Abstract
Resilience to developing emotional disorders is critical for adolescent mental health, especially following childhood trauma. Yet, brain markers of resilience remain poorly understood. By analyzing brain responses to angry faces in a large-scale longitudinal adolescent cohort (IMAGEN), we identified two functional networks located in the orbitofrontal and occipital regions. In girls with high genetic risks for depression, higher orbitofrontal-related network activation was associated with a reduced impact of childhood trauma on emotional symptoms at age 19, whereas in those with low genetic risks, lower occipital-related network activation had a similar association. These findings reveal genetic risk-dependent brain markers of resilience (GRBMR). Longitudinally, the orbitofrontal-related GRBMR predicted subsequent emotional disorders in late adolescence, which were generalizable to an independent prospective cohort (ABCD). These findings demonstrate that high polygenic depression risk relates to activations in the orbitofrontal network and to resilience, with implications for biomarkers and treatment.
Similar content being viewed by others
Introduction
Resilience, which is crucial for mental health, refers to the capacity for positive adaptation in coping with stress1. Childhood trauma (e.g., emotional abuse, physical abuse and sexual abuse), affecting over a billion people globally2, heightens the risk of emotional disorders such as depression and anxiety3. These disorders have been linked to dysfunctions in the brain’s emotion processing system (e.g., brain regions activated during emotion perception and emotion regulation)4, which is influenced by genetics during adolescent brain development5,6. Advanced knowledge of the genetic influences on the brain’s role in resilience can enhance the prediction of emotional disorders and aid in accurately identifying vulnerable individuals to facilitate early intervention.
In population-based neuroimaging studies, a brain marker of resilience is typically identified by its association with fewer emotional symptoms following childhood trauma1,7. Emotional disorders are characterised by dysfunctions in the brain’s emotional circuits, especially for processing negative emotional information8. Accordingly, many previous studies have focused on brain responses to negative emotional stimuli (e.g., angry and fearful faces) in predefined regions of interest9,10,11,12, including the orbitofrontal cortex (OFC), medial prefrontal cortex, anterior cingulate cortex, and amygdala13. These regions have been employed as candidate regions for identifying brain markers of resilience1. Following childhood trauma, maladaptive responses in these regions (e.g., hyper amygdala reactivity14, weaker medial prefrontal cortex activity15, etc.) to negative emotional stimuli are associated with susceptibility to emotional disorders, whereas adaptive responses indicate resilience16. These adaptive responses are considered brain markers of resilience. However, these regional findings remain inconclusive. For example, the role of the amygdala in resilience appears inconsistent in the literature, with both hyper-17 and hypo-14 amygdala responses to negative emotional stimuli linked to resilience. Emerging evidence suggests that brain networks, rather than isolated regions, provide more robust associations with emotion processing18. This implies that resilience may be a property of functional networks as a whole. Therefore, functional networks for emotion processing in adolescent brains may serve as better candidate networks for identifying brain markers of resilience.
Meanwhile, sex differences in the brain markers of resilience have also been reported in the literature19,20,21. For instance, resilience is associated with stronger spontaneous OFC activation in boys, but weaker activation in girls19, as well as larger prefrontal volume in boys and smaller volume in girls20. Such sex differences have also been observed in the temporal and frontal volumes21. Although boys and girls may share the same brain networks for emotion processing, sex differences have been observed in the maturation processes of these networks22. Therefore, these brain networks may play different functional roles in resilience between boys and girls.
These previous studies have primarily focused on the association between a brain feature, as a marker of resilience, and reduced emotional symptoms in individuals exposed to trauma. However, this association does not exclude the possibility that the brain feature could correlate with fewer emotional symptoms independent of childhood trauma, i.e., a trauma-independent form of protection. A marker of resilience may instead be defined by a two-way interaction between a brain feature (e.g., higher / lower activations) and childhood trauma (e.g., exposure / non-exposure), where this brain feature reduces the impact of childhood trauma on emotional symptoms, i.e., a trauma -related form of protection. Studying resilience based on interactions between childhood trauma and brain markers is rare in the literature, but it is important to distinguish between trauma -related and trauma -independent forms of protection when defining the brain marker of resilience to developing emotional disorders following childhood trauma1. This is crucial, as emotional symptoms during childhood and adolescence have been associated with an elevated risk of developing major depressive disorders (MDD) in adulthood23,24. Thus, such a two-way interaction could enhance the ability to predict emotional disorders.
In another line of research, the diathesis-stress model25 suggests that genetic predispositions, which may cause maladaptive brain changes following childhood trauma, can increase the risk of developing emotional disorders. A classic example is the depression-related 5-HTTLPR short variant, where carriers exhibit negative associations between amygdala responses to emotional faces and life stress, while non-carriers show positive associations26. A recent example is that following negative life events, frontal and parietal volumes decreased (i.e., adaptive changes following environmental stress) in healthy controls but increased (i.e., maladaptive changes) in MDD patients27. Particularly, lower amygdala responses to looming faces are significantly associated with higher resilience, as measured by the Connor-Davidson resilience scale, in nondepressed young adults with, but not in those without, a family history of depression28. Therefore, we hypothesised that the roles of brain networks in resilience might differ between subpopulations of individuals with varying genetic risk profiles, such as higher or lower polygenic risk scores for MDD (PRSMDD)29. To assess whether PRSMDD moderates these roles, a three-way interaction involving brain networks, childhood trauma, and PRSMDD should be tested. Identifying such an interaction could define a genetic risk-dependent brain marker of resilience (GRBMR), which is associated with fewer emotional symptoms following childhood trauma within a genetic risk-stratified subpopulation only, but does not indicate universal resilience in the whole population. However, previous studies have struggled to detect significant three-way interactions due to limited sample sizes. Recently, the IMAGEN study, a neuroimaging cohort of adolescents30, provided a uniquely large sample size to detect this interaction effect.
Based on these studies and considerations, we hypothesise that the adolescent brain’s emotional processing networks, rather than any single brain region, are more suitable candidates for identifying resilience markers that are predictive of subsequent emotional disorders. Importantly, the roles of these networks in resilience should be examined in a genetically dependent manner and analysed separately for boys and girls. To test this hypothesis, we aim to answer the following four main questions (Fig. 1): (1) Can we isolate functional networks in the adolescent brain’s emotion processing system as candidate networks for identifying markers for resilience? (2) Can we identify the GRBMR by detecting significant three-way interactions among these functional networks, childhood trauma and PRSMDD, when analysed separately for boys and girls? (3) Can these identified GRBMR predict subsequent emotional disorders? (4) Are these predictions generalisable to other developmental stages and independent datasets?
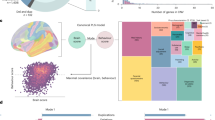
a Longitudinal cohorts included in the study. b Distinct functional networks were identified as candidate networks whose adaptive responses to angry faces may indicate resilience, using sparse non-negative matrix factorisation (sNMF) applied to brain responses to angry faces. c Genetic risk-dependent brain markers of resilience (GRBMR) were identified by testing a three-way interaction among childhood trauma, PRSMDD and candidate networks (shown in b) in relation to emotional symptoms at age 19, analysed separately for boys and girls. The error bands denote 95% confidence intervals around the regression lines. d The predictive utility of GRBMR was assessed using machine learning models. e Generalisability of the prediction was evaluated across developmental stages and in an independent dataset.
Results
Summary of experimental steps
Instead of using brain areas as candidate regions for identifying markers of resilience, the first analysis was to isolate functional networks within the brain’s emotion processing system as better candidates. Using a large neuroimaging sample of adolescents (19.02 ± 0.75 years; N = 809, 430 girls; the IMAGEN cohort30; Table 1), we decomposed brain responses to angry faces into distinct functional networks by sparse non-negative matrix factorisation (sNMF). These networks were further characterised by their neuroanatomy, function, development, and sex differences. Second, for each of these candidate networks, we evaluated the interaction effect between its response to angry faces and childhood trauma on emotional symptoms separately for boys and girls. To identify GRBMRs, we further examined three-way interactions on emotional symptoms, involving the candidate networks, childhood trauma and PRSMDD. Third, we conducted longitudinal analyses to assess predictive values of the identified GRBMRs in genetically stratified populations. We built prediction models using the data collected at age 14 to predict emotional disorders at age 19. Finally, we tested the generalisability of the prediction models using both the latest follow-up data at age 23 in the IMAGEN cohort and another independent cohort, namely the Adolescent Brain Cognitive Development (ABCD) cohort31 (Fig. 1).
Candidate networks for identifying brain markers of resilience
The brain’s emotion processing system was activated by an fMRI face task30. We analysed the angry>neutral contrast map for activations responding to angry faces higher than those to neutral faces (Figs. 1b, 2a). By applying the sNMF with optimal parameters to these brain activation data (Supplementary Fig. S2), we identified two distinct functional networks, including the orbitofrontal- and occipital-related networks (Fig. 2b). The orbitofrontal-related network mainly covered the lateral orbitofrontal cortex (OFC), ventromedial prefrontal cortex (vmPFC), medial superior prefrontal cortex, anterior cingulate cortex (ACC), precuneus, posterior cingulate cortex and dorsolateral prefrontal cortex (dlPFC) (Supplementary Table S2). The occipital-related network was mainly located in visual cortical regions: the lingual gyrus, cuneus, part of the inferior occipital gyrus (including the occipital face area, OFA), fusiform gyrus (including the fusiform face area, FFA), insula, amygdala, and Heschl’s gyrus (Fig. 3a and Supplementary Table S3). Using a database of brain functions (i.e., the NeuroSynth32), we found that the orbitofrontal-related network was mainly related to high-level cognitive terms, such as episodic memory, memory retrieval and self-reference, while the occipital-related network showed associations with perceptual terms, such as vision and perception (Fig. 3b).
a Brain responses to angry faces were decomposed into factors (i.e., functional networks) and factor weights (i.e., network activation) using sparse non-negative matrix factorisation. b Brain maps show the orbitofrontal-related and occipital-related networks. The colour scale indicates voxel-wise factor values, with brighter colours representing higher contributions to the spatial profile of each network. Source data are provided in the Source Data file.
a Proportion of voxels within AAL2 regions covered by the orbitofrontal- and occipital-related networks. b NeuroSynth decoding of the two networks. The lollipop charts show the correlation coefficients for each network with the top 10 functional terms. CING, cingulate cortex; INS, insula; SM, sensorimotor. Source data are provided as a Source Data file.
Sex differences in these networks
We found significant sex differences in these two networks at age 19 years and in their developmental trajectories between ages 14 and 19 years. Compared with boys at age 19, we found that the network activation (i.e., the factor weight) of the occipital-related network was smaller in girls (\(\beta \)=− 0.230, 95%CI = [− 0.369, − 0.090], p = 0.001; Supplementary Table S7). During the 5-year follow-up period, we found that the activation of the orbitofrontal-related network increased in both boys (\({\eta }_{p}^{2}\) = 0.012, F = 4.509, p = 0.034) and girls (\({\eta }_{p}^{2}\) = 0.010, F = 4.142, p = 0.042). Meanwhile, the activation of the occipital-related network significantly increased in boys (\({\eta }_{p}^{2}\) = 0.012, F = 4.593, p = 0.033) but not in girls (p = 0.643; Supplementary Tables S8, 9).
Genetic moderations of the brain networks’ roles in resilience
As expected, higher levels of childhood trauma were associated with more emotional symptoms at age 19 in both boys (\(\,\beta \) = 0.205, 95% CI = [0.091, 0.319], p = 0.0004, N = 379) and girls (\(\,\beta\, \)= 0.146, 95%CI = [0.059, 0.234], p = 0.001, N = 430; Fig. 4a).
Childhood trauma was dichotomised based on clinical cut-offs (see “Methods”). a Girls with childhood trauma (N = 100) exhibited more emotional symptoms than those without exposure (N = 330; t = 5.139, ptwo-sided = 8.18\(\times \)10−7; two-sample t test). b No significant two-way interactions between trauma and either PRSMDD or network activations. c,d For illustration, PRSMDD was binarised by median split. After controlling for main effects and all two-way interactions, significant three-way interactions were observed among trauma, PRSMDD and activations of the orbitofrontal- and occipital-related networks. e,f Network activations were similarly binarised. Group differences in symptom levels were assessed using two-sample t tests. e In girls with high PRSMDD, those with low orbitofrontal-related activation and trauma exposure (N = 32) had more symptoms than those with low activation but without exposure (N = 73; t = 5.764, ptwo-sided = 3.19\(\times \)10−7), and also more than those with high activation despite exposure (N = 21; t = 2.152, ptwo-sided = 0.037). f In girls with low PRSMDD, those with high occipital-related activation and exposure (N = 25) had more symptoms than those with high activation but without exposure (N = 81; t = 2.929, ptwo-sided = 0.004), and also more than those with low activation despite exposure (N = 22; t = 2.082, ptwo-sided = 0.043). In a, e and f, the upper and lower whiskers represent the Q3 + 1.5 × IQR and Q1 − 1.5 × IQR, respectively. The upper and lower edges of a box represent the Q3 and Q1, and the central line represents the median. In b-d, the error bands represent the 95% confidence intervals of the linear fitted models. *p < 0.05; **p < 0.01; ***p < 0.001; ns, non-significant. Source data are provided in the Source Data file.
For both boys and girls, we found no significant two-way interactions between childhood trauma and either PRSMDD or activations of the two networks identified above (Fig. 4b). These findings suggest that neither PRSMDD nor network activations per se are sufficient to independently indicate resilience.
In girls, we identified two GRBMRs as defined by two significant three-way interactions between childhood trauma, PRSMDD, and the activations of both the orbitofrontal-related (Cohen’s f 2 = 0.058, \(\beta \,\)= − 0.128, 95%CI = [− 0.224, − 0.031], p = 0.009; Fig. 4c) and occipital-related (Cohen’s f 2 = 0.067, \(\beta \)=− 0.148, 95%CI = [− 0.253, − 0.043], p = 0.005; Fig. 4d) networks in predicting emotional symptoms at age 19 (Supplementary Table S10 and 11). The Shapiro-Wilk normality test33 confirmed the applicability of these linear models with three-way interaction terms by testing the normality of model residuals (all W > 0.96 and p > 0.05).
As binarized for illustrative purpose in Fig. 4e, among girls with higher PRSMDD, we observed a two-way interaction between the orbitofrontal-related network activation and childhood trauma (\(\beta \)=− 0.147, 95%CI = [− 0.262, − 0.032], p = 0.012, N = 215), where higher network activation reduced the association between childhood trauma and increased emotional symptoms, and thereby defined a brain marker of resilience for girls with higher PRSMDD. However, this two-way interaction was not significant among girls with lower PRSMDD.
As illustrated in Fig. 4f, among girls with lower PRSMDD, we also found a two-way interaction between the occipital-related network activation and childhood trauma (\(\, \beta \,\)= 0.183, 95%CI = [0.015, 0.351], p = 0.011, N = 215), where lower network activation reduced the association between childhood trauma and increased emotional symptoms, and thereby defined another brain marker of resilience for girls with lower PRSMDD. However, this two-way interaction was not significant among girls with higher PRSMDD.
No such three-way interactions were significant in boys; thus, we focused on girls in the following analyses.
Sensitivity analyses
The three-way interactions identified above remained significant in the following sensitivity analyses. First, we confirmed that our current sample provided enough statistical power to detect those three-way interactions. To detect a three-way interaction with a small-to-medium effect size (Cohen’s f 2 = 0.058) at a significance level of 0.05, and a desired power of 0.8, a sample size of 358 was required, as indicated by the power analysis in the R package ‘pwr’ (version 1.3.0). Second, these interactions were confirmed when the childhood trauma was binarised by clinical cut-offs (Supplementary Table S15). Third, these interactions remained significant after additionally controlling for the age, childhood neglect, IQ and substance use (Supplementary Table S16). Fourth, these interactions were specific to emotional symptoms only and were not significant for the other four types of behavioural problem scores in the SDQ. Fifth, these interactions on the emotional symptoms were specific to PRSMDD and were not significant for either PRSADHD or PRSSCZ (Supplementary Table S17).
Prediction of emotional disorders
Next, we assessed the predictability of the two GRBMRs identified above for emotional disorders. Separately for girls with the higher and lower PRSMDD, we built machine learning models using data collected at age 14 to predict emotional disorders at age 19 (N = 430), and compared the model performance by the 5-fold cross-validation with 10 repetitions (see “Methods”). Among girls with higher PRSMDD, we found that the GRBMR model using the interaction term between the orbitofrontal-related network activation and childhood trauma outperformed the network model without using the interaction term, which in turn outperformed the baseline model without using the network activation. These findings were consistent across various thresholds for the higher PRSMDD (Table 2; see “Methods”). These findings were not significant for either girls with lower PRSMDD or when using the occipital-related network.
Generalisability of the prediction model
Using the latest follow-up data collected in the IMAGEN cohort, we found that the predictability of the GRBMR model extended into early adulthood. Specifically, the GRBMR model with the orbitofrontal-related network activation at age 19 significantly improved the prediction of emotional disorders at age 23 in girls with higher PRSMDD (Table 3).
In the independent ABCD cohort31, the emotional n-back (EN-back) task34 is an emotional face paradigm used to probe the brain’s emotion processing system. We used the fMRI data from the EN-back task to obtain the corresponding network activations for the two networks identified above in the IMAGEN cohort. Again, we demonstrated that the GRBMR models using the orbitofrontal-related network activation at age 10 enhanced the prediction of emotional disorders at age 11 among girls with higher PRSMDD (Table 4).
Discussion
Guided by our hypotheses, this study investigated the functional role of the adolescent brain’s emotional processing networks in resilience in a genetically dependent manner, with analyses separately for boys and girls. First, we isolated two candidate networks, the orbitofrontal- and occipital-related networks, for identifying markers of resilience. These networks exhibited different developmental patterns and significant sex differences. Second, building on our established two-way interaction approach for identifying brain markers of resilience (i.e., a brain marker is associated with a reduced trauma-symptom association), we hypothesised distinct neural mechanisms of resilience in subpopulations carrying different genetic risk profiles. Indeed, our three-way interaction analyses identified two GRBMRs: (1) Within the group of girls carrying high PRSMDD exposed to childhood trauma, higher orbitofrontal-related network engagement during angry-face processing is associated with fewer emotional symptoms, a sign of resilience; (2) Within the group of girls carrying low PRSMDD exposed to childhood trauma, lower occipital-related network reactivity to angry faces is associated with fewer emotional symptoms, a sign of resilience. Thus, we have identified two brain markers of resilience, strengthened regulatory engagement and reduced threat reactivity, for girls carrying high and low polygenic depression risks, respectively, where resilience refers to fewer emotional symptoms following childhood trauma within their respective genetic contexts. Third, we found that among girls with higher PRSMDD, the orbitofrontal-related GRBMR at age 14 significantly improved the prediction of emotional disorders at age 19. Fourth, this predictive improvement was validated using the latest follow-up data collected at age 23 in the IMAGEN cohort and was generalisable to another independent cohort (i.e., ABCD). These findings highlight the genetic influences on orbitofrontal cortex function related to resilience, suggesting how markers for resilience can be used, and having implications for targeting treatment to appropriate individuals at risk.
Our findings revealed two separable and interacting networks processing angry facial expressions in adolescents. The existing literature has hypothesised that there are multiple interconnected emotional circuits in the brain for facial emotion processing35, and these systems have hierarchically developmental trajectories during adolescence36. Here, combining a longitudinal functional neuroimaging sample of the emotional face task for adolescents with an advanced matrix factorisation approach, we identified a two-network system underlying angry face processing. Many key parts of the orbitofrontal-related network, including the vmPFC37, the ACC38 and the lateral OFC39, have long been implicated in the neural representations of negative emotion40. Notably, this network covering more than 80% of the lateral OFC but less than 23% of the medial OFC (Supplementary Table S2) provided strong evidence supporting the theory of a positive-to-negative gradient in the medial-to-lateral OFC41. Meanwhile, the occipital-related network, which is in fact a ventral cortical stream network that includes the FFA, that is involved in visual perception, is well supported by a 2022 meta-analysis of 141 fMRI studies showing the occipital cortex as a key part of the emotion processing system42. Longitudinally, the medial prefrontal activity in the orbitofrontal-related network implicated in emotion regulation grows throughout adolescence43, while the occipital activity, including those in the face-selective regions (i.e., the fusiform gyrus) in the occipital-related network often shows substantial developmental changes before adolescence44. These changes in the two-network emotion processing system may confer some adaptive advantages, such as greater flexibility in adjusting one’s intrinsic motivations and goal priorities amidst changing social contexts in adolescence.
The current findings emphasise that different brain systems can have different functional roles related to resilience to developing emotional disorders following childhood trauma within subpopulations carrying distinct genetic risk profiles. Our hypotheses and analyses focus on the resilience, referring to fewer emotional symptoms following childhood trauma within each genetic risk-stratified subgroup. This is different from previous studies investigating the resilience that is universal in the whole population45. For example, following childhood trauma, individuals with the high orbitofrontal-related network activity had fewer emotional symptoms when compared with their peers within the subgroup carrying high PRSMDD; however, this did not hold when compared across the genetic subgroups, i.e., with low-PRSMDD individuals following childhood trauma. The functional networks (i.e., the orbitofrontal- and occipital-related networks), where we identified the GRBMRs, covered the brain regions that have been implicated in resilience in the literature1, such as the OFC and ACC in the orbitofrontal-related network and the amygdala in the occipital-related network. Different from previous studies, we could not uncover any brain marker of resilience on the whole population despite the large size of the IMAGEN sample. Instead, our finding of genetic moderation of the resilience-brain network relationship provided a possible explanation for the inconsistent findings reported in the literature. For example, following stressful life events, genetically high-risk individuals (i.e., carriers with the depression-related 5-HTTLPR short variant) displayed elevated amygdala activation in response to fearful faces, while the low-risk individuals showed inverse activation patterns26. Such moderation is not so surprising, as the genetic risks for depression have already been associated with both structures and functions of the brain’s emotion processing system46. Furthermore, our finding of the resilience-related advanced maturation in orbitofrontal function provided new evidence for the stress acceleration hypothesis of resilience47, suggesting that it should account for individuals with varying genetic risks. The stronger function of the orbitofrontal-related network, including the dlPFC, OFC and hippocampus, may be linked to resilience through a better neurocognitive function of the top-down suppression of traumatic memories48. This link was further supported by a clinical rTMS study of patients with MDD, where depression symptoms were ameliorated through enhanced activations in both OFC and hippocampus49. This is also supported by the overlap between this network and the default mode network (DMN), particularly medial frontoparietal regions, which have been implicated in remembering the past and self-referencing50. In an imaging genetic study, the alterations of the DMN have been associated with both childhood trauma and the gene expression of SLC6A451. Furthermore, our enrichment finding of the dopaminergic synapse pathway (Method S5, Supplementary Figs. S4, 5) provided a neurobiological link between the orbitofrontal-related network and the dopaminergic signature of resilience52.
Our finding of non-significant three-way interactions in boys may be due to the fact that boys exhibit fewer emotional symptoms at age 19 when compared with girls53. Furthermore, girls have been found to exhibit greater developmental reorganisation of the depression-related brain system during adolescence than boys, a process modulated by expression of X chromosome genes22. This heightened plasticity may increase girls’ neural sensitivity to environmental stressors like childhood trauma54. In addition, sex differences in depression risk mechanisms are evident: postmortem brain studies reveal distinct molecular changes between male and female MDD patients55, and animal models demonstrate that female-specific resilience genes (e.g., LINC00473) are downregulated in depressed female mice but not males56. Together, these literatures suggest that girls’ dynamic neurodevelopment and stronger genetic susceptibility create a biological context where gene-environment-brain interactions are more readily detectable. In contrast, boys’ neurodevelopmental trajectories may be less influenced by such interactions due to their lower baseline emotional vulnerability, reduced depression-related neural plasticity, and/or sex-specific genetic buffering mechanisms, potentially explaining the absence of detectable resilience markers in our study.
Our findings also have significant clinical implications for promoting adolescent mental health. In this study, we also examined whether three-way interactions between activations of two identified networks, childhood trauma and PRSMDD were associated with emotional symptoms at age 14 in the IMAGEN cohort and at age 10 in the ABCD cohort. We found that these interactions had the same trend as the main finding at age 19 but did not reach a statistical significance level (Supplementary Table S18). This result might be understood by the developmental change from childhood to adolescence22. Despite the lack of replication in three-way interactions in different age groups, the prediction of subsequent emotional disorders using the interaction term between orbitofrontal-related network activation and childhood trauma demonstrated generalisability. This suggests that while cross-sectional interactions may be age-specific, the underlying predictive mechanism is developmentally robust, and the window for building resilience by enhancing the function of this network may extend from preadolescence to late adolescence. The predictive value might come from the significant maturation processes of this network during adolescence, as the occipital-related network with non-significant change during the same period was not predictive. Recently, neurofeedback training, such as the real-time fMRI feedback training of OFC57 and amygdala58, have been used to enhance emotion regulation skills and reduce emotional symptoms. However, the intervention results are mixed59. Our findings suggest that the OFC-targeted interventions might be particularly effective for those individuals carrying high genetic risks for depression. Therefore, the genetic-informed and neuroimaging-targeted approach might offer a promising way of promoting adolescent mental health.
The current study is not without limitations. First, we focused only on the brain function involved in facial emotion processing. Future studies are needed to test the generalisability of our findings to other types of emotional processing, which might lead to the discovery of additional brain markers for resilience. Second, data on childhood trauma exposure were collected retrospectively at age 19. Future prospective cohort studies are needed to exclude the potential for recall bias. Third, although our main findings in the IMAGEN cohort were generalisable to the independent ABCD cohort, these two cohorts mainly cover the White population with middle-to-high socioeconomic status. Therefore, future studies are needed to test whether the main findings could generalise to broader populations with diverse socioeconomic or racial/ethnic backgrounds. Fourth, apart from the covariates considered in the current study, many other psychosocial, cognitive and environmental factors (e.g., intervention programme, school engagement, etc.) can also contribute to the recovery from the exposure to childhood trauma60. Future researches with comprehensively characterised information of these factors are needed to assess the effects of these factors on resilience. Fifth, recent literature highlights that resilience may manifest across multiple domains of functioning61. While our study focused on mental health symptoms, future studies could examine other social, academic and cognitive domains of functioning. Sixth, we only found significant results for emotional symptoms in girls. Future studies could investigate externalising symptoms, which may be more prevalent in boys, to identify brain markers of resilience for them. Seventh, future pre-registered studies should validate the finding that the relationship between childhood trauma and emotional symptoms would differ as a function of orbitofrontal-related network activation in a sex-dependent fashion. Finally, the clinical value of building resilience through the genetic-informed and neuroimaging-targeted intervention strategy needs to be tested by randomised clinical trials.
Taken together, our study uncovered distinct brain markers, associated with fewer emotional symptoms following childhood trauma—whose effects differed between two subpopulations stratified by polygenic risk for depression (high vs. low), as revealed by a significant three-way interaction.
Methods
Participants
Participants were drawn from the IMAGEN project, a multicenter longitudinal study of adolescent brain development and mental health that recruited 2000 participants at age 14 in Europe and the UK30. Among them, 1335 participants had neuroimaging data at both ages 14 and 19. Following the previous work62, we selected 1269 participants with consistent activation patterns by examining the similarity of brain activations across research sites. Since genotype data were only available for Caucasian participants, this study included 809 Caucasian adolescents (430 girls) with complete neuroimaging, mental health, childhood trauma, and genome data (Table 1 and Supplementary Fig. S1). Notably, the included sample did not differ significantly from the excluded IMAGEN participants in key demographic or psychological variables (Supplementary Table S1). The local research ethics committees approved this study, and written consent was obtained from each participant and a parent or guardian.
Measurements
Behavioural and emotional problems
The Strengths and Difficulties Questionnaire (SDQ63) is a valid and reliable assessment and is often used to measure the emotional and behavioural problems in adolescents, including emotional symptoms, conduct problems, hyperactivity/inattention, peer relationship problems, and prosocial behaviour. SDQ questionnaires gathered directly from adolescents themselves are more reliable than those from their parents, especially for the emotional symptom subscale64. Therefore, the self-reported versions of the SDQ at ages 14 and 19 were used in this study.
Childhood trauma measurements
The Childhood Trauma Questionnaire (CTQ65) is a 28-item self-report inventory used to assess the history of abuse and neglect before the age of 19 years. Given that the IMAGEN study is based on a population cohort, the severity of reported abuse may be underestimated. In this study, three abuse subscales (i.e., emotional abuse, physical abuse and sexual abuse) were summed to generate a composite measure of childhood trauma66. The higher the composite score, the greater the severity of childhood trauma.
Polygenic risk scores
Since emotional disorders are not single-gene diseases, it is promising to use PRS to reflect the complex genetic architecture in the context of environment-gene-brain interactions7. We used GWAS summary data from European-ancestry participants (135,458 cases and 344,901 controls) provided by the Psychiatric Genomics Consortium67 as the discovery sample. Cases were required to meet international consensus criteria (DSM-IV, ICD-9, or ICD-10)68,69,70 for a lifetime diagnosis of MDD, while controls were screened for the absence of lifetime MDD. 493,592 single-nucleotide polymorphisms (SNPs) were shared by the discovery sample and the IMAGEN cohort. After the quality control measures (Method S1), a total of 123,481 SNPs were selected to compute the PRSMDD in our sample using the genetic analysis tool PLINK (version 2.0). The means of the PRSs at 7 p-value thresholds (i.e., 0.001, 0.05, 0.10, 0.20, 0.30, 0.40, and 0.50) were used in the current study in keeping with a previous study62. The principal component analysis of ancestral information was performed, and the first 8 principal components (PCs) were used as covariates when PRS was considered as the predictor in the models.
Nuisance covariates
Pubertal status was assessed using the Pubertal Development Scale. A total neglect score was generated from the summation of two types of neglect (i.e., emotional neglect and physical neglect) in the CTQ. Socioeconomic status was rated according to the total score derived from all the 16 items related to family stress of the Development and Well-being Assessment, with each item offering three response options: 0 (‘No, or does not apply’), 1 (‘A little’), and 2 (‘A lot’). Therefore, a lower score represents a better socioeconomic condition. The IQ score of each participant was calculated as the total score derived from the Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV). Substance use was measured using the European School Survey Project on Alcohol and Drugs (ESPAD) as ever/never smoking cigarettes, drinking alcohol, or using illicit drugs.
The face task and fMRI preprocessing
The face task paradigm was used to elicit strong activation in the brain’s emotion processing system. In this task, participants passively watched 18 s blocks of either a face movie (presenting faces with angry, happy or neutral expressions) or a control stimulus (concentric circles). Details can be found in the initial report on this paradigm71. In this study, we explored the neural reactivity associated with angry expressions, as neuroimaging data on these expressions was available at both ages 14 and 19. After the fMRI pre-processing (Method S2), the contrast map of angry vs. neutral faces was obtained for each participant. The angry > neutral (i.e., the activations responding to angry faces were higher than those to neutral faces) activations were used to measure the activation of the emotion processing system in the brain responding to angry faces. Although the mechanisms underlying the neutral > angry activations remained unclear, we still examined such activations in the supplementary materials to enhance the comprehensiveness of our study. The voxels within the automated anatomical labelling (AAL2) template72 for grey matter were considered in the following analyses (47,640 voxels).
Matrix decomposition
We constructed an activation matrix for the angry>neutral activations. The activation matrix has a number of rows equal to the voxel count (m = 47,640) and a number of columns corresponding to the number of subjects (n = 809). Sparse non-negative matrix factorisation (sNMF) was employed to decompose the activation matrix at age 19 into a factor matrix and a weight matrix (Fig. 2a). To facilitate meaningful sparse representation, we explicitly incorporated ℓ0 -sparseness constraints73 on the columns of the factor matrix. Meanwhile, each row of the factor matrix can have only one non-zero value to ensure that no overlapping voxels among the latent factors are obtained by the decomposition (Method S3). To determine the optimal parameter for sparsity (\(\lambda=L/m\), L is the maximal number of non-zeros voxels in each factor, m is the total number of voxels) and the optimal number of factors, we tested both the reconstruction error and the reproducibility of the obtained decompositions by a random half split for 80 times (Method S4).
Characterisation analysis of the functional networks
Neuroanatomical characterisation
We identified the respective positions of the non-zero values in each column of the factor matrix (i.e., each latent factor) within 47,640 voxels in the AAL2 template.
Functional characterisation
As recommended by the previous work74, we compared the spatial pattern of the networks (i.e., factors) to the functional anatomy of the human brain using NeuroSynth (http://www.neurosynth.org/)32, an online platform for meta-analysis of functional neuroimaging literature. Specifically, we sorted all correlation coefficients for each network in descending order and adopted the top ten terms to characterise each network. Similar terms (e.g., “percept” and “perception”) were merged into a base form to avoid selecting repetitive terms.
Sex difference
We built a linear regression model to test the association between sex and the activation of each network (i.e., the weights of each factor) at age 19. In this model, we adjusted for the following essential covariates (i.e., research sites, socioeconomic status, BMI at age 1975 and handedness62) in this analysis. These essential covariates were also used in the following analyses where applicable.
Developmental trajectory
We applied the NMF back-reconstruction algorithm to compute the activation of each network of each participant at age 14 (Method S6). Next, for boys and girls separately, we carried out repeated measures analyses of variance (ANOVAs) to investigate the developmental trajectories of the network activations. The age 14 and age 19 network activations were the within-subject variables. In addition to the essential covariates, we incorporated pubertal status as an additional covariate, considering the relationship between pubertal maturation and the reactivity of the brain’s emotion processing system during early adolescence76.
Moderation analysis
For boys and girls separately, associations were assessed by a linear regression model between emotional symptoms at age 19 and childhood trauma before age 19. Next, to identify the GRBMR, we examined the three-way interaction among PRSMDD, the activations of the above identified functional networks, and childhood trauma, in relation to emotional symptoms at age 19 in a linear regression model. In this model, we used 25 predictors, including 3 for main effects, 3 for two-way interaction effects, 1 for the three-way interaction effect, 7 dummy variables for research sites, 8 PCs for PRSMDD, BMI, handedness and socioeconomic status. The coefficients (standardised \(\beta \)) of the linear regression models and their 95% confidence intervals (CIs) are reported. A significant three-way interaction indicates that PRSMDD moderates the association between a higher level of this brain marker and fewer emotional symptoms following childhood trauma.
Sensitivity analyses
We tested whether the three-way interaction remained significant when the childhood trauma score was binarised using the following cut-offs as recommended in the literature77, including a cut-off of 8 for emotional abuse, 7 for physical abuse, and 5 for sexual abuse. If any type of the above abuse occurred, childhood exposure to trauma was scored as “1”; if not, a score of “0” was recorded. We also included age, childhood neglect, IQ or substance use as an additional covariate in the moderation models to examine their potential confounding effects. To investigate the specificity of the moderation effects, we reran the models while (1) replacing the emotional symptom scores with behavioural problem scores from the other four dimensions in the SDQ; (2) replacing the PRSMDD with the PRSADHD or the PRSSCZ.
Prediction models for late-adolescence emotional disorders
Using the GRBMRs identified in the three-way interaction analysis, we built prediction models for emotional disorders at age 19 for girls with higher and lower PRSMDD separately. To reduce the potential bias of using specific thresholds for partitioning genetic risk groups, we defined higher- and lower-risk groups using a range of cutoffs based on the PRSMDD distribution, including the median, the highest and lowest tail cut-offs (45%, 40%, 35%, 30%, 25%, 20%), and the mean ± standard deviation. The emotional disorders were indicated by an emotional symptom score above a clinical cut-off of 4, which has been recommended to favour the instrument’s (i.e., SDQ) sensitivity in identifying depression and generalised anxiety78. We considered the following three kinds of models: (1) The baseline model considered the following variables: childhood trauma, emotional symptom score, sites of data collection, handedness, pubertal status, socioeconomic status, and BMI. (2) A network model incorporated the activation of one brain network identified above into the baseline model. (3) A GRBMR model further included the interaction terms between the network activation and childhood trauma into a network model. These models were implemented using the Python package scikit-learn (version 1.3.2). To evaluate model performance, we repeated a 5-fold cross-validation 10 times to obtain the mean area under the curve (AUC). To assess the predictability of the GRBMR, the paired t test was used to test the significance of the difference in AUC between the GRBMR models and the network models, as well as between the network models and the baseline models.
Generalisability of the prediction models
Generalisability in early adulthood
Using the latest follow-up data at age 23 in the IMAGEN study, we tested the model performance among 256 girls. We applied the aforementioned trained models, without retraining (i.e., fixed weights), to see whether emotional disorders at age 23 can be predicted by the model using measurements at age 19.
Generalisability in an independent dataset
To test whether the GRBMR models could be generalised to an independent dataset, we used the data from the ABCD cohort (the ABCD data used in this study came from Data Release 5.0, https://doi.org/10.15154/8873-zj65) to rerun the prediction models. This independent dataset recruited 11,875 children between 9 and 10 years of age from 21 sites across the United States31. The negative > neutral activations during 0 back in the EN-back task79 were used. To ensure homogeneity of the datasets, only self-reported white people in the ABCD cohort were included. We applied the NMF back-reconstruction algorithm again to compute the activations of the functional networks for each participant. After quality control (the same as the IMAGEN cohort), 1478 participants with complete neuroimaging data, PRSMDD, adverse childhood experiences (ACEs)80, and the essential covariates at baseline, as well as the internalising symptoms of the Child Behaviour Checklist81 at both baseline and the 1-year follow-up were analysed. The emotional disorders were indicated by an internalising symptom t-score above a cut-off of 6082. Similarly, we first built the baseline model using the baseline measurements to predict emotional disorders at the 1-year follow-up for both the high and low genetic risk groups. Next, we added the network activation to construct the network model and further incorporated its interaction with ACEs to form the GRBMR model.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The IMAGEN data are available by application to the consortium coordinator, Dr. Schumann (http://imagen-europe.com), after evaluation according to an established procedure. The ABCD data are publicly released on an annual basis through the National Institute of Mental Health (NIMH) data archive (NDA, https://nda.nih.gov/abcd). The ABCD study data are openly available to qualified researchers for free. Access can be requested at https://nda.nih.gov/abcd/request-access. All the processed data used in this study are available from the corresponding author upon reasonable request. All data needed to evaluate the conclusions in this study are present in the paper and/ or the Supplementary Information. Source data are provided in this paper.
Code availability
The code used by the current study is made available at the following webpage: https://github.com/hanluyt/modulation_emotionalBrain.
References
Eaton, S., Cornwell, H., Hamilton-Giachritsis, C. & Fairchild, G. Resilience and young people’s brain structure, function and connectivity: A systematic review. Neurosci. Biobehav Rev. 132, 936–956 (2022).
Hillis, S., Mercy, J., Amobi, A. & Kress, H. Global prevalence of past-year violence against children: A systematic review and minimum estimates. Pediatrics 137, https://doi.org/10.1542/peds.2015-4079 (2016).
Kessler, R. C., Berglund, P. & Demler, O. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 593–602 (2005).
Fischer, A. S., Hagan, K. E. & Gotlib, I. H. Functional neuroimaging biomarkers of resilience in major depressive disorder. Curr. Opin. Psychiatry 34, 22–28 (2021).
Feder, A., Fred-Torres, S., Southwick, S. M. & Charney, D. S. The biology of human resilience: Opportunities for enhancing resilience across the life span. Biol. Psychiatry 86, 443–453 (2019).
Gee, D. G. Early adversity and development: Parsing heterogeneity and identifying pathways of risk and resilience. Am. J. Psychiatry 178, 998–1013 (2021).
Holz, N. E., Tost, H. & Meyer-Lindenberg, A. Resilience and the brain: a key role for regulatory circuits linked to social stress and support. Mol. Psychiatry 25, 379–396 (2020).
Jiang, Y. A theory of the neural mechanisms underlying negative cognitive bias in major depression. Front. Psychiatry 15, 1348474 (2024).
Heitzeg, M. M., Nigg, J. T., Yau, W. Y., Zubieta, J. K. & Zucker, R. A. Affective circuitry and risk for alcoholism in late adolescence: differences in frontostriatal responses between vulnerable and resilient children of alcoholic parents. Alcohol Clin. Exp. Res. 32, 414–426 (2008).
Nimarko, A. F., Garrett, A. S., Carlson, G. A. & Singh, M. K. Neural correlates of emotion processing predict resilience in youth at familial risk for mood disorders. Dev. Psychopathol. 31, 1037–1052 (2019).
Rodman, A. M., Jenness, J. L., Weissman, D. G., Pine, D. S. & McLaughlin, K. A. Neurobiological markers of resilience to depression following childhood maltreatment: The role of neural circuits supporting the cognitive control of emotion. Biol. Psychiatry 86, 464–473 (2019).
Wiggins, J. L. et al. Neural markers in pediatric bipolar disorder and familial risk for bipolar disorder. J. Am. Acad. Child Adolesc. Psychiatry 56, 67–78 (2017).
Nestler, E. J. & Russo, S. J. Neurobiological basis of stress resilience. Neuron 112, 1911–1929 (2024).
Wymbs, N. F. et al. Social supports moderate the effects of child adversity on neural correlates of threat processing. Child Abuse Negl. 102, 104413 (2020).
Kaldewaij, R. et al. Anterior prefrontal brain activity during emotion control predicts resilience to post-traumatic stress symptoms. Nat. Hum. Behav. 5, 1055–1064 (2021).
Norbury, A., Seeley, S. H., Perez-Rodriguez, M. M. & Feder, A. Functional neuroimaging of resilience to trauma: convergent evidence and challenges for future research. Psychol. Med. 53, 3293–3305 (2023).
Yamamoto, T. et al. Increased amygdala reactivity following early life stress: a potential resilience enhancer role. BMC Psychiatry 17, 27 (2017).
Pessoa, L. Understanding emotion with brain networks. Curr. Opin. Behav. Sci. 19, 19–25 (2018).
Wang, S. et al. Sex-linked neurofunctional basis of psychological resilience in late adolescence: a resting-state functional magnetic resonance imaging study. Eur. Child Adolesc. Psychiatry 29, 1075–1087 (2020).
Pan, N. et al. Sex differences in the relationship between brain gray matter volume and psychological resilience in late adolescence. Eur. Child Adolesc. Psychiatry 33, 1057–1066 (2024).
Cornwell, H. et al. Identifying structural brain markers of resilience to adversity in young people using voxel-based morphometry. Dev. Psychopathol. 35, 2302–2314 (2023).
Dorfschmidt, L. et al. Sexually divergent development of depression-related brain networks during healthy human adolescence. Sci. Adv. 8, eabm7825 (2022).
Pine, D. S., Cohen, E., Cohen, P. & Brook, J. Adolescent depressive symptoms as predictors of adult depression: moodiness or mood disorder?. Am. J. Psychiatry 156, 133–135 (1999).
Wilson, S., Vaidyanathan, U., Miller, M. B., McGue, M. & Iacono, W. G. Premorbid risk factors for major depressive disorder: are they associated with early onset and recurrent course?. Dev. Psychopathol. 26, 1477–1493 (2014).
Zubin, J. & Spring, B. Vulnerability: A new view of schizophrenia. J. Abnorm. Psychol. 86, 103–126 (1977).
Alexander, N. et al. Interaction of the serotonin transporter-linked polymorphic region and environmental adversity: increased amygdala-hypothalamus connectivity as a potential mechanism linking neural and endocrine hyperreactivity. Biol. Psychiatry 72, 49–56 (2012).
Thomas-Odenthal, F. et al. Neural foundation of the diathesis-stress model: longitudinal gray matter volume changes in response to stressful life events in major depressive disorder and healthy controls. Mol. Psychiatry 29, 2724–2732 (2024).
Barbour, T. et al. Elevated amygdala activity in young adults with familial risk for depression: A potential marker of low resilience. Biol. Psychiatry. Cogn. Neurosci. Neuroimaging 5, 194–202 (2020).
Peyrot, W. J. et al. Effect of polygenic risk scores on depression in childhood trauma. Br. J. Psychiatry 205, 113–119 (2014).
Schumann, G. et al. The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol. Psychiatry 15, 1128–1139 (2010).
Casey, B. J., et al. The adolescent brain. Ann. N. Y. Acad. Sci. 32, 43–54 (2018).
Yarkoni, T., Poldrack, R. A., Nichols, T. E., Van Essen, D. C. & Wager, T. D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods 8, 665–670 (2011).
Shapiro, S. S. & Wilk, M. B. An analysis of variance test for normality (Complete Samples). Biometrika 52, 591–611 (1965).
Barch, D. M. et al. Function in the human connectome: task-fMRI and individual differences in behavior. Neuroimage 80, 169–189 (2013).
Rolls, E. T. Two what, two where, visual cortical streams in humans. Neurosci. Biobehav. Rev. 160, 105650 (2024).
Birnie, M. T. & Baram, T. Z. Principles of emotional brain circuit maturation. Science 376, 1055–1056 (2022).
Grabenhorst, F. & Rolls, E. T. Value, pleasure and choice in the ventral prefrontal cortex. Trends Cogn. Sci. 15, 56–67 (2011).
Rolls, E. T., Deco, G., Huang, C. C. & Feng, J. The human orbitofrontal cortex, vmPFC, and anterior cingulate cortex effective connectome: emotion, memory, and action. Cereb. Cortex 33, 330–356 (2022).
Rolls, E. T. The orbitofrontal cortex and emotion in health and disease, including depression. Neuropsychologia 128, 14–43 (2019).
Rolls, E. T. Emotion, motivation, decision-making, the orbitofrontal cortex, anterior cingulate cortex, and the amygdala. Brain Struct. Funct. 228, 1201–1257 (2023).
Rolls, E. T., Cheng, W. & Feng, J. The orbitofrontal cortex: reward, emotion and depression. Brain Commun. 2, fcaa196 (2020).
Peng, S., Xu, P., Jiang, Y. & Gong, G. Activation network mapping for integration of heterogeneous fMRI findings. Nat. Hum. Behav. 6, 1417–1429 (2022).
Crone, E. A. & Dahl, R. E. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat. Rev. Neurosci. 13, 636–650 (2012).
Grill-Spector, K., Golarai, G. & Gabrieli, J. Developmental neuroimaging of the human ventral visual cortex. Trends Cogn. Sci. 12, 152–162 (2008).
Lowe, S. R. Embracing complexity in resilience research. Nat. Ment. Health 3, 391–392 (2025).
Matosin, N., Halldorsdottir, T. & Binder, E. B. Understanding the molecular mechanisms underpinning gene by environment interactions in psychiatric disorders: The FKBP5 model. Biol. Psychiatry 83, 821–830 (2018).
Callaghan, B. L. & Tottenham, N. The stress acceleration hypothesis: Effects of early-life adversity on emotion circuits and behavior. Curr. Opin. Behav. Sci. 7, 76–81 (2016).
Mary, A. et al. Resilience after trauma: The role of memory suppression. Science 367, https://doi.org/10.1126/science.aay8477 (2020).
Han, S. et al. Orbitofrontal cortex-hippocampus potentiation mediates relief for depression: A randomized double-blind trial and TMS-EEG study. Cell Rep. Med. 4, 101060 (2023).
Buckner, R. L. The brain’s default network: origins and implications for the study of psychosis. Dialogues Clin. Neurosci. 15, 351–358 (2013).
Tian, T. et al. Default mode network alterations induced by childhood trauma correlate with emotional function and SLC6A4 expression. Front. Psychiatry 12, 760411 (2021).
Willmore, L., Cameron, C., Yang, J., Witten, I. B. & Falkner, A. L. Behavioural and dopaminergic signatures of resilience. Nature 611, 124–132 (2022).
Rutter, M., Caspi, A., Fau, -, Moffitt, T. E. & Moffitt, T. E. Using sex differences in psychopathology to study causal mechanisms: unifying issues and research strategies. J. Child Psychol. Psychiatry 44, 1092–1115 (2003).
Malhi, G. S., Das, P., Bell, E., Mattingly, G. & Mannie, Z. Modelling resilience in adolescence and adversity: a novel framework to inform research and practice. Transl. Psychiatry 9, 316 (2019).
Seney, M. L., Glausier, J. & Sibille, E. Large-scale transcriptomics studies provide insight into sex differences in depression. Biol. Psychiatry 91, 14–24 (2022).
Issler, O. et al. Sex-specific role for the long non-coding RNA LINC00473 in depression. Neuron 106, 912–926 (2020).
Scheinost, D. et al. Orbitofrontal cortex neurofeedback produces lasting changes in contamination anxiety and resting-state connectivity. Transl. Psychiatry 3, e250 (2013).
Herwig, U. et al. Training emotion regulation through real-time fMRI neurofeedback of amygdala activity. Neuroimage 184, 687–696 (2019).
Tschentscher, N. et al. The clinical impact of real-time fMRI Neurofeedback on emotion regulation: A systematic review. Brain Sci. 14, https://doi.org/10.3390/brainsci14070700 (2024).
Levine, S. Psychological and social aspects of resilience: a synthesis of risks and resources. Dialogues Clin. Neurosci. 5, 273–280 (2003).
Miller-Graff, L. E. The multidimensional taxonomy of individual resilience. Trauma Violence Abus. 23, 660–675 (2022).
Xie, C. et al. A shared neural basis underlying psychiatric comorbidity. Nat. Med. 29, 1232–1242 (2023).
Goodman, R. The strengths and difficulties questionnaire: A Rsearch Note. J. Child Psychol. Psychiatry 38, 581–586 (1997).
Becker, A., Hagenberg, N., Roessner, V., Woerner, W. & Rothenberger, A. Evaluation of the self-reported SDQ in a clinical setting: do self-reports tell us more than ratings by adult informants?. Eur. Child Adolesc. Psychiatry 13, II17–II24 (2004).
Bernstein, D. P., Ahluvalia, T., Pogge, D. & Handelsman, L. Validity of the childhood trauma questionnaire in an adolescent psychiatric population. J. Am. Acad. Child Adolesc. Psychiatry 36, 340–348 (1997).
Luo, Q. et al. Association of a schizophrenia-risk nonsynonymous variant with putamen volume in adolescents: A voxelwise and genome-wide association study. JAMA Psychiatry 76, 435–445 (2019).
Wray, N. R. et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 50, 668–681 (2018).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, Washington, DC) (1994).
World Health Organization. International Classification of Diseases. (1978).
World Health Organization. International Classification of Diseases.(1992).
Grosbras, M. H. & Paus, T. Brain networks involved in viewing angry hands or faces. Cereb. Cortex 16, 1087–1096 (2006).
Rolls, E. T., Joliot, M. & Tzourio-Mazoyer, N. Implementation of a new parcellation of the orbitofrontal cortex in the automated anatomical labeling atlas. Neuroimage 122, 1–5 (2015).
Peharz, R. & Pernkopf, F. Sparse nonnegative matrix factorization with l0-constraints. Neurocomputing 80, 38–46 (2012).
Shan, X. et al. Mapping the heterogeneous brain structural phenotype of Autism Spectrum disorder using the normative model. Biol. Psychiatry 91, 967–976 (2022).
Sahle, B. W. et al. Association between depression, anxiety and weight change in young adults. BMC Psychiatry 19, 398 (2019).
Forbes, E. E., Phillips, M. L., Silk, J. S., Ryan, N. D. & Dahl, R. E. Neural systems of threat processing in adolescents: role of pubertal maturation and relation to measures of negative affect. Dev. Neuropsychol. 36, 429–452 (2011).
Bernstein, D., Fink, L. & Bernstein, D. Childhood Trauma Questionnaire: A Retrospective Self-Report Manual. (1998).
Fleitlich, B., Cortázar, P. G. & Goodman, R. Questionário de capacidades e dificuldades (SDQ). Infanto 8, 44–50 (2000).
Cohen, A. O. et al. When is an adolescent an adult? Assessing cognitive control in emotional and nonemotional contexts. Psychol. Sci. 27, 549–562 (2016).
Hoffman, E. A. et al. Stress exposures, neurodevelopment and health measures in the ABCD study. Neurobiol. Stress 10, 100157 (2019).
Achenbach, T. M. The Achenbach system of empirically based assessment (ASEBA): Development, findings, theory, and applications. (University of Vermont, Research Center for Children, Youth, & Families, 2009).
Pandolfi, V., Magyar, C. I. & Dill, C. A. An initial psychometric evaluation of the CBCL 6-18 in a sample of youth with Autism Spectrum disorders. Res. Autism Spectr. Disord. 6, 96–108 (2012).
Acknowledgements
This study was partially supported by grants from the National Key Research and Development Programme of China (No. 2023YFE0109700), the National Natural Science Foundation of China (No. 82272079 and 32441107), the Programme of Shanghai Academic Research Leader (No. 23XD1423400), and the Shanghai Municipal Science and Technology Major Project (No.s: 2018SHZDZX01 and 2021SHZDZX0103). This work also received support from the following sources: the European Union-funded FP6 Integrated Project IMAGEN (Reinforcement-related behaviour in normal brain function and psychopathology) (LSHM-CT- 2007-037286), the Horizon 2020 funded ERC Advanced Grant ‘STRATIFY’ (Brain network based stratification of reinforcement-related disorders) (695313), Human Brain Project (HBP SGA 2, 785907, and HBP SGA 3, 945539), the Medical Research Council Grant ‘c-VEDA’ (Consortium on Vulnerability to Externalising Disorders and Addictions) (MR/N000390/1), the National Institute of Health (NIH) (R01DA049238, A decentralised macro and micro gene-by-environment interaction analysis of substance use behaviour and its brain biomarkers), the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, the Bundesministeriumfür Bildung und Forschung (BMBF grants 01GS08152; 01EV0711; Forschungsnetz AERIAL 01EE1406A, 01EE1406B; Forschungsnetz IMAC-Mind 01GL1745B), the Deutsche Forschungsgemeinschaft (DFG grants SM 80/7-2, SFB 940, TRR 265, NE 1383/14-1), the Medical Research Foundation and Medical Research Council (grants MR/R00465X/1 and MR/S020306/1), the National Institutes of Health (NIH) funded ENIGMA (grants 5U54EB020403-05 and 1R56AG058854-01), NSFC grant 82150710554 and European Union funded project ‘environMENTAL’, grant no: 101057429. Further support was provided by grants from: - the ANR (ANR-12-SAMA-0004, AAPG2019 - GeBra), the Eranet Neuron (AF12-NEUR0008-01 - WM2NA; and ANR-18-NEUR00002-01-ADORe), the Fondation de France (00081242), the Fondation pour la Recherche Médicale (DPA20140629802), the Mission Interministérielle de Lutte-contre-les-Drogues-et-les-Conduites-Addictives (MILDECA), the Assistance-Publique-Hôpitaux-de-Paris and INSERM (interface grant), Paris Sud University IDEX 2012, the Fondation de l’Avenir (grant AP-RM-17-013), the Fédération pour la Recherche sur le Cerveau; the National Institutes of Health, Science Foundation Ireland (16/ERCD/3797), U.S.A. (Axon, Testosterone and Mental Health during Adolescence; RO1 MH085772-01A1) and by NIH Consortium grant U54 EB020403, supported by a cross-NIH alliance that funds Big Data to Knowledge Centres of Excellence. The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, and U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/ consortium_members/. ABCD consortium investigators designed and implemented the study and/or provided data, but did not necessarily participate in the analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from the Data Release 5.0 (https://doi.org/10.15154/8873-zj65).
Author information
Authors and Affiliations
Consortia
Contributions
Q.L. has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. H.L. and H.J.L. designed and implemented the experiments. H.L., E.T.R. and Q.L. wrote the manuscript. T.J., C.X. and S.X. conducted data analysis. Q.L. and G.S. secured funding. D.J.S., B.J.S., R.E., N.W. and J.F. provided expertise and feedback. T.B., A.L.W.B., S.D., H.F., A.G., H.G., A.H., R.B., J.-L.M., M.-L.P.M., E.A., F.N., D.P.O., H.L., L.P., S.H., N.H., J.H.F., M.N.S., N.V., H.W., R.W. and G.S. were the principal investigators of the IMAGEN Consortium; Imaging, genetic and behavioural data in the IMAGEN project were acquired and provided by the IMAGEN Consortium.
Corresponding author
Ethics declarations
Competing interests
Dr Banaschewski served in an advisory or consultancy role for eye level, Infectopharm, Lundbeck, Medice, Neurim Pharmaceuticals, Oberberg GmbH, Roche, and Takeda. He received conference support or a speaker’s fee by Janssen, Medice and Takeda. He received royalties from Hogrefe, Kohlhammer, CIP Medien, Oxford University Press; the present work is unrelated to these relationships. Dr Poustka served in an advisory or consultancy role for Roche and Viforpharm and received speaker’s fee by Shire. She received royalties from Hogrefe, Kohlhammer and Schattauer. The present work is unrelated to the above grants and relationships. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Michael Pluess, Gabriela Suarez and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lu, H., Rolls, E.T., Liu, H. et al. Genetic risk-dependent brain markers of resilience to childhood Trauma. Nat Commun 16, 6219 (2025). https://doi.org/10.1038/s41467-025-61471-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-61471-0
This article is cited by
-
Resilience in management of chronic diseases: a review of the strategies, approaches, and interventions
Discover Public Health (2025)