Abstract
The M5 muscarinic acetylcholine receptor (M5 mAChR) represents a promising therapeutic target for neurological disorders. However, the high conservation of its orthosteric binding site poses significant challenges for drug development. While selective positive allosteric modulators (PAMs) offer a potential solution, a structural understanding of the M5 mAChR and its allosteric binding sites remains limited. Here, we present a 2.8 Å cryo-electron microscopy structure of the M5 mAChR complexed with heterotrimeric Gq protein and the agonist iperoxo, completing the active-state structural characterization of the mAChR family. To identify the binding site of M5-selective PAMs, we implement an integrated approach combining mutagenesis, pharmacological assays, structural biology, and molecular dynamics simulations. Our mutagenesis studies reveal that selective M5 PAMs bind outside previously characterized M5 mAChR allosteric sites. Subsequently, we obtain a 2.1 Å structure of M5 mAChR co-bound with acetylcholine and the selective PAM VU6007678, revealing an allosteric pocket at the extrahelical interface between transmembrane domains 3 and 4 that is confirmed through mutagenesis and simulations. These findings demonstrate the diverse mechanisms of allosteric regulation in mAChRs and highlight the value of integrating pharmacological and structural approaches to identify allosteric binding sites.
Similar content being viewed by others
Introduction
The M5 muscarinic acetylcholine receptor (mAChR) belongs to the class A G protein-coupled receptor (GPCR) family. As one of five mAChR subtypes (M1-M5), it responds to the endogenous neurotransmitter acetylcholine (ACh)1. Despite its low expression levels in the central nervous system (CNS), the M5 mAChR plays crucial roles, primarily localizing to dopamine-containing neurons in the substantia pars nigra and ventral tegmental areas2,3,4. Historically, drug discovery efforts targeting the M5 mAChR concentrated on antagonists and negative allosteric modulators (NAMs). This focus stemmed from evidence that M5 mAChR inactivation can modulate dopaminergic signalling in the CNS5,6, suggesting therapeutic potential for substance addiction, depression, and anxiety7,8,9,10,11,12,13,14,15. However, M5 mAChR activation may offer equally promising therapeutic applications. Studies using M5 mAChR knockout mice revealed that this receptor mediates CNS vasculature dilation, thereby regulating cerebral blood flow16,17. This finding suggests that selective M5 mAChR activation could benefit conditions such as Alzheimer’s disease, schizophrenia, and ischaemic stroke by enhancing CNS circulation and blood flow.
The development of selective M5 mAChR activators faces significant challenges, primarily due to the highly conserved orthosteric acetylcholine binding site shared across all five mAChR subtypes18,19. This conservation has historically hindered the development of subtype-selective orthosteric ligands. To overcome this limitation, researchers shifted focus to allosteric modulators, which target spatially distinct binding sites20. This strategy proved promising, with several selective allosteric modulators successfully developed for the M5 mAChR21,22,23,24,25,26. The selectivity of these modulators likely results from non-conserved residues present in the allosteric binding sites, distinguishing them from the highly conserved orthosteric site.
M5-selective positive allosteric modulators (PAMs) were initially discovered and developed based on a bromo isatin scaffold, as exemplified by the PAM VU011949827. VU0119498 enhanced ACh signalling in a calcium assay at all Gαq-coupled mAChRs (M1, M3, M5) and was therefore chosen as a tool compound for further M5 mAChR PAM optimization. A range of M5 mAChR PAMs were developed based on VU0119498, and these all show M5 mAChR selectivity over all mAChR subtypes with varying levels of potency and PAM activity21,22,24. Unfortunately, all M5 mAChR selective PAMs based on the isatin scaffold exhibit a poor drug metabolism and pharmacokinetic (DMPK) profile that was hard to overcome due to the intractability of the isatin scaffold in medicinal chemistry optimization1,24.
To overcome these limitations, high-throughput screening identified a scaffold amenable to modification25. This effort led to the development of 1-((1H-indazol-5-yl)sulfoneyl)-N-ethyl-N-(2-(trifluoromethyl)benzyl)piperidine-4-carboxamide (ML380), which emerged as the most potent M5-selective PAM at the time25. ML380’s ability to penetrate the CNS made it valuable for studying molecular mechanisms of allosteric modulation and as a template for further derivatives26,28,29. However, poor partition coefficients ultimately limited its in vivo utility25. Despite these incremental advances in developing M5-selective PAMs, their precise allosteric binding site remained unknown, significantly limiting rational, structure-based drug design. The M5 mAChR contains at least three known allosteric sites: the extracellular vestibule (ECV), the amiodarone-binding site30, and the extrahelical EH4 pocket recognized by the M5-selective NAM ML37531. However, none of these sites were structurally confirmed as the binding location for M5-selective PAMs. Understanding the location of the M5-selective PAM binding site will accelerate the development of improved compounds for in vivo use32. Therefore, we initiated studies to determine the binding site of M5-selective PAMs, beginning with ML380.
Here, we determine the binding site of M5-selective PAMs through an integrated approach combining mutagenesis, pharmacological assays, structural biology, and molecular dynamics simulations. We present cryo-EM structures of the active M5 mAChR and reveal an allosteric binding site at the extrahelical interface between transmembrane domains 3 and 4 that binds selective PAMs. Our findings reveal the structural basis for M5-selective allosteric modulation, providing a foundation for rational, structure-based drug design targeting this therapeutically important receptor.
Results
ML380 does not bind to known M5 mAChR allosteric binding sites
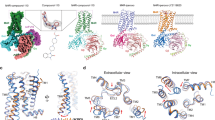
To identify ML380’s binding site, we first investigated its interaction with known allosteric sites through functional inositol monophosphate (IP1) accumulation assays. We tested ML380’s activity on both wild-type (WT) M5 mAChR and mutants targeting two known allosteric sites: (1) the ECV allosteric site33,34, using alanine mutants, and (2) the EH4 pocket used by the M5-selective NAM ML37531, using mutations that convert non-conserved residues to their M2 mAChR equivalents (A1133.35V, G1524.47A, and L1564.51V; superscript refers to the Ballesteros and Weinstein scheme for conserved class A GPCR residues35). These experiments measured three key parameters: ML380’s affinity (pKB), its efficacy in the system (log τ), and its functional cooperativity with ACh (log αβ)36. Consistent with previous studies, ML380 demonstrated both agonism and positive cooperativity with ACh at the WT M5 mAChR28,29 and thus acts as an agonist-PAM (Ago-PAM, Fig. 1a). The EH4 pocket mutant maintained ML380 function with no significant change in affinity compared to WT (Fig. 1a, c, d, Supplementary Table 1). While some ECV alanine mutants showed significant differences in ML380’s affinity, efficacy, and cooperativity parameters, the compound retained its ability to bind, activate, and modulate receptor function in all cases (Fig. 1b, c–e, Supplementary Fig. 1, Supplementary Table 1). No significant differences in receptor expression were observed at these mutants31. These results indicated that ML380 binds neither to the ECV allosteric site nor to the EH4 pocket used by ML375, leading us to use cryo-electron microscopy (cryo-EM) to identify its binding site.
a ML380-ACh interaction in IP1 accumulation assay at WT M5 mAChR (left) or M5 EH4 pocket mutant (right) in CHO cells. Data points are mean ± SEM values from 7 (WT M5 mAChR) and 3 (M5 EH4 mutant) experiments performed in duplicate. An operational model of allosterism was fit to the grouped data. b Effects of the M5 mAChR mutations on the pKB of ML380. Data shown are the mean ± SEM of the affinity estimates derived from a global least-squares fit of an allosteric model to three independent experiments for all conditions, except for WT M5 mAChR (n = 7). Pharmacology parameters were shared across experiments to yield a single best estimate of each parameter and its associated standard error, as derived from the nonlinear regression algorithm. This global pooled analysis approach ensured model convergence in all instances. *, significantly different from WT, p < 0.05, one-way ANOVA, Dunnett’s post hoc test. Parameters obtained are listed in Supplementary Table 1. c M5 mAChR mutated residues shown on receptor structure. d EH4 pocket residues are shown as purple sticks. e ECV residues are shown as red sticks. f Consensus cryo-EM map of M5 mAChR-mGαq/Gβ1γ2 complex with iperoxo at 2.8 Å (FSC 0.143). Model colouring: dark blue (receptor), red (mGαq), green (Gβ1), yellow (Gγ1). g Cryo-EM density for iperoxo in orthosteric site (local refined map, contour level 0.36). h Iperoxo-orthosteric site interactions. Interaction colouring: pink (charge-charge); black (hydrogen bonds); purple (cation-π). Source data are provided as a Source Data file.
Structure determination of the M5 mAChR in an active state
Active state structures of the M1-M4 mAChRs have been determined by cryo-EM34,37,38, leaving the M5 mAChR as the last remaining subtype. To determine an active state M5 mAChR structure, we engineered a modified receptor construct with several key alterations: removal of intracellular loop 3 (ICL3) residues 237-421, addition of an N-terminal HA signal sequence, and incorporation of an anti-Flag epitope tag. This modified receptor was then fused to mini-GαsqiN (hereafter referred to as mGαq), resulting in a chimeric construct39,40. The receptor-G protein complex was prepared through a multi-step process including detergent solubilization for purification, stabilization with scFv1641, addition of apyrase to hydrolyze GDP, and inclusion of 10 µM iperoxo. Prior to cryo-EM analysis, we incubated the iperoxo-bound M5 mAChR-mGαq complex with 10 µM ML380 overnight on ice, followed by freezing and imaging using single-particle cryo-transmission electron microscopy (TEM) on a Titan Krios microscope. The resulting structure achieved a global resolution of 2.8 Å, providing sufficient EM density maps to position the receptor and mGαqβ1γ2 complex (Fig. 1f, Supplementary Fig. 2-3, Supplementary Table 2). However, due to poor density, the scFv16 component was excluded from subsequent data analysis and modelling.
Analysis of the active state M5 mAChR
The iperoxo-bound M5 mAChR-mGαq complex revealed iperoxo bound to the canonical mAChR orthosteric binding site, which is characterized by several key aromatic residues (Fig. 1g, h). The rotamer toggle switch residue W4556.48 forms the binding site’s floor, while three tyrosine residues (Y1113.33, Y4586.51, and Y4817.39) move inward during activation to create a tyrosine lid, separating the orthosteric site from the ECV. Consistent with previous M1-M4 mAChR structures33,34,37,38, iperoxo was positioned between these aromatic residues (Fig. 1h). Additional binding site residues include the aromatic residues W1624.57 and Y4857.43, along with non-aromatic residues D1103.32, N1153.37, T1945.39, T1975.42, A2015.46, N4596.52, L188ECL2, and C4847.42. Two residues play crucial roles in iperoxo recognition. Residue N4596.52 forms hydrogen bonds with iperoxo, while D1103.32 engages in a charge-charge interaction with iperoxo’s quaternary nitrogen.
The active state M5 mAChR displays all the hallmarks of an active state class A GPCR. Relative to the inactive state, tiotropium bound M5 mAChR, there is an 8.1 Å outward movement of transmembrane 6 (TM6) and 4.4 Å inward movement of TM7 (Supplementary Fig. 4). At the extracellular face, there is an outward movement of TM5 and inward movement of TM6 and extracellular loop 3 (ECL3) that lead to a contraction of the ECV (Supplementary Fig. 4b). These global changes in TM and ECL movements are mediated by changes in the conserved class A activation motifs including the D3.49R3.50Y3.51, P5.50V3.40F6.44, and N7.49P7.50xxY7.53 motifs (Supplementary Fig. 4d)42.
Our active iperoxo-bound M5 mAChR structure enables the comprehensive analysis of the entire mAChR family in its active state. Comparing this M5 mAChR structure with other iperoxo-bound mAChR structures reveals remarkable similarity, with root mean squared deviation (RMSD) values of 0.49–0.77 Å (Fig. 2a)33,34,37,38. The TM domains, ECL domains, orthosteric site residues, and iperoxo positioning are nearly identical across all mAChR subtypes (Fig. 2b–d). At the receptor-G protein interface, Gαq- and Gαi/o-coupled mAChRs show distinct α5 helix insertion angles into the TM bundle (Fig. 2f, g). In Gαq-coupled receptors (M1, M3, and M5 mAChRs), the α5 helix top rotates toward TM6, while in Gαi/o-coupled receptors (M2 and M4 mAChRs), it rotates toward TM2. This pattern, however, does not extend to the G protein N-terminus (Fig. 2e), possibly due to the varying use of scFv16, Nb35, and N-terminal chimeras across different structures. Additionally, the Gαq-coupled M1, M3, and M5 mAChR structures display a more extended and resolved TM5.
a Overall view of the M1 to M5 mAChRs complexed to Gα and bound to iperoxo. Model colouring: purple (M1; PDB: 6OIJ), orange (M2; PDB: 6OIK), cyan (M3; PDB: 8E9Z), red (M4; PDB: 7TRK), dark blue (M5; PDB: 9EK0). b Extracellular view comparing ECLs and TM regions. c Intracellular view (G protein removed) comparing ICLs and TM regions. d Overlay of iperoxo and orthosteric binding site residues. e Side view comparing the αN movement of Gα. f Intracellular view comparing the α5 insertion of Gα. g Intracellular view comparing the α5 rotation of Gα. Changes are indicated by arrows.
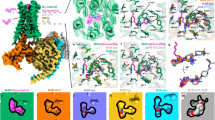
In line with our mutagenesis results (Fig. 1a, b), no cryo-EM density was observed for ML380 in the ECV allosteric site or in the EH4 binding pocket (Fig. 3a, b). Following focused refinement with a mask, some cryo-EM density was observed parallel to TM1 and TM7 in the ECV (coloured green Fig. 3c); however, this density was commonly observed in other mAChR structures and is likely a lipid molecule43. We also observed partial density directly below the EH4 pocket at the bottom of the TM2,3,4 interface (coloured orange in Fig. 3b, c). To investigate whether this partial density reflects ML380 occupancy at its allosteric binding site, we conducted radioligand binding experiments using M5/M2 TM chimera mutants31. We measured interactions between ACh and [3H]-N-methyl scopolamine ([3H]-NMS) with increasing ML380 concentrations. If ML380 binds at the TM2,3,4 interface, we would expect complete loss of its allosteric modulation in both M5/M2 TM2,3,4 and M5/M2 TM3,4,5 chimeras, given ML380’s selectivity profile25. Indeed, swapping TM2-5 completely abolished ML380’s ability to modulate ACh binding (Fig. 3d, Supplementary Table 3). The M5/M2 TM1,7,h8 chimera showed increased ML380 cooperativity, confirming that the density parallel to TM1 and TM7 is not ML380, while suggesting that exchanging these TMs affects the receptor’s global activation dynamics (Fig. 3d, Supplementary Table 3).
a–c Local refined M5 mAChR cryo-EM map (contour level 0.3). No ML380 density was observed in a ECV or b EH4 pocket. c Potential ML380 density observed parallel to TM1/TM7 (green) and the bottom of the TM2,3,4 interface (orange). d [3H]-NMS equilibrium binding studies between [3H]-NMS, ACh and ML380 at WT M5 mAChR and M5-M2 TM chimeras. Insets show receptor cartoons (blue: M5 domains; red: M2 domains). Data points are mean ± SEM values of three duplicate experiments. An allosteric ternary complex model was fit to the data. Parameters obtained are listed in Supplementary Table 3. RMSDs (Å) of e iperoxo in orthosteric pocket and f ML380 at the potential allosteric site at the bottom of the TM2,3,4 interface from GaMD simulations of the cryo-EM structure. Three simulation replicates are shown in different colours. Source data are provided as a Source Data file.
Although the TMs 2-5 mutagenesis results were promising, the density at the TM2,3,4 interface coincides with a common cholesterol binding site in class A GPCRs44,45, suggesting it might represent cholesterol or another lipid. For further analysis, we performed all-atom Gaussian accelerated Molecular Dynamics (GaMD) simulations on the modelled structure of the iperoxo-M5 mAChR-mGαq complex with ML380 bound at the TM2,3,4 interface (Fig. 3e, f). While iperoxo maintained its cryo-EM conformation with RMSD values mostly below 2 Å (Fig. 3e), ML380 showed significant fluctuations with RMSD values of ~3–8 Å compared to the initial cryo-EM pose (Fig. 3f). The combination of mutagenesis data, GaMD simulations, and ambiguous cryo-EM density did not provide convincing support for assigning ML380 to this putative allosteric binding site.
Use of an improved PAM, VU6007678, for structure determination
To discover the allosteric binding site for selective PAMs at the M5 mAChR, we investigated VU600767826, an optimized derivative of ML380’s indanyl core. VU6007678 demonstrated enhanced M5 mAChR affinity, better positive cooperativity with ACh compared to ML38029, and significantly improved GPCR-G protein complex stabilization. To increase our chances of obtaining a PAM-bound complex, we implemented several biochemical and pharmacological modifications. We supplemented Nb35 with scFv16 during purification for enhanced complex stability, used the endogenous agonist ACh instead of iperoxo, and maintained VU6007678 at 10 µM throughout purification rather than adding it before grid freezing as done with ML380. These modifications improved protein purification efficiency, yielding a sample at 18 mg/mL. When applied to Au grids, the sample produced a complex resolved to 2.1 Å from 418,794 particles (Fig. 4a, Supplementary Fig. 2-3).
a Consensus cryo-EM map of M5 mAChR-mGαq/Gβ1γ2 complex with ACh and VU6007678 at 2.1 Å resolution (FSC 0.143). Model colouring: light blue (receptor); red (mGαq), green (Gβ1), yellow (Gγ1), light grey (scFv16), dark grey (Nb35). b M5 mAChR model showing ACh (green) at the orthosteric site and VU6007678 (orange) at the allosteric site between TM3/TM4 and above ICL2. Cryo-EM density (local refined receptor map, contour 0.43) for c iperoxo at the orthosteric site and d VU6007678 at the allosteric site. e Comparison of orthosteric site residues. Stick colouring: light blue (ACh-bound residues), dark blue (Ipx-bound residues), light green (ACh), dark green (Ipx). f Interactions of ACh with the orthosteric binding site. Interaction colouring: pink dotted lines (charge-charge), black (hydrogen bonds), purple (cation-π); red spheres (water molecules). g 2D interaction plot of ACh with the orthosteric binding site; grey lines (hydrophobic interactions). h RMSDs (Å) of ACh relative to the starting conformation during GaMD simulations (three replicates, different colours). i VU6007678 allosteric site interactions: black (hydrogen bonds), purple (cation-π), green (π-π). j VU6007678 site comparison between the active complex (light blue) and inactive tiotropium-bound M5 (salmon, PDB:6OL9); VU6007678 (orange). k 2D VU6007678 interaction plot. Same colouring as g with green dashed lines representing π-π interactions. l RMSDs (Å) of VU6007678 relative to the starting conformation during GaMD simulations (three replicates, different colours). Source data are provided as a Source Data file.
The high-quality cryo-EM density maps enabled precise placement of the receptor, mGαq, β1γ2, Nb35, and scFv16, with clear sidechain orientations for most amino acids. The ACh-VU6007678 structure closely resembles the iperoxo structure, with an RMSD value of 0.49 Å (Supplementary Fig. 5a–d). The orthosteric binding pocket shows well-resolved density for ACh positioned beneath the tyrosine lid residues and above W4556.48 (Fig. 4b, c). While ACh engages the same orthosteric binding pocket residues as iperoxo with similar orientations, W4556.48 adopts a more horizontal and planar orientation with ACh-bound, similar to observations in the M4 mAChR with ACh and iperoxo34 (Fig. 4e). ACh forms key interactions within the orthosteric binding pocket through its quaternary ammonium group and acetyl moiety (Fig. 4f, g). The positively charged quaternary ammonium establishes a cation-π interaction with the aromatic cage formed by Y1113.33, W4556.48, Y4586.51, and Y4817.39, while also engaging in a charge-charge interaction with D1103.32. The acetyl group of ACh forms a hydrogen bond with a water molecule that coordinates with N4596.52 (Fig. 4g), altogether anchoring ACh in an orientation that promotes receptor activation.
Clear, well-defined density unambiguously corresponding to VU6007678 was detected at the intracellular interface of TM3,4 and above ICL2 (Fig. 4a, b, d). The extended binding site accommodates VU6007678 through multiple interactions with the M5 mAChR (Fig. 4i, k). The indanyl core, positioned at the top of the allosteric binding site, forms hydrophobic interactions with M1504.45, F1263.48, and V1233.45, while engaging in an edge-to-face π-interaction with F1303.52. The propyl chain extends toward TM4, forming a hydrophobic interaction with I1494.44. Hydrogen bonding occurs between the carboxamide with R1464.41 and between the sulfonyl and K141ICL2. The indazole of VU6007678 forms a π-π interaction with F1303.52, a cation-π interaction with R1343.56 and at the bottom of TM3, and a hydrophobic interaction with Y1293.51. Other residues that make up the VU6007675 binding site include Y682.42 and Y138ICL2. These extensive interactions likely explain why VU6007678 has high cooperativity with ACh and its high affinity for the M5 mAChR active state. Given the low affinity of VU6007678 for the inactive state M5 mAChR, as observed in radioligand binding with antagonist [3H]-NMS29, we hypothesized that these binding site residues undergo substantial rearrangement during activation. Superimposition of the ACh-VU6007678 structure with the inactive tiotropium-bound M5 mAChR crystal structure18 revealed significant conformational changes in the allosteric binding site. ICL2 remains ordered and adopts an α-helical conformation in both the inactive and active states of the M5 mAChR (Fig. 4j, Supplementary Fig. 4c). However, upon activation, ICL2 undergoes a significant shift toward the TM core, primarily driven by the inward movement of F1303.52, Y138ICL2, and K141ICL2 whilst R1343.56 moves outward (Fig. 4j). These coordinated conformational changes that occur upon receptor activation create the allosteric binding site (Supplementary Fig. 5e,f) enabling VU6007678 binding and explaining why VU6007678 has high affinity for the M5 mAChR active state and low affinity for the M5 mAChR inactive state. Consequently, this selectivity for the active state explains why VU6007678 acts as a PAM of agonists as opposed to a NAM. Comparison of the allosteric binding site between our active iperoxo-bound structure and the ACh-VU6007678 structure reveals the allosteric binding site is highly similar (Supplementary Fig. 5a,d), indicating the allosteric site is preformed in the M5 mAChR when bound to an orthosteric agonist and that VU6007678 stabilises the M5 mAChR active state.
We performed GaMD simulations on the ACh-VU6007678-bound M5 mAChR-mGαq complex and with the PAM removed (ACh-bound M5 mAChR-mGαq) to validate the binding pose of VU6007678 and to examine dynamic interactions with the receptor. Both ACh and VU6007678 maintained stable positions in both simulations, with ACh showing minimal fluctuations (RMSD = 1.70 ± 0.28 Å) and VU6007678 displaying moderate mobility while remaining in its binding pocket (RMSD = 4.85 ± 0.56 Å) (Fig. 4h, l, Supplementary Fig. 6).
The VU6007678 binding site contains several residues that vary across mAChR subtypes (Fig. 5a). While Y682.42 is unique to M5, appearing as phenylalanine in other mAChRs, R1343.56 and R1464.41 are conserved among M1, M3, and M5 but differ in M2 and M4. V1233.45 varies between leucine, isoleucine, and valine across M1-M4, while I1494.44 alternates between leucine, methionine, and valine in these subtypes. The ICL2 lysine is conserved in M1-M3 but appears as arginine in M4. In contrast, F1263.48, F1303.52, T1333.55, Y138ICL2, and M1504.45 remain fully conserved across all five mAChR subtypes. This partially non-conserved binding site architecture provided an opportunity to further validate and characterize the binding site through mutagenesis studies and functional assessment using the TruPath G protein activation assay46.
a Comparison of VU6007678 allosteric binding site residues (stick representation) across the M1-M5 mAChRs. Colouring: purple (M1; PDB: 6OIJ), orange (M2; PDB: 6OIK), light blue (M3; PDB: 8E9Z), red (M4; PDB: 7TRK), and dark blue (M5; PDB: 9EJZ). VU6007678-ACh interaction in Trupath Gq activation assay at b WT M5 mAChR or c M5/M2 swap in CHO cells. Data points are mean ± SEM of three to eight individual experiments performed in duplicate. WT M5 mAChR n = 8, M5/M2 swap mutant n = 3. An operational model of allosterism was fitted to the grouped data to derive the key pharmacological parameters (d–f). Pharmacology parameter values were shared across experiments to yield a single best estimate of the mean of each parameter and its associated standard error, as derived from the nonlinear regression algorithm. This global pooled analysis approach ensured model convergence in all instances. *, significantly different from WT, p < 0.05, one-way ANOVA, Dunnett’s post hoc test. WT M5 mAChR n = 8; M5/M2 swap, F1303.52M, T1333.55A, R1343.56A, R1343.56K mutants n = 3; Y682.42F, V1233.45I, K141ICL2A, R1464.41M mutants n = 4. g VU6007678 affinity (pKB) and h log affinity cooperativity (logαβ) between ACh and VU6007876 at WT M5 mAChR and mutants from [3H]-NMS equilibrium binding studies. Data points represent mean ± SEM values of pharmacological parameters determined by fitting an allosteric ternary complex model to the grouped data, as described in (d–f). All pharmacology parameters (d–h) are listed in Supplementary Table 4. WT M5 mAChR n = 3, F1303.52M, R1464.41M n = 4, M5/M2 swap n = 5. Time courses of distances (Å) from VU6007678 to i F1303.52, j R1464.41, k M1504.45 calculated from GaMD simulations performed with three separate replicates as indicated through different coloured traces. Source data are provided as a Source Data file.
At WT M5 mAChR, VU6007678 demonstrated robust positive functional cooperativity with ACh signalling and exhibited significant allosteric agonism (Fig. 5b) in a Gq TruPath experiment. Given the selectivity of VU6007678 for the M5 mAChR over the M2 mAChR and its extensive ICL2 interactions, we first investigated non-conserved residues at the bottom of TMs 3, 4, and ICL2 by mutating them to their M2 mAChR counterparts. A construct containing multiple mutations (S1313.53C, I1323.54V, R1343.56K, R139ICL2P, A140ICL2V, P1444.39T, R1464.41M, I1494.44M, G1524.47A, and L1534.48A (referred to as the M5/M2 swap) caused a complete loss in the ability of VU6007678 to modulate ACh signalling and to display allosteric agonism (Fig. 5c). Individual residue analysis revealed that the R1464.41M mutation reduced both functional modulation and allosteric agonism, while the F1303.52M mutation completely abolished the affinity, functional modulation, and agonism of VU6007678 (Fig. 5d–f, Supplementary Fig. 7). These effects align with our structural data with R1464.41 forming a hydrogen bond with the carboxamide group of VU6007678, and a π-interaction between F1303.52 and the indanyl core of VU6007678 (Fig. 4i). Other single mutations (Y682.42F, V1233.45I, T1333.55A, R1343.56A, R1343.56K and K141ICL2A) showed no significant changes in affinity, functional modulation, or allosteric agonism (Fig. 5d–f, Supplementary Fig. 7).
Radioligand binding studies of F1303.52M, R1464.41M, and the M5/M2 swap showed no significant change in VU6007678 affinity (Fig. 5g, Supplementary Fig. 8, Supplementary Table 4). While the binding cooperativity (log α) was reduced in these constructs (Fig. 5h, Supplementary Fig. 8, Table 4), the values were not significantly different from WT M5 mAChR, likely due to higher uncertainty in the parameter calculations for the F1303.52M and M5/M2 swap constructs. The degree of efficacy modulation (β) by VU6007678 on ACh can be calculated by subtracting the binding modulation (log α) from the functional modulation (log αβ)34. This calculation at WT M5 mAChR yielded a log β value of 0.4 ± 0.2, indicating that VU6007678’s allosteric effect is primarily mediated through binding cooperativity, with a smaller contribution from efficacy modulation. This value could only be compared to the R1464.41M mutant, as the F1303.52M and M5/M2 swaps showed no functional modulation. The R1464.41M mutant yielded a log β value of −0.7 ± 0.3, suggesting impaired efficacy modulation by VU6007678. These findings are supported by our previous characterisation of VU6007678 in receptor alkylation studies, which revealed modest efficacy modulation in functional IP one assays29. Interestingly, this observation was unique to VU6007678 in the structure-activity relationship (SAR) study. Collectively, these data highlight the importance of the VU6007678 binding site residues in mediating both functional and efficacy modulation. VU6007678 directly interacts with F1263.48, Y1293.51, F1303.52, residues adjacent to D1273.49 and R1283.50 that form the DRY activation motif. Additionally, VU6007678 mediated stabilisation of ICL2 facilitates interactions between L136ICL2 and R139ICL2 with the α5 and αN helices of the G protein, respectively. Together, these interactions explain how VU6007678 functions as an Ago-PAM by stabilising key receptor activation motifs and G protein interactions.
Further validation of the allosteric binding site through GaMD simulations revealed key interactions between VU6007678 and receptor residues. The PAM formed stable interactions with F1303.52, R1464.41, and M1504.45 (Fig. 5i–k), consistent with both the cryo-EM structure and mutagenesis data. In contrast, hydrogen bond interactions between VU6007678 and receptor residues R1343.56, T1333.55, and K141ICL2 showed greater variability with larger distances and higher fluctuations (Supplementary Fig. 9), aligning with our experimental observations where mutations of these residues did not significantly affect VU6007678 function. In addition, comparison of the ACh- and ACh-VU6007678-bound GaMD simulations revealed that VU6007678 led to an increase in the helical turn of ICL2 by one residue, and reduced fluctuations in ICL2 residues K141ICL2 and R142ICL2 (Supplementary Fig. 6g, h). Distinct conformations of ICL2 have been linked to G protein bias47 and allosteric activation48. Together, these results highlight how VU6007678 engages key residues involved in the activation of the M5 mAChR, specifically via stable interactions with F1303.52 and R1464.41, while stabilizing part of ICL2 via interactions with K141ICL2 and R142ICL2.
Discussion
Allosteric modulators for the mAChRs have long been pursued for selective targeting of a specific mAChR subtype. Whilst selective allosteric modulators for the M5 mAChR have been discovered, the development and application of these have lagged behind those of allosteric modulators selective for other mAChRs, particularly the M1 and M4 mAChRs. This partly reflects the limited structural knowledge of the M5 mAChR overall, as well as the specific lack of insight into the allosteric binding site for M5-selective PAMs. Here we present a cryo-EM structure of the M5 mAChR bound to the orthosteric agonist iperoxo that ‘completes’ the active state structure ensemble for all five mAChR subtypes. Initial attempts to solve the co-bound structure of an allosteric modulator with ML380 and iperoxo were unsuccessful. Yet through the use of analytical pharmacology to determine the optimum orthosteric and allosteric ligand combination and improved biochemical techniques, we obtained a high-resolution structure of the M5 mAChR co-bound to the endogenous orthosteric agonist ACh and the selective PAM VU6007678. Our data begins to explain several facets of selective allosteric modulation at the M5 mAChR, including 1) the historic difficulties in developing subtype-selective PAMs for this mAChR subtype, 2) how changes to ML380 led to the improved PAM VU6007678, 3) subtype selectivity, and 4) the mechanism of action.
The in vivo translation of M5 mAChR-selective PAMs has been hindered by issues related to DMPK and suboptimal partition coefficients (Kp). The observation that the allosteric binding site for VU6007678 is located in the transmembrane (TM) bundle partially explains this, as modulators must display a high degree of lipophilicity to reach this allosteric binding site. Despite this, the VU6007678 scaffold offers numerous opportunities for modification, and our structure will aid in the process of developing improved allosteric modulators, as it provides information on the molecular interactions that occur between VU6007678 and the M5 mAChR at its allosteric binding site. Specifically, Y682.42 is the only residue within the allosteric binding site that differs across all M1-M4 mAChR subtypes. It may therefore be possible to introduce and optimize the presence of various polar functional groups on the indanyl core to promote hydrogen bonding between the ligand and receptor and enhance affinity whilst reducing the compound’s lipophilicity. Note that the compounds reported in the previous SAR series all had a fluorine functional group attached to the indanyl core26. Enhancing the selectivity of PAMs for the M5 mAChR will be crucial, in part, to the future clinical success of M5 mAChR PAMs. All M5 PAMs discovered to date display activity at the M1 and M3 mAChRs, whilst they are most selective against the M2 and M4 mAChRs, where they display very little to no activity. Analysis of the residues within the VU6007678 allosteric binding site may explain the basis for this, as R1343.56 and R1464.41 are fully conserved between the M5 mAChR and M1/M3 mAChRs and non-conserved between the M5 mAChR and the M2/M4 mAChRs.
Despite the absence of an ML380-bound M5 mAChR structure, given that VU6007678 is a derivative of ML380, predictions on how ML380 interacts with this site can be made. This allows for an explanation of how the changes to VU6007678 led to an improved PAM. The extension of the ethyl present in ML380 into a propyl group at VU6007678 gives rise to an extra interaction with I1494.44. In addition, the substitution of the trifluoromethylbenzyl group at ML380 with the indanyl group gives rise to more hydrophobic interactions and greater engagement with the top of the allosteric site consisting of V1233.45, F1263.48 and M1504.45 where edge-to-face π-interaction takes place with F1263.48.
Our structures offer key insights into the mechanism of action for PAMs at the M5 mAChR. The allosteric binding site of VU6007678 is distant from the orthosteric site but positioned near the highly conserved DRY activation motif, suggesting that PAM binding stabilizes the active state of the M5 mAChR. This is in contrast to other allosteric modulators at the mAChRs. The mAChR family has served as a model receptor family for the study of allosteric modulation at GPCRs19. Despite a wealth of mAChR allosteric modulators with different scaffolds available, this is only the second site to be confirmed by structural biology studies at the mAChRs33,34 (Fig. 6a). All PAMs discovered to date bind to the mAChRs at what is termed the ECV allosteric site in a solvent accessible vestibule on top of the orthosteric binding site. Here, PAMs exert their mechanism of action through two mechanisms: (1) trapping the orthosteric ligand in the orthosteric site through stabilising the active state and (2) sterically hindering orthosteric ligand dissociation49,50. Due to the distant location of the VU6007678 binding site to the mAChR orthosteric site, our findings suggest that PAMs at this site act exclusively by stabilising the active state. Numerous allosteric modulators at other GPCRs have been structurally confirmed to bind at the same site as VU6007678 at the M5 mAChR, with diverse mechanisms of action (Fig. 6b)51,52,53,54,55,56,57,58,59. Structures of the M5 mAChR have an ordered ICL2 in an α-helical conformation in both the inactive and active states, irrespective of VU6007678 binding, although GaMD simulations suggest VU6007678 binding stabilizes an additional turn of the ICL2 α-helix. Conversely, at the β2-adrenergic receptor (β2AR), ICL2 is disordered in the inactive state and becomes α-helical upon receptor activation. Similar to VU6007678, the β2AR PAM Cmpd-6FA stabilises ICL2, but unlike VU6007678, Cmpd-6FA does influence the allosteric site by changing the orientation of interacting residues52. In contrast, at the free fatty acid receptor 1 (FFAR1), ICL2 only becomes ordered in the presence of the PAM AP848,56. Together, these structures illustrate that despite all binding to the extrahelical interface of TM2,3,4, the manner through which they engage with ICL2 differs.
a Model of the ACh-VU6007678 bound M5 mAChR showing the allosteric binding site discovered for VU6007678 (orange spheres) and the ECV allosteric site occupied by LY2119620 (salmon spheres). ACh is shown as green spheres in the orthosteric binding site. b Allosteric modulator structures overlaid on the VU allosteric site, with ligands shown as ball-and-stick models. D1:LY3154207:cholesterol in dark slate grey and slate grey, respectively (PDB:7X2F). β2AR:Cmpd-6FA in light sea green (PDB:6N48). FFAR1:AP8 in violet (PDB:5TZY). GPBAR1:INT-177 in purple (PDB:7CFN). CASR:NDT9513727 in salmon (PDB:6C1Q). C5aR:avacopan in green (PDB:6C1R). c Cryo-EM density (contour level 0.47) for lipid molecules observed at the bottom of TM2,3,4.
Hydrogen-deuterium exchange mass spectrometry and NMR studies suggest that the conformational dynamics of ICL2 are important for receptor interactions with the Gα protein and G protein specificity47,60,61,62,63. Thus, targeting the ICL2 allosteric site could facilitate the design of biased allosteric modulators64. Interestingly, we observed that the K141ICL2A mutant increased the Emax of ACh-mediated Gq activation in the presence of VU6007678 compared to WT M5 mAChR. In GaMD simulations, K141ICL2 was relatively dynamic, though less so when bound to VU6007678. Mutation of K141ICL2 to alanine likely alters the conformational dynamics of ICL2, leading to increased signalling when bound to VU6007678. Future studies investigating the dynamics of ICL2 at different GPCRs when bound to PAMs will aid our understanding of G protein signalling and the design of biased allosteric modulators.
Though less reported, NAMs at the extrahelical interface of TMs 3,4 are reported to inhibit receptor activation by inhibiting helical movement required for receptor activation58,59. NAM structures identified to date suggest NAMs occupy a more confined site at the centre of the TM2,3,4 interface. This raises an interesting possibility: NAMs may primarily target allosteric sites at the centre of the extrahelical interface to restrict the conformational changes necessary for receptor activation, whereas PAMs engage closer to the cytosol, ICL2, and activation motifs to stabilize the active state. The discovery of a methionine (M4.45) residue within the VU6007678 allosteric binding site enables deeper investigation into allosteric mechanisms. Spectroscopic analysis of PAM activity at a methionine-labelled M5 mAChR, as done for LY2119620 at the M2 mAChR65, will further elucidate their mode of action.
At the bottom of the TM2,3,4 interface of the ACh-VU6007678 structure, we observed well-defined densities that likely correspond to three cholesterol molecules (Fig. 6c). Note that in our iperoxo structure, this site was occupied by a partial density that we cautiously hypothesised could be ML380. Considering the much higher resolution of the ACh-VU6007678 structure, and assignment of the VU6007678 binding site at the bottom of the extrahelical interface at TMs3-4, it is likely that the density present in the iperoxo-bound structure represents cholesterol molecules. Especially given that this site is recognised as a common cholesterol site within class A GPCRs44,66 and that neurosteroids and steroid hormones, including derivatives of cholesterol, have displayed modulatory properties at the M5 mAChR67,68. Due to the lower density and map quality in this region, it was difficult to conclusively assign this density to either ML380 or cholesterol in the iperoxo structure, highlighting the map quality required to assign density to small molecules. Particularly at extrahelical regions where a number of lipid and cholesterol molecules may be present.
Altogether and more broadly, the discovery of this extrahelical allosteric binding site at the M5 mAChR adds to the knowledge of allostery at the mAChR, specifically on how allosteric modulators engage with the mAChRs, and provides an additional avenue through which to target these highly conserved proteins.
Methods
Chemical Probe Statement
ML380 is a positive allosteric modulator for the Gαq/11 coupled mAChRs subtypes (M1, M3, M5) with a binding affinity for the ACh-bound M5 mAChR of ~575 nM (determined in this study and others25,28). VU6007678 is an improved chemical analogue with ~30 nM affinity (determined in this study and others29) for the ACh-bound M5 mAChR and 150 nM affinity for the M1 and M3 mAChRs (5-fold selectivity, determined in other studies29). We used ML380 and VU6007678 at concentration ranges that are appropriate for in vitro pharmacology studies and cryo-EM. In the development of ML380, the (S) and (R) enantiomers were tested for activity, with the (S)-enantiomer being inactive25. Both ML380 and VU6007678 are the (R)-enantiomers. Both probes are active on receptors expressed in cells. We did not use the inactive (S)-enantiomer probe in this study, as our study was designed for the validation of the allosteric binding site using cryo-EM. As previously reported, these molecules likely need optimisation before being used in animal models25,28,29.
Receptor & G protein co-expression
A modified M5 mAChR construct was used where residues 237-421 of ICL3 were removed and HA signal sequence and anti-Flag epitope tag were added to the N terminus. Modified M5 mAChR was fused to a mGαq chimeric construct that is a mini-Gαs substituted with Gαq residues at the receptor interface and the αN of Gαi39,40. A 3 C protease recognition site and GGGS linker were used to separate the receptor and G protein. The fused M5ΔICL3mGαq/construct was cloned into a pFastbac baculovirus transfer vector. G protein β1 and γ2 subunits were cloned into a pVL1392 baculovirus transfer vector with the β subunit modified to contain a carboxy (C)-terminal 8× histidine tag. Trichoplusia ni (Hi5) insect cells were grown in ESF 921 serum-free media (Expression Systems) and infected at a density of 4.0 × 106 cells per millilitre with a 1:1 ratio of M5ΔICL3mGαq to Gβ1γ2 viruses and shaken at 27 °C for 48–60 hours. Cells were harvested by centrifugation, and the cell pellets were flash-frozen using liquid nitrogen and stored at −80 °C. Sf9 and Hi5 cells were not tested for mycoplasma.
Single-chain stabilising fragment expression and purification
A single-chain construct of Fab16 (scFv16)41, tagged with an 8× histidine sequence at the C-terminus, was cloned into a modified pVL1392 baculovirus transfer vector for secreted expression in Trichoplusia ni (Hi5) insect cells (Expression Systems). The cells were cultured in serum-free ESF 921 media (Expression Systems), infected at a density of 4.0 × 106 cells per millilitre, and incubated with shaking at 27 °C for 48-72 hours. For purification, the pH of the supernatant from the baculovirus-infected cells was adjusted with Tris pH 8.0. Chelators were neutralized by adding 5 mM CaCl2 and stirring the solution for 1 hour at 25 °C. Precipitates were cleared by centrifugation, and the supernatant was then applied to Ni-NTA resin. The column was washed with a solution of 20 mM Hepes pH 7.5, 50 mM NaCl, and 10 mM imidazole, followed by a second wash containing the same buffer with 100 mM NaCl. The scFv16 protein was eluted using the low-salt buffer with 250 mM imidazole. SDS-PAGE with Coomassie staining was used to assess the purity of the eluted protein. Finally, the sample was concentrated, flash-frozen in liquid nitrogen, and stored at −80 °C.
Nb35 expression and purification
Nb35 was expressed in the periplasm of the BL21(DE3) Rosetta Escherichia coli cell line using an autoinduction approach69. Transformed cells were cultured at 37 °C in a modified ZY medium containing 50 mM phosphate buffer (pH 7.2), 2% tryptone, 0.5% yeast extract, 0.5% NaCl, 0.6% glycerol, 0.05% glucose, and 0.2% lactose, with 100 μg/ml carbenicillin and 35 μg/ml chloramphenicol. When the culture reached an OD600 of 0.7, the temperature was reduced to 20 °C for approximately 16 hours, after which cells were harvested by centrifugation and stored at −80 °C. To purify, cells were lysed in ice-cold buffer (0.2 M Tris pH 8.0, 0.5 M sucrose, 0.5 M EDTA) at a ratio of 1 g of cell pellet to 5 mL of lysis buffer for 1 hour at 4 °C. 2x volume of ice-cold MQ was added and incubated for an additional 45 minutes at 4 °C. Lysate was centrifuged to remove cell debris, and supernatant containing Nb35 was spiked with 20 mM HEPES pH 7.5, 150 mM NaCl, 5 mM MgCl2, 5 mM Imidazole and applied to Ni-NTA resin, followed by 90 minutes incubation at 4 °C. The column was washed with 40 column volumes (CV) of wash buffer (20 mM HEPES pH 7.5, 500 mM NaCl, 5 mM Imidazole) and eluted with elution buffer (20 mM HEPES pH 7.5, 100 mM NaCl, 250 mM Imidazole). Elute was concentrated and stored at −80 °C.
Complex purification
Iperoxo-bound M5 mAChR-mGαq complex
M5ΔICL3-mGαq co-expressed with Gβ1γ2 was thawed and lysed in 20 mM HEPES pH 7.4, 5 mM MgCl2, 1 μM Ipx and protease inhibitors (500 µM PMSF, 1 mM LT, 1 mM benzamidine). The sample was rotated at room temperature for 15 minutes and spiked with apyrase towards the end of the 15 minutes. The pellet was spun down by centrifugation and solubilized in 20 mM HEPES pH 7.4, 100 mM NaCl, 5 mM MgCl2, 5 mM CaCl2, 0.5% LMNG (Anatrace, Maumee, OH, USA), 10 μM Ipx, and protease inhibitors (500 µM PMSF, 1 mM LT, 1 mM benzamidine). The resuspended pellet was homogenised in a Dounce homogeniser, and complex formation was initiated through addition of scFv16. The sample was incubated with stirring at 4 °C for 2 hours followed by centrifugation to remove insoluble material. Solubilised complex was bound to equilibrated M1 anti-Flag affinity resin through batch binding at room temperature for 1 hour. The resin was packed into a glass column and washed with 20 mM HEPES pH 7.4, 100 mM NaCl, 5 mM MgCl2, 5 mM CaCl2, 0.01% LMNG, and 1 μM Ipx until no more protein was coming off the column as determined by Bradford. Complex was eluted using 20 mM HEPES pH 7.4, 100 mM NaCl, 5 mM MgCl2, 0.01% LMNG, and 1 μM Ipx in the presence of 5 mM EDTA and 0.1 mg/ml Flag peptide. Eluted complex was concentrated in an Amicon Ultra-15 100 kDa molecular mass cut-off centrifugal filter unit (Millipore, Burlington, MA, USA) and purified by size exclusion chromatography (SEC) on a Superdex 200 Increase 10/300 GL (Cytiva, Marlborough, MA, USA) in 20 mM HEPES pH 7.4, 100 mM NaCl, 5 mM MgCl2, 0.01% LMNG, and 1 μM Ipx. Fractions containing complex (as determined by SDS-page and Coomassie staining) were pooled and concentrated to 3.5 mg/mL, flash frozen using liquid nitrogen and stored at −80 °C.
ACh-VU6007678-bound M5 mAChR-mGαq complex
The ACh-VU6007678 M5 mAChR sample was purified in an identical manner with the following changes; (1) complex formation was initiated through addition of scFv16 and Nb35, (2) ACh was included in all buffers at 100 μM until SEC where a concentration of 10 μM was utilised, (3) VU6007678 was present throughout the purification at a concentration of 10 μM. The final purified product was concentrated to 18 mg/mL.
Vitrified sample preparation and data collection
For the iperoxo-M5 mAChR sample, EMAsian - TiNi 200 mesh 1.2/1.3 grids were glow discharged using Pelco EasyGlow for 90 seconds with 15-mA current. Prior to grid freezing, the iperoxo-M5 mAChR sample was spiked with 30 μM of ML380 and incubated overnight at 4 °C. 3 μL of this spiked sample was applied and flash frozen in liquid ethane using a Vitrobot markIV with a blot force of 4 and blot time of 2 seconds at 100% humidity and 4 °C. Data were collected on a Titan Krios (Thermo Fisher Scientific) 300 kV electron microscope equipped with a K3 detector, 50 μm C2 aperture, no objective aperture inserted, indicated magnification x 130 000 in nanoprobe TEM mode, a slit width of 10 eV, pixel size 0.65 Å, exposure rate 10.57 counts per pixel per second, exposure time 2.68 s, total exposure 60 e Å-2, and 60 frames. In total, 9104 movies were collected.
For the ACh-VU6007678 M5 mAChR sample, 3 µL of sample was applied to a glow-discharged (15 mA, 180 s) UltrAufoil R1.2/1.3 300 mesh holey grid (Quantifoil) and was frozen in liquid ethane using a Vitrobot mark IV (Thermo Fisher Scientific) at 100% humidity and 4 °C with a blot time of 2 s and blot force of 10. The sample was collected similarly, except at 105kX magnification with a pixel size of 0.82 Å, and 7489 movies were collected.
Image Processing
For the iperoxo-M5 mAChR sample, 9104 movies were collected and adjusted for beam-induced motion by MotionCor270. Non-dose-weighted micrographs were used for CTF estimation using Gctf71. 8341 micrographs were identified as having a ctf fit resolution below 4 Å, and these were selected for further examination. 3,833,583 particles were autopicked using Gautomatch (https://www2.mrc-lmb.cam.ac.uk/research/locally-developed-software/zhang-software/#gauto). The particles were extracted with relion-3.172 and then imported into CryoSparc73 for rounds of 2D classification, ab initio 3D and 3D refinement to obtain a 3.04 Å model. Particles were taken to Relion3.1 for polishing and subsequent 3D refinement back in Cryosparc yielded a final model of 2.75 Å based on the gold standard Fourier shell correlation cut-off of 0.143 from 426,714 particles. A further local refinement was performed to generate a receptor-focused map (2.67 Å).
The ACh-VU6007678 M5 mAChR sample was processed similarly. 7489 movies were collected and adjusted for beam-induced motion by MotionCor270. Non-dose weighted micrographs were used for CTF estimation using Gctf71. 4,436,835 particles were autopicked using Gautomatch (https://www2.mrc-lmb.cam.ac.uk/research/locally-developed-software/zhang-software/#gauto). The particles were extracted with relion-3.172 and then imported into CryoSparc73 for rounds of 2D-classification and heterogeneous refinement. A set of particles (~900k) was polished in relion3.1 and a final round of 3D-classification (no alignment) was performed. This final set of 418,794 particles was finally subjected to non-uniform refinement with CTF-refinement in CryoSparc, resulting in a final map of 2.06 Å based on the gold standard Fourier shell correlation cut-off of 0.143. A further local refinement was performed to generate a receptor-focused map (2.11 Å).
Model building and refinement
An initial receptor model was generated from the cryo-EM structure of the M4 mAChR receptor (PDB: 7TRP). An initial model for the G protein (mGαq:Gβ1Gγ2:ScFv16) was generated from the CCK1:mGαq complex (PDB: 7MBY). Initial models were placed in the EM maps using UCSF ChimeraX74 and rigid-body-fit using PHENIX75. Models were refined with iterative rounds of manual model building in Coot76 and ISOLDE, and real-space refinement in PHENIX. Ligands ACh and iperoxo were obtained from the monomer library, while the initial model and restraints for VU6007678 were generated using the GRADE web server (https://grade.globalphasing.org). Model validation was performed with MolProbity77 and the wwPDB validation server78. Figures were generated with UCSF ChimeraX and PyMOL (Schrödinger).
Cell culture
FlpIn Chinese hamster ovary (CHO) cells (Thermo Fisher Scientific) stably expressing M5 mAChR constructs were cultured at 37 °C in 5% CO2 using Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen) supplemented with 5% foetal bovine serum (FBS; ThermoTrace). At confluence, media was removed, and cells were washed with phosphate-buffered saline (PBS) and harvested from tissue culture flasks using Versene (PBS with 0.02% EDTA). The cells were pelleted by centrifugation at 350 g for three minutes and then resuspended in DMEM with 5% FBS. Subsequently, the cells were either plated for an assay or reseeded into a tissue culture flask. CHO cells were regularly tested to ensure they were free from mycoplasma.
Inositol Monophosphate (IP1) Accumulation Assay
FlpIn CHO cells stably expressing either WT or mutant hM5 mAChR were seeded in clear, flat bottom 96-well plates at a density of 10,000−25,000 cells per well (depending on the cell line) one day prior to the assay. The optimal cell density for each line was chosen based on achieving an IP1 response that fell within the linear range of the IP1 standard curve. On the assay day, the medium was replaced with stimulation buffer (Hanks’ balanced salt solution (HBSS) containing 10 mM HEPES, 1.3 mM CaCl2, and 30 mM LiCl, pH 7.4) and allowed to incubate for 60 minutes at 37 °C before ligand stimulation. After this pre-incubation, the buffer was replaced, and cells were exposed to ligands for 60 minutes at 37 °C in a 5% CO2 atmosphere, with a total assay volume of 100 μL. Following the 60-minute stimulation, ligands were removed by rapid removal of buffer. Cells were lysed by freeze-thawing in 30 μL of stimulation buffer. IP1 accumulation was then quantified using the HTRF IP-One assay kit (Cisbio), with fluorescence measured on an EnVision multilabel plate reader (PerkinElmer).
TruPath – G protein Activation Assay
Upon reaching 60-80% confluence, FlpIn CHO cells stably expressing WT or mutant hM5 mAChR were transiently transfected using Polyethylenimine (PEI; Sigma-Aldrich). For each well, 10 ng of each plasmid (pcDNA5/FRT/TO-Gαq-RLuc8, pcDNA3.1-β3, and pcDNA3.1-Gγ9-GFP2) was added in a 1:1:1 ratio, totalling 30 ng. These plasmids were generously provided by Prof. Bryan Roth from the University of North Carolina. The cells were then plated at 30,000 cells per well into 96-well Greiner CELLSTAR white-walled plates (Sigma-Aldrich). After 48 hours, the cells were washed with 200 μL PBS and replaced with 1x HBSS supplemented with 10 mM HEPES. The cells were incubated for 30 minutes at 37 °C before adding 10 μL of 1.3 μM Prolume Purple coelenterazine (Nanolight Technology, Pinetop, AZ). Following a further 10-minute incubation at 37 °C, bioluminescence resonance energy transfer (BRET) measurements were performed using a PHERAstar FSX plate reader (BMG Labtech) with 410/80-nm and 515/30-nm filters. Four baseline measurements were taken before adding drugs or vehicle, bringing the final assay volume to 100 μL, followed 10 more minutes of readings. The BRET signal was calculated as the ratio of 515/30-nm emission to 410/80-nm emission. This ratio was vehicle-corrected using the initial four baseline measurements and then baseline-corrected again using the vehicle-treated wells. Data were normalized to the maximum ACh response to allow for grouping of results.
Radioligand Binding
FlpIn CHO cells stably expressing WT hM5 mAChR or mutants were plated at 25,000 cells per well in 96-well isoplates (PerkinElmer Life Sciences) and incubated overnight at 37 °C in a 5% CO2 incubator. The following day, the cells were washed with PBS and incubated in 20 mM HEPES, 100 mM NaCl, 10 mM MgCl2, pH 7.4. For saturation binding experiments, the cells were incubated with varying concentrations of the orthosteric antagonist [3H]-N-methylscopolamine ([3H]-NMS; specific activity, 70 Ci/mmol, Perkin Elmer) in a final volume of 100 μL for 6 hours at room temperature. For interaction experiments between orthosteric agonist and allosteric modulator, competition binding was performed between a KD concentration of [3H]-NMS and varying concentrations of an orthosteric drug in the presence of different concentrations of an allosteric modulator, in a total volume of 100 μL binding buffer. For all experiments, non-specific binding was defined using 10 μM of atropine. The assay was terminated by the rapid removal of the radioligand, followed by two 100 μL washes with ice-cold 0.9% NaCl buffer. Radioactivity was measured by adding 100 μL of Optiphase Supermix scintillation fluid (PerkinElmer) and counted using a MicroBeta2 Plate Counter (PerkinElmer Life Sciences).
Data analysis
All data were analysed using GraphPad Prism 10 (GraphPad Software, San Diego, CA). The interaction between orthosteric agonist and allosteric modulator in functional assays was analysed using an operational model of allosterism to determine functional modulation (log αβ) and affinity (pKB) parameters36. Radioligand saturation binding experiments with [3H]-NMS to determine Bmax and pKD values were determined using a one site – specific binding equation in Prism 1031. For the radioligand binding interaction of orthosteric agonist with various concentrations of allosteric modulator, the data were fit to an allosteric ternary complex model to derive pKB and α binding cooperativity parameters79. All affinity, potency, cooperativity, and efficacy parameters were estimated as logarithms. To ensure model convergence in all instances, allosteric parameters were determined from the respective allosteric models, which were globally fitted to all individual datasets for each allosteric modulator at each receptor construct. These parameters were constrained to be shared across these datasets. The resulting reported parameter estimates thus represent the least-squares best-fit value of each parameter, with its associated standard error as reported from the nonlinear regression programme, based on the pooled analysis of the individual datasets. Statistical analysis between different treatment conditions was performed using one-way ANOVA, with a p-value of <0.05 considered significant.
Gaussian accelerated Molecular Dynamics (GaMD) simulations anD Simulation Analysis
GaMD is an enhanced sampling method that works by adding a harmonic boost potential to reduce the system energy barriers. Details of the method have been described in previous studies80,81. A summary is provided here. Consider a system with N atoms at positions \({r}^{ \rightharpoonup }=\left\{{{r}^{ \rightharpoonup }}_{1},\cdots,{{r}^{ \rightharpoonup }}_{N}\right\}\). When the system potential \(V({r}^{ \rightharpoonup })\) is lower than a reference energy E, the modified potential \({V}^{*}({r}^{ \rightharpoonup })\) of the system is calculated as:
where k is the harmonic force constant. The two adjustable parameters E and k are automatically determined based on three enhanced sampling principles. The reference energy needs to be set in the following range:
where Vmax and Vmin are the system minimum and maximum potential energies. To ensure that Eq. (3) is valid, k has to satisfy: \(k\le \frac{1}{{V}_{\max }-{V}_{\min }}\) Let us define \(k\equiv {k}_{0}\frac{1}{{V}_{\max }-{V}_{\min }}\), then \({0 < k}_{0}\le 1\). The standard deviation of \(\Delta V\) needs to be small enough (i.e., narrow distribution) to ensure proper energetic reweighting: \({\sigma }_{\Delta V}=k\left(E-{V}_{{avg}}\right){\sigma }_{V}\le {\sigma }_{0}\) where \({V}_{{avg}}\) and \({\sigma }_{V}\) are the average and standard deviation of the system potential energies, \({\sigma }_{\Delta V}\) is the standard deviation of \(\Delta V\) with \({\sigma }_{0}\) as a user-specified upper limit (e.g., 10kBT) for proper reweighting. When E is set to the lower bound E=Vmax,\(\,{k}_{0}\) can be calculated as:
Alternatively, when the threshold energy E is set to its upper bound\(\,E={V}_{\min }+\frac{1}{k}\),\({k}_{0}\) is set to:
if \({k{\prime\prime} }_{0}\) is found to be between 0 and 1. Otherwise,\(\,{k}_{0}\) is calculated using Eq. (4).
The cryo-EM structures of the ACh-VU6007678-bound M5 mAChR-mGαq complex and the iperoxo-bound M5 mAChR-mGαq complex were used to set up initial simulation systems. For simulations with ACh-M5 mAChR-mGαq, VU6007678 was manually removed from the structure.The initial model of the iperoxo-ML380-bound M5 mAChR-mGαq complex was created by docking ML380 into the bottom of the TM2,3,4 interface. As in our previous studies34, the intracellular loop 3 (ICL3) of the receptor and the α-helical domain of the G protein, which were missing in the cryo-EM structure, were not modelled. The MD simulation systems were prepared by inserting the ACh-VU6007678-bound M5 mAChR-mGαq and the iperoxo-bound M5 mAChR-mGαq complexes into a POPC (palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine) lipid bilayer using VMD (Visual Molecular Dynamics). In each simulation system, the protein and lipid bilayer were solvated with TIP3P water molecules in a box of 12.5 nm x 12.5 nm x 14.0 nm with the periodic boundary condition. The system charge was neutralized with 150 mM NaCl. The AMBER FF14SB force field82 was applied for the proteins, while the AMBER LIPID21 force field83 was used for the lipids. The general amber force field (GAFF2) parameters84 for ACh, iperoxo, ML380, and VU6007678 were generated with ANTECHAMBER. The two simulation systems were first energy minimized for 5,000 steps with constraints on the heavy atoms of the proteins and phosphor atom of the lipids. The hydrogen-heavy atom bonds were constrained using the SHAKE algorithm and the simulation time step was set to 2.0 fs. The particle mesh Ewald (PME) method85 was employed to compute the long-range electrostatic interactions and a cutoff value of 9.0 Å was applied to treat the non-bonded atomic interactions. The temperature was controlled using the Langevin thermostat with a collision frequency of 1.0 ps-1. Each system was equilibrated using the constant number, volume, and temperature (NVT) ensemble at 310 K for 250 ps and under the constant number, pressure, and temperature (NPT) ensemble at 310 K and 1 bar for another 1 ns with constraints on the heavy atoms of the protein, followed by 10 ns short conventional MD (cMD) without any constraint.
The GaMD module implemented in the GPU version of AMBER1880,81,86 was then applied to simulate the ACh-VU6007678-bound M5 mAChR-mGαq and iperoxo-bound M5 mAChR-mGαq complexes. The GaMD simulations included an 8-ns short cMD run to collect the potential statistics for calculating GaMD acceleration parameters, followed by a 56-ns GaMD equilibration after adding the boost potential. Finally, three independent 500-ns GaMD production simulations were conducted for each system with randomized initial atomic velocities. The average and standard deviation (SD) of the system potential energies were calculated every 800,000 steps (1.6 ns). All GaMD simulations were performed at the “dual-boost” level, where the reference energy was set to the lower bound. One boost potential was applied to the dihedral energetic term and the other to the total potential energetic term. The upper limit of the boost potential SD (σ0) was set to 6.0 kcal/mol for both the dihedral and the total potential energetic terms.
For each system, the three GaMD production trajectories were combined for analysis. The CPPTRAJ software tool87 was applied to calculate the time-courses of the root-mean-square derivations (RMSDs) of agonists and PAMs relative to the simulation starting structure, as well as the distances between VU6007678 and key interacting residues in the receptor, including F1303.52, R1464.41, M1504.45, F1263.48, R1343.56, V1233.45, T1333.55 and K141ICL2.
Use of AI-assisted writing
The AI tool Claude 5 Sonnet (Anthropic, 2025) was used for proofreading of the manuscript, checking for consistency in spelling, grammar, and clarity of the text. It was not used for generating ideas, content, figures, or data.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Atomic coordinates were deposited in the Protein Data Bank (PDB) under accession codes 9EK0 (iperoxo-bound M5 AChR-mGq-scFV16); 9EJZ (ACh-VU6007678-bound M5 AChR-mGq-scFv16-Nb35 complex). Cryo-EM maps were deposited in the Electron Microscopy Data Bank under the accession codes EMD-48111 (consensus map for iperoxo-bound M5 AChR-mGq-scFV16); EMD-48109 (receptor focus map for iperoxo-bound M5 AChR-mGq-scFV16); EMD-48110 (consensus map for ACh-VU6007678-bound M5 AChR-mGq-scFv16-Nb35 complex); EMD-48108 (receptor focus map for ACh-VU6007678-bound M5 AChR-mGq-scFv16-Nb35 complex). Atomic coordinates for previously determined structures can be accessed via accession codes: 6OIJ, 6OIK,8E9Z, 7TRK, 7X2F, 6N48,5TZY, 7CFN, 6C1R, 6C1Q.
Code availability
Initial coordinate, simulation input files, and combined imaged trajectory files for GaMD simulations of the ACh-VU6007678-bound, iperoxo-ML380-bound and ACh-bound M5 mAChR-mGαq complexes in the public repository Figshare. The following are the links: [https://doi.org/10.6084/m9.figshare.28673348], [https://doi.org/10.6084/m9.figshare.28673351], [https://doi.org/10.6084/m9.figshare.29162954]. Source data are provided with this paper.
References
Bender, A. M., Garrison, A. T. & Lindsley, C. W. The Muscarinic Acetylcholine Receptor M5: Therapeutic implications and allosteric modulation. ACS Chem. Neurosci. 10, 1025–1034 (2019).
Vilaro, T. M., Palacios, J. M. & Mengod, G. Localization of m5 muscarinic receptor mRNA in rat brain examined by in situ hybridization histochemistry. Neurosci. Lett. 114, 154–159 (1990).
Weiner, D. M., Levey, A. I. & Brann, M. R. Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc. Natl. Acad. Sci. 87, 7050–7054 (1990).
Yasuda, R. P. et al. Development of antisera selective for m4 and m5 muscarinic cholinergic receptors: Distribution of m4 and m5 receptors in rat brain. Mol. Pharmacol. 43, 149–157 (1993).
Foster, D. J. et al. M5 receptor activation produces opposing physiological outcomes in dopamine neurons depending on the receptor’s location. J. Neurosci. 34, 3253–3262 (2014).
Forster, G. L., Yeomans, J. S., Takeuchi, J. & Blaha, C. D. M5 Muscarinic receptors are required for prolonged accumbal dopamine release after electrical stimulation of the pons in mice. J. Neurosci. 21, (2002).
Garrison, A. T. et al. Development of VU6019650: A potent, highly selective, and systemically active orthosteric antagonist of the M5 Muscarinic Acetylcholine receptor for the treatment of opioid use disorder. J. Med. Chem. 65, 6273–6286 (2022).
Orsi, D. L. et al. Discovery of a potent M5 antagonist with improved clearance profile. Part 2: Pyrrolidine amide-based antagonists. Bioorg. Medicinal Chem. Lett. 78, 129021 (2022).
Fink-Jensen, A. et al. Role for M5 muscarinic acetylcholine receptors in cocaine addiction. J. Neurosci. Res. 74, 91–96 (2003).
Berizzi, A. E. et al. Muscarinic M5 receptors modulate ethanol seeking in rats. Neuropsychopharmacology 43, 1510–1517 (2018).
Gunter, B. W. et al. Selective Inhibition of M5 Muscarinic Acetylcholine Receptors attenuates cocaine self-administration in rats. Addictive Biol. 23, 1106–1116 (2018).
Gould, R. W. et al. Acute negative allosteric modulation of M5 muscarinic acetylcholine receptors inhibits oxycodone self-administration and cue-induced reactivity with no effect on antinociception. ACS Chem. Neurosci. 10, 3740–3750 (2019).
Nunes, E. J. et al. Examining the role of muscarinic M5 receptors in VTA cholinergic modulation of depressive-like and anxiety-related behaviors in rats. Neuropharmacology 108089 (2020).
Walker, L. C. et al. Muscarinic M4 and M5 receptor subtypes in the ventral subiculum differentially modulate alcohol seeking vs consumption in male alcohol preferring rats. Br. J. Pharmacol. 178, 3730–3746 (2021).
Basile, A. S. et al. Deletion of the M5 muscarinic acetylcholine receptor attenuates morphine reinforcement and withdrawal but not morphine analgesia. Proc. Natl. Acad. Sci. 99, 11452–11457 (2002).
Yamada, M. et al. Cholinergic dilation of cerebral blood vessels is abolished in M5 muscarinic acetylcholine receptor knockout mice. Proc. Natl. Acad. Sci. 98, 14096–14101 (2001).
Araya, R. et al. Loss of M5 muscarinic acetylcholine receptors leads to cerebrovascular and neuronal abnormalities and cognitive deficits in mice. Neurobiol. Dis. 24, 334–344 (2006).
Vuckovic, Z. et al. Crystal structure of the M5 muscarinic acetylcholine receptor. Proc. Natl. Acad. Sci. 116, 26001–26007 (2019).
Burger, W. A. C., Sexton, P. M., Christopoulos, A. & Thal, D. M. Toward an understanding of the structural basis of allostery in muscarinic acetylcholine receptors. J. Gen. Physiol. 150, 1360–1372 (2018).
Foster, D. J. & Conn, P. J. Allosteric modulation of GPCRs: New insights and potential utility for treatment of schizophrenia and other CNS disorders. Neuron 94, 431–446 (2017).
Bridges, T. M. et al. Discovery of the first highly M5-preferring muscarinic acetylcholine receptor ligand, an M5 positive allosteric modulator derived from a series of 5-trifluoromethoxy N-benzyl isatins. J. Medicinal. Chem. 52, 3445–3448 (2009).
Bridges, T. M. et al. Chemical lead optimization of a pan Gq mAChR M1, M3, M5 positive allosteric modulator (PAM) lead. Part I: Development of the first highly selective M5 PAM. Bioorg. Med. Chem. Lett. 20, 558–562 (2010).
Gentry, P. R. et al. Discovery of the first M5-selective and CNS penetrant negative allosteric modulator (NAM) of a muscarinic acetylcholine receptor: (S)-9b-(4-chlorophenyl)-1-(3,4-difluorobenzoyl)-2,3-dihydro-1 H -imidazo[2,1- a]isoindol-5(9b H)-one (ML375). J. Medicinal Chem. 56, 9351–9355 (2013).
Gentry, P. R. et al. Discovery of ML326: The first sub-micromolar, selective M5 PAM. Bioorg. Med. Chem. Lett. 23, 2996–3000 (2013).
Gentry, P. R. et al. Development of a highly potent, novel M5 positive allosteric modulator (PAM) demonstrating CNS exposure: 1-((1H-indazol-5-yl)sulfoneyl)-N-ethyl-N-(2-(trifluoromethyl)benzyl)piperidine-4-carboxamide (ML380). J. Med. Chem. 57, 7804–7810 (2014).
Bender, A. M. et al. Discovery and optimization of potent and CNS penetrant M5-preferring positive allosteric modulators derived from a novel, Chiral N-(Indanyl)piperidine Amide Scaffold. ACS Chem. Neurosci. 9, 1572–1581 (2018).
Marlo, J. E. et al. Discovery and characterization of novel allosteric potentiators of M1 Muscarinic receptors reveals multiple modes of activity. Mol. Pharmacol. 75, 577–588 (2009).
Berizzi, A. et al. Molecular mechanisms of action of M5 Muscarinic Acetylcholine receptor allosteric modulators. Mol. Pharmacol. 90, 427–436 (2016).
Berizzi, A. E. et al. Structure-activity relationships of Pan-Gαq/11 coupled muscarinic acetylcholine receptor positive allosteric modulators. ACS Chem. Neurosci. 9, 1818–1828 (2018).
Stahl, E. & Ellis, J. Novel allosteric effects of Amiodarone at the Muscarinic M5 receptor. J. Pharmacol. Exp. Therapeutics 334, 214–222 (2010).
Burger, W. A. C. et al. Identification of a Novel allosteric site at the M5 Muscarinic Acetylcholine receptor. ACS Chem. Neurosci. 12, 3112–3123 (2021).
Congreve, M., Oswald, C. & Marshall, F. H. Applying Structure-Based Drug Design Approaches to Allosteric Modulators of GPCRs. Trends Pharmacol. Sci. 38, 837–847 (2017).
Kruse, A. C. et al. Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature 504, 101–106 (2013).
Vuckovic, Z. et al. Pharmacological hallmarks of allostery at the M4 muscarinic receptor elucidated through structure and dynamics. eLife 12, e83477 (2023).
Ballesteros, J. A. & Weinstein, H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci. 25, 366–428 (1995).
Leach, K., Sexton, P. M. & Christopoulos, A. Allosteric GPCR modulators: taking advantage of permissive receptor pharmacology. Trends Pharmacol. Sci. 28, 382–389 (2007).
Maeda, S., Qu, Q., Robertson, M. J., Skiniotis, G. & Kobilka, B. K. Structures of the M1 and M2 muscarinic acetylcholine receptor/G-protein complexes. Science 364, 552–557 (2019).
Zhang, S. et al. Molecular basis for selective activation of DREADD-based chemogenetics. Nature 612, 1–9 (2022).
Kim, K. et al. Structure of a Hallucinogen-activated Gq-coupled 5-HT2A Serotonin receptor. Cell 182, 1574–1588.e19 (2020).
Mobbs, J. I. et al. Structures of the human cholecystokinin 1 (CCK1) receptor bound to Gs and Gq mimetic proteins provide insight into mechanisms of G protein selectivity. PLoS Biol. 19, 1–28 (2021).
Maeda, S. et al. Development of an antibody fragment that stabilizes GPCR/G-protein complexes. Nat. Commun. 9, 3712 (2018).
Zhou, Q. et al. Common activation mechanism of class A GPCRs. eLife 8, e50279 (2019).
Brown, A. J. H. et al. From structure to clinic: Design of a muscarinic M1 receptor agonist with potential to treatment of Alzheimer’s disease. Cell 184, 5886–5901 (2021).
Hanson, M. A. et al. A specific cholesterol binding site is established by the 2.8 Å structure of the human β2-Adrenergic Receptor. Structure 16, 897–905 (2008).
Thal, D. M., Glukhova, A., Sexton, P. M. & Christopoulos, A. Structural insights into G-protein-coupled receptor allostery. Nature 559, 45–53 (2018).
Olsen, R. H. J. et al. TRUPATH, an open-source biosensor platform for interrogating the GPCR transducerome. Nat. Chem. Biol. 16, 841–849 (2020).
Casiraghi, M. et al. Structure and dynamics determine G protein coupling specificity at a class A GPCR. Sci. Adv. 11, eadq3971 (2025).
Powers, A. S. et al. A non-canonical mechanism of GPCR activation. Nat. Commun. 15, 9938 (2024).
Dror, R. O. et al. Structural basis for modulation of a G-protein-coupled receptor by allosteric drugs. Nature 503, 295–299 (2013).
Burger, W. A. C., Draper-Joyce, C. J., Valant, C., Christopoulos, A. & Thal, D. M. Positive allosteric modulation of a GPCR ternary complex. Sci. Adv. 10, eadp7040 (2024).
Zhang, L., Mobbs, J. I., May, L. T., Glukhova, A. & Thal, D. M. The impact of cryo-EM on determining allosteric modulator-bound structures of G protein-coupled receptors. Curr. Opin. Struct. Biol. 79, 102560 (2023).
Liu, X. et al. Mechanism of β 2 AR regulation by an intracellular positive allosteric modulator. Science 364, 1283–1287 (2019).
Teng, X. et al. Ligand recognition and biased agonism of the D1 dopamine receptor. Nat. Commun. 13, 3186 (2022).
Zhuang, Y. et al. Mechanism of dopamine binding and allosteric modulation of the human D1 dopamine receptor. Cell Res. 31, 593–596 (2021).
Xiao, P. et al. Ligand recognition and allosteric regulation of DRD1-Gs signaling complexes. Cell 184, 943–956.e18 (2021).
Lu, J. et al. Structural basis for the cooperative allosteric activation of the free fatty acid receptor GPR40. Nat. Struct. Mol. Biol. 24, 570–577 (2017).
Ho, J. D. et al. Structural basis for GPR40 allosteric agonism and incretin stimulation. Nat. Commun. 9, 1645 (2018).
Robertson, N. et al. Structure of the complement C5a receptor bound to the extra-helical antagonist NDT9513727. Nature 553, 111–114 (2018).
Liu, H. et al. Orthosteric and allosteric action of the C5a receptor antagonists. Nat. Struct. Mol. Biol. 25, 472–481 (2018).
Kim, H. R. et al. Structural mechanism underlying primary and secondary coupling between GPCRs and the Gi/o family. Nat. Commun. 11, 3160 (2020).
Chung, K. Y. et al. Conformational changes in the G protein Gs induced by the β2 adrenergic receptor. Nature 477, 611–615 (2011).
Du, Y. et al. Assembly of a GPCR-G protein complex. Cell 177, 1232–1242.e11 (2019).
Ma, X. et al. Analysis of β2AR-Gs and β2AR-Gi complex formation by NMR spectroscopy. Proc. Natl. Acad. Sci. 117, 23096–23105 (2020).
Slosky, L. M., Caron, M. G. & Barak, L. S. Biased allosteric modulators: new frontiers in GPCR drug discovery. Trends Pharmacol. Sci. 42, 283–299 (2021).
Xu, J. et al. Structural and dynamic insights into supra-physiological activation and allosteric modulation of a muscarinic acetylcholine receptor. Nat. Commun. 14, 376 (2023).
Taghon, G. J., Rowe, J. B., Kapolka, N. J. & Isom, D. G. Predictable cholesterol binding sites in GPCRs lack consensus motifs. Structure 29, 499–506.e3 (2021).
Dolejší, E. et al. Neuroactive steroids, WIN-compounds and cholesterol share a common binding site on muscarinic acetylcholine receptors. Biochem. Pharmacol. 192, 114699 (2021).
Dolejší, E. et al. Neurosteroids and steroid hormones are allosteric modulators of muscarinic receptors. Neuropharmacology 199, (2021).
Studier, F. W. Stable expression clones and auto-induction for protein production in E. coli. in Structural Genomics: General Applications (ed. Chen, Y. W.) 17–32 (Humana Press, Totowa, NJ, 2014).
Zheng, S. Q. et al. MotionCor2: Anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zhang, K. Gctf: Real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: Algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Pettersen, E. F. et al. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: Recent developments in Phenix. Acta Crystallogr. Sect. D: Struct. Biol. 75, 861–877 (2019).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. Sect. D: Biol. Crystallogr. 66, 486–501 (2010).
Williams, C. J. et al. MolProbity: More and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018).
Berman, H., Henrick, K. & Nakamura, H. Announcing the worldwide Protein Data Bank. Nat. Struct. Biol. 10, 980–980 (2003).
Christopoulos, A. & Kenakin, T. G protein-coupled receptor allosterism and complexing. Pharmacol. Rev. 54, 323–374 (2002).
Wang, J. et al. Gaussian accelerated molecular dynamics: Principles and applications. Wiley Interdiscip. Rev. Computational Mol. Sci. 11, e1521 (2021).
Miao, Y., Feher, V. A. & McCammon, J. A. Gaussian accelerated molecular dynamics: unconstrained enhanced sampling and free energy calculation. J. Chem. Theory Comput. 11, 3584–3595 (2015).
Maier, J. A. et al. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 11, 3696–3713 (2015).
Dickson, C. J., Walker, R. C. & Gould, I. R. Lipid21: Complex lipid membrane simulations with AMBER. J. Chem. Theory Comput. 18, 1726–1736 (2022).
Wang, J., Wolf, R. M., Caldwell, J. W., Kollman, P. A. & Case, D. A. Development and testing of a general amber force field. J. Computational Chem. 25, 1157–1174 (2004).
Darden, T., York, D. & Pedersen, L. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993).
D.A. Case et al. Amber 2022. (University of California, San Francisco, 2022).
Roe, D. R. & Cheatham, T. E. PTRAJ and CPPTRAJ: Software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 9, 3084–3095 (2013).
Acknowledgements
This work was funded by the Australian Research council (ARC) discovery Project DP190102950 (CV, AC) and a National Health and Medical Research Council of Australia (NHMRC) Programme Grant APP1150083 (AC), NHMRC Project Grant APP1138448 (DMT), an NHMRC early career investigator Grant APP1196951 (DMT), a Discovery Early Career Researcher Award DE170100152 (DMT), and a US National Institutes of Health Grant R01GM132572 (YM). PRG was a Sir Keith Murdoch Fellow of the American Australian Association. CWL was funded by the William K. Warren Foundation. Cryo-EM imaging and sample vitrification were carried out at the Monash University Ramaciotti Centre for Cryo-Electron Microscopy. Data processing and storage of the cryo-EM datasets were supported by the Monash University MASSIVE high-performance computing facility and its supercomputing resources. This work used supercomputing resources with allocation awards TG-MCB180049 and BIO210039 through the US NSF-funded Advanced Cyberinfrastructure Coordination Ecosystem: Services & Support (ACCESS) programme and project M2874 through the US DOE National Energy Research Scientific Computing Center (NERSC).
Author information
Authors and Affiliations
Contributions
D.M.T. designed the overall research. W.A.C.B., J.I.M. and D.M.T. designed, expressed, and purified protein samples. J.I.M, H.V. performed sample vitrification and cryo-EM imaging. W.A.C.B., J.I.M., D.M.T. processed the E.M. data and generated and analysed atomic models. JW., K.J., and Y.M. designed, performed, and analysed MD simulations. W.A.C.B., B.R, P.R.G. and M.Y. generated DNA constructs and performed pharmacology experiments. W.A.C.B., B.R, P.R.G., M.Y., A.C., C.V., and D.M.T. analysed pharmacology data. A.M.B. and C.W.L. provided chemical tools. Y.M., A.C., C.V., and D.M.T. provided supervision. W.AC.B., J.I.M. and D.M.T. wrote the manuscript with contributions and input from all authors.
Corresponding authors
Ethics declarations
Competing interests
AC is a co-founder and holds equity in Septerna Inc. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Cheng Zhang, and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Burger, W.A.C., Mobbs, J.I., Rana, B. et al. Cryo-EM reveals an extrahelical allosteric binding site at the M5 mAChR. Nat Commun 16, 7046 (2025). https://doi.org/10.1038/s41467-025-62212-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-62212-z