Abstract
Alkali metal doping has been widely utilized to regulate metal halide perovskites and improve their luminescence performance. However, due to the discordant tolerance factor caused by the smaller size of potassium and rubidium ions, it is still debatable whether they can be incorporated in the cesium perovskite crystal lattice. Here we provide unambiguous evidence for the formation of Rb+ and K+ substitutionally doped stable perovskite cubic crystal structure in the form of quantum wires embedded in nanoporous alumina template. The suppressed inner defects and enhanced exciton binding energy lead to a reduced non-radiative recombination in the co-doped perovskite quantum wires. The perovskite light-emitting diodes with a maximum external quantum efficiency of 17.5%, 21.2%, 24.9% and 30.1% and a maximum luminance of 1638 cd m−2, 3365 cd m−2, 13,483 cd m−2 and 31,706 cd m−2 for electroluminescence peak of 476 nm (primary-blue), 483 nm (sky-blue), 490 nm (sky-blue) and 512 nm (green) are fabricated respectively. Surprisingly, all devices emit high-color purity light with narrow linewidth of ≤16 nm.
Similar content being viewed by others
Introduction
The ease of compositional engineering for metal halide perovskite (MHPs) materials results in a wide spectrum of opto-electrical properties, facilitating their application in various research fields such as solar cells, photodetectors, light-emitting diodes and so forth1,2,3,4,5,6,7. Specifically, introducing alkali metal (Li+, Na+, K+, Rb+ and Cs+) into perovskites, characterized by a typical crystal structure with the chemical formula of ABX3, has been proven to be an effective approach to tailor their properties2,8,9,10,11,12,13. Nevertheless, only cesium cation possesses a tolerance factor value situated within the range of 0.8–1.01 among these alkali metals, ensuring the formation of a stable cubic perovskite structure. As the neighboring alkali metal cation of Cs+, the tolerance factors of Rb+ and K+ cations are ~0.78 and 0.751, respectively, which are below the range in which the stable cubic perovskite structure can be formed. This indicate that it is difficult for them to substitute A-site cation in the perovskite crystal lattice. However, controversy remains regarding whether Rb+ and K+ cations can enter the lattice.
Indeed, noteworthy improvements on the opto-electric device performance have been made by introducing Rb+ and K+ cations into perovskites, which regulates the crystal lattice, reduces trap density, modulates the film morphology and so on2,5,11,14,15,16,17. Nonetheless, a scarcity of reports provides substantial evidence to reveal their specific position and how they affect the device performance. Currently, investigations have suggested the existence of three possible scenarios: 1. Rb+ and K+ cations are unable to enter the lattice and they instead reside at the grain boundaries in the form of Rb+-rich or K+-rich compounds2,11,16,18,19,20. 2. Rb+ and K+ cations mainly occupy the interstitial sites in the lattice15,17,21,22. 3. Rb+ and K+ cations can partially replace A-site cation in the lattice1,5,23,24,25,26. Generally, there is no definitive or universally accepted understanding regarding the specific location of Rb+ and K+ cations within the lattice and its connection to the device performance.
Here, we present substantial evidence supporting the partial substitution of A-site Cs+ cation with Rb+ and K+ cations in all-inorganic perovskite quantum wires (PeQWs) arrays. The established fact is derived from a comprehensive analysis involving multiple experimental techniques and theoretical calculations. According to the density functional theory (DFT) calculation, the co-doped PeQWs lattice structure is stabilized by the bonding between perovskite and nanoporous alumina template (NAT). Both experimental and theoretical results illustrate that doping Rb+ and K+ cations greatly suppress inner defects and enhance the exciton binding energy, which originate from an ordered perovskite lattice and a weakened Anderson localization. Consequently, perovskite light-emitting diodes (PeLEDs) based on the co-doped PeQWs arrays exhibit a peak external quantum efficiency (EQE) of 17.5%, 21.2%, 24.9% and 30.1% and a maximum luminance of 1638 cd m−2, 3365 cd m−2, 13,483 cd m−2 and 31,706 cd m−2 for blue or green emission at the electroluminescence (EL) peak of 476 nm (primary-blue), 483 nm (sky-blue), 490 nm (sky-blue) and 512 nm (green). Intriguingly, the EL full width at half maximum (FWHM) values are 14.3 nm (primary-blue), 14.8 nm (sky-blue), 15.0 nm (sky-blue) and 16.0 nm (green) which are narrow among high-efficiency PeLEDs reported in literation, to our best knowledge. These results substantiate that incorporating Rb+ and K+ cations in PeQWs arrays ensures excellent efficiency, luminance and color purity simultaneously.
Results
Substituting Cs with alkali metals at A-site in perovskite
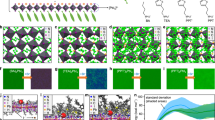
Initially, we fabricate all-inorganic PeQWs arrays through a template-assisted solution method27. The perovskite precursor solutions are prepared by mixing ABr and PbX2 (A = Rb, K or Cs; X = Br or Cl) in dimethyl sulfoxide (DMSO). The fabrication process is a high throughput single-step spin-coating process (Fig. 1a). As expected, the cross-sectional scanning electron microscopy (SEM) image in Fig. 1a reveals the presence of vertically aligned PeQWs arrays with a thickness of ~100 nm. Additionally, transmission electron microscopy (TEM) is also employed to examine the crystal orientation of single PeQW (Supplementary Fig. 1a–d). Observation reveals a high-crystallinity PeQW with a consistent orientation spanning a length of 40–50 nm, strongly suggesting the likelihood of single PeQW being single crystal.
a Schematic illustration of the fabrication of alkali metals doped PeQW arrays and substitution of Cs with Rb and K at A-site in PeQW. Inset, cross-sectional TEM image of PeQW arrays. Scale bar, 200 nm. b Atomically resolved low-dose HRTEM image of single pristine CsPbBr3 QW with atomic structure model projected along <100> direction. Scale bar, 2 nm. Inset, fast Fourier transform diffractograms showing the cubic lattice structure. Scale bar, 2 nm−1. c, d Zoom-in low-dose HRTEM image of perovskite lattice in the edge and middle of pristine CsPbBr3 QW. Scale bar, 1 nm. e Rb 3 d and K 2p XPS core-level spectra of Rb+, K+: CsPbBr3 QWs. f XRD pattern of pristine CsPbBr3 QW, Rb+: CsPbBr3 QWs and Rb+, K+: CsPbBr3 QWs. g GIWAXS pattern and in-plane intensity profiles extracted from the GIWAXS of pristine CsPbBr3 QW, Rb+: CsPbBr3 QWs and Rb+, K+: CsPbBr3 QWs. h Atomically resolved low-dose HRTEM image of single pristine CsPbBr3 QW with atomic structure model projected along <110> direction. Scale bar, 2 nm. Inset, fast Fourier transform diffractograms showing the cubic lattice structure. Scale bar, 5 nm−1. i, j Zoom-in low-dose HRTEM image of perovskite lattice in the edge and middle of Rb+, K+: CsPbBr3 QWs. Scale bar, 1 nm.
Several techniques are then utilized to determine the precise occupying locations of Rb+ and K+ cations in the PeQW, including low-dose high-resolution transmission electron microscopy (HRTEM), grazing-incidence wide-angle X-ray scattering (GIWAXS), X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS). Firstly, the pristine CsPbBr3 QWs are set as control samples. Figure 1b displays the HRTEM image of pristine CsPbBr3 QWs, demonstrating their typical cubic perovskite structure projected along <100> direction, along with the corresponding atomic structure. The fast Fourier transform (FFT) diffractogram in the inset of Fig. 1b confirms the presence of the plane in pristine CsPbBr3 QWs while the average interplane spacing between two neighboring (100) planes is measured to be 6.06 Å. The crystal lattice in the edge and middle of a single pristine CsPbBr3 QW are depicted in the selected zoom-in HRTEM images presented in Fig. 1c, d. In addition to three-dimensional cubic CsPbBr3 phase, the existence of zero-dimensional Cs4PbBr6 phase is also observed in pristine PeQWs, as shown in Supplementary Fig. 2a–d.
For target PeQWs, Rb+ and K+ cations are doped into the pristine CsPbBr3 QWs. To verify the success of Rb+ and K+ cations doping, XPS analysis and energy-dispersive X-ray spectroscopy (EDS) mapping of Rb+, K+:CsPbBr3 QWs arrays were collected (Fig. 1e and Supplementary Fig. 3a–l). The Rb 3 d spectrum for Rb+, K+:CsPbBr3 PeQWs exhibits two dominant peaks located at 110.20 eV and 111.51 eV, corresponding to 3d5/2 and 3d3/2 orbitals, respectively. In the K 2p spectrum, two K binding energy peaks are observed, which are associated with the 2p3/2 (293.29 eV) and 2p1/2 (296.10 eV) levels. In stark contrast, the elemental profiles of the Rb (Supplementary Fig. 3i) and K (Supplementary Fig. 3j) elements are evident in the profiles of the Rb+,K+:CsPbBr3 PeQWs arrays, whereas these profiles were not detected in the pristine CsPbBr3 PeQWs arrays (Supplementary Fig. 3c, d). Some studies point out that Rb+ and K+ cations tend to accumulate at the grain boundaries in the form of Rb+-rich or K+-rich compounds, such as RbPbX3, K2PbX4, and KX2,16,18,19,20,28. However, in our case, the XRD peak corresponding to these compounds is not identifiable in the XRD patterns depicted in Fig. 1f, which means these compounds don’t exist in the Rb+,K+:CsPbBr3 PeQWs arrays. Meanwhile, the distinctive peaks, which are classified to (100), (110) and (200) planes, undergo a noticeable shift towards a larger angle as the Rb+ and K+ cations are sequentially doped in PeQWs. This proves that the Rb+ and K+ cations doping leads to a lattice contraction which is consistent with previous studies that support Rb+ and K+ cations substituting A-site cation1,5,23,25. Moreover, the GIWAXS patterns and radial integration of 2D GIWAXS exhibit the same trend as that observed in the XRD patterns (Fig. 1g). From the HRTEM image of Rb+,K+:CsPbBr3 PeQWs (Fig. 1h), a perfect cubic structure projected along <110> direction is pictured. The average interplane distance of two neighboring (100) planes decreases to 5.97 Å which coincides with the XRD and GIWAXS results. Although some researchers also claim that interstitial occupancy of Rb+ and K+ cations is dominant15,17,21,22,26, there is no sign of Rb+ or K+ cations positioning at the interstitial site in the crystal lattice (Fig. 1i, j). In addition, the intensity profiles of various atoms at different positions are provided, as illustrated in Supplementary Fig. 4a–d. And yet, these atoms are precisely in their designated positions, devoid of occupying the interstitial site. Generally, the available evidence strongly indicates that Rb+ and K+ cations can replace Cs+ cation at A-site in the PeQWs.
The formation energy of alkali metals substituting Cs
We then perform DFT calculations to bolster our rationale and elucidate the impact it has on material properties. Firstly, a computational model of CsPbBr3 without Al2O3 (Fig. 2a) is constructed to calculate the formation energy of Rb and K atoms replacing Cs atom at A-site. The detailed simulation processes are shown in Supplementary Fig. 5. Since Rb and K atoms are smaller than Cs atom and beyond the tolerance of the perovskite structure, the formation energy of Rb and K atoms displacing Cs atom at A-site is 0.303 eV and 0.537 eV (Fig. 2b), respectively. Such a high formation energy implies that Rb and K cannot be easily dissolved in the perovskite structure. Then Al2O3 is introduced to the computational model of CsPbBr3 (Fig. 2c)29. In the simulation processes (Supplementary Figs. 6, 7), Cs atom in the bulk and at the interface between CsPbBr3 and Al2O3 was substituted by Rb and K atoms. Surprisingly, as shown in Fig. 2d, e and Supplementary Fig. 7b, the formation energy of Rb and K atoms replacing Cs atom at A-site has been reduced significantly compared with the model without Al2O3, making it possible for Rb and K atoms to be incorporated in the perovskite structure. This result is consistent with our experimental observation that Rb and K cations can reside at A-site in the perovskite lattice of all-inorganic PeQWs arrays. It is known that replacing Cs atom in CsPbBr3 at A-site by using Rb or K atom introduces large distortion of the cubic lattice, causing lattice instability and phase transformation19,20. However, in our scenario, when CsPbBr3 is embedded in the NAT, Br-Al and Pb-O bonds are formed (Supplementary Figs. 6, 7) at the Al2O3/CsPbBr3 interface27,29. These bonds help to stabilize the perovskite lattice upon doping of Rb or K, even though there is a lattice mismatch. As the PeQW has a very small diameter (~6.4 nm), this interface effect can dominate the entire PeQW, making Rb and K doping more stable inside PeQW as compared to that inside a bulk/film of perovskite30,31. Based on the prior report32, the interference between electron’s multiple scatterings by random defects in the potential will induce Anderson localization. Rb and K atoms staying at the interstitial site will definitely destroy the ordering of perovskite lattice, resulting in Anderson localization. On the contrary, Rb and K atoms replacing Cs atom at A-site will not damage the orderliness of perovskite lattice and contribute to positive impact on the material properties which will be demonstrated subsequently.
a Schematic drawing of Rb or K replacing Cs at A-site in the model without Al2O3. b Formation energy of Rb or K replacing Cs at A-site. c Schematic drawing of Rb or K replacing Cs at A-site in the model with Al2O3. d Formation energy of Rb or K replacing Cs at A-site in the bulk of CsPbBr3. e Formation energy of Rb or K replacing Cs at A-site at the interface of CsPbBr3 and Al2O3.
Properties of alkali metals doped perovskite quantum wires
Intriguingly, we have not only achieved green emission but also extended our approach to include blue emission simply through the process of chloride doping. XRD and GIWAXS measurements (Fig. 3a and Supplementary Fig. 8) show diffraction peaks indexed to the cubic perovskite structure. The XPS analysis is studied to confirm the existence of each chemical element (Supplementary Fig. 9). The corresponding steady state PL spectra of Rb+, K+:CsPbBr3 and Rb+, K+:CsPb(Br1-xClx)3 QWs are shown in Fig. 3b, exhibiting peaks at 476 nm (primary-blue), 483 nm (sky-blue), 490 nm (sky-blue) and 512 nm (green), respectively. Surprisingly, the photoluminescence (PL) FWHMs of corresponding PeQWs are measured to be 15.1 nm, 15.4 nm, 15.7 nm, 17.1 nm, showcasing an exceptional level of color purity. In comparison, the FWHM is ~17.6 nm and 18.0 nm for pristine PeQWs (Supplementary Fig. 10a, b). The Ultraviolet–visible (UV-Vis) absorbance spectra are also summarized in Supplementary Fig. 10. The transient absorption (TA) spectra exhibit a pronounced single bleaching peak and the peak position remains nearly constant over time (Supplementary Figs. 11–13 and Supplementary Table 1). The Urbach energy (Eu) is calculated from the absorption coefficient spectra (Supplementary Fig. 14) extracted from the absorbance spectra as follows:
where α is the absorption coefficient and hν is the photon energy33. The reduction in the Eu value from >30 meV to ~20 meV indicates a low electronic disorder in the band edges34 for Rb+ and K+ doped PeQWs which can be partially attributed to the elimination of zero-dimensional Cs4PbBr6 phase whose presence cannot be found in the HRTEM images.
a XRD patterns, b Normalized photoluminescence spectra (\({\lambda }_{{ex}}=350{nm}\)), c TRPL decay curves and d PLQYs (\({\lambda }_{{ex}}=365{nm}\)) measured at corresponding photoluminescence peak of as-synthesized alkali metals doped PeQWs. e Environmental photoluminescence stability under ambient condition for alkali metal doped PeQWs.
A time-resolved photoluminescence (TRPL) measurement is performed to examine the lifetime of excited charge carriers (Fig. 3c). The PL decay curves are fitted into two regions, with a short lifetime (\({\tau }_{1}\)) associated with trap-mediated nonradiative recombination, and a longer lifetime (\({\tau }_{2}\)) ascribed to radiative recombination (Supplementary Fig. 15 and Supplementary Table 2)35. The incorporation of Rb+ and K+ leads to an extension of both \({\tau }_{1}\) and \({\tau }_{2}\) in compared with the pristine PeQWs, implying Rb+ and K+ doped PeQWs possess a lower defect density and an enhanced radiative recombination. To investigate the defect density and the radiative recombination in Rb+ and K+ doped PeQWs, space charge-limited current (SCLC) measurements (Supplementary Figs. 16–18) are conducted based on hole-only and electron-only devices. Additionally, temperature-dependent steady-state PL spectra measurements (Supplementary Fig. 19) are also carried out. The equation as below is used to calculate the total trap-state density inside the PeQWs:
where nt is the trap state density, VTFL is the trap-filled limit voltage, L is the thickness of the perovskites, e is the elementary charge, ε0 and ε are the vacuum permittivity and relative permittivity, respectively36. The significant reduction in the trap-state density provides evidence that the inclusion of RbBr and KBr in PeQWs effectively heal the Cs and halide vacancies, thereby contributing to an increased luminescence efficiency. The confirmation of improved radiative recombination is achieved through temperature-dependent steady-state PL analysis. The exciton binding energy (Eb) is estimated to be 352 meV, 343 meV, 332 meV and 236 meV for Rb+, K+: CsPb(Br1-xClx)3 and Rb+,K+: CsPbBr3 QWs, significantly surpassing those reported in our previous study27 and in other literatures employing perovskite as emissive layer. Such a high Eb value indicates Rb+ and K+ doped PeQWs experience a strong quantum confinement effect due to the ultra-small size. The reduction in defect density and the contraction of perovskite lattice after doping also contribute to the enhanced exciton binding energy. The fluence-dependent TA measurements are performed to study the charge-carries dynamics for cross-check (Supplementary Fig. 20 and Supplementary Table 3). Consistent with the results in another paper37, the k1 of PeQWs is two orders of magnitude higher than that of bulk perovskite. The k1 of PeQWs is dominated by geminate exciton recombination while the k1 of bulk perovskite is dominated by trap-assisted Shockley–Read–Hall (SRH) nonradiative recombination. Moreover, the PeQWs exhibit higher radiative recombination rate compared with bulk perovskite. Because of enhanced exciton-exciton interactions, the obtained Auger recombination rate of PeQWs is one order of magnitude higher. Consequently, Rb+ and K+ doped PeQWs show an increased photoluminescence quantum yield (PLQY) of 66.2%, 71.5%, 81.6% and 96.0% for different emission wavelengths (Fig. 3d).
In strongly coupled material systems such as quantum dots or nanoplates38,39, the variation in size and interdot/interplate distance, quantum dots/nanoplates organization and defects on interface will generate bandtail states and mid-gap trap states, leading to a strong Anderson localization. However, even though the PeQWs diameter has a large variation, and they are not perfectly vertical aligned on the substrate, the coupling between PeQWs is weak since they are embedded in NAT and the pitch of NAT pores is more than 10 nm40. Hence, the adverse effect of Anderson localization in strongly coupled material system can be avoided. Moreover, with Rb and K atoms replacing Cs atom at A-site instead of situating at the interstitial site as well as healing Cs and halide defects at the interface, the lattice order is restored, resulting in a weak Anderson localization. Furthermore, previous study also shows that the formation of Cs and alkali metal cation alloy alters the supersaturation of Cl-rich domains and/or increases the formation energy of perovskites, leading to an excellent compositional homogeneity41. Consequently, the excellent compositional homogeneity results in a narrower emission linewidth. In general, we conclude that the narrow emission linewidth and high exciton binding energy can be attributed to the weakened Anderson localization and the improved compositional homogeneity based on the above discussion.
To evaluate the environmental PL stability under ambient condition (24 °C and 10–20% relative humidity) of the Rb+ and K+ doped PeQWs arrays, time-dependent normalized PL tests are performed (Fig. 3e). Remarkably, Rb+ and K+ doped PeQWs arrays have an extradentary T95 PL lifetime of over 1000 h.
High-performance perovskite light-emitting diodes
Encouraged by the benefits from Rb+ and K+ cations doping, we successfully manufacture PeLEDs based on the Rb+ and K+ doped PeQWs arrays (Supplementary Fig. 21). The device comprises a layered structure of conducting indium tin oxide (ITO)/ 1,4,5,8,9,11-Hexaazatriphenylenehexacarbonitrile (HAT-CN)/ 1,3,5-tris (bromomethyl) benzene (TBB) doped 1,1-Bis[(di-4-tolylamino)phenyl]cyclohexane (TAPC)/PeQWs arrays/aluminum oxide (Al2O3)/aluminum (Al) as schematically shown in Fig. 4a, b. The flat-band energy band diagram, derived from the ultraviolet photoelectron spectroscopy (UPS) measurements (Supplementary Fig. 22 and Supplementary Table 4), is illustrated in Fig. 4c. In contrast to the pristine PeQWs, the Rb+ and K+ doped PeQWs demonstrate a shallower valence band level, favoring the hole injection from TAPC to PeQWs. The EL spectra, corresponding Commission Internationale de l’éclairage (CIE) chromaticity coordinate diagram and angular electroluminescence distribution are depicted in Fig. 4d, e and Supplementary Fig. 23. Remarkably, the PeLEDs based on the Rb+ and K+ doped PeQWs arrays show an EL peak centered at 476 nm (primary-blue), 483 nm (sky-blue), 490 nm (sky-blue) and 512 nm (green), with a narrow FWHM of 14.3 nm, 14.8 nm, 15.0 nm and 16.0 nm, respectively. These FWHM values are quite narrow among high-efficiency PeLEDs (Supplementary Fig. 24), to our best knowledge. The corresponding CIE chromaticity coordinates are (0.101,0.122), (0.077, 0.183), (0.047, 0.323) and (0.040, 0.780), exhibiting high color-purity with a calculated value of as high as 99.1% (Supplementary Fig. 25 and Supplementary Table 5).
a Schematic of alkali metals doped PeQWs-based LED architecture. b Cross-sectional SEM image of a device. The thickness of each layer, which can be confirmed by SEM, are shown below: Al electrode/Al2O3 hole blocking layer (~5 nm)/PeQWs emission layer (90 nm)/TAPC hole transporting layer (20 nm)/HAT-CN hole injecting layer (20 nm)/ITO transparent electrode (90 nm). Scale bar, 200 nm. c Energy band diagram of each layer in PeLEDs. d Normalized electroluminescence spectra of blue and green PeLEDs. e The corresponding CIE coordinates of blue and green PeLEDs. f Current density (J)-voltage (V)-luminance (L) curves. g, h External quantum efficiency (EQE)-luminance (L) curves. i The EQEs distribution collected from 72 devices. The average peak EQEs are 15.8%, 18.2%, 20.2% and 26.2% with relative standard deviations of 7.7%, 7.3%, 11.1% and 7.3%, respectively.
Furthermore, the finite-difference-time-domain (FDTD) simulations (Supplementary Figs. 26–28 and Supplementary Table 6) verify that our PeLEDs can reach a light out-coupling efficiency (OCE) of 85% which is 4 times higher than planar structure PeQWs with only ~20% OCE27,42. The detailed current density (J)-voltage (V)-luminance (L) characteristics are summarized in Fig. 4f, Supplementary Fig. 29 and Supplementary Tables 7, 8. The PeLEDs show a maximum luminance of 1638 cd m−2 (primary-blue), 3365 cd m−2 (sky-blue), 13,483 cd m−2 (sky-blue) and 31,706 cd m−2 (green). From the external quantum efficiency (EQE)-luminance (L) curves (Fig. 4g, h), the PeLEDs attain a peak EQE value of 17.5% (primary-blue), 21.2% (sky-blue), 24.9% (sky-blue) and 30.1% (green). Besides, the EQEs distribution is reported in Fig. 4i and Supplementary Fig. 30, showing great reproducibility.
Finally, the devices stability is analyzed by separately subjecting them to different electric bias and a constant driving current. The EL spectra remain unchanged under varying applied voltages up to 12 V (Supplementary Fig. 31), suggesting that the devices have extraordinary spectral stability against high working voltage. To expedite the aging process, the initial luminance is deliberately set to 1000 cd m−2. The operating half lifetimes (T50) are 38 s (primary-blue), 58 s (sky-blue), 370 s (sky-blue) and 1325 s (green), respectively. Impressively, the EL wavelength and FWHM keep consistent throughout the operation.
Discussion
In conclusion, we reveal concrete evidence, including multiple experimental techniques and theoretical calculations, on that Rb and K cations can reside at A-site in the perovskite lattice of all-inorganic PeQWs arrays, preserving the order of perovskite lattice and avoiding detrimental effects caused by Anderson localization. In virtue of DFT calculation results, we attribute the stabilized lattice structure of co-doped PeQWs to the bonding between perovskite and NAT. The improved perovskite quality enables reduced trap density, narrowed emission linewidth, enhanced exciton binding energy and increased luminous efficiency. As a result, simultaneously ultrapure and efficient PeLEDs with narrow linewidth of ≤16 nm and maximum external quantum efficiency exceeding 20% (blue) and 30% (green) are demonstrated. This fundamental progress represents a significant advancement toward achieving both high color purity and high efficiency in PeLEDs, providing a clear pathway for PeLEDs suitable for wide-color-gamut display applications.
Methods
Materials
Cesium bromide (CsBr, 99.999%), rubidium bromide (RbBr, 99.6%), potassium bromide (KBr, ≥99%), lead bromide (PbBr2, 99.999%), lead chloride (PbCl2, 99.999%), 1,3,5-tris (bromomethyl) benzene (TBB, 97%), chlorobenzene (C6H5Cl, 99.8%) and dimethyl sulfoxide (DMSO, ≥99.9%) were purchased from Sigma Aldrich. 1,1-Bis[(di-4-tolylamino)phenyl]cyclohexane (TAPC, >99.5%) and 1,4,5,8,9,11-Hexaazatriphenylenehexacarbonitrile (HAT-CN, >99%) were purchased from Ossila. Note that all materials were used without further processing.
Preparation of the perovskite solution
The alkali metal doped perovskite precursors (K+, Rb+: CsPb(Br1-xClx)3) were prepared by dissolving stoichiometric ratios of each of KBr, RbBr, CsBr, PbBr2 and PbCl2 into DMSO at a concentration of 0.1 M. The molar ratio is KBr:RbBr:CsBr:PbBr2:PbCl2 = 0.2:0.3:1:1-x:x, where x is 0, 0.5, 0.6 and 0.7 for different emission wavelength. In the case of precursor solutions for control samples, the molar ratio is CsBr:PbBr2:PbCl2 = 1:1-x:x. The precursor solutions were stirred at 60 °C for 12 h in an N2-filled glovebox and filtered by 0.45-μm poly(tetrafluroethylene) filters before use.
Nanoporous alumina templates fabrication
The NAT were fabricated through a low-voltage anodic anodization process which can be found in our earlier work27,29,40. Briefly, aluminum chips of high purity with an area of 2 × 2.5 cm2 were sonicated in acetone and 2-propanol for 10 min each sequentially. Afterward, the aluminum chips were electro-polished at room temperature in a blended solution comprising 25 vol% perchloric acid and 75 vol% absolute ethanol at 18 V for 3 min to achieve a smooth surface. Subsequently, anodization at 5 V took place in a 5 vol% H2SO4 solution for 8 min to form 90 nm-thick porous aluminum oxide with ~6.4 nm pore diameter. Finally, the NAT were washed with deionized water and dried using compressed air.
Perovskite light-emitting diodes fabrication
Following the fabrication of NAT, the pristine 2 × 2.5 cm2 NAT were transferred into a N2-filled for PeLEDs fabrication. The filtered perovskite precursor solutions were dropped on NAT and then spin-coated at 5000 rpm for 50 s, followed by baking at 80 °C for 10 min. After annealing, the TBB doped TAPC solution (in chlorobenzene) was spin-coated on NAT filled with perovskite at 3000 rpm for 30 s. Samples were then transfered to the vacuum chamber for 20 nm HAT-CN evaporation (1 Å s−1). Finally, a 90 nm layer of ITO was magnetically sputtered at a rate of 1 Å s−1 using 70 W of RF sputtering. The active device area of 0.04 cm2 was defined by a shadow mask employed throughout the ITO sputtering process.
Characterization
A Dual Beam FIB/SEM system (FEI Helios G4 UX) was used to obtain Top-view SEM images. TEM images were taken from a transmission electron microscope (TEM) JEOL (2010F). XRD patterns were collected from a Bruker D8 X-ray diffractometer. The PL spectrum, PLQY and TRPL were measured on an Edinburgh Instruments FLS920P. J.A. Woollam M-2000VI Spectroscopic Ellipsometer and Lambda 1050+ (PerkinElmer) was used to obtain Complex refractive indices (n, k) and UV-vis absorption of sample. The TA absorption spectrum was recorded on a Helios (Ultrafast Systems LLC) spectrometer equipping a sapphire laser source (Coherent Legend, 800 nm, 150 fs, 5 mJ per pulse, and 1 kHz repetition rate). A BaB2O4 (BBO) crystal was used to double the frequency of 75% of the 800 nm output pulse, generating 400 nm pump light. At the same time, to produce white light continuum (420–780 nm) probe light, 25% of the output pulse was concentrated into a sapphire window. The 400 nm pump beam with a beam waist of about ∼360 μm was focused on the sample and the power intensity was fixed at 40 μJ cm−2. XPS and UPS spectrum were characterized from a Kratos Axis Ultra DLD multi-technique surface analysis system. Temperature-dependent PL measurements were performed on the QE-Pro spectrometer (Ocean Insight) and Newton 920 CCD (Oxford Instruments). Atomic-scale structure imaging and chemical analysis of the samples were taken from a double Cs-corrected (S) TEM systems (FEI Themis Z, ThermoFisher Scientific) equipped with EDS (Super-X EDS system). GIWAXS measurements were accomplished with a Xeuss 2.0 WAXS/SAXS laboratory beamline using a Cu X-ray source (8.05 keV, 1.54 Å) and a Pilatus3R 300 K detector. The surface roughness of samples was measured in ambient condition via an atomic force microscope (AFM) (Bruker Dimension XR, United States) using a back side Al 100 nm coating tip (Bruker: OTESPA-R3; k: 26 N/m; f0: 300 kHz).
Perovskite light-emitting diodes measurement
The performance of the fabricated PeLEDs was measured in ambient air without encapsulation using a Keithley 2450 source-meter unit and a calibrated Ocean Optics Flame spectrometer with an integrating sphere. The operational lifetime was monitored under constant-current conditions by the same set-up mentioned above. Cross-checking of device’s EL characteristics was also conducted with a luminance meter (Konica Minolta, CS-200) and an integrated system with sphere and photomultiplier tubes (Enlitech, LQ-50X-EL).
Data availability
The data that support the findings of this study are provided in the main text and the Supplementary Information. More data are available from the corresponding author upon request.
References
Saliba, M. et al. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science 354, 206–209 (2016).
Abdi-Jalebi, M. et al. Maximizing and stabilizing luminescence from halide perovskites with potassium passivation. Nature 555, 497–501 (2018).
Kausar, A. et al. Advent of alkali metal doping: a roadmap for the evolution of perovskite solar cells. Chem. Soc. Rev. 50, 2696–2736 (2021).
Li, Y. -h. et al. Review on the promising roles of alkali metals toward highly efficient perovskite light-emitting diodes. J. Mater. Chem. C. 11, 2011–2025 (2023).
Wang, Z. et al. Suppressed phase segregation for triple-junction perovskite solar cells. Nature 618, 74–79 (2023).
Zhou, Y. et al. An ultrawide field-of-view pinhole compound eye using hemispherical nanowire array for robot vision. Sci. Robot. 9, eadi8666 (2024).
Qiu, X. et al. A tetrachromatic sensor for imaging beyond the visible spectrum in harsh conditions. Device 2, 100357 (2024).
Yi, C. et al. Entropic stabilization of mixed A-cation ABX3 metal halide perovskites for high performance perovskite solar cells. Energy Environ. Sci. 9, 656–662 (2016).
Qiao, L., Fang, W.-H., Long, R. & Prezhdo, O. V. Extending carrier lifetimes in lead halide perovskites with alkali metals by passivating and eliminating halide interstitial defects. Angew. Chem. Int. Ed. 59, 4684–4690 (2020).
Li, Y. et al. Lattice modulation of alkali metal cations doped Cs1-xRxPbBr3 halides for inorganic perovskite solar cells. Sol. RRL 2, 1800164 (2018).
Wang, Y.-K. et al. All-inorganic quantum-dot LEDs based on a phase-stabilized α-CsPbI3 perovskite. Angew. Chem. Int. Ed. 60, 16164–16170 (2021).
Guo, Z. et al. Promoting energy transfer via manipulation of crystallization kinetics of quasi-2D perovskites for efficient green light-emitting diodes. Adv. Mater. 33, 2102246 (2021).
Cai, L. et al. Efficient and bright pure-blue all-inorganic perovskite light-emitting diodes from an ecofriendly alloy. J. Phys. Chem. Lett. 12, 1747–1753 (2021).
Nam, J. K. et al. Potassium Incorporation for enhanced performance and stability of fully inorganic cesium lead halide perovskite solar cells. Nano Lett. 17, 2028–2033 (2017).
Zhang, C. et al. Dual metal-assisted defect engineering towards high-performance perovskite solar cells. Adv. Funct. Mater. 32, 2208077 (2022).
Yang, J.-N. et al. Potassium bromide surface passivation on CsPbI3-xBrx nanocrystals for efficient and stable pure red perovskite light-emitting diodes. J. Am. Chem. Soc. 142, 2956–2967 (2020).
Hoang, M. T. et al. Potassium doping to enhance green photoemission of light-emitting diodes based on CsPbBr3 perovskite nanocrystals. Adv. Optical Mater. 8, 2000742 (2020).
Abdi-Jalebi, M. et al. Potassium- and rubidium-passivated alloyed perovskite films: optoelectronic properties and moisture stability. ACS Energy Lett. 3, 2671–2678 (2018).
Kuai, L. et al. Passivating crystal boundaries with potassium-rich phase in organic halide perovskite. Sol. RRL 3, 1900053 (2019).
Wang, L. et al. Potassium-induced phase stability enables stable and efficient wide-bandgap perovskite solar cells. Sol. RRL 4, 2000098 (2020).
Cao, J., Tao, S. X., Bobbert, P. A., Wong, C.-P. & Zhao, N. Interstitial occupancy by extrinsic alkali cations in perovskites and its impact on ion migration. Adv. Mater. 30, 1707350 (2018).
Kim, D. et al. Investigation of potassium doping and defect healing mechanism in core–shell CsPbBr3/SiO2 quantum dots. J. Mater. Chem. C. 12, 6395–6405 (2024).
Liu, Y. et al. Considerably enhanced exciton emission of CsPbCl3 perovskite quantum dots by the introduction of potassium and lanthanide ions. Nanoscale 10, 14067–14072 (2018).
El Ajjouri, Y., Chirvony, V. S., Sessolo, M., Palazon, F. & Bolink, H. J. Incorporation of potassium halides in the mechanosynthesis of inorganic perovskites: feasibility and limitations of ion-replacement and trap passivation. RSC Adv. 8, 41548–41551 (2018).
Shao, G. et al. KxCs1-xPbBr3 NCs glasses possessing super optical properties and stability for white light emitting diodes. Chem. Eng. J. 375, 122031 (2019).
Chu, Y. et al. Unveiling the photoluminescence regulation of colloidal perovskite quantum dots via defect passivation and lattice distortion by potassium cations doping: not the more the better. J. Colloid Interface Sci. 596, 199–205 (2021).
Cao, Y. B. et al. High-efficiency, flexible and large-area red/green/blue all-inorganic metal halide perovskite quantum wires-based light-emitting diodes. Nat. Commun. 14, 4611 (2023).
Hu, Y., Aygüler, M. F., Petrus, M. L., Bein, T. & Docampo, P. Impact of rubidium and cesium cations on the moisture stability of multiple-cation mixed-halide perovskites. ACS Energy Lett. 2, 2212–2218 (2017).
Fu, Y. et al. Strongly quantum-confined perovskite nanowire arrays for color-tunable blue-light-emitting diodes. ACS Nano 16, 8388–8398 (2022).
Waleed, A. et al. All inorganic cesium lead iodide perovskite nanowires with stabilized cubic phase at room temperature and nanowire array-based photodetectors. Nano Lett. 17, 4951–4957 (2017).
Gu, L. et al. Significantly improved black phase stability of FAPbI3 nanowires via spatially confined vapor phase growth in nanoporous templates. Nanoscale 10, 15164–15172 (2018).
Schwartz, T., Bartal, G., Fishman, S. & Segev, M. Transport and Anderson localization in disordered two-dimensional photonic lattices. Nature 446, 52–55 (2007).
Han, D. et al. Tautomeric mixture coordination enables efficient lead-free perovskite LEDs. Nature 622, 493–498 (2023).
Subedi, B. et al. Urbach energy and open-circuit voltage deficit for mixed anion–cation perovskite solar cells. ACS Appl. Mater. Interfaces 14, 7796–7804 (2022).
Fu, Y. et al. Scalable all-evaporation fabrication of efficient light-emitting diodes with hybrid 2D–3D perovskite nanostructures. Adv. Funct. Mater. 30, 2002913 (2020).
Kim, J. S. et al. Ultra-bright, efficient and stable perovskite light-emitting diodes. Nature 611, 688–694 (2022).
Xing, G. et al. Transcending the slow bimolecular recombination in lead-halide perovskites for electroluminescence. Nat. Commun. 8, 14558 (2017).
Kagan, C. R. & Murray, C. B. Charge transport in strongly coupled quantum dot solids. Nat. Nanotechnol. 10, 1013–1026 (2015).
Chen, M.-Y. et al. Strongly coupled tin-halide perovskites to modulate light emission: tunable 550–640 nm light emission (FWHM 36–80 nm) with a quantum yield of up to 6.4%. Adv. Mater. 30, 1706592 (2018).
Zhang, D. et al. Large-scale planar and spherical light-emitting diodes based on arrays of perovskite quantum wires. Nat. Photonics 16, 284–290 (2022).
Luo, X. et al. Effects of local compositional heterogeneity in mixed halide perovskites on blue electroluminescence. Matter 7, 1054–1070 (2024).
Zhang, Q. et al. Light out-coupling management in perovskite LEDs—what can we learn from the past?. Adv. Funct. Mater. 30, 2002570 (2020).
Acknowledgements
This work was financially supported by Hong Kong Research Grant Council (General Research Fund Project No. 16205321, 16309018, and 16214619), HKUST Fund of Nanhai (Grant No. FSNH-18FYTRI01), Guangdong-Hong Kong-Macau Intelligent Micro-Nano Optoelectronic Technology Joint Laboratory (Grant No. 2020B1212030010), Foshan Innovative and Entrepreneurial Research Team Program (2018IT100031), NSFC/RGC Joint Research Scheme Funding N_HKUST657/22. National Natural Science Foundation of China (NSFC-RGC 62261160392 and 52131304), Zhongshan Municipal Science and Technology Bureau (ZSST21EG05). The authors also acknowledge the support from the Material Characterization and Preparation Facility (MCPF) and the Center for 1D/2D Quantum Materials and the State Key Laboratory on Advanced Displays and Optoelectronics Technologies at HKUST.
Author information
Authors and Affiliations
Contributions
Z.F. and Y.C. initiated the study and designed the experiments. Y.C. carried out PeQWs and LED device fabrication, optical characterization and measured device’s performance. X.Q., W.T., Q.S. and H.Z. carried out SEM and TEM characterizations. J.L. and D.K. performed TA measurements. K.Z. and Y.L. conducted XRD measurements. Z.J. and J.W. conducted the temperature-dependent PL measurements. P.C. and X.L. carried out GIWAXS measurements. F.Y. and Y.X. did theoretical calculations. Y.C. and Q.Z. performed FDTD simulation. X.Q. and X.S. provided support for characterization of the materials. F.X. performed aluminum sputtering. B.R. and D.Z. assisted with the measurement of LED devices. Y.C. and Y.Z analyzed data and organized the figure. Y.C drafted the first version of the manuscript, with assistance from Y.Z., Y.F., D.Z., Y.D. and Z.F. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Jiajun Luo and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cao, Y.B., Fu, Y., Zhou, Y. et al. Ultrapure and efficient electroluminescence in alkali metal doped inorganic perovskite quantum wires arrays. Nat Commun 16, 6053 (2025). https://doi.org/10.1038/s41467-025-61085-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-61085-6