Abstract
Difluorocarbene, an important reactive intermediate in organic synthesis, exhibits intriguing properties and synthetic versatility. However, great challenges in modulating reaction pathways limit its widespread application in synthetic chemistry. While metal-catalyzed difluorocarbene transfer offers a promising strategy but remains a formidable challenge. Herein, we disclose a copper-mediated multicomponent reaction of amine, aldehyde and BrCF2CO2K for synthesis of α- aminoamide derivatives, wherein copper-difluorocarbene serve as carbonyl source. Control experiments and DFT calculations support the pathway initiated by formation of a copper-difluorocarbene from BrCF2CO2K, followed by nucleophilic attack of the amine to produce an ammonium ylide, interception of the ylide with imine, and defluorination via carbonyl migration. This transformation demonstrates broad substrate scope, accommodating not only aromatic aldehydes but also alkyl aldehydes and drug-modified arylamines, highlighting its synthetic applicability. Furthermore, the method provides a practical and ideal alternative to classical Ugi or Strecker reactions, circumventing the need for toxic cyanide salts or unstable isonitriles.
Similar content being viewed by others
Introduction
Difluorocarbene represents a versatile synthetic building block, readily accessible from commercially available and inexpensive halodifluoroalkyl reagents1,2,3,4,5,6,7,8,9,10,11, with wide applications in organic synthesis1,2,3,4,5, drug development6,7,8,9,10, and advanced functional materials11. As a singlet carbene, difluorocarbene is intrinsically electrophilic due to the existence of an empty p-orbital and exhibits conventional carbene reaction properties1, such as cycloaddition reactions with alkenes or alkynes for formation of gem-difluorocyclopropanes12,13,14,15,16,17, the Wittig reactions with carbonyl to generate gem-difluoroalkenes, etc (Fig. 1a, left)18,19,20. In addition, the difunctional reactions of difluorocarbene3, in which two chemical bonds are simultaneous formed at the carbene carbon center via reacting with nucleophile, followed by coupling with an electrophile, enable difluorocarbene to be a bipolar CF2 linker and production of the difluoroalkylated compounds (Fig. 1a, left)21,22,23,24,25,26,27,28,29. Compared to these conventional CF₂-containing compounds constructing, unconventional transformations of difluorocarbene involving deconstructive functionalization of C-F bonds, beyond its role as a difluoromethyl synthon, have garnered significant attention4,30. The reactions are initiated by the electron-deficient characteristics of difluorocarbene that cause the C-F bond scission, enabling difluorocarbene as a versatile C1 synthon for the assembly of valuable N-containing compounds31,32,33,34,35,36, heterocycles31,32, and aliphatic ethers36 via different intermediates such as isocyanides32, cyano anions33, formamides34, and others (Fig. 1a, right)31,35,36. However, despite its thought-provoking properties and fascinating application potential, the elusive reactivity of difluorocarbene poses great challenges to the control of the reaction pathways and limits its widespread use in synthetic chemistry, resulting in the restricted reaction types.
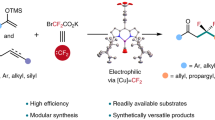
In contrast to free difluorocarbenes, metal difluorocarbenes ([M] = CF2) in which transition metals coordinate with carbene carbon to modulate the reactivity offer a promising approach to overcome the limitations mentioned above37,38,39,40. However, transition-metal catalyzed reactions involving metal–difluorocarbene complexes remain severely underdeveloped, although various [M] = CF2 have been synthesized and characterrized over the past 40 years37,38,39,40,41,42,43,44,45. Recently, Zhang’s group successfully synthesized, isolated, and characterized a [Pd⁰]=CF₂ complex for the first time and disclosed its application in the palladium-catalyzed coupling reaction of difluorocarbene with arylboronic acids (Fig. 1b)46,47,48. Following this breakthrough, several catalytic reactions involving palladium-difluorocarbene complexes have been reported, where metal difluorocarbenes exhibit varying reactivity controlled by the valence state of palladium49,50,51,52,53,54,55,56. Among them, the strongly nucleophilic [Pd⁰]=CF₂ can be protonated to produce PdII–CF₂H, which can subsequently couple with aryl boronic acids57 and terminal alkynes (Fig. 1b, left)49,50. While the electrophilic [PdII]=CF₂ undergoes hydrolysis with water to generate CO, serving as a CO surrogate in carbonylation reactions (Fig. 1b, right)51,52,53,54,55,56,57. Although [Cu]=CF₂ complexes were proposed by Burton decades ago and later suggested by Ichikawa in 2016, the development has significantly lagged behind that of [Pd]=CF₂ complexes58,59,60. Until 2023, the isolation and structural characterization of [CuI]=CF₂ complex was first achieved by Zhang’s group61.The study demonstrated the electrophilic nature of [CuI]=CF₂ complex, which allows it to be attacked by silyl enol ethers, thereby enabling the catalytic modular synthesis of fluorinated compounds (Fig. 1c)60,61,62,63. The intriguing discovery of difluorocarbene presents an exciting opportunity to expand the fluorine chemical space. However, catalytic transformations involving [CuI]=CF₂ are still in their infancy, and application of such electrophilic copper difluorocarbene complexes to organic synthesis remains a significant challenge.
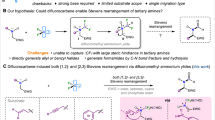
Multicomponent reactions (MCRs) are regarded as one of the strategies that most closely approach the ‘ideal synthesis’, which are flexible, selective, convergent, and atom-efficient processes to construct complex molecules by a single step64,65,66,67,68,69. Our research group has been dedicated to the development of multicomponent reactions involving metal carbene, which proceed through the interception of active ylide/zwitterionic intermediates by various electrophiles, enabling the synthesis of a series of multifunctional molecules68,69,70,71,72,73,74,75. However, these reactions predominantly focus on donor-acceptor carbene intermediates. As our continuing interest in multicomponent reactions and considering the intriguing chemical properties of metal difluorocarbene, we propose that metal difluorocarbene could be attacked by nucleophilic reagents to generate a ylide intermediate, which is subsequently captured by an electrophile, thereby enabling a catalytic multicomponent reaction60,68,69. This approach is initiated by formation of a difluorocarbene metal complex where metal control the reactivity of difluorocarbene. Thus, it would overcome the limitations of direct difunctionalization of free difluorocarbene that can only couple with limited nucleophiles such as organometallic regents, halide ions and phosphines3.
Herein, we report a multicomponent reaction of arylamine, aldehyde and BrCF2CO2K as difluorocarbene precursor under the catalysis of copper, providing cost-efficient access to multifunctional amide (Fig. 1d). The reaction is proposed to involve copper difluorocarbene intermediate that is attacked by amine to form copper associated ammonium ylide. Subsequent interception of the active ylide intermediate occurs and is accompanied by carbonyl migration with fluorine elimination. In addition, besides aromatic aldehydes, alkyl aldehydes and drug modified arylamine can also be tolerated in this MCRs, which demonstrates the practical applicability of this method. Moreover, this process could serve as an effective and ideal alternative to the Ugi or Strecker reaction, addressing key limitations such as the reliance on highly toxic cyanide salts or the use of toxic and unstable isonitriles76.
Results
We initiated our research with the reaction of 4-bromoaniline (1a), BrCF2COOK (2a), and benzaldehyde (3a) under different reaction conditions (Table 1). The multicomponent defluorination product 4a was obtained in 57% yield, rather than CF₂-containing compounds, when a 2.5:3:1 mixture of 1a, 2a and 3a was treated with CuCl and racemic BINOL-derived phosphoric acid (PPA) in acetonitrile at 50 °C for 12 h under argon atmosphere (Table 1, entry 1). After evaluating a series of copper catalysts (Table S1, in Supplementary Information), Cu(CH3CN)4PF6 was selected as the optimal one, leading the 4a in 78% yield (Table 1, entry 2). Subsequently, various solvents were tested, and it was found that acetonitrile was the ideal solvent for this model reaction (Table S1, in Supplementary Information). When BrCF2COOK 2a was replaced by TMSCF2Br (2b) or BrCF2COOEt (2c), no product was detected (Table 1, entries 3, 4). The reaction could proceed smoothly with the lower yield when ClCF2COONa (2 d) was employed as difluorocarbene precursor (Table 1, entry 5). The addition of a Brønsted acid was found to influence the yield of the reaction (Table 1, entries 6–11, and Table S2, in Supplementary Information). Among the acids tested, p-toluenesulfonic acid (TsOH) proved to be the most effective, affording 4a with in 83% yield. (Table 1, entry 11). The optimal reaction temperature was determined to be 50 °C. When the temperature was lowered, even with extended reaction times, the starting materials remained unchanged (Table 1, entries 12, 13). Additionally, increasing the temperature did not lead to a significant increase in the yield of the reaction (Table 1, entry 14). When the reaction occurred in air atmosphere, the yield of multicomponent product 4a would slightly decrease (Table 1, entry 15). Attempts at the asymmetric synthesis of the target product 4a were systematically explored. However, no significant stereoselectivity was observed in the transformation (Table S3, S4, in Supplementary Information).
With the optimized reaction conditions in hand, we explored the scope of the multi-component reaction by investigating aromatic amines 2 (Fig. 2). The reaction with substrates containing para-substituents on the aromatic ring of aniline proceeded smoothly, and whether halogen (4a-4c) and trifluoromethyl (4 d) substituents with electron withdrawing properties or alkyl (4 f, 4 h and 4i), methoxy (4 g), and phenyl (4j) substrates with electron withdrawing properties gave the corresponding products in middle to good yields. The structure of 4e, derived from benzaldehyde, aniline, and BrCF2COOK, was unambiguously confirmed by X-ray crystallographic analysis. In addition, meta-substituents on the aromatic ring of aniline were well tolerated in such transformation, affording the multi-component products (4k-4n). It is worth noting that the ortho-substituted products (4o and 4p) were not detected, likely due to steric hindrance from the functional groups. Both disubstituted aromatic amines and β-naphthylamine were suitable for this reaction system (4q-4t). When halogen substituted aromatic aldehydes are used as substrates, aniline substituted with chlorine, methoxy, and trifluoromethoxy are also suitable substrates, yielding the corresponding products in good yields (4u-4w). Furthermore, aliphatic amines such as benzylamine (BnNH2) and 2,2,2-trifluoroethylamine (CF3CH2NH2) were also evaluated as substrates in our reactions, and the reactions underwent well to react with BrCF2CO2K and imine as the reaction partner. The desired products were obtained in 36% and 32% yield, respectively (4x and 4 y), albeit with competitive formation of aniline-derived products 4a. Excitingly, drug Prezista as HIV-1 protease inhibitor could also be applied to this transformation, and the target compound was obtained in 55% yield (4z).
The scope of aldehydes was then investigated (Fig. 3). When aniline was used as the substrate, both monosubstituted 4-trifluoromethylbenzaldehyde and disubstituted 3,4-dichlorobenzaldehyde were well tolerated in this reaction system, to afford the target compounds (4aa and 4ab). When 4-bromoaniline participated in the reaction, aromatic aldehyde derivatives with electron-donating or electron-withdrawing functional groups on the para-position of aromatic ring, including halo, trifluoromethyl, nitro, methyl, methoxy, phenyl, and thiomethyl were harmonious with such catalytic system, yielding corresponding products (4ac-4aj). Once ortho- and meta-substituted aromatic aldehydes were applied to the reaction, the yields of the corresponding products (4ak-4aq) were favorable without significant decrease, which indicated that the steric hindrance of aromatic aldehydes did not interfere with the reaction yield. Among them, the structure of 4ap was unambiguously confirmed by X-ray crystallographic analysis. Heterocyclic aromatic aldehydes, polycyclic aromatic aldehyde and fused heterocyclic aromatic aldehydes were well tolerated in such reaction system, giving the multi-component products (4ar-4aw) in middle to good yields. To our delight, non-aromatic aldehydes such as cyclohexanecarbaldehyde, tetrahydropyran-4-carbaldehyde, and glyoxylic esters are also suitable substrates, affording desired products (4ax-4az). Moreover, aldehydes 3ab and 3ac derived from Gemfibrozil and Ciprofibrate were also suitable for this reaction system, to give the desired products (4 A and 4B), which demonstrated the utility of this reaction.
Moreover, to highlight the synthetic value of such approach, when 4-methoxybenzaldehyde was scaled up to 6 mmol, the desired amide 4ah was smoothly obtained in 67% yield, and the amides could be easily further functionalized (Fig. 4). First, compound 4ah was converted into reduced product 5 with 92% yield under the action of NaBH4. Then, compound 4ah could smoothly generate cyclization products with 90% yield in the presence of DBU. In addition, when amide 4 g was used as starting material, the reaction yielded the product 7 with 55% yield, which was deprotected from the p-methoxyphenyl group (PMP) with the assistance of ceric ammonium nitrate (CAN). Of note, when aldehydes were replaced by N, N-dibenzyl-1-methoxymethanamine 8, corresponding multi-component products 9 was obtained with 58% isolated yield.
In order to better understanding the pathway for this transformation, several validation experiments were conducted. Firstly, 100 μL oxygen-18 water was added to template reaction, the yield of multi-component products was not affected, and no 18O labeled products were observed by HRMS. The standard template reaction results in roughly the same yield with or without water (100 μL). These results indicated that water is not involved in the reaction and the water molecules generated in situ were not the oxygen source of the product in multi-component reactions (Fig. 5a). Secondly, when benzaldehyde was omitted from the multi-component reactions, p-tert-butylaniline 1i was reacted with BrCF2COOK under the standard conditions, leading to formylation products 10a formed via N-H insertion followed by defluorination and direct amidation product 11a with nearly 1:1 ratio in yield (Fig. 5b). Notably, isonitrile was not detected by LC-MS at any stage of the reaction. According to the literature research, isonitriles can only be obtained in the presence of organic or inorganic bases. However, our optimal reaction conditions were not conducive to the generation of isonitriles30. Therefore, we speculated that there was no involvement of isocyanide intermediates in such transformation. Then, compounds 10a and 11a were added to the model reaction of 4-bromoaniline 1a, BrCF2COOK 2a, and benzaldehyde 3a, only the target product 4a was obtained, with no detectable formation of cross-multicomponent products bearing 4-tert-butyl substitution (Fig. 5c). This observation suggests that neither the formylation product 10a nor the amidation product 11a serve as intermediates in this transformation, and exclude a stepwise mechanism for this multicomponent reaction.
Next, the role of the copper in the catalytic process was further investigated. The control experiments of 4-bromoaniline 1a, BrCF2COOK 2a, and benzaldehyde 3a were performed under the standard conditions in the absence of copper or with alternative Lewis acidic metal catalysts, including Sc(OTf)3, Yb(OTf)3, Zn(OTf)2, Ni(OTf)2 and AgSbF6. Notably, no significant formation of the target product was observed in any of these cases, even at elevated temperatures (Table S5, in Supplementary Information). These findings demonstrate that the copper catalyst fulfills a critical and distinctive role beyond conventional Lewis acid catalysis. Previous studies have unequivocally demonstrated that free difluorocarbenes readily undergo cycloaddition with silyl enol ethers to form gem-difluorocyclopropanes, whereas copper difluorocarbene species exhibit complete incapacitation of cyclopropanation activity60,61. Capitalizing on this distinct reactivity profile, the competitive experiments of BrCF2COOK, 4-bromoaniline and silyl enol ether 13a with or without copper catalyst were carried out. The formylation product 10b was obtained in 37% yield with no detectable cyclopropanation product 12a under the catalysis of Cu(CH3CN)4PF6. In contrast, in the absence of copper catalyst, ¹⁹FNMR analysis revealed the formation of difluorocyclopropane 12a in 19% yield, alongside a significant reduction in formylation product yields (10b < 5%, Fig. 5e). In addition, the template reaction of BrCF2COOK, 4-bromoaniline and benzaldehyde was also conducted under the previously reported conditions for transformations involving a copper-difluorocarbene intermediate (copper salt, 2,9-diMe-1,10-phen, CH3CN, 50 °C.)60,62, yielding the desired multi-component product in 60% yield (Figure S5, in Supplementary Information). These results are consistent with prior reports, corroborating the generation of a copper-difluorocarbene intermediate in our reaction system, and supporting the potential involvement of a copper-difluorocarbene species in the present multi-component reaction. We performed density functional theory (DFT) calculations to illustrate the formation of the intermediate IV as shown in Fig. 6. The free difluorocarbene I preferentially coordinate with [CuI]⁺ to form the copper-difluorocarbene complex II, which is energetically favorable (−15.3 kcal/mol) compared to the direct interaction with ArNH₂, yielding a weakly bound van der Waals complex II’ (4.9 kcal/mol). Subsequent coordination of ArNH₂ to copper-difluorocarbene complex II generates intermediate III (−15.0 kcal/mol), which undergoes C-N bond formation via transition state TS1 (−4.2 kcal/mol) to afford the copper-associated ammonium ylide intermediate IV (−24.1 kcal/mol). The overall process for formation the intermediate IV via copper difluorocarbene isexergonic, releasing 24.1 kcal/mol, indicating a thermodynamically favorable pathway. In the absence of copper catalyst, formation of a weakly bound van der Waals intermediate II’ from free difluorocarbene with ArNH2 exhibits significantly slower kinetics (4.9 kcal/mol versus −15.3 kcal/mol for the copper-mediated pathway). Computational attempts to locate a transition state for formation of C-N bond from II’ were unsuccessful, excluding the possibility of intermediate IV forming directly from free difluorocarbene (Figs. S7, S8, in Supplementary Information). The DFT calculations highlight the crucial role of copper(I) in the transformation, which initiates with coordination of copper(I) to difluorocarbene, generating a key copper-difluorocarbene intermediate. The key intermediate subsequently undergoes C-N bond formation through nucleophilic attack by ArNH₂, ultimately affording intermediate IV.
On the basis of the above results and previous works30,68,69, the plausible subsequently reaction pathway involving ylide interception, defluorination and carbonyl migration of the multicomponent reaction is proposed and demonstrated its energetic feasibility through DFT calculations (Fig. 6). The nucleophilic ammonium ylide intermediate IV is captured by the activated imine via transient state TS2 (1.6 kcal/mol), in which p-toluenesulfonic acid and bromodifluoroacetate ion facilitate the proton transfer, leading to the product VII (−54.4 kcal/mol) with releasing the copper(I). The resulting product VII containing a fragment of CF2 adjacent to nitrogen atom is vulnerable, and the consecutive scission of Csp3-F bond occurs under the assistant of BrCF2COOK. According to the DFT, the monofluoroimine species VIII (−33.3 kcal/mol) is formed via nucleophilic substitution of bromodifluoroacetate ion with VII through transition state TS3 (−33.1 kcal/mol), followed by the second Csp3-F bond cleavage to generate intermediate IX (−21.3 kcal/mol). Intramolecular carbonyl migration of intermediate IX via TS5 (−20.6 kcal/mol) with a barrier of 0.7 kcal/mol and subsequent deprotonation eventually render α-aminoamide products.
Discussion
In summary, we have developed copper-catalyzed MCRs for synthesis of multifunctional amide derivatives from amine, aldehyde and BrCF2COOK without the need for any ligands. The mild reaction conditions, non-toxic nature, and use of readily available raw materials demonstrate that this reaction serves as an effective alternative strategy to the Strecker or Ugi reactions, enabling the synthesis of versatile and valuable products. Additionally, the high functional group tolerance, accommodating not only aromatic aldehydes but also alkyl aldehydes and even complex drug-like molecules, underscores the practical applicability of this method. Control experiments and DFT calculations systematically support that the copper difluorocarbene complex serves as the key intermediate in this transformation and acts as the carbonyl source for the formation of the amide group, and exclude the formation of isonitriles under the reaction conditions. The reaction is proposed to through the formation of copper difluorocarbene, nucleophilic attack by the amine to produce a copper-associated ammonium ylide, interception of the active ylide intermediate with imine, and subsequent defluorination via carbonyl migration. This sequence accounts for the overall high efficiency and distinctiveness of the reaction.
Methods
General
All 1H NMR (500 MHz, 600 MHz) and 13C NMR (125 MHz, 150 MHz) and 19F NMR (471 MHz) spectra were recorded on 500 or 600 MHz spectrometers in in CDCl3, DMSO-d6 and Methanol-d4. Chemical shifts were reported in ppm with the solvent signal as reference, and coupling constants (J) were given in Hertz. The peak information was described as: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad. High-resolution mass spectrometry (HRMS) was recorded on a commercial apparatus (ESI Source). Single crystal X-ray diffraction data were recorded on Bruker-AXS SMART APEX II single crystal X-ray diffractometer.
General procedure for synthesis of product 4
To an oven-dried 10 mL Schlenk tube equipped with a stir bar was added Cu(CH3CN)4PF6 (7.5 mg, 0.02 mmol, 10.0 mol%), TsOH (6.9 mg, 0.04 mmol, 20.0 mol%), aromatic amines 1 (0.5 mmol, 2.5 equiv), BrCF2COOK 2a (128 mg, 0.6 mmol, 3.0 equiv), and aldehydes 3 (0.2 mmol, 1.0 equiv), and suspended in CH3CN (3.0 mL) under dry argon atmosphere. The resulting mixture was stirred at 50 °C for 12 hours. The progress of the reaction was monitored by TLC. After the reaction was complete, the reaction was cooled to room temperature and concentrated under reduced pressure. The residue was purified by flash column chromatography (eluent: EA:PE = 1/20 ~ 1/5) to give the pure product 4.
General procedure for synthesis of product 9
To an oven-dried 10 mL Schlenk tube equipped with a stir bar was added Cu(CH3CN)4PF6 (7.5 mg, 0.02 mmol, 10.0 mol%), TsOH (6.9 mg, 0.04 mmol, 20.0 mol%), aromatic amine 1a (0.3 mmol, 1.5 equiv), BrCF2COOK 2a (128 mg, 0.6 mmol, 3.0 equiv), and N,N-dibenzyl-1-methoxymethanamine 8 (0.2 mmol, 1.0 equiv), and suspended in CH3CN (2.0 mL) under dry argon atmosphere. The resulting mixture was stirred at 50 °C for 12 hours. The progress of the reaction was monitored by TLC. After the reaction was complete, the reaction was cooled to room temperature and concentrated under reduced pressure. The residue was purified by flash column chromatography (eluent: EA:PE = 1/20 ~ 1/10) to give the pure product 9 (58% yield).
Data availability
All data supporting the findings described in this manuscript are available in the the main text and Supplementary Information. For full characterization data of new compounds and experimental details, see Supplementary Methods. 1H NMR, 13C NMR, and 19F NMR spectra are supplied for all new compounds. The cartesian coordinates of the optimized structures in this study are provided in Source Data file. Source data are provided with this paper. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Center, under deposition numbers CCDC2393043 (4e) and CCDC2393055 (4ap). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. All other data are available from the corresponding authors upon request. Source data are provided with this paper.
References
Brahms, D. L. S. & Dailey, W. P. Fluorinated carbenes. Chem. Rev. 96, 1585–1632 (1996).
Ni, C. & Hu, J. Recent advances in the synthetic application of difluorocarbene. Synthesis 46, 842–863 (2014).
Dilman, A. D. & Levin, V. V. Difluorocarbene as a building block for consecutive bond-forming reactions. Acc. Chem. Res. 51, 1272–1280 (2018).
Ma, X. & Song, Q. Recent progress on selective deconstructive modes of halodifluoromethyl and trifluoromethyl-containing reagents. Chem. Soc. Rev. 49, 9197–9219 (2020).
Xie, Q. & Hu, J. A journey of the development of privileged difluorocarbene reagents TMSCF2X (X = Br, F, Cl) for organic synthesis. Acc. Chem. Res. 57, 693–713 (2024).
Sap, J. B. I. et al. [18F] Difluorocarbene for positron emission tomography. Nature 606, 102–108 (2022).
Müller, K., Faeh, C. & Diederich, F. Fluorine in pharmaceuticals: Looking beyond intuition. Science 317, 1881–1886 (2007).
Hagmann, W. K. The many roles for fluorine in medicinal chemistry. J. Med. Chem. 51, 4359–4369 (2008).
Zhou, Y. et al. Next generation of fluorine-containing pharmaceuticals, compounds currently in phase II–III clinical trials of major pharmaceutical companies: new structural trends and therapeutic areas. Chem. Rev. 116, 422–518 (2016).
Bhutani, P. et al. FDA Approved drugs from 2015-June 2020: a perspective. J. Med. Chem. 64, 2339 (2021).
Hudlicky, M. & Pavlath, A. E. Chemistry of Organic Fluorine Compounds II; American Chemical Society: WA, (1995).
Dolbier, W. R. & Battiste, M. A. Structure, synthesis and chemical reactions of fluorinated cyclopropanes and cyclopropenes. Chem. Rev. 103, 1071–1098 (2003).
Rulliere, P., Cyr, P. & Charette, A. B. Difluorocarbene addition to alkenes and alkynes in continuous flow. Org. Lett. 18, 1988–1991 (2016).
Wang, F. et al. Chloride ion-catalyzed generation of difluorocarbene for efficient preparation of gem-difluorinated cyclopropenes and cyclopropanes. Chem. Commun. 47, 2411–2413 (2011).
Li, L., Wang, F., Ni, C. & Hu, J. Synthesis of gem-difluorocyclopropa(e)nes and O-, S-, N-, and P-difluoromethylated compounds with TMSCF2Br. Angew. Chem., Int. Ed. 52, 12390–12394 (2013).
Liu, R. & Hu, J. Synthesis of aryl perfluorocyclopropyl ethers via [2 + 1] cyclopropanation using TMSCF2Br reagent. Org. Lett. 24, 3589–3593 (2022).
Wang, F. et al. Synthesis of gem-difluorinated cyclopropanes and cyclopropenes: trifluoromethyltrimethylsilane as a difluorocarbene source. Angew. Chem., Int. Ed. 50, 7153–7157 (2011).
Faqua, S. A., Duncan, W. G. & Silverstein, R. M. A one-step synthesis of 1,1-difluoroolefins from aldehydes by a modified wittig synthesis. Tetrahedron Lett. 5, 1461–1463 (1964).
Hu, M., Ni, C., Li, L., Han, Y. & Hu, J. Gem-difluoroolefination of diazo compounds with TMSCF3 or TMSCF2Br: transition-metal-free crosscoupling of two Carbene precursors. J. Am. Chem. Soc. 137, 14496–14501 (2015).
Zhang, Z. et al. Reaction of diazo compounds with difluorocarbene: an efficient approach towards 1,1-difluoroolefins. Angew. Chem. Int. Ed. 55, 273–277 (2016).
Dolbier, W. R., Wang, F., Tang, X., Thomoson, C. S. & Wang, L. Three step procedure for the preparation of aromatic and aliphatic difluoromethyl ethers from phenols and alcohols using a chlorine/fluorine exchange methodology. J. Fluor. Chem. 160, 72–76 (2014).
Zhu, J., Liu, Y. & Shen, Q. Direct difluoromethylation of alcohols with an electrophilic difluoromethylated sulfonium ylide. Angew. Chem. Int. Ed. 55, 9050–9054 (2016).
Liu, A., Ni, C., Xie, Q. & Hu, J. TMSCF2 Br-enabled fluorination-aminocarbonylation of aldehydes: modular access to α-fluoroamides. Angew. Chem. Int. Ed. 61, e202115467 (2022).
Liu, A., Ni, C., Xie, Q. & Hu, J. Transition-metal-free controllable single and double difluoromethylene formal insertions into C-H bonds of aldehydes with TMSCF2Br. Angew. Chem. Int. Ed. 62, e202217088 (2023).
Xie, Q. et al. Efficient difluoromethylation of alcohols using TMSCF2Br as a unique and practical difluorocarbene reagent under mild conditions. Angew. Chem. Int. Ed. 56, 3206–3210 (2017).
Miller, T. G. & Thanassi, J. W. The preparation of aryl difluoromethyl ethers. J. Org. Chem. 25, 2009–2012 (1960).
Faqua, S. A., Duncan, W. G. & Silverstein, R. M. A one-Step synthesis of 1,1-cifluoroolefins from aldehydes by a modified wittig synthesis. Tetrahedron Lett. 5, 1461–1463 (1964).
Yuan, W.-J., Tong, C.-L., Xu, X.-H. & Qing, F.-L. Copper-mediated oxidative chloro- and bromodifluoromethylation of phenols. J. Am. Chem. Soc. 145, 23899–23904 (2023).
Fier, P. S. & Hartwig, J. F. Synthesis of difluoromethyl ethers with difluoromethyltriflate. Angew. Chem. Int. Ed. 52, 2092–2095 (2013).
Ma, X., Su, J. & Song, Q. Unconventional transformations of difluorocarbene with amines and ethers. Acc. Chem. Res. 56, 592–607 (2023).
Su, J., Hu, X., Huang, H., Guo, Y. & Song, Q. Difluorocarbene enables to access 2-fluoroindoles from ortho-vinylanilines. Nat. Commun. 12, 4986 (2021).
Wang, Y., Zhou, Y., Ma, X. & Song, Q. Solvent-dependent cyclization of 2-alkynylanilines and ClCF2COONa for the divergent assembly of N-(quinolin-2-yl)amides and quinolin-2(1H)-ones. Org. Lett. 23, 5599–5604 (2021).
Yu, C., Ma, X. & Song, Q. Palladium-catalyzed cyanation of aryl halides with in situ generated CN- from ClCF2H and NaNH2. Org. Chem. Front. 7, 2950–2954 (2020).
Su, J., Ma, X., Ou, Z. & Song, Q. Deconstructive functionalizations of unstrained carbon-nitrogen cleavage enabled by difluorocarbene. ACS Cent. Sci. 6, 1819–1826 (2020).
Su, J., Li, C., Hu, X., Guo, Y. & Song, Q. Deaminative arylation and alkenylation of aliphatic tertiary amines with aryl and alkenylboronic acids via nitrogen ylides. Angew. Chem. Int. Ed. 61, e202212740 (2022).
Sheng, H., Su, J., Li, X. & Song, Q. Deconstrutive difunctionalizations of dyclic ethers enabled by difluorocarbene to access difluoromethyl ethers. CCS Chem. 4, 3820–3831 (2022).
Brothers, P. J. & Roper, W. R. Transition-metal dihalocarbene complexes. Chem. Rev. 88, 1293–1326 (1988).
Feng, Z., Xiao, Y.-L. & Zhang, X. Transition-metal (Cu, Pd, Ni)-catalyzed difluoroalkylation via cross-coupling with difluoroalkyl halides. Acc. Chem. Res. 51, 2264–2278 (2018).
Zhou, W. et al. Transition-metal difluorocarbene complexes. Chem. Commun. 57, 9316–9329 (2021).
Clark, G. R., Hoskins, S. V., Jones, T. C. & Roper, W. R. Oxidation state control of the reactivity of a transition metal carbon double bond. synthesis, X-ray crystal structure, and reactions of the zerovalent difluorocarbene complex [Ru(CF2)(CO)2(PPh3)2]. J. Chem. Soc., Chem. Commun. 719-721 (1983).
Trnka, T. M., Day, M. W. & Grubbs, R. H. Olefin metathesis with 1,1-difluoroethylene. Angew. Chem. Int. Ed. 40, 3441–3444 (2001).
Hughes, R. P. et al. A simple route to difluorocarbene and perfluoroalkylidene complexes of Iridium. J. Am. Chem. Soc. 127, 15020–15021 (2005).
Harrison, D. J., Gorelsky, S. I., Lee, G. M., Korobkov, I. & Baker, R. T. Cobalt fluorocarbene complexes. Organometallics 32, 12–15 (2013).
Harrison, D. J., Daniels, A. L., Korobkov, I. & Baker, R. T. d10 Nickel cifluorocarbenes and their cycloaddition reactions with tetrafluoroethylene. Organometallics 34, 5683–5686 (2015).
Takahira, Y. & Morizawa, Y. Ruthenium catalyzed olefin cross-metathesis with tetrafluoroethylene and analogous fluoroolefins. J. Am. Chem. Soc. 137, 7031–7034 (2015).
Feng, Z., Min, Q.-Q., Fu, X.-P., An, L. & Zhang, X. Chlorodifluoromethane-triggered formation of difluoromethylated arenes catalysed by palladium. Nat. Chem. 9, 918–923 (2017).
Feng, Z., Min, Q.-Q. & Zhang, X. Access to difluoromethylated arenes by Pd-catalyzed reaction of arylboronic acids with bromodifluoroacetate. Org. Lett. 18, 44–47 (2016).
Deng, X.-Y., Lin, J.-H. & Xiao, J.-C. Pd-catalyzed transfer of difluorocarbene. Org. Lett. 18, 4384–4387 (2016).
Zhang, X.-Y. et al. Reductive catalytic difluorocarbene transfer via palladium catalysis. Angew. Chem. Int. Ed. 62, e202306501 (2023).
Zhang, X.-Y., Fu, X.-P., Zhang, S. & Zhang, X. Palladium difluorocarbene involved catalytic coupling with terminal alkynes. CCS Chem. 2, 293–304 (2020).
Liu, X., Sheng, H., Zhou, Y. & Song, Q. Pd-Catalyzed assembly of fluoren-9-ones by merging of C-H activation and difluorocarbene transfer. Org. Lett. 23, 2543–2547 (2021).
Sheng, H., Chen, Z. & Song, Q. Palladium-catalyzed difluorocarbene transfer enabled divergent synthesis of γ-butenolides and ynones from iodobenzene and terminal alkynes. J. Am. Chem. Soc. 146, 1722–1731 (2024).
Wang, L., Hu, C., Yang, X., Fu, Y. & Du, Z. Pd-catalyzed synthesis of aryl esters involving difluorocarbene transfer carbonylation. Eur. J. Org. Chem. 27, e202400032 (2024).
Zuo, D. et al. Palladium-catalyzed regioselective [5 + 1] annulation of vinyl aziridines/epoxides with ClCF2COONa. Org. Lett. 24, 4630–4634 (2022).
Hu, C. et al. Synthesis of N-substituted phthalimides via Pd catalyzed [4 + 1] cycloaddition reaction. Chem. Commun. 59, 14839–14842 (2023).
Nie, Z. et al. Palladium-catalyzed difluorocarbene transfer enables access to enantioenriched chiral spirooxindoles. Nat. Commun. 15, 8510 (2024).
Fu, X.-P. et al. Controllable catalytic difluorocarbene transfer enables access to diversified fluoroalkylated arenes. Nat. Chem. 11, 948–956 (2019).
Wiemers, D. M. & Burton, D. J. Pregeneration, spectroscopic detection and chemical reactivity of (triˬluoromethyl) copper, an elusive and complex species. J. Am. Chem. Soc. 108, 832–834 (1986).
Yang, Z.-Y., Wiemers, D. M. & Burton, D. J. Trifluoromethylcopper: a useful difluoromethylene transfer reagent: a novel double insertion of diluoromethylene into pentafluorophenylcopper. J. Am. Chem. Soc. 114, 4402–4403 (1992).
Fuchibe, K., Aono, T., Hu, J. & Ichikawa, J. Copper(I)-catalyzed [4 + 1] cycloaddition of silyl dienol ethers with sodium bromodiˬluoroacetate: access to β,β-difluorocyclopentanone derivatives. Org. Lett. 18, 4502–4505 (2016).
Zeng, X., Li, Y., Min, Q.-Q., Xue, X.-S. & Zhang, X. Copper catalyzed difluorocarbene transfer enables modular synthesis. Nat. Chem. 15, 1064–1073 (2023).
Zeng, X. et al. Copper difluorocarbene enables catalytic difluoromethylation. J. Am. Chem. Soc. 146, 16902–16911 (2024).
Tan, T. D. et al. Catalytic difluorocarbene insertion enables access to fluorinated oxetane isosteres. Nat. Chem. 17, 719–726 (2025).
Coppola, G. A., Pillitteri, S., Eycken, E. V. V., You, S.-L. & Sharma, U. K. Multicomponent reactions and photo/electrochemistry join forces: atom economy meets energy efficiency. Chem. Soc. Rev. 51, 2313–2382 (2022).
Sharma, U. K., Prabhat, R., Eycken, E. V. V. & You, S.-L. Sequential and direct multicomponent reaction (MCR)-based dearomatization strategies. Chem. Soc. Rev. 49, 8721–8748 (2020).
Neochoritis, C. G., Zhao, T. & Dömling, A. Tetrazoles via multicomponent reactions. Chem. Rev. 119, 1970–2042 (2019).
Wang, Q., Wang, D., Wang, M. & Zhu, J. Still unconquered: enantioselective passerini and ugi multicomponent reactions. Acc. Chem. Res. 51, 1290–1300 (2018).
Guo, X. & Hu, W. Novel multicomponent reactions via trapping of protic onium ylides with electrophiles. Acc. Chem. Res. 46, 2427–2440 (2013).
Zhang, D. & Hu, W. Asymmetric multicomponent reactions based on trapping of active intermediates. Chem. Rec. 17, 739–753 (2017).
Qian, Y. et al. Enantioselective multifunctionalization with Rh carbynoids. J. Am. Chem. Soc. 145, 26403–26411 (2023).
Kang, Z. et al. Ternary catalysis enabled three-component asymmetric allylic alkylation as a concise track to chiral α,α-disubstituted ketones. J. Am. Chem. Soc. 143, 20818–20827 (2021).
Kang, Z. et al. Asymmetric counter-anion-directed aminomethylation: synthesis of chiral β-amino acids via trapping of an enol intermediate. J. Am. Chem. Soc. 141, 1473–1478 (2019).
Yu, S. et al. An enantioselective four-component reaction via assembling two reaction intermediates. Nat. Commun. 13, 7088 (2022).
Ma, C. et al. Synthesis and characterization of donor–acceptor iron porphyrin carbenes and their reactivities in N–H insertion and related three-component reaction. J. Am. Chem. Soc. 145, 4934–4939 (2023).
Qiu, H. et al. Highly enantioselective trapping of zwitterionic intermediates by imines. Nat. Chem. 4, 733–738 (2012).
Akritopoulou-Zanze, I. Isocyanide-based multicomponent reactions in drug discovery. Curr. Opin. Chem. Biol. 12, 324–331 (2008).
Acknowledgements
Financial support from Natural Science Foundation of Guangdong Province (2023A1515110403 T.J., 2024A1515030037 Z.K.) is gratefully acknowledged. We also acknowledge financial support from the China Postdoctoral Science Foundation under Grant (NO. 2023M743925 T.J.), Natural Science Foundation of Henan Province (242300420201 K.W., 252300420796 J.L.) and Natural Science Foundation of Zhejiang Province (LQN25B020014 X.Z.) for their financial support.
Author information
Authors and Affiliations
Contributions
J.L., B.W. and T.L. contributed equally to this work. Z.K. and J.L. contributed to the conception and design of the experiments. Z.K. directed the project. B.W., T.L., Q.W. and T.J. performed the experiments and analyzed the data. X.Z and X.F. contributed to density functional theory (DFT) calculations. Z.K., J.L. and W.H. wrote the manuscript. Y.P. and K.W. provided valuable suggestions to the project. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Donghui Wei and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, J., Wang, B., Liu, T. et al. Multi-component reactions via copper(I) difluorocarbene as carbonyl source for constructing α—aminoamide derivatives. Nat Commun 16, 6643 (2025). https://doi.org/10.1038/s41467-025-61947-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-61947-z