Abstract
The design and screening of sgRNA in CRISPR-dependent gene knock-in is always laborious. Therefore, a universal and highly efficient knock-in strategy suitable for different sgRNA target sites is necessary. In our mouse embryo study, we find that the knock-in efficiency guided by adjacent sgRNAs varies greatly, although similar indel frequency. MMEJ-biased sgRNAs usually lead to high knock-in efficiency, whereas NHEJ-biased sgRNAs result in low knock-in efficiency. Blocking MMEJ repair by knocking down Polq can enhance knock-in efficiency, but inhibiting NHEJ repair shows variable effects. We identify a compound, AZD7648, that can shift DSBs repair towards MMEJ. Finally, by combining AZD7648 treatment with Polq knockdown, we develop a universal and highly efficient knock-in strategy in mouse embryos. This approach is validated at more than ten genomic loci, achieving up to 90% knock-in efficiency, marking a significant advancement toward predictable and highly efficient CRISPR-mediated gene integration.
Similar content being viewed by others
Introduction
Genome editing in zygotes by CRISPR/Cas9 system is a convenient approach for germline modification, facilitating the creation of animal disease models and potentially enabling genome editing therapies in human embryos for incurable diseases1,2. Cas9-induced DNA double-strand breaks (DSBs) are repaired through multiple DNA repair pathways, namely non-homologous end joining (NHEJ)3,4,5, microhomology-mediated end joining (MMEJ)6, and homology-directed repair (HDR)7,8. The ability to create precise gene knock-in at target loci primarily depends on HDR repair. However, achieving efficient HDR in mammalian cells is often challenging9. Cultured cells allow for the isolation of edited clones via drug selection or flow cytometry, a feature lacking in embryos. Mammalian embryos represent a limited and highly valuable resource compared to cultured cells, underscoring the critical importance of enhancing gene knock-in efficiency. Enhancing HDR efficiency in embryos is thus a pressing issue in the field of genetic engineering and developmental biology.
Recent studies have enhanced our understanding of the indel outcomes resulting from the repair pathways of CRISPR/Cas9-induced DSBs. It has been proved that specific sgRNAs induce regular patterns in different cell lines10,11,12,13,14,15,16. Inhibition of key components in the NHEJ or MMEJ pathways suggests that the regular indel profiles at certain target sites likely arise from the concurrent engagement of both the NHEJ and MMEJ repair mechanisms10,15,16,17,18, demonstrating these repair pathways are mutually exclusive and mechanistically distinct. To improve HDR rates, various methods have been proposed, including strategies to circumvent cell cycle restrictions of HDR and to modulate key components of the NHEJ or MMEJ pathways9,19,20,21,22,23,24,25. Notably, DNA-PKcs, a key regulator of NHEJ, is targeted pharmacologically to promote HDR. Among the inhibitors developed, AZD7648, a highly potent and selective DNA-PKcs inhibitor, gains considerable attention for its ability to significantly enhance HDR efficiency in both transformed cell lines and primary human cells26,27,28. Other DNA-PKcs inhibitors, such as M3814, are also reported to influence repair pathway choice, though with varying specificity and effects on cellular toxicity28,29. Additionally, DNA polymerase theta (Polθ) plays a crucial role in MMEJ30, and recent studies demonstrate that combined inhibition of DNA-PK and Polθ not only enhances HDR efficiency and improves precise genome integration but also mitigates the large DNA deletions29,31. However, these studies are conducted exclusively in cell lines, and the mechanisms governing DNA repair in mammalian embryos remain largely unexplored.
During CRISPR-based genome editing, the design and selection of sgRNA is a necessary procedure. Usually, sgRNAs are selected based on their “cutting efficiency”, which is determined by indel frequency32,33. However, consist with previous studies34, our study reveals that knock-in efficiency varies significantly even among adjacent sgRNAs, despite similar indel rates. Moreover, effective HDR remains challenging in many genome loci, even when sgRNAs exhibit high indel frequency. Considering that rare sgRNA candidates could be designed in some specific HDR scenarios35,36, developing a universal and efficient HDR method independent of sgRNA screening is valuable.
To address this limitation, we systematically analyze DNA repair outcomes across multiple genomic loci in mouse embryos. We discover that, the repair pattern of sgRNAs biased toward MMEJ results in higher knock-in efficiency, whereas sgRNA biased toward NHEJ are associated with reduced knock-in efficiency. In our previous study, we develop a methodology (CATI) in which Polq silencing via the RNA editor CasRx significantly enhances HDR-mediated DNA integration37. However, blocking MMEJ repair by Polq knockdown selectively improves the knock-in efficiency of MMEJ-biased sgRNAs, while having little impact on NHEJ-biased sgRNAs. Through compound screening, we identify AZD7648, which re-orients NHEJ repair toward MMEJ repair. Based on this finding, we develop ChemiCATI, a broadly applicable knock-in strategy, by combining AZD7648 treatment and Polq knockdown. This strategy is validated at more than ten genomic loci, achieving knock-in efficiencies up to 90%, marking a significant advancement toward predictable and highly efficient CRISPR-mediated gene integration.
Results
Knock-in efficiency of dsDNA donor positively correlates with MMEJ repair patterns in mouse embryos
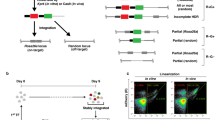
Prior research has indicated that distinct target sites manifest specific sequence repair patterns, and these repair patterns may exert significant influences on gene knock-in efficiency34. To investigate the relationship between repair mode and gene knock-in, we conducted a comprehensive analysis of DNA repair patterns at various sgRNA-targeted sites. Integrating the comparison of previous classification methods and our indel data obtained in embryos15,16,34,38, we categorized the DNA repair pattern initiated by CRISPR/Cas9 into three distinct types: wild-type (WT), i.e. uncut or precisely repaired; NHEJ repair pattern (≥ 1 bp insertions; deletions without microhomology arm (MH); deletions with 1 bp MH), and MMEJ repair pattern (deletions with ≥ 2 bp MH) (Supplementary Fig. 1A). To minimize distractions, such as chromatin structure, we selected three sgRNAs for each of the Actb and Cdx2 loci, respectively, within 90 bp which shorter than the length of DNA entangling nucleosome (~147 bp) (Fig. 1A). Our observations indicated that all tested sgRNAs demonstrated high indel frequency, exceeding 70% (Fig. 1B, C). We conducted a detailed analysis of the repair outcomes associated with these sgRNAs using ICE analysis and observed that each sgRNA exhibits a distinct ratio of NHEJ to MMEJ repair patterns (Fig. 1D, Supplementary Fig. 1B). Subsequently, we designed donors for each sgRNA and performed micro-injections of knock-in mixtures into zygotes for mCherry CDS (~800 bp) integration (Fig. 1E, Supplementary Fig. 1C). Intriguingly, we observed a notable negative correlation between the knock-in efficiency (ratio of mCherry-positive embryos) and the NHEJ/MMEJ (hereafter referred to as N/M) ratio. For instance, Actb-sgRNA3 (N/M = 1.00) and Cdx2-sgRNA6 (N/M = 0.23) exhibited relatively higher knock-in efficiencies. In contrast, sgRNAs with higher N/M ratios, such as Actb-sgRNA7 (N/M = 2.07) and Cdx2-sgRNA3 (N/M = 2.38), demonstrated the lowest level of mCherry integration, not only in 1-cell but also in 2-cell injections at both the Actb and Cdx2 sites (Fig. 1F, Supplementary Fig. 1D).
A Schematic of three sgRNAs targeting the mouse Actb and Cdx2 loci. “Stop” marks the stop codon. “d” indicates the distance (in bp) from the sgRNA cut site (blue triangle) to the donor DNA insertion site (green triangle), as shown. B Workflow of CRISPR reagent injection and embryo repair pattern analysis. Zygote, blastocyst, and CRISPR icons were created in BioRender. Hongyu, C. (2025) https://BioRender.com/qxdvotz. C Indel frequencies for different sgRNAs at Actb and Cdx2 loci. Each dot represents a blastocyst; n values and exact p-value are shown. Data represent mean ± SEM. Two-sided Kruskal-Wallis test was used. D Pie charts show DNA repair pattern distributions and NHEJ/MMEJ ratios per sgRNA. E Schematic of the dsDNA knock-in strategy. Figure was partially created in BioRender. Hongyu, C. (2025) https://BioRender.com/qxdvotz. F Representative fluorescence images and quantification of mCherry knock-in at Actb and Cdx2 loci (scale bars, 100 μm). Each dot: n = 3 experiments with > 20 embryos per condition. Bars: mean ± SEM. Two-sided unpaired t-tests; p-value in figure. G Correlation between knock-in efficiency and indel/MMEJ/NHEJ repair ratios. Each dot: one sgRNA. Red line: linear regression. R² and p-value shown in figure. Full statistics in Source Data. Source data are provided as a Source Data file.
To further investigate the general principles that dictate how sgRNA repair patterns affect gene knock-in efficiency, we administered injections of 11 sgRNAs targeting 9 loci, along with mCherry donors, into embryos. Subsequently, upon analysis of the repair patterns, we observed a positive correlation between gene knock-in efficiency and the level of indels (Fig. 1G). This finding aligns with previous research indicating that a higher indel frequency is conducive to enhancing knock-in frequency39. Furthermore, we endeavored to evaluate the correlation between knock-in levels and MMEJ or NHEJ repair patterns. The repair pattern profile of each sgRNA was analyzed in the absence of the mCherry donor (Supplementary Fig. 2). To ensure the reliability of the ICE-based repair pattern analysis, we compared its results with those obtained from deep sequencing (deep-seq). The consistency between ICE and deep-seq outcomes supports the validity of ICE as an effective tool for repair profile assessment (Supplementary Fig. 3). Remarkably, we observed a slightly stronger correlation between MMEJ repair patterns and gene knock-in events (R2 = 0.258) compared to that of NHEJ repair patterns and knock-in events (R2 = 0.003) (Fig. 1G). Our findings suggest that the efficiency of double-stranded DNA (dsDNA) knock-in is predominantly influenced by the MMEJ repair pattern.
The ssDNA donor-mediated targeted method also exhibits higher knock-in efficiency with MMEJ-biased sgRNA
To date, both dsDNA and ssDNA (single-stranded DNA) can serve as exogenous DNA templates in knock-in processes. The ssDNA-mediated knock-in strategy is ideal for evaluating precise knock-in efficiency in embryos, due to its convenience in PCR amplification and sequencing. Thus, we decided to perform ssDNA-mediated knock-in assay to confirm the relationship between MMEJ repair pattern and knock-in efficiency. First, we selected two human pathogenic mutations, A4V and G93A, in the Sod1 gene, which result in a progressive neurodegenerative disease—amyotrophic lateral sclerosis (ALS)—as targets in mouse embryos40. Similar to dsDNA-mediated knock-in, we designed three sgRNAs within a 70 bp surrounding the G93 and A4 sites, respectively (Fig. 2A), and assessed their indel frequency (Fig. 2B) as well as DNA repair patterns (Fig. 2C, Supplementary Fig. 4A). In order to minimize the impact of the spatial proximity between cleavage sites and modification sites on knock-in efficiency, all sgRNAs cleavage sites were confined to a distance of no more than 20 base pairs. Most selected sgRNAs for each of the G93 and A4 sites displayed similar editing capacity to evaluate their gene knock-in level (Fig. 2B). We observed that the presence of ssDNA led to a diminished total indel frequency and MMEJ-mediated indel frequency compared to experiments conducted without ssDNA (Supplementary Fig. 4B, C). This observation is consistent with the competitive relationship between HDR and MMEJ, as evidenced by previous findings in dsDNA-donor experiments38. Drawing from these findings, we hypothesize that the knock-in process entails a transition from MMEJ to HDR.
A Schematic of three sgRNAs targeting the mouse Sod1-A4 and Sod1-G93 loci. “Stop” marks the stop codon. “d” indicates the distance (in bp) from the sgRNA cut site (blue triangle) to the donor DNA insertion site (green triangle), as shown. B Indel frequencies at sgRNA target sites within the Sod1-A4 and Sod1-G93 loci. Each dot represents a single blastocyst from three independent experiments. Statistical significance was determined using a two-sided Kruskal–Wallis test. C The pie chart illustrates the distribution of DNA repair pattern (N/M = NHEJ: MMEJ). D A schematic representation of CRISPR/Cas9-mediated ssDNA gene knock-in. E Percentage of gene knock-in embryos out of the total embryos using different sgRNAs. F HDR repair proportions per knock-in embryo. Fractions above each group show HDR-positive over total injected embryos (n/N). G Distribution of DNA repair outcomes (HDR, random indels, WT) across all blastocysts analyzed in (F), summarized per sgRNA to show overall repair profiles. H A schematic representation of the classification criteria for different sgRNAs. I The “N/M” ratio of each indicated sgRNA. J Percentage of gene knock-in embryos out of the total embryos across sgRNAs categorized as MMEJ-Bias or NHEJ-Bias. K HDR repair proportion per gene knock-in embryo at indicated loci for different sgRNAs. L Correlation between knock-in efficiency and indel/MMEJ/NHEJ repair proportions (without donor). Each dot represents one sgRNA. R² and p-value indicate correlation strength; A linear regression line is overlaid in red. M Schematic of Cas9-induced DSB repair outcomes. Without donor: NHEJ and MMEJ; with donor: NHEJ, MMEJ, and HDR (facilitated by MMEJ). E, J Each dot represents one independent experiment (n = 3, > 10 embryos per group). F, K Each dot represents a single blastocyst from three independent experiments. Statistical significance was assessed using unpaired two-sided t-tests (E, J) and two-sided Mann–Whitney U tests (F, K). B, F, K Red bars or dashed lines indicate the mean ± SEM. D, M CRISPR, and DNA icons were created with BioRender. Created in BioRender. Hongyu, C. (2025) https://BioRender.com/qxdvotz. Other elements were designed by the authors. Sample sizes and exact p-value are shown in the figure. Source data are provided as a Source Data file.
The sgRNAs with lower N/M ratios (A4-sgRNA5, N/M = 0.21; G93-sgRNA2, N/M = 0.47) exhibited higher gene knock-in efficiency, as indicated by the proportion of knock-in embryos among all injected embryos (Fig. 2D, E). Among these knock-in embryos, sgRNAs showed a greater proportion of HDR-mediated repair events relative to other repair patterns (Fig. 2F, G), which is consistent with observations made using dsDNA donors. To provide a more precise delineation of the repair pattern characteristics of each sgRNA, we classified the sgRNAs based on the proportional representation of different repair outcomes. The categorization of repair patterns was defined as follows: NHEJ-bias (N/M ratio ≥ 1.2), MMEJ-bias (N/M ratio < 0.8), and NHEJ/MMEJ-balance (0.8 ≤ N/M ratio < 1.2) (Fig. 2H). Among six additional tested gene loci, MMEJ-biased sgRNAs (Dnmt1-sgRNA2, Eif4g1-sgRNA3, Fbp1-sgRNA1) exhibited significantly higher knock-in efficiency and a greater proportion of HDR repair pattern within knock-in embryos, compared to NHEJ-biased sgRNAs (Mapre2-sgRNA1, Npm2-sgRNA2, Map1b-sgRNA2) (Fig. 2I–K).
Furthermore, we examined 12 sgRNAs targeting 9 genomic loci for knock-in using ssDNA templates. As expected, we observed that an increased proportion of MMEJ repair patterns correlates with higher knock-in efficiency (R2 = 0.681), while NHEJ events negatively correlate with the knock-in level (R2 = 0.452) (Fig. 2L). These results indicate that the MMEJ repair pattern is beneficial to the efficiency of gene knock-in (Fig. 2M), further supporting a positive correlation between successful gene knock-in and MMEJ repair pattern in embryos.
Knock-in efficiency could be largely improved by inhibiting MMEJ repair pathways in mouse embryos
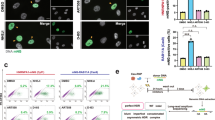
When DSBs occur, the NHEJ, MMEJ, and HDR repair pathways can compete. There are key factors that regulate each pathway (Fig. 3A)41. According to previous studies, we plan to knock down or inhibit the activity of the key regulatory factors of the NHEJ (e.g., Ku70/80, Ligase IV, DNA-PKcs, and Poll)9,41,42,43 or MMEJ pathways (e.g., Parp1, Polq)37,44 and tested the HDR efficiency mediated by each sgRNA (Fig. 3B, C, Supplementary Fig. 2). Our data have suggested that the efficiency of long dsDNA gene knock-in is generally lower than that of short ssDNA (Figs. 1G, 2L). Therefore, we evaluated the efficiency based on dsDNA knock-in using different methods in following tests. Besides, our data have demonstrated significant positive correlations between MMEJ and knock-in enhancement. This observation suggests a potential transition from MMEJ to HDR in repair patterns during knock-in procedures. However, significant MMEJ activity persists following knock-in, indicating ongoing competition between MMEJ and HDR mechanisms (Supplementary Fig. 4C). Therefore, it is assumed that blocking MMEJ could further facilitate the transition from MMEJ to HDR, thereby potentially augmenting knock-in efficiency. In our prior research, we developed a methodology (CATI) wherein silencing Polq expression via the RNA editor CasRx resulted in a significant enhancement of HDR-mediated DNA integration efficiency37. Given that RNA editors like CasRx have been reported to exhibit collateral activity45, potentially affecting the embryonic transcriptome, we assessed transcriptome-wide changes in Polq knockdown (KD-Polq) embryos compared to controls. Our analysis confirmed that CasRx-mediated Polq knockdown did not induce significant transcriptomic alterations, ensuring that the observed knock-in effects were due to Polq silencing rather than off-target transcriptome disruptions (Supplementary Fig. 5A–D). This is likely because Polq expression levels in embryos were relatively low (FPKM < 10)46, which is insufficient to trigger CasRx’s collateral activity (Supplementary Fig. 5E). However, we found that KD-Polq cannot improve knock-in efficiency at all sgRNA targeting sites, even with high indel frequency37. In this study, we found that KD-Polq significantly enhances knock-in efficiency for sgRNAs biased toward MMEJ, with minimal impact on those biased toward NHEJ. For NHEJ/MMEJ-balanced sgRNAs, KD-Polq partially enhanced knock-in efficiency (Fig. 3D, E, Supplementary Fig. 6). As widely documented, genes involved in MMEJ, aside from Polq, have been extensively characterized47,48. In pursuit of alternative target genes to facilitate an efficient MMEJ-to-HDR transition, we conducted CasRx-mediated knockdown experiments targeting Parp1. However, knockdown of Parp1 failed to enhance HDR efficiency and, in certain instances, led to impaired development of the blastocyst (Supplementary Fig. 7). Our findings suggest that KD-Polq is effective in improving knock-in efficiency with MMEJ-biased sgRNAs, owing to its capacity to facilitate the transition from MMEJ to HDR in the presence of a donor template.
A Schematic of Cas9-induced DSB repair outcomes with ssDNA or dsDNA donors. Key NHEJ regulators include Ku70/80, Ligase IV, DNA-PKcs, and Poll; MMEJ is regulated by Parp1 and Polq. B Strategy to inhibit NHEJ or MMEJ to promote HDR. C N/M ratios (NHEJ: MMEJ) of each sgRNA. D, F, H Relative knock-in efficiency of mCherry under control (KD-Ctrl), Polq knockdown (KD-Polq), Ku70 knockdown (KD-Ku70), or NU7441 treatment at indicated loci. Each dot represents one independent experiment (n = 3, >20 blastocysts per group). Bars indicate the mean ± SEM. E, G, I Knock-in efficiencies across sgRNAs classified as MMEJ-Bias, NHEJ/MMEJ-Balance, or NHEJ-Bias (as defined in D, F, H). Each dot represents one independent experiment (n = 6–12, as indicated). Box plots show the interquartile range, median (center line), and whiskers extend to min and max. J Schematic conclusion showing that only Polq knockdown significantly enhanced HDR efficiency, whereas NHEJ inhibition strategies did not. Statistical significance was assessed using unpaired two-sided t-tests (D–I) or Mann–Whitney U tests where appropriate (E, H). Sample sizes and exact p-values are indicated in the figure. Schematic elements in the panels were created with BioRender (Created in BioRender. Hongyu, C. (2025) https://BioRender.com/qxdvotz). A, B, J; all other elements were designed by the authors. Source data are provided as a Source Data file.
It is well-known that NHEJ repair predominates and plays a significant role in mammalian cell, with many previous studies aiming to enhance HDR efficiency by inhibiting the NHEJ pathway. While numerous strategies have successfully improved HDR efficiency in specific cell types or at particular loci, these enhancements often fail to be replicated across different systems9,49,50,51. Subsequently, we investigated the effect of inhibiting the NHEJ pathway by knocking down the critical protein Ku709. Our results indicate that Ku70 knockdown marginally enhanced knock-in levels for NHEJ-biased sgRNAs (p = 0.0932). Furthermore, Ku70 reduction had little impact on the knock-in efficiency for MMEJ-biased (p = 0.735) and NHEJ/MMEJ-balanced (p = 0.995) sgRNA target sites (Fig. 3F, G, Supplementary Fig. 8). Similarly, inhibiting DNA-PKcs activity with the small-molecule inhibitor NU7441 resulted in a modest increase in knock-in efficiency (Fig. 3H, I, Supplementary Fig. 9). Additionally, knocking down the Poll using CasRx or inhibiting DNA ligase IV with SCR7 did not improve gene knock-in efficiency in tested sites (Supplementary Fig. 10A, B). Vikash P. Chauhan and colleagues developed vCas9 and suggested that the breaks induced by vCas9 are primarily repaired through pathways that use homologous sequences, particularly MMEJ and HDR in HEK293T cells52. However, in mouse embryos, we found that the cutting frequency of vCas9 is low (Supplementary Fig. 10C, D). These findings suggest that the NHEJ repair pathway face greater challenges in transitioning to HDR compared to MMEJ in embryos (Fig. 3J). Therefore, to develop a more universal tool suited for different type of sgRNAs, it is necessary to facilitate the efficient transition of both NHEJ and MMEJ repair pattern to HDR.
Identification of compound AZD7648 as a regulator in shifting DSBs repair pattern from NHEJ to MMEJ
Given the ineffectiveness of the NHEJ-to-HDR transition and the effectiveness of the MMEJ-to HDR transition, we propose a broadly applicable strategy where NHEJ is initially converted into MMEJ, followed by the transition from MMEJ to HDR facilitated by KD-Polq. Therefore, we first need to demonstrate a method for NHEJ-to-MMEJ transition. To date, several small molecular compounds have been extensively used to modulate the DNA damage repair process9,42,53,54,55,56,57,58,59. Consequently, we screened 8 compounds reported in previous research and identified variations in the repair patterns of 3 sgRNAs—Sod1-A4-sgRNA1 (N/M = 0.87), Sod1-A4-sgRNA3 (N/M = 1.39), and Sod1-G93-sgRNA3 (N/M = 0.95) (Fig. 4A, B, Supplementary Fig. 11A). Compared to DMSO treatment, compound AZD764853 has been observed to shift the repair outcomes of all three sgRNAs from NHEJ-bias or NHEJ/MMEJ-balance to MMEJ-bias (Fig. 4B, C). Additionally, we found that Olaparib54,55, SCR742,56, Nu744157, ART55858, and Farrerol59 cannot alter sgRNA repair outcomes from NHEJ to MMEJ pattern in all sites (Fig. 4B, C). Furthermore, Olaparib and ART558 were found to impact embryo development in a dose-dependent manner, as shown by the graded concentration treatments (0.5 µM, 1 µM, 5 µM, and 10 µM) (Fig. 4D, Supplementary Fig. 11B–D). Notably, treatment with AZD7648 exhibited significantly higher proportion of HDR repair pattern at all tested sgRNA target sites, especially for NHEJ-biased sgRNAs (Fig. 4E, F, Supplementary Fig. 12). This improvement was also observed using dsDNA donor templates (Fig. 4G). In conclusion, our findings demonstrate the efficacy of the small compound AZD7648 in inducing the transition of the DNA repair pattern from NHEJ to MMEJ, subsequently enhancing knock-in levels, particularly at NHEJ-biased sgRNA-targeted sites.
A Workflow of compound screening in embryo gene editing and DNA repair pattern analysis. Zygote, blastocyst, and sequencing icons were created with BioRender (Created in BioRender. Hongyu, C. (2025) https://BioRender.com/qxdvotz), and other elements were designed by the authors. B Heatmap showing NHEJ/MMEJ (N/M) ratios in edited blastocysts treated with various compounds at different sgRNA sites. C Distribution of DNA repair outcomes (WT, NHEJ, MMEJ) under DMSO, Farrerol, or AZD7648 treatment. D Blastocyst development efficiency following compound treatment. E HDR frequencies in individual knock-in embryos at Sod1-A4 and Sod1-G93 loci using different sgRNAs under DMSO or AZD7648 treatment. Each dot represents one embryo; red dashed lines indicate group means. n/N indicates HDR-positive over total injected embryos. Data are from three independent experiments. F Summary of DNA repair pattern distribution (HDR, random indels, WT) for each sgRNA, based on embryos analyzed in (E). G Representative fluorescence images and quantification of mCherry-positive blastocysts following dsDNA-mediated knock-in. White arrows indicate mCherry-positive blastocysts. Scale bars, 100μm. Bar graphs show mean ± SEM (n = 3 experiments, > 20 embryos per group). Statistical significance was determined using two-sided Mann–Whitney U test (E) and unpaired two-sided t-test (G). p-value are indicated in the figure. Source data are provided as a Source Data file.
ChemiCATI as a universal strategy for enhancing gene knock-in efficiency
After the addition of AZD7648, a notable shift toward an MMEJ-biased repair pattern was observed. In theory, the concurrent utilization of chemical compounds and Polq knockdown presents a promising avenue to further enhance knock-in efficiency. We have designated the strategy of using Chemical-enhanced CasRx-assisted targeted integration (ChemiCATI). Specifically, in the context of ssDNA-mediated gene knock-in, the combined application of the ChemiCATI techniques enhances gene knock-in efficiency at most of our tested sites (e.g., for Sod1-G93-sgRNA5, from 38.46% (10/26) to 96.15% (25/26); for Sod1-G93-sgRNA3, from 24.14% (7/29) to 80.65% (25/31); for Sod1-G93-sgRNA2, from 50% (16/32) to 68% (17/25); for Sod1-A4-sgRNA3, from 45% (9/20) to 91.67% (22/24); for Sod1-A4-sgRNA1, from 32% (9/25) to 93.33% (14/15) (Fig. 5A–C). The HDR repair pattern proportion is significantly improved, particularly at NHEJ-biased and NHEJ/MMEJ-balanced sgRNA-targeted sites (Fig. 5A–F). Additionally, we observed a reduction in the proportion of random indels at five out of six tested sites (Fig. 5D–F; Supplementary Fig. 13A). Consistent with ssDNA, In the context of dsDNA-mediated gene knock-in, we also found significantly higher mCherry knock-in efficiency with ChemiCATI than DMSO and KD-Polq (CATI) groups, especially in NHEJ-biased sgRNAs (e.g., for Dppa3-sgRNA1, from 15% to 80%; for Actb-sgRNA2, from 10% to 40%; for Tubb5-sgRNA2, from 10% to 30%;) (Fig. 5G, H; Supplementary Figs. 13B, C). Nevertheless, in the MMEJ-biased sgRNAs, although the ChemiCATI method did not further improve knock-in efficiency compared to the CATI group, the addition of the AZD7648 compound did not impact the function of CATI method (Supplementary Fig. 13C).
A–C HDR frequencies in individual HDR-positive embryos at the Sod1-A4 and Sod1-G93 loci under DMSO, CATI, or ChemiCATI treatment. Each dot represents one HDR-positive embryo; red dashed lines indicate group means. n/N indicates HDR-positive over total injected embryos. D–F Repair pattern distribution (HDR, random indel, WT) for each sgRNA, based on embryos in (A–C), analyzed using ICE. G Representative fluorescence images of mCherry-labeled dsDNA knock-in blastocysts. White arrows indicate mCherry-positive embryos. Scale bars, 100 μm. H Quantification of mCherry knock-in efficiency under different treatments. Each dot represents one experiment (n = 3; > 20 embryos/replicate). I Workflow for generating gene-edited pups using ChemiCATI-treated 2-cell embryos. Icons created with BioRender (Created in BioRender. Hongyu, C. (2025) https://BioRender.com/qxdvotz). J Slbp locus: schematic of S23 > A23 editing, pup birth ratio, HDR frequency, and representative Sanger sequencing. KCenpe locus: schematic of dual editing (S2377 > A2377, S2380 > A2380), pup birth ratio, HDR frequency, and sequencing validation. Red lines indicate group means. Error bars represent mean ± SEM. Statistical tests: two-sided Mann–Whitney U test (A–C, K), unpaired two-sided t-test (H), Welch’s t-test ( J). p-value are indicated in the figure. Source data are provided as a Source Data file.
To further explore the potential of alternative DNA repair modulators in enhancing knock-in efficiency, we examined the effects of other DNA-PKcs inhibitors (M381428,29,31, NU702629) and the Polq inhibitor PolQi2. Among them, M3814 partially shifted NHEJ repair toward MMEJ, increasing knock-in efficiency, but its high toxicity to embryos limits its practical application (Supplementary Fig. 14A–D). In contrast, while NU7026 and PolQi2 did not affect embryo development, NU7026 failed to effectively shift NHEJ to MMEJ and did not enhance knock-in efficiency (Supplementary Fig. 14A–D). Notably, PolQi2 successfully promotes HDR efficiency and exhibited no apparent toxicity (Supplementary Fig. 14D). We further compared the effects of AZD7648 combined with either CasRx-mediated Polq knockdown or PolQi2-mediated Polq inhibition. Interestingly, the AZD7648 + PolQi2 combination had a more pronounced impact on embryo development, suggesting that careful optimization of compound concentrations, knock-in efficiency, and safety profiles is necessary for this approach in embryo (Supplementary Fig. 14E). In contrast, the ChemiCATI strategy caused minimal embryonic damage while maintaining high knock-in specificity. Meanwhile, we also assessed the off-target effects of the ChemiCATI strategy and found no detectable activity at the predicted off-target sites (Supplementary Fig. 15). Additionally, we analyzed the occurrence of large deletions at target sites and observed that while ChemiCATI effectively reduces large deletion frequencies compared to the control, it does not introduce any additional risk of large deletions beyond that observed with CATI (Supplementary Fig. 16). These findings suggest that ChemiCATI maintains high knock-in specificity while preserving genome integrity.
To further test whether the ChemiCATI method can improve gene knock-in efficiency at NHEJ-biased sgRNA-targeted sites in newborn mice, we targeted key regulatory genes for early embryo development, Slbp and Cenpe, at the zygote stage and produced gene-edited mice via implantation of 2-cell embryos (Fig. 5I). Slbp is crucial for histone mRNA processing, essential for DNA replication and cell division, while Cenpe is involved in chromosome movement and alignment during mitosis. Initially, we introduced a serine-to-alanine mutation (Ser23 > Ala23) in the Slbp gene using an ssDNA donor. We found that using the ChemiCATI method not only improved the birth rate but also enhanced knock-in efficiency, with some pups even reaching 100% knock-in efficiency compared to the control group (Fig. 5J). Following this, we challenged the method by introducing serine-to-alanine mutations at two serine sites (Ser2377 > Ala2377; Ser2380 > Ala2380) in Cenpe simultaneously. Consistent with Slbp, the knock-in efficiency with ChemiCATI was higher than the control group, and in some pups even reached 100% (Fig. 5K, Table 1). To further expand the validation of ChemiCATI across diverse genomic loci, we additionally targeted Grin2a and Trio, two genes involved in neurodevelopmental processes (Supplementary Fig. 17, Table 1). Similar to our previous observations, ChemiCATI significantly enhanced knock-in efficiency at these loci, confirming its effectiveness in a broader genomic context. These results further demonstrate that ChemiCATI is applicable to a wide range of target sites, including those critical for early development and neuronal function.
Because all four genes we tested are functionally important, disrupting their coding sequences by CRISPR/Cas9 could impact key biological processes, potentially affecting embryo viability and birth rates. However, the ChemiCATI method not only improved gene knock-in efficiency but also increased the birth rate, further validating its efficiency and safety (Fig. 5J, K; Supplementary Fig. 17). Together, we developed a broadly applicable knock-in strategy termed ChemiCATI, which is effective at universal sgRNA-targeted sites and can contribute to further basic research and cell therapy.
Discussion
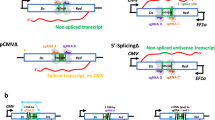
Cellular variability in the activity of repair processes and DNA sequence composition significantly influences the outcomes of DSB repair mechanisms60. Our study conducted a comprehensive analysis of various sgRNAs and examined the indels they induced in mouse embryos. When DNA is cleaved by CRISPR/Cas9, the genomic DNA initiates a repair process. In the absence of a template, the predominant repair patterns are NHEJ and MMEJ. Besides, introducing donor templates results in the presence of NHEJ, MMEJ, and HDR repair patterns. When we introduced the donor template combined with Polq knockdown, we observed a substantial shift from MMEJ to HDR Moreover, the combined use of AZD7648 and Polq knockdown (ChemiCATI) promotes a shift from the NHEJ and MMEJ pathways to HDR, thereby supporting high-efficiency gene knock-in (Fig. 6).
This schematic illustrates the potential transformation of DNA repair outcomes following various treatments. When DNA is cleaved by CRISPR/Cas9, the genomic DNA initiates a repair process. In the absence of templates, the predominant repair patterns are NHEJ and MMEJ. Introducing donor templates results in the presence of NHEJ, MMEJ, and HDR repair patterns. When donor templates are provided and Polq is simultaneously knocked down, there is no significant change in NHEJ frequency; however, a substantial shift from MMEJ to HDR is observed. Furthermore, the concurrent administration of donor templates, AZD7648, and Polq knockdown (ChemiCATI) facilitates the transition from NHEJ and MMEJ patterns to HDR. Red arrows indicate a transition in repair pattern from MMEJ to HDR, and yellow arrows indicate a transition from NHEJ to MMEJ. Figure created in BioRender. Hongyu, C. (2025). https://BioRender.com/qxdvotz.
Multiple strategies have been explored to enhance HDR efficiency. Genetic studies in Drosophila indicate that the absence of DNA ligase IV, a key component of the NHEJ pathway, can substantially increase the frequency of HDR61. Vikash P. Chauhan and colleagues developed vCas9 and suggested that the breaks induced by vCas9 are primarily repaired through pathways that use homologous sequences, particularly MMEJ and HDR52. However, in mouse embryos, we found that the editing efficacy of vCas9 is low. Extensive research has been conducted on enhancing the efficacy of HDR to support higher levels of gene knock-in efficiency through the use of small molecule inhibitors or activators23. Therefore, we evaluated several compounds that have been previously published. Olaparib62, a well-known PARP inhibitor, has shown efficacy in inhibiting MMEJ and enhancing HDR. However, it has been observed that Olaparib exhibits considerable toxicity, with embryonic development efficiency reaching only 70% (Fig. 4D, Supplementary Fig. 5B). ART558, an inhibitor of DNA Polymerase θ (Polθ), can alter its polymerase activity52. Our findings also indicate that treating embryos with ART558 adversely affects their development (Fig. 4D, Supplementary Fig. 9B). These underscore the advantage of leveraging our CasRx-based approach for transient knockdown of Polq gene expression, which does not impact embryonic development37. SCR7 (an inhibitor of DNA ligase IV)42,56, RS1 (an agonist of Rad51)49, Nu7441 (an inhibitor of DNA-PKcs)56,63, and Farrerol (an inhibitor of the AKT and NF-κB signaling pathways)59 have all been documented to enhance the efficacy of gene knock-in. However, in our system, RS1 and SCR7 are unable to convert the NHEJ repair pattern to MMEJ at all three detection sites. NU7441 and Farrerol compounds can convert the NHEJ repair pattern to MMEJ at some detection sites but not all (Fig. 4B). These results also elucidate why certain gene knock-in techniques are effective at some target sites but not at others63,64,65. We speculate that the emergence of such differences may be attributable to various target DNA sequences attracting different repair factors. The compounds employed are functional only for repair factors that bind to specific target sequences. The principle of matching between target site sequences and repair factors requires further verification by researchers. Notably, during the preparation of our work, two similar studies were published that aimed to enhance gene knock-in efficiency by simultaneously inhibiting DNA-PKcs and Polq29,31. These studies were conducted exclusively in cell lines and did not extend their testing to embryo systems. In Sandra Wimberger’s29 and Joost Schimmel’s58 research, the inhibition of Polθ was achieved using small molecules such as ART558 and PolQ2i. Our findings indicate that ART558 adversely affects embryo development. Similarly, Stephan Riesenberg’s study employed a siRNA mix to inhibit Polq31; However, we have demonstrated that siRNA is ineffective in embryo systems37. Despite these differences in methodology, our conclusions align with those of the aforementioned studies. However, we cannot fully exclude the possibility that off-target gene regulation by CasRx may have contributed to the observed gene expression changes.
In conclusion, our ChemiCATI strategy led to an obvious enhancement in gene knock-in efficiency across various sgRNA target sites we tested. The successful application of the ChemiCATI method could radically transform the current situation of generally low gene knock-in efficiencies. It would further accelerate the development of humanized animal models, broaden the application scope of CRISPR tools, and provide additional therapeutic options for genetic diseases that were previously difficult to treat using knock-in methods, especially those requiring high-efficiency and multi-gene knock-in.
Methods
Ethics statement
All animal experiments involving mice were approved by the Animal Care and Use Committee of the Institute of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences (Shanghai, China), under permit numbers NA-042-2019 and NA-042-2022. All procedures were performed in accordance with institutional guidelines and relevant national regulations. Mice were housed in a specific pathogen-free (SPF) facility accredited by the Assessment and Accreditation of Laboratory Animal Care, under a 12 h light/dark cycle with controlled temperature (20 °C) and relative humidity (50%). All animals were housed in the sunny room.
Mouse zygote collection and culturing
The mouse zygotes used in this study were derived from B6D2F1 (C57BL/6J × DBA/2N) female mice aged 3 or 8 weeks. The use and care of animals complied with the guidelines of the Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences. For zygote collection, superovulated B6D2F1 female mice (3 or 8 weeks old) were injected with pregnant mare serum gonadotropin (PMSG; 110914564, San-sheng Biotechnology) (5 IU per mouse for 3-week-old mice, 10 IU per mouse for 8-week-old mice), followed 48 h later by human chorionic gonadotropin (hCG; 110911282, San-sheng Biotechnology) (5 IU for 3-week-old mice, 10 IU for 8-week-old mice), and then mated with adult B6D2F1 males. Zygotes were collected from the oviducts at 20 h post-hCG injection and cultured in KSOM medium (MR-101-D, Sigma-Aldrich) supplemented with amino acids at 37 °C under 5% CO₂.
Mouse embryo injection and embryo transplantation
The gene editing reagents were injected into the cytoplasm of fertilized eggs with clearly visible pronuclei at the volume of 1–3 pL. Microinjection was performed in a droplet of M2 medium (M7167, Sigma-Aldrich) containing 5 μg/mL cytochalasin B (CB; C6762, Sigma-Aldrich) using a Piezo-driven micromanipulator (Prime Tech). The injected embryos were then cultured in KSOM medium supplemented with amino acids. For chemical treatments, the following compounds were used: DMSO (Sigma-Aldrich, D8418), AZD7648, Nu7441, Olaparib, Ku5593365, SCR7, RS1, ART558, and Farrerol (all from MedChemExpress; catalog numbers HY-111783, HY-11006, HY-10162, HY-12016, HY-107845, HY-19793, HY-141520, and HY-N0344, respectively). Zygotes were pre-cultured in KSOM medium containing the indicated concentration of compounds for 2 h, followed by injection with editing reagents. After injection, embryos were cultured in KSOM medium containing the same compounds at 37 °C in 5% CO₂ for 15–18 h, after which the medium was replaced with fresh KSOM. E4.5 blastocysts were collected for fluorescence imaging or genotyping analysis. For knock-in mouse generation, 2-cell stage embryos were transferred into oviducts of pseudopregnant ICR females at 0.5 days post coitum (dpc), with 20 embryos per surrogate.
Production of linearized donors
To produce linearized p2A-mCherry donors for the mouse genes Actb, Dppa5, Cfl1, H3.3b, Cdx2, H2afZ, Cdk4, Lmna, Dppa3, Gata6, and Tubb5, we use Actb as an example to describe the procedure in detail. The donor was generated by PCR amplification of an Actb donor plasmid containing a left homology arm (HAL, ~800 bp), a p2A-mCherry reporter cassette, and a right homology arm (HAR, ~800 bp). The PCR product was subsequently purified using a PCR Extraction Kit (Magen, D2121-03).
Production of Cas9/CasRx mRNA and sgRNA
A T7 promoter sequence was added to the 5′ end of the Cas9 or CasRx coding region by PCR amplification using the indicated primer. The resulting T7-Cas9/CasRx PCR product was purified and used as a template for in vitro transcription (IVT) using the mMESSAGE mMACHINE T7 ULTRA Kit (Invitrogen, AM1345). Similarly, a T7 promoter was added to the sgRNA template by PCR amplification. The T7-gRNA PCR product was purified and used as a template for IVT using the MEGAshortscript T7 Kit (Invitrogen, AM1354). Both the mRNA and sgRNA were subsequently purified using the MEGAclear Kit (Invitrogen, AM1908) and eluted in RNase-free water. All related primers are listed in Supplementary Data file 1.
Preparation of injection reagents
All injection mixtures were prepared in a final volume of 10 μL using RNase-free water, reagents, and consumables, according to the following concentrations: Cas9 and CasRx at 100 ng/μL; sgRNA (Cas9) and gRNA (CasRx) at 50 ng/μL per synthesized RNA; linearized donor at 100 ng/μL; and ssDNA donor at 30 ng/μL.
Embryo and mouse genotyping
For embryo genotyping, single blastocysts were transferred into PCR tubes containing 5 μL of lysis buffer from the Mouse Direct PCR Kit (PD101-01, Vazyme). Samples were incubated at 56 °C for 30 min, followed by heat inactivation of proteinase K at 95 °C for 5 min. Genomic DNA was then amplified using random primers in a 30 μL PCR reaction consisting of 0.5 μL rTaq polymerase (R001, TaKaRa), 10 μL random primer mix, 1.5 μL of 2.5 mM dNTPs, 3 μL 10× buffer, and nuclease-free water to a final volume of 30 μL. The primary PCR cycling conditions were as follows: 1 cycle at 95 °C for 5 min; 50 cycles of 95 °C for 1 min, 37 °C for 2 min, and 55 °C for 4 min; and a final extension at 55 °C for 4 min. A secondary PCR was performed using 1 μL of the primary PCR product and specific primers. The 50 μL reaction included 1 μL KOD-FX DNA polymerase (KFX-101, TOYOBO), 25 μL KOD buffer, 10 μL dNTP mix, 1.5 μL of 10 mM forward and reverse primers, and 1 μL of DNA template from the first-round PCR. The final volume was adjusted to 50 μL with nuclease-free water. Touchdown PCR conditions were: 1 cycle at 94 °C for 2 min; 10 cycles of 98 °C for 10 s, 65 °C for 15 s, and 68 °C for 50 s; followed by 34 cycles of 98 °C for 10 s, 55 °C for 15 s, and 68 °C for 50 s; and a final extension at 68 °C for 5 min. Specific PCR products were gel-purified and subjected to Sanger sequencing. For mouse genotyping, genomic DNA was extracted from toe biopsies using the Mouse Direct PCR Kit. PCR amplification was performed using primers specific to the targeted junctions. KOD-FX DNA polymerase was used under the same conditions as described above. The amplified products were gel-purified and sequenced. All primers used are listed in Supplementary Data file 1.
Analysis of editing efficiency and identification of repair patterns
Collected embryos and cells were lysed for Sanger sequencing, followed by ICE CRISPR Analysis, reliability of which has been confirmed by several studies (https://www.synthego.com/publications). To analyze HDR efficiency, we should also provide HDR donor sequences. After output of editing results, we analyzed all shown events per sample. However, since ICE does not distinguish between MMEJ and NHEJ within the indel category, we manually classified all editing patterns based on predefined criteria, as detailed in the manuscript and Supplementary Fig. S1. Then, the frequencies of indel, HDR, MMEJ, and NHEJ were calculated by averaging corresponding values of sequenced embryos, edited embryos, and knock-in embryos. All information of DNA repair patterns is listed in Table 2 and Table 3. The key terms used to assess gene knock-in efficiency in this study are defined as follows. (1) “Indel (%)” refers to the proportion of insertion and deletion (indel) events at the gene-editing target site within a single blastocyst, determined by targeted amplicon sequencing. (2) “mCherry (%)” represents the percentage of embryos with successful mCherry knock-in at the target site among all injected embryos, assessed by fluorescence imaging. Embryos exhibiting red fluorescence were classified as knock-in positive, while non-fluorescent embryos were considered knock-in negative. Due to the integration of mCherry using a long donor template with homology arms, PCR-based genotyping was not feasible. (3) “Percentage of gene knock-in embryos out of total embryos (%)” indicates the proportion of successfully edited embryos, determined by PCR amplification and sequencing of the target locus, relative to the total number of injected embryos when ssDNA was used as the donor template. (4) “HDR repair proportion per gene knock-in embryo (%)” represents the fraction of HDR repair pattern within an individual knock-in embryo when ssDNA was used as the donor template. (5) “Distribution of DNA repair patterns among all gene knock-in embryos (%)” describes the overall distribution of distinct DNA repair patterns across all gene knock-in embryos, determined through targeted sequencing when ssDNA was used as the donor template. (6) “Blastocyst (%)” refers to the proportion of fertilized embryos that successfully developed into blastocysts, calculated as the number of blastocysts divided by the total number of fertilized embryos.
Embryo collection and RNA-seq library sequencing
RNA-seq experiments were outsourced to a commercial sequencing service provider (Shanghai Applied Protein Technology Co. Ltd., Shanghai, China). Briefly, a lysis mixture was prepared by combining 3 μL Cell Lysis Buffer with 1 μL RNase Inhibitor (3335399001, Roche) to a final volume of 4 μL. Five embryos were transferred into the lysis buffer per sample, and the volume was adjusted to 10 μL with nuclease-free water. Subsequently, 1 μL Oligo(dT) RT Primer and 1 μL dNTP Mix were added, and the mixture was incubated at 72 °C for 5 min. Reverse transcription was performed using a proprietary reaction mix provided by the service provider (containing RT buffer, TSO primer II, RNase inhibitor, and reverse transcriptase), under the following conditions: 42 °C for 90 min, 70 °C for 10 min. cDNA amplification was performed using a commercial 2× PCR master mix and primers. The exact composition of these reagents was not disclosed by the service provider. Amplified full-length cDNA was purified using magnetic beads. Library preparation was conducted using the TruSeq RNA Sample Preparation Kit v2 (Illumina) according to the manufacturer’s instructions. Libraries were sequenced on an Illumina platform using TruSeq Cluster Kit v3 and TruSeq SBS Kit v3.
RNA-seq data analysis
Raw reads were firstly processed through Trim Galore (v0.6.10) with the default parameters. In this step, clean reads were obtained by removing reads containing adapter and low-quality reads from raw data. Reference genome (GRCm38) and gene model annotation files (Release M25) were downloaded from GENCODE database (http://gencodegenes.org/mouse/release_M25.html). Paired-end clean reads were aligned to the reference genome using STAR (v2.7.3a). The abundance of transcripts and genes was estimated from the aligned reads using RSEM (v1.2.28). RSEM provided quantification of gene expression metrics including expected read counts, TPM (transcripts per million), and FPKM (fragments per kilobase of transcript per million mapped reads). The expected read counts generated by RSEM were utilized by DESeq2 (v1.40.2) to identify significantly differentially expressed genes (DEGs). Only genes with adjusted P < 0.05 & |log2 (fold change) |≥1 were defined as significant DEGs and used for further analysis. Heatmaps were generated by pheatmap (v1.0.12) using log10(TPM + 1).
Statistics and reproducibility
Sample sizes were based on standard practices in the field and were consistent across independent biological replicates. Embryos were randomly assigned to experimental groups. In all experiments, microinjection and data analysis were performed by different individuals in a double-blinded manner. No data were excluded from the analyses. Detailed statistical methods are provided in the relevant figure legends. Sex was not considered as a biological variable in this study. Female mice were used for superovulation and embryo transfer due to biological necessity. For embryo experiments, early-stage embryos (zygote to blastocyst) were used, during which sex cannot be morphologically distinguished. For gene-edited offspring, sex was not recorded or analyzed, as it was not relevant to the study objectives. Comparison between two groups was performed using unpaired two-tailed Student’s t-tests, while comparisons among three or more groups were conducted using one-way ANOVA. GraphPad Prism 6 was used for data analysis. Figures were generated using R packages including ggplot2 (v3.4.4) and pheatmap (v1.0.12).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All source data supporting the findings of this study, including underlying numerical data for graphs and summary statistics, are provided in the Source Data file. The RNA-seq data generated in this study have been deposited in Gene Expression Omnibus (GEO) database under accession code GSE297792. Source data are provided with this paper.
Code availability
The customized scripts developed for high-throughput data analysis in this study are available on GitHub (https://github.com/JieBest/Chemi-CATI_mouse_embryo).
References
Hsu, P. D., Lander, E. S. & Zhang, F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278 (2014).
Zhang, X., Li, T., Ou, J., Huang, J. & Liang, P. Homology-based repair induced by CRISPR-Cas nucleases in mammalian embryo genome editing. Protein Cell 13, 316–335 (2022).
Kim, H. & Kim, J. S. A guide to genome engineering with programmable nucleases. Nat. Rev. Genet. 15, 321–334 (2014).
Carroll, D. Genome engineering with targetable nucleases. Annu Rev. Biochem. 83, 409–439 (2014).
Lieber, M. R. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev. Biochem. 79, 181–211 (2010).
McVey, M. & Lee, S. E. MMEJ repair of double-strand breaks (director’s cut): deleted sequences and alternative endings. Trends Genet. 24, 529–538 (2008).
Ira, G. et al. DNA end resection, homologous recombination and DNA damage checkpoint activation require CDK1. Nature 431, 1011–1017 (2004).
Saleh-Gohari, N. Helleday T. Conservative homologous recombination preferentially repairs DNA double-strand breaks in the S phase of the cell cycle in human cells. Nucleic Acids Res. 32, 3683–3688 (2004).
Chu, V. T. et al. Increasing the efficiency of homology-directed repair for CRISPR-Cas9-induced precise gene editing in mammalian cells. Nat. Biotechnol. 33, 543–548 (2015).
van Overbeek, M. et al. DNA repair profiling reveals nonrandom outcomes at Cas9-mediated breaks. Mol. Cell 63, 633–646 (2016).
Allen, F. et al. Predicting the mutations generated by repair of Cas9-induced double-strand breaks. Nat. Biotechnol. 37, 64–72 (2018).
Chakrabarti, A. M., Henser-Brownhill, T., Monserrat, J., Poetsch, A. R., Luscombe, N. M. & Scaffidi, P. Target-specific precision of CRISPR-mediated genome editing. Mol. Cell 73, 699–713 e696 (2019).
Chen, W. et al. Massively parallel profiling and predictive modeling of the outcomes of CRISPR/Cas9-mediated double-strand break repair. Nucleic Acids Res. 47, 7989–8003 (2019).
Leenay, R. T. et al. Large dataset enables prediction of repair after CRISPR-Cas9 editing in primary T cells. Nat. Biotechnol. 37, 1034–1037 (2019).
Shen, M. W. et al. Predictable and precise template-free CRISPR editing of pathogenic variants. Nature 563, 646–651 (2018).
Taheri-Ghahfarokhi, A. et al. Decoding non-random mutational signatures at Cas9 targeted sites. Nucleic Acids Res. 46, 8417–8434 (2018).
Brinkman, E. K., Chen, T., de Haas, M., Holland, H. A., Akhtar, W. & van Steensel, B. Kinetics and fidelity of the repair of Cas9-induced double-strand DNA breaks. Mol. Cell 70, 801–813.e806 (2018).
Ferreira da Silva, J. et al. Genome-scale CRISPR screens are efficient in non-homologous end-joining deficient cells. Sci. Rep. 9, 15751 (2019).
Lin, S., Staahl, B. T., Alla, R. K. & Doudna, J. A. Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. Elife 3, e04766 (2014).
Lomova, A. et al. Improving gene editing outcomes in human hematopoietic stem and progenitor cells by temporal control of DNA repair. Stem Cells 37, 284–294 (2019).
Charpentier, M. et al. CtIP fusion to Cas9 enhances transgene integration by homology-dependent repair. Nat. Commun. 9, 1133 (2018).
Gutschner, T., Haemmerle, M., Genovese, G., Draetta, G. F. & Chin, L. Post-translational regulation of Cas9 during G1 enhances homology-directed repair. Cell Rep. 14, 1555–1566 (2016).
Yeh, C. D., Richardson, C. D. & Corn, J. E. Advances in genome editing through control of DNA repair pathways. Nat. Cell Biol. 21, 1468–1478 (2019).
Shao, S. et al. Enhancing CRISPR/Cas9-mediated homology-directed repair in mammalian cells by expressing Saccharomyces cerevisiae Rad52. Int J. Biochem Cell Biol. 92, 43–52 (2017).
Liu, M. et al. Methodologies for Improving HDR Efficiency. Front Genet 9, 691 (2018).
Cullot, G. et al. Genome editing with the HDR-enhancing DNA-PKcs inhibitor AZD7648 causes large-scale genomic alterations. Nat. Biotechnol. (2024).
Fok, J. H. L. et al. AZD7648 is a potent and selective DNA-PK inhibitor that enhances radiation, chemotherapy and olaparib activity. Nat. Commun. 10, 5065 (2019).
Selvaraj, S. et al. High-efficiency transgene integration by homology-directed repair in human primary cells using DNA-PKcs inhibition. Nat. Biotechnol. 42, 731–744 (2024).
Wimberger, S. et al. Simultaneous inhibition of DNA-PK and Polϴ improves integration efficiency and precision of genome editing. Nat. Commun. 14, 4761 (2023).
Schimmel, J., Kool, H., van Schendel, R. & Tijsterman, M. Mutational signatures of non-homologous and polymerase theta-mediated end-joining in embryonic stem cells. EMBO J. 36, 3634–3649 (2017).
Riesenberg, S. et al. Efficient high-precision homology-directed repair-dependent genome editing by HDRobust. Nat. Methods 20, 1388–1399 (2023).
Bak, R. O., Dever, D. P. & Porteus, M. H. CRISPR/Cas9 genome editing in human hematopoietic stem cells. Nat. Protoc. 13, 358–376 (2018).
Asmamaw, M. & Zawdie, B. Mechanism and applications of CRISPR/Cas-9-mediated genome editing. Biologics 15, 353–361 (2021).
Tatiossian, K. J., Clark, R. D. E., Huang, C., Thornton, M. E., Grubbs, B. H. & Cannon, P. M. Rational selection of CRISPR-Cas9 guide RNAs for homology-directed genome editing. Mol. Ther. 29, 1057–1069 (2021).
DeWitt, M. A. et al. Selection-free genome editing of the sickle mutation in human adult hematopoietic stem/progenitor cells. Sci. Transl. Med. 8, 360ra134 (2016).
Pavel-Dinu, M. et al. Gene correction for SCID-X1 in long-term hematopoietic stem cells. Nat. Commun. 10, 1634 (2019).
Chen, H. et al. CATI: an efficient gene integration method for rodent and primate embryos by MMEJ suppression. Genome Biol. 24, 146 (2023).
Fu, Y. W. et al. Dynamics and competition of CRISPR-Cas9 ribonucleoproteins and AAV donor-mediated NHEJ, MMEJ and HDR editing. Nucleic Acids Res. 49, 969–985 (2021).
Yin, S. et al. Engineering of efficiency-enhanced Cas9 and base editors with improved gene therapy efficacies. Mol. Ther. 31, 744–759 (2023).
Hough, M. A. et al. Dimer destabilization in superoxide dismutase may result in disease-causing properties: structures of motor neuron disease mutants. Proc. Natl. Acad. Sci. USA 101, 5976–5981 (2004).
Danner, E., Bashir, S., Yumlu, S., Wurst, W., Wefers, B. & Kuhn, R. Control of gene editing by manipulation of DNA repair mechanisms. Mamm. Genome 28, 262–274 (2017).
Hu, Z. et al. Ligase IV inhibitor SCR7 enhances gene editing directed by CRISPR-Cas9 and ssODN in human cancer cells. Cell Biosci. 8, 12 (2018).
Nemec, A. A. et al. Estrogen drives cellular transformation and mutagenesis in cells expressing the breast cancer-associated R438W DNA polymerase lambda protein. Mol. Cancer Res 14, 1068–1077 (2016).
Pismataro, M. C. et al. Small molecules targeting DNA polymerase theta (POLtheta) as promising synthetic lethal agents for precision cancer therapy. J. Med. Chem. 66, 6498–6522 (2023).
Shi, P. et al. Collateral activity of the CRISPR/RfxCas13d system in human cells. Commun. Biol. 6, 334 (2023).
Xiong, Z. et al. Ultrasensitive Ribo-seq reveals translational landscapes during mammalian oocyte-to-embryo transition and pre-implantation development. Nat. Cell Biol. 24, 968–980 (2022).
Feldman, T. et al. Recurrent deletions in clonal hematopoiesis are driven by microhomology-mediated end joining. Nat. Commun. 12, 2455 (2021).
Hussmann, J. A. et al. Mapping the genetic landscape of DNA double-strand break repair. Cell 184, 5653–5669 e5625 (2021).
Pinder, J., Salsman, J. & Dellaire, G. Nuclear domain ‘knock-in’ screen for the evaluation and identification of small molecule enhancers of CRISPR-based genome editing. Nucleic Acids Res. 43, 9379–9392 (2015).
Leahy, J. J. et al. Identification of a highly potent and selective DNA-dependent protein kinase (DNA-PK) inhibitor (NU7441) by screening of chromenone libraries. Bioorg. Med. Chem. Lett. 14, 6083–6087 (2004).
Munck, J. M. et al. Chemosensitization of cancer cells by KU-0060648, a dual inhibitor of DNA-PK and PI-3K. Mol. Cancer Ther. 11, 1789–1798 (2012).
Chauhan, V. P., Sharp, P. A. & Langer, R. Altered DNA repair pathway engagement by engineered CRISPR-Cas9 nucleases. Proc. Natl. Acad. Sci. USA 120, e2300605120 (2023).
Rose EP, Osterberg VR, Banga JS, Gorbunova V, Unni VK. Alpha-synuclein regulates the repair of genomic DNA double-strand breaks in a DNA-PK(cs)-dependent manner. bioRxiv (2024).
Santiago-O’Farrill, J. M. et al. Poly(adenosine diphosphate ribose) polymerase inhibitors induce autophagy-mediated drug resistance in ovarian cancer cells, xenografts, and patient-derived xenograft models. Cancer 126, 894–907 (2020).
Campillo-Marcos, I. & Lazo, P. A. Olaparib and ionizing radiation trigger a cooperative DNA-damage repair response that is impaired by depletion of the VRK1 chromatin kinase. J. Exp. Clin. Cancer Res. 38, 203 (2019).
Robert, F., Barbeau, M., Ethier, S., Dostie, J. & Pelletier, J. Pharmacological inhibition of DNA-PK stimulates Cas9-mediated genome editing. Genome Med. 7, 93 (2015).
Feng, W. et al. Marker-free quantification of repair pathway utilization at Cas9-induced double-strand breaks. Nucleic Acids Res. 49, 5095–5105 (2021).
Schimmel, J. et al. Modulating mutational outcomes and improving precise gene editing at CRISPR-Cas9-induced breaks by chemical inhibition of end-joining pathways. Cell Rep. 42, 112019 (2023).
Zhang, W. et al. A high-throughput small molecule screen identifies farrerol as a potentiator of CRISPR/Cas9-mediated genome editing. Elife 9, e56008 (2020).
Gallagher, D. N. & Haber, J. E. Repair of a site-specific DNA cleavage: old-school lessons for Cas9-mediated gene editing. ACS Chem. Biol. 13, 397–405 (2018).
Beumer, K. J., Trautman, J. K., Mukherjee, K. & Carroll, D. Donor DNA utilization during gene targeting with zinc-finger nucleases. G3 3, 657–664 (2013).
Jiang Y, Yam JC, Chu WK. Poly ADP ribose polymerase inhibitor olaparib targeting microhomology end joining in retinoblastoma protein defective cancer: analysis of the retinoblastoma cell-killing effects by olaparib after inducing double-strand breaks. Int. J. Mol. Sci. 22, 10687 (2021).
Zhang, J. P. et al. Efficient precise knockin with a double cut HDR donor after CRISPR/Cas9-mediated double-stranded DNA cleavage. Genome Biol. 18, 35 (2017).
Yu, C. et al. Small molecules enhance CRISPR genome editing in pluripotent stem cells. Cell Stem Cell 16, 142–147 (2015).
Agnihotri, S. et al. ATM regulates 3-methylpurine-DNA glycosylase and promotes therapeutic resistance to alkylating agents. Cancer Discov. 4, 1198–1213 (2014).
Acknowledgements
We thank all members of Liu lab for helpful discussions. This work is supported in part by National Key R&D Program (2021YFA1101804 to Z. Liu), Shanghai Municipal Science and Technology (22JC1403101 to Z. Liu), Shanghai Municipal Science and Technology Major Project, to Z. Liu, the Talent Plan of Shanghai Branch, Chinese Academy of Sciences, to Z. Liu, The Yangfan Project of Shanghai Science and Technology Commission (24YF2752300 to H.C.), National Key R&D Program—Synthetic Biology Special Project (2024YFA0916602 to H.C.), the Shanghai Committee of Science and Technology, China (22JC1403101 to Z. Liu)
Author information
Authors and Affiliations
Contributions
Conceptualization, Z. Liu; Investigation, H.C. and Z. Lu; H.C., Q.T., and J.F. mainly performed molecular and cellular experiments; H.C., L.L., and L.X.L. mainly performed embryo experiments; W.Z., J.L., S.L., H.L., Y.W., Y.S., and Q.S. provided expert technical assistance. H.C. wrote the paper with inputs from all authors. Z. Liu managed the project.
Corresponding authors
Ethics declarations
Competing interests
Z. Liu, H.C., Z. Lu, and Q.T. have submitted a patent application to the China National Intellectual Property Administration pertaining to the gene-editing approach for modeling aspects of this work (application number 202510073394.1; status: under review). The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, H., Tan, Q., Li, L. et al. Refined DNA repair manipulation enables a universal knock-in strategy in mouse embryos. Nat Commun 16, 6502 (2025). https://doi.org/10.1038/s41467-025-61696-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-61696-z