Abstract
While copper-catalyzed asymmetric propargylic substitution has emerged as a versatile tool for constructing chiral propargylic frameworks, its application in forging cyclic chiral quaternary carbon centers remains a significant synthetic challenge. This work demonstrates a dinuclear copper-catalyzed asymmetric [3 + 2] annulation strategy between tertiary propargyl carbonates and diverse C,O-bisnucleophiles under mild conditions. The protocol enables efficient synthesis of chiral dihydrofurans featuring cyclic quaternary stereocenters with broad substrate compatibility. The stereochemical control may arise from the unique coordination geometry of dinuclear copper-allenylidene intermediates, which spatially aligns the electrophilic site within the chiral ligand environment. This spatial arrangement overcomes inherent steric challenges in the stereoselective formation of quaternary carbons bearing dual alkyl substituents, offering mechanistic insights into cooperative dinuclear catalysis for asymmetric transformations.
Similar content being viewed by others
Introduction
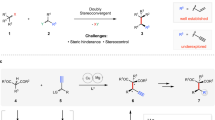
The asymmetric catalytic synthesis of chiral molecules bearing all-carbon quaternary stereocenters is a longstanding challenge for the synthetic community (Fig. 1a)1,2,3,4,5,6. Since the pioneering studies from van Maarseveen7 and Nishibayashi8 groups in 2008, respectively, Cu-catalyzed asymmetric propargylic substitution has been developed as an important method for the construction of chiral propargylic skeletons9,10,11,12,13,14,15,16. In particular, catalytic sequential propargylation/cycloisomerization reactions of propargylic esters, used as C3- or C2-bis-electrophiles, with various bis-nucleophiles constitute a powerful strategy for the synthesis of a diverse range of chiral cyclic frameworks17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32. However, Cu-catalyzed asymmetric propargylic substitution are largely limited to the substrates of secondary propargylic esters and only substrates with both an aryl and an oxygen-containing moiety at the prochiral carbon of the resulting Cu–allenylidene intermediates gave good results33,34,35,36,37,38,39. Very recently, Zhou40,41 and coworkers developed sterically confined PYBOX ligands with a bulky C4 shielding group and relaying groups, allowing highly enantioselective Cu-catalyzed propargylic amination of simple ketone-derived propargylic carbonates.
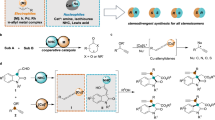
a Selected examples of important molecules with cyclic chiral quaternary carbon center. b Cu-catalyzed propargylation/cycloisomerization reactions. c Proposed binuclear Cu-allenylidene intermediates. d This work: dinuclear copper-catalyzed [3 + 2] annulation of tertiary propargylic esters with a diverse set of C,O bis-nucleophiles.
Despite these advances, the development of enantioselective Cu-catalyzed propargylation/annulation for rapid construction of cyclic frameworks with a chiral quaternary carbon center is still challenging and appealing42,43,44. For example, dihydrofuran derivatives are a class of naturally occurring bioactive compounds45,46,47,48,49,50,51,52,53 and Cu-catalyzed sequential propargylation/cycloisomerization reactions of propargylic esters with 1,3-C,O-bis-nucleophiles provides an efficient strategy to access these important cyclic molecules54,55,56. Nevertheless, the produced chiral dihydrofuran derivatives bearing an exocyclic double bond may easily undergo isomerization to furans57, due to the lack of a cyclic chiral quaternary carbon center. To the best of our knowledge, the synthesis of dihydrofuran derivatives featuring a chiral quaternary carbon center remains largely unexplored using problematic dialkyl propargylic alcohol derivatives, probably due to the poor chiral discrimination between two alkyl groups and the lack of directing factors in the resulting Cu–allenylidene intermediates (Fig. 1b).
Over the past decade, in situ generated binuclear copper species have been frequently implicated as active catalysts in copper-catalyzed asymmetric propargylic substitution reactions, with bridged dicopper allenylidene complexes commonly proposed as the key reactive intermediates24,34,42,43,58,59,60,61,62,63,64. Recently, we have disclosed the successful development of a series of binuclear copper catalysts, supported by chiral benzo[c]cinnoline dioxazoline frameworks, and their applications in catalytic asymmetric propargylic substitution reactions65,66. According to the proposed mechanism, the binuclear Cu core can play a bifunctional role in the coordination with acetylide and allenylidene species via a σ and a π interactions. Considering the potential dinuclear synergistic effect67,68,69,70,71, we envisioned that this type of coordination mode may bring the electrophilic site of the copper-allenylidene intermediates close to the chiral pocket, making it more prone to achieve a stereocontrolled formation of the quaternary carbon center when the problematic and challenging dialkyl propargylic alcohol derivatives are applied (Fig. 1c).
Herein, we report a strategy for the synthesis of enantioenriched dihydrofuran derivatives with a chiral quaternary carbon center via a dinuclear copper-catalyzed [3 + 2] annulation of tertiary propargylic esters with a diverse set of C,O bis-nucleophiles (Fig. 1d). The methodology is found to be quite general with respect to the substrate scope, since benzoylacetonitrile, 4-hydroxy-2-quinolinone, 4-hydroxy-1-methyl-2-quinolinone, 4-hydroxycoumarin, 4-hydroxythiocoumarin, alkyl and aryl-substituted 4,6-dihydroxypyrimidine, and multisubstituted 2,4-dihydroxypyridines, as well as dimedone, are found amenable to the catalysis, leading to the formation of various chiral dihydrofuran derivatives in good yields with good to high enantioselectivities. Notably, this work further demonstrates that the binuclear Cu catalysis can be used as an efficient strategy to solve the challenges in the asymmetric construction of chiral quaternary carbon centers via propargylic substitution of simple ketone-derived propargylic alcohol derivatives.
Results
Reaction discovery
At the initial studies, a tertiary propargyl carbonate (1a) was chosen as the model substrate in the [3 + 2] reaction with benzoylacetonitrile (2a), and a variety of Cu complexes of chiral nitrogen ligands were examined as the catalysts (Fig. 2). A careful survey of the reaction parameters using the binuclear copper catalyst C1 revealed that the reaction proceeded well in CF3CH2OH at room temperature for 24 h, affording the desired product 3 in 66% yield with 67% ee (see the Supplementary Information, Table S1–S5). Under the otherwise identical reaction conditions, several other bicopper catalysts C2-C9 with different substituents on the oxazolyl units or on the cinnoline backbone of the ligands were further investigated. The reaction using catalyst C2, containing four more phenyl substituents on the oxazolyl units, resulted in the formation of 3 with a slightly lower yield and ee value. Using catalyst C3 with the ligand derived from 2-aminoindanol, the reaction gave the desired product 3 with 60% ee. Further increasing the steric hindrance of the oxazoline units led to a boost of the ee up to 91% using catalyst C9. In a comparative study, chiral Pybox ligands (L1 and L2) were also examined together with CuI in the model reaction. Unfortunately, only very low ee values of the product were obtained in these cases. The reactions using some privileged bidentate chiral ligands, including bisoxazoline ligands L3-L5, Phox L6 and BINAP, only afforded product 3 with low to moderate ee values. Upon further evaluation of the privileged chiral P,N,N ligand L7 developed by Hu, it was observed that the annulation of tertiary propargyl carbonates proceeded smoothly with low enantioselectivity. These survey results of ligands L1-L7 contrast strikingly with those using the dinuclear copper catalysts, highlighting the effective catalytic performance of the dinuclear Cu catalysts in the current reaction.
Substrate Scope
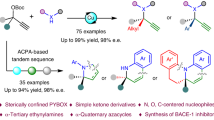
Under the optimized reaction conditions, the scope of the benzoylacetonitriles 2 was first explored in the reactions with 1a using C9 as the catalyst, and the results were summarized in Fig. 3a. To our delight, this catalytic system tolerated quite well with various benzoylacetonitriles bearing electronic donating para-substituents (-OMe, -Me, -iPr), furnishing the corresponding target products 3-6 in good yields with excellent enantioselectivities (87-91% ee). In addition, the substrates bearing electron-withdrawing groups (–F, –Cl, –Br, –CF₃, –CN, and –NO₂) at the para-position of the aryl ring delivered the desired products 7–12 with slightly lower enantioselectivities (65–81% ee), which may be attributed to the reduced nucleophilicity of the electron-deficient nucleophiles. The absolute configuration of 9 was determined as (S) by X-ray crystallographic studies (Fig. S8 and Table S6). Substrates with a meta-methoxy or meta-fluorine substituted aryl ring were also amenable to the reaction, affording the corresponding products 13 and 14 in good yields (94% and 75%) with high ee values (88% and 80%). The reactions of the substrates with an ortho-chloro, ortho-methyl or ortho-methoxy substituent on the aryl ring of the benzoylacetonitriles afforded the corresponding products 15-17 in good yields (79-81%) with good enantioselectivities (76-89% ee). The system was further demonstrated to be effective for the reaction of a poly-substituted benzoylacetonitrile, leading to the corresponding product 18 in 79% yield with 87% ee. Furthermore, aroylacetonitrile substrates with a β-naphthyl, α-furyl, or α-thienyl group were also amenable to the reaction, giving the corresponding products 19-21 in 66-94% yields with 84-88% ee.
a Substrate scope of the benzoylacetonitrile and its analogues. b Substrate scope of the quinolinones. c Substrate scope of the 2,4-dihydroxypyridine and its analogues. d Substrate scope of the tertiary propargylic esters. e Various C,O bis-nucleophiles. aGeneral conditions: 1 (0.1 mmol), bis-nucleophile (0.2 mmol), NaHCO3 (1.5 equiv), (R, R)-C9 (2.0 mol%), CF3CH2OH (1 mL), NH4I (2 equiv), 40 oC, 24 h. b tBuOLi (1 equiv), (S, S)-C1 (4.0 mol%), CF3CH2OH (1 mL), NH4I (20 mol%), rt, 24 h. c tBuOLi (1 equiv), (S, S)-C6 (4.0 mol%), CF3CH2OH (1 mL), NH4I (20 mol%), rt, 24 h. d 4-methylmorpholine (1 equiv), (S, S)-C1 (4.0 mol%), CF3CH2OH (1 mL), NH4I (20 mol%), rt, 24 h. e tBuOLi (1 equiv), (S, S)-C1 (4.0 mol%), CF3CH2OH (1 mL), rt, 24 h. f Na2CO3 (2 equiv), (S, S)-C1 (2.0 mol%), CH3OH (1 mL), rt, 24 h.
Notably, the methodology was applicable to the reaction of 1a with other types of 1,3-C,O-bis-nucleophiles such as quinoline-2,4(1H,3H)-diones (Fig. 3b). Various quinolin-diones bearing either electronic donating (-Me, -iPr, and -OMe) or withdrawing (-F) groups at the 8-position of the quinolindiones were tolerated quite well in the reactions, resulting in formation of the target products 22-26 in good yields with excellent enantioselectivities (87-91% ee). The 6-methyl and 6-fluorine substituted quinoline-2,4(1H,3H)-diones were also amenable to the reaction, affording the corresponding products 27 and 28 with high ee values (86% and 87%), albeit in moderate yields (51% and 55%). Notably, pyridyl-derived binucleophiles such as 2,4-dihydroxypyridine and its analogues were also applicable in the reaction using 4.0 mol% C1 as the catalyst (Fig. 3c). While the reaction of 2,4-dihydroxypyridine with 1a provided the corresponding product 29 in 46% yield with 84% ee, the reaction of ortho-methyl substituted pyridine gave product 30 in 60% yield with moderate enantioselectivity (76% ee). It was worth mentioning that the reaction using Gimeracil, a component in an anticancer therapy, afforded the corresponding product 31 in 52% yield with 90% ee. For the reactions of other alkyl substituted pyridine substrates, the corresponding cycloadducts 32-34 were obtained in 73-79% yields with 77-81% ee.
Encouraged by the efficiency of the binuclear Cu catalysts, we further evaluated the tertiary propargylic substrates in this asymmetric annulation with 2, as a quaternary stereogenic center can be installed along with the creation of the cyclic frameworks. To our delight, the reactions of propargylic carbonates derived from aliphatic ketones such as 2-butanone, 4-methyl-2-pentanone and cyclohexylacetone proceeded smoothly to give the corresponding cycloaddition products 35-37 bearing a quaternary chiral carbon, respectively, in 68-80% yields with 77-83% ee (Fig. 3d). Notably, using a tertiary propargyl carbonate with a methyl and an ethyl group, the reaction still afforded the desired cyclic product 35 in 80% yield with 80% ee, indicating the excellent chiral discrimination of the dinuclear Cu catalyst between these similar alkyl groups. This may arise from the unsymmetric coordination mode adopted by the dinuclear copper complex with allenylidene unit. Such coordination likely positions the electrophilic site of allenylidene unit in close proximity to the chiral environment created by the ligands, thereby enhancing stereocontrol even in cases involving minimally differentiated substituents. Branched propargylic carbonates bearing chlorocyclopropyl, cyclohexyl, cyclopentyl and tetrahydropyranyl moieties were also viable substrates, giving the corresponding cyclization products 38-41 in 64–84% yields with 82–90% ee. The absolute configuration of 39 was determined as (R) by X-ray crystallographic studies (Fig. S9 and Table S7), which is consistent with the use of the dinuclear copper catalyst (S, S)-C1. In addition, various benzylacetone-derived propargylic carbonates reacted with 4-hydroxy-2-quinolinone smoothly under the standard conditions to afford products 42-44 in 68–80% yields with 77–80% ee, irrespective of the nature and position of the phenyl substituent. Other types of tertiary propargylic substrates were also examined. To our delight, the reaction of substrates bearing 3-pyridyl and methyl groups with 4-hydroxy-2-quinolone afforded the desired product 45 in 90% yield with 87% ee. In addition, cyclic tertiary propargylic substrates also underwent the annulation smoothly, delivering the corresponding cycloaddition product 46 in 54% yield with 69% ee. Moreover, 4-hydroxy-1-methyl-2-quinolinone was also a compatible bis-nucleophile for the reaction, leading to the desired product 47 in 70% yield with 90% ee (Fig. 3e-i). Additionally, 4-hydroxy-2-chromenones were also surveyed in this annulation to further explore the substrate generality, and the desired products 48 and 49 were generated in 54-58% yields with high ee values (90% and 86%, respectively) (Fig. 3e-ii). 4-hydroxy-2H-thiochromen-2-one was also a compatible bis-nucleophile for the reaction, leading to the desired product 50 in 48% yield with 85% ee (Fig. 3e-iii). 4,6-dihydroxy-2-phenylpyrimidine and 4,6-dihydroxy-2-methylpyrimidine were also found as suitable nucleophiles and the reactions provided the corresponding products 51 and 52 in 50-52% yields with 78-80% ee (Figs. 3e-iv and 3e-v). It was worth mentioning that the reaction using dimedone afforded the corresponding product 53 in 71% yield with 82% ee (Fig. 3e-vi). These findings highlight the potential of the present binuclear copper catalysts in enabling cascade propargylic substitution–cyclization reactions of challenging tertiary propargylic esters, offering an efficient approach to access valuable tricyclic scaffolds bearing a quaternary stereogenic center and an exocyclic double bond.
Synthetic applications
In order to show the practical utility of this methodology, a gram-scale reaction of tertiary propargyl carbonate 1a and 4-hydroxy-2-quinolinone was performed under the standard conditions (Fig. 4-i), and the desired product 22 was isolated in 78% yield (1.0 g) with excellent enantioselectivity (90% ee). Subsequent investigations were carried out to demonstrate the synthetic utilities of product 22. By treatment with Tf2O or POCl3, 22 was transformed into 54 or 55, respectively, in high yields with excellent ee values (Fig. 4-ii and 4-iii). Amide reduction of 22 with LiAlH4, delivered quinoline derivative 56 in 46% yield, which is also a useful structural motif occurring in many natural products (Fig. 4-iv). The reaction of 22 with Lawesson’s reagent gave thioamide 57 in 68% yield with 90% ee (Fig. 4-v). Intriguingly, regioselective bromination of 22 gave rise to the alkenyl rather than aryl brominated product 58 in 68% yield (Fig. 4-vi). Treatment of 22 with Togni-I and TMSN3 using CuI/Pybox as catalyst afforded the product 59 in 64% yield with 90% ee, whose absolute configuration was unambiguously determined by the X-ray crystallographic analysis (Fig. 4-vii, Fig. S10 and Table S8). Reduction of 22 with H2 using Rh(PPh3)3Cl as the catalyst provided 60 in 98% yield with 90% ee (Fig. 4-viii). 60 was further reacted with (Boc)2O or BnBr to afford the N-Boc or N-Bn-substituted dihydrofuro [3,2-c] quinolinones 61 and 62 in good yields (85% and 73%, respectively). As a result of structural rigidity of the quaternary carbon moiety, the absolute configuration of the stereogenic center in 22 was retained over all these transformations.
i Gram-scale synthesis. ii–x various synthetic transformations (For details, see the Supplementary Information, Fig. S2).
To gain insight into the nature of the reactive intermediates involved in the binuclear copper catalysis, ESI-HRMS analysis was performed on the reaction mixture of tertiary propargyl carbonate (1a) and benzoylacetonitrile (2a). Distinct signals at m/z 767.2273 and 1367.2878 were observed, corresponding to the formation of a binuclear copper–acetylide complex derived from C1 and 1a, and the complex of C9 with 1a, respectively. Although the detailed structures of these two copper-acetylide species detected by HRMS are unknown, these results suggested that the catalytic intermediates featured by a binuclear Cu core may function throughout the catalytic cycle (Fig. 5a). Furthermore, a DFT computational study was carried out on the reaction of 1a using catalyst C9 to evaluate the viability of the plausible coordination modes of the two Cu centers with the allenylidene moiety65,66. Associated with the elimination of –OCOC6F5 moiety, distinct types of copper-allenylidene intermediates might be generated, such as an allenylidene α-carbon bridged binuclear Cu intermediate C9-INT II’24,34,42,43,58,59,60,61,62,63,64 or allenylidene α,β-bound Cu2-intermediate C9-INT II65,66,72,73 (Fig. 5b). In allenylidene intermediate C9-INT II’ (Fig. 5b left, Fig. S4 and Data S1), the two alkyl groups on Cγ were on the plane perpendicular to the plane of the catalyst backbone and the electrophilic site Cγ is far from the chiral oxazoline units. Therefore, the nucleophiles can attack Cγ from both left and right sides, which may result in poor ee values. Conversely, detailed structural analysis of the binuclear Cu–allenylidene intermediate C9-II revealed dihedral angles of –144° for N1–Cu1–Cu2–Cα and –156° for N1–Cu1–Cα–Cβ, which implies that the allene moiety is positioned below the plane of the catalyst backbone as a result of coordination with the –OCOC₆F₅ moiety (Fig. 5b right, Fig. S5 and Data S1). The surface distance projection color maps74 shown in Fig. 5c provided direct perspectives on the distinct types of intermediates. For INT C9-II’, the area around prochiral center is drawn in yellow (located at −2 Å) while those for the two chiral substituents of the ligand are in green (located at −4 Å), indicating that the prochiral center is located spatially relatively distal to the ligand and thus less influenced by the chiral pocket (Figs. 5c left and S6). On the other hand, the indenyl groups in binuclear Cu intermediate INT C9-II occupy the upper-left and lower-right sections of the ligand plane. As a result of the steric repulsion of the –OCOC6F5 moiety, the attack of the C,O-bis-nucleophile from the top is sterically hindered while the lower-right attack would be relatively less encumbered (Figs. 5c right and S7). Such an asymmetric coordination mode may provide a rationale for the stereoselectivity, as the dinuclear copper-allenylidene intermediate may position the electrophilic site closer to the chiral ligand environment, thereby facilitating effective stereoselective control over the quaternary carbon center. Therefore, we proposed a plausible catalytic cycle for the dinuclear copper-catalyzed asymmetric [3 + 2] propargylation/annulation (Fig. 5d). The catalytic cycle is initiated by activating the terminal alkyne of racemic tertiary propargyl carbonate 1 by the chiral dinuclear copper catalyst in the presence of a base. The corresponding alkynyl anion, which coordinates to the two Cu centers through both σ- and π-bonding interactions, forming the Cu-acetylide species I. The potential coordination of the iodide anion to the copper center in these cationic intermediates I cannot be conclusively excluded. The Cu-promoted elimination of the –OCOC6F5 group removes the stereochemical information at the C3 position, leading to the formation of a binuclear copper species II, in which an allenylidene ligand is coordinated to one copper atom and simultaneously binds in a π bond mode with the second copper center. Following this, a stereoselective nucleophilic attack by the C,O bis-nucleophile takes place, where the carbon atom preferentially attacks the γ-position of the allenylidene ligand, resulting in the formation of intermediate III. The elimination of C6F5CO2H from III by a base produces intermediate IV. Subsequently, an intramolecular nucleophilic attack by the oxygen atom on the β-carbon leads to the formation of intermediate V containing a dihydrofuran ring. Proton transfer between V and terminal alkyne substrate 1 yields the final product while regenerating intermediate I. Alternatively, protonation of intermediate IV followed by an intramolecular cyclization may also occur to deliver the final product.
a ESI-MS analysis of the reaction mixture under standard conditions. b Proposed transition state models. The blue shaded planes indicate the π-conjugated systems involved in the transition state geometry. c Surface distance projection maps of intermediates INT II and INT II’. d Proposed catalytic mechanism.
Discussion
In summary, we have developed an efficient method for the asymmetric [3 + 2] annulation of tertiary propargylic esters with diverse C,O bis-nucleophiles using well-defined chiral dinuclear copper complexes as the catalysts. The reaction is featured by mild conditions, excellent regioselectivity and high stereoselectivity. The reaction exhibits broad substrate scope with respect to various C,O-bis-nucleophiles, including benzoylacetonitrile, quinolinone, 4-hydroxy-1-methyl-2-quinolinone, 4-hydroxycoumarin, 4-hydroxythiocoumarin, alkyl- and aryl-substituted 4,6-dihydroxypyrimidine, multisubstituted 2,4-dihydroxypyridines and dimedone. It should be highlighted that the bifunctional role of the binuclear Cu and its unique coordination mode might be the key to the success of the excellent chiral control of the quaternary chiral carbon center.
Methods
General procedure
A typical experimental procedure for the preparation of (S)−4-isopropyl-2-(4-methoxyphenyl)−4-methyl-5-methylene-4,5-dihydrofuran-3-carbonitrile (3) is described below. C9 (2.8 mg, 0.002 mmol, 2.0 mol%), NH4I (29.0 mg, 2.0 equiv) and benzoylacetonitrile (35.1 mg, 0.20 mmol) were placed in a 10 mL Schlenk flask and a dry Ar atmosphere was established. Then, propargylic ester 1a (30.6 mg, 0.1 mmol), NaHCO3 (12.6 mg, 1.5 equiv) and CF3CH2OH (1 mL) were added, and the mixture was stirred at 40 °C for 24 h. The mixture was concentrated under reduced pressure and the residue was purified by silica gel chromatography with n-hexane and EtOAc (n-hexane/EtOAc = 4/1-1/1) as eluent to give 3 as a yellow solid (24.9 mg, 92% yield, 91% ee).
Data availability
The data supporting the findings of this study are available within the article and Supplementary Information files, and are also available from the corresponding author upon request. Crystallographic data coordinates for structures reported in this article has been deposited at the Cambridge Crystallographic Data Center (CCDC), under deposition numbers CCDC 2457310 (product 9), CCDC 2321726 (product 39), and CCDC 2327248 (product 59). These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/structures/.
References
Christoffers, J. & Baro, A. Quaternary stereocenters: challenges and solutions for organic synthesis. Adv. Synth. Catal. 348, 593–593 (2006).
Stockdale, T. P. & Williams, C. M. Pharmaceuticals that contain polycyclic hydrocarbon scaffolds. Chem. Soc. Rev. 44, 7737–7763 (2015).
Ling, T. & Rivas, F. All-carbon quaternary centers in natural products and medicinal chemistry: recent advances. Tetrahedron 72, 6729–6777 (2016).
Liu, Y., Han, S.-J., Liu, W.-B. & Stoltz, B. M. Catalytic enantioselective construction of quaternary stereocenters: assembly of key building blocks for the synthesis of biologically active molecules. Acc. Chem. Res. 48, 740–751 (2015).
Talele, T. T. Opportunities for tapping into three-dimensional chemical space through a quaternary carbon. J. Med. Chem. 63, 13291–13315 (2020).
Xu, P., Zhou, F., Zhu, L. & Zhou, J. Catalytic desymmetrization reactions to synthesize all-carbon quaternary stereocentres. Nat. Synth. 2, 1020–1036 (2023).
Detz, R. J., Delville, M. M. E., Hiemstra, H. & van Maarseveen, J. H. Enantioselective copper-catalyzed propargylic amination. Angew. Chem., Int. Ed. 47, 3777–3780 (2008).
Hattori, G., Matsuzawa, H., Miyake, Y. & Nishibayashi, Y. Copper-catalyzed asymmetric propargylic substitution reactions of propargylic acetates with amines. Angew. Chem., Int. Ed. 47, 3781–3783 (2008).
Ljungdahl, N. & Kann, N. Transition-metal-catalyzed propargylic substitution. Angew. Chem., Int. Ed. 48, 642–644 (2009).
Detz, R. J., Hiemstra, H. & Van Maarseveen, J. H. Catalyzed propargylic substitution. Eur. J. Org. Chem. 2009, 6263–6276 (2009).
Miyake, Y., Uemura, S. & Nishibayashi, Y. Catalytic propargylic substitution reactions. ChemCatChem 1, 342–356 (2009).
Ding, C.-H. & Hou, X.-L. Catalytic asymmetric propargylation. Chem. Rev. 111, 1914–1937 (2011).
Zhang, D.-Y. & Hu, X.-P. Recent advances in copper-catalyzed propargylic substitution. Tetrahedron Lett. 56, 283–295 (2015).
Roy, R. & Saha, S. Scope and advances in the catalytic propargylic substitution reaction. RSC Adv. 8, 31129–31193 (2018).
Sakata, K. & Nishibayashi, Y. Mechanism and reactivity of catalytic propargylic substitution reactions via metal–allenylidene intermediates: a theoretical perspective. Catal. Sci. Technol. 8, 12–25 (2018).
Tsuji, H. & Kawatsura, M. Transition-metal-catalyzed propargylic substitution of propargylic alcohol derivatives bearing an internal alkyne group. Asian J. Org. Chem. 9, 1924–1941 (2020).
Hattori, G., Miyake, Y. & Nishibayashi, Y. Copper-catalyzed diastereo- and enantioselective sequential reactions of propargylic acetates with (E)-2,4-pentadienylamine. ChemCatChem 2, 155–158 (2010).
Zhang, C. et al. Highly diastereo- and enantioselective Cu-Catalyzed [3+3] cycloaddition of propargyl esters with cyclic enamines toward chiral bicyclo [n.3.1] Frameworks. J. Am. Chem. Soc. 134, 9585–9588 (2012).
Zhang, D.-Y., Shao, L., Xu, J. & Hu, X.-P. Copper-catalyzed asymmetric formal [3+2] cycloaddition of propargylic acetates with hydrazines: enantioselective synthesis of optically active 2-pyrazolines. ACS Catal. 5, 5026–5030 (2015).
Wang, Q. et al. Catalytic asymmetric [4+1] annulation of sulfur ylides with copper–allenylidene intermediates. J. Am. Chem. Soc. 138, 8360–8363 (2016).
Li, T.-R. et al. A copper-catalyzed decarboxylative amination/hydroamination sequence: switchable synthesis of functionalized indoles. Angew. Chem., Int. Ed. 55, 12422–12426 (2016).
Liu, Z.-T., Wang, Y.-H., Zhu, F.-L. & Hu, X.-P. Enantioselective Copper-Catalyzed Formal [4+2] cycloaddition of o -aminophenol derivatives with propargylic esters for synthesis of optically active 3,4-Dihydro-2H−1,4-benzoxazines. Org. Lett. 18, 1190–1193 (2016).
Song, J., Zhang, Z. & Gong, L. Asymmetric [4+2] annulation of C1 ammonium enolates with copper-allenylidenes. Angew. Chem. Int. Ed. 56, 5212–5216 (2017).
Shao, W. & You, S. Highly diastereo- and enantioselective synthesis of tetrahydro-5H-Indolo[2,3-b]quinolines through copper-catalyzed propargylic dearomatization of indoles. Chem. Eur. J. 23, 12489–12493 (2017).
Lu, X. et al. Enantioselective cascade reaction for synthesis of quinolinones through synergistic catalysis using Cu–pybox and chiral benzotetramisole as catalysts. Chem. Eur. J. 23, 7689–7693 (2017).
Zhang, Y.-C., Zhang, Z.-J., Fan, L.-F. & Song, J. Enantioselective decarboxylative propargylation/hydroamination enabled by organo/metal cooperative catalysis. Org. Lett. 20, 2792–2795 (2018).
Ji, D., Wang, C. & Sun, J. Asymmetric [4+2]-cycloaddition of copper–allenylidenes with hexahydro-1,3,5-triazines: access to chiral tetrahydroquinazolines. Org. Lett. 20, 3710–3713 (2018).
Jiang, F. et al. Asymmetric [3+3] annulation of copper–allenylidenes with pyrazolones: synthesis of chiral 1,4-dihydropyrano[2,3-c]pyrazoles. Org. Lett. 20, 5278–5281 (2018).
Kong, H.-H. et al. Remote enantioselective [4+1] annulation with copper-vinylvinylidene intermediates. J. Am. Chem. Soc. 144, 21347–21355 (2022).
Li, L., Chen, X.-S. & Hu, X.-P. Intramolecular copper-catalyzed asymmetric propargylic [4+2]-cycloaddition toward optically active tetrahydroisoindolo[2,1-a]quinoxalines. Org. Lett. 24, 5433–5438 (2022).
Qu, B.-L. et al. Photoinduced carbene transfer for copper-catalyzed asymmetric [4+1] cycloadditions: an entry to chiral indolines bearing quaternary stereocenters. Org. Chem. Front. 10, 3498–3503 (2023).
Du, J. et al. Copper-catalyzed (3+2) annulation of 5-ethynyloxazolidine-2,4-diones with benzoylacetonitriles: synthesis of dihydrofuran derivatives. ChemistrySelect 8, e202303126 (2023).
Hattori, G., Yoshida, A., Miyake, Y. & Nishibayashi, Y. Enantioselective ring-opening reactions of racemic ethynyl epoxides via copper−allenylidene intermediates: efficient approach to chiral β-amino alcohols. J. Org. Chem. 74, 7603–7607 (2009).
Tsuchida, K., Senda, Y., Nakajima, K. & Nishibayashi, Y. Construction of chiral tri- and tetra-arylmethanes bearing quaternary carbon centers: copper-catalyzed enantioselective propargylation of indoles with propargylic esters. Angew. Chem., Int. Ed. 55, 9728–9732 (2016).
Detz, R. J., Abiri, Z., Remi, G., Hiemstra, H. & van Maarseveen, J. H. Enantioselective copper-catalysed propargylic substitution: synthetic scope study and application in formal total syntheses of (+)-Anisomycin and (−)-Cytoxazone. Chem. Eur. J. 17, 5921–5930 (2011).
Gómez, J. E., Guo, W., Gaspa, S. & Kleij, A. W. Copper-catalyzed synthesis of γ-amino acids featuring quaternary stereocenters. Angew. Chem., Int. Ed. 56, 15035–15038 (2017).
Tian, L., Gong, L. & Zhang, X. Copper-catalyzed enantioselective synthesis of β-amino alcohols featuring tetrasubstituted tertiary carbons. Adv. Synth. Catal. 360, 2055–2059 (2018).
Guo, W., Zuo, L., Cui, M., Yan, B. & Ni, S. Propargylic amination enabled the access to enantioenriched acyclic α-quaternary α-amino ketones. J. Am. Chem. Soc. 143, 7629–7634 (2021).
Liu, T., Ni, S. & Guo, W. Practical asymmetric amine nucleophilic approach for the modular construction of protected α-quaternary amino acids. Chem. Sci. 13, 6806–6812 (2022).
Zhang, Z. et al. Enantioselective propargylic amination and related tandem sequences to α-tertiary ethynylamines and azacycles. Nat. Chem. 16, 521–532 (2024).
Gong, Y. et al. Trifluoroethanol-assisted asymmetric propargylic hydrazination to α-tertiary ethynylhydrazines enabled by sterically confined pyridinebisoxazolines. Nat. Commun. 16, 4571–4582 (2025).
Xu, Y.-W., Li, L. & Hu, X.-P. Enantioselective copper-catalyzed [3+3] cycloaddition of tertiary propargylic esters with 1H-Pyrazol-5(4H)-ones toward optically active spirooxindoles. Org. Lett. 22, 9534–9538 (2020).
Gannarapu, M. R., Imai, T., Iwaki, K., Tsuzuki, S. & Shibata, N. Construction of poly-N-heterocyclic scaffolds via the controlled reactivity of Cu-allenylidene intermediates. Commun. Chem. 4, 158 (2021).
Shen, L., Lin, Z., Guo, B. & Zi, W. Synthesis of cycloheptanoids through catalytic enantioselective (4+3)-cycloadditions of 2-aminoallyl cations with dienol silyl ethers. Nat. Synth. 1, 883–891 (2022).
Nazrullaev, S. S., Bessonova, I. A. & Akhmedkhodzhaeva, K. S. Estrogenic activity as a function of chemical structure in haplophyllum quinoline alkaloids. Chem. Nat. Compd. 37, 551–555 (2001).
Zhang, M.-Z. et al. Microwave-assisted synthesis and antifungal activity of novel fused Osthole derivatives. Eur. J. Med. Chem. 124, 10–16 (2016).
Rueping, M., Parra, A., Uria, U., Besselièvre, F. & Merino, E. Catalytic asymmetric domino michael addition−alkylation reaction: enantioselective synthesis of dihydrofurans. Org. Lett. 12, 5680–5683 (2010).
Fan, L.-P. et al. Facile domino access to chiral mono-, bi-, and tricyclic 2,3-dihydrofurans. J. Org. Chem. 75, 8716–8719 (2010).
Mei, R.-Q. et al. Asymmetric Michael/cyclization tandem reaction of 4-hydroxycoumarin with β-nitroalkenes catalyzed by chiral bifunctional thioureas. Org. Biomol. Chem. 11, 1286 (2013).
Hack, D. et al. Combining silver catalysis and organocatalysis: a sequential michael addition/hydroalkoxylation one-pot approach to annulated coumarins. Org. Lett. 16, 5188–5191 (2014).
Xia, A.-B. et al. One-pot asymmetric synthesis of a spiro[dihydrofurocoumarin/pyrazolone] scaffold by a Michael addition/I2-mediated cyclization sequence. Org. Biomol. Chem. 15, 5709–5718 (2017).
Modrocká, V., Veverková, E., Baran, R. & Šebesta, R. Enantioselective synthesis of 2,3-dihydrofurocoumarins by squaramide-catalyzed michael addition/cyclization of 4-hydroxycoumarins with β-nitrostyrenes. ChemistrySelect 3, 1466–1471 (2018).
Zhou, Y., Zhu, F.-L., Liu, Z.-T., Zhou, X.-M. & Hu, X.-P. Chiral ferrocenyl P,N-ligands for palladium-catalyzed asymmetric formal [3+2] cycloaddition of propargylic esters with β-ketoesters: access to functionalized chiral 2,3-dihydrofurans. Org. Lett. 18, 2734–2737 (2016).
Zhu, F., Wang, Y., Zhang, D., Xu, J. & Hu, X. Enantioselective synthesis of highly functionalized dihydrofurans through copper-catalyzed asymmetric formal [3+2] cycloaddition of β-ketoesters with propargylic esters. Angew. Chem., Int. Ed. 53, 10223–10227 (2014).
Shao, L., Wang, Y., Zhang, D., Xu, J. & Hu, X. Desilylation-activated propargylic transformation: enantioselective copper-catalyzed [3+2] cycloaddition of propargylic esters with β-naphthol or phenol derivatives. Angew. Chem., Int. Ed. 55, 5014–5018 (2016).
Rohilla, S., Shah, S. & Singh, V. K. Copper-catalyzed asymmetric propargylic [3+2] cycloaddition: synthesis of enantioenriched dihydrofuro [3,2-c] coumarins and its quinolinone and thiocoumarin analogues. Org. Lett. 25, 3733–3738 (2023).
Raut, V. S. et al. Enantioselective syntheses of furan atropisomers by an oxidative central-to-axial chirality conversion strategy. J. Am. Chem. Soc. 139, 2140–2143 (2017).
Hattori, G. et al. Copper-catalyzed enantioselective propargylic amination of propargylic esters with amines: copper−allenylidene complexes as key intermediates. J. Am. Chem. Soc. 132, 10592–10608 (2010).
Nakajima, K., Shibata, M. & Nishibayashi, Y. Copper-catalyzed enantioselective propargylic etherification of propargylic esters with alcohols. J. Am. Chem. Soc. 137, 2472–2475 (2015).
Li, R.-Z. et al. Enantioselective propargylation of polyols and desymmetrization of meso 1,2-diols by copper/borinic acid dual catalysis. Angew. Chem., Int. Ed. 56, 7213–7217 (2017).
Cheng, L.-J., Brown, A. P. N. & Cordier, C. J. Enantioselective propargylic [1,3]-rearrangements: copper-catalyzed O-to-N migrations toward C–N bond formation. Chem. Sci. 8, 4299–4305 (2017).
Li, R.-Z., Liu, D.-Q. & Niu, D. Asymmetric O-propargylation of secondary aliphatic alcohols. Nat. Catal. 3, 672–680 (2020).
Garcia-Roca, A. et al. Comprehensive mechanistic scenario for the Cu-mediated asymmetric propargylic sulfonylation forging tertiary carbon stereocenters. J. Am. Chem. Soc. 145, 6442–6452 (2023).
Ma, J.-S. et al. Copper-catalysed convergent regio- and enantioselective alkynylallylic substitution. Nat. Synth. 2, 37–48 (2022).
Cai, Q. et al. Well-defined chiral dinuclear copper complexes in enantioselective propargylic substitution: For a long-standing supposition on binuclear mechanism. Chem 10, 265–282 (2024).
Lan, Y. et al. Well-defined chiral dinuclear copper-catalyzed tandem asymmetric propargylic amination–carboxylative cyclization sequence toward chiral 2-oxazolidinone derivatives. Org. Chem. Front. 11, 6319–6326 (2024).
Park, J. & Hong, S. Cooperative bimetallic catalysis in asymmetric transformations. Chem. Soc. Rev. 41, 6931 (2012).
Fricke, C., Sperger, T., Mendel, M. & Schoenebeck, F. Catalysis with Palladium(I) Dimers. Angew. Chem., Int. Ed. 60, 3355–3366 (2021).
Mankad, N. P. Selectivity effects in bimetallic catalysis. Chem. Eur. J. 22, 5822–5829 (2016).
Farley, C. M. & Uyeda, C. Organic reactions enabled by catalytically active metal–metal bonds. Trends Chem. 1, 497–509 (2019).
Campos, J. Bimetallic cooperation across the periodic table. Nat. Rev. Chem. 4, 696–702 (2020).
Jin, L., Tolentino, D. R., Melaimi, M. & Bertrand, G. Isolation of bis(copper) key intermediates in Cu-catalyzed azide-alkyne “click reaction”. Sci. Adv. 1, e1500304 (2015).
Worrell, B. T., Malik, J. A. & Fokin, V. V. Direct evidence of a dinuclear copper intermediate in Cu(I)-catalyzed azide-alkyne cycloadditions. Science 340, 457–460 (2013).
Lu, T. & Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Acknowledgements
The authors acknowledge financial support from the National Key R&D Program of China (No. 2022YFA1503200), the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB0610000), the National Natural Science Foundation of China (92256303, 21821002), the Shanghai Science and Technology Committee (23ZR1482400), the Natural Science Foundation of Ningbo (2023J034), Open Research Fund of School of Chemistry and Chemical Engineering, Henan Normal University and the Research Funds of Hangzhou Institute for Advanced Study, UCAS (2024HIAS-p003).
Author information
Authors and Affiliations
Contributions
L. S., Q. C. and X. W. directed the project and designed the experiments; L. S., Z. F., Q. C., P. L. and Y. L. performed all the experiments and analyzed all the data with X. W.; Z. F. performed all the computational studies and wrote the part of calculations. Q. C. and X. W. wrote the manuscript with contributions from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shao, L., Fang, Z., Liu, P. et al. Enantioselective construction of cyclic quaternary stereocenters via dinuclear copper catalyzed asymmetric [3 + 2] propargylation/annulation. Nat Commun 16, 7191 (2025). https://doi.org/10.1038/s41467-025-62564-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-62564-6
This article is cited by
-
Bidentate mixed-ligand strategy in dinuclear gold photoredox catalysis
Nature Communications (2025)