Abstract
Substituted 1,3,4-thiadiazoles find extensive use in pharmaceutical, agricultural, and materials chemistry. The incorporation of adaptable heterocyclic pharmacophores results in tunable hybrid molecules with diverse medicinal properties. In this study, the direct coupling of primary nitroalkanes and acyl hydrazines (hydrazides) is achieved simply by the mild action of S8 and Na2S. This method now delivers wide varieties of multi-functionalized 1,3,4-thiadiazoles in excellent yields. The reaction is scalable, shows a broad substrate scope, and tolerates a wide range of functional groups. The power of this method is exemplified with over twenty acyl hydrazines, spanning a diverse range of nitroalkane substrate classes, as well as the concise and scalable synthesis of 1,3,4-thiadiazole derivatives of over ten distinct types of drugs and peptides.
Similar content being viewed by others
Introduction
The 1,3,4-thiadiazole structural unit is often used as a heteroaromatic bioisostere of an amide bond. While improving hydrolytic stability, this amide replacement tends to retain the hydrogen bonding network within the acceptor site and is widely selected as a key motif in drug design targeting antibacterial agents, anticancer agents, anti-convulsants and anti-inflammatory agents1,2,3,4. Representative drug molecules are shown in Fig. 1a, including: cefazedone, a cephalosporin antibiotic used to treat bacterial infections5; sulfamethizole, a sulfonamide antibiotic used to treat a wide variety of susceptible bacteria6; Glybuzole, an antihyperglycemic antidiabetic7; MK-8189, a unique candidate in Phase II clinical development for the treatment of schizophrenia8; and BI-3231, a potential targeted drug to treat for NASH and liver disease9. Heterocyclic grafting with the structural alteration of amino acids as heterocycles, such as oxazoles and thiazoles, has been shown to improve the passive membrane permeability of macrocycles10,11,12,13. In addition to pharmaceutical applications, 1,3,4-thiadiazoles have shown great promise in agriculture, including herbicides, fungicides, insecticides, and plant growth regulators14, as exemplified by the herbicide, flufenacet, which can control annual grass weeds, sedges, and small broadleaf weeds15. In the field of optoelectronic materials the 1,3,4-thiadiazole structure has also been adopted to provide excellent electron-accepting, thermal, and chemical stabilities16,17,18.
The synthesis of 1,3,4-thiadiazoles is traditionally based on indirect condensation methods via sulfuration of the corresponding 1,4-dicarbonyl19 or acyl precursor20 using phosphorus sulfide reagents such as P2S5 or Lawesson-types of reagents (Fig. 1b). These methods require harsh electrophilic sulfurizing reagents with low functional group compatibility and selectivity, making them inappropriate for the incorporation of 1,3,4-thiadiazoles in drug molecules and chemical biology. To fully exploit the potential of 1,3,4-thiadiazoles in medicinal chemistry research, we realized that it would be essential to design a more direct ligation strategy to access a diverse array of 1,3,4-thiadiazole structures. This strategy should: (1) utilize readily accessible substrates; (2) operate under mild conditions; (3) tolerate a wide range of functional groups and give good overall yields; (4) avoid the use of expensive noble metals; (5) be scalable; and (6) be modular to facilitate the straightforward synthesis of disubstituted 1,3,4-thiadiazoles with varied structures. Herein, we describe an efficient method for synthesizing 1,3,4-thiadiazole structural units that meet all the above criteria (Fig. 1c)21.
Results and discussion
Reaction discovery and optimization
Primary nitroalkanes are readily available for synthesis and used as carbonyl precursors to form carboxylic22,23, amides24,25,26,27, esters28. Elemental sulfur (S8) is abundant in nature, non-toxic, non-volatile, non-hygroscopic and odorless, making it an ideal sulfur source29,30,31,32,33. Stemming from our recent thioamidation study of primary nitroalkanes with elemental sulfur in the presence of Na2S, we soon realized that nitroalkanes can be made to behave as a masked thioacylating species, which would be able to condense with acyl hydrazines to afford 1,3,4-thiadiazole products34. At the outset, we selected commercially available benzoyl hydrazine 1a and nitroethane 2a as simple starting materials. In initial experiments, when S8 and Na2S were used, we were pleased to isolate the disubstituted thiadiazole 3a in 76% yield (Table 1, entry 1). Here we found the chemical yield of 3a was affected dramatically by the work up procedure (see Supplementary Table 1 for details). Considering the price, Na2S·5H2O and Na2S·9H2O were both investigated, which gave similar results to non-hydrated Na2S (cf. entries 1-3). Encouraged by these results, further optimization was carried out using Na2S·9H2O to improve the chemical yield of 3a. A survey of different solvents soon demonstrated DMF and THF to deliver better yields of 3a (cf. entries 4-7 and entry 2) and the strong base NaOtBu proceeded comparably well to Na2S·9H2O (cf. entry 8-9). Notably, the reported methods to make 1,3,4-thiadiazoles are principally conducted under anhydrous conditions in non-polar solvent systems4. This greatly precludes the synthesis of polar drugs and more complex peptides. We therefore tested the polar solvent system of DMF/H2O at various ratios for 1,3,4-thiadiazole formation (entry 10–11). The use of Na2S·9H2O in a mixture of DMF/H2O (9:1) at room temperature proceeded excellently (entry 10), although yields decreased with higher water content, for example using DMF/H2O (5:1) (entry 11).
Generality of 1,3,4-Thiadiazole Formation using Elemental Sulfur
With high yielding conditions under various solvent systems in hand with S8/Na2S·9H2O, the substrate scope of various acyl hydrazines were first investigated with nitroethane in DMF (Fig. 2a). Acyl hydrazines carrying aliphatic, aromatic, alcohol, CF3, amine, furan, thiophene, pyridine, pyrimidine, aromatic amine, electron-deficient amine, free acid, and OH units were all tolerated well under the reaction conditions, providing the 1,3,4-thiadiazole products in high yields (3a–3r). Importantly, the α-amino product 3r was produced with complete stereochemical integrity. No epimerization of potentially labile α-stereocenters was observed (see Supplementary Fig. 1 for HPLC analyses). Changing the primary nitroalkane to bear valuable functionalities such as hydroxyl group, ester, amide, ketone, acetal and triazole, similarly furnished the corresponding thiadiazoles 4a–4j from benzoyl hydrazine 2a in good yields (Fig. 2b). Notably, Cbz amino protecting groups were tolerated well under our reaction conditions (4f). When using chiral nitroalkanes, reactions proceeded in moderate yield with minimal epimerization at −10 ˚C with iPrO-S-S-OiPr (e.g., 4k, 96% ee). (See Supplementary Table 2 and Supplementary Fig. 2) As anticipated, this method was found to be extremely useful for the modular synthesis of different thiadiazole-containing bioactive compounds, for example, the marketed drug sulfamethizole (5a) can be readily prepared in good to excellent yield, as well as various highly bioactive thiadiazole-derivatives 5b-i (Fig. 2c).
The introduction of heterocyclic grafting such as oxazole and thiazole units, into well-established medicinally active peptides or cyclic peptides, results in an increase in structural diversity of peptides and importantly improves the passive membrane permeability of macrocycles10,11,12,13,35. We thus explored the thiadiazole-grafting of peptides from their respective nitro- and hydrazide-bearing peptide fragments in DMF. Importantly, the peptide hydrazides (6) can be conveniently prepared through Fmoc-based solid phase peptide synthesis (SPPS; Supplementary Fig. 3)36 and nitromethyl peptides (7) can be easily prepared via Boc-based peptide synthesis (Supplementary Fig. 6). Coupling of fragments 6 and 7 in the presence of elemental sulfur furnished the 2,5-substituted 1,3,4-thiadiazoles 8a–8c in good to excellent yields. (Fig. 3a, Supplementary Fig. 7 for HPLC and MS analysis of 8c). Under our current conditions, peptides containing unprotected cysteine and histidine units were found to be incompatible. However, fluorophores and biotin were found to be compatible under mild conditions, for example, providing 8d and 8e. We further explored our coupling strategy in the construction of cyclic peptides, as inspired by the introduction of oxazole units, which can improve the activity and stability of peptides10,11,12,13,35. (Fig. 3b) Here, the nitro-hydrazine cyclization precursor 9 was readily synthesized via solid peptide synthesis (Supplementary Fig. 3 and Supplementary Fig. 5), then treated with our standard conditions. To our delight, a 20-membered cyclic peptide 10 was obtained in 50% yield. (Fig. 3c)
As an important structural unit widely present in various drug molecules, traditional approaches to introduce the 1,3,4-thiadiazole unit occur at an early stage of a synthesis followed by subsequent functionalization. These approaches, however, tend to show poor selectivity and low efficiency for complex drug molecules and peptides9. A representative example is BI-3231, which is a potent and selective HSD17B13 inhibitor that is under in-vivo development by Boehringer-Ingelheim (Fig. 4a)9. In the previous synthetic route from thio-semi-carbazide, 7 steps were required, including excess amounts of copper bromide and a palladium catalyst. Now, the direct hetero-annulation between the aroyl hydrazine 13 and nitroalkane 12 gave BI-3231 in excellent overall yield over 3 steps, whereby the nitroalkane 12 can be readily prepared via simple bromide substitution of 11 by nitrite (Supplementary Fig. 9 for the preparing 11 from 5-methyluracil).
Another example is MIK-8189, which is under Phase II development by Merck for the treatment of schizophrenia and Alzheimer’s disease (Fig. 4b)8. In the previous synthetic route from α-amino hydrozine, 6 steps were required. Here, the direct coupling of the acyl hydrazine 14 and nitroethane in the presence of elemental sulfur gave the thiadiazole 15 which, when treated with the cyclopropyl-methyl alcohol 16 by a known procedure, afforded MIK-8189 in excellent overall yield, (Supplementary Fig. 10 for the preparing 14 from 4,6-dichloro-2-methylpyrimidine). To further demonstrate the advantages of this direct 1,3,4-thiadiazole coupling method, we compared it to the O-S exchange reaction with Lawson’s reagent. At Idorsia Pharmaceuticals, ethyl 5-(2,4-difluorophenyl)-1,3,4-thiadiazole-carboxylate 19 is a key intermediate to making ACKR3 modulators37. For example, the first generation of 19 involved the acylation of commercially available 2,4-difluorobenzoic acid hydrazide 17 with ethyl chloro-oxoacetate, followed by cyclization of 18 with Lawesson’s reagent (Fig. 4c)38. This route suffered from handling the malodorous Lawesson’s reagent and removing its lipophilic byproducts, and the final product had to be purified by column chromatography. In the second-generation route, designed for large-scale synthesis, excess amounts of CuBr were required to make 21 from 20 via a Sandmeyer reaction, and Pd-coupling with boric acid 22 produced the diary ester 19. On a 20 g scale, we found the direct coupling of commercially available 2,4-difluorobenzoic acid hydrazide 17 and commercially available α-nitro acetate 23 with elemental sulfur and base furnished 19 in excellent yield without the need for further purification after a simple aqueous acid work up. Collectively, these examples emphasize the current thia-annulation method as a practical tool for the mild, late-stage installation of 1,3,4-thiadiazole moieties into highly functionalized molecules.
Mechanistic Investigations
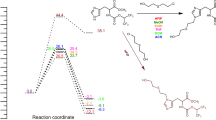
Control experiments to confirm the likely reaction mechanism are summarized in Fig. 5. First, when acyl hydrazine 1a was mixed with elemental sulfur and Na2S for 24 hours, the acyl hydrazine 1a was completely recovered (Fig. 5a, eq. (1)). Second, when ethyl nitroacetate was mixed with elemental sulfur and Na2S in d6-DMSO, the bis-thioacid sodium salt 24 was obtained quantitively by NMR (Fig. 5a, eq. (2); see Supplementary Fig. 12-13 for NMR and HRMS analysis). Third, when the bis-thioacid sodium salt 24 was mixed with acylhydrazine 1a in the presence of elemental sulfur and Na2S, no reaction was observed, which indicated 24 to not be the likely intermediate (Fig. 5a, eq. (3)). In addition, when nitroethane was mixed with acylhydrazine 1a in the presence of elemental sulfur and Na2S, the uncyclized thioacylated hydrazide 25 mostly formed together with trace amounts of desired thiadiazole 3a, prior to silica gel chromatographic purification (Fig. 5a, eq. (4)). After testing different work up procedures, the acyclic intermediate 25 was conveniently annulated to 3a in excellent yield through mild aqueous acid work-up in DMF (Fig. 5a, eq. (5)). Alternatively, intermediate 25 can be converted into the 1,3,4-oxadiazole counterpart of 3a by treatment with MeI (see Supplementary Table 3 for detail). Based on the above control reactions and our previous report34, a plausible reaction pathway is proposed in Fig. 5b. Thus, by the mild action of elemental sulfur and Na2S, nitroalkane 2 first converts to its thioacyl nitrate derivative 26, which then reacts with the aroyl-hydrazine 1 to afford the thioacylated hydrazide 27 that readily heteroaromatizes to the 1,3,4-thiadiazole 3 during mild aqueous work-up. (Fig. 5b).
In summary, we have developed a direct and modular synthesis of 1,3,4-thiadiazoles with utility in complex chemical environments for hetero-annulations. The method is operationally simple, exhibits excellent chemoselectivity with diverse functional groups, maintains stereochemical integrity even with epimerizable substrates and products, and eliminates the need for extensive protection/deprotection steps. With the ready availability of nitroalkanes and hydrazides with broad structural diversity and complexity, numerous applications and uses are anticipated across the chemical and biological sciences. For now, we have demonstrated its utility in making thiadiazole chemical libraries and medicinal analogs, in scaling the synthesis of marketed drugs and key thiadiazole intermediates, in the late-stage terminus capping and grafting of peptides, and in the intramolecular annulation of a cyclic peptide precursor.
Methods
Representative procedure
Nitroalkane (0.4 mmol) and acyl hydrazine (0.2 mmol) are added to S8 (0.4 mmol) and Na2S·9H2O (0.36 mmol, 1.8 equiv.) in DMF (2 mL) under nitrogen. The reaction mixture is stirred at room temperature and monitored by TLC until the acyl hydrazine is consumed, typically after 24 h, then 2 N HCl solution is added and the reaction stirred for a further 2 h. The crude residue is then purified by silica-gel flash-column chromatography, if needed.
Data availability
All experimental procedures, characterization data, and NMR spectra are available in the supplementary materials. All data are available from the corresponding author upon request.
References
Han, X., Yu, Y. L., Hu, Y. S. & Liu, X. H. 1,3,4-Thiadiazole: a privileged scaffold for drug design and development. Curr. Top. Med. Chem. 21, 2546–2573 (2021).
Khalili Ghomi, M. et al. 1,2,4triazolo3,4-b1,3,4thiadiazole derivatives as new therapeutic candidates against urease positive microorganisms: design, synthesis, pharmacological evaluations, and in silico studies. Sci. Rep. 13, 10136 (2023).
Pryde, D. C. et al. New selective inhibitors of neutral endopeptidase for the treatment of female sexual arousal disorder. Synthesis and activity of functionalized glutaramides. J. Med. Chem. 49, 4409–4424 (2006).
Jain, A. K., Sharma, S., Vaidya, A., Ravichandran, V. & Agrawal, R. K. 1,3,4-Thiadiazole and its derivatives: a review on recent progress in biological activities. Chem. Biol. Drug. Des. 81, 557–576 (2013).
Russell, A. D. & Rogers, D. T. In vitro activity of cefazedone, a new cephalosporin antibiotic. J. Antimicrob. Chemother. 6, 288–291 (1980).
Watanabe, H. & Hastings, J. W. Inhibition of bioluminescence in Photobacterium phosphoreum by sulfamethizole and its stimulation by thymine. Biochim. Biophys. Acta 1017, 229–234 (1990).
Otsuka, M., Ofusa, T. & Matsuda, Y. Physicochemical characterization of glybuzole polymorphs and their pharmaceutical properties. Drug Dev. Ind. Pharm. 25, 197–203 (1999).
Layton, M. E. et al. Discovery of MK-8189, a highly potent and selective PDE10A inhibitor for the treatment of schizophrenia. J. Med. Chem. 66, 1157–1171 (2023).
Thamm, S. et al. Discovery of a novel potent and selective HSD17B13 Inhibitor, BI-3231, a well-characterized chemical probe available for open science. J. Med. Chem. 66, 2832–2850 (2023).
Saunders, G. J. & Yudin, A. K. Property-driven development of passively permeable macrocyclic scaffolds using heterocycles. Angew. Chem. Int. Ed. 61, e202206866 (2022).
Saunders, G. J. & Yudin, A. K. A focus on the discovery of potent and selective cyclic peptide scaffolds for drug development. Chem. Sci. 13, 12942–12944 (2022).
Appavoo, S. D., Huh, S., Diaz, D. B. & Yudin, A. K. Conformational control of macrocycles by remote structural modification. Chem. Rev. 119, 9724–9752 (2019).
Frost, J. R., Scully, C. C. G. & Yudin, A. K. Oxadiazole grafts in peptide macrocycles. Nat. Chem. 8, 1105–1111 (2016).
Gilden, R. C., Huffling, K. & Sattler, B. Pesticides and Health Risks. J. Obst. Gyn. Neo. 39, 103–110 (2010).
Lechelt-Kunze, C., Meissner, R. C., Drewes, M. & Tietjen, K. Flufenacet Herbicide Treatment Phenocopies the Fiddlehead Mutant in Arabidopsis Thaliana. Pest. Manag. Sci. 59, 847–856 (2003).
Selivanova, G. A. Azo chromophores for nonlinear-optical application. Russ. Chem. Bull. 70, 213–238 (2021).
Budziak-Wieczorek, I. et al. Spectroscopic Characterization and Assessment of Microbiological Potential of 1,3,4-Thiadiazole Derivative showing ESIPT Dual Fluorescence Enhanced by Aggregation Effects. Sci. Rep. 12, 22140 (2022).
Kuo, H.-M., Li, S.-Y., Sheu, H.-S. & Lai, C. K. Symmetrical mesogenic 2,5-bis(6-naphthalen-2-yl)−1,3,4-thiadiazoles. Tetrahedron 68, 7331–7337 (2012).
Kaleta, Z., Makowski, B. T., Soós, T. & Dembinski, R. Thionation using fluorous Lawesson’s reagent. Org. Lett. 8, 1625–1628 (2006).
Matheau-Raven, D. & Dixon, D. J. General α-Amino 1,3,4-Oxadiazole Synthesis via Late-Stage Reductive Functionalization of Tertiary Amides and Lactams. Angew. Chem. Int. Ed. 60, 19725–19729 (2021).
Hu, Y., Li, C.-Y., Wang, X.-M., Yang, Y.-H. & Zhu, H.-L. 1,3,4-Thiadiazole: synthesis, reactions, and applications in medicinal, agricultural, and 1,3,4-Thiadiazole: synthesis, reactions, and applications in medicinal, agricultural, and materials chemistry. Chem. Rev. 114, 5572–5610 (2014).
Kornblum, N., Blackwood, R. K. & Mooberry, D. D. The reaction of aliphatic nitro compounds with nitrite esters. J. Am. Chem. Soc. 78, 1501–1504 (1956).
Kornblum, N. et al. A new method for the synthesis of aliphatic nitro compounds. J. Am. Chem. Soc. 78, 1497–1501 (1956).
Shen, B., Makley, D. M. & Johnston, J. N. Umpolung reactivity in amide and peptide synthesis. Nature 465, 1027–1032 (2010).
Schwieter, K. E., Shen, B., Shackleford, J. P., Leighty, M. W. & Johnston, J. N. Umpolung amide synthesis using substoichiometric N-iodosuccinimide (NIS) and oxygen as a terminal oxidant. Org. Lett. 16, 4714–4717 (2014).
Schwieter, K. E. & Johnston, J. N. A one-pot amidation of primary nitroalkanes. Chem. Commun. 52, 152–155 (2016).
Li, J. et al. Oxidative amidation of nitroalkanes with amine nucleophiles using molecular oxygen and iodine. Angew. Chem. Int. Ed. 54, 12986–12990 (2015).
Li, J., Lear, M. J. & Hayashi, Y. Autoinductive conversion of α,α-diiodonitroalkanes to amides and esters catalysed by iodine byproducts under O2. Chem. Commun. 54, 6360–6363 (2018).
Nguyen, T. B. Recent advances in organic reactions involving elemental sulfur. Adv. Synth. Catal. 359, 1066–1130 (2017).
Chung, W. J. et al. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat. Chem. 5, 518–524 (2013).
Lee, T., Dirlam, P. T., Njardarson, J. T., Glass, R. S. & Pyun, J. Polymerizations with elemental sulfur: from petroleum refining to polymeric materials. J. Am. Chem. Soc. 144, 5–22 (2022).
Tang, H. et al. Direct synthesis of thioesters from feedstock chemicals and elemental sulfur. J. Am. Chem. Soc. 145, 5846–5854 (2023).
Saito, M., Murakami, S., Nanjo, T., Kobayashi, Y. & Takemoto, Y. Mild and chemoselective thioacylation of amines enabled by the nucleophilic activation of elemental sulfur. J. Am. Chem. Soc. 142, 8130–8135 (2020).
Wang, X. et al. Nitroalkanes as thioacyl equivalents to access thioamides and thiopeptides. Nat. Commun. 14, 4626 (2023).
He, J. & Nitsche, C. Biocompatible synthesis of macrocyclic Thiazol(in)e peptides. Chem. Eur. J. 30, e202401716 (2024).
Fang, G.-M. et al. Protein chemical synthesis by ligation of peptide hydrazides. Angew. Chem. Int. Ed. 50, 7645–7649 (2011).
Rick, R. et al. Modulators of ACKR3 and uses thereof as drugs. PCT/EP2023/055469 (2023).
Schäfer, G., Fleischer, T., Ahmetovic, M. & Abele, S. Development of a scalable route for a key thiadiazole building block via sequential sandmeyer bromination and room-temperature Suzuki–Miyaura coupling. Org. Process Res. Dev. 24, 228–234 (2020).
Acknowledgements
Generous support from Xi’an Jiaotong University is acknowledged. We are thankful to the National Natural Science Foundation of China (22471206). We thank Miss Lu Bai and Miss Chao Feng at the Instrument Analysis Center of Xi’an Jiao tong University for their assistance with HRMS and NMR analysis. We also thank the University of Lincoln for its support.
Author information
Authors and Affiliations
Contributions
J.L. conceived the idea and supervised the whole project. X.N.W., X.W.Y., and R.X.Q. performed all the synthetic experiments and analyzed results. X.N.W., S.L.X., W.X.H., M.J.L., and J.L. co-wrote the manuscript. All authors approved the final version of the manuscript for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, X., Yu, X., Qian, R. et al. Chemoselective synthesis of 1,3,4-thiadiazoles from acyl hydrazines and nitroalkanes using elemental sulfur. Nat Commun 16, 6127 (2025). https://doi.org/10.1038/s41467-025-61359-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-61359-z