Abstract
Ideal laminar membrane reactors (LMR) necessitate the rational construction of 2D nanoconfined channels and regulation of microenvironment. The intrinsic structure-activity relationship of laminar membrane reactors essentially remains unclear. Herein, amorphous/crystalline HTiNbO5-X nanosheets (NS) with abundant oxygen vacancy (VO) are successfully prepared and stacked as 2D sub-nanoconfined membrane flow reactors with different interlayer spacings. When the interlayer spacing is decreased to 11.0 Å, the representative HTiNbO5-X membrane exhibits impressive conversion of ≈ 100% within 26.6 s and turnover number of up to 306.42 for the esterification of benzyl alcohol at 23 °C. The combination of structural characterizations and theoretical calculations reveals that the synergistic effect of VO and sub-nanoscale confinement greatly promotes the adsorption and orbital symmetry matching of reactants, and reduces the overall energy barrier. Furthermore, the HTiNbO5-X LMR is accessible for other alcohol derivatives, indicating the efficient and green synthesis of acetate ester flavours under mild conditions. This work demonstrates the promising potential of the metal oxide nanosheets to create inorganic laminar membrane reactors for continuous organic synthesis.
Similar content being viewed by others
Introduction
Efficient, environmental, and economically viable chemical processes or methodologies are in great demand for those widely used organic compounds. Acetate esters (e.g., benzyl acetate, anisyl acetate, cinnamyl acetate) are extensively used as flavours and fragrances in food, cosmetics and pharmaceuticals industries which are in high demand (as high as 10,000 tons per annum for benzyl acetate)1. Besides direct extraction from plant or fruit sources, these ester flavours are typically obtained through esterification reactions or enzymatic processes. Over the past few decades, various catalytic materials, including solid acids, ionic liquids and inorganic salts, have been explored to replace mineral acids (e.g., H2SO4) for serious corrosion and contamination issues2,3. Recently, the growing traction for sustainable processing has resulted in a transition from conventional batch reactors to advanced catalysis reactors4. Membrane reactors, as a continuous flow technology, offers many advantages including absence catalyst separation, precise temperature control and reduced power consumption5.
Confinement can play a defining role in interface properties and chemical catalysis6. As the materials are assembled/confined to nanoscale, their geometric and electronic structures in the confined state operate as thermodynamically and kinetically more critical factors than those in the bulk state, which can result in unusual physicochemical and catalytic properties7. Over the past decade, tremendous efforts have been devoted to preparing various porous nanomaterials from 1D to 3D (e.g., carbon nanotubes, graphene oxide, metal-organic frameworks), while only a small portion of work have successfully constructed nanoconfined systems or materials8,9,10. For example, Al/Ni-Pt/Ti nanotube was reported to show remarkably high efficiency in nitrobenzene hydrogenation due to the instant transfer of intermediates and the tailored electronic properties of confined nanochannels9. However, these materials mainly participate in catalytic reactions through batch reactors and the reaction efficiencies are still limited by the disordered motion of molecules and the inefficient diffusion of molecules between the bulk solution and the nanochannels of porous materials, especially for molecular-scale channels10. Moreover, current study of catalytic materials are mainly focused on the design of nanomaterials with hierarchical channels and the degree of confinement for these systems are localized within several to tens of nanometers considering heat and mass transfer, and thereby the intrinsic structure−activity relationship of sub-nanoscale confinement has attracted intense interests6.
In natural enzymes, reactants diffuse into the active sites with the binding sites and catalytic sites to break and form bonds11. The synergy of multiple sites contribute to their extremely high activity and selectivity. There is a great challenge to apply this concepts into membrane flow reactors with confined channels in sub-nanometer scale, even though it is at the forefront of catalytic chemistry. Recently, many 2D materials, such as graphene oxide (GO), covalent organic framework (COF), layered double hydroxide (LDH), and transition metal oxides/chalcogenides (TMO/C), have been employed for the fabrication of 2D material membranes, and these membranes have been effectively practiced in separation and purification12,13,14,15,16. Actually, 2D materials have atomic-level thicknesses and unique physicochemical properties, and they are also very promising platforms to develop new nanoconfined flow reactors for organic synthesis17,18. For instance, we have controlled the chemical structure of 2D graphene oxide membranes effectively, and realized rapid Knoevenagel condensation and synthesis of aspirin recently19,20. Through tuning the interlayer distances and interface properties of nanochannels, the intrinsic characteristics of the reaction, e.g., mass transfer, reaction kinetics, and selectivity, can be effectively dominated under mild process conditions. It is thus attractive to implement laminar membrane reactors for continuous pharmaceutical and fine chemical synthesis18. However, the difficulty in preparing of uniform, large sized and ultrathin 2D nanosheets (e.g., COF, LDH) to create effective confined nanochannels greatly restrict the development of LMR17. In addition, the currently reported 2D materials lack proper and stable catalytic active sites for specific reactions (esterification in this work). In contrast to 2D carbonaceous materials like GO with abundant oxygen-containing groups, 2D metallic compounds (e.g., TMO) are difficult to modulate the microenvironment nearby catalytic sites21. Oxygen vacancies in TMO have demonstrated to be beneficial for many applications due to their unsaturated coordination and distinct reactivity22. However, achieving precise control of these defects remains a significant challenge, and the role of vacancies to the adsorption and activation of reactants, especially under 2D confined conditions, remains largely unknown23.
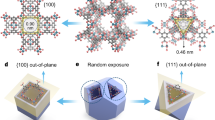
Here, we report an outstanding laminar membrane reactors prepared by amorphous/crystalline HTiNbO5-X nanosheets enriched with oxygen vacancies, which enables rapid and efficient synthesis of acetate ester flavours (Fig. 1a, b). The Brønsted acid sites and oxygen vacancies are engineered onto the HTiNbO5-X nanosheets by acid etching (Fig. 1c–f). The sub-nanoconfined channels of HTiNbO5-X membranes are controlled from 14.4 to 11.0 Å by mild thermal treatment (Fig. 1g, h). As a result, alcohol molecules move through the sub-nanoconfined channels directionally and react with acetic anhydride (Ac2O) molecules efficiently under mild conditions (Fig. 1i, j). When the interlayer spacing is decreased to 11.0 Å, the esterification of benzyl alcohol (BnOH) is realized with conversion of ≈ 100% in an unparalleled short time and turnover number (TON) of up to 28 times higher than that of bulk reaction at 23 °C (Fig. 1k). The synergy of multiple sites and confined spatial structures are realized in this bioinspired LMR, contributing to the modulation of reactant adsorption, molecular polarization, orbital symmetry matching and reaction thermodynamics. We have adjusted the membrane thicknesses, pressure difference, reaction temperatures and reactant concentrations, and further expanded the substrate scope and stability test, proving the rationality and superiority of the laminar membrane reactors to conventional batch reactors.
a The esterification of benzyl alcohol with acetic anhydride catalyzed by HTiNbO5-X membranes in CH3CN at 23 °C. b Fabrication of the HTiNbO5-X nanosheets and HTiNbO5-X membrane. c TEM image of HTiNbO5-X nanosheets. d AFM image and corresponding height profile of HTiNbO5-X nanosheets. e Pyridine IR spectra and f NH3 TPD profiles of HTiNbO5-X nanosheets and KTiNbO5. g Cross-sectional SEM image of HTiNbO5-X membrane (Inset show the photograph of the as-prepared HTiNbO5-X membrane). h XRD patterns measured for the reaction-state of HTiNbO5-X membranes obtained by different annealing temperatures. i Scheme of the HTiNbO5-X membrane reactor showing the esterification of benzyl alcohol with acetic anhydride. Driven by the pressure difference, the alcohol and anhydride molecules react efficiently within 2D nanochannels. j Symmetry matching between the HOMO of the benzyl alcohol and the LUMO of protonated acetic anhydride in the nanochannels with different interlayer spacing. k Comparison of the conversion and reaction time for bulk and confined reactions. Confined reaction condition: HTiNbO5-X membrane, benzyl alcohol (0.2 M) and acetic anhydride (0.3 M) in CH3CN (10 mL) at 23 °C. Bulk reaction condition: HTiNbO5-X nanosheets (10 mg), benzyl alcohol (0.2 M) and acetic anhydride (0.3 M) in CH3CN (10 mL) at 23 °C for 24 h. Error bars represent standard deviation of three measurements.
Results
The synthesis and characterization of HTiNbO5-X membrane
The ultrathin HTiNbO5-X NS were successfully obtained by calcination, acid etching, and exfoliation steps (Fig. 1b)22,24. It is noteworthy that KTiNbO5 was treated in HCl aqueous solution for multiple times. This step not only facilitated sufficient protonation of KTiNbO5 introducing Brønsted acid sites, but also created abundant oxygen vacancy (VO)25,26. After that, a small amount of tetrabutylammonium cations (TBA+) was used to expand the interlayer spacing of bulk HTiNbO5-X for exfoliation and to stabilize the interlayer distances of HTiNbO5-X membrane. The morphology of the samples was investigated by scanning electron microscopy (SEM), transmission electron microscopy (TEM) and atomic force microscopy (AFM). Both KTiNbO5 and bulk HTiNbO5-X presented closely compacted layered structure (Supplementary Fig. 1). The size of these lamellar particles reaches several micrometers. As shown in Supplementary Fig. 2, the colloidal dispersions of the HTiNbO5-X NS are stable over one month, implying its well dispersion and uniformity. The TEM images of HTiNbO5-X NS depicted in Fig. 1c and Supplementary Fig. 3 clearly show a large area 2D ultrathin nature and the average size is about 0.5 ± 0.1 μm, indicating its suitability for constructing laminar membrane reactors. Energy-dispersive X-ray spectroscopy (EDX) elemental mapping of the HTiNbO5-X nanosheets reveals the uniform distributions of Ti, Nb and O across the sample, confirming the successful synthesis of HTiNbO5-X nanosheets (Supplementary Fig. 4 and Supplementary Table 1). The thickness of the exfoliated HTiNbO5-X nanosheets was measured by AFM with the corresponding height profile ( ≈ 1.2 nm) presented in Fig. 1d. Considering the thickness of single-crystal nanosheet and surface adsorption of solvents or guest species (TBA+ cations), the as-prepared HTiNbO5-X nanosheets consist of monolayer22,24. The microstructure of the samples were also investigated by powder X-ray diffraction (XRD). In Supplementary Fig. 5a, all the characteristic peaks of the KTiNbO5 are indexed to a layered structure with an orthorhombic crystal KTiNbO5 phase (JCPDS 72-1076). Interestingly, the characteristic diffraction peaks for bulk HTiNbO5-X show obvious shift after replacement of K+ with H+, while the intensity of HTiNbO5-X nanosheets become very weak. The weak and absence of characteristic peaks for HTiNbO5-X nanosheets indicates the decreased crystallinity and presence of the amorphous phases27,28.
The surface properties were identified by the Fourier transform infrared (FTIR), pyridine FTIR spectra and NH3 temperature programmed desorption (TPD). As shown in Supplementary Fig. 5b, it was revealed that both HTiNbO5-X NS and bulk HTiNbO5-X had two distinctive signals at 3600–3100 cm−1 and 1600 cm−1 which can be assignable to the stretching modes and bending modes of hydroxyl groups (OH), respectively29,30,31,32,33. This indicates that there is a considerable amount of hydroxyl groups on the surfaces of HTiNbO5-X nanosheets. The acidity distribution was investigated using pyridine FTIR spectroscopy. As shown in Fig. 1e and Supplementary Fig. 6a–c, the absorption bands at 1445–1454 cm−1 indicate the presence of coordinately bonded pyridine at Lewis acid sites, while the bands at 1546 cm−1 are assigned to pyridine adsorbed at Brønsted acid sites32,33,34. The pyridine FTIR spectra prove that HTiNbO5-X NS have both Lewis acid and Brønsted acid sites. Even after evacuation at 200 °C, the absorption bands of Brønsted acid sites could still be detected and the Brønsted/Lewis acid ratio is 0.184 for HTiNbO5-X nanosheets, indicating these samples have good stability and catalytic potential (Supplementary Table 2).The overall acidity of the samples was further probed by NH3 TPD (Fig. 1f and Supplementary Fig. 6d). For bulk HTiNbO5-X, desorption of NH3 occurred from 150 to 650 °C, indicating a wide range of acid sites33,34. The broad desorption signal could be divided into two peaks centered at 208 and 525 °C, representing sites of medium and strong acidity. For HTiNbO5-X nanosheets, the peaks in the range of 200-400 °C belongs to sites of medium acidity, and peaks centered at 492 °C represents strong acidity sites. The strong acidity sites should be ascribed to Brønsted acid sites at Ti(OH)Nb sites, and a slight decrease of peaks centered at 492 °C is found for HTiNbO5-X nanosheets since bulk HTiNbO5-X was soaked in TBAOH dilute solution for exfoliation. The overall acidity of these samples was calculated in the following order: KTiNbO5 (0.013 mmol/g) <HTiNbO5-X nanosheets (3.885 mmol/g) <bulk HTiNbO5-X (4.567 mmol/g). These results consolidate that HTiNbO5-X nanosheets have abundant Brønsted acid sites and Lewis acid sites induced by acid treatment.
The 2D HTiNbO5-X laminar membranes were prepared by stacking HTiNbO5-X nanosheets onto a nylon substrate through vacuum filtration (Supplementary Fig. 7). It was demonstrated that the interlayer spacing of the layered metal oxide can be adjusted by changing the cations and thermal treatment18. The protons are partially exchanged by appropriate organic cations (TBA+), leading to chemical exfoliation of bulk HTiNbO5-X and modulation of interlayer distance of the HTiNbO5-X membranes. The as-prepared membranes were air dried at room temperature (RT) or thermally treated in vacuum oven at certain temperature, which are named Y °C dry (where Y is the corresponding temperature). In the Supplementary Fig. 8a, the XRD patterns of HTiNbO5-X membranes in dry-states exhibit sharp (002) diffraction peaks, which also indicates that the HTiNbO5-X membranes possessed a highly ordered stacking arrangement. Using the Bragg’s law, the interlayer distances of the dried HTiNbO5-X membranes were decreased from 1.37 nm to 1.02 nm after increasing the heat-treatment temperature. With increasing calcination temperature, the H2O gradually evaporates from the interlayer and the TBA+ cations can turn into different geometric configuration and sizes (Supplementary Fig. 9). This can be evidenced by the weakening of the infrared absorption peak at 3600–3100 cm−1 and 1600 cm−1 with increasing temperature (Supplementary Fig. 8b). As displayed in Supplementary Figs. 10 and 11, both SEM images and TEM images of the cross-section of membranes revealed the well-ordered lamellar structure of the HTiNbO5-X membrane. In Supplementary Fig. 11, the alternating dark and light pattern shows the average thickness of one layer of lamellar is 0.7 nm, which matches the theoretical thickness of the HTiNbO5-X nanosheet. The esterification of benzyl alcohol with acetic anhydride was carried out as the model reaction to study the catalytic properties of HTiNbO5-X membranes. Before the reaction, corresponding molecular sizes are all predicted (Supplementary Figs. 9, 12). Through comparison and analysis, the thicknesses of molecules involved in esterification are all less than that of TBA+ cations. Thus, the membranes are effectively developed into different interlayer spacings allowing the continuous flow of reactants and products.
The catalysis performance of HTiNbO5-X membrane
The esterification of benzyl alcohol with acetic anhydride was carried out in a microfiltration device (Supplementary Fig. 7b). After clamping HTiNbO5-X membranes, the reaction solution of alcohol and acetic anhydride permeates through the HTiNbO5-X membranes driven by the pressure difference (0.9 atm), and the reactants react within the two-dimensional sub-nanochannels at RT, and the products flow into the collection bottle with the solvent. As mentioned above, five types of HTiNbO5-X membranes with different interlayer distances were utilized for the confined benzyl acetate synthesis. After more than 12 h of reaction, the reaction-state interlayer spacing of membranes was tested by XRD immediately. In the Fig. 1h and Supplementary Table 3, the interlayer spacings of the reaction-state HTiNbO5-X membranes decrease monotonically, and are calculated to be 14.4, 12.3, 12.1, 11.9 and 11.0 Å by Bragg’s law, respectively. The XRD patterns of HTiNbO5-X membranes in both dry-state and reaction-state exhibit strong diffraction peak (002), confirming that their orderly layered structures are maintained in the process of reactions. The composition and conversion efficiency of the permeation solution was determined by 1H NMR spectroscopy (Fig. 2a and Supplementary Fig. 13). Surprisingly, the characteristic signals of benzyl alcohol faded away along with the interlayer spacings decreasing, whereas those for benzyl acetate enhanced and the conversion of benzyl alcohol gradually improved from 28.2% to 100% (Fig. 2a, b). When the interlayer spacing turned to 11.0 Å, the characteristic signals of benzyl alcohol completely disappeared, and nearly complete conversion ( ≈ 100%) was achieved at 23 °C in a very short reaction time of 26.6 s. Additionally, the flow volume of the reaction solution was observed to be linear with time, reflecting that the volumetric flow rate was steady (Supplementary Fig. 14). It was found that the velocity of the permeation solution gradually decreased with the decreasing of the interlayer spacing of the HTiNbO5-X membranes, whereas reaction time (tR) were calculated to be increased from 12.8 s to 26.6 s. The slightly increase in reaction time is attributed to the more severe spatial constraints to the reaction solution.
a 1H NMR spectra measured for the permeation solution through HTiNbO5-X membranes with different interlayer spacing (d). b The conversion and reaction time for HTiNbO5-X membranes with different interlayer spacing. c The conversion and reaction time for HTiNbO5-X membranes (interlayer spacing of 12.1 Å) with different thicknesses. d Comparison of turnover number (TON) for bulk reaction and confined reaction. Confined reaction condition: HTiNbO5-X membrane, benzyl alcohol (0.2 M) and acetic anhydride (0.3 M) in CH3CN (10 mL) at 23 °C. Bulk reaction condition: HTiNbO5-X nanosheets (10 mg), benzyl alcohol (0.2 M) and acetic anhydride (0.3 M) in CH3CN (10 mL) at 23 °C for 24 h. e Comparison of reaction temperature, reaction time, conversion and TON of HTiNbO5-X membranes with those of other catalysts. All the references associated with this plot are tabulated in Supplementary Table 4. Error bars represent standard deviation of three measurements.
Considering that the reaction time increases with the decrease of interlayer spacings, the increased conversion in HTiNbO5-X membranes may be related to both reaction time and confinement effects. Thereby, the reaction time of reaction solution in the HTiNbO5-X laminar membranes were further studied by adjusting the membrane thicknesses, and the corresponding conversions of benzyl alcohol were tested. The HTiNbO5-X membranes with different loadings were thermally treated in same temperature (60 °C dry) to maintain at a nearly constant interlayer spacing (d = 12.1 Å) but different thicknesses in Fig. 2c. The volume of the reaction mixture passing through is still linearly related to reaction time, and the velocity was decreased with the increased membrane thicknesses (Supplementary Fig. 15). As the thicknesses increased from 2.6 μm to 10.4 μm, the conversions of benzyl alcohol improved from 23.6% to 100%, but the reaction time greatly increased from 7.9 s to 63.5 s. This is mainly attributed to the weakened confinement effect. Moreover, the effect of reaction times on the conversion is further investigated by adjusting the operating pressure difference (Supplementary Fig. 16). When the pressure difference decreased to 0.3 atm and 0.6 atm, the reaction mixture had a lower flux and a higher reaction time through the HTiNbO5-X membranes (d = 14.4 Å). Even though the reaction time greatly increased to 27.5 s and 48.1 s, the conversions of benzyl alcohol only improved to 53.2% and 65.6% (Supplementary Fig. 16b). This results indicates that extending reaction time slightly increase benzyl alcohol conversion, but reducing interlayer spacing significantly promotes esterification reactivity. Besides, the confined reactions of HTiNbO5-X membranes are also performed at different temperatures in Supplementary Fig. 17. When the temperature comes to 30 °C, the conversion was greatly improved from 20.4% for HTiNbO5-X membranes (d = 14.4 Å) to 58.3% for HTiNbO5-X membranes (d = 11.0 Å) in Supplementary Figs. 18, 19. But the reaction time was calculated to show slight difference from 13.7 s to14.3 s. These results all proved that the prolonged reaction time has a slight impact on the conversion, whereas interlayer spacing has a more significant impact.
In comparison, HTiNbO5-X nanosheets were also utilized as heterogenous catalysts for benzyl acetate synthesis. The bulk reaction was conducted under the same conditions. As shown in the Figs. 1k, 2d, the catalytic performance for HTiNbO5-X nanosheets is limited by the disordered motion of molecules. Through increasing the reaction time from 24 h to 48 h, the reaction efficiencies of HTiNbO5-X nanosheets in the bulk system are raised from 21.3% to 38.4%. However, this enhancement is still inferior to that of the confined system. The catalyst dosage of HTiNbO5-X NS in bulk condition is 10 mg, whereas that of HTiNbO5-X membranes in confined system is calculated to be only 1.68 mg. Combining with the overall acidity probed by NH3 TPD, the turnover number for HTiNbO5-X membranes at 23 °C increases from 86.41 to 306.42 as the interlayer spacings decrease (Fig. 2d). The optimum results are about 28 times higher than that of bulk reaction (10.96). Over the past two decades, various homogeneous and heterogeneous catalysts, including solid-acids, inorganic salts and ionic liquids, have been applied to benzyl acetate synthesis (Supplementary Table 4). In order to achieve a better catalytic performance, most of the reported works adopted high reaction temperature or extended reaction time or catalyst dosages, and there were few studies for the efficient synthesis of benzyl acetate (Fig. 2e). Ulteriorly, three types of commercial solid acid catalysts, HZSM-5 (zeolite molecular sieves), H3O40PW12·xH2O (heteropoly acids) and amberlyst-15 (acidic resins), were purchased for comparison. Under the premise of the same concentrations, the performances of H3O40PW12·xH2O and amberlyst-15 are superior to HZSM-5 and
HTiNbO5-X nanosheets in the bulk reaction (Supplementary Table 5). This is mainly attributed to their stronger acidity. However, the H3O40PW12·xH2O was dissolved in the product benzyl acetate, and the collected benzyl acetate exhibits a light golden colour (Supplementary Fig. 46). The Amberlyst-15 ion-exchange resins were easily separated from solutions, but the collected benzyl acetate shows a noticeable orange color after reaction. This may be due to the unstable structure of the amberlyst-1535,36. These results demonstrate the advantages of HTiNbO5-X membranes in catalyst separation and product purity.
Structural analysis of HTiNbO5-X nanosheets
Since the HTiNbO5-X membranes are fabricated by vacuum filtration of HTiNbO5-X nanosheets, the origin of its outstanding performance should be related to the structures of HTiNbO5-X nanosheets. To gain further insights into the electronic structure and chemical states for HTiNbO5-X membranes, several other analytical techniques, including X-ray photoelectron spectroscopy (XPS) and Raman spectra, were performed. The XPS data for KTiNbO5 shows characteristic Ti 2p peaks for Ti4+ and Nb 3 d peaks for Nb5+, but in the case of bulk HTiNbO5-X and HTiNbO5-X nanosheets these peaks shift to lower binding energy (Supplementary Fig. 20). This likely results from the presence of Nb4+ and Ti3+ due to the acid etching37,38,39,40. In addition, O 1 s can be divided into two fitting peaks. As shown in Supplementary Fig. 21, the main peak of lattice oxygens are shifted from 530.3 eV to 529.7 eV, which can be ascribed to the oxygen defects or vacancies present in the lattice of HTiNbO5-X40. The shoulder peak at 531.3 eV typically corresponds to adsorbed oxygen around oxygen vacancy sites27,38. As for HTiNbO5-X membranes, the position of these peaks hold the stable during the vacuum thermal treatment (Supplementary Fig. 22). Notably, when the temperature increases, the content of oxygen vacancies gradually decreases from 29.2% to 22.5% (Supplementary Table 6). To gain insights into the role of oxygen vacancy, the nanosheets of HTiNbO5-X membranes after thermal treatment at 100 °C were collected for bulk reaction. Under the same dosages, the performances of the nanosheets decreases from 21.3% to 8.6%. Thus, it can be confirmed that oxygen vacancies play a key role during the reaction process. The Raman spectra were further collected to identify structural changes of HTiNbO5-X membranes, because it is very sensitive to the structure and bond order of metal oxides, especially in the region relating to metal-oxygen stretching modes (Supplementary Fig. 23)41,42. The Raman shift in the range of 590–720 cm–1 belongs to the stretching vibration of the Ti–O–Ti bond in the TiO6 octahedron, and the shift in the range of 480-570 cm–1 belongs to the stretching vibration of the Nb–O–Nb bond in the NbO6 octahedron41. It is worth noting that the peaks for HTiNbO5-X nanosheets and HTiNbO5-X membranes both showed evidently broad and enhanced absorption at around 885 and 934 cm–1, and these peak is typically regarded as the unsaturated Ti=O and Nb=O surface groups, compared with the shift of KTiNbO5. The above results suggest the prepared HTiNbO5-X nanosheets have partial structural changes which can be attributed to acid etching, and thereby the metal occupation at tetrahedral and octahedral sites might have slight differences38,41.
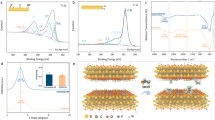
Ulteriorly, the selected area electron diffraction (SAED) pattern of HTiNbO5-X nanosheets shows clear diffraction rings (Fig. 3a), indicating that the HTiNbO5-X nanosheets are etched by hydrochloric acid resulting partially disordered atomic arrangement26,27. Notably, the High-resolution TEM image (HRTEM) reveals that the prepared HTiNbO5-X nanosheets are typically composed of amorphous and crystalline domains (Fig. 3b). The amorphous structural regions are relatively larger than crystalline regions, demonstrating the low crystallinity of HTiNbO5-X nanosheets (Supplementary Fig. 24).This is consistent with XRD results (Supplementary Fig. 5a). Additionally, low-temperature electron paramagnetic resonance (EPR) was employed to validate the generation of Nb4+/ Ti3+/VO pairs since this technology was very sensitive to paramagnetic species containing unpaired electrons (Fig. 3c and Supplementary Fig. 25). From Fig. 3c, it can be seen that HTiNbO5-X nanosheets show distinctive EPR signals at about g = 2.253 and g = 2.028 assigned to Nb4+ and Ti3+, respectively43,44,45. In Supplementary Fig. 25, signal with g = 4.252 could be assigned to Nb3+ species43. After that, the indicator of g values at 2.067 and 2.002 should be attributed to the corresponding oxygen vacancies in the lattices37. The abundant oxygen vacancies break the octahedral symmetry of NbO6 and TiO6, which derive Nb4+ and Ti3+ with unpaired electron. Besides, the thermogravimetric analysis (TGA) curve shows that the HTiNbO5-X nanosheets has a small mass loss in nitrogen atmosphere, but the decomposition rate is decreased in oxygen atmosphere (Fig. 3d). This feature also serves as an indirect evidence for the existence the oxygen vacancy, in agreement with the XPS, Raman, EPR and HRTEM findings above. The created oxygen defects as highly active low-coordination sites can break the inherent crystal arrangement causing amorphous domains and readjust the local electron structure (Supplementary Fig. 26)28,29. As such, the adsorption and activation of reactants may be optimized and thus boosting the reaction performance.
a TEM (Inset show the SAED pattern) and b HRTEM images of HTiNbO5-X nanosheets. c Low temperature EPR spectra of HTiNbO5-X NS. d Thermogravimetric curve of HTiNbO5-X NS in nitrogen and oxygen environment. e The adsorption energy of BnOH intercalating into the interlayer of HTiNbO5 membranes and HTiNbO5-X membranes in bulk and confined states. f The adsorption energy of Ac2O intercalating into the interlayer of HTiNbO5 membranes and HTiNbO5-X membranes in bulk and confined states. For comparison, the different interlayer free distances represent bulk states (40 Å) and confined states (4 Å). More details in supplementary information.
Theoretical calculation for catalytic mechanism
In view of the extensive oxygen vacancies in the confined nanochannel, an effective modulation of interface properties of HTiNbO5-X membranes is expected. Before theoretical calculations, we undertook Raman studies to understand the intricate interaction between reactant molecules and oxygen vacancies (Supplementary Fig. 27). The strong electrophilic properties of the oxygen vacancy are beneficial to its adsorption with oxygen containing groups of acetic anhydride and benzyl alcohol. As the experiment progresses, benzyl alcohol and acetic anhydride molecules gradually move through and react within the HTiNbO5-X membranes. In Supplementary Fig. 27, the peak of Nb=O gradually shifted from 940 cm-1 to 948 cm-1, whereas other peaks show no obvious shift. This can be attributed to the adsorption of reactant molecules at oxygen vacancies around Nb sites21. Therefore, the oxygen vacancies at Nb sites were considered for calculation29,30. The potential distribution is explored by electrostatic potential simulation to study the surface electronic state like bonding nature of HTiNbO5-X nanosheets with reactant molecules (Supplementary Fig. 26). The obvious potential difference can be seen around the oxygen vacancy, revealing the formation of local polarization and constructing strong local electric field46,47. The strong electrophilic properties of the oxygen vacancy are beneficial to molecular polarization and transition state stabilization48. Thus, the adsorption energies (Eads) of BnOH and Ac2O intercalating between the nanosheets of HTiNbO5 (no defects) and HTiNbO5-X were both calculated using density functional theory (DFT). Considering the reaction-state interlayer spacing (11.0 Å) and thickness of monolayer nanosheets (0.7 nm), the interlayer free distances (dfree) of 4 Å were taken to simulate the adsorption behavior of confined reaction (Supplementary Figs. 28, 29). For bulk reaction, the 40 Å vacuum space is used to simulate the surface environment, ensuring that the slab model remains sufficiently isolated15,49. This distance is large enough compared to the size of the reactants (benzyl alcohol: 4.02 × 7.00 × 9.10 Å; acetic anhydride: 4.01 × 5.34 × 8.72 Å) in the Supplementary Fig. 12. The calculated results proved that the adsorption energy (dfree = 40 Å) of BnOH and Ac2O in HTiNbO5-X were both higher than that of HTiNbO5, indicating the strong adsorption on oxygen vacancy (Eads = −0.800 eV for BnOH, Eads = −0.584 eV for Ac2O). Interestingly, these energies were further improved greatly when the dfree decreased to 4 Å, especially for HTiNbO5-X (Eads = −1.270 eV for BnOH, Eads = −1.133 eV for Ac2O). Specifically, benzyl alcohol and acetic anhydride exhibit strong electrostatic interactions with oxygen vacancies in HTiNbO5-X membranes through benzene ring, hydroxyl oxygen and carbonyl oxygen, whereas in HTiNbO5 membranes, the interactions are mediated by weak hydrogen bonds (Fig. 3e, f, Supplementary Figs. 28, 29). Therefore, the synergistic effects of oxygen vacancies and confinement effect are identified, which benefits to the adsorption and activation of the reactants.
ATR FTIR measurements were performed to gain further insights into the reaction progression (Supplementary Fig. 30). These investigations enabled the monitoring of the chemical transformations occurring within the HTiNbO5-X membranes. After a specific time of reaction (0 h, 0.5 h, 1 h and 12 h), reaction-state membranes were collected and tested by ATR FTIR spectra immediately. For acid anhydride, the peaks at 1756 cm−1 and 1828 cm−1 are assigned to the stretching mode of C = O. As the experiment progresses, these peaks are red shifted to 1752 cm−1 and 1822 cm−1, which should attribute to the protonation of acetic anhydrides50,51. After 12 h of confined flow reaction, benzyl acetate (product) is enriched inside the membrane. The stretching mode of C = O and C-O for the ester is significantly enhanced at around 1709 cm−1 and 1232 cm−1. These are direct observations of reaction intermediates and products.
To reveal the underlying mechanisms of bulk and confined reactions, we investigated the free energy of the reaction paths through DFT calculations, based on the spatial confinement and the chemical structure of catalysts. The esterification in both the bulk and confined systems is divided into the following three steps, that is protonation, nucleophilic addition, and deprotonation. As illustrated in Supplementary Fig. 31, Brønsted acid sites on the surface of HTiNbO5-X nanosheets are employed as the proton donor, and the acetic anhydrides are easily protonated to the first intermediate (IM1) in both bulk and confined systems due to the good electronegativity of carbonyl oxygen. This could promote the electron-withdrawing property of oxygen atom weakening C-O bond, and further increases the positive charge on the carbon atom. Subsequently, the lone pair electrons of alcohol hydroxyl oxygen in benzyl alcohol attack the empty antibonding π* orbital of carbon atom to generate the second intermediate (IM2) through the first transition state (TS1). Notably, the Gibbs free energies from IM1 to TS1 are demonstrate as the maximum energy barrier in both the bulk and confined systems. The energies from IM1 to TS1 for the bulk and confined conditions respectively are calculated to be 42.0 kJ/mol and 28.8 kJ/mol, demonstrating a great decrease of the energy barrier (Fig. 4c, d). For the bulk reaction, the energy barrier mainly derives from the disordered motion and inefficient diffusion of molecules to active sites reflected by its low turnover number (Supplementary Fig. 35). In order to obtain higher reaction efficiency, the reaction time and temperature need to be increased (Fig. 4a, b). For the confined reaction, as the channel dimension decreases to nanoscale (especially angstrom scale), the disordered motions of reactant molecules are greatly constrained. In nucleophilic addition reactions, the symmetry match of molecular orbitals determines whether the reaction can proceed efficiently, mainly involving the direction of orbital interaction between the nucleophile and the substrate. This has been confirmed by the higher conversion rate of the confined reaction (Fig. 4b). The conversion are raised from 7.8% (24 h) in bulk system to 56.2% in confined system at 10 °C. This value is close to the conversion (58.4%) of bulk reaction at 60 °C (Fig. 4a). As shown in the Supplementary Fig. 32, the reactants are reorganized and become more orderly within the 2D confined sub-nanochannels. When the interlayer distance decreases from 14.4 Å to 11.0 Å, the best symmetric matching state between the highest occupied molecular orbital (HOMO) of benzyl alcohol and the lowest unoccupied molecular orbital (LUMO) of protonated acetic anhydride is achieved, favoring the nucleophilic addition reaction. Besides the confinement effect, the intrinsic chemical and electronic structure of catalysts can also affect the molecular reactivity. Specifically, the oxygen vacancies on the surface of HTiNbO5-X nanosheets are considered as Lewis acid sites that also can attract carbonyl oxygen or hydroxyl oxygen assisting molecular polarization, and promote the formation of IM2, as evidenced by the increased adsorption energy of HTiNbO5-X membrane to BnOH and Ac2O. As shown in Supplementary Figs. 33, 34, Lewis acid sites at Nb sites of HTiNbO5-X nanosheets are also counted as active sites for calculation. In this case, the free energy from IM1 to TS1 also delivers an obvious decrease from 47.7 kJ/mol to 39.8 kJ/mol for confined conditions. Compared to that of bulk system, the lower activation energy barriers for reactions in the confined space give rise to a higher-efficiency reaction performance inevitably.
a Bulk reaction at different temperatures using HTiNbO5-X nanosheets as the catalyst. b Comparison of the conversion and reaction time of bulk and confined reactions at 10 °C and 23 °C. Bulk reaction condition: HTiNbO5-X nanosheets (10 mg), alcohol (0.2 M) and acetic anhydride (0.3 M) in CH3CN. Confined reaction condition: HTiNbO5-X membrane (d = 11.0 Å), alcohol (0.2 M) and acetic anhydride (0.3 M) in CH3CN. Free energy profiles for the esterification of benzyl alcohol with acetic anhydride in c bulk and d confined conditions calculated by DFT. Bronsted acid sites are taken as active sites acting as proton sources. The labels TS and IM indicate the transition state and intermediate of the reaction, respectively. The reaction rate is primarily determined by the step from IM1 to TS1, in which the confined energy barrier is clearly smaller than the bulk energy barrier. Error bars represent standard deviation of three measurements.
To determine the generality and expansibility of membrane reaction, we further adjust the reactant concentrations, and expand solvents and alcohol derivatives using HTiNbO5-X membranes (d = 11.0 Å) as mentioned above. In Supplementary Fig. 36, the flow volumes are linear with time, and the permeance of reaction solution fall slightly with the concentration increasing (0.1, 0.2, 0.6 and 1.0 M). The experimental results show that the conversion of confined reaction decreases gradually when the concentrations come to 0.6 M and 1.0 M (Supplementary Table 7). This should be ascribed to the saturation of active sites to reactant molecules, since the catalyst dosage of HTiNbO5-X membranes is only 1.68 mg. For bulk reactions, the conversion of benzyl alcohol delivers a completely opposite trend, mainly because the increased probability of molecular collision at high concentrations. In spite of that, the performances of confined reaction are still superior to that of the bulk reaction at the same concentrations. In addition, we have made a comparison of different solvents in terms of boiling point, conversion and flow rate of the reaction solution. When cyclohexane, toluene and chloroform are used as solvents, they also exhibit good conversion rates both in bulk and confined reactions (Supplementary Tables 8, 9). However, flow rates for cyclohexane and toluene are much smaller than acetonitrile in confined reactions, and the reaction time are significantly longer (Supplementary Fig. 37). This can be attributed to the larger size of cyclohexane and toluene, which obstructs their flow within the membranes (Supplementary Fig. 38). Moreover, the HTiNbO5-X membranes are also applied to other alcohol derivatives for corresponding acetate ester flavours, because these ester flavours have attracted special interests in many industrial sectors (Fig. 5, Supplementary Figs. 39–44, Supplementary Table 11). From the Fig. 5 and Supplementary Table 10, these alcohol derivatives exhibited different catalytic activities because of the differences in molecular size, geometric configuration and nucleophilicity. The performance of 1-phenylethyl alcohol and isoamyl alcohol was unsatisfactory, which was speculated to be steric hindrance effect of molecules themselves and mismatch between 2D nanochannel and reactants. Obviously, both the conversion and reaction time of the confined reaction exceed that of bulk reaction, implying HTiNbO5-X membranes are extendable to other different alcohols.
a Substrate scope. b The conversion and reaction time of bulk and confined reactions of different alcohol substrates are compared. Bulk reaction condition: HTiNbO5-X nanosheets (10 mg), alcohol (0.2 M) and acetic anhydride (0.3 M) in CH3CN at 40 °C for 24 h. Confined reaction condition: HTiNbO5-X membrane (d = 11.0 Å), alcohol (0.2 M) and acetic anhydride (0.3 M) in CH3CN at 23 °C. Error bars represent standard deviation of three measurements.
To determine the stability of the membranes, the HTiNbO5-X membranes are tested for four cycles of recyclability test. As shown in Supplementary Fig. 45, the membrane exhibited high stability in both the permeance and conversion of the solution. The conversion of benzyl alcohol remained unchanged at almost 100% for all operation cycles. In Supplementary Fig. 46, we conducted a continuous confined reaction for evaluating reactivity and practicality of the membrane. After more than 13 days of reaction, the products were collected in Supplementary Fig. 46a. According to the theoretical expected product quality, the yield was calculated about 87.5%. The loss of products mainly comes from the processes of filtration reaction and sample collection. The products were also analyzed by 1H NMR spectroscopy, and characteristic signals of benzyl alcohol completely disappeared which means a 100% conversion. The benzyl acetate collected by the confined reaction is almost colorless. To gain insights into stability of oxygen vacancy, XPS spectra of HTiNbO5-X membranes were performed after the long-term stability test. As shown in Supplementary Fig. 47, O 1 s can be divided into three fitting peaks that is lattice oxygen at 530.4 eV, oxygen vacancy at 531.3 eV and organic oxygen at 532.9 eV. Due to the relatively mild reaction conditions, the signal peak of oxygen vacancies did not show any signs of weakening or disappearing. Therefore, it can be confirmed that HTiNbO5-X membranes are very stable during the reaction process.
Discussion
In summary, HTiNbO5-X LMR with sub-nanoconfined channels was successfully prepared by stacking VO-enriched amorphous/crystalline HTiNbO5-X nanosheets. Such an ingenious atomic architectures endows the HTiNbO5-X LMR with unique electronic and spatial structures along with adjustment of adsorption energy of reactants, molecular polarization and orbital symmetry matching, and reaction thermodynamics. Experimental and theoretical studies clearly revealed the synergistic effect of the oxygen vacancy and the sub-nanoscale confinement to its outstanding performances. Consequently, the HTiNbO5-X membrane reactor exhibits ≈ 100% conversion of benzyl alcohol within 26.6 s under mild conditions, and shows satisfactory stability. Furthermore, this method is applicable for to other alcohol derivatives. This work unraveled the importance of the rational design of metal oxide laminar membrane reactors via synergistically surface defect and spatial confinement strategies. Our work should encourage the adoption of laminar membrane reactors for sustainable chemical synthesis.
Methods
Materials
Niobium pentoxide (Nb2O5, AR), titanium dioxide (TiO2, AR) and cinnamyl alcohol ( >98.0%) were commercially supplied by Sigma-Aldrich. Potassium carbonate (K2CO3, AR), acetic anhydride ( >99%), acetonitrile and hydrochloric acid (HCl) were supplied by Sinopharm Chemical Reagent. Amberlyst-15 (Acid siteså 4.7 eq/kg) was supplied by Aladdin. HZSM-5 (Mole ratio of SiO2/Al2O3 ≈ 38-40) and H3O40PW12·xH2O (AR) was supplied by Macklin. Benzyl alcohol ( >99.0%), 4-methylbenzyl alcohol ( >99.0%), 4-methoxybenzyl alcohol ( >98.0%), phenylethyl alcohol ( >98.0%), 1-phenylethyl alcohol ( >98.0%), 3-methyl-1-butanol ( >99.0%) and tetrabutyl ammonium hydroxide (TBAOH, 10 wt%) were purchased from TCI. Dimethyl sulfoxide-d6 (99.8 atom% D, contains 0.03% v/v TMS) was purchased from J&K Scientific. Deionized water (resistivity above 18 MΩ∙cm) was collected from a Mili-Q Biocel system. All solvents and reagents were used as received from commercial suppliers unless otherwise indicated.
Preparation of HTiNbO5-X nanosheets and HTiNbO5-X membranes
The HTiNbO5-X nanosheets were prepared by heating, acid etching, protonation, and exfoliation. K2CO3 (10.64 g), TiO2 (6.15 g), and Nb2O5 (10.23 g) were mixed in the mortar. The mixture was annealed in the muffle furnace at 1000 °C for 4 h. After that, the solid sample was collected and ground into powder. The obtained powder was annealed for another 20 hours to fabricate layered KTiNbO5. The KTiNbO5 (2 g) was stirred in HCl (500 mL, 1 M) solution for 3 day, and the solid product was recovered by centrifugation, washed thoroughly with deionized water to remove excess acid and then dried under vacuum. The bulk HTiNbO5-X was obtained via proton exchange and acid etching of KTiNbO5 with HCl aqueous solution for three times. To prepare HTiNbO5-X nanosheets, HTiNbO5-X (500 mg) was soaked in TBAOH dilute solution (100 mL, 1 wt%) for 6 h, and redispersed into 500 mL deionized water, and then exfoliated into nanosheets by mechanical shaking for two weeks. To obtain monolayer HTiNbO5-X nanosheet dispersion ( ≈ 0.6 mg/mL), the above solution was centrifuged at 3000 revolutions per minute (845 × g, 5 min) to remove unstripping HTiNbO5-X. The HTiNbO5-X nanosheet dispersion was centrifuged at 12000 revolutions per minute (13523 × g, 8 min) to obtain HTiNbO5-X nanosheets powder. The HTiNbO5-X nanosheet powder was thermally treated in vacuum ovens at 60 °C for 12 h.
The HTiNbO5-X membranes were fabricated by vacuum filtration of the HTiNbO5-X nanosheet dispersion. 20 mL HTiNbO5-X nanosheet dispersion ( ≈ 0.6 mg/mL) was diluted to 100 mL( ≈ 0.12 mg/mL), after homogeneous dispersion, and was filtrated with a nylon membrane (47 mm in diameter, pore size of 0.22 μm) as the substrate. The as-prepared HTiNbO5-X membranes were air-dried at 23 °C for 12 h. To regulate interlayer spacing, the membranes were further thermally treated in vacuum ovens at certain temperature (i.e., 40 °C, 60 °C, 80 °C or 100 °C) for 12 h. The membranes with different thicknesses (HTiNbO5-X loadings) were also prepared to investigate their catalytic activity.
Catalytic evaluation of HTiNbO5-X membranes for the confined reaction
The catalytic reaction was performed in microfiltration devices at room temperature (23 °C) with a measuring cylinder diameter of 15 mm. First, HTiNbO5-X membranes were cut to octagonal shape and fixed onto the surface of a porous glass support, clamping the upper measuring cylinder and the glass support to ensure sealing. Then, acetonitrile (CH3CN) solution containing alcohol (0.2 M) and acetic anhydride (0.3 M) was added to the measuring cylinder, and the measuring cylinder was covered with aluminum foil. The solution flowed through interlayer 2D nanochannels of the membranes driven by a pressure difference (0.9 atm), the reactants reacted in the interlayer, and the products flowed out with the solvent. After removing the solvent from the mixture obtained through membranes by rotary evaporation, dissolving the concentrate with DMSO-D6, analyzing its composition by 1H NMR spectroscopy, and calculating the conversion, the reactivity of membrane reactor was compared.
The relevant comparative experiments included the membrane interlayer distances, membrane thicknesses (HTiNbO5-X NS loadings), reaction temperatures, pressure differences, reactant concentrations, solvents and substrates, and overall experiments were conducted analogously.
Catalytic evaluation of HTiNbO5-X nanosheets for the bulk reaction
The catalytic reaction was performed in 25 mL glass flasks. HTiNbO5-X nanosheet powder (10 mg) was added to acetonitrile solution(10 mL) of alcohol (0.2 M) and acetic anhydride (0.3 M), and the reaction flask was then sealed, stirred and heated at certain temperatures (i.e., 10 °C, 23 °C, 40 °C or 60 °C) for 72 h. Every 24 hours, a specific volume (1 mL) of reaction solution was taken out for testing. After removing the solids by filteration and removing the solvent by rotary evaporation, the obtained concentrate was dissolved in DMSO-D6 and analyzed by 1H NMR spectroscopy.
The relevant comparative experiments included the reaction temperatures, reactant concentrations, solvents and substrates, and overall experiments were conducted analogously.
Recyclability test
The experimental procedures were the same as confined reaction: HTiNbO5-X membranes (d = 11.0 Å), benzyl alcohol (0.2 M) and acetic anhydride (0.3 M) in acetonitrile (10 mL). After the complete filtration of 10 mL of reaction solution (about 100 h), substances flowing through the membranes were collected and analyzed by 1H NMR spectroscopy for each cycle. Acetonitrile (2 mL) was utilized to rinse the bottom surface of the membrane and dissolve the substances. The solvent was removed under reduced pressure on a rotary evaporator and the obtained concentrate was dissolved in DMSO-D6. After that, the HTiNbO5-X membranes (d = 11.0 Å) was tested for another cycle, by adding acetonitrile solution (10 mL) of alcohol (0.2 M) and acetic anhydride (0.3 M).
Data availability
The data that support the findings of this work are available within the article, its supplementary information file (Supplementary Figs. 1–47, Supplementary Tables 1–11), as well as the source data file. The data are also available from the corresponding authors. Source data are provided with this paper.
References
Sá, A. G. A. et al. A review on enzymatic synthesis of aromatic esters used as flavour ingredients for food, cosmetics and pharmaceuticals industries. Trends Food Sci. Technol. 69, 95–105 (2017).
Lee, A. F. et al. Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification. Chem. Soc. Rev. 43, 7887–7916 (2014).
Choi, K. R. et al. A microbial process for the production of benzyl acetate. Nat. Chem. Eng. 1, 216–228 (2024).
Yao, P. et al. Greener and higher conversion of esterification via interfacial photothermal catalysis. Nat. Sustain 5, 348–356 (2022).
Xiao, Q. et al. Ultrafast complete dechlorination enabled by ferrous oxide/graphene oxide catalytic membranes via nanoconfinement advanced reduction. Nat. Commun. 15, 9607 (2024).
Grommet, A. B. et al. Chemical reactivity under nanoconfinement. Nat. Nanotechnol. 15, 256–271 (2020).
Schreyer, L. et al. Confined acids catalyze asymmetric single aldolizations of acetaldehyde enolates. Science 362, 216–219 (2018).
Wang, C. et al. Measuring and modulating substrate confinement during nitrogen-atom transfer in a Ru2-based. Met.-Org. Framew. J. Am. Chem. Soc. 141, 19203–19207 (2019).
Ge, H. et al. A tandem catalyst with multiple metal oxide interfaces produced by atomic layer deposition. Angew. Chem. Int. Ed. 55, 7081–7085 (2016).
Lei, F. et al. Metallic tin quantum sheets confined in graphene toward high-efficiency carbon dioxide electroreduction. Nat. Commun. 7, 12697 (2021).
Nothling, M. D. et al. Synthetic catalysts inspired by hydrolytic enzymes. ACS Catal. 9, 168–187 (2019).
Xu, X. et al. High-efficiency CO2 separation using hybrid LDH-polymer membranes. Nat. Commun. 12, 3069 (2021).
Tang, L. et al. Confinement catalysis with 2D materials for energy conversion. Adv. Mater. 31, 1901996 (2019).
Zou, Y. et al. Enhanced organic photocatalysis in confined flow through a carbon nitride nanotube membrane with conversions in the millisecond regime. ACS Nano 15, 6551–6561 (2021).
Meng, C. et al. Angstrom-confined catalytic water purification within Co-TiOx laminar membrane nanochannels. Nat. Commun. 13, 4010 (2022).
Lu, N. et al. Tempospatially confined catalytic membranes for advanced water remediation. Adv. Mater. 36, 2311419 (2024).
Fu, Q. et al. Surface chemistry and catalysis confined under two-dimensional materials. Chem. Soc. Rev. 46, 1842–1874 (2017).
Gutmann, B. et al. Continuous-flow technology-a tool for the safe manufacturing of active pharmaceutical ingredients. Angew. Chem. Int. Ed. 54, 6688–6728 (2015).
Pang, S. et al. Regulating interlayer spacing of aminated graphene oxide membranes for efficient flow reactions. Matter 6, 1173–1187 (2023).
Li, X. et al. Efficient flow synthesis of aspirin within 2D sub-nanoconfined laminar annealed graphene oxide membranes. Adv. Mater. 36, 2310954 (2024).
Verma, R. et al. Defects tune the acidic strength of amorphous aluminosilicates. Nat. Commun. 15, 6899 (2024).
Yang, M. et al. Disorder engineering in monolayer nanosheets enabling photothermic catalysis for full solar spectrum (250–2500nm) harvesting. Angew. Chem. Int. Ed. 58, 3077–3081 (2019).
Bi, F. et al. Engineering triple O-Ti-O vacancy associates for efficient water-activation catalysis. Nat. Commun. 16, 851 (2025).
Shen, L. et al. Ultrathin Ti2Nb2O9 nanosheets with pseudocapacitive properties as superior anode for sodium ion batteries. Adv. Mater. 30, 1804378 (2018).
Wang, Y. et al. Ammonia electrosynthesis from nitrate using a stable amorphous/crystalline dual-phase Cu catalyst. Nat. Commun. 16, 897 (2025).
Li, R. et al. Amorphization-induced surface electronic states modulation of cobaltous oxide nanosheets for lithium-sulfur batteries. Nat. Commun. 12, 3102 (2021).
Qin, Q. et al. Constructing interfacial oxygen vacancy and ruthenium Lewis acid-base pairs to boost the alkaline hydrogen evolution reaction kinetics. Angew. Chem. Int. Ed. 63, e202317622 (2024).
Guo, Z. et al. Crystallinity engineering for overcoming the activity-stability tradeoff of spinel oxide in Fenton-like catalysis. Proc. Natl Acad. Sci. USA. 120, e2220608120 (2023).
Chen, X. et al. Effect of adsorbed water molecules on the surface acidity of niobium and tantalum oxides studied by mas NMR. J. Phys. Chem. C. 125, 9330–9341 (2021).
Nakajima, K. et al. Titania as an early transition metal oxide with a high density of Lewis. acid sites workable water J. Phys. Chem. C. 117, 16028–16033 (2013).
Zhang, X. et al. Adsorption and separation of ethyl mercaptan from methane by hydroxyl groups on the surface of HTiNbO5-nanosheets. Langmuir 38, 1497–1508 (2022).
Zhang, H. et al. A combo Zr-HY and Al-HY zeolite catalysts for the one-pot cascade transformation of biomass-derived furfural to γ-valerolactone. J. Catal. 375, 56–67 (2019).
Zhang, J. et al. Control of interfacial acid–metal catalysis with organic monolayers. Nat. Catal. 1, 148–155 (2018).
Du, X. et al. Selective nucleophilic α-C alkylation of phenols with alcohols via Ti=Cα intermediate on anatase TiO2 surface. Nat. Commun. 14, 4479 (2023).
Sripada, S. et al. Continuous catalytic esterification using a solid acid activated carbon monolith: Comparison of granular and monolith forms with a commercial catalyst. Chem. Eng. J. 476, 146586 (2023).
Su, F. et al. Advancements in solid acid catalysts for biodiesel production. Green. Chem. 16, 2934 (2014).
Lu, Q. How to correctly analyze 2p X-ray photoelectron spectra of 3d transition-metal oxides: pitfalls and principles. ACS Nano 18, 13973–13982 (2024).
Nakajima, K. et al. Nb2O5·nH2O as a heterogeneous catalyst with water-tolerant lewis acid sites. J. Am. Chem. Soc. 133, 4224–4227 (2011).
Siddiki, S. M. A. H. et al. Lewis acid catalysis of Nb2O5 for reactions of carboxylic acid derivatives in the presence of basic inhibitors. ChemCatChem 11, 383–396 (2019).
Kreissl, H. T. et al. Structural studies of bulk to nanosize niobium oxides with correlation to their acidity. J. Am. Chem. Soc. 139, 12670–12680 (2017).
Xin, Y. et al. Correlation of the catalytic performance with Nb2O5 surface properties in the hydrodeoxygenation of lignin model compound. J. Catal. 375, 202–212 (2019).
Yang, L. et al. Stabilizing Pt electrocatalysts via introducing reducible oxide support as reservoir of electrons and oxygen species. ACS Catal. 12, 13523–13532 (2022).
Brayner, R. et al. Niobium pentoxide prepared by soft chemical routes: morphology, structure, defects and quantum size effect. Phys. Chem. Chem. Phys. 5, 1457–1466 (2003).
Yu, X. et al. Highly enhanced photoactivity of anatase TiO2 nanocrystals by controlled hydrogenation-induced surface defects. ACS Catal. 3, 2479–2486 (2013).
Livraghi, S. et al. Nature of reduced states in titanium dioxide as monitored by electron paramagnetic resonance. II. rutile brookite cases. J. Phys. Chem. C. 118, 22141–22148 (2014).
Dai, J. et al. Spin polarized Fe1-Ti pairs for highly efficient electroreduction nitrate to ammonia. Nat. Commun. 15, 88 (2024).
Pfriem, N. et al. Role of the ionic environment in enhancing the activity of reacting molecules in zeolite pores. Science 372, 952–957 (2021).
Ji, P. et al. Strongly lewis acidic metal-organic frameworks for continuous flow catalysis. J. Am. Chem. Soc. 141, 14878–14888 (2019).
Xue, Z. et al. Tuning the adsorption energy of methanol molecules along Ni-N-doped carbon phase boundaries by the mott-schottky effect for gas-phase methanol dehydrogenation. Angew. Chem. Int. Ed. 57, 2697–2701 (2018).
Collins, S. E. et al. Acylating capacity of the phosphotungstic Wells-Dawson heteropoly acid: intermediate reactive species. J. Phys. Chem. C. 115, 700–709 (2011).
Bachiller-Baeza, B. et al. FTIR and reaction studies of the acylation of anisole with acetic anhydride over supported HPA catalysts. J. Catal. 228, 225–233 (2004).
Acknowledgements
This work was supported by the National Key R&D program of China (Grant No. 2021YFA1200402 to X.Z.), the Beijing Natural Science Foundation (Grant No. 2252057 to X.Z.), and the National Natural Science Foundation of China (Grant Nos. 52373219 to X.Z. and 21988102 to L.J.).
Author information
Authors and Affiliations
Contributions
X. Zhang and L. Jiang conceived the idea and directed the study. Z. Fang performed the main experiments and drafted the manuscript. X. Li, Y. Zhang and G. He participated in the experiments for sample characterization. Y. Bai and D. Peng contributed to the computational studies. Y. Bai, S. Pang and F. Xia helped with data analysis and manuscript polishing. All authors participated in the interpretation of the data and production of the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Milad Abolhasani, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fang, Z., Li, X., Bai, Y. et al. Amorphous/crystalline HTiNbO5-X membranes for efficient confined flow synthesis of acetate ester flavours. Nat Commun 16, 6012 (2025). https://doi.org/10.1038/s41467-025-61411-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-61411-y