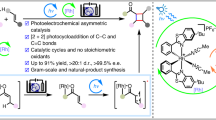
Abstract
Asymmetric dearomative photocycloaddition has emerged as a transformative strategy for the enantioselective construction of complex three-dimensional molecular architectures from simple planar aromatic precursors. While significant progress has been made in this field, the scope has largely been confined to electron-rich and electron-neutral aromatic systems. Herein, we present a breakthrough with the development of the direct asymmetric dearomative photocycloaddition involving electron-deficient isoquinolines. Our approach employs a quaternary carbon formation strategy to effectively suppress potential aromatization pathways. By establishing a synergistic photoredox and chiral hydrogen-bonding catalysis system, we achieve highly regioselective reactions between various acyclic and cyclic terminal enones and internal enones with the azaaryl ring of isoquinolines. This methodology facilitates the efficient synthesis of pharmaceutically relevant complex benzotropane derivatives, yielding satisfactory results in terms of yield, ee, and dr. Notably, this system demonstrates remarkable versatility in constructing three or four consecutive stereocenters, including challenging all-carbon quaternary stereocenters.
Similar content being viewed by others
Introduction
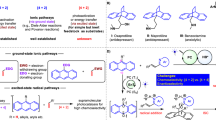
Photocycloaddition has emerged as a transformative strategy for constructing diverse pharmaceutically relevant (hetero)cyclic molecules1,2,3 by efficiently converting photon energy into chemical energy, activating inert molecules, and enabling transformations under mild conditions4,5,6,7. When combined with dearomatization strategies, this process can convert planar aromatic molecules into complex three-dimensional structures2,8,9,10,11, aligning with the concept of escape from flatland in pharmaceutical chemistry12,13. The development of innovative asymmetric catalytic systems has further expanded the synthetic utility of these transformations, leading to the successful implementation of enantioselective manifolds with various electron-rich14,15,16,17 (e.g., benzofurans, indoles) and electron-neutral18,19,20,21 (e.g., anthracenes, naphthalenes) substrates (Fig. 1A). Notably, the potential of imine-containing azaarenes remains largely untapped in asymmetric dearomative photocycloaddition22, despite their established utility in photoredox catalytic asymmetric Minisci-type reactions for constructing enantioenriched azaarene derivatives.
A Representative (aza)arenes in asymmetric dearomative photocycloadditions. B Racemic dearomative [3 + 2] photocycloaddition of isoquinoline N-oxides via SET. C Racemic dearomative [3 + 2] photocycloaddition of 4-substituted quinolines via SET. D Asymmetric dearomative [3 + 2] photocycloaddition of isoquinolines via SET (this design). DPZ = 5,6-bis(5-methoxythiophen-2-yl)pyrazine-2,3-dicarbonitrile. HE Hantzsch ester, CPA chiral phosphoric acid, LED light-emitting diode.
Recently, energy transfer (EnT) approaches have been increasingly applied to directly activate imine-containing bicyclic azaarenes, such as quinolines and isoquinolines, for cycloadditions involving their arene rings23,24,25,26. In contrast, we have pioneered a distinct strategy centered on the participation of azaaryl rings, enabling a dearomative [3 + 2] photocycloaddition between α-amino acids and isoquinoline N-oxides, through photocatalytic single-electron transfer (SET) strategy in 2019 (Fig. 1B)27. The pre-oxidation of isoquinolines to their N-oxide derivatives was critical, as the ammonium salts could effectively promote the addition of α-amino radicals to the imine moiety while simultaneously preventing product aromatization through tertiary amine formation. In 2020, Dixon and Duarte contributed the SET platform to conduct the dearomative [3 + 2] photocycloaddition between N-arylimine and quinolines, thereby diverting the reductive Minisci reaction, which is contingent upon the presence of a substituent at the 4-position of quinolines (Fig. 1C)28. Building on these exciting results, we hypothesize extending this strategy to isoquinolines by incorporating suitable substituents at the 1-position to enhance the thermodynamic stability of the dearomative cycloaddition products, as the formed quaternary carbon position would prevent further aromatization. However, steric constraints pose formidable challenges in achieving viable reactivity and precise selectivity during photocycloaddition processes8. To circumvent these limitations, we propose chiral acid catalysis as a robust platform for enantioselective synthesis, a strategy that could bypass the inherent restrictions of ammonium salt-mediated approaches.
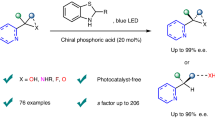
Herein, we report the asymmetric dearomative photocycloaddition reactions of isoquinolines, achieved through a synergistic photoredox and chiral Brønsted acid catalysis platform (Fig. 1D)29,30,31,32,33. By employing an amine as the terminal reductant, the reactions deliver a diverse array of enantioenriched [3 + 2] cycloaddition products—structurally attractive tropane derivatives—in high yields with high enantioselectivities and diastereoselectivities. A broad range of acyclic and cyclic terminal enones and internal enones as reaction partners were demonstrated to be compatible, enabling the precise construction of three or even four consecutive stereocenters on the molecular scaffolds. Notably, in addition to the uniform aza-quaternary carbon stereocenter, an all-carbon quaternary stereogenic center can be concurrently and precisely assembled.
Results
We initiated our investigation by selecting α-benzoylstyrene 1 as the model substrate to react with isoquinoline, based on the premise that the enolate radical derived from 1 via single-electron reduction34 has been shown to readily engage with electron-deficient double bonds (Table 1A). We postulated that the α-phenyl group of the enolate would enhance the stability of the enolate form, thereby facilitating the desired cyclization. However, initial attempts using simple isoquinoline as the substrate, in the presence of various photosensitizers, reductants, and chiral Brønsted acids, failed to yield the desired cyclic adduct, highlighting the inherent reactivity challenges. To address this, we proposed introducing an ester group at the 1-position of isoquinoline to suppress potential aromatization, which we suspected was inhibiting the cyclization. Indeed, when employing 1.0 mol% DPZ as the photosensitizer, 10 mol% SPINOL-derived chiral phosphoric acid (CPA) C1, and N-benzyl-N-ethylaniline H1 as the reductant under 3 W blue LED irradiation, a trace amount of product 7 was detected using phenyl ester as the 1-substituent of isoquinoline (2). This encouraging result prompted us to explore a range of ester substituents (3–6). Substrate 5, featuring a methyl ester, afforded product 10 in 61% yield with 73% enantiomeric excess (ee), while isoquinoline 6, containing an ethyl ester, delivered product 11 with 56% yield and a significantly improved ee of 76%, identifying the ethyl ester as the optimal substituent. Notably, the use of isopropyl (3) and tert-butyl (4) ester substituents did not yield more favorable outcomes, producing products 8 and 9 with inferior results. These findings underscore the critical role of the ester substituent in modulating reactivity and enantioselectivity.
We next systematically optimized the reaction conditions for the transformation between enone 1 and isoquinoline 6 (Table 1B). Pleasingly, the reaction conducted in CPME at −10 °C for 36 h, employing 1.0 mol% DPZ as the photosensitizer, 15 mol% chiral phosphoric acid C1, and 2.0 equivalents of NaH2PO4 as an additive, delivered product 11 in 76% yield with 94% ee and >19:1 dr (0.2 mmol scale, entry 1). The substituents at the 6,6′-positions of the SPINOL backbone were found to significantly influence enantioselectivity; for instance, catalyst C2 afforded 11 with 88% ee (entry 2). Comparative studies with catalysts C3 and C4 revealed that SPINOL-derived CPAs outperformed those derived from H8-BINOL (C3) and BINOL (C4) (entries 3–4). Replacing reductant H1 with tertiary amine H2 or secondary amine H3 resulted in 11 with 68% ee and 85% ee, respectively (entries 5–6). We also evaluated alternative Ru(II)- and Ir(III)-based photosensitizers (entries 7–8), with the latter providing 11 in 74% yield and 91% ee. Notably, the absence of NaH2PO4 led to a significant reduction in yield (entry 9), underscoring its role in promoting the reaction. Control experiments confirmed that the chiral catalyst C1 is indispensable for the transformation, as its omission nearly halted the reaction, highlighting its critical role in establishing an enantiocontrol environment (entry 10).
Further control experiments verified that the photosensitizer DPZ, photon irradiation, the reductant, and an oxygen-free atmosphere are all essential for the successful formation of product 11 (entry 11). Furthermore, treatment of isoquinoline carboxylic acid esters 2 or 6 with 1 under the optimized reaction conditions did not result in any improvement in the resulting enantioselectivities (entries 12 and 13).
With the optimized conditions established, we explored the scope of this asymmetric dearomative [3 + 2] photocycloaddition (Fig. 2). Initially, a series of acyclic α-substituted vinyl phenylketones were reacted with 6, yielding the corresponding adducts 11–36 in 42–90% yields with 80–95% ee and >19:1 drs. The reaction demonstrated broad tolerance for diverse substitution patterns at the α-position, including aryl groups (11–20), fused rings (21), various alkyl groups (22–25), and even hydrogen (26). Notably, the isopropyl-substituted ester 4 emerged as the optimal partner for α-alkyl vinyl phenylketones. Subsequently, the introduction of electron-withdrawing or electron-donating groups at different positions on the phenyl ring of the acyl benzene (27–35), or the replacement of the phenyl ring with a heteroaromatic ring (36), afforded the corresponding benzotropanes with consistently high yields and enantioselectivities. Furthermore, exocyclic terminal enones, including 1-tetralones (37–41), 4-chromanone (42), and other five- to seven-membered exocyclic enones (43–45), were evaluated as reaction partners with 3, delivering the desired products with high yields and good to excellent enantioselectivities under slightly modified conditions. The versatility of this platform was further underscored by the successful reactions of α-benzoylstyrene 1 with various isoquinoline-1-carboxylic acid ethyl esters bearing diverse substituents at different positions of the isoquinoline ring (46–52). The absolute configuration of the cycloaddition adducts was determined based on the structure of 34 using single-crystal X-ray diffraction analysis (CCDC: 2416627) (see the Supporting Information for details). Notably, ethyl quinoline-4-carboxylate underwent smooth conversion under standard conditions, providing the dearomative [3 + 2] cycloaddition product 53 in 65% yield, with 45% ee and >19:1 dr. However, when the ester group was positioned at the 2-position of the quinoline ring, as exemplified by ethyl quinoline-2-carboxylate, the reaction became unselective. Unfortunately, substituting the ethyl ester at the 1-position of isoquinoline with electron-deficient groups such as CF₃, CN, acetyl, and benzoyl did not yield the desired dearomative [3 + 2] cycloaddition products. The CF₃-substituted analog showed no reactivity, while 1-cyanoisoquinoline underwent reductive coupling to form an ipso-substitution product, consistent with previous reports35. In contrast, the acetyl- and benzoyl-substituted variants were reduced, producing the corresponding alcohol derivatives, as documented in earlier studies36.
The reaction was performed on a 0.20 mmol scale. bC2 was used along with 2.0 equiv. of H3 in THF at 25 °C. c10 mol% of C2 was used along with 2.0 equiv. of H3 in EtOAc at −20 °C for 48 h. DPZ = 5,6-bis(5-methoxythiophen-2-yl)pyrazine-2,3-dicarbonitrile. CPME Cyclopentyl methyl ether, LED light-emitting diode.
Encouraged by the success of the initial studies, we sought to extend this methodology to the more challenging transformation of β-substituted enones37 with isoquinolines, a process that entails the simultaneous generation of four stereogenic centers, including one or more quaternary stereocenters, within a single product. Employing C2 as the chiral catalyst and H1 as the terminal reductant in dimethoxymethane (DME), the reaction between chalcone and 6 proceeded smoothly, delivering the desired product in 42% yield with 30% ee and >19:1 dr (see the Supporting Information for details). This preliminary result prompted further investigation into the influence of isoquinoline-1-carboxylic acid-substituted esters on reactivity and stereochemical control. Through systematic optimization, 2-methoxyphenyl isoquinoline-1-carboxylate was identified as the optimal substrate. Under modified conditions—using BINOL-derived chiral phosphoric acid C4 as the catalyst and H18 as the tertiary amine in THF at −20 °C for 20 h—the dearomative [3 + 2] photocycloaddition of chalcone with 2-methoxyphenyl isoquinoline-1-carboxylate afforded product 54 in 75% yield with 90% ee and >19:1 dr.
As summarized in Fig. 3, a wide range of β-substituted vinyl arylketones, featuring diverse substitution patterns at the β-position (54–71) or varied substituents on the phenyl ring (72–82), proved compatible with the optimized conditions, yielding products in 47–90% yields with 84–94% ees and >20:1 drs. Adduct 59, which exhibited favorable crystallinity, had its absolute configuration elucidated through X-ray crystallography (CCDC: 2416687) (see the Supporting Information for details). Notably, this method successfully incorporated bioactive natural compounds such as L-menthol (61) and abietic acid (77) into the benzotropane framework, delivering the corresponding cycloadducts with high efficiency and stereocontrol, thereby highlighting its potential for pharmaceutical applications. While enones bearing two substituents exhibited reduced reactivity, products 81 and 82 were obtained in good yields and stereoselectivities after extending the reaction time to 40 or 60 h, utilizing C2 as the chiral catalyst. These results highlight the robustness and versatility of this methodology for constructing complex, polycyclic architectures with multiple stereocenters.
The reaction was conducted on a 0.20 mmol scale. b10 mol% of C4 was used along with 1.2 equiv. of H12 at −20 °C for 12 h. c10 mol% of C2 was used along with 2 equiv. of H5 at −10 °C for 60 h. d10 mol% of C2 was used along with 1.2 equiv. of H12 at −20 °C for 40 h. DPZ = 5,6-bis(5-methoxythiophen-2-yl)pyrazine-2,3-dicarbonitrile. CPME Cyclopentyl methyl ether, LED light-emitting diode.
As previously described, the [3 + 2] dearomatization cycloaddition adducts of isoquinolines hold substantial value in the pharmaceutical industry (Fig. 1D)38,39,40,41,42. To demonstrate the practical utility of this approach, the reaction between 1 and 6 was performed on a 3.0 mmol scale. To our delight, the reaction proceeded smoothly, affording adduct 11 in 72% yield with 94% ee and >20:1 dr after 90 h (Fig. 4). Subsequent investigations on 11 revealed its potential for diversification. Upon treatment with sodium borohydride, 11 could afford polycyclic compound 84a in 92% yield while retaining its enantiomeric excess. The reaction of 11 with acetyl chloride in the presence of triethylamine and pyridine resulted in the protection of the secondary amine, producing compound 84b in 81% yield with >19:1 dr and 90% ee. Additionally, treating adduct 11 with 1-chloro-4-isocyanato-2-(trifluoromethyl)benzene (83) in the presence of N,N-diisopropylethylamine (DIPEA) yielded polycyclic urea 84c in 78% yield with 94% ee and >19:1 dr. These transformations not only underscore the versatility of the developed method but also highlight its potential for generating structurally complex and pharmacologically relevant scaffolds.
To elucidate the mechanism of this dearomative [3 + 2] photocycloaddition, a series of mechanistic experiments were conducted, using the reaction of 1 with 6 as a representative case. Initially, UV-vis spectra of 1, 6, chiral catalyst C1, and photosensitizer DPZ were measured (Fig. 5A). The results revealed that neither the substrates nor the chiral catalyst C1 could be excited by the photon source employed, whereas DPZ exhibited strong absorption, suggesting its pivotal role in initiating the photoredox cycle. This was further supported by control experiments as shown in Table 1B (entry 11), which showed no reaction in the absence of DPZ, confirming the necessity of photoredox catalysis mediated by DPZ. Stern-Volmer quenching experiments indicated that the catalytic cycle of DPZ (DPZ*/DPZ•− = +0.91 V vs SCE) is likely initiated by reductive quenching with H1 (E1/2ox = +0.80 V vs SCE) (Fig. 5B). The measured quantum yield (Φ = 0.48) ruled out the possibility of a radical chain process, suggesting a closed catalytic cycle42. Further insights were gained by conducting the reaction under standard conditions in the presence of pyrene or anthracene. Although photoexcited DPZ (ET = 2.01 eV) is susceptible to quenching by pyrene (ET = 2.0 eV)43 or anthracene (ET = 1.8 eV)43 via triplet-triplet energy transfer (EnT), both reactions yielded product 11 with comparable yields, ee, and drs (Fig. 5C). These results strongly suggest that energy transfer (EnT) is not the primary pathway for this transformation, further supporting the involvement of a photoredox catalytic process.
A UV-Vis absorption spectroscopy. B Stern-Volmer quenching plot of DPZ. C Reaction with triplet quencher. D Reaction with radical trapping reagent. E Reaction of 1 or 6. F Reaction with 1-phenylisoquinoline. G Crossover experiment. H Proposed mechanism. DPZ = 5,6-bis(5-methoxythiophen-2-yl)pyrazine-2,3-dicarbonitrile. CPME Cyclopentyl methyl ether, LED light-emitting diode, TEMPO 2,2,6,6-tetramethylpiperidinoxy, ET triplet energy, SET single-electron transfer, PCET proton-coupled electron transfer.
To further probe the reaction mechanism, we conducted the reaction in the presence of 1.0 equivalent of 2,2,6,6-tetramethylpiperidinoxy (TEMPO), a well-known radical scavenger (Fig. 5D). The formation of product 11 was completely inhibited, and high-resolution mass spectrometry (HRMS) analysis detected the potential formation of adduct 85 (see the Supporting Information for details), which is likely derived from the trapping of the putative enolate radical intermediate by TEMPO. This observation supports the generation of an enolate radical intermediate (1-I) during the reaction. The existence of such an intermediate was further corroborated by a homocoupling reaction of 1 under the established conditions, using unreactive pyridine instead of 6 to mimic the acid-base environment (Fig. 5E). Furthermore, no reaction involving 6 was observed under these conditions. Notably, although 1-phenylisoquinoline 87 exhibits poor oxidative ability (E1/2red = −2.09 V vs SCE), its reaction with 1 still afforded product 88 in 12% yield (Fig. 5F). A comparison of the reaction outcomes of isoquinoline, 1-phenylisoquinoline, and 1-esterisoquinoline demonstrates the substantial influence of the ester substituent at the 1-position on the dearomatization process and its notable impact on stereoselectivity.
Additionally, a crossover experiment involving 6 and two enones—one with stronger oxidative ability (1) and the other featuring a methoxy group on the aromatic ring—yielded two products, 11 and 34. The lower yield of 34 compared to 11 can be attributed to the reduced electrophilicity of the methoxy-substituted enone (Fig. 5G). These results collectively provide robust evidence for the formation of enolate radicals through the single-electron reduction of enones, which subsequently undergo radical addition to the 1-position of isoquinolines to form the products17. Importantly, alternative pathways, such as the single-electron reduction of isoquinolines followed by radical addition to enones or radical cross-coupling of two distinct species, could be excluded based on these experimental findings.
Of note, the evaluation of the relationship between the ee value of C1 and product 11 revealed a linear correlation (see the Supporting Information for details), suggesting the involvement of a single molecule of the chiral catalyst in the formation of the new bond. In this context, we propose a mechanistic rationale for the dearomative [3 + 2] photocycloaddition, as depicted in Fig. 5H. Our mechanistic investigation suggests that photoexcitation of DPZ initiates a single-electron transfer to H1, generating the radical ion pair DPZ•− and H1•+. Subsequent deprotonation and secondary oxidation of H1•+ by *DPZ yields H1+ and DPZ•−, with H1+ undergoing hydrolysis to afford H0, which has been unequivocally characterized by NMR spectroscopy42. Furthermore, enone 1 undergoes conversion to ketyl 89 through a proton-coupled electron transfer (PCET)37,44,45,46 process mediated by C1, owing to the high oxidative potentials of 1. While tertiary amine cations have been previously reported to facilitate ketone reduction via PCET, the strict requirement for C1 in this transformation strongly implicates the acid catalyst as the key mediator of this process. Protonation of 6 facilitates the radical addition of enolate radical 90 through a plausible ternary transition state (Int-1), generating radical intermediate 9128, which can be efficiently reduced by DPZ•− (DPZ•−/DPZ = −1.42 V vs. SCE), thereby yielding the corresponding enamine. Subsequent tautomerization followed by a Mannich-type reaction between the enolate and the resulting imine completes the annulation process, affording the enantioenriched product 11.
Discussion
To summarize, we have successfully achieved the asymmetric dearomative photocycloaddition of isoquinolines. The success of this [3 + 2] cycloaddition with enones is facilitated by a dual catalytic system: photoredox catalysis initiates radical addition at the 1-position of isoquinolines, ensuring high regioselectivity, while a chiral Brønsted acid catalyst simultaneously activates the radical addition process and provides precise enantiocontrol. Crucial to the reaction’s success is the strategic introduction of substituents at the 1-position of isoquinolines, which effectively suppresses undesired aromatization pathways. This methodology enables the synthesis of a diverse array of pharmaceutically relevant benzotropane derivatives, featuring three or even four consecutive stereocenters, with high yields, enantioselectivities, and diastereoselectivities. We anticipate that this work will stimulate further exploration of asymmetric photocycloaddition reactions involving imine-containing azaarenes, which hold significant potential for drug discovery due to the prevalence of nitrogen-containing dearomatized structures in pharmaceuticals and bioactive molecules.
Methods
General procedure for asymmetric dearomative [3 + 2] photocycloaddition between terminal ketene and isoquinoline-1-carboxylic acid-substituted ester
For products 11-21, 27-36, 46-53: 200.0 μL (0.002 mmol, 0.01 equiv) of DPZ (7 mg DPZ in 2 mL toluene) was added into a 25 mL Schlenk tube, and then the solvent was removed in vacuo. Subsequently, terminal ketene (0.4 mmol, 2.0 equiv.), isoquinoline-1-carboxylic acid ethyl ester (0.2 mmol, 1.0 equiv.)/quinoline-4-carboxylic acid ethyl ester for 53, H1 (0.4 mmol, 2.0 equiv.), NaH2PO4 (0.4 mmol, 2.0 equiv.), C1 (0.03 mmol, 0.15 equiv.), and CPME (8 mL) were sequentially added and degassed three times using the freeze-pump-thaw method. The reaction mixture was stirred under an argon atmosphere at −10 °C in the dark for 30 min, followed by exposure to a 3 W blue LED (λ = 450–455 nm) from a distance of 0 cm for another 36 h. After removing the solvent, the resulting residue was loaded onto a short silica gel column and subjected to gradient elution with a PE/acetone mixture (50/1‒10/1 ratio). The corresponding product was obtained by evaporating the solvent under vacuum.
For products 22–26: 200.0 μL (0.002 mmol, 0.01 equiv) of DPZ (7 mg DPZ in 2 mL toluene) was added into a 25 mL Schlenk tube, and then the solvent was removed in vacuo. Subsequently, terminal ketene (0.4 mmol, 2.0 equiv.), isoquinoline-1-carboxylic acid isopropyl ester (0.2 mmol, 1.0 equiv.) for 22–25/isoquinoline-1-carboxylic acid ethyl ester (0.2 mmol, 1.0 equiv.) for 26, H3 (0.4 mmol, 2.0 equiv.), NaH2PO4 (0.4 mmol, 2.0 equiv.), C2 (0.03 mmol, 0.15 equiv.), and THF (16 mL) were sequentially added and degassed three times using the freeze-pump-thaw method. The reaction mixture was stirred under an argon atmosphere at 25 °C in the dark for 30 min, followed by exposure to a 3 W blue LED (λ = 450–455 nm) from a distance of 0 cm for another 36 h. After removing the solvent, the resulting residue was loaded onto a short silica gel column and subjected to gradient elution with a PE/acetone mixture (50/1‒10/1 ratio). The corresponding product was obtained by evaporating the solvent under vacuum.
For 37–44: 200.0 μL (0.002 mmol, 0.01 equiv) of DPZ (7 mg DPZ in 2 mL toluene) was added into a 25 mL Schlenk tube, and then the solvent was removed in vacuo. Subsequently, terminal ketene (0.4 mmol, 2.0 equiv.), isoquinoline-1-carboxylic acid tertiary butyl ester (0.2 mmol, 1.0 equiv.), H3 (0.4 mmol, 2.0 equiv.), C2 (0.02 mmol, 0.10 equiv.), and EtOAc (8 mL) were sequentially added and degassed three times using the freeze-pump-thaw method. The reaction mixture was stirred under an argon atmosphere at −20 °C in the dark for 30 min, followed by exposure to a 3 W blue LED (λ = 450–455 nm) from a distance of 0 cm for another 48 h. After removing the solvent, the resulting residue was loaded onto a short silica gel column and subjected to gradient elution with a PE/acetone mixture (50/1‒10/1 ratio). The corresponding product was obtained by evaporating the solvent under vacuum.
General procedure for enantioselective dearomative [3 + 2] photocycloaddition between internal ketene and isoquinoline-1-carboxylic acid-substituted ester
For 54–60, 62–68, 72–76, and 78–79: 200.0 μL (0.002 mmol, 0.01 equiv) of DPZ (7 mg DPZ in 2 mL toluene) was added into a 25 mL Schlenk tube, and then the solvent was removed in vacuo. Subsequently, chalcones (0.24 mmol, 1.2 equiv.), 2-methoxyphenyl isoquinoline-1-carboxylate (0.2 mmol, 1.0 equiv.), H18 (0.4 mmol, 2.0 equiv.), C4 (0.04 mmol, 0.2 equiv.), and THF (10 mL) were sequentially added and degassed three times using the freeze-pump-thaw method. The reaction mixture was stirred under an argon atmosphere at −40 °C in the dark for 30 min, followed by exposure to a 3 W blue LED (λ = 450–455 nm) from a distance of 0.5 cm for another 20 h. After removing the solvent, the resulting residue was loaded onto a short silica gel column and subjected to gradient elution with petroleum ether/ethyl acetate mixture (20/1‒5/1 ratio). The corresponding product was obtained by evaporating the solvent under vacuum.
For 61, 77, 80, and 82: 200.0 μL (0.002 mmol, 0.01 equiv) of DPZ (7 mg DPZ in 2 mL toluene) was added into a 25 mL Schlenk tube, and then the solvent was removed in vacuo. Subsequently, ketenes (0.24 mmol, 1.2 equiv.), isoquinolines (0.2 mmol, 1.0 equiv.), H12 (0.24 mmol, 1.2 equiv.), C4 (0.02 mmol, 0.1 equiv. for 61 and 80)/C2 (0.02 mmol, 0.1 equiv. for 77 and 82) and THF (4 mL) were sequentially added and degassed three times using the freeze-pump-thaw method. The reaction mixture was stirred under an argon atmosphere at −20 °C in the dark for 30 min, followed by exposure to a 3 W blue LED (λ = 450–455 nm) from a distance of 0.5 cm for suitable time (12 h for 61 and 80, 40 h for 77 and 82). After removing the solvent, the resulting residue was loaded onto a short silica gel column and subjected to gradient elution with petroleum ether/ethyl acetate mixture (10/1‒5/1 ratio). The corresponding product was obtained by evaporating the solvent under vacuum.
For 69–71 and 81: 200.0 μL (0.002 mmol, 0.01 equiv) of DPZ (7 mg DPZ in 2 mL toluene) was added into a 25 mL Schlenk tube, and then the solvent was removed in vacuo. Subsequently, ketene (0.24 mmol, 1.2 equiv.), tert-butyl isoquinoline-1-carboxylate (0.2 mmol, 1.0 equiv.), H5 (0.4 mmol, 2 equiv.), C2 (0.02 mmol, 0.1 equiv.), and THF (6 mL) were sequentially added and degassed three times using the freeze-pump-thaw method. The reaction mixture was stirred under an argon atmosphere at −10 °C in the dark for 30 min, followed by exposure to a 3 W blue LED (λ = 450–455 nm) from a distance of 0.5 cm for another 60 h. After removing the solvent, the resulting residue was loaded onto a short silica gel column and subjected to gradient elution with petroleum ether/ethyl acetate mixture (10/1‒5/1 ratio). The corresponding product was obtained by evaporating the solvent under vacuum.
Data availability
All data are available from the corresponding authors upon request. The authors declare that all data supporting the findings of this study are available in the paper and its Supplementary Information files. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2416627 (34) and CCDC 2416687 (59). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Moyano, A. & Rios, R. Asymmetric organocatalytic cyclization and cycloaddition reactions. Chem. Rev. 111, 4703–4832 (2011).
Remy, R. & Bochet, C. G. Arene–alkene cycloaddition. Chem. Rev. 116, 9816–9849 (2016).
Yadav, R. N. et al. Transition metal complexes: a new era of photosensitizers for dearomative photocycloaddition/annulation via energy and electron transfer photocatalysis. Coord. Chem. Rev. 520, 216136 (2024).
Xuan, J. & Xiao, W.-J. Visible-light photoredox catalysis. Angew. Chem. Int. Ed. 51, 6828–6838 (2012).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013).
Romero, N. A. & Nicewicz, D. A. Organic photoredox catalysis. Chem. Rev. 116, 10075–10166 (2016).
Shaw, M. H., Twilton, J. & MacMillan, D. W. C. Photoredox catalysis in organic chemistry. J. Org. Chem. 81, 6898–6926 (2016).
Cheng, Y.-Z., Feng, Z., Zhang, X. & You, S.-L. Visible-light induced dearomatization reactions. Chem. Soc. Rev. 51, 2145–2170 (2022).
Zhu, M., Zhang, X., Zheng, C. & You, S.-L. Energy-transfer-enabled dearomative cycloaddition reactions of indoles/pyrroles via excited-state aromatics. Acc. Chem. Res. 55, 2510–2525 (2022).
Escolano, M. et al. Recent strategies in the nucleophilic dearomatization of pyridines, quinolines, and isoquinolines. Chem. Rev. 124, 1122–1246 (2024).
Ji, P. et al. Photochemical dearomative skeletal modifications of heteroaromatics. Chem. Soc. Rev. 53, 6600–6624 (2024).
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Lovering, F. Escape from Flatland 2: complexity and promiscuity. MedChemComm 4, 515–519 (2013).
Hu, N. et al. Catalytic asymmetric dearomatization by visible-light-activated [2+2] photocycloaddition. Angew. Chem. Int. Ed. 57, 6242–6246 (2018).
Sun, N. et al. Enantioselective [2+2]-cycloadditions with triplet photoenzymes. Nature 611, 715–720 (2022).
Guo, J. et al. Chemogenetic evolution of diversified photoenzymes for enantioselective [2 + 2] cycloadditions in whole cells. J. Am. Chem. Soc. 146, 19030–19041 (2024).
Hou, L. et al. Catalytic asymmetric dearomative [2 + 2] photocycloaddition/ring-expansion sequence of indoles with diversified alkenes. J. Am. Chem. Soc. 146, 23457–23466 (2024).
Stegbauer, S., Jandl, C. & Bach, T. Enantioselective Lewis acid catalyzed ortho photocycloaddition of olefins to phenanthrene-9-carboxaldehydes. Angew. Chem. Int. Ed. 57, 14593–14596 (2018).
Li, M. et al. Gd(III)-catalyzed regio-, diastereo-, and enantioselective [4 + 2] photocycloaddition of naphthalene derivatives. J. Am. Chem. Soc. 146, 16982–16989 (2024).
Tian, D. et al. Catalytic asymmetric [4 + 2] dearomative photocycloadditions of anthracene and its derivatives with alkenylazaarenes. Nat. Commun. 15, 4563 (2024).
Yan, P. et al. Enantioselective Intramolecular ortho photocycloaddition reactions of 2-acetonaphthones. Angew. Chem. Int. Ed. 63, e202318126 (2024).
Yin, Y., Zhao, X. & Jiang, Z. Advances in the synthesis of imine-containing azaarene derivatives via photoredox catalysis. ChemCatChem 12, 4471–4489 (2020).
Ma, J. et al. Photochemical intermolecular dearomative cycloaddition of bicyclic azaarenes with alkenes. Science 371, 1338–1345 (2021).
Guo, R. et al. Photochemical dearomative cycloadditions of quinolines and alkenes: scope and mechanism studies. J. Am. Chem. Soc. 144, 17680–17691 (2022).
Ma, J. et al. Facile access to fused 2D/3D rings via intermolecular cascade dearomative [2 + 2] cycloaddition/rearrangement reactions of quinolines with alkenes. Nat. Catal. 5, 405–413 (2022).
Kleinmans, R. et al. ortho-Selective dearomative [2π + 2σ] photocycloadditions of bicyclic aza-arenes. J. Am. Chem. Soc. 145, 12324–12332 (2023).
Liu, X., Yin, Y. & Jiang, Z. Photoredox-catalysed formal [3+2] cycloaddition of N-aryl α-amino acids with isoquinoline N-oxides. Chem. Commun. 55, 11527–11530 (2019).
Leitch, J. A., Rogova, T., Duarte, F. & Dixon, D. J. Dearomative photocatalytic construction of bridged 1,3-diazepanes. Angew. Chem. Int. Ed. 59, 4121–4130 (2020).
Li, J. et al. Formal enantioconvergent substitution of alkyl halides via catalytic asymmetric photoredox radical coupling. Nat. Commun. 9, 2445 (2018).
Silvi, M. & Melchiorre, P. Enhancing the potential of enantioselective organocatalysis with light. Nature 554, 41–49 (2018).
Lv, X., Xu, H., Yin, Y., Zhao, X. & Jiang, Z. Visible light-driven cooperative DPZ and chiral hydrogen-bonding catalysis. Chin. J. Chem. 38, 1480–1488 (2020).
Yin, Y., Zhao, X., Qiao, B. & Jiang, Z. Cooperative photoredox and chiral hydrogen-bonding catalysis. Org. Chem. Front. 7, 1283–1296 (2020).
Yao, W., Bazan-Bergamino, E. A. & Ngai, M.-Y. Asymmetric photocatalysis enabled by chiral organocatalysts. ChemCatChem 14, e202101292 (2022).
Kong, M. et al. Catalytic reductive cross coupling and enantioselective protonation of olefins to construct remote stereocenters for azaarenes. J. Am. Chem. Soc. 143, 4024–4031 (2021).
Kong, M. et al. Radical cross coupling and enantioselective protonation through asymmetric photoredox catalysis. Adv. Sci. 11, 2307773 (2024).
Qiao, B., Li, C., Zhao, X., Yin, Y. & Jiang, Z. Enantioselective reduction of azaarene-based ketones via visible light-driven photoredox asymmetric catalysis. Chem. Commun. 55, 7534–7537 (2019).
Li, Y. et al. Catalytic asymmetric reductive azaarylation of olefins via enantioselective radical coupling. J. Am. Chem. Soc. 144, 7805–7814 (2022).
Monn, J. A., Thurkauf, A., Mattson, M. V., Jacobson, A. E. & Rice, K. C. Synthesis and structure-activity relationship of C5-substituted analogs of (+-)-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine [(+-)-desmethyl-MK801]: ligands for the NMDA receptor-coupled phencyclidine binding site. J. Med. Chem. 33, 1069–1076 (1990).
Grynkiewicz, G. & Gadzikowska, M. Tropane alkaloids as medicinally useful natural products and their synthetic derivatives as new drugs. Pharmacol. Rep. 60, 439 (2008).
Cohen-Kaminsky, S. et al. Preparation of novel dizocilpine derivatives as peripheral NMDA receptor antagonists. WO Patent 2017/216159 A1 (2017).
Afewerki, S., Wang, J.-X., Liao, W.-W. & Córdova, A. in The Alkaloids: Chemistry and Biology Vol. 81 (ed. Knölker, H.-J.) 151−233 (Academic Press, 2019).
McCarthy, T., Milne, G., Moechel, T., Naylor, A. & Miller, D. N-Phenylaminocarbonyl pyridino-, pyrimidino and benzo-tropanes as modulators of GPR65 and their preparation. WO Patent 2021/245427 A1 (2021).
Ghosh, I., Shaikh, R. S. & König, B. Sensitization-initiated electron transfer for photoredox catalysis. Angew. Chem. Int. Ed. 56, 8544–8549 (2017).
Gentry, E. C. & Knowles, R. R. Synthetic applications of proton-coupled electron transfer. Acc. Chem. Res. 49, 1546–1556 (2016).
Cao, K. et al. Catalytic enantioselective addition of prochiral radicals to vinylpyridines. J. Am. Chem. Soc. 141, 5437–5443 (2019).
Murray, P. R. D. et al. Photochemical and electrochemical applications of proton-coupled electron transfer in organic synthesis. Chem. Rev. 122, 2017–2291 (2022).
Acknowledgements
Grants from the National Natural Science Foundation of China (21925103, Z.J., 22271077, B.Q., 21901062, B.Q.), Natural Science Foundation of Henan (232300421078, B.Q., 222301420046, B.Q.), Program for Science & Technology Innovation Talents in Universities of Henan Province (24HASTIT001, B.Q.), China Postdoctoral Science Foundation (2021M690890, B.Q.), and Henan University are gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
B.Q. and Z.J. conceived and designed the experiments. J.H., S.Y., and M.X. performed the experiments. B.Q., Z.J., and M.K. analyzed and interpreted the results. J.H., S.Y., and B.Q. prepared the Supplementary Information. B.Q. and Z.J. wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Soumitra Maity and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huo, J., Yang, S., Kong, M. et al. Photoredox catalytic asymmetric dearomative [3 + 2] cycloaddition of isoquinolines with enones. Nat Commun 16, 7520 (2025). https://doi.org/10.1038/s41467-025-62876-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-62876-7