Abstract
Mitochondrial Rho GTPase (MIRO) features N- and C-terminal GTPase domains (nGTPase and cGTPase) flanking two pairs of EF-hands, and functions as a master scaffold on the outer mitochondrial membrane. It regulates mitochondrial motility by recruiting trafficking kinesin-binding protein (TRAK), which in turn recruits kinesin-1 and dynein-dynactin. The MIRO-TRAK interaction remains incompletely understood. Here, we describe the cryo-electron microscopy structure of TRAK1569-623 bound to MIRO1. The complex forms a dimer, mediated by interactions through the second EF-hand pair, cGTPase, and TRAK1. TRAK1569-623 binds in a cleft between the nGTPase and first EF-hand pair, inserting side chains into hydrophobic pockets of both domains. Another MIRO1-binding site involves TRAK1425-428, which binds in a pocket between the second EF-hand pair and cGTPase. Both binding sites are validated by mutagenesis and binding assays, showing no clear dependence on cofactor conditions (calcium or nucleotide). In cells, both sites contribute to TRAK1’s mitochondrial localization.
Similar content being viewed by others
Introduction
Mitochondrial Rho GTPase (MIRO) functions as a master regulator of mitochondrial dynamics, coordinating their transport, homeostasis, degradation, morphogenesis, inheritance, and contacts with the endoplasmic reticulum1,2,3,4,5,6,7,8. From N- to C-terminus, MIRO features a GTPase domain (nGTPase), two pairs of EF-hands, a second GTPase domain (cGTPase), and a transmembrane helix that inserts into the outer mitochondrial membrane (Fig. 1a). MIRO’s EF-hand pairs are referred to as ELM domains (ELM1 and ELM2), because of their unique architecture consisting of a canonical Ca2+-binding EF-hand, a “hidden” EF-hand that does not bind Ca2+, and a ligand mimic helix that occupies the hydrophobic pocket of the pair9,10. Consistent with its domain organization, studies in vitro and in cells suggest that MIRO’s control of mitochondrial dynamics depends on its nucleotide and Ca2+-bound states1,2,4,11,12,13,14,15,16. MIRO, including two paralogs (MIRO1 and MIRO2), recruits numerous proteins to mitochondria17. Among these, myosin-19 mediates actin-based mitochondrial dynamics18,19,20, while trafficking kinesin-binding protein (TRAK) recruits the microtubule-based motors kinesin-1 and dynein-dynactin for long-range motility11,13,21,22,23,24,25,26,27,28,29.
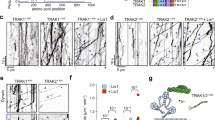
a Domain diagrams of human MIRO1 and TRAK1, highlighting the conserved regions CR2 and CR4, which contain the MIRO1 binding Site-1 and Site-2, respectively. Secondary structure elements within Site-2 are shown in a cartoon representation below the sequence. Each EF-hand ligand mimic (ELM) domain consists of two EF-hands and a ligand mimic helix, with only the first EF-hand binding Ca2+. b Two perpendicular views of the cryo-EM map of the MIRO1-TRAK1 dimer, colored and labeled by domains (as in part a). Bound cofactors (GTP, Mg2+, and Ca2+) are highlighted in the ribbon diagram on the right. c Close-up views of specific interactions between MIRO1 and Site-2 of TRAK1, numbered 1 to 6 from N- to C-terminus. MIRO1 is shown as a transparent electrostatic surface. The image in the center shows the cryo-EM map extracted around Site-2 and indicates the location of each close-up view (labeled 1-6).
TRAK, also including two paralogs (TRAK1 and TRAK2), features a conserved N-terminal coiled-coil domain that binds and activates kinesin-1 and dynein-dynactin11,13,21,24,26,29,30, and a less conserved, mostly unstructured C-terminal region (Fig. 1a, Supplementary Fig. 1a). In vitro motility assays previously indicated that TRAK11-532 is sufficient for MIRO1-TRAK1 complex formation and co-migration along microtubules, driven by either kinesin-1 or dynein-dynactin27. Inspired by these findings, we previously characterized an interaction between TRAK1399-431 and a MIRO1 fragment comprising the ELMs and cGTPase (ELMs-cGTPase)28.
Here, this interaction is specifically mapped to TRAK1425-428 and is referred to as Site-1 (Fig. 1a). However, other studies using co-immunoprecipitation12 and an AlphaFold prediction combined with yeast two-hybrid screening31 suggested that MIRO1 interacts with a fragment C-terminal to Site-1, namely TRAK2476-700. Consistent with these observations, we describe here a second MIRO1-binding site, TRAK1569-623 (TRAK2553-605), referred to as Site-2 (Fig. 1a). We present the cryo-electron microscopy (cryo-EM) structure of Site-2 in complex with Ca2+- and GTP-bound cytosolic MIRO11-591, revealing a dimer with TRAK1569-623 occupying a cleft between the nGTPase and ELM1 of each MIRO1 monomer. Contrary to previous findings4,11,12,13,14,16, mutagenesis and biochemical experiments show that MIRO1 binds Site-1 and Site-2 under all cofactor conditions (calcium or nucleotide). Both binding sites are important for efficient TRAK1 recruitment to mitochondria in cells.
Results
Cryo-EM structure of the MIRO1-TRAK1 complex
TRAK2476-700 (corresponding to TRAK1489-700), which co-immunoprecipitates with MIRO112, contains three highly conserved regions among vertebrate TRAK1/2 sequences (Supplementary Fig. 1a). These regions are designated as conserved regions (CRs) 3, 4, and 5, following the nomenclature used in our prior study on CR1-328. We previously showed that CR3, predicted to form a coiled coil, does not participate in MIRO1 binding28, whereas CR5 harbors the binding site for O-GlcNAc transferase (OGT), which regulates mitochondrial dynamics through glucose-dependent O-GlcNAcylation of TRAK30. Based on these considerations, CR4 (TRAK1569-623) appeared more likely to contain a MIRO-binding site. After confirming this prediction in pulldown assays (see below), we determined the 3.57-Å resolution cryo-EM structure of human TRAK1569-623 in complex with full-length cytosolic human MIRO1 (MIRO11-591) (Fig. 1, Supplementary Figs. 2–4, Supplementary Movie 1, Table 1, Methods). The structure was determined in the presence of 1 mM CaCl2, 1 mM MgCl2, and 100 µM GTP. Consistent with previous structures9,10, Ca2+ was bound to the first but not the second EF-hand of each ELM (Supplementary Fig. 4a). The nucleotide in both GTPases resides in low-resolution regions of the cryo-EM map (Supplementary Fig. 3e), so neither its identity nor the presence of Mg²⁺ can be definitively established from the map alone. However, because the cryo-EM sample was prepared under conditions that favor GTP binding to both GTPases (see below), we infer that GTP is bound in both cases (Supplementary Fig. 4b). In the nGTPase, the density is also consistent with the presence of Mg²⁺, whereas no corresponding density is observed in the cGTPase.
Crystal structures have been determined for human MIRO1’s nGTPase32 and ELMs-cGTPase10. Individually, these structures superimpose remarkably well with the current cryo-EM structure, with RMSDs of ~1 Å in each case. However, these two regions are connected by a long and flexible linker, suggesting that they can move semi-independently, which may explain why the full-length cytosolic domain has not been crystallized. In the current structure, TRAK1 residues L570-R613 occupy a large cleft at the interface between the nGTPase and ELM1, helping to stabilize their relative positions (Fig. 1b). We designated this MIRO-binding site in TRAK as Site-2, to distinguish it from our previously characterized interaction involving CR228, referred to as Site-1 (Fig. 1a).
Site-2 displays three regions of secondary structure (Fig. 1a, b): (a) residues Q575-V577 from the two TRAK1 molecules interact to form an anti-parallel β-sheet, (b) residues A584-A593 form an α-helix that interacts with the nGTPase, and (c) residues G606-K610 add as an additional strand to the six-stranded mixed β-sheet of the nGTPase Rossmann fold. Other regions of TRAK1 form a series of loops that meander through the cleft between the nGTPase and ELM1, contacting primarily the nGTPase.
From N- to C-terminus, the first interaction with MIRO1 involves TRAK1 residue E572, which forms a salt bridge with R36 of switch-I of the nGTPase (Fig. 1c). This salt bridge suggests a potential pathway for nucleotide state-dependent regulation of the interaction. Next, the side chains of TRAK1 residues I576, V577, K578, L580, L586, W589, and L592 stack against a large hydrophobic patch on the nGTPase formed by residues L24, V25, I40, T41, I42, P43, V46, F159, and Y160 (Fig. 1c). TRAK1 residue W589, in particular, inserts deeply into a hydrophobic pocket created by these residues on the nGTPase surface (Supplementary Movie 1).
Following the α-helix, TRAK1 bends toward ELM1, inserting the side chains of L597 and I600 into a hydrophobic pocket formed by MIRO1 residues F217, L221, V229, V232, L254, L257, and F258 (Fig. 1c). The TRAK1 chain then turns back toward the nGTPase, making additional hydrophobic contacts through V607 and V608, which face M128, M135, C144, and V145 on the nGTPase (Fig. 1c). The last interaction, before exiting the MIRO1 cleft, involves TRAK1 residue K610, which makes a hydrogen bond with the main chain oxygen of MIRO1 residue M135 (Fig. 1c).
Overall, the two proteins interact extensively, with contact surface areas of 1915 Å2 on MIRO1 and 2060 Å2 on TRAK1 (Fig. 2a, b). The interaction involves numerous hydrophobic contacts among highly conserved residues in both TRAK and MIRO paralogs, suggesting a high degree of specificity (Supplementary Fig. 1). For both the nGTPase and ELM1, the interaction diverges from the classical binding mechanisms of Ras GTPases33 and EF-hand proteins34, as TRAK1 makes only one contact with switch-I and does not engage the canonical hydrophobic pocket of ELM1, which is occupied by the ligand mimic helix. Consequently, any observed dependence of this interaction on the nucleotide or Ca²⁺-bound states1,2,4,11,12,13,14,16 may be allosteric in nature or mediated by factors not present in the structure.
a Structure of the MIRO11-591-TRAK1569-623 dimeric complex, with MIRO1 monomers shown in cyan and gray and Site-2 of TRAK1 in red. Red cylinders indicate the likely location of the transmembrane helices as they extend from the structure. b Contact surfaces (yellow) of Site-2 on MIRO1 (1915 Å2, top) and MIRO1 on Site-2 (2065 Å2, bottom). c MIRO1 dimerization interface shown as a surface representation (cyan, left) and a ribbon diagram (right). ELM2, ELM2-cGTPase linker, and cGTPase are colored orange, magenta, and blue, respectively. Contact surface areas were calculated using the SPPIDER server (https://sppider.cchmc.org/). d Anti-parallel β-sheet formed by TRAK1 residues Q575-V577. e The last observed MIRO1 residue in the structure, L587, is positioned <20 Å from its counterpart in the other dimer subunit, suggesting that the transmembrane helices are closely spaced as they insert into the outer mitochondrial membrane. f Representative mass photometry data for MIRO11-591 alone and with TRAK1569-623 from fraction 16 (see also Supplementary Fig. 5). Mass photometry and cryo-EM samples were prepared using GraFix36. Data are presented as histograms normalized to the bin with the highest number of counts (bin width = 6.1 kDa). The average mass, standard deviation, and percent of counts are derived from Gaussian fits.
Dimerization of the MIRO1-TRAK1 complex
In crystal structures, the ELMs-cGTPase was found to be monomeric9,10 while the nGTPase formed a dimer32. It was proposed that the nGTPase dimerization interface might mediate both the dimerization of full-length MIRO and/or interactions with binding partners32. The cryo-EM structure supports the latter prediction, as part of the Site-2-binding interface overlaps with the nGTPase dimerization interface. However, the MIRO1-TRAK1 complex dimerizes through a different interface, involving ELM2, the cGTPase, and TRAK1 (Fig. 2c, d).
On MIRO1, the dimerization interface covers an area of 785 Å2, involving both hydrogen bonds through residues Q310, K411, Q413, Q415, N417, Q467, and Y579, and hydrophobic contacts by residues H303, L307, R403, I407, and Y465 (Fig. 2c). Residues at the dimerization interface are highly conserved among 188 vertebrate MIRO1/2 sequences (Supplementary Fig. 1b). Given the relatively small contact surface area, dimerization through this interface alone is likely weak. However, two additional factors may enhance the stability of the MIRO1-TRAK1 dimer: (a) clustering of MIRO1 molecules on the outer mitochondrial surface via interactions involving the C-terminal transmembrane helix, as two such helices are predicted to come into close proximity as they emerge from the current structure (Fig. 2a, e), and (b) binding to TRAK1, which is itself a dimer. Dimerization through TRAK1 may involve not only its coiled-coil regions but also the β-sheet formed by residues Q575-V577 of the two TRAK1 molecules in the structure (Fig. 2d).
To assess whether TRAK1 binding contributes to MIRO1 dimerization in solution, we used mass photometry35 in combination with GraFix (glycerol gradient with glutaraldehyde fixation)36, following the same approach used for cryo-EM sample preparation (Methods). GraFix ensures that MIRO1-MIRO1 dimers and MIRO1-TRAK1 complexes present in the sample do not dissociate at the low concentrations used in mass photometry (~50 nM). Alone, MIRO11-591 exhibited a mass distribution consisting of a prominent peak, followed by a trailing tail, observed in all purification fractions around the maximum UV absorbance peak used in cryo-EM analysis (Fig. 2f and Supplementary Fig. 5). Although the accuracy of mass photometry measurements decreases considerably for proteins and complexes <100 kDa35, the large peak had an average mass consistent with a MIRO11-591 monomer (68.4 ± 8.5 kDa) and accounted for 80.2% of the total mass measurements. The higher-mass species corresponded to either a dimer, observed as a separate minor peak in several of the measurements, or higher-order oligomers and aggregates. The addition of TRAK1569-623 resulted in two key effects: (a) the formation of a well-defined second peak and (b) a rightward shift in the average mass of the first peak. The average mass of the first peak (79.3 ± 14.1 kDa) was consistent with a mixture of MIRO11-591 monomers and monomeric MIRO11-591-TRAK1569-623 complexes, while that of the second peak (162.3 ± 8.3 kDa) indicated a combination of dimers of MIRO11-591 and MIRO11-591-TRAK1569-623. These data suggest that TRAK1 contributes to the formation of the MIRO1-TRAK1 dimer observed in the structure, and as noted earlier, this effect is likely further enhanced for the full-length proteins on the outer mitochondrial membrane.
Lack of a cofactor effect on the MIRO1-TRAK1 interaction
We previously established that Site-1 binds MIRO1 in a nucleotide- and Ca²⁺-independent manner28. Given that Site-2 makes extensive contacts with both the nGTPase and ELM1, we asked whether these cofactors could regulate its interaction. To assess the ability of MIRO11-591 (comprising both GTPase domains), MBP-MIRO11-180 (comprising the nGTPase), and MIRO1177-591 (comprising the cGTPase) to bind GTP or GDP, we used reverse phase high performance liquid chromatography28. MIRO11-591 was prepared in the presence of 100 µM GTP or GDP (10-fold molar excess to protein) in all buffers during purification, followed by 15-min incubation with a 35-fold molar excess nucleotide. Under GTP preparation conditions, MIRO11-591 contained 98% GTP bound, whereas under GDP conditions, it had 60% GDP bound (Fig. 3a, b). To confirm whether this represented the maximum GDP incorporation achievable, we performed an experiment where MIRO11-591 was purified and incubated in the presence of 1 mM GDP (100-fold molar excess to protein) and obtained the same result (Supplementary Fig. 6a, b). Therefore, to minimize cost, we proceeded with the initial protocol. Under these conditions, the constructs comprising the nGTPase and cGTPase bound 99% and 100% GTP, or 45% and 78% GDP, respectively (Fig. 3a, b). Notably, the nGTPase, purified from bacteria without added nucleotide, also contains approximately 99% GTP (Supplementary Fig. 6c). Note also that the average GDP incorporation percentages for the nGTPase and cGTPase closely match that observed for MIRO11-591.
a, b HPLC analysis and quantification of nucleotide bound to MIRO11-591, MBP-MIRO11-180, and MIRO1177-591. Proteins were purified and incubated for 15 min, respectively, with 10- and 35-fold molar excesses of nucleotide (GDP, pink trace; GTP, green trace). The normalized maximum absorbance at 256 nm is plotted as a function of retention time. c, e Representative SDS-PAGE analyses of MIRO11-591 pulled down by either MBP-TRAK1569-623 or MBP-TRAK1416-446,561-623 prepared under GTP or GDP conditions (as described in part a), with either 1 mM CaCl2 or 5 mM EGTA added during incubation. From left to right, the gel lanes correspond to the MIRO11-591 load control and amylose pulldowns of MIRO11-591, TRAK1 constructs, and MIRO11-591 + TRAK1 constructs. d, f Densitometric quantification of the pulldowns. Three biological replicates (N = 3), each comprising three technical replicates, were performed using different MIRO11-591 preparations. Data from each biological replicate are represented by distinct symbols (triangles, circles, or squares), with their corresponding averages outlined in black. For each MBP-TRAK1 construct and cofactor condition, replicates are normalized to the average of the CaCl2/GTP condition for that replicate. Colored bars (matching the gel contours in parts c, e) and brackets indicate the mean ± SD for the biological replicates. Statistical analysis was performed using a right-tailed one-way ANOVA with Tukey’s multiple comparisons test, revealing no statistically significant differences among the experiments (p > 0.05). Full gels and quantifications are provided in the Source Data file.
Due to the limited solubility of MIRO11-591, attempts to quantitatively characterize its interaction with TRAK1 Site-2 using fluorescence anisotropy or isothermal titration calorimetry (ITC), as previously done for Site-1 and MIRO1177-59128, were unsuccessful. Therefore, we turned to pulldown assays, assessing the ability of MBP-TRAK1569-623 to pull down MIRO11-591, prepared under GTP or GDP conditions as described above, and with either CaCl2 (1 mM) or ethylene glycol tetraacetic acid (EGTA, 5 mM) added during incubation. Pulldowns were analyzed by SDS-PAGE and densitometry, with results normalized to the corresponding CaCl2/GTP condition for each replicate to account for variability among MIRO1 preparations. Regardless of nucleotide or cation conditions, no statistically significant differences were observed (Fig. 3c, d). Notably, while the maximum GDP incorporation achieved by the nGTPase was 45% (Fig. 3b), the average pulldown amount under all conditions was greater than 90% of that observed with CaCl2/GTP.
We then asked if the nucleotide and Ca²⁺ states allosterically regulate the MIRO1-TRAK1 interaction when both Site-1 and Site-2 are present. We previously mapped Site-1 to the CR2 region of TRAK1 (Q399-S431) and showed that only the conserved C-terminal portion of CR2 is involved in the interaction (Fig. 1a and Supplementary Fig. 1a), with the mutant 425IPG427 ⟶ AAA abolishing MIRO1 binding28. Consistently, AlphaFold337 predicts that the binding motif comprises residues 425IPGS428 (see below). We further demonstrated that CR3, located between Site-1 and Site-2, does not bind MIRO1, and is predicted to form a coiled coil, which obscures pulldown interpretation. Based on these insights, we designed an MBP-fused Site 1-2 construct excluding CR3 and the N-terminal portion of CR2, consisting of TRAK1 residues R416-R446 and G561-Y623. This construct (MBP-TRAK1416-446,561-623) pulled down MIRO11-591, with no statistically significant differences observed across all cofactor conditions (Fig. 3e, f). We conclude that, at least under the conditions tested here, the MIRO1-TRAK1 interaction is not significantly affected by cofactor conditions.
Mutagenesis analysis of the MIRO1-TRAK1 interaction
Guided by the structure and sequence conservation, we examined the effect on MIRO11-591 binding of two TRAK1 Site-2 mutants, W589D and L597D. Both W589 and L597 are highly conserved (Fig. 4a and Supplementary Fig. 1a) and insert into hydrophobic pockets in the nGTPase and ELM1, respectively (Fig. 1c). In pulldown assays, both Site-2 mutants completely abolished the binding of GTP- and Ca2+-bound MIRO11-591 (Fig. 4b, c). However, when introduced into the hybrid Site 1-2 construct, W589D showed a more significant reduction in MIRO11-591 pulldown than L597D (Fig. 4d,e). This result suggests that, in the presence of Site-1, TRAK1’s interaction with the nGTPase is more critical than its interaction with ELM1 for the formation of the MIRO1-TRAK1 complex. It may also explain why the L594A mutation in mouse TRAK1 (equivalent to L597 in human TRAK1) abolished the interaction between Site-2 and MIRO1 in a yeast two-hybrid assay, whereas the same mutation did not noticeably affect the localization of full-length TRAK1 to mitochondria in cells31.
a Sequence alignment of a subgroup of TRAK1/2 from 183 vertebrate sequences (Supplementary Fig. 1a), highlighting conserved region 4 (CR4). Amino acids conserved in 85–97% and 97–100% of the sequences are shaded in light and dark blue, respectively. Residues W589 and L597, which were mutated to aspartate (W589D and L597D), are marked with stars. b, d Representative SDS-PAGE analyses of MIRO11-591 pulldown by wild-type (WT), W589D, and L597D variants of MBP-TRAK1569-623 and MBP-TRAK1416-446,561-623 (n = 4). Pulldowns were performed in the presence of 1 mM CaCl2 and 50 µM GTP. From left to right, the gel lanes correspond to the MIRO11-591 load control, and amylose pulldowns of MIRO11-591, MBP-TRAK1 constructs, and MIRO11-591 + MBP-TRAK1 constructs. c, e Densitometric quantification of the pulldowns. Colored bars (matching the gel contours in parts b and d) and brackets represent the mean ± SD for each pulldown condition. Data are normalized to the average of the corresponding WT MBP-TRAK1 construct. Statistical analyses were performed using a right-tailed one-way ANOVA with Tukey’s multiple comparisons test. P-values: part c (WT vs W589D p < 0.0001, WT vs L597D p < 0.0001, W589D vs L597D p = 0.9754), part e (WT vs W589D p < 0.0001, WT vs L597D p = 0.0019, W589D vs L597D p = 0.0001). P-values are also indicated in the figures, with ns for p > 0.05. Gels and quantifications are provided in the Source Data file.
Identification of the Site-1 binding pocket on MIRO1
AlphaFold337 was used to predict the binding pocket of TRAK1 Site1 on MIRO128, using as input the cytosolic domain of MIRO1 (MIRO11-591) and a TRAK1 fragment generously encompassing both Site-1 and Site-2 (TRAK1394-700). Consistent with our previous mutagenesis and binding studies28, AlphaFold3 predicted that Site-1 could be more narrowly defined as consisting of residues 425IPGS428, which form a loop that inserts into a MIRO1 pocket at the interface of ELM2 and the cGTPase (Fig. 5a and Supplementary Fig. 7). Similar predictions were obtained assuming monomeric or dimeric MIRO1–TRAK1 complexes and TRAK1 fragments of varying length. While the dimeric complexes generally agreed with the experimental structure, the orientation of the nGTPase relative to the ELMs–cGTPase deviated substantially from the cryo-EM structure and varied from model to model.
a Surface representation of the MIRO11-591-TRAK1569-623 dimeric complex, highlighting the pocket (yellow) where AlphaFold337 predicts TRAK1 Site-1 (shown in all-atom representation) binds at the interface between ELM2 and cGTPase. The per-residue confidence of this prediction, as indicated by the predicted local distance difference test (pLDDT) score and the predicted aligned error (PAE), is shown in Supplementary Fig. 7a, b. A close-up view displays details of the interaction using surface and all-atom representations, with MIRO1 residue D344 (mutated to lysine to validate this interaction) shown in red. b, c ITC titrations of MBP-TRAK1416-431 into MIRO1177-591 wild-type and mutant D344K in the presence of 1 mM CaCl2 and 50 µM GTP (left) or 5 mM EGTA and 50 µM GDP (right). The experimental conditions and fitting parameters (stoichiometry, N; dissociation constant, Kd) are indicated with each graph.
The MIRO1 residues predicted to line the Site-1-binding pocket include: P340, W341, G342, P343, D344, V345, T348, Q365, L368, T369, L372, D373, R376, Y380, R420, S455, Y456, Y457, H474, and A485. To validate this prediction, we tested whether mutating this pocket would disrupt Site-1 binding. For this, we used a more narrowly defined 16-amino acid Site-1 peptide (R416-S431) fused to MBP (MBP-TRAK1416-431) for easier purification and enhanced solubility. As before28, ITC was used to measure the binding of this peptide to both wild-type MIRO1177-591 and mutant D344K, which inverts the charge of a key amino acid in the predicted Site-1 binding pocket. Under both CaCl2/GTP and EGTA/GDP conditions, MBP-TRAK1416-431 bound wild-type MIRO1177-591 with 1:1 stoichiometry and dissociation constant (Kd) ranging from 10.9 to 15.9 µM (Fig. 5b), similar to previous measurements with the longer construct MBP-TRAK1342-43128. In contrast, no binding was observed with mutant D344K (Fig. 5c), confirming the AlphaFold3 prediction and the lack of cofactor effects on the Site-1 interaction.
Role of Sites 1 and 2 in TRAK1 mitochondrial recruitment
We assessed the contribution of Site-1 and Site-2 to TRAK1 recruitment to mitochondria, both separately and together. Wild-type and mutant variants of Halo-TRAK11-640 were expressed in HeLa cells. The following mutants were examined: 425IPG427 ⟶ AAA, W589D, L597D, W589D/L597D, and 425IPG427 ⟶ AAA/W589D/L597D (Fig. 6). None of the Halo-TRAK11-640 constructs localized to mitochondria unless co-expressed with Myc-MIRO1, known to be essential for the mitochondrial localization of exogenously expressed TRAK112,28 (Supplementary Fig. 8a–d). Myc-MIRO1 co-expression levels did not differ significantly among cells expressing the various Halo-TRAK1 constructs and mutants (Supplementary Fig. 8e, f).
a, c Representative maximum-intensity projections of Halo-tagged TRAK11-640 wild-type (WT) and Site-2 mutants (W589D, L597D, and W589D + L597D) or Site-1/Site-2 mutants (425IPG427 ⟶ AAA, W589D + L597D, and 425IPG427 ⟶ AAA + W589D + L597D), co-expressed in HeLa cells with Myc-MIRO1 and Mito-DsRed2. Scale bars represent 15 μm. b, d Mitochondrial-to-cytoplasmic intensity ratios of Halo-tagged TRAK11-640 WT and the mutants shown in (a, c). Data points are color-coded by experimental replicate (N = 3 biological replicates, n = 10 cells), with average values outlined in black. The center line and bars represent the mean ± SD of the three biological replicates. Statistical significance was determined using a right-tailed one-way ANOVA with Tukey’s multiple comparisons test. P-values: b (WT vs W589D p = 0.0696, WT vs L597D p = 0.8494, WT vs W589D + L597D p = 0.0192, W589D vs L597D p = 0.5728, W589D vs W589D + L597D p = 0.9965, L597D vs W589D + L597D p = 0.2345), d (WT vs 425IPG427⟶AAA p = 0.1111, WT vs W589D + L597D p = 0.0195, WT vs 425IPG427⟶AAA + W589D + L597D p = 0.0112, 425IPG427⟶AAA vs W589D + L597D p < 0.0001, 425IPG427⟶AAA vs 425IPG427⟶AAA + W589D + L597D p < 0.0001, W589D + L597D vs 425IPG427⟶AAA + W589D + L597D p > 0.9999). P-values are also indicated in the figures, with ns for p > 0.05. All data points and statistical tests are provided in the Source Data file.
We previously showed that when co-expressed with Myc-MIRO1, a significant fraction of Halo-TRAK11-440, lacking Site-2, localizes to mitochondria, and this localization was disrupted by the Site-1 mutation 425IPG427 ⟶ AAA28. However, we observed here that this mutation on its own does not significantly disrupt mitochondrial localization of the longer Halo-TRAK11-640 construct, which is likely recruited via Site-2 (Fig. 6c, d). Moreover, while individually the Site-2 mutations W589D and L597D appeared to reduce the mitochondrial localization of Halo-TRAK11-640, the effect was not statistically significant (Fig. 6a, b). Only when these two mutations were combined (mutant W589D/L597D) was the mitochondrial localization of Halo-TRAK11-640 significantly reduced, indicating that the interactions with both the nGTPase and ELM1 are critical for the formation of the MIRO1-TRAK1 complex. Similarly, the combined Site-1/Site-2 mutant 425IPG427 ⟶ AAA/W589D/L597D significantly decreased Halo-TRAK11-640 mitochondrial localization (Fig. 6c, d). These results suggest that both Site-1 and Site-2 contribute to TRAK1 localization to mitochondria via interaction with MIRO1, with Site-2 playing a predominant role.
Discussion
MIRO mediates the recruitment of numerous proteins to the outer mitochondrial membrane, including actin- and microtubule-based motors that drive mitochondrial dynamics7,8,17. This study describes the structure of the cytosolic domain of MIRO1, with Ca2+ and GTP bound to its ELM and GTPase domains, respectively. Structural, biochemical, and cellular data provide further insights into MIRO1’s interaction with the kinesin and dynein-dynactin motor adaptor TRAK1. This includes the characterization of two TRAK1-binding sites on MIRO1 and two MIRO1-binding sites on TRAK1, located C-terminal to the motor-binding region and ~140 amino acids apart. The structure also reveals that the MIRO1-TRAK1 complex assembles into a dimer, mediated by interactions between both proteins (Fig. 7). While this study focused on MIRO1 and TRAK1, the findings are also likely applicable to MIRO2 and TRAK2, as the identified contact surfaces are highly conserved across paralogs and species (Supplementary Fig. 1)28. These findings have broad implications for our understanding of MIRO1 regulation, dimerization, target recognition, and oligomerization on the mitochondrial membrane.
The MIRO1-TRAK1 complex forms a dimer at the outer mitochondrial membrane, with the dimerization interface involving MIRO1’s ELM2-cGTPase and TRAK1. TRAK1 Site-1 (I425-S428) and Site-2 (L570-R613) bind at the interface of MIRO1’s ELM2-cGTPase and nGTPase-ELM1, respectively. In vitro, both interactions are independent of cofactor conditions (Ca2+ or nucleotide), though their regulation in cells may involve other factors, such as microtubule-based motors and/or MIRO1 clustering on the mitochondrial membrane.
MIRO’s modular organization, comprising two GTPase domains and two EF-hand pairs, immediately suggests that its interactions with partners may be regulated by the nucleotide- and Ca2+-bound states. Consistent with this view, numerous studies have shown that these cofactors influence MIRO’s ability to recruit TRAK to mitochondria and, indirectly, affect motor-driven mitochondrial motility. However, the precise mechanism of regulation has remained elusive. For example, studies implicating MIRO’s nGTPase in TRAK binding disagree on whether the bound nucleotide regulates their interaction12,16 or not2. Other studies emphasize the role of the EF-hands in regulating the MIRO-TRAK interaction, noting that increases in local [Ca²⁺] halt mitochondrial motility in cells38,39,40, and that this effect is abolished by mutations disrupting Ca²⁺ binding to the EF-hands4,11,13,14. One study, however, reported that MIRO and TRAK interact and comigrate independently of Ca2+ 27, although we now know that the TRAK1 construct used in that study (TRAK11-532) lacked Site-2.
How does this work align with these sometimes-conflicting findings? Previously, we had concluded that the MIRO-TRAK interaction occurred independently of MIRO’s nucleotide and Ca2+ states28. However, like Canty et al.27, we used TRAK1 constructs lacking Site-2. Following further characterization here, we can now conclude that Site-1 mediates a relatively low-affinity interaction (Kd ~ 10 µM) between TRAK1 residues 425IPGS428 and a pocket at the ELM2-cGTPase interface of MIRO1 (Fig. 5a). This interaction is not regulated by nucleotide or Ca2+ 28, and may be distinct to TRAK, although we cannot rule out MIRO’s other partners or regulators binding in that pocket17. Because both MIRO and TRAK form dimers, the effective affinity of this interaction is likely enhanced through avidity on the mitochondrial membrane. TRAK may thus be recruited to mitochondria via Site-1 but remain folded in an autoinhibited conformation24, with activation and recruitment of microtubule-based motors requiring additional interactions via Site-2.
Site-2 of TRAK1 contacts both the nGTPase and ELM1, suggesting the interaction might be regulated. However, both the nGTPase and ELM1 are non-canonical1,2,9,10,32. Classical Ras GTPases function as nucleotide-dependent on/off switches in signaling cascades33. In the GTP-bound state, switches I and II form part of the target-binding interface, undergoing a conformational change upon nucleotide hydrolysis and γ-phosphate release that weakens the interaction with downstream effectors. The contacts between MIRO1’s nGTPase and Site-2 differ from this model in several ways. First, it remains unclear whether the nGTPase can hydrolyze GTP32 or whether nucleotide exchange occurs in cells, given that mammalian MIRO has no identified GAP or GEF41. Moreover, the nGTPase purifies from bacteria with nearly 100% GTP bound (Supplementary Fig. 6c), and even in the presence of 35-fold molar excess GDP, the maximum GDP incorporation observed was 45% (Fig. 3a, b), suggesting a strong preference for GTP and/or slow hydrolysis. Given these observations, and the fact that the cellular concentration of GTP is approximately 10-fold higher than GDP42, the nGTPase is likely GTP-bound in cells. Second, crystal structures of MIRO fragments have not revealed any nucleotide- or cation-dependent conformational changes9,10,32. Third, we do not observe extensive contacts between switches I and II of the nGTPase and Site-2. The only contact detected is a salt bridge between switch-I residue R36 and TRAK1 residue E572 (Fig. 1c), which appears to play a relatively minor role in Site-2 binding, which is primarily driven by hydrophobic interactions.
The contacts between MIRO1’s ELM1 and Site-2 also differ from those of classical EF-hand proteins. In most EF-hand proteins, Ca²⁺ binding induces a conformational change that exposes a hydrophobic pocket for target binding34. In MIRO, however, the ELMs are constitutively open, with their hydrophobic pockets occupied by the ligand mimic helix9,10. Furthermore, only the first EF-hand of each ELM binds Ca²⁺ and has the potential to be regulated. Finally, TRAK1’s interaction with ELM1 is both less extensive than its interaction with the nGTPase and involves a non-canonical hydrophobic pocket formed by the ligand mimic helix and both EF-hands (Fig. 1c). Based on these considerations, as we concluded for Site-1, Site-2 also appears unlikely to be regulated by the nucleotide- or Ca²⁺-bound states. Consistent with this conclusion, our pulldown experiments show that MIRO1 and TRAK1 interact under all cofactor conditions (Fig. 3c–f).
Unlike Site-1, which may be distinct to TRAK, the cleft at the nGTPase-ELM1 interface could serve as a common binding site for multiple MIRO effectors, including myosin-19, CENP-F, VPS13D, Parkin, mitofusin, and DISC1, as recently suggested31. However, their specific interactions likely differ, as these proteins do not share a clearly defined MIRO-binding motif. Furthermore, their ability to bind MIRO may be differentially regulated by Ca²⁺, nucleotide state, and interactions with other proteins. In TRAK, for instance, binding to microtubule-based motors may disrupt autoinhibitory interactions24, making Site-2 accessible for MIRO binding.
Effector binding may also be interdependent with MIRO dimerization. MIRO has two distinct dimerization interfaces that, while not mutually exclusive, cannot occur simultaneously. One interface, involving the nGTPase32, is incompatible with effector binding to the nGTPase-ELM1 cleft and may thus represent an autoinhibited state. The other interface, observed in the cryo-EM structure, involves ELM2-cGTPase and is enhanced by TRAK binding (Fig. 2c, d, f). Dimerization through either interface, as well as effector binding, may be influenced by multiple factors, including clustering on the mitochondrial membrane6, interactions between MIRO’s transmembrane helices, competition among MIRO effectors, effector dimerization, [Ca²⁺], and the nucleotide state4,11,12,13,14,16. In summary, while we have not found direct in vitro evidence that Ca2+ or nucleotide regulate the MIRO-TRAK interaction, our findings do not rule out other mechanisms that may modulate this interaction and its role in mitochondrial dynamics in cells.
Methods
Proteins
The DNA for human MIRO1 (Addgene, plasmid #127613), human TRAK1 (GenScript, E. coli codon-optimized), and TRAK1416-446,561-623 (GenScript, E. coli codon-optimized) were used as templates for cloning (primers listed in Source Data file). MIRO11-591 and MIRO1177-591 were cloned into Novagen’s pRSFDuet-1 and pETDuet-1 vectors, respectively. MBP-MIRO11-180, MBP-TRAK1569-623, and MBP-TRAK1416-446,561-623 were cloned into vector pMAL-c6T (New England Biolabs). TRAK1569-623 was cloned into vector pTYB12 (Addgene). The QuickChange mutagenesis kit (Agilent Technologies) was used to introduce the D344K mutation into MIRO1177-591, and the W589D and L597D mutations into MBP-TRAK1569-623 and MBP-TRAK1416-446,561-623. Purification tags were either included in the expression vectors or introduced during cloning.
Proteins were expressed in ArcticExpress (DE3) cells (Agilent Technologies), grown in terrific broth medium at 37 °C to an A600 of 1.5–2.0. Expression was induced with 0.35 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) after cooling the culture to 9 °C. After 24 h, cells were pelleted by centrifugation, resuspended in their respective buffers (detailed below) supplemented with 1 mM phenylmethylsulfonyl fluoride (PMSF) and 3 mM benzamidine hydrochloride hydrate, and lysed using a microfluidizer (Microfluidics). Lysates were clarified by centrifugation, and the resulting supernatants were loaded onto the corresponding affinity columns.
TRAK1-derived proteins were prepared in TRAK1 buffer (20 mM HEPES pH 7.4 and 200 mM NaCl). TRAK1569-623 was expressed as a Sce VMA intein/chitin domain fusion and affinity purified on a chitin column (New England Biolabs) using 20 mM HEPES pH 7.4, 500 mM NaCl, and 1 mM ethylenediaminetetraacetic acid (EDTA). Self-cleavage of the intein was induced by incubating the column in the same buffer supplemented with 40 mM dithiothreitol (DTT), and the protein was further purified on a SD200HL 26/60 column (GE HealthCare) in TRAK1 buffer. MBP-fused constructs (WT and mutants) were affinity purified on a Ni-NTA column (G-Biosciences) in TRAK1 buffer with 30 mM imidazole and eluted with 300 mM imidazole. Proteins were then further purified using a Strep-Tactin Sepharose column (IBA Lifesciences) and eluted in TRAK1 buffer supplemented with 40 mM biotin (pH 8.0).
MIRO1-derived proteins were prepared in MIRO1 buffer (20 mM HEPES, 300 mM NaCl, 1 mM MgCl2, and 1 mM CaCl2). Wash and elution buffers contained either no nucleotide or a 10-fold molar excess of GDP or GTP. MIRO11-591 was obtained in MIRO1 buffer supplemented with 500 mM NaCl, 5% (w/v) sucrose, 5% (w/v) glycerol, 30 mM imidazole, and 3 mM benzamidine hydrochloride hydrate. It was purified via Ni-NTA and Strep-Tactin affinity chromatography and eluted in the same buffer with 40 mM biotin (pH 8.0). MBP-MIRO11-180 was prepared in MIRO1 buffer without CaCl2, purified on an amylose affinity column, and eluted with 10 mM maltose. It was further purified on a Strep-Tactin Sepharose column and eluted in the same buffer with 40 mM biotin (pH 8.0). MIRO1177-591 (WT and D344K mutant) was prepared in MIRO1 buffer with 30 mM imidazole and purified using a Ni-NTA column.
Glycerol gradient ultracentrifugation and GraFix
A 5–30% glycerol gradient was prepared by layering 2 mL of light solution (20 mM HEPES pH 7.4, 300 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 100 µM GTP, 3 mM benzamidine hydrochloride hydrate, and 5% glycerol) over 2 mL of heavy solution (identical to the light solution but with 30% glycerol). For GraFix runs36, 0.125% (v/v) glutaraldehyde was added to the heavy solution. The gradient was formed using a Gradient Master 107 (BioComp Instruments). Samples (250 µL) containing either 25 µM MIRO11-591, 50 µM TRAK1569-623, and 100 µM GTP for cryo-EM or 10 µM MIRO11-591 and 100 µM GTP ± 40 µM TRAK1569-623 for mass photometry, were centrifuged at 165,000 g for 16 h at 4 °C in an SW-60 rotor and fractionated. Fractions without crosslinker were analyzed by SDS-PAGE, while GraFix samples were quenched with 80 mM glycine hydrochloride (pH 7.5) and used for cryo-EM or mass photometry.
Sample preparation and cryo-EM data collection
GraFix samples of Ca2+- and GTP-bound MIRO11-591 in complex with TRAK1569-623 were prepared as described above. Peak fractions were pooled, concentrated using a 10-kDa Amicon Ultra-4 centrifugal filter, and dialyzed for 30 min against 20 mM HEPES pH 7.5 and 200 mM NaCl to remove glycerol. Decyl maltose neopentyl glycol (DMNG) was added to a final concentration of 0.008 mM to improve particle distribution. Samples (3 µL) were pipetted onto glow-discharged (1 min, PELCO easiGLO) 300-mesh R1.2/1.3 Quantifoil holey carbon grids (Electron Microscopy Sciences). Grids were blotted with Whatman 41 filter paper for 2.5 s at blot force 7 and plunged into liquid ethane using a Vitrobot Mark IV (ThermoFisher Scientific).
Datasets were collected over three sessions using EPU software (Thermo Fisher Scientific) on a FEI Titan Krios transmission electron microscope operating at 300 kV. The microscope was equipped with a Gatan K3 direct electron detector and a quantum energy filter. Movies were recorded in super-resolution mode at a magnification of 105,000×, yielding a pixel size of 0.43 Å, with a defocus range of −0.5 to −2.5 μm.
Cryo-EM data processing
Movies (14314) were imported into CryoSPARC v4.6.043 and binned by two during patch motion correction, resulting in a pixel size of 0.86 Å. Patch contrast transfer function (CTF) estimation was performed, and micrographs with CTF > 8 Å were excluded, leaving 14034 accepted micrographs. Blob picking and 2D classification were performed on a subset of 788 micrographs to obtain well-defined particles, which were then used to train Topaz, a neural network-based particle-picking software44. After training, Topaz identified 2991759 particles, which were extracted with a box size of 300 pixels (258 Å) and subjected to 2D classification. Accepted particles (821667) were used to generate an ab initio volume, while rejected particles were used to generate a ‘junk’ volume (Supplementary Fig. 2).
The accepted particles underwent multiple rounds of heterogeneous refinement using different combinations of the ab-initio and ‘junk’ volumes as input. In each iteration, particles assigned to the ‘junk’ class(es) were discarded, and the global map quality was assessed to determine which class(es) contained particles suitable for further classification.
The final stack of 328,097 particles was subjected to reference-based motion correction and non-uniform refinement with C2 symmetry, yielding the final map. Map quality was assessed using cryoEF45 and 3DFSC46, with cryoEF reporting an efficiency (Eod) of 0.75 and 3DFSC reporting a sphericity of 0.979, both indicative of high-quality orientation distribution coverage. Local resolution estimation was performed in CryoSPARC. The cryo-EM workflow, map quality, and model statistics are presented in Supplementary Figs. 2 and 3 and Table 1.
Model refinement
Model building and refinement were performed in Coot47 and Phenix48, respectively, beginning with crystal structures of MIRO1 fragments (6D71 and 5KSZ). The real-space refinement function of Phenix was used to refine individual atomic positions and temperature factors. Extra density observed near MIRO1 cysteine residues 175, 322, and 350 was not modeled, but may be attributable to glutaraldehyde conjugation to the sulfhydryl groups49. Final model quality and refinement statistics are provided in Supplementary Fig. 3 and Table 1. Figures were prepared using PyMOL (Schrödinger, LLC) and ChimeraX50. For figures showing cryo-EM map density around specific areas of interest (Fig. 1c and Supplementary Fig. 4a), images were generated in ChimeraX by selecting the relevant regions of the model and extracting the corresponding cryo-EM map density using the command volume zone near [selection].
Mass photometry
Experiments were performed using a Refeyn TwoMP-0220 instrument, with data collection and analysis conducted using AcquireMP v2022 R1 and DiscoverMP v2022 R1 software. The instrument was calibrated with bovine serum albumin (MW: 66 kDa, 132 kDa) and Arp2/3 complex (MW: 224 kDa) as references. Three replicates (n = 3) of GraFix-derived36 MIRO11-591 ± TRAK1569-623 samples were analyzed, selecting the maximum UV-absorbance peak fractions (14, 16, and 18) for mass measurements. Samples were diluted to ~50 nM in 20 mM HEPES pH 7.4 and 200 mM NaCl to ensure well-separated landing events on the measurement surface. Gaussian curves were fitted to the sample histogram distributions using DiscoverMP software, and the masses and normalized counts were determined. Fits were accepted if the MW of the peak was within 25% of the expected mass of the species (MIRO11-591: 71 kDa, TRAK1569-623: 6.6 kDa), considering that mass photometry is less reliable for proteins with MW < 100 kDa and glutaraldehyde can increase the MW of crosslinked complexes. For each fit, the bin width was set to 6.1 kDa, and data were reported as the percentage relative to the bin with the highest number of counts. Histograms and Gaussian fits were rendered using Prism v10.2.2.
Analysis of bound nucleotide
The nucleotide bound to MIRO11-591, MBP-MIRO11-180, and MIRO1177-591 was analyzed using reverse-phase high-performance liquid chromatography (HPLC)28. Samples were prepared either without added nucleotide or with a 10- and 35-fold molar excess of GDP or GTP during purification and incubation, respectively. Prior to analysis, unbound nucleotide was removed using a Sephadex G-25 PD10 column (Global Life Science Solutions) equilibrated in 20 mM Tris pH 7.5. The peak fraction was denatured with 2.5 µL of 10% perchloric acid, neutralized with 2.5 µL of 4 M sodium acetate, and centrifuged to remove precipitates. The absorbance profiles of buffer controls treated in the same manner were subtracted. Supernatant (200 µL) was mixed with 100 µL of HPLC loading buffer (100 mM KH2PO4, 100 mM K2HPO4, and 10 mM tetrabutylammonium bromide, pH 6.5), and a 250 µL sample was injected and run isocratically using the HPLC loading buffer supplemented with 8.5% acetonitrile on a Vydac 208MS C8 column (VWR). Nucleotide content was determined by comparison to GDP and GTP standards analyzed under identical HPLC conditions.
Pulldown assays
Amylose resin (New England Biolabs) was washed with 20 mM HEPES (pH 7.5) and 200 mM NaCl, then resuspended as a 1:1 (v/v) slurry in the same buffer. Aliquots (200 µL) were stored in 1.5 mL microcentrifuge tubes. Pulldown buffers contained 20 mM HEPES (pH 7.5), 300 mM NaCl, 1 mM MgCl2, and the specified cofactor combination: 1 mM CaCl2 or 5 mM ethylene glycol tetraacetic acid (EGTA) with 175 µM GDP or GTP for cofactor pulldowns (Fig. 3) or 1 mM CaCl2 with 50 µM GTP for WT/mutant pulldowns (Fig. 4). For cofactor pulldowns, MIRO11-591 was purified and incubated with GDP or GTP as described above, with the addition of either 5 mM EGTA or 1 mM CaCl2. For WT/mutant pulldowns, freshly purified MIRO11-591 was pre-incubated with pulldown buffer containing 1 mM CaCl2 and 50 µM GTP, resulting in GTP-bound MIRO11-591 (Supplementary Fig. 6b). After a 15-min incubation, MIRO11-591 (5 nmol) and MBP-TRAK1569-623 or MBP-TRAK1416-446,561-623 (WT or mutants) (2.5 nmol) were added to the resin slurry. The total incubation volume was adjusted to 1 mL with pulldown buffer. Samples were incubated on ice for 10 min, then washed three times by pelleting and resuspending in 1 mL of pulldown buffer. The resin was resuspended in 150 µL of pulldown buffer supplemented with 20 mM maltose and incubated for 10 min. A 100 µL elution was collected, mixed with 33 µL of loading dye containing a reducing agent, and analyzed by SDS-PAGE. Protein gels were imaged using GENESys V1.5.6.0 (Genesys Limited), and band intensities were quantified with ImageLab (Bio-Rad). To account for variability among MIRO11-591 preparations, pulldown data were normalized to the average intensity of the CaCl2/GTP condition for cofactor pulldowns or the WT condition for WT/mutant pulldowns. All full images of protein gels are included in the Source Data file, and quantifications are shown in Figs. 3 and 4.
Isothermal titration calorimetry
Proteins were co-dialyzed for three days against 20 mM HEPES pH 7.5, 300 mM NaCl, 1 mM MgCl2, 5% w/v sucrose, and 0.125 mM TCEP, in the presence of either 1 mM CaCl2 and 50 μM GTP or 5 mM EGTA and 50 μM GDP. ITC experiments were performed using a VP-ITC instrument (MicroCal). MBP-TRAK1416-431 in the syringe was titrated into MIRO1177-591 (WT or mutant D344K) in the 1.44 mL cell (protein concentrations specified in Fig. 5b,c). A total of 29 injections (10 μL each and lasting 10 s) were performed at 25 °C, with 200-s intervals between injections. The heats of binding were corrected by subtracting the heats of control injections into buffer. Data were analyzed using Origin software (OriginLab).
Recruitment of Halo-TRAK1 to mitochondria in HeLa cells
HeLa-M cells (A. Peden, Cambridge Institute for Medical Research) were maintained in a 37 °C incubator with 5% CO2 in Dulbecco’s modified Eagle’s medium (Corning) supplemented with 10% fetal bovine serum (HyClone) and 1% GlutaMAX (Thermo Fisher Scientific)28. The cells were authenticated by short tandem repeat profiling by the University of Pennsylvania DNA Sequencing Core and confirmed negative for mycoplasma contamination.
Cells were plated on 35 mm glass-bottom dishes (MatTek) and transfected using FuGENE Transfection Reagent (Promega). After 24 h, cells were fixed in warm PBS containing 4% paraformaldehyde and 4% sucrose for 10 min, then washed three times with PBS. To label Halo-tagged proteins, cells were incubated at 37 °C for 20 min with 100 nM JaneliaFluor 646-Halo ligand (Promega) in PBS, followed by a 30-min wash in PBS at 37 °C. Cells were then permeabilized for 15 min with 0.2% Triton X-100 in PBS, washed three times with PBS, and blocked for 1 h in PBS containing 5% goat serum and 1% bovine serum albumin. For immunostaining, cells were incubated overnight at 4 °C with a 1:1000 dilution of α-Myc antibody (Invitrogen, R950–25, lot #2669480) in blocking buffer. After three washes with PBS, cells were incubated for 1 h with Alexa Fluor 488 goat anti-mouse IgG (H + L) (Invitrogen, A11029, lot ##2747581) diluted 1:1000 in blocking buffer, followed by three final PBS washes.
Cells were imaged using a Nikon Eclipse Ti microscope equipped with a V3 spinning disk confocal system and an Apochromat 100× 1.49 NA oil immersion objective (Nikon). Images were acquired using VisiView software (Visitron, https://www.visitron.de/products/visiviewr-software.html) on a Hamamatsu ORCA-Fusion digital CMOS camera. Z-stacks were acquired with 200-nm step-size increments. Maximum intensity projections for each channel were generated in ImageJ (NIH, https://imagej.net/ij/download.html) and used to create binarized masks of the mitochondrial network using the pixel classification module of Ilastik51. The mean fluorescence intensity of Halo-TRAK1 fragments and Myc-MIRO1 was then measured in each cell, distinguishing between the mitochondrial network and other regions.
Statistics
Statistical analyses were performed using Prism v10.2.2. Pulldown data assessing cofactor dependence (Fig. 3) and TRAK1 mutations (Fig. 4) were analyzed using a right-tailed one-way ANOVA with Tukey’s multiple comparisons test (N = 3, n = 3 and N = 1, n = 4, respectively). Identical statistical tests were applied to both raw and normalized data. For Halo-TRAK1 mitochondrial recruitment assays (Fig. 6, Supplementary Fig. 8), means of mitochondrial-to-cytoplasmic ratios for Halo-TRAK1 and Myc-MIRO1 from biological replicates (N = 3, n = 10) were analyzed using a right-tailed one-way ANOVA. All p < 0.05 are shown in the corresponding figures (or marked as ns if p > 0.05), with all data points and p-values provided in the Source Data file. Prior to analysis, pulldown and mitochondrial localization data passed normality and log-normality tests.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The cryo-EM map was deposited in the Electron Microscopy Data Bank under accession code EMD-47460. Atomic coordinates were deposited in the Protein Data Bank under accession code 9E2P. Unprocessed cryo-EM micrographs were deposited in the Electron Microscopy Public Image Archive under accession code EMPIAR-12448. The PDB accession codes for the initial models used in map interpretation and refinement were 6D71 and 5KSZ. Source Data are provided as a Source Data file. Source data are provided with this paper.
References
Fransson, A., Ruusala, A. & Aspenstrom, P. Atypical Rho GTPases have roles in mitochondrial homeostasis and apoptosis. J. Biol. Chem. 278, 6495–6502 (2003).
Fransson, S., Ruusala, A. & Aspenstrom, P. The atypical Rho GTPases Miro-1 and Miro-2 have essential roles in mitochondrial trafficking. Biochem. Biophys. Res. Commun. 344, 500–510 (2006).
Weihofen, A., Thomas, K. J., Ostaszewski, B. L., Cookson, M. R. & Selkoe, D. J. Pink1 forms a multiprotein complex with Miro and Milton, linking Pink1 function to mitochondrial trafficking. Biochemistry 48, 2045–2052 (2009).
Saotome, M. et al. Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc. Natl. Acad. Sci. USA 105, 20728–20733 (2008).
Kanfer, G. et al. Mitotic redistribution of the mitochondrial network by Miro and Cenp-F. Nat. Commun. 6, 8015 (2015).
Modi, S. et al. Miro clusters regulate ER-mitochondria contact sites and link cristae organization to the mitochondrial transport machinery. Nat. Commun. 10, 4399 (2019).
Eberhardt, E. L., Ludlam, A. V., Tan, Z. & Cianfrocco, M. A. Miro: a molecular switch at the center of mitochondrial regulation. Protein Sci. 29, 1269–1284 (2020).
Aspenstrom P. Miro GTPases at the Crossroads of Cytoskeletal Dynamics and Mitochondrial Trafficking. Cells 13, (2024).
Klosowiak, J. L. et al. Structural coupling of the EF hand and C-terminal GTPase domains in the mitochondrial protein Miro. EMBO Rep. 14, 968–974 (2013).
Klosowiak, J. L. et al. Structural insights into Parkin substrate lysine targeting from minimal Miro substrates. Sci. Rep. 6, 33019 (2016).
Macaskill, A. F. et al. Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron 61, 541–555 (2009).
MacAskill, A. F., Brickley, K., Stephenson, F. A. & Kittler, J. T. GTPase dependent recruitment of Grif-1 by Miro1 regulates mitochondrial trafficking in hippocampal neurons. Mol. Cell Neurosci. 40, 301–312 (2009).
Wang, X. & Schwarz, T. L. The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility. Cell 136, 163–174 (2009).
Chang, K. T., Niescier, R. F. & Min, K. T. Mitochondrial matrix Ca2+ as an intrinsic signal regulating mitochondrial motility in axons. Proc. Natl. Acad. Sci. USA 108, 15456–15461 (2011).
Babic, M. et al. Miro’s N-terminal GTPase domain is required for transport of mitochondria into axons and dendrites. J. Neurosci. 35, 5754–5771 (2015).
Davis, K., Basu, H., Izquierdo-Villalba, I., Shurberg, E. & Schwarz, T. L. Miro GTPase domains regulate the assembly of the mitochondrial motor-adaptor complex. Life. Sci. Alliance 6, e202201406 (2022).
Li, Y., Yang, Z., Zhang, S. & Li, J. Miro-mediated mitochondrial transport: A new dimension for disease-related abnormal cell metabolism?. Biochem. Biophys. Res. Commun. 705, 149737 (2024).
Oeding S. J. et al. Identification of Miro1 and Miro2 as mitochondrial receptors for myosin XIX. J. Cell Sci. 131, jcs219469 (2018).
Bocanegra, J. L. et al. The MyMOMA domain of MYO19 encodes for distinct Miro-dependent and Miro-independent mechanisms of interaction with mitochondrial membranes. Cytoskeleton77, 149–166 (2020).
Coscia S. M. et al. Myo19 tethers mitochondria to endoplasmic reticulum-associated actin to promote mitochondrial fission. J. Cell Sci. 136, jcs260612 (2023).
Smith, M. J., Pozo, K., Brickley, K. & Stephenson, F. A. Mapping the GRIF-1 binding domain of the kinesin, KIF5C, substantiates a role for GRIF-1 as an adaptor protein in the anterograde trafficking of cargoes. J. Biol. Chem. 281, 27216–27228 (2006).
Glater, E. E., Megeath, L. J., Stowers, R. S. & Schwarz, T. L. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J. Cell Biol. 173, 545–557 (2006).
Brickley, K. & Stephenson, F. A. Trafficking kinesin protein (TRAK)-mediated transport of mitochondria in axons of hippocampal neurons. J. Biol. Chem. 286, 18079–18092 (2011).
van Spronsen, M. et al. TRAK/Milton motor-adaptor proteins steer mitochondrial trafficking to axons and dendrites. Neuron 77, 485–502 (2013).
Randall, T. S., Moores, C. & Stephenson, F. A. Delineation of the TRAK binding regions of the kinesin-1 motor proteins. FEBS Lett. 587, 3763–3769 (2013).
Fenton, A. R., Jongens, T. A. & Holzbaur, E. L. F. Mitochondrial adaptor TRAK2 activates and functionally links opposing kinesin and dynein motors. Nat. Commun. 12, 4578 (2021).
Canty, J. T., Hensley, A., Aslan, M., Jack, A. & Yildiz, A. TRAK adaptors regulate the recruitment and activation of dynein and kinesin in mitochondrial transport. Nat. Commun. 14, 1376 (2023).
Baltrusaitis, E. E. et al. Interaction between the mitochondrial adaptor MIRO and the motor adaptor TRAK. J. Biol. Chem. 299, 105441 (2023).
Brickley, K., Smith, M. J., Beck, M. & Stephenson, F. A. GRIF-1 and OIP106, members of a novel gene family of coiled-coil domain proteins: association in vivo and in vitro with kinesin. J. Biol. Chem. 280, 14723–14732 (2005).
Pekkurnaz, G., Trinidad, J. C., Wang, X., Kong, D. & Schwarz, T. L. Glucose regulates mitochondrial motility via Milton modification by O-GlcNAc transferase. Cell 158, 54–68 (2014).
Covill-Cooke, C. et al. Shared structural features of Miro binding control mitochondrial homeostasis. EMBO J. 43, 595–614 (2024).
Smith, K. P. et al. Insight into human Miro1/2 domain organization based on the structure of its N-terminal GTPase. J. Struct. Biol. 212, 107656 (2020).
Mozzarelli, A. M., Simanshu, D. K. & Castel, P. Functional and structural insights into RAS effector proteins. Mol. Cell 84, 2807–2821 (2024).
Yap, K. L., Ames, J. B., Swindells, M. B. & Ikura, M. Diversity of conformational states and changes within the EF-hand protein superfamily. Proteins 37, 499–507 (1999).
Kratochvil, J. et al. Best practice mass photometry: A guide to optimal single molecule mass measurement. BioRxiv (2024).
Stark, H. GraFix: stabilization of fragile macromolecular complexes for single particle cryo-EM. Methods Enzymol. 481, 109–126 (2010).
Abramson, J. et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 630, 493–500 (2024).
Yi, M., Weaver, D. & Hajnoczky, G. Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J. Cell Biol. 167, 661–672 (2004).
Brough, D., Schell, M. J. & Irvine, R. F. Agonist-induced regulation of mitochondrial and endoplasmic reticulum motility. Biochem. J. 392, 291–297 (2005).
Quintana, A. et al. Sustained activity of calcium release-activated calcium channels requires translocation of mitochondria to the plasma membrane. J. Biol. Chem. 281, 40302–40309 (2006).
Suzuki, M., Danilchanka, O. & Mekalanos, J. J. Vibrio cholerae T3SS effector VopE modulates mitochondrial dynamics and innate immune signaling by targeting Miro GTPases. Cell Host Microbe 16, 581–591 (2014).
Traut, T. W. Physiological concentrations of purines and pyrimidines. Mol. Cell Biochem. 140, 1–22 (1994).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Bepler, T. et al. Positive-unlabeled convolutional neural networks for particle picking in cryo-electron micrographs. Nat. Methods 16, 1153–1160 (2019).
Naydenova, K. & Russo, C. J. Measuring the effects of particle orientation to improve the efficiency of electron cryomicroscopy. Nat. Commun. 8, 629 (2017).
Tan, Y. Z. et al. Addressing preferred specimen orientation in single-particle cryo-EM through tilting. Nat. Methods 14, 793–796 (2017).
Casanal, A., Lohkamp, B. & Emsley, P. Current developments in Coot for macromolecular model building of Electron Cryo-microscopy and Crystallographic Data. Protein Sci. 29, 1069–1078 (2020).
van Zundert, G. C. P., Moriarty, N. W., Sobolev, O. V., Adams, P. D. & Borrelli, K. W. Macromolecular refinement of X-ray and cryoelectron microscopy structures with Phenix/OPLS3e for improved structure and ligand quality. Structure 29, 913–921.e914 (2021).
Migneault, I., Dartiguenave, C., Bertrand, M. J. & Waldron, K. C. Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 37, 790–796 798-802 (2004).
Pettersen, E. F. et al. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Berg, S. et al. ilastik: interactive machine learning for (bio)image analysis. Nat. Methods 16, 1226–1232 (2019).
Acknowledgements
Supported by National Institutes of Health (NIH) grant RM1 GM136511 (R.D. and E.L.F.H.), R01 GM073791 (R.D.), and T32 GM132039 (E.E.B.).
Author information
Authors and Affiliations
Contributions
E.E.R., E.E.B. and R.D. conceptualized the project. E.E.R. and E.E.B. obtained proteins and performed biochemical experiments. E.E.R., E.E.B. and K.R.B. determined the cryo-EM structure. T.A.P., A.R.F. and E.L.F.H. performed the cell biology experiments. E.E.R., E.E.B. and R.D. prepared figures and wrote the manuscript. All authors participated in the analysis of the data and reviewed the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Ahmet Yildiz and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ravitch, E.E., Baltrusaitis, E.E., Perez, T.A. et al. Structural-functional characterization of the MIRO1-TRAK1 complex. Nat Commun 16, 6173 (2025). https://doi.org/10.1038/s41467-025-61174-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-61174-6