Abstract
Carbon dioxide (CO2) is an ideal chemical feedstock due to its abundance, low cost, low toxicity and its role as a greenhouse gas. Telomerization with butadiene give rise to functional small molecules and polymers with significant CO2 content, but the fossil origin of the olefin offsets sustainability benefits. Here, we present a palladium-catalyzed telomerization of CO2 with isoprene, two of the most prevalent organic compounds in the atmosphere, yielding “COOIL”, an ideally 100% renewable δ-lactone containing 24 wt% CO2, with high selectivity and turnover numbers above 100. A combination of a Pd catalyst, acetate, and controlled water promoted selectivity and conversion. Density functional theory calculations reveal reductive elimination as the rate-limiting and selectivity-determining step, preceded by isoprene dimerization. The head-tail pathway is the kinetic pathway while the tail-tail product is the thermodynamic product. This functionalized lactone also shows promise for polymerization under Lewis acid-promoted conditions, opening avenues for sustainable polymers from CO2 and bio-derived feedstocks.
Similar content being viewed by others
Introduction
Developing methods for the chemical fixation of carbon dioxide (CO2) offers significant potential to reduce its atmospheric levels, addressing a key contributor to climate change. This approach not only utilizes an abundant and inexpensive feedstock but also creates a CO2-negative process1. Despite CO2 being an ideal C1 feedstock due to its global availability, low cost, and low toxicity, its use in organic synthesis is challenged by its low reactivity and high oxidation state2.
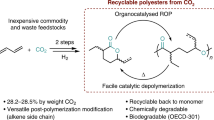
3-Ethylidene-6-vinyltetrahydro-2H-pyran-2-one (EVP), a six-membered lactone synthesized from two butadiene units and CO2, has gained attention as a versatile monomer for polymers with high CO2 content (Fig. 1a)3,4. EVP is accessible through an atom-economical palladium-catalyzed telomerization of butadiene under a CO2 atmosphere5,6. In the past decade, EVP’s potential as a monomer for sustainable CO2-based polymers has been extensively explored7. Various polymerization procedures have been investigated, including free radical polymerization, controlled radical polymerization8, coordination-insertion polymerization9, ring-opening polymerization10,11,12,13,14, amongst others2,3,4. In parallel, improved homogeneous and heterogeneous protocols have been developed to access EVP in high yields (Fig. 1b)7,15,16,17,18,19.
Isoprene, a C5 hydrocarbon, is the most abundantly emitted hydrocarbon in the atmosphere after methane20,21. It is estimated that emissions release 400‒500 teragrams of carbon (Tg C) from plants per year22,23, approximately matching the amount of biogenic methane emissions22,24. In contrast to butadiene, which is derived from fossil resources, isoprene is a feedstock with the inherent potential for renewable sourcing independent of petrochemical processes, albeit current production routes from biomass are not competitive in terms of energy utilization25. Despite its abundance and the versatility of its 1,3-diene motive, isoprene’s use as a renewable carbon source is largely focused on natural rubber and adhesive production26,27,28. This underutilization of such an abundant resource underscores the need to explore its potential for sustainable polymer production. Sequestering the two ubiquitous climate gases, CO2 and isoprene, into an EVP analog and its derived polymers could be a transformative step towards a more sustainable polymer economy.
Telomerization of substituted 1,3-dienes like isoprene, which contains an internal methyl group, has been challenging due to reduced reactivity from increased steric hindrance7. Although some telomerization reactions with alcohols and amines have been reported29,30,31,32,33, telomerization with CO2 remains an outstanding challenge. In 1995, Dinjus and Leitner reported the isolation of two lactone isomers from isoprene and CO2 in 1% yield (TON = 14) by preparatory thin-layer chromatography (pTLC)34, in sharp contrast to up to 86% yield achieved with butadiene (Fig. 1b)16,17,18,19. In addition, achieving selectivity over several other lactone, acid, and terpene products, as well as controlling the formation of up to 4 regioisomers while maintaining high turnover numbers, complicates the reaction development31.
In 2014, our group showed that a mixture of isoprene and butadiene could result in a poly-lactone material via a one-pot two-step process, though the isoprene content was not quantified7. These materials exhibited a lower glass transition temperature (Tg = 63 °C) than poly-EVP (Tg = 178–192 °C), which only consisted of butadiene-derived monomers, possibly due to the introduced methyl groups. Further lowering the Tg of these isoprene-derived materials would impart them with favorable properties distinct from poly-EVP. Clearly, further catalyst development is required to establish an effective pathway for lactone production from cheap and available isoprene and CO2 to tap into the vast potential of the isoprene economy.
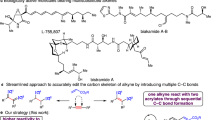
In this study, we present a palladium-based telomerization catalyst for isoprene and CO2, generating CO2-Isoprene-Lactone “COOIL” (1) with 24 wt% CO2 with turnover numbers >100 (Fig. 1c). The success of this reaction was dependent on a combination of a palladium(II) precursor, a triarylphosphine ligand, tetrabutylammonium acetate, and co-catalytic amounts of water. The resulting lactone isomers could be converted into low-molecular-weight polymers under Lewis acid-promoted conditions with the lactone units intact.
Results and discussion
We initiated developing the telomerization reaction by screening phosphine and amine ligands in the presence of 0.07 mol% Pd(acac)2 in acetonitrile under 2 MPa of CO2 pressure (see Supplementary Information (SI) for details). All evaluated ligands resulted in low isoprene conversion and predominantly afforded mono- and sesquiterpenes without CO2 incorporation, as determined by GC/MS analysis. Using the diamine ligand TEEDA, the desired δ-lactone 1 was generated with high selectivity, albeit only at trace amounts (0.1% isolated yield based on isoprene). Two of four possible regioisomers, 1a and 1b, were observed in a 1:3 ratio, with isomer 1b, featuring an α-oxygen quaternary center, as the predominant isomer (Fig. 1c). The same two isomers were detected by Dinjus and Leitner in earlier work, albeit with the opposite regioselectivity34.
The use of tetrabutylammonium acetate (TBAAc) as an additive, as reported by Bao et al.35 enhanced the formation of the desired lactone products, reaching 2% yield (as determined by GC) while maintaining good selectivity (50‒70% over other isoprene-derived products by GC). The hygroscopic reagent TBAAc readily absorbed ambient moisture during experiment preparation, which was mitigated by prolonged drying of the assembled autoclave under vacuum before charging the liquid reagents and conducting the reaction. Increasing the catalyst loading to 0.25 mol% and extending the reaction time allowed the isolation of 1 in 5% yield (TON = 19) in a regioisomer ratio (r.r.) of 1:11, favoring isomer 1b.
With these initial results in hand, we further optimized the conditions to increase the yield of lactone 1. Reducing the catalyst loading to 0.10 mol% and adding PPh3 improved the yield to 12% and the lactone selectivity to 69% with a r.r. of 1:3 (Table 1, entry 1). Using the slightly more electron-rich ligand P(p-Tol)3 further boosted the yield to 17% (1: 2.0 r.r.) (entry 2). In contrast, other phosphine and NHC ligands previously reported for the telomerization of butadiene with CO2 were ineffective (entry 3 and SI). Increasing CO2 pressure to 4 MPa marginally increased the yield (entry 5), while further adjustments to catalyst loading or temperature were ineffective (see SI). Testing different palladium precursors revealed that Pd(acac)2 was optimal, with other Pd(II) complexes yielding less product (entries 6‒7). Meanwhile, a Pd(0) precursor was incompatible with this reaction (entry 8).
Ammonium salts, in conjunction with a reductant, are known to promote the formation of nanoparticles from Pd(II) precursors36. Wang et al. recently reported that in situ generated Pd nanoparticles from Pd(acac)2 and TBAAc are highly active in the telomerization of butadiene and CO2, achieving yields comparable with homogeneous protocols18,35. We investigated whether nanoparticles were the active catalyst in our transformation. However, using colloidal Pd nanoparticles synthesized from Pd(acac)2 and anhydrous TBAAc37, no reaction occurred, irrespective of the presence of additional TBAAc and phosphine (entry 9, SI). In addition, using heterogenized Pd on ceria support38, or in the form of a palladium-containing perovskite39, resulted in no conversion (entries 10‒11). These findings suggest that the active species are likely monomeric or small aggregates of homogeneous palladium species. We generally obtained homogeneous clear solutions after the reaction was completed, indicating no Pd black formation.
Having established that the combination of Pd(acac)2, TBAAc, and P(p-Tol)3 presents the optimal catalyst system for the isoprene telomerization, we addressed the variable moisture content due to the hygroscopic nature of TBAAc. Anhydrous TBAAc absorbed significant amounts of water when exposed to ambient air (25 °C, 60% relative humidity) for two hours, as discernable by IR and 1H NMR measurements (see Fig. S6-9, SI). We rigorously dried the previously used commercial TBAAc batch by prolonged heating at 80 °C under vacuum, removing the bound water, as corroborated by IR measurements. Remarkably, using this anhydrous TBAAc no trace of 1 but only terpenes were produced (entry 12). Notably, when anhydrous TBAAc was allowed to age under ambient conditions for 15 min (20 °C, 80% rel. humidity), 1 was formed in 9%, implicating the beneficial effect of water in the reaction (entry 13).
To determine the optimal water amount for the reaction, we subsequently employed anhydrous TBAAc and added defined amounts of degassed water to the catalyst solution under an inert atmosphere. Without added water, the reaction produced only terpene products (Table 2, entry 1). Conversely, with the addition of 0.2 mol% of water (entry 2), equivalent to twice the amount of palladium species, we reproduced the optimal result with hydrated TBAAc (Table 1, entry 2). Thus, we obtained an isolated combined yield of 19% (TON = 93) of 1a and 1b and 77% selectivity. Omission of ligand or acetate shut down the reaction (entries 3–4). Wei and co-workers reported that a base is necessary to generate well-defined Pd(0) species and found that TBAAc, in conjunction with co-stoichiometric amounts of water, was more effective than other bases40. They also found that B2pin2 to be a more efficient reductant to produce Pd(0) species within short times. However, in this reaction, substituting TBAAc/water by B2pin2 did not promote the telomerization (entry 5). We could further decrease the reaction temperature to 50 °C by extending the reaction time (entries 6‒8), achieving a yield of 23% (TON = 114), the highest in this work, which is a magnitude larger than state-of-the-art (1%, TON = 14)34. The conversion of isoprene, as determined by 1H NMR analysis, lied in the range of 50–60%. The optimized conditions were scalable, enabling a gram-scale synthesis of a mixture of 1a and 1b (1.63 g in total using 0.125 mmol of Pd, see SI).
Thus, water has a potential effect on the formation of lactone 1 over terpenes, although its exact role remains unclear. The beneficial effect of trace moisture in palladium-catalyzed coupling reactions has been well documented40,41,42,43,44, including examples of the telomerization of butadiene/CO26. We hypothesize that a small amount of water might facilitate the reduction of the Pd(II) species to active Pd(0) by concurrent phosphine oxidation. Supporting this, we detected the corresponding phosphine oxide after the reaction by GC analysis. While reduction of Pd is generally assumed to occur readily in the presence of phosphine ligands and trace moisture41,45,46, the fate of the presumed Pd(0) species remains unexplored in the telomerization literature.
Beyond solely mediating precatalyst reduction, water appears to play other beneficial roles in this reaction. For instance, using a diborane as alternative reductant did not result in any catalytic activity (Table 2, entry 5). One potential additional role of water as a hydrogen bonding donor is increasing solubility and/or activating CO2, as has been widely documented in the contexts of CO2 adsorbents47,48,49.
To gain insight into the mechanism of this transformation, we performed density functional theory (DFT) calculations on the pathways leading to the four possible lactone isomers. The proposed catalytic cycle consists of three principal stages: oxidative dimerization of two isoprene units, migratory insertion of CO2, and reductive elimination of the C–O bond to form the lactone products (Fig. 2). Finally, outer-cycle isomerization leads to the more stable conjugated lactone products 1a and 1b.
The free energy diagram of the two predominant pathways, head-to-tail (HT) and tail-to-tail (TT), is depicted in Fig. 3. The two isoprene units can each coordinate in head- or tail-fashion, leading to three distinct Pd(0) diolefin complexes (INT1) by symmetry32,50,51,52. Oxidative dimerization (TS1) proceeds with a relatively low barrier resulting in four possible palladacycles (INT2), and exists in a fast pre-equilibrium prior to the subsequent catalytic steps. The head-to-head (HH) dimer was found to have both the lowest transition state barrier and ground state energy due to reduction of steric repulsion, while the TT transition state (TS) barrier was the highest. In all cases a η1-η3-coordination mode of the isoprene dimer was preferred to η3-η3-mode to relieve ring strain as previously found.
Outer-sphere approach by CO2 is succeeded by migratory insertion and C-C bond formation (INT3). The initial approach is calculated to be energetically uphill under standard conditions, and the insertion transition states (TS2) are reactant-like. Notably, insertion into the HH palladacycle leads to a comparably less stable intermediate, which rationalizes the experimental absence of lactone 1d. Conversely, the post-insertion intermediates (INT4) are low in energy for the HT, tail-to-head (TH) and TT pathways.
Following CO2 insertion, rotation of the carboxylate sets up the reductive elimination step (INT5). The C-O reductive elimination (TS3) to furnish the lactone exhibits the highest transition state barriers among all elementary steps in the catalytic cycle, ranging from 29.6 kcal/mol for the HT pathway to 33.2 kcal/mol for the TT isomer to 37.4 kcal/mol for the TH isomer. This high energy barrier for ring closure is consistent with the moderate overall conversion observed experimentally, indicating that reductive elimination is the overall rate-limiting step. Further, the lack of formation of the TH-pathway derived lactone 1c can be rationalized by the high barrier of reductive elimination compared to the other isomers. The reaction is thermodynamically favorable overall upon ligand exchange and release of the lactone product (ΔG HT: −5.7 kcal/mol; ΔG TT: −11.1 kcal/mol). Finally outer-cycle isomerization of the double bond generates the experimentally observed lactones 1a (ΔG = −16.8 kcal/mol) and 1b (ΔG = −11.9 kcal/mol).
The product regioselectivity is governed by the relative free energy barriers of the reductive elimination transition states leading to the different lactone isomers and the relative thermodynamic stabilities of the final lactone products. Specifically, our calculations with the Pd/P(p-Tol)3 catalyst system show that the pathway forming lactone 1b’ (derived from head-to-tail coupling) is kinetically favored due to a lower reductive elimination barrier compared to the pathway forming 1a’ (derived from TT coupling). However, lactone 1a’ is calculated to be the thermodynamically more stable product. This is in alignment with experimental observations showing that the selectivity ratio of 1a relative to 1b increases with increasing reaction conversion (cf. Table 2, entries 2 versus 4 and SI). Conversely, the product distribution follows Curtin-Hammett conditions: it is governed by the relative barriers of the reductive elimination transition states, but not by the relative stabilities or formation rates of the initial dimeric intermediates.
Given the diverse polymerization pathways available for butadiene-derived EVP3,4,53, we explored the potential of converting isoprene-derived monomer 1 into macromolecules with high CO2 content. Unlike butadiene-derived EVP, 1 showed no reactivity with a free radical initiator, in all attempts under neat conditions leading to monomer recovery. This diminished reactivity of 1 can be rationalized by the tetrasubstituted double bond, that is even more sterically hindered than the tiglate moiety in butadiene-derived EVP. Similarly, emulsion polymerization and organocatalytic ring-opening polymerization protocols resulted in no conversion.
However, when ZnCl2 as a promoter in ethyl carbonate (EC) solvent was employed in addition to V-40 as a radical initiator, full conversion of 1 into a polymer was observed according to 1H NMR analysis (Table 3, entry 1). Size-exclusion chromatography (SEC) analysis revealed that the material obtained under these conditions was oligomeric (Mn = 0.7 kg/mol, Mw/Mn = 1.98). Employing AIBN increased Mn and reduced dispersity, but at the same time, reduced the amount of recovered material (entry 2). Raising the reaction temperature reduced control over the polymerization (entry 3), while apolar solvents resulted in poor material recovery (entry 4). As noted, ZnCl2 was essential to initiate polymerization (entry 5). Interestingly, polymerization still occurred in the absence of a radical initiator (entry 6). On a smaller scale, polymer 2 with higher molecular weight and lower polydispersity could be isolated and characterized (Mn = 3.9 kg/mol, Mw/Mn = 1.28) (Table 3, entry 7 and Fig. 4d). The glass-transition temperature (Tg) of the obtained material was 44 °C, in line with the trend that the addition of methyl substituents significantly decreases Tg.
The repeating unit of 2 was deduced using IR and NMR spectral data (Fig. 4a–c) and by comparison with butadiene-derived poly-EVP and reference compounds (Fig. 4e, see SI for details). The 13C NMR chemical shift of the carbonyl at 169 ppm, along with the IR data rule out bicyclic structure α. In the 1H NMR spectrum, the signal at 4.3 ppm is assigned to the α-alkoxy proton in structure β arising from polymerization of the allylic ester in 1a (R2 = H). For 1b, all signals can be assigned in the aliphatic region. Structures γ and δ can be ruled out as well, both from NMR data and because polymerization of tetrasubstituted olefins is generally disfavored7,54.
In summary, we have achieved the catalytic telomerization of isoprene with CO2, two of the most abundant carbon-containing molecules in the atmosphere. This accomplishment overcomes the long-standing challenge of telomerization of substituted 1,3-dienes with CO2. The key to achieving consistent catalyst performance was selecting a suitable palladium/ligand combination and controlling the amount of co-catalytic water in the reaction.
Given the tremendous market potential of valorizing the abundant, renewable, and underutilized carbon-feedstocks of isoprene and CO2, our work is poised to stimulate further research into this sustainably sourced lactone and its potential applications of sustainable polymers.
Data availability
Details about the materials and methods, experimental procedures, characterization data, and computational studies, are included in this article and its supplementary files. Cartesian coordinates of structures for DFT experiments are provided in a separate .xlsx file as Supplementary Data. All additional data are available from the corresponding author upon request.
References
Appel, A. M. et al. Frontiers, opportunities, and challenges in biochemical and chemical catalysis of CO2 fixation. Chem. Rev. 113, 6621–6658 (2013).
Liu, Y. & Lu, X.-B. Current challenges and perspectives in CO2-based polymers. Macromolecules 56, 1759–1777 (2023).
Eagan, J. M. The divergent reactivity of lactones derived from butadiene and carbon dioxide in macromolecular synthesis. Macromol. Rapid Commun. 44, 2200348 (2023).
Tang, S. et al. Sustainable copolymer synthesis from carbon dioxide and butadiene. Chem. Rev. 124, 3590–3607 (2024).
Sasaki, Y., Inoue, Y. & Hashimoto, H. Reaction of carbon dioxide with butadiene catalysed by palladium complexes. Synthesis of 2-ethylidenehept-5-en-4-olide. J. Chem. Soc. Chem. Commun. 15, 605–606 (1976).
Musco, A., Perego, C. & Tartiari, V. Telomerization reactions of butadiene and CO2 catalyzed by phosphine Pd(0) complexes: (E)-2-ethylideneheptden-5alide and octadienyl esters of 2-ethylidenehepta-4,6-dienoic acid. Inorg. Chim. Acta 28, L147–L148 (1978).
Nakano, R., Ito, S. & Nozaki, K. Copolymerization of carbon dioxide and butadiene via a lactone intermediate. Nat. Chem. 6, 325–331 (2014).
Chen, L. et al. Chemoselective RAFT polymerization of a trivinyl monomer derived from carbon dioxide and 1,3-butadiene: from linear to hyperbranched. Macromolecules 50, 9598–9606 (2017).
Tang, S., Zhao, Y. & Nozaki, K. Accessing divergent main-chain-functionalized polyethylenes via copolymerization of ethylene with a CO2/butadiene-derived lactone. J. Am. Chem. Soc. 143, 17953–17957 (2021).
Garcia Espinosa, L. D., Williams-Pavlantos, K., Turney, K. M., Wesdemiotis, C. & Eagan, J. M. Degradable polymer structures from carbon dioxide and butadiene. ACS Macro Lett. 10, 1254–1259 (2021).
Yue, S. et al. Ring-opening polymerization of CO2-based disubstituted δ-valerolactone toward sustainable functional polyesters. ACS Macro Lett. 10, 1055–1060 (2021).
Rapagnani, R. M., Dunscomb, R. J., Fresh, A. A. & Tonks, I. A. Tunable and Recyclable Polyesters from CO2 and Butadiene. Nat. Chem. 14, 877–883 (2022).
Anderson, R. J., Fine, R. L., Rapagnani, R. M. & Tonks, I. A. Ring-opening copolymerizations of a CO2-derived δ-valerolactone with ε-caprolactone and l-lactide. Macromolecules 57, 6248–6254 (2024).
Zhang, J. et al. Bifunctional and recyclable polyesters by chemoselective ring-opening polymerization of a δ-lactone derived from CO2 and butadiene. Nat. Commun. 15, 8698 (2024).
Dai, Y., Feng, X., Wang, B., He, R. & Bao, M. Preparation and application of air-stable P,N-bidentate ligands for the selective synthesis of δ-lactone via the palladium-catalyzed telomerization of 1,3-butadiene with carbon dioxide. J. Organomet. Chem. 696, 4309–4314 (2012).
Sharif, M., Jackstell, R., Dastgir, S., Al-Shihi, B. & Beller, M. Efficient and selective palladium-catalyzed telomerization of 1,3-butadiene with carbon dioxide. ChemCatChem 9, 542–546 (2017).
Balbino, J. M., Dupont, J. & Bayón, J. C. Telomerization of 1,3-butadiene with carbon dioxide: a highly efficient process for δ-lactone generation. ChemCatChem 10, 206–210 (2018).
Wang, J. et al. The ultrasmall palladium nanoparticles catalyzed telomerization of CO2 with 1,3-butadiene at room temperature: selective synthesis of δ-lactone. ChemistrySelect 5, 9404–9408 (2020).
Yang, Z., Shen, C. & Dong, K. Hydroxyl group-enabled highly efficient ligand for Pd-catalyzed telomerization of 1,3-butadiene with CO2. Chin. J. Chem. 40, 2734–2740 (2022).
Wells, K. C. et al. Satellite isoprene retrievals constrain emissions and atmospheric oxidation. Nature 585, 225–233 (2020).
Paulot, F. et al. Unexpected epoxide formation in the gas-phase photooxidation of isoprene. Science 325, 730–733 (2009).
Guenther, A. et al. Estimates of global terrestrial isoprene emissions using MEGAN (model of emissions of gases and aerosols from nature). Atmos. Chem. Phys. 6, 3181–3210 (2006).
Hantson, S., Knorr, W., Schurgers, G., Pugh, T. A. M. & Arneth, A. Global isoprene and monoterpene emissions under changing climate, vegetation, CO2 and land use. Atmos. Environ. 155, 35–45 (2017).
Guenther, A. B. et al. The model of emissions of gases and aerosols from nature Version 2.1 (MEGAN2.1): an extended and updated framework for modeling biogenic emissions. Geosci. Model. Dev. 5, 1471–1492 (2012).
Liang, C., Gracida-Alvarez, U. R., Hawkins, T. R. & Dunn, J. B. Life-Cycle assessment of biochemicals with clear near-term market potential. ACS Sustain. Chem. Eng. 11, 2773–2783 (2023).
Matos, C. T., Gouveia, L., Morais, A. R. C., Reis, A. & Bogel-Łukasik, R. Green metrics evaluation of isoprene production by microalgae and bacteria. Green. Chem. 15, 2854–2864 (2013).
Morais, A. R. C. et al. Chemical and biological-based isoprene production: green metrics. Catal. Today 239, 38–43 (2015).
Global Isoprene Market Report, History and Forecast 2016–2027, Breakdown Data by Manufacturers, Key Regions, Types and Application. Research Reports World. https://www.researchreportsworld.com/global-isoprene-market-18440678. Accessed 2025-05-01.
Keim, W. & Roeper, M. Terpene amine synthesis via palladium-catalyzed isoprene telomerization with ammonia. J. Org. Chem. 46, 3702–3707 (1981).
Maddock, S. M. & Finn, M. G. Palladium-catalyzed head-to-head telomerization of isoprene with amines. Organometallics 19, 2684–2689 (2000).
Jackstell, R., Grotevendt, A., Michalik, D., El Firdoussi, L. & Beller, M. Telomerization aNd Dimerization of Isoprene by in Situ Generated Palladium–carbene Catalysts. J. Organomet. Chem. 692, 4737–4744 (2007).
Colavida, J. et al. Regioselectivity control in Pd-catalyzed telomerization of isoprene enabled by solvent and ligand selection. ACS Catal. 10, 11458–11465 (2020).
Desai, S. P., Xiang, Z., McEnally, C. S., Moore, C. M. & Yang, X. Isoprene telomers for biodiesel applications. Energy Fuels 38, 11006–11011 (2024).
Dinjus, E. & Leitner, W. New insights into the palladium-catalysed synthesis of δ-lactones from 1,3-dienes and carbon dioxide. Appl. Organomet. Chem. 9, 43–50 (1995).
Song, J. et al. Selective synthesis of δ-lactone via palladium nanoparticles-catalyzed telomerization of CO2 with 1,3-butadiene. Tetrahedron Lett. 57, 3163–3166 (2016).
Astruc, D., Lu, F. & Aranzaes, J. R. Nanoparticles as recyclable catalysts: the frontier between homogeneous and heterogeneous catalysis. Angew. Chem. Int. Ed. 44, 7852–7872 (2005).
Reetz, M. T. & Maase, M. Redox-controlled size-selective fabrication of nanostructured transition metal colloids. Adv. Mater. 11, 773–777 (1999).
Huang, Y. et al. Bimetallic synergy in supported Ni–Pd catalyst for selective hydrogenolysis of C–O bonds in epoxy resins. Nat Commun 16, 1188 (2025).
Andrews, S. P., Stepan, A. F., Tanaka, H., Ley, S. V. & Smith, M. D. Heterogeneous or homogeneous? A case study involving palladium-containing perovskites in the Suzuki reaction. Adv. Synth. Catal. 347, 647–654 (2005).
Wei, C. S. et al. The impact of palladium(II) reduction pathways on the structure and activity of palladium(0) catalysts. Angew. Chem. Int. Ed. 52, 5822–5826 (2013).
Amatore, C. et al. Kinetic data on the synergetic role of amines and water in the reduction of phosphine-ligated palladium(II) to Palladium(0). Eur. J. Org. Chem. 2014, 4709–4713 (2014).
Rosner, T., Le Bars, J., Pfaltz, A. & Blackmond, D. G. Kinetic studies of heck coupling reactions using palladacycle catalysts: experimental and kinetic modeling of the role of dimer species. J. Am. Chem. Soc. 123, 1848–1855 (2001).
Rosner, T., Pfaltz, A. & Blackmond, D. G. Observation of unusual kinetics in heck reactions of aryl halides: the role of non-steady-state catalyst concentration. J. Am. Chem. Soc. 123, 4621–4622 (2001).
Chernyshev, V. M. et al. Revealing the unusual role of bases in activation/deactivation of catalytic systems: O–NHC coupling in M/NHC catalysis. Chem. Sci. 9, 5564–5577 (2018).
Amatore, C., Jutand, A. & M’Barki, M. A. Evidence of the formation of zerovalent palladium from Pd(OAc)2 and triphenylphosphine. Organometallics 11, 3009–3013 (1992).
Amatore, C., Carre, E., Jutand, A. & M’Barki, M. A. Rates and mechanism of the formation of zerovalent palladium complexes from mixtures of Pd(OAc)2 and tertiary phosphines and their reactivity in oxidative additions. Organometallics 14, 1818–1826 (1995).
Kolle, J. M., Fayaz, M. & Sayari, A. Understanding the effect of water on CO2 adsorption. Chem. Rev. 121, 7280–7345 (2021).
Warren, J. J. Examining the importance of hydrogen bonding and proton transfer in iron porphyrin-mediated carbon dioxide upconversion. Acc. Chem. Res. 57, 2512–2521 (2024).
Liang, X.-M., Ruan, Z.-J., Guo, G.-Q., Lin, J.-Q. & Zhong, D.-C. CO2 Reduction by transition-metal complex systems: effect of hydrogen bonding on the second coordination sphere. ChemCatChem 17, e202401394 (2025).
Benn, R., Jolly, P. W., Mynott, R. & Schenker, G.Intermediates in the Palladium-catalyzed Reactions of 1,3-dienes. 1. (η3,η3-Dodecatrienediyl)palladium [Pd(η3,η3-C12H18)]. Organometallics 4, 1136–1138 (1985).
Huo, C.-F., Jackstell, R., Beller, M. & Jiao, H. Mechanistic study of palladium-catalyzed telomerization of 1,3-butadiene with methanol. J. Mol. Model. 16, 431–436 (2010).
Jabri, A. & Budzelaar, P. H. M. DFT study of Pd(PMe3)/NMe3-catalyzed butadiene telomerization of methanol. Organometallics 30, 1374–1381 (2011).
Rapagnani, R. M. & Tonks, I. A. 3-Ethyl-6-vinyltetrahydro-2 H -pyran-2-one (EVP): a versatile CO2-derived lactone platform for polymer synthesis. Chem. Commun. 58, 9586–9593 (2022).
Iio, K., Kobayashi, K. & Matsunaga, M. Radical polymerization of allyl alcohol and allyl acetate. Polym. Adv. Technol. 18, 953–958 (2007).
Acknowledgements
M.D.R.L. acknowledges that this work was supported by the Swiss National Science Foundation (Postdoc.Mobility P500PN_ 214255). The computational studies were performed using resources of the Research Center for Computational Science, Okazaki, Japan (Project: 22-IMS-C017).
Author information
Authors and Affiliations
Contributions
M.D.R.L.: Conceptualization, Methodology, Investigation, Formal Analysis, Writing—Original Draft, Funding acquisition. F.K.: Methodology, Investigation, Writing—Review & Editing. K.M.: Validation, Review & Editing. K.N.: Conceptualization, Supervision, Writing—Review & Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lutz, M.D.R., Kracht, F., Marumoto, K. et al. Gram-scale selective telomerization of isoprene and CO2 toward 100% renewable materials. Nat Commun 16, 7326 (2025). https://doi.org/10.1038/s41467-025-62409-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-62409-2