Abstract
(+)-cis-12-oxo-phytodienoic acid (cis-OPDA) is a biosynthetic precursor of the plant hormone (+)−7-iso-Jasmonoyl-L-isoleucine (JA-Ile). It was proposed as an endogenous chemical signal independent of the JA-Ile receptor COI1-JAZ in Arabidopsis thaliana. The bioactive form of cis-OPDA that induces COI1-JAZ-independent gene expression remains unknown. Here we demonstrate that the downstream metabolites of cis-OPDA, especially tetranor-cis-OPDA (tn-cis-OPDA) and 7-iso-4,5-didehydro-JA (4,5-ddh-JA), upregulate the expression of the OPDA marker genes such as ZAT10 and ERF5. These downstream metabolites function independently of the JA-Ile-COI1-JAZ-MYCs canonical jasmonate signaling module, and their electrophilic α, β-unsaturated carbonyl functionalities are essential for their bioactivity. These results raise a possibility that the downstream metabolites of cis-OPDA would function as endogenous chemical signal in A. thaliana.
Similar content being viewed by others
Introduction
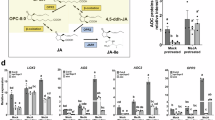
Jasmonic acid and related fatty acid-derived oxylipins are collectively referred to as jasmonates. ( + )−7-iso-jasmonoyl-L-isoleucine (JA-Ile; Fig. 1), is a lipid-derived plant hormone that regulates growth, reproduction, and defense responses against pathogens and chewing insects1,2,3. In plant cells, JA-Ile is synthesized from α-linolenic acid via the biosynthetic precursor (+)-cis-12-oxo-phytodienoic acid (cis-OPDA) (Fig. 1A). cis-OPDA is reduced to OPC-8 by OPDA reductase 3 (OPR3), followed by oxidation to naturally occurring (3 R, 7S)-7-iso-jasmonic acid (JA, a cis-form) through three rounds of β-oxidation. Successive conjugation of JA with L-isoleucine (Ile) by GH3 enzyme jasmonic acid-resistant 1 (JAR1) or AtGH3.10 produce JA-Ile4,5,6,7,8. Recently, an OPR3-independent route for JA-Ile biosynthesis from cis-OPDA was reported (Fig. 1B)9. In this bypassing route, three rounds of β-oxidation occurred from cis-OPDA to dinor-cis-OPDA (dn-cis-OPDA), tetranor-cis-OPDA (tn-cis-OPDA), and 7-iso-4,5-didehydro JA (4,5-ddh-JA), which is then reduced to JA by OPR2 (Fig. 1B). An increase in JA-Ile levels in plant cells leads to the triggering of protein-protein interactions between the F-box protein CORONATINE INSENSITIVE 1 (COI1) and jasmonate-ZIM domain (JAZ) repressors, causing proteasomal degradation of JAZ to derepress transcription factors, such as MYC2, and activating gene expression10,11,12,13.
A Canonical OPR3-dependent synthesis of JA-Ile. In this route, cis-OPDA is reduced to OPC-8 by OPR3, followed by oxidization to JA through three β-oxidation and conjugation with Ile by JAR1. (B) OPR3-independent synthesis of JA-Ile. Through three β-oxidation, cis-OPDA is oxidized to 4,5-ddh-JA, which is then reduced to JA by OPR2.
Several reports have demonstrated JA-Ile-independent biological activities of the biosynthetic intermediate cis-OPDA in Arabidopsis thaliana, tomato, and maize14,15,16,17,18,19,20,21,22,23,24. Taki et al. compared the gene expression mediated by exogeneous cis-OPDA and exogeneous JA to demonstrate cis-OPDA-specific expression of genes, including genes encoding ZINC FINGER OF ARABIDOPSIS THALIANA 10 (ZAT10), ETHYLENE REPONSIVE ELEMENT BINDING FACTOR 5 (ERF5), DRE-BINDING PROTEIN 2 A (DREB2A), ZINC FINGER OF ARABIDOPSIS THALIANA 12 (ZAT12), and ARABIDOPSIS FE-DEFICIENCY INDUCED TRANSCRIPTION FACTOR 1 (FIT1)16. ZAT10 encodes a transcription factor (TF) involved in salt tolerance16, and ERF5 encodes an ethylene-responsive TF involved in wounding and cold stress responses25. DREB2A encodes a TF involved in drought and salt stress26,27. ZAT12 encodes a TF in cold stress and salt tolerance28,29. FIT1 encodes a TF in iron acquisition30. Biochemical, yeast two-hybrid (Y2H), and pull-down assays demonstrated that cis-OPDA is not recognized by Arabidopsis COI1-JAZ1/3/9 co-receptor pairs12,31, suggesting that cis-OPDA functions via a COI1-independent mode of action (MOA). However, in previous studies in A. thaliana, the biological activities of exogeneous cis-OPDA have been distinguished from those of the JA-Ile, which biosynthesized from exogeneous cis-OPDA, using the Arabidopsis mutants opr3-1 and jar1-1. In these mutants, the biosynthetic genes of JA-Ile (OPR3 or JAR1) are impaired, and thus, cis-OPDA is considered not to be converted into JA-Ile22,32. Nevertheless, recent work has revealed that the residual JA-Ile detected in opr3-1 and jar1-1 is not explained solely by the leakiness of these alleles; rather, Arabidopsis harbors an alternative OPR3-independent bypassing route that converts cis-OPDA to JA via tetranor-cis-OPDA (tn-cis-OPDA) and 7-iso-4,5-didehydro-JA(4,5-ddh-JA) before conjugation to JA-Ile (Fig. 1B)9,33. In parallel, a JAR1-independent route can also supply JA-Ile in jar1-1 plants34. As a result, the participation of cis-OPDA in JA-Ile-independent signaling pathways in A. thaliana remains debatable35,36. To date, the COI1-independent MOA of cis-OPDA has been reliably reported only in the thermotolerance of Marchantia polymorpha, in which the electrophilic α,β-unsaturated cyclopentenone moiety of cis-OPDA plays an essential role37.
In this paper, we investigated the function of cis-OPDA in WT A. thaliana and the non-leaky mutant lines coi1-138 and opr2-1 opr3-39 (Fig. 1) with impaired JA signaling and biosynthesis to identify the genuine bioactive form of cis-OPDA in A. thaliana.
Results
cis-OPDA mediated expression of OPDA marker genes is independent of the canonical COI1-JAZ-MYC signaling pathway
cis-OPDA (Fig. 1) was chemically synthesized using previously reported methods39,40. The subsequent experiments were performed using synthetic cis-OPDA of naturally occurring stereochemistry. Gene expression analysis was performed based on the information from a previously reported comprehensive DNA microarray analysis16: the impact of JA (Supplementary Fig. 1) or cis-OPDA was respectively evaluated on the expression of three JA marker genes, OPR3, JAZ8, and MYC2 (Fig. 2A–C), and five cis-OPDA-specific marker genes, ZAT10, ERF5, DREB2A, ZAT12, and FIT1 (Fig. 2D–H)16,32.
A–H Gene expression analysis by RT-qPCR in 10-day-old wild-type (WT, Col-0; white bars) and coi1-1 mutant (gray bars) seedlings treated with 30 μM ( − )-JA or cis-OPDA for 30 min, or mock-treated. Gene expression levels were normalized to UBQ10. Data are presented as mean ± SD, with n = 4 biologically independent samples per condition. Statistical significance was determined using one-way ANOVA followed by Tukey-Kramer post hoc test (two-sided). Different letters represent a significant difference at P < 0.05. The experiment was repeated independently three times with similar results. I Evaluation of the affinity between cis-OPDA and COI1-JAZs. Pull-down assay of GST-AtCOI1 with Fl-AtJAZPs in the presence of JA-Ile (1 µM) or cis-OPDA (1/30 µM). Fl-AtJAZ13 was used as a negative control because JAZ13 has no canonical JAZ degron sequence, which is necessary for JA-Ile perception. GST-AtCOI1 bound to Fl-AtJAZPs was pulled down with anti-fluorescein antibody and Protein G magnetic beads and analyzed by immunoblotting (anti-GST-HRP conjugate for detection of GST-AtCOI1). Arrowheads indicate GST-AtCOI1 (95 kDa). Experiments were repeated three times with similar results (shown in Supplementary Figs. 2–3).
In all experiments, cis-OPDA affected the expression of JA and OPDA marker genes in Col-0 (Fig. 2A–H). cis-OPDA and JA induced the expression of JA marker genes, OPR3, JAZ8, and MYC2 in Col-0, and their expression was impaired in coi1-1, indicating their COI1-dependence (Fig. 2A–C). In contrast, the expression of OPDA-marker genes, ERF5, DREB2A, ZAT12, and FIT1, by cis-OPDA was not or slightly affected in coi1-1, confirming their COI1-independence (Fig. 2D–G). However complex effects were observed regarding ZAT10 expression (Fig. 2H). ZAT10 expression was induced by all compounds in Col-0. In the coi1-1 mutant, ZAT10 expression was not induced by (-)-JA, whereas moderate ZAT10 expression was observed for cis-OPDA treatment (Fig. 2H). These findings imply that the expression of ZAT10 by JA is COI1-dependent, whereas that of cis-OPDA depends on two distinct pathways, COI1-dependent and COI1-independent. To further confirm that the activity of cis-OPDA is independent of the COI1-JAZ co-receptor, a pull-down assay was performed to examine the affinity of cis-OPDA for all functional COI1-JAZ co-receptor pairs. As a result, cis-OPDA showed no affinity for the functional COI1-JAZ1-6/9-12 co-receptor pairs in the pull-down assay (Fig. 2I, Supplementary Figs. 2–3), confirming that cis-OPDA is not a ligand for functional COI1-JAZ co-receptors.
We also examined the effect of cis-OPDA on the expression of JA and OPDA marker genes in the myc2myc3myc4 triple mutant, in which the master TFs of JA signaling, MYC2/MYC3/MYC4, were impaired41 (Supplementary Fig. 4). The expression of the JA marker genes OPR3 and JAZ8 was suppressed in myc2myc3myc4 (Supplementary Fig. 4A–B). Different effects were observed for the five OPDA marker genes, ZAT10, ERF5, DREB2A, ZAT12, and FIT1; expressions of ZAT10 and DREB2A were moderately suppressed, and that of ERF5, ZAT12, and FIT1 were enhanced or not affected (Supplementary Fig. 4C–G), respectively. The current results demonstrated that cis-OPDA mediated the expression of OPDA marker genes independently of the canonical COI1-JAZ-MYC signaling pathway.
cis-OPDA induces the expression of OPDA-marker genes independent of the conversion into JA-Ile
Given that cis-OPDA is a major biosynthetic precursor of JA-Ile, we examined whether cis-OPDA-induced gene expression depends on the in planta conversion into JA-Ile. This was investigated using the synthetic cis-OPDA-d5 (Fig. 3A and Supplementary Note 1). To investigate the conversion of cis-OPDA into JA-Ile, we employed the Arabidopsis opr2-1 opr3-3 double mutant9, which is genetically deficient in this biosynthetic step. Consistent with its genetic background, we verified that this mutant is entirely incapable of converting cis-OPDA into JA-Ile. UPLC-MS/MS analysis demonstrated that cis-OPDA-d5 was converted into JA-d5-Ile within 30 min in Col-0, whereas this conversion was impaired in opr2-1opr3-3 (Fig. 3A).
A Schematic representation of the experimental design to assess the conversion of deuterium-labeled cis-OPDA (cis-OPDA-d₅) to JA-d₅-Ile in 10-day-old wild-type (WT, Col-0) and opr2-1 opr3-3 double mutant seedlings. Seedlings were treated with 30 μM cis-OPDA-d₅ for 30 min, and the accumulation of JA-d₅-Ile was measured in pmol per gram fresh weight. Data are presented as mean ± SD, with n = 4 biologically independent samples per genotype. B–G Gene expression analysis by RT-qPCR in 10-day-old wild-type (WT, Col-0; white bars) and opr2-1 opr3-3 (blue bars) seedlings treated with 30 μM ( − )-JA, 30 μM cis-OPDA, or mock-treated for 30 min. Expression levels were normalized to UBQ10. Data are presented as mean ± SD, with n = 4 biologically independent samples per condition. Statistical significance was assessed using one-way ANOVA followed by Tukey-Kramer post hoc test (two-sided). Different letters represent a significant difference at P < 0.05. The experiment was repeated independently three times with similar results.
Then we examined the cis-OPDA- or JA-mediated marker gene expression in opr2-1opr3-3. For the JA marker gene, cis-OPDA-induced expression of JAZ8 in Col-0 was significantly reduced in opr2-1opr3-3 (Fig. 3B), whereas JA-mediated JAZ8 expression in Col-0 was not affected by opr2-1opr3-3 (Fig. 3B). In contrast, the expression of OPDA-marker genes, ZAT10, ERF5, DREB2A, ZAT12, and FIT1, was not affected or increased in opr2-1opr3-3 (Fig. 3C–G). And the JA-induced expression of ZAT10 in opr2-1opr3-3 (Fig. 3C) suggested that expression of ZAT10 expression is also depends on canonical JA-signaling pathway, as shown in Fig. 2H. The undetectable level of JA-d5-Ile in cis-OPDA-d5-treated opr2-1opr3-3 in Fig. 3A9 implies that cis-OPDA induces the expression of OPDA maker genes independently of their conversion to JA-Ile.
cis-OPDA import into peroxisome is required for full signaling activity
Next, we examined whether cis-OPDA itself is a genuine bioactive form by using the Arabidopsis cis-OPDA transporter mutant. cis-OPDA is converted to JA in the peroxisomes, and the peroxisomal ATP-binding cassette (ABC) transporter COMATOSE (CTS) is involved in the import of cis-OPDA into the peroxisomes in A. thaliana (Fig. 4A, left)36. In the cts1 mutant line, in which CTS is impaired, wound-induced accumulation of JA-Ile was significantly reduced but not completely abolished42,43,44,45. In the cts1 mutant line, the level of cis-OPDA was significantly increased (Fig. 4A, right), however, to our surprise, cis-OPDA-induced expressions of ZAT10, ERF5, DREB2A, ZAT12, and FIT1 were reduced in cts1 (Fig. 4B). These results strongly supported that cis-OPDA import into peroxisome is required for full signaling activity.
A Left: Conversion of cis-OPDA into JA-Ile. The CTS transporter is localized on the peroxisomal membrane and involved in the import of cis-OPDA into the peroxisome. In OPR3-dependent pathway, cis-OPDA is reduced to OPC-8 and suffered three-rounds of β-oxidation and finally converted to JA-Ile. In OPR3-independent pathway, cis-OPDA is oxidized to dn-cis-OPDA, tn-cis-OPDA, and 4,5-ddh-JA in the peroxisome and finally converted to JA-Ile. Right: Accumulation of deuterium-labeled cis-OPDA-d₅ (pmol/ g fresh weight) in 10-day-old wild-type (WT, Ler-0; white bars) and cts1 mutant (orange bars) seedlings treated with 30 μM cis-OPDA-d₅ for 30 min. Data are presented as mean ± SD, with n = 4 biologically independent samples per genotype. Statistical significance was assessed using a two-sided unpaired Student’s t-test, p = 0.003452. B Gene expression analysis by RT-qPCR in 10-day-old WT (Ler-0; white bars) and cts1 mutant (orange bars) seedlings treated with 30 μM cis-OPDA or mock for 30 min. Expression levels were normalized to UBQ10. Data are presented as mean ± SD, with n = 4 biologically independent samples per condition. Statistical significance was assessed using one-way ANOVA followed by Tukey-Kramer post hoc test (two-sided). Different letters represent a significant difference at P < 0.05. The experiment was repeated independently three times with similar results.
In the peroxisome, cis-OPDA is converted into JA by the canonical OPR3-dependent or OPR3-independent route via several downstream metabolites (Figs. 1 and 3A). Considering the attenuation of marker gene expressions in cts1 mutant, these findings suggest that downstream metabolites of cis-OPDA may represent candidates of bioactive form.
The downstream metabolites of cis-OPDA, dn-cis-OPDA, tn-cis-OPDA, and (+)-7-iso-4,5-didehydrojasmonic acid, are bioactive forms of cis-OPDA
When the increased expression of ERF5, DREB2A, ZAT12, and FIT1 by cis-OPDA treatment was compared between Col-0 and opr2-1opr3-3, the expression was significantly higher in opr2-1opr3-3, especially for ERF5, DREB2A (Fig. 3D–G). This finding implies that metabolites located downstream of cis-OPDA and upstream of JA in the OPR3-independent bypassing route (Fig. 1B) are potential candidates for the bioactive form of cis-OPDA. To test this hypothesis, we performed UPLC-MS/MS analyses of the downstream metabolites of cis-OPDA in the canonical OPR3-dependent (OPC-4, Fig. 1A) and OPR3-independent routes (dn-cis-OPDA, tn-cis-OPDA, and 4,5-ddh-JA; Fig. 1B) using Col-0 and opr2-1opr3-3 (Fig. 5). For UPLC-MS/MS analysis, Col-0 and opr2-1opr3-3 were treated with cis-OPDA-d5. The accumulation level of cis-OPDA-d5 in opr2-1opr3-3 is slightly higher than that in Col-0 (Fig. 5). This observation suggests that the turnover or consumption of cis-OPDA in opr2-1 opr3-3 is marginally slower than in WT, but the impact on the accumulation levels of downstream metabolites is likely small. Compared to Col-0, cis-OPDA-d5-treatment enhanced the accumulation of 4,5-ddh-JA-d5 and tn-cis-OPDA-d5 in the opr2-1opr3-3 mutant but did not affect the level of dn-cis-OPDA-d5 (Fig. 5). The accumulation of tn-cis-OPDA-d5 (ca.4200 pmol/g FW in opr2-1opr3-3 and ca. 2400 pmol/g FW in Col-0) and 4,5-ddh-JA-d5 (ca.3700 pmol/g FW in opr2-1opr3-3 and ca. 1400 pmol/g FW in Col-0) was significantly higher than dn-cis-OPDA-d5 (ca.400 pmol/g FW). In contrast, the accumulation of OPC-4-d5, a downstream metabolite of the OPR3-dependent route, was below the detection limit in opr2-1opr3-3 (Fig. 5). In addition, we measured the accumulation of dn-cis-OPDA-d5, tn-cis-OPDA-d5, and 4,5-ddh-JA-d5 in the cis-OPDA-d5-treated cts mutant. Their accumulation was also significantly decreased in cts mutant (Supplementary Fig. 5).
10-day-old wild-type (WT, Col-0) and opr2-1 opr3-3 seedlings were treated with 30 μM cis-OPDA-d₅ for 30 min. The accumulation of metabolites was measured and is presented as mean ± SD, with n = 4 biologically independent samples per genotype. Statistical significance was assessed using one-way ANOVA followed by Tukey-Kramer post hoc test (two-sided). Different letters represent a significant difference at P < 0.05. The experiment was repeated independently three times with similar results.
Considering the correlation between increased (opr2-1opr3-3, Fig. 3C–G) and decreased (cts, Fig. 4B) abundance of OPDA metabolites and marker expression, metabolites such as tn-cis-OPDA and 4,5-ddh-JA are candidates of the genuine bioactive form of cis-OPDA because of the enhanced accumulation in opr2-1opr3-3.
The downstream metabolites of cis-OPDA were similarly effective as cis-OPDA on the expression of OPDA-marker genes
Next, we examined the effects of dn-cis-OPDA, tn-cis-OPDA, and 4,5-ddh-MeJA, the methyl ester of 4,5-ddh-JA (Fig. 6A and Supplementary Notes 2, 3), on the expression of OPDA marker genes in A. thaliana. Tn-cis-OPDA and 4,5-ddh-MeJA were equally effective in upregulating expression of ZAT10, ERF5, DREB2A, ZAT12, and FIT1 in the opr2-1opr3-3 mutant (Figs. 6B, C, and Supplementary Fig. 5A). Tn-cis-OPDA and 4,5-ddh-MeJA upregulated the expression of OPDA marker genes in a concentration-dependent manner (Figs. 6D, E). However, in this experiment, conversion from dn-cis-OPDA to tn-cis-OPDA or 4,5-ddh-JA and tn-cis-OPDA to 4,5-ddh-JA occurred (Fig. 5). Thus, it is necessary to consider the possibility that the bioactivity of dn-cis-OPDA and tn-cis-OPDA may depend on the in planta conversion to their downstream metabolites.
A OPR3-independent bypassing route and chemical structure of 4,5-ddh-JA and 4,5-ddh-MeJA. B, C Gene expression analysis by RT-qPCR in 10-day-old wild-type (WT, Col-0; white bars) and opr2-1 opr3-3 (blue bars) treated with 30 μM cis-OPDA, 30 μM tn-cis-OPDA, or 30 μM 4,5-ddh-MeJA for 30 min. Mock represents untreated samples. Expression levels were normalized to UBQ10. Data are presented as mean ± SD, with n = 4 biologically independent samples per condition. Statistical significance was assessed using one-way ANOVA followed by Tukey-Kramer post hoc test (two-sided). Different letters represent a significant difference at P < 0.05. The experiment was repeated independently three times with similar results. D, E Dose-dependent gene expression analysis by RT-qPCR in 10-day-old opr2-1 opr3-3 treated with 1, 10, 30, or 100 μM of either cis-OPDA (left), tn-cis-OPDA (middle), or 4,5-ddh-MeJA (right) for 30 min. Mock represents untreated samples. Expression levels were normalized to UBQ10. Data are presented as mean ± SD, with n = 3 biologically independent samples per condition. Statistical significance was assessed using one-way ANOVA followed by Tukey-Kramer post hoc test (two-sided). Different letters represent a significant difference at P < 0.05. The experiment was repeated independently three times with similar results.
The downstream metabolites of cis-OPDA caused gene expression through their electrophilic property
Monte et al. reported that cis-OPDA and dn-cis-OPDA upregulate the expression of HSP genes in A. thaliana and M. polymorpha in a COI1-independent manner37. In addition, the upregulated gene expression depends on the electrophilic properties of cis-OPDA and dn-cis-OPDA because dn-iso-OPDA, which is an isomer of dn-cis-OPDA and less reactive as an electrophile, is significantly less effective than dn-cis-OPDA. Therefore, we compared the effects of tn-cis-OPDA and tn-iso-OPDA (Figs. 7A), 4,5-ddh-MeJA and 3,7-ddh-MeJA (Fig. 7A), corresponding to an iso-isomer of 4,5-ddh-MeJA, on the expression of ZAT10, ERF5, and HSP genes. The effect on the expression of DREB2A, ZAT12, and FIT1 genes was also investigated (Supplementary Fig. 6A-C). As shown in Fig. 7 and Supplementary Fig. 6A-C, tn-cis-OPDA and 4,5-ddh-MeJA upregulated the expression of ZAT10 (Fig. 7B), ERF5 (Fig. 7C), DREB2A (Supplementary Fig. 6A), ZAT12 (Supplementary Fig. 6B), and FIT1 (Supplementary Fig. 6C) whereas tn-iso-OPDA and 3,7-ddh-MeJA did not. Similarly, cis-OPDA, tn-cis-OPDA, and 4,5-ddh-MeJA upregulated the expression of HSP, whereas tn-iso-OPDA and 3,7-ddh-MeJA did not (Fig. 7D–F). This suggests that the electrophilic nature of tn-cis-OPDA and 4,5-ddh-JA might be responsible for upregulating ZAT10, ERF5, DREB2A, ZAT12, FIT1, and HSPs. These results suggest their function as protein modifier in the plant cell.
A Chemical structures of tn-iso-OPDA, 3,7-ddh-JA, and 3,7-ddh-MeJA. B–F Time-course gene expression analysis by RT-qPCR. B, C Gene expression analysis in 10-day-old wild-type (WT, Col-0; white bars) and opr2-1 opr3-3 (blue bars) seedlings treated with 30 μM tn-cis-OPDA, tn-iso-OPDA, 4,5-ddh-MeJA, or 3,7-ddh-MeJA for 30 min, or mock. Expression levels were normalized to UBQ10. Data are presented as mean ± SD, with n = 3 biologically independent samples per condition. Statistical significance was assessed using one-way ANOVA followed by Tukey-Kramer post hoc test (two-sided). Different letters represent a significant difference at P < 0.05. The experiment was repeated independently three times with similar results. D–F Gene expression analysis in the same genotypes treated with 30 μM cis-OPDA, tn-cis-OPDA, tn-iso-OPDA, 4,5-ddh-MeJA, or 3,7-ddh-MeJA for 180 min, or mock. Expression levels were normalized to UBQ10. Data are presented as mean ± SD, with n = 4 biologically independent samples per condition. Statistical significance was assessed using one-way ANOVA followed by Tukey-Kramer post hoc test (two-sided). Different letters represent a significant difference at P < 0.05. The experiment was repeated independently three times with similar results.
Discussion
Previous studies reported that cis-OPDA is a bioactive jasmonate in A. thaliana14,15,16,17,18,19,20,21. Two cis-OPDA derivatives such as cis-OPDA amino acid conjugates (cis-OPDA-AAs) including cis-OPDA-Ile (Fig. 1)32,46,47,48 or modified OPDA (mo-OPDA)49 which is an unidentified metabolites of cis-OPDA generated by dioxygenase JID1 are previously reported as possible bioactive form of cis-OPDA. However, cis-OPDA-AA was shown as a storage form of cis-OPDA and the genuine bioactive form of cis-OPDA-AA is cis-OPDA50, and mo-OPDA was identified as an artifact produced through Michael addition of cis-OPDA51. Thus, genuine bioactive form of cis-OPDA remains unknown.
Here, we reexamined the bioactivities of cis-OPDA and concluded that cis-OPDA metabolites in the OPR3-independent biosynthetic route of JA-Ile (Fig. 1) are the predominant active forms responsible for the expression of OPDA-marker genes. This conclusion is supported by three complementary lines of evidence. (i) In the opr2-1 opr3-3 double mutant, marked accumulation of tn-cis-OPDA and 4,5-ddh-JA and a concomitant enhancement of OPDA-marker transcript levels were observed. (ii) In the cts1 transporter mutant, impaired peroxisomal import of cis-OPDA suppressed the formation of downstream catabolites and proportionally attenuated marker-gene expression. (iii) Structure-activity relationship study showed that an electrophilic α,β-unsaturated carbonyl of downstream catabolites is required for bioactivity.
The finding that the expression of OPDA marker genes was attenuated in the cts1 mutant line44, in which cis-OPDA transport to peroxisomes was decreased, strongly supports our conclusion (Fig. 4). This finding indicated that the downstream metabolites of cis-OPDA, and not cis-OPDA itself, were genuine bioactive forms. Next, we focused on the fact that cis-OPDA caused higher expression of ERF5, DREB2A, ZAT12, and FIT1 in opr2-1opr3-3 mutant compared to Col-0 (Fig. 3D–G). This result may reflect a modified accumulation of cis-OPDA metabolites that would in turn impinge on gene expression. Chini et al. reported that the OPR3-independent JA biosynthetic pathway operates weakly in Col-0 and is predominant in opr2-1opr3-3 and opr3-3 mutants9. In opr2-1opr3-3, the downstream metabolites of the OPR3-independent route, tn-cis-OPDA and 4,5-ddh-JA, accumulated more than in Col-0, whereas no accumulation was observed for the downstream metabolites of cis-OPDA in the canonical OPR3-dependent route (Fig. 5). The accumulation of metabolites in opr3-3 was previously reported using deuterium-labeled α-linolenic acid9, however, we used deuterium-labeled cis-OPDA-d5 of naturally occurring stereochemistry to exclude the effect of metabolites from the branched metabolic routes of α-linolenic acid, such as hydroperoxide lyase/isomerase, epoxyalcohol synthase, divinyl ether synthase, and 9-LOX routes4,52. The results indicate that downstream metabolites of cis-OPDA via the OPR3-independent pathway can act as bioactive compounds affecting cis-OPDA-induced marker gene expression. Unfortunately, we could not identify one of them or all of them function as endogenous chemical signals. Identification of the genuine endogenous signal must await the target identification and subsequent confirmation of binding of cis-OPDA metabolites to it. UPLC-MS/MS analysis revealed that tn-cis-OPDA and 4,5-ddh-JA accumulated at higher levels in opr2-1 opr3-3 compared to Col-0, while dn-cis-OPDA levels remained unchanged (Fig. 5). These findings suggest that tn-cis-OPDA and 4,5-ddh-JA are likely endogenous signaling molecules responsible for inducing the enhanced expression of OPDA-marker genes in opr2-1 opr3-3 (Fig. 3C–G). However, our current data do not allow us to completely rule out the possibility that dn-cis-OPDA functions as an endogenous chemical signal. Dn-cis-OPDA is converted into tn-cis-OPDA and then 4,5-ddh-JA through β-oxidation in the peroxisome and this conversion cannot be impaired because of the lack of a mutant line (Fig. 1). Thus, the information on the localization of these downstream metabolites will help the identification of genuine bioactive form of cis-OPDA. On this point, previous report have shown that the conversion of 4,5-ddh-JA to JA occurs in the cytosol by cytosol-localized OPR2 reductase, suggesting the localization of 4,5-ddh-JA in cytosol (Fig. 1)9. In addition, Mekkaoui et al. clearly demonstrated that 4,5-ddhJA is released from peroxisomes into the cytosol by using a trans-organellar complementation approach53,54. A previous study demonstrated the difference in substrate specificity between OPR1 and OPR2: OPR1 could reduce cis-OPDA with low efficiency and could not reduce 4,5-ddh-JA, whereas OPR2 could not reduce cis-OPDA and could reduce 4,5-ddh-JA9. Based on this difference, they expressed OPR1 or OPR2 linked to localization signal peptides targeted to cytosol, to the peroxisome, and to the mitochondria in opr2-1opr3-3 background. Major jasmonate responses, fertility and normal flower development, are impaired in opr2-1opr3-355, thus, the restored biosynthesis of JA-Ile in these mutant lines will restore these traits. Complementation experiments showed that OPR2 in the opr2-1opr3-3 background could restore these traits, suggesting that 4,5-ddh-JA might be the compound released from peroxisome. In contrast, OPR1 in the opr2-1opr3-3 background could restore these traits when localized in peroxisomes, suggesting the plausible compartmentation of endogenously generated cis-OPDA in plastids and peroxisomes. This conclusion strongly reinforces our conclusions with cts1 mutant line (Fig. 4). However, there is no evidence supporting the peroxisomal localization or cytosolic transport of dn-cis-OPDA and tn-cis-OPDA. Combining with our results, the exogenously applied cis-OPDA is either detoxified by conjugation to GSH56 or rapidly converted to 4,5-ddh-JA of which level reaches to ca.3700 pmol / g FW, and released into cytosol to function as signaling molecule.
Two independent approaches by us and Mekkaoui et al. 54 reached the same conclusion that not cis-OPDA itself, but the downstream metabolite function as the genuine bioactive signal to induce the expressions of OPDA-marker genes. Mekkaoui et al. examined the wound-induced induction of some of cis-OPDA-marker genes in opr2-1opr3-3 mutant54, while our results are based on the experiments by exogenous application of cis-OPDA which have been discussed in previous studies on the JA-independent functions of cis-OPDA. In addition, our current conclusions are primarily based on the expression analyses of several marker genes following treatment with exogenously applied chemicals, while Mekkaoui et al. reported the RNA sequence analysis of the wounded opr2-1 opr3-3 and aos mutants. Mekkaoui et al. also reported that all these OPDA-marker genes were also wound-induced in aos, in which OPDA and JA are deficient. It is interesting that the transcriptomes of the wounded opr2-1 opr3-3 and aos mutants were highly similar, despite the lower cis-OPDA accumulation in the latter54. This may indicate distinct effects between exogenously applied cis-OPDA and endogenous cis-OPDA; however, it could also be attributed to a significant decrease in endogenous cis-OPDA accumulation in both opr2-1opr3-3 and aos mutants compared with Col-0, resulting from the lack of positive feedback to cis-OPDA biosynthesis54. Consequently, the effects of endogenous cis-OPDA and its downstream metabolites may also be severely decreased in these mutants. Thus, careful interpretation is required when evaluating the role of endogenous cis-OPDA in plants. Based on the current results, it can be hypothesized that wound-induced JA-independent gene expression is at least partially mediated by downstream metabolites of cis-OPDA, such as 4,5-ddh-JA, which possesses electrophilic properties and is released into the cytosol. These responses may include oxidative stress, such as the production of reactive oxygen species (ROS)57 and γ-aminobutyric acid (GABA)58, which mediate at least part of the wound response, resulting in the induction of general stress-responsive genes. Mekkaoui et al. discussed that low level of endogenous 4,5-ddh-JA in the wounded opr2-1opr3-3 mutant (500 pmol/g FW9) is insufficient to induce the expression of marker genes54. However, dn-cis-OPDA and tn-cis-OPDA, in addition to 4,5-ddh-JA, can function as similar signaling molecules, although it is unclear whether they are localized in the cytosol. Definitive conclusions can be made once the unidentified non-COI1 target involved in the expression of OPDA marker genes is identified.
In addition, we have another evidence suggesting that downstream metabolites of cis-OPDA is the genuine bioactive signal. Our findings indicated that the electrophilic reactivity of 4,5-ddh-JA and tn-cis-OPDA may be responsible for the expression of OPDA marker genes ZAT10 and ERF5. Monte et al. demonstrated that cis-OPDA and dn-cis-OPDA upregulate MpCOI1-independent expression of HSP genes in M. polymorpha through electrophilic reactivities37. They concluded that electrophilic reactivity is essential for HSP expression because it is not induced by dn-iso-OPDA, an isomer of dn-cis-OPDA with significantly lower electrophilic reactivity, due to the tetra-substituted α,β-unsaturated ketone moiety37,59. Similarly, tn-iso-OPDA and 3,7-ddh-JA, iso-isomers of tn-cis-OPDA and 4,5-ddh-JA with little electrophilic reactivity, did not induce the expression of OPDA maker genes (Fig. 7 and Supplementary Fig. 6). These results suggest that these downstream metabolites of OPDA may act as protein modifiers through Michael addition.
Downstream metabolites of cis-OPDA function as chemical signals that cause the upregulation of OPDA marker genes independently of COI1 and MYCs (Fig. 2 and Supplementary Fig. 4). COI1-independent gene upregulation may be attributed to a non-COI1-target, such as cyclophilin 20-3 (CYP20-3) and glutathione S-transferase 19, which are proposed putative targets of cis-OPDA (Fig. 1)18,60. cis-OPDA has reported to bind to CYP20-3, which resides in chloroplast, promoting the formation of a cysteine synthase complex involved in sulfur assimilation18,61,62. Importantly, decreased transport of cis-OPDA to the peroxisome using the cts mutant has minimal impact on this plastid-localized mechanism. Thus, the possibility that cis-OPDA itself functions as an endogenous signal within a plastid-localized mechanism cannot be entirely excluded.
Our current findings suggest that tn-cis-OPDA and 4,5-ddh-JA, in conjunction with cis-OPDA, can function as endogenous bioactive substances. Further studies identifying the non-COI1-target will enable detailed studies of the unknown MOA of cis-OPDA.
Methods
Plant materials
A. thaliana ecotype Col-0 and Ler-0 seeds were surface-sterilized in 5% sodium hypochlorite with 0.3% Tween 20 and vernalized for 2–3 d at 4 °C. All seedlings were grown (118 μmol m-2 s-1) under a 16 h light/8 h dark cycle at 22 °C in a CL-301 growth chamber (Tomy Seiko Co., Ltd., Japan). Seedlings were grown in 1/2 Murashige and Skoog (MS) liquid medium. The mutant lines used in this study were coi1-138, opr2-1opr3-39, myc2myc3myc441, and cts142. The seeds of coi1-1 and opr2-1opr3-3 mutants were kindly gifted from Professor Roberto Solano and Dr. Andrea Chini (CNB-CSIC, Spain). The cts1 mutant was purchased from the Nottingham Arabidopsis Stock Centre (NASC). To select homozygous coi1-1, heterozygous coi1-1 seeds were geminated on a 1/2 MS plate containing 10 µM JA, and well-grown 4 d seedlings (homozygous coi1-1) were transferred to a 1/2 MS liquid medium. After 6 d of culture, the seedling was treated with each compound ((‒)-JA, cis-OPDA, cis-OPDA-d5, dn-cis-OPDA, tn-cis-OPDA, 4,5-ddh-MeJA, tn-iso-OPDA, 3,7-ddh-MeJA). For cts1, surface-sterilized seeds were pipetted onto 1/2 MS plate medium, and seed coats were disrupted using sterile tweezers to stimulate germination43.
Chemical synthesis
Synthesis of (+)-cis-OPDA-d 5
(+)-12-(R)-Hydroxy-phytodienoic alcohol-d5 was synthesized as described in the Supplementary Notes. To a solution of the diol (+)-12-(R)-hydroxyphytodienoic alcohol-d5 (32.7 mg, 0.11 mmol) in acetone (9.8 mL), Jones reagent (4.0 M solution) at −20 °C was added until the orange color of the reagent persisted (8 drops). After 10 min of stirring at −20 °C, i-PrOH was added to quench the remaining reagent. AcOEt/n-hexane (1:1) and H2O were then added, and the water layer was extracted with AcOEt. The combined organic layers were washed with saturated aqueous NaCl, dried over Na2SO4, and concentrated under reduced pressures. The residue was purified by medium-pressure chromatography (Isolera, eluent: 0.1:88:12 AcOH/n-hexane/EtOAc to 0.1:99.9 AcOH/EtOAc) to obtain cis-OPDA-d5 (14.4 mg, 42%) as a colorless oil. [α]D23 + 127.2 (c 0.34, CHCl3). 1H NMR (400 MHz, CDCl3) δH: 7.74 (dd, J = 6.0, 2.8 Hz, 1H), 6.19 (dd, J = 6.0, 2.0 Hz, 1H), 5.32–5.45 (m, 2H), 2.93–3.02 (m, 1H), 2.40–2.56 (m, 2H), 2.36 (t, J = 7.8 Hz, 2H), 2.08–2.19 (m, 1H), 1.09–1.82 (m, 12H); 13C NMR (100 MHz, CDCl3) δC: 211.1, 179.7, 167.3, 132.9, 132.5, 127.0, 49.8, 44.3, 34.0, 30.7, 29.6, 29.1, 28.9, 27.6, 24.6, 23.8, 19.3–20.3 (m), 12.4–13.3 (m); IR (neat) cm–1: 2929, 2222, 1707, 1213; HRMS (ESI, negative) m/z [M-H]- calcd for C18H22D5O3: 296.2279, found: 296.2268.
Synthesis of tn-cis-OPDA
( + )-8-(R)-hydroxyphytodienoyl alcohol was synthesized as described in the Supplementary Notes. To a solution of (+)-8-(R)-hydroxyphytodienoyl alcohol (18.0 mg, 81.1 µmol) in acetone (7 mL), Jones reagent (4.0 M solution) was added at −20 °C dropwise until the color of the reagent persisted (7 drops). i-PrOH and AcOEt/n-hexane (1:1) were then added to quench the remaining reagents. The mixture was extracted using EtOAc. The organic layer was washed with saturated aqueous NaCl, dried over Na2SO4, and then filtered. The reaction mixture was purified using medium-pressure chromatography (Isolera, eluent: 88:12:0.1 n-hexane/EtOAc/AcOH to 100:0.1 EtOAc/AcOH) to obtain tn-cis-OPDA as a colorless oil (18.5 mg, 96%). [α]D23 + 207.2 (c 0.14, CHCl3); 1H NMR (400 MHz, CDCl3) δH: 7.75 (dd, J = 5.8, 2.8 Hz, 1H), 6.21 (dd, J = 5.8, 1.8 Hz, 1H), 5.52-5.27 (m, 2H), 3.09–2.92 (m, 1H), 2.65–2.30 (m, 4H), 2.27–1.93 (m, 3H), 1.89–1.59 (m, 3H), 1.34–1.11 (m, 1H), 0.97 (t, J = 7.6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δC: 210.56, 178.52, 166.28, 133.21, 132.91, 126.71, 49.67, 44.04, 33.96, 30.19, 23.79, 22.76, 20.80, 14.01; IR (neat) cm–1: 3050, 1732, 1702, 1109; HRMS (ESI negative) m/z [M-H]- calcd for C14H19O3: 235.1340, found: 235.1328.
Synthesis of 4,5-ddh-MeJA
( + )-Methyl 4,5-ddh-6-epi-cucurbate was synthesized as described in the Supplementary Notes. To a solution of (+)-methyl 4,5-ddh-6-epi-cucurbate (9.8 mg, 43.7 µmol) in acetone (4.5 mL), Jones reagent (4.0 M solution) was added at −20 °C until the orange color of the reagent persisted (16 drops). After 10 min of stirring at −20 °C, i-PrOH was added to quench the remaining reagent. Subsequently, n-hexane and H2O were added, and the water layer was extracted with n-hexane. The combined organic layers were washed with saturated aqueous NaCl, dried over Na2SO4, and concentrated under reduced pressures. The residue was purified by medium-pressure chromatography (Isolera, eluent: 98:2 n-hexane/EtOAc to 80:20 n-hexane/EtOAc) to obtain 4,5-ddh-cis-MeJA (5.3 mg, 55%) as a colorless oil. Diastereomeric purity of 4,5-ddh-cis-MeJA was > 99% by 1H NMR spectroscopy (δH = 7.71 (dd, J = 5.7, 2.7 Hz, 1H) for 4,5-ddh-cis-MeJA; 7.63 (dd, J = 5.7, 2.4 Hz, 1 H) for the trans isomer). [α]D24 + 127.4 (c 0.26, CHCl3). 1H NMR (400 MHz, CDCl3) δH: 7.71 (dd, J = 5.7, 2.7 Hz, 1H), 6.22 (dd, J = 5.7, 2.0 Hz, 1H), 5.45 (dtt, J = 10.9, 7.4, 1.8 Hz, 1H), 5.32 (dddt, J = 10.9, 6.8, 5.0, 1.4 Hz, 1H), 3.72 (s, 3H), 3.55-3.45 (m, 1H), 2.76 (dd, J = 16.4, 5.8 Hz, 1H), 2.57 (dt, J = 6.8, 5.0 Hz, 1H), 2.53 (brdt, J = 15.7, 5.0 Hz, 1H), 2.21 (dd, J = 16.4, 10.1 Hz, 1H), 2.13 (brdt, J = 15.7, 6.8 Hz, 1H), 2.05 (quintet, J = 7.4 Hz, 2H), 0.97 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δC: 210.03, 172.34, 165.87, 133.66, 133.19, 126.17, 51.87, 48.32, 40.41, 34.44, 24.39, 20.79, 13.85; IR (film) cm–1:2961, 1733, 1717, 1198; HRMS (ESI, positive) m/z [M+Na]+ calcd for C13H18NaO3: 245.1154, found: 245.1148.
Synthesis of tn-iso-OPDA and 3,7-ddh-MeJA
tn-iso-OPDA63 and 3,7-ddh-MeJA64 were synthesized according to the previously reported methods.
Gene expression analyses
Surface sterilized A. thaliana seeds were germinated (118 μmol m−2 s−1) under a 16 h light/8 h dark cycle at 22 °C in a CL-301 growth chamber (TOMY SEIKO Co., Ltd., Japan) after vernalization in the dark at 4 °C for 2 d. 10-day-old seedlings (5–6 seedlings/sample) grown in 1/2 MS liquid medium were treated with each compound ((-)-JA, cis-OPDA, dn-cis-OPDA, tn-cis-OPDA, 4,5-ddh-MeJA, tn-iso-OPDA, 3,7-ddh-MeJA). Based on the results of previously reported comprehensive DNA microarray analyses16, we performed gene expression analyses by adding cis-OPDA to Arabidopsis WT (Col-0) and the coi1-1 mutant. Arabidopsis WT and coi1-1 plants were treated with (-)-JA (a mixture of cis and trans = 5/95; Supplementary Fig. 1) or cis-OPDA, to examine their effects on the expression of three JA marker genes, OPR3, JAZ8, and MYC2, as well as cis-OPDA-specific marker genes, ZAT10, ERF5, DREB2A, ZAT12, and FIT116,32. Each sample was frozen after treatment for 30 min, and total RNA was extracted using ISOGEN (NIPPON GENE, Japan). First-strand cDNA was obtained using ReverTra Ace® reverse transcriptase (TOYOBO, Japan) with oligo-dT primers. A StepOnePlus Real-Time PCR System (Life Technologies, USA) was used for quantitative PCR (qPCR). PCR conditions were followed: an initial hold at 95 °C for 30 s, followed by a two-step PCR program of 95 °C for 5 s and 60 °C for 30 s for 40 cycles. Primer sequences used in this study are listed in Supplementary Table 1. Polyubiquitin 10 was used as the reference gene.
UPLC-MS/MS analysis of cis-OPDA metabolites
Surface sterilized A. thaliana seeds were germinated (118 μmol m-2 s-1) under a 16 h light/8 h dark cycle at 22 °C in a CL-301 growth chamber (TOMY SEIKO Co., Ltd., Japan) after vernalization in the dark at 4 °C for 2 d. Four independent biological replicates, each consisting of five 10-day-old seedlings grown on 1/2 MS liquid medium, were treated with 30 µM cis-OPDA-d5. Mock-treated samples (EtOH) were included as negative controls, with the same number of biological replicates. The frozen plant materials were homogenized and extracted using 600 µL of ice-cold (pre-cooled at −20 °C) 25% MeOH/H2O. The samples were sonicated for 10 min and extracted for 30 min at 4 °C using a rotator. After centrifugation at 15,000 g for 15 min, supernatants were collected. The pooled supernatants were purified by one-step reversed-phase polymer-based solid-phase extraction using Oasis® HLB cartridges (Waters, USA). Before sample loading, the SPE sorbent was conditioned with 1 mL of 100% MeOH and equilibrated with 1 mL of 0.1% HCOOH/H2O (v/v). After the samples had been loaded onto the Oasis® HLB column, interfering compounds were removed by washing with 1 mL of 25% MeOH/H2O, following which the pre-concentrated analytes on the sorbent were eluted with 2 mL of 50% MeOH/H2O and 2 mL 100% MeOH. The eluent solution was evaporated to dryness at 30 °C under reduced pressure through freeze-drying. The samples were then resuspended in 100 μL of 100% MeOH.
The samples were examined by UPLC-MS/MS using a TripleTOF 5600 system (AB SCIEX, USA) operating in the negative mode. Liquid chromatography separation was performed with an Eclipse Plus C18 RRHD 1.8 µm (Φ1.05 × 50 mm, Waters, USA) at 35 °C and a flow rate of 0.3 mL/min. The elution was performed using a gradient of water (solvent A) and MeOH (solvent B), both containing 0.1% formic acid (v/v). The proportion of solvent B in the eluent was increased linearly from 10% to 60% for 2 min and from 60% to 90% for 8 min of the elution phase, followed by maintaining a flow of 90% B for 1 min. The column was re-equilibrated with 10% solvent B for 3 min. Data were acquired in multiple reaction monitor (MRM) mode with mass transitions (precursor ions/product) as followed: 327.2338 > 130.0874 for JA-Ile-d5, 214.1497 > 59.0418 for JA-d5, 244.1956 > 226.1850 for OPC-4-d5 296.2280 > 170.1599 for cis-OPDA-d5, 268.1967 > 170.1599 for dn-cis-OPDA-d5, 242.1799 > 224.1693 for tn-cis-OPDA-d5 and 212.1341 > 168.1442 for 4,5-ddh-JA-d5. The Ion Source Gas1 and Ion Source Gas 2, curtain gas were set at 15, 30, and 25 psi, respectively. The ion spray voltage was -4500 V in negative mode. The temperature (TEM) was 450 °C. Collision Energy Spread, Ion Release Delay and Ion Release Width were set at 5, 30, and 15 V, respectively. Calibration curves of compounds were made with standards. The SCIEX OS software (version 2.0.0.45330, AB SCIEX, MA, USA) was applied for metabolite identification and concentration calculation. Statistical analysis was performed using Microsoft Excel (version 2504, Microsoft Corporation, USA). An F-test was first conducted to assess the equality of variances between groups. Based on the result, an unpaired two-tailed Student’s t-test (assuming equal or unequal variances as appropriate) was subsequently applied to determine statistical significance.
Pull-down assay
For the pull-down experiments using fluorescein-tagged JAZ peptides (Fl-AtJAZPs), purified GST-AtCOI1 (5 nM), Fl-AtJAZP (10 nM), and each compound (JA-Ile or cis-OPDA) in 350 µL of incubation buffer (50 mM Tris-HCl buffer, pH 7.8, 100 mM NaCl, 10% glycerol, 0.1% Tween20, 100 nM inositol-1,2,4,5,6-pentakisphosphate (IP5)) were combined with anti-fluorescein antibody (0.2 µL, GeneTex, GTX26644, USA), and incubated for 10–15 h at 4 °C with rotation. After incubation, the samples were combined with SureBeadsTM Protein G (10 µL in 50% incubation buffer slurry; Bio-Rad, Hercules, CA, USA). After 3 h of incubation at 4 °C with rotation, the samples were washed three times with 350 µL of wash buffer (phosphate-buffered saline containing 0.1% Tween 20). The washed beads were resuspended in 35 µL of SDS-PAGE loading buffer containing dithiothreitol (DTT, 100 mM). After heating for 10 min at 60 °C, the samples were subjected to SDS-PAGE and analyzed by western blotting. The bound GST-COI1 protein was detected using an anti-GST HRP conjugate (RPN1236, GE Healthcare, USA, 5,000-fold dilution in blocking buffer (Nakalai Tesque, Inc., Japan)). Anti-fluorescein is a goat polyclonal IgG. Anti-GST is a goat polyclonal IgG and conjugated with HRP. Anti-mouse IgM is a goat polyclonal IgG and conjugated with HRP.
Statistical analyses
Samples were analyzed in triplicate, and the data are presented as the mean ± standard deviation (SD). An analysis of variance (ANOVA) was used for data analysis. Different letters within a column indicate statistically significant differences by the Tukey-Kramer multiple range test (p < 0.05, CoStat version 6.400). A Student’s t-test was performed to examine differences between the two groups.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data generated in this study are provided in the Supplementary Information and Source Data file. Source data are provided with this paper.
References
Wasternack, C. & Hause, B. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. an update to the 2007 review in Annals of Botany. Ann. Bot. 111, 1021–1058 (2013).
Ueda, M., Kaji, T. & Kozaki, W. Recent advances in plant chemical biology of jasmonates. Int J. Mol. Sci. 21, 1124 (2020).
Howe, G. A., Major, I. T. & Koo, A. J. Modularity in jasmonate signaling for multistress resilience. Annu Rev. Plant Biol. 69, 387–415 (2018).
Wasternack, C. & Feussner, I. The oxylipin pathways: biochemistry and function. Annu Rev. Plant Biol. 69, 363–386 (2018).
Staswick, P. E., Tiryaki, I. & Rowe, M. L. Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell 14, 1405–1415 (2002).
Staswick, P. E. & Tiryaki, I. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 16, 2117–2127 (2004).
Delfin, J. C. et al. AtGH3.10 is another jasmonic acid-amido synthetase in Arabidopsis thaliana. Plant J. 110, 1082–1096 (2022).
Schaller, F., Biesgen, C., Mussig, C., Altmann, T. & Weiler, E. W. 12-Oxophytodienoate reductase 3 (OPR3) is the isoenzyme involved in jasmonate biosynthesis. Planta 210, 979–984 (2000).
Chini, A. et al. An OPR3-independent pathway uses 4,5-didehydrojasmonate for jasmonate synthesis. Nat. Chem. Biol. 14, 171–178 (2018).
Wasternack, C. & Song, S. Jasmonates: biosynthesis, metabolism, and signaling by proteins activating and repressing transcription. J. Exp. Bot. 68, 1303–1321 (2017).
Chini, A. et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448, 666–671 (2007).
Thines, B. et al. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448, 661–665 (2007).
Yan, J. et al. The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell 21, 2220–2236 (2009).
Maynard, D., Groger, H., Dierks, T. & Dietz, K. J. The function of the oxylipin 12-oxophytodienoic acid in cell signaling, stress acclimation, and development. J. Exp. Bot. 69, 5341–5354 (2018).
Dave, A. & Graham, I. A. Oxylipin Signaling: A Distinct Role for the Jasmonic Acid Precursor cis-(+)-12-Oxo-Phytodienoic Acid (cis-OPDA). Front Plant Sci. 3, 42 (2012).
Taki, N. et al. 12-oxo-phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in Arabidopsis. Plant Physiol. 139, 1268–1283 (2005).
Dave, A. et al. 12-oxo-phytodienoic acid accumulation during seed development represses seed germination in Arabidopsis. Plant Cell 23, 583–599 (2011).
Park, S. W. et al. Cyclophilin 20-3 relays a 12-oxo-phytodienoic acid signal during stress responsive regulation of cellular redox homeostasis. Proc. Natl Acad. Sci. USA 110, 9559–9564 (2013).
Scalschi, L. et al. Silencing of OPR3 in tomato reveals the role of OPDA in callose deposition during the activation of defense responses against Botrytis cinerea. Plant J. 81, 304–315 (2015).
Varsani, S. et al. 12-Oxo-Phytodienoic Acid Acts as a Regulator of Maize Defense against Corn Leaf Aphid. Plant Physiol. 179, 1402–1415 (2019).
Goetz, S. et al. Role of cis-12-oxo-phytodienoic acid in tomato embryo development. Plant Physiol. 158, 1715–1727 (2012).
Stintzi, A., Weber, H., Reymond, P., Browse, J. & Farmer, E. E. Plant defense in the absence of jasmonic acid: the role of cyclopentenones. Proc. Natl Acad. Sci. USA 98, 12837–12842 (2001).
Dave, A., Vaistij, F. E., Gilday, A. D., Penfield, S. D. & Graham, I. A. Regulation of Arabidopsis thaliana seed dormancy and germination by 12-oxo-phytodienoic acid. J. Exp. Bot. 67, 2277–2284 (2016).
Yi, R., Li, Y. & Shan, X. OPDA/dn-OPDA actions: biosynthesis, metabolism, and signaling. Plant Cell Rep. 43, 206 (2024).
Fujimoto, S. Y., Ohta, M., Usui, A., Shinshi, H. & Ohme-Takagi, M. Arabidopsis ethylene-responsive element binding factors act as transcriptional activators or repressors of GCC box-mediated gene expression. Plant Cell 12, 393–404 (2000).
Stockinger, E. J., Gilmour, S. J. & Thomashow, M. F. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc. Natl Acad. Sci. USA 94, 1035–1040 (1997).
Liu, Q. et al. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10, 1391–1406 (1998).
Meissner, R. Michael AJ. Isolation and characterisation of a diverse family of Arabidopsis two and three-fingered C2H2 zinc finger protein genes and cDNAs. Plant Mol. Biol. 33, 615–624 (1997).
Kreps, J. A. et al. Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol. 130, 2129–2141 (2002).
Colangelo, E. P. & Guerinot, M. L. The essential basic helix-loop-helix protein FIT1 is required for the iron deficiency response. Plant Cell 16, 3400–3412 (2004).
Fonseca, S. et al. +)−7-iso-Jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nat. Chem. Biol. 5, 344–350 (2009).
Arnold, M. D. et al. The recently identified isoleucine conjugate of cis-12-oxo-phytodienoic acid is partially active in cis-12-oxo-phytodienoic acid-specific gene expression of Arabidopsis thaliana. PloS one 11, e0162829 (2016).
Chehab, E. W. et al. Intronic T-DNA insertion renders Arabidopsis opr3 a conditional jasmonic acid-producing mutant. Plant Physiol. 156, 770–778 (2011).
Yan, J. et al. Endogenous Bioactive Jasmonate Is Composed of a Set of (+)-7-iso-JA-Amino Acid Conjugates. Plant Physiol. 172, 2154–2164 (2016).
Liu, W. & Park, S. W. 12-oxo-phytodienoic acid: a fuse and/or switch of plant growth and defense responses?. Front Plant Sci. 12, 724079 (2021).
Jimenez Aleman, G. H., Thirumalaikumar, V. P., Jander, G., Fernie, A. R. & Skirycz, A. OPDA, more than just a jasmonate precursor. Phytochemistry 204, 113432 (2022).
Monte, I. et al. An ancient COI1-independent function for reactive electrophilic oxylipins in thermotolerance. Curr. Biol. 30, 962–971 e963 (2020).
Xie, D. X., Feys, B. F., James, S., Nieto-Rostro, M. & Turner, J. G. COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280, 1091–1094 (1998).
Wang, J., Sakurai, H., Kato, N., Kaji, T. & Ueda, M. Syntheses of dinor-cis/iso-12-oxo-phytodienoic acid (dn-cis/iso-OPDAs), ancestral jasmonate phytohormones of the bryophyte Marchantia polymorpha L., and their catabolites. Sci. Rep. 11, 2033 (2021).
Nonaka, H., Ogawa, N., Maeda, N., Wang, Y. G. & Kobayashi, Y. Stereoselective synthesis of epi-jasmonic acid, tuberonic acid, and 12-oxo-PDA. Org. Biomol. Chem. 8, 5212–5223 (2010).
Fernandez-Calvo, P. et al. The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. Plant Cell 23, 701–715 (2011).
Russell, L., Larner, V., Kurup, S., Bougourd, S. & Holdsworth, M. The Arabidopsis COMATOSE locus regulates germination potential. Development 127, 3759–3767 (2000).
Footitt, S. et al. Control of germination and lipid mobilization by COMATOSE, the Arabidopsis homologue of human ALDP. Embo J. 21, 2912–2922 (2002).
Theodoulou, F. L. et al. Jasmonic acid levels are reduced in COMATOSE ATP-binding cassette transporter mutants. Implications for transport of jasmonate precursors into peroxisomes. Plant Physiol. 137, 835–840 (2005).
Footitt, S. et al. Analysis of the role of COMATOSE and peroxisomal beta-oxidation in the determination of germination potential in Arabidopsis. J. Exp. Bot. 57, 2805–2814 (2006).
Flokova, K. et al. A previously undescribed jasmonate compound in flowering Arabidopsis thaliana - The identification of cis-(+)-OPDA-Ile. P hytochemistry 122, 230–237 (2016).
Wasternack, C. & Hause, B. OPDA-Ile - a new JA-Ile-independent signal?. Plant Signal Behav. 11, e1253646 (2016).
Mik, V. et al. Synthetic and analytical routes to the L-amino acid conjugates of cis-OPDA and their identification and quantification in plants. Phytochemistry 215, 113855 (2023).
Yi, R. et al. Dioxygenase JID1 mediates the modification of OPDA to regulate jasmonate homeostasis. Cell Discov. 9, 39 (2023).
J. Široká. et al. Amide conjugates of the jasmonate precursor cis-(+)-12-oxo-phytodienoic acid regulate its homeostasis during plant stress responses. Plant Physiol. 197, kiae636 (2025).
Nishizato, Y. et al. Identification of “modified OPDA (mo-OPDA)” as a Michael adduct of cis-OPDA. Biosci., Biotechnol., Biochem. 88, 885–891 (2024).
Brash, A. R. Mechanistic aspects of CYP74 allene oxide synthases and related cytochrome P450 enzymes. Phytochemistry 70, 1522–1531 (2009).
Mehrshahi, P. et al. Transorganellar complementation redefines the biochemical continuity of endoplasmic reticulum and chloroplasts. Proc. Natl Acad. Sci. USA 110, 12126–12131 (2013).
Mekkaoui, K. et al. Transcriptomics and trans-organellar complementation reveal a limited signaling capacity of 12-cis-oxo-phytodienoic acid in wounded Arabidopsis. Nat. Commun. In press (2025).
Browse, J. The power of mutants for investigating jasmonate biosynthesis and signaling. Phytochemistry 70, 1539–1546 (2009).
Ohkama-Ohtsu, N. et al. 12-oxo-phytodienoic acid-glutathione conjugate is transported into the vacuole in Arabidopsis. Plant Cell Physiol. 52, 205–209 (2011).
Li, X. et al. Wounding induces a peroxisomal H(2) O(2) decrease via glycolate oxidase-catalase switch dependent on glutamate receptor-like channel-supported Ca(2+) signaling in plants. Plant J. 116, 1325–1341 (2023).
Scholz, S. S., Reichelt, M., Mekonnen, D. W., Ludewig, F. & Mithofer, A. Insect herbivory-elicited GABA accumulation in plants is a wound-induced, direct, systemic, and jasmonate-independent defense response. Front Plant Sci. 6, 1128 (2015).
Jackson, P. A., Widen, J. C., Harki, D. A. & Brummond, K. M. Covalent modifiers: a chemical perspective on the reactivity of alpha,beta-unsaturated carbonyls with thiols via hetero-michael addition reactions. J. Med Chem. 60, 839–885 (2017).
Dixon, D. P. & Edwards, R. Selective binding of glutathione conjugates of fatty acid derivatives by plant glutathione transferases. J. Biol. Chem. 284, 21249–21256 (2009).
Muller, S. M. et al. The redox-sensitive module of cyclophilin 20-3, 2-cysteine peroxiredoxin and cysteine synthase integrates sulfur metabolism and oxylipin signaling in the high light acclimation response. Plant J. 91, 995–1014 (2017).
Maynard, D. et al. The in vitro interaction of 12-oxophytodienoic acid and related conjugated carbonyl compounds with thiol antioxidants. Biomolecules 11, 457 (2021).
Monte, I. et al. Ligand-receptor co-evolution shaped the jasmonate pathway in land plants. Nat. Chem. Biol. 14, 480–488 (2018).
Kaji, T. et al. Delta(4)-dn-iso-OPDA, a bioactive plant hormone of Marchantia polymorpha. iScience 27, 110191 (2024).
Acknowledgements
Seeds of the opr2-1opr3-3 double mutant and coi1-1 mutant were provided by Professor Roberto Solano and Dr. Andrea Chini (CNB-CSIC, Spain). We thank Dr. Y.T. and Ms. M.N. (Tohoku University) for their technical assistance. And we also thank to Prof. Bettina Hause (Leibniz Institute for Plant Biochemistry, Germany) for valuable discussion on our manuscript. This work was financially supported by Grants-in-Aid for Scientific Research from JSPS, Japan (Nos. 23H00316, 23K17967, 22KK0076, 21K19037, 20H00402, JPJSBP120229905, and JPJSBP120239903 to MU) and a Grant-in-Aid for Transformative Research Areas (A) “Latent Chemical Space” [JP23H04880 and JP23H04883] for MU from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Contributions
Conceptualization, M.U.; methodology, M.U., N.K.; validation, M.U. and N.K.; formal analysis, R.S., Y.N., N.K., T.K.; investigation, R.S., Y.N., N.K., T.K.; resources, R.S., Y.N., N.K., T.K.; data curation, M.U. and N.K.; writing—original draft preparation, M.U.; writing—review and editing, M.U., N.K., Y.N., R.S.; visualization, Y.N., R.S., N.K.; supervision, M.U.; project administration, M.U.; funding acquisition, M.U. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Thierry Heitz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ueda, M., Saito, R., Nishizato, Y. et al. Downstream metabolites of (+)-cis-12-oxo-phytodienoic acid function as noncanonical bioactive jasmonates in Arabidopsis thaliana. Nat Commun 16, 6683 (2025). https://doi.org/10.1038/s41467-025-61072-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-61072-x
This article is cited by
-
Transcriptomics and trans-organellar complementation reveal limited signaling of 12-cis-oxo-phytodienoic acid during early wound response in Arabidopsis
Nature Communications (2025)
-
HY5 integrates light and electrical signaling to trigger a jasmonate burst for nematode defense in tomato
Nature Communications (2025)