Abstract
Photoinduced electron transfer is fundamental to both biological and synthetic processes; however, modulating back electron transfer (BET) remains a formidable challenge in achieving more efficient photocatalytic transformations. In this work, we present a strategy to regulate electron transfer dynamics via ligand-to-metal charge transfer (LMCT) catalysis, wherein the rapid β-scission of alkoxy radicals is harnessed to suppress BET, thereby facilitating the efficient transfer of reducing equivalents to drive transition metal-mediated reductive cross-coupling reactions. By strategically utilizing a diverse array of alcohol reductants, such as methanol and pinacol, we employ a cerium benzoate catalyst to enable reductive processes not through modulation of redox potentials, but by promoting synchronized electron transfer. Detailed mechanistic investigations reveal that the photoinduced electron relay process, governed by LMCT-BET, plays a pivotal role in effectively delivering reducing equivalents to catalytic sites, underscoring its significance in optimizing catalytic efficiency.
Similar content being viewed by others
Introduction
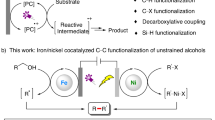
Electron transfer, the movement of electrons between donors and acceptors, is crucial in both biological and chemical processes1. The dynamic interplay between forward and backward electron transfer (BET) plays a central role in determining reaction efficiency. Photosynthesis in nature provides an elegant example of sophisticated electron relay mechanisms that rapidly transfer reducing equivalents to catalytic sites, effectively outcompeting BET through fast, successive redox-equivalent transfers2. Despite significant advances in photocatalytic organic synthesis, where electron transfer typically governs the catalytic generation of radical intermediates, synthetic chemists have yet to effectively regulate back electron transfer (BET, or charge recombination), a process that is believed to occur at diffusion-controlled rates and remains difficult to apprehend3. While considerable progress has been made in tuning redox potentials, absorption/excitation efficiencies, and excited-state lifetimes, back electron transfer remains an underexplored factor that limits photocatalytic efficiency (Fig. 1A)4,5,6,7,8,9. Only a few isolated strategies have successfully mitigated this recombination process, a notable example being recently demonstrated by Knowles and Moore through structural modifications of Ir-based photocatalysts5. Ligand-to-metal charge transfer (LMCT) excitation offers a distinctive approach to generating radical intermediates, such as alkoxy radicals, through the bond homolysis of photoexcited metal complexes10,11,12,13,14,15,16. Although BET remains a persistent challenge, LMCT catalysis offers exciting opportunities to tailor electron transfer kinetics, diverging from traditional outer-sphere electron transfer processes. In this work, we demonstrate an LMCT-enabled electron relay that utilizes alcohols, including methanol, as sources of reducing equivalents to drive transition metal-mediated cross-coupling reactions. By strategically modulating BET through the β-scission of radicals, we reveal that variations in alcohol structure significantly enhance reductive processes—not through changes in redox potential, but by enabling synchronized electron transfer.
With the advancement of nickel catalysis, reductive cross-electrophile coupling has emerged as a synthetically versatile platform for forging carbon-carbon bonds between readily accessible and operationally convenient carbon-centered electrophiles17,18,19,20. The necessity of an external electron source is pivotal for generating low-valent nickel complexes capable of activating aryl and alkyl halides and modulating the oxidation states of nickel for selective C–C bond formation21,22,23,24,25,26,27. Accordingly, diverse reductive systems including chemical reductants and photochemical as well as electrochemical approaches have been devised to broaden the synthetic scope of cross-electrophile coupling (XEC) reactions28,29,30,31. While inexpensive Zn or Mn powder (E~–1.5 to –1.0 V vs SCE) is frequently employed, the importance of tunable reduction systems in developing broadly applicable XEC reactions has recently been highlighted32. Organic electron donors, such as tetraaminoethylenes, offer a distinct advantage by enabling precise tuning of the reductant’s redox capacity through structural diversification33. Notably, compounds like tris(trimethylsilyl)silane, amines, and Hantzsch ester, despite their redox potentials not aligning with those required for nickel reductions, have shown remarkable efficacy in selective reductive couplings via photocatalyzed electron relay34,35,36. It is believed that the reduction of nickel species serves as the turnover-limiting steps in nickel-catalyzed XEC reaction21,37,38,39,40. However, employing a more reducing reductant or catalyst does not always yield straightforward improvements, as the reaction kinetics are often complicated by BET and competing reductive side reactions with electrophiles32. Despite these challenges, the potential to fine-tune the kinetics for matched and synergistic electron transfer processes remained largely unexplored (Fig. 1B).
Feedstock alcohols such as methanol and pinacol are frequently employed as reductants in transition metal catalysis due to their wide availability, benign nature, and non-disruptive oxidation by-products like formaldehyde and acetone. However, their application as electron sources in reductive cross-electro-phile couplings is significantly constrained by their high oxidation potential (E > 2 V vs SCE) and the potential for nucleophilic hydroxyl groups to form ether by-products41,42. To date, only one report has demonstrated success using 1-phenylethanol and an iridium photocatalyst in Ni catalytic XEC reaction, which produced acetophenone as a by-product43. We have recently developed a Ce-LMCT catalytic system that facilitates the oxidative activation of various aliphatic alcohols, leveraging hydrogen atom transfer and β-scission processes of alkoxy radicals for selective C–H and C–C bond cleavage in alkane and alcohol feedstocks44,45,46,47,48,49,50. A critical challenge in achieving efficient catalysis was the turnover of the Ce catalyst (E1/2 = 0.40 V vs SCE). This issue was resolved by introducing the electron shuttle catalyst DPA (9,10-diphenyl anthracene, E1/2 = 1.13 V vs SCE), which facilitates the energetically demanding electron transfer between radical intermediates and Ce(III) in a highly synergistic manner51,52,53,54. The inherent back electron transfer (BET), or geminate recombination of the O-centered radical with Ce(III), was identified as a key factor that, while limiting the quantum efficiency and reducing overall radical generation, simultaneously enhances the selectivity of productive radical generation48,50. This observation prompted us to investigate the Ce/DPA catalytic system as a tunable electron relay mechanism, utilizing alcohol reductants for open-shell reductive transformations by leveraging the β-scission of alkoxy radicals to suppress BET. We wonder whether the modular selection of alcohols reductants can be harnessed to drive Ni-catalyzed cross-electrophile coupling processes in a straightforward “gear-shifting” fashion52,55,56. Key challenges remain threefold: (i) whether a relatively slow β-scission process (e.g., k = 19 s−1 for ethoxy radical57) can effectively compete with the diffusion-controlled BET, (ii) the potential interference of LMCT-generated O- or C-centered radicals with cross-electrophile coupling, and (iii) whether the structural diversity of aliphatic alcohols, despite their similar oxidation potentials, could significantly influence Ni reduction kinetics.
Herein, we report a catalytic strategy to regulate electron transfer dynamics via ligand-to-metal charge transfer (LMCT) catalysis, wherein the rapid β-scission of alkoxy radicals is harnessed to suppress BET, thereby facilitating the efficient transfer of reducing equivalents to drive nickel catalytic reductive cross-coupling reactions (Fig. 1C).
Results
Reaction development
We began our investigation by evaluating the cross-coupling reaction between bromobenzene (1) and ethyl 4-bromobutanoate (2). Initial evaluation of the reaction conditions with premade dinuclear [Ni(diPhbpy)Br2(MeCN)]2 catalyst, DPA (E* = –1.79 V vs SCE) as electron shuttle, methanol as the reductant, Na3PO4 as the base, 4-methyl-pyridine as the additive led to the formation of the desired product in a 44% yield (Fig. 2A). Screening of ligand revealed that sterically less hindered benzoate and 2,4,6-triisopropyl benzenesulfonate gave inferior results. It was then found that the cross-coupling was completely shut down when 4,4’-bipyridine ligand or no ancillary ligand was added. Fluctuating yields were obtained using the catalyst generated from in situ coordination of CeCl3/2,4,6-triisopropylbenzoate, presumably owing to the competing coordination with bipyridine or pyridine additive. These results suggest the importance of structurally stable coordination for cerium in achieving high catalytic performance. With a focus on enhancing the catalytic performance and elucidating the coordination pattern of cerium benzoate complex, we prepared a well-defined Ce(III) complex with the 2,4,6-triisopropyl benzoate ligand. Treating 3 equivalent of potassium 2,4,6-triisopropyl benzoate with CeCl3 in tBuOH/H2O (1:1) for 6 h followed by recrystallization in DMSO/MeOH (1:1) resulted in the facile isolation of crystallized catalyst Ce(TRIPCO2)3(DMSO)3 (Fig. 2B). X-ray crystallography (CCDC number: 2418044) revealed that the cerium center was coordinated by three benzoate ligands in the κ2 chelating mode with the Ce−O bond length ranging from 2.44 Å to 2.63 Å and three solvent molecules of DMSO. Notably, this catalyst is found to be air-stable and moisture-insensitive.
Reactions were performed on a 0.1 mmol scale for 20 hours under a nitrogen atmosphere. The yields were determined by GC-FID. A Evaluation of supporting ligands for cerium. B Preparation of Ce(TRIPCO2)3(DMSO)3 catalyst. C Control experiments using Ce(TRIPCO2)3(DMSO)3 catalyst. aIsolated yield. D Evaluation of other alcohol reductants.
To our delight, this robust cerium catalyst demonstrated high catalytic performance, affording cross-coupling product 3 in a 74% yield under otherwise identical conditions. Importantly, the reaction demonstrated consistent efficiency under this reaction conditions using 24-well parallel photoreactor (Fig. S1). Notably, the dimerization products from two electrophiles were detected merely in trace amounts under optimized conditions. A slight loss in yield (52%) was observed when the 4-methyl pyridine additive was omitted (Fig. 2C, entry 2)21 We ascribe the beneficial effect of pyridine additive to a soluble organic co-base in the reaction. Moreover, control experiments revealed that cerium catalyst, nickel catalyst, DPA, light, and methanol are all essential for the desired reactivity (entries 3-7). Having identified the optimal reaction conditions, we next set out to assess other simple alcohol reductants in mediating the XEC reactions. The use of ethanol, isopropanol, 1,2-propanediol, or 2,3-butanediol in place of methanol gave the desired product 3 in comparably good yields, and corresponding aldehydes and ketones were detected in the reaction mixtures12,58. Furthermore, bulky diols such as pinacol and acetophenonepinacol were found to be effective reductants in promoting the desired cross-coupling. (Fig. 2D).
Substrate scope
Subsequently, we explored the generality of this protocol by using methanol, a commonly used organic solvent, as the practical reductant. The scope with regards to the alkyl electrophiles was first evaluated to furnish the products 4-18 in moderate to good yields (Fig. 3). A broad range of alkyl electrophiles bearing various substituents including, nitrile (6), silyl ether (7), sulfinate ester (8), phthalimide (9), alkene (10), alkyl cyclic rings (11-13), amide (14) were well-tolerated. Benzyl chloride, and α-chloroamides, were found to be suitable coupling partners to react with bromobenzene to afford the desired products (15-17) in good yields. The catalytic system could be successfully extended to secondary alkyl electrophiles (18). Next, a variety of C(sp2) bromides were investigated as coupling partners. Aryl bromides containing electron-withdrawing and electron-neutral substituents at the different positions of aromatic rings reacted smoothly to afford the desired products (19-25). Notably, vinyl bromides were readily alkylated with moderate efficiencies (26-27) with no methoxy radical addition or hydrogen atom fun-ctionalization product observed.
General reaction conditions: aryl halide (0.2 mmol), alkyl halide (0.1 mmol), Ce(TRIPCO2)3(DMSO)3 (0.01 mmol), DPA (0.002 mmol), [Ni(diPhbpy)Br2(MeCN)]2 (0.002 mmol), 4-Me-pyridine (0.04 mmol), Na3PO4 (0.3 mmol) and alcohol (0.5 mmol) in 0.5 mL MeCN, LEDs (λmax = 395 nm, 0.8 W/cm2) at 20 °C for 20 h. Isolated yields were given.
While applying the standard conditions for electron-rich aryl bromides, we observed in general, diminished coupling efficiencies. In these cases, aryl electrophiles were observed with rather low conversions as compared to alkyl halides, with considerable amounts of protodehalogenation byproducts detected. Prolonging the reaction time failed to improve the coupling efficiency, and only increased dehalogenation products were observed. The decreased reactivity might arise from the unfavored oxidative addition of electron-rich Csp2- electrophiles to low-valent nickel intermediate, leading to the bleaching of the catalyst and consequently mismatched kinetics of cross coupling processes40,43,59,60,61,62,63. Isolated effort using additives has been made to improve the efficacy of the oxidative addition of aryl halides to low-valent nickel species59. Intrigued by these persistent challenges associated with nickel catalysis, we turned our attention to investigate the possibility of tuning the reductive processes of our system by using different alcohol reductants. A simple swap of alcohol reductants demonstrates that significantly higher yields can be achieved with diols, which are more prone to undergo β-scission, even at lower light intensities (0.4 W/cm2). For example, coupling product 28 was produced in 26% yield with methanol and a 41% yield with 2,3-butanediol. The yield could be dramatically enhanced to 74% by using pinacol as the reductant, and protodehalogenation product from alkyl halide was observed with low yield. The switch of coupling efficiency has also been proved effective in the coupling reaction of aryl halides bearing different electron-donating substituents with alkyl bromides (29-36).
When strained spirocyclic alkyl bromides were employed as coupling partners, the desired product 37 was obtained in a modest yield of 59% with pinacol as the reductant, with the mass balance largely consisting of unreacted alkyl bromide. This suggests that while the reduction of Ni species was achievable, it was not synchronized with alkyl radical generation and coupling, resulting in overall low efficiency. Remarkably, by switching to acetophenonepinacol as the reductant, the yield was significantly boosted to 91%. This improvement was further demonstrated when aryl halides with multi-electron donating groups (38-40) were used, where acetophenonepinacol also enhanced coupling efficiencies, shifting the yield from moderate to highly satisfactory levels. This trend of yield enhancement continued with more electron-donating substituents, such as morpholine (41) and dihydroindole (42), installed on aryl bromides, highlighting the remarkable “gear-shifting” tunability of this approach in driving challenging cross-coupling reactions.
The broad functional group tolerance of this protocol encouraged us to explore its application in the late-stage functionalization of biologically relevant molecules (Fig. 4). Moderate to excellent yields of coupling products were achieved with complex molecules derived from L-borneol (43), pregnenolone (44), ibuprofen (45), lenalidomide (46), celecoxib (47), loratadine (48), PARA7 inhibitors analog (49). Notably, the utilization of homogeneous and economical alcohols as reductants, as compared with metal reductants, facilitates the effortless scale-up of the XEC reaction in a homemade circulation flow setup by replacing insoluble Na3PO4 with 4-methoxy pyridine to neutralize the acids generated in the reaction64. For instance, transferring the reaction into the continuous flow conditions enables facile gram-synthesis of fluorinated loratadine 50 in a 75% yield. The operational simplicity and broad applicability allow the direct application of reaction system in the Csp2-Csp3 cross coupling with N-tosyl aziridine, and 1,2-alkyl arylation of vinyl boronic ester, obviating the need for modification of reaction conditions (51-52)65,66. Moreover, the synergistic reaction system showed high level control of both the reactivity and enantioselectivity, enabling the asymmetric reductive arylation of α-chloroboronates (53) and α-chlorophosphonates (54) using chiral bioxazoline ligands36,67.
Unless otherwise noted, reactions were performed with aryl halide (0.2 mmol), alkyl halide (0.1 mmol), [Ce] (0.01 mmol), DPA (0.002 mmol), [Ni] (0.002 mmol), 4-Me-pyridine (0.04 mmol), Na3PO4 (0.3 mmol) and pinacol (0.5 mmol) in 0.5 mL MeCN, LEDs (λmax = 395 nm, 0.8 W/cm2) at 20 oC. Isolated yields were given, er values were determined by HPLC analysis. bMeCN/THF (4/1, 0.2 M). c2 eq. acetonephenonepinacol. dMeCN/THF (3/2, 0.1 M) was used. e8 mol% Ni(acac)2, 8 mol% chiral ligand, 6 mol% DPA.
Mechanistic studies
To investigate the electron relay mechanism of this triple catalytic system, we first employed spectroscopic and electrochemical techniques to characterize the ground-state redox properties (Fig. 5). UV-vis measurements revealed that the Ce(III)(TRIPCO₂)₃(DMSO)₃ catalyst primarily absorbs in the ultraviolet region (<350 nm). In contrast, the in situ-formed complex Ce(IV)(OR)ₓLy (ROH = pinacol; L = 2,4,6-triisopropyl benzoate, Fig. S2) displayed a characteristic LMCT absorption band extending to 450 nm, overlapping significantly with the absorption range of DPA (300–415 nm) under LED irradiation. Cyclic voltammetry revealed that while the Ce(IV) benzoate complexes (E1/2 = 0.65 V vs SCE) and their alkoxide-ligated derivatives (Ep/2 = 0.72 V vs SCE) have redox potentials insufficient for direct electron transfer to DPA (E1/2 = 1.13 V vs SCE in MeCN), single-electron transfer between the oxidized DPA species and Ce(III) intermediates remains feasible (Fig. S4).
The photoinduced electron transfer of Ce(IV) alkoxide complexes was further investigated by steady photolysis experiments under 375 nm laser irradiation (Fig.S6). The gradual attenuation of characteristic LMCT absorption bands confirmed that Ce(IV) alkoxide species undergo photolysis to form Ce(III) intermediates (Fig. 5A). Crucially, the generation of alkoxy radicals through LMCT-induced homolysis was validated by operando EPR experiments. Upon irradiation of the Ce(IV) alkoxide complex in the presence of 5,5-dimethyl-1-pyrroline N-oxide (DMPO), a spin-adduct signal characteristic of DMPO–alkyl (AN = 14.8 G, AHβ = 24.0 G) was observed, corroborating the formation of α-hydroxy radicals via β-scission of alkoxy radicals derived from pinacol. Concurrently, acetone—formed alongside the α-hydroxy radicals—was detected by GC-FID, providing further evidence of selective alkoxy radical generation. Notably, the absence of carboxyl radicals underscores the supportive role of the benzoate ligand, which does not directly participate in the photoexcitation and electron transfer process.
Intriguingly, steady-state photolysis experiments with various Ce(IV) alkoxide complexes under otherwise identical conditions revealed dramatically varied quantum efficiencies. As shown in Fig. 5A, the Ce(IV) complex derived from pinacol exhibited a much faster photolysis rate (56% quantum yield), approximately ten times more rapid than that of the cerium methoxide complex (5% quantum yield). Given their similar absorption intensities and structures, we reasoned that this significant difference was not due to the LMCT homolysis process itself, but rather to differences in the BET kinetics (Fig. S6). Our previous study with the Ce(IV) methoxide complex has elucidated the BET process between Ce(III) and alkoxy radicals, where the geminate recombination of these species immediately following homolysis regenerates the ground-state Ce(IV) methoxide, thereby reducing the quantum efficiency of the LMCT process48,50. This trend in quantum yields of Ce(IV) alkoxide complexes aligns with their propensity to undergo β-scission of the corresponding alkoxy radicals. When vicinal diols were employed, the propensity to generate energetically stabilized α-hydroxy radicals significantly enhanced β-scission, effectively removing the LMCT-homolyzed alkoxy radicals from the dynamic interplay of forward and backward electron transfer, thereby facilitating the net accumulation of Ce(III). For the Ce(IV) tert-butoxide complex, the improved quantum efficiency observed at diluted concentrations (increasing from 17% to 26% upon dilution from 6 × 10⁻⁴ M to 8 × 10⁻⁵ M, Fig. S7) also suggests that the suppression of BET through dilution may, to some extent, contribute to the enhanced net electron transfer4.
Stern-Volmer (SV) quenching experiments were conducted to probe the electron transfer between DPA* and cerium complexes as well as nickel species. Steady-state SV quenching between DPA* (Eox1/2 = –1.79 V vs SCE) and Ni(II) [(2-tol)Ni(II)(dtbpy)Br (E1/2 = –1.47 V vs SCE)] indicated a photoinduced electron transfer process, which oxidizes DPA to the corresponding radical cation intermediate (Fig. 5B, KSV = 2909 M⁻¹). Despite the relatively low quenching constant (KSV = 88 M⁻¹), Ce(III) species were also found to quench the excited DPA. Comparing the relative KSV values of Ni(II) species to Ce(III), we deduce that 4 mM of Ni(II) species quench the excited DPA emission by 92.1%, indicating that Ni(II) is the primary quencher under catalytic conditions. Given that KSV = kqτ₀, where τ₀ is the natural (unquenched) lifetime of DPA* (τ₀ = 8.9 ns, Fig. S10), the rate constants for quenching by Ni(II) calculated to be 3.2 × 10⁵ M⁻¹s⁻¹. Additionally, steady-state fluorescence quenching experiments (Fig. S15, 16) ruled out direct electron transfer between DPA* and the two substrates.
To probe the final missing piece of the electron relay mechanism—whether the radical cation of DPA (DPA•+) could oxidize Ce(III) efficiently to refurbish the LMCT cycle—we found that DPA•+can be easily generated via anodic oxidation, exhibiting a broad characteristic absorption band in the 500–800 nm range (Fig. 5C)68. Under anhydrous and anaerobic conditions, the intense blue color of the DPA•+ solution persisted for several hours, indicative of the long lifetime of this oxidative species. This persistence enabled us to directly observe its signal via EPR spectroscopy (g = 2.0025, see Fig. S9) and probe the electron transfer between DPA•+ and Ce(III) through simple titration experiments. As demonstrated in the UV-visible absorption spectrum (Fig. 5C), upon increasing the concentration of Ce(III), the characteristic absorption band of DPA•+ gradually decayed, accompanied by the recovery of DPA’s characteristic absorption signals in the 300–400 nm region. This provided direct evidence for the electron transfer between DPA•+ and Ce(III) species. Furthermore, we were able to estimate the kinetics of the electron transfer process (k = 1.2 × 105 M−1s−1) employing stopped-flow experiments (Fig. S19). The closely matched kinetics of the two electron transfer steps, between excited DPA and nickel species and between DPA•+ and Ce(III), enable a synchronized electron relay between cerium and nickel catalytic cycles, where the formation of Ce(III) serves as the key driving force and LMCT-BET as the critical regulatory factor for transferring reducing equivalents to catalytic sites.
Proposed reaction mechanism
In light of these observations and literature reports, a plausible reaction mechanism is outlined in Fig. 5D. We envision that a Ce(IV) species, generated by SET oxidation of Ce(III) by DPA•+, could coordinate with alcohols in situ to produce Ce(IV)-alkoxide which undergo photo-induced LMCT to generate the alkoxy radical and a Ce(III) intermediate. The ensuing β-scission and/or HAT of alkoxy radicals could modulate BET and synchronize electron transfer, regulating the kinetics of the electron relay from alcohols to the DPA catalytic cycle. The SET reduction of DPA•+ by α-hydroxy radical to regenerate DPA and carbonyl compound, consequently supplying another electron to the catalytic system. Concurrently, the photoexcited DPA can sequentially reduce Ni(III) aryl dibromide complex, generated via oxidative addition of aryl halide to LNi(I)Br, to afford the Ni(I)-aryl species and DPA radical cation, while the possibility of SET reduction of Ni(III) aryl dibromide complex by α-hydroxy radical cannot be excluded at current stage. Finally, the activation of alkyl bromide by Ni(I)-aryl species affords the alkyl radical and the LNi(II)(Ar)(Br), which then combines and proceeds via reductive elimination to afford the product and regenerate the LNi(I)Br species.
In summary, we have developed an operationally simple protocol employing a cerium benzoate catalyst for nickel-catalyzed XEC reactions. By harnessing an LMCT catalytic cycle to transfer reducing equivalents from alcohols rather than directly activating substrates, this synergistic catalytic system enables efficient coupling of challenging, electron-rich aryl halides through simple modulation of the alcohol reductants. Leveraging the β-scission of alkoxy radicals as a tunable mechanism to modulate BET allows for synchronized electron transfer, further emphasizing the potential of integrating LMCT-driven electron relay mechanisms to enable advanced reductive transformations.
Methods
General procedure for the batch reactions
In a nitrogen-filled glovebox, an 8 mL vial was charged with substrates aryl bromide (2 equiv.), alkyl bromide (1 equiv.), Ce(TRIPCO2)3(DMSO)3 (0.1 equiv.), [Ni(diPhbpy)Br2(MeCN)]2 (0.02 equiv.), DPA (0.02 equiv.), alcohol (5 equiv.), Na3PO4 (3 equiv.) and MeCN (0.5 mL). The vial was sealed with a PTFE-lined septum, and the suspension was allowed to stir for 10 minutes. The mixture was then irradiated with LED (λmax = 395 nm, 0.8 W/cm2) and stirred at 20 °C for 20 hours. After the reaction, the resulting mixture was concentrated in vacuo and purified by flash column chromatography.
Data availability
Crystallographic data for the structure reported in this work have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under the deposition number no. CCDC2418044 (Ce(TRIPCO2)3(DMSO)3), and CCDC2418042 ([Ni(diPhbpy)Br2(MeCN)]2). Copies of the crystal data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/ cif. All other data supporting the findings of this study are available in the main text and supplementary files, or from the corresponding author upon request.
References
Houmam, A. Electron transfer initiated reactions: bond formation and bond dissociation. Chem. Rev. 108, 2180–2237 (2008).
Blankenship, R. E. Molecular Mechanisms of Photosynthesis, Wiley-Blackwell, 2nd edn, 2014.
Kavarnos, G. J. & Turro, N. J. Photosensitization by Reversible electron transfer: theories, experimental evidence, and examples. Chem. Rev. 86, 401–449 (1986).
Ruccolo, S., Qin, Y., Schnedermann, C. & Nocera, D. G. General Strategy for Improving the Quantum Efficiency of Photoredox Hydroamidation Catalysis. J. Am. Chem. Soc. 140, 14926–14937 (2018).
Sayre, H. et al. PCET-based ligand limits charge recombination with an Ir(III) Photoredox Catalyst. J. Am. Chem. Soc. 143, 13034–13043 (2021).
Zhao, H. & Leonori, D. Minimization of back-electron transfer enables the elusive sp3 C−H Functionalization of secondary anilines. Angew. Chem., Int. Ed. 60, 7669–7674 (2021).
Earley, J. D. et al. Ion-pair reorganization regulates reactivity in photoredox catalysts. Nat. Chem. 14, 746–753 (2022).
Draper, F. et al. Maximizing photon-to-electron conversion for atom. Effic. Photoredox Catal. J. Am. Chem. Soc. 146, 26830–26843 (2024).
Wang, C., Li, H., Bürgin, T. H. & Wenger, O. S. Cage escape governs photoredox reaction rates and quantum yields. Nat. Chem. 16, 1151–1159 (2024).
Abderrazak, Y., Bhattacharyya, A. & Reiser, O. Visible-light-induced homolysis of earth-abundant metal-substrate complexes: a complementary activation strategy in photoredox catalysis. Angew. Chem., Int. Ed. 60, 21100–21115 (2021).
Tsurugi, H. & Mashima, K. Renaissance of homogeneous cerium catalysts with unique Ce(IV/III) couple: redox-mediated organic transformations involving homolysis of Ce(IV)–ligand covalent bonds. J. Am. Chem. Soc. 143, 7879–7890 (2021).
Chang, L., An, Q., Duan, L., Feng, K. & Zuo, Z. Alkoxy radicals see the light: new paradigms of photochemical synthesis. Chem. Rev. 122, 2429–2486 (2022).
Juliá, F. Ligand-to-Metal Charge Transfer (LMCT) photochemistry at 3D-metal complexes: an emerging tool for sustainable organic synthesis. ChemCatChem 14, e202200916 (2022).
An, Q., Chang, L., Pan, H. & Zuo, Z. Ligand-to-Metal Charge Transfer (LMCT) Catalysis: Harnessing simple cerium catalysts for selective functionalization of inert C–H and C–C bonds. Acc. Chem. Res. 57, 2915–2927 (2024).
May, A. M. & Dempsey, J. L. A new era of LMCT: Leveraging ligand-to-metal charge transfer excited states for photochemical reactions. Chem. Sci. 15, 6661–6678 (2024).
West, J. G. Building catalytic reactions one electron at a time. Acc. Chem. Res. 57, 3068–3078 (2024).
Ehehalt, L. E. et al. Cross-electrophile coupling: principles, methods, and applications in synthesis. Chem. Rev. 124, 13397–13569 (2024).
Pang, X., Su, P.-F. & Shu, X.-Z. Reductive cross-coupling of unreactive electrophiles. Acc. Chem. Res. 55, 2491–2509 (2022).
Poremba, K. E., Dibrell, S. E. & Reisman, S. E. Nickel-catalyzed enantioselective reductive cross-coupling reactions. ACS Catal. 10, 8237–8246 (2020).
Everson, D. A. & Weix, D. J. Cross-electrophile coupling: principles of reactivity and selectivity. J. Org. Chem. 79, 4793–4798 (2014).
Everson, D. A., Jones, B. A. & Weix, D. J. Replacing conventional carbon nucleophiles with electrophiles: nickel-catalyzed reductive alkylation of aryl bromides and chlorides. J. Am. Chem. Soc. 134, 6146–6159 (2012).
Wang, X., Wang, S., Xue, W. & Gong, H. Nickel-catalyzed reductive coupling of aryl bromides with tertiary alkyl halides. J. Am. Chem. Soc. 137, 11562–11565 (2015).
Wang, X. et al. Ni-Catalyzed reductive coupling of electron-rich aryl iodides with tertiary alkyl. Halides J. Am. Chem. Soc. 140, 14490–14497 (2018).
Hamby, T. B., LaLama, M. J. & Sevov, C. S. Controlling Ni redox states by dynamic ligand exchange for electroreductive Csp3–Csp2 Coupling. Science 376, 410–416 (2022).
Twilton, J. et al. Quinone-mediated hydrogen anode for non-aqueous reductive electrosynthesis. Nature 623, 71–76 (2023).
Huang, Z., Akana, M. E., Sanders, K. M. & Weix, D. J. A decarbonylative approach to alkylnickel intermediates and C(sp3)-C(sp3) bond formation. Science 385, 1331–1337 (2024).
Kim, S., Goldfogel, M. J., Gilbert, M. M. & Weix, D. J. Nickel-catalyzed cross-electrophile coupling of aryl chlorides with primary alkyl chlorides. J. Am. Chem. Soc. 142, 9902–9907 (2020).
Charboneau, D. J., Hazari, N., Huang, H., Uehling, M. R. & Zultanski, S. L. Homogeneous organic electron donors in nickel-catalyzed reductive transformations. J. Org. Chem. 87, 7589–7609 (2022).
Yi, L., Ji, T., Chen, K.-Q., Chen, X.-Y. & Rueping, M. Nickel-catalyzed reductive cross-couplings: new opportunities for carbon-carbon bond formations through photochemistry and electrochemistry. CCS Chem. 4, 9–30 (2022).
Liu, Y., Li, P., Wang, Y. & Qiu, Y. Electroreductive Cross-Electrophile Coupling (eXEC) reactions. Angew. Chem., Int. Ed. 62, e202306679 (2023).
Wang, H. & Xu, T. Dual Nickel- and Photoredox-catalyzed carbon-carbon bond formations via reductive cross-coupling involving organohalides. Chem. Catal. 4, 100952 (2024).
Su, Z.-M., Deng, R. & Stahl, S. S. Zinc and manganese redox potentials in organic solvents and their influence on nickel-catalysed cross-electrophile coupling. Nat. Chem. 16, 2036–2043 (2024).
Charboneau, D. J. et al. Tunable and practical homogeneous organic reductants for cross-electrophile coupling. J. Am. Chem. Soc. 143, 21024–21036 (2021).
Duan, Z., Li, W. & Lei, A. Nickel-catalyzed reductive cross-coupling of aryl bromides with alkyl bromides: Et3N as the terminal reductant. Org. Lett. 18, 4012–4015 (2016).
Zhang, P., Le, C. C. & MacMillan, D. W. C. Silyl radical activation of alkyl halides in metallaphotoredox catalysis: a unique pathway for cross-electrophile coupling. J. Am. Chem. Soc. 138, 8084–8087 (2016).
Wang, H., Zheng, P., Wu, X., Li, Y. & Xu, T. Modular and facile access to chiral α-aryl phosphates via dual Nickel- and photoredox-catalyzed reductive cross-coupling. J. Am. Chem. Soc. 144, 3989–3997 (2022).
Lin, Q. & Diao, T. Mechanism of Ni-catalyzed reductive 1,2-Dicarbofunctionalization of alkenes. J. Am. Chem. Soc. 141, 17937–17948 (2019).
Lin, Q., Fu, Y., Liu, P. & Diao, T. Monovalent Nickel-mediated radical formation: a concerted halogen-atom dissociation pathway determined by electroanalytical studies. J. Am. Chem. Soc. 143, 14196–14206 (2021).
Ben-Tal, Y. & Lloyd-Jones, G. C. Kinetics of a Ni/Ir-photocatalyzed coupling of ArBr with RBr: Intermediacy of ArNiII(L)Br and rate/selectivity factors. J. Am. Chem. Soc. 144, 15372–15382 (2022).
Ting, S. I., Williams, W. L. & Doyle, A. G. Oxidative addition of aryl halides to a Ni(I)-Bipyridine complex. J. Am. Chem. Soc. 144, 5575–5582 (2022).
MacQueen, P. M., Tassone, J. P., Diaz, C. & Stradiotto, M. Exploiting ancillary ligation to enable nickel-catalyzed C–O cross-couplings of aryl electrophiles with aliphatic alcohols. J. Am. Chem. Soc. 140, 5023–5027 (2018).
Yang, L. et al. Light-promoted nickel catalysis: etherification of aryl electrophiles with alcohols catalyzed by a NiII-Aryl complex. Angew. Chem., Int. Ed. 59, 12714–12719 (2020).
Li, Z. et al. Metallaphotoredox-catalyzed enantioselective cross-electrophile coupling using alcohols as reducing agents. Angew. Chem. Int. Ed. 62, e202305889 (2023).
Guo, J.-J. et al. Photocatalytic C−C bond cleavage and amination of Cycloalkanols by Cerium(III) chloride complex. Angew. Chem., Int. Ed. 55, 15319–15322 (2016).
Hu, A. et al. δ-selective functionalization of alkanols enabled by visible-light-induced ligand-to-metal charge transfer. J. Am. Chem. Soc. 140, 1612–1616 (2018).
Hu, A., Guo, J.-J., Pan, H. & Zuo, Z. Selective functionalization of methane, ethane, and higher alkanes by cerium photocatalysis. Science 361, 668–672 (2018).
Zhang, K., Chang, L., An, Q., Wang, X. & Zuo, Z. Dehydroxymethylation of alcohols enabled by cerium photocatalysis. J. Am. Chem. Soc. 141, 10556–10564 (2019).
An, Q. et al. Cerium-catalyzed C–H functionalizations of alkanes utilizing alcohols as hydrogen atom transfer agents. J. Am. Chem. Soc. 142, 6216–6226 (2020).
Chen, Y., Du, J. & Zuo, Z. Selective C-C bond scission of ketones via visible-light-mediated cerium. Catal. Chem. 6, 266–279 (2020).
An, Q. et al. Identification of alkoxy radicals as hydrogen atom transfer agents in Ce-catalyzed C–H functionalization. J. Am. Chem. Soc. 145, 359–376 (2023).
Hu, A. et al. Cerium-catalyzed formal cycloaddition of cycloalkanols with alkenes through dual photoexcitation. J. Am. Chem. Soc. 140, 13580–13585 (2018).
Chen, Y., Wang, X., He, X., An, Q. & Zuo, Z. Photocatalytic Dehydroxymethylative arylation by synergistic Cerium and nickel catalysis. J. Am. Chem. Soc. 143, 4896–4902 (2021).
Du, J. et al. Photocatalytic aerobic oxidative ring expansion of cyclic ketones to macrolactones by Cerium and Cyanoanthracene catalysis. Angew. Chem., Int. Ed. 60, 5370–5376 (2021).
Chinchole, A., Henriquez, M. A., Cortes-Arriagada, D., Cabrera, A. R. & Reiser, O. Iron(III)-light-induced homolysis: a dual photocatalytic approach for the hydroacylation of alkenes using acyl radicals via direct HAT from aldehydes. ACS Catal. 12, 13549–13554 (2022).
Jaber, M., Ozbay, Y., Chefdeville, E., Tran, G. & Amgoune, A. Unified photocatalytic strategy for the cross-coupling of alcohols with aryl halides enabled by synergistic nickel and iron LMCT catalysis. ACS Catal. 14, 12757–12768 (2024).
Nsouli, R. et al. Decarboxylative cross-coupling enabled by Fe and Ni Metallaphotoredox catalysis. J. Am. Chem. Soc. 146, 29551–29559 (2024).
Rauk, A., Boyd, R. J., Boyd, S. L., Henry, D. J. & Radom, L. Alkoxy radicals in the gaseous phase: β-scission reactions and formation by radical addition to carbonyl compounds. Can. J. Chem. 81, 431–442 (2003).
Matsuo, T., Sano, M., Sumida, Y. & Ohmiya, H. Organic Photoredox-catalyzed unimolecular PCET of benzylic alcohols. Chem. Sci. (2025).
Gisbertz, S., Reischauer, S. & Pieber, B. Overcoming limitations in dual photoredox/nickel-catalysed C–N cross-couplings due to catalyst deactivation. Nat. Catal. 3, 611–620 (2020).
Kawamata, Y. et al. Electrochemically driven, Ni-catalyzed aryl amination: scope, mechanism, and applications. J. Am. Chem. Soc. 141, 6392–6402 (2019).
Lau, S. H. et al. Ni/Photoredox-catalyzed enantioselective cross-electrophile coupling of styrene oxides with aryl iodides. J. Am. Chem. Soc. 143, 15873–15881 (2021).
Till, N. A., Oh, S., MacMillan, D. W. C. & Bird, M. J. The application of pulse radiolysis to the study of Ni(I) intermediates in Ni-catalyzed cross-coupling reactions. J. Am. Chem. Soc. 143, 9332–9337 (2021).
Ni, S. et al. C–heteroatom coupling with electron-rich aryls enabled by nickel catalysis and light. Nat. Catal. 7, 733–741 (2024).
Regnier, M., Vega, C., Ioannou, D. I. & Noël, T. Enhancing electrochemical reactions in organic synthesis: the impact of flow chemistry. Chem. Soc. Rev. 53, 10741–10760 (2024).
Steiman, T. J., Liu, J., Mengiste, A. & Doyle, A. G. Synthesis of β-Phenethylamines via Ni/Photoredox Cross-Electrophile coupling of aliphatic Aziridines and aryl iodides. J. Am. Chem. Soc. 142, 7598–7605 (2020).
Sun, S.-Z., Duan, Y., Mega, R. S., Somerville, R. J. & Martin, R. Site-selective 1,2-Dicarbofunctionalization of vinyl boronates through dual catalysis. Angew. Chem., Int. Ed. 59, 4370–4374 (2020).
Zheng, P. et al. Dual Ni/Photoredox-catalyzed asymmetric cross-coupling to access chiral benzylic boronic esters. Nat. Commun. 12, 1646 (2021).
Sioda, R. E. Electrolytic oxidation of 9,10-Diphenylanthracene and properties of its free radical cation and anion. J. Phys. Chem. 72, 2322–2330 (1968).
Acknowledgements
We thank the National Key R&D Program of China (No. 2021YFA1500100, Z.Z.), National Natural Science Foundation of China (No. 22125111, 21971163, Z.Z.), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB0610000, Z.Z.), the Shanghai Pilot Program for Basic Research – Chinese Academy of Science, Shanghai Branch, and SIOC, China Postdoctoral Science Foundation (2022M723285, S.W.) for financial support.
Author information
Authors and Affiliations
Contributions
S.W., W.Z. contributed equally to this work. Z.Z. conceived and directed the research; Z.Z., S.W., W.Z., Q.A. and L.D. designed the experiments; S.W., W.Z. and Q.A. performed and analyzed the reactions; Z.Z., S.W. and W.Z. prepared the manuscript, which was approved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Haohua Huo and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, S., Zeng, W., An, Q. et al. LMCT-driven electron relay unlocks alcohols as tunable reductants for nickel-catalyzed cross-electrophilic couplings. Nat Commun 16, 6162 (2025). https://doi.org/10.1038/s41467-025-61414-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-61414-9