Abstract
Insulin deficiency from β-cell dysfunction underpins both type 1 and type 2 diabetes. However, the regulatory pathways underlying β-cell function remain incompletely understood. Here, we identify that March5 and Trim28 as key modulators of β-cell function. March5 is downregulated and Trim28 upregulated in islets from human or mouse with impaired glucose tolerance. Loss of March5 in β-cells impairs insulin production and glucose tolerance, while its overexpression improves both. Mechanistically, March5 inhibits Trim28 by targeting it for ubiquitination, thereby preventing Trim28-mediated Kindlin-2 degradation, which elevates MafA and insulin expression in male mice. Trim28 deletion in β-cells rescues glucose intolerance in March5-deficient male mice, highlighting their joint regulatory pathway. Furthermore, March5 and Kindlin-2 double haploinsufficiency significantly impair insulin production and glucose tolerance, underscoring their shared pathway. Importantly, islet transplantation with March5-overexpressing or Trim28-deficient β-cells effectively ameliorates glucose intolerance in streptozotocin-induced diabetic male mice. In conclusion, our results suggest that targeting the March5/Trim28/Kindlin-2/MafA pathway may offer a promising therapeutic strategy to restore β-cell function in diabetes.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) is a chronic metabolic disorder affecting over 500 million people worldwide, characterized by impaired insulin secretion or peripheral insulin resistance, leading to hyperglycemia and related complications1,2,3. Type 1 diabetes (T1D) results from autoimmune destruction of insulin-producing β-cells, resulting in a complete lack of insulin. Type 2 diabetes (T2D) involves insufficient insulin secretion relative to insulin resistance, progressing to β-cell dysfunction and eventual insulin deficiency. Progressive loss of β-cell function is a key common feature of both T1D and T2D3,4,5,6. While exogenous insulin administration and oral hypoglycemic agents manage hyperglycemia and delay DM-related complications, they fail to prevent the progressive decline in β-cell function. Recently, islet transplantation has emerged as a promising therapeutic approach for DM, aiming to restore endogenous insulin secretion and maintain glucose homeostasis7,8. Despite showing improved metabolic outcomes and reduced severe hypoglycemia compared to conventional insulin therapy, islet transplantation faces several challenges9,10,11,12, including limited donor availability and reduced insulin secretory capacity of isolated islets, etc.13,14. Mikaël Chetboun et al. reported a 5-year cumulative incidence of unsuccessful islet transplantation at 70.7%, with an inverse and linear correlation with primary graft function12,15,16,17. The success of islet transplantation depends on acquiring islets with normal or enhanced insulin expression and secretion capabilities. Understanding the mechanisms governing β-cell function and identifying key molecular determinants to bolster β-cell function presents significant therapeutic opportunities for diabetes and islet transplantation.
Membrane-associated RING-CH-type finger 5 (March5), also known as MITOL (Mitochondrial ubiquitin ligase), is a pivotal E3 ligase implicated in mitochondrial dynamics and quality control18,19,20,21, as well as antiviral signaling, cell proliferation, and drug-induced cell death, which are independent of its mitochondria-related functions22,23,24,25,26. March5 is associated with diseases such as Parkinson’s disease27,28, Alzheimer’s disease29, amyotrophic lateral sclerosis30,31, ischemia-reperfusion injury32, sepsis33, and liver diseases22,34. Two recent studies demonstrate that March5 is closely associated with the regulation of glucose and lipid metabolism. Simon T Bond et al. discovered that March5 expression in adipose tissue was significantly downregulated in obese individuals with elevated fasting blood glucose levels, mediated by peroxisome proliferator-activated receptor γ (PPARγ), a key regulator of adipogenesis35. Another study revealed that glucotoxicity upregulated March5 expression in cardiomyocytes, resulting in mitochondrial dysfunction and subsequent cardiomyocyte apoptosis36. Tripartite motif 28 (Trim28), a canonical RING-type E3 ubiquitin ligase, is intricately involved in numerous biological processes, such as cell proliferation, cellular energy homeostasis, and apoptosis, by modulating degradation of key proteins37,38,39. Trim28 deficiency in adipocytes promotes obesity but maintains glucose tolerance40, indicating a potential role in glucose homeostasis. However, the roles of March5 and Trim28 in modulating β-cell function remain unexplored.
Focal adhesion (FA) proteins are specialized subcellular structures that bridge the cell surface and extracellular matrix (ECM). FA proteins mediate cell-ECM signaling by coupling integrin receptors on the plasma membrane with ECM components41,42. Previous studies have demonstrated that the activation status of FA proteins at β-cell-capillary interfaces in pancreatic islets is crucial for regulating calcium channel opening on the plasma membrane and insulin granule fusion-release processes following glucose stimulation43,44. Of note, as a key FA protein, Kindlin-2 downregulation leads to impaired insulin secretion in β-cells45. β-cell differentiation and function are intricately regulated by factors like MafA and Pdx1, etc.45,46,47,48, with MafA being central to insulin gene expression49. Kindlin-2 also regulates insulin gene expression in mice by stabilizing MafA protein45.
In this study, we employ a comprehensive evaluation encompassing control mice alongside β-cell-specific March5 knockout or overexpression mice and establish the essential role of March5 in preserving β-cell function. Furthermore, the deleterious effects of HFD-induced progression of T2D are aggravated in mice lacking March5 in β-cells. Mechanistically, March5 directly ubiquitinates Trim28, inhibiting Trim28-mediated Kindlin-2 ubiquitination, resulting in increased MafA and insulin expression. We demonstrate that Trim28 expression in islets exhibits an opposite trend to that of March5. Genetic deletion of Trim28 in β-cells improves glucose homeostasis and effectively rescues the impaired glucose tolerance and diminished GSIS in March5-deficient mice. Importantly, islet transplantation with March5 overexpression or Trim28 deficiency in β-cells effectively ameliorates blood glucose levels in a streptozotocin (STZ)-induced diabetic mouse model. Notably, MARCH5 upregulation or TRIM28 downregulation enhances insulin production and secretion in human islets. In conclusion, our findings underscore the critical role of the March5/Trim28/Kindlin-2/MafA axis in modulating β-cell insulin expression and secretion, highlighting its potential as a therapeutic target for pancreatic β-cell dysfunction in the treatment of diabetes.
Results
March5 is abundantly expressed in islets and is positively correlated with insulin expression
To study the potential role of March5 in pancreatic islet β-cells, we assessed the expression levels of March5 and insulin under various physiological and pathological conditions. Both protein and mRNA levels of MARCH5 and insulin were markedly reduced in islets from aged mice (18-month-old) (Supplementary Fig. 1a–c), mice fed a high-fat diet (HFD) (Supplementary Fig. 1d–f), and leptin-deficient obese ob/ob mice (Supplementary Fig. 1g–i), compared to their respective controls. Moreover, a similar decline in MARCH5 and INSULIN expression was observed in human pancreatic islets from elderly individuals compared to young subjects (Fig. 1a–d). These findings suggest a potential positive association between March5 expression and islet β-cell function. We next analyzed the expression of MARCH5 and INSULIN in pancreatic islets of T2D patients using publicly available transcriptomic data from the NCBI GEO database (GSE25724)50. Both MARCH5 and INSULIN expression were significantly reduced in islets from T2D patients compared to non-diabetic controls (Fig. 1e).
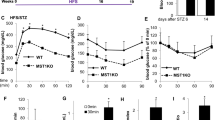
a–d Immunofluorescence (IF) staining, Western blotting, and qPCR analyses of March5 and insulin expression in islets from human individuals with different ages. Scale bar, 50 μm. N = 5 per group (a, b). N = 3 biologically independent samples (c, d). e Expression of MARCH5 and INSULIN genes in β-cell of non-diabetic (Non-DM) and type 2 diabetic (T2D) patients. f, g IF staining and quantitative data of pancreatic sections derived from 3-month-old March5 cKO or CON mice with an anti-March5 and anti-Insulin antibody. Scale bar, 50 μm. h Growth curve of male mice. i Glucose tolerance test (GTT). 10-week-old male mice were subjected to GTT assay. j Area under the curve (AUC) was calculated based on the data presented in (i). N = 7 for CON group and N = 8 for cKO group (f–j). k In vivo glucose-stimulated insulin secretion assay (GSIS). 12-week-old male mice were subjected to GSIS. N = 7 per group. l Growth curve of male mice fed a high-fat diet (HFD). N = 7 per group. m GTT. 18-week-old male mice (treated with HFD for 12 weeks) were subjected to GTT. n Area under the curve (AUC) was calculated based on the data presented in (m). N = 8 for CON group and N = 9 for cKO group (m, n). o GSIS. 20-week-old male mice (fed a HFD for 14 weeks) were subjected to GSIS. N = 7 per group. p Growth curve of male mice. q GTT. 10-week-old male mice were subjected to GTT. r AUC was calculated based on the data presented in (q). s GSIS. 14-week-old male mice were subjected to GSIS. N = 5 for each group (p–s). Data are expressed as mean ± standard deviation (s.d). Data were analyzed by two-tailed unpaired Student’s t-tests for comparisons between two groups and one-way ANOVA for multiple groups, followed by Tukey’s post hoc test. Source data are provided as a Source Data file.
March5 deficiency in β-cells induces diabetic phenotypes
The observed downregulation of March5 and insulin expression prompted us to explore the potential role of March5 in regulating the islet β-cells function. We generated β-cell-specific March5 knockout mice by crossing floxed March5 (March5 fl/fl) mice with the Ins2-Cre mice, resulting in β-cell-specific March5 knockout mice, referred to as March5 fl/fl; Ins2-Cre mice (cKO). The March5 fl/fl mice served as controls (referred to as CON). The mating strategy is illustrated in Supplementary Fig. 2a. The cKO mice were born in accordance with Mendelian inheritance patterns. Immunofluorescent (IF) staining of pancreatic tissues with antibodies against March5 and insulin revealed a significant reduction in both March5 and insulin expression within islet β-cells of cKO mice compared to CON mice (Fig. 1f, g). Additionally, Western blotting (WB) and quantitative real-time RT-PCR (qPCR) analyses demonstrated a substantial decrease in March5 expression at both protein and mRNA levels in isolated islets of cKO mice relative to CON mice, while March5 expressions in other organs, including the heart, liver, spleen, kidney, and brain, remained unaltered (Supplementary Fig. 2b, c). Subsequent IF staining demonstrated that March5 is also expressed in α- and δ-cells. However, its expression in these cell types remained largely unchanged in cKO mice. Comparable co-localization of March5 with glucagon or somatostatin was observed in pancreatic islets from both control and cKO mice (Supplementary Fig. 2d, e), confirming that March5 deletion was specific and efficient in islet β-cells of the cKO mice.
Long-term body weight monitoring from birth revealed growth retardation in both male (Fig. 1h) and female (Supplementary Fig. 3a) cKO mice starting at 2–3 weeks of age. To further investigate the impact of March5 deficiency on glucose homeostasis, we conducted a glucose tolerance test (GTT). Following an overnight fast, mice received intraperitoneal (i.p.) administration of glucose (2 g/kg body weight), with blood samples collected at 0, 15, 30, 60, 90, and 120 min for blood glucose measurement. Results indicated that blood glucose levels were significantly elevated in 10-week-old male cKO mice compared to sex- and age-matched CON mice at 30, 60, and 90 min (Fig. 1i, j). The blood glucose monitoring and insulin tolerance tests (ITT) revealed no significant differences in random blood glucose (RBG), fasting blood glucose (FBG), and insulin sensitivity between cKO mice and CON mice (Supplementary Fig. 3b–d). A similar pattern of glucose intolerance was observed in 3-month-old female cKO mice (Supplementary Fig. 3e–i). Subsequently, male mice were exclusively utilized to minimize the overall number of animals used.
Next, we performed glucose-stimulated insulin secretion (GSIS) assays to investigate whether March5 deficiency affects insulin secretion. Blood insulin levels were measured at 0, 3, 6, 10, and 30 min after intraperitoneal (i.p.) injection of glucose (2 g/kg body weight). The results showed that insulin secretion was markedly impaired at 0 and 3 min in cKO mice compared to CON mice (Fig. 1k). Although GTT assays showed an unchanged level of fasting blood glucose between cKO and CON mice, the level of fasting blood insulin in cKO mice was significantly reduced due to March5 deficiency (Fig. 1k and Supplementary Fig. 3j).
High-fat diet (HFD) treatment exacerbates the diabetic phenotypes caused by March5 deficiency in β-cells
To investigate the role of March5 in diabetes progression, 6-week-old male cKO and CON mice were fed a high-fat diet (HFD) to induce a T2D model. Long-term monitoring of body weight revealed that cKO mice developed more pronounced obesity compared to CON mice (Fig. 1l). After 12 weeks of HFD feeding, both groups exhibited RBG levels exceeding 11.1 mM, confirming the successful induction of T2D. Of note, both RBG and FBG levels were significantly elevated in cKO mice relative to CON mice (Supplementary Fig. 3k). GTT revealed markedly higher blood glucose levels at all time points in cKO mice compared to sex- and age-matched CON mice (Fig. 1m, n), accompanied by more severe defects in insulin secretion (Fig. 1o). These impairments were more pronounced than those observed under the normal chow diet (NCD) conditions (Fig. 1i, j). ITT further showed a modest but significant reduction in peripheral insulin sensitivity in cKO mice (Supplementary Fig. 3l, m). Together, these data indicate that March5 deficiency in islet β-cells accelerates the onset and progression of T2D.
March5 overexpression in β-cells increases insulin expression and enhances glucose clearance
We further generated β-cell-specific March5 overexpression mouse model by crossing March5OE mice with Ins2-Cre mice, yielding March5OE; Ins2-Cre mice (hereafter referred to as M5OE). Littermate March5OE mice lacking the Cre transgene were used as control mice (hereafter referred to as CON). IF staining and Western blot analysis confirmed markedly increased March5 expression in islet β-cells of M5OE mice compared with CON mice (Supplementary Fig. 3n–p). Long-term monitoring of body weight showed no significant differences between M5OE and CON mice (Fig. 1p). While RBG and FBG levels were comparable between 10-week-old male M5OE and CON mice (Supplementary Fig. 3q), GTT assays showed significantly enhanced glucose clearance in M5OE mice at 30 and 60 min post-glucose administration (Fig. 1q, r). Notably, GSIS assays revealed a substantial elevation in insulin release in M5OE mice compared with CON mice (Fig. 1s). ITT results showed no significant differences in peripheral insulin sensitivity between M5OE and CON mice (Supplementary Fig. 3r, s). A similar improvement in glucose tolerance was also observed in 4-month-old female M5OE mice (Supplementary Fig. 3t–x). To reduce animal usage, subsequent experiments were conducted using male mice only. Together, these findings indicate that March5 overexpression in β-cells promotes insulin secretion and improves systemic glucose clearance.
March5 Ablation in β-cells reduces insulin expression and secretion without affecting β-cell mass
The in vivo GSIS assays suggested impaired insulin secretion caused by β-cell March5 deficiency. To further confirm the requirement for March5 for insulin release, primary islets isolated from cKO and CON mice were treated with either 2.8- or 16.7-mM glucose in vitro. Insulin protein levels in the culture supernatants were quantified and normalized to the DNA content of the islets. In CON islets, high-glucose stimulation led to an ~6-fold increase in insulin secretion, whereas the response in cKO islets was significantly blunted, with only an ~4-fold increase (Fig. 2a). Furthermore, perifusion assays revealed that both the first- and second phase of insulin secretion were dramatically attenuated in islets from cKO mice compared to controls (Fig. 2b, c). These results suggest that impaired in vivo insulin secretion in cKO mice is attributed to insulin release. Since Ca2+ influx in β-cells is a critical step for the exocytosis of insulin secretory vesicles, we next assessed glucose- and KCl-induced Ca2+ release. Results showed that March5 deficiency impaired both glucose- and KCl-induced Ca2+ release from cKO islets. Under stimulation with 16.7 mM glucose, Ca2+ release was significantly reduced in cKO islets compared to CON islets (Fig. 2d). Similarly, the amplitude of Ca2+ transients induced by 25 mM KCl was also significantly decreased in cKO islets compared to CON islets (Fig. 2d). Next, we used transmission electron microscopy (TEM) to observe and quantify insulin granules in single β-cells from CON and cKO mice. High-resolution transmission electron microscopy (TEM) analysis revealed a diminished number of insulin secretory granules, including docked granules, in islets from cKO mice compared to controls (Fig. 2e, f). These findings are consistent with the reduced insulin levels observed in cKO islets (Fig. 1f), indicating that the impaired in vivo insulin secretion also attributed to impaired insulin production in cKO mice. Taken together, these data demonstrate that March5 is essential for insulin expression and granule exocytosis in β-cells.
a In vitro GSIS. Islets isolated from 10-week-old male mice were treated with 2.8- or 16.7-mM glucose. N = 3 biologically independent samples. b, c Biphasic GSIS determined by the perifusion assays (b) using isolated islets from 10-week-old CON and cKO mice, along with corresponding area under the curve (AUC) analyses (c) for first phase (6–12 min) and second phase (14–36 min) of insulin secretion. N = 3 biologically independent samples. d Ca2+ influx. Representative results from CON and cKO mice islets are provided. N = 15 islets per group. The quantification of 16.7 mM glucose and 25 mM KCl stimulation in islets. N = 3 biologically independent samples. e Representative electron micrographs of islets showing β-cell insulin granules. Dotted lines indicate the plasma membrane. Docked granules are marked by arrowheads. Upper scale bar 1 μm. Bottom scale bar, 0.2 μm. f Insulin granules density and Number of docked insulin granules in the plasma membrane. N = 5 mice per group. g QPCR analyses of total RNA isolated from islets of 2-month-old male cKO and CON mice. h Western blot analyses using isolated islets from 2-month-old male cKO and CON mice with indicated antibodies. N = 3 biologically independent samples (g, h). i, j IHC staining and quantitative data for MafA. Scale bar, 50 μm. N = 5 per group. k, l Western blot analyses of INS1 cells. Quantitative data from three independent experiments (l). m In vitro GSIS. Islets isolated from 12-week-old male mice were treated with 2.8- or 16.7-mM glucose. n QPCR analyses of total RNA isolated from islets of 12-week-old male mice. N = 3 biologically independent samples (m, n). o, p Western blot analyses using isolated islets from 12-week-old male CON and M5OE mice with indicated antibodies. Quantitative data from three independent experiments (p). q, r Western blot analyses of INS1 cells. Quantitative data from three independent experiments (q). All mRNA levels were normalized to Gapdh mRNA level. Data are expressed as mean ± s.d. Data were analyzed by two-tailed unpaired Student’s t-tests for comparisons between two groups and one-way ANOVA for multiple groups, followed by Tukey’s post hoc test. Source data are provided as a Source Data file.
In addition to the decrease in insulin expression by islet β-cells, the reduction in total insulin levels could also be attributed to increased apoptosis or decreased proliferation of β-cells51,52,53,54. We next investigated whether March5 regulates β-cell area. Firstly, we performed IHC staining using an insulin-specific antibody and measured the β-cell area-to-pancreas ratio and β-cell mass. Results showed no significant differences in β-cell area or β-cell mass between cKO and CON mice (Supplementary Fig. 4a–c). Additionally, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining revealed no significant differences in β-cell apoptosis between cKO and CON mice (Supplementary Fig. 4d–f). Consistently, insulin expression was significantly reduced in islets from cKO mice (Fig. 1f). These results suggest that the loss of March5 directly reduces insulin expression without affecting β-cell mass.
Next, we examined the expression of genes involved in insulin production, insulin, β-cell identity, including Ins1, Ins2, Exoc6, Styl3, Glut2, MafA, Pdx1, and Nkx6.145,46,47,48. QPCR analysis revealed significant downregulation of Ins1, Ins2, Exoc6, and Styl3 in islets from cKO mice, whereas the expression of MafA, Pdx1, and Nkx6.1 remained unchanged compared to CON islets (Fig. 2g). Given that MafA is a key transcriptional regulator of insulin gene expression49, we next determined whether March5 modulates MafA protein levels. IHC staining and WB analyses demonstrated a dramatic reduction in MafA protein levels in the islets of cKO mice (Fig. 2h–j). Similarly, siRNA-mediated knockdown of March5 in INS1 cells led to a significant decrease in both MafA and insulin protein levels (Fig. 2k, l). In contrast, March5 overexpression resulted in enhanced in vivo GSIS and opposite gene expression pattern compared to cKO mice (Fig. 2m, n). Consistently, MafA and insulin protein levels were significantly increased in the islets of M5OE mice and in March5-overexpressing INS1 cells, compared to their respective controls (Fig. 2o–r and Supplementary Fig. 4g–j). Similarly, β-cell mass was comparable between M5OE and CON mice (Supplementary Fig. 4k–m). Collectively, these findings suggest that March5 enhances insulin expression primarily through upregulation of MafA protein levels, without altering its transcription, in islet β-cells.
March5 deficiency in β-cells has no effect on mitochondrial morphology and activity
Considering the close association of March5 with mitochondrial dynamics, we performed a detailed analysis of mitochondrial morphology using TEM. No significant differences were observed in mitochondrial length or area between β-cells from CON and cKO mice (Supplementary Fig. 5a–c). These findings were further supported by IF staining with Mitotracker in March5-knockdown INS1 cells (Supplementary Fig. 5d–f). Moreover, measurements of cellular energy status revealed no significant differences in either relative ATP levels or ATP/ADP ratios between March5-deficient and control INS1 cells (Supplementary Fig. 5g, h), indicating that March5 deficiency does not impair mitochondrial function in β-cells.
March5 modulation of β-cell function is mediated by Kindlin-2
To investigate how March5 modulates MafA protein levels and insulin dynamics, we conducted mRNA sequencing on islets from 10-week-old CON and cKO mice. KEGG pathway analysis revealed enrichment in pathways related to insulin secretion, ECM-receptor interaction, focal adhesion (FA), and cell adhesion (Fig. 3a), all linked to FA proteins. Notably, FA proteins, such as Kindlin-2 and Talin-1, are key regulators of various cellular processes. We previously reported that β-cell-specific deletion of Talin-1 results in altered β-cell mass without affecting insulin secretion55. Kindlin-2 has been shown to stabilize MafA and promote insulin expression, and its deficiency markedly impairs insulin secretion, probably by disrupting cytoskeletal architecture and extracellular matrix (ECM)–receptor interactions essential for insulin granule trafficking and release45,56,57,58. Given that March5 knockout in β-cells led to reduced MafA and insulin protein levels as well as impaired insulin secretion, we hypothesized that these effects might be mediated through Kindlin-2. To explore this possibility, we first assessed Kindlin-2 expression in pancreatic sections and isolated islets using IHC staining and western blotting. We observed a substantial reduction in Kindlin-2 protein in islets of cKO mice compared with CON mice, whereas March5-overexpressing (M5OE) mice displayed significantly elevated Kindlin-2 levels (Fig. 3b–g). Consistently, siRNA-mediated knockdown of March5 in INS1 cells markedly decreased Kindlin-2 protein levels (Fig. 3h, i), while March5 overexpression resulted in a marked increase (Fig. 3j, k). Notably, qPCR analyses revealed that Kindlin-2 mRNA levels remained unchanged following March5 knockdown or expression (Fig. 3l–n), suggesting post-transcriptional regulation. Importantly, the decrease in MafA protein caused by March5 knockdown was effectively reversed by Kindlin-2 overexpression, and conversely, the enhanced MafA expression resulting from March5 overexpression was abrogated by Kindlin-2 knockdown (Fig. 3o–r), further supporting a mechanistic link between March5 and MafA via Kindlin-2.
a Enrichment of cell transport and cell adhesion-associated KEGG signaling pathways in islets from cKO mice compared to CON mice. b–e IHC staining for Kindlin-2. Scale bar, 50 μm. Quantitative data (c, e). N = 5 mice per group. f, g Western blot analyses of isolated islets from 12-week-old mice. h, i INS1 cells were co-transfected with plasmids expressing siMarch5 and then subjected to western blot analyses with indicated antibodies. j, k Western blot analyses. INS1 cells were co-transfected with plasmids expressing March5 and then subjected to western blot analyses with indicated antibodies. N = 3 biologically independent samples (f–k). l, m QPCR analyses of total RNA isolated from the primary islets of 10-week-old male mice. N = 3 mice per group. n QPCR analyses of total RNAs isolated from INS cells, after co-transfected with the plasmids of siMarch5. N = 3 biologically independent samples. All mRNA levels were normalized to Gapdh mRNA. o–r INS1 cells were transiently co-transfected with indicated plasmids for 48 h. Cell lysates were subjected to western blot analyses with indicated antibodies. Quantitative data from three independent experiments (p, r). s, t Cycloheximide (CHX) experiments. INS1 cells with and without March5 knockdown were treated with 100 μg/mL CHX for the indicated times, followed by western blot analyses to assess Kindlin-2 expression. Quantitative data from three independent experiments (t). u, v Western blot analyses of MG132 or Chloroquine (CQ) treated INS1 cells. Quantitative data from three independent experiments (v). w March5 knockdown increases endogenous Kindlin-2 ubiquitination in INS1 cells. N = 3 biologically independent samples. were transiently transfected with control siRNAs or siMarch5. At 48 h post-transfection, cells were treated with MG132 (10 μM) for 6 h, followed by immunoprecipitation (IP) and immunoblotting (IB) with the indicated antibodies. Data are expressed as mean ± s.d. Data were analyzed by two-tailed unpaired Student’s t-tests for comparisons between two groups and one-way ANOVA for multiple groups, followed by Tukey’s post hoc test. Source data are provided as a Source Data file.
Taken together, these findings strongly suggest that March5 regulates MafA and insulin protein levels, as well as insulin secretion, through a Kindlin-2–dependent mechanism.
March5 regulates Kindlin-2 protein stability
We next investigated the potential mechanisms underlying the regulation of Kindlin-2 protein levels by March5. Cycloheximide (CHX) experiments in INS1 cells revealed a significant acceleration in Kindlin-2 protein degradation following March5 knockdown (Fig. 3s, t). To identify the degradation pathway involved, we treated INS1 cells with MG132 or Chloroquine (CQ), inhibitors of the proteasome and lysosome pathways, respectively. WB analyses demonstrated a notable increase in Kindlin-2 protein levels upon MG132 treatment, while CQ treatment had no discernible effect on Kindlin-2 protein levels (Fig. 3u, v), suggesting that Kindlin-2 degradation primarily occurs via the proteasome pathway. Moreover, March5 knockdown in INS1 cells markedly increased the ubiquitination of endogenous Kindlin-2 (Fig. 3w). Taken together, these results suggest that March5 deficiency accelerates Kindlin-2 protein degradation through enhancing its ubiquitination and subsequent proteasomal degradation.
Simultaneous haploinsufficiency of the March5 and Kindlin-2 genes in β-cells causes diabetic phenotypes in mice
We further investigated the role of Kindlin-2 in mediating the impact of March5 deficiency on diabetic phenotypes in vivo by generating mice with specific deletion of one allele of March5 and/or Kindlin-2 genes in β-cells using Ins2-Cre mice. This resulted in the generation of singly heterozygous mice for March5 or Kindlin-2 (M5 fl/+; Ins2-Cre or K2 fl/+; Ins2-Cre) and double heterozygous mice (i.e., M5 fl/+; K2 fl/+; Ins2-Cre), with Ins2-Cre mice as controls. No significant difference in growth was observed among these four genotypes (Supplementary Fig. 6a). GTT assays conducted on 2-month-old male mice of these four genotypes indicated that M5 fl/+; Ins2-Cre or K2 fl/+; Ins2-Cre mice displayed no discernible variations in blood glucose clearance compared to control Ins2-Cre mice. However, M5 fl/+; K2 fl/+; Ins2-Cre mice exhibited notably compromised glucose clearance compared to the other three groups (Supplementary Fig. 6b, c). Furthermore, ITT assays showed no significant difference in peripheral insulin sensitivity among the four groups (Supplementary Fig. 6d, e). GSIS assays demonstrated impaired insulin secretion in March5 and Kindlin-2 double heterozygous mice compared to singly heterozygous or control mice (Supplementary Fig. 6f). Additionally, no significant difference in β-cell mass was observed among mice among the four groups (Supplementary Fig. 6g). IF staining revealed a marked decrease in March5 signal in the islets of M5 fl/+; Ins2-Cre and M5 fl/+; K2 fl/+; Ins2-Cre mice, but no change in K2 fl/+; Ins2-Cre mice compared to control Ins2-Cre mice (Supplementary Fig. 6h, i). IHC staining showed that Kindlin-2 expression was markedly reduced in the islets of both M5 fl/+; Ins2-Cre and K2 fl/+; Ins2-Cre mice, with further reduction observed in the islets of M5 fl/+; K2 fl/+; Ins2-Cre mice, relative to control Ins2-Cre mice (Supplementary Fig. 6h, j). MafA (Supplementary Fig. 6h, k) and insulin (Supplementary Fig. 6h, l) expression remained unchanged in the islets of M5 fl/+; Ins2-Cre and K2 fl/+; Ins2-Cre mice, but were significantly decreased in the islets of M5 fl/+; K2 fl/+; Ins2-Cre mice, compared to control Ins2-Cre mice. These findings are consistent with a previous report showing that Kindlin-2 heterozygosity in β-cells does not affect glucose clearance45. Collectively, these data suggest that March5 functions upstream of Kindlin-2 and MafA, with March5 regulating β-cell function by modulating Kindlin-2 expression and MafA activity within the same genetic pathway.
March5 regulates Kindlin-2 protein stability by modulating Trim28 expression
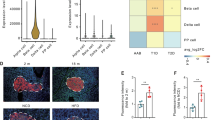
Based on the results of our previous experiments and the known function of March5 as an E3 ubiquitin ligase, we hypothesized the involvement of another E3 ubiquitin ligase in the regulation of Kindlin-2 by March5. To identify potential E3 ubiquitin ligases involved in Kindlin-2 protein degradation, we conducted mass spectrometry and identified Trim28 as the only E3 ubiquitin ligase found to interact with Kindlin-259. Subsequent IF staining demonstrated the co-localization of Trim28 and Kindlin-2 in INS1 cells (Fig. 4a). Additionally, co-immunoprecipitation (co-IP) assays validated the protein interaction between Trim28 and Kindlin-2 (Fig. 4b, c). These findings indicate that Trim28 may function as the E3 ubiquitin ligase responsible for the ubiquitination of Kindlin-2. Next, we investigated the relationship between March5, Trim28, and Kindlin-2. IHC staining revealed a significant increase in Trim28 expression in the islets of cKO mice, whereas Trim28 levels were significantly decreased in the islets of M5OE mice (Fig. 4d–g). Notably, Trim28 expression was significantly upregulated upon March5 knockdown, and silencing Trim28 effectively reversed the decrease in Kindlin-2 levels caused by March5 knockdown in HEK293T cells (Fig. 4h, i). Conversely, Trim28 expression was markedly reduced by March5 overexpression, and Trim28 overexpression sufficiently counteracted the increase in Kindlin-2 levels induced by March5 overexpression in HEK293T cells (Fig. 4j, k). Furthermore, IF staining demonstrated the co-localization of Marhc5 and Trim28 in INS1 cells (Fig. 4l), while co-IP assays validated the protein interaction between March5 and Trim28 in HEK293T cells (Fig. 4m). Collectively, these results indicate that March5 promotes Kindlin-2 protein levels by inhibiting the expression of Trim28.
a IF staining. INS1 cells were subjected to IF staining with an anti-March5 antibody (green) and an anti-Trim28 antibody (red). Scale bar, 10 μm. N = 3 biologically independent samples. b, c Co-IP assays. INS1 cells were transiently transfected with plasmids expressing Kindlin-2 or Trim28, and the cell lysates were collected for IP and IB with the indicated antibodies. N = 3 biologically independent samples. d–g IHC staining for Trim28. Pancreatic sections from CON and cKO, CON and M5OE mice were subjected to IHC staining for Trim28. Scale bar, 50 μm. Quantitative data from 5 mice per group (f, g). h–k Western blot analyses. HEK293T cells were transiently transfected with the indicated plasmids for 48 h. Cell lysates were then subjected to western blot analyses with the indicated antibodies. Quantitative data from three independent experiments (i, k). l IF staining. INS1 cells were subjected to IF staining with an anti-March5 antibody (red), an anti-Trim28 antibody (green), and DAPI (blue). Scale bar, 10 μm. N = 3 biologically independent samples. m Co-IP assays. INS1 cells were transiently transfected with plasmid expressing Trim28 for 48 h, the cell lysates were then collected for IP and IB with the indicated antibodies. N = 3 biologically independent samples. Data are expressed as mean ± s.d. Data were analyzed by two-tailed unpaired Student’s t-tests for comparisons between two groups and one-way ANOVA for multiple groups, followed by Tukey’s post hoc test. Source data are provided as a Source Data file.
March5 promotes Trim28 protein degradation via ubiquitination
Next, we determined the mechanisms underlying March5 regulation of Trim28. Knockdown of March5 expression in vivo and in vitro led to a significant increase in Trim28 protein levels, as revealed by WB analyses (Fig. 5a–c), while the mRNA levels of Trim28 remained unaltered, as demonstrated by qPCR analyses (Fig. 5d, e). Conversely, a marked reduction in Trim28 protein levels, without changes in mRNA expression, was observed in isolated islets from M5OE mice compared to CON mice (Supplementary Fig. 7a, b). CHX experiments revealed that March5 knockdown significantly decelerated Trim28 protein degradation (Fig. 5f, g). WB analyses demonstrated a notable increase in Trim28 protein accumulation following MG132 treatment (Fig. 5h, i), suggesting that Trim28 degradation is predominantly mediated through the proteasome pathway. Notably, March5 knockdown significantly decreased the ubiquitination level of Trim28 (Fig. 5j). Conversely, March5 overexpression effectively enhanced the ubiquitination level of Trim28 (Fig. 5k).
a Western blot analyses for March5, Trim28, and actin expression in isolated islets from 10-week-old male cKO and CON mice. b, c Western blot analyses. INS1 cells were co-transfected with siMarch5 plasmids for 48 h, the protein extracts were then subjected to western blot analyses with the indicated antibodies. N = 3 biologically independent samples (a–c). d QPCR analyses. Total RNA isolated from the primary islets of 2-month-old male cKO and CON mice was subjected to qPCR for Trim28 mRNA expression. N = 3 per group. Trim28 mRNA was normalized to Gapdh mRNA. e QPCR analyses. Total RNA isolated from INS1 cells, as treated in (b), was subjected to qPCR for Trim28 expression. All mRNA levels were normalized to Gapdh mRNA. N = 3 biologically independent samples. f, g CHX experiments. HEK293T cells with or without March5 knockdown were treated with 100 μg/mL of CHX for the indicated times, followed by western blot analyses for expression of Trim28. Quantitative data from three independent experiments (g). h, i Western blot analyses. Effect of MG132 on Trim28 protein expression in INS1 cells. j March5 knockdown decreases endogenous Trim28 ubiquitination. k March5 overexpression increases endogenous Trim28 ubiquitination. l Loss of function of March5 (H43W) decreases Trim28 ubiquitination. N = 3 biologically independent samples (h–l). m, n CHX experiments. HEK293T cells with or without Trim28 knockdown were treated with 100 μg/mL of CHX for the indicated times, followed by western blot analyses for Kindlin-2 expression. Quantitative data from three independent experiments (n). o Trim28 knockdown decreases endogenous Kindlin-2 ubiquitination. HEK293T cells were transiently transfected with control siRNAs or siTrim28. At 48 h post-transfection, cells were treated with MG132 (10 μM) for 6 h, then IP and IB with the indicated antibodies were performed. p Trim28 knockdown abrogates the increased endogenous Kindlin-2 ubiquitination by March5 deficiency. q Trim28 overexpression restores the decreased endogenous Kindlin-2 ubiquitination by March5 overexpression. N = 3 biologically independent samples (o–q). Data are expressed as mean ± s.d. Data were analyzed by two-tailed unpaired Student’s t-tests for comparisons between two groups and one-way ANOVA for multiple groups, followed by Tukey’s post hoc test. Source data are provided as a Source Data file.
To further investigate whether the E3 ligase activity of March5 is necessary for Trim28 protein ubiquitination, we mutated histidine 43 (H43W) in the RING domain of March5, which is essential for its ubiquitin-transfer function22,60. We then assessed the degradation status of Trim28 protein under different conditions in HEK293T cells. Notably, there were no significant differences in the expression and degradation levels of Trim28 protein between March5 with the H43W mutation and the Flag control, while the ubiquitination of Trim28 was markedly increased under wild-type March5 overexpression (Fig. 5l and Supplementary Fig. 7c, d). These findings suggest that March5 directly interacts with and ubiquitinates Trim28.
Trim28 promotes Kindlin-2 protein degradation via ubiquitination
Next, we determined the mechanisms underlying Trim28 regulation of Kindlin-2. Since Kindlin-2 mRNA expression was unaltered by March5 deficiency (Fig. 3l–n), and Trim28 is known as an E3 ubiquitin ligase, we hypothesized that Trim28 regulates Kindlin-2 protein stability via ubiquitination. Our IF staining and co-IP assays already demonstrated the co-localization and protein interaction between Trim28 and Kindlin-2 (Fig. 4a–c). CHX experiments revealed that Trim28 knockdown significantly attenuated Kindlin-2 protein degradation in HEK293T cells (Fig. 5m, n). Moreover, Trim28 knockdown significantly reduced the ubiquitination of Kindlin-2 protein (Fig. 5o). Notably, Trim28 knockdown effectively reversed the elevated ubiquitination level of Kindlin-2 caused by March5 knockdown in HEK293T cells (Fig. 5p). Conversely, Trim28 overexpression reversed the decreased ubiquitination level of Kindlin-2 caused by March5 overexpression in HEK293T cells (Fig. 5q).
Taken together, these findings demonstrate that March5 promotes the ubiquitination of Trim28, thereby inhibiting the Trim28-mediated degradation of Kindlin-2. This leads to the accumulation of Kindlin-2 and MafA, ultimately enhancing β-cell function.
Deletion of Trim28 in β-cells improves glucose tolerance in mice by enhancing β-cell function
Since the role of Trim28 in β-cells in regulating glucose homeostasis is unknown, we initially detected the expression of Trim28 in mouse or human islets under different conditions. We observed a notable increase in Trim28 signal in islets of aged mice (Supplementary Fig. 8a, b). Similarly, Trim28 levels were markedly enhanced in islets from HFD-fed mice and leptin-deficient obese ob/ob mice (Supplementary Fig. 8c–f). Moreover, IF staining revealed a significant increase in TRIM28 and a concomitant decline in INSULIN in human pancreatic islets from elderly individuals compared to younger subjects (Supplementary Fig. 8g, h).
Next, we generated β-cell-specific Trim28 deletion mouse model to study the in vivo role of β-cell Trim28 in regulating glucose homeostasis (Supplementary Fig. 9a, b). No significant differences were observed in the growth, RBG, and FBG of Trim28fl/fl and Trim28fl/fl; Ins2-Cre mice (Supplementary Fig. 9c, d). GTT assays conducted on 12-week-old male mice indicated that Trim28fl/fl; Ins2-Cre mice had better blood glucose clearance than control Trim28fl/fl mice at 30 and 60 min after glucose injection (Fig. 6a, b). GSIS assays demonstrated significantly higher insulin secretion in Trim28fl/fl; Ins2-Cre mice at 3 and 6 min following glucose administration compared to control mice (Fig. 6c). Consistently, in vitro GSIS analyses showed an ~7.6-fold increase in insulin secretion in islets from Trim28fl/fl; Ins2-Cre mice, compared to a 6-fold increase in control islets (Fig. 6d). ITT assays showed no significant differences in peripheral insulin sensitivity between the two groups (Supplementary Fig. 9e, f), and β-cell mass was also comparable (Supplementary Fig. 9g). Western blotting, qPCR, and IHC staining revealed markedly increased expression of Kindlin-2, MafA, and insulin in islets from Trim28fl/fl; Ins2-Cre mice relative to controls, while March5 expression remained unchanged (Fig. 6e–g and Supplementary Fig. 9h–l). Collectively, these results further indicate that March5 functions upstream of the Trim28/Kindlin-2/MafA axis.
a GTT. 12-week-old Trim28fl/fl and Trim28fl/fl; Ins2-Cre male mice were subjected to GTT. b Area under the curve (AUC) was calculated based on the data presented in (a). c GSIS. 16-week-old male mice were subjected to GSIS. N = 5 per group (a–c). d In vitro GSIS. Islets isolated from 14-week-old male mice were treated with 2.8- or 16.7-mM glucose. Insulin secretion levels in the supernatants were measured by ELISA and normalized to the total islet DNA contents. e, f Western blot and qPCR analyses for indicated proteins (e) and mRNA expressions (f) in isolated islets from 14-week-old male Trim28fl/fl (CON) and Trim28fl/fl; Ins2-Cre (Trim28KO) mice. N = 3 biologically independent samples (d–f). g IF and IHC staining for March5, Trim28, Kindlin-2, MafA, and insulin in pancreatic sections. Scale bar, 50 μm. N = 5 per group. h GTT. 10-week-old Ins2-Cre, M5 fl/fl; Ins2-Cre and M5 fl/fl; Trim28fl/fl; Ins2-Cre male mice were subjected to GTT. P value in black font, M5 fl/fl; Ins2-Cre versus Ins2-Cre. P value in blue font, M5 fl/fl; Trim28fl/fl; Ins2-Cre versus M5 fl/fl; Ins2-Cre. i Area under the curve (AUC) was calculated based on the data presented in (h). j GSIS. 12-week-old male mice were subjected to GSIS. N = 5 per group (h–j). k In vitro GSIS. Islets isolated from 12-week-old male mice were treated with 2.8- or 16.7-mM glucose. Insulin secretion levels in the supernatants were measured by ELISA and normalized to the total islet DNA contents. l, m Western blot and qPCR analyses for indicated proteins (l) and mRNA (m) expression in isolated islets from 12-week-old male Ins2-Cre (CON), M5 fl/fl; Ins2-Cre (M5KO) and M5 fl/fl; Trim28fl/fl; Ins2-Cre (DKO) mice. N = 3 biologically independent samples (k–m). n IF and IHC staining for March5, Trim28, Kindlin-2, MafA, and insulin in pancreatic sections. Scale bar, 50 μm. N = 5 for each group. Data are expressed as mean ± s.d. Data were analyzed by two-tailed unpaired Student’s t-tests for comparisons between two groups and one-way ANOVA for multiple groups, followed by Tukey’s post hoc test. Source data are provided as a Source Data file.
Collectively, these findings indicate the critical role of Trim28 in modulating islet β-cell function
Deletion of Trim28 in β-cells completely reverses the diabetic phenotype observed in mice with β-cell-specific March5 deficiency
We next investigated whether genetic deletion of Trim28 in β-cells could reverse the diabetic phenotypes caused by March5 deficiency by generating mice with simultaneous deletion of both March5 and Trim28 genes in β-cells using Ins2-Cre mice. Notably, Trim28 deletion completely reversed the growth retardation (Supplementary Fig. 9m), glucose intolerance, and decreased insulin secretion observed in March5 knockout mice (Fig. 6h–k). Consistently, Western blotting, qPCR, and IHC staining revealed that the decreased expression of Kindlin-2, MafA, and insulin resulting from March5 deletion was efficiently reversed by Trim28 deletion (Fig. 6l–n and Supplementary Fig. 9n–r). Similarly, no significant differences were observed in RBG, FBG, peripheral insulin sensitivity, and β-cell mass among the three groups (Supplementary Fig. 9s–v).
Collectively, these findings further demonstrate that March5 maintains β-cell function by targeting Trim28.
Adult mice with March5 deficiency in β-cells exhibit diabetic phenotypes
We further evaluated the impact of March5 deficiency on glucose homeostasis in adult mice using a mouse model with conditional March5 alleles (March5 fl/fl) and an Ins1-CreERT transgene, which allows for tamoxifen (TM)-inducible Cre recombinase expression specifically in β-cells. Two-month-old mice received TM injections (100 mg/kg body weight) for five consecutive days to induce deletion of March5 expression in β-cells (hereafter referred to as March5 fl/fl; Ins1-CreERT) (Supplementary Fig. 10a). At 30 days post-TM injections, March5 fl/fl; Ins1-CreERT mice exhibited significant impairment in glucose clearance and insulin secretion, as indicated by GTT and GSIS assays, respectively (Supplementary Fig. 10b–d). There were no significant differences in β-cell mass or peripheral insulin sensitivity between March5 fl/fl; Ins1-CreERT and control March5 fl/fl mice (Supplementary Fig. 10e–g). Consistent with findings from cKO mice (March5 fl/fl; Ins2-Cre mice), IF and IHC staining revealed a marked reduction in the expression of March5, Kindlin-2, MafA, and insulin in the islets of March5 fl/fl; Ins1-CreERT mice compared to control March5 fl/fl littermates (Supplementary Fig. 10h–l). In contrast, Trim28 expression was markedly elevated in the islets of March5 fl/fl; Ins1-CreERT mice relative to control littermates (Supplementary Fig. 10h, m).
Collectively, these findings indicate that March5 is crucial for maintaining glucose homeostasis in adult mice and could serve as a potential therapeutic target for diabetes.
March5 overexpression or Trim28 inhibition in β-cells enhances islet transplation efficiency in STZ-induced diabetic mice
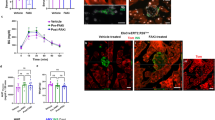
Based on our previous findings, we identified March5 as a key facilitator and Trim28 as a potent suppressor of insulin expression and secretion in islet β-cells. We then initiated investigations to determine whether enhancing March5 or inhibiting Trim28 expression could be viable strategies for diabetes treatment. Initially, syngeneic islet transplantation experiments were conducted to evaluate whether islets overexpressing March5 in β-cells could improve metabolic control. Streptozotocin (STZ) was administered to induce diabetes in C57BL/6 WT mice. One-week post-STZ treatment, fasting glucose concentrations in all mice exceeded 11.1 mM, confirming the establishment of the STZ-induced diabetic mouse model. Subsequently, primary islets were isolated from 3-month-old CON and M5OE mice. Our previous findings demonstrated that March5 knockout (Supplementary Fig. 4a–c) or overexpression (Supplementary Fig. 4k–m) did not affect β-cell mass. Six hundred primary islets from CON and M5OE mice were intramuscularly injected into the hindlimb muscle of STZ-induced diabetic recipient mice. IF staining of insulin and TUNEL assays indicated a significant increase in TUNEL-positive β-cell numbers in STZ-induced diabetic mice compared to control mice (Fig. 7a, b). Insulin expression was dramatically decreased in STZ-treated mice compared to control mice (Fig. 7a, c), further confirming the successful establishment of the STZ-induced diabetic mouse model. IF staining of insulin demonstrated the viability and functionality of the transplanted islets in the hindlimb muscle of recipient mice (Fig. 7d). Fasting (4 h) blood glucose measurements showed that diabetic recipient mice transplanted with islets from CON (STZ-CON) or M5OE (STZ-M5OE) mice achieved glycemic control within ~2 weeks (Fig. 7e). Remarkably, mice transplanted with islets from M5OE mice (STZ-M5OE) displayed lower blood glucose levels compared to mice in the STZ-CON group. We next investigated whether inhibition of Trim28 in β-cells enhances glucose tolerance in STZ-induced diabetic mice. Similarly, islets from both Trim28fl/fl and Trim28fl/fl; Ins2-Cre mice successfully decreased blood glucose levels within ~2 weeks post-transplantation (Fig. 7f–j). Notably, from day 24 post-transplantation, mice receiving islets from Trim28fl/fl; Ins2-Cre mice displayed significantly lower blood glucose levels compared to the Trim28fl/fl group (Fig. 7j).
a–c IF staining of insulin and TUNEL in pancreatic sections. Scale bar, 50 μm. Quantitative data (b, c). d IF staining of insulin in mice hindlimb muscle after transplantation with islets derived from CON or M5OE mice. Scale bar, 50 μm. e Fasting blood glucose. N = 5 mice per group (b–e). f–h IF staining of insulin and TUNEL in pancreatic sections. Scale bar, 50 μm. Quantitative data (g, h). i IF staining of insulin in mice hindlimb muscle after transplantation with islets derived from Trim28fl/fl or Trim28fl/fl; Ins2-Cre mice. Scale bar, 50 μm. j Fasting blood glucose. N = 5 mice per group (f–j). k, l WB analyses of MARCH5 or TRIM28 in isolated islets from human donors after infection with lentiviruses overexpressing human MARCH5 (M5OE) or inhibiting human TRIM28 (TRIM28KD), or negative control (NC). m, n In vitro GSIS. Islets isolated from human donors were treated as described in (k, l), followed by exposure to 2.8- or 16.7-mM glucose. Insulin secretion levels in the supernatants were measured by ELISA and normalized to the total DNA content. The relative insulin secretions were normalized to that of NC islets treated with 2.8 mM glucose. N = 3 biologically independent samples (k–n). o Schematic representation of the March5/Trim28/Kindlin-2/MafA pathway, highlighting its potential as a therapeutic target to enhance β-cell function. Cartoon representations of Kindlin-2, MafA, Trim28, and March5 were generated using Cellscape (MIT license, https://github.com/josyr/Cellscape). Data are expressed as mean ± s.d. Data were analyzed by two-tailed unpaired Student’s t-tests for comparisons between two groups and one-way ANOVA for multiple groups, followed by Tukey’s post hoc test. Source data are provided as a Source Data file.
Taken together, these findings suggest that islet transplantation with either March5-overexpressing or Trim28-deficient β-cells significantly enhances glucose clearance capacity of diabetic mice.
MARCH5 overexpression or TRIM28 inhibition enhances insulin expression and secretion in human islets
To further investigate whether MARCH5 overexpression or TRIM28 inhibition in human islets enhances insulin expression and secretion, we induced MARCH5 overexpression (M5OE) or TRIM28 inhibition (TRIM28KD) in freshly isolated human islets via lentiviral transduction (Fig. 7k, l), followed by GSIS assays. Upon exposure to 16.7 mM glucose, insulin secretion rose markedly, with an ~6-fold increase in NC islets (Fig. 7m), a 10-fold increase in M5OE islets (Fig. 7m), and an 8-fold increase in TRIM28KD islets (Fig. 7n).
Overall, these findings highlight the potential benefits of enhancing March5 or inhibiting Trim28 signaling in islet β-cells for improving insulin expression and secretion, which is of direct relevance to clinical islet transplantation where donor islets are limited in supply.
Ins2-Cre expression does not alter feeding behavior
Although Ins2-Cre is widely used for β-cell-specific gene manipulation, previous studies have noted ectopic expression in the hypothalamus61, a key center for regulating feeding and satiety. To address the potential impact of such off-target recombination, we performed whole-brain western blot and qPCR analyses, which revealed no significant differences in March5 protein or mRNA levels between β-cell-specific March5 knockout mice and littermate controls (Supplementary Fig. 2a, b), nor in Trim28 expression between β-cell-specific Trim28 knockout mice and controls (Supplementary Fig. 9a, b). Nonetheless, the use of whole-brain lysates may mask region-specific alterations, subtle changes within the hypothalamus cannot be definitively excluded. To further assess this possibility, we assessed feeding behavior. Daily food intake was comparable between β-cell-specific knockout mice and their corresponding controls (Supplementary Fig. 11), suggesting that any potential ectopic Ins2-Cre activity in the hypothalamus does not significantly influence the observed metabolic phenotypes.
Discussion
In the present study, we elucidate a mechanism regulating β-cell function and blood glucose homeostasis, involving the E3 ligases March5 and Trim28. Our findings demonstrate that March5 enhances β-cell insulin expression and secretion by facilitating the ubiquitination of Trim28, which in turn inhibits Trim28-mediated ubiquitination of Kindlin-2. Consequently, MafA protein levels are increased, leading to upregulated insulin expression and secretion. We provide compelling evidence supporting the pivotal roles of March5 and Trim28 in modulating β-cell insulin expression and secretion. Notably, our study reveals that enhancing March5 or inhibiting Trim28 expression in islets from both mice and human donors significantly improves β-cell function. These findings hold substantial importance, as impaired β-cell insulin expression and secretion are fundamental pathological features of diabetes.
Our investigation represents the inaugural demonstration of the E3 ligase March5 as a positive regulator of insulin expression and secretion. This conclusion is supported by multiple lines of evidence: (1) reduced expression of March5 in islet β-cells of individuals with impaired glucose homeostasis in both mice and in humans; (2) attenuated insulin expression and secretion in β-cells due to March5 deficiency; (3) pronounced glucose intolerance caused by inducible deletion of March5 in islet β-cells of adult mice; and (4) significant enhancement of insulin expression and secretion upon upregulation of March5 expression in human or mouse islet β-cells. These findings unveil for the first time that March5 is a prospective therapeutic target for improving β-cell function. Although several studies have highlighted the critical role of March5 in maintaining mitochondrial integrity and function18,19,20,21, our findings suggest that its role in maintaining β-cell function, as demonstrated in the present study, operates independently of its effects on mitochondrial dynamics. This conclusion is supported by our TEM and ATP/ADP analyses, which revealed no significant differences in mitochondrial morphology or activity between β-cells from CON and cKO mice.
In this study, we reveal that March5 enhances β-cell insulin expression and secretion through promoting Kindlin-2/MafA signaling. Firstly, March5 deficiency decreases, while March5 overexpression increases, both Kindlin-2 and MafA protein levels without affecting their mRNA levels in β-cells, both in vivo and in vitro. Secondly, the reduced expression of MafA protein caused by March5 deficiency can be reversed by Kindlin-2 overexpression, and vice versa. Thirdly, March5 deficiency accelerates the ubiquitin-mediated degradation of Kindlin-2 protein. Finally, the simultaneous haploinsufficiency of both March5 and Kindlin-2 genes, rather than either gene alone, in β-cells of mice significantly diminishes insulin expression and secretion, consequently leading to glucose intolerance. Thus, our study provides the first in vivo evidence highlighting the collaborative action of both March5 and Kindlin-2 within the same genetic pathway in β-cells, which is crucial for the regulation of β-cell function and glucose homeostasis in mice.
March5 is an E3 ubiquitin ligase responsible for the ubiquitination and subsequent degradation of target proteins. The positive correlation between Kindlin-2 protein levels and March5 expression suggests the involvement of an additional E3 ubiquitin ligase that mediates the regulation of Kindlin-2 protein by March5. Our study provides compelling evidence that Trim28 is the E3 ubiquitin ligase responsible for this regulation. Firstly, mass spectrometry identifies Trim28 as the sole E3 ligase interacting with Kindlin-2. Secondly, co-IP assays and IF staining confirm the interaction and co-localization of Trim28 with Kindlin-2, and Trim28 with March5. Thirdly, March5 ablation elevates Trim28 protein levels by reducing its ubiquitination, while March5 overexpression reduces Trim28 protein levels by increasing its ubiquitination, without affecting Trim28 mRNA expression. Fourthly, Trim28 knockdown increases Kindlin-2 protein levels, and the reduction in Kindlin-2 caused by March5 knockdown can be rescued by Trim28 silencing, and vice versa. Fifthly, Trim28 depletion stabilizes Kindlin-2 protein by reducing its ubiquitination, and the increased ubiquitination of Kindlin-2 due to March5 knockdown can be effectively abolished by Trim28 knockdown, and vice versa. Finally, and most importantly, specific deletion of Trim28 in β-cells greatly improves glucose tolerance in mice by enhancing insulin expression and secretion, and completely reverses the diabetic phenotype observed in March5 cKO mice.
Initially identified as a nuclear protein, Trim28 was primarily recognized as a transcriptional regulator that modulates diverse biological processes through its interactions with heterochromatin protein 1 (HP1) and involvement in histone deacetylation pathways62. Recent studies highlight the role of Trim28 as an E3 ubiquitin ligase, promoting the proteasome-dependent degradation of a variety of nuclear and cytoplasmic proteins, including p27, AMPK, BCL2A1, etc.37,38,39. Consistent with this, our study demonstrates that Trim28 functions as an E3 ubiquitin ligase in β-cells, regulating the protein stability of Kindlin-2 without affecting its mRNA expression (Fig. 5m–o and Fig. 6e–g), indicating a post-translational mechanism rather than transcriptional control.
Based on results of our study, we propose a working model elucidating the mechanisms by which March5 and Trim28 regulate β-cell function (Fig. 7o). Specifically, March5 interacts with and ubiquitinates Trim28, thereby accelerating its degradation. In turn, Trim28 interacts with and ubiquitinates Kindlin-2, leading to the degradation of Kindlin-2. Thus, the ubiquitination of Trim28 by March5 leads to the accumulation of Kindlin-2 protein. This increase in Kindlin-2 protein levels stabilizes MafA, a master transcription factor governing insulin expression, ultimately enhancing insulin expression and secretion.
Islet transplantation represents an established therapeutic approach for patients with diabetes characterized by impaired β-cell function and absolute insulin deficiency. However, the functionality of transplanted islet grafts diminishes over time, necessitating strategies to enhance β-cell function and improve long-term outcomes. Our study demonstrates that augmenting March5 or inhibiting Trim28 expression significantly enhances glucose homeostasis and improves outcomes post-islet transplantation. Specifically, islets with March5 overexpression or Trim28 knockdown in β-cells greatly improve glucose tolerance in STZ-induced diabetic mice compared to control islets. Furthermore, MARCH5 overexpression or TRIM28 inhibition in islets from human donors substantially enhances β-cell function, as evidenced by greatly increased insulin expression and secretion. These findings indicate that manipulating MARCH5 or TRIM28 expression in β-cells may offer therapeutic opportunities for diabetes through islet transplantation by addressing insulin deficiency.
In this study, we primarily employed mouse models utilizing the Ins2-Cre transgene, also known as RIP-Cre, which is widely used to achieve β-cell-specific gene deletion or overexpression45. Although Ins2-Cre is considered β-cell–selective, previous studies have reported ectopic expression in the hypothalamus61, a key center for regulating feeding and satiety. To address the potential confounding effects of hypothalamic Ins2-Cre activity on metabolic phenotypes, we conducted whole-brain WB and qPCR analyses. These assays reveal no significant differences in March5 protein or mRNA expression between β-cell-specific March5 knockout mice and controls (Supplementary Fig. 2a, b), nor in Trim28 expression between β-cell-specific Trim28 knockout mice and their respective controls (Supplementary Fig. 9a, b). However, given that whole-brain lysates may dilute region-specific alterations, subtle changes in the hypothalamus cannot be entirely ruled out. To further exclude hypothalamic contributions, we assessed feeding behavior. Food intake measurements demonstrate no significant differences between β-cell-specific knockout mice and controls (Supplementary Fig. 11). Collectively, these data suggest that while minimal Ins2-Cre activity in the hypothalamus may occur, it is unlikely to confound the β-cell-specific phenotypes observed in this study.
We acknowledge that there are certain limitations to our study. One important limitation is that the therapeutic efficacy of the engineered islets was evaluated exclusively in the STZ-induced diabetic male mouse model. To more comprehensively assess the translational potential of the pathway identified in this study, further investigations employing additional models, such as the STZ-induced diabetic female mouse model and the HFD-induced diabetic mouse model, will be necessary. It is also worth noting that the diabetic phenotype observed in March5 cKO mice appears to be less severe than that reported for Kindlin-2 knockout mice45. In the study by Zhu et al., Kindlin-2 deletion led to a marked reduction in insulin expression and secretion, which is consistent with our findings. However, their study also reported a significant loss of β-cell mass following Kindlin-2 deficiency45. In contrast, our results indicate that neither the deletion nor the overexpression of March5 in β-cells alters β-cell mass. This discrepancy may be due to the residual presence of Kindlin-2 protein following March5 deletion. Alternatively, given that March5 functions as an E3 ubiquitin ligase, it is possible that it also regulates other substrates involved in β-cell mass control. This possibility warrants further investigations in future study.
In conclusion, our study identifies the March5/Trim28/Kindlin-2/MafA axis as critical for preserving islet β-cell function. Targeted overexpression of March5 or inhibition of Trim28 in islet β-cells could enhance β-cell function in male mice and human islets, highlighting it may serve as a promising therapeutic target for diabetes.
Methods
Animal study
Mice with conditional knockout were generated using CRISPR/Cas9 for gene modification. For March5 fl/fl mice, loxP sites were inserted flanking exon 2 (Shanghai Model Organisms Center). For Trim28fl/fl mice, loxP sites were inserted flanking exons 4–17 (Cyagen Biosciences), enabling Cre recombinase-mediated deletion of this essential genomic segment. This strategy allows tissue-specific gene ablation upon Cre recombinase expression. We established β-cell-specific March5 knockout mice by crossing March5 fl/fl mice with Ins2-Cre transgenic mice (RIP-Cre; Jackson laboratory, USA). The offspring with March5 fl/fl; Ins2-Cre (hereafter referred to as cKO) was obtained as β-cell-specific March5 knockout mice. To generate March5 overexpression (March5OE) mice, a CMV-loxp-stop-loxp-March5 construct was inserted into the Rosa26 locus via CRISPR/Cas9-mediated gene targeting, allowing Cre-dependent tissue-specific expression (Shanghai Model Organisms Center). To achieve inducible deletion of March5 in β-cells of adult mice, we mated March5 fl/fl mice with transgenic Ins1-Cre/ERT mice to obtain March5 fl/fl; Ins1-CreERT. At 2 months of age, these mice received daily intraperitoneal Tamoxifen (Sigma, T5648) injection at 100 mg/kg for 5 consecutive days. We have previously established the Kindlin-2fl/fl mice63. To establish mice with March5 and/or Kindlin-2 haploinsufficiency, Kindlin-2fl/fl mice were mated with March5 fl/fl; Ins2-Cre mice or C57BL/6J mice, yielding genotypes such as March5 fll+; Ins2-Cre mice, Kindlin-2fl/+; Ins2-Cre mice, Kindlin-2fl/+; March5 fll+; Ins2-Cre mice, among others. Similarly, β-cell-specific double knockout mice for March5 and Trim28 (March5 fl/fl; Trim28fl/fl; Ins2-Cre) were generated using the same Cre-loxP approach. To ensure experimental consistency and minimize animal usage, only male mice were utilized for most experiments. To induce a type 2 diabetes (T2D) model, 6-week-old male cKO and control mice were subjected to a high-fat diet (HFD; 60% fat calories; Research Diets, D12492) for 14 weeks.
All experimental mouse strains were derived from the C57BL/6J genetic line. The animals were bred under strict specific pathogen-free (SPF) environment with temperatures ranging between 20–24 °C and a consistent 12-h light/dark cycle. Fresh water and standard chow (Beijing HFK Bioscience, 1035) were fully provided to all animals. Animal health was checked daily. Predefined humane endpoints for CO2 euthanasia included ≥20% loss of initial body weight, failure to thrive or overt developmental abnormality, pronounced lethargy, external bleeding, obvious infection, or inability to obtain food or water. All protocols were approved by the Institutional Animal Care and Use Committees of the Southern University of Science and Technology (SUSTech-JY202505042).
Human pancreatic specimens
Human pancreatic surgical samples, including tumor-adjacent tissues, as well as fresh human pancreas samples, were obtained from patients undergoing Whipple surgery at Sun Yat-sen Memorial Hospital, Sun Yat-sen University. These samples were used for IHC staining and islet isolation, respectively. The procedure of isolating human islets was similar to that used for mouse pancreas. This research received approval from the Ethics Committee of Sun Yat-sen Memorial Hospital, Sun Yat-sen University (SYSKY-2024-155-01), with all participants providing written consent prior to their involvement in this study. The basic information is listed in Supplementary Table 2. Participants age ≥60 were classified as Group Old, while those <60 were Group Young.
Cell culture
HEK 293T cells and Rat INS1 832/13 cells (provided by Prof. Feng Rao, Southern University of Science and Technology, Shenzhen, China) were cultured in a humidified atmosphere of 5% CO2/95% air at 37°C in RPMI-1640 medium (GIBCOTM, C11875500BT) supplemented with 10% fetal bovine serum (FBS; GIBCOTM, 10091148) along with a standard antibiotic cocktail containing 100 U/mL penicillin and 100 μg/mL streptomycin.
Glucose tolerance test (GTT), insulin tolerance test (ITT), and glucose-stimulated insulin secretion assay (GSIS)
GTTs were performed following a 16-h fast. Mice received glucose (2 g/kg body weight) via an intraperitoneal (i.p.) injection45. At intervals of 0, 15, 30, 60, 90, and 120 min, tail vein blood samples were taken for blood glucose level assessment with a blood glucose meter (YASEE, GLM-76). For ITTs, mice underwent a 4-h fast before receiving an i.p. injection of 1 U/kg body weight recombinant human insulin (ProSpec, cyt-270). Blood glucose levels were monitored at identical intervals as GTTs. For in vivo GSIS, the animals were fasted for 16 h before receiving a glucose injection (i.p., 2 g/kg of body weight). Blood samples were taken at baseline (0 min) and subsequent time points (3, 6, 10, and 30 min) to measure insulin concentrations by ELISA kits (EZassay, MS200).
Isolation of pancreatic islets and insulin release measurement
Mouse pancreatic islets were prepared according to the protocol from Dong-Sheng Li et al.64. Briefly, we injected 3 mL of ice-cold collagenase V solution (Sigma, C9263, 1 mg/mL in Hanks balanced salt solution [HBSS]) into the pancreas’ common bile duct. Post-injection, the solution was extracted and subjected to digestion at a controlled temperature of 37 °C for a duration of 15 min, while being agitated every 2–3 min. Islets were hand-picked after termination with ice-cold Hanks’ buffer, which supplemented with 0.2% bovine serum albumin (BSA; Sigma, A8806). Then we used RPMI-1640 supplemented with 10% FBS along with 100 μg/mL streptomycin and 100 U/mL penicillin as the culture medium for isolated islets. For in vitro GSIS assays, isolated islets were cultured for 1 -h in Krebs buffer with 2.8- or 16.7-mM glucose. Supernatants and islets were then collected, and insulin concentrations in the supernatants were measured using an ELISA kit (EZassay, MS300) following the manufacturer’s instructions. Insulin levels were normalized to total DNA content. For perifusion assays, 30 islets of similar sizes and morphology were loaded into a temperature-controlled perifusion chamber. After a 30-min preincubation period in Krebs buffer with 2.8 mM glucose at 37 °C, we continuously perifused the islets with the same glucose concentration for an additional 5-min. Subsequently, we applied high-glucose stimulus (16.7 mM) glucose to stimulate islets at a flow rate of 200 μL /min flow for half an hour. Throughout the experiment, the effluent was gathered every 2 min for analysis. After the assay, islets were retrieved for DNA quantification. Insulin secretion at each time point was assessed by ELISA (EZassay, MS300) and normalized to DNA content.
Human islet isolation procedure was similar to that used for mouse pancreas. We utilized RMPI-1640, supplemented with 10% FBS and 5.5 mM glucose as the culture medium for human islets. Human islets were also cultured under 37 °C conditions and a 5% CO2 atmosphere. After infection with adenovirus overexpressing human MARCH5, we used Krebs buffer with 2.8- or 16.7-mM glucose to incubate islets for an hour. Islets and supernatant were then collected. Islets proteins were obtained via sonication using 300 μL of acidified ethanol (0.18 M HCl dissolved in 95% ethanol). The collected supernatants and extracts were used for insulin quantification by ELISA (Sangon Biotech, D711309) and normalized to DNA content.
Lentivirus package
HEK193T cell line was used to produce lentivirus65. We used lentiviral vectors (15 μg), including pLv-NC-GFP, pLv-MARCH5-GFP, or pLv-shTRIM28-GFP, along with Paspx2 vector and PMD2.G vector to transfect with HEK293T cells in a 10 cm dish. Post-transfection, the culture medium spun at 80,000 × g for 2-h to isolate lentivirus after 48 h and 72 h. The lentiviruses were harvested and diluted in 100 μL PBS (Solarbio Life Science, P1022) at a titer of 2 × 109 PFU/mL.
Transmission electron microscopy (TEM)
The transmission electron microscope (TEM) analysis of islets was performed following the protocol of Xiaozhe Zhang et al.66. Briefly, pancreatic samples from CON and cKO mice at age 2 months were initially preserved with 2.5% glutaraldehyde for at room temperature for an hour, followed by additional overnight fixation in fresh 2.5% glutaraldehyde at 4 °C. Samples were fixed with 1% osmium tetroxide in PBS 2 h at 4 °C, then dehydrated via ethanol and propylene oxide washes. We used Eponate 12 (TEPELLA-18010) to embed and overnight curing samples at 60 °C. Subsequently, Ultracut microtome (Leica) was applied to generate 80 nm ultrathin sections, which were then stained with 1% uranyl acetate in methanol. Finally, 0.2% lead citrate was used to further treated the sections and then the sections dried overnight. Samples were analyzed using Transmission electron microscope (Hitachi TEM-HT7700) at 100 kV.
Measurement of Ca2+ influx in primary islets
Ca2+ recording was conducted as previously reported66. In brief, islets cultured overnight on fibronectin-coated cover slips were transferred to REC buffer (119 mM NaCl, 2.5 mM CaCl2, 4.7 mM KCl, 10 mM Hepes,1.2 mM MgSO4, 1.2 mM KH2PO4, and 2.8 mM glucose) supplemented with 5 µM FURA2 (Beyotime, S1052) for 30-min at 37 °C. Following this, the islets were transferred to a fresh batch of REC buffer to get a read on baseline Ca2+ levels. 16.7 mM glucose was added to stimulate Ca2+ influx at 4 min. 25 mM KCl was added to further stimulate Ca2+ influx at 6 min. Metafluor system (Molecular Device) was used to record the A340 nm/A380 nm ratio.
β-cell mass measurement
β-cell mass was measured as previously reported45,67. Pancreatic tissues were prepared into paraffin slices, yielding at least six 5-µm sections were obtained from each mouse throughout the entire pancreas. Each pancreatic section underwent immunohistochemical (IHC) staining to detect insulin expression. We used a multifunctional digital pathology scanner (Aperio VERSA 8) to scan each slide for further analysis. The total insulin-positive area relative to the total pancreatic area determined the β-cell ratio. β-cell mass was then estimated as this fraction multiplied by the pancreas weight.
Western blot analysis
Western blotting was conducted following established protocols68. We used ice-cold RIPA buffer containing 0.1% protease inhibitor cocktail (YEASEN, 20124ES10) to collect lysed cells or tissues samples. Then we used SDS-PAGE to separate proteins and subsequently blotted proteins onto PVDF membranes (Thermo Scientific™, 88518). After that, membranes were blocked with 5% nonfat milk within TBST initial for an hour. Subsequently, we incubated the membranes with primary antibody overnight at 4 °C. The next day, we first washed the membranes to remove any unbound antibodies, then probed them with HRP-conjugated secondary antibody for an hour at 37 °C. Finally, we used a Western Blotting Detection Kit (Mei5bio, MF-078-01) and a chemiluminescence imaging system (LAS4000, ImageQuant) to capture the signal from protein bands. Antibodies details are listed in Supplementary Table 1.
Quantitative real-time RT-PCR (qPCR) analysis
Trizol reagent (TransGen Biotech, ET111-01) was used to isolated total RNA from cells or tissues. Then we used the Titanium One-Step RT-PCR Kit (Takara, 639504) to reverse transcription of RNA samples. For the quantitative PCR analysis, we utilized the SYBR Green dye (Bio-Rad, 1725125) within the Analytik Jena AG qTOWER3 Real-time PCR instrument. Relative mRNA abundance was quantified via the ΔΔCT approach following normalization to the expression level of Gapdh. Detailed primer sequences of this study can be found in Supplementary Tables 1 and 3–5.
Immunohistochemistry (IHC) and Immunofluorescence (IF) staining
IHC and IF were conducted following established protocols68. Samples underwent 4% paraformaldehyde fixation, paraffin embedding, and 5-μm sections. For IHC, sections underwent deparaffinization and antigen retrieval before blocking, followed by overnight primary antibody incubation at 4 °C. PBST washed preceded secondary antibody application. DAB Kit (Vectorlabs, SK-4100) was used to determine expressed signal. We scanned all slides by using a Multifunctional digital pathology scanner (Aperio VERSA 8). For IF, 5-μm sections underwent permeabilized via 0.2% Triton X-100 and DAPI for 5 min. Next, we used 2% BSA to block the tissues from each slide for an hour and then incubated the whole tissues with primary antibody (Supplementary Table 1) at 4 °C overnight. After washing the sections with PBS, we then incubate the tissues with secondary antibody for an hour at room temperature. Finally, imaging was performed utilizing a confocal microscope (Zeiss, LSM 980) to capture the images, and ImageJ software was applied for signal quantification. Briefly, each group included 5 mice, with 3 randomly chosen tissue sections taken from each mouse. The section’s depth was standardized across all samples for consistency. For every section analyzed, the mean fluorescence intensity or grayscale value was determined by averaging measurements from 5 distinct areas. All data were then adjusted relative to the control group.
Co-immunoprecipitation (Co-IP), ubiquitination, and cycloheximide (CHX) assay
Co-IP assays were conducted following established protocols69. We first transfected the targeted plasmids into cells using a transfection reagent (Thermo). 48 h later, we used ice-cold IP buffer containing 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.5% sodium deoxycholate, 1% NP-40, and a protease inhibitor cocktail and phosphatase inhibitor cocktail to sustain protein stable to lysis the cells. To augment the signal, the cell lysates were pre-cleared with Protein A/G-agarose beads (Thermo) and then incubated overnight at 4 °C with the appropriate antibody. Finally, we pulled down the immunocomplexes and used them to western blot analyses, probing with the target primary and corresponding secondary antibody to reveal combinations between different proteins.
We co-transfected cells with HA-ubiquitin vectors alongside the target plasmids to perform the ubiquitination assay. After 48-h transfection, we treated the cells with 10 μM MG132 for 6-h to block proteasomal degradation. After that, ice-cold IP buffer containing 1% sodium dodecyl sulfate (SDS) was used to lysis the cells before undergoing IP as previously described. Finally, polyubiquitinated proteins were identified through immunoblot analysis using the indicated antibody.
For the CHX assay, we treated the cells expressing the indicated proteins with 100 μg/mL CHX to halt protein synthesis and harvested at the indicated time points. Then we performed immunoblotting to determine protein degradation.
RNA sequencing
Islets were isolated from CON and cKO mice and cultured at a density of ~50 islets per well in a 48-well plate. Islets were initially pre-incubated in Krebs-Ringer bicarbonate buffer, which contains with 0.5% BSA and 2.8 mM glucose for an hour at standard room temperature. After that, we incubated islets in Krebs buffer containing 16.7 mM glucose for another 30-min. Then we extracted total RNA by using TRIzol Reagent (TransGen Biotech, ET111-01) before treating it with DNase I to eliminate residual genomic DNA. Ribosomal RNA (rRNA) was removed by hybridizing complementary DNA oligonucleotides, followed by RNase H (Beyotime, D7089), selective degradation of the resulting RNA:DNA hybrids. The enriched mRNA fraction was chemically fragmented and converted into sequencing libraries, whose quality was verified on the Agilent 2100 Bioanalyzer (Agilent Technologies). Final libraries underwent rolling circle amplification with phi29 DNA polymerase (Beyotime, D7053S) to generate DNA nanoballs (DNBs), each containing over 300 copies of a single template molecule. DNBs were arrayed on a patterned nanoarray and sequenced by utilizing the BGISEQ-500 platform (BGI, Shenzhen) to produce 150 bp reads. The gene expression levels were normalized, and a differential expression analysis then generated with the DEGseq2 package (v1.4.5, Bioconductor). Genes that showed a fold change of 1.5 or higher and a P value ≤ 0.05 were defined as differentially expressed. Subsequently, we used the clusterProfiler package to perform Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis on those differentially expressed genes that had been annotated.
Liquid chromatography–tandem mass spectrometry (LC-MS/MS) protein identification
Mass spectrometry analysis was conducted as previously described59. Briefly, Kindlin-2 Co-IP was conducted in HEK293T cells using an anti-Kindlin-2 antibody. The resulting immunocomplexes (n = 1 biologically independent sample) were subjected to trypsin digestion. The generated peptides were then analyzed by LC-MS/MS. Acquired spectra were processed and searched against the appropriate protein database using PD/MASCOT software (V2.5) to identify associated proteins, including E3 ligases.
Statistics and reproducibility
Sample sizes for each experiment were determined on the basis of our prior experimental experience and established experimental standards in our laboratory. GraphPad Prism 8 (Version 8.0) was applied to perform statistical analyses. Two-tailed unpaired Student’s t-tests were used for comparisons between two groups and one-way ANOVA for multiple groups, followed by Tukey’s post hoc test. Values are presented as mean ± standard deviation (s.d.). Statistical significance was defined as P value < 0.05. Mice were allocated to experimental or controls by random assignment. All tissue samples underwent histological, IHC, and IF analyses, were conducted and assessed using a double-blinded protocol. All in vivo experiments were performed with a minimum of five biologically independent animals per group, with all replication attempts were successful. In vitro experiments were independently repeated at least three times, with consistent reproducibility observed across all trials.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The publicly available transcriptomic datasets for human pancreatic islets that were re-analyzed are obtained from the National Centre for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) site under accession number GSE2572450 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE25724) for the analysis of gene expression. The RNA-seq data have been deposited in the NCBI Sequence Read Archive (SRA) database with the accession BioProject ID: PRJNA1283382. The mass spectrometry proteomics data have been deposited to the ProteomeXChange Consortium via the PRIDE partner repository with the dataset identifier PXD066332. All other data generated in this study are provided in the Article, Supplementary information, or Source Data file. Source data are provided with this paper.
References
Dirice, E. et al. Increased beta-cell proliferation before immune cell invasion prevents progression of type 1 diabetes. Nat. Metab. 1, 509–518 (2019).
Fasolino, M. et al. Single-cell multi-omics analysis of human pancreatic islets reveals novel cellular states in type 1 diabetes. Nat. Metab. 4, 284–299 (2022).
Ng, M. et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384, 766–781 (2014).
Wang, Z., York, N. W., Nichols, C. G. & Remedi, M. S. Pancreatic beta cell dedifferentiation in diabetes and redifferentiation following insulin therapy. Cell Metab. 19, 872–882 (2014).
Zhang, X. et al. IL18 signaling causes islet beta cell development and insulin secretion via different receptors on acinar and beta cells. Dev. Cell 57, 1496–1511 e1496 (2022).
Lee, J. H. et al. Protection from beta-cell apoptosis by inhibition of TGF-beta/Smad3 signaling. Cell Death Dis. 11, 184 (2020).
Vantyghem, M. C., de Koning, E. J. P., Pattou, F. & Rickels, M. R. Advances in beta-cell replacement therapy for the treatment of type 1 diabetes. Lancet 394, 1274–1285 (2019).
Lablanche, S. et al. Islet transplantation versus insulin therapy in patients with type 1 diabetes with severe hypoglycaemia or poorly controlled glycaemia after kidney transplantation (TRIMECO): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 6, 527–537 (2018).
Brennan, D. C. et al. Long-term follow-up of the edmonton protocol of islet transplantation in the United States. Am. J. Transpl. 16, 509–517 (2016).
Bachul, P. J. et al. BETA-2 score is an early predictor of graft decline and loss of insulin independence after pancreatic islet allotransplantation. Am. J. Transpl. 20, 844–851 (2020).
Lablanche, S. et al. Ten-year outcomes of islet transplantation in patients with type 1 diabetes: data from the Swiss-French GRAGIL network. Am. J. Transpl. 21, 3725–3733 (2021).
Chetboun, M. et al. Association between primary graft function and 5-year outcomes of islet allogeneic transplantation in type 1 diabetes: a retrospective, multicentre, observational cohort study in 1210 patients from the Collaborative Islet Transplant Registry. Lancet Diabetes Endocrinol. 11, 391–401 (2023).
Bellin, M. D. & Dunn, T. B. Transplant strategies for type 1 diabetes: whole pancreas, islet and porcine beta cell therapies. Diabetologia 63, 2049–2056 (2020).
Niu, F. et al. β-cell neogenesis: a rising star to rescue diabetes mellitus. J. Adv. Res. 62, 71–89 (2024).
Vantyghem, M. C. et al. Erratum. Ten-year outcome of islet alone or islet after kidney transplantation in type 1 diabetes: a prospective parallel-arm cohort study. Diabetes Care 2019;42:2042-2049. Diabetes Care 43, 1164 (2020).
Vantyghem, M. C. et al. Primary graft function, metabolic control, and graft survival after islet transplantation. Diabetes Care 32, 1473–1478 (2009).
Ryan, E. A. et al. Beta-score: an assessment of beta-cell function after islet transplantation. Diabetes Care 28, 343–347 (2005).
Yonashiro, R. et al. A novel mitochondrial ubiquitin ligase plays a critical role in mitochondrial dynamics. EMBO J. 25, 3618–3626 (2006).
Karbowski, M., Neutzner, A. & Youle, R. J. The mitochondrial E3 ubiquitin ligase MARCH5 is required for Drp1 dependent mitochondrial division. J. Cell Biol. 178, 71–84 (2007).
Shiiba, I., Takeda, K., Nagashima, S. & Yanagi, S. Overview of mitochondrial E3 ubiquitin ligase MITOL/MARCH5 from molecular mechanisms to diseases. Int. J. Mol. Sci. 21, 3781 (2020).
Park, Y. Y. et al. Loss of MARCH5 mitochondrial E3 ubiquitin ligase induces cellular senescence through dynamin-related protein 1 and mitofusin 1. J. Cell Sci. 123, 619–626 (2010).
Yoo, Y. S. et al. Mitochondria ubiquitin ligase, MARCH5 resolves hepatitis B virus X protein aggregates in the liver pathogenesis. Cell Death Dis. 10, 938 (2019).
Lei, W. et al. MARCH5 restores endothelial cell function against ischaemic/hypoxia injury via Akt/eNOS pathway. J. Cell. Mol. Med. 25, 3182–3193 (2021).
Haschka, M. D. et al. MARCH5-dependent degradation of MCL1/NOXA complexes defines susceptibility to antimitotic drug treatment. Cell Death Differ. 27, 2297–2312 (2020).
Huang, A. S. et al. Mitochondrial E3 ubiquitin ligase MARCHF5 controls BAK apoptotic activity independently of BH3-only proteins. Cell Death Differ. 30, 632–646 (2023).
Liu, R. et al. PD-1 signaling negatively regulates the common cytokine receptor gamma chain via MARCH5-mediated ubiquitination and degradation to suppress anti-tumor immunity. Cell Res. 33, 923–939 (2023).
Toyofuku, T., Okamoto, Y., Ishikawa, T., Sasawatari, S. & Kumanogoh, A. LRRK2 regulates endoplasmic reticulum-mitochondrial tethering through the PERK-mediated ubiquitination pathway. EMBO J. 39, e100875 (2020).
Tanaka, A. et al. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J. Cell Biol. 191, 1367–1380 (2010).
Takeda, K. et al. Mitochondrial ubiquitin ligase alleviates Alzheimer’s disease pathology via blocking the toxic amyloid-beta oligomer generation. Commun. Biol. 4, 192 (2021).
Kim, H. J. et al. HDAC6 maintains mitochondrial connectivity under hypoxic stress by suppressing MARCH5/MITOL dependent MFN2 degradation. Biochem. Biophys. Res. Commun. 464, 1235–1240 (2015).
Yonashiro, R. et al. Mitochondrial ubiquitin ligase MITOL ubiquitinates mutant SOD1 and attenuates mutant SOD1-induced reactive oxygen species generation. Mol. Biol. Cell 20, 4524–4530 (2009).
Lu, L. et al. Irisin attenuates myocardial ischemia/reperfusion-induced cardiac dysfunction by regulating ER-mitochondria interaction through a mitochondrial ubiquitin ligase-dependent mechanism. Clin. Transl. Med. 10, e166 (2020).
Xiong, X. et al. Irisin attenuates sepsis-induced cardiac dysfunction by attenuating inflammation-induced pyroptosis through a mitochondrial ubiquitin ligase-dependent mechanism. Biomed. Pharmacother. 152, 113199 (2022).
Yu, Z., Li, Q., Wang, Y. & Li, P. A potent protective effect of baicalein on liver injury by regulating mitochondria-related apoptosis. Apoptosis 25, 412–425 (2020).
Bond, S. T. et al. The E3 ligase MARCH5 is a PPARgamma target gene that regulates mitochondria and metabolism in adipocytes. Am. J. Physiol. Endocrinol. Metab. 316, E293–E304 (2019).
Zhang, L. et al. TRAP1 inhibits MARCH5-mediated MIC60 degradation to alleviate mitochondrial dysfunction and apoptosis of cardiomyocytes under diabetic conditions. Cell Death Differ. 30, 2336–2350 (2023).
Pineda, C. T. et al. Degradation of AMPK by a cancer-specific ubiquitin ligase. Cell 160, 715–728 (2015).
Zhang, R. Y. et al. UBE2S interacting with TRIM28 in the nucleus accelerates cell cycle by ubiquitination of p27 to promote hepatocellular carcinoma development. Signal Transduct. Target. Ther. 6, 64 (2021).
Lionnard, L. et al. TRIM17 and TRIM28 antagonistically regulate the ubiquitination and anti-apoptotic activity of BCL2A1. Cell Death Differ. 26, 902–917 (2019).
Bond, S. T. et al. Deletion of Trim28 in committed adipocytes promotes obesity but preserves glucose tolerance. Nat. Commun. 12, 74 (2021).
Dong, L. et al. Oriented cellulose hydrogel: Directed tissue regeneration for reducing corneal leukoplakia and managing fungal corneal ulcers. Bioact. Mater. 41, 15–29 (2024).
Kanchanawong, P. & Calderwood, D. A. Organization, dynamics and mechanoregulation of integrin-mediated cell-ECM adhesions. Nat. Rev. Mol. Cell Biol. 24, 142–161 (2023).
Jevon, D. et al. Local activation of focal adhesion kinase orchestrates the positioning of presynaptic scaffold proteins and Ca(2+) signalling to control glucose-dependent insulin secretion. eLife 11, e76262 (2022).
Gan, W. J. et al. Local integrin activation in pancreatic beta cells targets insulin secretion to the vasculature. Cell Rep. 24, 2819–2826 e2813 (2018).
Zhu, K. et al. Kindlin-2 modulates MafA and beta-catenin expression to regulate beta-cell function and mass in mice. Nat. Commun. 11, 484 (2020).
Edlund, H. Pancreatic organogenesis-developmental mechanisms and implications for therapy. Nat. Rev. Genet. 3, 524–532 (2002).
Gradwohl, G., Dierich, A., LeMeur, M. & Guillemot, F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc. Natl. Acad. Sci. USA 97, 1607–1611 (2000).
Melloul, D. Transcription factors in islet development and physiology: role of PDX-1 in beta-cell function. Ann. N. Y. Acad. Sci. 1014, 28–37 (2004).
Wang, H., Brun, T., Kataoka, K., Sharma, A. J. & Wollheim, C. B. MAFA controls genes implicated in insulin biosynthesis and secretion. Diabetologia 50, 348–358 (2007).
Dominguez, V. et al. Class II phosphoinositide 3-kinase regulates exocytosis of insulin granules in pancreatic beta cells. J. Biol. Chem. 286, 4216–4225 (2011).
Boland, B. B., Rhodes, C. J. & Grimsby, J. S. The dynamic plasticity of insulin production in beta-cells. Mol. Metab. 6, 958–973 (2017).
Yong, J., Johnson, J. D., Arvan, P., Han, J. & Kaufman, R. J. Therapeutic opportunities for pancreatic beta-cell ER stress in diabetes mellitus. Nat. Rev. Endocrinol. 17, 455–467 (2021).
Mezza, T. et al. Beta-cell fate in human insulin resistance and type 2 diabetes: a perspective on islet plasticity. Diabetes 68, 1121–1129 (2019).
Tiano, J. P. & Mauvais-Jarvis, F. Importance of oestrogen receptors to preserve functional beta-cell mass in diabetes. Nat. Rev. Endocrinol. 8, 342–351 (2012).
Hou, X. et al. Talin-1 inhibits Smurf1-mediated Stat3 degradation to modulate beta-cell proliferation and mass in mice. Cell Death Dis. 14, 709 (2023).
Fan, F. et al. Dynamin 2 regulates biphasic insulin secretion and plasma glucose homeostasis. J. Clin. Investig. 125, 4026–4041 (2015).
Naumann, H., Rathjen, T., Poy, M. N. & Spagnoli, F. M. The RhoGAP Stard13 controls insulin secretion through F-actin remodeling. Mol. Metab. 8, 96–105 (2018).
Zhu, X. et al. Microtubules negatively regulate insulin secretion in pancreatic beta cells. Dev. Cell 34, 656–668 (2015).
Ma, G. et al. Endothelial-to-Osteoblast Conversion maintains bone homeostasis through Kindlin-2/Piezo1/TGFβ/Runx2 axis. Protein Cell 16, 497–502 (2025).
Yoo, Y. S. et al. The mitochondrial ubiquitin ligase MARCH5 resolves MAVS aggregates during antiviral signalling. Nat. Commun. 6, 7910 (2015).
Mori, H. et al. Critical role for hypothalamic mTOR activity in energy balance. Cell Metab. 9, 362–374 (2009).
Nielsen, A. L. et al. Interaction with members of the heterochromatin protein 1 (HP1) family and histone deacetylation are differentially involved in transcriptional silencing by members of the TIF1 family. EMBO J. 18, 6385–6395 (1999).
Wu, C. et al. Kindlin-2 controls TGF-beta signalling and Sox9 expression to regulate chondrogenesis. Nat. Commun. 6, 7531 (2015).
Li, D. S., Yuan, Y. H., Tu, H. J., Liang, Q. L. & Dai, L. J. A protocol for islet isolation from mouse pancreas. Nat. Protoc. 4, 1649–1652 (2009).
Ding, Z. et al. Targeting miR-29 mitigates skeletal senescence and bolsters therapeutic potential of mesenchymal stromal cells. Cell Rep. Med. 5, 101665 (2024).
Zhang, X. et al. 5-IP(7) is a GPCR messenger mediating neural control of synaptotagmin-dependent insulin exocytosis and glucose homeostasis. Nat. Metab. 3, 1400–1414 (2021).
Weng, Q. et al. STAT3 dictates beta-cell apoptosis by modulating PTEN in streptozocin-induced hyperglycemia. Cell Death Differ. 27, 130–145 (2020).
Liu, Z. et al. A novel sprayable thermosensitive hydrogel coupled with zinc modified metformin promotes the healing of skin wound. Bioact. Mater. 20, 610–626 (2023).
Wang, Z. et al. The CK1delta/epsilon-AES axis regulates tumorigenesis and metastasis in colorectal cancer. Theranostics 11, 4421–4435 (2021).
Acknowledgements
We thank the Core Research Facilities of the Southern University of Science and Technology (SUSTech) for technical support. We are grateful to Dr. Yucong Xie and Dr. Zan Li at the SUSTech Testing Center for their assistance. We thank Prof. Kaosheng Lv (Hunan University) for kindly providing the Trim28 floxed mice, and Mr. Jialiang Zhu (Amphira LLC) for help with the schematic illustrations. This work was supported, in part, by the National Key R&D Program of China (2024YFA0919203, to H.C.), the National Natural Science Foundation of China Grants (82372476, 82350710800, and 824B2074, to H.C.), Guangdong Provincial Science and Technology Innovation Council Grant (2017B030301018, to H.C.).
Author information
Authors and Affiliations
Contributions
Y.C., G.M., and H.C. conceived and designed the study; Y.C., W.P., Y.Y., Z.X., Y.C., Z.D., L.C., X.H., and G.M. conducted the study, collected and analyzed the data; Y.C., G.M., and H.C. interpreted the data. Y.C., G.M., and H.C. drafted the manuscript. G.M. and H.C. are responsible for the integrity of the data analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Jianjie Ma, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, Y., Pang, W., Ma, G. et al. March5-mediated Trim28 degradation preserves islet β-cell function in mice. Nat Commun 16, 7073 (2025). https://doi.org/10.1038/s41467-025-62587-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-62587-z