Abstract
Wheat tillering is an important agronomic trait influencing grain yield. Here, we identify an NHL repeat-containing protein, TaNHLP1, which positively regulates tiller number in wheat. We discovered that the core components of the abscisic acid (ABA) signaling pathway, type 2C protein phosphatase TaPP2C and SNF1-related protein kinase TaSnRK2, interact with TaNHLP1 to regulate its abundance. Furthermore, TaNHLP1 interacts with the Receptor for Activated C Kinase 1 (TaRACK1A), an ABA pathway negative regulator, and influences its subcellular localization. Importantly, both the TaNHLP1 and TaRACK1A mutations promote ABA accumulation in the shoot bases and tiller buds. Notably, the NHLP1-RACK1 module is conserved across monocots and eudicots, and natural variations in the promoter of TaNHLP1-A enhance its transcriptional activity, leading to increased tiller number and yield. Collectively, these findings elucidate the genetic mechanism of NHLP1-mediated tillering regulation and highlight its potential as a target for improving crop plant architecture.
Similar content being viewed by others
Introduction
Common wheat (Triticum aestivum L.), as a major staple food crop globally, plays a critical role in ensuring global food security1. To satisfy the future demand for food supplies due to the growing population and reduced farmland, a net increase in grain yield is essential2. Wheat plant architecture, which is determined by plant height, tiller number and angle, spike morphology, leaf structure, and root systems, is crucial for crop yield3,4. Among these factors, tillering capacity is commonly regarded as the key factor affecting the yield potential of cereal crops directly, since tillers are specialized branches bearing spikes5,6. However, excessive tillers are not always beneficial because young and weak tillers consume nutrients without producing spikes7. Therefore, elucidating the molecular mechanisms of tillering will contribute to the sustained increase in crop yield.
Tiller number is a crucial agronomic trait in wheat. To date, several tiller inhibition genes (tin1, tin2, tin3, TIN4, TIN5, ftin, and dmc) and a high‑tillering dwarf mutant (NAUH167) have been reported in wheat6,8,9,10,11,12,13. Moreover, numerous wheat tillering quantitative trait loci (QTL) have subsequently been revealed4,14,15,16,17. However, only a few genes, such as the tiller inhibition gene (tin), encoding a cellulose-synthase-like protein, have been isolated through the map-based cloning approach18. Tiller number 1 (TN1) encodes an ankyrin repeat protein with a transmembrane domain (ANK-TM) and promotes tiller bud outgrowth probably through inhibiting ABA biosynthesis and signaling19. According to reverse genetics and the analysis of functional validation of homologous genes, some reports have recently demonstrated that TaD27-B, tae-miR156, TaSPL1, and TaPIN1s can effectively regulate the tiller number in wheat20,21,22,23.
Various plant hormones, such as Indole-3-acetic acid, Cytokinins, Strigolactones (SL), ABA, and Gibberellins, are involved in the occurrence and development of plant branching/tillering24,25. ABA is a pivotal hormone that orchestrates plant responses to a variety of stress conditions and plays a complex role in the regulation of growth and development, notably in the inhibition of tillering/branching26,27. ABA exerts its function by binding to its receptors, the pyrabactin resistance (PYR)/pyrabactin resistance-like (PYL)/regulatory component of ABA receptors (RCAR) proteins, which then interact with type 2C protein phosphatases (PP2Cs) to release sucrose non-fermenting-1 (SNF1)-related kinases (SnRK2s), thereby initiating downstream signaling pathways28. In rice, ABA primarily suppresses the formation of higher-order tillers by reducing the expression of key genes involved in SL biosynthesis, leading to decreased levels of SL and the regulation of tillering29. In wheat, the oligo-tillering mutant ot1 shows an upregulation of ABA pathway genes, including those involved in its biosynthesis genes 9-cis-epoxicarotenoid dioxigenase 3 (NCED3) and NCED5 and signal transduction, such as SnRK2s and PP2Cs30. This suggests a correlation between ABA signaling and the reduced tillering phenotype. Furthermore, the scaffold protein Receptor for Activated C Kinase 1 (RACK1) is a negative regulator of ABA-mediated pathways31, modulating endogenous ABA levels and impacting seed germination32. In rice, the Osrack1a mutant exhibits reduced tiller numbers and shorter heights33. These insights highlight the multifaceted influence of ABA and its associated proteins in the intricate hormonal network that governs plant architecture and growth.
The complexity of the common wheat genome has been a barrier to gene cloning studies. However, the diploid wheat species Triticum urartu, as the progenitor of the wheat A-genome, provides a simpler genomic model for research. Our study isolated a T. urartu mutant, tiller inhibition 7 (tin7), which has a reduced tiller number. Map-based cloning revealed an NHL (NCL-1, HT2A and LIN-41)-repeat protein, NHLP1, which regulates tiller number. Overexpression and knock-out studies in wheat confirmed its role. The effect of the tin7 mutation on tiller buds is likely due to increased ABA accumulation and signaling. Importantly, we found an allele, TaNHLP1-A-Hap1, associated with higher tiller counts and yield in common wheat, indicating its potential for wheat improvement.
Results
Map-based cloning of the causal gene underlying low-tiller phenotype
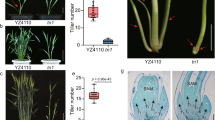
We isolated a low-tiller mutant, tin7 (Fig. 1a), from an ethyl methanesulfonate (EMS)-mutagenized population of the genome-sequenced T. urartu accession G1812. Under field conditions, the tiller number of the tin7 mutant was reduced by 55% compared to the wild type (WT) at the grain filling stage (Fig. 1b, c). This reduction may be attributed to the inhibition of tiller bud outgrowth (Fig. 1d). Additionally, the mature tin7 mutants displayed notably shorter internodes (Supplementary Fig. 1a) and a significant reduction in plant height compared to WT plants (Fig. 1c and Supplementary Fig. 1b). The architecture of the tin7 mutant was more compact and erect compared to WT (Fig. 1c and Supplementary Fig. 1c, d). Furthermore, the tin7 mutant exhibits a reduced spikelet number (Supplementary Fig. 1e, f) and displays a delayed flowering phenotype, with flowering occurring 5–7 days later under field conditions compared to WT. Together, these results suggest that the causal gene responsible for the tin7 phenotype regulates multiple important agronomic traits, particularly tiller number.
a Morphology of WT (G1812) and the tin7 mutant grown in the greenhouse for 30 days. Scale bar = 5 cm. Tiller number comparison b and plant morphology c between WT and the tin7 mutant at the filling stage. Data in b are presented as mean ± SD (n = 15 individual plants) and were analyzed using Student’s t test (two-sided). d Tiller buds from WT and the tin7 mutant are shown, with white arrows indicating the positions of the first primary tiller. Scale bar = 10 mm. e Fine mapping using F2 and F3 recombinants narrowed down the candidate gene to a 387 kb interval between markers IN13 and IN5. The numbers below the markers indicate the number of recombinants. Genes annotated within the IN13-IN5 interval of the T. urartu v2.0 reference genome. The red arrow indicates TuNHLP1. f Gene structure and sequence variation of the TuNHLP1 gene. Black boxes represent exons, and black horizontal lines represent introns. Nucleotide change and mutation position between WT (black) and the tin7 mutant (red) are highlighted, with the coordinates starting from the initial ATG of the open reading frame. g Schematic diagram of the predicted TuNHLP1 protein structure. SP, signal peptide; NHL, NHL (NCL-1, HT2A, and LIN-41) repeat domain; TM, transmembrane helix region. Amino acid change and mutation position between WT (black) and the tin7 mutant (red) are highlighted, with the coordinates starting from the initial Met. Source data are provided as a Source Data file.
To isolate the causal gene responsible for the low-tiller phenotype of tin7, we performed crosses between tin7 and T. urartu accession G3146, generating a mapping population. A high-tiller phenotype was observed in all F1 plants, suggesting that the tiller inhibition phenotype is recessive. Self-pollination of these F1 plants resulted in a large F2 population comprising 1458 individuals. Subsequently, 268 F2 individual plants were randomly selected for genetic analysis and initial mapping, and the tiller number exhibited a continuous distribution (Supplementary Fig. 2a). Given that the tin7 mutant typically possesses an average of 20 tillers accompanied by reduced plant height (Fig. 1b, c), the F2 population was categorized into two groups: low-tiller plants (with one to 20 tillers, and low plant height) and multi-tiller plants (with more than 20 tillers, and high plant height). The F2 plants segregated into 194 multi-tiller and 74 low-tiller plants, conforming to the expected 3:1 ratio (χ² = 0.975, P > 0.05). The F2:3 families segregated into 65 homozygous multi-tiller plants, 129 segregating plants, and 74 homozygous low-tiller plants, fitting a 1:2:1 segregation ratio (χ² = 0.978, P > 0.05). These results indicate that the low-tiller phenotype of the tin7 mutant is controlled by a single recessive nuclear gene.
To elucidate the genetic basis underlying the tin7 phenotype, 168 polymorphic markers distributed across the genome of T. urartu13 were employed to genotype these 74 homozygous low-tiller F2 individuals. The target gene was initially mapped to the long arm of chromosome Tu6, between markers 6T-48 and 6T-36 (Supplementary Data 1). Subsequent fine mapping, utilizing newly developed markers, narrowed the target region to a 387-kb interval between markers IN13 and IN5 (Fig. 1e and Supplementary Fig. 2b). Within this refined region, two high-confidence genes were annotated using the T. urartu v2.0 reference genome (Supplementary Data 2). Sequencing analysis of both candidate genes, encompassing their coding sequences and untranslated regions (1.8 kb upstream of the start codon and 700 bp downstream of the stop codon), revealed a single C-to-A nucleotide substitution at position 455 relative to the start codon of TuG1812G0600003205 (Fig. 1f). This mutation results in an amino acid change from threonine to lysine at position 152 in the deduced protein sequence (Fig. 1g). Additionally, we observed that TuG1812G0600003205 is significantly upregulated in the tin7 mutant compared to WT (Supplementary Fig. 2c). In contrast, no sequence polymorphisms or expression differences were identified in TuG1812G0600003204 (Supplementary Fig. 2c). TuG1812G0600003205 encodes a six-bladed beta-propeller protein containing two NHL (NCL-1, HT2A, and LIN-41) domains, as predicted by the Conserved Domain Database (NCBI, https://www.ncbi.nlm.nih.gov/); therefore, we designated TuG1812G0600003205 as NHL-repeat protein 1 (TuNHLP1). The Thr152Lys substitution occurs within the boundary region of the second NHL domain (Fig. 1g). Moreover, the N-terminal region of TuNHLP1 is predicted to contain a signal peptide (Supplementary Fig. 3a), while the central region is predicted to harbor a transmembrane domain (Supplementary Fig. 3b). Consequently, we considered TuNHLP1 as the most likely candidate for the tin7 mutation.
To assess the functional implications of the T152K amino acid substitution, we first analyzed the protein structure of TuNHLP1 and the T152K mutant, Tunhlp1, using the AlphaFold Protein Structure Database (https://alphafold.ebi.ac.uk/) and Missense3D (http://missense3d.bc.ic.ac.uk/missense3d/). Structural predictions suggested that the T152K mutation in TuNHLP1 may expose an otherwise buried residue, potentially disrupting the native hydrogen bonding network (Supplementary Fig. 4). Next, we investigated the subcellular localization of TuNHLP1 and Tunhlp1 by co-expressing the constructs 35Spro:TuNHLP1-green fluorescent protein (GFP) or 35Spro:Tunhlp1-GFP, together with the cis-Golgi marker 35Spro:GmMan1-mCherry, in the epidermal cells of Nicotiana benthamiana leaves. Confocal microscopy revealed that the fluorescence of both TuNHLP1-GFP and Tunhlp1-GFP fusion proteins overlapped with the red fluorescence of GmMan1-mCherry (Supplementary Fig. 5a), indicating that both TuNHLP1 and Tunhlp1 are localized to the cis-Golgi network. Despite this, we observed that TuNHLP1-GFP formed numerous punctate aggregates, whereas no such aggregates were detected in Tunhlp1-GFP (Supplementary Fig. 5a). One possible explanation is that the specific mutations in Tunhlp1-GFP prevent the formation of such aggregates, whereas the wild-type TuNHLP1-GFP can readily form higher-order structures. These results suggest that the T152K substitution does not alter the subcellular localization of TuNHLP1 within the Golgi apparatus, but it may affect its functionality. Additionally, quantification of protein levels revealed a reduced accumulation of Tunhlp1-GFP compared to TuNHLP1-GFP in N. benthamiana leaves (Supplementary Fig. 5b), indicating that the T152K substitution could impair protein stability. This provides further evidence that TuNHLP1 is the most likely candidate for the tin7 mutation.
Characterization of TaNHLP1
Given that no robust genetic transformation system has been established for the diploid T. urartu, we functionally characterized the homoeologous genes of TuNHLP1 in common wheat. Bread wheat is a hexaploid species containing A, B, and D sub-genomes; thus, there are three TuNHLP1 homoeologs in the common wheat accession Chinese Spring (CS): TaNHLP1-A (TraesCS6A02G291600, where “A” represents the A genome copy), TaNHLP1-B (TraesCS6B02G322100), and TaNHLP1-D (TraesCS6D02G272900) (Supplementary Fig. 6). These homoeologs encode proteins that share 99.80%, 98.01%, and 98.01% amino acid identity, respectively, with TuNHLP1 (Supplementary Fig. 6), based on the IWGSC RefSeq Annotation v1.1. To elucidate the spatial expression profiles of TuNHLP1 and its homolog TaNHLP1, we conducted reverse transcription quantitative PCR (RT-qPCR) analysis using gene-specific primers across various developmental tissues. The results revealed that TuNHLP1 exhibited robust expression in the tiller bud and spike (Supplementary Fig. 7a). Similarly, TaNHLP1 displayed high expression levels in the shoot base, tiller bud, and spike, while comparatively lower expression was observed in the sheath and leaf tissues (Supplementary Fig. 7b). Notably, TaNHLP1-B exhibited the highest expression in the examined tissues compared to TaNHLP1-A and TaNHLP1-D (Supplementary Fig. 7b). Additionally, RNA in situ hybridization revealed prominent accumulation of TaNHLP1 transcripts in the shoot apical meristem and florets (Supplementary Fig. 7c–f), suggesting that TaNHLP1 may play a critical role in the development and maintenance of wheat plant architecture.
TaNHLP1 positively regulates tiller number in wheat
To investigate the biological function of TaNHLP1, we generated Tanhlp1 mutants using CRISPR/Cas9-mediated genome editing. Single guide RNAs (sgRNAs) targeting the conserved region of the fourth exon in TaNHLP1 were designed (Fig. 2a) and introduced into the hexaploid wheat cultivar “Fielder”. Through screening, three independent lines, Tanhlp1-1, Tanhlp1-2, and Tanhlp1-3, exhibiting homozygous frameshift mutations in the A, B, and D sub-genomes were obtained (Fig. 2b). In the T2 generation, these mutant lines showed a significant reduction in tiller number and plant height compared to Fielder at the grain filling stage (Fig. 2c, d). In addition, we introduced the full-length coding sequence of TaNHLP1-A, which shows the highest similarity to TuNHLP1, into common wheat “Kenong199” (KN199) under the control of the ubiquitin promoter using Agrobacterium-mediated transformation method. These TaNHLP1-A-overexpressing lines exhibited significantly more tiller numbers than KN199 at the mature plant stage (Fig. 2e, f). Collectively, these results suggest that TaNHLP1 regulates tiller development in wheat.
a Schematic representation of the TaNHLP1 gene structure with the designated target sites for the single guide RNA (sgRNA) highlighted in red. The corresponding sgRNA sequence is displayed below, with the protospacer-adjacent motif (PAM) indicated in red. Black boxes represent exons, and black horizontal lines represent introns. b Illustration of the mutation types induced in the TaNHLP1 gene generated by CRISPR/Cas9-mediated gene editing. The PAM site is marked in red, nucleotide insertions are denoted by blue letters, and deleted nucleotides are represented by ‘.’. The symbols “−” and “+” signify nucleotide deletions and insertions, respectively, with the numerical positions of these mutations provided thereafter. Comparative phenotypes (c) and tiller number (d) of the common wheat cultivar Fielder (serving as a negative control) and CRISPR/Cas9-generated Tanhlp1 mutants (T2 generation). p = 0.9650 (Tanhlp1-1 vs. Tanhlp1-2), 0.8937 (Tanhlp1-1 vs. Tanhlp1-3), 0.9953 (Tanhlp1-2 vs. Tanhlp1-3), <0.0001 for other comparisons. Scale bar, in (c) is 15 cm. Comparative phenotypes (e) and tiller number (f) of the common wheat cultivar KN199 (negative control) and T2 generation transgenic wheat plants overexpressing TaNHLP1-A. p = 0.0039 (KN199 vs. #1), 0.1443 (KN199 vs. #2), 0.4036 (#1 vs. #2). Scale bar, 10 cm in (e). Data in parts (d) and (f) are presented as the mean ± SD, different letters above the bars indicate significant differences at P < 0.05 in one-way ANOVA with Tukey’s multiple comparisons range tests. Individual data points are plotted, with the sample size (n) is listed at the bottom of the figure. Source data are provided as a Source Data file.
TaNHLP1 interacts with TaRACK1A and regulates tillering through ABA signaling
To elucidate the role of TaNHLP1 in wheat tiller development, we conducted a split-ubiquitin membrane yeast two-hybrid (Y2H) screen using the full-length TaNHLP1-A protein as bait to identify potential interacting proteins from a wheat complementary DNA library derived from tiller buds, leaves, and young spikes. Among the candidate interactors identified (Supplementary Data 3), TraesCS1A02G378000, which is an ortholog of the RACK1A in wheat and was designated as TaRACK1A-A (where “A” denotes the A genome copy), was prioritized for further validation (Fig. 3a), given the well-documented role of its ortholog in tillering regulation in rice33. The interaction between TaNHLP1-A and TaRACK1A-A was subsequently confirmed by luciferase complementation imaging (LCI) (Fig. 3b) and co-immunoprecipitation (Co-IP) assays (Fig. 3c).
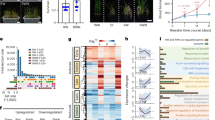
a Interaction between TaNHLP1-A and TaRACK1A-A was demonstrated using a split-ubiquitin yeast two-hybrid assay. Yeast cells were first selected on synthetic dropout (SD) medium lacking Leu and Trp (SD-LW) and then transferred to SD-Leu/-Trp/-His/-Ade (SD-LWHA) selective medium with 25 mM 3-AT for protein interaction analysis. b Luciferase complementation imaging assays conducted in N. benthamiana leaves confirmed the interaction between TaNHLP1-A and TaRACK1A-A. c In vivo interaction between MYC-TaNHLP1-A and HA-TaRACK1A-A was revealed through co-immunoprecipitation assays. Following immunoprecipitation with anti-MYC magnetic beads, the precipitated proteins were probed using anti-HA and anti-MYC antibodies, respectively. Two independent experiments were performed with similar results. d Morphological observations of the shoot base and tiller buds (highlighted with red lines), which were utilized for RNA-seq and analysis of ABA levels. Scale bar, 10 mm. e Quantification of ABA contents in the shoot base and tiller buds of the Fielder and the Tanhlp1-1 mutant (T3 generation, n = 4). f Heatmap depicting the expression levels of differentially expressed genes in the shoot base and tiller buds shared between the wheat variety Fielder (negative control) and the Tanhlp1-1 mutant. g Subcellular localization of TaRACK1A-A-mCherry with or without TaNHLP1-A-GFP in N. benthamiana leaves. White and blue arrows indicate nucleus and punctate dots, respectively. Three independent experiments were performed with similar results. Scale bars, 20 μm. Representative phenotypes h and tiller number i of Fielder (negative control) and CRISPR/Cas9-generated Tarack1a mutants. p = 0.8392 (Tarack1a#1 vs. Tarack1a#2), 0.9621 (Tarack1a#1 vs. Tarack1a#3), 0.9856 (Tarack1a#2 vs. Tarack1a#3), <0.0001 for other comparisons. The sample size (n) for each group is listed at the bottom of the rectangular column; scale bar in (h) is 15 cm. j Quantification of ABA contents in the shoot base and tiller buds of the Fielder and the Tarack1a#1 mutant (T3 generation, n = 4). Data are presented as mean ± SD. The P-values in (e) and (j) were determined using a two-tailed unpaired Student’s t test, and the different letters indicate significant differences between groups in i, as determined by one way ANOVA with Tukey’s multiple comparisons range tests (p < 0.01). Source data are provided as a Source Data file.
Given that OsRACK1A is known to negatively regulate endogenous ABA levels32, and elevated ABA levels can inhibit the growth of tiller buds in wheat and rice19,29, we hypothesize that TaNHLP1-A may be implicated in the ABA signaling pathway through its interaction with TaRACK1A-A. Consequently, transcriptomic and hormone analyses were conducted on the shoot bases and tiller buds of both the Tanhlp1-1 mutant and WT (Fielder) plants (Fig. 3d). Interestingly, the ABA content in the shoot bases and tiller buds of the Tanhlp1-1 mutant was threefold higher than that of Fielder (Fig. 3e). Additionally, genes associated with the ABA signaling pathway, such as TaPYR/PYL/RCAR, TaPP2C, and TaSnRK2, were significantly upregulated in the Tanhlp1-1 mutant, while TaRACK1A was downregulated (Fig. 3f and Supplementary Fig. 8). These findings lead us to hypothesize that the elevated ABA content in the shoot bases and tiller buds may be responsible for the reduced tiller number observed in the Tanhlp1-1 mutant. To test this hypothesis, both the Tanhlp1-1 mutant and Fielder were treated with varying concentrations of exogenous ABA. We observed that a 20 µM concentration of exogenous ABA substantially inhibited tiller bud outgrowth in Fielder (Supplementary Fig. 9a). Conversely, treatment with the ABA biosynthesis inhibitor sodium tungstate partially ameliorated the low-tiller phenotype in the Tanhlp1-1 mutant (Supplementary Fig. 9b). In addition, similar results were also observed in T. urartu tin7 mutant and WT (G1812) plants (Supplementary Fig. 9). These results suggest that the increased ABA levels in the shoot bases and tiller buds contribute, at least in part, to the reduced tiller number observed in the tin7 and Tanhlp1 mutants.
To elucidate the functional role of TaRACK1A in wheat, we comprehensively analyzed its temporal and spatial expression patterns. Through RT-qPCR analysis, we determined that TaRACK1A exhibits high expression levels in the shoot base, tiller bud, spike, and stem (Supplementary Fig. 10). Subsequently, our results revealed that TaRACK1A-A-mCherry is predominantly localized in the cytoplasm and nucleus in the epidermal cells of N. benthamiana leaves (Fig. 3g). Furthermore, co-expression of TaNHLP1-A-GFP with TaRACK1A-A-mCherry led to a significant redistribution of TaRACK1A-A-mCherry, shifting from a uniform cytoplasmic distribution to punctate dots that co-localizes with TaNHLP1-A-GFP, while its nuclear localization remained largely unaffected (Fig. 3g), suggesting that TaNHLP1 may modulate the function of TaRACK1A by altering its subcellular localization. To further assess the potential function of TaRACK1A in wheat, we generated three independent TaRACK1A mutants by simultaneously knocking out the A, B, and D homeologs of TaRACK1A in the Fielder background using CRISPR/Cas9-mediated genome editing. These knockout mutants, designated as Tarack1a#1-#3 (Supplementary Fig. 11), exhibited a significant reduction in tiller number and plant height compared to the wild-type Fielder (Fig. 3h, i). Additionally, similar to the Tanhlp1-1 mutant (Fig. 3e), the shoot bases and tiller buds of the Tarack1a#1 mutant displayed elevated ABA content compared to Fielder (Fig. 3j). Collectively, these findings suggest that the TaNHLP1-TaRACK1A pathway is likely involved in the regulation of tiller number in wheat through the ABA signaling.
TaPP2C-7A directly dephosphorylates TaNHLP1-A, which is phosphorylated by TaSnRK2-1A in vitro, thereby mitigating its degradation
The core of the ABA signaling network comprises PYR1/PYL/RCAR proteins, type 2C protein phosphatases (PP2Cs), and SnRK2-type protein kinases34, whose activities are tightly controlled by ABA. Here, we further investigated whether the regulation of tiller development by TaNHLP1-A is also linked to the core signaling components of the ABA pathway, which were significantly upregulated in the Tanhlp1 mutant (Fig. 3f). Using in vitro Y2H assays, as well as in vivo LCI and Co-IP assays, we confirmed that TaNHLP1-A physically interacts with both TaSnRK2-1A (TraesCS1A02G270800) and TaPP2C-7A (TraesCS7A02G241800) (Fig. 4a–c).
a Split-ubiquitin yeast two-hybrid assays confirmed the interaction between TaNHLP1-A and TaSnRK2-1A and TaPP2C-7A. Yeast cells were first selected on synthetic dropout (SD) medium lacking Leu and Trp (SD-LW) and then transferred to SD-Leu/-Trp/-His/-Ade (SD-LWHA) selective medium with 25 mM 3-AT for protein interaction analysis. b TaNHLP1-A interacts with TaSnRK2-1A and TaPP2C-7A, respectively, in Luciferase complementation imaging assays conducted in N. benthamiana leaves. c The interactions between MYC-TaNHLP1-A, HA-TaSnRK2-1A, and HA-TaPP2C-7A were revealed through co-immunoprecipitation assays. Following immunoprecipitation with anti-HA magnetic beads, the precipitated proteins were probed using anti-HA and anti-MYC antibodies, respectively. d TaSnRK2-1A phosphorylates TaNHLP1-A and is negatively regulated by TaPP2C-7A in vitro. Kinase assays were performed for 90 min at 30 °C. Prior to the addition of TaSnRK2-1A, TaNHLP1-A, and ATP, TaPP2C-7A was incubated with TaPYL-1D and ABA on ice for 20 min. The phosphorylation status of MBP-△TaNHLP1-A was analyzed using a Phos-tag gel assay (top, anti-MBP). The phosphorylated (MBP-p△TaNHLP1-A) and unphosphorylated (MBP-△TaNHLP1-A) forms of TaNHLP1-A are indicated by the red and black arrows, respectively. Red stars indicate non-specific bands. e TaPP2C-7A directly dephosphorylates TaNHLP1-A in vitro. MBP-△TaNHLP1-A and Flag-TaSnRK2-1A plasmids were co-expressed in Escherichia coli strain BL21, then the cell lysates were incubated with GST-TaPP2C-7A in a protein phosphatase reaction buffer for 90 min. The phosphorylated MBP-△TaNHLP1-A, indicated by the red arrow, were detected by Phos-tag gel (top, anti-MBP). △TaNHLP1-A refers to TaNHLP1-A with the signal peptide (1-27 aa) and transmembrane domain (241–263 aa) removed. f Subcellular localization of TaNHLP1-A-GFP with or without HA-TaSnRK2-1A in N. benthamiana leaves. GmMan1-mCherry was used as a cis-Golgi marker protein. Scale bars, 50 μm. g TaSnRK2-1A triggers TaNHLP1-A degradation in N. benthamiana, possibly by influencing its phosphorylation. Red star indicates non-specific bands. The experiments shown in (c–e) and (f, g) were performed independently two and three times, respectively, with similar results. Source data are provided as a Source Data file.
As TaSnRK2-1A is a serine/threonine protein kinase, we next performed in vitro phosphorylation assays using MBP-tagged TaNHLP1-A (MBP-△TaNHLP1-A, which lacks the signal peptide and transmembrane domain) as the substrate. As expected, Flag-tagged TaSnRK2-1A (Flag-TaSnRK2-1A) effectively phosphorylated MBP-△TaNHLP1-A (Fig. 4d, lanes 1 and 2). However, in the presence of GST-tagged TaPP2C-7A (GST-TaPP2C-7A), phosphorylation of MBP-△TaNHLP1-A was inhibited, suggesting that TaPP2C-7A either inhibits TaSnRK2-1A activity or actively dephosphorylates MBP-△TaNHLP1-A (Fig. 4d, lanes 5 and 8). Previous studies have documented that, upon ABA binding to the PYL family of receptors, these receptors bind to the catalytic site of PP2Cs, inhibiting their enzymatic activity and thereby promoting SnRK2 activation34. Here, we validated the interaction between TaPP2C-7A and TaPYL-1D (TraesCS1D02G126900) as well as TaSnRK2-1A using an LCI assay (Supplementary Fig. 12). Subsequently, we found that the inhibition of MBP-△TaNHLP1-A phosphorylation by TaPP2C-7A was reversed when 15 μM ABA and Flag-tagged TaPYL-1D (Flag-TaPYL-1D) were added to the assay (Fig. 4d, lanes 3-8). Given the physical interaction between TaNHLP1-A and TaPP2C-7A (Fig. 4a–c), we investigated whether TaPP2C-7A could directly dephosphorylate TaNHLP1-A. To test this, we co-expressed MBP-△TaNHLP1-A and Flag-TaSnRK2-1A in Escherichia coli strain BL21, then incubated the cell lysates with GST-TaPP2C-7A in a protein phosphatase reaction buffer. The results showed that GST-TaPP2C-7A directly and actively dephosphorylates MBP-△TaNHLP1-A (Fig. 4e).
To investigate whether TaNHLP1-A phosphorylation affects its subcellular localization, we transformed plasmids expressing TaNHLP1-A-GFP, GmMan1-mCherry, or HA-TaSnRK2-1A into N. benthamiana leaf epidermal cells. We found that all combinations showed similar subcellular localization patterns, suggesting that phosphorylation may not alter the subcellular localization of TaNHLP1-A-GFP (Fig. 4f). We then explored whether TaNHLP1-A protein levels could be influenced by TaSnRK2-1A. Co-expression of TaNHLP1-A-GFP and HA-TaSnRK2-1A in N. benthamiana leaf epidermal cells revealed that the TaNHLP1-A-GFP levels were significantly lower compared to control samples expressing TaNHLP1-A-GFP alone (Fig. 4g). These results indicate that TaSnRK2-1A affects the stability of TaNHLP1-A protein, possibly by influencing its phosphorylation. Therefore, we concluded that TaSnRK2-1A and TaPP2C-7A, which are the core of the ABA signaling network, antagonize each other and affect the protein stability of TaNHLP1-A, possibly through phosphorylation or dephosphorylation.
The NHLP1-RACK1 module is evolutionarily conserved in regulating tillering/branching across plant species
Phylogenetic analysis indicated that TaNHLP1-A is conserved among both monocots and eudicots (Supplementary Fig. 13 and Supplementary Data 4). In rice, three OsNHLP1 proteins have been identified: OsNHLP1a (LOC_Os02g49770), OsNHLP1b (LOC_Os06g15820), and OsNHLP1c (LOC_Os02g13950), which exhibit amino acid sequence identities of 88.37%, 76.16%, and 41.00% with TaNHLP1-A, respectively (Supplementary Fig. 14). To investigate whether OsNHLP1 also regulates tillering in rice, we generated Osnhlp1 mutants using CRISPR/Cas9-mediated gene editing in the Japonica cultivar Zhonghua 11 (ZH11) and indica cultivar Zhefu802 (ZF802). The Osnhlp1a mutants displayed a reduced tiller number compared to ZH11 (Fig. 5a, b and Supplementary Fig. 15a). In addition, both Osnhlp1a and Osnhlp1b mutants showed a significant reduction in tillering than that of ZF802 at the mature plant stage, and the double mutant Osnhlp1aOsnhlp1b exhibited impaired growth, reduced tillers, and sterility (Fig. 5c, d and Supplementary Fig. 15b). Previous research has demonstrated that both Osnhlp1c, formerly referred to FUWA35, and Osrack1a mutants repress rice tillering33. Because TaNHLP1-A was found to physically interact with TaRACK1A-A (Fig. 3a–c), we next asked whether OsNHLP1 likewise interacts with OsRACK1A in rice. As we speculated, LCI and Co-IP assays in N. benthamiana leaves confirmed that OsNHLP1a and OsNHLP1b can interact with OsRACK1A (Fig. 5e, f).
Representative phenotypes (a) and tiller number comparison (b) of ZH11 (negative control) and Osnhlp1a mutants. p = 0.196 (Osnhlp1a-1 vs. Osnhlp1a-2), <0.0001 for other comparisons. Scale bar, 10 cm in (a). Representative phenotypes c and tiller number comparison d of ZF802 (negative control) and CRISPR/Cas9-generated Osnhlp1a and Osnhlp1b mutants. p = 0.4539 (Osnhlp1a vs. Osnhlp1b), 0.0017 (Osnhlp1b vs. Osnhlp1aOsnhlp1b), <0.0001 for other comparisons. Scale bar, 10 cm in (c). e OsRACK1A interacts with OsNHLP1a and OsNHLP1b, respectively, in a split-luciferase complementation assay. Different combinations of OsNHLP1a-nLUC, OsNHLP1b-nLUC, and cLUC-OsRACK1A were co-expressed in N. benthamiana leaves, and luciferase activity was detected after 2 days of infiltration. f Co-immunoprecipitation assays showed that OsRACK1A interacts with OsNHLP1a and OsNHLP1b, respectively, in N. benthamiana. OsNHLP1a-MYC and OsNHLP1b proteins were immunoprecipitated (IP) by HA beads. The black triangle represents the target bands. Representative phenotypes g and branch numbers h of the wild-type Arabidopsis thaliana Col-0 (negative control) and Atnhlp1a mutants, assessed approximately 65 days post-germination. The quantification of branch numbers encompasses the sum of the main stem, primary stem branches, secondary rosette branches, and primary rosette leaf branches. p = 0.1343 (Atnhlp1a−6 vs. Atnhlp1a−8), <0.0001 for other comparisons. The scale bar in (g) is 5 cm. i, j AtNHLP1a interacts with AtRACK1A, AtRACK1B, and AtRACK1C, respectively, according to split-luciferase complementation assays and co-immunoprecipitation assays in N. benthamiana. Data in (b, d, and h) are presented as mean ± SD (T2 generation). Different letters indicate significant differences between groups, as determined by one-way ANOVA with Tukey’s multiple comparisons range tests (p < 0.01). These experiments in (f, i) were repeated three times independently with similar results. Individual data points are plotted, with the sample size (n) for each group is listed at the bottom of the rectangular column. Source data are provided as a Source Data file.
In Arabidopsis, three homologous proteins of TaNHLP1-A have been identified, with amino acid identities not exceeding 57.20% (Supplementary Fig. 14). Consequently, we selected AT1G70280, which shows the highest identity to TaNHLP1-A, for further investigation and designated it as AtNHLP1a. Phenotypic analysis revealed that AtNHLP1a-knockout mutants (Atnhlp1a-6 and -8), generated by CRISPR/Cas9-mediated gene editing, had fewer shoot branches compared to Col-0 (Fig. 5g, h and Supplementary Fig. 16). The Arabidopsis genome contains three distinct RACK1 genes (RACK1A, RACK1B, and RACK1C), and the rack1 mutants (rack1a rack1b and rack1a rack1c) exhibit reduced branching36. Thus, we examined whether AtNHLP1a physically interacts with AtRACK1. As expected, LCI assays conducted in N. benthamiana leaves demonstrated that AtNHLP1a can interact with AtRACK1 (Fig. 5i). To further validate this interaction, we performed Co-IP assays in N. benthamiana leaves, which showed that AtNHLP1a physically interacts with AtRACK1 (Fig. 5g). These results indicated that nhlp1 mutants in rice and Arabidopsis exhibit phenotypes akin to those observed in wheat Tanhlp1 and T. urartu tin7 mutant, suggesting that NHLP1 may have conserved functions in regulating tillering/branching across plant species.
A natural TaNHLP1-A beneficial haplotype enhances the tiller number and grain yield
To explore the natural variation of TaNHLP1-A, we examined its sequence polymorphism using whole-genome re-sequencing data from 855 common wheat cultivars/lines (Supplementary Data 5). Genomic analysis revealed that TaNHLP1-A harbors one synonymous mutation in the exon, as well as three and seven SNPs distributed across introns and a 1.5-kb promoter region, respectively (Supplementary Data 5). These tandem SNPs in the promoter region were subsequently categorized into three haplotypes (Fig. 6a). Given that only two accessions belonged to the TaNHLP1-A-Hap3 type, subsequent analyses focused exclusively on TaNHLP1-A-Hap1 and TaNHLP1-A-Hap2. RNA-seq analysis demonstrated that varieties carrying TaNHLP1-A-Hap1 exhibit significantly higher expression levels of TaNHLP1-A compared to those with TaNHLP1-A-Hap2 (Fig. 6b). Phenotypic analysis showed that wheat varieties containing the TaNHLP1-A-Hap1 had a higher number of tillers compared to those possessing the TaNHLP1-A-Hap2 across four different environments (Fig. 6c and Supplementary Data 6). Additionally, wheat varieties carrying TaNHLP1-A-Hap1 demonstrated increased grain yield per plant (Fig. 6d). Consistently, the TaNHLP1-A-overexpressing lines resulted in enhanced tiller number, with an additional 1.4 tillers per plant (Fig. 2e, f) and greater single-plant grain productivity (Fig. 6e), as averaged across two overexpression lines. Therefore, the TaNHLP1-A-Hap1 beneficial haplotype propelled tiller development and grain yield potential, similar to over-expression lines.
a The schematic presentation and distribution of tandem single nucleotide polymorphisms (SNPs) in the promoter region of the TaNHLP1-A gene. Positions of SNPs are coordinates starting from the initial ATG of the open reading frame. Comparative analysis of expression levels (b), tiller number (c) and grain yield per plant d between varieties with the TaNHLP1-A-Hap1 and TaNHLP1-A-Hap2. The expression levels in (b) are represented as transcripts per million (TPM) from RNA-seq. Data in (c) and (d) are shown as mean ± SD and obtained from four distinct environments (E1 to E4) during the 2013–2014 cropping season. Statistical significance between TaNHLP1-A-Hap1 and TaNHLP1-A-Hap2 was determined for each environment using a two-way ANOVA analysis. e Comparative grain yield per plant of the common wheat cultivar KN199 (negative control) and T2 generation transgenic wheat plants overexpressing the TaNHLP1-A gene. Significant differences were observed in grain yield per plant between negative control plants (KN199) and transgenic plants (pUbi::TaNHLP1-A), averaged across the two TaNHLP1-A-overexpression lines. The sample size (n) for each group is listed at the bottom of the figure. Data in (b) and (e) are shown as mean ± SD, with statistical significance determined by a two-tailed unpaired Student’s t test. Individual data points are plotted, with corresponding P-values indicated at the top of the panels. Geographic distributions of TaNHLP1-A haplotypes in a worldwide wheat collection, including 853 accessions f and in a Chinese wheat collection, including 532 accessions (g). The Yellow and Huai River Valleys Facultative Wheat Zone and the Northern Winter Wheat Zone in (g) are highlighted with white circles. h Comparison of the number of wheat landraces and cultivars harboring TaNHLP1-A-Hap1 (red) and TaNHLP1-A-Hap2 (blue) in Chinese wheat accessions. Source data are provided as a Source Data file.
A comprehensive analysis of haplotype variation and distribution of TaNHLP1 was conducted using a diverse collection of 576 cultivars and 279 landraces from geographically distinct regions (Supplementary Data 7). The results revealed that the TaNHLP1-A-Hap1 haplotype is widely prevalent in wheat germplasm from China, Europe, and the United States. Specifically, in China, TaNHLP1-A-Hap1 was predominantly identified in the Yellow and Huai River Valleys Facultative Wheat Zone and the Northern Winter Wheat Zone (Fig. 6g). Further geographic distribution analysis of Chinese accessions demonstrated a significantly higher frequency of the advantageous TaNHLP1-A-Hap1 haplotype in modern cultivars compared to landraces (Fig. 6h). This pattern suggests that TaNHLP1-A-Hap1 may have been subject to selective pressure during the breeding process in China.
Discussion
Tillers are specialized branches that arise from axillary buds in wheat, usually at the basal nodes of the stem and each bears a spike. Previous studies have shown that plant hormones such as SL, ABA, and GA are involved in tillering regulation24,25,37. Among them, ABA plays a key role in inhibiting tiller bud outgrowth, as evidenced in Arabidopsis26, rice29,38, and wheat19. ABA exerts its function by binding to its receptors, the PYR/PYL/RCAR proteins, which then interact with PP2Cs to release SnRK2s, thereby initiating downstream signaling pathways34. Recent studies have shown that activated SnRK2s kinases phosphorylate TILLER ENHANCER (TE), inhibiting the activity of ANAPHASE-PROMOTING COMPLEX/CYCLOSOME (APC/C)TE. The phosphorylated TE weakens its interaction with the key tillering promoter MONOCULM1 (MOC1), thereby stabilizing MOC1 and ultimately promoting tillering39. Additionally, RACK1A, a negative regulator of ABA responses, has been shown to reduce tiller and branch numbers when mutated in Arabidopsis36 and rice33. However, it remains unclear how RACK1A participates in or regulates tillering/branching through the ABA signaling pathway, or whether there is a connection between RACK1A and the core components (SnRK2s and PP2Cs) of the ABA signaling pathway.
In this study, through map-based gene cloning, we identified TuNHLP1 as the causal gene responsible for the low-tiller phenotype of the tin7 mutant. Sequencing analysis revealed a C-to-A single-nucleotide variation at position 455 from the start codon of TuNHLP1 in the tin7 mutant, leading to a single amino acid change from Thr to Lys at the 152nd amino acid of the deduced protein (Fig. 1f, g). Notably, TuNHLP1 exhibited an uneven, punctate distribution when expressed as TuNHLP1-GFP in N. benthamiana leaves (Supplementary Fig. 5a). Moreover, Tunhlp1 showed decreased accumulation compared with TuNHLP1-GFP when expressed in N. benthamiana leaves (Supplementary Fig. 5b). These results suggest that the Thr152 amino acid residue plays a critical role in controlling the stability, function, or higher-order structure of TuNHLP1. Furthermore, the ABA level was elevated in the Tanhlp1-1 mutant (Fig. 3e), and its low-tiller phenotype could be partially reversed by treatment with sodium tungstate, an inhibitor of ABA biosynthesis (Supplementary Fig. 9). Notably, both TaPP2C and TaSnRK2, core components of the ABA signaling pathway, were found to interact with TaNHLP1 and regulate its protein stability (Fig. 4a–g), indicating that TaNHLP1 is an important regulator within the ABA signaling pathway. More importantly, we discovered that TaNHLP1 interacts with TaRACK1A and recruits it from the cytoplasm to the Golgi apparatus (Fig. 3g), suggesting that TaNHLP1 may modulate TaRACK1A function by altering its subcellular localization. Additionally, TaRACK1A expression is downregulated in the Tanhlp1-1 mutant (Fig. 3f), and Tarack1a mutants exhibit abnormal ABA accumulation in tiller buds and nodes (Fig. 3j), indicating that TaNHLP1 may indirectly affect ABA levels through regulation of TaRACK1A expression (Fig. 6). Given that TaNHLP1-GFP is localized to the Golgi apparatus, which is usually involved in protein processing, transport, and secretion, we propose that TaNHLP1 may indirectly regulate TaRACK1A expression by modulating the activity or localization of an unknown transcription factor. This dual regulation of TaRACK1A by TaNHLP1 at both the protein and transcriptional levels likely reflects the fine-tuning required for precise ABA signaling.
Considering that TaNHLP1 interacts with both upstream core components of the ABA signaling pathway (TaPP2C and TaSnRK2) and TaRACK1A, and given that in Arabidopsis, ABSCISIC ACID INSENSITIVE5 (ABI5) is phosphorylated by SnRK2s kinases to activate downstream signaling and repress the expression of RACK140, we speculate that TaNHLP1 may act upstream of TaRACK1A, bridging TaSnRK2 and TaRACK1A. This hypothesis is further supported by the observation that RACK1 acts downstream of WITH NO LYSINE (K) PROTEIN KINASE 8 (WNK8) in flowering pathways in Arabidopsis, and RACK1 is phosphorylated by WNK8, whose phosphatase activity is strictly controlled by PP2C(A)41. Future genetic evidence is needed to confirm this hypothesis. Therefore, our findings highlight a sophisticated signaling pathway that provides a previously unidentified example in wheat to explain the essential role of TaSnRK2 and TaRACK1A in regulating tillering. In addition, given that ABA is a pivotal hormone orchestrating plant responses to various stress conditions, and SnRK2 and RACK1A function under abiotic stress42,43, we hypothesize that TaNHLP1 might also play a crucial role in stress responses, which merits further investigation.
In summary, this study supports a hypothetical model for TaNHLP1 in the regulation of wheat tiller number via ABA signaling (Fig. 7). In the absence of ABA, the phosphatase activity of TaPP2C inhibits TaSnRK2, resulting in minimal phosphorylation of TaNHLP1. Meanwhile, TaPP2C directly dephosphorylates TaNHLP1, maintaining a dynamic balance of the protein. Consequently, TaNHLP1 facilitates the translocation of a substantial quantity of TaRACK1A from the cytoplasm to the Golgi apparatus. This alteration in subcellular localization likely enhances the functional efficacy of TaRACK1A or enables TaRACK1A to function properly, ultimately promoting tillering. However, when ABA is present in high concentrations, ABA binds to TaPYL and TaPP2C, releasing TaPP2C’s inhibition on TaSnRK2. This results in the sustained phosphorylation of TaNHLP1 by activated TaSnRK2, while dephosphorylation of TaNHLP1 by TaPP2C is attenuated. Consequently, the stability of TaNHLP1 is compromised, leading to a reduced capacity to recruit TaRACK1A to the Golgi apparatus. Moreover, the transcriptional level of TaRACK1A is downregulated. Under this dual regulation at both the protein and transcriptional levels, the functionality of TaRACK1A is impaired, thereby suppressing tillering.
(Left) and (right) in (a) and (b) are known roles of RACK1A in the ABA response and the core ABA signaling pathway, respectively. Our identified connections between NHLP1 and the ABA signaling pathway are indicated in red. A detailed description of the model is provided in the discussion. Arrowhead, activation; perpendicular arrowhead, inhibition; line, unknown regulation; dashed line, indirect regulation.
With strict regulations on genetically modified methods, marker-assisted selection (MAS) has become a practical strategy for targeted wheat improvement44,45. Our research shows a strong correlation between specific haplotypes of TaNHLP1-A and yield-related traits. Notably, TaNHLP1-A-Hap1 increases tiller number without reducing thousand-grain weight (Fig. 6c, Supplementary Fig. 17a), unlike TaNHLP1-A-Hap2. Additionally, TaNHLP1-A-Hap1 is associated with favorable plant architecture, including reduced plant height, thicker stems, and slightly shorter leaves, without affecting leaf width or heading days (Supplementary Fig. 17b–f). These results suggest that TaNHLP1-A-Hap1 can optimize plant structure and boost grain yield, as evidenced by its positive selection in Chinese wheat breeding programs (Fig. 6h).
Additionally, overexpression of TaNHLP1-A increases both tiller number and plant height (Fig. 2e, f and Supplementary Fig. 18), which contrasts with the reduced plant height observed in TaNHLP1-A-Hap1 varieties (Fig. 6b and Supplementary Fig. 17b). This suggests a complex relationship, possibly due to other genes near the TaNHLP1-A locus (Chr6A: 525 Mb) influencing plant height. Indeed, association analysis reveals a significant height-associated peak near TaNHLP1-A, but its position varies across environments (Supplementary Fig. 19). Future research may identify height-related mutations to clarify this issue. Another possibility is that the ubiquitin promoter used for TaNHLP1-A overexpression drives expression differently from the native TaNHLP1-A promoter in TaNHLP1-A-Hap1 plants. Using the native TaNHLP1-A promoter (Hap1) for overexpression studies could help resolve these discrepancies. Overall, marker-assisted selection for TaNHLP1-A-Hap1 shows promise for improving wheat plant structure.
The TaNHLP1 protein contains two NHL domains, named after the proteins NCL-1, HT2A, and LIN-4146,47. While NHL domain-containing proteins play crucial roles in various biological processes such as cell growth, development, and immune responses in animals48,49, their functions in plants remain relatively underexplored. For instance, in rice, the NHL domain-containing protein FUWA is involved in tillering, plant height, and panicle and grain shape. Notably, homologs of FUWA from sorghum and maize can rescue the fuwa mutant phenotype, indicating a highly conserved function among grass species35. In this study, we found that loss-of-function mutations in NHLP1 across T. urartu, wheat, rice, and Arabidopsis consistently result in reduced tiller/branch numbers and plant height (Figs. 1, 2 and 5). The Osnhlp1a mutant exhibits a phenotype similar to that of the fuwa mutant, despite only 31.2% amino acid similarity between OsNHLP1a and FUWA. These findings collectively suggest that NHL domain-containing proteins have highly conserved functions across both dicotyledonous and monocotyledonous plants. Given these conserved functions, future efforts could leverage gene editing technologies to modify NHL genes in major crops, potentially improving plant architecture and enhancing yield.
Methods
Plant materials and growth conditions
A tiller inhibition mutant (M3 generation) was developed from an EMS-mutagenized population of T. urartu accession G1812 (PI 428198), which had previously been used for genome assembly50. Subsequently, we conducted backcrossing between G1812 and the mutant. Finally, a homozygous low-tillering mutant was selected from the BC2F3 population and named tin7. To create a mapping population (F2), the tin7 mutant was crossed with T. urartu accession G3146 (PI 538737), known for its loose plant type, high plant height, and robust tillering ability (about 45 tillers). The F2:3 families were produced through self-pollination of recombinant individuals selected from the F2 population and were employed for fine mapping. Both the F2 population and the F2:3 families were evaluated in Zhaoxian County (37°50′N, 114°49′E), Hebei Province, China, during the wheat growing seasons of 2017–2018 and 2018–2019, respectively. Seeds of T. urartu materials were sown in one-meter row plots with six plants per row, spaced 20 cm apart within rows and 25 cm between rows, and covered with plastic films during winter. Additionally, seedlings of T. urartu were cultivated in the greenhouse at 18 ± 2 °C with a long-day cycle (16 h light/8 h dark). Transgenic wheat experiments utilized the hexaploid wheat varieties Fielder and KN199. These transgenic plants were planted in Zhaoxian County during the 2021–2023 cropping seasons for phenotypic analysis. Each line was sown in two-row plots (1.0 m long, 0.25 m apart) at a sowing density of 11 seeds per row. Field management adhered to local wheat production practices. For association analysis, a total of 300 winter wheat accessions were collected and cultivated in double-row plots with three replications during the 2013–2014 wheat growing season in Zhaoxian County, with various fertilizer treatments applied. Detailed information on four trials is provided in Supplementary Data 6 (designated as E1-4).
In order to obtain the Osnhlp1 mutant, we performed genetic transformations using Oryza sativa subsp. japonica variety ZH11 and indica cultivar ZF802 from China, respectively. Rice plants were grown under natural conditions in Beijing (40° 16′ N, 116° 24′ E), China, from June to October. For Arabidopsis experiments, seeds from the Col-0 background were sown on half-strength MS medium and germinated at 22 °C under long-day conditions (16 h light/8 h dark) for 7 days. The 7-day-old seedlings were then transferred to soil and grown in a greenhouse under the same long-day conditions (22 °C; 16 h light/8 h dark). Additionally, N. benthamiana plants were cultivated in a greenhouse at 24 ± 2 °C with a long-day cycle (16 h light/8 h dark).
Phenotypic and genetic analysis
The tiller numbers of all materials/lines utilized in this study were recorded approximately 15–20 days before harvest, and only effective tillers were counted. For the mapping population, individuals were visually assessed and categorized into two distinct groups: mutant and wild types. Specifically, individuals with 20 or fewer tillers and reduced plant height (less than 96.0 cm) were classified as mutant types, whereas those with more than 20 tillers were categorized as wild types. The frequency distributions of tiller numbers were subsequently analyzed, and a chi-squared (χ²) test was performed using Microsoft Office Excel. For the association analysis of wheat accessions, measurements were taken from the main spike, flag leaves, and third internodes from mature plants to evaluate plant height, grain weight, leaf lenght/width, and stem diameter, respectively. Additionally, grain yield per plant and thousand grain weight were measured after drying. Data were collected from five plants per replicate, and the results were averaged across three replicates for each accession. The quantification of branch numbers in Arabidopsis experiments is the sum of the main stem, primary stem branches, secondary rosette branches, and primary rosette leaf branches. Detailed information on branch structure is provided in Supplementary Fig. 16b.
Marker development, gene mapping, and isolation
Genomic DNA was extracted from young seedlings using the cetyltrimethylammonium bromide method51. We previously designed a low-density physical map comprising 168 InDel markers covering all seven chromosomes of T. urartu, based on the polymorphic sequences of G3146 and G181213. Given that G3146 exhibits a high tillering phenotype (with approximately 45 tillers), we selected it for crossing with the tin7 mutant in this study. F1 plants from the cross between the tin7 mutant and G3146 were self-pollinated to produce an F2 population. From this F2 segregating population, plants exhibiting short stature and a compact plant architecture were selected as extreme plants (mutant type). Genotype analysis was conducted on the extreme plants using the 168 polymorphic markers between G1812 and G314613, and the data were combined with corresponding phenotype information to achieve preliminary mapping of the causal gene. New marker development and application were carried out following the methodology described previously13. Briefly, the PCR reactions were performed in a 10 μL system consisting of 5 μL of 2× Taq PCR Starmix (GenStar, China), 3 μL ddH2O, 1 μL of 80 ng/μL genomic DNA, and 0.5 μL of 10 μM each primer. To identify the genetic mutation associated with the tin7 phenotype, we cloned, sequenced, and analyzed the coding sequences, 3′-UTR regions (~500 bp), and roughly 1500 bp promoter sequences of candidate genes within the mapping interval from both G1812 and tin7 plants. The primer sequences used for gene mapping are listed in Supplementary Data 1.
Constructs and plant transformation
To knock out TaNHLP1 and TaRACK1A in wheat, conserved single guide RNA (sgRNA) target sequences were designed on the exon sequences of these genes using the CCTop-CRISPR/Cas9 target online predictor (https://cctop.cos.uni-heidelberg.de:8043/). The pMT1T2 vectors were amplified using two pairs of primers containing the sgRNAs and then cloned into the CRISPR-Cas9 vector pBUE411. All these recombinant plasmids were separately transformed into the hexaploid wheat cultivar Fielder via Agrobacterium-mediated gene transformation, following the procedure outlined previously52. In addition, to generate TaNHLP1-A-overexpressing transgenic plants, the full-length coding sequence (CDS) of TaNHLP1-A was amplified and inserted into the pWMB110 vector under the control of the maize ubiquitin promoter53. Then, this construct was transformed into immature embryos of the Chinese common wheat cultivar KN199 using the method described previously52. The transgenic plants of TaNHLP1-A were identified using the primer OE-TaNHLP1-A, and the expression level of the homozygous positive transgenic plants of generation T2 was detected by qPCR technology. To generate knockout mutants of OsNHLP1a (LOC_Os02g49770) and OsNHLP1b (LOC_Os06g15820), sgRNA target sequences were developed and separately inserted into the CRISPR/Cas9 vector pC1300-Cas9, respectively; and then transferred into japonica variety ZH11 or indica cultivar ZF802 from China using the Agrobacterium-mediated transformation system54, respectively. To generate knockout mutants of Arabidopsis AtNHLP1a (AT1G70280), a specific sgRNA sequence was designed and cloned into the pCAMBIA1300-pYAO-Cas9 vector55. The resulting plasmids were transformed into Arabidopsis Col-0 plants using the floral dip method via Agrobacterium-mediated transformation56. Specific primers were designed and used to clone the target genes, and homozygous frameshift mutations were obtained and used for phenotypic analysis. Primers used for vector construction are listed in Supplementary Data 1.
RNA isolation, RNA-sequencing, and RT-qPCR analyses
Total RNA was extracted from wheat tissues using the RNA Prep Pure Plant Kit according to the manufacturer’s instructions (Invitrogen, USA). RNA sequencing was performed on shoot bases (1 cm from the base of the bud) and tiller buds (2-2.5 cm) from common wheat Fielder and Tanhlp1-1 mutant, with each sample consisting of three independent biological replicates. The sequencing was carried out on the BGISEQ-500 platform at the Beijing Genomics Institute. High-quality reads were aligned to the wheat reference genome sequence IWGSC RefSeq v1.0 using Salmon (v1.8.0) with the parameters described previously57. Differentially expressed genes were analyzed following the method described previously58. RT-qPCR was conducted using the LightCycler 480 platform (Roche, Switzerland) with SYBR Green PCR Premix (RR820A, TaKaRa, Japan). The RT-qPCR conditions included an initial denaturation at 95 °C for 3 min, followed by 40 cycles of 30 s at 95 °C, 30 s at 58 °C, and 30 s at 72 °C, with a final extension at 72 °C for 5 min. TaACTIN1 or TaGAPDH served as the internal control. Each experiment was conducted with at least three biological replicates, and the relative expression level of the gene was calculated using the 2−ΔΔCt method. Primers used for RT-qPCR are listed in Supplementary Data 1.
Subcellular localization
The CDSs of TuNHLP1, Tunhlp1 (the T152K mutant), and TaNHLP1-A were separately amplified and cloned into the pMDC43-GFP vector to generate TuNHLP1-GFP, Tunhlp1-GFP, and TaNHLP1-A-GFP expression vectors. Different vector combinations were expressed in N. benthamiana epidermal leaf cells through Agrobacterium-mediated transient expression method59. GmMan1-mCherry was co-expressed as a cis-Golgi marker. The infiltrated plants were then grown in a greenhouse at 24 °C for 48–60 h. Fusion protein localization was visualized in leaf epidermal cells using a Zeiss LSM 710 confocal microscope. Details of the primers used are provided in Supplementary Data 1.
Western blotting
For immunoblot analysis, total proteins were extracted from N. benthamiana leaves expressing the indicated constructs. The leaves were first ground in liquid nitrogen and then homogenized in a 2× loading buffer containing 0.25 mg/mL bromophenol blue, 25% glycerol, 5% SDS, and 1 M Tris-HCl (pH 7.5). The suspensions were subsequently boiled at 90 °C for 5 min and centrifuged at 12,000 rpm for 3 min. A volume of 25 µL of the resulting supernatant was subjected to SDS-PAGE electrophoresis. The separated proteins were then transferred onto a nitrocellulose membrane for 100 min at 300 mA. The membrane was incubated with antibodies anti-GFP (1:5000; M20004S, Abmart) and anti-β-Actin (1:5000; BE0027, Basybio). Detection was performed using Super Pierce ECL Western Blotting Substrate (ab65623, Abcam) and visualized with a cooled CCD camera (Tanon-4500).
Phylogenetic analysis
The TaNHLP1-A polypeptide sequence was used as a query for BLASTP searches against the Ensembl Plants non-redundant protein database, using default parameters and a similarity threshold of 40% amino acids. Homologous protein sequences of TaNHLP1-A from various plant species were selected for phylogenetic analysis. These species included monocots such as T. urartu, T. aestivum, wild emmer, T. turgidum subsp. durum, Aegilops tauschii subsp. tauschii, Hordeum vulgare, Oryza sativa, Zea mays, Brachypodium distachyon, Setaria italica, Panicum miliaceum, and Sorghum bicolor, as well as dicots like Glycine max, Arabidopsis thaliana, and Solanum lycopersicum. A total of 54 sequences, including TaNHLP1-A, were used for the phylogenetic analysis (Supplementary Data 4). The phylogenetic tree was constructed using the neighbor-joining method with 1000 bootstrap replicates, implemented via MEGA 11.
Yeast two-hybrid assay
The split-ubiquitin Yeast Two-Hybrid (Y2H) assay was employed to identify interacting proteins of TaNHLP1 using the DUALhunter system (Dualsystems Biotech). The full-length coding sequence of TaNHLP1-A was amplified from Chinese Spring and cloned into the pBT3-SUC vector, generating the bait construct SUC-TaNHLP1-A-Cub. Library screening, plasmid isolation, and sequencing were performed by ZOON-BIO BIOTECHNOLOGY as described previously60. Briefly, for library screening, a yeast cDNA library derived from tiller buds, leaves, and young spikes of the hexaploid wheat cultivar Fielder was used. Approximately 10 μg of the yeast cDNA library plasmid was cotransformed with 5 μg SUC-TaNHLP1-A-Cub vector into the yeast strain NMY-51. Transformants were selected on synthetic dropout (SD) medium lacking tryptophan, leucine, histidine, and adenine (SD-LWHA) supplemented with 10 mM 3-amino-1,2,4-triazole (3-AT, a competitive inhibitor of the HIS3 gene product) at 30 °C. After incubation for three to 6 days, positive yeast colonies were amplified using the primers pSUC-select and sequenced. The sequences of positive yeast clones were then subjected to BLAST analysis on the National Center for Biotechnology Information platform (https://blast.ncbi.nlm.nih.gov/) to obtain functional annotations of putative interacting proteins of TaNHLP1-A. For point-to-point Y2H assays, the coding sequences of TaRACK1A-A, TaSnRK2-1A, and TaPP2C-7A were inserted into the prey vector pPR3-Nx, respectively. The indicated combinations of plasmids were cotransformed into the yeast strain NMY-51. Yeast cells were first selected on SD medium lacking Leu and Trp (SD-LW) and then transferred to SD-LWHA selective medium with 25 mM 3-AT for protein interaction analysis. Details of the primers used are provided in Supplementary Data 1.
Luciferase complementation imaging assay
The luciferase complementation assay was carried out in N. benthamiana leaves as described previously59. The CDSs of TaNHLP1-A, OsNHLP1, AtNHLP1a, TaPYL-1D, and TaPP2C-7A were separately inserted into the vector pCambia-nLuc, while the coding sequence of TaRACK1A-A, OsRACK1A (LOC_Os01g49290), AtRACK1A (AT1G18080), AtRACK1B (AT1G48630), AtRACK1C (AT3G18130), TaSnRK2-1A, and TaPP2C-7A were inserted into the vector pCambia-cLuc, respectively. These vectors were separately transformed into Agrobacterium cells (GV3101), and then the indicated combinations of nLUC and cLUC constructs were co-infiltrated into N. benthamiana leaves. After 48 h, the leaves were treated with 0.5 mM luciferin (Promega, E1605) and imaged for 5 min using a charge-coupled device imaging system (Berthold, LB985).
Co-immunoprecipitation
The co-immunoprecipitation experiments were conducted as described previously61. The full-length coding sequences of TaNHLP1-A, OsNHLP1, and AtRACK1 were individually inserted into the pCAMBIA1300-221-MYC vector, while the CDSs of TaRACK1A-A, TaSnRK2-1A, TaPP2C-7A, OsRACK1A, and AtNHLP1a were cloned into the pCAMBIA1300-221-HA vector, respectively. These vectors were separately transformed into A. tumefaciens strain GV3101 and co-expressed in N. benthamiana leaves for transient expression with indicated combinations. Total proteins were extracted using extraction buffer (BB-31245, BESTBIO), and then the lysates were incubated with anti-HA magnetic beads (M180-11, MBL BEIJING BIO) or anti-MYC magnetic beads (P2118, BEYOTIME) for 0.5 h at 4 °C with rotation. After that, the obtained immunoprecipitated samples were washed more than 5 times with a buffer (150 mM NaCl, 50 mM Tris-HCl (pH 7.4), 1 mM EDTA, 10% (v/v) glycerol, 0.5% (v/v) Triton X-100, and 1× protease inhibitor cocktail), and then boiled at 90 °C for 5 min in 50 μL 1× SDS-loading buffer. The samples were then separated by 10% SDS-PAGE and detected by anti-HA (1:5000; Ab236632, Abcam) and anti-MYC (1:5000; M20002, Abmart) antibodies, respectively. The protein signals were detected using the eECL Western Blot Kit (Ab65623, Abcam) and analyzed using the Tanon-4500 gel imaging system as per the manufacturer’s instructions. The primer sequences are listed in Supplementary Data 1.
Measurement of endogenous ABA level
For the measurement of endogenous ABA level, shoot bases (1 cm from the base of the bud) and tiller buds (2–2.5 cm) from common wheat Fielder, Tanhlp1-1 and Tarack1a#1 mutant were used, with each sample consisting of four independent biological replicates. The samples were ground to a fine powder in liquid nitrogen. Subsequently, 0.1 g of the powdered material was weighed and used for ABA determination. The extraction and quantification of endogenous ABA were carried out by Wuhan Metware Biotechnology Co., Ltd, Wuhan, China, and the detailed main parameters were as described previously62. The content of ABA was determined using the internal standard method and is expressed as ng/g FW.
Chemical treatments of wheat seedlings
Seeds of common wheat Fielder and Tanhlp1-1 mutant, and T. urartu tin7 mutant and G1812 were planted in 18 × 18 × 18 cm pots filled with vermiculite for chemical treatments. Stock solutions of ABA (Sigma-Aldrich, USA) and sodium tungstate (Sangon Biotech, CHN) were prepared in ethanol and water, respectively, and then diluted with Hoagland solution to achieve the final concentrations. An equivalent volume of ethanol or water was used as a control treatment. For ABA treatments, 7-day-old uniform seedlings were exposed to 0, 10, and 20 µM ABA solutions, with 500 mL of solution applied to each pot. Treatments were administered every 3 days. After three rounds of treatment, the seedlings were grown for an additional 10 days. The seedlings were maintained in an LED-supplemented greenhouse equipped with LX602C LED Grow Lights from Heliospectra (more information available at https://www.heliospectra.com/led-grow-lights/elixia/), under conditions of 22 h light/2 h dark (22 °C/ 17 °C, 65% relative humidity). For sodium tungstate treatments, 7-day-old uniform seedlings were treated with 0, 10, 20, and 30 µM sodium tungstate solutions, with 500 mL of solution applied to each pot. Treatments were repeated every 3 days. After two rounds of treatment, the seedlings were grown for an additional 14 days, under conditions of 16-h light/8-h dark cycle, at 24 °C/22 °C, 65% relative humidity. Subsequently, the samples were photographed with a digital camera, and the tiller number was measured.
Phosphorylation and dephosphorylation in vitro analysis
The Flag-TaSnRK2-1A and Flag-TaPYL-1D plasmids were constructed by individually cloning the coding sequences of TaSnRK2-1A and TaPYL-1D into the pETnT vector, respectively. The CDS of TaPP2C-7A was cloned into the pGEX4T-1 vector. To generate the MBP-△TaNHLP1-A plasmid, the signal peptide and transmembrane helix region in the CDS of TaNHLP1-A were removed, and the construct was then inserted into the pMALC2 vector. All plasmids were transformed into E. coli strain BL21 (DE3) for expression and purification of fusion proteins. Kinase assays were performed as previously described41. For in vitro phosphorylation assays, MBP-△TaNHLP1-A was incubated with Flag-TaSnRK2-1A or GST-TaPP2C-7A in kinase reaction buffer (1 mM DTT, 10 mM MgCl2, 25 mM Tris-HCl [pH 7.5], and 50 mM ATP) at 30 °C for 90 min. To investigate the inhibitory effect of TaPP2C-7A on TaSnRK2-1A, GST-TaPP2C-7A and Flag-TaPYL-1D were incubated on ice for 30 min with or without 15 μM (±) ABA (Sigma-Aldrich, USA), before the addition of TaSnRK2-1A and MBP-△TaNHLP1-A. After incubation, samples were supplemented with kinase reaction buffer and incubated for an additional 30 min. To verify whether TaPP2C-7A can directly dephosphorylate TaNHLP1-A, Flag-TaSnRK2-1A and MBP-△TaNHLP1-A plasmids were co-transformed into E. coli strain BL21 (DE3). The resulting cell lysates were incubated with GST-TaPP2C-7A in the protein phosphatase reaction buffer (4 mM MgCl2, 25 mM Tris-HCl, 0.02% β-mercaptoethanol, 0.1 mg/mL BSA, 0.1 mM EDTA, 0.2 mM DTT) at 30 °C for 90 min. Reactions were terminated by boiling with 5× SDS loading buffer and subjected to 10% SDS-PAGE gel electrophoresis, containing 75 μM MnCl2 and 75 μM Phos-Tag (304-93521, Wako). Proteins were detected by anti-GST (1:5000; M20007, Abmart), anti-MBP (1:5000; E8032, NEB), and anti-Flag (1:5000; M20008, Abmart) antibodies.
Haplotype analyses
To investigate the genetic variation of TaNHLP1-A, we utilized 855 published hexaploid wheat genome re-sequencing datasets63,64,65,66,67, and analyzed the genomic variant call format (VCF) file for each sample (Supplementary Data 5). These 855 hexaploid wheat samples were sourced from different countries and included 576 cultivars and 279 landraces (Supplementary Data 5). For the expression analysis of the two TaNHLP1-A haplotypes, RNA-seq data generated from 300 common wheat varieties in a single experiment under uniform conditions were used68. These 300 wheat lines were grown in a greenhouse with a 14-h light/10-h dark photoperiod (22 °C/18 °C, 65% relative humidity). Each line had three biological replicates. At the two-week seedling stage, leaf tissues from the three biological replicates were pooled for RNA extraction and sequencing, yielding 2 × 150 bp reads. The transcript abundance was quantified using the Salmon software (v1.8.0)69, which employs pseudoalignment techniques on RNA-seq reads against reference gene models, and the resulting quant.sf files were imported into R using the tximport package (v3.21) to summarize transcript-level abundances to gene-level Transcripts Per Million (TPM) values for downstream analyses. In addition, to visualize the geographic distribution of the TaNHLP1-A allele, we generated proportional symbol maps using R (version 4.3.1) with the packages ggplot2, sf, scatterpie, rnaturalearth, and ggspatial. The basemap data included global country boundaries from the rnaturalearth package, as well as shapefile vector layers for Chinese provincial boundaries and the nine-dash line. Haplotypes were visualized as pie charts based on their proportions.
Statistics and reproducibility
Quantitative results are expressed as mean ± SD from three biologically independent replicates. Between-group differences were evaluated by two-tailed unpaired Student’s t test for two conditions, one-way or two-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons. Sample sizes were empirically determined based on historical effect sizes and field standards; no a priori power analysis was performed. Experimental design incorporated random allocation and investigator blinding during intervention and outcome assessment to mitigate allocation and ascertainment bias; no outliers were excluded.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data in this study are available in the article or in the Supplementary Information. The RNA-seq data generated from the current study have been deposited in the Genome Sequence Archive in the National Genomics Data Center, China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences database under accession number CRA026051. Source data are provided with this paper.
References
FAOSTAT. Worldwide cereal production comparisons. Food and Agriculture Organization of the United Nations. https://www.fao.org (2021).
Shewry, P. R. & Hey, S. J. The contribution of wheat to human diet and health. Food Energy Secur. 4, 178–202 (2015).
Xu, T. et al. Characterization of a common wheat (Triticum aestivum L.) high-tillering dwarf mutant. Theor. Appl. Genet. 130, 483–494 (2017).
Wang, Z. et al. Comprehensive transcriptomics, proteomics, and metabolomics analyses of the mechanisms regulating tiller production in low-tillering wheat. Theor. Appl. Genet. 132, 2181–2193 (2019).
Naruoka, Y. et al. Identification of quantitative trait loci for productive tiller number and its relationship to agronomic traits in spring wheat. Theor. Appl. Genet. 123, 1043–1053 (2011).
Kuraparthy, V., Sood, S., Dhaliwal, H. S., Chhuneja, P. & Gill, B. S. Identification and mapping of a tiller inhibition gene (tin3) in wheat. Theor. Appl. Genet. 114, 285–294 (2007).
Kebrom, T. H. et al. Inhibition of tiller bud outgrowth in the tin mutant of wheat is associated with precocious internode development. Plant Physiol. 160, 308–318 (2012).
Spielmeyer, W. & Richards, R. A. Comparative mapping of wheat chromosome 1AS which contains the tiller inhibition gene (tin) with rice chromosome 5S. Theor. Appl. Genet. 109, 1303–1310 (2004).
Peng, Z., Yen, C. & Yang, J. L. Genetic control of oligo-culms in common wheat. Wheat Inf. Serv. 26, 19–24 (1998).
Zhang, J. et al. Genetic mapping of a fertile tiller inhibition gene, ftin, in wheat. Mol. Breed. 31, 441–449 (2013).
An, J. et al. The miRNA-mRNA networks involving abnormal energy and hormone metabolisms restrict tillering in a wheat mutant dmc. Int. J. Mol. Sci. 20, https://doi.org/10.3390/ijms20184586 (2019).
Wang, Z. et al. Fine mapping of the tiller inhibition gene TIN4 contributing to ideal plant architecture in common wheat. Theor. Appl. Genet. 135, 527–535 (2022).
Si, Y. et al. Fine mapping of the tiller inhibition gene TIN5 in Triticum urartu. Theor. Appl. Genet. 135, 2665–2673 (2022).
Liu, J. et al. A novel, major, and validated QTL for the effective tiller number located on chromosome arm 1BL in bread wheat. Plant Mol. Biol. 104, 173–185 (2020).
Moeller, C., Evers, J. B. & Rebetzke, G. Canopy architectural and physiological characterization of near-isogenic wheat lines differing in the tiller inhibition gene tin. Front. Plant Sci. 5, 617 (2014).
Nasseer, A. M., Martin, J. M. & Heo, H. Y. Impact of a quantitative trait locus for tiller number on plasticity of agronomic traits in spring wheat. Crop Sci. 56, 595–602 (2016).
Ren, T. et al. Utilization of a Wheat55K SNP array for mapping of major QTL for temporal expression of the tiller number. Front. Plant. Sci. 9, 333 (2018).
Hyles, J. et al. Repeat-length variation in a wheat cellulose synthase-like gene is associated with altered tiller number and stem cell wall composition. J. Exp. Bot. 68, 1519–1529 (2017).
Dong, C. et al. Tiller Number1 encodes an ankyrin repeat protein that controls tillering in bread wheat. Nat. Commun. 14, 3523 (2023).
Zhao, B. et al. TaD27-B gene controls the tiller number in hexaploid wheat. Plant Biotechnol. J. 18, 513–525 (2019).
Liu, J., Cheng, X., Liu, P. & Sun, J. miR156-targeted SBP-box transcription factors interact with DWARF53 to regulate Teosinte Branched1 and Barren Stalk1 expression in bread wheat. Plant Physiol. 174, 1931–1948 (2017).
Yao, F. Q. et al. Down-expression of TaPIN1s increases the tiller number and grain yield in wheat. BMC Plant Biol. 21, 443 (2021).
Zhang, L. et al. PIL transcription factors directly interact with SPLs and repress tillering/branching in plants. N. Phytol. 233, 1414–1425 (2022).
Guo, W., Chen, L., Herrera-Estrella, L., Cao, D. & Tran, L. P. Altering plant architecture to improve performance and resistance. Trends Plant Sci. 25, 1154–1170 (2020).
Wang, B., Smith, S. M. & Li, J. Genetic regulation of shoot architecture. Annu. Rev. Plant Biol. 69, 437–468 (2018).
Yao, C. & Finlayson, S. A. Abscisic acid is a general negative regulator of Arabidopsis axillary bud growth. Plant Physiol. 169, 611–626 (2015).
Zhan, J. et al. The miR164-GhCUC2-GhBRC1 module regulates plant architecture through abscisic acid in cotton. Plant Biotechnol. J. 19, 1839–1851 (2021).
Chen, K. et al. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 62, 25–54 (2020).
Liu, X. et al. ζ-Carotene isomerase suppresses tillering in rice through the coordinated biosynthesis of strigolactone and abscisic acid. Mol. Plant 13, 1784–1801 (2020).
Bai, J. et al. Strigolactone and abscisic acid synthesis and signaling pathways are enhanced in the wheat oligo-tillering mutant ot1. Mol. Breed. 44, 12 (2024).
Denver, J. B. & Ullah, H. miR393s regulate salt stress response pathway in Arabidopsis thaliana through scaffold protein RACK1A mediated ABA signaling pathways. Plant Signal. Behav. 14, 1600394 (2019).
Zhang, D. P. et al. OsRACK1 is involved in abscisic acid- and H2O2-mediated signaling to regulate seed germination in rice (Oryza sativa, L.). PLOS One 9, e97120 (2014).
Chen, K. et al. Translational regulation of plant response to high temperature by a dual-function tRNAhis guanylyltransferase in rice. Mol. Plant 12, 1123–1142 (2019).
Soon, F.-F. et al. Molecular mimicry regulates ABA signaling by SnRK2 kinases and PP2C phosphatases. Science 335, 85–88 (2012).
Chen, J. et al. An evolutionarily conserved gene, FUWA, plays a role in determining panicle architecture, grain shape and grain weight in rice. Plant J. 83, 427–438 (2015).
Wang, W. et al. Integration of RACK1 and ethylene signaling regulates plant growth and development in Arabidopsis. Plant Sci. 280, 31–40 (2019).
González-Grandío, E. et al. Abscisic acid signaling is controlled by a BRANCHED1/HD-ZIP I cascade in Arabidopsis axillary buds. Proc. Natl. Acad. Sci. USA 114, E245–E254 (2017).
Abuauf, H. et al. The Arabidopsis DWARF27 gene encodes an all-trans-/9-cis-β-carotene isomerase and is induced by auxin, abscisic acid and phosphate deficiency. Plant Sci. 277, 33–42 (2018).
Lin, Q. et al. The APC/CTE E3 ubiquitin ligase complex mediates the antagonistic regulation of root growth and tillering by ABA and GA. Plant Cell 32, 1973–1987 (2020).
Li, Z., Zhang, D., Liang, X. & Liang, J. Receptor for Activated C Kinase 1 counteracts ABSCISIC ACID INSENSITIVE5-mediated inhibition of seed germination and post-germinative growth in Arabidopsis. J. Exp. Bot. 75, 3932–3945 (2024).
Waadt, R. et al. Modulation of ABA responses by the protein kinase WNK8. FEBS Lett. 593, 339–351 (2019).
Li, G. et al. Overproduction of OsRACK1A, an effector-targeted scaffold protein promoting OsRBOHB-mediated ROS production, confers rice floral resistance to false smut disease without yield penalty. Mol. Plant 15, 1790–1806 (2022).
Belda-Palazón, B. et al. A dual function of SnRK2 kinases in the regulation of SnRK1 and plant growth. Nat. Plants 6, 1345–1353 (2020).
Niu, Z. et al. Targeted introgression of a wheat stem rust resistance gene by DNA marker-assisted chromosome engineering. Genetics 187, 1011–1021 (2011).
Gordeeva, E. et al. The strategy for marker-assisted breeding of anthocyanin-rich spring bread wheat (Triticum aestivum L.) cultivars in Western Siberia. Agronomy 10, 1603 (2020).
Wulczyn, F. G., Cuevas, E., Franzoni, E. & Rybak, A. MiRNA need a TRIM regulation of miRNA activity by Trim-NHL proteins. Adv. Exp. Med. Biol. 700, 85–105 (2010).
Tocchini, C. & Ciosk, R. TRIM-NHL proteins in development and disease. Semin. Cell Dev. Biol. 47-48, 52–59 (2015).
Loedige, I. et al. The crystal structure of the NHL domain in complex with RNA reveals the molecular basis of Drosophila brain-tumor-mediated gene regulation. Cell Rep. 13, 1206–1220 (2015).
Nishi, K. et al. ROS-induced cleavage of NHLRC2 by caspase-8 leads to apoptotic cell death in the HCT116 human colon cancer cell line. Cell Death. Dis. 8, 3218 (2017).
Ling, H.-Q. et al. Genome sequence of the progenitor of wheat A subgenome Triticum urartu. Nature 557, 424–428 (2018).
Allen, G. C., Flores-Vergara, M. A., Krasnyanski, S., Kumar, S. & Thompson, W. F. A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nat. Protoc. 1, 2320–2325 (2006).
Shan, Q., Wang, Y., Li, J. & Gao, C. Genome editing in rice and wheat using the CRISPR/Cas system. Nat. Protoc. 9, 2395–2410 (2014).
Christensen, A. H. & Quail, P. H. Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res. 5, 213–218 (1996).
Hiei, Y., Ohta, S., Komari, T. & Kumashiro, T. Efficient transformation of rice (Oryza Sativa L.) mediated by Agrobacterium and sequence-analysis of the boundaries of the T-DNA. Plant J. 6, 271–282 (1994).
Yan, L. et al. High-efficiency genome editing in Arabidopsis using YAO promoter-driven CRISPR/Cas9 system. Mol. Plant 8, 1820–1823 (2015).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Patro, R., Duggal, G., Love, M. I., Irizarry, R. A. & Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419 (2017).
Wang, L., Feng, Z., Wang, X., Wang, X. & Zhang, X. DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 26, 136–138 (2010).
Yuan, H. et al. The powdery mildew effector CSEP0027 interacts with barley catalase to regulate host immunity. Front. Plant Sci. 12, 733237 (2021).
Xu, Y. et al. OsCNGC13 promotes seed-setting rate by facilitating pollen tube growth in stylar tissues. PLOS Genet. 13, e1006906 (2017).
Cui, Y. et al. Four IVa bHLH transcription factors are novel interactors of FIT and mediate JA inhibition of iron uptake in Arabidopsis. Mol. Plant 11, 1166–1183 (2018).
He, Y. et al. UDP-glucosyltransferase OsUGT75A promotes submergence tolerance during rice seed germination. Nat. Commun. 14, 2296 (2023).
Niu, J. et al. Whole-genome sequencing of diverse wheat accessions uncovers genetic changes during modern breeding in China and the United States. Plant Cell 35, 4199–4216 (2023).
Hao, C. et al. Resequencing of 145 landmark cultivars reveals asymmetric sub-genome selection and strong founder genotype effects on wheat breeding in China. Mol. Plant 13, 1733–1751 (2020).
Zhou, Y. et al. Triticum population sequencing provides insights into wheat adaptation. Nat. Genet. 52, 1412–1422 (2020).
Ma, S. et al. A comprehensive map of DNA-segment copy number variation in 491 genomes of common wheat uncovers genes associated with multiple agronomic traits. Plant Commun. 6, 101226 (2025).
Wu, J. et al. Genomics-driven discovery of superior alleles and genes for yellow rust resistance in wheat. Nat. Genet. https://doi.org/10.1038/s41588-025-02259-2 (2025).
Zhang, Z. et al. Population-scale gene expression analysis reveals the contribution of expression diversity to the modern wheat improvement. Preprint at bioRxiv https://doi.org/10.1101/2025.02.18.638840 (2025).
Patro, R. et al. Salmon provides fast and bias-aware1105 quantification of transcript expression. Nat. Methods 14, 417–419 (2017).
Acknowledgements
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA24010104 to H.-Q.L.), the National Natural Science Foundation of China (Grant No. 31971877 to S.S.Z., 31991211 to H.-Q.L.), the Major Basic Research Program of Shandong Natural Science Foundation (ZR2019ZD15 to H.-Q.L.) and the China Postdoctoral Science Foundation (2022M710163 to Y.Q.S.).
Author information
Authors and Affiliations
Contributions
H.-Q.L., Y.S., S.Z., and F.W. conceived and designed the study. Y.S. performed most of the experiments and drafted the manuscript. Y.S., S.T., Q.L., J.N., Q.S., Z.Z., T.D., H.W., and J.L. conducted fieldwork, protein interaction experiments, and analyzed the data. S.M. and S.Z. performed bioinformatics analysis. H.W. and X.Z. provided scientific support and advice. H.-Q.L., S.Z., F.W., and S.T. revised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. (A peer review file is available).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Si, Y., Tian, S., Niu, J. et al. The TaNHLP1-TaRACK1A module regulates tillering via abscisic acid signaling in wheat. Nat Commun 16, 7336 (2025). https://doi.org/10.1038/s41467-025-62654-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-62654-5
This article is cited by
-
AtGADD45A forms a complex with the scaffold protein AtRACK1A in response to DNA damage
Plant Cell Reports (2025)