Abstract
Cortical descending modulation of sensory processing in the spinal cord has been revealed, the role of cortico-brainstem projections in sensory and motor regulation remains poorly investigated. Here, we identified two previously unappreciated subpopulations in the primary sensorimotor cortex (SM1), which are anatomically and electrophysiologically distinct but functionally similar, projecting to the periaqueductal gray (PAG) and rostroventral medulla (RVM), respectively. Postsynaptic PAG glutamatergic neurons then project to the noradrenergic (NA) neurons in the locus coeruleus (LC) and 5-HT neurons in the RVM to drive spinal NA and 5-HT anti-nociception, while RVM enkephalinergic neurons that receive SM1 projections directly drive spinal opioid analgesia. Intriguingly, silencing these two SM1 subpopulations impair motor behaviors and motor-induced analgesia, but does not affect behavioral responses to cutaneous sensory stimuli. Our findings indicate that these brainstem-projecting SM1 subpopulations are crucial for cortical descending pain modulation, which could be exploited to optimize pain relief approaches.
Similar content being viewed by others
Introduction
The primary sensorimotor cortex (SM1) includes the primary somatosensory cortex (S1) and primary motor cortex (M1), which have long been presumed to process somatosensory perception and control motor behaviors, respectively1,2. However, there is increasing evidence that both S1 and M1 regulate sensory and motor functions through descending projections3,4. For example, in addition to bottom-up somatosensory perception, S1 regulates the sensory processing in the spinal cord through top-down control4, drives whisker retraction5, participates in motor adaptation of the forelimbs6, and modulates the speed of locomotion in mice7. Furthermore, M1 responds to painful stimulation and undergoes plastic changes during chronic pain8,9. The interactions between sensory and motor functions are important in cortical stimulation-induced analgesia. Both human and animal studies have shown that stimulation of SM1, and especially M1, can effectively reduce pathological pain10,11,12,13. However, the cellular and circuitry-level substrates underlying the functional diversity and complexity of S1 and M1 remain poorly understood in the context of sensory and motor regulation.
In mammals, cortex-mediated sensory and motor control are primarily regulated by descending projection neurons within SM14,14,15,16. Various axon projections from layer 5–6 pyramidal neurons in SM1 descend to the thalamus, brainstem, dorsal column nucleus, and spinal cord17,18. Ablation of somatosensory corticospinal neurons impaired behavioral responsiveness to light touch, and attenuated tactile allodynia in neuropathic pain mice4. Also, impairments of corticofugal neurons in the SM1 may affect tactile signal transmission in the spinal dorsal horn and cuneate nucleus, resulting in poor motor performance19,20. Multiple lines of evidence suggest that the midbrain periaqueductal gray (PAG) and rostroventral medulla (RVM) participate in motor control and sensory modulation21,22,23. Such regions have also been implicated in sensorimotor cortical stimulation-induced analgesia3,24,25,26. However, the circuitry connecting SM1 to the PAG and RVM is unclear; there is also a lack of clarity concerning the mechanisms by which they mediate cortical stimulation-induced analgesia and regulate sensation and movement.
To address the above questions, we selectively labeled PAG- and RVM-projecting neurons within SM1, then explored their modulatory roles in sensory and motor activity under physiological and pathological conditions. Our results showed that selective activation of these two SM1 neuronal subpopulations, as well as the SM1–PAG and SM1–RVM projection pathways, produced a robust analgesic effect during chronic pain; inhibition of these neurons impaired motor behaviors under normal physiological conditions. Our study also revealed the cellular and molecular mechanisms underlying descending pain inhibition, and it showed that two previously obscure subpopulations of SM1 neurons (and their corresponding midbrain/medullary projections) have equivalent and synergistic analgesic effects under conditions of chronic pain.
Results
Identification of PAG- and RVM- projecting neurons in the sensorimotor cortex
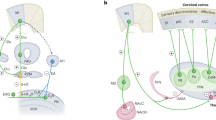
To explore cortico–midbrain/medulla connections arising from S1 and M1, we injected adeno-associated viruses (AAVs) carrying synaptophysin-mRuby (AAV-hsyn-synaptophysin-mRuby-T2A-GFP) into S1 or M1 (Fig. 1a). Both S1 and M1 axon terminals labeled with synaptophysin-mRuby were similarly distributed in the PAG and RVM (Fig. 1b, c, Supplementary Fig. 1a–c). To determine the location of cortical neurons projecting to the PAG and RVM, we simultaneously injected retrograde AAVs carrying GFP (AAV2/retro-GFP) and tdTomato (AAV2/retro-tdTomato) into the PAG and RVM (Fig. 1d, Supplementary Fig. 1d). PAG-projecting (green) and RVM-projecting (red) cortical neurons were identified in S1, M1, and M2; they were especially prominent in S1 and M1 (Fig. 1e, f). GFP-labeled PAG-projecting SM1 (SM1PAG) neurons were predominantly distributed in the posterior (caudal) part of S1 and M1 (SM1), whereas tdTomato-labeled RVM-projecting SM1 (SM1RVM) neurons were mostly observed in SM1 anterior to bregma (Fig. 1e, g, h). These two subgroups of neurons primarily resided within layers 5 and 6 of SM1; the SM1RVM neurons were located deeper than the SM1PAG neurons (Fig. 1i, Supplementary Fig. 1e). Surprisingly, very few GFP or tdTomato double-labeled neurons were detected ( < 3%), implying that SM1PAG and SM1RVM neurons were nonoverlapping subpopulations in SM1 (Fig. 1h, j). The distribution pattern of these two neuronal subpopulations in SM1 was further confirmed by cholera toxin subunit B (CTB) 488 and CTB 546 retrograde tracing (Supplementary Fig. 1f–h). Moreover, RNAscope fluorescence in situ hybridization (FISH) revealed that > 95% of SM1PAG and SM1RVM neurons were vGlut1+ neurons (Fig. 1k, l). Overall, these data indicate that SM1PAG and SM1RVM neurons are anatomically separable glutamatergic subpopulations in the deep layers of SM1.
a Sagittal schematic showing the strategy to label cortical projection terminals. b and c Brain sections of virus injection site and projection terminals in PAG and RVM of S1 (b) and M1 (c). Scale bars, 200 μm. Both experiments were independently repeated three times from three mice with similar results. d Schematic showing the dual retro-virus labeling strategy. e Localization of retro-virus labeled neurons in sensorimotor cortex. Green and Red dots represent labeled SM1PAG and SM1RVM neurons. f Relative proportion of labeled cells within S1, M1, and M2. g Cell density of SM1PAG and SM1RVM throughout the AP extent of SM1. h SM1 sections showing SM1 subpopulations localization. Scale bars, 100 μm. This experiment was independently repeated four times from four mice with similar results. i Sections showing the distribution (left) and relative proportion (right) of labeled cells in layer structure of SM1. Scale bars, 100 μm. j Schematic showing the proportion of labeled PAG- and RVM-projecting neurons in SM1. k and l RNAscope FISH showing colocalization and proportion of vGlut1 or vGat mRNA with SM1PAG (k) and SM1RVM (l) in the SM1. Scale bars, 100 μm. All data are expressed as mean ± sem. Source data are provided as a Source Data file.
Distinct axon collateral-targeting and electrophysiological characteristics of SM1PAG and SM1RVM neuronal subpopulations
Although the SM1PAG and SM1RVM subgroups of neurons are distinguished based on their projections to the PAG and RVM, respectively, they may also project to other brain regions through axon collaterals. To label the axon collaterals of SM1PAG and SM1RVM neurons, we unilaterally injected retrograde AAVs carrying Cre (AAV2/retro-Cre) into the PAG or RVM, and Cre-dependent AAVs expressing Synaptophysin-mRuby (AAV-DIO-Synaptophysin-mRuby) into ipsilateral SM1, ensuring mRuby labeling of axon terminals of SM1PAG and SM1RVM neurons throughout the brain (Supplementary Figs. 2 and 3). Quantitative analysis of mRuby labeling showed that SM1PAG and SM1RVM neurons had axon collaterals projecting to many regions of the forebrain, midbrain, and hindbrain (several of which are implicated in motor and sensory modulation) including the striatum, thalamus, hypothalamus, and medulla (Supplementary Figs. 2 and 3). Although the axon collaterals of the two subgroups of neurons targeted similar brain regions, their projection density varied. For example, the axon collaterals of SM1PAG neurons showed dense projections to the ipsilateral posterior thalamic nucleus, zona incerta, and the superior colliculus, whereas SM1RVM neurons had relatively few projections to these brain regions (Supplementary Fig. 3d). Intriguingly, we also observed that both SM1PAG and SM1RVM neurons had axon collaterals projecting to the spinal cord (Supplementary Figs. 2 and 3). The extensive collateral projections of these two subgroups of SM1 neurons imply that they have complex roles in sensory and motor modulation.
We also compared electrophysiological characteristics, including intrinsic excitability and membrane properties, between the SM1PAG and SM1RVM neuronal subpopulations via whole cell patch-clamp recording in SM1 slices. We selectively labeled SM1PAG and SM1RVM neurons by retrograde injection of AAV2/retro-GFP and AAV2/retro-tdTomato into the PAG and RVM, respectively (Supplementary Fig. 4a). Action potentials (APs) were evoked by a superimposed positive current. Compared with SM1RVM neurons, SM1PAG neurons had fewer AP firings and a higher rheobase (Supplementary Fig. 4b–d). Analysis of single APs evoked by depolarization current stimulation showed that SM1RVM neurons had a more hyperpolarized AP threshold, narrower half-width, and slightly higher AP amplitude (Supplementary Fig. 4e–h). No differences in AP latency, resting membrane potential, input resistance, time constant, or after-hyperpolarization potential were identified between the SM1PAG and SM1RVM subgroups (Supplementary Fig. 4i–m). Correlations between the electrophysiological characteristics of SM1PAG and SM1RVM neurons are presented in Supplementary Fig. 4n. These results suggest that the intrinsic excitability of SM1RVM neurons is higher than that of SM1PAG neurons at the single-neuron level; these two subpopulations of SM1 neurons may be distinguishable on the basis of their electrophysiological characteristics.
Activation of SM1PAG and SM1RVM neuronal subpopulations produces similar analgesic effects in mice with neuropathic pain
Considering that the PAG and RVM are key nuclei within the descending pain inhibitory system21,27, we investigated the roles of the two subgroups of SM1 neurons in pain processing. We injected AAV2/retro-Cre-EGFP into the PAG or RVM, along with AAVs carrying the Cre-dependent Gq-coupled hM3D receptor (AAV-DIO-hM3Dq-mCherry) or Gi-coupled hM4D receptor (AAV-DIO-hM4Di-mCherry) into SM1. Intraperitoneal (i.p.) injection of clozapine-N-oxide (CNO, 3 or 5 mg/kg) was performed specifically to activate and inhibit hM3Dq- and hM4Di-expressing SM1PAG and SM1RVM neurons, respectively. The von Frey threshold and Hargreaves latency were tested before and after CNO injection under normal physiological conditions and during neuropathic pain (Fig. 2a). Immunofluorescence staining revealed specific expression of mCherry in GFP+, SM1PAG, and SM1RVM neurons (Supplementary Fig. 5a, b). The efficiency of hM3Dq-mediated activation and hM4Di-mediated inhibition was confirmed by c-Fos immunofluorescence staining or whole-cell patch clamp recording (Fig. 2b, Supplementary Fig. 5c). Chemogenetic inhibition of SM1PAG or SM1RVM neurons did not affect the mechanical or thermal threshold under normal physiological conditions or during neuropathic pain (Supplementary Fig. 5d, e). Notably, chemogenetic activation of SM1PAG or SM1RVM neurons effectively alleviated the mechanical allodynia and thermal hyperalgesia induced by chronic constriction injury (CCI) of the sciatic nerve, without sex difference (Fig. 2c–f, Supplementary Fig. 5f, g). However, it did not affect the mechanical or thermal thresholds under normal physiological conditions (Fig. 2g, h), suggesting that the two neuronal subpopulations are important for controlling nociceptive hypersensitivity under pathological conditions but not under physiological conditions. Moreover, using real-time conditional place preference (RT-CPP) test, an operant measurement of spontaneous or ongoing pain that does not rely on reflex behaviors28,29, we observed that optogenetic activation of SM1PAG or SM1RVM neurons resulted in an obvious CPP, marked by the increased time spent in the blue light-paired chamber (Supplementary Fig. 5h–k).
a Schematic of the experimental design. b Immunofluorescence stain of cFos (green) and hM3Dq+ neurons (red) after Saline and CNO injection. Right: percentage of hM3Dq+ neurons and cFos co-localization (P = 1.13 × 10-7). Scale bars, 200 μm. c and d Schematic showing the strategy to label SM1PAG (c) and SM1RVM (d) neurons with hM3Dq receptors or mCherry. e and f Effects of activating SM1PAG (e von Frey, Groups, P = 0.0002, Hargreaves, Groups, P = 0.0234) or SM1RVM (f von Frey, Groups, P = 0.0003, Hargreaves, Groups, P = 0.0248) neurons on von Frey threshold (left) and Hargreaves latency (right) in CCI mice. g and h Effects of activating SM1PAG (g) or SM1RVM (h) neurons on von Frey threshold (left) and Hargreaves latency (right) in naive mice. i Schematic showing the strategy to activate both SM1PAG and SM1RVM neurons. j Effects of co-activating SM1PAG and SM1RVM neurons on von Frey threshold (left, Groups, P = 3.2 × 10-6) and Hargreaves latency (right, Groups, P = 0.0019) in CCI mice. k Co-activating of SM1PAG and SM1RVM neurons increased von Frey threshold (vFT, left, P = 0.0001) and Hargreaves latency (HL, right, P = 0.0297). l Schematic showing the viral strategy for labeling the SM1non-PAG/RVM neurons with DO-hM3Dq virus. Scale bar, 200 μm. m–p Chemogenetic activation of SM1non-PAG/RVM neurons raised the responses to dynamic (m P = 0.0312) and punctate (n Groups, P < 1 × 10−15) mechanical stimuli, and induced mechanical allodynia (o) and thermal hyperalgesia (p). All data are expressed as mean ± sem, and (b) were analyzed by two-tailed unpaired t test, (e, g, f, h, j, n) were analyzed by two-way RM ANOVA, (k) were analyzed by Brown-Forsythe ANOVA test, (m, o, p) were analyzed by two-tailed paired t test. Sample sizes are indicated in the figures, with detailed statistics provided in Supplementary Data 1. *P ≤ 0.05, **P ≤ 0.01. BL, baseline. Source data are provided as a Source Data file.
To determine whether these two neuronal subpopulations have synergistic or additive analgesic effects in CCI mice, we injected AAV2/retro-Cre into both the PAG and RVM, along with AAV-DIO-hM3Dq into SM1 (Fig. 2i). Simultaneous activation of SM1PAG and SM1RVM neurons produced a stronger inhibitory effect on mechanical allodynia and thermal hyperalgesia in CCI mice (Fig. 2j, k), suggesting that SM1PAG and SM1RVM neurons exert equivalent and additive anti-nociceptive effects on neuropathic pain. Additionally, we examined the effect of activating SM1 neurons that did not project to the PAG or RVM on noxious and innocuous cutaneous stimuli. A Cre-off hM3Dq (AAV-DO-hM3Dq-eGFP) virus was injected into SM1, followed by the injection of retro-Cre virus into the PAG and RVM to activate all SM1 neurons except SM1PAG and SM1RVM neurons (Fig. 2l, Supplementary Fig. 5l). Interestingly, chemogenetic activation of non-SM1PAG and non-SM1RVM neurons led to sensory hypersensitivity and spontaneous pain-like behaviors in the hindpaw but not forepaw or whisker pad of naïve mice, confirming the attribution of SM1 hindlimb region (Fig. 2m–p, Supplementary Fig. 5m, and Supplementary Movie 1).
Activation of SM1–PAG and SM1–RVM pathways ameliorates neuropathic pain
Considering the axonal collaterals of SM1PAG and SM1RVM neurons may also project to other brain regions, we next carried out circuit-specific manipulation experiments. First, we used fiber photometry to measure changes in Ca2+ signals in PAG and RVM neurons expressing the genetically encoded Ca2+ marker GCaMP6f during photoactivation of SM1 excitatory pyramidal neurons. For this purpose, we injected CaMK2a-activating AAVs carrying channelrhodopsin-2 (ChR2) into SM1, and AAV-hsyn-GCaMP6f into the PAG and RVM (Fig. 3a, Supplementary Fig. 6a). Optogenetic stimulation of SM1 excitatory neurons increased calcium signaling in both the PAG and RVM (Fig. 3b–e), indicating that PAG and RVM neurons can be activated by SM1–PAG and SM1–RVM excitatory projections. Next, we injected retro-Cre virus into the PAG or RVM, along with AAVs carrying Cre-dependent ChR2 or halorhodopsin (eNpHR) into SM1, then placed optical fibers into the PAG or RVM to selectively manipulate the axonal terminals of SM1PAG and SM1RVM neurons (Fig. 3f; Supplementary Fig. 6b, c). Optogenetic activation (473 nm, 20 Hz, 5 mW), but not inhibition (580 nm, 5 mW, continuous), of SM1 terminals in the PAG or RVM significantly reduced CCI-induced mechanical allodynia and thermal hyperalgesia (Fig. 3g, h; Supplementary Fig. 6d, e). Consistent with the effect of activating SM1PAG and SM1RVM neurons, activation of SM1 terminals in the PAG or RVM also produced similar CPP in CCI mice (Supplementary Fig. 6f–i). To strengthen the evidence for the specific role of SM1-PAG and SM1-RVM pathways, we injected retro-Cre virus into the PAG or RVM, AAV-DIO-hM3Dq-mCherry virus into SM1, and implanted cannulas into the PAG or RVM to allow local infusion of CNO at the axonal terminals of SM1PAG and SM1RVM neurons (Fig. 3i). Circuit-specific chemogenetic activation of SM1 terminals by local infusion of CNO into PAG or RVM replicated the anti-nociceptive effects induced by the optogenetic activation of SM1-PAG or SM1-RVM pathways (Fig. 3j, k).
a Schematic of fiber photometry recording. b Heatmap (left) and mean fluorescence trace (right) showing increased PAG neuronal activity. Blue column indicates laser stimulation period. c Peak of PAG Ca2+ fluorescence, P = 0.0152. d Heatmap (left) and mean fluorescence trace (right) showing increased RVM neuronal activity. Blue column indicates laser stimulation period. e Peak of RVM Ca2+ fluorescence, P = 0.0252. f Schematic showing optogenetic activating strategy. g Representative image showing the fiber placement within PAG (left), and optogenetic activating SM1PAG-PAG alleviated mechanical allodynia (middle, P = 0.0045) and thermal hyperalgesia (right, P = 2.08 × 10−5) in CCI mice. Scale bar, 200 μm. h Representative image showing the fiber placement within RVM (left), and optogenetic activating SM1RVM-RVM alleviated mechanical allodynia (middle, P = 0.0313) and thermal hyperalgesia (right, P = 0.0054) in CCI mice. Scale bar, 200 μm. i Schematic showing chemogenetic activating strategy of SM1PAG or SM1RVM terminals. j Representative image showing the cannula placement in the PAG (left), and local infusion of CNO alleviated mechanical allodynia (middle, P = 0.0017) and thermal hyperalgesia (right, P = 0.0004) in CCI mice. Scale bar, 200 μm. k Representative image showing the cannula placement in the RVM (left), and local infusion of CNO alleviated mechanical allodynia (middle, P = 0.0079) and thermal hyperalgesia (right, P = 3.49 × 10−5) in CCI mice. Scale bar, 200 μm. l Schematic showing the strategy to activate post-synaptic neurons in PAG or RVM. m Representative image showing the labeled PAGSM1 neurons (left), and chemogenetic activating PAGSM1 neurons alleviated mechanical allodynia (middle, P = 0.0074) and thermal hyperalgesia (right, P = 0.0418) in CCI mice. Scale bar, 200 μm. n Representative image showing the labeled RVMSM1 neurons (left), and chemogenetic activating RVMSM1 neurons alleviated mechanical allodynia (middle, P = 0.0065) and thermal hyperalgesia (right, P = 0.0493) in CCI mice. Scale bar, 200 μm. All data are expressed as mean ± sem, and (c, e, g, h) were analyzed by two-tailed paired t test. (j, k, m, n) were analyzed by two-tailed unpaired t-test or Mann Whitney test. Sample sizes are indicated in the figures, with detailed statistics provided in Supplementary Data 1. *P ≤ 0.05, **P ≤ 0.01. Source data are provided as a Source Data file.
To further confirm the effects of SM1–PAG and SM1–RVM projections, we injected anterograde trans-synaptic AAV2/1-hsyn-Cre into SM1, and AAV-DIO-hM3Dq-mCherry (or AAV-DIO-hM4Di-mCherry) into the PAG or RVM, with the goal of specifically transducing postsynaptic PAG or RVM neurons (Fig. 3l, Supplementary Fig. 6j). Chemogenetic activation of postsynaptic PAG or RVM neurons effectively alleviated CCI-induced mechanical allodynia and thermal hyperalgesia, whereas inhibition of these neurons had no effect (Fig. 3m, n, Supplementary Fig. 6k, l). These results indicate that the SM1–PAG and SM1–RVM pathways mediate the inhibition of CCI-induced mechanical allodynia and thermal hyperalgesia via SM1PAG and SM1RVM neurons, respectively.
As mentioned above, both SM1PAG and SM1RVM neurons project to the spinal cord through axonal collaterals. A previous study has been demonstrated that somatosensory cortical neurons directly project to spinal cord via the corticospinal tract. To address whether there were differences between the spinal cord projecting cortical neurons identified by Liu et al.4 and PAG or RVM projecting SM1 neurons, we simultaneously injected retrograde AAV2/retro-mCherry into spinal cord and AAV2/retro-GFP into the PAG or RVM (Supplementary Fig. 7a, b). All the corticospinal projecting neurons (red) and SM1PAG or SM1RVM neurons (green) were identified in SM1 (Supplementary Fig. 7c–f. Despite that SM1PAG and SM1RVM neurons had axonal collaterals to the spinal cord, only few GFP and mCherry double-labeled neurons ( ~ 6% of SM1PAG and 12% of SM1RVM neurons) were detected, implying that cortico-midbrain/medulla and corticospinal neurons were nonoverlapping subpopulations in SM1 (Supplementary Fig. 7c–f). Different from previous report that ablation of somatosensory corticospinal neurons impaired behavioral responsiveness to light touch, and attenuated tactile allodynia in neuropathic pain mice4, chemogenetic inhibition of SM1PAG and SM1RVM spinal collaterals by intrathecal administration of CNO did not affect the responses to tactile (von Frey and brush) and noxious heat stimuli in naïve mice (Supplementary Fig. 7g–p). Also, either inhibition or activation of those spinal collaterals of SM1PAG and SM1RVM neurons did not affect CCI-induced mechanical allodynia (Supplementary Fig. 7q–t), suggesting that SM1PAG and SM1RVM-driven anti-nociception was not mediated by collaterals projections to the spinal cord.
Postsynaptic PAGvGlut2 and RVMPenk neurons of SM1 contribute to SM1PAG- and SM1RVM-mediated anti-nociception, respectively
RNAscope FISH showed that >65% of SM1 postsynaptic PAG neurons were vGlut2+ glutamatergic neurons (Fig. 4a–d); this result was confirmed using Cre-dependent retrograde trans-monosynaptic tracing rabies virus in vGlut2-Cre mice (Supplementary Fig. 8a, b). Additionally, we performed voltage-clamp recording in vGlut2+ PAG (PAGvGlut2) neurons, revealing that optogenetic activation of SM1 CaMK2a+ presynaptic terminals in the PAG evoked optoactivated excitatory postsynaptic currents (oEPSCs) in 5/12 of PAGvGlut2 neurons. The oEPSCs were completely abolished by the voltage-gated Na+ channel blocker tetrodotoxin (TTX, 1 μM) and rescued by the voltage-gated K+ channel blocker 4-aminopyridine (4-AP, 100 μM) in the presence of TTX (Fig. 4e, f), indicating that the oEPSCs were elicited by direct synaptic connections between SM1 CaMK2a+ excitatory neurons and PAGvGlut2 neurons. To determine the effects of SM1–PAGvGlut2 pathway activation on CCI-induced allodynia and hyperalgesia, we implemented a double-recombinase strategy that involved retrograde injection of AAV2/retro-Flpo and AAV-DIO-eNpHR3.0 into the PAG, and Flpo-dependent AAV-fDIO-hM3Dq-mCherry into SM1, in vGlut2-Cre mice (Fig. 4g, Supplementary Fig. 8c, d). As shown in Fig. 4h, i, the anti-nociceptive effects induced by chemogenetic activating SM1PAG were significantly abolished by yellow light inhibition of PAGvGlut2 neurons in CCI mice. Optogenetic inhibition of PAGvGlut2 per se did not affect CCI-induced allodynia or hyperalgesia (Supplementary Fig. 8e). Furthermore, we injected an anterograde trans-synaptic AAV2/1-FLEX-Flpo virus into SM1, and AAV-fDIO-hM3Dq-mCherry into PAG, in vGlut2-Cre mice (Fig. 4j, Supplementary Fig. 8f) to selective activation of the postsynaptic PAGvGlut2 neurons, which effectively ameliorated CCI-induced mechanical allodynia and thermal hyperalgesia (Fig. 4k). However, chemogenetic activation of the postsynaptic PAGvGat neurons has no impact on CCI-induced allodynia and hyperalgesia (Supplementary Fig. 8g, h). These findings imply that postsynaptic PAGvGlut2 neurons may play an important role in mediating anti-nociceptive effect of SM1PAG neurons.
a Schematic of labeling strategy (left), and section of labeled PAGSM1 neurons (right). Scale bars, 200 μm. b–d RNAscope FISH showing colocalization (b) and proportion of vGlut2 (c) or vGat (d) mRNA with PAGSM1 neurons. Scale bars, 50 μm. e Schematic of recording configuration in vGlut2-cre mice. f Sample traces showing the SM1 terminals activating evoked oEPSC in PAGvGlut2 neurons, Blue arrow indicates optogenetic stimulation. g Schematic of SM1PAG activation combined with PAGvGlut2 inhibition. h and i Effects of inhibiting PAGvGlut2 on von Frey threshold (h P = 7.92 × 10−5) and Hargreaves latency (i P = 9.84 × 10−5). j Schematic of activating postsynaptic PAGvGlut2 neurons (left), and section of labeled PAGvGlut2 neurons (right). Scale bars, 200 μm. k Activating of postsynaptic PAGvGlut2 neurons increased the mechanical threshold (left, P = 0.0022) and thermal threshold (right, P = 0.0062) in CCI mice. l Schematic of labeling strategy (left), and section of labeled RVMSM1 neurons (right). Scale bars, 200 μm. m and n Immunofluorescence staining and RNAscope FISH showing colocalization (m) and proportion (n) of TPH, vGat and Penk mRNA with RVMSM1 neurons. Scale bars, 50 μm. o Proportion of Penk mRNA within vGat+ RVMSM1 neurons. p Schematic of recording configuration in Penk-cre mice. q Sample traces showing the SM1 terminals activating evoked oEPSC in RVMPenk neurons, Blue arrow indicates optogenetic stimulation. r Schematic of SM1RVM activation combined with RVMPenk inhibition. s and t Effects of inhibiting RVMPenk neurons on von Frey threshold (s P = 0.0001) and Hargreaves latency (t P = 0.0003). u Schematic of activating postsynaptic RVMPenk neurons. v Activating of postsynaptic RVMPenk neurons increased the mechanical threshold (left, P = 0.0195) and thermal threshold (right, P = 0.0003) in CCI mice. All data are expressed as mean ± sem, and (h, i, s, t) were analyzed by Brown-Forsythe ANOVA test or Kruskal-Wallis test followed by Dunn or Dunnett’s multiple comparisons test, (k and v) were analyzed by two-tailed unpaired t test or Mann Whitney test. Sample sizes are indicated in the figures, with detailed statistics provided in Supplementary Data 1. *P ≤ 0.05, **P ≤ 0.01. Source data are provided as a Source Data file.
We also investigated the role of SM1 postsynaptic RVM neurons using a strategy similar to the approach described above (Fig. 4l). Immunohistochemistry showed that a few SM1 postsynaptic RVM neurons were tryptophan hydroxylase (TPH)-immunoreactive (a 5-TH neuronal marker) neurons (Fig. 4m, n). RNAscope FISH revealed that approximately 76% of postsynaptic RVM neurons were vGat+, suggesting that GABAergic neurons are the dominant SM1 postsynaptic neurons in the RVM (Fig. 4l–n). More than 80% of these postsynaptic GABAergic neurons were Penk+ neurons (Penk gene products encode proenkephalins) (Fig. 4m–o). Enkephalinergic/GABAergic RVM neurons reportedly project to the spinal dorsal horn and participate in pain modulation30. Therefore, we investigated the roles of RVMPenk and RVMvGat neurons in SM1RVM-driven anti-nociception. The direct synaptic connections between SM1RVM and RVMPenk neurons were confirmed by electrophysiological recordings and Cre-dependent retrograde trans-monosynaptic tracing using rabies virus (Fig. 4p, q, Supplementary Fig. 8i, j). Optogenetic inhibition of RVMPenk neurons completely eliminated the anti-nociceptive effects induced by chemogenetic activation of SM1RVM neurons in CCI mice (Fig. 4r–t, Supplementary Fig. 8k, l). Selective chemogenetic activation of the postsynaptic RVMPenk neurons significantly reversed CCI-induced mechanical allodynia and thermal hyperalgesia (Fig. 4u, v, Supplementary Fig. 8m). Consistently, photoinhibition of RVMvGat neurons also abolished the anti-nociceptive effects of SM1RVM neurons (Supplementary Fig. 8n). Collectively, the above data suggest that the postsynaptic PAGvGlut2 and RVMPenk neurons may be involved in the anti-nociceptive effects driven by the SM1–PAG and SM1–RVM pathways, respectively.
Spinal monoamine and opioid signaling are involved in the descending pain modulation associated with SM1PAG and SM1RVM neurons, respectively
It is clear that ventral PAG output neurons project to the RVM and locus coeruleus (LC), and then to the spinal dorsal horn, to modulate nociceptive transmission22,31,32. Thus, we mapped descending projections of PAG neurons that receive SM1 inputs through virus tracing. Consistent with previous reports31, SM1 postsynaptic PAG neurons (PAGSM1) had direct projections to the RVM and LC, but not to the spinal cord (Fig. 5a–d, Supplementary Fig. 9a–d). Next, we injected trans-synaptic AAV2/1-Cre into SM1, and Cre-dependent synaptophysin-mRuby (AAV-DIO-synaptophysin-mRuby) into the PAG, to trace the SM1–PAG–LC/RVM pathways. mRuby+ synaptic boutons of PAGSM1 neurons are found on the soma and dendrites of LC noradrenergic (NA) neurons and RVM5-HT neurons (Fig. 5e–g, Supplementary Movie 2). Chemogenetic activation of PAGSM1 neurons significantly increased c-Fos expression in LCNA neurons and RVM5-HT neurons (Fig. 5h–j), suggesting that the anti-nociceptive effects driven by PAGSM1 neurons may be mediated by RVM5-HT and LCNA neurons. RVMSM1 neurons directly projected to the spinal dorsal horn (Supplementary Fig. 9e). Previous studies found that multiple subtypes of 5-HT receptors participated in pain descending modulation, such as 5-HT7 receptors33,34 and 5-HT3 receptors35,36. Thus, we separately blocked the 5-HT7, 5-HT3, and α2A-adrenergic receptors to examine the role of 5-HT and NA descending system in SM1PAG and SM1RVM neurons-driven anti-nociceptive effects. Intrathecal (i.t.) injection of 5-HT7 receptor antagonist SB-269970 (10 μg), 5-HT3 receptor antagonist ondansetron (10 μg), and the α2A-adrenergic receptor antagonist yohimbine (5 μg) significantly reversed the anti-nociceptive effects induced by activating SM1PAG rather than SM1RVM neurons in CCI mice (Fig. 5k–m, Supplementary Fig. 9g, h). This result supports the notion that SM1–PAG pathway-driven anti-nociception is mediated by LCNA and RVM5-HT neurons, as mentioned above. Additionally, we examined the roles of the opioid system in SM1PAG- and SM1RVM-driven anti-nociception. I.t. injection of the non-selective opioid receptor antagonist naloxone (10 μg) or the mu-opioid receptor antagonist D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP, 5 μg) effectively prevented SM1RVM- but not SM1PAG-mediated anti-nociceptive effects (Fig. 5k–m, Supplementary Fig. 9g, h), further supporting the involvement of SM1 postsynaptic RVMPenk neurons in the anti-nociceptive effects of SM1RVM neurons. Taken together, these findings imply that the anti-nociceptive effects driven by SM1PAG and SM1RVM neurons are achieved through separate molecular, cellular, and circuit mechanisms.
a Schematic of labeling strategy. Scale bars, 200 μm. b and c Representative image showing the axon of PAGSM1 in LC (b) and RVM (c). Scale bars, 200 μm. This experiment was independently repeated 3 times from 3 mice with similar results. d Representative image showing no projection of PAGSM1 was found in the spinal cord. Scale bars, 200 μm. This experiment was independently repeated 3 times from 3 mice with similar results. e Schematic of labeling the presynaptic terminal of PAGSM1. Scale bars, 200 μm. f and g Representative image showing the synaptic boutons of PAGSM1 neurons colocalize with TH+ LC neurons (f pointed by white arrow) and TPH+ RVM neurons (g pointed by white arrow). Scale bars, Left: 100 μm, Right: 20 μm. This experiment was independently repeated 3 times from 3 mice with similar results. h Schematic of chemogenetic activation of PAGSM1 neurons. i and j Representative images of cFos immunohistochemistry in TH+ LC neurons (i P = 1.10 × 10-5) and TPH+ RVM neurons (j P = 2.97 × 10−6) after chemogenetic activation of PAGSM1 neurons. Right: percentage of neurons that colocalize with green cFos signal. Scale bars, 100 μm. k Schematic of SM1PAG or SM1RVM neurons activation combined with intrathecal administration of drugs. l Effects of activating SM1PAG neurons on CCI-induced mechanical allodynia (left, P = 7.61 × 10-5) and thermal hyperalgesia (right, P = 0.0002) after intrathecal administration of SB269970 (5-HT7 receptor antagonist), ondansetron (5-HT3 receptor antagonist), yohimbine (α2A-adrenergic receptor antagonist), naloxone (opioid receptor antagonist), and CTAP (μ opioid receptor antagonist). m Effects of activating SM1RVM neurons on CCI-induced mechanical allodynia (left, P = 4.72 × 10-7) and thermal hyperalgesia (right, P = 1.24 × 10-5) after intrathecal administration of SB269970, ondansetron, yohimbine, naloxone and CTAP. All data are expressed as mean ± sem, and (i and j) were analyzed by two-tailed unpaired t test, (l and m) were analyzed by one-way RM ANOVA test or Friedman test followed by Dunn or Dunnett’s multiple comparisons test. Sample sizes are indicated in the figures, with detailed statistics provided in Supplementary Data 1. *P ≤ 0.05, **P ≤ 0.01. Source data are provided as a Source Data file.
SM1PAG and SM1RVM neurons are involved in motor coordination and skilled motor behaviors under normal physiological conditions
As mentioned above, SM1PAG and SM1RVM neurons modulate the allodynia and hyperalgesia associated with neuropathic pain but do not participate in the regulation of physiological nociception. We examined the responses of these two subgroups of SM1 neurons to sensory and motor stimuli under physiological conditions at the single-neuron level. We injected AAV2/retro-Cre virus into the PAG or RVM, along with Cre-dependent GCaMP6m into SM1 (Fig. 6a, Supplementary Fig. 10a). Calcium signals of the SM1 neuronal subpopulations were recorded in real time via in vivo miniaturized two-photon (2 P) microscopy during innocuous and noxious mechanical stimulation and motor processing (Fig. 6b–d). In total, 56 SM1PAG and 59 SM1RVM neurons were recorded. A few neurons (SM1PAG, 19/56; SM1RVM, 8/59) in freely moving mice responded to at least one of the sensory stimuli, such as an innocuous brush, mechanical force (using a 0.6 g von Frey filament), or a noxious pinprick (Fig. 6e–g, Supplementary Fig. 10b, c). Intriguingly, when mice were forced to run at a constant speed on a rotarod, the calcium event rates of most neurons (SM1PAG, 46/56; SM1RVM, 39/59) significantly increased (Fig. 6h–l; Supplementary Movie 3). In particular, an increase in calcium signaling was observed in 22 SM1PAG and 18 SM1RVM neurons at the onset of forced running (Fig. 6m–o, Supplementary Fig. 10b–e).
a Schematic showing the viral strategy for labeling the SM1PAG or SM1RVM neurons with GCaMP6m. b and c Schematic showing the two-photon recording setup in free moving mice (b), and the specific localization of recording windows within SM1 (c). d Example image of recorded SM1 neurons (left), and corresponding representative calcium traces from 10 neurons of interest (right, color-matched to the left). Scale bars, 100 μm. This experiment was independently repeated 6 times from 6 mice with similar results. e Schematic showing recording strategy for sensory stimuli. f Heatmaps (up) and averaged ΔF/F traces of example neuron (down) illustrating the three types of sensory stimuli response kinetics (brush, 0.6 g von Frey, pinprick) of SM1PAG neurons. g Heatmaps (up) and averaged ΔF/F traces of example neuron (down) illustrating the three types of sensory stimuli response kinetics (brush, 0.6 g von Frey, pinprick) of SM1RVM neurons. h Schematic showing recording strategy for forced running. i and j, Representative traces (i) and summarized data (j P = 1.87 × 10-8) showing that the Ca2+ transient of recorded SM1PAG neurons increased during forced running. k and l Representative traces (k) and summarized data (l P = 0.002) showing that the Ca2+ transient of recorded SM1RVM neurons increased during forced running. m Heatmaps (up) and example neuron traces (down) of averaged ΔF/F traces illustrating the response kinetics to forced running of SM1PAG (left) and SM1RVM (right) neurons. n and o Proportions of recorded SM1PAG (n) or SM1RVM (o) neurons that sensitive to forced running (motor ensembles), running onset, and sensory stimuli (sensory ensembles). All data are expressed as mean ± sem, and (j and l) were analyzed by two-tailed Wilcoxon matched-pairs signed rank test. Sample sizes are indicated in the figures, with detailed statistics provided in Supplementary Data 1. **P ≤ 0.01. Source data are provided as a Source Data file.
Next, we evaluated the roles of SM1PAG and SM1RVM neurons in motor and sensory modulation under normal physiological conditions. We injected AAV2/retro-Cre into either the PAG or the RVM, as well as AAV-DIO-hM4Di into SM1, to specifically inhibit these neurons (Fig. 7a–c). A series of motor behaviors and behavioral responses to tactile and noxious stimuli were tested (Supplementary Fig. 11a). Chemogenetic inhibition of SM1PAG or SM1RVM neurons did not alter the behavioral responses of mice to punctate mechanical force (von Frey filaments; Fig. 7d), innocuous dynamic tactile brushing (Fig. 7e), sticky tape on the plantar (Fig. 7f), or noxious thermal stimuli (Fig. 7g, h), suggesting that SM1PAG and SM1RVM neurons are not required to modulate the behavioral responses induced by tactile and noxious stimuli in naïve mice. Motor behavioral tests showed that silencing of both SM1PAG and SM1RVM neurons impaired general motor coordination in the accelerating rotarod test, as indicated by a shorter mean latency to fall from the rod (Fig. 7i). However, in the elevated beam test, SM1PAG but not SM1RVM silencing led to a higher number of slips (Fig. 7j, Supplementary Fig. 11b, Supplementary Movie 4), suggesting that SM1PAG neurons are important for fine motor control. We also tested the skilled motor behavior of mice using a horizontal ladder. The results showed that silencing of SM1PAG or SM1RVM neurons significantly decreased the likelihood of correct forelimb or hindlimb placement (Fig. 7k, l, Supplementary Fig. 11b, Supplementary Movie 5). However, in the open field test, silencing of either SM1PAG or SM1RVM neurons did not affect the total distance traveled by the mice, implying that these neurons are not involved in regulating mouse gross motor function (Fig. 7m). Additionally, silencing of SM1PAG or SM1RVM neurons did not affect the movement speed of the mice in the beam, ladder, or open field tests (Supplementary Fig. 11c). These results indicate that normal mouse SM1PAG and SM1RVM neurons might be important for fine motor control and coordination (Supplementary Fig. 11d).
a and b Schematic showing the viral strategy (left), and representative imaging of hM4Di-mCherry virus infection (right) of SM1PAG (a) or SM1RVM (b) neurons. Scale bars, 200 μm. c Representative traces (left) and summarized data (right) showing APs were suppressed by bath CNO in SM1 hM4Di+ neurons. d–h Effects of silencing SM1PAG or SM1RVM neurons on von Frey frequency (d Groups, P = 0.4468), brush (e SM1PAG, P = 0.8236. SM1RVM, P = 0.8309), tape removal (f SM1PAG, P = 0.7570. SM1RVM, P = 0.4098), Hargreaves (g SM1PAG, P = 0.3746. SM1RVM, P = 0.2782), and hotplate (h SM1PAG, P = 0.9372. SM1RVM, P = 0.2015) test. i–m Effects of silencing SM1PAG or SM1RVM neurons in rotarod (i SM1PAG, P = 0.0006. SM1RVM, P = 0.0423), beam (j SM1PAG, P = 0.0267. SM1RVM, P = 0.6174), ladder (k SM1PAG, P = 0.0565. SM1RVM, P = 0.0470, and l SM1PAG, P = 0.0418. SM1RVM, P = 0.1551), and open field (m SM1PAG, P = 0.3857. SM1RVM, P = 0.8275) test. All data are expressed as mean ± sem, and (d) were analyzed by two-way ANOVA test. (e–m) were analyzed by two-tailed unpaired t test or Mann Whitney test. Sample sizes are indicated in the figures, with detailed statistics provided in Supplementary Data 1. *P ≤ 0.05, **P ≤ 0.01. Source data are provided as a Source Data file.
SM1PAG and SM1RVM neurons are required for motor-induced analgesia
As mentioned above, both SM1PAG and SM1RVM neurons participate in motor control, and the activation of these two subgroups and their descending projections effectively alleviated CCI-induced allodynia and hyperalgesia. Both subgroups may be involved in regulating nociception and locomotion. Previous studies have shown that exercise and motor learning can decrease pain and restore motor function37,38,39. To determine whether the performance of certain motor movements reduces nociceptive responses through SM1PAG and SM1RVM neurons, we tested the von Frey threshold and Hargreaves latency after a 5 min rotarod task in naïve and CCI mice (Fig. 8a). CCI-induced mechanical allodynia and thermal hyperalgesia were significantly reduced at 10 min after the rotarod task, then gradually faded at 40 min (Fig. 8b, Supplementary Fig. 12a–c). The basal nociceptive response thresholds of naïve mice were unaffected at the same rotarod task (Fig. 8c). In order to rule out the tiredness effect of rotarod motor task, we performed open field test immediately after the motor task. The total distance and movement speed of the mice in open field were not affected by the rotarod task (Supplementary Fig. 12d, e). Next, we used an optogenetic approach to test whether SM1PAG or SM1RVM neurons participate in motor-induced anti-nociception. We injected AAV2/retro-Cre into the PAG or RVM, as well as AAV-DIO-eNpHR into SM1, and implanted optical fibers in SM1 to inhibit SM1PAG or SM1RVM neurons. As shown in Fig. 8d–g, motor task-induced analgesia was completely blocked by optogenetic inhibition of SM1PAG or SM1RVM neurons (Fig. 8d–g), suggesting the motor-induced analgesic effect may be mediated by increased excitatory activity of SM1PAG and SM1RVM neurons. Thus, we further examined the effects of the rotarod challenge on neuronal activity of SM1PAG and SM1RVM neurons by fiber photometry and in vivo two-photon calcium imaging. Intriguingly, the calcium signals in both SM1PAG and SM1RVM neurons increased immediately following rotarod task onset and returned to basal level after task completion (Supplementary Fig. 12f–k). Combined with above optogenetic inhibition results, these data indicate that SM1PAG and SM1RVM neurons are necessary for motor-induced analgesia but do not need to maintain continuous activity. We propose that the prolonged analgesic effect observed after motor task may result from SM1 population activity-induced postsynaptic events and neuroplasticity mechanisms. Together, these results imply that, during chronic pain, SM1PAG and SM1RVM neurons facilitate motor task execution by suppressing nociception; alternatively, they might alleviate nocifensive responses through effecting movement control.
a Schematic showing the experiment design for motor induced analgesia. b and c Effects of locomotion on von Frey threshold (left) and Hargreaves latency (right) in CCI (b von Frey, P = 0.0130, Hargreaves, P = 0.0162) or naïve (c von Frey, P = 0.8834, Hargreaves, P = 0.4848) mice. d Schematic showing the viral strategy for optogenetic blocking SM1PAG neurons. e Optogenetic inhibition of SM1PAG neurons blocked motor induced analgesia (P = 0.0196). f Schematic showing the viral strategy for optogenetic blocking SM1RVM neurons. g Optogenetic inhibition of SM1RVM neurons blocked motor induced analgesia (P = 0.0011). All data are expressed as mean ± sem, and (b and c) were analyzed by two-tailed unpaired t test or Mann Whitney test, (e and g) were analyzed by Brown-Forsythe ANOVA test followed by Dunnett’s multiple comparisons test. Sample sizes are indicated in the figures, with detailed statistics provided in Supplementary Data 1. *P ≤ 0.05, **P ≤ 0.01. Source data are provided as a Source Data file.
Discussion
SM1 controls multiple sensory and motor behaviors in both humans and mice, and it undergoes plastic changes during chronic pain. This region has become a key target for neuromodulation used to treat refractory chronic pain10,11,12,13. However, it is unclear how cortical regions drive analgesic effects at the molecular, cellular, or circuitry levels. In this study, we identified two SM1 subpopulations, SM1PAG and SM1RVM, where, SM1PAG included a group of M1PAG neurons identified by Gan, et al.3. Furthermore, we revealed two parallel descending circuits corresponding to the two SM1 subpopulations: SM1vGlut1−PAGvGlut2 and SM1vGlut1−RVMPenk, which induce equivalent and synergistic anti-nociceptive effects during chronic pain through 5-HT/NA and opioid signaling in the spinal cord, respectively (Supplementary Fig. 13).
Early retrograde and anterograde tracing experiments revealed that projections to PAG mainly arouse from media prefrontal cortex (mPFC), anterior cingulate cortex (ACC), orbitofrontal cortex (OFC) and insular cortex (IC) in rat40,41. Projections from caudal mPFC and ACC terminated predominantly in dlPAG, whereas projections from the rostroventral mPFC, OFC and IC innervated predominantly the vlPAG41. In a recent study, Gan and colleagues found direct projections from M1 to the PAG (lPAG/vlPAG), which mediate the analgesic effect of M1 stimulation3. The present study further observed that both M1 and S1 project to lPAG and vlPAG, and drove the anti-nociception in neuropathic pain mice. Although early studies have identified that RVM, especially raphe magnus, receives direct projections from cortical regions, including medial and lateral precentral cortices, prefrontal and insular cortices42,43, our findings reveal that SM1 descending projections innervate the RVM in a parallel pattern and synergize analgesia with the SM1-PAG pathway.
Studies from the Brain Initiative Cell Census Network showed that SM1 forms broad and complex projections throughout the brain and spinal cord18,44,45,46. Among these cortical descending projections, the roles of corticospinal neurons (CSNs) have been well studied. Evidence showed that CSNs, the principle output neurons of sensorimotor cortex, directly descend to the spinal cord and formed synaptic connections with diverse spinal interneurons involved in patterning motor output and shaping sensory feedback19,47,48. In particular, a pioneer work by He’s laboratory found that a subset of CSNs originating in the sensory cortex contribute to both innocuous tactile processing in normal conditions and the generation of mechanical allodynia in pathological states4. Intriguingly, the two subgroups of SM1 neurons identified in our study did not participate in regulating tactile sensitivity in normal mice, although they also have collaterals to the spinal cord. Chemogenetic inhibition or activation of spinal collaterals of SM1PAG and SM1RVM neurons did not affect CCI-induced mechanical allodynia. These behavioral results, combined with our retrograde tracing data, indicate that SM1PAG or SM1RVM neurons and CSNs are functional and anatomical distinct subpopulations.
In the current study, SM1PAG and SM1RVM neurons were located throughout layers 5–6 of M1 and S1; activation of either of these SM1 neuronal subpopulations produced a strong analgesic effect in CCI mice. Differently, Gan et al. found that activation of M1-PAG or M1-zona incerta pathway led to robust analgesic effect, whereas activating S1 cannot produce analgesia similar to those associated with M1 activation. The distinct effects of S1 activation between the two studies highlight the functional complexity of SM1, especially regarding the S1 region. Indeed, S1 has roles in sensory information processing, pain chronicity49,50,51,52,53, and the regulation of motor behavior7. The activities of layer 2, 3, and 5 excitatory neurons in S1 reportedly increase during chronic pain50,51,52. However, activation of layer 5 neurons in S1 induced allodynia in naïve mice14. These inconsistent reports suggest that SM1 comprises numerous cell subpopulations that mediate different, or even opposing, functions. In support of these findings, we demonstrated that activating SM1 neurons other than the SM1PAG and SM1RVM subpopulations via the Cre-off system elicited allodynia, hyperalgesia, and spontaneous pain-like behaviors in naïve mice in complete contrast to the effects of activating SM1PAG and SM1RVM neurons. Many brain regions may receive the projections from non-PAG/RVM projecting SM1 neurons, which may mediate behavioral hypersensitivity driven by these SM1 subgroups (non-PAG/RVM projecting). Multiple pieces of evidence showed that sensorimotor cortex forms direct synaptic connections with neurons that receive and gate sensory input at the spinal and brainstem level19,20,54,55; activation of those neurons or their target projections facilitates sensory inputs and increase sensory sensitivity4,54. Using single-cell transcriptomics, Lui et al. investigated how specific transcriptomic types of mouse PFC projection neurons related to axonal projections and encoding properties across multiple cognitive tasks. They found that different cell classes preferentially contributed to different aspects of task encoding, suggesting that each transcriptomic type makes quantitatively different contributions to behavior56. Thus, in future study, integration of transcriptome and specific neuronal subpopulations (e.g. SM1 neuronal subpopulations with different axon projection patterns) analysis with signal encoding in a dynamic behavioral context will be a great challenge.
Some human and animal studies have shown that the serotonin and endogenous opioid systems contribute to the analgesia induced by cortical stimulation24,26,57,58. However, it remains unclear how activity in these systems modulates chronic pain. The role of the RVM in descending control of pain is well-known. In particular, the RVM descending serotonergic system is involved in both the descending inhibition and facilitation of pain21,59,60. However, using trans-synaptic tracing, we showed that SM1 postsynaptic RVM neurons are largely GABAergic; only a few are TPH-positive 5-HT neurons. Considering the functional heterogeneity of RVM GABAergic neurons61,62,63, we identified the postsynaptic GABAergic neurons as Penk-positive GABAergic neurons. As a primary source of endogenous opioid peptides, Penk+ neurons in many brain regions, including the RVM, participate in antinociceptive effects30,64. In the present study, inhibition of RVMPenk neurons or i.t. pretreatment with opioid receptor antagonists effectively blocked the analgesic effects induced by activation of SM1RVM neurons. This finding implies that SM1 postsynaptic RVMPenk neurons release enkephalin into the spinal dorsal horn to mediate SM1RVM-driven anti-nociception. Additionally, we found that postsynaptic PAG neurons of SM1 are mainly vGlut2-positive excitatory neurons. The inhibition of PAGvGlut2 neurons blocked the analgesic effects induced by activation of SM1PAG neurons, supporting previous reports of anti-nociception in association with PAG glutamatergic neuron activity22,23. Excitatory projections from the PAG to the RVM give rise to serotonergic projections to the spinal dorsal horn21,22,27,65. In our study, viral tracing of postsynaptic PAG neurons of SM1 confirmed that there are projections from PAGSM1 to RVM5-HT and from PAGSM1 to LCNA. LC catecholaminergic neurons, the main source of spinal cord NA, have been implicated in descending pain modulation31,32,65. Consistent with this implication, we observed considerable c-Fos expression in LCNA and RVM5-HT neurons after PAGSM1 stimulation in the current study. Moreover, blockade of spinal α2-ARs or 5-HT3/7 receptors partially reversed the analgesic effects induced by SM1PAG activation, suggesting that LCNA and RVM5-HT neurons relay pain-related information from SM1PAG to the spinal cord via NA and serotonergic signaling, respectively. Taken together, our results provide insight concerning the cellular mechanisms and circuitry underlying SM1-mediated analgesia, where SM1PAG and SM1RVM neuronal subgroups exert their functions independently and synergistically through distinct descending pathways and neurotransmitters.
Notably, our in vivo 2 P imaging data showed that normal physiological conditions, both SM1PAG and SM1RVM neurons are sensitive to rotarod motor behaviors, but have a lower proportion of response to cutaneous sensory stimulation. Because running mice have more foot plants with more pressure, we cannot exclude the possibility that the increase in calcium events during forced running may be due to the increased plantar pressure. Considering the adaptive characteristics of tactile pressure receptors, the sustained increase in calcium signals in SM1 neurons during running in mice can still be attributed to motor task. In addition, although we stimulate the mouse hindpaw while recording the calcium signals in the hindlimb region of the contralateral SM1, von Frey and pinprick stimulation only covered a very small hindlimb receptive field, and the recording window of calcium imaging could only record a limited number of cells. These may explain why the proportion of SM1 neurons responding to sensory stimuli was lower than their response to forced running. It is worth mentioning that chemogenetic silencing of either of these two SM1 neuronal subpopulations impaired motor behaviors, but not touch or nociceptive behavioral responses, in naïve mice. Several studies indicate that sensorimotor cortical projections targeting spinal cord and cuneate nucleus neurons receiving peripheral sensory inputs are crucial for gating tactile signal and controlling fine motor behaviors19,20,54. Impairments of these projections led to poor motor performance and abnormal tactile sensation. However, either SM1PAG or SM1RVM neurons and their spinal collaterals were not involved in regulating tactile sensitivity under normal conditions, therefore, these subpopulations are distinct from SM1 neurons previously reported to be involved in tactile sensation and fine motor control4,19,20,54. At present, we are unable to explain why silencing SM1PAG or SM1RVM neurons impaired motor performance but did not affect the responses to cutaneous sensory stimuli (innocuous and noxious). Relevant mechanisms remain future investigations.
The SM1PAG and SM1RVM neuronal subpopulations identified here simultaneously participate in the modulation of chronic pain and motor control. Their functional significance warrants investigation. As mentioned above, when mice were forced to run on a rotarod, most SM1PAG and SM1RVM neurons were robustly activated; together with the activation of their descending projections, this neuronal activation significantly alleviated CCI-induced allodynia and hyperalgesia. Furthermore, 5 min rotarod task effectively suppressed CCI-induced allodynia and hyperalgesia, which can be blocked by inhibition of these neuronal subpopulations. Thus, it is reasonable to speculate that activation of SM1PAG and SM1RVM neurons by performing motor behaviors may aid in the accomplishment of certain motor tasks (especially those vital for survival) by suppressing pain. The dual control of motor behaviors and nociceptive reflex exerted by SM1PAG and SM1RVM neurons may also contribute to regular exercise-induced analgesia. Physical activities and regular exercise have positive effects on a range of physical and mental health outcomes; these include chronic pain and anxiety66,67,68,69, the central mechanisms of which may involve the PAG and RVM37,39,70.
It should be emphasized that our experimental approach specifically focused on SM1PAG and SM1RVM neurons located in the hindlimb representation area of SM1, and sensory-related behavioral assessments were conducted in the hindlimb of mice. Given that SM1 neurons projecting to the RVM and PAG were distributed across widespread SM1 territories, including regions with receptive fields representing other body regions, we speculate that the analgesic effects of activating SM1PAG and SM1RVM neurons may be universal in different body regions. However, further in-depth investigation is required to validate this possibility.
In conclusion, we distinguished two SM1 neuronal subpopulations based on their projection targets in the brainstem. Activation of these two subpopulations, as well as their descending projections, independently and synergistically alleviated CCI-induced behavioral hypersensitivity. Our results imply that a sophisticated and redundant system of neural organization to ensure functional resilience is crucial for survival. From an evolutionary perspective, such system might provide safety benefits for animals by helping to accomplish vital motor behaviors and suppressing pain. Our study provides insight into the interactions between motor and sensory systems, and suggests that the approaches targeting SM1-brainstem pathways and serotonergic, NA, and opioidergic signaling may improve therapies for chronic pain.
Methods
Animals
Adult C57BL/6 J mice (8–16 weeks old; Shanghai Experimental Animal Center of Chinese Academy of Science, Shanghai, China) and transgenic Penk-IRES-Cre (JAX# Stock No. 025112; Jackson Laboratory, Bar Harbor, ME, USA), vGlut2-IRES-Cre (JAX# Stock No. 016963; Jackson Laboratory), and vGat-IRES-Cre (JAX# Stock No. 028862; Jackson Laboratory) mice were used in this study. Both male and female mice were used, and no sex difference were observed in this study. All mice were housed under a 12 h light/dark cycle at 22 ± 1 °C, with food and water provided ad libitum. We randomly assigned the mice to experimental and control groups; the tests were carried out by an investigator blinded to the mouse genotypes, treatments, and group assignments. All procedures were approved by the Animal Care and Use Committee of Fudan University (Permit No. SYXK 2009-0082) and conducted in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the International Association for the Study of Pain.
CCI surgery
CCI of the sciatic nerve was performed in accordance with a previously published protocol71. Briefly, mice were anesthetized with sodium pentobarbital (50 mg/kg, i.p.), and the left sciatic nerve was exposed at the mid-thigh level via blunt dissection of the biceps femoris. For CCI, two ligatures with polyglycolic-co-lactic acid sutures (5-0) were loosely tied around the nerve, proximal to its trifurcation and ~2–3 mm apart. For sham surgery, the sciatic nerve was isolated but not ligated. After CCI or sham surgery, the overlying muscles and skin were closed in layers.
Stereotaxic injection
Mice were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and fixed on a stereotaxic device (51725D; Stoelting, Woo Lane, IL, USA). The skull plane was adjusted to ensure that the bregma and lambda were oriented horizontally. A craniotomy hole was made using a dental drill, and 100–300 nL of virus (determined according to the purpose of the experiment and the viral titer) were injected into the target brain regions using a glass micropipette connected to a microinjection pump (Nanoliter 2010; World Precision Instruments Inc., Sarasota, FL, USA) at a rate of 30–50 nL/min. After injection, the micropipette remained in place for 10 min to avoid virus overflow. The stereotaxic coordinates of the target brain regions were as follows: S1: anteroposterior (AP) −0.50 mm, mediolateral (ML) ± 1.50 mm, dorsoventral (DV) −0.50 mm (from the brain surface); M1: AP −0.02 mm, ML ± 1.00 mm, DV −0.50 mm (from the brain surface); SM1: AP −0.25/0.90 mm, ML ± 1.00/1.50 mm, DV −0.50 mm (from the brain surface); PAG: AP −4.50 mm, ML ± 0.50 mm, DV −2.75 mm; and RVM: AP −5.80 mm, ML 0.00 mm, DV −5.90 mm. For the intra-spinal injections, we used a published protocol72. Briefly, mice were anesthetized, and lumbar spinal cord was exposed. We injected virus bilaterally into the spinal dorsal horn at 2 injection sites each side at a vertical depth of 300 μm from dorsal surface and at a horizontal distance of 400 μm from midline of spinal cord. Virus were infused at a rate of 40 nL/min by a glass micropipette. The micropipette was held in the injection site for additional 5 min after each viral injection for diffusion. After injections, the incision was closed, and the mice were allowed to recover from the anesthesia on a heating blanket before returning to their home cages. At the end of each experiment, brains or spinal cords were sectioned to confirm the injection site, and mice with off-target localization were excluded from the analysis.
Viral vectors
AAV2/retro-CAG-GFP (5.09 × 1012 vg/ml), AAV2/retro-CAG-tdTomato (6.10 × 1012 vg/ml) were purchased from Sunbio Medical Biotechnology (Shanghai, China) Co. AAV2/retro-hSyn-EGFP (7.38 × 1012 vg/ml), AAV2/retro-hSyn-tdTomato (4.78 × 1012 vg/ml), AAV2/retro-hSyn-mCherry (7.38 × 1012 vg/ml), AAV2/retro-hSyn-EGFP-P2A-Cre (5.79 × 1012 vg/ml), AAV2/retro-hSyn-Cre-mCherry (5.209 × 1012 vg/ml), AAV2/1-hSyn-Cre (1.03 × 1013 vg/ml), AAV2/retro-hSyn-Flpo (4.88 × 1012 vg/ml), AAV2/1-hSyn-Flpo (1.11 × 1013 vg/ml), AAV2/9-CaMK2a-hChR2-mCherry (5.64 × 1012 vg/ml), AAV2/9-CaMK2a-hChR2-EYFP (4.56 × 1012 vg/ml), AVV2/9-EF1a-DIO-mCherry (5.14 × 1012 vg/ml), AAV2/9-EF1a-DIO-hChR2-mCherry (3.16 × 1012 vg/ml), AAV2/9-EF1a-DIO-eNpHR3.0-mCherry (3.48 × 1012 vg/ml), AAV2/9-EF1a-DIO-eNpHR3.0-EYFP (4.38 × 1012 vg/ml), AAV2/9-EF1a-DIO-hM3D(Gq)-mCherry (5.54 × 1012 vg/ml), AAV2/9-EF1a-DIO-hM4D(Gi)-mCherry (5.45 × 1012 vg/ml), AAV2/9-hSyn-DO-hM3D(Gq)-eGFP (5.24 × 1012 vg/ml), AAV2/9-hSyn-fDIO-hM3D(Gq)-mCherry (2.73 × 1012 vg/ml), AAV2/9-hSyn-GCaMP6f (5.00 × 1012 vg/ml) AAV2/9-Ef1a-DIO-GCaMP6m (5.00 × 1012 vg/ml), AAV2/9-hSyn-DIO-mGFP-T2A-Synaptophysin-mRuby (5.20 × 1012 vg/ml), AAV2/8-EF1a-DIO-H2B-EGFP-T2A-TVA (5.52 × 1012 vg/ml), AAV2/8-EF1a-DIO-oRVG (5.08 × 1012 vg/ml), RV-CVS-ENVA-N2C(ΔG)-tdTomato (2 × 108 IFU/ml) were purchased from BrainVTA (Wuhan, China) Co. AAV2/9-hSyn-Synaptophysin-mRuby-T2A-mGFP (1.77 × 1013 vg/ml), AAV2/9-Ef1a-fDIO-mCherry (5.34 × 1012 vg/ml), AAV2/1-CAG-FLEX-Flpo (1.00 × 1013 vg/ml) were purchased from Taitool Bioscience (Shanghai, China) Co. Further details of the viruses are provided in Supplementary Table 1. All viral vectors were stored in aliquots at −80 °C until use.
Slice preparation and electrophysiological recordings
Briefly, mice were deeply anesthetized with sodium pentobarbital. Coronal slices of SM1 (300 μm), the PAG (280 μm), and the RVM (280 μm) were cut using a vibrating microtome (VT1200S; Leica, Wetzlar, Germany) after intracardial perfusion with ice-cold cutting solution containing (in mM) 92 N-methyl-d-glucamine, 2.5 KCl, 1.2 NaH2PO4, 20 HEPES, 30 NaHCO3, 25 glucose, 5 Na-ascorbate, 3 Na-pyruvate, 2 thiourea, 10 MgSO4, and 0.5 CaCl2 (oxygenated with 95% O2 and 5% CO2). The slices were immediately transferred to artificial cerebrospinal fluid (ACSF) containing (in mM) 119 NaCl, 2.3 KCl, 1 NaH2PO4, 26.2 NaHCO3, 12 glucose, 1.3 MgSO4, and 2.5 CaCl2 (oxygenated with 95% O2 and 5% CO2, 300–310 mOsm) for recovery at room temperature (RT) over 30 min. The recording chamber was continuously perfused with well-oxygenated ACSF at a rate of 2–3 mL/min at RT. Cells were visualized with a 60× water immersion objective on an upright fluorescent microscope (BX51WI; Olympus, Tokyo, Japan), which was equipped with a charge-coupled device imaging system operating in infrared differential interference contrast mode.
Whole-cell patch-clamp recordings were made using a MultiClamp 700B amplifier (Molecular Devices, Sunnyvale, CA, USA). Signals were collected, filtered at 2 kHz, and digitized at 10 kHz using a Digidata 1550B digitizer (Molecular Devices). Data were collected and analyzed using pCLAMP 10.6 software (Molecular Devices). Recording electrodes made by borosilicate pipettes were pulled using a horizontal micropipette puller (P-1000; Sutter Instruments, Novato, CA, USA). To record current-evoked APs, current-clamp recordings were obtained using a solution containing (in mM) 135 potassium gluconate, 15 KCl, 0.5 EGTA, 10 HEPES, 10 phosphocreatine, 2 Mg-ATP, and 0.5 Na-GTP (pH 7.3, 290–300 mOsm). With this solution, the recording electrodes exhibited a resistance of 3–8 MΩ.
For light-evoked oEPSCs, voltage-clamp recordings were performed by filling a pipette with a solution containing (in mM) 127.5 Cs-methanesulfonate, 7.5 CsCl, 2.5 MgCl2·6H2O, 0.6 EGTA, 10 HEPES, 10 phosphocreatine, 4 Na-ATP, and 0.4 Na-GTP (pH 7.3, 290–300 mOsm); the membrane potential was maintained at −70 mV. Optical stimulation (470 nm; light intensity, 10 mW) was delivered by a light-emitting diode (X-Cite 110LED Illumination System; Excelitas Technologies Corp., Pittsburgh, PA, USA) over 10 ms. To determine whether postsynaptic currents were elicited by direct synaptic connections, 1 μM TTX and 100 μM 4-AP were added to the ACSF.
Immunohistochemistry
Mice were deeply anesthetized with sodium pentobarbital and intracardially perfused with normal saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Each mouse brain was dissected and immersed in sucrose (10–30%) at 4 °C until it was isotonic. Coronal sections (30 μm) were cut using a cryostat microtome system (model 1950; Leica). For immunofluorescence, sections were blocked for 2 h at RT with phosphate-buffered saline containing 10% donkey serum and 0.3% Triton X-100, then incubated for 24 or 48 h at 4 °C with the following primary antibodies: rabbit anti-cFos (1:1,000; Abcam, Cambridge, UK), guinea pig anti-cFos (1:1,000; Synaptic Systems, Göttingen, Germany), rabbit anti-TPH (1:1,000; Novus Biologicals, Littleton, CO, USA), rabbit anti-TH (1:1,000; Cell Signaling Technology, Danvers, MA, USA), rabbit anti-RFP (1:1,000; Rockland Immunochemicals, Pottstown, PA, USA), and chicken anti-GFP (1:1,000; Aves Labs, Davis, CA, USA). The sections were then incubated with Alexa Fluor 488/546/647-conjugated secondary antibodies (1:500; Invitrogen, Waltham, MA, USA) for 2 h at 4 °C or 4′,6-diamidino-2-phenylindole (1:10,000; Invitrogen) for 2 min at RT. Further details of the antibodies are provided in Supplementary Table 1. The stained sections were observed under an Olympus VS120 microscope.
FISH
FISH was performed using the RNAscope system (Advanced Cell Diagnostics [ACD], Newark, CA, USA), in accordance with the manufacturer’s instructions. Sections (15–20 μm) were pretreated with hydrogen peroxide, target-retrieval reagents, and protease III, then treated for 2 h with Penk (CAT# 318761; ACD), vGlut1 (CAT# 416631; ACD), vGat (CAT# 319191; ACD), and vGlut2 (CAT# 319171; ACD) probes followed by AMP1, AMP2, and AMP3 successively for 15–30 min. The sections were then incubated in horseradish peroxidase (HRP)-C1/2/3 for 15 min and Opal 520/570/690 (1:1,500; PerkinElmer, Waltham, MA, USA) for 30 min to fluorescently label the probes. Finally, an HRP blocker was added 15 min after fluorescent labeling of each channel. All hybridization and incubation steps were performed at 40 °C in a wet box within a hybridization oven. Stained sections were imaged using a Nikon confocal microscope with a 40× objective (AX; Nikon, Tokyo, Japan).
Behavioral tests
The following behavioral assays were performed by experimenters who were blinded to the treatments. Mice were habituated to the testing environment for at least 30 min per day for 3 days before testing.
von Frey test
The paw withdrawal threshold was measured with a series of von Frey filaments (0.02–1.4 g; Stoelting). Each mouse was placed in a chamber on an elevated metal mesh floor, and von Frey filaments were applied to the central region of the plantar surface of the hindpaw. A withdrawal response was considered valid only if the hindpaw completely lifted off the mesh bottom. Each filament was tested 10 times at 10 s intervals in ascending order of force to determine the response frequency. The PWT was defined as the lowest force that caused at least 5 withdrawals during 10 consecutive applications.
Hargreaves’ test
The thermal nociceptive threshold was assessed by measuring the paw withdrawal latency in response to a radiant heat source (390 G, IITC Life Science, Woodland Hills, CA. USA). Mice were individually placed in Plexiglas chambers on an elevated glass platform, and radiant heat was applied from underneath the platform to the glabrous surface of the hindpaw through the glass plate. The heat source was switched off when paw withdrawal occurred, or after 15 s of heat emission. The time between the onset of radiant heat application and hindpaw withdrawal was regarded as the PWL.
Real-time conditional place preference test
RT-CPP was evaluated by using a three-chambers device: two large conditioning chambers (20 cm × 20 cm × 20 cm) and a smaller connecting chamber (8 × 8 × 20 cm, length × width × height). The two conditioning chambers were characterized by distinct visual and tactile stimuli. Mice were allowed to explore freely all chambers for 15 min on day 1 (pre-conditioning day), and subsequently tested 15 min on next day (conditioning day). During the test, blue light stimulation (473 nm, 5 mW, 20 Hz, 10 ms) was delivered whenever the mouse entered or stayed in the paired chamber and turned off when the animal exited the paired chamber. The time mice spent on the blue light simulation side (paired chamber) were recorded during 15 min test on days 1 and 2.
Brush
Mice were individually placed in a chamber on an elevated metal mesh floor, and mechanical sensitivity was measured by lightly brushing (velocity, ~1.5 cm/s) the lateral plantar region of the hindpaw from heel to toe with a paint brush (Princeton Artist Brush Co., Princeton, NJ, USA). Withdrawal or flinching of the paw was regarded as a positive response. The test was repeated 10 times at 2 min intervals, and the values from the 10 trials were averaged.
Tape removal
Mice were individually placed in a plexiglass chamber, and a 5 mm-diameter circular adhesive label (Diversified Biotech, Dedham, MA, USA) was attached to the plantar surface of the hindpaw. The latency of licking or biting to remove the tape was measured. The test was repeated three times at 10 min intervals, and the values from the three trials were averaged.
Hotplate test
Mice were placed on a hot plate (IITC Life Science), with the plate temperature set to 52 °C. The latency of flinching or licking the hindpaw was regarded as the hot plate response latency.
Rotarod
Locomotor coordination was assessed using an accelerating rotarod (IITC Life Science). One day before the test, mice were habituated and trained three times to run on the rotarod, at a fixed speed of 5 rpm, until they were able to remain on it for 300 s. Mice unable to remain on the rotarod for 300 s after three training trials were excluded from the experiment. On the day of the experiment, locomotor coordination was evaluated using a rotarod that accelerated from 5 to 40 rpm over 300 s. Three trials were repeated at 15 min intervals, and the average latency to fall across three sessions was recorded for each mouse.
Beam test
The elevated beam test was used to evaluate fine motor coordination. The test was conducted on a wooden beam with a width of 10 mm, length of 1 m, and height of 60 cm. Before the test, mice were trained to cross the beam and reach an enclosed box at the end within 60 s. Three trials per day were completed on 2 consecutive days. On the third day, three additional consecutive trials were recorded using a high-speed camera (a6300; Sony, Tokyo, Japan) at 100 fps, and the number of foot slips was measured.
Ladder test
To assess skilled paw placement during walking, mice were placed on a horizontal ladder (1 m long) with 3 mm rungs at 1 cm intervals. The home cage was located at the end of the ladder. Mice were trained to anticipate the position of the rungs over several trials until they were able to cross the ladder and reach the home cage within 60 s. On the test day, three consecutive trials were recorded using a high-speed camera (a6300; Sony) at 100 fps; the videos were used to detect foot placement and analyze the coordination of forelimbs and hindlimbs.
Open field test
Locomotor activity was assessed using the open field test. Each mouse was placed in the center of the open field (40 × 40 cm) and allowed to freely explore for 10 min. The movements of the mice were recorded with a digital camera (3206, Zhongwei Inc., Shenzhen, China). Video tracking software (EthoVision XT v11.5; Noldus, Leesburg, VA, USA) was used to determine the distance traveled in the field.
Chemogenetic manipulation
Three weeks after chemogenetic virus injections, clozapine N-oxide (CNO) was intraperitoneally injected 30 min before each behavioral test. For hM3d (Gq) activation, 3 mg/kg CNO was used, whereas 5 mg/kg CNO was injected for hM4D (Gi) inhibition. For intra-PAG or RVM infusion, CNO (300 ng/site) was applied using a pre-implanted cannula. And for intrathecal administration, CNO (2 μg/mouse), were intrathecally infused into the L5-6 intervertebral space using a 20-gauge needle.
Optogenetic manipulation
Two weeks after optogenetic virus injection, optical fibers (200 μm core diameter, 0.37 numerical aperture; Newdoon, Hangzhou, China) were implanted 200 μm above the virus injection site in the targeted regions (SM1 and the RVM). For the PAG, optical fibers were implanted at the following coordinates (relative to bregma): AP −4.50, ML ± 0.67 (lateral-to-medial angle = 10°), DV −2.40. After surgery, mice were allowed to recover for at least 1 week. Before optogenetic stimulation, the laser source was adjusted to the appropriate intensity and connected to the implanted optical fiber through a fiber cable. Behavioral tests were performed during optogenetic activation (473 nm, 5 mW, 20 Hz, 10 ms) or inhibition (580 nm, 5 mW, continuous). At the end of the experiments, the brains of all mice were sectioned to confirm the placement of the optical fibers.
In vivo pharmacology combined with chemogenetic activation
Three weeks after chemogenetic virus injections, mice were habituated to the test environment. CNO was intraperitoneally injected 30 min before each behavioral test; 15 min after CNO injection, yohimbine, SB-269970, ondansetron, naloxone, CTAP, or NS (vehicle control) was delivered into the cerebrospinal fluid space between the L5 and L6 vertebrae through a lumbar puncture made by a 30-gauge needle.
Fiber photometry
Two weeks after GCaMP6 virus injection, optical fibers (200 μm core diameter, 0.37 NA; Newdoon Technology) were implanted. Mice were allowed to recover for at least 1 week. Calcium-dependent fluorescent signals were recorded in GCaMP6-expressing neurons using the 470 nm laser of a fiber photometry system (FPS-MC-LED; ThinkerTech Nanjing BioScience Inc., Nanjing, China). To avoid photobleaching, the light intensity of the fiber tip was adjusted to 30–35 μW. The average value of repeated trials was regarded as the calcium signal for each mouse. For optogenetic activation of presynaptic neurons, and to record postsynaptic neuronal activity, we defined an event as the onset of optogenetic light stimulation, measured through its transistor–transistor logic signal. Calcium signal data were normalized to the Z-scores (ΔF/σF) of fluorescence changes, where σF is the standard deviation of basal fluorescence (2 s before the event). Data were analyzed in the MATLAB environment (R2020a; MathWorks, Natick, MA, USA). At the end of the experiment, all mice were euthanized and optical fiber placement was verified.
Miniature 2 P calcium imaging in vivo
For miniature 2 P calcium imaging, AAV-Ef1a-DIO-GCaMP6m was injected into SM1, whereas AAV2/retro-Cre was injected into the PAG or RVM. Two weeks after virus injection, the mice were anesthetized, and a 4 mm-diameter window was made on the skull above the target cortical area for imaging. One week after recovery from craniotomy, an imaging baseplate was positioned over the skull window and cemented with denture base resins. Before the imaging experiments, mice were allowed to adapt to the imaging environment for at least 2 days (30 min per day) to minimize stress during the imaging session. A miniature 2 P microscope (FHIRMTPM V2.0; Beijing Transcend Vivoscope Co., Ltd., Beijing, China) was installed on the baseplate, and image acquisition was performed using imaging software (OGLFMTPM-V2.0; Transcend Vivoscope). A 512 × 512-pixel frame was used to acquire images at a frame rate of 9.6 Hz. The field of view was 420 × 420 μm2. To assess the neuronal response to forced movement, each mouse was placed on a rotarod (IITC Life Science) and forced to run at a speed of 10 rpm for 30 s (3–5 trials at 30 s intervals). For imaging of GCaMP6m-expressing neurons in response to sensory stimuli, each mouse was allowed to freely move in a transparent plastic chamber (10 × 10 × 15 cm); various sensory stimuli, including brushing, 0.6 g von Frey hair stimulation, and a noxious pinprick, were applied to the contralateral hindpaw of recorded cortical regions (5 trials, 30 s interval). During all imaging experiments, the behaviors of the mice were recorded using an infrared camera (FHIRMTPM V2.0; Transcend Vivoscope) at 25 fps.
After the imaging session, motion correction, image registration, cell detection, and signal extraction were performed using Suite2p, followed by manual correction. Calcium signals were converted to the relative change in fluorescence intensity, using the following formula: ΔF/F0 = (F - F0)/F0, where F0 is the mean signal over the entire movie for a given recording day. To identify neurons activated by each stimulus, we compared the ΔF/F0 of the stimulation period with that of the equivalent baseline period (5 s for forced running, 2 s for other stimuli); neurons with peak signals more than five times the standard deviation of the baseline were classified as activated neurons.
Motor-induced analgesia
Motor-induced analgesia was assessed using an accelerating rotarod (IITC Life Science). One the day before the test, mice were placed on a rod with a fixed speed of 5 rpm for three sessions. On the testing day, mice were placed on an accelerating rotarod (10–19 rpm with an acceleration of 0.06 rpm/s) for 150 s (a single trial). All animals were subjected to 2 trials with a 60 s interval. The von Frey test and Hargreaves test were performed after the rotarod task to evaluate the analgesic effect of motor training.
Statistical analysis
All data are presented as means ± standard errors of the mean, and statistical analyses were conducted using Prism 8.0 software (GraphPad Software Inc., San Diego, CA, USA). Before the analysis, all data were assessed for normality and homogeneity of variance using the Shapiro–Wilk and Brown–Forsythe tests; no data were transformed. Anatomical, behavioral, and electrophysiological data were analyzed using the paired t-test, Student’s t test, or the Mann–Whitney U test (nonparametric data) when comparing two groups; they were analyzed by one-way analysis of variance (ANOVA), two-way ANOVA, or the Kruskal–Wallis H test (nonparametric data), followed by the Sidak or Dunn post hoc test, when comparing three or more groups. All analyses were two-tailed, and P-values < 0.05 were considered statistically significant. A summary of the statistical analyses is provided in Supplemental Data 1. Sample sizes and replicates are shown in the figures.
Data availability
All data needed to evaluate the conclusions in this paper are presented in the results, supplementary materials and Source Data File. Source data are provided with this paper.
Code availability
The codes that used to analyze the fiber photometry data of this study were provided by ThinkerTech BioScience Inc. (Nanjing, China), and deposited in the Zenodo database: https://zenodo.org/records/14545435.
References
Ebbesen, C. L. & Brecht, M. Motor cortex - to act or not to act? Nat. Rev. Neurosci. 18, 694–705 (2017).
Vierck, C. J., Whitsel, B. L., Favorov, O. V., Brown, A. W. & Tommerdahl, M. Role of primary somatosensory cortex in the coding of pain. Pain 154, 334–344 (2013).
Gan, Z. et al. Layer-specific pain relief pathways originating from primary motor cortex. Science 378, 1336–1343 (2022).
Liu, Y. et al. Touch and tactile neuropathic pain sensitivity are set by corticospinal projections. Nature 561, 547–550 (2018).
Matyas, F. et al. Motor control by sensory cortex. Science 330, 1240–1243 (2010).
Mathis, M. W., Mathis, A. & Uchida, N. Somatosensory cortex plays an essential role in forelimb motor adaptation in mice. Neuron 93, 1493–1503.e1496 (2017).
Karadimas, S. K. et al. Sensory cortical control of movement. Nat. Neurosci. 23, 75–84 (2020).
Frot, M., Magnin, M., Mauguière, F. & Garcia-Larrea, L. Cortical representation of pain in primary sensory-motor areas (S1/M1)-a study using intracortical recordings in humans. Hum. Brain Mapp. 34, 2655–2668 (2013).
Peyron, R. et al. An fMRI study of cortical representation of mechanical allodynia in patients with neuropathic pain. Neurology 63, 1838–1846 (2004).
Garcia-Larrea, L. Non-invasive cortical stimulation for drug-resistant pain. Curr. Opin. Support Palliat. Care 17, 142–149 (2023).
Hamani, C. et al. Motor cortex stimulation for chronic neuropathic pain: results of a double-blind randomized study. Brain 144, 2994–3004 (2021).
Knotkova, H. et al. Neuromodulation for chronic pain. Lancet 397, 2111–2124 (2021).
Ostergard, T., Munyon, C. & Miller, J. P. Motor cortex stimulation for chronic pain. Neurosurg. Clin. N. Am. 25, 693–698 (2014).
Jin, Y. et al. A somatosensory cortex input to the caudal dorsolateral striatum controls comorbid anxiety in persistent pain. Pain 161, 416–428 (2020).
Yang, W., Kanodia, H. & Arber, S. Structural and functional map for forelimb movement phases between cortex and medulla. Cell 186, 162–177 (2023).
Inagaki, H. K. et al. Neural algorithms and circuits for motor planning. Annu Rev. Neurosci. 45, 249–271 (2022).
Yao, M. et al. Long-range connectome of pyramidal neurons in the sensorimotor cortex. iScience 26, 106316 (2023).
Winter, C. C. et al. A transcriptomic taxonomy of mouse brain-wide spinal projecting neurons. Nature 624, 403–414 (2023).
Bourane, S. et al. Identification of a spinal circuit for light touch and fine motor control. Cell 160, 503–515 (2015).
Conner, J. M. et al. Modulation of tactile feedback for the execution of dexterous movement. Science 374, 316–323 (2021).
Millan, M. J. Descending control of pain. Prog. Neurobiol. 66, 355–474 (2002).
Huang, J. et al. A neuronal circuit for activating descending modulation of neuropathic pain. Nat. Neurosci. 22, 1659–1668 (2019).
Yin, J. B. et al. dmPFC-vlPAG projection neurons contribute to pain threshold maintenance and antianxiety behaviors. J. Clin. Invest. 130, 6555–6570 (2020).
Pagano, R. L. et al. Motor cortex stimulation inhibits thalamic sensory neurons and enhances activity of PAG neurons: possible pathways for antinociception. Pain 153, 2359–2369 (2012).
Tan, L. L. et al. Gamma oscillations in somatosensory cortex recruit prefrontal and descending serotonergic pathways in aversion and nociception. Nat. Commun. 10, 983 (2019).
Lopes, P. S. S., Campos, A. C. P., Fonoff, E. T., Britto, L. R. G. & Pagano, R. L. Motor cortex and pain control: exploring the descending relay analgesic pathways and spinal nociceptive neurons in healthy conscious rats. Behav. Brain Funct. 15, 5 (2019).
Heinricher, M. M., Tavares, I., Leith, J. L. & Lumb, B. M. Descending control of nociception: specificity, recruitment and plasticity. Brain Res. Rev. 60, 214–225 (2009).
King, T. et al. Unmasking the tonic-aversive state in neuropathic pain. Nat. Neurosci. 12, 1364–1366 (2009).
Sun, L. et al. Parabrachial nucleus circuit governs neuropathic pain-like behavior. Nat. Commun. 11, 5974 (2020).
Zhang, Y. et al. Identifying local and descending inputs for primary sensory neurons. J. Clin. Invest. 125, 3782–3794 (2015).
Lubejko, S. T. et al. Inputs to the locus coeruleus from the periaqueductal gray and rostroventral medulla shape opioid-mediated descending pain modulation. Sci. Adv. 10, eadj9581 (2024).
Suárez-Pereira, I. et al. The role of the locus coeruleus in pain and associated stress-related disorders. Biol. Psychiatry 91, 786–797 (2022).
Nemoto, W. et al. Monoaminergic mediation of hyperalgesic and analgesic descending control of nociception in mice. Pain 164, 1096–1105 (2023).
Brenchat, A. et al. Pharmacological activation of 5-HT7 receptors reduces nerve injury-induced mechanical and thermal hypersensitivity. Pain 149, 483–494 (2010).
Song, Z., Meyerson, B. A. & Linderoth, B. Spinal 5-HT receptors that contribute to the pain-relieving effects of spinal cord stimulation in a rat model of neuropathy. Pain 152, 1666–1673 (2011).
Hayashida, K. et al. Ondansetron reverses antihypersensitivity from clonidine in rats after peripheral nerve injury: role of γ-aminobutyric acid in α2-adrenoceptor and 5-HT3 serotonin receptor analgesia. Anesthesiology 117, 389–398 (2012).
Lima, L. V., Abner, T. S. S. & Sluka, K. A. Does exercise increase or decrease pain? Central mechanisms underlying these two phenomena. J. Physiol. 595, 4141–4150 (2017).
Rice, D. et al. Exercise-induced hypoalgesia in pain-free and chronic pain populations: state of the art and future directions. J. Pain. 20, 1249–1266 (2019).
Sluka, K. A., Frey-Law, L. & Hoeger Bement, M. Exercise-induced pain and analgesia? Underlying mechanisms and clinical translation. Pain 159, S91–s97 (2018).
An, X., Bandler, R., Ongür, D. & Price, J. L. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J. Comp. Neurol. 401, 455–479 (1998).
Floyd, N. S., Price, J. L., Ferry, A. T., Keay, K. A. & Bandler, R. Orbitomedial prefrontal cortical projections to distinct longitudinal columns of the periaqueductal gray in the rat. J. Comp. Neurol. 422, 556–578 (2000).
Gallager, D. W. & Pert, A. Afferents to brain stem nuclei (brain stem raphe, nucleus reticularis pontis caudalis and nucleus gigantocellularis) in the rat as demonstrated by microiontophoretically applied horseradish peroxidase. Brain Res. 144, 257–275 (1978).
Hermann, D. M., Luppi, P. H., Peyron, C., Hinckel, P. & Jouvet, M. Afferent projections to the rat nuclei raphe magnus, raphe pallidus and reticularis gigantocellularis pars alpha demonstrated by iontophoretic application of choleratoxin (subunit b). J. Chem. Neuroanat. 13, 1–21 (1997).
Peng, H. et al. Morphological diversity of single neurons in molecularly defined cell types. Nature 598, 174–181 (2021).
BRAIN Initiative Cell Census Network (BICCN). A multimodal cell census and atlas of the mammalian primary motor cortex. Nature 598, 86–102 (2021).
Muñoz-Castañeda, R. et al. Cellular anatomy of the mouse primary motor cortex. Nature 598, 159–166 (2021).
Ueno, M. et al. Corticospinal circuits from the sensory and motor cortices differentially regulate skilled movements through distinct spinal interneurons. Cell Rep. 23, 1286–1300.e1287 (2018).
Nelson, A., Abdelmesih, B. & Costa, R. M. Corticospinal populations broadcast complex motor signals to coordinated spinal and striatal circuits. Nat. Neurosci. 24, 1721–1732 (2021).
Zhu, X. et al. Distinct thalamocortical circuits underlie allodynia induced by tissue injury and by depression-like states. Nat. Neurosci. 24, 542–553 (2021).
Cichon, J., Blanck, T. J. J., Gan, W. B. & Yang, G. Activation of cortical somatostatin interneurons prevents the development of neuropathic pain. Nat. Neurosci. 20, 1122–1132 (2017).
Kim, W., Kim, S. K. & Nabekura, J. Functional and structural plasticity in the primary somatosensory cortex associated with chronic pain. J. Neurochem. 141, 499–506 (2017).
Eto, K. et al. Inter-regional contribution of enhanced activity of the primary somatosensory cortex to the anterior cingulate cortex accelerates chronic pain behavior. J. Neurosci. 31, 7631–7636 (2011).
Yan, Y. et al. Thalamocortical circuit controls neuropathic pain via up-regulation of HCN2 in the ventral posterolateral thalamus. Neurosci. Bull. 39, 774–792 (2023).
Paixão, S. et al. Identification of spinal neurons contributing to the dorsal column projection mediating fine touch and corrective motor movements. Neuron 104, 749–764 (2019).
Abraira, V. E. et al. The cellular and synaptic architecture of the mechanosensory dorsal horn. Cell 168, 295–310 (2017).
Lui, J. H. et al. Differential encoding in prefrontal cortex projection neuron classes across cognitive tasks. Cell 184, 489–506 (2021).
Maarrawi, J. et al. Motor cortex stimulation for pain control induces changes in the endogenous opioid system. Neurology 69, 827–834 (2007).
Maarrawi, J. et al. Brain opioid receptor density predicts motor cortex stimulation efficacy for chronic pain. Pain 154, 2563–2568 (2013).
Wei, F. et al. Molecular depletion of descending serotonin unmasks its novel facilitatory role in the development of persistent pain. J. Neurosci. 30, 8624–8636 (2010).
Kim, Y. S. et al. Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron 81, 873–887 (2014).
François, A. et al. A brainstem-spinal cord inhibitory circuit for mechanical pain modulation by GABA and enkephalins. Neuron 93, 822–839 (2017).
Jiao, Y. et al. Molecular identification of bulbospinal ON neurons by GPER, which drives pain and morphine tolerance. J. Clin. Invest 133, e154588 (2023).
Nguyen, E. et al. Medullary kappa-opioid receptor neurons inhibit pain and itch through a descending circuit. Brain 145, 2586–2601 (2022).
Tang, Y. L. et al. Green light analgesia in mice is mediated by visual activation of enkephalinergic neurons in the ventrolateral geniculate nucleus. Sci. Transl. Med. 14, eabq6474 (2022).
Kuner, R. & Kuner, T. Cellular circuits in the brain and their modulation in acute and chronic pain. Physiol. Rev. 101, 213–258 (2021).
Vaegter, H. B. & Jones, M. D. Exercise-induced hypoalgesia after acute and regular exercise: experimental and clinical manifestations and possible mechanisms in individuals with and without pain. Pain. Rep. 5, e823 (2020).
Grace, P. M. et al. Prior voluntary wheel running attenuates neuropathic pain. Pain 157, 2012–2023 (2016).
Dobson, J. L., McMillan, J. & Li, L. Benefits of exercise intervention in reducing neuropathic pain. Front. Cell Neurosci. 8, 102 (2014).
Zhang, X. Y. et al. A role for the cerebellum in motor-triggered alleviation of anxiety. Neuron 112, 1165–1181 (2024).
Brito, R. G., Rasmussen, L. A. & Sluka, K. A. Regular physical activity prevents development of chronic muscle pain through modulation of supraspinal opioid and serotonergic mechanisms. Pain. Rep. 2, e618 (2017).
Han, M. et al. SIP30 is required for neuropathic pain-evoked aversion in rats. J. Neurosci. 34, 346–355 (2014).
Luo, H. et al. Interleukin-17 regulates neuron-glial communications, synaptic transmission, and neuropathic pain after chemotherapy. Cell Rep. 29, 2384–2397 (2019).
Acknowledgements
We thank Dr. J.-N. Zhu (Nanjing University) for his suggestion on the manuscript. We thank Dr. T.-W. Huang (Shenzhen Institutes of Advanced Technology, CAS) for giving some Penk-Cre mice. We also thank all the members of the Zhang Laboratory for their support and discussions. This work was supported by Science and Technology Innovation (STI) 2030-Major Projects (2021ZD0203200-05 to Y.-Q.Z.), and National Natural Science Foundation of China (82130032 and 82021002 to Y.-Q.Z.).
Author information
Authors and Affiliations
Contributions
Y.-Q.Z. and G.-H.W. conceived the project and designed the experiments. Y.-Q.Z. supervised the project. G.-H.W. and X.-Y.H. performed most experiments and data analysis. Z.-R.Z., H.-Z.L., S.-S.L., L.-X.Y. and H.L. participated in some of the behavior experiments and data analysis. S.-S.L. and H.L. participated in the whole-cell patch recording. G.-H.W. and Y.-Q.Z. analyzed the data. All authors contributed to data interpretation. G.-H.W. and Y.-Q.Z. wrote the manuscript. All authors reviewed the manuscript and approved its submission.
Corresponding author
Ethics declarations
Competing interests
All the authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, GH., Hou, XY., Liu, HZ. et al. Descending projection neurons in the primary sensorimotor cortex regulate neuropathic pain and locomotion in mice. Nat Commun 16, 5918 (2025). https://doi.org/10.1038/s41467-025-61164-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-61164-8
This article is cited by
-
Mechanisms and Clinical Application Progress of Exercise in the Treatment of Neuropathic Pain
Cellular and Molecular Neurobiology (2026)