Abstract
Hair cells within the inner ear cochlea are specialized mechanoreceptors required for hearing. Hair cells are not regenerated in mammals, and their loss is a leading cause of deafness in humans. Cochlear supporting cells in newborn mice have the capacity to regenerate hair cells, but persistent Notch signaling, presumably activated by the Notch ligand Jagged1, prevents supporting cells from converting into hair cells. Employing a cochlear organoid platform, we show that while Jagged1 participates in hair cell-fate repression, Jagged1’s primary function is to preserve the progenitor-like characteristics of supporting cells. Transcriptomic and mechanistic studies reveal that Jagged1/Notch signaling maintains progenitor and metabolic gene expression in supporting cells and sustains pro-growth pathways, including phosphoinositide-3-kinase/Akt /mammalian target of rapamycin signaling, a function that is Notch1 and Notch2-receptor mediated. Finally, we show that Jagged1/Notch signaling stimulation with Jagged1-Fc peptide enhances the hair cell-forming capacity of supporting cells in cochlear explants and in vivo.
Similar content being viewed by others
Introduction
Notch signaling is an evolutionarily conserved signaling pathway that controls key aspects of vertebrate development through juxtacrine interactions of its transmembrane receptors and ligands (reviewed in ref. 1). Mammals express four Notch receptors (Notch1-4) and five canonical Notch ligands, Delta-like 1, 3, 4 (DLL1, 3, 4) and Jagged1, 2 (JAG1, 2). Upon ligand binding, the Notch receptor protein undergoes a conformational change, triggering proteolytic cleavages by ADAM and γ-secretase enzymes, which frees the Notch intracellular domain (NICD) to translocate into the nucleus. In the nucleus, NICD binds to the RBPJ-MAML complex to drive the expression of Notch target genes (reviewed in ref. 2).
Notch signaling has two contrasting functions during the development of the auditory sensory organ. Low Notch receptor signaling strength is necessary to specify and maintain the pool of progenitors (termed pro-sensory cells) from which two types of mechano-sensory hair cells (HCs), termed inner and outer HCs, and several subtypes of surrounding supporting cells (SCs) derive (see schematic Fig. 1a). By contrast, high Notch receptor signaling strength is required to repress an HC-fate and limit the number of HCs produced during differentiation (reviewed in ref. 3). These two distinct modes of Notch receptor signaling rely on different sets of Notch ligands. The Notch ligand JAG1, which in the murine cochlea is initially expressed at the medial border of the pro-sensory domain and later throughout the pro-sensory domain, elicits low levels of Notch1 receptor signaling4,5, which is critical for maintaining the expression of SOX26, a transcription factor essential for pro-sensory cell fate specification7. In addition, JAG1/Notch signaling positively regulates the expression of the transcriptional repressors HEY18,9 and HES110, which help to maintain pro-sensory cells in an undifferentiated state. Highlighting JAG1’s critical role in maintaining a pro-sensory cell fate, early otic deletion of Jag1 results in a near-complete loss of outer HCs and surrounding SCs6,11. By contrast, the Notch ligands DLL1 and JAG2 are essential for HC-fate repression. DLL1 and JAG2 are expressed by nascent HCs and are capable of activating high levels of Notch1 receptor signaling4,5, leading to the induction of HES512,13,14 in neighboring pro-sensory cells. Together with other members of the HES/HEY family of transcriptional repressors, HES515,16 antagonizes the function of ATOH1, a transcription factor essential for HC-fate determination17, limiting these pro-sensory cells to an SC fate. Consistent with their HC-repressive role, early otic deletion of Dll1 and or Jag2 results in supernumerary HCs11,18,19. Similarly, early otic deletion of Notch1 results in massive overproduction of HCs, indicating that the Notch1 receptor is critical for HC-fate repression but dispensable for Notch signaling’s earlier function in pro-sensory cell specification or maintenance19.
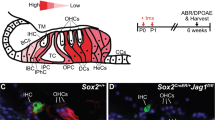
a Schematic of early postnatal murine cochlear sensory epithelium. SC-subtypes (He, Hensen cells; DC1-3 Deiter’s cells 1–3, OP, outer pillar cell; IP, inner pillar cell; IPh, inner phalangeal cells) highly express SOX2 (bright green nuclei). KCs express lower levels of SOX2 (faint green nuclei). Inner HC (IHC) and three outer HCs (OHC) are labeled red. b Experimental scheme (c–j). Cochlear epithelial cells from stage P2 mice were used to establish control and Jag1 cKO organoid cultures, which were analyzed on day 7 of expansion. c Representative confocal images of control and Jag1 cKO organoids stained for JAG1 protein (red). Hoechst labels nuclei (blue). Scale bars = 50 µm. d RT-qPCR of relative Jag1 mRNA expression in control (Ctrl, blue bar) and Jag1 cKO (red bar) organoids (n = 3 animals per group, three independent experiments). e Bright field (BF) images of control and Jag1 cKO organoids. Scale bars = 200 µm. f, g Organoid forming efficiency (f) and organoid diameter (g) for (e) (n = 8 animals per group, three independent experiments). h Cell proliferation in control and Jag1 cKO organoids on day 7. Hoechst labels cell nuclei (blue). Scale bars = 50 µm. i Percentage of EdU+ cells in (h) (n = 6 animals per group, three independent experiments). j RT-qPCR of Notch target Hes1, SC markers (Fgf20, Sox2, S100a1, Lfng) and HC markers (Atoh1, Pou4f3, Gfi1, Myo7a) in control and Jag1 cKO organoids (n = 3 animals per group). k Experimental scheme (l, m). FACS-purified p27-GFP(+) SCs and p27-GFP(−) KCs from stage P2 mice were used to establish control and Jag1 cKO organoid cultures, which were analyzed on day 7 of expansion. l BF images of control and Jag1 cKO organoid cultures established from GFP(-) KCs and GFP(+) SCs. Scale bars = 200 µm. m, n Colony (organoid) forming efficiency (m) and organoid diameter (n) for (e) (n = 3 independent biological replicates per group, two independent experiments). Graphed are individual data points and mean ± standard deviation. A two-tailed, unpaired Student’s t test was used to calculate exact p-values. Source data are provided as a Source Data file. N.s. not significant. Created in BioRender. Doetzlhofer, A. (2025) https://BioRender.com/vo09ioe.
In mice, cochlear HCs and SCs are formed between embryonic day (E) 14.5 and E18.5, after which they undergo a 2-week-long maturation process that ends with the onset of hearing at around postnatal day 13 (P13)20. During the early phase of postnatal development (P0–P3), Notch signaling is highly active in cochlear SCs, and inhibition of Notch signaling using a small molecule γ-secretase inhibitor (GSI) is sufficient to convert SCs into HCs21. Even in the absence of HCs, the presence of GSI triggers massive conversion of SCs into HCs, indicating that Notch signaling remains active in cochlear SCs following HC loss22,23. At later stages (P5 and beyond), the ability of murine cochlear SCs to form HCs in response to GSI treatment sharply declines24; nevertheless, GSI drug treatment has been reported to yield new HCs and improve hearing after acoustic trauma in adult mice23.
A candidate for preventing SC-to-HC conversion in the absence of HCs is the Notch ligand JAG1, which is expressed in both developing and mature cochlear SCs25. Recent studies in mice revealed that conditional deletion of Jag1 in cochlear SCs at birth leads to hearing deficits, which is accompanied by the loss of Hensen cells, a SC subtype located at the lateral edge of the sensory epithelium26 and defects in inner HC stereocilia development27, highlighting a critical role for JAG1 in the maturation and maintenance of cochlear SCs and HCs. However, JAG1’s role in SC-mediated HC formation/regeneration is poorly understood.
Using cochlear epithelial-derived organoid cultures, we show that the Notch ligand JAG1 has two contrasting functions in cochlear HC regeneration. On the one hand, JAG1 acts as a mild HC-fate repressor. On the other hand, JAG1 is critical for preserving progenitor-like characteristics of cochlear SCs, a feature we previously found necessary for cochlear HC regeneration28,29,30. Our transcriptomic and mechanistic studies reveal that JAG1 maintains the expression of pro-growth genes and pathways (e.g., PI3K–Akt–mTOR) in cochlear SCs and identify Notch1 and Notch2 as mediating JAG1’s pro-growth function. Supporting a positive role in HC regeneration, we find that during cochlear maturation (P5 and P7), when cochlear SCs are already resistant to HC-fate-inducing signals, stimulation of JAG1/Notch signaling using JAG1 peptide restores the ability of SCs to generate HCs in response to HC-fate-inducing cues in cochlear explants and in vivo. The positive effect of JAG1 peptide on HC-formation can be blocked by mTORC1 inhibitor rapamycin, revealing a molecular link between JAG1/Notch signaling and mTOR signaling.
Results
The Notch receptor ligand JAG1 enhances the mitotic potential of cochlear SCs
Organoid-type cultures are well-suited for characterize the mitotic and regenerative potential of cochlear SCs29 and the SC-like Kölliker’s cells31 (alternative name: greater epithelial ridge cells). Kölliker’s cells (KCs) are a transient group of epithelial cells located medial to the inner HCs (IHC) and their surrounding SCs32 (see schematic Fig. 1a). While inner and outer HCs (IHCs, OHCs) rapidly die upon dissociation of the cochlear sensory epithelium, SC subtypes and KCs are able to survive dissociation, and can be propagated in 3D-extracellular matrix (Matrigel) in a cocktail of growth factors and small molecule inhibitors28. For our experiments, we expanded organoids in culture media containing high concentrations of the growth factors EGF and FGF2, valproic acid (VPA, HDAC inhibitor), TGFBR1 inhibitor (616452), and GSK-3β inhibitor (CHIR99021, activates Wnt-signaling) (Fig. 1 b).
To determine whether the loss of Jag1 affects the mitotic potential of cochlear SCs and KCs, we acutely deleted Jag1 in cochlear organoids using a doxycycline-inducible Cre strategy. Briefly, we isolated cochlear epithelial cells (containing both SCs and KCs) from stage P2 TetO-Cre; R26rtTA*M2/+; Jag1f/f mice and littermates that lacked the TetO-Cre transgene (control) and cultured the cells as organoids in the presence of doxycycline (dox) (Fig. 1b). We confirmed Jag1 deletion using RT-qPCR and anti-JAG1 immunostaining, which showed high expression of JAG1 protein in control organoids, whereas cells in Jag1 cKO organoids lacked JAG1 protein (Fig. 1c). Similarly, RT-qPCR showed that Jag1 transcript in Jag1 cKO cultures was more than 1000-fold less abundant than in control cultures (Fig. 1d). Furthermore, consistent with JAG1 function being disrupted, we found that the expression of the transcription factor SOX2, a target of JAG1 regulation, was reduced in Jag1 cKO organoids compared to control organoids (Supplementary Fig. 1a). While control organoids contained a mix of SOX2-high (67%) and SOX2-low (33%) expressing cells, nearly all cells (94%) in Jag1 cKO organoids expressed SOX2 at a low level (Supplementary Fig. 1b).
After 7 days of expansion, we analyzed organoid formation efficiency (number of organoids per input cells) and organoid size (organoid diameter) in control cultures and Jag1 cKO cultures (Fig. 1e). We found that organoid formation efficiency in control cultures was about 2-fold higher compared to Jag1 cKO cultures (Fig. 1e, f), and the diameter of organoids in control cultures is about 1.5-fold larger compared to Jag1 KO cultures (Fig. 1e, g). 1-h EdU pulse experiments revealed a 1.6-fold lower rate of EdU incorporation in Jag1 cKO organoid cultures compared to control cultures, suggesting that the smaller organoid size in Jag1 cKO organoid cultures is due, at least in part, to a reduction in cell proliferation (Fig. 1h, i). RT-qPCR analysis revealed that loss of Jag1 significantly reduced mRNA expression of Fgf20, Sox2, and Hes1 and modestly increased Atoh1 and Pou4f3 mRNA expression (Fig. 1j). During cochlear development Fgf20, Sox2 and Hes1 are critical for pro-sensory cell specification (Fgf20, Sox2)7,33,34 and proliferation (Fgf20, Hes1) and preventing premature HC-fate induction (Hes1)35.
To determine whether loss of Jag1 has a differential effect on SCs versus KCs, we established control and Jag1 cKO organoid cultures with FACS-purified SCs and KCs (Fig. 1k). We made use of p27-GFP transgene, which is highly expressed in SCs but only weakly in KCs, to fractionate cochlear epithelial cells into GFP(+) SCs and GFP(−) KCs36 (see Supplementary Fig. 2a for gating). We found that deletion of Jag1 in KC-derived organoid cultures did not alter organoid formation (Fig. 1l, m, GFP(−)) but significantly reduced average organoid size (Fig. 1l, n, GFP(−)). By contrast, deletion of Jag1 in SC-derived organoid cultures resulted in both significantly fewer (Fig. 1l, m, GFP(+)) and significantly smaller organoids (Fig. 1l, n, GFP(+)). Our cell type-specific analysis reveals that both SCs and KCs require JAG1 for organoid growth. However, in contrast to SCs, which depend on JAG1 function for organoid formation, KCs do not require JAG1. The difference in JAG1 dependency and the higher rate of organoid formation observed for KC-derived cultures is likely because at stage P2, KCs are still proliferating at a low rate in vivo, while cochlear SCs have permanently withdrawn from the cell cycle and are post-mitotic.
Notch ligand JAG1 participates in cochlear HC-fate repression
Studies in mice found that inhibition of Notch signaling with GSIs in the HC-damaged cochlea stimulates trans-differentiation of immature and, to a much lesser extent, mature cochlear SCs into HCs22,23. To determine whether loss of Jag1 enhances the rate of HC formation in cochlear organoids, we established control and Jag1 cKO cochlear organoid cultures from cochlear epithelial cells and, after 11 days of expansion, switched to differentiation media to induce HC formation (Fig. 2a). Wnt activation (e.g., CHIR99021) in combination with Notch inhibition (e.g., LY411575) is typically used to induce HC formation in perinatal cochlear explants and organoids28,37. To avoid masking JAG1’s potentially HC-repressive function, LY411475 was omitted from our differentiation media (CHIR99021 only) (Fig. 2a).
a Experimental scheme (b–d). Cochlear epithelial cells from stage P2 mice were used to establish control and Jag1 cKO organoid cultures. HC formation was analyzed using RT-qPCR and immuno-staining after 5 days of differentiation. b RT-qPCR was used to analyze relative mRNA expression of early HC genes (Atoh1, Pou4f3, Myo7a) in control and Jag1 cKO organoids (n = 3 animals per group). c Confocal images of MYO7A (magenta) and SOX2 (green) stained control and Jag1 cKO organoids. Note MYO7A and SOX2 co-staining labels new HCs. Control organoids only contain a few scattered new HCs, whereas Jag1 cKO organoids contain clusters of new HCs. Scale bars = 50 µm. d Quantification of the percentage of HCs per control and Jag1 cKO organoid in (c) (n = 3 animals per group, two independent experiments). e Experimental scheme (f–i). FACS-purified P27-GFP(+) SCs and p27-GFP(−) KCs from stage P2 mice were used to establish control and Jag1 cKO organoid cultures. HC formation was analyzed by RT-qPCR and immuno-staining after 5 days of differentiation. f RT-qPCR was used to analyze relative mRNA expression of early HC-specific transcription factors (Atoh1, Gfi1, Pou4f3) in control and Jag1 cKO organoids (n = 3 independent biological replicates per group, two independent experiments). g Confocal images of KC-derived (GFP(−)) and SC-derived (GFP(+)) control and Jag1 cKO organoids stained for MYO7A (magenta) and SOX2 (green) Scale bars = 100 µm. h Percentage of HC-containing organoids in control and Jag1 cKO organoid cultures (n = 4-6 independent biological replicates per group, two independent experiments). i Percentage of HCs in HC-containing organoids in control and Jag1 cKO organoid cultures (n = 12 independent biological replicates for control and n = 8 independent biological replicates for Jag1 cKO groups). Graphed are individual data points and mean ± standard deviation. A two-tailed, unpaired Student’s t test was used to calculate exact p-values. Source data are provided as a Source Data file. N.s. not significant. Created in BioRender. Doetzlhofer, A. (2025) https://BioRender.com/vo09ioe.
Consistent with JAG1 participating in HC-fate repression, we found that at day five of differentiation, Jag1 cKO organoids expressed the transcripts of HC-specific Myo7a gene at more than threefold higher level and the HC-specific Pou4f3 gene at a more than 10-fold higher level than control organoids (Fig. 2b), indicating a higher rate of HC production in Jag1 cKO organoid cultures compared to control cultures. Next, we used immunostaining against MYO7A and SOX2 to visualize newly formed HCs in organoids. Nascent cochlear HCs co-express MYO7A and SOX2, which distinguishes them from existing HCs lacking SOX2 expression38. We found that in Jag1 cKO cultures, organoids contained large clusters of cells that upregulated SOX2 and co-expressed MYO7A, identifying them as newly formed HCs. In Jag1 cKO cultures, MYO7A+ SOX2+ HCs constituted 20% of total cells per organoid, whereas in control cultures, organoids contained only a few scattered MYO7A+ SOX2+ HCs (Fig. 2c, d).
To determine whether Jag1 deficiency differentially affects HC formation by SCs and KCs, we established control and Jag1 cKO organoids with p27-GFP(+) SCs and p27-GFP(−) KCs (Fig. 2e), and after five days of differentiation, we analyzed HC formation using RT-qPCR and immunostaining. We found that in the absence of Jag1, SC-derived cultures expressed early HC-specific transcripts (Atoh1, Pou4f3, Gfi1) 2-3-fold higher than corresponding control cultures (Fig. 2f, GFP(+)). By contrast, deletion of Jag1 in KC-derived cultures resulted only in a modest increase of Atoh1 and Pou4f3 expression (Fig. 2f, GFP(−)). MYO7A and SOX2 immunostaining revealed that the percentage of organoids that contained HCs was similar across the four conditions (Fig. 2h). However, as anticipated, the percentage of HCs per organoid was significantly higher in both SC and KC-derived Jag1 cKO organoid cultures compared to control cultures, and only Jag1 cKO organoids contained small to mid-size clusters of HCs (Fig. 2g, i).
To further investigate the role of JAG1 in HC-fate repression, we analyzed whether loss of Jag1 increases the rate of HC regeneration in early postnatal cochlear explants. We harvested cochleae from stage P2 TetO-Cre; Jag1f/f; R26M2-rtTA/+ mice and Jag1f/f; R26M2-rtTA/+ control littermates that received dox starting at E18.5 and used microdissection to isolate the cochlear sensory epithelium and underlying mesenchyme and spiral ganglion (Supplementary Fig. 3a). To ablate HCs in control and Jag1 cKO cochlear explants gentamicin was added for the first 15 h of culture. We used a dose of 100 µg/mL gentamicin, which is highly effective in killing cochlear HCs throughout the mid-apical to basal cochlea without damaging surrounding SCs22. After 15 h, gentamicin-containing culture media were removed and Jag1 cKO and control explants were cultured with CHIR99021 or DMSO (0.05%; vehicle control) for three more days (Supplementary Fig. 3b). On day four (~P6), explants were stained for SOX2 and MYO7A and EdU incorporation, and the formation of new HCs (MYO7A+ SOX2+) was analyzed in the mid-apex. We found that the rate of spontaneous HC regeneration was low in both DMSO-treated Jag1 cKO and control explants (4–6 HCs/200 µm = ~4–6% of total HCs) (Supplementary Fig. 3c, d). Moreover, the number of SCs that incorporated EdU was low in DMSO-treated control and Jag1 cKO explants (Supplementary Fig. 3c, e), and none of the newly formed HCs incorporated EdU, indicating that the few spontaneously regenerated HCs were generated through non-mitotic mechanisms (Supplementary Fig. 3c, f). The presence of CHIR99021 (CHIR) increased the rate of cochlear SC proliferation in both control and Jag1 cKO explants to a similar extent (~30 SOX2+ EdU+ cells/200 µm = ~16% of total SCs) (Supplementary Fig. 3c, e). Furthermore, the presence of CHIR99021 modestly increased HC-production in Jag1 cKO explants (~8 HC/200 µm = ~8% of total HCs) (Supplementary Fig. 3c, d) and about half of the newly formed HCs in Jag1 cKO cultures derived from dividing cells (Supplementary Fig. 3c, f). Our data, taken together, indicate that JAG1/Notch signaling participates in HC-fate repression. However, the effect of Jag1 deletion on new HC-formation is modest, hinting at a more complex role in this process.
Notch ligand JAG1 maintains metabolic and progenitor genes in cochlear SCs and KCs
To identify genes and pathways that are regulated by JAG1/Notch signaling in cochlear organoids, we established control and Jag1 cKO organoids from P2 cochlear epithelial cells and analyzed their transcriptome after 7 days of expansion using bulk RNA sequencing (RNA_seq) (Fig. 3a) (Supplementary Data 1). We first examined the expression of Notch ligands and Notch receptors in control organoids. Jag1, Notch1, Notch2, and Notch3 transcripts were highly expressed. Consistent with organoids being close to void of HCs, HC-specific ligand Dll1 transcript was close to undetectable, and HC-specific ligand Jag2 transcript was more than 100-fold lower expressed than Jag1 (Supplementary Fig. 4a). Next, we used the data to identify differentially expressed genes (DEG). Our analysis identified a total of 802 DEGs (q-value 0.01), with 160 genes being upregulated and 642 genes being downregulated in Jag1 CKO organoids compared to control organoids (Supplementary Data 2). As expected, transcripts for Jag1 and effector genes of Notch signaling (Hes1, Hey1, HeyL, Sox2) were among the top down-regulated transcripts in Jag1 cKO organoid cultures compared to control organoid cultures (Fig. 3b, d) (Supplementary Fig. 4b). Moreover, transcripts of growth-promoting genes (e.g., Fgf10, Igf2, Igfbp3, Ntf3), and genes regulating cell metabolism and metabolite transport (e.g., Me2, Eif4ebp1, Slc16a3, Slc7a5) were significantly downregulated in Jag1 CKO organoids compared to control organoids (Fig. 3b) (Supplementary Data 2). In contrast, several genes that were significantly upregulated in Jag1 CKO organoids included those expressed in HCs (e.g., Tunar39, Pou4f3, Scx40) and genes expressed in interdental cells (e.g., Smoc239 and Otoa41), which are a group of cochlear epithelial cells critical for the production of tectorial membrane proteins (Fig. 3b) (Supplementary Data 2). To identify the pathways and biological processes affected by the loss of JAG1-mediated signaling, we conducted gene ontology enrichment analysis on the list of downregulated genes using Metascape, a web-based portal42. As expected, the Notch signaling pathway and regulation of nervous system development were among the top 20 pathways and biological processes (Fig. 3c) (Supplementary Data 3). However, five out of the ten top-scoring pathways and biological processes were linked to cell stress responses (response to endoplasmic reticulum stress, HIF-1 pathway) and cell metabolism (PI3K-Akt-signaling pathway, metabolism of carbohydrates, amino acid metabolic process) (Supplementary Fig. 5a–c) (Fig. 3c) (Supplementary Data 3). This suggests that loss of Jag1 disrupted PI3K-Akt-mTOR signaling in cochlear SCs and KCs.
a Experimental scheme. Cochlear epithelial cells from stage P2 mice were used to establish control and Jag1 cKO organoid cultures, which were analyzed on day 7 of expansion. b Volcano plot of RNA-seq data. Plotted is the beta-value (x-axis) versus −log10 q-value (y-axis). Transcripts that are significantly upregulated in response to Jag1 cKO are marked in red dots, and transcripts that are significantly downregulated are marked in blue dots. c Biological processes and pathways associated with Jag1 cKO downregulated genes ranked by adjusted p-value (q-value). d RT-qPCR was used to analyze relative mRNA expression of Jag1, Hey1, Id1, Eif4ebp1, Minar2, Nupr1, Dkk3, Scx in control and Jag1 cKO organoids (n = 4 animals per group, two independent experiments). e Immunoblots of p-Akt, Akt, p-S6, p-4EBP1, 4EBP1, JAG1 and β-actin proteins in control and Jag1 cKO organoids. f Normalized p-Akt, p-S6, and p-4EBP1 protein levels in (e) (n = 3 animals per group, from one representative experiment, two independent experiments). Graphed are individual data points and mean ± standard deviation. A two-tailed, unpaired Student’s t test was used to calculate exact p-values. Source data are provided as a Source Data file. N.s. not significant. Created in BioRender. Doetzlhofer, A. (2025) https://BioRender.com/vo09ioe.
Notch ligand JAG1 maintains PI3K-Akt-mTOR signaling in cochlear SCs and KCs
To investigate whether PI3K–Akt–mTOR signaling is downregulated in Jag1 cKO organoids, we analyzed the expression levels of phosphorylated (p) forms of the kinase Akt (p-Akt, Ser473), the ribosomal protein S6 (p-S6, Ser240/244), and the eukaryotic initiation factor 4E binding protein 1 (p-4EBP1, Thr37/46) in control and Jag1 cKO organoid protein extracts by immunoblotting. An increase in the protein levels of the p-S6 (Ser240/244) and p-4EBP1 (Thr37/46)43 correlates with increased mTOR activity, while p-Akt (Ser473) serves as a marker for maximal Akt activation downstream of PI3K44. Our analysis revealed that protein levels of p-AKT and p-4EBP1 were significantly reduced in Jag1 cKO organoids compared to control, identifying JAG1 as a positive regulator of PI3K-AKT-mTOR signaling (Fig. 3e, f).
JAG1 peptides containing the extracellular domain of human JAG1 fused in frame with Fc sequence (JAG1-Fc) have been shown to effectively activate Notch signaling in various contexts45,46,47. To determine whether extracellular JAG1 can rescue the defects in organoid formation and growth observed in Jag1 CKO organoids, we established P2 Jag1 cKO and control organoids and cultured them with (5, 50, 500 ng/mL) or without (0 ng/mL) JAG1-Fc peptide (Ser32-Ser1046)48 (Fig. 4a). We found that the rate of organoid formation (Fig. 4b, c) and organoid growth (Fig. 4b, d) was significantly increased with exposure to the JAG1-Fc peptide in both control and Jag1 cKO organoid cultures. However, likely due to endogenous JAG1 ligand competing for Notch receptor binding, a higher concentration of JAG1-Fc peptide was required to elicit a pro-growth response in control organoids (50 ng/mL) compared to Jag1 cKO organoids (5 ng/mL) (Fig. 4b–d). Correspondingly, JAG1-Fc peptide treatment resulted in an increase in the abundance of p-Akt and p-4EBP1 proteins in both control and Jag1 cKO organoids (Fig. 4e). Jag1 expression in cochlear SCs is positively regulated by Notch signaling49. Consistent with JAG1-Fc activating Notch receptor signaling, exposure to JAG1-Fc peptide led to a dose-dependent increase in endogenous JAG1 protein expression in control organoids (Fig. 4e, control).
a Experimental scheme. Cochlear epithelial cells from stage P2 mice were used to establish control and Jag1 cKO organoid cultures, which were analyzed on day 10 of expansion. Organoids were treated with JAG1-Fc peptide on day 3 of expansion and cultured for seven additional days. b BF images of control and Jag1 cKO organoids treated with or without JAG1-Fc peptide on day 10 of expansion. Scale bars = 400 µm. c, d Quantification of organoid forming efficiency (c) and organoid diameters (d) for (b) (n = 4 animals per group, two independent experiments). e Immunoblots of p-Akt, pS6, p-4EBP1, JAG1, and β-actin protein in control and JAG1 peptide-treated organoids on day 10 of expansion (three independent experiments). Graphed are individual data points and mean ± standard deviation. One-way ANOVA with Tukey’s correction was used to calculate exact p-values. Source data are provided as a Source Data file.
To further confirm that stimulating JAG1-Notch signaling promotes organoid formation and growth, we overexpressed full-length murine JAG1 protein in P2 wild-type cochlear organoids using a lentiviral strategy. To mark infected cells, control, and JAG1 expressing lentiviruses expressed red-fluorescent protein mCherry (Supplementary Fig. 6a). Our analysis revealed that JAG1 overexpression significantly increased organoid size and organoid formation efficiency (Supplementary Fig. 6b–d) and increased the rate of cell proliferation (EdU+ mCherry+ cells/organoid) (Supplementary Fig. 6e, f). In summary, our data indicate that JAG1 positively regulates the mitotic capacity of early postnatal cochlear SCs and KCs.
Qualitatively similar positive effects on organoid growth and organoid formation were observed with low activation of Notch1 receptor signaling. To activate Notch1 receptor signaling, we infected P2 wild-type cochlear epithelial cells (SC and KC) with lentivirus that expressed the Notch intracellular domain (NICD) under the control of dox and expanded the cells as organoids for 7 days with no dox (0), low dox (0.25, 0.5 μg/mL) or high dox (5, 10 μg/mL) (Fig. 5a). We found that a relatively low concentration of dox significantly increased organoid size (Fig. 5b, c) and organoid formation efficiency (Fig. 5b, d) compared to no dox (0 μg/mL) while a high concentration of dox (10 μg/mL) had an adverse effect on organoid growth and formation, significantly decreasing organoid size and organoid formation efficiency (Fig. 5b–d). The higher rate of organoid formation and growth observed in low dox (0.5 µg/mL) was accompanied by an increase in mTOR activity. In particular, the low dox-induced 2-fold increase in NICD protein led to a 5 to 10-fold increase in p-Akt, p-S6, and p-4EBP1 protein levels in cochlear organoids (Supplementary Fig. 7a, b).
a Experimental scheme (b–d). Cochlear epithelial cells from stage P2 wild-type mice were infected with a lentivirus that expressed the intracellular domain of Notch1 (NICD) in a dox-dependent manner. Dox (0.25, 0.5, 5, 10 µg/mL) was added to the culture medium on day 2, and organoids were analyzed after 7 days of expansion. b Bright field (BF) and green fluorescent (GFP) images of control (0 dox) and NICD expressing organoids. GFP labels organoids infected with lentivirus. Scale bars = 400 µm. c, d Organoid diameter (c) and organoid forming efficiency (d) for (b) (n = 6 independent biological replicates, two independent experiments). e Experimental scheme (f, g). f Bright field (BF) images of DMSO (vehicle control) and LY411575-treated organoids on day 7 of expansion. LY411575 or DMSO (0.2%) was added to the culture medium on day 2 of expansion. Scale bars = 400 µm. g, h Organoid diameter (g) and organoid forming efficiency (h) for (f) (n = 6 independent biological replicates, two independent experiments). i Cell proliferation in control (DMSO) and LY411575-treated organoids. A single EdU pulse was given on day 7 of expansion, and EdU incorporation (red) was analyzed 1 hour later. Hoechst labels cell nuclei (blue). Scale bars = 25 µm. j Percentage of EdU+ cells in (i) (n = 8 animals, two independent experiments). Graphed are individual data points and mean ± standard deviation. One-way ANOVA with Tukey’s correction was used to calculate exact p-values. Source data are provided as a Source Data file.
Next, we examined whether inhibition of Notch receptor signaling with LY411575 (GSI) reduces organoid formation and growth. We established organoids from P2 wild-type cochlear epithelial cells and expanded organoids in the presence of DMSO (0.2%, vehicle control) or the presence of increasing concentrations of LY411575 (5, 10, 20 μM) (Fig. 5e) for four days. We found that 5 μM LY411575, a dosage commonly used to induce SC-to-HC conversion in perinatal cochlear organoids or explants, did not affect organoid formation or growth. However, two and four-fold higher concentrations of LY411575 (10, 20 μM) significantly decrease the average organoid size (Fig. 5f, g) and organoid formation in a dose-dependent manner (Fig. 5f, h). EdU pulse experiments showed that cells in organoids cultured with 10 or 20 μM LY411575 incorporated EdU at a significantly lower rate than control organoids (DMSO) or organoids cultured with 5 μM LY411575 (Fig. 5i, j). In summary, these data indicate that low levels of Notch1 receptor signaling promote cochlear organoid formation and growth, while high levels of Notch1 receptor signaling are inhibitory. Furthermore, these data reveal that 5 μM of LY411575, a concentration used to induce SC-to-HC conversion, does not affect cochlear organoid formation and growth.
Loss of Notch1/2 reduces PI3K-Akt-mTOR signaling in cochlear SCs and KCs
Early postnatal cochlear SCs and KCs cells co-express the Notch receptors Notch1, Notch2, and Notch339, suggesting that one or more of these three Notch receptors mediate the positive effects of JAG1 on organoid formation, growth, and PI3K–Akt–mTOR signaling. To identify the Notch receptor(s) through which JAG1 signals, we established P2 organoid cultures with Notch1, Notch2, or Notch3-deficient cochlear epithelial cells (SCs and KCs) and corresponding control cochlear epithelial cells. To analyze the function of Notch3, we used Notch3 heterozygous (Notch3+/−) (control) and Notch3 homozygous mutant (Notch3−/−) mice (Supplementary Fig. 8a)50. Analysis of cochlear HC phenotype in early postnatal Notch3−/− mice and Notch3−/+ littermates (control) revealed no developmental defects. Both control and Notch3-deficient cochlear sensory epithelia contained a single row of inner HCs and three rows of outer HCs, surrounded by SCs (Supplementary Fig. 8b), indicating that Notch3 function in vivo is not required for pro-sensory cell maintenance, nor is Notch3 required for HC fate repression. We next established organoid cultures with stage P2 Notch3−/− and Notch3+/− cochlear epithelial cells and expanded them for seven days (Supplementary Fig. 8a). Our analysis of colony-forming efficiency (organoid formation) and organoid size (diameter) revealed no differences between Notch3−/+ and Notch3−/− organoid cultures (Supplementary Fig. 8c–e). RT-qPCR experiments revealed that Notch3−/− organoids expressed Notch target genes Hes1, Hes5, and Sox2 at lower levels than Notch3−/+ (control organoids), however Atoh1 expression remained unchanged (Supplementary Fig. 8f). Furthermore, immunoblots showed that p-Akt, p-S6, and p-4EBP1 protein expression in Notch3−/− organoids were unchanged compared to Notch3−/+ organoids (Supplementary Fig. 8g, h).
Previous studies have demonstrated that Notch1 plays a critical role in the repression of HC fate. However, the function of Notch1 signaling in the proliferation of cochlear SCs and KCs remains unclear, particularly regarding its necessity for PI3K-Akt-mTOR signaling. To analyze Notch1 function, we established cochlear organoids using cochlear epithelial cells from stage P2 Sox2CreER/+; Notch1f/f mice and Notch1f/f littermates (control). In the early postnatal cochlea, the tamoxifen-inducible Sox2CreER transgene is highly expressed in SCs and KCs51. To induce Notch1 deletion, 4-hydroxy-tamoxifen (4-OH TM) was included in the culture media. We also established additional control cultures with DMSO (0.02%, vehicle control) to control Sox2 haploinsufficiency51 (Supplementary Fig. 9a). Our analysis revealed that organoid formation and growth in Notch1 cKO organoid cultures (Sox2CreER/+; Notch1f/f + 4-OH TM) was not significantly different compared to control cultures (Sox2CreER/+; Notch1f/f + DMSO, or Notch1f/f + DMSO, or Notch1f/f + 4-OH TM) (Supplementary Fig. 9b–d). Furthermore, analysis of p-S6, p-Akt, and p-4EBP1 protein expression in Notch1 cKO and control organoids revealed no significant differences (Supplementary Fig. 9e, f).
Next, we analyzed the function of the Notch2 receptor using a cochlear-specific Cre line (Emx2Cre/+)52 to delete Notch2 by itself or in combination with Notch1 conditionally. Analysis of the cochlear HC phenotype of stage P4 Notch2-deficient mice revealed no obvious HC patterning defects. Like control cochlear tissue (Cre negative), the cochlear sensory epithelia that lacked both Notch2 alleles (Emx2Cre/+; Notch2f/f; Notch1fl/+) contained a single row of inner HCs and three rows of outer HCs (Supplementary Fig. 10a), indicating that Notch2 function in the developing cochlea is not required for the maintenance of pro-sensory cells, nor is Notch2 required for HC fate repression. By contrast, cochlear sensory epithelia that lacked both Notch1 alleles (Emx2Cre/+; Notch2f/+; Notch1f/f) showed severe HC patterning defects, containing many newly formed HCs (MYO7A+ SOX2+), which is consistent with Notch1’s known role in HC-fate repression (Supplementary Fig. 10a).
To determine whether loss of Notch2 alters the mitotic capacity of cochlear SCs and KCs, we established organoid cultures with cochlear epithelial cells isolated from stage P2 Emx2Cre/+; Notch2f/f mice and control litter mates and analyzed organoid growth and formation after 10 days of expansion (Supplementary Fig. 10b). Our analysis revealed that both organoid formation efficiency and organoid size were significantly reduced in Notch2 deficient (Emx2Cre/+; Notch2f/f) organoid cultures compared to control (Notch2f/f) (Supplementary Fig. 10c–e). However, immunoblots analyzing p-Akt, p-S6, and p-4EBP1 protein expression revealed no reduction in PI3K-Akt-mTOR signaling in Notch2-deficient organoids (Supplementary Fig. 10f, g) or acutely isolated Notch2-deficient cochlear sensory epithelia compared to control (Supplementary Fig. 10h, i).
To determine whether Notch1 may have compensated for the loss of Notch2, we made use of Notch1fl/fl; Notch2fl/fl mice to acutely knock out both Notch1 and Notch2 (Notch1/2 DKO) in cochlear organoids. We isolated cochlear epithelial cells from stage P2 Notch1f/f; Notch2fl/f mice and infected the cells with control lentivirus or Cre-expressing lentivirus, after which we expanded them as organoids for ten days (Fig. 6a). To track transduced cells, both the control and the Cre-expressing lentivirus also expressed the red fluorescent protein mCherry (Fig. 6b). RT-qPCR revealed that Cre-infected organoid cultures (Notch1/2 DKO) expressed Notch1, Notch2, and Notch effector genes Hes1, Hey1, and HeyL at a 2–3-fold lower level than organoid cultures infected with control virus (Control), confirming successful disruption of Notch1/2 signaling (Fig. 6c). Moreover, we found that Notch1/2 DKO cultures formed 2-fold fewer organoids than control cultures (Fig. 6b, d), and the diameter of organoids in Notch1/2 DKO cultures was about 1.5-fold smaller compared to organoids in control cultures (Fig. 6b, e). Furthermore, analysis of p-S6, p-Akt and p-4EBP1 levels in protein extracts obtained from control and Notch1/2 DKO cultures revealed significantly lower protein expression of p-Akt and p-4EBP1 in Notch1/2 DKO organoids than control organoids (Fig. 6f, g), suggesting that Notch1 and Notch2 have redundant functions in regulating PI3K-Akt-mTOR signaling in cochlear SCs and KCs.
a Experimental scheme. Organoid cultures were established with cochlear epithelial cells from stage P2 Notch1f/f Notch2f/f mice. Cells were infected with Cre–mCherry and mCherry only (control) expressing lentivirus (LV) prior to plating, and organoids were expanded for 10 days. b Bright field (BF) and red fluorescent (mCherry) images of control and Notch1/2 double knockout (DKO) organoids were analyzed on day 10 of expansion. Scale bars = 400 µm. c RT-qPCR analyzing relative mRNA expression of Notch1, Notch2, and Notch effectors (Hes1, Hey1, and HeyL) in control and Notch1/2 DKO organoids on day 10 of expansion (n = 3 independent biological replicates, two independent experiments). d, e Organoid (colony) forming efficiency (d) and organoid diameter (e) were analyzed for the experiment shown in (b) (n = 5 independent biological replicates, two independent experiments). f Representative immunoblot of Notch1 (N1), NICD1, cleaved N1, Notch2 (N2), NICD2, p-Akt, Akt, p-S6, p-4EBP1, 4EBP1 and β-actin proteins in pooled samples of control and Notch1/2 dKO cochlear organoids on day 10 of expansion. g Relative protein levels of Notch1 (N1), NICD1, cleaved N1, Notch2 (N2), NICD2, p-Akt, Akt, p-S6, p-4EBP1, 4EBP1 in control and Notch1/2 dKO cochlear organoids on day 10 of expansion (n = 4 independent biological replicates obtained from two independent experiments). Graphed are individual data points and mean ± standard deviation. Two-tailed, unpaired Student’s test was used to calculate exact p-values. Source data are provided as a Source Data file. Created in BioRender. Doetzlhofer, A. (2025) https://BioRender.com/vo09ioe.
To further confirm the growth-inhibitory effects of Notch1 and/or Notch2 deletion, we knocked down Notch1 and/or Notch2 expression in SC-derived organoids using short hairpin RNAs (shRNAs) (Supplementary Fig. 11a). We designed three shRNA constructs each for targeting Notch1 or Notch2 and used RT-qPCR to analyze their knockdown efficiency in pilot experiments. Based on these experiments, we selected shNotch1-2 and shNotch2-2 to knock down Notch1 and or Notch2 in subsequent experiments (Supplementary Fig. 11b, c). We isolated cochlear SCs from stage P2 Lfng-GFP transgenic mice using FACS as previously described29 (see Supplementary Fig. 2b for gating). Following lentiviral infection, Lfng-GFP(+) cochlear SCs were expanded as organoids for 7 days (Supplementary Fig. 11d). Consistent with our Notch1 and or Notch2 knockout experiments that used unfractionated cochlear epithelial cells to establish organoids, we found that Notch2 knockdown and, to a larger extent, combined Notch1 and Notch2 knockdown (Notch1/2) significantly reduced organoid formation efficiency in cochlear SC-derived organoids (Supplementary Fig. 11d, e). Furthermore, we found that Notch2 knockdown and Notch1/2 knockdown significantly reduced the organoid size of cochlear SC-derived organoids (Supplementary Fig. 11d, f). By contrast, Notch1 knockdown did not significantly reduce organoid formation, nor did it reduce organoid size compared to control (Supplementary Fig. 11d–f).
Exogenous JAG1 enhances the mitotic and HC-forming potential of cochlear SCs
During cochlear maturation, the ability of SCs to form HCs sharply declines, which is accompanied by a reduction in responsiveness to Notch inhibition and an overall reduction in Notch1 signaling strength5,24. To characterize potential changes in Jag1 expression during cochlear maturation, we isolated SCs from stage P1, P5, and P13 Lfng-GFP transgenic mice using FACS (see Supplementary Fig. 2b for gating) and analyzed Jag1 mRNA abundance using RT-qPCR (Fig. 7a). Our analysis revealed that Jag1 mRNA expression in Lfng-GFP(+) cochlear SCs declines between stages P1 and P5 by 2-fold and by more than 5-fold between stages P1 and P13, which is around the time when mice start to hear (Fig. 7b).
a Experimental scheme for isolating Lfng-GFP(+) cochlear SCs used in (b). b RT-qPCR analysis of Jag1 mRNA expression in stage P1, P5, and P13 FACS-purified cochlear SCs (n = 2 for P1, n = 3 for P5 and n = 2 for P13. N = independent biological replicates that were obtained from at least 2 independent experiments). c Experimental scheme for (d–g). Cochlear explants from P2 or P5 Atoh1-nGFP tg mice were cultured with JAG1-Fc peptide or without JAG1-Fc peptide (control) in the presence of CHIR99021 and LY411575 for 4 days. EdU was added on day 1. d Representative confocal images of the HC layer taken at the cochlear mid-apical turn following culture. Newly formed HCs (white asterisks) are labeled by MYO7A (magenta) and SOX2 (blue) immuno-staining and Atoh1-nGFP (green) expression. EdU (red) labels actively and previously dividing cells. Scale bars = 25 µm. e–g Quantification of SC proliferation (EdU+SOX2+) (e), mitotic HC formation (EdU+ MYO7A+) (f), and the total number of newly formed HCs (MYO7A+SOX2+Atoh1-nGFP+) (g) for (d) (n = 5 animals per group, two independent experiments). h Experimental scheme for (i, j). Cochlear explants from stage P4 Atoh1-nGFP tg mice were cultured with JAG1-Fc peptide or without JAG1-Fc peptide (control) in the presence of rapamycin (4 ng/mL) or DMSO (0.05%, vehicle control) for 5 days. CHIR99021 and LY411575 were added on day 1 to induce SC-to-HC conversion. i Representative confocal images of the HC layer taken at the cochlear mid-apical turn following culture. Newly formed HCs are marked by MYO7A (magenta) and SOX2 (blue) immuno-staining and Atoh1-nGFP (green) expression. Scale bars = 25 µm. j Quantification of newly formed HCs in (i) (n = 4 animals per group, two independent experiments). Graphed are individual data points and mean ± standard deviation. Two-way ANOVA with Tukey’s correction was used to calculate exact p-values. Source data are provided as a Source Data file. N.s. not significant.
To determine whether increasing JAG1/Notch signaling restores the mitotic and or HC-forming capacity of cochlear SCs, we isolated cochlear tissue from stage P2 and P5 Atoh1-GFP transgenic mice and cultured them as cochlear explants with JAG1-Fc peptide or without JAG1-Fc peptide (control) in the presence of CHIR99021 (Wnt activation) and LY411575 (GSI, Notch inhibitor) (Fig. 7c). EdU was added to the culture on day one to analyze cell proliferation. After 4 days of culture, cochlear explants were stained for MYO7A, SOX2, and EdU and analyzed for SC proliferation (EdU+ SC+) and mitotic (EdU+ MYO7A+) and non-mitotic HC formation (MYO7A+ Atoh1-nGFP+ SOX2+) (Fig. 7d). We found that in stage P2 cochlear explants, the addition of JAG1-Fc peptide did not significantly increase cochlear SC proliferation (Fig. 7e, P2) or HC formation (Fig. 7f, g, P2) compared to control. By contrast, in stage P5 cochlear explants, the presence of JAG1-Fc peptide significantly increased overall cell proliferation (Fig. 7e), mitotic HC formation (Fig. 7 f), and the total number of newly formed HCs (39.7 new HCs /200 µm = 40% of total HCs) compared to control (14.1 new HCs/ 200 µm = 14% of total HCs) (Fig. 7 e-g, P5). The newly formed HCs were localized in the outer HC region, suggesting that Deiter’s cells and pillar cells (see Fig. 1 a), may have formed the new HCs. No ectopic cell proliferation and/or HC formation was observed in the GER (Fig. 7 d, P5).
To determine the source of the newly formed HCs, we conducted a lineage tracing experiment using cochlear tissue from stage P5 Fgfr3iCreERT2; R26LSL tdTomato/+; Atoh1-nGFP transgenic mice that received 4-OH tamoxifen at P3 to permanently mark Deiter’s cells and pillar cells as tdTomato+ cells (Supplementary Fig. 12a). One cochlea per animal received JAG1-Fc peptide at plating, the other functioned as control. To stimulate SC-to-HC conversion, both JAG1-Fc treated, and control explants were cultured in the presence of CHIR99021 and LY411575. After 4 days of culture, we used Atoh1-nGFP reporter expression, which marks nascent HCs, to analyze the number of tdTomato+ SCs that transformed into new HCs at the cochlear mid-apex (Supplementary Fig. 12b). Our analysis revealed that in control cultures, only the most lateral Deiter’s cells (D3) responded to CHIR99021 and LY411575 and induced Atoh1-nGFP (9.6 new HCs/ 200 µm =10% of total HCs and 7% of total Deiter’s cells and pillar cells), while in JAG1-Fc peptide treated explants both Deiter’s cells and pillar cells upregulated Atoh1-nGFP expression in response to CHIR99021 and LY411575 (29.2 new HCs/ 200 µm =30% of total HCs and 21% of total Deiter’s cells and pillar cells) (Supplementary Fig. 12 c).
We next determined whether mTOR signaling is required for JAG1’s enhancing effect on HC formation. To that end, we established cochlear explant cultures from stage P4 wild-type mice, pre-treated explants with or without JAG1-Fc peptide in the presence of rapamycin (mTORC1 inhibitor) or DMSO (0.05%, vehicle control) and the following day added CHIR99021 and LY411575 to the culture media to induce SC-to-HC conversion (Fig. 7h). After 4 days, we stained for EdU, MYO7A and SOX2 and quantified the number of newly formed HCs (MYO7A+ SOX2+ Atoh1-nGFP+) (Fig. 7i). We found that rapamycin reduced the number of new HCs that formed in JAG1-Fc peptide treated cultures by 3-fold (Fig. 7i and j), indicating that JAG1-Fc peptide treatment stimulates new HC formation at least in part through activating mTOR signaling.
Next, we analyzed how HC loss affects JAG1 expression in the cochlea. To selectively ablate cochlear HCs in vivo, we utilized Pou4f3DTR/+ transgenic mice, which allows for HC ablation using diphtheria toxin (DT). We injected stage P1 Pou4f3DTR/+ transgenic mice with DT, and 48 hours later, immuno-stained their cochlear sensory epithelia for JAG1 protein. As a control, we examined JAG1 protein expression in stage P3 wild-type cochlear sensory epithelia (Fig. 8 a, wild-type, undamaged). We found that JAG1 expression in cochlear SCs and KCs was largely maintained following mild HC loss; however, JAG1 expression was nearly absent in cochlear SCs after severe HC loss, while expression in KCs was preserved. To determine whether stimulating JAG1/Notch signaling enhances the mitotic and or HC-regenerative capacity of cochlear SCs after severe HC loss, we prepared stage P5 wild-type cochlear explants. These explants were treated with gentamicin for 15 hours and then cultured with or without JAG1-Fc peptide in the presence of CHIR99021 and LY411575 (Fig. 8 b). After 5 days of culture, we stained the control and JAG1-Fc peptide treated cochlear explants for EdU, MYO7A, and SOX2 and quantified the number of newly formed HCs (MYO7A+ SOX2+) and proliferating SCs (EdU+ SOX2+) in the cochlear mid-apex and base (Fig. 8c). Our analysis revealed that at the cochlear mid-apex the presence of JAG1-Fc peptide allowed for some modest cell proliferation (5.6 EdU+SOX2+ cells = 3% of total SCs) (Fig. 8 c and d) and increased HC regeneration (42 new HCs/ 200 µm =42% of total HCs) (Fig. 8 c, e) compared to the control group (23.6 new HCs/ 200 µm =24%). At the cochlear base, JAG1-Fc peptide treatment (5.3 HCs/ 200 µm =5% of total HCs) increased HC regeneration compared to the control (1.3 HCs/ 200 µm =1% of total HCs), but the effect was minimal and not statistically significant (Fig.8 c, f).
a Representative confocal image of JAG1 expression in undamaged cochlear sensory epithelia from stage P3 wild-type mice, and in HC-damaged cochlear sensory epithelia from stage P3 Pou4f3DTR/+ transgenic mice that received diphtheria toxin (DT) at P1 (two independent experiments). Scale bars=50 µm. b Experimental scheme for (c-f). Cochlear explants from stage P5 wild-type mice were cultured with gentamicin to ablate HCs, followed by culturing them with JAG1-Fc peptide or without JAG1-Fc peptide (control) in the presence of CHIR99021 and LY411575. EdU was added to the culture media on day two to analyze cell proliferation. c Representative confocal images of the HC layer taken at the cochlear mid-apex and base following culture. Newly formed HCs are marked by MYO7A (magenta) and SOX2 (blue) immuno-staining, indicated by yellow asterisks. EdU (green) labels actively dividing SOX2+ SCs (white asterisks) and previously dividing MYO7A+ SOX2+ HCs (red asterisks). Scale bars=25 µm. d-f Quantification of EdU+ SOX2+ cells in HC layer (HCs and SCs) (d), new HCs (MYO7A+SOX2+) in mid-apex (e) and new HCs in base (f) for c (n = 4 animals per group, two independent experiments). Graphed are individual data points and mean ± standard deviation. Two-tailed, unpaired Student’s t test was used to calculate exact p-values. Source data are provided as a Source Data file. N.s. not significant.
Next, we investigated whether stimulation of JAG1/Notch signaling enhances the formation of cochlear HCs in vivo. During perinatal stages, ATOH1 overexpression in cochlear SCs or KCs is highly effective in triggering their conversion into HCs, but starting at around P7/P8, only a limited number of new inner HCs are formed in response to ectopic ATOH1 expression53. To determine whether JAG1-peptide treatment can increase ATOH1-induced SC-to-HC conversion, we co-injected ATOH1-EGFP expressing AAV with JAG1-Fc peptide through the round window membrane (RWM) into the cochlea of 7-day old wild-type mice. For control, we co-injected AAV expressing EGFP with JAG1-Fc peptide and co-injected AAV expressing ATOH1-EGFP with PBS (vehicle control). We used the AAV-ie (AAV-inner ear) variant, which infects cochlear HCs, SCs, and KCs54. Seven days later at P14, we harvested the AAV-infected cochlear tissue and analyzed new HC formation in the cochlear apex and base. As expected, cochleae that received JAG1-Fc peptide along with EGFP-expressing control AAV (JAG1-Fc + AAV-EGFP) contained many infected cells, including EGFP expressing HCs. However, none of these HCs expressed SOX2, indicating that they were pre-existing HCs. Consistent with previous reports, ATOH1 overexpression (PBS + AAV-ATOH1-EGFP) resulted in only a few new HCs (0.5 new HCs/ 200 µm= 0.5% of total HCs) within the inner HC region of the cochlear apex, and almost none in the base. In contrast, cochleae that received JAG1-Fc peptide along with ATOH1-EGFP expressing AAV (JAG1-Fc + AAV-ATOH1-EGFP) contained a significantly higher number of new HCs in the cochlear apex (10.5 new HCs/ 200 µm = 10.5% of total HCs) and in the cochlear base (2.5 new HCs/ 200 µm= 2.5% of total HCs) compared to the other two conditions (Fig.9 b, c).
a Experimental scheme. AAV expressing ATOH1 and EGFP (or EGFP only) was injected with or without JAG1-Fc peptide into the cochlea of P7 wild-type mice through the round window membrane. 7 days later, injected cochleae were immuno-stained for SOX2 (blue) and MYO7A (magenta) to identify newly formed HCs (MYO7A+ SOX2+). EGFP (green) marks infected cells. b Representative confocal images of the HC layer at the cochlear apex and base that received JAG1-Fc peptide (JAG1-Fc peptide + AAV-EGFP) or ATOH1 expressing AAV (PBS + AAV-ATOH1-EGFP) (control) or both JAG1-Fc peptide and ATOH1 expressing AAV (JAG1-FC peptide + AAV-ATOH1-EGFP). Scale bar=25 μm. c Quantification of infected cells that formed HCs (SOX2+MYO7A+) in the cochlear apex and base in (b) (graphed are mean ± SD, n = 6 mice per group, three independent experiments). One-way ANOVA with Tukey’s correction was used to calculate exact p-values. Source data are provided as a Source Data file. N.s. not significant. Created in BioRender. Doetzlhofer, A. (2025) https://BioRender.com/m18j826.
Discussion
The safety and efficacy of a gamma secretase inhibitor as a drug treatment for sensorineural hearing loss has been recently investigated in a phase I/IIa clinical trial55. However, surprisingly, little is known about how Notch signaling operates in the HC-damaged cochlea.
The complexity of Notch ligand and receptor interactions, coupled with the limited availability of cells and the dynamic and stochastic nature of HC regeneration make it challenging to gain mechanistic insights into how Notch signaling operates during cochlear HC regeneration. The recent developed organoid culture models, in which cochlear SCs can be easily manipulated and propagated and then re-differentiated into HCs, overcome many of these obstacles. Applying such an organoid culture model, we demonstrate two opposing functions for JAG1 in cochlear HC regeneration: (1) a negative role with JAG1 participating in HC-fate repression and (2) a positive role with JAG1 maintaining progenitor-like features of cochlear SCs, which we argue are necessary for mitotic and non-mitotic HC regeneration.
We show that Jag1 deficiency at perinatal stages leads to an increased rate of HC formation in cochlear organoids and HC-damaged cochlear explants, indicating that JAG1 participates in HC fate repression. How does JAG1/Notch signaling restrict HC fate induction? Our transcriptomic data indicates that JAG1 function is necessary to maintain the expression of Hes1, HeyL, and Hey1 in cochlear SCs and KCs. HES1, HEYL, and HEY1 are members of the HES/HEY family of transcriptional repressors56, which are highly expressed in cochlear SCs21 and are thought to prevent spontaneous conversion of SCs into HCs35. However, deletion of Jag1 in HC-depleted early postnatal cochlear explants by itself is insufficient to trigger SC-to-HC conversion. We find that only in the presence of the GSK3 inhibitor CHIR99021, which activates Wnt signaling, does the loss of Jag1 enhance cochlear HC regeneration. This finding contrast sharply with the high rate of cochlear HC regeneration induced by pharmacological inhibition of Notch signaling with GSIs22,23 or the deletion of Notch157, indicating a more complex role for JAG1 in cochlear HC regeneration.
Our investigation reveals that, outweighing its role in HC-fate repression, JAG1’s function is essential for maintaining the ‘progenitor-like’ characteristics of cochlear SCs. We hypothesize that maintaining or acquiring a progenitor-like state is a general prerequisite for HC regeneration by SCs. This hypothesis is supported by recent findings showing that in chicks and adult zebrafish, auditory and vestibular SCs de-differentiate and activate a progenitor-like state during the early phase of natural HC regeneration58,59.
We find that acute deletion of Jag1 or exposure to high dosages of GSI reduces cell cycle re-entry (organoid formation) and reduces continued cell proliferation (organoid size). Conversely, stimulating JAG1/Notch signaling through lentiviral expression of Jag1, low N1ICD expression, or JAG1-Fc peptide treatment increases both the frequency of cell cycle re-entry and proliferation in cochlear organoid culture. A similar pro-growth function for Notch signaling was observed when N1ICD was ectopically expressed in the murine cochlea in vivo at pro-sensory stages60.
How does JAG1/Notch signaling promote cochlear SC proliferation and HC formation? Our transcriptomic and mechanistic data indicate that JAG1/Notch1/2 signaling positively regulates PI3K-Akt-mTOR signaling in cochlear SCs and KCs. There is mounting evidence that mTOR signaling plays a critical role in cochlear SC proliferation and HC regeneration28,61,62. We recently showed that inhibiting mTOR activity with rapamycin reduces cochlear SC proliferation and HC formation at perinatal stages and interferes with LIN28B-induced reprogramming of cochlear SCs into progenitor-like cells28. Similarly, a study by Shu et al. found that at an adult stage reprogramming of cochlear SCs with MYC and N1ICD to a developmental younger stage was sensitive to the mTOR inhibitor rapamycin and the authors showed that MHY1485, a small molecule mTOR activator, could partially replace the need for MYC in cochlear SC reprogramming61. However, the above-mentioned study did not examine whether Notch signaling plays a role in MYC-induced activation of mTOR in cochlear SCs. Our study identifies the Notch ligand JAG1 and the Notch receptors Notch1 and Notch2 as crucial regulators of PI3K-Akt-mTOR signaling pathway in cochlear SCs (and KCs). We demonstrate using cochlear organoids that treatment with JAG1-Fc-peptide or low induction of NICD enhances PI3K-Akt-mTOR activity, while loss of Jag1, or combined loss of Notch1 and Notch2 significantly reduced PI3K-Akt-mTOR activity in cochlear SCs and KCs.
Interestingly, the loss of Notch2 did not affect PI3K-Akt-mTOR activity in cochlear organoids; however, it significantly reduced the rate of organoid formation and growth. This suggests the involvement of other JAG1-Notch2-dependent mechanisms in the regulation of cochlear organoid formation and growth. Our transcriptomic data indicate that lactate production/ transport may be reduced in the absence of JAG1 (and potentially Notch2). A recent study discovered that disrupting glycolysis and lactate production decreases cochlear progenitor cell proliferation in vivo, whereas adding exogenous lactate increases cell proliferation and HC formation in cochlear organoid and explant culture63. Future studies are warranted to explore the potential role of JAG1/Notch2 signaling in regulating cochlear glucose metabolism.
Which Notch receptors mediate the function of JAG1 during embryonic cochlear development? We have previously shown that deleting Jag1 during early embryonic cochlear development using Emx2Cre/+ leads to defects in pro-sensory specification and maintenance, resulting in the absence of the lateral sensory domain (which includes outer hair cells and surrounding supporting cells)49. In contrast, our research and that of others have indicated that the deletion of Notch1 does not impact pro-sensory specification or maintenance64. Here, we investigated whether the previously uncharacterized Notch receptors, Notch2 or Notch3, play a role in mediating JAG1’s pro-sensory function. Our findings reveal that deletion of Notch2 using Emx2Cre/+, or global deletion of Notch3, does not change the patterning of cochlear hair cells and supporting cells. This suggests that both pro-sensory specification and the repression of hair cell fate remain unaffected in these single knockout mice. Future studies employing double and triple knockout approaches will be necessary to clarify the roles of Notch1, Notch2, and Notch3 in pro-sensory specification and maintenance in the developing inner ear.
Studies conducted in chicken and zebrafish revealed that the strength and duration of Notch signaling are dynamically regulated during HC regeneration3. Initially downregulated in response to damage, Notch signaling is critical for maintaining the proper spatial and temporal patterns of SC proliferation while restricting HC-fate induction65,66,67,68,69. Numerous studies have shown that sustained high levels of Notch signaling inhibit HC regeneration70,71. Our research provides evidence that insufficient JAG1/Notch signaling similarly compromises the ability of murine cochlear SCs to proliferate and form HCs. We found that Jag1 mRNA expression in murine cochlear SCs declines more than five fold between P1 and P13, which coincides with the onset of hearing. Furthermore, we show that JAG1 protein expression in cochlear SCs nearly disappears following severe HC loss. We hypothesize that such decline in JAG1 expression reduces JAG1/Notch signaling strength below the threshold needed for SC proliferation and HC formation. Supporting this hypothesis, we find that stimulating JAG1/Notch signaling with JAG1-Fc peptide enhances SC proliferation and HC formation in cochlear explants, but only after cochlear maturation has begun and it enables ATOH1-induced HC formation by cochlear SCs in vivo in one week-old mice. Recent studies have shown that ectopic ATOH1 expression in cochlear SCs is ineffective due to HC-specific gene loci becoming inaccessible to ATOH172. Future research will investigate whether activating JAG1/Notch signaling can promote cochlear HC formation in adults and whether this signaling pathway affects the chromatin accessibility of HC-specific gene loci in cochlear SCs.
Importantly, JAG1/Notch signaling is not inhibited by the gamma-secretase inhibitor (GSI) dosages typically used to induce SC-to-HC conversion. This suggests that co-injecting JAG1-Fc peptide alongside a low dosage of GSI may be a promising strategy for stimulating cochlear HC regeneration in later, adult stages. Overall, our study offers mechanistic insights into the intricate role of JAG1/Notch signaling in cochlear HC regeneration and identifies JAG1 as a promising target for future therapies aimed at regenerating HCs in individuals who have experienced severe HC loss.
Methods
Mouse breeding and genotyping
All mouse procedures were performed in accordance with the approved animal protocols from the Institutional Animal Care and Use Committees at the Johns Hopkins University School of Medicine (USA), protocol MO23M258 and Xi’an Jiaotong University (China), and all efforts were made to minimize the number of mice used and their suffering. Mice were maintained on a 14 h light/10 h dark cycle. Both male and female mice were used. The sex of mice, which age ranged from P2 to P14 (pre-weaning age), was not tracked. The exact age of mice is indicated in the figure legends. All mice were group-housed in pathogen-free facilities with regulated temperature (21 ± 2 °C) and humidity and given ad libitum access to food and water. Animal husbandry and health status monitoring were provided by the Office of Animal Resources staff at Johns Hopkins University School of Medicine (USA) and Xi’an Jiaotong University (China). The Atoh1-nGFP transgenic (tg) mice were obtained from J. Johnson (University of Texas Southwestern Medical Center, Dallas). P27/GFP tg mice were obtained from Neil Segil (University of Southern California). Lfng/GFP tg mice were obtained from Nathan Heintz (Rockefeller University). Fgfr3-iCreERT2 tg mice73 were obtained from W. Richardson (University College London, UK). Emx2Cre/+ mice were obtained from Shinichi Aizawa (RIKEN Kobe, Japan)52. Jag1 floxed mice were obtained from Julian Lewis (Cancer Research UK London Research Institute)11. Sox2CreER/+ (no. 17593), R26rtTA*M2 (no. 006965), R26LSLtdTomato/+ (Ai14; no. 007914), TetO-Cre tg (no. 006234), Notch1 floxed (f) (no. 007181), Notch2 floxed (no. 010525) and Notch3-/+ (no. 010547) and Pou4f3DTR/+ (no. 028673) mice were purchased from the Jackson Laboratories (Bar Harbor, ME). Mice were genotyped by PCR. Genotyping primers are listed in Supplementary Table 1. To ablate HCs, Pou4f3DTR/+ transgenic mice received a single dose of diphtheria toxin (DT) (6.25 ng/g; Sigma-Aldrich, no. D0564) by intraperitoneal injection at P1.
Viral vector and production
All plasmids were constructed using MonClone Hi-Fusion Cloning Mix V2 (Monad, no. MC40101M). The lentiviral expression construct for Cre was generated using LentiCRISPRv2Cre (Addgene, no. 82415) and pCDH-EF1a-eFFly-mCherry (Addgene, no. 104833) plasmids, replacing eFFly with Cre using XbaI (NEB (New England Biolabs, no. R0145) and EagI-HF (NEB (New England Biolabs, no. R3505) restriction sites. Lentiviral expression construct for murine Jag1 was generated by subcloning a fragment containing 3HA-Jag1, synthesized by Sangon Biotech, into pCDH-EF1a-eFFly-mCherry (Addgene, no. 104833), replacing eFFly. The pLVX-TetOne-EGFP-3HA-N1ICD plasmid, including the 3HA-N1ICD fragment, was constructed using plasmid pLVX-TetO-EGFP (FENGHUISHEWU, no. BR727), linearized with EcoRI-HF (NEB (New England Biolabs, no. R3101S). For Notch1 and Notch2 knockdown experiments, the plasmids for constructing the shRNAs were based on the pLV[shRNA]-mCherry/Puro-U6>Scramble shRNA#l plasmid (VectorBuilder, vector ID: VB900082.4613xkq). The sequence of shRNAs are as follows: scr-shRNA-mCherry, 5′-CCT AAG GTT AAG TCG CCC TCG-3′; Notch1-shRNA1-mCherry, 5’-ACG GCG TGA ATA CCT ACA ATT -3’; Notch1-shRNA2-mCherry,5’-CCG CTG TGA GAT TGA TGT TAA-3’; Notch1-shRNA3-mCherry, 5’-GCC AGG TTA TGA AGG TGT ATA-3’; Notch2-shRNA1-mCherry, 5’-CAC ACC AAC TTG CGC ATT AAA-3’; Notch2-shRNA2-mCherry, 5’- CGA GAC CCA GTA CAG TGA AAT -3’; Notch2-shRNA3-mChery, 5-TTC GCC TCC TGG ACG AGT ATA-3. For packaging, 293 T cells (ATCC, no. CRL-3216, RRID: CVCL_0063) were transfected with 10 μg of transfer plasmid, 2 μg of pMDLg/pRRE (Addgene, no. 12251), 2 μg of pRSV-Rev (Addgene, no.12253), and 2 μg of pCMV-VSV-G (Addgene, no.8454), respectively. After 48-72 h of culture, the supernatant was collected, centrifuged at 500 g for 10 min at 4 °C and filtered with a 0.45 μm PES membrane filter to remove cell debris. Lentiviral particles were concentrated using ultracentrifugation at 90,000g for 1.5 h at 4 °C. Pellet was dissolved with 100 µl DMEM/F12 media overnight at 4 °C and stored at −80 °C. The pAAV-CAG-EGFP-2A-Atoh1-3xFLAG-WPRE (7 × 1012 viral particles per mL) and pAAV-CAG-EGFP-P2A-3xFLAG-WPRE (7 × 1012 viral particles per ml) were purchased from OBiO Technology. The AAV particles were produced with an AAV packaging plasmid that expressed the AAV-inner ear (AAV-ie) variant54.
Fluorescence-activated cell sorting
For isolating p27-GFP(+) SCs and p27-GFP(−) KCs, cochlear epithelial cells from stage P2 TetO-Cre; R26 rtTA*M2; Jag1f/f; p27-GFP transgenic animals and control littermates were used as starting material. For isolating Lfng-GFP(+) SCs, micro-dissected cochlear tissue from stages P1, P2, P5, and P13 Lfng-GFP transgenic mice was used as starting material. Tissue was incubated in TrypLE solution (Thermo Fisher Scientific, no. 2604013), triturated, and filtered through a 35-μm filter. The resulting single cells were resuspended in an expansion medium and, after incubation with propidium iodide, sorted on a MoFlo Legacy sorter with a 100-μm nozzle tip.
Organoid culture
Organoid cultures were established as previously described. Briefly, dissociated cochlear epithelial cells, or FACS-purified p27-GFP(+) cochlear SCs or p27-GFP(−) KCs from stage P2 mice were used to establish organoid cultures. In a subset of experiments, cochlear epithelial cells or FACS-purified Lfng-GFP(+) cochlear SCs were mixed with lentiviral particles and centrifuged at 600g for 30 min prior to plating. Otherwise, cells were immediately resuspended in expansion medium and mixed 1:1 with Matrigel (Corning, no. 356231) into pre-warmed four-well plates (CELLTREAT, no. 229103) and cultured in expansion media [DMEM/F12 (Corning, no. 10–092-CV), N-2 supplement (1×, ThermoFisher, no. 17502048), B-27 supplement (1×, ThermoFisher, no. 12587010), EGF (50 ng/mL, Sigma-Aldrich, no. SRP3196), FGF2 (50 ng/mL, ThermoFisher, no. PHG0264), CHIR99021 (3 μM, Sigma-Aldrich, no. SML1046), VPA (1 mM, Sigma-Aldrich, no. P4543), 616452 (2 μM, Sigma-Aldrich, no. 446859-33-2) and penicillin (100 U/mL, Sigma-Aldrich, no. P3032) to stimulate organoid formation and growth. To induce HC formation, organoids were cultured in a differentiation medium [DMEM/F12, N2 (1×), B27 (1×), CHIR99021 (3 μM)]. Doxycycline hyclate (Sigma-Aldrich, no. D9891) was used at 10 μg/mL if not otherwise stated. 4-hydroxy tamoxifen (Sigma-Aldrich, no. H7904) was used at 20 ng/mL. Human Jagged 1 Fc Chimera (R&D Systems, no. 1277 JG) was used at 50 ng/mL if not otherwise stated. LY411575 (Sigma-Aldrich, no. SML0506) was used as indicated. Culture media was changed every other day. Control and experimental samples were included in each experiment.
Quantification of organoid-forming efficiency, organoid diameter
Low-power bright-field and fluorescent images of organoid cultures were captured with an Axiovert 200 microscope using 5x and 10x objectives (Carl Zeiss Microscopy). The total number of organoids per culture was counted to calculate organoid-forming efficiency, and values were normalized to the total number of cells plated. To calculate the organoid size, the diameter of organoids in three to four randomly chosen fields was measured per culture using ImageJ (https://imagej.nih.gov/ij/), and the average value was reported as an individual data point. For each genotype and or treatment, a minimum of three biologically independent organoid cultures were established and analyzed. At a minimum, two independent experiments were conducted and analyzed. All analyses were performed masked to genotype and or treatment.
Explant culture
Cochlear sensory epithelia, including innervating neurons (cochlear explants) from stages P2–P5 mice were isolated by microdissection, and explants were cultured on SPI-Pore membrane filters (Structure Probe, no. E1013-MB) in DMEM/F12 containing 1× N-2, EGF (5 ng/mL) and penicillin (100 U/ml; Sigma-Aldrich, no. P3032). Gentamicin sulfate (100 μg/ml; Sigma-Aldrich, no. G1272) was used to ablate HCs. Recombinant human Jagged1-Fc peptide (50 ng/mL) was used to stimulate JAG1/Notch signaling. CHIR99021 (3 μM) and LY411575 (5 μM; Sigma-Aldrich, no. SML0506) were used to induce SC-to-HC conversion. To block mTORC1 activity, organoids received rapamycin (4 ng/mL; Sigma-Aldrich, no. R0395). DMSO (0.05%) served as vehicle control. Culture media was changed every other day. For each animal, two cochlear explant cultures were established, and per genotype and condition, at least three cultures obtained from different animals were analyzed. Control and experimental samples were included in each experiment.
In vivo JAG1-Fc peptide experiment
Wild-type stage P7 mice were anesthetized by placing them on ice for 2–3 min. Once anesthetized, mice were placed under the microscope, and an incision was made below the left ear. The surrounding tissue and fat were carefully removed with forceps to expose the round window membrane of the cochlea. 500 nL JAG1-Fc peptide (50 μg/mL) dissolved in PBS or 500 nL PBS was injected together with 500 nL AAV-EGFP or 500 nL AAV-ATOH1-EGFP at the speed of 20 nL/s by a pressure-controlled motorized micro injector (RWD, R-480). An equal volume of AAV was used, and the total volume for each injection was 1 μl. After the injection, the skin incision was closed using veterinary tissue adhesive (Millpledge Ltd., UK). Pups were subsequently returned to their mother for continued nursing.
RNA extraction, RT-qPCR, and TaqMan assay
Lfng-GFP(+) cochlear SCs from stage P1, P5, and P13 Lfng-GFP transgenic mice were isolated by FACS as previously described29. Organoids were harvested using Cell Recovery Solution. Total RNA was extracted from acutely isolated SCs or cultured cochlear organoids/explants using the miRNeasy Micro Kit. MRNA was reverse transcribed into cDNA using the iScript cDNA synthesis kit. Q-PCR was performed on a CFX-Connect Real-Time PCR Detection System using SYBR Green Master Mix reagent. Gene-specific primers used are listed in Supplementary Table 2.
RNA sequencing and data analysis
For each condition, three independent cultures from three animals were established. All the samples were processed using Illumina’s TruSeq stranded Total RNA kit, per the manufacturer’s recommendations, using the UDI indexes. The samples were sequenced on the NovaSeq 6000, paired-end, 2 × 50 base pair reads. Kallisto (v0.46.1) was used to pseudo-align reads to the reference mouse transcriptome and to quantify transcript abundance. The transcriptome index was built using the Ensembl Mus Musculus v96 transcriptome. The companion analysis tool Sleuth was used to identify differentially expressed genes (DEGs). We performed a Wald test to produce a list of significant DEGs between Jag1 KO expressing sample and the control. These lists were then represented graphically using sleuth along with pheatmap and ggplot2 packages in R v1.3.1093. Gene identifier conversion, gene annotation, and enrichment analysis were conducted using Metascape42.
Cell proliferation
EdU (ThermoFisher Scientific, no. C10338) was added to the culture medium at a final concentration of 3 µM as indicated, after which cochlear organoids/explants were harvested and processed for EdU detection using the Click-iTPlus EdU Cell Proliferation Kit (ThermoFisher Scientific, no. C10637 and C10638) following the manufacturer’s recommendations.
Immunohistochemistry and imaging
Cochlear organoids/explants were fixed in 4% paraformaldehyde for 30 minutes, permeabilized and blocked with 0.25% Triton X-100/10% fetal bovine serum for 30 min, and immuno-stained as previously described28. Antibodies are listed in Supplementary Table 3. DAPI was used to visualize cell nuclei. Single plane and z-stack images were captured using an LSM 700 confocal microscope (Zeiss microscopy) using identical settings for control and experimental samples. Maximum intensity projections were generated using ZEN (Zeiss) or ImageJ (https://imagej.nih.gov/ij/) software.
Quantification of cell proliferation and HC formation
For in vivo experiments, three high-power confocal images of the HC-layer were taken per animal and treatment at defined positions within the apex and base and used to determine the average number of new HCs (MYO7A+SOX2+) per length unit. For cochlear explants, three high-power confocal images of the HC-layer were taken per genotype and or treatment at defined positions. HC formation and cell proliferation (EdU+ cells) were quantified in the cochlear mid-apex if not otherwise stated. At least three biologically independent cultures per group (treatment/genotype) were analyzed and reported as individual data points. The orientation of the shown cochlear sensory epithelium is such that the medial inner HC region is closest to the bottom edge, and the lateral outer HC region is closest to the top edge of the shown images. For each organoid culture, low-power and high-power confocal images of processed organoids were taken and used to calculate the average percentage of EdU+ cells and MYO7A+SOX2+ HCs per organoid. At least three biologically independent cultures per group (treatment/genotype) were analyzed and reported as individual data points. All analyses were performed masked to genotype and or treatment.
Protein lysis and immunoblotting
Cochlear organoids were lysed with RIPA buffer (Sigma-Aldrich, no. R0278) supplemented with protease inhibitor (Sigma-Aldrich, no. 11697498001), phosphatase Inhibitor cocktail 2 (Sigma-Aldrich, no. P5726) and phosphatase inhibitor cocktail 3 (Sigma-Aldrich, no. P0044). Protein separation and immunoblots were conducted as previously described28. The resulting chemiluminescence was captured using X-ray films or digitally using the LI-COR Odyssey imaging system. ImageJ (https://imagej.nih.gov/ij/) was used to quantify the protein levels by measuring the relative density of bands. The antibodies used are listed in Supplementary Table 4. Uncropped and unprocessed scans of immunoblots are provided in the Source Data file.
Statistics and reproducibility
All results were confirmed by at least two independent experiments. Control and experimental samples were included in each independent experiment. The sample size (n) represents the number of animals analyzed per group if not otherwise stated. Animals (biological replicates) were allocated into control or experimental groups based on genotype and/or type of treatment. Derived statistics correspond to the analysis of mean values across biological replicates. Masking was used during data analysis to avoid bias. Data were analyzed using GraphPad Prism 8.0. Relevant information for each experiment, including sample size, statistical tests, and reported p-values, is found in the legend corresponding to each figure. All results were confirmed by at least two independent experiments. In all cases, P values ≤ 0.05 were considered significant, and error bars represent standard deviation (SD). For the post hoc power analysis, we used G*Power (3.1.9.7) software74. Effect size was calculated using means and standard deviations (SDs) of study groups. For the post hoc power analysis, the following parameters were used: t-test, for the test family, means: difference between two independent means (two groups), for the statistical test tail (s) was set to two, and α err prob was set to 0.0575. The findings of the post hoc power study are listed in Supplementary Data 4.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The RNA sequencing data generated in this study have been deposited in the Gene Expression Omnibus data repository (https://www.ncbi.nlm.nih.gov/geo/) under accession number GSE266157. The remaining data are available within the Article, Supplementary Information or Source Data file. Source data are provided with this paper.
References
Artavanis-Tsakonas, S., Rand, M. D. & Lake, R. J. Notch signaling: cell fate control and signal integration in development. Science 284, 770–776 (1999).
Kopan, R. & Ilagan, M. X. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137, 216–233 (2009).
Daudet, N. & Żak, M. Notch Signalling: The Multitask Manager of Inner Ear Development and Regeneration. Adv. Exp. Med. Biol. 1218, 129–157 (2020).
Petrovic, J. et al. Ligand-dependent Notch signaling strength orchestrates lateral induction and lateral inhibition in the developing inner ear. Development 141, 2313–2324 (2014).
Murata, J., Tokunaga, A., Okano, H. & Kubo, T. Mapping of notch activation during cochlear development in mice: implications for determination of prosensory domain and cell fate diversification. J. Comp. Neurol. 497, 502–518 (2006).
Kiernan, A. E., Xu, J. & Gridley, T. The Notch Ligand JAG1 Is Required for Sensory Progenitor Development in the Mammalian Inner Ear. PLoS Genet. 2, e4 (2006).
Kiernan, A. E. et al. Sox2 is required for sensory organ development in the mammalian inner ear. Nature 434, 1031–1035 (2005).
Benito-Gonzalez, A. & Doetzlhofer, A. Hey1 and Hey2 control the spatial and temporal pattern of mammalian auditory hair cell differentiation downstream of Hedgehog signaling. J. Neurosci. 34, 12865–12876 (2014).
Hayashi, T. et al. Hesr1 and Hesr2 may act as early effectors of Notch signaling in the developing cochlea. Dev. Biol. 316, 87–99 (2008).
Murata, J. et al. Notch-Hes1 pathway contributes to the cochlear prosensory formation potentially through the transcriptional down-regulation of p27Kip1. J. Neurosci. Res. 87, 3521–3534 (2009).
Brooker, R., Hozumi, K. & Lewis, J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development 133, 1277–1286 (2006).
Petrovic, J., Gálvez, H., Neves, J., Abelló, G. & Giraldez, F. Differential regulation of Hes/Hey genes during inner ear development. Dev. Neurobiol. 75, 703–720 (2015).
Lanford, P. J., Shailam, R., Norton, C. R., Gridley, T. & Kelley, M. W. Expression of Math1 and HES5 in the cochleae of wildtype and Jag2 mutant mice. J. Assoc. Res. Otolaryngol. 1, 161–171 (2000).
Hartman, B. H. et al. Hes5 expression in the postnatal and adult mouse inner ear and the drug-damaged cochlea. J. Assoc. Res. Otolaryngol. 10, 321–340 (2009).
Li, S. et al. Hey2 functions in parallel with Hes1 and Hes5 for mammalian auditory sensory organ development. BMC Dev. Biol. 8, 20 (2008).
Tateya, T., Imayoshi, I., Tateya, I., Ito, J. & Kageyama, R. Cooperative functions of Hes/Hey genes in auditory hair cell and supporting cell development. Dev. Biol. 352, 329–340 (2011).
Bermingham, N. A. et al. Math1: an essential gene for the generation of inner ear hair cells. Science 284, 1837–1841 (1999).
Lanford, P. J. et al. Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat. Genet. 21, 289–292 (1999).
Kiernan, A. E., Cordes, R., Kopan, R., Gossler, A. & Gridley, T. The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear. Development 132, 4353–4362 (2005).
Alford, B. R. & Ruben, R. J. Physiological, behavioral and anatomical correlates of the development of hearing in the mouse. Ann. Otol., Rhinol., Laryngol. 72, 237–247 (1963).
Doetzlhofer, A. et al. Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti. Dev. Cell 16, 58–69 (2009).
Korrapati, S., Roux, I., Glowatzki, E. & Doetzlhofer, A. Notch signaling limits supporting cell plasticity in the hair cell-damaged early postnatal murine cochlea. PloS ONE 8, e73276 (2013).
Mizutari, K. et al. Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron 77, 58–69 (2013).
Maass, J. C. et al. Changes in the regulation of the Notch signaling pathway are temporally correlated with regenerative failure in the mouse cochlea. Front. Cell. Neurosci. 9, 110 (2015).
Morrison, A., Hodgetts, C., Gossler, A., Hrabé de Angelis, M. & Lewis, J. Expression of Delta1 and Serrate1 (Jagged1) in the mouse inner ear. Mechan. Dev. 84, 169–172 (1999).
Chrysostomou, E. et al. The Notch Ligand Jagged1 is required for the formation, maintenance, and survival of Hensen’s cells in the mouse cochlea. J. Neurosci. 40, 9401–9413 (2020).
Gilels, F. A., Wang, J., Bullen, A., White, P. M. & Kiernan, A. E. Deletion of the Notch ligand Jagged1 during cochlear maturation leads to inner hair cell defects and hearing loss. Cell Death Dis. 13, 971 (2022).
Li, X. J. & Doetzlhofer, A. LIN28B/let-7 control the ability of neonatal murine auditory supporting cells to generate hair cells through mTOR signaling. Proc. Natl. Acad. Sci. USA 117, 22225–22236 (2020).
Li, X. J., Morgan, C., Goff, L. A. & Doetzlhofer, A. Follistatin promotes LIN28B-mediated supporting cell reprogramming and hair cell regeneration in the murine cochlea. Sci. Adv. 8, eabj7651 (2022).
Li, X. J., Morgan, C., Nadar-Ponniah, P. T., Kolanus, W. & Doetzlhofer, A. TRIM71 reactivation enhances the mitotic and hair cell-forming potential of cochlear supporting cells. EMBO Rep. 24, e56562 (2023).
Kubota, M., Scheibinger, M., Jan, T. A. & Heller, S. Greater epithelial ridge cells are the principal organoid-forming progenitors of the mouse cochlea. Cell Rep. 34, 108646 (2021).
Chen, J., Gao, D., Sun, L. & Yang, J. Kölliker’s organ-supporting cells and cochlear auditory development. Front. Mol. Neurosci. 15, 1031989 (2022).
Hayashi, T., Ray, C. A. & Bermingham-McDonogh, O. Fgf20 is required for sensory epithelial specification in the developing cochlea. J. Neurosci. 28, 5991–5999 (2008).
Yang, L. M., Cheah, K. S. E., Huh, S. H. & Ornitz, D. M. Sox2 and FGF20 interact to regulate organ of Corti hair cell and supporting cell development in a spatially-graded manner. PLoS Genet. 15, e1008254 (2019).
Zheng, J. L., Shou, J., Guillemot, F., Kageyama, R. & Gao, W. Q. Hes1 is a negative regulator of inner ear hair cell differentiation. Development 127, 4551–4560 (2000).
White, P. M., Doetzlhofer, A., Lee, Y. S., Groves, A. K. & Segil, N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature 441, 984–987 (2006).
McLean, W. J. et al. Clonal expansion of Lgr5-positive cells from mammalian Cochlea and high-purity generation of sensory hair cells. Cell Rep. 18, 1917–1929 (2017).
Kempfle, J. S., Turban, J. L. & Edge, A. S. Sox2 in the differentiation of cochlear progenitor cells. Sci. Rep. 6, 23293 (2016).
Kolla, L. et al. Characterization of the development of the mouse cochlear epithelium at the single cell level. Nat. Commun. 11, 2389 (2020).
Mann, Z. F., Chang, W., Lee, K. Y., King, K. A. & Kelley, M. W. Expression and function of scleraxis in the developing auditory system. PloS ONE 8, e75521 (2013).
Lukashkin, A. N. et al. A mouse model for human deafness DFNB22 reveals that hearing impairment is due to a loss of inner hair cell stimulation. Proc. Natl Acad. Sci. USA 109, 19351–19356 (2012).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523 (2019).
von Manteuffel, S. R. et al. The insulin-induced signalling pathway leading to S6 and initiation factor 4E binding protein 1 phosphorylation bifurcates at a rapamycin-sensitive point immediately upstream of p70s6k. Mol. Cell. Biol. 17, 5426–5436 (1997).
Facchinetti, V. et al. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J. 27, 1932–1943 (2008).
Smyrlaki, I. et al. Soluble and multivalent Jag1 DNA origami nanopatterns activate Notch without pulling force. Nat. Commun. 15, 465 (2024).
Wang, S. et al. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron 21, 63–75 (1998).
Varnum-Finney, B. et al. The Notch ligand, Jagged-1, influences the development of primitive hematopoietic precursor cells. Blood 91, 4084–4091 (1998).
Nobta, M. et al. Critical regulation of bone morphogenetic protein-induced osteoblastic differentiation by Delta1/Jagged1-activated Notch1 signaling. J. Biol. Chem. 280, 15842–15848 (2005).
Campbell, D. P., Chrysostomou, E. & Doetzlhofer, A. Canonical Notch signaling plays an instructive role in auditory supporting cell development. Sci. Rep. 6, 19484 (2016).
Krebs, L. T. et al. Characterization of Notch3-deficient mice: normal embryonic development and absence of genetic interactions with a Notch1 mutation. Genes. (N. Y., N. Y.: 2000) 37, 139–143 (2003).
Atkinson, P. J. et al. Sox2 haploinsufficiency primes regeneration and Wnt responsiveness in the mouse cochlea. J. Clin. Investig. 128, 1641–1656 (2018).
Kimura, J. et al. Emx2 and Pax6 function in cooperation with Otx2 and Otx1 to develop caudal forebrain primordium that includes future archipallium. J. Neurosci. 25, 5097–5108 (2005).
Kelly, M. C., Chang, Q., Pan, A., Lin, X. & Chen, P. Atoh1 directs the formation of sensory mosaics and induces cell proliferation in the postnatal mammalian cochlea in vivo. J. Neurosci. 32, 6699–6710 (2012).
Tan, F. et al. AAV-ie enables safe and efficient gene transfer to inner ear cells. Nat. Commun. 10, 3733 (2019).
Schilder, A. G. M. et al. A phase I/IIa safety and efficacy trial of intratympanic gamma-secretase inhibitor as a regenerative drug treatment for sensorineural hearing loss. Nat. Commun. 15, 1896 (2024).
Fischer, A. & Gessler, M. Delta-Notch–and then? Protein interactions and proposed modes of repression by Hes and Hey bHLH factors. Nucleic Acids Res. 35, 4583–4596 (2007).
Wu, J. et al. The crosstalk between the Notch, Wnt, and SHH signaling pathways in regulating the proliferation and regeneration of sensory progenitor cells in the mouse cochlea. Cell Tissue Res. 386, 281–296 (2021).
Jimenez, E. et al. A regulatory network of Sox and Six transcription factors initiate a cell fate transformation during hearing regeneration in adult zebrafish. Cell Genomics https://doi.org/10.1016/j.xgen.2022.100170 (2022).
Matsunaga, M. et al. Stepwise fate conversion of supporting cells to sensory hair cells in the chick auditory epithelium. iScience 26, 106046 (2023).
Pan, W. et al. Ectopic expression of activated Notch or SOX2 reveals similar and unique roles in the development of the sensory cell progenitors in the mammalian inner ear. J. Neurosci. 33, 16146–16157 (2013).
Shu, Y. et al. Renewed proliferation in adult mouse cochlea and regeneration of hair cells. Nat. Commun. 10, 5530 (2019).
Zhang, Z. et al. Ti(3) C(2) T(x) MXene composite 3D hydrogel potentiates mTOR signaling to promote the generation of functional hair cells in Cochlea organoids. Adv. Sci. 9, e2203557 (2022).
Wu, M. et al. PKM2 controls cochlear development through lactate-dependent transcriptional regulation. Proc. Natl Acad. Sci. USA 122, e2410829122 (2025).
Basch, M. L. et al. Fine-tuning of Notch signaling sets the boundary of the organ of Corti and establishes sensory cell fates. eLife https://doi.org/10.7554/eLife.19921 (2016).
Ma, E. Y., Rubel, E. W. & Raible, D. W. Notch signaling regulates the extent of hair cell regeneration in the zebrafish lateral line. J. Neurosci. 28, 2261–2273 (2008).
Wibowo, I., Pinto-Teixeira, F., Satou, C., Higashijima, S. & López-Schier, H. Compartmentalized Notch signaling sustains epithelial mirror symmetry. Development 138, 1143–1152 (2011).
Romero-Carvajal, A. et al. Regeneration of sensory hair cells requires localized interactions between the Notch and Wnt pathways. Dev. Cell 34, 267–282 (2015).
Lush, M. E. et al. scRNA-Seq reveals distinct stem cell populations that drive hair cell regeneration after loss of Fgf and Notch signaling. eLife https://doi.org/10.7554/eLife.44431 (2019).
Thomas, E. D. & Raible, D. W. Distinct progenitor populations mediate regeneration in the zebrafish lateral line. eLife https://doi.org/10.7554/eLife.43736 (2019).
Daudet, N. et al. Notch regulation of progenitor cell behavior in quiescent and regenerating auditory epithelium of mature birds. Dev. Biol. 326, 86–100 (2009).
McGovern, M. M., Zhou, L., Randle, M. R. & Cox, B. C. Spontaneous Hair Cell Regeneration Is Prevented by Increased Notch Signaling in Supporting Cells. Front. Cell Neurosci. 12, 120 (2018).
Iyer, A. A. et al. Cellular reprogramming with ATOH1, GFI1, and POU4F3 implicate epigenetic changes and cell-cell signaling as obstacles to hair cell regeneration in mature mammals. eLife https://doi.org/10.7554/eLife.79712 (2022).
Young, K. M. et al. An Fgfr3-iCreER(T2) transgenic mouse line for studies of neural stem cells and astrocytes. Glia 58, 943–953 (2010).
Faul, F., Erdfelder, E., Lang, A. G. & Buchner, A. G. Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191 (2007).
Kang, H. Sample size determination and power analysis using the G*Power software. J. Educ. Eval. Health Prof. 18, 17 (2021).
Acknowledgements
We thank the members of the Doetzlhofer Laboratory for the help and advice provided throughout the course of this study. This work has been supported by the National Institute on Deafness and Other Communication Disorders grants R01DC011571 (A.D.), R01DC019359 (A.D.) and F31DC020882 (C.M.) as well as Action on Hearing Loss Discovery Grant G99 (A.D.), David M. Rubenstein Fund for Hearing Research (A.D.), the Natural Science Basic Research Plan in Shaanxi Province of China (Program No.2024JC-ZDXM-43) (X.J.L.), National Natural Science Fund for Excellent Young Scientists Fund Program (Program No.GYKP045) (X.J.L.) and the Fundamental Research Funds for the Central Universities (X.J.L.).
Author information
Authors and Affiliations
Contributions
Conceptualization: A.D., X.-J.L.; Methodology: A.D., X.-J.L., C.M.; Investigation: X.-J.L., C.M., L.L., W.-Y.Z., E.C.; Supervision: A.D., X.-J.L.; Writing—original draft: A.D., X.-J.L.; Writing—review & editing: A.D., X.-J.L., C.M.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Anthony Ricci, who co-reviewed with Shefin Sam George; Alan Cheng, and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, XJ., Morgan, C., Li, L. et al. The Notch ligand Jagged1 plays a dual role in cochlear hair cell regeneration. Nat Commun 16, 8169 (2025). https://doi.org/10.1038/s41467-025-63053-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-63053-6