Abstract
Once considered pristine forests, the mid-elevational forests of the eastern Andean flank are now known to have long histories of human occupation. Past habitations, such as the ‘Lost City of the Amazon’ in the Upano Valley of eastern Ecuador, were societally and temporally complex with sophisticated cultures emerging, flourishing, and disappearing. The cultures of the Upano Valley transformed local ecosystems, but whether lasting ecological changes from those activities persist in modern forests is not known. Here, using paleoecological reconstructions from Lake Cormorán, located immediately adjacent to the Upano Valley and within 10 km of an area of >300 km2 of abandoned mound complexes, we provide a timeline of human influence spanning the last 2770 years. We document the onset of maize cultivation c. 570 BCE, and changes in land use within the occupation phase that included slash-and-burn, slash-and-mulch, and silviculture. A gradual decline in forest exploitation presaged an apparent abandonment of the site c. 550 CE. A much later wave of land use that began about 1500 CE, coupled with abandonment and a succession influenced by a warmer and wetter climate, produced a distinctive forest composition unique to the last 120 years.
Similar content being viewed by others
Introduction
Where the tropical Andes slope toward the Amazon plain, steep environmental gradients support cloud-soaked montane forests and mountain streams that incise valleys through ancient flows of ash and tephra. Especially high levels of biodiversity and endemism characterize montane forests between elevations of c. 2500 and 1000 m above sea-level (hereafter masl)1,2. For many years, these mid-elevational Andean forests were regarded as inimical to human occupation. This belief was based on the perceived harshness of cold nighttime temperatures, high rainfall, frequent ground-level cloud, and steep slopes subject to landslides3,4,5. Nevertheless, paleoecological and archeological studies have revealed that people occupied the Andean foothills of Ecuador for at least the last 6000 years6,7. Here, we add detailed paleoecological data for the occupation and land use of a mid-elevation site on the eastern Andean flank of Ecuador. Our study area, the Upano Valley, is a well-known area of exceptional archeological interest8,9,10,11. While the archeological data inform our analysis, our aim is to provide fully independent, temporally-continuous paleoecological data that complement archeological studies in the region.
In the mid elevations, sites that lay in large river valleys, i.e., potential major trade routes, were occupied by c. 2500 years before the Common Era (hereafter BCE) (c. 4500 years ago)12,13. The crops cultivated at this time included maize, manioc, squash, peppers, and peanuts14,15. Fruit-bearing trees were planted and tended16,17, and silviculture was likely practiced on fast growing trees such as Alnus18. Alnus supports symbionts that increase nitrogen in the soil, is a good timber for construction19 and a source of renewable fuel20. Another common theme of these occupational histories is that periods of occupation differed in the cultivational style, i.e., raised-field cultivation, slash-and-burn, slash-and-mulch, and silviculture21,22. Occupations were frequently discontinuous, with perceived abandonments lasting centuries, and renewed occupation often marked by a change in land management style23,24. Whether these phases of occupation and cultivational style were largely driven by purely societal pressures or stimulated by climate change has been actively debated25,26,27. The principal climate changes influencing the equatorial Andes in the last 2700 years was the dryness of the medieval climate anomaly (c. 700–1200 CE)28, followed by a trend toward wetter conditions29.
The scale of occupations in the mid-elevation Andean forests varied widely from small-scale disturbances, perhaps attributable to a single extended family30, through to large ecological manipulations as structured populations built imposing fortifications as at Kuelap31 or temple-palaces as at Machu Picchu32. Over the past 30 years, a series of studies has drawn attention to a sprawling archeological site in the Upano Valley of Ecuador that was larger than Kuelap or Machu Picchu8,9,10,11. The archeological finds are dispersed across c. 20 km and occupy over 300 km2 of the valley, but are concentrated between c. 1300 and 1000 masl. The site has captured public imagination and was repeatedly described as the ‘Lost City’ of the Amazon33. A recent LiDAR (light detection and ranging) survey of 300 km2 in the Upano Valley revealed over 7000 structures34, many of which were raised mound homesites connected by roads and causeways to plazas and ceremonial centers. Near the Upano homesites, raised fields contained maize (Zea mays), beans (Phaseolus sp.), manioc (Manihot esculenta), and sweet potato (Ipomoea batatas)35. About 70 radiocarbon dates indicated that the valley was first occupied by the Upano people about 700 BCE, and that they began to construct mounds about 500 BCE34. The mound occupation was suggested to have ended at 300–600 CE with an abrupt abandonment8,9,10,11. This uncertainty in timing also extended to the cause of the abandonment. Initially, the end of the Upano occupation was attributed to a 40–70 cm thick ash layer found in the archeological exposures, attributed to the eruption of Volcan Sangay (Fig. 1)34. Other archeologists, however, failed to find evidence of the ash layer, leaving the abandonment unexplained. After several centuries, the Upano Valley was re-occupied by the Huapula, a culture that colonized the mounds c. 800 CE11. Although not adding to the mounds, and generally seen as a smaller and less influential occupation than the Upano, the Huapula lived at the site until c. 1200 CE9.
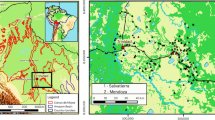
The location of Lake Cormorán, the Sangay Volcano, known archeological settlements in the Upano River Valley (open circles scaled by size)34, and the modern town of Macas are shown in relation to regional topography and water flow. The labeled botanical inventory was conducted by Toasa in 1999. The inset map shows the location of Lake Cormorán in relation to other sites mentioned in the text. Base map source is from ESRI (http://www.esri.com/data/ basemaps, © Esri, DeLorme Publishing Company).
A much-debated topic among archeologists and ecologists is the influence of past human activity on modern tropical forest systems, i.e., to what extent are modern forests manufactured landscapes produced by deliberate past forest manipulation36,37,38,39,40,41,42,43? Some basic predictions are that modern forests should reflect past occupation by having enhanced proportions of fruit-bearing trees prized by humans and being deficient in species cut for their timber44. When such modifications of the forest persist beyond the period of occupation, they are termed ecological legacies45. Here, through paleoecological analysis of the sediments of Lake Cormorán (2°04'20.59” S, 78°12'52.40” W), which lies within 10 km of the Upano mound complex (Fig. 1 and Supplementary Figs. 1–4), we provide a c. 2770-year continuous history of vegetation change. Our data reflect changes in land use in the Lake Cormorán watershed, including periods of extensive habitat modification through human activity. Our goal is to place the occupation of the Upano Valley into a context of environmental change and to determine the periods when human occupations affected the Lake Cormorán catchment. We document the period of Upano disturbance of the landscape, highlight hitherto undocumented impacts during the colonial period (c. 1500–1900 CE), and consider the relative legacy effects of these occupations on the modern forests.
Our study site, Lake Cormorán lies within the Sangay National Park, Ecuador, at an elevation of 1750 masl. The modern forests surrounding the lake are rich in Dictyocaryum lamarckianum (~40% of individuals near the lake; see Figs. S1, S2), Prumnopitys montana (Podocarpaceae), Bambusoideae, Euterpe (Arecaceae), Moraceae/Urticaceae, and Cecropia (Urticaceae) (Supplementary Notes 1; Supplementary Data 1 and 2). The lake lay c. 16 km upwind of Sangay Volcano, which is important because though occasionally impacted by the eruptions of this very active volcano, the majority of the ashfalls would be blown away from the lake. The prevailing easterly winds bring moisture year-round from Amazonia estimated to exceed 3800 mm per annum. Similarly, mean temperatures are even year-round at c. 21 °C.
In 2017, a 5.98 m-long core of sediment was raised from beneath 16 m of water in the center of the Lake Cormorán. The sediments were subsequently analyzed for their fossil pollen, phytolith and charcoal content. Dates were calibrated using the Intcal20 curve46 and a chronology was constructed from a Bayesian age-depth model47 based on eight 14C ages (Supplementary Notes 2).
Results
The oldest dated sediment recovered from Lake Cormorán was 454 BCE, but by extrapolation to the base of the core the oldest sediment in the core would have been c. 770 BCE (Table 1, Fig. 2). We observed that pollen and phytoliths of Zea mays (maize) were present from c. 670 BCE to 520 CE (Fig. 3, Supplementary Data 3 and 4). Between c. 500 BCE and 520 CE maize cultivation was coincident with high abundances of Poaceae pollen and a marked increase in the abundance of Alnus pollen (up to c. 34% of the pollen sum) with major peaks at c. 400 BCE and 0 CE. Evidence of fire (charcoal) occurred in two periods, from c. 200 BCE to100 CE and from c. 250–500 CE (Fig. 3, Supplementary Data 5). Notably Podocarpaceae, probably Prumnopitys, pollen persists throughout this interval.
Teal-colored points represent two ‘modern’ age that can be calibrated and 2017 when the core was raised. a Bayesian convergence iterations used to determine the most likely age of each depth; b accumulation rate (yr/cm) of samples within the core (mean and variance); c temporal autocorrelation analysis (memory) that assess how much sedimentation rates changes within the core (with 0 being no autocorrelation and changing slopes and 1 being a linear sedimentation throughout the core); d inferred depth-age relationship; e a simplified stratigraphy of the sedimentary core from Lake Cormorán. Colored bands approximate the actual colors found in the sedimentary gyttjas but cannot capture the fineness of laminae.
Zea pollen are from extended counts. Podocarpus is shown with a X10 exagerration. The periods of human activity associated with the Upano and ‘Late’ are shown. The Huapula occupation as per34 is also shown although no evidence was found of it influencing Cormorán. Source data are provided.
Forest elements increased in abundance after c. 200 CE alongside increases in taxa such as Euphorbiaceae, Solanaceae, Rubiaceae, Alchornea, Acalypha, Cecropia, Celtis, and Vallea (Supplementary Data 6). This period of forest recovery lasted until 1500 CE. The cultivation of maize around the lake, which apparently stopped at c. 550 CE, resumed at c. 1500 CE. Increased forest disturbance at this time was marked by elevated abundances of Hedyosmum pollen and depressed abundances of Moraceae/Urticaceae pollen. Abundances of Dictyocaryum (a canopy palm) pollen, which had remained at low levels throughout the bottom of the core, increased from 4% to 17% between c. 1780 and 2000 CE (Fig. 3, Supplementary Data 6).
Discussion
The steep bowl-shaped depression that formed the catchment of Lake Cormorán would have contributed all the crop pollen and phytoliths and the great majority of other pollen described here (Fig. 4). Pollen grains of Zea mays are large (>82 µm) and very poorly transported48 and phytoliths are similarly local in their provenance49.
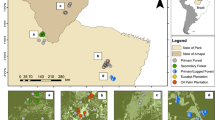
The flattest area to the southwest of the lake was probably the most likely place for clearance and cultivation, whereas steep portions of the basin probably always retained forest. The base map was created entirely by the authors using QGIS version 3.34.6-Prizren71 for geospatial processing, visualization, and layout design. Slope and contours were derived from the ASTER Global Digital Elevation Model (GDEM)72 with a spatial resolution of 30 m, which was downloaded from the USGS Earth Explorer platform (https://earthexplorer.usgs.gov/).
At the start of this record at c. 770 BCE the area around Lake Cormorán supported a largely undisturbed forest, but by 570 BCE deforestation and maize cultivation had begun (Fig. 3). Maize is absolutely indicative of humans cultivating the landscape, and this site provides evidence from both pollen and phytoliths that is temporally in keeping with many other records of maize cultivation from the Andes and Andean foothills7,50,51 (Fig. 5).
Comparisons of patterns found in the Cormorán sediment core with Pumacocha δ18O isotopic data29, and fossil maize pollen from Lakes Kumpaka 23 and Ayauchi 52, Ecuador. The non-pioneer forest pollen curve is a recalculation of all non-pioneer forest pollen, i.e., it excludes Alnus and Cecropia. Black outlined maize circles are from phytoliths, non-outlined are from pollen. Gray maize circles indicate possible slash-and-mulch cultivation (i.e., no charcoal was found in the sample), and black circles indicate possible slash-and-burn cultivation (i.e., charcoal was found in the sample) in the catchment. The pink box highlights our inference for Upano modification around Lake Cormorán. The gray box highlights the Huapula period (23). Purple line marks the 1 cm ashfall of 1285 CE.
The occurrence of maize pollen and phytoliths at Lake Cormorán is comparable with that seen in two high-resolution paleoecological records23,52 from lowland Ecuadorean lakes that lie ~90 km to the south and ~1400 m lower: Ayauchi and Kumpaka (Figs. 1, 5). These records provide detailed, but contrasting, insights into phases of occupation over the last 2700 years. Lake Ayauchi, which lies on a navigable tributary of the Marañon, shows a longer and more continuous history of maize cultivation and occupation than the nearby, but more isolated Lake Kumpaka 7,23,52 (Fig. 5). Lake Kumpaka shows a complex history of occupation and abandonment, with phases that temporally resemble the Upano and Huapula occupations of the Upano River valley. Kumpaka also showed evidence of slash-and-mulch cultivation in pre-Colonial periods, whereas at Ayauchi slash-and-burn appeared to be more important.
The occurrence of maize at Cormorán coincides with peaks of disturbed forest taxa such as Alnus, Begonia and Myrica, and the highest abundance of Poacaeae in the entire record occurs at 520 BCE (Fig. 3). Poaceae (grasses) seldom contribute more than 2–5% of the pollen sum in an undisturbed montane forest53, but aquatic grasses can increase this proportion. At Cormorán, the background input of all grasses appears to be about 4–8%, with values of 11–24% occurring during times of cultivation. Nevertheless, the continuous inputs of >50% non-pioneer arboreal pollen suggest that the area of cleared forest was relatively small (Fig. 5, Supplementary Data 7). The presence of Prumnopitys, a mature forest taxon suggests that the clearance was rather local and some mature forest remained. The Cormorán basin is relatively flat immediately south of the lake (Fig. 4, Figs. S3, S4), and this was probably the most likely area for maize cultivation, leaving the shadier, steeper slopes of the northern portion of the basin less affected.
The occurrence of Alnus (alder) pollen, a pioneer species, at c. 35% between c. 550 BCE and 500 CE is notable, in that it does not re-occur at such high values elsewhere in the core. The modern pollen representations of Alnus, and co-occurring montane taxa, Dictyocaryum and Hedyosmum, at sites greater than 500 masl and located in Ecuador, Peru, and Bolivia, closely track the abundances of those trees across elevation (Fig. 6, Supplementary Data 8 and 9). Only one species of Alnus grows in the equatorial Andes, A. acuminata, and although a common pollen type above 2200 m, the elevation of the lake at 1750 m is close to its ecological lower limit. The observed c. 35% abundance greatly exceeds prior observations of near-natural systems at this elevation (Fig. 6). In other Andean settings, where fossil Alnus pollen was found at atypically high abundances, it was suggested to have been the subject of silviculture18,21. Alnus is fast-growing and today is planted to provide firewood20. The timing of the Alnus peaks at Lake Cormorán was coincident with periods of Zea cultivation documented in the sediment core and the main phases of mound building by the Upano people that occurred within 10 km of the core site (Fig. 1). We thus infer that Alnus was being enriched around Lake Cormorán by human activity during this period.
The modern pollen and observed distribution of Dictyocaryum trees are similarly tightly related, and it can be seen that Lake Cormorán lies close to the upper limit of this taxon (Fig. 6). Indeed, a 3300-year record from Lake Palotoa at 1360 m elevation in southern Peru, revealed a continuous high-abundance record for this species in the middle of its elevational range54. The elevation of Lake Cormorán, by contrast, lies more centrally in the thermal, i.e., elevation dependent, range of Hedyosmum, and yet this genus does not increase markedly until c. 1500 CE as conditions get wetter (Fig. 5).
After 1600 CE, maize cultivation continued near Lake Cormorán, but the forest began a new successional trajectory. Prior to this time, Dictyocaryum pollen occurred as an occasional, rare, type. This palm is known to be patchy in its occurrence, being very abundant when it is present, but only occupying a small portion of what appears to be suitable habitat55. About a century after maize cultivation stopped at 1780 CE, Dictyocaryum surged in abundance, reaching 18% of the pollen sum. Dictyocaryum can be a long-term dominant in the middle of its ecological range54, but at Cormorán, it may have taken unusually wet, warm, conditions to allow it to become a major canopy component (Fig. 5). Another possibility is that a low-level of selective cutting had been a persistent pressure from before the start of the record and only at c. 1780 was there an opportunity for the trees to mature and their population to expand. This view, however, does not fit well with the general recovery of the forest from 550–1500 CE, nor with a broadly similar pattern exhibited by Hedyosmum, which would not have been harvested. Consequently, we favor a mixed indirect human legacy23 and climate-based explanation for the expansion of Dictyocaryum. A closely-allied palm, Iriartea deltoidea, which is an ecological equivalent of Dictyocaryum at elevations below 1200 masl, is known to be a fast-growing, mid-successional species on wet soils56. We hypothesize that the transition to a progressively wetter climate28 between c. 1500 and 1700 CE (Fig. 5) accounts for the increased abundance initially of Hedyosmum and latterly of Dictyocaryum. Both these taxa require moist soils and both are early to mid-successional elements in mid-elevational moist wet areas. As the climate became wetter in the 1600 s and 1700 s, Dictyocaryum was able to establish populations. The large modern populations were built after humans abandoned the site. Once established, these palms were probably favored by the warming conditions of the last 200 years28 as this would have brought the elevation of the lake more solidly into their niche space (Fig. 6). Our 2019 floral survey estimated that 40% of stems (16% of pollen sum) reaching the canopy around Lake Cormorán were of Dictyocaryum, and that recruitment was occurring. We suggest that the increase in Dictyocaryum and the formation of modern forest is a legacy of past human activity in that the disturbance allowed a new succession to take place, but that climate change caused the species that were dominant in that succession to differ from those of drier times.
Our data support the archeological interpretation of a peak in the impacts of the Upano occupation between c. 500 BCE and 200 CE but offer three additional insights into past human occupations of the mid-elevation cloud forests on the eastern Andean slopes. The first is that the scale of the Upano occupation was larger than suggested by the current LIDAR data, and that people exerted strong modifications of the landscape as much as 10 km beyond the known mound complex. Second, as suggested by archeologists, the Upano occupation could be divided into multiple phases8,57. Our data add to that understanding by identifying different adaptations to cultivate the land that may align with those archeological phases: slash-and-mulch with maize and probably Alnus enrichment (c. 500–350 BCE); slash-and-burn in which maize was cultivated, but Alnus was not (c. 350–100 BCE), slash-and-mulch with maize and probably Alnus enrichment (c. 100 BCE − 200 CE) and a continuation of this phase, but with shrinking land use (200–550 CE) marked by increased occurrence of forest pollen types (Fig. 5). Lastly, and perhaps most importantly, we found no evidence of an abrupt abandonment, but rather of a protracted decline. The lake sediment did not contain evidence of a catastrophic ashfall terminating the Upano period. Ashfall into lake sediment should make a clear layer, and indeed a small event was found at 1285 CE, but there was no major ash layer at c. 550 CE. What was evident was that between c. 200 CE and 550 CE there was a gradual return of forest, consistent with reduced occurrences of Zea phytoliths and charcoal, which is interpreted to indicate a gradual decline in human activity. Our data raise a fundamental and yet unanswered question as to why a site with so much invested effort was gradually abandoned.
Our data show that the plant assemblages of apparently undisturbed forests, such as those currently around Lake Cormorán, may be as young as 120 years, and result from complex ecological histories that can include long periods of occupation, abandonment, recovery, and climate change. We show that modern forests on the eastern Andean slopes may be a result of past human land use, but the altered successional paths were a product of human activity and climate change, rather than solely a product of either. A long period of forest recovery largely eradicated the influence of Pre-Columbian occupations, whereas Indigenous forest use in the Colonial period that ended about 200 years ago left a lasting legacy on the modern system. These data, as well as empirical data from the Amazonian lowlands23 indicate that the composition of modern forests is a unique mosaic that is driven by environmental conditions, ecological gradients, climate change, and various forms, frequencies, and types of human land use; as the contingency of historical events dynamically shapes modern forests.
Methods
Work was conducted under permit number 08-2017-IC-FLO-DNB/MA issued by the Ecuadorean Government. Importation of sediments was conducted under USDA APHIS permit P330-15-00190. Lake Cormorán lies at 1750 masl (2° 4'9.93“S, 78°12'54.35“W, Fig. 1). Archeological sites, containing the remains of the Upano and Huapula societies, are located as little as 5 km west of Cormorán, on the Eden Plateau9,58. The lake lies within the Parque Nacional Sangay, and the modern scene appears to be largely undisturbed (Supplementary Figs. 1–4, Supplementary Notes 1). Sangay Volcano lies approximately 16 km away, downwind of the lake (Fig. 1).
A total of 5.98 m of sediment was collected from a lake depth of 16 m in July 2017 using a Colinvaux-Vohnout piston coring rig59. Sediment cores were shipped back to the Florida Institute of Technology and stored at 4 °C. Core 1 was kept as an archive and all analyses were conducted on Core 2, which was the longest core collected at Lake Cormorán. Core 2 was split longitudinally and subsamples were removed for 14C, charcoal and pollen analysis, with a further set shipped to the University of Amsterdam for phytolith analysis.
Age model
Bulk sediment samples of a least 1 cm3 (N = 12) were dried and sent to the NOSAMS lab at Woods Hole Oceanographic Institute or the Direct AMS lab for dating (Table 1). The 14C ages of the sediment were calibrated using the Intcal2046 and the NH1 postbomb calibration curve60. A chronology was constructed from nine accepted ages calibrated based on a Bayesian age-depth model47 using the rBacon package61 (see Supplementary Notes 2).
Pollen analysis
The sediment from Lake Cormorán was generally organic rich, with thick layers of gyttja (Fig. 2). Samples to be processed for pollen were taken every c. 10 cm (N = 38) for a resolution of ~50–80 years. Each pollen sample was 0.5 cm3 of sediment. Processing followed Faegri and Iversen62, with Lycopodium spore tablets added as an exotic marker. Processed samples were counted at x6300, using a Zeiss AxioImager.M2 microscope. At each level, pollen identification continued until 300 individual pollen grains were counted. Pollen was identified using pollen atlases produced by Hooghiemstra63 and the Neotropical Pollen Database64, and the modern pollen reference collection of the Florida Institute of Technology paleoecology lab. Percentages of pollen types were calculated (Supplementary Data 6).
Phytolith analysis
Subsamples of 1 cm3 in volume were collected ca. every 10 cm throughout the core for phytolith analysis (N = 61). Exotic markers (15 μm microspheres) were added to each subsample, and the subsamples were then treated with HCl, H2O2, and KMnO4 to remove organics, carbonates, and humic acids. Heavy liquid flotation using bromoform (CHBR3, specific gravity 2.3 g/cm3) was used to extract the phytoliths from the remaining soil material12. Phytolith extracts were mounted on slides using Naphrax. A total of 250 phytoliths or 2000 microspheres were identified or counted for each sample using a Zeiss Axioscope.A1 at 400x and 630x magnification. Percentages of each phytolith morphotype for each sample were calculated by dividing the number of the morphotype by the total number of phytoliths counted for that sample. Phytoliths were identified using guides that were published at the time of analysis in 201949,65,66 and the reference material at the University of Amsterdam. The relative percentage of grass, palm, arboreal phytoliths were calculated for each sample, and extended scanning for the presence of maize phytoliths were performed on each sample.
Charcoal analysis
Subsamples of 0.5 cm3 from the Lake Cormorán cores were collected continuously at 1 cm increments for charcoal analysis (c. 5-year resolution) until 306 cm (c. 730 CE), where they were collected every two centimeters (c. 10-year resolution, N = 439). Each charcoal sample was filtered at 180 µm67,68. The retained material was transferred to a petri dish using distilled water and viewed under a Zeiss Stemi 2000-C stereoscope. Charcoal particles were identified and photographed at x20, x25, and x32 magnification. The surface area of the charcoal particles (cm2/cm3) was calculated using Image J69.
Modern vegetation and pollen comparison
Modern vegetation inventories were performed by members of our team in 2017 and herbarium specimens were collected from the area in 1999 by Germán Toasa (Supplementary Notes 1, Supplementary Data 1 and 2). All modern vegetation records for Hedyosmum, Alnus, and Dicytocaryum were downloaded from the Global Biogeographic Information Facility on 8/13/2024 (Supplementary Data 8). Data were downloaded for Bolivia, Ecuador and Peru and filtered by elevation (500–4000 m above sea level). Pollen data were derived for the same genera, elevation, and countries, from Bush et al.70(Supplementary Data 9).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The paleoecological and modern vegetation data both raw and processed in this have been deposited in Figshare database under accession code https://doi.org/10.6084/m9.figshare.26970589 and are provided in the Supplementary Data file. The Sediment cores are archived at the Florida Institute of Technology and are available under restricted access due to the small amount of material available, access can be obtained by contacting the corresponding author.
Code availability
Code is provided in Supplementary Notes 2 that generate the age/depth model. Code is provided in Supplementary Notes 3 that generate the downloads for the GBIF occurrences of Alnus, Dictyocaryum, and Hedyosmum.
References
Swenson, J. J. et al. Plant and animal endemism in the eastern Andean slope: challenges to conservation. BMC Ecol. 12, 1 (2012).
Myers, N., Mittermeier, R. A., Mittermeier, C. G., da Fonseca, G. A. B. & Kent, J. Biodiversity hotspots for conservation priorities. Nature 403, 853–858 (2000).
Bonavia, D. in The Inca world: The development of pre-Columbian Peru. (ed Laura Laurencich Minelli) 121–131 (University of Oklahoma Press, 2000).
Lanning, E. P. Chronological and cultural relationships of early pottery styles in ancient Peru. Vol. 2 (University of California, Berkeley, 1960).
Steward, J. H. Handbook of South American Indians. Vol. 3. The tropical forest tribes. (Smithsonian Institution, 1948).
Valdez, F. Primeras Sociedades de la Alta Amazonía: La cultura mayo Chinchipe-Marañón. INPC-IRD, 84 p., 2013, 978-9942-955-02.〈 ird-01347343〉. (2013).
Bush, M. B., Piperno, D. R. & Colinvaux, P. A. A 6000 year history of Amazonian maize cultivation. Nature 340, 303–305 (1989).
Rostain, S. Secuencia arqueológica en montículos del valle del Upano en la Amazonía ecuatoriana. Bulletin de l’Institut Français D'études Andines 28 (1999).
Rostain, S. Between Sierra and Selva: landscape transformations in upper Ecuadorian Amazonia. Quat. Int. 249, 31–42 (2012).
Salazar, E. De vuelta al Sangay-Investigaciones arqueológicas en el Alto Upano, amazonía ecuatoriana. Bulletin de l’Institut français d'études andines 27 (1998).
Salazar, E. Pre-Columbian mound complexes in the Upano river valley, lowland Ecuador. The Handbook of South American Archaeology, 263–278 (2008).
McMichael, C. et al. 30,000 years of landscape and vegetation dynamics in a mid-elevation Andean valley. Quat. Sci. Rev. 258, 106866 (2021).
Church, W. B. & Von Hagen, A. in The Handbook of South American Archaeology 903–926 (Springer, 2008).
Pearsall, D. M. in The Handbook of South American Archaeology (eds Silverman, H. & Isbell, W. H.) 105–120 (Springer New York, 2008).
Piperno, D. R. in Foraging Theory and the Transition to Agriculture (eds Kennett, D. & Winterhalder, B.) 137–166 (University of California Press, 2006).
Clement, C. R. et al. Disentangling domestication from food production systems in the Neotropics. Quaternary 4, 4 (2021).
Levis, C. et al. How people domesticated Amazonian forests. Front. Ecol. Evol. 5, 171 (2018).
Chepstow-Lusty, A. & Winfield, M. Inca agroforestry: Lessons from the past. Ambio 29, 322–328 (2000).
Carlson, P. & Dawson, J. Soil nitrogen changes, early growth, and response to soil internal drainage of a plantation of Alnus jorullensis in the Colombian highlands. Turrialba 35, 141–150 (1985).
Dunn, W., Morgan, P. & Lynch, A. Production of alder (Alnus jorullensis) to meet fuelwood demand in the Sierra of Ecuador. Agrofor. Syst. 10, 199–211 (1990).
Bush, M. B., Mosblech, N. A. S. & Church, W. Climate change and the agricultural history of a mid-elevation Andean montane forest. Holocene 25, 1522–1532 (2015).
Iriarte, J., Maezumi, S., Robinson, M., Travassos, D. & Gonda, R. What can pre-Columbian polyculture agroforestry systems tell us about sustainable Amazonian futures? Tales from Amazonian Dark Earths and the ‘Geoglyph Builders. Hum. Environ. Sustain. 127, 127–129 (2018).
Åkesson, C. et al. Long-term ecological legacies in western Amazonia. J. Ecol. 1, 432–446 (2021).
de Novaes Nascimento, M. et al. Fire in the clouds: How changing land use shaped an Andean biodiversity hotspot. Quat. Sci. Rev. 317, 108278 (2023).
Kolata, A. L., Binford, M. W. & Ortloff, C. Environmental thresholds and the empirical reality of state collapse: a response to Erickson (1999). Antiquity 74, 424 (2000).
Mosely, M. E. & Richardson, J. B. III Doomed by natural disaster. Archaeology 45, 44–45 (1992).
Erickson, C. L. Neo-environmental determinism and agrarian ‘collapse’ in Andean prehistory. Antiquity 73, 634 (1999).
Ledru, M.-P. et al. The Medieval climate anomaly and the Little Ice Age in the eastern Ecuadorian Andes. Climate 9, 307–321 (2013).
Bird, B. W. et al. A 2,300-year-long annually resolved record of the South American summer monsoon from the Peruvian Andes. Proc. Natl Acad. Sci. 108, 8583–8588 (2011).
Sales, R. et al. Wet and dry events influenced colonization of a mid-elevation Andean forest. Quat. Sci. Rev. 327, 108518 (2024).
Righetti, G., Serafini, S., Rueda, F. B., Church, W. B. & Garnero, G. in Computational Science and Its Applications–ICCSA 2021: 21st International Conference, Cagliari, Italy, September 13–16, 2021, Proceedings, Part VI 21. 613-628 (Springer).
Burger, R. L. & Salazar, L. C. Machu Picchu: unveiling the mystery of the Incas. (Yale University Press, 2004).
Science, A. A. Lost City development in the Upper Amazon. Science 383, 1 (2024).
Rostain, S. et al. Two thousand years of garden urbanism in the Upper Amazon. Science 383, 183–189 (2024).
Pagán Jiménez, J. R. & Rostain, S. in Antes de Orellana. Actas del 3er Encuentro Internacional de Arqueología Amazónica Vol. 313–322 (ed S. Rostain) (IFEA, FLACSO, Embajada de los EEUU, 2014).
Levis, C. et al. Persistent effects of pre-Columbian plant domestication on Amazonian forest composition. Science 355, 925–931 (2017).
Levis, C. et al. Historical Human Footprint on Modern Tree Species Composition in the Purus-Madeira Interfluve, Central Amazonia. PloS One 7, e48559 (2012).
Levis, C. et al. Contributions of human cultures to biodiversity and ecosystem conservation. Nat. Ecol. Evol. 8, 866–879 (2024).
Bush, M. B. et al. Anthropogenic influence on Amazonian forests in pre-history: An ecological perspective. J. Biogeogr. 42, 2277–2288 (2015).
McMichael, C. N. H. Ecological legacies of past human activities in Amazonian forests. N. Phytol. 229, 2492–2496 (2020).
McMichael, C. N. H., Bush, M. B., Jiménez, J. C. & Gosling, W. D. Perspective Article: Past human-induced ecological legacies as a driver of Amazonian forest resilience. People Nat. 5, 1415–1429 (2023).
Balée, W., Swanson, T., Zurita-Benavides, M. G. & Macedo, J. C. R. Evidence for landscape transformation of ridgetop forests in amazonian Ecuador. Lat. Am. Antiq. 34, 842–856 (2023).
Balée, W. Sowing the Forest: A Historical Ecology of People and Their Landscapes. (University of Alabama Press, 2023).
Ferreira, M. J., Levis, C., Iriarte, J. & Clement, C. R. Legacies of intensive management in forests around pre-columbian and modern settlements in the Madeira-Tapajós interfluve, Amazonia. Acta Bot. Bras. 33, https://doi.org/10.1590/0102-33062018abb33060339 (2019).
Turner, M. G. Disturbance and landscape dynamics in a changing world. Ecology 91, 2833–2849 (2010).
Reimer, P. J. et al. The IntCal20 Northern Hemisphere radiocarbon age calibration curve (0–55 cal kBP). Radiocarbon 62, 725–757 (2020).
Blaauw, M. & Christen, J. A. rbacon: Age-depth modelling using Bayesian statistics. R Package Version 2.4. (2018).
Lane, C. S., Cummings, K. E. & Clark, J. J. Maize pollen deposition in modern lake sediments: A case study from Northeastern Wisconsin. Rev. Palaeobot. Palynol. 159, 177–187 (2010).
Piperno, D. R. Phytoliths: A comprehensive guide for archaeologists and paleoecologists. (Alta Mira Press, 2006).
Athens, J. S. et al. Early prehistoric maize in Northern Highland Ecuador. Lat. Am. Antiq. 27, 3–21 (2016).
Pearsall, D. M., Chandler-Ezell, K. & Zeidler, J. A. Maize in ancient Ecuador: results of residue analysis of stone tools from the Real Alto site. J. Archaeological Sci. 31, 423–442 (2004).
Åkesson, C. M., McMichael, C. N., León-Yánez, S. & Bush, M. B. Late-Holocene maize cultivation, fire, and forest change at Lake Ayauchi, Amazonian Ecuador. Holocene 33, 550–561 (2023).
Grabandt, R. A. J. Pollen rain in relation to vegetation in the Colombian Cordillera Oriental, University of Amsterdam, (1985).
Schiferl, J. D., Bush, M. B., Silman, M. R. & Urrego, D. H. Vegetation responses to late Holocene climate changes in an Andean forest. Quat. Res. 89, 60–74 (2018).
Henderson, A. Flora Neotropica, monograph 53: Arecaceae. Part I. Introduction and the Iriarteinae., (The New York Botanical Garden, 1990).
Losos, E. Habitat specificity of two palm species: experimental transplantation in Amazonian successional forests. Ecology, 2595–2606 (1995).
Pazmiño, E. M. Monumentality and Social Complexity in the Upano Valley, Upper Amazon of Ecuador. The Archaeology of the Upper Amazon: Complexity and Interaction in the Andean Tropical Forest, 129–147 (2021).
Salazar, E. in The Handbook of South American Archaeology 263–278 (Springer, 2008).
Colinvaux, P., de Oliveira, P. E. & Moreno, P. J. E. Amazon Pollen Manual and Atlas. (Harwood Academic Publishers, 1999).
Hua, Q., Barbetti, M. & Rakowski, A. Z. Atmospheric radiocarbon for the period 1950–2010. Radiocarbon 55, 2059–2072 (2013).
Blaauw, M. & Christen, J. A. Flexible paleoclimate age-depth models using an autoregressive gamma process. Bayesian Anal. 6, 457–474 (2011).
Faegri, K. & Iversen, J. Textbook of pollen analysis. 4th edn, 328 (Wiley, 1989).
Hooghiemstra, H. Vegetational and climatic history of the high plain of Bogota, Colombia. (Dissertaciones Botanicae 79, J.Cramer, 1984).
Bush, M. B. & Weng, C. Introducing a new (freeware) tool for palynology. J. Biogeogr. 34, 377–380 (2007).
Morcote-Ríos, G., Bernal, R. & Raz, L. Phytoliths as a tool for archaeobotanical, palaeobotanical and palaeoecological studies in Amazonian palms. Bot. J. Linn. Soc. 182, 348–360 (2016).
Huisman, S., McMichael, C. H. & Bush, M. B. Four centuries of local-scale vegetation change in a fire-free mid-elevation Andean forest. Veg. Hist. Archaeobot. 28, 679–689 (2019).
Clark, J. S. & Hussey, T. C. Estimating the mass flux of charcoal from sedimentary records: effects of particle size, morphology, and orientation. Holocene 6, 129–144 (1996).
Whitlock, C. & Larsen, C. Charcoal as a fire proxy. Tracking environmental change using lake sediments, 75–97 (2002).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671 (2012).
Bush, M. B. et al. Modern pollen assemblages of the Neotropics. J. Biogeogr. 48, 231–241 (2021).
QGIS Development Team, 2025. QGIS Geographic Information System. Open Source Geospatial Foundation Project. URL: http://qgis.org.
Abrams, M., Crippen, R. & Fujisada, H. ASTER global digital elevation model (GDEM) and ASTER global water body dataset (ASTWBD). Remote Sens. 12, 1156 (2020).
Acknowledgements
We are indebted to the people of Ecuador and the Minesterio del Ambiente in Macas for allowing us to conduct our fieldwork. We thank Majoi Nascimento, Seringe Huisman Jorge Celi, Jairo Cabrera, John Lima and Carlos for their assistance in the field. Annemarie Philip provided laboratory assistance. This project was funded by the Belmont Forum funded project VULPES (VULnerability of Populations under Extreme Scenarios, Project ID: ANR-15-MASC-0003), and National Science Foundation grants EAR −1624207 and HEGS- 2148984 to MBB.
Author information
Authors and Affiliations
Contributions
Design and implementation of the project (M.B.B., C.N.H.M.), Manuscript preparation (M.B.B., C.N.H.M., R.K.S, S.L.-Y., B.G.V.), Vegetation survey (D.N.), Pollen, phytolith and charcoal analyses (R.K.S., M.B.B., W.S., A.S., BT.G., K.L., I.B., C.N.H.M.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Daniel Contreras, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.”
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bush, M.B., Sales, R.K., Neill, D. et al. Ecological legacies and recent footprints of the Amazon’s Lost City. Nat Commun 16, 7408 (2025). https://doi.org/10.1038/s41467-025-62315-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-62315-7