (Supplementary Fig. 5e, f), where USP13 was overexpressed. While, USP13 overexpression did not affect the transcription level of Stat1 mRNA in cardiomyocytes (Fig. 3j). These data suggest that USP13 increases STAT1 level through preventing STAT1 degradation.
USP13 regulates the deubiquitination and stability of STAT1 at residue K379 via its active site C343
Next, we examined the effects of USP13 on STAT1 ubiquitination. Hypertrophic stimuli led to an increase in STAT1 ubiquitination in heart tissues (Supplementary Fig. 6a). USP13 overexpression decreased the STAT1 ubiquitination in HL-1 cells (Fig. 4a). As expected, USP13 deficiency increased the STAT1 ubiquitination in NRCMs (Fig. 4b). Furthermore, we replaced HA-Ub with mutants retaining only the K48 or K63 sites, the two most common sites in polyubiquitination. We observed that USP13 reduced STAT1 ubiquitination specifically when transfected with HA-K48, but not with HA-K63, in NIH/3T3 cells (Fig. 4c). This result suggests that USP13 regulates STAT1 deubiquitination in a K48-linked manner.
a HL-1 cells were transfected with Flag-USP13 and then subjected to Ang II (1 μM, 24 h) and MG132 (20 μM, 2 h). Lysates were subjected to Co-IP with anti- STAT1, which was followed by western blot of Ub, Flag-USP13 and STAT1. b NRCMs were transfected with siUSP13 and then subjected to Ang II (1 μM, 24 h) and MG132 (20 μM, 2 h). Lysates were subjected to Co-IP with anti- STAT1, which was followed by western blot of Ub, USP13 and STAT1. c STAT1, HA-Ub, or its mutant reserving only K48 (HA-K48) or K63 (HA-K63) were transfected into NIH/3T3 with or without Flag-USP13 and then subjected to 20 μM MG132 for 2 h. Co-IP assays were performed with anti-STAT1 and followed by western blot of HA, Flag-USP13 and STAT1. d Schematic illustration of USP13 active site (C343). e STAT1 and HA-Ub were transfected into NIH/3T3 with or without Flag-USP13 (WT or C343A) followed by MG132. Co-IP assays were performed with anti-STAT1 and followed by western blot of HA, Flag and STAT1. f Representative western blot of STAT1 and Flag-USP13 in NIH/3T3 expressing Flag-USP13 (WT or C343A) with CHX (25 μg/mL) pulse-chase stimulation and the quantitative analysis of STAT1 (n = 3 independent experiments, adjusted P values were determined by two-way ANOVA with Bonferroni’s correction and data are presented as mean ± s.e.m). g Schematic illustration of the STAT1 ubiquitinated-lysine residue (K379). h STAT1 (WT or K379R) and HA-Ub were transfected into NIH/3T3 with or without Flag-USP13 and followed by MG132. Ubiquitinated STAT1 was enriched with anti-STAT1 and then was detected with HA, Flag and STAT1. i Representative western blot of Flag-USP13 and STAT1 in NIH/3T3 expressing Flag-USP13 and STAT1 (WT or K379R). j Scheme for the mechanism of USP13 deubiquitinates STAT1.
The cysteine at position 343 (C343) has been reported to be a catalytic motif in the deubiquitinating function of USP1316. C343 residue and its surrounding residues were conserved in many species, including Mus musculus, Homo sapiens and Rattus norvegicus (Fig. 4d). We constructed a mutant USP13 plasmid with C343A (mutation of cysteine to alanine at C343) to examine the active site of USP13. The ability of USP13(C343A) to remove ubiquitin molecules from STAT1 was decreased (Fig. 4e), although USP13(C343A) still binds to STAT1 (Supplementary Fig. 6b). We also observed that the inactive USP13(C343A) failed to maintain the stability of STAT1 in NIH/3T3 (Fig. 4f) and HL-1 cells (Supplementary Fig. 6c), compared with USP13(WT). These data revealed the importance of C343 in USP13 deubiquitinating and stabilizing STAT1.
To determine the deubiquitination site of STAT1 mediated by USP13, we conducted an affinity-based ubiquitinated peptide enrichment ubiquitylome analysis (Supplementary Fig. 7a, b). Through comprehensively analyzing the ubiquitylome (Supplementary Fig. 7b) and an ubiquitination site prediction for STAT1 (Supplementary Fig. 7c), we identified 4 potential ubiquitination lysine residues K379, K592, K673, and K375 in STAT1 protein. Among these four residues, the evolutionarily conserved K379 site located in the DNA binding domain (DBD) of STAT1 has been identified as the ubiquitination site for the E3 ligase enzyme RNF217. Therefore, we selected K379 and generated a K379R mutant (lysine-to-arginine mutation at position 379) of STAT1 (Fig. 4g). As shown in Supplementary Fig. 7d, K379R mutation did not affect the binding between USP13 and STAT1. However, the polyubiquitination of STAT1(K379R) was much lower than STAT1(WT) and was not further reduced by USP13 (Fig. 4h), indicating that the lysine residue K379 of STAT1 is involved in USP13-mediated STAT1 deubiquitination. As expected, the ability of USP13 to stabilizing STAT1 was also abolished in STAT1(K379R) (Fig. 4i). Collectively, USP13 reduces K48-linked ubiquitination of STAT1 at position K379 through its active site C343 (Fig. 4j).
USP13 promotes the nuclear translocation of STAT1, thereby upregulating anti-hypertrophic gene transcription in cardiomyocytes
STAT1 is an important transcriptional regulator. Upon phosphorylation, STAT1 translocates into the nucleus and binds to specific promoter sequences to initiate the transcription of target genes18. Firstly, we aimed to explore whether USP13 promotes the nuclear translocation of STAT1. USP13 overexpression (USP13OE) enhanced Ang II-mediated nuclear translocation of P-STAT1 in HL-1cells (Fig. 5a–c) and NRCMs (Supplementary Fig. 8a, b), as a result of the increased total STAT1 protein. Meanwhile, the USP13 inhibitor Spautin-119 (USP13i) reduced the nuclear level of P-STAT1 (Fig. 5a–c and Supplementary Fig. 8a, b). USP13cKO decreased the P-STAT1 nuclear translocation in heart tissues of TAC mice (Supplementary Fig. 8c).
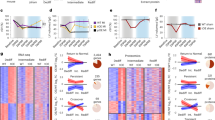
a–g HL-1 were transfected with plasmids of empty vector (EV) or USP13 (USP13OE) followed by Ang II stimulation (1 μM, 24 h). HL-1 were pretreated with Spautin-1 (USP13i, 10 μM, 1 h) followed by Ang II stimulation. a, b The P-STAT1 nuclear translocation was detected by immunofluorescence and the corresponding quantitative analysis (n = 6 independent experiments, adjusted P values were determined by one-way ANOVA with Bonferroni’s correction; black column: Ang II + EV, purple column: Ang II+Veh). c Levels of P-STAT1 in nuclear were detected by western blot. Lamin B was used as loading control. d Schematic of CUT&Tag to map the genomic occupancy of STAT1. e Distribution of STAT1 binding peaks around the gene transcriptional start site (TSS, within 3 kb (kb=1000 bp)) from CUT&Tag-sequence of Ang II-induced HL-1 cells with or without USP13OE. f Genome distribution of STAT1‑binding peaks from CUT&Tag-sequence of Ang II-induced HL-1 cells with or without USP13OE. g CUT&Tag-qPCR assay of the binding of STAT1 at Nppb promoter regions (n = 6 independent experiments, adjusted P values were determined by one-way ANOVA with Bonferroni’s correction). h HL-1 cells were co-transfected with the luciferases and pcDNA3.1-STAT1 plasmid for 48 h. Dual luciferase reporter assay detected the luciferase activation driven by the wild type (WT) or mutant (Mut) of Nppb promoter after normalization to Renilla luciferase (n = 6 independent experiments, adjusted P values were determined by one-way ANOVA with Bonferroni’s correction). i STAT1 knockdown HL-1 cells achieved by short hairpin RNA (shSTAT1) were transfected with plasmids of EV or USP13OE followed by Ang II. RT-qPCR analysis the mRNA level of Nppb (n = 6 independent experiments, adjusted P values were determined by one-way ANOVA with Bonferroni’s correction). j The schematic shows that USP13 promotes the nuclear translocation and promoter regions (e.g. Nppb) binding of STAT1, and then transcriptionally regulates gene expression via STAT1 in cardiomyocytes. For b and g-i, data are presented as mean ± s.e.m.
Next, to examine the genomic occupancy of STAT1, we performed a Cleavage Under Targets and Tagmentation (CUT&Tag) assay (Fig. 5d) and then subjected to a high-throughput sequencing. As shown in Fig. 5e, f, USP13OE increased the binding of STAT1 to the promoter regions, especially the promoter regions within 1000 bp (1 kb) of the gene transcriptional start site (TSS) (Fig. 5f, from 24.17% to 28.94%). These results suggested that USP13 could promote the transcriptional function of STAT1. Subsequently, we investigated the USP13OE- mediated up-regulation of STAT1 peaks and observed that it was significantly increased in the promoter region of the hypertrophy-associated gene Nppb (Supplementary Fig. 8d, e). As a biomarker of heart failure, the Nppb gene encodes the secreted protein BNP, which exerts biological functions including natriuresis, vasorelaxation, and inhibition of renin and aldosterone secretion. Thus, we hypothesize that USP13-STAT1 axis protects against cardiac hypertrophy through upregulating Nppb transcription and BNP expression. Using CUT&Tag-qPCR assay, we confirmed that STAT1 directly bound to the promoter regions of Nppb, which was significantly increased by USP13OE and decreased by USP13i Spautin-1 (Fig. 5g). Further, we analyzed the peak annotation results of CUT&Tag-sequence (Supplementary Fig. 8d, e) and found two potential STAT1 binding sites, which are clustered in the −369 ~ −381 bp and −912 ~ −927 bp of the Nppb promoter regions. Thus, we cloned the mutants of these two fragments (Nppb-Mut) and performed the luciferase promoter assay. As shown in Fig. 5h, STAT1 increased wildtype (WT) Nppb promotor-drived luciferase activity, while Nppb-Mut abolished the binding of STAT1 to Nppb promotor (Fig. 5h).
USP13OE significantly increased Nppb gene expression in Ang II-challenged HL-1 cells, while STAT1 knockdown abrogated the Nppb up-regulation induced by USP13OE (Fig. 5i), validating that USP13 increased the expression of Nppb gene via STAT1. Consistently, loss of USP13 in cardiomyocytes decreased the expression of Nppb transcript in vitro (Supplementary Fig. 8f) and in vivo (Supplementary Fig. 8g). Together, these results reveal that the USP13-STAT1 axis protects against cardiac hypertrophy by promoting STAT1 binding to the Nppb gene promoter and upregulating Nppb transcription and BNP expression (Fig. 5j).
USP13 improves mitochondria function via increasing mitochondrial STAT1 level in cardiomyocytes
Zhen C et al. reported that STAT1 promotes mitochondrial ATP production, thereby improving myocardial dysfunction15. Thus, we speculated that USP13 might also regulate mitochondrial function to protect cardiomyocyte through STAT1. We first showed that USP13OE increased the mitochondrial translocation of P-STAT1 and STAT1 in Ang II-induced HL-1 cells (Fig. 6a, b and Supplementary Fig. 9a). Similarly, TAC-induced mitochondrial translocation of P-STAT1 was decreased in heart tissues of USP13cKO mice (Supplementary Fig. 9b). As shown in Fig. 6c, d, Ang II-induced down-regulation of mitochondrial membrane potential (MMP) was reversed in USP13 in HL-1 cells, but further decreased by USP13i Spautin-1. Moreover, transmission electron microscopy (TEM) analysis confirmed that USP13 knockout aggravated myocardial mitochondrial ultrastructural defect induced by Ang II in mouse heart (Supplementary Fig. 9c).
a–d, j, k HL-1 were transfected with plasmids of empty vector (EV) or USP13 (USP13OE) followed by Ang II stimulation (1 μM, 24 h). HL-1 were pretreated with Spautin-1 (USP13i, 10 μM, 1 h) followed by Ang II stimulation. a Levels of P-STAT1 and STAT1 in mitochondria was detected by western blot. Tom 20 was used as loading control. b The P-STAT1 mitochondria translocation was detected by immunofluorescence. c, d Representative images of JC-1 staining (c; green: JC-1 monomers meaning decreased MMP; red: JC-1 aggregates meaning increased MMP) and the quantitative analysis (d; n = 6 independent experiments, adjusted P values were determined by one-way ANOVA with Bonferroni’s correction; black column: Ang II + EV, purple column: Ang II+Veh). e GSEA enrichment analysis of transcriptome of heart tissues from Ang II- induced USP13cKO mice and USP13f/f mice (NES: normalized enrichment score; FDR: false discovery rate). f–i Complex I activity and ATP levels were detected in heart tissues from Ang II- (f, g) or TAC- (h, i) induced USP13f/f mice and USP13cKO mice (n = 6, adjusted P values were determined by one-way ANOVA with Bonferroni’s correction). j, k Complex I activity (j) and ATP levels (k) were detected in HL-1 (n = 6 independent experiments, adjusted P values were determined by one-way ANOVA with Bonferroni’s correction; black column: Ang II + EV, purple column: Ang II+Veh). l, m STAT1 knockdown HL-1 cells were transfected with plasmids of EV or USP13OE followed by Ang II. Complex I activity (l) and ATP levels (m) were detected (n = 6 independent experiments, adjusted P values were determined by one-way ANOVA with Bonferroni’s correction). For (d, f–m), data are presented as mean ± s.e.m.
To further characterize the detailed mechanism by which the USP13-STAT1 axis regulates mitochondrial function, we performed transcriptomic analysis of heart tissues from Ang II-challenged USP13cKO mice and USP13f/f mice. Gene set enrichment analysis (GSEA) revealed multiple differentially regulated mitochondrial signaling pathways (Supplementary Table 2), especially focusing on mitochondrial respiratory complex I activity and ATP synthesis (Fig. 6e). Thus, we further measured the complex I activity and ATP level in heart tissues and found that USP13cKO decreased complex I activity and ATP synthesis in Ang II- (Fig. 6f, g) and TAC- (Fig. 6h, i) induced hearts. Likewise, the down-regulation of complex I activity and ATP level induce by Ang II was improved by overexpressing USP13 but further decreased by USP13i in vitro (Fig. 6j, k). Next, we investigated the mitochondrial bioenergetic function. Endogenous mitochondrial respiration was significantly increased in HL-1 cells overexpressing USP13 (Supplementary Fig. 9d). USP13OE increased complex I-dependent respiration but had no effect on complex II/III- or IV- dependent respiration (Supplementary Fig. 9e). As shown in Fig. 6l, m, USP13OE significantly improved the complex I activity and ATP level in Ang II-challenged HL-1 cells, while STAT1 knockdown abrogated these changed induced by USP13OE. Similarly, the USP13-mediated upregulation of MMP was reversed in STAT1-deficient HL-1 cells (Supplementary Fig. 9f), validating that USP13 improves mitochondrial function via STAT1. In conclusion, USP13 promotes the STAT1 mitochondria translocation and improves mitochondrial complex I activity and ATP synthesis in cardiomyocytes.
Cardiomyocyte-specific overexpression of USP13 ameliorates TAC-induced established cardiac dysfunction by regulating STAT1
Finally, we examined whether overexpression of USP13 or STAT1 exerts a therapeutic effect on pre-established myocardial hypertrophy. Wildtype (WT) and USP13cKO mice were subjected to TAC for 6 weeks (experimental timeline shown in Fig. 7a). Based on the heart-targeting property of AAV9 and our previous experience8, the effect of AAV9 injection typically occurs two weeks after the injection. Therefore, we administered AAV9 vectors overexpressing USP13 (USP13OE), STAT1 (STAT1OE), or empty vehicle (EV), respectively, at the end of two weeks after TAC; then, USP13 and STAT1 were overexpressed since TAC 4-week. Western blot assay confirmed the upregulation of Flag-USP13 expression in heart tissues from mice since TAC 4-week (Supplementary Fig. 10a). Echocardiography verified the presence of cardiac dysfunction 4 weeks after TAC (Fig. 7b), and animals were euthanized 6 weeks post-TAC. We examined the overexpression efficiency of AAV9s and showed increased protein levels of Flag-USP13 (Supplementary Fig. 10b) and Flag-STAT1 (Supplementary Fig. 10c) in heart tissues. As expected, USP13OE decreased the STAT1 ubiquitination (Supplementary Fig. 10d) and increased the STAT1 protein level in heart tissues (Supplementary Fig. 10e).
a Wildtype (WT) and USP13cKO mice were subjected to TAC for 6 weeks. AAV9 cardiomyocyte-specific overexpressing USP13 (USP13OE), STAT1 (STAT1OE), or vehicle (EV) were injected at the end of 2nd week after TAC (2E + 11 v.g.). Echocardiography was performed at different stages (0, 4, and 6 weeks) after TAC. Mice were harvested 6 weeks after TAC. b Ejection fraction (EF) and fractional shortening (FS) at TAC 0, 4, 6 weeks. c–e M-mode echocardiographic images of left ventricle, EF and FS at TAC 6 week. f Representative images of gross-heart at TAC 6 week. g The ratio of heart weight (HW) to tibial length (TL). h Representative images of heart sections stained with H&E at TAC 6 week. i, j Representative images of heart sections stained with WGA and corresponding quantitative analysis at TAC 6 week. k, l Representative Masson’s trichrome stained images of heart sections and corresponding quantitative analysis at TAC 6 week. n = 7 for each group; For (d, e, g, j, l), adjusted P values were determined by one-way ANOVA with Bonferroni’s correction and data are presented as mean ± s.e.m.
As shown in Fig. 7b–e and Supplementary Table 3, USP13OE significantly alleviated established cardiac dysfunction, and STAT1OE showed similar therapeutic effects against cardiac dysfunction at TAC 6 week. It is worth noting that USP13cKO + STAT1OE group did not show worse cardiac dysfunction than WT + STAT1OE group, suggesting that STAT1 is the main substrate of USP13 in hypertrophic hearts. Serum ANP level showed similar changing trends (Supplementary Fig. 10f). The examinations on whole heart size (Fig. 7f, g), cardiomyocyte size (Fig. 7h–j), and interstitial fibrosis (Fig. 7k–l) also indicated that both USP13OE and STAT1OE protected hearts from established remodeling. Importantly, these data showed that USP13cKO failed to aggravate cardiac hypertrophy when STAT1 is overexpressed, indicating that USP13 functions by STAT1 (Fig. 7f–l). Mechanistically, either USP13OE or STAT1OE could up-regulate the mRNA level of Nppb (Supplementary Fig. 10g) and improve mitochondrial function (Supplementary Fig. 10h, i) in TAC mouse heart tissues.
Further, we administered an AAV9-cTNT-shRNA vector to silence STAT1 expression in cardiomyocytes (shSTAT1). Mice were injected with AAV9-cTNT-shSTAT1 and subsequently subjected to TAC for 6 weeks. Two weeks post-TAC, mice were received AAV9 vectors to overexpress USP13 (USP13OE). Western blot analysis confirmed the down-regulation of STAT1 in heart tissues (Supplementary Fig. 11a). As shown in Supplementary Fig. 11b–f, USP13OE significantly improved cardiac dysfunction and hypertrophy induced by TAC. However, STAT1 knockdown abrogated the beneficial effects of USP13OE on mouse heart (Supplementary Fig. 11b–f), validating the crucial role of STAT1 in the USP13-mediated antihypertrophic effect in vivo. Together, USP13 exerts a therapeutic effect on myocardial hypertrophy via regulating STAT1, and USP13- or STAT1-targeted gene therapies can be potential strategies for cardiac hypertrophy.
Discussion
In this study, we showed that USP13 was increased in hypertrophic myocardium, and up-regulated USP13 was predominantly distributed in cardiomyocytes. Cardiomyocyte-specific USP13 knockout aggravated TAC- or Ang II-induced myocardial dysfunction and hypertrophy. Correspondingly, AAV9-mediated USP13 overexpression in hearts had a therapeutic effect on established cardiac hypertrophy and dysfunction. Using interactomes, we identified STAT1 as the crucial substrate of USP13 in hypertrophic cardiomyocyte. Mechanistically, USP13 increased deubiquitination and stability of STAT1 via its active site C343. Then, USP13 promoted the transcription of STAT1-targeted Nppb gene and improved mitochondrial function in cardiomyocytes. Finally, we overexpressed STAT1 in mouse hearts and validated that STAT1 worked as the crucial substrate for USP13 to treat TAC-induced hypertrophic cardiomyopathy.
Given that cardiomyocytes are terminally differentiated, mammals do not have sufficient capacity to regenerate lost cardiomyocytes4. Therefore, maintain the intracellular protein quality control might be a vital factor in determining cardiac pathophysiology. DUBs could maintain the stability of substrate proteins through the removal of ubiquitin chains6. Several DUBs display beneficial roles in cardiac hypertrophy, including USP420, USP1821 and A2022. Recently, our team also found that USP257 and JOSD28 protect hearts from hypertrophic response via maintain the stability of the key Ca2+-handling protein SERCA2a. This study identified cardiomyocyte-derived USP13 as a vital factor involved in cardiac hypertrophy. Although USP13 has been reported to perform multiple functions in other organs12,13, we show the role of USP13 in cardiomyocytes and cardiac pathophysiology. Importantly, we found that the up-regulated USP13 was mainly distributed in cardiomyocytes, especially hypertrophic cardiomyocytes. Given the specificity of USP13 in pathological cardiomyocytes, we propose that cardiac-specific gene therapy targeting USP13, such as AAVs encoding a cardiac-specific promoter (e.g., cTNT or Myh6) and the Usp13 gene, could serve as a promising therapeutic approach for cardiac hypertrophy.
Currently, there are some substrates regulated by USP13 in different pathophysiological processes12,13. Here, we performed interactomes and identified STAT1 as a direct substrate of USP13 in hypertrophic cardiomyocyte. Generally, K48-linked ubiquitination promotes proteasome-mediated degradation of STAT1, while K63-linkage mainly regulate the configuration and function23,24. Elevating the K63-linked polyubiquitination of STAT1 could promote its phosphorylation25. Our results revealed that K48-linkage is involved in USP13-regulated STAT1 deubiquitination and protein stability. To date, several ubiquitin-modified residues in STAT1 have been reported, such as K11025 and K37917. We identified that the K379 of STAT1 is involved in USP13-mediated STAT1 deubiquitination by ubiquitylome. It’s worth noting that K379 site locates at the DBD domain of STAT1 and polyubiquitination at this position may hinder the association of STAT1 with target gene promoters17. Here, we reported that USP13 promoted the nuclear translocation and gene promoter region binding of STAT1 to up-regulate target gene transcription. Therefore, we speculate that, in addition to increasing nuclear STAT1 level, USP13-mediated K397 deubiquitination of STAT1 may also contribute to the function of STAT1 as a transcriptional factor.
As a transcription factor, activated STAT1 initiates the transcription of target genes through binding to the gene promoters under different pathophysiological conditions26. For example, IFN-induced phosphorylated STAT1 translocates into the nucleus and binds IFN-stimulated response elements to initiate the transcription of IFN-stimulated genes18. It has been reported that STAT1 prevents the cardiac hypertrophy in TAC-induced mice15, while the transcriptional regulation mechanism of STAT1 in cardiomyocytes is unknown. We found that nuclear STAT1 initiates the transcription of the cardiac hormone gene Nppb via directly binding to its promoter regions. Nppb gene encodes BNP protein, which is both a biomarker and a beneficial feedback modulator of hypertrophy. The recombinant human BNP protein (rhBNP or Nesiritide) has been approved for clinical treatment of heart failure for many years27. Recent studies have underscored that BNP suppresses cardiac hypertrophy28,29. USP13 promotes the BNP expression via deubiquitinating and stabilizing STAT1. Under pathological conditions, BNP levels are elevated as a compensatory response; however, this increase is insufficient to halt the progression of heart failure, necessitating exogenous supplementation with BNP. Further, our study demonstrates that exogenously USP13 significantly protected against cardiac hypertrophy through up-regulating STAT1 in cardiomyocytes. However, STAT1 may not be an ideal therapeutic target for cardiac hypertrophy, given its widespread distribution across organs and its systemic influence on immunity30. Moreover, achieving selectivity among STAT family members through small-molecule is challenging. Here, we demonstrate a cardiomyocyte-specific USP13-STAT1 axis in cardiac hypertrophy that effectively circumvents the potential systemic side effects associated with targeting STAT1 alone. This suggests that targeting USP13 represents a more specific and promising strategy for cardiac hypertrophy therapy.
Elevated intracellular STAT1 level resulting from USP13 upregulation leads to increased mitochondrial STAT1, thereby alleviating mitochondrial dysfunction. In fact, the role of mitochondria-localized STAT1 in maintaining mitochondrial function and protecting cardiomyocytes has been extensively validated in myocardial hypertrophy15,31. Specifically, Meier J. A. et al. proposed that mitochondrial-localized STAT1 regulates the transcription of mitochondrial-encoded RNAs and both the heavy and light strands of the mitochondrial genome, thus affecting the activity of the oxygen respiration chain32. These results might elucidate our observation that the USP13-STAT1 axis modulates the activity of complex I in the mitochondrial respiration chain. Our study confirms that USP13 protects mitochondrial function and alleviates cardiac hypertrophy through increasing mitochondrial STAT1 level.
Collectively, our findings demonstrate that USP13 deubiquitinates and stabilizes STAT1. Additionally, we reveal the protective role of the cardiomyocyte-specific USP13-STAT1 axis in myocardial hypertrophy, indicating that cardiac-specific gene therapy targeting USP13 could represent a promising therapeutic strategy for cardiac hypertrophy.
Methods
Mice experiments
All mice experimental schemes were approved by the Experimental Animal Ethics Committee Center of Wenzhou Medical University (approval document No. wydw2024-0164). The USP13cKO mice were developed by GemPharmatech Co., Ltd (Nanjing, China). The USP13cKO genotype was preserved through the crossbreeding of the C57BL/6JGpt-H11em1Cin(Myh6-Cre)/Gpt mouse (Myh6-Cre, strain No. T004713) with the C57BL/6JGpt-Usp13em1Cflox/Gpt mouse (USP13f/f, strain No. T005058).
-
(1)
Pressure overload- induced cardiac hypertrophic model was established by a modified TAC operation33. Six- to eight-week-old male mice were anesthetized using isoflurane (2%) via an anesthesia ventilator. After a partial thoracotomy, aortic arch was released by blunt separation. Next, the aortic arch was ligated using a 6-0 nylon suture and a 27 G needle. For the sham group, the same procedure was performed without inducing aortic constriction. Mice were subjected to either TAC or sham surgery, and were monitored for 4 weeks.
-
(2)
The Ang II-induced cardiac hypertrophy model was established through subcutaneous implantation of an osmotic mini pump (1004, Alzet, Calif, USA). Six- to eight-week-old male mice were anesthetized using isoflurane (2%) via an anesthesia ventilator. The osmotic pump was subcutaneously implanted in the mouse back. Mice were administered either 1000 ng/kg/min Ang II (HY-13948, MedChemExpress, New Jersey, USA) or saline for 4 weeks. The systolic blood pressure (SBP) in the mice were measured by a non-invasive blood pressure monitoring device (BP-2010A, Softron, Japan).
-
(3)
USP13 (USP13OE) or STAT1 (STAT1OE) cardiomyocyte-specific overexpression was established by recombinant AAV9. AAV9s carrying Usp13 cDNA (NM_001013024) or Stat1 cDNA (NM_009283) as well as a cardiac- specific promoter cTNT were developed by Genechem Co., Ltd. AAV9s were injected through tail vein (2E + 11 v.g.). The control group received an injection of empty vector (EV, 2E + 11 v.g., equal volume). The AAV9 vector, which includes a cTNT promoter driving a shRNA targeting the Stat1 gene sequence (GCTGTTACTTTCCCAGATATT), was applied.
After evaluating the cardiac function using a Vevo 3100 ultrasound imaging system, mice were euthanized under sodium pentobarbital anesthesia. Subsequently, myocardial tissues and serum were harvested for next analysis.
scRNA-seq
Cardiac tissues were collected from TAC-induced mice at 0 and 6 weeks, and subsequently dissociated into single cells. For each experimental group, single-cell suspensions derived from three-four hearts were pooled to create one sample. These samples were then processed by loading them onto the 10x Chromium system for single-cell capture using the 10x Genomics Chromium Single-Cell 3’ Library kit. The subsequent steps of cDNA amplification and library construction were carried out by LC-BIO Technologies (Hangzhou, China). Libraries were sequenced on an Illumina NovaSeq 6000 sequencing system (paired-end multiplexing run, 150 bp) at a minimum depth of 20,000 reads per cell. CellRangerv6.0 pre-analyzes the off-machine single-cell data (FASTQ format) and compares it with the Mus musculus GRCm38.96 reference genome. Then, the expression matrix file of each cell was generated in the format of mtx, tsv, etc. Seuratv4.0 (https://satijalab.org/seurat/) was used for further follow-up analysis of single-cell expression matrices. Dimensionality reduction was achieved through the application of tSNE or UMAP clustering algorithms to group the cells. Cell populations were manually identified based on marker genes.
Histological analysis
To investigate the cellular origin of USP13 in the heart, OCT-embedded sections (5 μm thick) were co-incubated with primary antibodies against USP13 (1:200, sc-514416, Santa Cruz) and α-actinin (1:200, ab68194, Abcam). The sections were then incubated with secondary antibodies labeled with TRITC (1:1000, Abcam) or Alexa Fluor 488 (1:1000, Abcam). The sections were subsequently counterstained with DAPI, and images were captured using a laser confocal microscope (C2si, Nikon, Japan).
To access histological analysis, paraffin-embedded sections (5 μm thick) were stained with H&E (G1120, Solarbio, Beijing, China). To evaluate cardiomyocyte area, OCT-embedded sections were stained with WGA (GTX01502, GeneTex, Texas, USA). For the analysis of cardiac fibrosis, paraffin-embedded sections were colored using Masson’s trichrome staining (G1340, Solarbio, Beijing, China). To assess cardiomyocyte death, TUNEL staining (A113-03, Vazyme, Nanjing, China) was performed on OCT-embedded sections. All histological images were captured using fluorescence or optical microscope (80i, Nikon, Japan).
Cell culture and cell transfection
HL-1 and NIH/3T3 cells were acquired from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). NRCMs were isolated from ventricle of neonatal SD rats34,35. HL-1, NIH/3T3 and NRCMs were maintained in DMEM (Gibco, Germany) supplemented with 10% fetal bovine serum, along with 100 U/ml streptomycin and penicillin. Adult mouse ventricular cardiomyocytes were isolated from the ventricle of USP13cKO and USP13f/f mice following a Langendorff- free, simplified method36.
Usp13 gene was silenced by siRNA (GGAGAACAATGCGAATGCA for si-rat-USP13, RIBOBIO, China). Usp13 gene silence was achieved by LipofectAMINE 2000 (11668500, Thermo Fisher, German). STAT1 knockdown was achieved by shRNA (the corresponding siRNA sequence: GCTGTTACTTTCCCAGATATT, Hanbio, Shanghai, China). HL-1 cells were infected with the specified lentiviruses and subsequently selected using 10 μg/mL puromycin over a period of 2 weeks. Following this, single stable cell clones were obtained. Expression plasmids (Flag-USP13 (Rat or Mouse), Flag-USP13-C343A (Mouse), STAT1 (Mouse), STAT1-K379R (Mouse), HA-Ub (Mouse), HA-K63 (Mouse) and HA-K48 (Mouse)) were constructed by Genechem (Shanghai, China). The expressing plasmid transfections were performed by LipofectAMINE 3000 (L3000150, Thermo Fisher, German).
Interactome
Cell samples were subjected to co-IP using anti-Flag antibodies and protein G-Sepharose beads. The interacting proteins were eluted from the co-IP beads using SDT lysis buffer and digested into peptides via the FASP method. LC-MS/MS analysis was carried out by PTM Bio Co., Ltd (for NRCMs; Zhejiang, China) or BIOPROFILE (for HL-1; Shanghai, China). Potential substrate proteins were identified based on the intensity of the detected proteins.
Ubiquitylome
Protein was extracted from HL-1 cells and digested with trypsin in two rounds to obtain peptides. These peptides were then used for ubiquitin peptide enrichment by dissolving them in IAP buffer and incubating with anti-K-Ubiquitin antibody beads (5562, CST) overnight at 4 °C. LC-MS/MS analysis and the subsequent ubiquitylome were performed by BIOPROFILE (Shanghai, China).
Determination of cardiomyocyte surface area
The surface area of NRCMs was assessed using rhodamine phalloidin (CA1610, Solarbio, Beijing, China). Nuclei were stained with DAPI, and images were captured using a fluorescence microscope (TI-S, Nikon, Japan).
P-STAT1 nuclear or mitochondria translocation
For P-STAT1 nuclear translocation, cells were incubated with anti-P-STAT1 (1:200, 7649, CST) overnight at 4 °C. Then, above cells were incubated with TRITC-labeled secondary antibody (Abcam, 1:1000) for 1 hour at room temperature (RT). Nuclei were stained with DAPI, and images were captured using a fluorescence microscope (TI-S, Nikon, Japan).
P-STAT1 mitochondria translocation was determined by double-immunofluorescence staining with anti-Tom20 (ab186735, Abcam, 1:200) and anti-P-STAT1 (1:200, 7649, CST). Nuclei were stained with DAPI, and images were captured using a laser confocal microscopy (C2si, Nikon, Japan).
CUT & Tag
A NovoNGS CUT&Tag 3.0 High-Sensitivity Kit (N259-YH01, Novoprotein, China) was used to perform CUT&Tag. Briefly, cells were collected and resuspended in 90 μL wash buffer and 10 μL ConA beads for 10 min at RT. Then, cell samples were incubated with anti-STAT1 for 2 h at RT and were subsequently incubated with the corresponding secondary antibody for 1 h at RT. Subsequently, the transposome pA-Tn5 was introduced and incubated with the cell samples. The DNA fragments were then isolated using Tagment DNA extract beads and resuspended in 37 μL of TE buffer. The DNA was amplified using N5 and N7 primers and further analyzed by qPCR (the primer sequences for CUT&Tag are listed in Supplementary Table 4) and next generation sequencing.
The following next-generation sequencing was carried out by Sangon Biotech (Shanghai, China). Library preparation was performed using a DNA Library Prep Kit (12201, YEASEN, Shanghai, China). The concentration and size of the libraries were verified using Qubit 4.0. Subsequently, the libraries were combined and sequenced. Raw reads including adapter sequences, as well as those with low-quality or ambiguous bases at the start or end, were processed by Trimmomatic. Qualified reads were mapped to the reference genome assembly using BWA with default settings. Peaks were identified using MACS2 with a p-value threshold of <1e-5 and annotated to gene functional regions.
Luciferase reporter assay
The wildtype (WT) or mutant (Mut) DNA fragments of the Nppb promoter were amplified and subsequently cloned into the pGL3-Basic luciferase reporter vector. pcDNA3.1-STAT1 plasmid was obtained from Genechem (Shanghai, China). Cells were co-transfected with pcDNA3.1-STAT1 and the luciferase. Subsequently, the Nppb promoter reporter activity was assessed using firefly luciferase and normalized to Renilla luciferase activity.
Mitochondrial membrane potential (MMP)
JC-1 staining (C2006, Beyotime, China). was used to assess MMP in HL-1 cells. JC-1 staining reversibly changes from red (aggregate form) to green (monomeric form) as MMP decreases. Images were captured using a fluorescence microscope (TI-S, Nikon, Japan).
Determination of ultrastructural defects in heart tissue
Ultrastructural defects in heart tissue were determined by TEM. Heart tissues were initially fixed in 2.5% glutaraldehyde for 18–20 h, followed by secondary fixation in 1% osmium tetroxide for 1 h. The samples were then dehydrated and embedded in epoxy resin. Subsequently, ultrathin sections (60 nm) were prepared and stained with 2% uranyl acetate and lead tartrate. Ultrastructural visual images were taken by an electron microscope (H-7500, Hitachi, Japan).
Transcriptome
Transcriptome was carried out in heart tissues from USP13f/f and USP13cKO mice undergoing Ang II infusion. Total RNA was extracted from heart tissues using TRIzol reagent (Thermo Fisher). Library preparation, sequencing, and GSEA analysis were carried out by LC-Bio Technologies (Hangzhou, China).
Measurement of mitochondrial respiratory chain complex I activity and ATP levels
The complex I activity was detected by a Complex I assay Kit (KTB1850, Abbkine CheKine™). Cell or tissue lysis was centrifuged and the precipitate was resuspended with the corresponding kit reagent as the mitochondrial suspension. According to the product datasheet, the absorbance of A1 and A2 was measured at 340 nm for 0 min and 2 min (ΔA = A1-A2), respectively. For cell samples, the activity of Complex I (nmol NADH/min/104 cells)=1.46×ΔA. For tissue samples, the activity of Complex I (nmol NADH/min/mg protein)=0.731×ΔA÷sample weight. The ATP level in tissues or cells was measured by an ATP assay kit (S0026, Beyotime, Shanghai, China) according to the instructions. Luminescence signal is detected by Agilent BioTek Synergy Neo2 hybrid multi-mode microplate reader.
Determination of VO2
Endogenous VO2 in HL-1 was measured by the Oroboros oxygraph-2k system (O2K, Innsbruck, Austria). Approximately 0.6–1.4 million cells were added into the respirometry chamber, and 3.5 μL oligomycin (Oligo, 0.1 mg/mL) or 3 μL carbonyl cyanide p-trifluoromethoxy phenylhydrazone (FCCP, 0.1 mM) was sequentially added to detect the mitochondrial proton leak and maximal respiration. For mitochondrial complex-dependent oxygen respiration, 3–6 million cells were collected and treated with 1 μL 2% digitonin for 45 s, 25 μL of glutamate (1 M) and malate (1 M), 1.5 μL rotenone (100 μM), 12.5 μL succinate (1 M), 3 μL antimycin A (10 mM), 10 μL ascorbic acid (1 M) and 15 μL TMPO (4 mM), and 3 μL NaN3 (4 M) were sequentially added into the chamber for the evaluation of complex-dependent respiration.
Co-IP and western blot
For co-IP experiments, cell or tissue lysates were incubated with the primary antibody (overnight at 4 °C), while a portion of the lysate was reserved as the input sample. Subsequently, the protein lysate was precipitated using Protein G-Sepharose beads (6–12 h at 4 °C). Following washes with PBS, the Protein G-Sepharose beads were subjected to Western blot.
Nuclear proteins or mitochondria were extracted with their extraction kits (P0028 for nuclear proteins, C3601 for mitochondria, Beyotime, Shanghai, China). The routine procedures of western blot were carried out34,35. Proteins were loaded and separated using SDS-PAGE, followed by transfer onto PVDF membranes. These membranes were then incubated with 5% Bovine Serum Albumin to block non-specific binding sites and subsequently probed with primary antibodies at 4 °C overnight. Following this, the membranes were treated with secondary antibodies for 1 hour and visualized using an enhanced chemiluminescence detection system. Antibodies against USP13 (1:1000, 16840-1-AP), Flag (1:1000, 20543-1-AP) and HA (1:1000, 51064-2-AP) were procured from Proteintech. Antibodies against Lamin B (1:1000, ab133741) and Tom20 (1:1000, ab186735) were purchased from Abcam. Antibodies against GAPDH (1:1000, 5174), STAT1 (1:1000, 14994) and P-STAT1 (1:1000, 7649) were obtained from CST.
Human samples
Human myocardial tissue samples were obtained from patients with cardiac hypertrophy undergoing pacemaker electrode replacement surgery. Control samples were collected from patients with arrhythmia but without cardiac hypertrophy undergoing the same surgical procedure. The clinical characteristics of these patients are summarized in Supplementary Table 5. Written informed consent was obtained from all participants, and the experiments involving human samples were approved by the Ethics Committee of 1st Affiliated Hospital of Wenzhou Medical University (Wenzhou, China; approval number KY2022-156).
RT-qPCR
Total RNA of mouse heart tissues, human myocardial samples or culture cells was extracted by TRIzol reagent (Thermo Fisher). Subsequently, RNA was reverse-transcribed into cDNA using the PrimeScript RT Kit (11201ES03, Yeasen). RT-PCR was carried out using the SYBR Green reagent kit (R223-00, Vazyme). The primers were synthesized by Sangon Biotech (Shanghai, China), and the sequences are listed in Supplementary Table 3. The levels of target mRNAs were normalized to β-actin.
Statistics and reproducibility
All in vitro or in vivo data are reported as means ± standard error (SEM) in this study. For western blot analysis, each experiment was independently repeated at least three times with similar results. To identify differences among groups, Student’s t-test was used for comparisons involving two groups, while one-way analysis of variance (ANOVA) with multiple comparisons (Bonferroni’s correction) was applied for studies with more than two groups. Two-way ANOVA with Bonferroni’s correction was employed when analyzing more than two variables. These analyses were performed using GraphPad Prism 8.0 (GraphPad, San Diego, CA). A result was considered statistically significant when the P value was less than 0.05.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data are included within the article or Supplementary Information or available from the authors on request. Source data are provided with this paper. The transcriptomic datasets generated in this study, including both bulk and single cell, have been deposited in the Gene Expression Omnibus (GEO) database under accession code: GSE236650 and GSE271946. The transcriptomic datasets used in this study from previous publications (accession code: GSE2213967, GSE284286 and GSE20308337). Source data are provided with this paper.
References
Nakamura, M. & Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 15, 387–407 (2018).
Mishra, S. & Kass, D. A. Cellular and molecular pathobiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 18, 400–423 (2021).
McKinsey, T. A. & Kass, D. A. Small-molecule therapies for cardiac hypertrophy: moving beneath the cell surface. Nat. Rev. Drug Discov. 6, 617–635 (2007).
Karbassi, E. et al. Cardiomyocyte maturation: advances in knowledge and implications for regenerative medicine. Nat. Rev. Cardiol. 17, 341–359 (2020).
Popovic, D., Vucic, D. & Dikic, I. Ubiquitination in disease pathogenesis and treatment. Nat. Med. 20, 1242–1253 (2014).
Mevissen, T. E. T. & Komander, D. Mechanisms of Deubiquitinase Specificity and Regulation. Annu. Rev. Biochem. 86, 159–192 (2017).
Ye, B. et al. USP25 Ameliorates Pathological Cardiac Hypertrophy by Stabilizing SERCA2a in Cardiomyocytes. Circulation Res. 132, 465–480 (2023).
Han, J. et al. Deubiquitinase JOSD2 improves calcium handling and attenuates cardiac hypertrophy and dysfunction by stabilizing SERCA2a in cardiomyocytes. Nat. Cardiovascular Res. 2, 764–777 (2023).
Zhang, Y. H., Zhou, C. J., Zhou, Z. R., Song, A. X. & Hu, H. Y. Domain analysis reveals that a deubiquitinating enzyme USP13 performs non-activating catalysis for Lys63-linked polyubiquitin. PloS one 6, e29362 (2011).
Sun, H. et al. USP13 negatively regulates antiviral responses by deubiquitinating STING. Nat. Commun. 8, 15534 (2017).
Xie, W., Jin, S. & Cui, J. The NEDD4-USP13 axis facilitates autophagy via deubiquitinating PIK3C3. Autophagy 16, 1150–1151 (2020).
Wang, Q. et al. Role of USP13 in physiology and diseases. Front. Mol. Biosci. 9, 977122 (2022).
Li, X. et al. USP13: Multiple Functions and Target Inhibition. Front. cell developmental Biol. 10, 875124 (2022).
Ren, Z. et al. Single-Cell Reconstruction of Progression Trajectory Reveals Intervention Principles in Pathological Cardiac Hypertrophy. Circulation 141, 1704–1719 (2020).
Zhen, C., Liu, H., Gao, L., Tong, Y. & He, C. Signal transducer and transcriptional activation 1 protects against pressure overload-induced cardiac hypertrophy. FASEB J.: Off. Publ. Federation Am. Societies Exp. Biol. 35, e21240 (2021).
Zhao, X., Fiske, B., Kawakami, A., Li, J. & Fisher, D. E. Regulation of MITF stability by the USP13 deubiquitinase. Nat. Commun. 2, 414 (2011).
Liu, S. et al. Nuclear RNF2 inhibits interferon function by promoting K33-linked STAT1 disassociation from DNA. Nat. Immunol. 19, 41–52 (2018).
Hu, X., Li, J., Fu, M., Zhao, X. & Wang, W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct. Target. Ther. 6, 402 (2021).
Liu, J. et al. Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell 147, 223–234 (2011).
He, B. et al. Ubiquitin-Specific Protease 4 Is an Endogenous Negative Regulator of Pathological Cardiac Hypertrophy. Hypertension 67, 1237–1248 (2016).
Ying, X. et al. Novel Protective Role for Ubiquitin-Specific Protease 18 in Pathological Cardiac Remodeling. Hypertension 68, 1160–1170 (2016).
Huang, H. et al. Tumor suppressor A20 protects against cardiac hypertrophy and fibrosis by blocking transforming growth factor-beta-activated kinase 1-dependent signaling. Hypertension 56, 232–239 (2010).
Yuan, C., Qi, J., Zhao, X. & Gao, C. Smurf1 protein negatively regulates interferon-γ signaling through promoting STAT1 protein ubiquitination and degradation. J. Biol. Chem. 287, 17006–17015 (2012).
Lawrence, D. W. & Kornbluth, J. E3 ubiquitin ligase NKLAM ubiquitinates STAT1 and positively regulates STAT1-mediated transcriptional activity. Cell. Signal. 28, 1833–1841 (2016).
Guo, X. et al. RNF220 mediates K63-linked polyubiquitination of STAT1 and promotes host defense. Cell death Differ. 28, 640–656 (2021).
Ramana, C. V., Chatterjee-Kishore, M., Nguyen, H. & Stark, G. R. Complex roles of Stat1 in regulating gene expression. Oncogene 19, 2619–2627 (2000).
Keating, G. M. & Goa, K. L. Nesiritide: a review of its use in acute decompensated heart failure. Drugs 63, 47–70 (2003).
Gardner, D. G. Natriuretic peptides: markers or modulators of cardiac hypertrophy? Trends Endocrinol. Metab.: TEM 14, 411–416 (2003).
Volpe, M., Rubattu, S. & Burnett, J. Jr. Natriuretic peptides in cardiovascular diseases: current use and perspectives. Eur. heart J. 35, 419–425 (2014).
Ivashkiv, L. B. IFNγ: signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 18, 545–558 (2018).
Boengler, K., Hilfiker-Kleiner, D., Heusch, G. & Schulz, R. Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res. Cardiol. 105, 771–785 (2010).
Meier, J. A. & Larner, A. C. Toward a new STATe: the role of STATs in mitochondrial function. Semin. Immunol. 26, 20–28 (2014).
Richards, D. A. et al. Distinct Phenotypes Induced by Three Degrees of Transverse Aortic Constriction in Mice. Sci. Rep. 9, 5844 (2019).
Han, J. et al. GSDMD (Gasdermin D) Mediates Pathological Cardiac Hypertrophy and Generates a Feed-Forward Amplification Cascade via Mitochondria-STING (Stimulator of Interferon Genes) Axis. Hypertension 79, 2505–2518 (2022).
Ye, B. et al. Gasdermin D mediates doxorubicin-induced cardiomyocyte pyroptosis and cardiotoxicity via directly binding to doxorubicin and changes in mitochondrial damage. Transl. Res. 248, 36–50 (2022).
Ackers-Johnson, M. et al. A Simplified, Langendorff-Free Method for Concomitant Isolation of Viable Cardiac Myocytes and Nonmyocytes From the Adult Mouse Heart. Circulation Res. 119, 909–920 (2016).
Lv, J. et al. Downregulation of MLF1 safeguards cardiomyocytes against senescence-associated chromatin opening. Nucleic Acids Res. 53, gkae1176 (2025).
Acknowledgements
This study was supported by National Natural Science Foundation of China (82370244 and 82361138563 to Y.W., 82271347 to G.W., and U24A20814 to G.L.). We appreciate Lingli Hou and Yanni Dong from the Scientific Research Center of Wenzhou Medical University for their help in echocardiography.
Author information
Authors and Affiliations
Contributions
L.L., Z.F., J.H., D.X., L.W., X.H., B.Y., X.L., and J.M. performed the experiments. J.H., Y.W., and G.L. contributed to literature review and study design. J.H., Y.W., and G.L. were involved in drafting the manuscript. J.H., L.L., and Z.F. participated in data collection and analysis. G.L., G.W., and Y.W. provided essential reagents or tools.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Soumitra Pal, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Han, J., Lin, L., Fang, Z. et al. Cardiomyocyte-derived USP13 protects hearts from hypertrophy via deubiquitinating and stabilizing STAT1 in male mice. Nat Commun 16, 5927 (2025). https://doi.org/10.1038/s41467-025-61028-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-61028-1
This article is cited by
-
Identification of the E3 ligase TRIM21 as a crucial regulator of STAT1 in metabolic dysfunction—associated steatohepatitis
Cell Communication and Signaling (2026)
-
USP13 promotes colorectal cancer progression by deubiquitinating and stabilizing HIF-1α
Cell Communication and Signaling (2025)
-
Ubiquitin-specific protease 13 promotes colorectal cancer progression by stabilizing mitogen-activated protein kinase kinase 3
Molecular Biomedicine (2025)