Abstract
The apelin receptor (APJR) plays a pivotal role in regulating cardiovascular and metabolic health1,2. Understanding the mechanisms of biased agonism at APJR is crucial for drug discovery, as stimulation of the β-arrestin pathway may lead to some adverse effects3. Structural analyses of APJR-Gi complexes have clarified the structural basis of receptor dimerization and activation4,5, yet the absence of structural data on APJR-arrestin complexes has impeded a comprehensive understanding of APJR stoichiometry in the dual signaling pathways and biased agonism. Here, we present APJR-β-arrestin1 structures bound to a clinical drug analog, revealing 2:2 and 2:1 stoichiometries associated with differential β-arrestin recruitment. Through comparison of the two transducer-coupled APJR structures bound to the same ligand, we identify key residues and motifs crucial for directing biased signaling. These findings highlight APJR’s versatile stoichiometry in coupling with β-arrestin and Gi proteins, establishing a framework for understanding biased agonism and guiding the development of therapeutics.
Similar content being viewed by others
Introduction
The apelin receptor (APJR), a crucial member of the class A G protein-coupled receptor (GPCR) family, plays an integral role in the regulation of diverse cardiovascular functions. Upon activation by the endogenous ligand apelin, APJR signaling through Gi proteins enhances cardiac contractility and produces vasodilatory effects that mitigate angiotensin-II-induced atheroma formation6,7,8,9,10. Notably, apelin-deficient mice exhibit a progressive decline in cardiac function, culminating in heart failure11. Beyond its cardiovascular roles, APJR is also pivotal in metabolic regulation such as glucose homeostasis, lipid metabolism, insulin sensitivity, and muscle generation, thereby influencing energy balance and overall metabolic health2,12,13,14. Dysregulation of APJR signaling has been linked to several metabolic disorders, including muscle loss, obesity, and type 2 diabetes15,16,17,18,19, demonstrating its potential as a therapeutic target in these conditions. BioAge Inc. has recently disclosed the robust efficacy profile of its clinical-stage drug BGE-105 (originally from Amgen named as AMG986) in promoting weight loss and muscle preservation19. Importantly, activation of Gi proteins via apelin elicits cardioprotective responses, whereas stimulation of the β-arrestin pathway can lead to cardiac hypertrophy3. The desensitization and internalization of APJR caused by the β-arrestin pathway might also be undesirable. This dual signaling pathway highlights the therapeutic potential for developing Gi protein-biased APJR agonists for treating heart failure or other cardiovascular diseases. Recent studies have advanced our understanding of biased signaling mechanisms by elucidating the structures of APJR-Gi complexes with biased agonists20. Nevertheless, the absence of direct structural information on APJR-β-arrestin complexes hinders detailed analysis of conformational changes necessary for comprehensive insight into APJR-biased signaling. Thus, structural studies are imperative to fully elucidate the mechanisms supporting APJR signaling bias and to foster the development of targeted therapeutic strategies.
Furthermore, existing structural studies of class A GPCR-β-arrestin complexes have predominantly revealed monomeric interactions with β-arrestin. The potential existence and interaction mechanisms of dimeric class A GPCR-β-arrestin complexes remain underexplored and poorly defined. Previous research has revealed APJR dimerization may occur at low receptor concentrations in living cells and demonstrated that APJR can engage Gi proteins in both dimeric and monomeric forms4,5, with a small dimerization interface (FGXXF motif) characterized and a “dimer-switch” mutation (F1013.24A) identified. However, the interaction mechanisms with β-arrestin are not yet fully elucidated. This gap in understanding necessitates further investigation to comprehend the structural and functional implications of β-arrestin interactions with APJR.
Given the importance of APJR in drug discovery, numerous peptides, small molecules and biologics drug molecules have been reported. We have previously uncovered molecular mechanism for ligand recognition across a series of APJR complexes including: the inactive-like-state APJR (through x-ray crystallography)21 as well as the APJR-Gi complexes (through cryo-EM) for the two endogenous peptides—apelin and ELA peptides4, the apelin-mimetic peptide21, the agonist and antagonist nanobody5, as well as a super potent small molecule cmpd644 (((1R,2S)-N-(4-(2,6-dimethoxyphenyl)−5-(6-methylpyridin-2-yl)−4H-1,2,4-triazol-3-yl)−1-hydroxy-1-(5-methylpyrimidin-2-yl) propane-2-sulfonamide). It’s worth mentioning that cmpd644 was originally developed by Amgen, exhibiting great potency on both Gi-protein and β-arrestin signaling pathways, and is a close analog to the above-mentioned clinical drug candidate BGE-105 (AMG986)22,23 (Supplementary Fig. 1a). However, cmpd644 bound to APJR-β-arrestin complex has not been elucidated, impeding the understanding of the biased signaling mechanism and the guidance for development of G-protein biased drugs with desired therapeutic profile.
In this work, to advance our understanding of the modulation of receptor signaling by β-arrestin and structure-function relationship of receptor: β-arrestin stoichiometry—thereby facilitating the discovery of biased ligands specifically tailored for APJR, we determine the cryo-electron microscopy (cryo-EM) structures of three distinct APJR-β-arrestin1 (βarr1) complexes. We successfully obtain two 3.5 Å resolution maps of the wild-type APJR-βarr1 complex activated by the small molecule agonist cmpd644. These maps depict the APJR-βarr1 complex at stoichiometries of 2:2 and 2:1, respectively. Additionally, we acquire a high-resolution 3.2 Å resolution map of the monomeric mutant APJRF101A-βarr1 complex at a 1:1 stoichiometry. These structures provide a detailed depiction of the interaction patterns between the receptor and βarr1 across different stoichiometric arrangements. Comprehensive functional analysis further uncovers the full and partial activation mechanisms achieved by different βarr1 coupling stoichiometries. Together, our study establishes a comprehensive framework for understanding the mechanisms underlying biased signaling through APJR.
RESULTS
APJR-βarr1 complexes with distinct stoichiometry
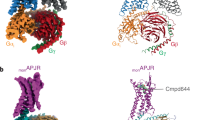
To promote the assembly of a functional complex, we followed an established protocol for GPCR-βarr1 structural determination24. Briefly, the C-terminal segment (residues C331-D380) of the APJR was replaced with the corresponding C-terminal sequence (residues A343–S371) from the vasopressin type 2 receptor (V2R) (termed APJR(V2RC)) (Supplementary Fig. 1b). We employed a cysteine-deficient βarr1 variant containing seven mutations. Furthermore, we substituted its C-terminal segment (comprising residues I377 to R418) with the scFv30 antibody24. The recruitment of βarr1 was conducted to verify the enhancement effect mediated by the V2R tail. The efficacy (Emax) of APJR-V2R was found to be approximately 1.6-fold greater than that of the APJR-WT (Supplementary Fig. 1c and Supplementary Table 1). Moreover, the addition of the V2R tail had minimal impact on the Gi signaling pathways (Supplementary Fig. 1c and Supplementary Table 1). The APJR(V2RC)-βarr1-scFv30 (hereafter abbreviated as APJR-βarr1 complex unless specified) complex was achieved by co-expressing APJR and βarr1 together with GPCR kinase 2 (GRK2), and subsequently co-purified in the presence of the small molecule agonist cmpd644. The previously reported structures of the class A GPCR-arrestin complexes all exhibit GPCR: arrestin at a 1:1 stoichiometry. However, in our APJR-βarr1 structures, the receptor-βarr1 interactions can adopt both 2:2 and 2:1 ratios (Fig. 1a, b, Supplementary Table 2, and Supplementary Figs. 2 and 3). Unlike the APJR-Gi complexes, the monomeric form was not observed during cryo-EM data processing, suggesting distinct stoichiometry for different transducer coupling by APJR. It is remarkable that a class A GPCR can engage with transducers in a 2:2 stoichiometry, a similar configuration was reported in the recent mGluR3 (class C GPCR dimers) study25.
a–c Cryo-EM maps and structure models of all three APJR-βarr1 complexes reported in this study, shown in two orientations and colored by chain: APJR-βarr1 complex at a 2:2 stoichiometry (yellow, APJR; pink, βarr1; gray, scFv30; light purple, cmpd644) (a); APJR-βarr1 at a 2:1 stoichiometry (yellow, APJR coupled with βarr1; green, APJR uncoupled with βarr1; pink, βarr1; gray, scFv30; light purple, cmpd644) (b); APJRF101A-βarr1 complex (blue, APJRF101A-βarr1; pink, βarr1; gray, scFv30; orange, cmpd644) (c).
Subsequently, we examined the dimer interface of the APJR-βarr1 complex in both 2:2 and 2:1 stoichiometric ratios. The analysis revealed that the complex adopts a conformation analogous to that observed in the dimeric APJR-Gi protein complex (PDB ID: 7W0L) (Supplementary Fig. 3c). To further elucidate the interaction between monomeric APJR and βarr1, we introduced the dimer-switch mutation F1013.24A4 (Supplementary Fig. 1b). The structure of cmpd644-bound monAPJRF101A(V2RC)-βarr1-scFv30 complex primarily adopts a monomeric 1:1 arrangement (hereafter abbreviated as APJRF101A-βarr1 complex unless specified). The protein sample underwent cryo-EM single-particle analysis, yielding a map at 3.2 Å global resolution (Fig. 1c, Supplementary Table 2, and Supplementary Figs. 4 and 5). The high-resolution map allows for the accurate determination of the ligand binding features in the APJRF101A-βarr1 structure, along with the detailed structure information of the majority of residues within the receptor transmembrane domain and βarr1.
Structural and functional characterization of APJR dimerization in β-arrestin1 signaling
Our study reveals the intriguing ability of the APJR dimer to associate with either one or two βarr1 molecules, with both configurations occurring in an approximately 1:1 ratio according to the single particle classifications (Supplementary Fig. 2). We initially superimposed the two APJR-βarr1 complexes, revealing that these structures share an overall similar conformation (Supplementary Fig. 6a–c). The interface between APJR and βarr1 in the two complexes appeared nearly identical (Supplementary Fig. 6d). Notably, helix 8 (H8) in ProtB of APJR-βarr1 (2:1) adopts an inverted conformation compared to that in the APJR-βarr1 (2:2) (Supplementary Fig. 6e). This structural shift results in a significant clash between H8 in ProtB of APJR-βarr1 (2:1) and the βarr1 finger loop. Intriguingly, while the ProtB is uncoupled to βarr1, our structural analysis suggests that the inverted H8 may partially assume a role analogous to that of a transducer to stabilize the ProtB in an active-like conformation characterized by the outward shift of TM6 and downward movement of toggle switch W2616.48 (Supplementary Fig. 6g, h). These findings align with previous observations in the cmpd644-bound dimAPJR-Gi complex structures, where ProtB similarly exhibited an inverted H8 while maintaining an active-like conformation without G protein coupling4. Together, these results indicate that ProtBs within the APJR dimer, when bound to the agonist, can indeed adopt an active-like conformation even in the absence of downstream transducers. This insight enhances our understanding of the functional dynamics of these complexes and the structural basis for APJR activation.
We further examined the functional effect of receptor dimerization on APJR’s βarr1 recruitment. Through alignment of the APJRF101A-βarr1 complex with the APJR-βarr1 (2:2), we observed a broadly similar receptor architecture, with variations at ECL2/3 (less than 3.0 Å displacement) (Fig. 2a). The interaction between the βarr1 finger loop and APJR in both monomeric and dimeric forms is primarily mediated by a similar hydrophobic network, including residues I1313.54, P13434.50, V13534.51, H2315.68, F2325.69, and L2466.33 of APJR, along with residues in the finger loop (Fig. 2b). However, a substantial difference was found in the positioning of the βarr1 finger loop: in the APJRF101A-βarr1 complex, the finger loop inserted slightly deeper into the receptor core cavity compared to its position in the APJR-βarr1 complex (Fig. 2b). This deeper insertion facilitates an additional interaction with the key residue R1273.50 of APJR (Fig. 2b). Consistent with this structural finding, mutagenesis of R1273.50A largely decreased βarr1 recruitment to APJR induced by cmpd644 (Fig. 2c and Supplementary Table 3). We therefore, designate the R1273.50A mutation as a βarr1-functional switch on APJR.
a Structural comparison between APJRF101A (1:1, blue/pink) and APJR-βarr1 (2:2, yellow/gray) in overall side view. b Interactions of the finger loop of βarr1 with APJR in both its dimeric and monomeric forms. The key residues involved in these interactions are depicted as stick representations. The downward movement of the finger loop of βarr1 in the APJR-βarr1 (2:2) complex is indicated by a red arrow. c Effects of cmpd644 on WT and R1273.50A mutants in βarr1 recruitment and Gi signaling pathways using BRET assays to assess the βarr1 recruitment and Gαi-Gγ dissociation. Data are represented as mean ± SEM (n = 3 independent experiments). See also Supplementary Table 3 for details. d Schematic diagram depicting the experimental setup for detecting βarr1 recruitment to APJR dimers using modified BRET biosensor assay. APJR dimers are labeled with LgBiT and HiBiT fragments of Nluc, respectively. βarr1 is fused to Venus. An increase of BRET signal between Nluc and Venus can be observed when βarr1 is recruited to APJR dimer. e Quantification of βarr1 recruitment to APJR dimers as measured by BRET in response to cmpd644. The recruitment is depicted as a percentage of the maximum BRET response observed with WT APJR dimers. Data are represented as mean ± SEM (n = 4 independent experiments). See also Supplementary Table 4 for details.
The intriguing observation of two distinct APJR-βarr1 complex stoichiometries (2:2 and 2:1) prompted further investigation into the functional distinctions between these two states. We employed the above-characterized R1273.50A βarr1-functional switch on either protomer to elucidate these differences. We developed a luciferase complementation assay combined with BRET biosensor to specifically measure βarr1 recruitment to the APJR dimer (Fig. 2d). In this experiment, APJR was labeled with LgBiT and HiBiT, respectively, creating a linked configuration that facilitates the formation of a functional Nluc molecule when the two protomers (APJRLgBiT and APJRHiBiT) are brought together. This configuration allows for the detection of BRET signaling upon the recruitment of Venus-tagged βarr1 to the APJR dimer labeled with complementary Nluc tags (Fig. 2d). Interestingly, our results revealed a significant reduction in βarr1 recruitment when one of the protomers was mutated at R1684.64A (impairing ligand binding, Supplementary Fig. 6g)21 and R1273.50A (impairing βarr1 coupling). This suggests that although one protomer is sufficient to activate βarr1 recruitment, both protomers are necessary for the full activation of the APJR dimer (Fig. 2e and Supplementary Table 4). This study provides crucial insights into the functional dynamics of APJR dimerization underlying βarr1 recruitment.
Cmpd644 binding pocket across different functional states of APJR structures
The development of small molecule ligands targeting the APJR holds significant scientific and therapeutic promise, potentially offering several advantages over peptide ligands. Cmpd644 is a close analog to the clinical-stage drug BGE-105 (AMG986) for cardiovascular and metabolic regulations22,23 (Supplementary Fig. 1a). To understand the binding characteristics of cmpd644, we compared its binding modes across various functional states of APJR, using structures elucidated in our previous and current studies4,21. First, we analyzed the binding pocket of cmpd644 in three distinct APJR-βarr1 complexes. In APJR-βarr1 (2:2), cmpd644 was observed in each protomer of the complex. Intriguingly, in APJR-βarr1 (2:1), the ligand was also present in ProtB (receptor uncoupled with βarr1) likely owing to its exceptionally high potency and the potentially promoting role of the inverted H8 as discussed above. Cmpd644 exhibited almost identical binding poses in all three complex structures (Supplementary Fig. 6h–j).
Subsequently, we compared the binding pocket of cmpd644 in different transducer-coupled states. The high-resolution APJRF101A-βarr1 complex was selected as a representative model to examine the binding modes of cmpd644 in the βarr1-coupled state. Notably, cmpd644 occupies a comparable position in both the βarr1-coupled and Gi-coupled states (Fig. 3a). However, in the βarr1-coupled state, cmpd644 is slightly deeper within the orthostatic pocket compared to the Gi-coupled state, a phenomenon similarly observed in the FUB-bound CB1 structures in both transducer-coupled states26. Specifically, the methylpyrimidine group of cmpd644 exhibits a downward shift of 1.1 Å in the βarr1-coupled state (Fig. 3b). This shift at the extracellular portion leads to further downward movement of residues Y2716.58, M2887.32, and F2917.35 (Fig. 3c). The Y2716.58–F2917.35 motif notably forms a sandwich clamp, stabilizing cmpd644 within the pocket. Mutagenesis assays showed that the F2917.35A substitution completely abolished both βarr1 recruitment and Gi protein activation; while the Y2716.58A substitution resulted in a ten to twenty-fold reduction in both Gi signaling and βarr1 recruitment, confirming the essential role of this Y2716.58–F2917.35 sandwich motif in modulating the signaling (Fig. 3d and Supplementary Table 5). Moreover, the conformational changes induced by cmpd644’s methylpyridine moiety on the lower portion may promote a subtle inward and downward shift of surrounding residues. This rearrangement may enhance the compactness of interactions with cmpd644, particularly involving residues I1093.32, T2957.39, and Y2997.43 (Fig. 3e). This observation aligns with the recently identified hotspots in APJR signaling bias20. Our findings also suggest that minor conformational changes in these residues can lead to significant alterations in signaling output.
a Superimposition of APJR structures bound to comp644 in Gi- (APJR: green, cmpd644: purple) and βarr1-coupled (APJR: blue, cmpd644: orange) states. b Zoomed-in view of the binding pose of cmpd644. Red arrow indicates the downward shift of methylpyrimidine group in cmpd644 in the βarr1-coupled state. c The detailed interactions of Y2716.58, M2886.32, and F2917.35 with cmpd644 in the binding pocket of APJR. The relative movements of residues in the βarr1-coupled APJR, as compared to its Gi-coupled state, are highlighted by red arrows. d Mutagenesis analysis of the Y2716.58–F2917.35 motif on cmpd644-induced βarr1 recruitment (left graph) and Gi activation (right graph) was performed using BRET assays. Data are presented as net BRET (% of WT Emax) and are represented as mean ± SEM (n = 3 independent experiments). See also Supplementary Table 5 for details. e The detailed interactions at the bottom of the APJR orthosteric pocket. Relative movements of residues in the βarr1-coupled APJR, as compared to its Gi-coupled state, are indicated by red arrows.
Comparative analysis of Gi- and βarr1-bound states of APJR
To elucidate the distinct coupling mechanisms between the two transducer-coupled states, we performed a comparative analysis focusing on the transducer-binding interfaces. This analysis was conducted between the cmpd644 bound monomeric Gi-coupled APJR complex (PDB ID: 7W0M) and the higher-resolution cmpd644 bound βarr1-coupled APJRF101A complex. For the transducer-binding interfaces, we observed the finger loop inserting into the receptor core cavity in the similar manner with α5 helix of Gi protein (Supplementary Fig. 7a, b). The cleft in the βarr1-coupled state is wider than in Gi-coupled state (Supplementary Fig. 7a, b). Conversely, the extent to which the Gi protein and βarr1 engage with the cytoplasmic cavity of the APJR is nearly indistinguishable in the monomeric form (Supplementary Fig. 7c). Additionally, the larger finger loop expands the volume of the intracellular binding pocket, resulting in a more pronounced outward expansion of TM6 compared to its interaction with Gi proteins (Supplementary Fig. 7a and d). Furthermore, in the βarr1-coupled structure, TM7 of APJR exhibits an inward shift towards the TM3 and finger loop of βarr1 (Supplementary Fig. 7e), while the TM5 moves towards TM6 (Supplementary Fig. 7d). We observed shared residues that engage both Gi and βarr1, including D1263.49, R1273.50, I1313.54 in TM3, P13434.50 in ICL2, I2285.65, H2315.68, F2325.69 in TM5, and L2466.33 in TM6. However, differential interactions are also noted, including I2496.36 and I2506.37 that form hydrophobic or van der Waals contacts with the α5 helix of Gαi but not with βarr1 (Supplementary Fig. 7f, g). Of note, the L2466.33A decreases the efficacy in Gi signaling slightly while greatly enhances the potency and efficacy of βarr1 recruitment (10-fold improvement on EC50 and 288% on Emax) (Supplementary Fig. 7h and Supplementary Table 6). It appears that the bulky side chain of L2466.33 in TM6 may interfere with the interaction between APJR and βarr1.
Rearrangement of key motifs in the βarr1-coupled state and implication to biased signaling mechanism
The three structures of APJR-βarr1 complex may offer a valuable opportunity to unravel the molecular basis of biased signaling for APJR, which is highly demanded from the drug discovery perspective. We thus compared APJR conformational transitions between the βarr1-coupled state (according to the APJRF101A-βarr1 complex structure, given its higher-resolution) and Gi-coupled state (ProtA from the dimAPJRcmpd644-Gi structure, PDB: 7W0L) activated by cmpd644 (Fig. 4a). Here, we focused on the conformational changes in APJR’s conserved activation motifs when coupled with βarr1 or Gi proteins. We noted the subtle conformational change of the toggle switch W2616.48 and F2576.44 (Fig. 4b), accompanied by the slight downward movement of these two residues when coupled with βarr1.
a Overlay of cmpd644-bound APJR structures in the Gi-coupled (green) and βarr1-coupled (blue) states. Key regions and residues involved in ligand binding and receptor activation are highlighted. The ligand is depicted in purple. b–e The conformational changes during βarr1 coupling in the key activation motifs of APJR: the toggle switch W2616.48 and P5.50I3.40F6.44 motif (b); the rearrangement of the sodium ion-binding pocket (c); the N7.49P7.50xxY7.53 motif (d); and the D3.29R3.50Y3.51 motif (e). Movements of APJR residues upon βarr1 coupling are indicated by red arrows. f Molecular interactions within the intracellular pocket surrounding residue Y3097.53 of APJR in coupling to βarr1. Key interactions include a hydrophobic network formed by the L3.43Y5.58L6.40 motif, along with contributions from residues L1203.43, Y2215.58, and L2536.40. The left panel diagrammatically represents the spatial relationships among these interacting residues. g Functional assays were conducted to evaluate the impact of various substitutions within the L3.43Y5.58L6.40 motif on βarr1 recruitment and Gi protein signaling, quantified as net BRET (% of WT Emax) and data are represented as mean ± SEM (n = 3 independent experiments). See also Supplementary Table 8 for details. h, Schematic representation of the βarr1-bound conformations of APJR. The key residues and motifs contributing to biased signaling are marked with different colors (purple for the Y2716.58–F2917.35 motif; orange for the sodium ion-binding pocket; green for the L3.43Y5.58L6.40 motif and Y7.53). Movements of residues are indicated by red arrows.
The sodium ion is widely recognized as an endogenous negative allosteric modulator (NAM) of class A GPCR activation, stabilizing the receptor’s inactive conformation and undergoing rearrangement upon receptor activation27,28,29,30. Recent structural analyses of the APJR-Gi complex bound with G protein-biased ligands have proposed the sodium ion-binding pocket as a key motif in the APJR biased signaling mechanism20. Our study revealed significant changes concerning the sodium ion-binding pocket in the APJR-βarr1 complex. Specifically, we observed an upward displacement of N461.50 and a movement towards TM7, resulting in a polar interaction between N461.50 and S3027.46. Additionally, the shift of N3057.49 towards TM2 was noted, enhancing its interaction with residue D752.50 (Fig. 4c). This interaction network potentially constrains the relative positions of TM1 and TM7. These structural observations are supported by the reported mutagenesis assays showing that the N461.50A substitution abolished βarr1 recruitment but preserved Gi protein activation, indicating the importance of N461.50 in βarr1 recruitment20.
We next investigated the N7.49P7.50xxY7.53 motif, which has been proposed to contribute to the selectivity of downstream transducers31. While Y3097.53 within the N7.49P7.50xxY7.53 motif maintains a similar rotamer conformation in both the βarr1-coupled and Gi-coupled states (Fig. 4d), a displacement (1.7 Å) of N3057.49 towards TM2 and TM3 was observed during βarr1 coupling (Fig. 4c, d). We conducted BRET assays on N3057.49A and Y3097.53A mutants to assess their effects on βarr1 recruitment and Gi protein signaling pathway activation. The results indicated that the N3057.49A mutation notably abolished βarr1 recruitment while only slightly affecting Gi protein activity (Supplementary Fig. 7i and Supplementary Table 7). These findings suggest that mutations in the N7.49P7.50xxY7.53 motif, particularly at the N7.49 position, disrupt critical interaction network necessary for effective βarr1 coupling.
Inspired by the CB1-βarr1 structure, where a T3.46Y5.58Y7.53 triad motif is identified as critical in stabilizing the TM core of CB1 in a specific conformation preferentially coupling to βarr126, we investigated this motif further within our APJR-βarr1 complex structures. Despite APJR lacking the threonine at position 3.46 on TM3, Y3097.53 likely facilitates the formation of a hydrophobic network at the equivalent region. This network involves residues L1203.43 on TM3, Y2215.58 on TM5, and L2536.40 on TM6, which we have designated as the L3.43Y5.58L6.40 motif (Fig. 4f). The displacement of the Gi α5 helix by the bulkier finger loop of βarr1 results in the inward movement of TM5 towards TM6, accompanied by the shift of the Y2215.58 side chain towards Y3097.53 on TM7 (Fig. 4f and Supplementary Fig. 7d). Additionally, the residue L1203.43 exhibits a slight upward displacement. Mutations within this motif, especially L1203.43A and Y2215.58A, showed a more pronounced inhibitory effect on βarr1 recruitment compared to Gi protein activation (Fig. 4g and Supplementary Table 8). These observations indicate the critical role of the L3.43Y5.58L6.40 motif in modulating selective downstream transducers for APJR. On the other hand, the classical activation-switch motif D3.49R3.50Y3.51 exhibits a similar conformation between the two transducer-coupled states (Fig. 4e). These analyses characterized the key motifs such as N7.49P7.50xxY7.53 and L3.43Y5.58L6.40 that contribute to the differential coupling of two transducers and may offer critical insight into the structural basis for functional selectivity.
Discussion
This study provides insights into the structural basis of APJR’s differential coupling mechanisms with β-arrestin and Gi proteins. One notable discovery is the variable stoichiometries (2:2 and 2:1) observed in the APJR-βarr1 complexes, suggesting a flexible binding mechanism that diverges from the typically monomeric interactions noted in other class A GPCR-β-arrestin complexes. By presenting cryo-EM structures of distinct APJR-βarr1 complexes, our research significantly enhances the understanding of APJR’s versatile signaling profiles. This study also represents a substantial advancement beyond the previously characterized APJR-Gi complexes with a range of ligands: (1) For the dimeric APJR, it can only engage a single copy of Gi proteins, presumably because the Gi proteins partially occupies the binding site of ProtB. In contrast, the dimeric APJR-βarr1 structure reveals that both protomers can couple with βarr1, forming a 2:2 complex, while the binding of βarr1 to each protomer could be dynamic (Fig. 5). (2) In addition, although we characterized a monomeric APJR-Gi structure from the wild-type APJR-Gi sample, the monomeric APJR-βarr1 complex might be in minority, if any, in the wild-type sample. Instead, by employing the F1013.24A dimer-switch mutation, we were able to determine a high-resolution structure of the monomeric APJRF101A-βarr1 complex (1:1 stoichiometry). (3) Most importantly, our investigation into the functional distinctions between different stoichiometries of the APJR-βarr1 complex revealed the functional dynamics between the protomers. Our luciferase complementation assay results indicate that while one protomer can initiate βarr recruitment, the full activation of the APJR dimer requires the coordinated effort of both protomers (Fig. 3). Consequently, the dynamic interplay between the protomers is crucial for effective βarr1 recruitment and the activation of signaling pathways. Together with our prior research into APJR dimerization, our findings reveal the following potential functions of APJR dimerization: First, dimer formation may inhibit the basal activity of APJR in the Gi signaling pathway; Second, ligand binding coupled with G protein interaction may promote dimer dissociation during activation; Third, through structural analysis and functional assays, we establish that 2:2 stoichiometry is essential for maximal β-arrestin recruitment for APJR.
Dimeric APJR Activation: The apo form of the APJR dimer (grey, PDB ID: 8XQI) transitions to a fully active state upon binding the agonist cmpd644 and coupling with Gi proteins (orange), forming a 2:1 APJR-Gi protein complex (PDB ID: 7W0L). On the other hand, the dimeric APJR (green) can couple with βarr1 (blue), leading to fully and partially active APJR-βarr1 complexes with 2:2 and 2:1 stoichiometric ratios, respectively. Red arrows indicate the further TM6 movements in the βarr1-coupled state, with key residues R3.50, L6.33, and Y7.53 highlighted.
Our APJR-βarr1 (2:2 and 2:1) and APJRF101A-βarr1 structures expand the structural landscape of class A GPCR-βarr1 complexes. We conducted comparative analysis using the higher-resolution APJRF101A-βarr1 complex structure. Consistent with other class A GPCRs, this complex adopts a core conformation primarily established through the interaction between the βarr1 finger loop and the receptor (Supplementary Fig. 8a). The finger loop of βarr1 fosters extensive interactions with the intracellular core of APJR, which involve both hydrophobic and electrostatic interactions (Supplementary Fig. 8b). In comparison to the other reported βarr1 complexes including the vasopressin hormone-V2 receptor (V2R), β1-adrenoceptor (β1AR), M2 muscarinic receptor (M2R) and cannabinoid receptor 1 (CB1), the finger loop in the APJRF101A-βarr1 complex adopts a similarly extended loop configuration (Supplementary Fig. 8a)26,32,33,34,35,36,37,38. Furthermore, in our APJRF101A-βarr1 complex, ICL2 of APJR adopts a helical configuration, in sharp contrast to the loop configuration observed in the Gi-bound state (Supplementary Fig. 8c). In this helical form, ICL2 is positioned between the C-loop and the middle loop (Supplementary Fig. 8c). During the revision of this manuscript, a class C GPCR, mGluR3 coupled with βarr1 study was reported (PDB ID: 9II2), showing the similar GPCR: arrestin stoichiometry at 2:1 or 2:2. The structure of mGluR3–βarr1-one (2:1) closely resembles that of mGluR3–βarr1-two (2:2). We thus aligned βarr1 from the mGluR3–βarr1-two (2:2) with our APJR-βarr1 (2:2) complexes, revealing a similar architecture of the arrestins. This architecture is also consistent with previously published GPCR-arrestin structures (Supplementary Fig. 8d). Additionally, overlaying the mGluR3 TMD onto APJR–arrestin complexes reveals a different orientation of βarr1, consistent with the comparative analysis with other class A GPCR-arrestin structures as mentioned in the mGluR3-βarr1 study, suggesting the diverse arrestin coupling landscape (Supplementary Fig. 8e).
The structure of APJR-βarr1 elucidated in this study provide crucial insight into the molecular mechanisms driving biased signal transduction. By comparing APJR in the βarr1-coupled state with its Gi-coupled state when bound to the same ligand (Fig. 4a), we sought to unravel the propagation of extracellular signals to intracellular domains. Firstly, the engagement of key residues within the top of the orthosteric binding pocket (extracellular domain), such as the Y2716.58–F2917.35 clamping motif formed by TM6 and TM7, underwent intricate shifts. Additionally, the sodium ion-binding pocket surrounded by D752.50 also underwent rearrangement. In particular, residues N461.50 and N3057.49 displayed significant conformational changes in the β-arrestin coupled state, suggesting its role in facilitating biased signaling through APJR. Additionally, the N7.49P7.50xxY7.53 motif appears to function as a critical switch in biased signaling. Finally, the hydrophobic interactions around Y3097.53 led to the identification of a conserved L3.43Y5.58L6.40 motif linked to biased signaling, with mutations in these residues resulting in altered signaling profiles, emphasizing their pivotal roles (Fig. 4h). These integrated analyses have delineated the molecular details of biased agonism in APJR, revealing key residues and motifs that govern the selective engagement of downstream signaling transducers. Additionally, the conservation of these motifs across class A GPCRs may suggest a general framework for understanding the structural basis of biased signaling.
Based on these structural findings, we propose several guidelines for drug design strategies: (1) Structural changes involving residues such as N461.50 in the sodium ion-binding pocket highlight its role in β-arrestin recruitment. By disrupting interactions at this site, it may be possible to shift the signaling bias towards G proteins, which is therapeutically desirable for mitigating cardiac hypertrophy. (2) Disruption of the L3.43Y5.58L6.40 hydrophobic network may preferentially activate G protein pathways, providing another avenue for biased signaling modulation. (3) The versatile stoichiometries (2:2 and 2:1) observed in the APJR-βarr1 complex structures, along with the comprehensive functional assessment, indicate that protomer interactions influence signaling outcomes. Drug designs that promote an APJR-β-arrestin 2:1 ratio could selectively dampen β-arrestin recruitment. By incorporating these structural insights into the design framework, future APJR ligand development can potentially achieve precise signaling profiles, offering therapeutic advantages.
In summary, this study elucidates the variable stoichiometries present in APJR-βarr1 complexes and uncovers the functional implications of the versatile regulation between the two APJR-βarr1 configurations. By mapping the detailed molecular interfaces that govern βarr1 interactions and identifying key conformational rearrangements within the ligand binding pocket and crucial activation motifs, we provide a comprehensive framework for understanding differential transducer coupling and biased signaling. Collectively, these findings significantly advance our understanding of APJR’s complex signaling mechanisms, offering insights into the broader dynamics of GPCR-arrestin interactions. By unraveling the molecular basis of biased signaling, this study lays the foundation for the development of next-generation, signaling-biased agonists targeting APJR to treat cardiovascular and metabolic disorders.
Limitations of the study
To obtain a stable APJR-βarr1 complex, we have engineered the receptor with fusion of the V2R C-tail to the C-terminus of APJR (Supplementary Fig. 1b). Consequently, the specific phosphorylation patterns of native APJR required for βarr1 recruitment remains uncharacterized. Notably, previous studies of GPCR-βarr1 using the same V2R fusion strategy did not observe the diverse GPCR:βarr1 stoichiometry, suggesting it is less likely that the various APJR:βarr1 stoichiometries are induced by the C-terminal modifications. Further investigation into native APJR-transducer stoichiometry in living cells is warranted. Additionally, the physiological role of APJR dimerization in disease contexts remains an intriguing question meriting future study.
Methods
Construct cloning and protein expression for cryo-EM study
To facilitate protein expression, the gene APLNR, encoding human WT APJR, was sub-cloned into the pFastBac1 vector with the N-terminal fusion consisting of a Haemagglutinin (HA) signal peptide, Flag tag, 10 × His tag, and BRIL fusion39. The C-terminal residues H331–F380 of APJR were replaced with the residues A343–S371 in the C terminus of V2R (generated by GenScript)40. The additional F101A mutation was introduced for the study of monAPJRF101A-βarr1 complex. A cysteine-free bovine βarr1 was generated by introducing the mutations C59A, C125S, C140I, C150V, C242V, C251V, and C269S to enhance the expression level and protein stability24,32,37. The pre-activated mutation R169E was also introduced to increase the activation level of βarr138,41. Furthermore, the C-terminal region of βarr1 (residues I377–R418) was replaced with an engineered single-chain Fab30 (scFv30) to stabilize the APJR-βarr1 complex, and a 6 × His tag was added to the C terminus. The modified APJR and βarr1 were co-expressed with GRK2 in Trichuplusia ni Hi5 insect cells using the Bac-to-bac system (Invitrogen, #B85502). The cells were infected with baculoviruses for APJR, βarr1-scFv30, and GRK2 at a 1:1:1 ratio, at a density of 2 × 106 cells per mL. Cells were cultured for 48 h at 27 °C following infection, then collected by centrifugation, and the resulting cell pellets were stored at −80 °C.
Complex formation and purification
The cell pellets expressing the APJR-βarr1 complex were thawed on ice and lysed in a hypotonic buffer containing 10 mM HEPES pH 7.5, 10 mM MgCl2, 20 mM KCl, and a protease inhibitor cocktail (MedChemExpress, #HY-K0010) by dounce homogenization.
To facilitate complex formation, 20 µM cmpd644 was added into the suspension, followed by 1 h incubation at room temperature. Post-centrifugation at 140,000 × g for 20 min at 4 °C, the resulting pellet was then solubilized by 50 mM HEPES pH 7.5, 100 mM NaCl, 0.5% (w/v) LMNG (Anatrace, #NG310), and 0.1% (w/v) CHS (Sigma-Aldrich, #C6512), supplemented with 20 µM cmpd644, and incubating for 2 h at 4 °C. The solubilized supernatant was collected and incubated with Talon superflow metal affinity resin (Clontech, #635670) with 20 mM imidazole (ABCONE, #I99222) added, followed by an overnight incubation at 4 °C. The mixture was then loaded onto a chromatography column and washed with 14 column volumes (CV) of washing buffer containing 50 mM HEPES pH 7.5, 100 mM NaCl, 5% glycerol, 0.01% (w/v) LMNG, 0.002% (w/v) CHS, 30 mM imidazole, and 20 µM cmpd644. Elution of the complex was performed with 4 CV of elution buffer containing 50 mM HEPES pH 7.5, 100 mM NaCl, 5% glycerol, 0.01% (w/v) LMNG, 0.002% (w/v) CHS, 200 mM imidazole, and 20 µM cmpd644. This mixture underwent size-exclusion chromatography on a Superdex 200 10/300 GL column (GE Healthcare, #28990944) with running buffer containing 20 mM HEPES pH 7.5, 100 mM NaCl, 0.00075% (w/v) LMNG, 0.0002% (w/v) CHS, and 2 µM cmpd644. The peak fractions were analyzed by SDS-PAGE and concentrated to a final protein concentration of 2 mg/mL for electron microscopy experiments.
Cryo-EM sample preparation and data collection
Holey carbon grid (Quantifoil, #R1.2/1.3Au300) was glow-discharged for 45 s with H2/O2. 3 μL complex was then applied on the grid, using the Vitrobot Mark IV (Thermo Fisher Scientific, USA) to prepare the sample. The chamber of Vitrobot was set to 100% humidity, 4 °C and the sample preparation parameters were set to blot time 3 s with blot force −1. The cryo-EM dataset was collected on a Titan Krios 300 kV electron microscope (Thermo Fisher Scientifics, USA). The calibrated magnification was 165,000 with the pixel size of 0.832 Å/pixel for APJR-βarr1 complex. Each movie consisted of 40 frames with a total dose of 60 e−/Å2, and the dose rate was 15 e−/Å2/s. Data collection was done using SerialEM v3.8.0 software with a defocus range of −0.7 μm to −2.2 μm.
Cryo-EM image processing and 3D reconstruction
For the structural analysis of the APJR-βarr1 complex, a dataset comprising 7253 movies was acquired and subsequently processed using software package of cryoSPARC v4.542. Beam-induced motion artifacts were corrected by applying the patch motion correction algorithm. The Contrast Transfer Function (CTF) parameters for each dose-weighted micrograph were determined using the patch CTF estimation module. Auto blob picking was processed on a small set of 500 images and yielded 245,380 particles. After 2 cycles 2D classification, 15,174 particles in good 2D feature were selected out to train in Topaz and then deep picker on whole set of images was processed to yield a total of 7,911,688 particles. These particles underwent 2D classification, resulting in the selection of 887,490 particles for the generation of initial models. These models served as a basis for further several cycles of 3D classification through heterogeneous refinement within cryoSPARC. A subsequent round of 3D classification yielded refined particle subsets: 209,447 particles for the APJR-βarr1 (2:2) complex and 213,186 particles for the APJR-βarr1 (2:1) complex. These subsets were subjected to final homogeneous refinement, non-uniform refinement, and local refinement in cryoSPARC, culminating in density maps with nominal resolutions of 3.49 Å for the APJR-βarr1 (2:2) complex and 3.57 Å for the APJR-βarr1 (2:1) complex. The resolutions were established based on the gold standard Fourier shell correlation (FSC) at the 0.143 threshold. Local resolution variations were assessed using the local resolution estimation tool in cryoSPARC. To enhance the local density of maps, automatic masking and local sharpening procedures were conducted utilizing DeepEMhancer43.
For the structural analysis of the APJRF101A-βarr1 complex, a dataset comprising 7750 movies was acquired and subsequently processed using software package of cryoSPARC v4.542. Beam-induced motion artifacts were corrected by applying the patch motion correction algorithm. The Contrast Transfer Function (CTF) parameters for each dose-weighted micrograph were determined using the patch CTF estimation module. Auto blob picking was processed on a small set of 500 images and yielded 244,796 particles. After 2 cycles 2D classification, 10,521 particles in good 2D feature were selected out to train in Topaz and then deep picker on whole set of images was processed to yield a total of 2,831,433 particles. These particles underwent 2D classification, resulting in the selection of 508,470 particles for the generation of initial models. After several rounds of 3D classification, 292,664 particles were selected out for final homogeneous refinement followed by non-uniform refinement and local refinement in cryoSPARC, culminating in density maps with nominal resolutions of 3.21 Å for the monAPJRF101A-βarr1 complex. The resolutions were established based on the gold standard Fourier shell correlation (FSC) at the 0.143 threshold. Local resolution variations were assessed using the local resolution estimation tool in cryoSPARC. To enhance the local density of maps, automatic masking and local sharpening procedures were conducted utilizing DeepEMhancer43.
Cryo-EM model building and refinement
Reference models with Protein Data Bank (PDB) identifiers 7W0L and 7W0M were utilized for model construction and iterative refinement against the EM density map. Components of the target models were initially positioned within the EM density map employing UCSF Chimera v1.1544, succeeded by manual modifications and iterative rebuilding via Coot v.0.9.8.545, and subsequent real-space refinement using Phenix v.1.20.146. Model quality was assessed and validated by MolProbity v4.247. Visualization and preparation of structural figures were achieved with UCSF Chimera v1.15, Chimera X v1.7.1, and PyMOL v2.5.8 (http://www.pymol.org). Comprehensive refinement metrics are detailed in Supplementary Table 2.
Bioluminescence resonance energy transfer (BRET) assays
For β-arrestin recruitment and G protein activity measurement, BRET assays were performed in 96-well plate48,49. The β-arrestin ebBRET sensor (βarr1-Rluc and Venus-CAAX, 30 ng and 40 ng/well, respectively), or Gi protein dissociation BRET sensor (Gαi1-Nluc, Gβ1, and Venus-Gγ2, 1.5 ng, 10 ng, and 10 ng/well, respectively) were transfected together with wild-type APJR (50 ng/well) or APJR mutants (50 ng/well) in HEK293 cells (ATCC, #CRL-1573) and seeded in 96-well plates. For β-arrestin recruitment to APJR dimer, LgBiT was fused to the C-terminus of APJR with a linker GSGGSGGGGSGSGSGSGS sequence; HiBiT was fused to the C-terminus of APJR with a linker GSSGGGGSGGGGSSG sequence. Cells were transfected with Venus-β-arrestin1 (30 ng/well) together with APJR-LgBiT (15 ng/well) and APJR-HiBiT (15 ng/well) or indicated mutants to form complementary APJR dimer. After 24 h transfection, cells were washed and starved in PBS at 37 °C for 1 h. BRET measurements were performed using PHERAstar FS (BMG Labtech, USA). The signals emitted by the donor (460–500 nm band-pass filter, Em 480) and the acceptor entity (510–550 nm band-pass filter, Em 530) were recorded after the addition of 5 µM coelenterazine (for Rluc) or 10 μM furimazine (for Nluc). The BRET signal was determined by calculating the ratio between the emission of acceptor and donor (Em 530/Em 480). The basal BRET ratio (BRETbasal) of cells was recorded before the stimulation with drugs or buffer. The change in BRET ratio (net BRET) was obtained by subtracting the BRET ratio between agonist treatment and the basal BRET.
Cell surface quantification by ELISA
ELISA was performed for detection of the Flag-tagged APJR WT and mutants at the cell surface. 24 h after transfection, the HEK293 cells were fixed with 4% paraformaldehyde, blocked with 10% FBS. Flag-tagged receptors were detected with the mouse monoclonal anti-Flag antibody (with horseradish peroxidase) M2 (Sigma Aldrich, #A8592) at 1.0 μg/mL. HA tagged constructs were detected with a monoclonal rat anti-HA antibody (with horseradish peroxidase) 3F10 (Roche, #12158167001) at 0.5 μg/mL. Bound antibodies coupled to horseradish peroxidase were detected by chemoluminescence using SuperSignal substrate (Pierce) and read using the Tecan infinite 200Pro (Swiss).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The cryo-EM density map generated in this study of the APJR-βarr1 (2:2), APJR-βarr1 (2:1), and APJRF101A-βarr1 have been deposited in the Electron Microscopy Data Bank (EMDB) under accession code EMD-62582, APJR-βarr1 (2:2), EMD-62583, APJR-βarr1 (2:1), and EMD- 62581, APJRF101A-βarr1. The atomic coordinates have been deposited in the Protein Data Bank (PDB) under accession codes 9KUW, APJR-βarr1 (2:2), 9KUX, APJR-βarr1 (2:1), and 9KUV, APJRF101A-βarr1, respectively. Previously reported PDB codes cited in this manuscript: 7W0L, 7W0M, 8XQI and 9II2. The source data underlying Figs. 2c, e, 3d and 4g and Supplementary Figs. 1c and 7h, i are provided as a Source Data file. Source data are provided with this paper.
References
Chapman, F. A., Maguire, J. J., Newby, D. E., Davenport, A. P. & Dhaun, N. Targeting the apelin system for the treatment of cardiovascular diseases. Cardiovasc. Res. 119, 2683–2696 (2023).
Bertrand, C., Valet, P. & Castan-Laurell, I. Apelin and energy metabolism. Front. Physiol. 6, 115 (2015).
Scimia, M. C. et al. APJ acts as a dual receptor in cardiac hypertrophy. Nature 488, 394–398 (2012).
Yue, Y. et al. Structural insight into apelin receptor-G protein stoichiometry. Nat. Struct. Mol. Biol. 29, 688–697 (2022).
Yue, Y. et al. Structural insights into the regulation of monomeric and dimeric apelin receptor. Nat. Commun. 16, 310 (2025).
Szokodi, I. et al. Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circ. Res. 91, 434–440 (2002).
Ashley, E. A. et al. The endogenous peptide apelin potently improves cardiac contractility and reduces cardiac loading in vivo. Cardiovasc. Res. 65, 73–82 (2005).
Jia, Y. X. et al. Apelin protects myocardial injury induced by isoproterenol in rats. Regul. Pept. 133, 147–154 (2006).
Siddiquee, K. et al. Apelin protects against angiotensin II-induced cardiovascular fibrosis and decreases plasminogen activator inhibitor type-1 production. J. Hypertens. 29, 724–731 (2011).
Chun, H. J. et al. Apelin signaling antagonizes Ang II effects in mouse models of atherosclerosis. J. Clin. Invest. 118, 3343–3354 (2008).
Kuba, K. et al. Impaired heart contractility in apelin gene-deficient mice associated with aging and pressure overload. Circ. Res. 101, e32–e42 (2007).
Higuchi, K. et al. Apelin, an APJ receptor ligand, regulates body adiposity and favors the messenger ribonucleic acid expression of uncoupling proteins in mice. Endocrinology 148, 2690–2697 (2007).
Vinel, C. et al. The exerkine apelin reverses age-associated sarcopenia. Nat. Med. 24, 1360–1371 (2018).
Zhou, Q., Chen, L., Tang, M., Guo, Y. & Li, L. Apelin/APJ system: a novel promising target for anti-aging intervention. Clin. Chim. Acta 487, 233–240 (2018).
Xu, S., Tsao, P. S. & Yue, P. Apelin and insulin resistance: another arrow for the quiver? J. Diabetes 3, 225–231 (2011).
Li, C. et al. The role of apelin-APJ system in diabetes and obesity. Front. Endocrinol. 13, 820002 (2022).
Mehri, K., Hamidian, G., Zavvari Oskuye, Z., Nayebirad, S. & Farajdokht, F. The role of apelinergic system in metabolism and reproductive system in normal and pathological conditions: an overview. Front. Endocrinol. 14, 1193150 (2023).
Hu, H., He, L., Li, L. & Chen, L. Apelin/APJ system as a therapeutic target in diabetes and its complications. Mol. Genet. Metab. 119, 20–27 (2016).
Arnold, C. After obesity drugs’ success, companies rush to preserve skeletal muscle. Nat. Biotechnol. 42, 351–353 (2024).
Wang, W. W. et al. Structure-based design of non-hypertrophic apelin receptor modulator. Cell 187, 1460–1475.e1420 (2024).
Ma, Y. et al. Structural basis for apelin control of the human apelin receptor. Structure 25, 858–866.e854 (2017).
Winkle, P. et al. A first-in-human study of AMG 986, a novel apelin receptor agonist, in healthy subjects and heart failure patients. Cardiovasc. Drugs Ther. 37, 743–755 (2023).
Trivedi, A. et al. Evaluation of the pharmacokinetics and safety of AMG 986 tablet and capsule formulations in healthy adult subjects: a phase I, open-label, randomized study. Drugs R. D. 22, 147–154 (2022).
Chen, K. et al. Tail engagement of arrestin at the glucagon receptor. Nature 620, 904–910 (2023).
Wen, T. et al. Molecular basis of β-arrestin coupling to the metabotropic glutamate receptor mGlu3. Nat. Chem. Biol. 21, 1262–1269 (2025).
Liao, Y. Y. et al. Snapshot of the cannabinoid receptor 1-arrestin complex unravels the biased signaling mechanism. Cell 186, 5784–5797.e5717 (2023).
Liu, W. et al. Structural basis for allosteric regulation of GPCRs by sodium ions. Science 337, 232–236 (2012).
Katritch, V. et al. Allosteric sodium in class A GPCR signaling. Trends Biochem. Sci. 39, 233–244 (2014).
Hori, T. et al. Na(+)-mimicking ligands stabilize the inactive state of leukotriene B(4) receptor BLT1. Nat. Chem. Biol. 14, 262–269 (2018).
Zhang, M. et al. G protein-coupled receptors (GPCRs): advances in structures, mechanisms, and drug discovery. Signal Transduct. Target Ther. 9, 88 (2024).
Suomivuori, C. M. et al. Molecular mechanism of biased signaling in a prototypical G protein-coupled receptor. Science 367, 881–887 (2020).
Staus, D. P. et al. Structure of the M2 muscarinic receptor-β-arrestin complex in a lipid nanodisc. Nature 579, 297–302 (2020).
Bous, J. et al. Structure of the vasopressin hormone-V2 receptor-β-arrestin1 ternary complex. Sci. Adv. 8, eabo7761 (2022).
Lee, Y. et al. Molecular basis of β-arrestin coupling to formoterol-bound β(1)-adrenoceptor. Nature 583, 862–866 (2020).
Kang, Y. et al. Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser. Nature 523, 561–567 (2015).
Yin, W. et al. A complex structure of arrestin-2 bound to a G protein-coupled receptor. Cell Res. 29, 971–983 (2019).
Huang, W. et al. Structure of the neurotensin receptor 1 in complex with β-arrestin 1. Nature 579, 303–308 (2020).
Cao, C. et al. Signaling snapshots of a serotonin receptor activated by the prototypical psychedelic LSD. Neuron 110, 3154–3167.e3157 (2022).
Chun, E. et al. Fusion partner toolchest for the stabilization and crystallization of G protein-coupled receptors. Structure 20, 967–976 (2012).
Thomsen, A. R. B. et al. GPCR-G protein-β-arrestin super-complex mediates sustained G protein signaling. Cell 166, 907–919 (2016).
Kovoor, A., Celver, J., Abdryashitov, R. I., Chavkin, C. & Gurevich, V. V. Targeted construction of phosphorylation-independent beta-arrestin mutants with constitutive activity in cells. J. Biol. Chem. 274, 6831–6834 (1999).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Sanchez-Garcia, R. et al. DeepEMhancer: a deep learning solution for cryo-EM volume post-processing. Commun. Biol. 4, 874 (2021).
Pettersen, E. F. et al. UCSF Chimera-a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D. Biol. Crystallogr. 66, 486–501 (2010).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D. Biol. Crystallogr. 66, 12–21 (2010).
Liu, J. et al. Biased signaling due to oligomerization of the G protein-coupled platelet-activating factor receptor. Nat. Commun. 13, 6365 (2022).
Xu, C. et al. Specific pharmacological and G(i/o) protein responses of some native GPCRs in neurons. Nat. Commun. 15, 1990 (2024).
Acknowledgements
We thank Qiwen Tan, Qiaoyun Shi, Lu Zhang, Junlin Liu, Suwen Hu, Na Chen, Ling Wang, Fangfang Zhou, Xiaoyan Liu, Pei Si for protein cloning, expression, and assay support; Li Wang, Dandan Liu, Qianqian Sun, Yuan Pei at the Bio-EM facility at ShanghaiTech University for technical support on cryo-EM data collection. This work was supported by the National Natural Science Foundation of China (grants W2412030 to F.X.; 32330049 to J.L.) and Innovative Research Team of High-Level Local Universities in Shanghai.
Author information
Authors and Affiliations
Contributions
F.X. and J.L. conceived the study. Y.Y. performed cloning and purification of APJR-βarr1 complexes with compound, performed cryo-EM sample preparation, data collection, and structure analysis. L.W. built and refined structural models. C.X. designed and guided BRET experiments of βarr recruitment and G protein activation. X.C., Y.S., and S.W. performed BRET experiments and C.X. prepared the related figures. X.L. and F.L. assisted in BRET assays. M.N., K.X., and L.X. assisted in cryo-EM sample preparation. A.W. and J.L. participated in the interpretation of the data. All authors analyzed the results. Y.Y., C.X., J.L., and F.X. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Sudarshan Rajagopal, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yue, Y., Xu, C., Wu, L. et al. Mechanistic insights into the versatile stoichiometry and biased signaling of the apelin receptor-arrestin complex. Nat Commun 16, 7403 (2025). https://doi.org/10.1038/s41467-025-62870-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-62870-z
This article is cited by
-
Molecular basis of antagonism of the dimeric human arginine vasopressin receptor 1A
Nature Communications (2026)