Abstract
Mitochondrial dynamics enable cellular adaptation to fluctuations in energy demand, such as those imposed on skeletal muscle by exercise, metabolic disorders, or aging. Here, we report a novel pathway that modulates mitochondria dynamics in skeletal muscle involving the scaffolding protein ankyrin-B. Rare variants in ankyrin-B, encoded by ANK2, increase risk for cardio-metabolic syndrome in humans and mice. We show that mice selectively lacking skeletal muscle ankyrin-B have reduced endurance exercise capacity without alterations in muscle strength or systemic glucose regulation. Muscle fibers in these mice have increased oxidative stress, reduced fatty acid oxidation, and enlarged and hyperconnected mitochondria. We found that ankyrin-B interacts with and is required for efficient mitochondria recruitment of fission modulators and sarcoplasmic reticulum-mitochondria coupling. Thus, we conclude that ankyrin-B enables substrate adaptability and bioenergetic homeostasis under energetic stress, and exercise capacity by promoting efficient mitochondrial fission in skeletal muscle.
Similar content being viewed by others
Introduction
Skeletal muscle (SKM) performs pivotal functions, from thermoregulation to maintenance of whole-body metabolic homeostasis1. Most notably, through its contractile properties, SKM drives movement and supports motor control, strength, endurance, posture, and autonomic tasks. SKM also serves as the principal insulin-stimulated sink for circulating glucose2 and increases glucose uptake in response to contraction in an insulin-independent manner3. Consequently, muscle dysfunction underlies detrimental diseases such as muscular dystrophies4, sarcopenia5, and type II diabetes (T2D)2, and is associated with higher morbidity6. Exercise-induced secreted factors, including myokines, have been proposed to improve cardiometabolic and immune health, and confer neuroprotection7,8. Thus, enhancing SKM function through exercise can improve these disease states and is prescribed as a primary form of intervention and prophylaxis9,10. Nonetheless, the molecular mechanisms that contribute to SKM adaptation during exercise and their connection to metabolic homeostasis and health are not fully understood.
Both an acute bout of endurance exercise and chronic training result in molecular and structural remodeling of SKM to match energy demand and protect its structural integrity11. Mitochondria adaptations are central to the remarkable plasticity that enables SKM to respond to mechanical and energetic stressors, including exercise12. Dysfunctional mitochondria can result in mitochondrial myopathies, fatigability, sarcopenia13,14, and insulin resistance (IR)15, and acute exercise alone is sufficient to upregulate mitochondrial biogenesis and turnover to support oxidative capacity12,16,17. Notably, mitochondrial dynamics –the remodeling of mitochondria via fusion and fission– is a key regulator of mitochondrial adaptations to exercise18. In humans and mice, acute exercise upregulates and activates key mediators of mitochondrial dynamics16,18,19, such as the GTPase dynamin-related protein 1 (DRP1), which constricts and separates the mitochondrial membrane at the points of fission20. In other cell types, additional elements such as the endoplasmic reticulum (ER)21 and components of the cytoskeleton help recruit, activate, and oligomerize DRP1 and its receptors, and provide force propagation to separate dividing mitochondria22,23. However, our understanding of the molecular players that contribute to mitochondrial dynamics in SKM is incomplete.
Ankyrin-B (AnkB), encoded by ANK2, is a ubiquitously expressed scaffolding protein that links the cytoskeleton to specialized membrane microdomains and is a trafficking adaptor for organelles, including mitochondria in neurons24,25,26,27. Deficiencies in AnkB have been associated with metabolic syndrome in humans and mice28,29,30. We reported that knock-in mice expressing cardiac arrythmia- and T2D-associated rare human AnkB variants, which cause AnkB deficiency in multiple tissues, develop metabolic syndrome characterized by primary pancreatic β-cell insufficiency, diet- or age-dependent obesity, and IR28. We further showed that obesity, systemic lipotoxicity, and IR presentations are in part caused by adipose tissue-autonomous increases in glucose uptake and de novo lipogenesis, due to impairments in AnkB-mediated membrane trafficking of the glucose transporter Glut429. While AnkB deficiency in knock-in mice eventually leads to glucose mishandling by SKM, whether it's restricted loss in SKM, the major sink for circulating glucose2,3,31, cell-autonomously affects systemic energy balance and glucose homeostasis has not been assessed. In other studies, whole-body neonatal AnkB knockout (KO) and acute shRNA knockdown of AnkB in the tibialis anterior (TA) of juvenile mice via local electroporation suggested that AnkB is required for the proper localization of dystrophin and β-dystroglycan to the sarcolemma. When subjected to exercise, mice with AnkB-depleted TA showed signs of muscle injury32,33, implying that AnkB organizes SKM structural components and helps preserve SKM integrity. However, whether these defects are also observed upon constitutive loss of AnkB in mature SKM is not known.
Here, we leveraged mice constitutively lacking the canonical full-length 220-kDa AnkB isoform selectively in SKM to assess the consequences of sustained AnkB deficiency on muscle structure and metabolism. We found that AnkB is a novel modulator of mitochondrial dynamics in SKM required for optimal endurance exercise capacity. Consistent with a premature fatigue phenotype, AnkB-deficient muscle fibers failed to properly remodel mitochondria through fission and to sustain respiratory capacity under energetic stress.
Results
Expression of 220-kDa AnkB in skeletal muscle is not required to maintain systemic glucose homeostasis
To determine the effects of constitutive AnkB loss on SKM structure and systemic metabolic homeostasis, we generated SKM-specific AnkB knockout (SKM-AnkB-KO) mice, driven by human skeletal muscle α-actin (HSA)-Cre (Fig. 1a), leading to loss of the canonical full-length 220-kDa AnkB (AnkB-220), the most abundant AnkB isoform in SKM (Supplementary Fig. 1a). SKM-specific expression of Cre recombinase in the HSA-Cre line starts at embryonic day 9 and persists throughout SKM development and adulthood34. We confirmed selective loss of AnkB-220 in flexor digitorum brevis (FDB) (Fig. 1b), soleus (SOL), TA, and gastrocnemius (GC) (Supplementary Fig. 1b) of adult SKM-AnkB-KO mice. Protein levels of AnkB-220 remained unchanged in other AnkB high-expressing tissues, including heart, brown adipose tissue, and brain (Supplementary Fig. 1b). In addition to AnkB-220, muscle tissue of SKM-AnkB-KO mice lacks 110-kDa AnkB (AnkB-110), an isoform of unknown roles without the membrane binding domain24,25,26,29 (Supplementary Fig. 1c, d). AnkB-220 loss was not compensated by changes in expression of other SKM AnkB isoforms (Supplementary Fig. 1c, d). Similarly, AnkB-220 deficiency did not affect the expression of ankyrin-G (AnkG), ankyrin-R (AnkR) or ankyrins’ binding partner βII-spectrin (Supplementary Fig. 1e–h), previously implicated in the maintenance of SKM integrity32,33. Likewise, the specific localization of AnkG to the sarcomeric z-lines was maintained in SKM-AnkB-KO GC muscle (Supplementary Fig. 1i).
a SKM-AnkB-KO mice were generated by SKM-specific recombination of an Ank2 floxed allele driven by HSA-Cre. b Images of FDB fibers isolated from 4-mo control and SKM-AnkB-KO mice stained with a pan-AnkB antibody and DAPI to label nuclei (representative of n = 3 independent experiments). Scale bar, 3 µm. Analysis of exercise performance of 4-mo male (control n = 5, SKM-AnkB-KO n = 4) and female (control n = 7, SKM-AnkB-KO n = 7) mice on a voluntary running wheel. c was created in BioRender. Voos, K. (2025) https://BioRender.com/vz1ekdw. Line pots of daily maximum (d) and average non-zero (f) speeds and area under the curve (A.U.C.) (e, g). Line pots of daily cumulative distance (h), calculated as total distance traveled and break time (j), assessed as total minutes in which the wheel was not moving, and A.U.C (I, k). l Experimental design to evaluate endurance running capacity on a mouse treadmill, and to measure lactate and glucose levels in blood. Created in BioRender. Voos, K. (2025) https://BioRender.com/bxi9clq. Maximum velocity achieved by mice using the hybrid endurance exercise protocol (m) and time to exhaustion (n) of 4-mo control (n = 14) and SKM-AnkB-KO (n = 15) male mice. o-r, Blood lactate (o, p) and glucose (q, r) levels of 4-mo control (n = 8) and SKM-AnkB-KO (n = 6) mice before and during endurance running. Error bars are mean ± S.D. Statistical differences were calculated separately for males and females by two-way ANOVA with Šídák’s multiple comparisons test or multiple two-tailed unpaired t-tests with a significance threshold of p < 0.05.
We first evaluated body weight and composition, and energy balance in 4-month-old (4-mo) and 20-mo mice, and 4-mo mice fed a high-fat diet for 15 weeks (4-mo+HFD) (Supplementary Fig. 2a). Surprisingly, SKM-AnkB-KO mice showed similar body weights to littermate controls across the lifespan or following HFD feeding (Supplementary Fig. 2b). Body composition was also similar between 4-mo mice regardless of genotype or diet treatment, except for a trending increase in lean mass in the 4-mo+HFD SKM-AnkB-KO group (Supplementary Fig. 2c, d). In addition, 20-mo SKM-AnkB-KO mice showed trends towards increased lean and decreased fat mass relative to age-matched controls (Supplementary Fig. 2c, d). Indirect calorimetry analysis of 4-mo and 4-mo+HFD mice revealed increases in non-adjusted energy expenditure (EE) in 4-mo + HFD AnkB-KO mice (Supplementary Fig. 2e). Trends towards elevated CO2 production (VCO2) were apparent in AnkB-KO mice under HFD feeding at the whole animal level (Supplementary Fig. 2k) but disappeared after adjusting for lean mass (Supplementary Fig. 2f, g, i, j, l, m). There were no significant differences in respiratory quotient (RER) or food intake (Supplementary Fig. 2n, o). Energy balance parameters of 20-mo SKM-AnkB-KO mice were also similar to controls, except for a trend towards increased food intake during the light cycle (Supplementary Fig. 2e-o). Notably, we detected decreases in cage activity in 4-mo SKM-AnkB-KO mice during the light cycle when fed a normal diet or during the dark cycle if fed an HFD (Supplementary Fig. 2p).
Given the previous connection between AnkB and Glut4 trafficking28,29, we next assessed systemic glucose regulation. Contrary to our findings in other models of AnkB deficiency28,29, selective loss of AnkB-220 in SKM did not alter glycemia after 4-, 6-, or 18-hour fasts (Supplementary Fig. 3a, f, k, p), or sensitivity to glucose or insulin administration across ages and diets, assessed via glucose (Supplementary Fig. 3b–e, l–o) or insulin (Supplementary Fig. 3g–j, q-t) tolerance tests. While we cannot fully exclude a role for SKM AnkB in modulating glucose uptake in SKM and systemic glucose control, our results indicate that loss of AnkB-220 expression in this tissue is not a major driver of the increased adiposity and glucose dysregulation observed in mutant AnkB knock-in mice28.
Loss of SKM AnkB reduces endurance exercise capacity
The diminished cage activity of 4-mo SKM-AnkB-KO mice (Supplementary Fig. 2p) prompted us to evaluate whether SKM AnkB is required for mouse physical activity and optimal exercise capacity. We first assessed the ability of 4-mo male and female mice to exercise voluntarily when given unrestricted access to running wheels (Fig. 1c). 4-mo SKM-AnkB-KO male mice reached similar maximum running speeds to controls (Fig. 1f, e), but showed a consistent trend towards running at slower speeds, for shorter distances, and taking cumulative longer break times per day (Fig. 1d–k). As expected, female mice ran at increased speeds, for longer distances, and took shorter break times than male mice regardless of genotype (Fig. 1d–k). Notably, the reductions in voluntary running wheel capacity detected in SKM-AnkB-KO male mice were also observed, and often potentiated, in SKM-AnkB-KO females (Fig. 1d–k).
We also evaluated endurance exercise capacity of 4-mo male mice running on a treadmill at increasing velocities until exhaustion (Fig. 1l). Notably, 4-mo SKM-AnkB-KO mice could not achieve the same maximum velocity during this test and became exhausted faster than littermate controls (Fig. 1m, n). We confirmed increases in blood lactate levels in both control and SKM-AnkB-KO mice after this exhaustion exercise regime (Fig. 1o). In line with a faster time to exhaustion, SKM-AnkB-KO mice accumulated blood lactate quicker than control mice (Fig. 1p). Lastly, we found that control and SKM-AnkB-KO mice maintained similar blood glucose levels throughout the exercise protocol (Fig. 1q, r). These results indicate that AnkB-220 expression in SKM is required to achieve optimal exercise capacity.
Constitutive AnkB loss induces mild, muscle-type-specific changes in sarcolemma dystrophin and muscle organization
The observed reduction in exercise capacity of 4-mo SKM-AnkB-KO male mice motivated us to investigate the genes and functional pathways associated with SKM AnkB deficiency. RNA-sequencing analysis from 4-mo AnkB-KO GC showed upregulation of transcripts related to muscle atrophy and regeneration after injury, including leucine-rich repeats and transmembrane domains 1 (Lrtm1), MyoD family inhibitor (Mdfi), and downregulation of dystrophin (Dmd) (Fig. 2a)4,35,36,37. Previous reports proposed that AnkB stabilizes the dystrophin/β-dystroglycan complex at the sarcolemma of SKM fibers32,33, which links the extracellular matrix to the actin cytoskeleton and is essential for maintaining SKM fiber integrity37. However, these studies were conducted in neonatal SKM from whole-body AnkB-KO mice or in juvenile 1-mo wildtype mice using acute knockdown of AnkB expression via in situ electroporation of shRNAs in SKM32,33. Thus, we assessed the long-term effects of SKM-specific, constitutive loss of AnkB on dystrophin levels and distribution, and on the organization of mature SKM fibers. Total dystrophin abundance in protein lysates was unaltered in GC (Fig. 2b, c) and reduced in SOL (Fig. 2d, e) of adult (4-mo) SKM-AnkB-KO mice. In contrast to previous findings, dystrophin was localized to the sarcolemma in muscle fibers from 4-mo SKM-AnkB-KO mice (Fig. 2f, k). Detailed analysis revealed that only 20% of SKM-AnkB-KO GC fibers lacked sarcolemma dystrophin (Fig. 2f asterisks, g), 80% of which were type IIb fast-twitch glycolytic fibers (Supplementary Fig. 4a, b), with no significant changes in dystrophin levels in the rest of GC fibers (Fig. 2h). AnkB-KO GC fibers with normal or almost absent sarcolemma dystrophin exhibited the same level of AnkB downregulation (Supplementary Fig. 4c, d). The mosaic loss of dystrophin in GC fibers persisted in 20-mo SKM-AnkB-KO mice (Supplementary Fig. 4e). In contrast, dystrophin localized to the sarcolemma of all SOL fibers from SKM-AnkB-KO mice, although at levels reduced by approximately 20% (Fig. 2k–m). Contrary to previous reports32, GC and SOL muscle fibers from mice with constitutive AnkB loss preserved β-dystroglycan sarcolemma localization (Supplementary Fig. 5a, b).
a Volcano plot of differentially expressed genes in GC from SKM-AnkB-KO mice (n = 6 mice/genotype). Blue and red dots, respectively, denote downregulated and upregulated transcripts. Western blots and quantification of total dystrophin levels in GC (b, c) and SOL (d, e) normalized to total protein stain, and graphed as fold-change over WT levels (n = 4 mice/genotype). Representative images of GC (f) and SOL (k) from 4-mo mice. Asterisks denote fibers lacking dystrophin. Arrowheads indicate centrally located nuclei (CLN). Scale bar, 200 µm. Percent of dystrophin-negative fibers (g, l), dystrophin membrane levels relative to WT (h, m), fiber size distribution (i, n), and percent of fibers with CLN (j, o), all quantified from GC (f-j) and SOL (k–o) sections from 4-mo male mice (n = 3 mice/genotype). Error bars are mean ± S.D. Statistical differences were determined by a two-tailed unpaired t-test with a significance threshold of p < 0.05.
We next evaluated whether these minor effects of persistent AnkB loss on sarcolemma dystrophin are accompanied by alterations in muscle organization and strength. Overall, loss of AnkB resulted in slightly disorganized muscle fascicles in both GC and SOL (Fig. 2f, k) without changes in muscle fiber size (Fig. 2i, n). Likewise, AnkB deficits and the mosaic loss of membrane dystrophin did not alter collagen deposition in GC, an indicator of fibrosis, assessed by Masson’s trichrome staining (Supplementary Fig. 4f, g). Notably, approximately 15% of SKM-AnkB-KO GC fibers developed centrally located nuclei (CLN), indicating previous muscle damage and subsequent regeneration (Fig. 2f, DAPI stain and arrowheads, j; Supplementary Fig. 4f, g, arrowheads). However, we found no correlation between loss of sarcolemma dystrophin and the presence of CLN in SKM-AnkB-KO GC fibers (Fig. 2f). Furthermore, despite a 20% reduction in membrane-associated dystrophin (Fig. 2k, m), AnkB-KO SOL fibers did not have increased CLN (Fig. 2k, o). Mild changes in sarcolemma dystrophin and muscle organization did not affect muscle strength of 4-mo or 20-mo SKM-AnkB-KO mice compared to age-matched littermate controls, assessed by hang-wire and grip strength tests (Supplementary Fig. 4h–l). In sum, we found that persistent loss of AnkB only mildly affects the long-term maintenance of dystrophin localization to the sarcolemma in a muscle type-specific manner without altering β-dystroglycan distribution. Our results that AnkB deficits are not sufficient to elicit substantial changes in SKM structural organization or strength support similar findings of preservation of dystrophin/β-dystroglycan localization, grip strength and peak force in mice with combined loss of all SKM ankyrins38.
AnkB promotes fatty acid oxidation to sustain SKM energy demand during endurance exercise
Endurance exercise requires optimal mitochondria levels, dynamics, and respiratory capacity to meet the sustained high energy demand of SKM fibers16,17,18. Therefore, we evaluated if deficits in mitochondrial content or function contribute to the premature exhaustion of 4-mo SKM-AnkB-KO mice. mRNA levels of critical regulators of mitochondrial biogenesis, including nuclear respiratory factor 1 (Nrf1) and peroxisome proliferator-activated receptors (Ppar) α, δ, and γ were reduced in GC muscle of 4-mo SKM-AnkB-KO mice (Supplementary Fig. 6a). However, we did not detect changes in mitochondria content, assessed as mitochondria to nuclear DNA ratio (Fig. 3a), or in levels of oxidative phosphorylation (OXPHOS) complexes (Supplementary Fig. 6b, c). Mitochondria content, biogenesis, and OXPHOS levels were not altered in SOL, except for a reduction in OXPHOS complex II (Fig. 3a, Supplementary Fig. 6d–f).
a Mitochondrial content of GC (n = 5 mice/genotype) and SOL (n = 6 mice/genotype), evaluated as mitochondria DNA to nuclear DNA ratio. b Representative images of FDB fibers isolated from 4-mo mice showing ROS levels at baseline and after antimycin A treatment. Scale bar, 20 µm. c quantification of ROS accumulation (MitoSOX signal) normalized to total mitochondria (MitoTracker Green FM) signal at baseline (n = 58 WT, n = 104 AnkB-KO fibers) and after treatment (n = 21 WT, n = 27 AnkB-KO fibers). d Mitochondrial membrane potential of FDB fibers (n = 37 WT, n = 38 AnkB-KO) measured using TMRE. e (left) Simplified schematic of the metabolic pathways that enable utilization of sugars or fatty acids as fuels for mitochondria OXPHOS. Created with elements of BioRender. Voos, K. (2025) https://BioRender.com/y1njcg9. (right) Curve showing the relationship between substrate utilization by SKM mitochondria and maximal oxygen consumption (VO2max). During low to moderate exercise intensity (VO2max < 65%) or prolonged exercise, SKM fibers mostly rely on β-oxidation of fatty acids to meet energy requirements. Glucose is the main energy substrate during high-intensity training. Representative high-resolution respirometry traces (e, g) of isolated mitochondria using palmitoylcarnitine (PC) (f) or pyruvate (PYR) (g) as energy substrates. Quantification of O2 flux after substrate addition (state 2 respiration) (h), ADP-stimulation (state 3 respiration) (i), FCCP addition (maximal-uncoupled respiration) (j), oligomycin inhibition (leak respiration) (k), and antimycin A inhibition (non-mitochondrial respiration) (l) assessed in mitochondria isolated from n = 7 mice/genotype. Data shows mean ± S.D. Statistical differences were determined by one-way ANOVA with Bartlett’s multiple comparison test or by two-tailed multiple unpaired t-test with a significance threshold of p < 0.05.
The fatigue resistance capacity of a muscle is determined by the relative abundance of distinct fiber types, each of which differ in contraction speed, mitochondria content, and oxidative capacity39. Interestingly, AnkB is enriched in fast-twitch type IIa and slow-twitch type I oxidative muscle fibers (Supplementary Fig. 7a–d, f–h), which are mitochondria-rich fibers that are resistant to fatigue40. AnkB loss increased the proportion of hybrid fibers in GC and of oxidative type IIa fibers in SOL, but it did not significantly reduce the abundance of any specific fiber type (Supplementary Fig. 7e, i). These findings confirm that exercise deficits of SKM-AnkB-KO mice are not due to changes in mitochondria content or in abundance of fiber types that promote endurance performance.
We next tested the effects of AnkB loss on mitochondrial health and respiratory capacity. Single, intact muscle fibers from the FDB, which are largely mitochondria-rich, oxidative type IIa fibers41, can be efficiently isolated and cultured to assess mitochondrial function ex vivo. FDB fibers isolated from 4-mo SKM-AnkB-KO mice showed increases in mitochondrial reactive oxygen species (ROS) at baseline, measured as the ratio of MitoSOX to MitoTracker Green FM intensity, which respectively label mitochondrial ROS and the mitochondria pool (Fig. 3b, c). Mitochondria in AnkB-KO FDB fibers also exhibited diminished membrane potential, determined by tetramethylrhodamine ethyl ester (TMRE) staining (Fig. 3d).
Under conditions of prolonged low- to moderate-intensity exercise (~25%-65% of maximal oxygen consumption (VO2max)), such as the regime we used to assess endurance running capacity of SKM-AnkB-KO mice (Fig. 1l), SKM uses fatty acids as the primary fuel source to meet energetic demands42,43 (Fig. 3e). Thus, we used high-resolution respirometry to evaluate the respiratory capacity of intact mitochondria isolated from SKM of 4-mo mice using palmitoylcarnitine (PC) or pyruvate (PYR) as energy substrates (Fig. 3e). We measured substrate-stimulated (state 2), coupled maximum (state 3, ADP-stimulated), proton leak (oligomycin-resistant), and uncoupled maximum (FCCP-stimulated) respiration, as well as non-mitochondrial oxygen consumption (antimycin A-resistant). Notably, AnkB-KO SKM mitochondria showed a selective reduction of ADP-stimulated respiration when PC was the fuel substrate, indicating impaired fatty acid β-oxidation (Fig. 3f–l). This impairment is not caused by altered carnitine palmitoyltransferase 1B (Cptb1) levels in SKM, which imports fatty acids into the mitochondrial matrix for oxidation (Supplementary Fig. 6g, h). These findings suggest that mitochondria in AnkB-deficient muscle are under stress at baseline and have a reduced capacity to oxidize fatty acids, which likely underlies their failure to maintain SKM energetic demands during moderate-intensity endurance exercise.
SKM lacking AnkB is more susceptible to endurance exercise-induced energetic stress
Given the baseline increases in ROS and lower mitochondrial membrane potential of SKM-AnkB-KO muscle fibers, we evaluated if AnkB loss induces stress mechanisms in SKM. Pathway analysis of differentially expressed genes (DEG) from RNA-seq data revealed that genetic components of stress signaling pathways, including eIF2 (eukaryotic translation initiation factor 2), mTOR (mammalian target of rapamycin), and AMPK (AMP-activated protein kinase), were differentially expressed in GC from non-exercised 4-mo SKM-AnkB-KO mice (Fig. 4a, b). Interestingly, the upregulated expression of adenylate kinases (Ak7, Ak4) and oxidative metabolism regulator forkhead box protein O6 (Foxo6) suggested increased baseline activation of AMPK in AnkB-KO SKM (Fig. 4b).
a Top canonical pathways enriched in differentially expressed gene sets from RNA-sequencing analysis of SKM from non-exercised 4-mo mice (n = 6 mice/genotype) obtained using Ingenuity Pathway Analysis (IPA). p-values were calculated using Fisher’s Exact test. b Protein-protein interaction map of differentially expressed genes associated with mTOR and AMPK pathways identified through IPA analysis. Blue and red, respectively, denote downregulated and upregulated transcripts. Nodes are presented based on fold-change (FC), indicated by color intensity, and on p-value, shown as node size. c-e, Western blots (c) and quantification of pAMPKT172 normalized to total AMPK levels at baseline (d) and after a single bout of moderate intensity endurance running (e). Western blots (f) and quantification of pRaptorS792 normalized to total Raptor levels at baseline (g) and after endurance running (h). Data in c–h was collected from n = 4 mice/genotype/treatment. Data shows mean ± S.D. Statistical differences were determined using multiple two-tailed unpaired t-tests with multiple comparisons using the two-stage step-up (Benjamini, Krieger, and Yekutieli) test with a significance threshold of p < 0.05.
To validate the transcriptomics data and determine the physiological effects of AnkB deficits on SKM energetic stress, we assessed AMPK activation in GC and SOL at baseline and in response to moderate-intensity endurance running (Fig. 1l). Exercise-linked energetic deficits increase AMP/ATP ratios and activate the energy sensor AMPK by 100-fold via its phosphorylation at Thr172 (pAMPKT172)44,45,46. GC and SOL from SKM-AnkB-KO mice showed reductions in AMPKT172 phosphorylation at baseline (Fig. 4c, d). In contrast, levels of active pAMPKT172 increased by 2-fold in GC and SOL of SKM-AnkB-KO mice in response to exercise relative to a milder surge in controls (Fig. 4c, e). Consistent with energetic stress, phosphorylation of regulatory-associated protein of mTOR (Raptor) at S792 (pRaptorS792) by active AMPK, which blunts mTORC1 activity47, was enhanced in AnkB-KO GC by endurance exercise (Fig. 4f–h). Thus, SKM lacking AnkB is more susceptible to energetic stress induced by a single bout of moderate-intensity endurance exercise.
Loss of AnkB-220 results in elongated and interconnected mitochondria in SKM
Changes in mitochondrial ultrastructure and morphology can regulate respiratory capacity and substrate oxidation independent of mitochondrial content48,49,50,51,52,53. Thus, we evaluated if altered mitochondrial morphology contributes to the exercise deficits, the reduced fatty acid oxidation, and the lower threshold for energetic stress observed in SKM-AnkB-KO mice. Transmission electron microscopy (TEM) images of GC and SOL cross-sections showed dense mitochondria cristae ultrastructure across genotypes (Fig. 5a, d). However, mitochondria in both GC and SOL from SKM-AnkB-KO mice were enlarged, appeared hyperconnected (Fig. 5a–h) based on continuous cristae, had increased cross-sectional area (Fig. 5b, g), and often exhibited a lobular appearance not observed in control SKM (Fig. 5a, d, Supplementary Fig. 8). While the area occupied by subsarcolemmal (SSM) mitochondria did not change (Fig. 5d, i), the number of SSM mitochondrion was reduced in AnkB-KO GC, corresponding with an increase in their mean size (Fig. 5e, j). Leveraging the preservation of the three-dimensional (3D) mitochondria network in isolated FDB fibers, composed primarily of AnkB-enriched type IIa fibers41, we confirmed the global presence of elongated mitochondria in AnkB-KO fibers stained for the mitochondria marker COX IV using super-resolution microscopy (Fig. 5k–l’). Color-coded 3D volumetric analysis of rendered z-stacks revealed a more complex and interconnected mitochondria network in AnkB-KO fibers (Fig. 5m–o’), characterized by a higher frequency of larger mitochondria and a lower fragmentation index (Fig. 5p-s).
a, f Representative TEM images of GC and SOL cross-sections from 4-mo male mice. Scale bar, 1 µm. Quantification of average mitochondrial cross-sectional area (CSA) (b, g), size frequency distribution (c, h), subsarcolemmal (SSM) area occupied by mitochondria (d, i), and density of SSM mitochondria (e, j) per mouse collected from n = 3 mice/genotype. Data in b, c was collected from n = 62 WT and n = 72 AnkB-KO mitochondria. Data in d was collected from n = 20 WT and n = 14 AnkB-KO images. Data in e was collected from n = 20 WT and n = 25 AnkB-KO images. Data in g, h was collected from n = 197 WT and n = 211 AnkB-KO mitochondria. Data in i was collected from n = 30 WT and n = 39 AnkB-KO images. Data in j was collected from n = 34 WT and n = 40 AnkB-KO images. k-l’, Representative single z-stack images (scale bar, 5 µm) k, l and high magnification insets of regions denoted by yellow dotted boxes (scale bar, 1 µm) (k’, l’). m, m’, z-stack maximum projections of mitochondria networks in FDB fibers from 4-mo mice. Scale bar, 5 µm. n, n’, Volume color-coded 3D rendering of mitochondria in FDB fibers. Scale bar, 6 µm. o, o’, Image of three individual 3D-rendered mitochondria from control (o) or one hyperfused mitochondria from AnkB-KO (o’) FDB fibers. Scale bar, 2 µm. Average mitochondrion volume per fiber (p), sum of mitochondria volume per fiber (q), frequency distribution of mitochondria volume (r), and mitochondria fragmentation index (s) of 3D-rendered mitochondria in control and SKM-AnkB-KO fibers (n = 3 mice/genotype). Data in p–s was collected from n = 18 WT and n = 28 AnkB-KO images. Data show mean ± S.D. Statistical differences were determined by two-tailed unpaired test with a significance threshold of p < 0.05.
To further assess if AnkB cell-autonomously regulates mitochondria network morphology in SKM cells, we generated AnkB-KO C2C12 myoblasts using CRISPR-Cas9 editing. We confirmed a selective loss of AnkB-220 in C2C12 cells, which was accompanied by a robust upregulation of AnkB-150, a low-abundance isoform that lacks most of the membrane binding domain (Fig. 6a, b). Notably, levels of AnkB-110, which was downregulated in SKM of AnkB-KO mice (Supplementary Fig. 1c, d), were unaltered in AnkB-KO C2C12 cells (Fi. 6a, b). Thus, we used AnkB-KO C2C12 myoblasts to further define the contribution of AnkB-220, the canonical and predominant AnkB isoform in SKM (Supplementary Fig. 1a), to the organization of the mitochondrial network. Consistent with our observations in SKM sections and isolated muscle fibers from SKM-AnkB-KO mice (Fig. 5), AnkB-220-deficient C2C12 cells showed elongated and more interconnected mitochondria (Fig. 6c–g). Mitochondrial volume, size, sphericity, and network connectivity (estimated using fragmentation index) were fully or significantly rescued by adenoviral expression of GFP-tagged AnkB-220 in AnkB220-KO myoblasts (Fig. 6c–g). Super-resolution imaging of AnkB220-KO C2C12 revealed the expected broad subcellular distribution of AnkB220-GFP54, including co-localization with mitochondria (Fig. 6h, yellow arrowheads). Thus, loss of AnkB-22O in SKM leads to hyperfused mitochondrial networks, which likely underlies the observed reduced exercise capacity and bioenergetic deficits of SKM-AnkB-KO mice.
Western blot (a) and quantification (b) of AnkB isoform levels in C2C12 myoblasts (n = 3/genotype). c Representative images of WT and AnkB220-KO C2C12 myoblasts transduced with GFP or AnkB220-GFP adenovirus for 60 hours. Scale bar, 10 µm. White line-demarked insets show GFP and mitotracker (mitochondria) signal. Lower insets show high magnification views of regions enclosed by a yellow dotted box. Scale bar, 1 µm. Average mitochondrial volume per cell (d), mitochondria surface area (e), mitochondria sphericity (f), and mitochondria fragmentation index (g) of C2C12 myoblasts (n = 56 WT + GFP, n = 58 KO + GFP, and n = 62 KO + AnkB220-GFP). h left, Images of AnkB220-KO C2C12 myoblasts expressing AnkB220-GFP (representative of n = 3 independent transfections). Scale bar, 4 µm. right, High magnification views of the region enclosed by a yellow dotted box. The top view shows overlaid AnkB220-GFP and mitotracker signals. Scale bar, 2 µm. The bottom views show individual signals. Scale bar, 1 µm. Yellow arrowheads indicate AnB220-GFP/mitotracker colocalization. Data show mean ± S.D. Statistical differences were determined by multiple two-tailed unpaired t-tests or by one-way ANOVA followed by Bartlett’s multiple comparison test with a significance threshold of p < 0.05.
AnkB-220 interacts with mitochondria and the mitochondrial fission machinery in SKM
AnkB co-transports with mitochondria in neurons27 and binds the outer mitochondria membrane (OMM) protein PGC-1α- and ERR-induced regulator in muscle 1 (PERM1) in SKM55. We confirmed that AnkB is enriched in mitochondria fractions from SKM isolated by subcellular fractionation (Fig. 7a), supporting its interaction with OMM proteins. To uncover the mitochondria-associated AnkB interactome in SKM, we conducted proteomic analysis of eluates from native immunoprecipitation (IP) of AnkB complexes from GC and SOL (Fig. 7b). We identified 325 proteins in complexes with AnkB in GC and 665 in SOL, with 235 of the hits present in IP eluates from both muscles (Fig. 7c). Gene ontology analysis revealed that 55 of the AnkB interactors found in both muscle types are mitochondria-associated proteins (Fig. 7c, green, Supplementary Fig. 9a). This list includes Samm50 (Fig. 7d), the key subunit of the OMM sorting and assembly machinery (SAM) complex, which binds Perm1 and the mitochondrial contact site and cristae organizing system (MICOS) complex to promote cristae structural integrity and assembly of respiratory complexes55,56. Our analysis also uncovered the OMM protein mitochondrial fission factor (MFF) as part of AnkB complexes (Fig. 7d). MFF is the primary mitochondrial receptor for Drp1, a key regulator of mitochondria fission (Fig. 7e)20,57. Consequently, the presence of enlarged mitochondria and a hyperconnected mitochondrial network in AnkB-220 KO muscle and myoblasts (Figs. 5, 6) led us to hypothesize that AnkB-220 promotes mitochondria fission via interactions with the MFF-Drp1 complex. To confirm the presence, and establish the subcellular distribution, of native AnkB/MFF/Drp1 complexes in SKM, we performed a proximity ligation assay (PLA) in FDB fibers isolated from SKM-specific MitoTag mice58, in which HSA-Cre drives endogenous targeting of GFP to the OMM (Fig. 7f). We confirmed PLA signal, which detects in situ nanoscale interactions between proteins that are less than 40 nm apart29,59, between AnkB and its established partner βII-spectrin60,61, some of which localized to the mitochondria (Fig. 7f”). We also detected the presence of mitochondria-associated complexes between AnkB and both Drp1 and Drp1’s receptor MFF in SKM fibers (Fig. 7f”’, f””).
a Western blot of total GC lysates and mitochondria fractions probed with antibodies specific to AnkB and markers of subcellular compartments. n = 3 fractionations. b IP-proteomics-MS/MS workflow used to uncover the AnkB interactome in GC and SOL and validation of AnkB pulldown from SKM (n = 3 AnkB-IP or control IgG-IP). Putative AnkB interactors showed at least a two-fold enrichment in AnkB-IgG IP eluates over control-IgG IP with a p < 0.05 determined by a one-tail t-test. Created with elements of BioRender. Voos, K. (2025) https://BioRender.com/iyaxrha. c, Total (left) and mitochondrial (right) AnkB interactome hits identified in GC and SOL IPs. d Protein-protein interaction map of mitochondrial hits shared between AnkB interactomes in GC and SOL. Nodes and edges respectively represent proteins and protein interactions. e Schematic depicting the role of MFF and Drp1 in mitochondrial fission. f Images showing in situ nanocomplexes between AnkB and either βII-spectrin (f”), MFF (f”’), or Drp1 (f””) in FDB fibers detected by a proximity ligation assay (PLA) (representative of n = 3 independent PLA experiments). A PLA using only anti-AnkB antibodies serves as a negative control (f’). Mitochondria are labeled by endogenous GFP expression. Insets show higher magnification of regions demarked by a yellow dotted box. Scale bar, 3 µm. g MFF-GFP and AnkB-HA co-expression in total lysates and complex formation in GFP-IP eluates from control and Drp1 knockdown HEK293T cells. h Quantification of AnkB-HA/MFF-GFP signal in GFP-IP eluates. i AnkB-GFP and mCherry-Drp1 co-expression in total lysates and complex formation in GFP-IP eluates from control and MFF knockdown HEK293T cells. j Quantification of mCherry-Drp1/AnkB-GFP signal in GFP-IP eluates. mCherry-Drp1 and MFF-GFP co-expression in total lysates and complex formation in GFP-IP eluates from control and AnkB knockdown HEK293T under crosslinking (k) or native IP (m) conditions. l, n Quantification of mCherry-Drp1 monomers or oligomers/MFF-GFP signal in GFP-IP eluates. Data in g–n was collected from n = 3 independent transfections and co-IP/group. Graphs show mean ± S.D. Statistical differences were determined by single or multiple two-tailed unpaired t-tests with a significance threshold of p < 0.05.
We validated that AnkB binds MFF using co-IP experiments in HEK293T cells co-expressing AnkB-HA and MFF-GFP and treated with the thiol-cleavable crosslinker dithiobis (succinimidylpropionate) (DSP) (Fig. 7g, h). The interaction between AnkB and MFF does not require Drp1, as it is unaltered by over 90% Drp1 loss (Fig. 7g, h, Supplementary Fig. 9b, c). Notably, AnkB also formed complexes with mCherry-Drp1, in both control cells and upon 70% knockdown of all MFF isoforms (Fig. 7i, j, Supplementary Fig. 9d, e). The ratio of mCherry-Drp1 oligomeric to monomeric species was increased in GFP-AnkB IP eluates relative to the input fraction, indicating a preferential association of AnkB with Drp1 oligomers (Supplementary Fig. 9f). Together, our data supports direct and independent interactions of AnkB with both Drp1 and MFF, which, together with our finding that AnkB-deficiency results in hyperfused mitochondria (Figs. 5, 6), suggest that AnkB is a modulator of mitochondria fission in SKM.
AnkB recruits Drp1 to mitochondria in skeletal muscle fibers to promote efficient fission
AnkB localizes and stabilizes macromolecular complexes at specialized membrane nanodomains24,25,26. Our findings showing that AnkB interacts with MFF and Drp1 suggest it is involved in positioning the mitochondria fission machinery in SKM fibers. However, AnkB loss did not change the abundance of MFF in SKM (Supplementary Fig. 10a–d). Thus, we evaluated whether AnkB recruits cytosolic Drp1 to the mitochondria or promotes Drp1 oligomerization to prime fission events. Site-specific phosphorylation of Drp1 influences its dynamic recruitment to the OMM and GTPase activity; hence, it serves as a proxy for measuring levels of “active” pDrp1S579 (corresponding to human pDrp1S616) and “less fission-effective” pDrp1S600 (corresponding to human pDrp1S637) (Fig. 8a)62,63,64,65. AnkB loss led to decreases in Drp1 (total antibody) protein levels in GC (Fig. 8b, c). It also reduced the density of Drp1 (total) puncta per cell, the intensity of Drp1 (total) signal per field of view (FOV), and the co-localization of Drp1 (total) with the OMM protein Tom20 in isolated FDB fibers Supplementary Fig. 10f–h). In contrast, despite a reduction in total Drp1, levels of pDrp1S600, both relative to the total Drp1 pool in AnkB-KO muscle (Fig. 8b, c) and co-localized with mitochondria in FDB fibers (Supplementary Fig. 10j, k) were increased, consistent with reports of Drp1 hyperphosphorylation at S600 in mice with enlarged and hyperfused SKM mitochondria65. AnkB loss also resulted in less recruitment of pDrp1S579 to mitochondria (Fig. 8d–g). Deficits in abundance of Drp1 were not due to changes in mRNA expression (Supplementary Fig. 11a, b), suggesting a posttranslational effect of AnkB on Drp1’s stability. Notably, the volumes of pDrp1S579 and pDrp1S600 puncta, an indicator of the growth and maturation of OMM-associated Drp1 oligomers, a crucial requirement for productive fission66, were reduced in AnkB-KO muscle fibers (Fig. 8i, Supplementary Fig. 10l), suggesting a deficit in Drp1 clustering.
a Drp1’s subcellular localization and GTP-ase activity are modulated by phosphorylation. Western blot (b) and quantification (c) of Drp1 (total), pDrp1S579/Drp1-total (S616 in humans), and pDrp1S600/Drp1-total (S637 in humans) levels in GC and SOL lysates from 4-mo mice (GC: n = 5 WT, n = 8 AnkB-KO mice; SOL n = 5 WT, n = 6 AnkB-KO mice). d Representative images of pDrp1S579 localization in FDB fibers from 4-mo mice co-stained with the OMM marker Tom20. Scale bar, 5 µm. Insets show higher magnification of the yellow dotted boxed regions. Scale bar, 1 µm. e–g pDrp1S579 colocalization with mitochondria measured using overlap coefficient (n = 3 mice/genotype). Data in e was collected from n = 47 WT and n = 44 AnkB-KO fibers. Data in f, g was collected from n = 27 WT and n = 23 AnkB-KO fibers. h, i. Per volume number (h) and volume (i) of pDrp1S579 puncta (n = 3 mice/genotype). Data in h was collected from n = 37 WT and n = 35 AnkB-KO fibers. Data in i was collected from n = 42 WT and n = 32 AnkB-KO fibers. j Representative images of pDrp1S579 and MFF localization in FDB fibers. Scale bar, 5 µm. Insets show higher magnification of yellow dotted boxed regions. Scale bar, 1 µm. k pDrp1S579 colocalization with MFF measured using overlap coefficient (n = 3 mice/genotype; n = 12 WT and n = 11 AnkB-KO fibers). Western blot (l) and quantification of MFF (m) and Drp1 (n) monomers and higher order species in either native (−DTT) or reduced (+DTT) lysates from 4-mo SOL (n = 3 WT, n = 4 KO mice). Data show mean ± S.D. Statistical differences were determined by single or multiple two-tailed unpaired t-test with a significance threshold of p < 0.05.
The decreased association of pDrp1S579 with MFF in AnkB-KO muscle fibers (Fig. 8j, k) indicates that AnkB promotes the recruitment of fission-competent Drp1 to the OMM. Importantly, AnkB is not required for the formation of the Drp1/MFF complex based on our data showing that AnkB knockdown in HEK293T cells did not disrupt the association of mCherry-Drp1 monomers or oligomers with MFF-GFP under protein crosslinking (Fig. 7 k, l, Supplementary Fig. 9g, h), and only led to a 10% reduction in mCherry-Drp1 monomers/MFF-GFP complexes under native IP conditions (Fig. 7 m, n). Likewise, AnkB knockdown did not affect the binding of Drp1 to its fission co-receptor MiD5167 (Supplementary Fig. 9i, j).
In non-reduced GC and SOL lysates, we detected Drp1 mostly as dimers and tetramers while MFF appeared as monomers and higher order multimers (Fig. 8l, Supplementary Fig. 10m). Notably, levels of MFF oligomers, which are required for Drp1/MFF interactions that stimulate Drp1’s GTPase activity and its effective recruitment to, and oligomerization at, OMMs57,68, were reduced in SOL of SKM-AnkB-KO mice (Fig. 8l, m, Supplementary Fig. 10n). Membrane-anchored MFF preferentially promotes the self-assembly of dimeric Drp1 into oligomers of enhanced activity, whereas the premature formation of Drp1 multimers in solution weakens functional interactions with MFF68,69. We identified increases in Drp1 tetramers, the predominant cytosolic Drp1 form70, in AnkB-KO SOL lysates (Fig. 8l, n, Supplementary Fig. 10m, o), which also supports a reduction in mitochondria-associated Drp1, and in fission-productive MFF-Drp1 complexes.
Mitochondria hyperfusion in SKM-AnkB-KO muscle fibers can also arise from upregulation of fusion events. Interestingly, both mRNA and protein levels of Opa1, which promotes mitochondrial inner membrane fusion71, were reduced in AnkB-KO GC (Supplementary Fig. 11a, c, e), suggesting a compensatory downregulation of fusion. Levels of Fis1, an alternative Drp1 receptor at the OMM, which promotes mitophagy in SKM, especially under cellular stress72, were elevated in AnkB-KO GC (Supplementary Fig. 11c, e). Together, our findings indicate that AnkB directly modulates mitochondrial fission in SKM by promoting efficient Drp1 localization and oligomerization at the OMM.
AnkB regulates sarcoplasmic reticulum-mitochondria contact formation and calcium homeostasis
Calcium (Ca2+) signaling emerged as a top transcriptionally dysregulated canonical pathway in AnkB-KO SKM (Fig. 4a). The set of differentially expressed genes involved in Ca2+ regulation encode sarcoplasmic reticulum (SR)- and triad-localized components of the excitation-contraction (EC) coupling and the Ca2+ release apparatus, including triadin (Trdn), ryanodine (Ryr3) and inositol triphosphate (Itpr3) receptors, the sarco/ER Ca2+-ATPase (Atp2a2, Atp2a3), the Na+/Ca2+ exchanger (Slc8a3, Slc8b1), and subunits of voltage-dependent Ca2+channels (Cacna2d1, Cacnab3, Cacnab4) (Fig. 9a). The physical contact and bidirectional crosstalk between the SR—the specialized ER of SKM—and mitochondria modulates intracellular Ca2+ flux and promotes mitochondria dynamics and respiratory capacity73. Therefore, we evaluated whether deficits in Ca2+ regulation and SR-mitochondria association also contribute to the impairments in mitochondria fission and energetic capacity of SKM-AnkB-KO muscle fibers. We found reduced baseline cytosolic and mitochondrial Ca2+ in live AnkB-KO FDB fibers, respectively measured as the ratio of Fluo-4 to Fura Red and of Rhod2-AM to MitoTracker fluorescence (Fig. 9b, c). Interestingly, AnkB loss caused a global disorganization of the SR, detected by TEM in muscle sections (Fig. 9d) and super-resolution imaging of FDB fibers labeled with ER-tracker (Fig. 9e). Triads—substructures composed of a transverse tubule with two flanking SR cisternae that coordinate EC coupling—were randomly distributed and often more closely clustered in AnkB-KO fibers (Fig. 9e, g, h). Triad disorganization was also detected in AnkB-KO fibers stained for the triad and SR-resident protein ryanodine receptor (RyR) (Fig. 9f, i). These deficits were accompanied by upregulation of the muscle-specific small ankyrin-R (AnkR) isoform sAnk1.5 (Fig. 9j, k), whose overexpression increases SERCA activity and SR Ca2+ reuptake74, further supporting impairments in Ca2+ signaling.
a Protein-protein interaction map of differentially expressed genes (DEGs) associated with Ca2+ signaling derived from RNA-seq analysis. Blue and red denote downregulated and upregulated genes, respectively. Nodes are presented based on fold change (FC), indicated by color intensity and on p-value, shown as node size. Triad- or SR-localized DEGs are indicated. Cytosolic (b) and mitochondrial (c) Ca2+ measured at baseline in 4-mo FDB fibers. Data in b was collected from n = 53 WT and n = 42 AnkB-KO fibers. Data in c was collected from n = 57 WT and n = 39 AnkB-KO fibers. d TEM images of longitudinal GC sections from 4-mo mice (representative of sections collected from n = 3 mice/genotype in one experiment). Scale bar, 1 µm. Insets show higher magnification of yellow dotted boxed regions. Scale bar, 500 nm. Arrows indicate the sarcoplasmic reticulum (SR). e FDB fibers stained with ER-tracker (representative of n = 3 independent experiments). Scale bar, 5 µm. Insets show higher magnification of yellow dotted boxed regions. White dashed lines show regions used for line intensity profile analysis shown in (g). Scale bar, 1 µm. f Ryanodine receptor (RyR) distribution in FDB fibers from 4-mo mice. Scale bar, 5 µm. Insets sh≤ow higher magnification of yellow dotted boxed regions. Arrowheads indicate RyR puncta spacing. Scale bar, 1 µm. g Line intensity plot of ER-tracker signal in (e). h Average distance between triads (n = 17 WT, n = 27 AnkB-KO fibers). i Quantification of triad spacing based on RyR distribution in (f) (n = 19 WT, n = 33 AnkB-KO fibers). Western blot analysis of levels of the muscle-specific AnkR isoform sAnk1.5 in GC (j) and quantification (k). Protein levels were normalized to total protein stain and graphed as relative fold-change over WT (control n = 7, KO = 6). l Representative images show ER-tracker and PKmito distribution in FDB fibers from 4-mo mice. Scale bar, 5 µm. Insets show higher magnification of the yellow dotted boxed regions. Scale bar, 1 µm. m-o, Mander’s overlap coefficient (m) and channel-specific overlap coefficients for mitochondria (n) and ER tracker (o). Data in m was computed from n = 46 WT and n = 81 AnkB-KO fibers. Data in n, o was computed from n = 22 WT and n = 29 AnkB-KO fibers. Data show mean ± S.D. Statistical differences were determined by two-tailed unpaired t-test with a significance threshold of p < 0.05.
ER contacts with mitochondria mark the sites of mitochondrial fission prior to Drp1 recruitment21. Consequently, changes in SR organization can reduce the frequency of its contact with mitochondria and diminish the number of fission events. Thus, we assessed global SR-mitochondria coupling by dual labeling live FDB fibers with ER tracker and PKmito-deep RED. Control mice exhibited a highly coupled SR-mitochondria network, in which all mitochondria were tightly associated with the SR (Fig. 9l–o). In contrast, in AnkB-KO fibers, SR-mitochondria coupling was reduced and sometimes absent, especially in areas with hyperconnected and enlarged mitochondria (Fig. 9l–o). Thus, AnkB is necessary for the proper organization of Ca2+ release units (CRUs) in SKM, which are required for maintaining Ca2+ homeostasis and mitochondrial health.
Exercise-induced mitophagy is impaired in SKM of AnkB KO mice
Acute endurance exercise triggers mitochondrial fission and mitophagy to remove damaged mitochondria75,76,77,78. To assess the contribution of AnkB-220 to exercise-induced mitochondrial turnover, we evaluated mitophagy flux during the post-acute exercise recovery period—when dysfunctional mitochondria are typically removed77,78—using isolated SKM mitochondria from sedentary (SED) or exercised (EXE) mice (Fig. 1l, Supplementary Fig. 11f). Notably, levels of AnkB-220 in the mitochondrial fraction increased after a single bout of acute exercise, suggesting its enhanced recruitment to mitochondria under energetic stress (Fig. 10a, b). Under energy deficits, the E3 ubiquitin ligase Parkin is recruited to damaged mitochondria, where it ubiquitinates OMM targets and recruits autophagy effectors and adaptors, including p62, to facilitate the enclosure and degradation of dysfunctional mitochondria by autophagosomes77. In control mice, mitochondrial Parkin and p62 levels were reduced three hours post-exercise, indicating normal mitophagy flux, whereas their sustained higher levels in AnkB-KO SKM mitochondria fractions were consistent with impaired mitochondrial removal due to defective mitophagy (Fig. 10c–e).
Western blots (a) and quantification (b) of AnkB-220 levels in isolated mitochondrial from sedentary (SED) and exercised (EXE) mice (n = 4/treatment). Western blots (c) and quantification of Parkin (d) and p62 (e) levels in isolated mitochondrial from SED and EXE mice. (WT SED n = 4, KO SED n = 4; WT EXE n = 6, KO EXE n = 5). Levels were normalized to total protein stain and represented as fold-change over WT SED mice. Data show mean ± S.D. Statistical differences were determined by two-tailed unpaired t-test or one-way ANOVA with Tukey’s multiple comparisons test, and a significance threshold of p < 0.05. f We propose that SKM AnkB modulates mitochondrial fission by synergistically recruiting fission-active Drp1 to the OMM and promoting SR organization and SR-mitochondria coupling. These functions enable efficient Ca2+ flux and remodeling of the mitochondrial network during energetic stress associated with endurance exercise and promote SKM respiratory capacity. Created in BioRender. Voos, K. (2025) https://BioRender.com/c46t297.
Together, our results show that AnkB enables SKM mitochondrial fission by synergistically promoting SR-mitochondria contacts and the recruitment of fission-competent Drp1 to OMMs (Fig. 10g). These AnkB-regulated functions enable SKM cellular respiration, Ca2+ flux, and the maintenance of a healthy mitochondrial network under energetic stress imposed by endurance exercise.
Discussion
Mitochondria remodeling in SKM is a central player during adaptation to cellular stress caused by heightened metabolic demands, such as physical exercise. By examining the SKM-autonomous role of membrane adaptor AnkB in glucose metabolism and SKM integrity, we uncovered a novel function of AnkB in the regulation of mitochondrial fission and energetics. We find that mitochondria in SKM lacking AnkB are enlarged and hyperconnected, and exhibit increased oxidative stress at baseline and reduced capacity to oxidize fatty acids. Consequently, SKM-AnkB-KO mice fatigue faster and show enhanced activation of cellular stress pathways during prolonged endurance running of moderate intensity. We demonstrate that AnkB forms complexes with mitochondria fission effectors Drp1 and MFF and promotes the efficient recruitment and oligomerization of fission-competent Drp1 to OMMs. We further show that AnkB loss in SKM causes broad disorganization of the SR and triads, concomitant with reductions in SR-mitochondria contacts, and in cytosolic and mitochondrial Ca2+ levels, all required for optimal mitochondria fission and respiratory function. Therefore, we propose that by regulating the interplay between the ER, the Ca2+ release machinery, and Drp1, SKM AnkB promotes mitochondria remodeling and enables bioenergetic and substrate fuel adaptability to prolonged energy demand and optimal exercise capacity.
We previously reported that AnkB regulates whole-body energy homeostasis by modulating Glut4 membrane trafficking and glucose uptake in adipocytes28,29. Surprisingly, given the major contribution of Glut4-dependent glucose uptake in SKM to systemic glucose levels, AnkB loss in SKM did not alter adiposity or overtly impair glucose handling in mice, regardless of age or diet. SKM-AnkB-KO mice also maintained blood glucose levels during running. On the other hand, SKM-AnkB-KO mice showed premature increases in blood lactate and reduced exercise capacity. Additionally, fibers lacking AnkB isolated from the FDB, primarily composed of fast oxidative IIa fibers40, exhibited lower membrane potential and higher ROS levels at baseline. Our finding that AnkB is most abundant in type IIa fibers that support endurance activities indicates an increased vulnerability of muscles with high density of fast oxidative fibers to AnkB deficiency and suggests fiber-type specific effects of AnkB on mitochondrial energetics and exercise capacity.
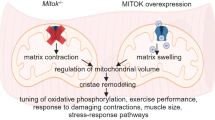
A prior study that characterized whole-body Perm1 KO mice proposed that AnkB’s binding to the Perm1-MICOS-mitochondrial intermembrane space bridging (MIB) complex links SSM mitochondria to the membrane of SKM fibers55. Our findings confirmed that AnkB associates with SKM mitochondria and forms complexes with Perm1 interactors and mitochondrial resident proteins, including the SAM and MIB component Sam50. However, unlike Perm1 KO, AnkB loss did not alter mitochondrial cristae organization, SSM mitochondria abundance, total mitochondria content, or levels of OXPHOS complex proteins. Future evaluations of the effects of disrupting Perm1 association with AnkB will clarify the contribution of the AnkB-Perm1 complex to mitochondria homeostasis and endurance exercise deficits of AnkB-KO and Perm1-KO mice79. Instead, SSM and intermyofibrillar mitochondria were enlarged and hyperconnected across muscle types in AnkB-KO mice despite downregulation of mitochondria fusion effector Opa1. The interconnected SKM mitochondrial network dilutes mtDNA damage and boosts membrane potential conduction and oxidative metabolism80. However, pathological mitochondrial hyperfusion propagates ROS accumulation and mitochondrial damage throughout the network and impairs fatty acid oxidation49,50,51,52,53. Unlike other cell types, the large size of SKM fibers and their crowded cytoskeleton limit the rapid transport and incorporation of new mitochondria needed to preserve a healthy network under endurance exercise-induced energetic stress. Consequently, SKM relies more extensively on effective fission to remove dysfunctional mitochondria and adapt the mitochondrial network to prolonged bioenergetic requirements81.
Mitochondrial connectivity and dynamics depend on muscle fiber type and the differentiation state of myofibers81. Here, we investigated AnkB-dependent molecular mechanisms that regulate SKM mitochondria remodeling using intact adult FDB fibers, which more closely preserve the unique 3D architecture and metabolic environment of SKM. A previous study linked AnkB to the regulation of mitochondrial fission in cortical neurons via interaction and auto-activation of brain-specific kinases 1/1 (Brsk1/2 or SadA/B), upstream of the destabilization of actin filaments, and the recruitment of Drp1 to the mitochondrion to enable scission82. However, deficits in SadA/B activity cannot explain the mitochondrial fission defects observed in AnkB-KO muscle fibers, as these kinases are not expressed in SKM83,84. Instead, we uncovered and validated that AnkB independently binds mitochondria fission regulators MFF and Drp1. Clustering of total and pro-fission pDrp1S579 with MFF and OMMs was reduced in AnkB-KO FDB fibers. Parallelly, levels and mitochondria clustering of less fission effective pDrp1S600 were increased, while the size of Drp1 puncta and the abundance of MFF oligomers were reduced, indicating less efficient oligomerization of fission-competent Drp157. However, AnkB is not required for the direct binding of Drp1 to MFF. SKM Drp1 levels and its phosphorylation at S579 (S616) promote endurance exercise performance in mice and humans77. SKM-AnkB-KO mice with decreased levels of mitochondria-associated pDrp1S579 exhibit impairments in fatty acid oxidation, endurance exercise, and mitophagy similar to SKM-specific Drp1 haploinsufficient mice51,75, but do not develop the severe muscle atrophy and loss of muscle strength seen upon more pronounced loss of SKM Drp175,85. Thus, it is possible that AnkB-dependent translocation of Drp1 to OMMs plays a more significant role in remodeling the mitochondrial network in muscles with high density of oxidative fibers, where AnkB is more abundant. Moreover, these roles may be essential under energetic stress, such as prolonged endurance exercise, when mitochondrial fission and mitophagy are rapidly and transiently upregulated77,78, and SKM relies on fatty acid oxidation for energy42,43.
AnkB loss also leads to disorganization of the SR of SKM fibers and reduced SR-mitochondria coupling. The interaction of peripheral ER tubules with mitochondria marks sites of constriction prior to Drp1 recruitment21. SR-mitochondria apposition together with increased phosphorylated Drp1 (S616) are observed in fatigued or electrically stimulated muscle86. Therefore, it is possible that reductions in SR-mitochondria contacts in AnkB-deficient SKM contribute to the fission impairment caused by direct loss of AnkB-Drp1 complexes. Besides its function as a mitochondria fission effector, Drp1 clusters and oligomerizes at the ER to promote ER tubulation87,88. It would be informative to define if, like in other cell types, ER-associated Drp1 promotes SKM SR organization and tubulation to potentiate SR-mitochondria contacts and fission initiation, and if these functions depend on AnkB. Future work should also clarify if, instead, deficits in ER-mitochondria coupling of AnkB-KO muscle fibers are secondary to the hyperfused mitochondrial network.
In line with a global SR disorganization, AnkB-KO fibers showed altered spacing of triad-localized Ca2+ release units (CRUs), marked by RyR, and reduced cytosolic and mitochondrial Ca2+. AnkB stabilizes the Na+/K+ ATPase, the Na+/Ca2+ exchanger, and the inositol phosphate receptor (IP3R) at a specialized T-tubule microdomain of cardiomyocytes required for cytosolic Ca2+ modulation, which is segregated from RyR-enriched T-tubule/SR dyads, but, importantly, it is absent in SKM89,90. The mechanisms through which AnkB controls Ca2+ levels in SKM and their contribution to mitochondria fission and energetics require further investigation. A potential direction is our observation that AnkB loss changed the expression of regulators of SR Ca2+ flux, including SR-tethered small Ank1.5, whose overexpression enhances SERCA activity and SR reuptake of cytosolic Ca2+74.
SKM-AnkB-KO mice showed deficits in endurance exercise without alterations in muscle strength or muscle mass. Our data is supported by a prior report that functional redundancy among all SKM ankyrins (AnkB, AnkG, AnkR) protects against muscle strength and force deficits, and raises the possibility that the muscle fatigue observed in SKM lacking all ankyrins is partially caused by AnkB deficency38. Loss of AnkB in neonatal muscle of whole-body AnkB-KO mice or acute shRNA-induced AnkB knockdown in juvenile muscle impairs the sarcolemma localization of dystrophin and β-dystroglycan, which can be rescued by exogenous expression of AnkB-22032,33. In contrast, we show that constitutive and persistent AnkB-220 loss throughout SKM development and adulthood resulted in only partial fiber type-specific reduction in dystrophin’s membrane localization without changes in β-dystroglycan distribution. A similar preservation of membrane dystrophin was detected in muscle-specific triple ankyrin KO mice38. These findings raise the possibility that developmental compensatory mechanisms enable the sarcolemma localization of the dystrophin-dystroglycan complex in AnkB-KO SKM, which might be absent in mature SKM fibers. Alternatively, AnkB might be required for localizing these membrane proteins upon acute injury of adult SKM. Future studies using inducible Cre lines to eliminate AnkB expression in adult SKM will compare developmental versus adult roles of AnkB loss in maintaining the sarcolemma organization of SKM fibers. The 20% of GC AnkB-KO fibers devoid of sarcolemma dystrophin are mostly type IIb fibers, which are more susceptible to muscle damage90,91,92. Severe dystrophin deficiency impairs contractile function and causes muscle fatigue in humans and animal models, whereas sub-threshold dystrophin decreases in a subset of fibers often does not yield adverse phenotypes93. The endurance exercise capacity deficits of SKM-AnkB-KO mice are unlikely to arise solely from loss of sarcolemma dystrophin in a small subset of glycolytic fibers, given that as low as 20% dystrophin expression rescues muscle fatigue in mouse models of dystrophin deficiency93,94.
In sum, our data support novel roles of SKM AnkB in modulating mitochondrial fission through direct interaction with MFF and Drp1 at the OMM and by promoting SR organization. We propose that these AnkB-dependent functions synergistically orchestrate efficient remodeling of the mitochondrial network during energetic stress to support respiration and endurance exercise. Importantly, our findings suggest that AnkB loss of function in SKM is not a major driver of the metabolic syndrome derailments caused by AnkB variants. However, the reduced exercise capacity caused by AnkB deficiency may lead to suboptimal therapeutic benefits of physical activity.
Methods
Mouse lines and animal care
Experiments were performed in accordance with the guidelines for animal care of the Institutional Animal Care and Use Committees (IACUC) of the University of North Carolina at Chapel Hill (animal protocol approval # 16-291) and the University of Pennsylvania (animal protocol approval # 807277). Mouse (Mus musculus) lines were maintained in the C57BL/6J background by regular backcrossing to the C57BL/6 J line (Jackson laboratory, stock number 000664). SKM-specific AnkB knockout (SKM-AnkB-KO) mice were generated by breeding homozygous floxed were crossed with heterozygous HSA-Cre mice to generate mice with SKM-specific targeting of GFP to the OMM for PLA evaluations. All studies were performed using 4-mo or 20-mo male mice housed at 22 ± 2 °C on 12-hr light/dark cycles and given food and water ad libitum. For high-fat diet (HFD) studies, mice were fed a rodent diet with 60 kcal% of fat (Research Diet, D12492) for 15 weeks starting a week after weaning.
Plasmids
AnkB-HA and AnkB-GFP were previously described29,33,54. mCherry-Drp1 (Addgene plasmid #49152) and GFP-Mff (Addgene plasmid #49153) were gifts from Dr. Gia Voeltz21. MiD51-GFP and MiD49-GFP were gifts from Dr. Henry Higgs95. The pCas9-2A-GFP-puro-gRNA plasmid was a generous gift from Dr. Dale Cowley. Plasmids were verified by full-length sequencing. To generate the AnkB220-GFP adenoviral plasmid, an AnkB220-GFP fragment was amplified by PCR from peGFPN3/AnkB-GFP27,29 with primers 5’-ctagcctcgaagatctatgatgaacgaagatgcagctcaga-3’ and 5’- tttggccccgcggccgcgcggccgctttact-3’. The PCR product was cloned into the pDUAL-CCM(+)-AdV shuttle (Vector Biolabs) linearized by BglII/NotI restriction digestion using In-Fusion® Snap Assembly Master Mix (Takara, 638947). All plasmids were verified by full-length sequencing.
Cell lines and cell culture
Muscle fibers were isolated from FDB muscle96. In brief, FDB muscle was digested in DMEM containing 0.2% collagenase (Sigma, 10103578001) for 2-hr with gentle agitation. Digested muscles were transferred to 35-mm culture dish containing 10% horse serum and triturated with a wide-bore Pasteur pipette to release individual myofibers. Fibers were seeded on Matrigel-coated (Corning, 358234) glass-bottom dishes (Cellvis, D35-20-1.5H) and cultured at 37 °C and 5% CO2 in fiber media (DMEM, 20% fetal bovine serum (FBS), 1% penicillin/streptomycin) for 24 hours. HEK 293 T/17 cells were obtained from ATCC (CRL-11268) and maintained in DMEM containing 10% FBS and 1% pen/strep at 37 °C with 5% CO2. The mouse C2C12 myoblast cell line was obtained from ATCC (CRL1772) and grown in DMEM containing 1% penicillin/streptomycin and 10% FBS. HEK 293 T/17 and C2C12 cells were authenticated by ATCC based on its short tandem repeat profile and have recurrently tested negative for mycoplasma contamination.
AnkB-KO C2C12 were generated using CRISPR-Ca9 editing. In brief, C2C12 were electroporated with 20 µg of pCas9-2A-GFP-puro-gRNA, either empty vector without gRNAs (control line), or containing gRNA sequences targeting exon 22 of mouse AnkB (Ank2) [mAnk2-E22-g1 (TCACCCCAGCCGCTTAGCAATCGCC); mAnk2-E22-g2 (CACCAACCTTCAGGGTGTCAACCA); mAnk2-E22-g3 (CACCCCTGAAGGTTGTGACTGAAG)]. GFP-expressing C2C12 cells were selected in culture media supplemented with puromycin (2 µg/mL) for two weeks. Cells passage numbers 5-8 were used for experimentation.
Protein extraction
Lysates from flash-frozen muscles or HEK 293 T/17 were prepared in homogenization buffer (25 mM Tris-HCl, 150 mM NaCl, 0.32 M sucrose, 1% Igepal (NP40) (v/v), 1% Triton X 100 (v/v), 0.5% CHAPS (wt/v), 5 mM EDTA, pH 7.4) supplemented with EDTA-free protease inhibitor cocktail (Sigma, cat# 4693159001). Total protein homogenates were centrifuged at 20,000 x g for 20 min at 4 °C and the soluble fraction collected for immunoprecipitation or western blot analysis.
Mitochondria isolation
Mitochondria were isolated from fresh GC muscle of 4-mo C57BL/6 J mice following a reported protocol97,97,. Briefly, muscle was minced in hypotonic isolation buffer (67 mM sucrose, 50 mM Tris/HCl, 50 mM KCl, 10 mM EDTA and 0.2% BSA, pH 7.4), digested in 0.05% trypsin for 30 min on ice, and homogenized with a Dounce homogenizer. Homogenates were cleared of nuclei and tissue debris by centrifugation at 700 g for 10 min at 4 °C, and mitochondria were purified by centrifugation at 8000 g at 4 °C for 10 min. The mitochondrial pellet was resuspended in 200 mM D-mannitol, 70 mM sucrose, 5 mM EDTA, and 10 mM Tris-HCl, pH 7.4, and further centrifuged at 8000 g at 4 °C for 10 min to remove residual BSA.
For respiration analysis, mitochondria were resuspended in MiRO5 respiration media (110 mM sucrose, 20 mM HEPES, 10 mM KH2PO4, 20 mM taurine, 60 mM K-lactobionate, 3 mM MgCl2•6H20, 0.5 mM EGTA, 1 g/L BSA (fraction V), pH 7.2). For western blot and BCA analysis, mitochondria were lysed in homogenization buffer (25 mM Tris-HCl, 150 mM NaCl, 0.32 M sucrose, 1% Igepal (NP40) (v/v), 1% Triton X 100 (v/v), 0.5% CHAPS (wt/v), 5 mM EDTA, pH 7.4) supplemented with EDTA-free protease inhibitor cocktail (Sigma, cat# 4693159001). Mitochondria lysates were further mixed with 5x SDS-PAGE buffer (5% SDS (wt/vol), 25% sucrose (wt/vol), 50 mM Tris pH 8, 5 mM EDTA, bromophenol blue) at 95 °C and sonicated to ensure complete solubilization. Samples were analyzed by western blot to evaluate purity and presence of ankyrin-B.
Immunoblots
Protein extracts were mixed at a 1:1 ratio with 5x SDS-PAGE buffer and heated for 15 min at 65 °C. Protein concentration was determined using a BCA assay (Thermo Fisher, 23225), and equal amounts of proteins were loaded onto acrylamide gels and resolved by SDS-PAGE. Immunoblots to evaluate SKM ankyrins were run using house-made 3.5-17.5% acrylamide gradient gels in Fairbanks Running Buffer (40 mM Tris pH 7.4, 20 mM sodium acetate, 2 mM EDTA, 0.2%SDS (wt/vol)). For all other immunoblots, samples were separated using 4-20% gels Novex™ Tris-Glycine Plus Midi Protein Gels (Thermo Fisher, WXP42020BOX) or 4–15% Criterion™ TGX™ Precast Midi Protein Gel (Bio-Rad, 5671084) using Tris-glycine running buffer (APEX, 18-238). Proteins were transferred overnight onto 0.45-μm nitrocellulose membranes (BioRad, 1620115) at 4 °C. Transfer efficiency and total protein content were determined by Ponceau-S stain (0.1% Ponceau wt/vol, 0.1% acetic acid), Revert 700 (LI-COR, 926-11011), or Revert 520 (LI-COR, 926-10011) total protein stain. Membranes were blocked in TBS containing 5% non-fat milk for one hour at RT and incubated overnight with primary antibodies diluted in antibody buffer (TBS, 5% BSA, 0.1% Tween-20). After three washes in TBST (TBS, 0.1% Tween-20), membranes were incubated with secondary antibodies diluted in antibody buffer for two hours at RT. Membranes were washed 3x for 10 min with TBST and 2x for 5 min in TBS. Protein-antibody complexes were detected using the Odyssey® M Imaging system (LI-COR) running LI-COR Acquisition Software version 1.1.0.61. Signal intensity in blots was quantified using the Empiria Studio Software version 2.2.0.141 and normalized to Ponceau S stain, Revert 700, or Revert 520 total protein stains.
Affinity-purified rabbit antibodies anti ankyrin-B, ankyrin-G, βII-spectrin, and GFP, used at a 1:1000 for western blot, were generated by Vann Bennett’s laboratory at Duke University and have been previously described27,28,29,30,32,33,54,59,60,61,. Other antibodies used for western blot analysis included rabbit anti-AnkR (Aviva Sys Bio, ARP42566-T100, 1:500), rabbit anti-dystrophin (Thermo Scientific, PA1-37587, 1:500), mouse anti-SERCA (Affinity Bioreagents, MA3-911, 1:1000), mouse anti-OPA1 (BD Transduction, 612606, 1:1000), mouse anti-p62 (Abnova, H00008878-M01, 1:500), and Abcam antibodies, mouse anti-total rodent OXPHOS antibody cocktail (ab110413, 1:1,000) and rabbit anti-GAPDH (ab9485, 1:1,000). We also used rabbit antibodies specific for total AMPK (2757), phospho-AMPKT172 (2535), total Raptor (2280), phospho-RaptorS792 (2083), HA epitope (3724), phospho-Drp1Ser616 (3455), phospho-Drp1Ser637 (4867), and total Drp1 (8570), as well as mouse anti-Lamin A/C (4777), and mouse anti-Parkin (4211S), all purchased from Cell Signaling Technology and used at a 1:1000 dilution. Commercial rabbit antibodies specific for COXIV (11242-I-AP), mCherry (26765-I-AP), MFF (17090-1-AP) and Fis1 (10956-1-AP) were obtained from Proteintech and used at 1:1000, except for CPT1B (22170-1-AP) which was used 1:500 dilution. Mouse anti-Na/K ATPase (48645), anti-MFN1 (166644), and anti-MFN2 (515647) antibodies were from Santa Cruz Biotechnology and used at 1:1000. Fluorescent signals in western blots were detected using IRDye® 680RD goat anti-mouse (926-68070) and IRDye® 800CW goat anti-rabbit (926-32211) from LiCOR at a 1:5000 dilution.
MFF and Drp1 oligomerization assessment
Oligomeric states of MFF and Drp11 were evaluated by western blot in total protein lysates prepared from GC and SOL diluted in SDS-PAGE buffer in the absence or presence of DTT (5 mg/mL).
Plasmid transfection and co-immunoprecipitation analysis
Transfections were conducted in triplicate in HEK 293 T/17 cells grown in 6-well culture plates using Lipofectamine RNAiMAX (Thermo Fisher, 13778075) or polyethylenimine (PEI). Interactions between AnkB with either Drp1 or MFF were evaluated in cells transfected with either control Dicer-Substrate Short Interfering RNAs (DsiRNA), IDT DNA, 51-01-08-22) or a cocktail of custom-ordered DsiRNA specific to human AnkB [ANK2: DsRNA1 (rGrArGrArArUrCrArCrArUrUrGrArUrGrUrUrGrUrArArAAT and rArUrUrUrUrArCrArArCrArUrCrArArUrGrUrGrArUrUrCrUrCrUrU), DsiRNA2 (rGrArUrArArGrArCrCrCrUrUrGrArArCrArArCrArArGrAAA and rUrUrUrCrUrUrGrUrUrGrUrUrCrArArGrGrGrUrCrUrUrArUrCrCrA, DsiRNA3 (rCrCrUrGrCrArUrGrUrGrGrCrGrUrUrUrArArArArArUrCAA and rUrUrGrArUrUrUrUrUrArArArCrGrCrCrArCrArUrGrCrArGrGrUrA)]; human Drp1 [DNM1L: DsiRNA1 (rCrArUrGrUrUrUrCrArCrArArGrArArGrArUrArArArCrGGA and rUrCrCrGrUrUrUrArUrCrUrUrCrUrUrGrUrGrArArArCrArUrGrGrA), DsiRNA2 (rGrArUrUrCrArUrArCrUrUrArArArCrCrUrGrArArArGrCAG and rCrUrGrCrUrUrUrCrArGrGrUrUrUrArArGrUrArUrGrArArUrCrArA), DsiRNA3 (rCrCrGrUrGrGrArUrGrArUrArArArArGrUrGrCrUrArCrUTT and rArArArGrUrArGrCrArCrUrUrUrUrArUrCrArUrCrCrArCrGrGrGrU)]; and human MFF [MFF: DsiRNA1 (rArCrUrCrArGrUrUrCrArUrGrCrGrUrGrArArCrArArUrUTA and rUrArArArUrUrGrUrUrCrArCrGrCrArUrGrArArCrUrGrArGrUrArA), DsiRNA2 (rGrArCrCrArGrCrArGrArUrCrUrUrGrArCrCrUrUrArUrUCA and rUrGrArArUrArArGrGrUrCrArArGrArUrCrUrGrCrUrGrGrUrCrUrU)]. 10 nM of each DsiRNA was mixed in Optimem together with 10 nM of TYE 563 DsiRNA transfection control (IDT DNA, 570381937). DsiRNA-lipofectamine RNAiMAX complexes were added to cells. 24-hours post-transfection of DsiRNA complexes, cells were co-transfected with either 2 μg of AnkB-GFP and 1 μg of mCherry-Drp1 plasmids; 2 μg of AnkB-3xHA and 1 μg of MFF-GFP plasmids; 1 μg of mCherry-Drp1 and 1 μg of MFF-GFP plasmids; or 1 μg of mCherry-Drp1 and 1 μg of MiD51-GFP plasmids using PEI. 48 hr after plasmid (72 hr post DsiRNA) transfections. HEK293T/17 cells were either directly collected for native IP or crosslinked with 2 mM DSP at RT for 30 min. The crosslinking reaction was quenched with 1 M Tris-HCl for 15 min at RT. Proteins were extracted in homogenization buffer and soluble fractions loaded to ChromoTek GFP-Trap® Magnetic Particles M-270 (gtd) (Proteintech). Beads were washed three times with TBS with 0.05 Triton-X100, and bound complexes were eluted in 5X SDS-PAGE buffer with DTT at 95 °C. Eluates were resolved by SDS-PAGE and Western blot.
Immunoprecipitation of AnkB complexes from SKM
Total protein lysates from GC and SOL muscle were incubated overnight at 4 °C with Protein A/G magnetic beads (Life Technologies, 88802) coupled to either rabbit anti-ankyrin-B-IgG (AnkB IP)27,28,29,30 or rabbit-IgG (control IP). Beads were sequentially washed with lysis (25 mM Tris-HCl, 300 mM NaCl, and 1 mM EDTA), TBS (10 mM Tris-HCl, 150 mM NaCl, 0.5 mM EDTA) containing 0.1% Triton-X 100, and TBS. Beads were rinsed with 50 mM ammonium bicarbonate and protein complexes eluted in 50 mM ammonium bicarbonate containing 0.1% Rapigest (Waters, 186001861).
LC-MS/MS interaction proteomics analysis
IP eluents were brought to 5% SDS, reduced for 15 min at 80 °C with 10 mM dithiolthreitol, and alkylated with 20 mM iodoacetamide for 30 min at RT. Samples were then supplemented with a final concentration of 1.2% phosphoric acid and 375 µL of S-Trap (Protifi) binding buffer (90% MeOH/100 mM TEAB). Proteins were trapped on the S-Trap micro cartridge, digested using 20 ng/µL sequencing grade trypsin (Promega) for 1 hour at 47 °C, and eluted using 50 mM TEAB, followed by 0.2% FA, and lastly using 50% ACN/0.2% FA. All samples were then lyophilized to dryness. Samples were resuspended in 40 μL of 1% TFA/2% acetonitrile with 12.5 fmol/µL of yeast ADH. Digested peptides were lyophilized to dryness and resuspended in 12 μL of 0.2% formic acid/2% acetonitrile. Each sample was subjected to chromatographic separation on a Waters NanoAquity UPLC equipped with a 1.7 µm HSS T3 C18 75 µm I.D. X 250 mm reversed-phase column (NanoFlow data). The mobile phase consisted of (A) 0.1% formic acid in water and (B) 0.1% formic acid in acetonitrile. 3 µL was injected and peptides were trapped for 3 min on a 5 µm Symmetry C18 180 µm I.D. X 20 mm column at 5 µl/min in 99.9% A. The analytical column was then switched in-line and a linear elution gradient of 5% B to 40% B was performed over 90 min at 400 nL/min. The analytical column was connected to a Fusion Lumos mass spectrometer (Thermo) through an electrospray interface operating in a data-dependent mode of acquisition. The instrument was set to acquire a precursor MS scan from m/z 375-1500 at R = 120,000 (target AGC 2e5, max IT 50 ms) with MS/MS spectra acquired in the ion trap (target AGC 1e4, max IT 100 ms). For all experiments, HCD energy settings were 30 v and a 20 s dynamic exclusion was employed for previously fragmented precursor ions.
Raw LC-MS/MS data files were processed in Proteome Discoverer (Thermo Scientific) and submitted to independent Mascot searches (Matrix Science) against a SwissProt Mouse protein database containing both forward (20260 entries) and reverse entries of each protein. Search tolerances were 5 ppm for precursor ions and 0.8 Da for-product ions using trypsin specificity with up to two missed cleavages. Carbamidomethylation (+57.0214 Da on C) was set as a fixed modification, whereas oxidation (+15.9949 Da on M) was considered a dynamic mass modification. All searched spectra were imported into Scaffold (v4.4, Proteome Software), and scoring thresholds were set to achieve a peptide false discovery rate of 1% using the PeptideProphet algorithm. Potential AnkB interactors were identified as hits showing at least a two-fold enrichment of normalized total spectral counts in AnkB-IP compared to control-IP eluates and a p-value < 0.05. Mitochondria interactors were identified using stringApp (https://apps.cytoscape.org/apps/stringapp) in Cytoscape (version 3.9.0).
RNA isolation and sequencing
Flash-frozen muscles were thawed on ice and lysed in QIAzol Lysis Reagent (Qiagen, 79306) using a bead homogenizer. RNA was isolated using the RNeasy Mini kit (Qiagen, 74106) with on-column DNAse digestion. RNA-sequencing was performed on a Novaseq 6000 platform by Novagene (Sacramento, CA). Differential expressed genes (DEG) were identified using DESeq2 based on a 1.15 log fold change and adjusted p-value > 0.05. Pathway analysis was performed in IPA (QIAGEN Inc., https://digitalinsights.qiagen.com/IPA) and data visualized using Cytoscape.
Quantitative PCR
cDNA synthesis from total RNA isolated from CG and SOL muscles was performed using the SuperScript® III First-Strand Synthesis System (Invitrogen). Quantitative PCR (qPCR) was performed with the QuantStudio 5 Real Time PCR Machine (ThermoFisher) using SYBR Green detection reagent. Transcript levels were normalized to Gapdh (forward 5’-TCAACGGGAAGCCCATCA-3’ and reverse 5’- CTCGTGGTTCACACCCATCA-3’). The following primers were used: Pgc1-α (forward 5’-GGAGCCGTGACCACTGACA-3’ and reverse 5’-GGAGCCGTGACCACTGACA-3’), ERR-α (forward 5’-GGAGGACGGCAGAAGTACAAA-3’ and reverse 5’-GCGACACCAGAGCGTTCAC-3’), NRF1 (forward 5’-TGGAGTCCAAGATGCTAATG-3’ and reverse 5’-AGAGCTCCATGCTACTGTTC -3’), NRF2 (forward 5’-TGAAGTTCGCATTTTGATGGC-3’ and reverse 5’-CTTTGGTCCTGGCATCTCTAC-3’), TFAM (forward 5’-CAAAGGATGATTCGGCTCAG-3’ and reverse 5’-AAGCTGAATATATGCCTGCTTTTC-3’), PPAR-α (forward 5’-AAGGCTATCCCAGGCTTTGC-3’ and reverse 5’-TTTAGAAGGCCAGGCCGATCTC-3’), PPAR-γ (forward 5’-AGGCCGAGAAGGAGAAGCTGTTG-5’ and reverse 5’-TGGCCACCTCTTTGCTCTGCTC-3’), PPAR-δ (forward 5’-GTATGCGCATGGGACTCAC-3’ and reverse 5’-GTCTGAGCGCAGATGGACT-3’), Opa1 (forward 5’-TCACCTCTGCGTTTATTTGAAGA-3’ and reverse 5’-GGGTAGAACGGGAGGAAAGG-3’), and Dnml1 (forward 5’-GGGCACTTAAATTGGGCTCC-3’ and reverse 5’-GGAGAAAAGGGAAGGCGATG-3’).
Mitochondrial to nuclear DNA ratio was determined by quantitative PCR in total DNA isolated from flash-frozen SKM using the QIAamp DNA minikit (Qiagen, 51304). Mitochondrial DNA was quantified using primers for mitochondria-encoded NADH dehydrogenase 1 (Nd1) (forward 5’-CTAGCAGAAACAAACCGGGC-3’ and reverse 5’-CCGGCTGCGTATTCTACGT-3’). Nuclear DNA was assessed using primers specific for nucleus-encoded hexokinase 2 (Hk2) (forward 5’-GCCAGCCTCTCCTGATTTTAGTGT-3’ and reverse 5’-GGGAACACAAAAGACCTCTTCTGG-3’).
Body composition and calorimetry analysis
Mouse weights were evaluated at 4-5 months or 20 months of age. Body composition was measured by magnetic resonance imaging. Mice were housed in individual indirect calorimetry cages (Phenomaster/Labmaster, TSE SYSTEMS, Chesterfield, MO) for continuous measurements of O2 consumption (VO2), CO2 production (VCO2), activity (total sum of X, Y, and Z beam break counts), and food and water consumption for 48 hours. Energy expenditure and respiratory exchange rate (RER (VCO2/VO2)) were also calculated using raw values or normalized to lean body mass as a covariate using an ANCOVA analysis.
Insulin and glucose tolerance tests
For a glucose tolerance test (GTT), glucose was administered at 2 g/kg body weight via intraperitoneal injection (IP) after either a four-hour or an overnight fast98,98,,99,99,. For an insulin tolerance test (ITT), recombinant insulin (Humulin-R, Eli Lilly) was administered at 1 U/kg body weight via IP after either a four-hour or a six-hour fast98,98,,99,99,. Blood glucose samples were taken from a tail clip at different post-injection timepoints, and glucose levels were measured using a handheld Accucheck glucometer.
Wire-hang and grip strength tests
For wire-hang assessments, an individual mouse was placed on a large metal cage lid. The lid was gently shaken to induce the mouse to grip onto the metal grid and then flipped over. The latency for the mouse to fall from the lid was recorded with a maximum time of 60 sec. The strength of paw grasp was evaluated using precision force gauges based on grasp of a metal grid by a mouse gently pulled by the tail. Digital force meters (Chatillon DFIS-10) were mounted on an acrylic platform (San Diego Instruments), with two different grids: a left-hand grid for the front paws, and a right-hand grid for all-four paws. Each grid was connected to a force transducer, which provided measures of peak force (N). Each mouse was given three trials, with at least one min of recovery between trials. Each trial measured front-paw measures from the left-hand grid immediately followed by evaluation of all-four-paw measures from the right-hand grid.
Voluntary running wheel
Mice were single-housed with free access to a running wheel connected to a computer to measure wheel speed and distance continually over a seven-day period. Maximal running wheel speed was calculated as the fastest wheel speed per day over the seven-day period. Average speed was estimated at the per-day average non-zero speed of each mouse. Break time was determined as the total zero-speed min per day.
Evaluations of exercise capacity and blood metabolites during endurance running
Mice ran on single-lane rodent treadmills equipped with individual shock grates to encourage running (Columbus Instruments). All experiments were done with the treadmill set at a 5% incline. Mice were first acclimated to the treadmill for three days by running at increasing speeds (0-14 m/min) for 15–17 min/day. Electric stimulation was employed on days 2–3 of acclimation. Mice rested for two days after acclimation before endurance running. To determine exhaustion time and maximum velocity during the endurance exercise test, mice ran at 10, 12, and 14 m/min for 10, 4, and 45 min respectively. This was followed by a 2 m/min increase in speed every 10 min until a mouse could no longer keep up with the speed of the treadmill, defined as the mouse failing to keep all four paws on the running belt for 15 seconds or longer. Once a mouse reached exhaustion, the shock stimulus was turned off. The exhaustion time, total running time, and maximum velocity achieved were recorded. Blood lactate and glucose levels were evaluated during exercise in mice running at a constant speed for four min, followed by a one-min rest to collect tail blood. The initial speed (5 m/min) was increased 2.5 m/min over a 60-min period, or until the time when blood lactate levels were at least 2-fold higher than pre-exercise baseline measurements. Blood lactate and glucose were respectively measured using a handheld Lactate Plus meter (Nova Biomedical) and an Accucheck glucometer.
High resolution respirometry
Mitochondria were isolated as described and resuspended in MiRO5 respiration media. Oxygen consumption was measured in mitochondria fractions isolated from SKM using the O2k Oxygraph high-resolution respirometer (Oroboros Inc.). Briefly, 80-100 µg of total mitochondria fraction proteins were transferred to an air-calibrated respirometer containing 2.2 ml of pre-warmed MiRO5 respiration medium. The following reagents were added sequentially to each chamber to determine the respiratory capacity of the electron transport chain (final concentrations listed): 2 mM malate, 10 mM pyruvate or 25 µM palmitoyl-carnitine, 2.5 mM ADP (adenosine diphosphate), 5 µM oligomycin, 2 µM FCCP, and 5 µM antimycin A. Oxygen consumption rate was normalized to total protein determined by a BCA assay (Thermo Fisher, 23225).
Evaluation of exercise-induced stress and mitophagy flux
To determine the activation of canonical stress pathways after exercise, mice were run to exhaustion using the moderate intensity endurance exercise test described above. Samples were collected immediately after exercise cessation for protein extraction and western blot analysis. To determine the recruitment of mitophagy effectors to mitochondria after acute exercise, mice were run to exhaustion using the same protocol. SKM was collected 3-hr post-exercise for mitochondrial isolation and western blot analysis.
Transmission electron microscopy
Mice were perfused with 2% PFA/2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) at RT. GC and SOL were dissected into ~2 mm strips and stored in fixative at 4 °C. Specimens were rinsed in phosphate buffer, post-fixed for one hour in 1% osmium tetroxide in sodium phosphate buffer, rinsed in deionized water, dehydrated through ethanol series (30%, 50%, 75%, 100%, 100%, 10 min each), and incubated twice for 15 min with propylene oxide. Specimens were sequentially infiltrated for two hours with a 1:1 mixture of propylene oxide:Polybed 812 epoxy resin (1 A:2B formulation, Polysciences, Inc.) and a 1:2 mixture of propylene oxide: Polybed 812 epoxy resin, followed by overnight incubation in the resin. Tissue was embedded in fresh epoxy resin and allowed to polymerize for 24 hours at 60 °C. Semi-thin (1 µm) tissue sections were cut with a diamond knife and stained with 1% toluidine blue to isolate the region of interest. Ultrathin cross and longitudinal sections (70-80 nm) were mounted on 200 mesh copper grids and stained with 4% uranyl acetate for 12 min and Reynold’s lead citrate for 8 min. TEM images were acquired with a JEOL 1230 transmission electron microscope at the Microscopy Service Laboratory at UNC-Chapel Hill. Mitochondrial morphology was assessed using images acquired at 25000X by measuring cross-sectional area using Fiji (version 2.1.0/1.53c). The scale was set according to the scale bar of the original images. Mitochondrial periphery was determined by the presence of a double membrane. Adjacent mitochondria with indistinguishable membranes were treated as one mitochondrion.
Histological preparation and staining of SKM sections
Muscles were fixed by mouse transcardial perfusion with 4% PFA in PBS. GC and SOL were dissected and postfixed overnight in 4 % PFA/PBS at 4 °C, washed in PBS, cryoprotected in 30% sucrose in PBS at 4 °C, and embedded in Epredia Cryomatrix embedding resin (Epridia, 6769006). 10 µm frozen longitudinal and cross sections were cut using a CryoStar NX50 Cryostat (Fisher Scientific). Evaluations of muscle structure were conducted in cryosections permeabilized for 30 min in 1% Triton-X 100 in PBS, blocked overnight at 4 °C in blocking solution (4% BSA, 0.5% Tween 20, PBS), and incubated overnight at 4 °C with rabbit anti-dystrophin (Thermo Scientific, PA1-37587, 1:500) alone or together with sheep anti-AnkB (house made, 1:200)29. AnkG distribution was evaluated in longitudinal sections incubated with rabbit-anti AnkG (house-made, 1:200)29 and mouse anti-α-actinin (1:500, ThermoFisher, MA1-22863) antibodies overnight at 4 °C. Sections were washed with PBS and incubated for one hour at RT with secondary antibody and/or fluorescently conjugated wheat-germ agglutin (WGA) (Vector Labs, FL-1021) diluted at 1:300 in blocking solution. Sections were washed with PBS, incubated DAPI (1 µg/mL in PBS), and mounted in ProLong™ Gold Antifade Mountant (ThermoFisher, P36930). For fiber typing analysis, cryosections were boiled in 2 mM EDTA for 10 min at 95 °C, cooled to RT, and stained as above. Samples were incubated with 5 µg/mL of mouse antibodies specific for Myh7 (type I fibers, BA-D5), Myh2 (type IIa fibers, SC-71), and Myh4 (type IIb fibers, BF-F3), all purchased from the Developmental Studies Hybridoma Bank, together with rabbit anti-AnkB (1:100)27,28,29,30,32,33,52,53,54 or anti-dystrophin antibodies. Sections were then washed and incubated with donkey anti-rabbit IgG (H + L) Alexa Fluor™ Plus 405 (A48258), in combination with goat anti-mouse IgG isotype-specific antibodies anti-IgG2b-Alexa Fluor™ 568 (A-21144), anti-IgG1-Alexa Fluor™ 647 (A-21240), and anti-IgM- Alexa Fluor™ 488 (A-21042) from ThermoFisher. Z-stacks covering the entire section thickness and tile scans were acquired for all muscle sections using a ZEISS LSM 980 confocal microscope with a 20x (0.8 NA) objective lens. LSM-Plus processing and tile stitching was performed in Zen Blue (ZEISS, version 3.5.093.00006). Maximum Z-stack projections and calculations of minimum Ferret’s diameter, dystrophin membrane localization, and quantification of fiber typing and AnkB fluorescence were performed in Fiji.
Masson’s Trichrome staining and analysis of collagen deposition
SKM specimens were dissected, fixed overnight in 4% PFA in PBS at RT, dehydrated through a series of ethanol solutions and xylene, and embedded in paraffin. 5 µm thick paraffin crossections were mounted onto Fisherbrand adhesion slides, dried overnight at 58 °C, deparaffinized through a series of xylene and ethanol solutions, hydrated in PBS, and stained using a standard Masson’s Trichrome staining protocol. Images were acquired using a 20x objective on a Zeiss Axio Observer 7 inverted microscope. Analysis of collagen quantification was performed on RGB images after color deconvolution using Fiji (version 2.1.0/1.53c).
Staining and imaging of FDB fibers
Evaluations of mitochondria network morphology, MFF and Drp1 cellular distribution, and ryanodine receptor localization were conducted in isolated FDB fibers fixed for 15 min with 4% PFA and permeabilized for 30 min with 0.1% Triton-X 100 in PBS, both at RT. Samples were blocked with blocking buffer and incubated overnight at 4 °C with primary antibodies diluted in blocking buffer. Samples were washed and incubated for two hours at RT with fluorescently conjugated secondary antibodies diluted in blocking buffer, washed, and mounted in ProLong™ Gold Antifade Mountant. Primary antibodies included Protein Tech rabbit antibodies specific for COXIV (11242-I-AP, 1:50) and Tom20 (11802-1-AP, 1:20), chicken anti-GFP (Aves Lab, GFP-1020, 1:100) and Santa Cruz Biotechnology mouse antibodies against MFF (sc-398617, 1:50) and Drp1 (sc-271583, 1:50). We also used mouse antibodies against Tom20 (Abcam, ab56783, 1:20) and ryanodine receptor (Affinity Bioreagents, MA3-916, 1:100) and rabbit antibodies from Cell signaling Technology specific for pDrp1S616 (3455) and pDrp1S637 (4867) at a 1:50 dilution. The following secondary antibodies from ThermoFisher were used at 1:100 dilution: donkey anti-rabbit IgG (H + L) Alexa Fluor™ 568 (A10042), donkey anti-mouse IgG (H + L) Alexa Fluor™ 488 (A32766), donkey anti-mouse IgG (H + L) Alexa Fluor™ 568 (A10037), and goat anti-chicken IgY (H + L) Alexa Fluor™ 488 (A11039). Alexa Fluor® 488 AffiniPure™ goat anti-rabbit IgG (H + L) (Jackson ImmunoResearch, 111-545-144) was used at a 1:100 dilution.
To globally assess SR organization and SR-mitochondria association, cultured FDB fibers were loaded with 1 µM ER-tracker (ThermoFisher, E3425) and PKmito DEEP RED (Cytoskeleton, Inc., CY-SC055,1:1000) for one hour at 37 °C in fiber media. Fibers were washed and live imaging performed on a Zeiss LSM 980 confocal microscope equipped with Airyscan 2, a temperature- and CO2-controlled incubation chamber, and a 63X (1.4 NA) objective lens. Z-stack images, acquired in Airyscan mode, were processed using the Airyscan joint deconvolution function of Zen Blue. Colocalization analysis was performed using Arivis.
Evaluation of mitochondria morphology and rescue experiments in C2C12 cells
Control and AnkB220-KO C2C12 cells were transduced for 60 hr with Ad-GFP (Vector Biolabs, 1060) or Ad-AnkB220-GFP adenoviruses (1 × 10^10 PFU/ml), packed and purified by Vector Biolabs. Cells were incubated with 250 nM Mitotracker CMXRos (Invitrogen, M7512) for 15 min at 37 °C, washed with PBS, fixed for 15 min with 4% PFA, and imaged in PBS. Airyscan z-stack images covering the entire cell volume were acquired using a ZEISS LSM 980 confocal microscope with a 63x objective lens. Airyscan processing was performed in Zen Blue (ZEISS, version 3.5.093.00006). Analysis of the volume, sphericity, and surface area of mitochondria was performed in Arivis (Version 4.1.0) using the blob finder analysis tool. The fragmentation index was determined by calculating the number of discrete mitochondria divided by the sum of mitochondrial volume per cell.
Proximity ligation assay
FDB fibers were fixed for 15 min with 4% PFA, permeabilized for 30 min with 0.5% Triton-X 100 in PBS, blocked for two hours in blocking solution, all at RT, and incubated overnight at 4 °C with primary antibodies diluted in blocking solution. The following antibody combinations were used to detect proximity ligation signal: rabbit anti-AnkB (1:100) and mouse anti-βII-spectrin (BD Transduction, 612563,1:50); rabbit-AnkB and mouse anti-MFF (Santa Cruz Biotechnology, sc-398617, 1:50); rabbit anti-AnkB and mouse anti-Drp1 (Santa Cruz Biotechnology, 271583, 1:50). Fibers were also incubated with chicken anti-GFP (Aves Lab, GFP-1020,1:100) to amplify signal from endogenous GFP-labeling of mitochondria. Fibers stained only with rabbit anti-AnkB and chicken anti-GFP antibodies served as PLA negative controls. Nanoscale protein complexes were detected using a Duolink® In Situ Red (PLA) kit (Sigma, DUO92101) and PLA probes specific for mouse (Sigma, DUO92004) and rabbit (Sigma, DUO92002) antibodies. Samples were sequentially washed and incubated with goat anti-chicken IgY (H + L) Alexa Fluor™ (ThermoFisher, 488A11039) and DAPI (1 µg/ mL), and mounted in ProLong™ Gold Antifade Mountant. Fiber PLA and GFP signals were imaged on a ZEISS LSM 980 confocal microscope with Airyscan 2 using a 63x (1.4 NA) objective lens. Z-stack images acquired in Airyscan mode were processed in Zen Blue.
Measurements of mitochondrial ROS and membrane potential
Levels of reactive oxygen species (ROS) were measured in FDB fibers loaded with 500 nM MitoSOX Red Superoxide Indicator (ThermoFisher, M36008) and 1 µM MitoTracker Green-FM (ThermoFisher, M7514) for one hour at 37 °C and washed prior to imaging. Live imaging was performed on a Zeiss LSM 980 confocal microscope equipped with a temperature- and CO2-controlled incubation chamber and a 20X (0.8 NA) objective lens. Images were acquired at baseline and after a 10 min incubation with 5 µM Antimycin A. The ratio of MitoSOX/MitoTracker fluorescence in FDB fibers was quantified in Fiji from non-nuclear regions. Mitochondrial membrane potential was evaluated in cultured FDB fibers loaded with 30 nM tetramethylrhodamine ethyl ester (TMRE) dye (ThermoFisher, T669) and 1 µM Mito Tracker Green FM for 15 min at 37 °C and washed prior to imaging. Live imaging was performed as indicated. The ratio of TMRE/MitoTracker fluorescence in fibers was quantified in Fiji.
Calcium imaging
Baseline cytosolic calcium levels were measured in FDB fibers cultured in fiber media at 37 °C and 5% CO2 incubation and loaded for one hour with 2 µM of each Fluo-4-AM (ThermoFisher, F14201), the Fura-Red (ThermoFisher, F3020) ratiometric calcium indicator, and 0.04% Pluronic F-127. Following media replacement, fibers were further incubated for 30 min at 37 °C with 5% CO2 to allow for AM-ester cleavage. Unbound calcium was determined by measuring Fura Red excitation (488 nm) and emission (660 nm). Bound calcium was assessed by measuring Fluo-4 excitation (488 nm) and emission (506 nm). Fluorescence for each cell area was calculated using Fiji. Cytosolic calcium was expressed as the ratio of bound to unbound signal.
Baseline mitochondrial calcium levels were measured in FDB fibers cultured under the same conditions as above and loaded for one hour with 1 µM MitoTracker Green FM to label the total mitochondrial pool and 5 µM Rhod2-AM (ThermoFisher, R1244) to label calcium in the mitochondrial matrix. Following media replacement, fibers were incubated for 30 minutes at 37 °C with 5% CO2 to allow for AM-ester cleavage. Mitochondria were segmented based on MitoTacker Green signal using Fiji, and the average Rhod2-AM fluorescence signal per mitochondrial pixel was calculated.
Statistical analysis
GraphPad Prism (GraphPad Software, v.8.4.3) was used for statistical analysis, unless otherwise stated. Two groups of measurements were compared by unpaired, two-tailed Student's t-test or multiple t-tests. Multiple groups were compared by one-way ANOVA followed by Tukey’s multiple comparisons tests. Two-way mixed effect model analysis was used to compare differences between timepoints of two or more groups for GTT, ITT, and exercise blood lactate and blood glucose measurements. Simple linear regression was used to correlate AnkB expression with fiber type fluorescence. Energy expenditure was analyzed using ANCOVA with lean body mass as a covariate using vassarstats.net.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
RNA-seq data is available at GEO under accession code GSE286564. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD057362. All other data is available upon request. Source data are provided with this paper.
References
Periasamy, M., Herrera, J. L. & Reis, F. C. G. Skeletal muscle thermogenesis and its role in whole body energy metabolism. Diab Metab. J. 41, 327–336 (2017).
Sylow, L. et al. The many actions of insulin in skeletal muscle, the paramount tissue determining glycemia. Cell Metab. 33, 758–780 (2021).
Lee, A. D., Hansen, P. A. & Holloszy, J. O. Wortmannin inhibits insulin-stimulated but not contraction-stimulated glucose transport activity in skeletal muscle. FEBS Lett. 361, 51–54 (1995).
Duan, D. et al. Duchenne muscular dystrophy. Nat. Rev. Dis. Prim. 7, 13 (2021).
Sayer, A. A. et al. Sarcopenia. Nat. Rev. Dis. Prim. 10, 68 (2024).
Linge, J. et al. Adverse muscle composition predicts all-cause mortality in the UK Biobank imaging study. J. Cachexia Sarcopenia Muscle 12, 1513–1526 (2021).
Chow, L. S. et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 18, 273–289 (2022).
Gupta, R., Khan, R. & Cortes, C. J. Forgot to exercise? Exercise derived circulating myokines in Alzheimer’s disease: A perspective. Front. Neurol. 12, 649452 (2021).
Robinson, M. M. et al. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metab. 25, 581–592 (2017).
Ashcroft, S. P. et al. Exercise induces tissue-specific adaptations to enhance cardiometabolic health. Cell Metab. 36, 278–300 (2024).
Furrer, R., Hawley, J. A. & Handschin, C. The molecular athlete: exercise physiology from mechanisms to medals. Physiol. Rev. 103, 1693–1787 (2023).
Smith, J. A. B. et al. Exercise metabolism and adaptation in skeletal muscle. Nat. Rev. Mol. Cell Biol. 24, 607–632 (2023).
Bua, E. et al. Mitochondrial DNA-deletion mutations accumulate intracellularly to detrimental levels in aged human skeletal muscle fibers. Am. J. Hum. Genet. 79, 469–480 (2006).
Vincent, A. E. et al. The spectrum of mitochondrial ultrastructural defects in mitochondrial myopathy. Sci. Rep. 6, 30610 (2016).
Lowell, B. B. & Shulman, G. I. Mitochondrial dysfunction and type 2 diabetes. Science 307, 384–387 (2005).
Picard, M. et al. Acute exercise remodels mitochondrial membrane interactions in mouse skeletal muscle. J. Appl. Physiol. 115, 1562–1571 (2013).
Oliveira, A. N. & Hood, D. A. Exercise is mitochondrial medicine for muscle. Sports Med. Health Sci. 1, 11–18 (2019).
Casuso, R. A. & Huertas, J. R. The emerging role of skeletal muscle mitochondrial dynamics in exercise and ageing. Ageing Res. Rev. 58, 101025 (2020).
Perry, C. G. et al. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J. Physiol. 588, 4795–4810 (2010).
Smirnova, E. et al. Drp1 is required for mitochondrial division in mammalian cells. Mol. Biol. Cell. 12, 2245–2256 (2001).
Friedman, J. R. et al. ER tubules mark sites of mitochondrial division. Science 334, 358–362 (2011).
Fung, T. S., Chakrabarti, R. & Higgs, H. N. The multiple links between actin and mitochondria. Nat. Rev. Mol. Cell Biol. 24, 651–667 (2023).
Huang, D. et al. Mitochondrial dynamics: Working with the cytoskeleton and intracellular organelles to mediate mechanotransduction. Aging Dis. 14, 1511–1532 (2023).
Bennett, V. & Lorenzo, D. N. Spectrin- and ankyrin-based membrane domains and the evolution of vertebrates. Curr. Top. Membr. 72, 1–37 (2013).
Bennett, V. & Lorenzo, D. N. An adaptable spectrin/ankyrin-based mechanism for long-range organization of plasma membranes in vertebrate tissues. Curr. Top. Membr. 77, 143–184 (2016).
Lorenzo, D. N. Cargo hold and delivery: Ankyrins, spectrins, and their functional patterning of neurons. Cytoskeleton 77, 129–148 (2020).
Lorenzo, D. N. et al. A PIK3C3-ankyrin-B-dynactin pathway promotes axonal growth and multiorganelle transport. J. Cell. Biol. 207, 735–752 (2014).
Lorenzo, D. N. et al. Ankyrin-B metabolic syndrome combines age-dependent adiposity with pancreatic beta cell insufficiency. J. Clin. Invest. 125, 3087–3102 (2015).
Lorenzo, D. N. & Bennett, V. Cell-autonomous adiposity through increased cell surface GLUT4 due to ankyrin-B deficiency. Proc. Natl. Acad. Sci. USA114, 12743–12748 (2017).
Healy, J. A. et al. Cholinergic augmentation of insulin release requires ankyrin-B. Sci. Signal. 3, ra19 (2010).
Ploug, T. & Ralston, E. Exploring the whereabouts of GLUT4 in skeletal muscle. Mol. Membr. Biol. 19, 39–49 (2002).
Ayalon, G. et al. An ankyrin-based mechanism for functional organization of dystrophin and dystroglycan. Cell 135, 1189–1200 (2008).
Ayalon, G. et al. Ankyrin-B interactions with spectrin and dynactin-4 are required for dystrophin-based protection of skeletal muscle from exercise injury. J. Biol. Chem. 286, 7370–7378 (2011).
Miniou, P. et al. Gene targeting restricted to mouse striated muscle lineage. Nucleic Acids Res. 27, e27 (1999).
Li, H. et al. LRTM1 promotes the differentiation of myoblast cells by negatively regulating the FGFR1 signaling pathway. Exp. Cell Res. 396, 112237 (2020).
Chen, C. M., Kraut, N., Groudine, M. & Weintraub, H. I-mf, a novel myogenic repressor, interacts with members of the MyoD family. Cell 86, 731–741 (1996).
Ibraghimov-Beskrovnaya, O. et al. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature 355, 696–702 (1992).
Zhang, C. et al. Ankyrin-dependent Na+ channel clustering prevents neuromuscular synapse fatigue. Curr. Biol. 31, 3810–3819.e4 (2021).
Plotkin, D. L. et al. Muscle fiber type transitions with exercise training: shifting perspectives. Sports 9, 27 (2021).
Kovács, B. et al. Lower leg muscle-tendon unit characteristics are related to marathon running performance. Sci. Rep. 10, 17870 (2020).
Tarpey, M. D. et al. Characterization and utilization of the flexor digitorum brevis for assessing skeletal muscle function. Skelet. Muscle 8, 14 (2018).
van Loon, L. J. et al. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J. Physiol. 536, 295 (2001).
Lundsgaard, A. M., Fritzen, A. M. & Kiens, B. Molecular regulation of fatty acid oxidation in skeletal muscle during aerobic exercise. Trends Endocrinol. Metab. 29, 18 (2018).
Reisman, E. G., Hawley, J. A. & Hoffman, N. J. Exercise-regulated mitochondrial and nuclear signalling networks in skeletal muscle. Sports Med. 54, 1097–1119 (2024).
Richter, E. A. & Ruderman, N. B. AMPK and the biochemistry of exercise: implications for human health and disease. Biochem. J. 418, 261–275 (2009).
Spaulding, H. R. & Yan, Z. AMPK and the adaptation to exercise. Annu. Rev. Physiol. 84, 209–227 (2022).
Gwinn, D. M. et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell. 30, 214–226 (2008).
Bell, M. B. et al. Adult skeletal muscle deletion of Mitofusin 1 and 2 impedes exercise performance and training capacity. J. Appl. Physiol. (1985) 126, 341–353 (2019).
King, W. T. et al. Dynamin-related protein 1 regulates substrate oxidation in skeletal muscle by stabilizing cellular and mitochondrial calcium dynamics. J. Biol. Chem. 297, 101196 (2021).
Monaco, C. M. F. et al. Altered mitochondrial bioenergetics and ultrastructure in the skeletal muscle of young adults with type 1 diabetes. Diabetologia 61, 1411–1423 (2018).
Zhou, Z. et al. Drp1 controls complex II assembly and skeletal muscle metabolism by Sdhaf2 action on mitochondria. Sci. Adv. 10, 14 (2024).
Song, J.-E. et al. Mitochondrial fission governed by Drp1 regulates exogenous fatty acid usage and storage in Hela cells. Metabolites 11, 322 (2021).
Ngo, J. et al. Mitochondrial morphology controls fatty acid utilization by changing CPT1 sensitivity to malonyl-CoA. EMBO J. 42, 11 (2023).
Qu, F. Ankyrin-B is a PI3P effector that promotes polarized α5β1-integrin recycling via recruiting RabGAP1L to early endosomes. eLife 5, e20417 (2016).
Bock, T. et al. PERM1 interacts with the MICOS-MIB complex to connect the mitochondria and sarcolemma via ankyrin B. Nat. Commun. 12, 4900 (2021).
Ott, C. et al. Sam50 functions in mitochondrial intermembrane space bridging and biogenesis of respiratory complexes. Mol. Cell Biol. 32, 1173–1188 (2012).
Liu, A., Kage, F. & Higgs, H. N. Mff oligomerization is required for Drp1 activation and synergy with actin filaments during mitochondrial division. Mol. Biol. Cell. 32, ar5 (2021).
Bayraktar, E. C. et al. MITO-Tag Mice enable rapid isolation and multimodal profiling of mitochondria from specific cell types in vivo. Proc. Natl. Acad. Sci. USA 116, 303–312 (2019).
Creighton, B. A. et al. Giant ankyrin-B mediates transduction of axon guidance and collateral branch pruning factor sema 3A. Elife 10, e69815 (2021).
Lorenzo, D. N. et al. βII-spectrin promotes mouse brain connectivity through stabilizing axonal plasma membranes and enabling axonal organelle transport. Proc. Natl. Acad. Sci. USA116, 15686–15695 (2019).
Cousin, M. A. et al. Pathogenic SPTBN1 variants cause an autosomal dominant neurodevelopmental syndrome. Nat. Genet. 53, 1006–1021 (2021).
Chang, C. R. & Blackstone, C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J. Biol. Chem. 282, 21583–21587 (2007).
Valera-Alberni, M. et al. Crosstalk between Drp1 phosphorylation sites during mitochondrial remodeling and their impact on metabolic adaptation. Cell Rep. 36, 109565 (2021).
Cereghetti, G. M. et al. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc. Natl. Acad. Sci. USA 105, 15803–15808 (2008).
Pfluger, P. T. et al. Calcineurin links mitochondrial elongation with energy metabolism. Cell Metab. 22, 838–850 (2015).
Ji, W. et al. Actin filaments target the oligomeric maturation of the dynamin GTPase Drp1 to mitochondrial fission sites. eLife 4, e11553 (2015).
Palmer, C. et al. Adaptor proteins MiD49 and MiD51 can act independently of Mff and Fis1 in Drp1 recruitment and are specific for mitochondrial fission. J. Biol. Chem. 288, 27584–27593 (2013).
Yu, R. et al. The molecular assembly state of Drp1 controls its association with the mitochondrial recruitment receptors Mff and MIEF1/2. Front Cell Dev. Biol. 9, 706687 (2021).
Clinton, R. et al. Dynamin-related protein 1 oligomerization in solution impairs functional interactions with membrane-anchored mitochondrial fission factor. J. Biol. Chem. 291, 478–921 (2016).
Michalska, B. A. et al. Insight into the fission mechanism by quantitative characterization of Drp1 protein distribution in the living cell. Sci. Rep. 8, 8122 (2018).
Noone, J., O’Gorman, D. J. & Kenny, H. C. OPA1 regulation of mitochondrial dynamics in skeletal and cardiac muscle. Trends Endocrinol. Metab. 33, 710–721 (2022).
Zhang, Z. et al. Fis1 deficiencies differentially affect mitochondrial quality in skeletal muscle. Mitochondrion 49, 217–226 (2019).
Castro-Sepulveda, M. et al. Mitochondria-SR interaction and mitochondrial fusion/fission in the regulation of skeletal muscle metabolism. Metabolism 144, 155578 (2023).
Pierantozzi, E. et al. Skeletal muscle overexpression of sAnk1.5 in transgenic mice does not predispose to type 2 diabetes. Sci. Rep. 13, 8195 (2023).
Moore, T. M. et al. The impact of exercise on mitochondrial dynamics and the role of Drp1 in exercise performance and training adaptations in skeletal muscle. Mol. Metab. 21, 51–67 (2019).
Dulac, M. et al. Drp1 knockdown induces severe muscle atrophy and remodelling, mitochondrial dysfunction, autophagy impairment and denervation. J. Physiol. 598, 3691–3710 (2020).
Díaz-Castro, F. et al. A single bout of resistance exercise triggers mitophagy, potentially involving the ejection of mitochondria in human skeletal muscle. Acta Physiol.240, e14203 (2024).
Laker, R. C. et al. Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat. Commun. 8, 548 (2017).
Cho, Y. et al. Perm1 regulates CaMKII activation and shapes skeletal muscle responses to endurance exercise training. Mol. Metab. 23, 88–97 (2019).
Glancy, B. et al. Mitochondrial reticulum for cellular energy distribution in muscle. Nature 30, 617–620 (2015).
Mishra, P. et al. Mitochondrial dynamics is a distinguishing feature of skeletal muscle fiber types and regulates organellar compartmentalization. Cell Metab. 22, 1033–1044 (2015).
Di Meo, D., Ravindran, P., Sadhanasatish, T., Dhumale, P. & Puschel, A. W. The balance of mitochondrial fission and fusion in cortical axons depends on the kinases SadA and SadB. Cell Rep. 37, 110141 (2021).
Hagiwara, A., Harada, K., Hida, Y., Kitajima, I. & Ohtsuka, T. Distribution of serine/threonine kinase SAD-B in mouse peripheral nerve synapse. Neuroreport 22, 319–325 (2011).
Kishi, M., Pan, Y. A., Crump, J. G. & Sanes, J. R. Mammalian SAD kinases are required for neuronal polarization. Science 307, 929–932 (2005).
Favaro, G. et al. DRP1-mediated mitochondrial shape controls calcium homeostasis and muscle mass. Nat. Commun. 10, 2576 (2019).
Lavorato, M. et al. Elongated mitochondrial constrictions and fission in muscle fatigue. J. Cell Sci. 131, 595 jcs221028 (2018).
Adachi, Y. et al. Drp1 tubulates the ER in a GTPase-independent manner. Mol. Cell. 80, 621–632.e6 (2020).
Ji, W. K. et al. Receptor-mediated Drp1 oligomerization on endoplasmic reticulum. J. Cell Biol. 216, 4123–4139 (2017).
Mohler, P. J., Davis, J. Q. & Bennett, V. Ankyrin-B coordinates the Na/K ATPase, Na/Ca exchanger, and InsP3 receptor in a cardiac T-tubule/SR microdomain. PLoS Biol. 3, e423 (2005).
Macaluso, F., Isaacs, A. W. & Myburgh, K. H. Preferential type II muscle fiber damage from plyometric exercise. J. Athl. Train. 47, 414–420 (2012).
Choi, S. J. Differential susceptibility on myosin heavy chain isoforms following eccentric-induced muscle damage. J. Exerc. Rehabil. 10, 344–348 (2014).
McGreevy, J. W. et al. Animal models of Duchenne muscular dystrophy: from basic mechanisms to gene therapy. Dis. Model Mech. 8, 195–213 (2015).
Hamm, S. E. et al. Prolonged voluntary wheel running reveals unique adaptations in mdx mice treated with microdystrophin constructs ± the nNOS-binding site. Front. Physiol. 14, 1166206 (2023).
Li, D., Yue, Y. & Duan, D. Preservation of muscle force in Mdx3cv mice correlates with low-level expression of a near full-length dystrophin protein. Am. J. Pathol. 172, 1332–1341 (2008).
Liu, A. et al. Fatty acyl-coenzyme A activates mitochondrial division through oligomerization of MiD49 and MiD51. Nat. Cell Biol. 26, 731–744 (2024).
Keire, P. et al. Isolation and culture of skeletal muscle myofibers as a means to analyze satellite cells. Methods Mol. Biol. 946, 431–468 (2013).
Boutagy, N. E. et al. Isolation of mitochondria from minimal quantities of mouse skeletal muscle for high throughput microplate respiratory measurements. J. Vis. Exp. 105, 53217 (2015).
Moro, C. & Magnan, C. Revisited guidelines for metabolic tolerance tests in mice. Lab Anim. 54, 16-23 (2025).
Carper, D., Coué, M., Laurens, C., Langin, D. & Moro, C. Reappraisal of the optimal fasting time for insulin tolerance tests in mice. Mol. Metab. 42,101058 (2020).
Acknowledgements
The authors thank Dr. Antonio Davila (University of Pennsylvania) for his assistance with high resolution respirometry, Dr. Saame Shaikh and Traci Davis (Genomics and Energy Metabolism Core, University of North Carolina at Chapel Hill (UNC-CH) Nutrition and Obesity Research Center (NORC)) for their help with body composition and indirect calorimetry measurements, Dr. Sheryl S. Moy and Viktoriya D. Nikolova (UNC-CH Mouse Behavioral Phenotyping Core) for their assistance with the hang-wire and grip strength tests, Drs. Paul Titchenell and James Davis (University of Pennsylvania Rodent Metabolic Phenotyping Core (RMPC, RRID: SCR_022427) for their assistance with additional grip strength evaluations. We also thank Dr. Andrea Stout (University of Pennsylvania Cell and Developmental Biology Microscopy Core (RRID: SCR_022373) for help with widefield image acquisition, Dr. Pablo Ariel and Kristen White (UNC-CH Microscopy Services Laboratory) for TEM sample preparation and assistance with TEM imaging, Drs. Jeremy Simon and Austin Hepperla (UNC-CH, Bioinformatics and Analytics Research Collaborative) for assistance with transcriptomics analysis, and Dr. Anastasia Kralli (Johns Hopkins University), Dr. Faye Mourkioti (University of Pennsylvania), and Dr. Beverly Koller (UNC-CH) for helpful discussions. D.N.L. and K.M.V. were supported by the American Diabetes Association (ADA) grant no. 1-19-JDF-081 and by the US National Institutes of Health (NIH) grants no. R01NS110810 and R01MH127848. K.M.V. was also supported by NIH grants no. F31DK132982 and no. T32GM119999. J.A.B. was supported by NIH grants no. R01HL165792 and R01DK098656. Research reported in this study was also supported by NIH grants no. 5P30DK124723 to the North Carolina Diabetes Research Center, no. P30DK19525 and no. S10-OD025098 to the University of Pennsylvania RMPC, no. P30DK056350 to the UNC-CH NORC, no. P30AR069619 to the Penn Center for Musculoskeletal Disorders at the University of Pennsylvania, and no. U54HD079124 to the Intellectual and Developmental Disabilities Research Center at the UNC-CH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the ADA and NIH.
Author information
Authors and Affiliations
Contributions
Conceptualization: K.M.V. and D.N.L. Investigation: K.M.V., J.T., P.P., H.E.C., T.P., S.R., S.S., J.A.B., E.J.S., D.N.L. Writing: K.M.V. and D.N.L. Editing: K.M.V., J.T., P.P., H.E.C., T. P., S.R., S.S., J.A.B., E.J.S., D.N.L. Funding acquisition: D.N.L. Supervision: D.N.L.
Corresponding author
Ethics declarations
Competing interests
J.A.B. has received research funding and/or materials from Pfizer, Elysium Health, Chromadex, and Metro International Biotech, and consulting fees from Pfizer, Elysium Health, Cytokinetics and Altimmune, and is an inventor on a patent for the use of NAD+ precursors to promote liver regeneration (US no. 16/078,446; “Methods for Enhancing Liver Regeneration”). All other authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Anna Czajkowska, Cedric Moro, and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Voos, K.M., Tzeng, J., Patel, P. et al. Ankyrin-B modulates mitochondrial fission in skeletal muscle and is required for optimal endurance exercise capacity. Nat Commun 16, 7671 (2025). https://doi.org/10.1038/s41467-025-62977-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-62977-3