Abstract
Mast cells (MC) serve as pivotal sentinels in the regulation of immune responses and inflammation, yet their function in lung adenocarcinoma (LUAD) remains largely neglected. To decode their heterogeneity, we perform single-cell transcriptomic analysis of LUAD-infiltrating MCs. Our study uncovers the complexity in MC composition and identifies 9 distinct states, including proinflammation, chemotaxis, and antigen presentation. The proinflammatory MC subset, characterized by high IL-18 expression, is associated with improved outcomes for LUAD patients. This pro-inflammatory property is regulated by the activation of NLRP3 inflammasome within MCs, resulting in the formation of GSDMD pores and successive pyroptosis. Moreover, these MCs enhance the innate-like anti-tumor activity of MAIT cells by upregulating NKG2D and IFN-γ through the cytokine-activation mechanism. Our results uncover an unappreciated state of MCs and describe an inflammasome-dependent, MC-mediated regulation of MAIT cells in LUAD. These findings diversify our understanding of the functional repertoire and mechanistic equipment of MCs and MAIT cells, and suggest a potential therapeutic target for cancer treatment.
Similar content being viewed by others
Introduction
Mast cells (MC), as evolutionarily conserved and ancient innate immune cells, are well-known for their crucial roles in IgE-mediated allergic disorders1. The specialized sentinel identity and potent immunomodulatory properties have sparked an increasing recognition of the importance of MCs in the context of tumors, extending beyond their traditional roles in allergies and parasitic infections2,3,4,5. The observation of robust infiltration of MCs across cancers has highlighted their involvement in the tumor microenvironment, such as tumor angiogenesis and microenvironment remodeling6,7,8. The exceptional advantage of MCs resides in the high content of cytoplasmic electron-dense secretory granules containing substantial preformed inflammatory mediators, peptides, proteases, cytokines, and chemokines, which are closely associated with the effects that MCs have on the tumor microenvironment in many instances9,10,11. Moreover, the intricate crosstalk between MCs and other immune cells, tumor cells, and stromal cells within the tumor microenvironment has deepened our understanding of the complex function of MCs12,13,14,15. Given their versatile repertoires, unique differentiation, and maturation trajectory occurring locally, and location proximity to blood vessels and peripheral nerves, MCs present a compelling target for therapeutic interventions in the tumor immunotherapy16.
Inflammasomes are critical sentinels of the innate immune system, forming supramolecular complexes composed of a cytosolic sensor protein, an adaptor protein, an apoptosis-associated speck-like protein containing a CARD (ASC), and a downstream effector protein, pro-caspase-117,18. The assembly of inflammasomes triggers the activation of caspase-1, leading to the maturation and secretion of proinflammatory IL-1β and IL-18, which in turn drive inflammation and pyroptosis, a form of inflammatory cell death19,20. Most studies on inflammasomes have primarily focused on macrophages, dendritic cells, and certain subsets of T cells21,22,23,24. Previous research has demonstrated that MCs can release appreciable amounts of IL-1β through NLRP3 inflammasome activation in various non-neoplastic conditions, including anaphylaxis, Cryopyrin-associated periodic syndromes (CAPS), experimental autoimmune encephalomyelitis (EAE)25,26,27,28,29. However, despite the recognized role of inflammasomes in tumor immunity30,31, no studies have yet investigated the involvement of inflammasome activation in MCs in the tumor microenvironment.
In this study, we investigate the infiltration patterns and function of MCs in lung adenocarcinoma (LUAD), based on a large single-cell RNA sequencing (scRNA-seq) cohort. We find that MCs exhibit complex and diverse transcriptional heterogeneity with 9 distinct cell states. In particular, proinflammatory MCs engage in the regulation of innate-like anti-tumor effects of MAIT cells via inflammasome-dependent secretion of IL-18. Our study unravels a new mechanism of MCs as well as an unconventional mode of cytokine secretion by MCs. Our findings also reveal the potential role of the crosstalk between MCs and MAIT cells in establishing an anti-tumor LUAD microenvironment and suggest that targeting the MCs-MAIT cell axis may provide potential therapeutic opportunities.
Results
MC infiltration patterns during LUAD progression
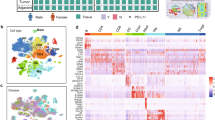
To decipher the infiltration levels and patterns of MCs in the LUAD microenvironment, we first evaluated eight immune infiltration deconvolution algorithms in our in-house FUSCC_1019 cohort (Fig. 1A and Supplementary Fig. 1A). CIBERSORT and xCell exhibited significant consistency in quantifying MC infiltration levels (Supplementary Fig 1B, Supplementary Data 1A, B), with validation performed in the independent FUSCC_150 cohort32 (Supplementary Fig 1C, D, Supplementary Data 1C, D). This reliability may be indicative of the unique biological and transcriptional signatures associated with MCs33. Integrating MCs infiltration profiling with genomic, radiologic, pathologic, and clinical features revealed significant variability in the distribution of MCs, which showed distinct patterns in relation to radiological and pathological characteristics (Fig. 1B). Specifically, elevated MC densities were identified in younger patients, females, never-smokers, and in cases exhibiting EGFR mutation along with wild-type KRAS and TP53 LUAD (Supplementary Fig. 1E).
A Schematics of study design. B The infiltration abundance of MCs among LUAD-infiltrating immune cells deconvoluted by CIBERSORT. The clinical features and mutation status for selected genes are shown at the bottom. C The dynamic infiltration pattern of MCs during LUAD progression in the FUSCC_1019 cohort (white, nLung; green, LUAD, n = 1019). The infiltration abundance of MCs was deconvoluted by CIBERSORT. Data were presented as mean + SEM. D t-SNE visualization and the proportion of MCs subsets. E Heatmap of marker genes in each MCs subset. F Enriched gene ontology terms of each MCs subset. Benjamini–Hochberg adjusted P values were shown. G The prognostic value of each MCs subset signature in the FUSCC_1019 cohort, the FUSCC-150 cohort, and OAK cohort, with numbers in each square showing the hazard ratio value. p values were determined by the log-rank test. NS not significant. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001. H Representative mIF staining of IL-18 (violet) and tryptase (green) for MCs in LUAD. Nuclei were counterstained with DAPI (gray). Yellow arrowhead, IL-18+ MCs; scale bars, 30 μm. Each group includes more than 3 replicates. I Survival analysis of IL-18+ MCs in FUSCC_TMA cohort using mIF. p values were determined by log-rank test. J Profiles of differentially expressed cytokines (left), chemokines (middle), and pathways (right) in each MCs subset. K Gene Set Enrichment Analysis of IL-18+ MC subset compared with other MC subsets using the hallmarker inflammatory response geneset form MSigDB. p value was generated by GSEA analysis, with permutation test as its fundamental inferential mechanism. NES, normalized enrichment score. See also Supplementary Fig. 1-2 and Supplementary Data 1-4 and Supplementary Table 1.
LUAD can be characterized by varying degrees of aggressiveness, with some cases exhibiting indolent behavior while others show invasive and metastatic potential34. Indolent LUAD typically remains stable for extended periods without progression, often manifesting as ground-glass opacity (GGO) in radiological evaluations, which may be associated with pathological conditions such as atypical adenomatous hyperplasia (AAH), adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), and lepidic adenocarcinoma35,36. We found that MC infiltration was elevated in indolent LUAD compared to normal lung tissue (nLung), whereas a substantial decrease was observed when LUAD evolved into an invasive stage (Fig. 1C). Flow cytometry was employed to accurately identify MCs, gated on CD45+ Lin- CD117+ FceR1α+ cells (Supplementary Fig. 1F, G), which demonstrated a similar trend along with LUAD progression (Supplementary Fig. 1H). These findings suggested that MCs may have a role in maintaining the indolent state of LUAD from progression.
Taken together, these results emphasized the variability in MC infiltration throughout LUAD progression and established a foundation for further investigation into the functional heterogeneity of MCs in the tumor microenvironment.
Single-cell transcriptome profiling of MCs
To perform comprehensive single-cell transcriptome analysis for infiltrating MCs, we generated an in-house scRNA-seq dataset (n = 24) and integrated them with published scRNA-seq data (Fig. 1D and Supplementary Fig. 2A). Following rigorous quality control and unsupervised clustering, we identified MC populations by retaining clusters that exhibited high expression of canonical MC marker genes (Supplementary Fig. 2B). The resulting MC data comprised 14,590 MCs derived from 115 samples, including 51 nLung, 11 benign lung inflammation, and 53 tumors, with 50.5% of the data originating from in-house datasets (Fig. 1D and Supplementary Fig. 2C). The average infiltration percentage of MCs across all samples was 3.07% (Fig. 1D).
The unbiased clustering analysis revealed nine distinct MC subsets with different maturation and functional states: IL-18+, FCERIA+, CD81+, RPL+, SLC24A3+, HSP90+, EEF1G+, CD74+, and CCL5+ MCs, according to the expression of canonical gene markers (Fig. 1D and Supplementary Fig. 2D, E, Supplementary Data 2). Notably, IL-18+ MCs represented a proinflammatory subset, predominantly enriched in tumor tissues, and were implicated in the regulation of proinflammatory responses and T cell activation (Fig. 1E, F, Supplementary Data 3). MC subsets with antigen-presenting (CD74+ MCs) and chemotactic (CCL5+ MCs) properties were also identified (Fig. 1E, F, Supplementary Data 3). Additionally, previously less-characterized MC subsets were identified. HSP90+ MCs exhibited a stress-response phenotype, marked by elevated expression of heat shock proteins and stress-related molecules (Fig. 1E, F, Supplementary Data 3), a feature similarly observed in other immune cell types37,38. RPL+ MCs, primarily derived from tumor tissues, were distinguished by high expression of ribosomal proteins (Fig. 1E, F, Supplementary Data 3). Ultimately, this atlas not only documented established MCs subpopulations but also uncovered potentially novel subsets that merit further investigation.
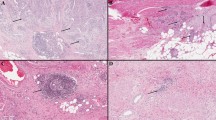
Based on the Ro/e analysis, we identified a prominent enrichment of IL-18+ and CCL5+ MCs within tumors, while FCER1A+, HSP90+, and CD74+ MCs exhibited a preference for nLung (Fig. 1D and Supplementary Fig. 2F). To examine the association between MC subsets with patient survival, we evaluated the MC subset signature scores (Supplementary Table 1) across three independent cohorts: the FUSCC_1019 cohort, the FUSCC_150 cohort, and the external OAK cohort39 (Fig. 1G and Supplementary Data 4). Among the subsets tested, HSP90+ and EEFG1+ MCs were correlated with poorer clinical outcomes, whereas IL-18+ and CD74+ MCs were associated with a favorable prognosis (Fig. 1G and Supplementary Fig. 2G, H). Additionally, the secretion of IL-18 by MCs was confirmed through multi-immunofluorescence (mIF) on LUAD tissue sections (Fig. 1H). The prognostic significance of was substantiated in the FUSCC_TMA cohort (Fig. 1I and Supplementary Fig 2I). Collectively, these findings underscored the potential role of tumor-enriched IL-18+ MCs with anti-tumor properties.
Proinflammatory characteristic of IL-18+ MCs
To elucidate the functional role of IL-18+ MCs within the tumor microenvironment, we integrated cytokine profiling and gene signature analysis. The IL-18+ MC subset was characterized by high expression of proinflammatory cytokines, including IL-6, IL-18, CSF-1, and chemokine CCL20 (Fig. 1J), as well as genes associated with Toll-like receptor (TLR) and NOD-like receptor (NLR) signaling pathways (Fig. 1J), which are vital for detecting pathogen-associated and damage-associated molecular patterns (PAMP and DAMP) and triggering the innate immune responses40. In addition, IL-18+ MCs exhibited elevated histamine secretion activity, further reinforcing their proinflammatory profile (Fig. 1J). A direct comparison between IL-18+ MCs and other MC subsets revealed significantly enhanced proinflammatory signatures in IL-18+ MCs, as evidenced by Gene Set Enrichment Analysis (Fig. 1K). Moreover, IL-18+ MCs displayed notable enrichment in lipid metabolism, as determined by the analysis of metabolic activity across MC subsets (Supplementary Fig. 2J, Supplementary Data 5). These findings collectively supported the proinflammatory nature of IL-18+ MCs, which likely contributed to the creation of an inflamed tumor microenvironment. In support of this, we observed that MCs infiltration was preferentially associated with inflammatory tumor microenvironments (Supplementary Fig. 2K), as opposed to tumor types characterized by lymphocyte depletion or immunologically silent features, consistent with the proinflammatory role of IL-18+ MCs.
To determine whether IL-18 expression is a general feature of MCs, we distinguished between two well-characterized MC types: mucosal MCs (MCT subset, expressing tryptase alone) and connective tissue MCs (MCTC subset, expressing both tryptase and chymase) based on the canonical markers TPSAB1 and CMA141. No significant differences in IL-18 expression were observed between the two MC types (Supplementary Fig. 2L), suggesting that IL-18 expression was not strictly associated with their classical dichotomy or tissue-specific localization. Moreover, analysis of IL-18 expression across all cell types within the microenvironment revealed that IL-18 was predominantly confined to MCs and myeloid cells, with MCs expressing a higher level of IL-18 than myeloid cells (Fig. 2A), which were previously assigned to IL-18-producing cells.
A Dot plot for the expression of IL1B and IL18 among all cell subtypes in LUAD. B The concentration of IL-18 in cell culture supernatants of MCs after stimulation with or without LPS and Nig, Brefeldin A by ELISA. Each group includes 6 replicates, p value was determined by two-tailed paired Student’s t-test. C Immunoblot analysis of cell lysates of MCs or macrophages (Mø) from nLung and LUAD, or LUVA cell line after stimulation with or without LPS and Nig. Each group includes more than 3 replicates. D The proportion of MCs subsets ranked by inflammasome complex assembly index (upper panel). MCs subsets ranked by inflammasome complex component (y axis) and inflammasome complex assembly index (x axis, lower panel). The dot size represents the IL-18 expression percentage of each MCs subset. E FPKM of inflammasome-associated gene expression in LUVA cell line or MCs from nLung and LUAD. Each group includes 3 replicates in MCs from nLung and LUAD and 2 replicates in LUVA cell line. F Immunoblot analysis of cell lysates of MCs or macrophages (Mø) from nLung and LUAD, or LUVA cell line after stimulation with or without LPS and Nig. Casp-1, Caspase 1. Each group includes more than 3 replicates. G Representative mIF staining of NLRP3 (green, upper), ASC (green, lower), and tryptase (violet) for MCs in LUAD sections. Nuclei were counterstained with DAPI (gray). Yellow arrowhead, NLRP3+ MCs (upper), ASC+ MCs (lower); scale bars, 30 μm. Each group includes more than 3 replicates. H Representative images of ASC (yellow) co-staining with NLRP3 (red) in nLung and LUAD MCs using imaging flow cytometry. BF, bright-field. Scale bars, 10 μm. Each group includes more than 3 replicates. I Immunoblot analysis of cell lysates of nLung and LUAD MCs after stimulation with or without LPS and Nig in the presence or absence of indicated inhibitors. Casp-1, Caspase 1. J The concentration of IL-18 in cell culture supernatants of MCs after stimulation with or without LPS and Nig in the presence or absence of indicated NLRP3 inhibitors by ELISA. Each group includes 6 replicates, p values were determined by two-tailed Student’s t-test. See also Supplementary Fig. 3 and Supplementary Table 2.
Taken together, these results indicated that, despite their well-established role in allergic responses, MCs in the tumor microenvironment served as a principal source of the inflammatory cytokine IL-18. We highlighted that the proinflammatory expression of IL-18 represented a distinct and critical function of MCs within the tumor microenvironment.
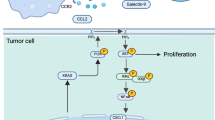
Regulation of IL-18 secretion by the NLRP3 inflammasome complex
The mechanism underlying IL-18 secretion by MCs remains incompletely understood. To address this, we first treated MCs with the protein transport inhibitor brefeldin A (BFA) and observed that IL-18 secretion was unaffected, a notable contrast to the suppression observed for conventional inflammatory cytokines (Fig. 2B). This finding suggested the existence of an unconventional, ER–Golgi-independent pathway for IL-18 release in MCs, aligning with previous reports in macrophages42. Moreover, stimulation of MCs (Fig. 2B) and MCs cell line LUVA (Supplementary Fig 3A) with lipopolysaccharides (LPS) and nigericin (Nig) induced a significant increase in IL-18 secretion, a combination known to trigger IL-18 cleavage and release in macrophages. Consequently, we then sought to determine whether IL-18 underwent intracellular processing within MCs. By analyzing both full-length and cleaved forms of IL-18 in MC lysates, we observed a preferential accumulation of the mature form of IL-18 in activated MCs derived from both nLung and LUAD tissues, as well as from LUVA cells (Fig. 2C). These findings implied that MCs may be equipped with the necessary molecular machinery for pro-IL-18 cleavage, enabling the extracellular release of this proinflammatory cytokine.
In macrophages, extracellular release of IL-18 has been linked to inflammasome activation43. Given this, we next sought to determine whether MCs in LUAD possess the molecular scaffold required for inflammasome assembly and activation. The assessment of inflammasome complex component and assembly signatures across MC subsets revealed that IL-18+ MCs exhibited the highest levels of both inflammasome component and assembly activity (Fig. 2D, Supplementary Table 2). Profiling inflammasome components [42, 43] in sorted MCs from nLung and LUAD revealed that NLRP3 and NLRP1 were expressed abundantly (Fig. 2E), in contrast to other inflammasome types, including NLRC4, AIM2, and Pyrin. Transcriptomic analysis of LUVA cells further confirmed high expression of NLRP3 and NLRP1, with no other members of the inflammasome sensor family detected (Fig. 2E). We subsequently validated the protein expression of NLRP3 and the adapter molecule ASC in both MCs and LUVA through various techniques, including immunoblotting (Fig. 2F), flow cytometry (Supplementary Fig. 3B), and mIF (Fig. 2G). The selective activation of the NLRP3 inflammasome by MCs was further supported by an induction of NLRP3 transcripts (Fig. 2E) and proteins (Fig. 2F) upon stimulation with LPS and Nig. Evidence of ongoing inflammasome activation in stimulated MCs was corroborated by the detection of colocalization between ASC and NLRP3 specks using ImageStream technology (Fig. 2H). Importantly, the processing and secretion of IL-18 were nearly completely inhibited by the specific NLRP3 inflammasome inhibitors MCC950 and CY-09 (Fig. 2I, J and Supplementary Fig. 3C, D). In summary, these findings provided valuable insights into the critical role of the NLRP3 inflammasome in modulating IL-18 secretion by MCs in the LUAD microenvironment.
Processing of Pro-IL-18 by Caspase 1
Caspase 1, along with caspase 4 and 5, constitutes the principal effector proteins within the human NLRP3 inflammasome complex44. In our study, we identified approximately 12.2% and 12.3% activated FLICA+ (fluorochrome-labeled inhibitor of caspases assay) MCs from nLung and LUAD (Fig. 3A and Supplementary Fig. 3E), suggesting the intrinsic presence of activated caspases in MCs influenced by the local microenvironment. The proportion of FLICA+ MCs increased two- to three-fold in the presence of NLRP3 inflammasome activators (Fig. 3A). Activation was similarly observed in macrophages but not in CD4+ and CD8+ T lymphocytes (Supplementary Fig. 3F). This finding was further supported by the inhibition of caspase-1 cleavage upon treatment with the NLRP3 inflammasome inhibitors MCC950 or CY-09 (Fig. 2I), indicating that pro-IL-18 processing was associated with caspase-1 cleavage events.
A Representative images of FLICA co-staining with PI in nLung and LUAD MCs after stimulation with or without LPS and Nig using flow cytometry (upper). Cumulative data for active caspase-1 in nLung and LUAD MCs (lower). Data are presented as mean + SEM, the heights of bars present the mean value and the error bars represent the SEM value. Each group includes 6 replicates, and p values were determined by two-tailed paired Student’s t-test. FMO fluorescence minus one. B Representative images of FLICA (green) co-staining with ASC (yellow), CD117 (purple), and NLRP3 (red) in LUVA cell line using imaging flow cytometry. BF, bright-field. Scale bars, 10 μm. Each group includes more than 4 replicates. C The concentration of IL-18 in cell culture supernatants of MCs after stimulation with or without LPS and Nig in the presence or absence of indicated caspases inhibitors. Each group includes 6 replicates, p values were determined by two-tailed Student’s t-test. D Immunoblot analysis of cell lysates of nLung and LUAD MCs after stimulation with or without LPS and Nig in the presence or absence of indicated inhibitors. Each group includes more than 3 replicates. E Representative images of FLICA staining in nLung and LUAD MCs after stimulation with or without LPS and Nig using flow cytometry in the presence or absence of indicated inhibitors (left). Cumulative data of FLICA+ cells (right). Each group includes 5 replicates, p values were determined by two-tailed paired Student’s t-test. F Heatmap showing the expression of GSDM family genes in LUVA cell line, nLung MCs (nMC), and LUAD MCs (tMC). G Representative mIF staining of Asp275 (cleaved GSDMD, orange), and tryptase (blue) for MCs in LUAD sections. Nuclei were counterstained with DAPI (gray). Scale bars, 30 μm. Each group includes more than 3 replicates. H The concentration of IL-18 in cell culture supernatants of LUVA clones (including wild-type (WT), two GSDMD KO clones: KO#10 and KO#15) after stimulation with or without LPS and Nig by ELISA. Data are presented as mean + SEM, the heights of bars present the mean value and the error bars represent the SEM value. Each group includes 8 replicates, p values were determined by two-tailed Student’s t-test. I Quantification of dead cells on the basis of PI staining in WT or CRISPR–Cas9 KO LUVA clones. Data are presented as mean + SEM, the heights of bars present the mean value and the error bars represent the SEM value. Each group includes 6 replicates, p values were determined by two-tailed Student’s t-test. J CytoTox 96 Non-Radioactive Cytotoxicity Assay from WT or CRISPR–Cas9 KO LUVA clones. Each group includes 4 replicates, p values were determined by two-tailed Student’s t-test. K Representative flow images of PI staining in caspase 1+ or caspase 1- MCs from nLung and LUAD (left). Cumulative data of PI+ cells in caspase 1+ or caspase 1- MCs from nLung and LUAD (right). Data are presented as mean + SEM, the heights of bars present the mean value and the error bars represent the SEM value. Each group includes 4 replicates, p values were determined by two-tailed paired Student’s t-test. L CytoTox 96 Non-Radioactive Cytotoxicity Assay from LUVA cell line in the presence or absence of indicated NLRP3 inhibitors. Each group includes 8 replicates, p values were determined by two-tailed Student’s t-test. M CytoTox 96 Non-Radioactive Cytotoxicity Assay from LUVA cell line in the presence or absence of indicated caspase inhibitors. Each group includes 8 replicates, p values were determined by two-tailed Student’s t-test. See Supplementary Fig. 3.
To further elucidate the role of caspase 1 in IL-18 processing, we utilized imaging flow cytometry to examine the expression and intracellular localization of FLICA, ASC, and NLRP3. Activated inflammasomes that formed large micrometer-sized complexes composed of inflammasome-ASC-caspase 1 specks (Fig. 3B) were observed within MCs. Given the nonspecific nature of the FLICA probe, we subsequently utilized selective caspase inhibitors to more precisely delineate the role of individual caspases in IL-18 secretion. Treatment with the pan-caspase inhibitor Z-VAD-FMK, the caspase 1 inhibitor Ac-YVAD-cmk, and the caspase 4 inhibitor Z-LEVD-FMK resulted in nearly complete inhibition of IL-18 secretion (Fig. 3C and Supplementary Fig. 3G, H). Interestingly, the caspase 1 inhibitor led to a more pronounced reduction in IL-18 cleavage compared to the caspase 4 inhibitor (Fig. 3D). Furthermore, the proportion of FLICA+ MCs was markedly reduced upon treatment with the respective caspase inhibitors, with varying degrees of inhibition observed (Fig. 3E). Collectively, these findings provided robust evidence that IL-18 production in MCs was critically dependent on caspase 1-mediated cleavage, a key step in the activation of the NLRP3 inflammasome.
GSDMD pore formation mediates IL-18 release and MCs pyroptosis
Gasdermin proteins, integral to a family of recently identified pore-forming effector molecules, facilitate the release of inflammatory mediators45. Our transcriptomic analysis revealed selective expression and upregulation of GSDMB and GSDMD in sorted MCs from both nLung and LUAD, as well as in LUVA cells (Fig. 3F), with no observable upregulation of other gasdermin family members. This finding was unexpected, as GSDMB has been reported to be cleaved by granzyme A from cytotoxic lymphocytes, triggering pyroptosis in target cells46. GSDMD, by contrast, is known to form membrane pores in innate immune cells, which serve as conduits for the extracellular release of alarmins47. To investigate the role of GSDMD in MCs, we assessed its expression at the protein level. The cleaved N-terminal, pore-forming form of GSDMD (Asp275) was detectable in LUAD tissue sections (Fig. 3G), indicating inflammasome activation in MCs within the tumor microenvironment. Next, we explored whether GSDMD pores facilitated the extracellular release of IL-18 from MCs. Utilizing CRISPR-Cas9 technology, we generated GSDMD knockout (GSDMD-/-) LUVA clones (Supplementary Fig. 3I) and assessed IL-18 release into the supernatant via ELISA. Notably, IL-18 secretion was significantly inhibited in two GSDMD-/- LUVA clones (Fig. 3H). Moreover, the cleavage of GSDMD could be restricted by both NLRP3 inflammasome inhibitors and caspase 1 inhibitors (Supplementary Fig. 3J, K), providing compelling evidence that GSDMD pore formation acted as a mechanism for IL-18 release by MCs.
Interestingly, the process of GSDMD pore formation does not consistently coincide with pyroptotic cell death, as some pore-forming cells build resilience against pyroptosis and remain hyperactive48. To investigate the fate of MCs after inflammasome activation, we evaluated their viability. To investigate the fate of post-inflammasome activation, we evaluated the cell viability of MCs. GSDMD-intact LUVA cells demonstrated a higher percentage of propidium iodide (PI) positivity compared to their GSDMD-deficient counterparts (Fig. 3I), correlating with a reduction in lactate dehydrogenase (LDH) release observed in two GSDMD-/- LUVA cells (Fig. 3J). Furthermore, the proportion of PI+ MCs was significantly elevated in the FLICA+ population, which exhibited signs indicative of inflammasome activation (Fig. 3K). Both NLRP3 and caspase-1 inhibitors were effective in obstructing LDH release (Fig. 3L, M), suggesting that pore-forming MCs may be more susceptible to pyroptosis. In summary, these findings established a close connection between IL-18 production and pyroptotic cell death of MCs mediated by GSDMD pore formation.
MCs-derived IL-18 triggers MAIT cell activation
As pivotal effector molecules of inflammasome activation, IL-18 amplifies inflammatory responses by promoting alarmin signals in conjunction with IL-1β 49,50. Unique feature of IL-18 in IL-1 family cytokine is the potent ability to induce IFN-γ production by cytotoxic lymphocytes. We then sought to investigate the functional mechanisms associated with IL-18-producing MCs, which were a major source of IL-18 in LUAD. To explore this, we re-clustered lymphocytes into 11 subclusters, including CD4+ T cells, CD8+ T cells, Tregs, γδT cells, NK cells, B cells, plasma cells, and MAIT cells (Supplementary Fig. 4A). A ligand-receptor crosstalk analysis revealed that IL-18+ MCs predominantly interacted with innate-like T cells, particularly γδT cells and MAIT cells (Fig. 4A). Among the identified ligand-receptor pairs, the IL-18/IL-18R interaction was significantly enriched (Fig. 4B). Flow cytometric analysis revealed that MAIT cells were the predominant innate-like T cell subset in LUAD, while γδT cells and NKT cells were relatively scarce (Fig. 4C and Supplementary Fig. 4B).
A The ranked IL-18+ MCs-lymphocytes crosstalk. B Heatmap showing the ligand-receptor pairs between IL-18+ MCs and lymphocyte subsets. P value was generated by CellphoneDB, with permutation test as its fundamental inferential mechanism. C Representative flow plots showing the frequency of innate-like T cells: NKT, γδT and MAIT cells in LUAD (upper). Cumulative data of the frequency of innate-like T cells (lower). Data are presented as mean ± SEM, the center line presents the mean value and the error bars represent the SEM values. Each group includes 6 replicates, p values were determined by two-tailed paired Student’s t-test. D The quantitative relationship between immune cell subsets (estimated by CIBERSOT) and IL-18+ MCs. P values were determined by Pearson Correlation Analysis. E The spatial distribution of MCs and MAIT cells in LUAD by mIF. Representative mIF staining of PanCK (green), TCRVa7.2 (yellow), and tryptase (violet) in LUAD sections and nuclei were counterstained with DAPI (gray). Scale bars, 30 μm. Each group includes more than 3 replicates. F The expression of IL-18R1 (left) and IL-18RAP (right) in tumor-infiltrating lymphocytes. G Representative flow plots showing the fluorescence intensity (left) and quantification (right) of IL-18Rα on intratumoral lymphocytes. Data are presented as mean + SEM, the heights of bars present the mean value and the error bars represent the SEM value. Each group includes 8 replicates from blood samples and 6 8 replicates from LUAD samples. FMO, fluorescence minus one. See Supplementary Fig. 4 and Supplementary Table 3 and Supplementary Data 6.
To further assess the quantitative relationship between IL-18+ MCs and lymphocyte infiltration, we analyzed immune cell profiles in the FUSCC_1019 cohort through immune cell deconvolution. The MAIT cell signatures (Supplementary Table 3), derived from three independent studies51,52,53, which demonstrated strong consistency in the assessment of MAIT cell infiltration (Supplementary Fig. 4C). MAIT cell signatures showed a strong positive correlation with the IL-18+ MC signature, suggesting that the infiltrating levels of these cells are closely linked (Fig. 4D and Supplementary Fig. 4D, Supplementary Data 6). This finding was also validated in the FUSCC_150 cohort (Supplementary Fig. 4C, D). Conversely, plasma cells and Tregs exhibited a negative correlation with IL-18+ MCs (Fig. 4D, Supplementary Data 6). Furthermore, spatial visualization revealed that MAIT cells preferentially localized to MC-rich areas in the tumor microenvironment (Fig. 4E). We next examined the expression of IL-18 receptors (IL18R1 and IL18RAP) on LUAD-infiltrating lymphocytes. As corroborated by previous studies, MAIT cells exhibited the highest expression levels of both IL18R1 and IL18RAP (Fig. 4F), a finding further confirmed by flow cytometry (Fig. 4G, Supplementary Fig. 4E, F). Collectively, these findings underscored the potential role of MCs in the preferential activation of MAIT cells through inflammasome-driven secretion of IL-18, highlighting the intricate interplay between these immune cell types in the tumor microenvironment.
Single-cell multi-omics of IL-18-activated MAIT cells
To investigate the characteristics of IL-18-activated MAIT cells, we conducted scCITE-seq and scTCR-seq on MAIT cells from eight matched LUAD and blood samples (Supplementary Fig. 5A). MAIT cells were identified as T cells that were double positive for TCRVa7.2 and CD161, as detected by CITE-seq following CD3 magnetic-activated cell sorting (MACS) enrichment (Fig. 5A). This approach facilitated an in-depth investigation of infiltrating MAIT cells in the LUAD microenvironment. The analysis of MAIT cells from these 8 matched LUAD-blood pairs revealed five distinct subclusters (Fig. 5B, Supplementary Data 7). Most MAIT cell clusters were primarily composed of cells from a single tissue type, which may be due to the influence of the local tumor microenvironment on MAIT cell transcriptional signature. CXCR4+IFNG+, FCER1G+IFI30+, and CD24+HSPB1+ MAIT cells were enriched in LUAD, whereas NCR3+KLRG1+ and CX3CR1+GNLY+ MAIT cells were more prevalent in blood samples (Fig. 5B, C). Specifically, MAIT cells in LUAD exhibited elevated expression levels of activation and tissue-resident genes and surface proteins, whereas their peripheral blood counterparts demonstrated a naïve-like profile (Supplementary Fig. 5B). This disparity underscored the active status of MAIT cells within the LUAD microenvironment.
A Schematics of MAIT cells identification and MAIT cells transcriptome, epitome, and TCR repertoire. B t-SNE visualization (left) and the proportion (right) of MAIT cell subsets. C t-SNE plot showing the canonical cell markers of MAIT cell subsets. D The cytokine-activation activity of MAIT cells scored by previously published signature. E Enriched gene ontology terms of CXCR4+IFNG+ MAIT cells subset. Benjamini–Hochberg adjusted P values were shown. F Differentiation state of MAIT cells subsets estimated by scTour. G Heatmap showing activity of the top 20 differentially active regulons between CXCR4+IFNG+ MAIT cells and non-CXCR4+IFNG+ MAIT cells. H The surface proteome of MAIT cells subsets. I Percentage of top 5 TRAJ gene usage generated from CXCR4+IFNG+ MAIT cells (yellow) and non-CXCR4+IFNG+ MAIT cells (gray). J Sequence logo generated from CXCR4+IFNG+ MAIT cells and non-CXCR4+IFNG+ MAIT cells CDR3β amino acid sequences of length 15. K The proportion of unique clonotype in CXCR4+IFNG+ MAIT cells and non-CXCR4+IFNG+ MAIT cells. L Shannon diversity index for CXCR4+IFNG+ MAIT cells clonotypes and non-CXCR4+IFNG+ MAIT cells clonotypes for each patient. The center of the boxes indicated the median value and upper and lower bounds of the boxes indicated the 25th and 75th percentile of data. Whiskers went from the minimum of data to the lower bounds of the box and the upper bounds of the box to the maximum of data. Each group includes 8 replicates and p value is computed using the Wilcoxon test methodology. See Supplementary Fig. 5 and Supplementary Data 7.
A comprehensive analysis of the transcriptional signatures and differential gene expression revealed that the CXCR4+IFNG+ MAIT cells, the predominant subcluster in LUAD, were distinguished by heightened expression of activation markers, including ICOS and IFN-γ (Supplementary Fig. 5C). MAIT cells can be activated by bacterial metabolites via MR1 in a TCR-dependent manner or by innate cytokine signals54,55, with IFN-γ expression serving as a hallmark of cytokine-activated MAIT cells51. We hypothesized that this subcluster of CXCR4 + IFNG + MAIT cells may represent cytokine-activated MAIT cells, which was substantiated by the observation of pronounced activity of the cytokine activation pathway in these cells (Fig. 5D). Furthermore, these cells exhibited the highest expression levels of specific genes and transcription factors elicited by cytokine stimulation51, such as IFNG, CD40LG, and IL-26 (Supplementary Fig. 5D). In alignment with these results, our gene set enrichment analysis indicated that pathways enriched in CXCR4+IFNG+ MAIT cells were closely associated with T cell activation and cytokine-mediated signaling pathway (Fig. 5E). Additionally, we observed continuous differentiation along MAIT cell clusters, with the terminal pseudotime value observed for MAIT cell clusters from LUAD (Fig. 5F). Notably, CXCR4+IFNG+ MAIT cells expressed high levels of IL-18 receptors, including IL-18R1 and IL-18RAP (Supplementary Fig. 5E), highlighting CXCR4 as a potential marker for cytokine-activated MAIT cells.
To further investigate potential transcription factor regulons associated with cytokine-activated MAIT cells, we conducted SCENIC analysis56. The results revealed that CXCR4+IFNG+ MAIT cells exhibited increased activity of key transcription factors such as IRF4, JUN, and FOS (Fig. 5G), highlighting the critical roles of these factors in regulating gene expression within cytokine-activated MAIT cells. Surface proteomic profiling (Fig. 5H) confirmed that the CXCR4+IFNG+ MAIT subset highly expressed an array of chemokine receptors (CCR4, CCR5, and CXCR3), tissue retention receptors (CD103 and CD49a), and activation markers (4-1BB, OX40, and ICOS), further supporting the active role of cytokine-activated MAIT cells in the context of LUAD.
In subsequent analyses, we sought to determine whether the TCR repertoire of MAIT cells induced by cytokines exhibited specific characteristics. The overall TCR repertoire characteristics were comparable between CXCR4+IFNG+ MAIT cells and other MAIT cell subtypes. TRAJ33, TRAJ20, and TRAJ12 were commonly utilized by both CXCR4+IFNG+ and other MAIT cell populations (Fig. 5I). Similarly, the usage of TRBV genes was biased toward 20-1, 6-1, and 6-4, although these patterns were comparable across MAIT cell subsets (Supplementary Fig. 5F). Furthermore, the variability in CDR3β amino acid length and sequence were greater than that observed for CDR3α. No significant differences were found between the MAIT cell subsets in this regard (Fig. 5J and Supplementary Fig. 5G, H). A small reduction in the frequency of unique clonotypes was observed in CXCR4+IFNG+ MAIT cells (Fig. 5K), although no differences were noted in the Shannon diversity index (Fig. 5L). In conclusion, our multi-omics analysis revealed the program of IL-18-activated MAIT cells, emphasizing their functional significance in LUAD.
Enhancement of innate-like anti-tumor effects of MAIT cells by MCs
To investigate the mechanisms underlying the cytotoxic activity of MAIT cells, we first analyzed the transcriptomic and epitope profiles of cytotoxic molecules and receptors. We found that CXCR4+IFNG+ MAIT cells exhibited high expression of natural killer receptor genes, including NKp46, NKp44, NKp30, NKG2A, and NKG2C, as well as surface proteins such as NKG2D, NKp46, KIR2DL1, KIR2DL3, and KIR3DL1 (Fig. 6A). In contrast, T cell-associated cytotoxic molecules did not demonstrate substantial upregulation (Fig. 6A). Consistent with prior studies57, MAIT cells displayed low levels of granzyme B and perforin (Fig. 6B and Supplementary Fig 6A). We further confirmed the expression and upregulation of NKG2D in MAIT cells derived from LUAD tissues (Fig. 6C). The application of IL-18 resulted in a marked activation of MAIT cells, indicated by the increased expressions of CD69 and CD25, and enhanced proliferation assessed by Ki-67 detection (Supplementary Fig. 6B). However, no significant changes in PD-1 expression were observed following cytokine stimulation (Supplementary Fig. 6B). Notably, IL-18 was shown to augment the secretion of IFN-γ and the expression of NKG2D in MAIT cells (Fig. 6D). In addition, stimulation of MCs from both nLung and LUAD samples with LPS and Nig induced MAIT cell activation, as measured by increased expression of 4-1BB, OX-40, HLA-DR, and CD25, along with enhanced proliferation (Fig. 6E and Supplementary Fig. 6C). To determine whether MC-mediated activation of MAIT cells is IL-18-dependent or MR1-dependent, we first assessed the expression of MR1 across various types of cells in LUAD. We found that macrophages exhibited the highest levels of MR1 expression, whereas the expression of MR1 on MCs was low, which was comparable to that on plasma cells, fibroblasts, and NK cells (Supplementary Fig. 6D). Then, we conducted in vitro blocking experiments using MR1 or IL-18 inhibitors (IL-18BP). IL-18BP effectively inhibited MCs-mediated activation of MAIT cells, as demonstrated by the reduced expression of 4-1BB and OX40. In contrast, blocking MR1 did not significantly affect MCs-mediated activation of MAIT cells (Supplementary Fig. 6E). Furthermore, activated MCs were found to upregulate the expression of IFN-γ and NKG2D in MAIT cells (Fig. 6F). Collectively, these findings suggested that MCs could enhance the innate-like cytotoxicity of MAIT cells via IFN-γ and NKG2D. In vitro co-culture assays indicated that treatment with IL-18 or a combination of IL-18 and IL-12 significantly potentiated the cytotoxic effects of MAIT cells against GFP-A549 and H1299 tumor cell lines (Fig. 6G and Supplementary Fig. 6F).
A Dot plot for the transcriptome (left) and heatmap for the surface proteome of cytotoxicity molecules and receptors (right) in MAIT cells. B Representative flow plots (left) and the percentage of (right) cytotoxicity molecules expression in MAIT cells. Each group includes 5 replicates, p values were determined by two-tailed paired Student’s t-test. C Representative flow plots (left) and the percentage of (right) NKG2D expression in MAIT cells. Each group includes 7 replicates, p value was determined by two-tailed paired Student’s t-test. D Quantification of IFN-γ secretion (left) and NKG2D expression (right) by MAIT cells with or without cytokine stimulation. Each group includes 7 replicates, p value was determined by two-tailed paired Student’s t-test. E Quantification of 4-1BB and Ki-67 expression by MAIT cells stimulated by MCs. Blue, nLung MCs, pink, LUAD MCs. Each group includes 5 replicates, p value was determined by two-tailed paired Student’s t-test. F Quantification of IFN-γ and NKG2D expression by MAIT cells stimulated by MCs. Blue, nLung MCs, pink, LUAD MCs. Each group includes 5 replicates, p value was determined by two-tailed paired Student’s t-test. G The proportion of surviving target cells GFP-A549 and H1299 cell lines by flow cytometry. Each group includes 6 replicates, p value was determined by two-tailed paired Student’s t-test. H Dot plot showing the relationship between immunotherapy response or survival and immune cell subsets using OAK cohort. I Survival analysis of MAIT cells (left) and IL-18+ MCs (right) in OAK cohort. p values were determined by the log-rank test. See Supplementary Fig. 6 and Supplementary Data 8.
To evaluate the prognostic significance of the MCs-MAIT cells axis in patients with LUAD, we performed a Kaplan-Meier survival analysis on two LUAD cohorts (the FUSCC_1019 cohort and the FUSCC_150 cohort). We found that higher MAIT cell infiltration was significantly associated with improved overall survival and recurrence-free survival (Supplementary Fig. 6G). Furthermore, multivariate analyses by the Cox regression model revealed that MCs infiltration was a significant prognostic factor for overall survival of patients with LUAD, which was independent of tumor stage (Supplementary Fig. 6H). To assess the implications of MAIT cells in the context of immunotherapy, we conducted survival analyses in the OAK immunotherapy cohort. Among various tumor-infiltrating immune cells, only MAIT cells, plasma cells, and dendritic cells (DC) demonstrated significant associations with therapeutic responsiveness (Fig. 6H) and favorable prognostic outcomes after immunotherapy (Fig. 6I and Supplementary Fig. 6I, J, Supplementary Data 8). In summary, our findings demonstrated that MCs enhanced the innate-like anti-tumor effects of MAIT cells, and that this interaction was associated with improved survival outcomes in LUAD patients, particularly in the context of immunotherapy.
Discussion
The transcriptional heterogeneity of MCs within the tumor-infiltrating innate immune compartment remains poorly understood, leaving their roles across various human cancers ambiguous2. In this study, we presented a comprehensive transcriptomic analysis of LUAD-infiltrating MCs, elucidating the previously unappreciated complexity of these cells in the tumor microenvironment. Notably, we identified a proinflammatory subset of MCs in LUAD characterized by the expression of IL-18. This subset engaged an NLRP3-dependent signaling cascade, leading to the formation of membrane pores via GSDMD and the subsequent release of IL-18. Importantly, this proinflammatory MC subset was found to activate MAIT cells in a TCR-independent manner, which, in turn, enhanced their innate-like anti-tumor activity, showcasing upregulation of NKG2D and IFN-γ, as elucidated through an integrative analysis of transcriptomics, epitopes, and TCR repertoires.
The evolutionary dynamics of the tumor microenvironment during LUAD progression remain largely unexplored12. To address this gap, we undertook a comprehensive assessment of immune profiling throughout LUAD progression by integrating a large sequencing cohort with a flow cytometry cohort. Our findings revealed a close relationship between MC infiltration and LUAD progression, with MCs preferentially enriched in indolent LUAD tumors, which can remain stable for extended periods. Consistent with previous studies58, we observed a decline in MC infiltration as LUAD progresses from indolent to invasive stages. Notably, MC infiltration was higher in indolent LUAD compared to normal lung tissue, while it was diminished in invasive LUAD. Future investigations should explore the mechanisms underpinning mastocytosis within the indolent LUAD microenvironment and investigate the role of resident MCs in shaping this phenotype12.
Despite significant advances in the understanding of tumor-infiltrating innate immune compartments, including macrophages and neutrophils59, the heterogeneity and functions of MCs within the tumor microenvironment have received limited attention. Our study presents a comprehensive analysis of tumor-infiltrating MCs, unveiling distinct functional states, including chemotaxis and antigen presentation, that align with previous literature60. A key finding from our research is the identification of a pro-inflammatory MC subset enriched in LUAD, characterized by IL-18 expression and traits of inflammasome activation, which corresponds to favorable prognostic outcomes. Notably, IL-18 expression and its cleavage by chymase have been previously implicated in allergic diseases61. Our findings extend this knowledge by revealing a novel cytokine secretion pathway and functional modality in LUAD-infiltrating MCs, thereby expanding the functional and mechanistic repertoire attributed to MCs in the tumor milieu. Besides, it is important to note that macrophages may serve as a significant source of IL-18 through inflammasome activation, potentially outnumbering MCs within the tumor microenvironment. Our study emphasizes the identification of inflammasome activation in MCs, which can nonspecifically activate MAIT cells via IL-18 secretion, thereby providing a valuable supplement in this field. Unlike macrophages, MCs in LUAD displayed high levels of IL-18, with minimal expression of IL-1β. Given the distinct biological roles of IL-1β and IL-18, we hypothesize that MCs and macrophages may mediate divergent immune responses in tumors following inflammasome activation. We propose that targeting inflammasome-related pathways may pave the way for the development of MCs-based immunotherapies.
Alterations in MAIT cell frequency, activation status, and cytotoxicity within tumor microenvironment have been well-documented, including lung cancers52,57,62,63,64. In our study, although MAIT cell density demonstrated prognostic relevance in the univariate analysis, it did not remain an independent prognostic factor in the Cox multivariate model. We believe this may be partly attributable to limitations in the deconvolution of MAIT cell infiltration using RNA-seq data. Specifically, the low infiltration levels of MAIT cells likely reduce the accuracy of transcriptome-based estimation. Additionally, the transcriptomic signatures of MAIT cells overlap substantially with those of other T cell subsets, further complicating their distinct quantification. Moreover, the characteristics cytokine-activated MAIT cells in LUAD, as explored through multi-omics approaches, remain insufficiently defined. Our study provides a detailed, high-dimensional single-cell multi-omics analysis of LUAD-infiltrating MAIT cells, integrating transcriptomics, epitopes, and TCR repertoires. We revealed a previously unrecognized interaction between MCs and MAIT cells. While interactions between MCs and T cells have been reported65,66, direct evidence linking MCs and MAIT cells has been lacking. Our results uncover a novel mechanism of MAIT cell activation by MCs, driven by IL-18, a cytokine known to activate MAIT cells in other contexts such as COVID-1967. We demonstrate that MC-derived IL-18 enhances MAIT cell activation via a cytokine-dependent pathway68,69. Activated MAIT cells upregulated IFN-γ, which was reported to enhance the anti-tumor function of NK cells70. Further endeavors are warranted to examine the interplay between MAIT cells and other immune cell types, in particular NK cells and MCs, as these insights may pave the way for innovative MAIT-centered immunotherapies70,71,72. For instance, in vitro stimulation of MAIT cells using IL-18 or MCs prior to CAR-MAIT delivery may represent a promising therapeutic strategy73. Moreover, in our study, we found that IL-18+ MCs also interacted closely with NK cells and NK cells also express high levels of IL-18 receptors (IL18R1 and IL18RAP). We speculated that MCs-derived IL-18 could also augment the activation and cytotoxicity of NK cells, in addition to MAIT cells.
In summary, our studyperformed comprehensive analysis of MCs and provides a multi-omics profile of MAIT cells in LUAD. We identified the presence of a pro-inflammatory MC subset exhibiting traits of inflammasome activation. We demonstrated that MC-derived IL-18 mediated the innate-like anti-tumor cytotoxicity of MAIT cells, through NLRP3 inflammasome activation, culminating in GSDMD pore formation. These findings not only diversified our understanding of the functional repertoire and mechanistic equipment of MCs but also highlighted potential therapeutic strategies targeting MCs and MAIT cells in cancer immunotherapy.
Methods
Patient cohort and cell line
This study encompassed multiple cohorts: the FUSCC_1019 cohort, FUSCC_150 cohort, FUSCC_CITE cohort, FUSCC_FCM cohort, FUSCC_TMA cohort, and OAK cohort. The FUSCC_1019 cohort consisted of 1019 LUAD patients who underwent surgery at the Department of Thoracic Surgery, Fudan University Shanghai Cancer Center. Paired tumor and nLung samples were obtained for whole genome sequencing (WGS) and bulk RNA-seq (Supplementary Fig. 1A). The FUSCC_150 cohort, also from our center, served as an independent validation cohort for WES and RNA-seq, and included 150 LUAD patients with paired tumor and nLung samples (Supplementary Fig. 1C)32. The FUSCC_CITE cohort comprised 8 paired peripheral blood and LUAD samples, collected at our institution between December 2023 and January 2024. scRNA-seq, scCITE-seq, and scTCR-seq were conducted on this cohort, with details outlined in Supplementary Fig. 5A. The FUSCC_FCM cohort included matched blood, LUAD, and nLung samples procured from Fudan University Shanghai Cancer Center to profile LUAD-infiltrating MCs. The FUSCC_TMA cohort consisted of pathological specimens from LUAD patients at the Fudan University Shanghai Cancer Center, organized on slides measuring 4 mm in thickness and 1.5 mm in diameter, with detailed information provided in Supplementary Fig. 2I. The OAK cohort was downloaded from the European Genome-phenome Archive (EGA) under accession number: EGAS00001005013.
GFP-labeled A549 and H1299 cell lines were cultured in RPMI-1640 medium (Gibco, Cat#11875093) supplemented with 10% fetal bovine serum (FBS, Thermo Fisher Scientific, Cat#A5256701). The LUVA cell line was obtained from Fuheng Biology (Cat# FH1301, Shanghai, China) and maintained in StemPro-34 medium (Gibco, Cat#10639011), supplemented with Glutamax (Gibco, Cat#35050061) and 1% penicillin-streptomycin. Two GSDMD knockout LUVA clones were generated using the CRISPR-Cas9 system from Corues Biotechnology (Nanjing, China).
All human LUAD specimens in this study were collected in compliance with the Declaration of Helsinki, following written informed consent from patients. The study was approved by the Ethics Committee and Institutional Review Boards (IRB) of Fudan University Shanghai Cancer Center (IRB number: 20082239).
Sample collection and processing
Tumor tissues and matched normal lung tissues were meticulously dissected and mechanically minced. These samples were subsequently enzymatically digested using RPMI1640 (Gibco, Cat# 11875093), supplemented with collagenase IV (1 mg/mL; Gibco, Cat#17104019) and DNase I (100 mg/mL, Sigma, AMPD1-1KT), and incubated for 1 h at 37 °C. The resulting mixture was then filtered to obtain a single-cell suspension using a 70 µm filter (Corning). Fresh peripheral blood samples were processed using Lymphoprep density gradient centrifugation to enrich for peripheral mononuclear cells, which were subsequently washed and resuspended in phosphate-buffered saline.
Immune cell infiltration deconvolution
To assess immune cell infiltration, we applied eight commonly used algorithms for immune cell deconvolution: CIBERSORT, TIMER, EPIC, ESTIMATE, MCPCounter, Quantiseq, IPS, and xCell, to data from the FUSCC_1019 and FUSCC_150 cohorts. Two of these algorithms, CIBERSORT and xCell, supported the quantification of MC levels in the tumor microenvironment. The results from these algorithms are summarized in Supplementary Data 1. In the case of CIBERSORT algorithm, the MC quantification was derived by calculating the sum of “Mast.cells.resting” and “Mast.cells.activated”. Similarly, other immune cell subsets were quantified as follows: B cells (B.cells.memory + B.cells.naive), CD4 T cells (T.cells.CD4.naive + T.cells.CD4.memory.resting + T.cells.CD4.memory.activated), NK cells (NK.cells.resting + NK.cells.activated), and dendritic cells (Dendritic.cells.activated + Dendritic.cells.resting), with the detailed results presented in Supplementary Data 6. To evaluate the prognostic value of each MCs subset signature (Supplementary Table 1), we performed ssGSEA analysis (Supplementary Data 4) and Cox univariate analysis in the FUSCC_1019 cohort, FUSCC_150 cohort, and OAK cohort. A p-value greater than 0.05 is considered indicative of no statistically significant difference. When the p-value was <0.05 and the hazard ratio (HR) was <1, the variable was considered a favorable factor; conversely, when the p-value was <0.05 and the HR was >1, it was considered an adverse factor.
Single-cell RNA-seq
The scRNA-seq data pertaining to MCs were sourced from both previously published datasets and our own in-house data. The detailed information of the public scRNA-seq datasets was outlined in the Supplementary Table 4. For data processing, we utilized the Cell Ranger Single-Cell Software Suite to align the scRNA-seq data to the GRCh38 human reference genome. The resulting unique molecular identifier (UMI) matrix was then analyzed using Seurat (version 4.3.0.1) for downstream analysis.
The DoubletFinder software (version 2.0.3) was utilized to detect doublets resulting from cell encapsulation or unintended pairings of cells, as well as to ensure adequate segregation throughout the sample generation process. Cell samples exhibiting fewer than 200 or more than 5000 expressed genes were excluded from the analysis to maintain data integrity. Additionally, cells with less than 80% RNA encoding proteins were discarded. To accommodate the heterogeneity observed across different samples, a dynamic adjustment strategy was employed for specific filtering parameters. Moreover, cells with a percentage of molecules mapping to mitochondrial genes exceeding 15% were also excluded from downstream analyses. After quality control, the data were normalized using the NormalizeData function from Seurat, and the logarithm-transformed normalized matrix was utilized for downstream analyses.
Data integration, dimension reduction, and unsupervised clustering
Principal Component Analysis (PCA) was conducted on the variable gene matrix to facilitate data integration. To correct for batch effects, Harmony was applied, using the top 30 principal components for further analysis. Batch effects across datasets and samples were mitigated by using the RunHarmony function with a parameter setting of lambda = 1. To construct a Shared Nearest Neighbor (SNN) graph, the FindNeighbors function of Seurat was used. Unsupervised clustering was then performed using the FindClusters function. For data visualization, dimensionality reduction was carried out using t-distributed stochastic neighbor embedding (t-SNE), which was performed through Seurat’s RunTSNE function with the parameters dims = 1:30 and reduction = ‘harmony’ to facilitate visualization of the integrated datasets.
Cell type hierarchical identification
For accurate cell type identification in the scRNA-seq data, we performed hierarchical clustering across three levels of cell classification. In the first level, cells were categorized into three major types: epithelial cells (EPCAM), stromal cells (MME, PECAM), and immune cells (PTPRC). In the second level, stromal cells were further subdivided into fibroblasts (DCN, C1R, COL1A1) and endothelial cells (PECAM1, RAMP2, CLDN5). Immune cells were classified into T cells (CD3D, CD3E), NK cells (NKG7), B cells (CD79A), plasma cells (IGKC), myeloid cells (LYZ, CD68), and mast cells (TPSB2, CPA3, MS4A2). In the third level, T cells were further categorized into CD4+ T cells, CD8+ T cells, and CD4- CD8- T cells based on the expression of CD4 and CD8. Regulatory T cells (Treg) were defined by the expression of IL2RA, FOXP3, IKZF2, TGFB1, TGFB3, TGFBI, and TGFBR1. CD8+ T cells were subdivided into multiple subclusters based on the expression of GZMK and GZMB. Additionally, a subcluster of exhausted cytotoxic T cells was identified using markers for exhaustion, such as LAG3, TIGIT, PDCD1, CTLA4, HAVCR2, and ENTPD1. NK cells were divided into CD56dim and CD56bright subpopulations based on the expression of NCAM1, FCGR3A, and FGFBP2. A comprehensive list of the specific markers used for each cell type is provided in Supplementary Table 5.
Pathway enrichment analysis
Differentially expressed genes (DEG) within each cell cluster were identified using the FindAllMarkers function in Seurat with the parameter only.pos = TRUE. These DEGs were then subjected to Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis. The enrichGO and enrichKEGG functions from the clusterProfiler package (version 4.8.1) were used for enrichment analysis, with a significance threshold set at p < 0.05 and p-adjustment method set to “BH” (Benjamini-Hochberg).
Gene signature scoring
Gene signatures were curated from previous studies and additional gene signatures relevant to MAIT cells and MCs were obtained from the Molecular Signatures Database (MSigDB). A comprehensive list of all gene signatures used in this study is available in the Supplementary Tables 2 and 3. To assess the activity of these gene signatures, the AddModuleScore function in Seurat was applied with default parameters. This function normalized the scores across all cells, and the mean score for each signature was computed for each subset of MAIT cells and MCs.
Trajectory inference and pseudotime analysis
For trajectory inference and pseudotime analysis, we utilized the scTour package (version 1.0.0), with parameters set to “percent = 0.6” and “nepoch = 100”. During the construction of the vector field, the background field was derived from the t-SNE map using the parameter “basis = ‘X_rnatsne’”. Pseudotime values were subsequently assigned to individual cells, allowing us to calculate and visualize the temporal distribution across different MAIT cell subgroups, as illustrated in Fig. 5F.
Cell-cell communication analysis
Cell-cell communication analysis was conducted using CellPhoneDB (version 3.1.0), a publicly available repository of ligand-receptor interactions. The significance of the interactions, including mean and communication significance (p < 0.05), was determined based on interaction frequencies and the normalized cell expression matrix obtained from Seurat normalization. Visualization of the communication network was achieved using the netVisual_circle function from the CellChat package (version 1.6.1), as depicted in Fig. 4A.
Tissue specificity of MCs
The tissue specificity of MCs was assessed using the Ro/e metric (observed/expected), as shown in Supplementary Fig. 2F. Specifically, Ro/e represents the ratio of the observed number of cells to the expected number of cells for a given combination of MC cluster and tissue type. The expected number of cells for each MC cluster and tissue combination was determined using a chi-squared test.
Tumor microenvironment classification using predefined gene signatures
To investigate the tumor microenvironment classification in the FUSCC_1019 cohort, we employed single-sample Gene Set Enrichment Analysis (ssGSEA) using predefined gene sets, as previously described74. The gene sets used for analysis included: CSF1_response (Macrophage activation), Liexpression_score (Lymphocyte infiltration), TGFB_score_21050467 (TGF-β response), Module3_IFN_score (IFN-γ response), and CHANG_CORE_SERUM_RESPONSE_UP (Wound healing). Hierarchical clustering was performed using the pheatmap package (version 1.0.12) in R, which generated heatmaps and visualized the clustering results. Six distinct clusters, each representing different biological roles, were identified across the tumor samples.
Single-cell CITE-seq and TCR-seq analysis
For scCITE-seq and scTCR-seq analysis, matched LUAD and peripheral blood samples (n = 8) were obtained from patients undergoing surgical resection at Fudan University Shanghai Cancer Center. The specific details of this cohort are provided in Supplementary Fig. 5A. Single-cell suspensions were prepared as previously described and enriched using magnetic bead separation for CD3+ T cells (Miltenyi Biotec, Cat#130-097-043). Prior to antibody staining, cells were incubated with Human TruStain FcX (BioLegend, Cat#422302) for 10 min at 4 °C. Subsequently, diluted TotalSeq-C Human Universal Cocktail V1.0 (BioLegend, Cat#399905) was added according to the manufacturer’s instructions. Hashtag antibodies (BioLegend, Cat#394667 and Cat#394669) were used for sample multiplexing and to trace the source of peripheral blood samples. Sequencing libraries were generated using the 10x Chromium Single-Cell Platform with 5’ Reagent Kits, following the manufacturer’s protocol.
For CITE-seq data analysis, MAIT cell populations were identified based on the expression of TCRAV7.2 and KLRB1, which are known markers for MAIT cells. The thresholds for these markers were established by analyzing distribution patterns and prior knowledge of MAIT cell prevalence in tissues, with thresholds set at TCRAV7.2 ≥ 5 and KLRB1 ≥ 10. For TCR-seq analysis, MAIT cells were defined by the expression of the TRAV1-2 TCRα chain and the presence of at least one TCRβ chain.
Cells sorting and stimulation
Single-cell suspensions derived from LUAD and paired nLung samples were stained with anti-CD117 antibodies and enriched using magnetic CD117 bead separation, following the manufacturer’s protocol (Miltenyi Biotec, Cat#130-091-332). Sorted MCs or LUVA cell lines were resuspended in StemPro-34 cell culture medium (Gibco, Cat#10639011) supplemented with Glutamax (Gibco, Cat#35050061) and 1% Penicillin-Streptomycin. Cells were stimulated with or without 1 μg/ml LPS (Sigma, Cat#L2880) for 9 h, and 10 μM/ml nigericin (MCE, Cat#HY-100381) was added for the last 30 min. In some experiments, cells were further treated with pharmacological inhibitors, including MCC950 (MCE, Cat#HY-12815, 10 μM/ml), CY-09 (MCE, Cat#HY-103666, 1 μM/ml), Z-VAD-FMK (MCE, Cat#HY-16658B, 50 μM/ml), Ac-YVAD-cmk (MCE, Cat#HY-16990, 50 μM/ml), and Z-LEVD-FMK (MCE, Cat#HY-128707, 20 μM/ml) where indicated.
Fresh peripheral blood samples were subjected to Lymphoprep density gradient centrifugation, washed, and resuspended in phosphate-buffered saline to isolate peripheral mononuclear cells. Cells were initially stained with TCRVa7.2 Biotin antibody (Biolegend, Cat#351724) and subsequently incubated with anti-Biotin MicroBeads (Miltenyi Biotec, Cat#130-090-485), followed by sorting with MS columns (Miltenyi Biotec, Cat#130-042-201). In some experiments, sorted MAIT cells were stimulated with IL-18 (R&D, Cat#9124-1L-010) or recombinant IL-12 (Gibco, Cat#200-12H-10UG) in combination with IL-18. Additionally, sorted MAIT cells were stimulated with either stimulated or unstimulated MCs, T-Activator CD3/CD28 Dynabeads (Gibco, Cat#11131D) or inhibitors (anti-MR1, Biolegend, 361110; IL-18BP, MCE, HY-P7211). The following antibodies were used: anti-CD3D BUV395 (BD, Cat#564001), anti-CD4 BV570 (Biolegend, Cat#300534), anti-CD8 AF700 (BD, Cat#557945), anti-CD25 APC (Biolegend, Cat#302610), anti-CD127 BV421 (Biolegend, Cat#351310), anti-CD19 PECy5 (Biolegend, Cat#302210), anti-CD56 BV711 (Biolegend, Cat#318336), anti-CD161 Biotin (Biolegend, Cat#339932), anti-TCRVa7.2 PE/Dazzle 594 (Biolegend, Cat#351730), anti-TCRγδ BV650 (BD, Cat#564156), anti-TCRVa24 BV510 (Biolegend, Cat#342918), anti-CD137 FITC (eBioscience, Cat#11-1379-42), anti-CD69 APCCy7 (BD, Cat#557756), anti-NKG2D PE (Biolegend, Cat#320806), anti-IFNγ BV786 (BD, Cat#563731), anti-Ki-67 PerCPCy5.5 (BD Pharmingen, Cat#561284), Streptavidin PECy5.5 (SouthernBiotech, Cat#7100-16S). The purity of the sorted cell populations, including MAIT cells and MCs, exceeded 80%.
WGS and RNA-seq of FUSCC_1019 cohort
Genomic DNA was extracted from nLung and LUAD samples using the QIAamp DNA Mini Kit (Qiagen, Germany). A total of 2 μg DNA was fragmented to approximately 350 bp, and libraries were constructed and sequenced on the Illumina NovaSeq 6000 platform, generating 150-bp paired-end reads. Total RNA was extracted using NucleoZOL (Macherey-Nagel, Germany) and the NucleoSpin RNA Set for NucleoZOL (Macherey-Nagel, Germany). Ribosomal RNA was removed using the KAPA Stranded RNA-Seq Kit with RiboErase (KK8481, Roche, Switzerland). Libraries were then prepared and sequenced on the Illumina NovaSeq 6000 platform.
Bulk-seq
Sorted MCs from LUAD or nLung, or LUVA cells, were stimulated with LPS (Sigma, Cat#L2880) and nigericin (MCE, Cat#HY-100381), or left unstimulated, and subsequently subjected to RNA-seq. RNA was extracted using Trizol reagent (Thermo Fisher Scientific, Cat#15596018). Library preparation was performed and sequencing was carried out using the Illumina NovaSeq 6000 platform.
Flow cytometry
Single-cell suspensions from tissues or peripheral blood were stained for viability using the Zombie Yellow Fixable Viability Kit (Biolegend, Cat#423104). Surface staining was performed by incubating cells with the appropriate antibodies for 20 min at room temperature. For intracellular staining, cells were fixed and permeabilized using the Fixation and Permeabilization Kit (eBioscience, Cat#00-5523-00) for 1 h at 4 °C, followed by incubation with intracellular antibodies for 30 min at 4 °C, as per the manufacturer’s instructions. Data acquisition was performed using an LSR Fortessa flow cytometer (BD Biosciences), and data analysis was conducted with FlowJo software (v10.5.3, TreeStar). A complete list of antibodies used is provided in the Supplementary Table 4.
FLICA assay
The FAM-FLICA Caspase 1 Assay Kit (FAM-YVAD-FMK, ImmunoChemistry Technologies, Cat#ICT-97) was used to detect the catalytically active form of caspase-1. Cells were incubated with 30× FAM-VAD-FMK for 30 min at 37 °C, followed by two washes with apoptosis wash buffer, according to the manufacturer’s instructions. Data were collected and analyzed by flow cytometry
Imaging flow cytometry
Imaging flow cytometry was performed using an ImageStream Mark II imaging flow cytometer (AMNIS, Merck Millipore) at 40× magnification, capturing a minimum of 10,000 cells per sample. The following antibodies were used: anti-CD117-APCCy7 (Biolegend, Cat#313228), anti-NLRP3-APC (Miltenyi Biotec, Cat#130-111-210), anti-ASC-PE (Biolegend, Cat#653904), and FAM-FLICA Caspase-1 (ImmunoChemistry Technologies, ICT-97). Data analysis was performed using IDEAS 6.0 software.
LDH activity assay
Lactate dehydrogenase (LDH) activity was measured using the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega, Cat#G1780). Supernatants from nLung and LUAD MCs or LUVA cells were collected, and LDH absorbance was measured at 490 nm. Relative LDH release was calculated using the formula: LDH release (%) = 100 × [(Experimental LDH release (OD490) − Unstimulated control (OD490))/(Lysis control (OD490) − Unstimulated control (OD490))].
Cytokine assays
MCs (2 × 105 cells per well) or LUVA cells (2 × 105 cells per well) were stimulated as indicated. Cell-free supernatants were collected by centrifugation, and IL-18 levels were quantified using an ELISA Kit (Neobioscience, Cat#EHC127.96) according to the manufacturer’s instructions.
Multiplex immunohistochemistry analysis
LUAD tissue sections or tissue microarrays were deparaffinized and rehydrated successively, followed by microwave antigen retrieval and incubation with 3% H2O2 to block endogenous peroxidase activity. Sections were serially stained with primary and secondary antibodies, after pre-incubation with 10% normal goat serum. The following antibodies were used: TCRVa7.2 (BioLegend, Cat#351702, paired with Opal 690), Tryptase (BioLegend, Cat#B208014, paired with Opal 570), PanCK (Novus, Cat#IHC-M067, paired with Opal 650), NLRP3 (Proteintech, Cat#68102-1-Ig, paired with Opal 690), ASC (Santa Cruz, Cat#sc-514414, paired with Opal 620), Asp275 (cleaved Gasdermin D, CST, Cat#36425, paired with Opal 690), and IL-18 (Proteintech, Cat#60070-1-Ig, paired with Opal 520), along with DAPI (BioLegend, Cat#422801). The signal for antibody complexes was visualized using corresponding Opal Fluorophore Reagents. The slides were scanned using the PerkinElmer Vectra3 platform, and data were analyzed using inForm (v2.3) software, as previously described12.
Immunoblotting
Cells were harvested and lysed in SDS Lysis Buffer (Beyotime, Cat#P0013G) supplemented with 1% phosphatase and protease inhibitors. Protein concentrations were quantified using the BCA Protein Quantification Kit (Beyotime, Cat#P0010S). Equal amounts of protein (40 μg) were separated by SDS-PAGE and transferred onto NC membranes (Millipore, Cat#HATF29325). Membranes were then blocked using Protein Free Rapid Blocking Buffer (Epizyme, Cat#S108P) for 30 minutes at room temperature. Subsequently, the membranes were incubated with diluted primary antibodies overnight at 4 °C. Following this, the membranes were washed four times with 1× TBST and incubated with HRP-conjugated secondary antibodies for 2 h at room temperature. After washing the membranes four more times, protein bands were visualized using the ECL Exposure System (Thermo Fisher Scientific).
Primary antibodies used in Western blotting are anti-Caspase 1/p20/p10 Polyclonal antibody (ProteinTech, 1:2000, Cat#22915-1-AP), anti-Cleaved-GSDMD (HUABIO, 1:1000, Cat#HA721144), anti-GSDMD (Abcam, 1:1,000, Cat#ab210070), anti-Cleaved-IL-18 (Abclonal, 1:1000, Cat#A24057), anti-IL-18 (ProteinTech, 1:1000, Cat#60070-1-Ig), anti-IL-1β(Abcam, 1:1000, Cat# Ab216995), anti-ASC(Santa Cruz, 1:1000, Cat#sc-514414), anti-NLRP3 (Cell Signaling Technology, 1:1000, Cat#15101), and anti-β-Actin(Cell Signaling Technology, 1:5000, Cat#4970).
Cytotoxicity assay
Sorted MAIT cells isolated from peripheral blood were aliquoted into 96-well U-bottom plates (1 × 105 cells per 100 μL per well) and stimulated with either IL-18 or IL-12 in combination with IL-18, as previously described. GFP-expressing A549 or H1299 cells were seeded in 96-well plates (1 × 105 cells per well) in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin. These tumor cells were co-cultured with either stimulated or unstimulated MAIT cells at a 1:1 ratio. After 48 h of incubation, cells were harvested and stained with Annexin V-APC and propidium iodide (PI) according to the manufacturer’s instructions. This staining was used to evaluate the survival of the tumor cells following co-culture with MAIT cells.
Statistical analysis
Statistical analyses were performed using R (version 4.1.0) and RStudio. A p-value of <0.05 was considered statistically significant. To compare differences between two groups, Student’s t-test or Wilcoxon rank-sum test was employed. For paired comparisons, paired t-tests were utilized. Differences among multiple groups were assessed using one-way or two-way ANOVA. For correlation analyses, both Spearman’s rho and Pearson’s r were used. Survival analyses were conducted using log-rank tests.
Data availability
The scRNA-seq data have been deposited in GSA database under the HRA009655. The data used in the figures in this study are provided in the Source Data file. All other data are available in the article and its Supplementary files or from the corresponding author upon request. Source data are provided with this paper. All data are included in the Supplementary Information or available from the authors, as are unique reagents used in this Article. The raw numbers for charts and graphs are available in the Source Data file whenever possible. Source data are provided with this paper.
Code availability
Custom codes for data analysis were stored in the GitHub repository: https://github.com/wangjun-hub/MAIT_code/.
References
Provine, N. M. & Klenerman, P. MAIT cells in health and disease. Annu. Rev. Immunol. 38, 203–228 (2020).
Marichal, T., Tsai, M. & Galli, S. J. Mast cells: potential positive and negative roles in tumor biology. Cancer Immunol. Res. 1, 269–279 (2013).
Majorini, M. T., Colombo, M. P. & Lecis, D. Few, but efficient: the role of mast cells in breast cancer and other solid tumors. Cancer Res. 82, 1439–1447 (2022).
Plum, T., Feyerabend, T. B. & Rodewald, H. R. Beyond classical immunity: mast cells as signal converters between tissues and neurons. Immunity 57, 2723–2736 (2024).
Kolkhir, P., Elieh-Ali-Komi, D., Metz, M., Siebenhaar, F. & Maurer, M. Understanding human mast cells: lesson from therapies for allergic and non-allergic diseases. Nat. Rev. Immunol. 22, 294–308 (2022).
Majorini, M. T. et al. Infiltrating mast cell-mediated stimulation of estrogen receptor activity in breast cancer cells promotes the luminal phenotype. Cancer Res. 80, 2311–2324 (2020).
Ligan, C., Ma, X. H., Zhao, S. L. & Zhao, W. The regulatory role and mechanism of mast cells in tumor microenvironment. Am. J. Cancer Res. 14, 1–15 (2024).
Somasundaram, R. et al. Tumor-infiltrating mast cells are associated with resistance to anti-PD-1 therapy. Nat. Commun. 12, 346 (2021).
Galli, S. J., Gaudenzio, N. & Tsai, M. Mast Cells in inflammation and disease: recent progress and ongoing concerns. Annu. Rev. Immunol. 38, 49–77 (2020).
Sulsenti, R. et al. Intracellular osteopontin promotes the release of tnfalpha by mast cells to restrain neuroendocrine prostate cancer. Cancer Immunol. Res. 12, 1147–1169 (2024).
Wernersson, S. & Pejler, G. Mast cell secretory granules: armed for battle. Nat. Rev. Immunol. 14, 478–494 (2014).
Fan, F. et al. Elevated mast cell abundance is associated with enrichment of CCR2+ Cytotoxic T cells and favorable prognosis in lung adenocarcinoma. Cancer Res. 83, 2690–2703 (2023).
Bodduluri, S. R. et al. Mast cell-dependent CD8(+) T-cell recruitment mediates immune surveillance of intestinal tumors in Apc(Min/+) Mice. Cancer Immunol. Res. 6, 332–347 (2018).
Guo, W. et al. Tumor draining lymph nodes connected to cold triple-negative breast cancers are characterized by Th2-associated microenvironment. Nat. Commun. 15, 8592 (2024).
Aller, M. A., Arias, A., Arias, J. I. & Arias, J. Carcinogenesis: the cancer cell-mast cell connection. Inflamm. Res. 68, 103–116 (2019).
Oldford, S. A. & Marshall, J. S. Mast cells as targets for immunotherapy of solid tumors. Mol. Immunol. 63, 113–124 (2015).
Barnett, K. C., Li, S., Liang, K. & Ting, J. P. Y. A 360° view of the inflammasome: Mechanisms of activation, cell death, and diseases. Cell 186, 2288–2312 (2023).
Yu, G., Choi, Y. K. & Lee, S. Inflammasome diversity: exploring novel frontiers in the innate immune response. Trends Immunol. 45, 248–258 (2024).
Coll, R. C. & Schroder, K. Inflammasome components as new therapeutic targets in inflammatory disease. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-024-01075-9 (2024).
Pandey, A., Shen, C., Feng, S. & Man, S. M. Cell biology of inflammasome activation. Trends Cell Biol. 31, 924–939 (2021).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
Zhivaki, D. et al. Correction of age-associated defects in dendritic cells enables CD4(+) T cells to eradicate tumors. Cell 187, 3888–3903.e3818 (2024).
Doitsh, G. et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 505, 509–514 (2013).
Chao, Y. Y. et al. Human T(H)17 cells engage gasdermin E pores to release IL-1alpha on NLRP3 inflammasome activation. Nat. Immunol. 24, 295–308 (2023).
Nakamura, Y. et al. Mast cells mediate neutrophil recruitment and vascular leakage through the NLRP3 inflammasome in histamine-independent urticaria. J. Exp. Med. 206, 1037–1046 (2009).
Nakamura, Y. et al. Critical role for mast cells in interleukin-1beta-driven skin inflammation associated with an activating mutation in the nlrp3 protein. Immunity 37, 85–95 (2012).
Russi, A. E., Walker-Caulfield, M. E. & Brown, M. A. Mast cell inflammasome activity in the meninges regulates EAE disease severity. Clin. Immunol. 189, 14–22 (2018).
Bonnekoh, H., Scheffel, J., Kambe, N. & Krause, K. The role of mast cells in autoinflammation. Immunol. Rev. 282, 265–275 (2018).
Mencarelli, A. et al. Anaphylactic degranulation by mast cells requires the mobilization of inflammasome components. Nat. Immunol. 25, 693–702 (2024).
Zitvogel, L., Kepp, O., Galluzzi, L. & Kroemer, G. Inflammasomes in carcinogenesis and anticancer immune responses. Nat. Immunol. 13, 343–351 (2012).
Karki, R. & Kanneganti, T. D. Diverging inflammasome signals in tumorigenesis and potential targeting. Nat. Rev. Cancer 19, 197–214 (2019).
Chen, H. et al. Genomic and immune profiling of pre-invasive lung adenocarcinoma. Nat. Commun. 10, 5472 (2019).
Plum, T. et al. Human mast cell proteome reveals unique lineage, putative functions, and structural basis for cell ablation. Immunity 52, 404–416.e405 (2020).
Zhang, Y., Fu, F. & Chen, H. Management of ground-glass opacities in the lung cancer spectrum. Ann. Thorac. Surg. 110, 1796–1804 (2020).
Fan, F. et al. Subsolid lesions exceeding 3 centimeters: the ground-glass opacity component still matters. Ann. Thorac. Surg. 113, 984–992 (2022).
Ye, T. et al. Lung adenocarcinomas manifesting as radiological part-solid nodules define a special clinical subtype. J. Thorac. Oncol. 14, 617–627 (2019).
Chu, Y. et al. Pan-cancer T cell atlas links a cellular stress response state to immunotherapy resistance. Nat. Med. 29, 1550–1562 (2023).
Ma, J. et al. A blueprint for tumor-infiltrating B cells across human cancers. Science 384, https://doi.org/10.1126/science.adj4857 (2024).
Rittmeyer, A. et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389, 255–265 (2017).
Brubaker, S. W., Bonham, K. S., Zanoni, I. & Kagan, J. C. Innate immune pattern recognition: a cell biological perspective. Annu. Rev. Immunol. 33, 257–290 (2015).
St John, A. L., Rathore, A. P. S. & Ginhoux, F. New perspectives on the origins and heterogeneity of mast cells. Nat. Rev. Immunol. 23, 55–68 (2023).
Evavold, C. L. et al. The pore-forming protein gasdermin D regulates Interleukin-1 secretion from living macrophages. Immunity 48, 35–44.e36 (2018).
Landy, E., Carol, H., Ring, A. & Canna, S. Biological and clinical roles of IL-18 in inflammatory diseases. Nat. Rev. Rheumatol. 20, 33–47 (2023).
Van Opdenbosch, N. & Lamkanfi, M. Caspases in cell death, inflammation, and disease. Immunity 50, 1352–1364 (2019).
Weindel, C. G., Ellzey, L. M., Martinez, E. L., Watson, R. O. & Patrick, K. L. Gasdermins gone wild: new roles for GSDMs in regulating cellular homeostasis. Trends Cell Biol. 33, 773–787 (2023).
Zhou, Z. et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science 368, https://doi.org/10.1126/science.aaz7548 (2020).
Xia, S. et al. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature 593, 607–611 (2021).
Liu, X., Xia, S., Zhang, Z., Wu, H. & Lieberman, J. Channelling inflammation: gasdermins in physiology and disease. Nat. Rev. Drug Discov. 20, 384–405 (2021).
Yamanishi, K. et al. Molecular mechanisms of IL18 in disease. Int. J. Mol. Sci. 24, https://doi.org/10.3390/ijms242417170 (2023).
Freeman, B. E., Hammarlund, E., Raue, H. P. & Slifka, M. K. Regulation of innate CD8+ T-cell activation mediated by cytokines. Proc. Natl. Acad. Sci. USA 109, 9971–9976 (2012).
Garner, L. C. et al. Single-cell analysis of human MAIT cell transcriptional, functional and clonal diversity. Nat. Immunol. 24, 1565–1578 (2023).
Ruf, B. et al. Tumor-associated macrophages trigger MAIT cell dysfunction at the HCC invasive margin. Cell 186, 3686–3705.e3632 (2023).
Yao, T., Shooshtari, P. & Haeryfar, S. M. M. Leveraging public single-cell and bulk transcriptomic datasets to delineate MAIT cell roles and phenotypic characteristics in human malignancies. Front. Immunol. 11, https://doi.org/10.3389/fimmu.2020.01691 (2020).
Kjer-Nielsen, L. et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 491, 717–723 (2012).
Legoux, F., Salou, M. & Lantz, O. MAIT cell development and functions: the microbial connection. Immunity 53, 710–723 (2020).
Aibar, S. et al. SCENIC: single-cell regulatory network inference and clustering. Nat. Methods 14, 1083–1086 (2017).
Duan, M. et al. Activated and exhausted MAIT cells foster disease progression and indicate poor outcome in hepatocellular carcinoma. Clin. Cancer Res. 25, 3304–3316 (2019).
Kammer, M. N. et al. Tumoral densities of T-Cells and mast cells are associated with recurrence in early-stage lung adenocarcinoma. JTO Clin. Res. Rep. 4, 100504 (2023).
Cheng, S. et al. A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell 184, 792–809.e723 (2021).
Stelekati, E. et al. Mast cell-mediated antigen presentation regulates CD8+ T cell effector functions. Immunity 31, 665–676 (2009).
Omoto, Y. et al. Human mast cell chymase cleaves pro-IL-18 and generates a novel and biologically active IL-18 fragment. J. Immunol. 177, 8315–8319 (2006).
Cheng, O. J. et al. MAIT cell-mediated inhibition of colorectal tumor growth is associated with increase in IL-5 and accumulation of eosinophils in the tumor microenvironment. J. Immunol. 210, 84.10–84.10 (2023).
Shi, L. et al. Clinicopathological and predictive value of MAIT cells in non-small cell lung cancer for immunotherapy. J. Immunother. Cancer 11, https://doi.org/10.1136/jitc-2022-005902 (2023).
Ouyang, L. et al. Mucosal-associated invariant T cells reduce and display tissue-resident phenotype with elevated IL-17 producing capacity in non-small cell lung cancer. Int. Immunopharmacol. 113, 109461 (2022).
Mantri, C. K. & St John, A. L. Immune synapses between mast cells and gammadelta T cells limit viral infection. J. Clin. Investig. 129, 1094–1108 (2019).
Dudeck, J. et al. Mast-cell-derived TNF amplifies CD8(+) dendritic cell functionality and CD8(+) T cell priming. Cell Rep. 13, 399–411 (2015).
Flament, H. et al. Outcome of SARS-CoV-2 infection is linked to MAIT cell activation and cytotoxicity. Nat. Immunol. 22, 322–335 (2021).
Lamichhane, R. et al. TCR- or Cytokine-Activated CD8(+) mucosal-associated invariant T cells are rapid polyfunctional effectors that can coordinate immune responses. Cell Rep. 28, 3061–3076.e3065 (2019).
Leng, T. et al. TCR and inflammatory signals tune human MAIT cells to exert specific tissue repair and effector functions. Cell Rep. 28, 3077–3091.e3075 (2019).
Petley, E. V. et al. MAIT cells regulate NK cell-mediated tumor immunity. Nat. Commun. 12, 4746 (2021).
Parrot, T. et al. Expansion of donor-unrestricted MAIT cells with enhanced cytolytic function suitable for TCR redirection. JCI Insight 6, https://doi.org/10.1172/jci.insight.140074 (2021).
Jensen, O. et al. A subset of follicular helper-like MAIT cells can provide B cell help and support antibody production in the mucosa. Sci. Immunol. 7, eabe8931 (2022).
Li, Y. R. et al. Mucosal-associated invariant T cells for cancer immunotherapy. Mol. Ther. 31, 631–646 (2023).
Thorsson, V. et al. The immune landscape of cancer. Immunity 48, 812–830.e814 (2018).
Acknowledgements
This work was supported by the National Natural Science Foundation of People’s Republic of China (82430099 and 81930073), the Cooperation Project of Conquering Major Diseases in Xuhui District (XHLHGG202101), the National Key R&D Program of China (2022YFA1103900), the Strategic Priority Research Program of the Chinese Academy of Sciences (No.XDB0940102), National Natural Science Foundation of China (No. U23A6010), National Natural Science Foundation of China (82203504), National Natural Science Foundation of China (T24B2017). We thank Lishuang Zhang, and Pengwei Zhang (ShanghaiTech University), Yingcheng Wu, Dongning Rao, and Yifei Cheng (Fudan University Zhongshan Hospital), Shan Jiang, Xixi Gu, Jianghong Yu, Fengyun Jia, Xiaoshan Liu, Yamin Chen, and Qian Sun (Shanghai Institute of Immunity and Infection), Ran Guo, Yuan Hui, Yawei Zhang, Jiaqing Xia, Xiaoyang Luo, Longsheng Miao, Longfei, Ma, Haiqing Chen, Haoxuan Wu, Jie, Wang, ShanBo, Zheng (Fudan University Shanghai Cancer Center), Tian Tian, Gege Yuan (Nanjing Medical University) for technical assistance. We thank the CFFF platform of Fudan University for providing the computing platform in this study.
Author information
Authors and Affiliations
Contributions
Conceptualization, F.F. Fan, X.M. Zhang, and H.Q. Chen; methodology, F.F. Fan, J. Wang, K. Liu, J. Gao, J.Q. Ma, T. Li, X.M. Zhang, H.Q. Chen; software, F.F. Fan, J. Gao, J. Wang, S.Y. Zhang, Z.W. Cao; validation, F.F. Fan, J. Wang, K. Liu, J. Gao, S.Y. Zhang, X.F. Li, H. Su, X.F. Yang; investigation, F.F. Fan, J. Wang, K. Liu, J. Wang, S.Y. Zhang, X.F. LI, H. Su, X.F. Yang; formal analysis, F.F. Fan, J. Wang, K. Liu, S.Y. Zhang; writing – original draft, F.F. Fan, J. Wang, K. Liu; writing – review & editing, F.F. Fan, Z.W. Cao, X.M. Zhang, H.Q. Chen; visualization, F.F. Fan, J. Wang, K. Liu, J. Wang, S.Y. Zhang; funding acquisition, Q.Y. Huang, X.M. Zhang, H.Q. Chen; sample collection, Y. Zhao, Q.Y. Huang, H. Han, Y.L. Zhang, Y.J. Pan, Ting Ye, H. Hu, Y.H. Sun, Y. Zhang, H.Q. Chen; and supervision, T. Li, F. Li, Z.W. Cao, Y. Zhang, X.M. Zhang, H.Q. Chen. K. Liu performed Immunoblotting; FF Fan, J Wang, SY Zhang performed bulk RNA-seq and scRNA-seq, scCITE-seq, and scTCR-seq analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fan, F., Wang, J., Liu, K. et al. Mast cells boost anti-tumor potency of MAIT cells via inflammasome-dependent secretion of IL-18. Nat Commun 16, 6074 (2025). https://doi.org/10.1038/s41467-025-61324-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-61324-w
This article is cited by
-
Review: Progress of the NLRP3 inflammasome in tumours and perspectives for cholangiocarcinoma
Cell Communication and Signaling (2025)