Abstract
Entamoeba histolytica, an anaerobic protozoan parasite, is the causative agent of amoebiasis, bloody diarrhea, and liver abscesses in humans. Amoebiasis is more predominant in tropical areas with poor sanitation conditions, and it remains the fourth leading cause of death due to a protozoan infection. E. histolytica life cycle spans between an infective ‘cyst stage’ and an active disease-causing ‘trophozoite stage’. We have isolated ribosomes from the trophozoite stage of E. histolytica. Here, we report single particle cryo-EM structures of the 53S ribosome large subunit (LSU), and 75S associated ribosomes, with P-tRNA, A/P and P/E tRNAs, and with paromomycin antibiotic, at 2.8 Å to 3.4 Å resolution. The E. histolytica possesses a reduced ribosome with a conserved core, and the periphery evolved with species-specific unique features. The most notable features are the presence of the rRNA triple helix near the peptide exit tunnel, the expansion segment H88ES102 near the exit site on LSU, and unique insertions in r-proteins. Furthermore, the 75S ribosome paromomycin complex structure provides the atomic details of its interactions. These structures provide insights into the evolutionary adaptation of the E. histolytica translational machinery and may be explored further for amoebicidal therapeutic intervention.
Similar content being viewed by others
Introduction
Entamoeba histolytica, as its name suggests (histo-lytic = tissue destroyer), is an anaerobic parasite responsible for intestinal infection that results in bloody diarrhea, known as invasive amoebiasis or invasive colitis1. Occasionally, the parasite reaches into the bloodstream and spreads through the body, most frequently ending up in the liver, where it causes liver abscesses. Rarely, it reaches the lung, brain, and spleen and causes abscesses, which can be fatal if untreated1,2,3. E. histolytica infects ~35–50 million people worldwide and roughly kills 55,000 people each year (https://www.cdc.gov/dpdx/amebiasis/index.html)4. The life cycle of E. histolytica spans the active disease-causing “trophozoite stage,” which exists only in the host, and the infective “cyst stage,” which survives outside the host. Infection occurs mainly via ingesting mature cyst from fecally contaminated food, water, or hands4.
Earlier studies showed that ribosomes from Entamoeba invadens, a non-pathogenic model organism for E. histolytica, assemble in a rod-shaped helical structure known as chromatoid bodies during the cyst stage5,6,7,8,9. In contrast, its trophozoite stage contains typical ribosomes having monosomes, ribosomal large subunit (LSU), and ribosomal small subunit (SSU)10. E. histolytica possesses a reduced ribosome as its monosome, LSU, and SSU, which sediments at 75S, 53S, and 36S, respectively (S—Svedberg unit)11 and their respective rRNA sediments at 25S, 17S, 5.8S, 5S, and 4S12. Conversely, human ribosomes monosome, LSU, and SSU sediments, at the 80S, 60S, and 40S, respectively, and possess four rRNA species 28S, 18S, 5.8S, and 5S13,14. Whereas the bacterial ribosome, monosome, LSU, and SSU sediments at the 70S, 50S, and 30S, respectively, and their rRNAs are 23S, 16S, and 5S14.
Protein synthesis in Entamoeba trophozoites was first reported by Carter et al.15. The purified ribosomes form helices of the cyst, and polysomes from growing trophozoites appeared identical16. However, the crude cyst extract was inefficient in protein synthesis, and it became efficient only after adding the soluble fractions from trophozoites16. Emetine was introduced as an amoebicidal drug that binds to the E. histolytica ribosome and inhibits protein synthesis17,18. However, within two decades, E. histolytica mutants resistant to emetine were also reported19. Currently, ribosome-targeting aminoglycoside antibiotic “paromomycin” and other drugs are used as amoebicidal20,21. The high-resolution ribosome structures from other protozoans22,23,24,25,26,27,28,29 have revealed the species-specific unique features of their architecture and protein synthesis. The atomic details for E. histolytica ribosome architecture are absent, as no structure is available.
Here, we report the single particle cryo-EM structures of E. histolytica 53S, ribosome large subunit, at 2.8 Å resolution, 75S translating ribosome with bound tRNA at P-site, 75S with bound tRNAs at A/P- and P/E-sites, and 75S with bound paromomycin antibiotic and tRNAs at A/P- and P/E-sites. All the ribosome structures are reported at 3.0–3.4 Å resolutions. These structures reveal several unique features specific to E. histolytica ribosome architecture, such as the rRNA triple helix motif, expansion segment defined as H88ES102, and unique insertion in r-proteins. Further, the structure of 75S with bound paromomycin provided an atomic illustration of its binding pocket.
Results and discussion
E. histolytica ribosome
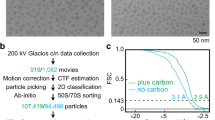
The E. histolytica ribosomes purified through sucrose density gradient centrifugation (SDGC) showed three prominent peaks (Supplementary Fig. 1a). The fractions analyzed on 1% agarose gel stained with ethidium bromide showed two prominent bands, suggesting that it contains ribosomal RNA (rRNA), most likely corresponding to the 25S and 17S rRNAs (Supplementary Fig. 1a), fractions corresponding to the peaks P1, P2, and P3 (Supplementary Fig. 1a), analyzed through negative staining electron microscopy, clearly showed that peak P1 contains irregular shape and size particles (Supplementary Fig. 1b), which might be degraded ribosomes. Peak P2 contains a ribosomal large subunit (LSU) like particle, which is 53S in Entamoeba11 (Supplementary Fig. 1c). Peak P3 contains the associated ribosome like particles (Supplementary Fig. 1d), which is 75S in Entamoeba11, and small helical segments were also visible (Supplementary Fig. 1d), which might be fragments of helical ribosomal assemblies, observed earlier by Morgan & Uzman6, Further, peak P2 sample screening in cryo-EM conditions showed a uniform ribosomal distribution with optimum ice thickness (Supplementary Fig. 1e), suggesting the samples are ideal for high-resolution cryo-EM data collection.
E. histolytica ribosomal large subunit contains a rRNA triple helix motif
For ribosomal large subunit (LSU), the single particle reconstruction performed using RELION 3.1.4 has yielded an overall 2.8 Å resolution cryo-EM map from finally selected 378,061 particles (Fig. 1a, Supplementary Fig. 2 and Supplementary Table 1). The local resolution calculated using RELION showed that the core of the ribosomal LSU has a resolution of up to 2.5 Å, whereas the surface has nearly 4.5 Å resolution (Supplementary Fig. 2). The cryo-EM map clearly shows the high-resolution features, such as resolved r-protein alpha helices and rRNA double helices (Fig. 1a, e, Supplementary Fig. 2, Supplementary Movie 1). Remarkably, we could clearly see the rRNA triple helix motif in the LSU cryo-EM map, which is located near the peptide exit tunnel (PET) (Fig. 1a). The rRNA triple helix is composed of three helices, two parallel and one antiparallel helix. The three nucleotides are on the same plane. The nucleotide bases of the middle helix that have purine nucleotide interact through Watson and Crick base pairing (–) with the nucleotide bases of one of the helices, forming a classical double helical structure. The third helix’s nucleotides interact with the double helix’s major groove through Hoogsteen base pairing (•). Therefore, it is a major groove RNA triple helix. Four such nucleotide triplets U3326–A3414•U3311, U3325–A3425•U3312, U3324–A3426•U3313, and C3323–G3417•U3314 constitutes the triple helix architecture (Fig. 1b–d, Supplementary Movie 1).
a The 2.8 Å resolution cryo-EM map of 53S ribosomal large subunit, rRNA (sky blue), r-proteins (royal blue), and third helix of rRNA triple helix (purple) are shown. The red circle shows the boundary of the triple helix. Central perturbance (CP), large subunit (LSU), and peptide exit tunnel (PET) are labeled. A dotted circle that is 50% transparent shows the location of PET in LSU. b, c rRNA triple helix, Watson and Crick pairing helices (sky blue), and third helix interacting to the major groove (purple), backbone in ribbon, and nucleotides in filled atoms are shown, and 5′ and 3′end are labeled. b, c are related by a rotation of 45° along the Y-axis. d A 2D diagram for the triple helix color code and labeling is the same as for (b, c). The − represents Watson and Crick base-paired nucleotide, the square box flanked by line represents Hoogsteen base-paired nucleotides, and circle flanked by a line represents nucleotides in close proximity. An animation is shown in Supplementary Movie 1. e Four base triples that constitute the triple helix are shown in the sticks, and cryo-EM density is shown on the surface with 85% transparency.
The overall architecture of the E. histolytica 75S ribosome
Some of the high-resolution features similar to the large subunit cryo-EM map (Fig. 1) were also visible in the 75S ribosome cryo-EM map (Figs. 2 and 3 and Supplementary Figs. 3–5). The 75S ribosome showed the presence of mRNA, nascent peptide, and bound tRNAs at P-site in one class and A/P and P/E-sites in another class (Figs. 2 and 3, and Supplementary Figs. 3–5, Supplementary Movies 2 and 3), suggesting that these translation factors were co-purified during ribosome purification and E. histolytica ribosomes were in an active translating state.
a, b Composite cryo-EM maps of the 75S P-tRNA ribosome reconstructed from the focused 3D refinement of LSU, SSU body, and SSU head. LSU rRNA (light blue) and SSU rRNA (light yellow), r-proteins (different colors), P-tRNA (red), and mRNA (purple) are shown. a, b are related by ~180° rotation along the Y-axis and 35° rotation along the Z-axis. c, d The cryo-EM maps of a, b are replaced by the atomic coordinates. e The composite map of the 75S ribosome with P-tRNA is sliced through the peptide exit tunnel, NP-nascent peptide (green), mRNA (purple), and P-tRNA (red) are shown. f Only the mRNA, P-tRNA, and NP of the 75S ribosome are shown; the second residue from the CCA end of the P-tRNA, which looks like Arginine, is also shown. An animation is provided in Supplementary Movie 2.
a Composite cryo-EM maps of 75S ribosome with A/P- and P/E-tRNAs and mRNA. LSU (sky blue) and SSU (khaki) with 80% transparency, A/P-tRNA (salmon), P/E-tRNA (orange), and mRNA (purple) are shown. b Only the translational factors with coordinates and cryo-EM map with 70% transparency are shown. c A comparison of A/P- and P/E-tRNAs from this study with A-, P-, and E-tRNAs from Giardia intestinalis (PDB ID 8G4S) is shown. An animation is provided in Supplementary Movie 3.
The LSU cryo-EM map at 2.8 Å resolution and 75S ribosome bound with P-tRNA focused refinement maps at 3.0–3.4 Å resolution were used for initial model building. The cryo-EM maps of 75S clearly showed two extra densities, one adjacent to H88 and another at the 3′ end of 25S rRNA after modeling the annotated 25S rRNA sequence 3459 nucleotides (nt) long in the NCBI database (GenBank: BDEQ01000001.1). We could unambiguously build nearly 70 nt in H88 and 44 extra nt at the 3′ end (Supplementary Fig. 6). The extra nt in H88 appears to be an E. histolytica-specific expansion segment situated near the L1 stalk and E-site of the LSU, and therefore, we named it H88ES102 (Figs. 2 and 4, Supplementary Fig. 7). We have reannotated the length of 25S rRNA to 3504 nt long after building 44 extra nt at the 3′ end.
a 2D representation of the 25S, 5.8S and 5S rRNAs colored by domains. D0 (Sandy brown), DI (purple dark), DII (blue), DIII (purple light), DIV (yellow), DV (pink), DVI (green), 5.8S (sienna), and 5S (light green) are shown and labeled with a Roman number. The rRNA that could not be modeled is shown in gray. Secondary structures were drawn using the R2DT tool from RNA Central and Adobe Illustrator, and human templates were obtained for comparison from http://apollo.chemistry.gatech.edu/RibosomeGallery/index.html. A detailed 2D diagram for 25S is provided in Supplementary Fig. 7, and rRNA nt building details are provided in Supplementary Table 2. b, c Comparative analysis of E. histolytica 25S rRNA with the human 28S rRNA (PDB ID: 4UG013). Conserved regions are shown in a color similar to that of (a). The rRNA helices present in humans, which are either missing or disordered in E. histolytica, are shown in dark gray. The triple helix and H88ES102 are also labeled. A comparison of individual E. histolytica 25S rRNA domains with the human 28S rRNA domains (gray) (PDB ID: 4UG013) is provided in Supplementary Fig. 9a.
Approximately 90.26% of 25S nt (3163 nt out of 3504 nt), 92.9% of 5.8S nt (144 nt out of 155 nt), and 100% 5S nt (117 nt) of LSU rRNAs were modeled in the cryo-EM map (Fig. 4, Supplementary Fig. 7 and Supplementary Table 2). Similarly, 74.06% of 17S nt (1442 nt out of 1947 nt) was modeled for SSU rRNA in cryo-EM map (Fig. 5, Supplementary Fig. 8 and Supplementary Table 3). Overall, E. histolytica rRNA retains a conserved core and an altered surface (Figs. 4b, c and 5b, c, Supplementary Fig. 9 and Supplementary Tables 2 and 3). The cryo-EM maps did not show any extra density that could be attributed to the 4S rRNA, as observed previously12; maybe its binding is highly dynamic.
a 2D representation of the 17S rRNAs colored by domains. 5′ (blue), C (brown), 3′M (pink), 3′m (green). The rRNA that could not be modeled is shown in gray. The secondary structures were drawn using the R2DT tool from RNA Central and Adobe Illustrator, and human templates for comparison were obtained from the RiboVision suite (http://apollo.chemistry.gatech.edu/RiboVision). A detailed 2D diagram for 25S is provided in Supplementary Fig. 8, and statistics in Supplementary Table 3. b, c Comparisons of E. histolytica 17S rRNA with the human 18S rRNA (PDB ID: 4UG013). Regions either missing or disordered in 17S rRNA are shown in dark gray; other color schemes are similar to (a). A detailed comparison of individual domains is provided in Supplementary Fig. 9b.
We were able to build 40 LSU and 31 SSU r-proteins using the focused refinement cryo-EM maps for LSU, SSU body, and SSU head of 75S ribosome with bound P-tRNA and LSU map of individual subunit (Fig. 2, Supplementary Figs. 10–13, Supplementary Tables 2 and 3). High-resolution features in our cryo-EM maps allow us to assign the correct isoform for r-protein (Supplementary Figs. 10–12) and to build r-protein extensions/insertions (Supplementary Fig. 13). There were discrepancies in the annotations of ribosomal protein in the whole genome sequence available at NCBI with GENBANK ID: BDEQ01000001.1 (https://www.ncbi.nlm.nih.gov/nuccore/BDEQ01000001.1). Such as protein eL13 has six protein IDs, and uL13 has one protein ID. Sequence alignment showed that four IDs annotated to eL13, out of the six IDs, show similarity to the uL13 r-protein. Thus, these IDs were misannotated as eL13 (Supplementary Fig. 10). The genome database does not annotate any protein ID for eL22 and eL29. However, after a sequence search, human eL22 and eL29 showed significant sequence identity with E. histolytica protein IDs EHI_141940, EHI_023220, respectively. Further, our cryo-EM maps clearly showed density that could be attributed to these two proteins (Fig. 2a–d). The eL29 is shorter (64 aa) and shares only 33% identity with its human (122 aa) counterpart. Similarly, the SSU r-protein eS8 and RACK1 were not annotated in the E. histolytica genome database. Based on sequence similarity with human counterpart and cryo-EM density in our map, we could assign the protein IDs GAT98092 and GAT91985 to eS8 and RACK1 E. histolytica r-proteins, respectively (Fig. 2a–d).
E. histolytica rRNA is reduced compared to its human counterpart
The LSU of E. histolytica consists of 25S, 5.8S, and 5S rRNAs with 3504, 155, and 117 nt in length, respectively12 (Fig. 4a, Supplementary Fig. 7, Supplementary Table 2). Its human counterpart has elaborate rRNAs, 28S, 5.8S, and 5S, with 5070, 157, and 121 nt in length, respectively (Supplementary Table 4)13. On comparing both, the overall architecture of the rRNA core is conserved, whereas the surface helices are reduced compared to their human counterpart (Fig. 4b, c, Supplementary Fig. 9a). The E. histolytica 25S rRNA can be subdivided into the domain system adopted for human 28S rRNA (http://apollo.chemistry.gatech.edu/RibosomeGallery/index.html)30,31, which comprises of conserved core termed as domain 0, along with six other domains (DI to DVI) (Supplementary Table 4).
Domain I of the 25S rRNA in E. histolytica, consisting of 1–769 nt, is shorter than the human counterpart, which is 1313 nt long (Supplementary Table 4). The difference in length is primarily due to the absence of ESs, H25ES7d, e, f, g, h, and the shortening of ESs, H25ES7a and b, which are ~96 nt long in E. histolytica as compared to ~245 nt in humans (Fig. 4, Supplementary Figs. 7 and 9a). The ES H25ES7c is shorter and adopts a different conformation than its human counterpart. The 25S rRNA H15 remains partially disordered in our cryo-EM maps, which is longer (87 nt) in E. histolytica compared to the 28S rRNA of the human ribosome (Fig. 4, Supplementary Figs. 7 and 9a).
Domain II of the 25S rRNA in E. histolytica is from 794 to 1566 nt (772 nt long), which is 257 nt shorter than human Domain II (from 1317 to 2346 nt) (Supplementary Table 4), and adopts a similar conformation. The ESs, 30ES9, 31ES9a, 45ES15, and 45ES15a are absent altogether, whereas H45, 30ES9, and H31are truncated in E. histolytica compared to human counterparts (Fig. 4, Supplementary Figs. 7 and 9a).
Domain III comprises 1587–2079 nt (492 nt in length) in E. histolytica,25S rRNA. This domain is ~33 nt longer than the domain III in human 28S rRNA, which is 459 nt in length (Supplementary Table 4). The longer size can be attributed to the longer H54ES20a, H55, and H47, whereas the H54ES20a and H55 remain partially disordered in our cryo-EM maps (Fig. 4, Supplementary Figs. 7 and 9a).
Domain IV spans from 2110 to 2408 nt (298 nt in length), which is drastically reduced in E. histolytica 25S rRNA compared to 977 nt long in its human (Supplementary Table 4). This domain is mainly composed of H63 and its expansion segment ES27. The enormous difference in size is due to the absence of 668 nt, constituting two major expansion segments 63ES27a and b (Fig. 4, Supplementary Figs. 7 and 9a). These ESs, 63ES27a and b, have been known to play a crucial role in recruiting factors at the peptide exit tunnel and ribosome assembly32,33,34, and their deletion is lethal in higher eukaryotes32.
Domain V, which harbors the peptidyl transferase center (PTC)35, spans from 2476 to 3121 nt (645 nt long) (Supplementary Table 4) and has a conserved PTC region. Although it has a similar nt number (654 nt in humans), it shows significant alterations in the L1 stalk region. The L1 stalk region is composed of H76, H77, and H78, which are disordered in our map, whereas the constituting ESs (ES30 and ES30a) are absent in E. histolytica rRNA. The r-protein, uL1, also remains disordered in our cryo-EM maps. The H79 and its ESs, ES31, ES31a, and ES31b, are shorter compared to their human counterpart (Fig. 4, Supplementary Figs. 7 and 9a). The H88 possesses a 70 nt long E. histolytica-specific ES, H88ES102 (Figs. 2 and 4, Supplementary Figs. 7 and 9a), and makes a unique interaction with the insertion segment of eL13 r-protein (Fig. 2c, d). The H88ES102 region remains disordered in our 53S LSU cryo-EM map, suggesting it gets ordered only after 75S formation. Recently, it has been suggested that the H88 plays a role in phase 2 of the 60S ribosomal subunit biogenesis, the assembly of the central protuberance (CP). H88 and the L1 stalk provide two nucleation sites for the compaction and assembly of the rRNAs H80-H87, and are involved in forming CP and 5S RNP36. Whether the E. histolytica H88 expansion H88ES102 has any role in modulating the CP assembly remains to be investigated.
Domain VI of E. histolytica comprises 3146 to 3504 nt (358 nt long), shorter than the human domain VI (497 nt long) (Supplementary Table 4). The reduction in length is mainly due to reduced H98 and H101 and their compact expansion segments, 98ES39, 98ES39a, 98ES39b, and 101ES41 (Fig. 4, Supplementary Fig. 7, 9a). The H98 adopts a different conformation in E. histolytica 25S rRNA, which leads to a unique rRNA motif formation, the triple helix rRNA motif (Fig. 4c, Supplementary Fig. 9a). The 5.8S and 5S are mostly conserved, with 155 nt and 117 nt in length, respectively. (Fig. 4a, Supplementary Fig. 7).
Similar to LSU 25S rRNA, the overall architecture of the E. histolytica SSU rRNA has a conserved core region (Fig. 5, Supplementary Figs. 8 and 9b). The peripheral helices are either missing or reduced in length compared to their human counterpart (Fig. 5b, c Supplementary Fig. 9b). The SSU rRNA is 17S12, and consists of 1947 nt. In contrast, humans have 18S rRNA, which has a length of 1869 nt (Supplementary Table 4). The 17S rRNA is also made up of four domains: 5′ domain (5′), central (C) domain, 3′ major (3′M) domain, and 3′ minor (3′m) domain (Fig. 5, Supplementary Figs. S8 and S9b). The 5′ domain spans from 1 to 602 nt, shorter than its human (658 nt long) counterpart (Supplementary Table 4), mainly because of missing expansion segments, h9es3c and b. Meanwhile, h16 and h17 remain disordered in our cryo-EM map (Fig. 5, Supplementary Figs. S8 and S9b). The 17S rRNA, C domain consists from 603 to 1162 nt (559 nt long), which is longer in E. histolytica compared to humans (534 nt long) 18S rRNA (Supplementary Table 4), mainly because of an insertion in surface helices 21es6c and 26es7, which remains disordered in our cryo-EM map. The 21es6b, which is shorter in 17S rRNA, also remains a disorder. The 3′M domain comprises 1171–1801 nt (630 nt in length) of 17S rRNA, which is exceptionally longer than humans (497 nt long) 18S rRNA (Supplementary Table 4). The longer length is mainly attributed to ESs present in h39, h40, and h41es10, which remain partially disordered in our cryo-EM map (Fig. 5, Supplementary Figs. 8 and 9b). The 3′m domain encompasses from 1802 to 1947 nt (145 nt in length) and is shorter than its human (169 nt long) counterpart (Supplementary Table 4). The shorter length is mainly because of h44, the major helix in the 3′m domain, which remains partially disordered in our cryo-EM maps (Fig. 5, Supplementary Figs. 8 and 9b). The 3′ M and 3′m are domains that constitute the decoding center on the SSU, where codon and anticodon interaction takes place during protein synthesis37, retaining a conserved core with alteration in surface helices in E. histolytica.
The ribosomal catalytic sites, the decoding center33, the peptidyl transferase center, and the polypeptide exit tunnel35 are highly conserved in E. histolytica. The tRNA binding sites, A-, P- and E-sites on both the subunits are also conserved (Figs. 2 and 3) with minor variations, particularly in the r-proteins; uS13, uS19, eS25, uL16, and eL42, which constitute the tRNA binding sites (Supplementary Fig. 14). Although the core of these proteins is conserved, the terminal end remains disordered compared to their human counterparts (Supplementary Fig. 15), even in the tRNA-bound state (Supplementary Fig. 14). Interestingly, the loop region of the uL16, which interacts with the acceptor arm of the P-tRNA adopts a slightly different conformation and remains partially disordered in both E. histolytica and humans despite having the same amino acid sequence (Supplementary Fig. 15d). C-terminus of eL42 is slightly shorter in E. histolytica than in humans (Supplementary Fig. 15e).
E. histolytica r-protein possesses species-specific insertions/extensions
E. histolytica r-protein possesses overall conserved domains compared to its human counterpart, with deletions and insertions/extensions in a few r-proteins (Supplementary Fig. 13). The uL3, uL18, eL6, eL14, eL29, eL37 and eS17 are truncated, whereas uL4, eL9, eL13 eL18 eL20, uL24, eL31, uS3, eS19, and eS21 carries E. histolytica-specific insertions/extensions (Supplementary Fig. 13). The cryo-EM density for these extensions/insertions were evident in the map (Fig. 2a–d). We could build these extensions/insertions (Supplementary Fig. 13). Intriguingly, uL24 of E. histolytica, which has a nearly ~80 amino acid long insertion, occupies the position of the human eL28 protein. The eL28 is absent in E. histolytica but present in humans (Supplementary Fig. 16a). The uL24 extension appears specific to E. histolytica as it is shorter in other eukaryotes and protozoa (Supplementary Fig. 16b). Similarly, a ~90 amino acid residue insertion in the eL13 seems species-specific (Supplementary Fig. 17a) and is absent in other eukaryotes and protozoa (Supplementary Fig. 17b).
The E. histolytica r-proteins, eL6, eS8, and uS17 loops adopt a slightly different conformation, despite sharing a conserved domain with humans (Supplementary Fig. 18). The uL10, uL11, eL28, eS31, and eS32 present in human ribosomes are absent in E. histolytica. Some Entamoeba-specific insertions/extensions in r-proteins, eS4, eS6, eS7, eS25, and eS26, could not be modeled in our cryo-EM map, maybe because of their flexible nature.
E. histolytica intersubunit bridges and conformational changes in LSU upon association
Intersubunit bridges at the interface side of the SSU and LSU27 hold the ribosomal subunits together. The bridges were analyzed using Chimera X. The residues within a 4 Å distance at the interface side of LSU and SSU were selected. The intersubunit bridges were compared with those of human and another protozoan, Giardia lamblia bridges13,27 (Fig. 6a and Supplementary Table 5). We were able to analyze 13 bonafide bridges, whereas the bridge eB13 remains disordered in the 75S ribosome cryo-EM map. The E. histolytica ribosome has retained most of the bacterial-like bridges, B1b/c, B2a, B2b, B2c, B2e, B3, B4, B5, B6, B7a, B7b/a, and B8, and also acquired a few eukaryotic-specific bridges, eB11, eB12. A notable difference was the distinct conformation of the C-terminus of uS17 (Supplementary Fig. 18a), which makes a unique interaction with eL19 r-protein near bridge eB12. This interaction appears to be E. histolytica-specific; therefore, we have named this an eB12a bridge. The eS32 protein is absent in E. histolytica; consequently, the bridge eB14 is missing. The bridge B1a, eB8, and eB13 could not be observed because the interacting residues remain disordered in our cryo-EM map.
a The ribosomal small subunit (left) and large subunit (right) are shown in light khaki and blue, respectively. The residues involved in intersubunit bridges are shown in the atomic sphere, and bridges are labeled. The bridges shown in gray color were not observed in the 75S ribosome. A detail of interacting residues is provided in Supplementary Table 5. b The LSU coordinates built using 75S cryo-EM maps (light blue) docked into the LSU Cryo-EM map (gray) are shown. The rRNA helices that become ordered upon association with the SSU are labeled.
Docking the LSU coordinates built in the 75S ribosome cryo-EM into the 2.8 Å cryo-EM map of the LSU alone, showed that the rRNA helices, mainly on the interface side, become ordered upon association with SSU (Fig. 6b). The most notable is the E. histolytica-specific surface ES H88ES102 and insertions in eL13, which become ordered upon association (Fig. 7). Other rRNA helices, H34, H43, H44, H69, H76, H79ES31b, H84, H101 also become ordered in the 75S associated ribosome. The H34, H69, and H101 lead to the formation of intersubunit bridges B4, B2a, and eB11, respectively, upon association with SSU (Fig. 6a).
The E. histolytica LSU is shown on the solvent side in the middle, and regions of rRNA and r-protein co-evolution are zoomed and shown in the surrounding area in roughly matching orientation. The rRNA (blue) is shown in ribbon and surface view with 80% transparency. The r-proteins in the ribbon (light blue) and extension/insertion (red) are shown. Central perturbance (CP) and peptide exit tunnel (PET) are labeled. A circle that is 50% transparent shows the location of PET in LSU. A full 3D model of r-protein with extensions is provided in Supplementary Fig. 13.
E. histolytica rRNA and r-protein have co-evolved
E. histolytica ribosomes have evolved with a conserved core and alterations mainly on the solvent-exposed surface. The co-evolution of r-protein and rRNA is a common feature observed earlier in the structure of eukaryotic ribosomes13,22,38,39. The r-proteins, such as uL4, uL9, eL13, eL18, eL20, uL24, and eL31, possess prominent E. histolytica-specific extensions/insertions in their sequences (Supplementary Fig. 13). These are mainly localized on the solvent side surface of the LSU (Fig. 7). On the other hand, the LSU 25S rRNA is compact and reduced in size compared to human 28S rRNA. The small size of 25S rRNA is mainly because some eukaryotic-specific expansion segments are missing (Fig. 4b, c, Supplementary Fig. 9a). To stabilize the reduced size of rRNA, which has opted a different conformation for surface helices, E. histolytica has evolved with extensions, which contain mostly positively charged amino acid residues and mainly adopts α helical secondary structure (Fig. 7). The rRNA helices sandwich these α helices, which are localized in the vicinity of the ribosomal surface (Fig. 7). For example, uL4 extension sandwich between H25ES7b and H25ES7c, eL9 insertion interacts with H42. Most intriguingly, the eL13 extension is sandwiched between H18ES43 and H25ES7a, with the E. histolytica-specific helix H88ES102. Similarly, the uL24 extension sandwich between H45 and H46, and eL31 interacts with the H94, H99 and H98ES39b, which constitutes the rRNA triple helix, an E. histolytica-specific rRNA motif (Fig. 7). In summary, it suggests that the E. histolytica LSU rRNA helices and r-proteins have co-evolved to provide a compact and stable architecture, which might be crucial for its survival.
E. histolytica possesses a triple helix motif in its 25S rRNA
RNA triple helix, naturally found in biological machineries, plays a vital role and provides a catalytic scaffold, structural stability, metal binding site, etc.40,41. RNA triple helix is found in telomerase, an RNP enzyme that maintains the chromosome end, where RNA triple helix is crucial for its catalytic activity42. Spliceosome and Group II introns RNA triple helix coordinate the catalytic Mg2+ ions during splicing43,44. In riboswitches, the RNA triple helix provides a ligand-binding scaffold45. It is also reported in humans that metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), which is endowed with a blunt end triple helix (no 3′overhang), inhibits the rapid phase of nuclear RNA decay and also upregulates translation46. Kaposi’s sarcoma-associated herpesvirus (KSHV) adopts a triple RNA helix motif in lytic phage and protects its genome from decay47. However, there are no reports of rRNA adopting a triple helical conformation.
Interestingly, we found a distinctly visible cryo-EM density for a triple helix in the E. histolytica LSU map (Figs. 1a–e and 4, Supplementary Fig. 7, 9a, Supplementary Movie 1). To the best of our knowledge, this is the first report of an RNA triple helix present in ribosomal RNA. The triple helix is present in DVI, towards the 3′ end of 25S rRNA, on the surface, solvent side, of the LSU near the PET (Figs. 4c and 8, Supplementary Figs. 7 and 9a). Compared with the human 28S rRNA, we observed that E. histolytica 3′ is shorter by 16 nt compared with its human counterpart (Fig. 8). The human 28S rRNA 3′ position overlaps with the E. histolytica triple helix position on 25S rRNA (Figs. 4c and 8, Supplementary Fig. 9a). The other eukaryotes, Saccharomyces cerevisiae and protozoans, Plasmodium falciparum, Leishmania donovani, and Giardia lamblia, also possess an extended 3′ end, and overall, a conserved architecture of domain VI to that of human rRNA domain VI (Supplementary Fig. 19).
a 25S rRNA domain VI from E. histolytica (green) and the triple helix motif (sky blue and magenta). b The 28S rRNA, domain VI from human (PDB ID: 4UG013) (gray), and the extra 3′ rRNA nucleotide (black) are shown, and major helices are labeled. A comparative analysis of Domain VI is provided in Supplementary Fig. 18.
As the 3′ end is a target site for 3′ to 5′ exonucleases, it is speculative to say that E. histolytica has remodeled its 25S rRNA by shortening its length and adopting a different conformation, the triple helix, to protect its rRNA from human 3′ to 5′ exonucleases. It will most likely be similar to what was reported earlier for KSHV47. However, it needs experimental evidence.
Cryo-EM structure of E. histolytica 75S ribosome bound with paromomycin
The cryo-EM single particle reconstruction yielded a final 3.2 Å resolution map after performing focused refinement of the SSU body (Supplementary Fig. 5). We could clearly see the cryo-EM density that could be attributed to paromomycin (PAR), an aminoglycoside (AG) antibiotic (Fig. 9 and Supplementary Fig. 5). The PAR binds to a conserved binding pocket, the major groove of h44 in the SSU body (Fig. 9b–d), as earlier reported in E. coli48 and Leishmania donovani24 (Fig. 10). PAR binding flips out the universally conserved residues A1902 and A1903 (A1492 and A1493 in E. coli numbering) (Figs. 9c, e and 10), which otherwise are flipped out only when there is a cognate codon-anticodon interaction at A-site of the decoding center. In normal situations, the flipped-out conformation of A1902 and A1903 (A1492 and A1493 in E. coli numbering) stabilizes the cognate tRNA at the A-site and facilitates the correct tRNA selection49. In PAR-bound state, these two nucleotides, A1902 and A1903 (A1492 and A1493 in E. coli numbering), remain flipped out permanently, thus facilitating the selection of both cognate and non-cognate (incorrect) tRNA at the A-site in the decoding center, leading to error-prone translation49. The PAR binding pocket is highly conserved, where ring 1 of PAR is involved in stacking interactions with G1901 (G1491 in E. coli numbering) and hydrogen bonds with G1901 and A1902. The ring II of PAR makes three hydrogen bonds with A1903, G1904, and U1905 (A1493, G1494, and U1495 in E. coli numbering), whereas ring III of PAR makes a single hydrogen bond with C1810 (G1407 in E. coli). The ring IV of PAR also makes a single hydrogen bond with G1808 (G1399 in E. coli numbering) (Figs. 9e, f and 10). The H69 of LSU and uS12 protein further stabilize the PAR binding pocket. The rRNA nucleotide modifications within the PAR binding pocket have been shown to play a role in stabilizing PAR binding24. However, we did not model any modifications in our cryo-EM map; thus, these observations could not be made. The PAR binding pocket is highly conserved in E. histolytica, L. donovani, and E. coli. In contrast, the corresponding pocket in humans is similar with a few notable differences (Fig. 10). The base stacking interaction between ring I of PAR with G1901 (G1491 in E. coli) appears to be the major stabilizing interaction, where G1901 makes Watson Crick base pair with C1812 (C1409 in E. coli) and provides a stable platform for base stacking interaction with ring I of PAR. Whereas in humans, the corresponding residue A1823 adopts a slightly different conformation and does not base pair with the opposite strand nucleotide, which is C1710 (Fig. 10). Therefore, in humans, the base stacking interaction would be disfavored upon PAR binding.
a Cryo-EM maps of 3D focus refinement of SSU body (olive), a thumbnail with 75S cryo-EM map in transparent on the left is shown. b The map of the (a) rotated by ~90° along the Y-axis with 70 % transparency, and atomic models of SSU body (olive), mRNA (red), anticodon stem-loop of A/P-tRNA (salmon), P/E-tRNA (orange), and paromomycin cryo-EM density (magenta) are shown. c A zoomed paromomycin binding pocket with transparent cryo-EM map is shown. d Paromomycin coordinates fitted in cryo-EM density (transparent magenta) are shown. e Paromomycin binding pocket residues within 4 Å distance are shown. f A 2D diagram of the paromomycin binding pocket residues within 4 Å is shown.
(Top panel) Paromomycin (purple) bound to the h44 binding pocket in ribosomes from E. histolytica (from this study), L. donovani (PDB ID: 6AZ1), and E. coli (PDB ID: 7K00) are shown. A corresponding region in humans (PDB ID: 8g61) is also shown in the left panel. (Bottom panel) A 2D diagram of helix 44 paromomycin binding pocket. Universally conserved residues A1492 and A1493 (E. coli numbering) are shown with a green background. Other crucial residues for paromomycin are shown with blue and red backgrounds.
In summary, the E. histolytica ribosomes isolated from the disease-causing trophozoite stage using sucrose density gradient centrifugation showed prominent peaks corresponding to the 75S ribosome and its 53S large subunit. Single particle reconstruction and model building have elucidated the atomic details of the 53S and 7SS ribosomes with tRNAs, mRNA, and nascent peptide and revealed E. histolytica-specific features such as triple helix motif, H88ES102, and insertion/extensions in r-proteins. The E. histolytica retains a conserved core with modifications in solvent-exposed surface. E. histolytica might have evolved with a triple RNA helix motif to protect its ribosome from host RNase attack. The cryo-EM structure of 75S ribosome in complex with paromomycin antibiotic has illustrated the atomic details of its binding pocket in E. histolytica. This study opens a new avenue to investigate protein synthesis in E. histolytica, which is vaguely understood, and to exploit the unique species-specific features of its translation machinery to design novel amoebicidal therapeutics.
Methods
Entamoeba histolytica cell growth
Trophozoites of E. histolytica strain HM1: IMSS clone-6 were grown axenically in TYI-S-33 medium at 35 °C as described earlier by Diamond et al.50. To get a sufficient amount of ribosomes, the culture was expanded into a 75 cm2 cell culture, plug seal flask (NEST) with 250 ml complete media following the earlier protocol by Gautam et al.51. In brief, TYI-S-33 media was prepared by mixing potassium phosphate dibasic 1.0 g, potassium phosphate monobasic 0.6 g, biosate peptone 30.0 g, glucose 10.0 g, sodium chloride 2.0 g, L-cysteine hydrochloride 1.0 g, ascorbic acid 1.0 g, and ferric ammonium citrate 22.8 mg in 700 ml of double distilled water and pH was adjusted to 6.8 using 5 N NaOH. The volume was made up to 900 ml (10 units) and filtered using a filter assembly (Corning 500 ml, filter system). The cells were grown in TYI-S-33 medium complemented with 15% adult bovine serum, 1X Diamond vitamin mix, 125 µl of 250 U/ml Benzylpenicillin, and 0.25 mg/ml Streptomycin per 90 ml of medium (complete media). After 60 h, the media was removed, and 1X Phosphate Buffer Saline (PBS pH 7.4) was added to the confluent culture, chilled on ice for 7 min, and then harvested by centrifugation at 1200 × g for 7 min at 4 °C.
Ribosome isolation and purification
Ribosome isolation was done similarly, as reported previously in ref. 52, with minor modifications. The E. histolytica cells were resuspended in lysis buffer (20 mM) HEPES-sodium salt buffer, pH 7.4, 40 mM KOAc, 10 mM Mg(OAc)2, 250 mM sucrose, 3 mM DTT, 1 mM PMSF, 1X Protease inhibitor cocktail (cOmplete, Roche), RNasin (Thermo,1:40 dilution) and flash frozen in liquid nitrogen. The cell lysis was performed using Retsch Mixer Mill MM500 at 30 Hz, 1 min/cycle, and a total of 8 cycles. 2 U/ml DNase I (Thermo Scientific) was added to the cell lysate and incubated on ice for 45 min. The cell debris was cleared by centrifugation at 18,626 × g for 45 min at 4 °C. The cleared cell lysate was layered on a 1.1 M sucrose cushion (20 mM HEPES-sodium salt buffer pH 7.4, 150 mM KOAc, 10 mM Mg(OAc)2, 1.1 M sucrose, 3 mM DTT) in equal volume, followed by ultra-centrifugation at 100,000 × g for 16 h at 4 °C (Hitachi P70AT2 rotor). The pellet was then resuspended in buffer A (20 mM HEPES-sodium salt buffer, pH 7.4, 150 mM KOAc, 10 mM Mg(OAc)2, 3 mM DTT) and centrifuged at 18,626 × g 30 min, 4 °C. The absorbance of the supernatant was measured at 260 nm, and 10 OD units were layered on a 10%–50% sucrose gradient in gradient buffer (20 mM HEPES-sodium salt buffer, pH 7.4, 150 mM KOAc, 10 mM Mg(OAc)2, 3 mM DTT) prepared using Gradient Master from Biocomp Instruments. Gradients were centrifuged at 256,400 × g for 8 h at 4 °C (Hitachi, P40ST rotor). Afterward, the gradients were fractionated using a Gilson Fractionator from BioComp Instruments (Supplementary Fig.1). The fractions were then analyzed using 1% agarose gel with 0.06% bleach (Supplementary Fig.1). The fractions corresponding to peaks were then pooled and concentrated using 100 kDa cut-off amicon from Merck Millipore, followed by buffer exchange with ribosome storage buffer (20 mM HEPES-sodium salt buffer pH 7.4, 100 mM KOAc, 10 mM Mg(OAc)2, 10 mM NH4OAc, 3 mM DTT). The concentration of the sample was determined by absorbance at 260 nm, and ribosomes were stored at −80 °C for future use.
Electron microscopy
The 300 mesh Copper grids with a thin carbon layer on the top (CF300-Cu) from Electron Microscopy Science (EMS) were glow-discharged for 50 s in a JEOL glow discharge unit. A 3 µl sample from peak 1(Supplementary Fig.1a) of sucrose gradient fractionation was applied on the grid’s carbon side and washed three times with Mili-Q water. The grid was stained with 1% uranyl acetate solution. The grids for peaks 2 and 3 (Supplementary Fig.1a) were prepared following a similar protocol. The grids were analyzed in a 120 kVa JEOL JEM1400 electron microscope at Advanced Technology Platform Centre (ATPC), Regional Centre for Biotechnology (RCB), Faridabad (Supplementary Fig. 1b–d).
Cryo-electron microscopy
A 3 µl of 53S ribosome (1 µg/µl, peak 1) sample was applied to a quantifoil Cu 1.2/1.3 grids from Ted Pella, Inc. and plunge freeze using GATAN CP3 plunger after 3-s blotting at 4 °C temperature and 80% humidity. The cryo-EM grid was mounted in a GATAN 626 cryo-holder and analyzed using a JEOL JEM2200FS microscope equipped with a GATAN K2 summit direct electron detector camera at ATPC, RCB, Faridabad. At optimum ice thickness, the data were collected in a Movie mode at 1.649 e−/Å2/frame with 30 frames per Movie stack.
After initial cryo-EM condition optimization at ATPC, RCB, the final high-resolution data were collected at a 300 kVa Titan Krios microscope equipped with Falcon 3 direct electron detector camera (ThermoFisher) at the National Electron Cryo-Microscopy Facility, Bangalore Life Science Cluster (BLiSc), Bangalore. The data sets were collected in an electron integration Movie mode. For the 53S ribosomal subunit, 3470 Movie stacks were collected with 25 frames per Movie stack at 75,000× magnification with 1.07 Å pixel and an electron dose of 1.34 e-/Å2/frame in electron integration mode using a Falcon 3 electron detector camera (Supplementary Fig. 2, Supplementary Table 1). For 75S ribosome, 5724 Movie stacks (dataset 1) and 6116 Movie stacks (dataset 2) were collected in a similar setting as that of 53S ribosomal subunit data collection, with a similar electron dose of 1.34 e-/Å2/frame 75,000× magnification with 1.07 Å pixel (Supplementary Fig. 3, Supplementary Table 1).
75S ribosome paromomycin complex EM data collection
The purified 75S ribosomes, stored at −80 °C, were thawed and spun at 20,000 × g for 10 min. 200 nM of 75S ribosomes were incubated with a 1000-time excess of PAR (0.2 mM) at 25 °C for 15 min and immediately used for EM analysis. Initial sample screening was performed by following steps similar to those of the associated 75S ribosome. The samples were screened at room temperature, negative staining using JEOL JEM1400 120 kVa electron microscope, and at cryo-condition using JEOL JEM2200FS 200 kVa electron microscope equipped with GATAN K2 camera at ATPC, RCB Faridabad. High-resolution cryo-EM data were collected using a 300 kVa Titan Krios microscope equipped with a Falcon 3 direct electron detector camera (ThermoFisher) at the National Electron Cryo-Microscopy Facility, Bangalore Life Science Cluster (BLiSc), Bangalore. A total of 4,688 Movie stacks were collected with 32 frames per Movie stack at 75,000× magnification with 1.07 Å pixel with an electron dose of 1.09 e-/Å2/frame in electron integration mode using a Falcon 3 electron detector camera (Supplementary Fig. 5, Supplementary Table 1).
Single particle reconstruction
RELION 3.1.453 was used for single particle reconstruction of all three, 53S, 75S and 75S-PAR, ribosome 3D reconstruction (Supplementary Figs. S2–S5, Supplementary Table 1). The Movie stacks were drift corrected using RELION’s own implementation, and single micrographs were generated. The micrographs were CTF corrected using CTFFIND454. Initially, particles were picked manually, and 2D reference images were generated for automatic particle picking. 763,731 auto-picked particles for the 53S ribosomal subunit were subjected to 2D classification, and the best classes with 554,832 particles were selected for 3D classification. A 60 Å low-pass filtered 80S ribosome cryo-EM map (EMDB ID 2660) from Plasmodium falciparum23 was used as a reference map. The three best 3D classes, consisting of 447,323 particles, were subjected to 3D refinement, which yielded a consensus map of 3.1 Å resolution after post-processing. The gold-standard FSC = 0.143 criterion55 was used for resolution estimation throughout data processing. These 447,323 particles were subjected to CTF refinement and Bayesian polishing, followed by 3D refinement and post-processing, which improved the overall map quality and resolution to 2.8 Å (Supplementary Fig. 2, Supplementary Table 1). Further, the particles were subjected to a 3D classification into 3 classes without alignment, and one class with 378,061 particles, which has homogeneous particles, was selected. The particles were 3D refined and post-processed to 2.8 Å resolution. The gold-standard FSC = 0.143 criterion55 was used for final resolution estimation. Local resolution was estimated using ResMap56, embedded in RELION. The final map was auto-sharpened using Phenix.auto_sharpen57.
Similar steps to those of 53S data processing were followed for the 75S ribosome refinement. For dataset 1, 100,475 particles were auto-picked using 2D reference images generated after manual particle picking and 2D classification. The auto-picked particles were subjected to 2D classification, and the best classes consisting of 45,379 particles were selected for 3D refinement, and a consensus cryo-EM map was generated at 3.6 Å resolution. A low-pass filtered to 60 Å cryo-EM map (EMDB ID 2660) from Plasmodium falciparum23 was used as a reference map in the 3D refinement. The particles were polished using CTF refinement and Bayesian polishing, which further improved the overall resolution to 3.3 Å. A 3D classification into 3 classes without alignment was performed on the polished particles. The best class with 37,355 (82%), was subjected to 3D refinement and post-processing, which yielded a 3.3 Å resolution cryo-EM map (Supplementary Fig. 3). For dataset 2, similar data processing steps were followed. 96,868 particles were selected after 2D and 3D classification, which yielded a 3.0 Å resolution map after particle polishing (Supplementary Fig. 3).
The polished particles from data 1 and data 2, a total of 142,247 particles, were merged, and a consensus map at 3.0 Å resolution was generated. Further, a 3D classification into 4 classes without alignment was performed. Two best classes emerged, class 1 having 39,958 (29%) particles with tRNA bound A/P- and P/E-sites of the ribosome, and another class (class 4) having 53,764 (38%) particles with tRNA bound at the P-site of the ribosome. Meanwhile, 29% of particles yield a low-resolution class, and 2% of particles remain unaligned. The 3D refinement and post-processing yielded a 3.1 Å resolution cryo-EM map for both classes. A partial signal subtraction followed by focused refinement58 was performed to further improve the quality and resolution of the LSU, SSU body, and SSU head map. After post-processing, the large subunit resolution improved to 3 Å for both classes. Whereas the class 1, SSU body and SSU head resolved to 3.2 Å and 3.4 Å resolution, respectively. Similarly, the class 4, SSU body and SSU head resolved to 3.1 Å and 3.4 Å resolution, respectively (Supplementary Fig. 3, Supplementary Table 1). The resolution was estimated using the gold-standard FSC = 0.143 criterion55 and local resolution was calculated using ResMap56 (Supplementary Fig. 4). The Phenix.auto_sharpen was used to auto-sharpen the final maps57.
The 75S-PAR data was processed by following similar steps. Finally, the selected 54,889 particles yielded a 3.0 Å resolution cryo-EM map after particle polishing and 3D classification without alignment. A partial signal subtraction followed by focused refinement58 for SSU body was performed59 to improve the local resolution (Supplementary Fig. 5 and Supplementary Table 1).
Model building and structure analysis
rRNA and r-protein coordinates were built by coalescing both approaches, which are template-based and de novo model building. The atomic coordinates of Toxoplasma gondii (PDB ID: 5XXB)25 and Homo sapiens (PDB ID: 4UG013) were used for template-based modeling, where ribosomal rRNAs, 28S, 5S, and 5.8S were rigid-body docked into 53S cryo-EM using Chimera60. By convention, 28S rRNA was divided into 6 domains, and each was rigid-body docked (Supplementary Table 4). The regions not fitting into the cryo-EM maps were deleted, and the nucleotide residues were mutated to the E. histolytica 25S rRNA, 5.8S, and 5S rRNA sequences using COOT v.0.9.361. The nucleotide sequences were manually built in the cryo-EM map using COOT for the remaining segments of rRNA. The r-protein modules were initially built using Model Angelo62 without the FASTA sequence, followed by threading of the Entamoeba sequence on the carbon backbone using PHYRE263. The iterative steps of manual model building, real-space refinement, and geometry regularization were done in COOT, then phenix.real_space_refinement was used for the flexible refinement64.
For model building in the 75S, the atomic coordinates of the 53S ribosomal subunit were rigid docked in cryo-EM map of the LSU and followed by flexible refinement using phenix.real_space_refinement. The atomic coordinates of the 36S ribosomal subunit were built by following steps similar to that of the 53S model building in the 53S cryo-EM map described above. The final coordinates of the 53S and 36S were rigid-body docked in the 75S cryo-EM map and followed by flexible refinement using phenix.real_space_refinement. The model-building statistics are given in Supplementary Table 1. Unless otherwise stated, all figures were prepared in Chimera and ChimeraX65. The RNA 2D diagram was prepared using the R2DT tool66 at RNA Central using Saccharomyces cerevisiae and Homo sapiens as templates, RNAPDBee67,68,69, and Adobe Illustrator were also used.
Similarly, for the model building of the 75S-PAR complex, the coordinates of individual subunits were docked, followed by rigid body and flexible fitting using Phenix. The coordinates of PAR were docked from the E. coli ribosome PAR complex, PDB 7K00. Figure 9f was generated using Discovery Studio70.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The cryo-EM maps have been deposited in the EMDB and the atomic coordinates have been deposited in the wwPDB (https://www.wwpdb.org) with accession codes: EMD-64694 and 9v1i for ribosomal large subunit (LSU), EMD-64713 and 9v21 for 75S ribosome with P-tRNA, EMD-64697 and 9V1L for large subunit (LSU) of 75S ribosome with P-tRNA, EMD-64695 and 9V1J for small subunit (body) of 75S ribosome with P-tRNA, EMD-64696 and 9V1K for small subunit (head) of 75S ribosome with P-tRNA, EMD-64716 and 9V24 for 75S ribosome with A/P- and P/E-tRNAs, EMD-64717 and 9V25 for large subunit (LSU) of 75S ribosome with A/P- and P/E-tRNAs, EMD-64719 and 9V27 for small subunit (body) of 75S ribosome with A/P- and P/E-tRNAs, EMD-64720 and 9V28 for small subunit (head) of 75S ribosome with A/P- and P/E-tRNAs, EMD-64718 and 9V26 for 75S ribosome with A/P- and P/E-tRNAs bound to antibiotic paromomycin and EMD-64721 and 9V29 for small subunit (body) of 75S ribosome with A/P- and P/E-tRNAs bound to antibiotic paromomycin.
References
Stanley, S. L. Amoebiasis. Lancet 361, 1025–1034 (2003).
Nespola, B. et al. First case of amebic liver abscess 22 years after the first occurrence. Parasite 22, 20 (2015).
Ryan, K. J., Ray, C. G. & Sherris, J. C. Sherris Medical Microbiology: An Introduction to Infectious Diseases https://hslic-unm.on.worldcat.org/oclc/52358530 (McGraw-Hill, 2004).
Shirley, D. A. T., Farr, L., Watanabe, K. & Moonah, S. A review of the global burden, new diagnostics, and current Therapeutics for amebiasis. Open Forum Infect. Dis. 5, ofy161 (2018).
Barker, D. C. & Deutsch, K. The chromatoid body of Entamoeba invadens. Exp. Cell Res. 15, 604–610 (1958).
Morgan, R. S. & Uzman, B. G. Nature of the packing of ribosomes within chromatoid bodies. Science 152, 214–216 (1966).
Morgan, R. S., Slayter, H. S. & Weller, D. L. Isolation of ribosomes from cysts of Entamoeba invadens. J. Cell Biol. 36, 45–51 (1968).
Lake, J. A. & Slayter, H. S. Three dimensional structure of the chromatoid body of Entamoeba invadens. Nature 227, 1032–1037 (1970).
Lake, J. A. & Slayter, H. S. Three-dimensional structure of the chromatoid body helix of Entamoeba invadens. J. Mol. Biol. 66, 271–282 (1972).
Morgan, R. S. Structure of ribosomes of chromatoid bodies: Three-dimensional Fourier synthesis at low resolution. Science 162, 670–81 (1968).
Price, M., Specht, C., Boudreau, R. E., Shaw, D. E. & Weller, D. L. Preliminary characterization of ribosomes of Entamoeba invadens. Mol. Biochem. Parasitol. 8, 137–143 (1983).
Albach, R. A., Prachayasittikul, V. & Heebner, G. M. Isolation and characterization of RNA of Entamoeba histolytica. Mol. Biochem. Parasitol. 12, 261–272 (1984).
Khatter, H., Myasnikov, A. G., Natchiar, S. K. & Klaholz, B. P. Structure of the human 80S ribosome. Nature 520, 640–645 (2015).
Nelson, D. L. & Cox, M. M. Lehninger Principles of Biochemistry 7th edn, Vol. 2 (W.H. Free Co., 2017).
Carter, W. A., Levy, H. B. & Diamond, L. S. Protein synthesis by amoebal ribosomes. Nature 213, https://doi.org/10.1038/213722b0 (1967).
Kusamrarn, T., Vinijchaikul, K. & Bailey, G. B. Comparison of the structure and function of polysomal and helical ribosomes from Entamoeba Invadens. J. Cell Biol. 65, 540–548 (1975).
ENTNER, N. & GROLLMAN, A. P. Inhibition of protein synthesis: a mechanism of amebicide action of emetine and other structurally related compounds. J. Protozool. 20, 160–163 (1973).
ENTNER, N. Emetine binding to ribosomes of Entamoeba histolytica—inhibition of protein synthesis and amebicidal action. J. Protozool. 26, 324–328 (1979).
Orozco, E., de la Cruz Hernández, F. & Rodríguez, M. A. Isolation and characterization of Entamoeba histolytica mutants resistant to emetine. Mol. Biochem. Parasitol. 15, 49–59 (1985).
Gonzales, M. L. M., Dans, L. F. & Sio-Aguilar, J. Antiamoebic drugs for treating amoebic colitis. Cochrane Database Syst. Rev. 2019, https://doi.org/10.1002/14651858.CD006085.pub3 (2019).
World Health Organization. WHO model prescribing information: drugs used in parasitic diseases, 2nd ed. World Health Organization. https://iris.who.int/handle/10665/41765 (1995).
Hashem, Y. et al. High-resolution cryo-electron microscopy structure of the Trypanosoma brucei ribosome. Nature 494, 385–389 (2013).
Wong, W. et al. Cryo-EM structure of the Plasmodium falciparum 80S ribosome bound to the anti-protozoan drug emetine. eLife 2014, e03080 (2014).
Shalev-Benami, M. et al. Atomic resolution snapshot of Leishmania ribosome inhibition by the aminoglycoside paromomycin. Nat. Commun. 8, 1589 (2017).
Li, Z. et al. Cryo-EM structures of the 80S ribosomes from human parasites Trichomonas vaginalis and Toxoplasma gondii. Cell Res. 27, 1275–1288 (2017).
Matzov, D. et al. Cryo-EM structure of the highly atypical cytoplasmic ribosome of Euglena gracilis. Nucleic Acids Res. 48, 11750–11761 (2020).
Hiregange, D. G. et al. Cryo-EM structure of the ancient eukaryotic ribosome from the human parasite Giardia lamblia. Nucleic Acids Res. 50, 1770–1782 (2022).
Majumdar, S. et al. Insights into translocation mechanism and ribosome evolution from cryo-EM structures of translocation intermediates of Giardia intestinalis. Nucleic Acids Res. 51, 3436–3451 (2023).
Eiler, D. R. et al. The Giardia lamblia ribosome structure reveals divergence in several biological pathways and the mode of emetine function. Structure https://doi.org/10.1016/j.str.2023.12.015 (2024).
Petrov, A. S. et al. Secondary structures of rRNAs from all three domains of life. PLoS ONE 9, e88222 (2014).
Petrov, A. S. et al. Secondary structure and domain architecture of the 23S and 5S rRNAs. Nucleic Acids Res. 41, 7522–7535 (2013).
Shankar, V. et al. RRNA expansion segment 27Lb modulates the factor recruitment capacity of the yeast ribosome and shapes the proteome. Nucleic Acids Res. 48, 3244–3256 (2020).
Fujii, K., Susanto, T. T., Saurabh, S. & Barna, M. Decoding the function of expansion segments in ribosomes. Mol. Cell 72, 1013–1020.e6 (2018).
Pfeffer, S. et al. Structure and 3D arrangement of endoplasmic reticulum membrane-associated ribosomes. Structure 20, 508–518 (2012).
Ban, N., Nissen, P., Hansen, J., Moore, P. B. & Steitz, T. A. The complete atomic structure of the large ribosomal subunit at 2.4 Å resolution. Science 289, 905–920 (2000).
Kater, L. et al. Construction of the Central Protuberance and L1 Stalk during 60S Subunit Biogenesis. Mol. Cell 79, 615–628.e5 (2020).
Wimberly, B. T. et al. Structure of the 30S ribosomal subunit. Nature 407, 327–339 (2000).
Jenner, L. et al. Crystal structure of the 80S yeast ribosome. Curr. Opin. Struct. Biol. 22, https://doi.org/10.1016/j.sbi.2012.07.013 (2012).
Wang, X. et al. Coevolution of ribosomal RNA expansion segment 7Land assembly factor Noc2p specializes the ribosome biogenesis pathway between Saccharomyces cerevisiae and Candida albicans. Nucleic Acids Res. 49, 4655–4667 (2021).
Conrad, N. K. The emerging role of triple helices in RNA biology. Wiley Interdiscip. Rev. RNA 5, https://doi.org/10.1002/wrna.1194 (2014).
Brown, J. A. Unraveling the structure and biological functions of RNA triple helices. Wiley Interdiscip. Rev. RNA 11, https://doi.org/10.1002/wrna.1598 (2020).
Nguyen, T. H. D. et al. Cryo-EM structure of substrate-bound human telomerase holoenzyme. Nature 557, 190–195 (2018).
Bertram, K. et al. Cryo-EM structure of a human spliceosome activated for step 2 of splicing. Nature 542, 318–323 (2017).
Qu, G. et al. Structure of a group II intron in complex with its reverse transcriptase. Nat. Struct. Mol. Biol. 23, 549–557 (2016).
Huang, L., Wang, J., Wilson, T. J. & Lilley, D. M. J. Structure of the Guanidine III Riboswitch. Cell Chem. Biol. 24, 1407–1415.e2 (2017).
Brown, J. A. et al. Structural insights into the stabilization of MALAT1 noncoding RNA by a bipartite triple helix. Nat. Struct. Mol. Biol. 21, 633–640 (2014).
Mitton-Fry, R. M., DeGregorio, S. J., Wang, J., Steitz, T. A. & Steitz, J. A. Poly(A) tail recognition by a viral RNA element through assembly of a triple helix. Science 330, 1244–1247 (2010).
Watson, Z. L. et al. Structure of the bacterial ribosome at 2 Å resolution. eLife 9, 1–62 (2020).
Carter, A. P. et al. Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature 407, 340–348 (2000).
Diamond, L. S., Harlow, D. R. & Cunnick, C. C. A new medium for the axenic cultivation of entamoeba histolytica and other entamoeba. Trans. R. Soc. Trop. Med. Hyg. 72, 431–432 (1978).
Gautam, G., Ali, M. S., Bhattacharya, A. & Gourinath, S. EhFP10: a FYVE family GEF interacts with myosin IB to regulate cytoskeletal dynamics during endocytosis in Entamoeba histolytica. PLoS Pathog. 15, e1007573 (2019).
Kumar, N., Sharma, S. & Kaushal, P. S. Cryo-EM structure of the mycobacterial 70S ribosome in complex with ribosome hibernation promotion factor RafH. Nat. Commun. 15, 638 (2024).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Rohou, A. & Grigorieff, N. CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Rosenthal, P. B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003).
Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local resolution of cryo-EM density maps. Nat. Methods 11, 63–65 (2014).
Terwilliger, T. C., Sobolev, O. V., Afonine, P. V. & Adams, P. D. Automated map sharpening by maximization of detail and connectivity. Acta Crystallogr. Sect. D Struct. Biol. 74, 545–559 (2018).
Bai, X. C., Rajendra, E., Yang, G., Shi, Y. & Scheres, S. H. W. Sampling the conformational space of the catalytic subunit of human g-secretase. eLife 4, e11182 (2015).
Nguyen, T. H. D. et al. Cryo-EM structure of the yeast U4/U6.U5 tri-snRNP at 3.7 Å resolution. Nature 530, 298–302 (2016).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Casañal, A., Lohkamp, B. & Emsley, P. Current developments in Coot for macromolecular model building of electron cryo-microscopy and crystallographic data. Protein Sci. 29, 1069–1078 (2020).
Jamali, K. et al. Automated model building and protein identification in cryo-EM maps. Nature 628, 450–457 (2024).
Kelley, L. A., Mezulis, S., Yates, C. M., Wass, M. N. & Sternberg, M. J. E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 10, 845–858 (2015).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. Sect. D Struct. Biol. 74, 531–544 (2018).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Sweeney, B. A. et al. R2DT is a framework for predicting and visualising RNA secondary structure using templates. Nat. Commun. 12, 3494 (2021).
Antczak, M. et al. New algorithms to represent complex pseudoknotted RNA structures in dot-bracket notation. Bioinformatics 34, 1304–1312 (2018).
Zok, T. et al. RNApdbee 2.0: multifunctional tool for RNA structure annotation. Nucleic Acids Res. 46, W30–W35 (2018).
Antczak, M. et al. RNApdbee—a webserver to derive secondary structures from pdb files of knotted and unknotted RNAs. Nucleic Acids Res. 42, W368–W372 (2014).
Miyata, T. Discovery studio modeling environment. Ensemble 17, 1–382 (2015).
Acknowledgements
We acknowledge the National Electron Cryo-Microscopy facility at BLiSc, C-CAMP, Bangalore, and a big thanks to Drs. Vinothkumar and Sucharita for cryo-EM data collection. We acknowledge the Advanced Technology Platform Centre at the Regional Centre for Biotechnology, Faridabad, and thank Dr. Reena and Mr. Madhava for help in the initial cryo-EM sample screening. We acknowledge the computing resource at the Indian Biological Data Centre (IBDC), Faridabad, used for data processing. We thank all PSK lab members for proofreading the manuscript and providing valuable insights. S.S. acknowledges the fellowship from DBT. S.M. acknowledges a Fellowship from UGC and ICMR. This work is supported by a research grant, BT/PR45101/DRUG/134/121/2022, awarded to S.G. and P.S.K. from the Department of Biotechnology, India.
Author information
Authors and Affiliations
Contributions
P.S.K. and S.G. conceived the research project. S.M. and S.S. grew the E. histolytica pellet. S.S. purified and characterized the ribosomes. P.S.K. and S.S. performed the single particle reconstruction, model building, and structure analysis. P.S.K. and S.S. wrote the manuscript with input from S.M. and S.G.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sharma, S., Mishra, S., Gourinath, S. et al. Cryo-EM structure of ribosome from pathogenic protozoa Entamoeba histolytica reveals unique features of its architecture. Nat Commun 16, 7758 (2025). https://doi.org/10.1038/s41467-025-62767-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-62767-x