Abstract
Chiral N-heterocycles such as piperidines and tetrahydroisoquinolines are privileged structural motifs in drug discovery and pharmaceutical industry. The development of efficient and practical asymmetric synthetic methods towards pharmaceutically important chiral N-heterocycles constitutes an important subject in synthetic chemistry. Asymmetric synthesis of 2,3-cis-disubstituted piperidines bearing two consecutive chiral centers is particularly challenging in terms of both diastereoselective and enantioselective control. In this work, a regiospecific and enantioselective cyclizative aminoboration is designed to tackle this problem by employing Cu/(S, S)-Ph-BPE as the chiral catalyst, leading to a series of 2,3-cis-disubstituted piperidines as well as C1-substituted tetrahydroisoquinolines in moderate to good yields and excellent enantioselectivities. The asymmetric six-membered ring-closing aminoboration features a broad substrate scope, mild reaction conditions, and excellent functional group compatibility. DFT calculation reveals the importance of noncovalent interactions between substrate and Cu catalyst in controlling the enantioselectivity. The synthetic utility and practicality of this cyclization protocol have been exemplified by the synthesis of the key chiral intermediates of avacopan and L-733,060.
Similar content being viewed by others
Introduction
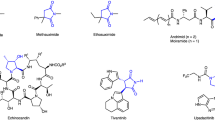
Chiral substituted N-heterocycles such as piperidines, tetrahydroquinolines, and tetrahydroisoquinolines exist widely in the structures of biologically important natural products1,2. They also serve as privileged structural motifs in medicinal chemistry and drug discovery, as demonstrated by a number of chiral N-heterocyclic therapeutic agents (Fig. 1A)3,4,5. Among them, the 2,3-cis-disubstituted piperidines belong to a class of important N-heterocycles existed in the structures of C5a receptor inhibitor avacopan6, NK1 antagonist L-733,0607 and vofopitant8. According to a recent search result from Sci-Finder, more than 6000 known natural products possess chiral 2,3-cis-disubstituted piperidine moieties, and over 300,000 therapeutic agents as well as intermediates contain such chiral motif, manifesting its privileged role in drug discovery and pharmaceutical industry. The prevalence and biological functions of such structures have rendered their syntheses an important research topic. However, most chiral 2,3-disubstituted piperidines and derivatives in pharmaceutical industry currently relied on chiral feedstock through multi-step synthesis9,10,11,12,13 or inefficient chemical resolution(Fig. 1B)14. A two-step strategy of asymmetric organocatalytic bromoaminocyclization of linear olefins was developed by Yeung to initially generate chiral trans-2-aryl-3-bromopiperidines and subsequently convert to 2,3-cis-subsituted piperidines15. The ideal asymmetric hydrogenation or transfer hydrogenation of substituted pyridinium salts with noble metal catalysts were recently accomplished by Zhang16 and Xiao17, respectively, to form directly chiral cis-2-aryl-3-aminopiperidines and cis-2-aryl-3-fluoropiperidines, albeit with limited substrate scope. Despite these advances, efficient asymmetric synthesis of chiral 2,3-cis-substituted piperidines with excellent diastereoselective and enantioselective control remains scarce but highly desirable. We envisioned that a ring-forming alkene cis-difunctionalization of a readily accessible unsaturated amine would form a 2,3-cis-disubsituted piperidines in a highly efficient fashion (Fig. 1C). The question is how to achieve high regioselectivity, diastereoselectivity, and enantioselectivity of such transformation.
Alkene difunctionalizations has become a straightforward approach to install two syn-functional groups across a carbon-carbon double bond18,19,20. The copper-catalyzed alkene aminoboration pioneered by Hirano and Miura utilizes syn-addition of a boron-copper(I) species over alkene followed by oxidative addition of acyloxyamine and reductive elimination, forming 2-borylated-1-amine product with perfectly syn relation21,22,23,24,25,26,27,28,29,30(Fig. 2A). The copper-catalyzed intramolecular cyclizative alkene aminoboration has been explored, providing exclusively 5-membered N-heterocycles on terminal olefinic substrates23. The excellent regioselectivity observed on boron-copper(I) addition is presumably governed by steric reason, with boron adding on the terminal carbon (Fig. 2B)31,32. However, regioselective formation of 6-membered N-heterocycles by copper-catalyzed alkene aminoboration has never been reported. We envisioned that incorporation of an aryl coordination during boron-copper(I) addition would alter the regioselectivity during boron-copper(I) addition and such coordinatively controlled cyclizative aminoboration would form cis-2-aryl-3-borylated piperidines in one step from an easily accessible arylalkene with acyloxyamine moiety (Fig. 2C). Herein we report an enantioselective copper-catalyzed cyclizative aminoborylation, forming cis-2-aryl-3-substituted piperidines in high yields and excellent enantioselectivity. The cyclizative aminoboration method is also extended to ortho-substituted styrene substrates, leading to a series of chiral C1-substituted tetrahydroisoquinolines in highly enantioselective fashion.
Results and discussion
The development of cyclizative aminoborylation protocol
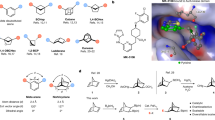
(E)-O-Benzoyl-N-benzyl-N-(5-phenylpent-4-en-1-yl)hydroxylamine (1a) was prepared as the substrate of study for copper-catalyzed intramolecular cyclizative alkene aminoboration. The reactions were carried out at rt in a designated solvent in the presence of a base (3 eq.) and B2pin2 (1.5 eq.) for 72 h with a copper precursor (10 mol %) and chiral ligand (10 mol %) (Table 1). The aminoboration products were directly treated with NaBO3 to form the corresponding alcohols 2a for ease of purification, whoses ee values were determined by chiral HPLC. The ligand effect for the asymmetric cyclizative aminoboration of 1a was evaluated with different types of chiral bidentate ligands (See Supplementary Information for details) and (S, S)-Ph-BPE turned out to be the best ligand, providing 2a in 47% yield and 87% ee (Table 1, entry 1) and no formation of 5-membered N-heterocycle side-product was observed. Subsequently, (S, S)-Ph-BPE was chose as the ligand to investigate the solvent effect of this reaction (Table 1, entries 2–4). When 1,4-dioxane was used as the solvent, 2a was obtained with comparable enantioselectivity to that observed in THF, but with an improved yield (61%). The use of toluene or chlorobenzene was found to be beneficial to enantioselectivity. Notably, when chlorobenzene was used as the solvent, the product was obtained in 95% ee. However, the reaction yield dropped dramatically to 35% due to the low reactivity. To improve the reaction yield, we used chlorobenzene as the solvent to explore the base effect (Table 1, entries 5–7) and the protecting group of hydroxylamines (Details were included in Supplementary Information). When substrate 1a’ was used and the base was switched from KOtBu to KOMe, the reaction yield increased to 50% (Table 1, entry 9) and the enantioselectivity was elevated to 96% ee. It should be noted that employment of 2 eq B2pin₂ and 4 eq. KOMe led to a decrease of the reaction yield (23%) (Fig. 3, entry 8), presumably due to substrate decomposition. Further optimization of reaction conditions indicated that [CuOTf]₂·benzene was a suitable copper source and product 2a was obtained in 70% yield and 96% ee with NaOMe as the base (Table 1, entries 10-12). It was noted that styrene substrate possessing other N-alkyl groups, such as n-butyl group, was also tolerated and the reaction gave product 2a” in 98% ee, albeit in decreased yield (35% yield) (entry 13).
With the optimized reaction conditions, we set out to investigate the scope of the cyclizative asymmetric aminoboration in the synthesis of chiral 2,3-disubstituted piperidines (Fig. 3). Firstly, substrates with various para-substituents on the aromatic ring, including methyl, tert-butyl, methoxy, phenyl, fluorine, and trifluoromethyl groups, were employed and all reactions yielded the corresponding cyclic products in moderate to good yields and with 91–98% ee (2b-2g). Substrates with different meta-substituents, including ester, phenyl, fluorine, chlorine, methyl, and methoxy groups, were all compatible to the aminoboration conditions (2h-2m). The reaction was also tolerable with substrates bearing two substituents on the benzene ring, producing the target products 2n-2p in good yields and 91–96% ee. Substrates containing various heteroaryl rings were all suitable as chiral piperidine products (2q-s, 2u) possessing benzofuran, benzothiophene, indole, and pyridine rings were obtained in good yields and high enantioselectivities. In addition, the reaction proceeded smoothly with sterically hindered ortho-substituted aryl alkenes and products 2t, 2v and 2w were all isolated with good yields and 94–96% ee. Notably, the cyclizative aminoboration reaction enabled the synthesis of 4-cyclopentylamine-substituted product 2x in 64% yield and 94% ee, which was the core scaffold of the anti-angiolipoma drug avacopan. Finally, 2,3-disubstituted pyrrolidine 2y was successfully synthesized in 78% yield and 96% ee under the current conditions from the corresponding acyclic olefin substrate.
Synthetic applications
To demonstrate the synthetic utilities of the asymmetric cyclizative aminoboration protocol, a scale-up experiment of substrate 1a’ was conducted at 2 mmol scale (Fig. 4A). The reaction proceeded smoothly and yielded chiral piperidine 2a, the key chiral intermediate of NK1 antagonist L-733,06011, in 62% yield and 96% ee. Furthermore, chiral piperidine derivatives 3 and 4 were successfully synthesized in good yields and high enantioselectivities by reacting the in situ formed 2a’ with furan-2-yl-lithium and thiophene-2-yl-lithium, respectively (Fig. 4B). A one-carbon elongation was accomplished from 2a’ under Matteson conditions and subsequent oxidation afforded primary alcohol 5 in 48% overall yield and 96% ee. Additionally, Zweifel-type olefination of 2a’ delivered the vinylation product 6 in 93% yield with 94% ee. Finally, compound 1x proceeded the cyclizative aminoboration under similar reaction conditions at 1.2 mmol scale, forming chiral alcohol 7 in 52% yield and 95% ee (Fig. 4C). Subsequent swap of the benzyl protecting group with Boc group successfully afforded compound 8, a key chiral intermediate for asymmetric synthesis of ent-avacopan33. The successful formation of key chiral intermediates of several therapeutic agents demonstrated the practicality and usefulness of this methodology.
Enantioselective synthesis of tetrahydroisoquinolines
Chiral C1-substituted tetrahydroisoquinolines exist widely in the structures of numerous biologically active natural products34,35,36. The enantioselective Cu-catalyzed cyclizative aminoboration could also extend to ortho-substituted styrene substrates 9. Asymmetric cyclizative aminoboration of 9 proceeded at rt in THF in the presence of LiOtBu (3 eq.) and B2pin2 (1.5 eq.) with Cu(CH3CN)4PF6 (10 mol %) and (S, S)-Ph-BPE (10 mol%) as the catalyst system, leading to a series of chiral C1-hydroxymethyl substituted tetrahydroisoquinolines in moderate to good yields and almost perfect enantioselectivities after NaBO3 oxidation (Fig. 5). The reaction was compatible with various substituents on the benzene ring including methyl, methoxy, fluorine, chlorine, and benzyloxy groups, affording products 10a–l in good yields (66-82%) and excellent enantioselectivities (91-99% ee’s). Substrates bearing anthracenyl, piperonyl, and 3,4-dimethoxy groups were all tolerable, providing 10m-10o in acceptable yields (61-65%) and high ee values (97-99%). Furthermore, styrenes with N-butyl and N-cyclopropylmethyl amine moieties were fine substrates, giving rise to 10p and 10q, respectively, in high ee’s (96-98%). The relatively low yields of 10p and 10q were presumably due to the instability of trialkyl tertiary amine products during NaBO3 oxidation. Moreover, when the boron source was changed to Bpin-Bdan, the reaction afforded the Bdan-substituted product 10r in 68% isolated yield without interfering the high enantioselectivity (99% ee). Additionally, isoindoline product 10s was successfully obtained in 57% yield and 96% ee, demonstrating the generality of this cyclization for the synthesis of chiral N-heterocyclic compounds.
Mechanistic studies
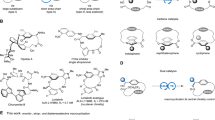
A plausible reaction mechanism was proposed for the current cyclizative aminoboration reaction based on related reports on Cu-catalyzed borylative reactions (Fig. 6A)21,29,37,38,39,40. An active copper-boron species is initially formed in the presence of base and B2pin2. This species then engages in the migratory insertion with the carbon-carbon double bond of substrate (borocupration process), generating an alkyl-copper intermediate C. The intermediate subsequently undergoes a cyclizative C-N bond formation to afford product 2 and regenerates the active copper catalyst. The borocupration process is conceived to be the enantio-determining step and DFT calculations were performed in order to illuminate the origin of enantioselectivity. As shown in Fig. 6B, the optimized structure of favored TS A, in which the two substituents of the trans olefin substrate would orientate towards the two vacant quadrants of the Cu/(S, S)-Ph-BPE catalyst (Fig. 6A), is stabilized by numerous C-H···H-C and C-H···π non-covalent interactions (NCIs) between the substrate and Cu species. On the contrary, notably fewer NCIs are observed for disfavored TS B (See supplementary Figs S3–S6 for detailed NCI analysis). The calculated difference in Gibbs free energy barrier between favored TS A which leads to piperidine product with observed configuration and disfavored TS B providing the opposite configuration is 1.5 kcal/mol, which is in line with the obtained enantioselectivity. In the synthesis of tetrahydroisoquinoline 10a from 9a, the bulky aryl moiety of olefin 8a would reside at one of the unshielded quadrants upon approaching the catalytic active site of Cu catalyst, while the Bpin unit would be accommodated at the other unhindered quadrant, giving TS D and TS E (Fig. 6C). The calculated difference in Gibbs free energy barrier between the two transition states is 3.2 kcal/mol. TS D is the favored transition state leading to 10a in 99% ee, while TS E is found to be higher in energy due to several repulsive NCIs between olefin substrate and ligand backbone, as well as within Cu-Bpin species (See supplementary Figs S3–S6 for detailed NCI analysis).
A Proposed catalytic cycle. B Computed borylcupration transition states with substrate 1b and proposed stereochemical models. C Computed borylcupration transition states with substrate 9a and proposed stereochemical models. Relative electronic energies (ΔE, bold decimals), Gibbs free energies (ΔG, bold italic decimals), interaction energy (ΔEint) and distortion energy (ΔEdist) in kcal/mol are presented. Calculated at M06/6-311 + G(d,p)/SDD//B3LYP/6-31 G(d)-D3/SDD level, with SMD solvation model (solvent = chlorobenzene).
In conclusion, we have developed a Cu-catalyzed asymmetric cyclizative aminoboration taking advantage of a coordinately controlled regioselective borocupration, providing a general and practical way for the synthesis of pharmaceutically important chiral 2,3-cis-disubstituted piperidines in moderate to good yields and excellent enantioselectivities. The method features mild reaction conditions, good functional group tolerance, excellent regioselectivity, diastereoselectity and enantioselectity with Cu/(S, S)-Ph-BPE as the catalyst system. Its broad substrate scope has also extended to the expeditious synthesis of C1-substituted tetrahydroisoquinolines and isoindolines in excellent enantioselectivities. DFT calculation reveals the importance of noncovalent interactions between substrate and Cu catalyst in controlling the enantioselectivity. The facile synthesis of key chiral piperidine intermediates of avacopan and L-733,060 has demonstrated the synthetic utilities and practicality of asymmetric cyclizative aminoboration for drug discovery and pharmaceutical development.
Methods
General procedure for the synthesis of chiral 2,3-disubstituted piperidines
To a sealed tube was added [CuOTf]2·PhH (5 mol%), (S, S)-Ph-BPE (10 mol%), NaOMe (3.0 eq.) and anhydrous PhCl (1.0 mL) in the glove box. The tube was stirred for 5 min before the addition of a solution of B2pin2 (1.5 eq.) in anhydrous PhCl (0.5 mL). After 15 min, the hydroxylamine ester 1(1 eq.) in anhydrous PhCl (0.5 mL) was added. Then the tube was sealed, taken out of the glove box and stirred at rt for 72 h before quenching with water (20 mL). The mixture was extracted with EtOAc (20 mL×3) and the organic phases were combined and concentrated. The residue was dissolved in THF/water (4 mL/2 mL), followed by addition of NaBO3·4H2O (5 eq.). After 16 h, the reaction was quenched with saturated Na2S2O3 (aq) (5 mL). The mixture was extracted with EtOAc (20 mL×3). The organic phases were combined and dried over Na2SO4. After filtration and concentration, the crude product was purified by silica gel column chromatography (A mixture of PE/EA with the appropriate ratio as the eluent).
General procedure for the synthesis of chiral tetrahydroisoquinolines
To a sealed tube was added Cu(CH3CN)4PF6 (10 mol%), (S, S)-Ph-BPE (10 mol%), LiOtBu (3.0 eq.) and anhydrous THF (1.0 mL). The tube was evacuated and refilled with argon and stirred for 5 min before the addition of a solution of B2pin2 (1.5 eq.) in anhydrous THF (0.5 mL). After 15 min, the hydroxylamine ester 9 (1 eq.) in anhydrous THF (0.5 mL) was added to the tube and the mixture was stirred at rt for 24 h before quenching with water (20 mL). The mixture was extracted with EtOAc (20 mL×3) and concentrated. The residue was dissolved in THF/water (4 mL/2 mL), followed by addition of NaBO3·4H2O (5 eq.). After 16 h, the reaction was quenched with saturated Na2S2O3 (aq) (5 mL). The mixture was extracted with EtOAc (20 mL×3). The organic phases were combined and dried over Na2SO4. After filtration and concentration, the product was purified by silica gel column chromatography (A mixture of PE/acetone with the appropriate ratio as the eluent).
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. X-ray crystallographic data for 10n are found in Fig. S1.
References
Kandepedu, N., Abrunhosa-Thomas, I. & Troin, Y. Stereoselective strategies for the construction of polysubstituted piperidinic compounds and their applications in natural products’ synthesis. Org. Chem. Front. 4, 1655–1704 (2017).
Scott, J. D. & Williams, R. M. Chemistry and biology of the tetrahydroisoquinoline antitumor antibiotics. Chem. Rev. 102, 1669–1730 (2002).
Chrzanowska, M., Grajewska, A. & Rozwadowska, M. D. Asymmetric Synthesis of Isoquinoline Alkaloids: 2004–2015. Chem. Rev. 116, 12369–12465 (2016).
Rosen, T. et al. Synthesis, in vitro binding profile, and autoradiographic analysis of [3H]-cis-3-[(2-Methoxybenzyl)amino]-2-phenylpiperidine, a highly potent and selective nonpeptide substance P receptor antagonist radioligand. J. Med. Chem. 36, 3197–3201 (1993).
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014).
Jayne, D. R. W. et al. Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J. Am. Soc. Nephrol. 28, 2756–2767 (2017).
Harrison, T., Williams, B. J., Swain, C. J. & Ball, R. G. Piperidine-ether based hNK1 antagonists 1: Determination of the relative and absolute stereochemical requirements. Bioorg. Med. Chem. Lett. 4, 2545–2550 (1994).
Diemunsch, P. L. Grelot, Potential of substance P antagonists as antiemetics. Drugs 60, 533–546 (2000).
Chaudhuri, S., Parida, A., Ghosh, S. & Bisai, A. Highly stereoselective syntheses of proline-derived vicinal amino alcohols through grignard addition onto N-tosylprolinal. Synlett 27, 215–220 (2016).
Bilke, J. L., Moore, S. P., O’Brien, P. & Gilday, J. Catalytic asymmetric synthesis of piperidines from pyrrolidine: Concise synthesis of L-733,060. Org. Lett. 11, 1935–1938 (2009).
Huy, P. H. & Koskinen, A. M. P. Efficient, stereodivergent access to 3-piperidinols by traceless P(OEt)3 cyclodehydration. Org. Lett. 15, 5178–5181 (2013).
Pansare, S. V. & Paul, E. K. Synthesis of (+)-L-733,060, (+)-CP-99,994 and (2S,3R)-3-hydroxypipecolic acid: Application of an organocatalytic direct vinylogous aldol reaction. Org. Biomol. Chem. 10, 2119–2125 (2012).
Yoon, Y. J. et al. Asymmetric synthesis of (+)-L-733,060. Tetrahedron Lett. 46, 739–741 (2005).
Cochi, A., Pardo, D. G. & Cossy, J. Synthesis of two neurokinin NK1 receptor antagonists: (+)-L-733,060 and (-)-L-733,061. Heterocycles 86, 89–116 (2012).
Zhou, L., Tay, D. W., Chen, J., Yiu, G. C. L. & Yeung, Y. Y. Enantioselective synthesis of 2-substituted and 3-substituted piperidines through a bromoaminocyclization process. Chem. Commun. 49, 4412–4414 (2013).
Zheng, L. S., Wang, F. Y., Ye, X. Y., Chen, G. Q. & Zhang, X. M. Asymmetric hydrogenation of 2-aryl-3-phthalimidopyridinium salts: Synthesis of piperidine derivatives with two contiguous stereocenters. Org. Lett. 22, 8882–8887 (2020).
Wu, J. et al. Synthesis of chiral piperidines from pyridinium salts via rhodium-catalysed transfer hydrogenation. Nat. Catal. 5, 982–992 (2022).
Hirano, K. & Miura, M. Hydroamination, aminoboration, and carboamination with electrophilic amination reagents: Umpolung-enabled regio- and stereoselective synthesis of N-containing molecules from alkenes and alkynes. J. Am. Chem. Soc. 144, 648–661 (2022).
Ji, C. L. & Gao, D. W. Recent advances in catalytic asymmetric synthesis of 1,2-bis(boronic) esters. Chin. J. Org. Chem. 44, 1385–1402 (2024).
Xi, Y. et al. Catalytic asymmetric diarylation of internal acyclic styrenes and enamides. J. Am. Chem. Soc. 144, 8389–8398 (2022).
Tobisch, S. Copper-catalysed aminoboration of vinylarenes with hydroxylamine esters—a computational mechanistic study. Chem. Eur. J. 23, 17800–17809 (2017).
Li, X. Y. & Hall, D. J. Synthesis and applications of β-aminoalkylboronic acid derivatives. Adv. Synth. Catal. 363, 2209–2223 (2021).
Matsuda, N., Hirano, K., Satoh, T. & Miura, M. Regioselective and stereospecific copper-catalyzed aminoboration of styrenes with bis(pinacolato)diboron and O-Benzoyl-N, N-dialkylhydroxylamines. J. Am. Chem. Soc. 135, 4934–4937 (2013).
Sakae, R., Hirano, K., Satoh, T. & Miura, M. Copper-catalyzed stereoselective aminoboration of bicyclic alkenes. Angew. Chem. Int. Ed. 54, 613–617 (2015).
Sakae, R., Hirano, K. & Miura, M. Ligand-controlled regiodivergent Cu-catalyzed aminoboration of unactivated terminal alkenes. J. Am. Chem. Soc. 137, 6460–6463 (2015).
Kato, K., Hirano, K. & Miura, M. Synthesis of β-Boryl-α-aminosilanes by copper-catalyzed aminoboration of vinylsilanes. Angew. Chem. Int. Ed. 55, 14400–14404 (2016).
Kato, K., Hirano, K. & Miura, M. Copper-catalyzed regio- and enantioselective aminoboration of unactivated terminal alkenes. Chem. - Eur. J. 24, 5775–5778 (2018).
Nishino, S., Nishii, Y. & Hirano, K. anti-selective synthesis of β-boryl-α-amino acid derivatives by Cu-catalysed borylamination of α, β-unsaturated esters. Chem. Sci. 13, 14387–14394 (2022).
Whyte, A., Torelli, A., Mirabi, B., Zhang, A. & Lautens, M. C. Copper-catalyzed borylative difunctionalization of π-systems. ACS Catal. 10, 11578–11622 (2020).
Wu, L. L. et al. Cu-catalyzed asymmetric aminoboration of E-vinylarenes with pivZPhos as the ligand. Org. Lett. 21, 8952–8956 (2019).
Semba, K., Fujihara, T., Terao, J. & Tsuji, Y. Copper-catalyzed borylative transformations of non-polar carbonecarbon unsaturated compounds employing borylcopper as an active catalyst species. Tetrahedron 71, 2183–2197 (2015).
Kubota, K., Yamamoto, E. & Ito, H. Copper(I)-catalyzed borylative exo-cyclization of alkenyl halides containing unactivated double bond. J. Am. Chem. Soc. 135, 2635–2640 (2013).
Trammel, G. L. et al. Arylboration of enecarbamates for the synthesis of borylated saturated N-heterocycles. Angew. Chem. Int. Ed. 61, e202212117 (2022).
Liu, W., Liu, S., Jin, R., Guo, H. & Zhao, J. Novel strategies for catalytic asymmetric synthesis of C1-chiral 1,2,3,4-tetrahydroisoquinolines and 3,4-dihydrotetrahydroisoquinolines. Org. Chem. Front. 2, 288–299 (2015).
Muthukrishnan, I., Sridharan, V. & Menéndez, J. C. Progress in the chemistry of tetrahydroquinolines. Chem. Rev. 119, 5057–5191 (2019).
Yang, H., Yu, H., Stolarzewicz, I. A. & Tang, W. Enantioselective transformations in the synthesis of therapeutic agents. Chem. Rev. 123, 9397–9446 (2023).
Hu, J., Ferger, M., Shi, Z. & Marder, T. B. Recent advances in asymmetric borylation by transition metal catalysis. Chem. Soc. Rev. 50, 13129–13188 (2021).
Hemming, D., Fritzemeier, R., Westcott, S. A., Santos, W. L. & Steel, P. G. Copper-boryl mediated organic synthesis. Chem. Soc. Rev. 47, 7477–7494 (2018).
Talbot, F. J. T. et al. Copper-catalyzed borylative couplings with C−N electrophile. Angew. Chem. Int. Ed. 59, 20278–20289 (2020).
Mei, P. et al. Chiral bisphosphine Ph-BPE ligand: a rising star in asymmetric synthesis. Chem. Soc. Rev. 53, 6735–6778 (2024).
Acknowledgements
We are grateful to National Key R&D Program of China 2022YFA1503700 (W. T.), NSFC 82188101 (W. T.), Science and Technology Commission of Shanghai Municipality 23ZR1476500 (H. Y.).
Author information
Authors and Affiliations
Contributions
D. Z. performed experiments. Q. Z. performed DFT calculations. W. T. and H. Y. conceived and directed the project and wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Yangyang Li, Guoyin Yin, and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, D., Yang, H., Zhou, Q. et al. Enantioselective synthesis of chiral 2,3-cis-disubstituted piperidines and C1-substituted tetrahydroisoquinolines by asymmetric Cu-catalyzed cyclizative aminoboration. Nat Commun 16, 6851 (2025). https://doi.org/10.1038/s41467-025-61736-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-61736-8