Abstract
Viral infection induces robust reprogramming of metabolic pathways in host cells. However, whether host metabolic enzymes detect viral components remains unknown. Our group and others previously identified O-GlcNAc transferase (OGT), an important glucose metabolic enzyme, as a crucial mediator of the antiviral immune responses. Here, by studying a mouse model with a catalytically impaired OGT, we discover a catalytic activity-independent function of OGT in restraining influenza A virus (IAV) infection in addition to its catalytic activity-dependent effect on MAVS-mediated antiviral immunity. Biochemical studies reveal a critical antiviral effect based on OGT interacting with IAV genomic RNA that requires its N-terminal tetracopeptide repeat-4 motif. This interaction causes the translocation of nuclear OGT to cytosolic lipid droplets (LDs) to destabilize LDs-coating perilipin 2, thereby limiting LDs accumulation and in turn virus replication. In sum, our findings reveal OGT as a multifaceted metabolic sensor that integrates MAVS signaling and lipid metabolism to combat viral infection.
Similar content being viewed by others
Introduction
During RNA virus infection, the innate immune system uses retinoic-acid inducible gene I (RIG-I)-like receptors (RLRs), including RIG-I and melanoma differentiation associated gene 5 (MDA5), to detect cytosolic viral RNA species and initiate a signaling cascade leading to immune activation1,2,3. Activation of RIG-I and MDA5 promotes the recruitment of the signaling adaptor protein MAVS and activation of downstream NF-κB and IRF3 antiviral signaling, causing the production of type I interferon (IFN-I) and IFN-stimulated genes (ISGs)4,5. In addition, viral genomic RNA can be detected by Z-DNA binding protein 1 (ZBP1), which initiates receptor-interacting serine/threonine-protein kinase 3 (RIPK3)-mediated necroptosis6,7,8,9,10. These two evolutionarily conserved sensing mechanisms constitute the front line of host defense responses against viral infection11,12,13,14.
Reprogramming of cellular metabolic activities has recently been demonstrated to play a critical role in the activation of host defense responses following viral infection15,16,17,18. Elevated catabolic activity in activated immune cells is required to provide biomolecules and energy for effective immune functions and virus clearance. Meanwhile, a variety of metabolic enzymes, metabolites or proteins involved in cell metabolism have been shown to play essential roles in orchestrating immune activation, which is required for host response against viral infection19. Therefore, metabolic system regulates immune cell function and inflammation through combined strategies. Indeed, though metabolic reprogramming is a hallmark feature of host antiviral defense responses, it remains unclear whether metabolism-coupled sensing mechanisms exist to alarm metabolic networks for reprogramming upon viral infection.
O-GlcNAc transferase (OGT) is an essential glycolytic enzyme that mediates the transfer of UDP-GlcNAc to serine or threonine residues of target proteins20,21,22,23,24. This unique posttranslational modification (PTM), known as protein O-GlcNAcylation, targets many nuclear and cytosolic proteins20,25. Previous studies have discovered important functions of OGT and O-GlcNAc signaling in many fundamental biological activities, including transcription, translation and signal transduction25,26,27,28,29,30. Recent studies from our group31 and others32,33,34,35,36 have shown OGT-dependent protein O-GlcNAcylation as an important cellular mechanism to promote antiviral innate immune response. OGT contains a C-terminal glycosyltransferase catalytic domain and an N-terminal tetratricopeptide repeats (TPR) motif involved in protein-protein interactions. Previously, most OGT studies have focused on its catalytic function (i.e., O-GlcNAcylation of target proteins affecting their functions such as translocation, kinase activity, and protein stability, etc.). Meanwhile, a noncatalytic function of OGT likely exists because the TPR motif has been well known to have a scaffolding function involving many proteins, such as antiviral IFIT proteins (interferon-induced protein with tetratricopeptide repeats)37. Indeed, one study reported a critical function of noncatalytic activity of OGT in regulating mammalian cell proliferation and survival38. Using a whole-body catalytically impaired mouse strain with missense variant of OGT that is associated with intellectual disability, one recent study reported that the catalytic activity of OGT is essential for normal skull morphology and brain size39. However, the relative contribution of catalytic versus noncatalytic effects of OGT in modulating antiviral responses remains unexplored.
In this study, we generated a conditional OGT catalytically impaired knock-in mouse model to investigate its catalytic versus noncatalytic effects on host antiviral responses. Surprisingly, our findings demonstrate an OGT catalytic activity-dependent effect on MAVS antiviral signaling and an OGT catalytic activity-independent antiviral effect via modulating lipid droplets (LDs) during influenza A virus (IAV) infection. Biochemical studies detected OGT interacting with IAV genomic RNA that requires its N-terminal TPR4 motif, which promotes a translocation of nuclear OGT to cytosolic LDs compartment and thereby limits viral replication. Therefore, our studies identified OGT as an important metabolic component of host antiviral defense through sensing viral infection and integrating innate immune signaling and cellular lipid metabolism.
Results
OGT inhibits IAV infection via both catalytic activity-dependent and -independent manner
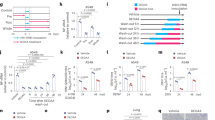
We and others have recently identified OGT as an essential antiviral protein that modifies MAVS to enhance its signaling capacity31,32,34. To determine if OGT-mediated antiviral effects are solely dependent on protein O-GlcNAcylation, we generated a conditional OGT catalytically impaired knock-in mouse model by replacing lysine 908, which is a residue critical for capturing substrate UDP-GlcNAc and for its enzymatic activity40,41,42, with alanine (Ogt-K908Afl/fl) (Supplementary Fig. 1a, b). Ogt-K908Afl/fl and Ogtfl/fl (Supplementary Fig. 1c, d)31 mice were then crossed with R26-Cre-ERT2 mice to achieve tamoxifen-inducible Ogt catalytically impaired (referred to as OgtK908A) and Ogt-knockout (referred to as OgtKO) models, respectively. Treatment with tamoxifen for 72 h caused efficient reduction of total O-GlcNAc signal in OgtK908A and OgtKO mouse embryonic fibroblasts (MEFs) (Supplementary Fig. 1e, f). Next, we infected OgtK908A and OgtKO MEFs with IAV (PR8) at a multiplicity of infection (MOI) of 143. OgtKO MEFs exhibited a significantly increased IAV infection, evidenced by elevated IAV transcripts NS1 and NP (encoding non-structural protein 1 and nucleoprotein of IAV, respectively) (Fig. 1a) and viral titers (Fig. 1b), compared to Ogtfl/fl control cells. Moreover, OgtKO MEFs contained a markedly increased amount of IAV protein hemagglutinin (HA) and neuraminidase (NA) following viral infection compared to Ogtfl/fl control cells (Fig. 1c). Despite being more permissive for viral replication than WT MEFs, OgtK908A MEFs showed an attenuated viral replication compared to OgtKO MEFs (Fig. 1a–c). When cells were treated with GFP-expressing IAV, we observed a similarly attenuated viral replication phenotype in OgtK908A MEFs compared to OgtKO MEFs (Fig. 1d and Supplementary Fig. 1g). These results suggest a possibility that OGT may regulate antiviral response through both catalytic activity-dependent and -independent mechanism.
a–g Mouse embryonic fibroblasts (MEFs) generated from Ogtfl/fl, Ogt-K908Afl/fl, Ogt KO and OgtK908A mice were infected with PR8 or PR8-GFP virus at a multiplicity of infection (MOI) = 1 for 12 h. Viral mRNAs (a), viral titers (b), expression of IAV HA and NA proteins (c), and virus infected cells (d) were measured by RT-PCR, TCID50 assays, immunoblotting with corresponding antibodies and flow cytometry respectively. e Gene transcripts including Ifna4, Ifnb1, Mx1, Isg15, Cxcl10, Il6, and Tnfa in the cells were measured with RT-PCR. f Immunoblotting of phosphorylated TBK1, IRF3, NF-κB signaling molecules and protein O-GlcNAcylation was performed with indicated antibodies. g Immunoprecipitated MAVS from the MEFs was assessed for O-GlcNAcylation with anti-O-GlcNAc antibody. Data are representative of three independent experiments (a, b and d, e). Statistical significance was determined by two-way ANOVA followed by Tukey’s test (a, b and d, e), P values of statistical comparisons are shown in each graph. The error bars represent SEM from n = 3 independent biological replicates (a, b and d, e). Source data are provided as a Source Data file.
We next sought to determine if impaired catalytic activity of OGT abolished MAVS-mediated innate immune activation and ISG production31. Following IAV infection, we found a significantly decreased production of multiple ISGs such as Ifna4, Ifnb1, Mx1, Isg15, Cxcl10 and genes encoding inflammatory cytokine Il6 and Tnfa in OgtK908A MEFs compared to Ogt-K908Afl/fl cells (Fig. 1e). Moreover, IAV-induced activation of MAVS antiviral signaling, evidenced by phosphorylation of IRF3, TBK1 and NF-κB p65, was markedly blunted in OgtK908A MEFs (Fig. 1f). These results were consistent with previous studies showing a critical function of OGT in the activation of innate immune signaling upon viral infection31,32,34. OgtK908A and OgtKO MEFs showed a comparable level of defect in ISG production and antiviral signaling activation (Fig. 1e, f), suggesting that impaired catalytical activity of OGT is sufficient to cause defective MAVS-mediated antiviral immune signaling in OgtK908A MEFs. O-GlcNAcylation of MAVS was enhanced in WT cells following IAV infection but was completely abolished in both OgtK908A and OgtKO MEFs (Fig. 1g). These results support the concept that OGT promotes MAVS innate immune signaling via its catalytic activity, as we recently reported31.
We further examined whether catalytic activity-dependent versus catalytic activity-independent antiviral effects of OGT also exist in human cells. OGT-KO HT29 cells were reconstituted with stable expression of OGT WT or K908A mutant construct, as reported in our recent study31. Reconstitution of OGT-KO cells with WT OGT caused a significant decrease in IAV transcripts NS1 and NP (Fig. 2a) and viral titers (Fig. 2b), compared to OGT-KO cells reconstituted with empty vector. In contrast, reconstitution of OGT-KO cells with OGT K908A mutant only partially inhibited viral replication compared to OGT-KO cells reconstituted with WT OGT (Fig. 2a, b). As expected, reconstitution of OGT-KO cells with WT OGT markedly elevated the production of multiple ISGs (Fig. 2c) and activation of antiviral innate immune signaling (Fig. 2d). However, the inhibitory effect of OGT K908A mutant on viral replication was unlikely related to MAVS signaling due to the lack of change in ISG production and no phosphorylation of IRF3, TBK1, or NF-κB p65 in OGT-KO cells reconstituted with OGT K908A mutant compared to empty vector (Fig. 2c, d).
a–d OGT-KO HT29 cells were reconstituted with empty vector (EV), GFP-tagged OGT (OGT-WT) or its K908A mutants, followed by the infection with PR8 virus at a MOI = 1 for 12 h. Viral mRNAs (a), and viral titers (b) were measured by RT-PCR, and TCID50 assays respectively. c Gene transcripts including IFNA4, IFNB, MX1, ISG15, IL6, and TNFA in the cells were measured with RT-PCR. d Immunoblotting of phosphorylated TBK1, IRF3, IKKα/β, IκBα, and NF-κB signaling molecules and protein O-GlcNAcylation was performed with indicated antibodies. e–h, MAVS-KO or IRF3-KO and WT control of HT29 EV, OGT-WT and OGT-K908A cells were infected with PR8 virus at a MOI = 1 for 12 h. e The expression of MAVS and IRF3 proteins in cells were assessed by immunoblotting. Viral mRNAs (f), and viral titers (g) were measured by RT-PCR and TCID50 assays respectively. h Gene transcripts including IFNB, MX1, ISG15, IL6, and TNFA in the cells were measured with RT-PCR. Data are representative of three independent experiments (a–c and f–h). Statistical significance was determined by two-way ANOVA followed by Tukey’s test (a–c and f–h), P values of statistical comparisons are shown in each graph. The error bars represent SEM from n = 3 independent biological replicates (a–c and f–h). Source data are provided as a Source Data file.
To determine if additional ISGs induced by MAVS signaling other than those examined (Fig. 2c) may contribute to antiviral effect of OGT K908A mutant, we compared antiviral effect of OGT WT and K908A mutant in cells with the depletion of MAVS (MAVS-KO) or IRF3 (IRF-KO). CRISPR/Cas9-based gene targeting strategy with the use of single guide RNA (gRNA) specific to human MAVS or IRF3 gene was employed in cells with various OGT genetic backgrounds to generate cells with single or double gene mutations (Fig. 2e). When MAVS or IRF3 was genetically depleted, we detected a comparable viral-restriction effect between OGT WT and K908A mutant, evidenced by similar levels of IAV transcripts (Fig. 2f) and viral titers (Fig. 2g). As expected, the deletion of MAVS or IRF3 abolished ISG production upon IAV infection despite OGT genetic background (Fig. 2h). In sum, these results from genetic studies indicate that OGT-mediated antiviral response relies on both catalytic activity-dependent and -independent mechanisms and that OGT catalytic activity-dependent antiviral effect requires MAVS-IRF3 innate immune signaling pathway.
Catalytic activity-dependent and -independent antiviral functions of OGT in vivo
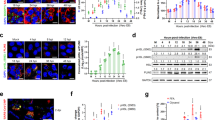
To examine catalytic activity-dependent and -independent antiviral effects of OGT in vivo, we generated mice with lung epithelial cell (LEC)-specific OGT deletion (referred to as OgtΔLEC) or catalytically impaired OGT (referred to as Ogt-K908AΔLEC) by crossing Ogtfl/fl or Ogt-K908Afl/fl with Foxj1-Scgb1a1-Cre mice44. Previous study has demonstrated that Foxj1-Scgb1a1-Cre system could efficiently induce gene deletion specifically in ciliated cells and columnar cells in the mouse respiratory tract, the two major cell compartments targeted by respiratory viral infection44. We infected mice intranasally with IAV at a sublethal dose of 10 TCID50. All Ogtfl/fl WT mice survived over the entire course of 20 days after infection, as we previously reported43, whereas all OgtΔLEC mice succumbed to infection by day 12 after infection (Fig. 3a). OgtΔLEC mice also experienced markedly exacerbated weight loss compared to Ogtfl/fl WT mice (Fig. 3b). In contrast, 50% of Ogt-K908AΔLEC mice survived over 20 days after infection and experienced moderately increased weight loss compared to Ogtfl/fl WT mice (Fig. 3a, b). These results indicate that impaired catalytic activity of OGT resulted in less severe disease outcomes compared to OGT deletion in lung epithelial cells.
a–g Ogtfl/fl, OgtΔLEC and Ogt-K908AΔLEC mice were infected with 10 TCID50 of PR8 virus. Survival (a) and changes in body weight (b) of mice were monitored every 2 days. Ogtfl/fl mice (n = 18), OgtΔLEC mice (n = 17) and Ogt-K908AΔLEC mice (n = 18). Viral mRNAs (c), and viral titers (d) in the lung were measured by RT-PCR and TCID50 assays respectively. e Gene transcripts including Ifna4, Ifnb1, Mx1, Isg15 and Cxcl10 in the lung were measured with RT-PCR. f IFN-β protein in the lung was measured with ELISA. g Histological analysis of the lung tissue collected from animals at day 2 after viral infection. Scale bars, 20 μm. Data are representative of three independent experiments (a–f). Statistical significance was determined by log-rank (Mantel-Cox) test survival analysis (a) or two-way ANOVA followed by Tukey’s test (b–f), P values of statistical comparisons are shown in each graph. The error bars represent SEM from n = 3 independent experiments (a–f), each dot represents the means of each of the three experiments (c–f). Source data are provided as a Source Data file.
We further examined viral replication and antiviral immune responses in lung tissue following IAV infection. Consistent with elevated viral replication in OgtKO cells, OgtΔLEC mice contained significantly higher amounts of virus transcripts (Fig. 3c) and viral titers (Fig. 3d) in lung tissues at day 1 and 2 after infection. Ogt-K908AΔLEC mice exhibited attenuated viral replication in lung tissue compared to OgtΔLEC mice, indicating both catalytic activity-dependent and -independent functions of OGT in limiting IAV replication in vivo. Furthermore, we detected robust induction of ISG gene transcription (Fig. 3e) and production of IFN-β protein (Fig. 3f) in the lung from WT mice at day 1 and 2 after infection, indicating efficient induction of antiviral immunity. In contrast, ISG induction and IFN-β production were significantly suppressed in both OgtΔLEC mice and Ogt-K908AΔLEC mice to the same extent compared to Ogtfl/fl WT mice (Fig. 3e, f). Histological analysis of the lung tissue from animals 2 days after viral infection showed greater infiltration of immune cells and injury in OgtΔLEC mice compared with WT mice (Fig. 3g). Less immune cell infiltration was observed in the lung from IAV- infected Ogt-K908AΔLEC mice compared to similarly treated OgtΔLEC mice, likely due to less viral replication. These findings indicate that OGT is crucial for limiting IAV viral replication in vivo through both catalytic activity-dependent and -independent mechanisms.
OGT interacts with IAV genomic RNA
The N-terminal region of OGT is composed of 13.5 TPR repeats that are involved in protein-protein interactions and substrate recognition45,46. It has been documented that critical antiviral effector molecules IFIT1 and IFIT5 recognize influenza and vesicular stomatitis virus RNA via their TPR motif47. Thus, we sought to determine whether OGT could recognize viral genomic RNA that requires its TPR motif and, if so, whether OGT interaction with viral RNA is important for its antiviral effect. Several steps of examination of OGT-viral genomic RNA interaction were performed. First, we overexpressed a full-length (FL) OGT, or its N-terminal or C-terminal truncations in 293 T cells (Fig. 4a, b), followed by IAV infection. Using IAV genomic RNA specific Uni-12 primer for RT-PCR assay48,49, we found that all eight viral genomic RNA (PA, PB2, NP, NA, HA, NS1, PB1 and M) were coimmunoprecipitated with OGT FL (Fig. 4c and Supplementary Fig. 2a). Coimmunoprecipitation of viral genomic RNA with OGT depends on its N-terminal, but not C-terminal (Fig. 4c). These results indicate that OGT interacts with viral genomic RNA that requires its N-terminal TPR motif. Second, we generated mutant OGT construct with the deletion of either TPR1-7 or TPR8-13.5 (Fig. 4d) and found that the lack of TPR1-7 abolished OGT coimmunoprecipitation with viral PA and PB2 genomic RNA (Fig. 4e). Third, we examined the role of TPR1-7 by deleting each repeat (Fig. 4f, g and Supplementary Fig. 2b) and found that the lack of TPR4 (ΔTPR4) abolished OGT-viral genomic RNA interaction (Fig. 4h). As controls, OGT failed to immuneprecipitate host-derived housekeeping transcripts ACTB and GAPDH (Fig. 4h). These results suggest that OGT interacts with IAV genomic RNA that requires its TPR4 motif.
a–h, 293T cells were transfected with indicated vectors. At 12 h after transfection, cells were infected with PR8 virus at MOI = 1 and cell lysates were harvested at 48 h after transfection and immunoprecipitated with anti-Myc Agarose. a Schematic diagram of full-length OGT and its truncated mutants. N-ter, N-terminal; C-ter, C-terminal; TPR, tetratricopeptide repeats motif; b, d Immunoblotting of full-length OGT and its truncated mutants in immunoprecipitation assay. c, e Viral PA and PB2 genomic RNAs in immunoprecipitation assay were quantified by RT-PCR. f Schematic diagram of full-length OGT and its TPR motif deletion mutants. g Immunoblotting of full-length OGT and its TPR motif deletion mutants in immunoprecipitation assay. h Viral PA, PB2 genomic RNAs and human housekeeping gene transcripts in immunoprecipitation assay were quantified by RT-PCR. i, j 293T cells were transfected with indicated vectors for 36 h, cell lysates were incubated with biotin-labeled viral PB2 genomic RNA and immunoprecipitated with streptavidin beads. Immunoblotting of Flag-RIG-I (Positive control) and OGT (i) or OGT and its mutants (j) in immunoprecipitation assay. k, l Electrophoretic mobility shift assay (EMSA) of in vitro transcribed viral PB2 genomic RNA in the presence of purified Flag-RIG-I (Positive control), Flag-OGT or its mutants. Mobility of viral PB2 genomic RNA (600 ng) in the presence of purified Flag-RIG-I (1, 2, and 4 μg) and Flag-OGT (1, 3, and 6 μg) (k) or in the presence of purified Flag-OGT and its mutants (4 μg for each sample) (l). Data are representative of three independent experiments (c, e, h). Statistical significance was determined by one-way ANOVA followed by Tukey’s test (c, e, h), P values of statistical comparisons are shown in each graph. The error bars represent SEM from n = 3 independent biological replicates (c, e, h). Source data are provided as a Source Data file.
A reciprocal coimmunoprecipitation assay was performed to further examine the interaction between OGT and viral genomic RNA. The PB2 genomic RNA of IAV generated via in vitro transcription was biotinylated and mixed with Streptavidin beads (Supplementary Fig. 2c). After the incubation with Flag-tagged OGT protein overexpressed in 293T cells, biotinylated RNA/beads mixture, but not unlabeled control sample, coimmunoprecipitated FL OGT (Fig. 4i). RIG-I, a well-established innate immune sensor for viral RNA, was also immunoprecipitated by biotinylated IAV PB2 genomic RNA. We further found that pulldown of OGT by IAV genomic RNA required the presence of TPR4 (Fig. 4j), suggest a critical role of TPR4 in the interaction between OGT and IAV genomic RNA.
To assess the viral genomic RNA-interacting properties of OGT, we measured the mobility of synthetic IAV PB2 genomic RNA in the absence or presence of OGT by electrophoretic mobility shift assay (EMSA)9,50. Purified FL OGT protein (Supplementary Fig. 2d) caused mobility retardations of IAV PB2 genomic RNA in a dose-dependent manner (Fig. 4k). RIG-I protein exhibited a similar inhibitory effect on PB2 genomic RNA mobility. Deletion of TPR4, but not TPR5, of OGT rescued the migration of PB2 genomic RNA compared to FL OGT (Fig. 4l and Supplementary Fig. 2e). Therefore, these results strongly support that OGT interacts with IAV genomic RNA that requires its TPR4 motif.
To probe the noncovalent interaction between OGT and viral genomic RNA, we performed isothermal titration calorimetry (ITC) assay, a widely used method to measure the binding of two molecules including protein-RNA binding51. It is worth noting that for ITC assay, production of OGT protein was carried out in prokaryotic system (BL21 cells) due to a greater expression yield compared to 293 T cells (Supplementary Fig. 3a). When adding OGT WT protein to the solution containing PB2 RNA, an enthalpy-driven (ΔH° = 36.9 ± 0.91 kcal/mol) binding with an apparent Kd of 3.95 μM was observed, which fit well to noncooperative binding model. With the entropic driving force (ΔS° = 148.4 cal/mol), the favorable complexation is likely driven by hydrophobic effect (Supplementary Fig. 3b). In contrast, an incremental addition of OGT ΔTPR4 protein to PB2 produced negligible exothermic heat, which indicated the absence of noncovalent complexation (Supplementary Fig. 3c). These findings indicate that OGT directly interacts with viral RNA and their interaction requires TPR4.
To determine whether OGT specifically interacts with viral RNA, we performed sequencing assay of OGT-immunoprecipitated RNA under eight experimental conditions, including both IP and input samples with and without IAV infection. We followed a similar method reported recently, which calculated the transcript enrichment of OGT versus empty vector control with and without IAV infection in IP and input samples52 (Supplementary Fig. 4a). All eight IAV genomic RNA species were detected in OGT precipitate after viral infection (Supplementary Fig. 4b, e). Meanwhile, we also observed the enrichment for OGT-bound human RNA species, such as ribosomal RNA (rRNA) pseudogene transcripts in nontreated cells (Supplementary Fig. 4c, g). OGT-human RNA interaction was attenuated after viral infection (Supplementary Fig. 4d, f). Importantly, we found that OGT dominantly immunoprecipitated viral RNA compared to human RNA in viral-infected cells (Supplementary Fig. 4d). Therefore, our results support that OGT interacts with viral RNA, which is more dominant compared to interaction with human RNA in viral-infected cells. The functional consequence of OGT interaction with host cell RNA species warrants further investigations.
OGT interaction with IAV genomic RNA inhibits viral replication independently of MAVS
Based on the identification of OGT ΔTPR4 as a mutant lacking the interaction with IAV genomic RNA, we sought to explore the functional importance of OGT-IAV genomic RNA interaction. Compared to OGT-KO cells reconstituted with either FL OGT or OGT ΔTPR5, reconstitution of OGT-KO cells with OGT ΔTPR4 caused a partial reduction of IAV transcripts NS1 and NP (Fig. 5a) and viral titers (Fig. 5b). These results indicate that TPR4-dependent OGT-IAV genomic RNA interaction contributes to antiviral function of OGT. Meanwhile, reconstitution with OGT ΔTPR4 caused a similar induction of ISGs (Fig. 5c) and activation of antiviral immune signaling (Fig. 5d) upon IAV infection compared to WT OGT or OGT ΔTPR5. In addition, no defect in total protein O-GlcNAcylation or MAVS O-GlcNAcylation was observed in OGT-KO cells reconstituted with OGT ΔTPR4, indicating an intact catalytic activity of OGT lacking TPR4 (Fig. 5e). We further genetically deleted MAVS in cells with FL OGT, OGT ΔTPR4, OGT ΔTPR5, or OGT-K908A to determine if TPR4-dependent OGT antiviral function requires MAVS signaling (Fig. 5f). In cells lacking MAVS, reconstitution of OGT-KO cells with FL OGT or OGT ΔTPR5 caused a significant reduction of IAV NS1 and NP transcripts (Fig. 5g) compared to empty vector, highlighting a critical virus-restriction capacity of OGT in the absence of MAVS signaling. In sharp contrast, despite efficient ablation of ISG induction (Fig. 5h), reconstitution with OGT ΔTPR4 caused no reduction in IAV transcripts compared to empty vector in cells lacking MAVS. In sum, these genetic results demonstrate TPR4-dependent OGT-IAV genomic RNA interaction as an essential antiviral response that is independent of OGT catalytic activity and MAVS signaling.
a–d OGT-KO HT29 cells were reconstituted with empty vector (EV), GFP-tagged OGT (OGT FL) or its indicated mutants, followed by the infection with PR8 virus at a MOI = 1 for 12 h. Viral mRNAs (a) and viral titers (b) were measured by RT-PCR and TCID50 assays, respectively. c Gene transcripts including IFNB, MX1, ISG15, IL6, and TNFA in the cells were measured with RT-PCR. d Immunoblotting of phosphorylated TBK1, IRF3, NF-κB signaling molecules was performed with indicated antibodies. e 293T cells were transfected with indicated vectors. After 48 h of transfection, cell lysates were harvested and immunoprecipitated with anti-Flag Agarose. Immunoprecipitated Flag-MAVS was assessed for O-GlcNAcylation with anti-O-GlcNAc antibody by immunoblotting. f–hMAVS-KO and WT control of HT29 EV, OGT-FL and its indicated mutant cells were infected with PR8 virus at a MOI = 1 for 12 h. f MAVS protein expression in cells was assessed by immunoblotting. Viral mRNAs (g), and gene transcripts including IFNB, MX1, and ISG15 in the cells (h) were measured with RT-PCR. Data are representative of three independent experiments (a–c and g, h). Statistical significance was determined by two-way ANOVA followed by Tukey’s test (a–c and g, h), P values of statistical comparisons are shown in each graph. The error bars represent SEM from n = 3 independent biological replicates (a–c and g, h). Source data are provided as a Source Data file.
OGT counteracts lipid droplets accumulation upon IAV infection
We next sought to determine the signaling mechanism by which TPR4-dependent OGT-IAV genomic RNA interaction suppresses IAV replication. Previous studies have reported that OGT can translocate between nuclear and cytoplasmic compartments in response to extracellular nutrient cues53,54,55. Following IAV infection, we observed a robust cytoplasmic translocation of nuclear OGT, which is independent of its catalytic activity (Fig. 6a). Surprisingly, loss of TPR4, but not TPR5, abolished IAV-induced OGT cytoplasmic translocation, highlighting a possibility that TPR4-dependent virus-restriction capacity of OGT may involve signaling occurring in the cytosol (Fig. 6b). Moreover, we isolated nuclear versus cytosolic compartment in noninfected and IAV-infected cells to monitor OGT translocation. IAV infection caused an increased accumulation of WT OGT protein in the cytosol, but decreased OGT in nuclear compartments, indicating the translocation of WT OGT from nuclear to cytosolic compartment (Supplementary Fig. 5a). This translocation depends on OGT TPR4 (ΔTPR4), but not TRP5 (ΔTPR5) or its catalytic activity (K908A). In sum, these results indicate a cytoplasmic translocation of nuclear OGT upon IAV infection.
a, b OGT-KO HT29 cells were reconstituted with empty vector (EV), GFP-tagged OGT (OGT-WT) or its indicated mutants, followed by the infection with PR8 virus at a MOI = 1 for 12 h. Representative confocal microscopy images and quantification of subcellular localizations of OGT-WT and its K908A mutant in cells (a), n = 50 cells in EV group, n = 49 cells in OGT-WT group, n = 46 cells in OGT-K908A group. Scale bar: 15 μm. Representative confocal microscopy images and quantification of subcellular localizations of OGT-WT and its indicated mutants in cells (b), n = 50 cells in EV group, n = 43 cells in OGT-WT group, n = 47 cells in OGT ΔTPR4 group, n = 46 cells in OGT ΔTPR5 group, n = 50 cells in OGT-K908A group. Scale bar, 15 μm. c Volcano Plot of differential protein level analysis and subcellular localization of the OGT interactome from raw MS assay count data comparing the OGT and OGT PR8 infection groups. Red: upregulated protein (log2 Fold Change > 1 and adjust p < 0.01; Gray: protein with no significant change (−1 < log2 Fold Change < 1 or adjust p > 0.01); Blue: downregulated protein (log2 Fold Change < -1 and adjust p < 0.01). Circle: nuclear protein; Triangle: non- nuclear protein. d–f OGT-WT or its indicated mutants HT29 cells were infected with PR8 virus at a MOI = 1 for 12 h. OGT-WT and its K908A mutant (d) or OGT-WT and its indicated mutants (e)-interacting proteins were assessed by immunoprecipitation assay and immunoblotting with indicated antibodies. f Lipid droplets levels in OGT-WT and its mutant cells were stained with LipidTOX Deep Red and assessed by flow cytometry. g–j PLIN2-KO and WT control of HT29 EV, OGT-FL and its indicated mutant cells were infected with PR8 virus at a MOI = 1 for 12 h. g PLIN2 protein expression in cells was assessed by immunoblotting. Viral mRNAs (h), viral titers (i), and gene transcripts including IFNB, MX1, and ISG15 in the cells (j) were measured with RT-PCR and TCID50 assays, respectively. Data are representative of three independent experiments (a, b, f and h–j). Statistical significance was determined by two-way ANOVA followed by Tukey’s test (a, b, f and h–j), P values of statistical comparisons are shown in each graph. The error bars represent SEM from n = 50 cells in EV group, n = 49 cells in OGT-WT group, n = 46 cells in OGT-K908A group in (a), n = 50 cells in EV group, n = 43 cells in OGT-WT group, n = 47 cells in OGT ΔTPR4 group, n = 46 cells in OGT ΔTPR5 group, n = 50 cells in OGT-K908A group in (b), and n = 3 independent biological replicates (f, h–j). Statistical significance was calculated using two-sided Wald test and adjusted with Benjamini and Hochberg method (c). Source data are provided as a Source Data file.
We next tested the hypothesis that OGT antagonizes viral replication through its interaction with cytoplasmic components upon IAV infection. To compare the interactome of OGT in the absence or presence of IAV infection, we immunoprecipitated OGT-containing protein complexes from cells reconstituted with GFP-tagged OGT or empty vector, followed by liquid chromatography coupled to tandem MS (LC-MS/MS) assay (Supplementary Data 1). A total of 459 proteins were detected that specifically coimmunoprecipitated with OGT-GFP, but not with empty vector. Importantly, we discovered perilipin 2 (PLIN2) and PLIN3 as enriched proteins in the precipitates of OGT-GFP from IAV- infected cells, showed at volcano plot analysis of OGT interactome (Fig. 6c). We cross-examined OGT-interacting proteins between this study and two other studies56,57. Twenty-two proteins were identified that were shared by three studies, including several well-known OGT-interacting proteins such as USP10 and TET3 (Supplementary Fig. 5b and Supplementary Table 3). The limited number of OGT-interacting proteins shared by three studies is presumably due to different cell types and stimulation approaches utilized in three studies. PLIN2 and 3 were identified as OGT-interacting proteins only in our study, indicating that OGT-PLIN2/3 interaction was specific in response to IAV challenge. Moreover, coimmunoprecipitation assay confirmed that the interaction between OGT and PLIN2 and PLIN3 was enhanced following IAV stimulation (Fig. 6d). OGT interaction with PLIN2 and PLIN3 was independent of its enzyme activity (Fig. 6d), but instead required TPR4 (Fig. 6e), which was consistent with TPR4-dependent OGT cytoplasmic translocation.
PLIN2 and PLIN3 are essential proteins that maintain the stability of cellular LDs58. Previous studies have well documented that LDs are important organelles downstream of fatty acid synthesis and promote viral replication59,60,61. Consistent with IAV-induced OGT interaction with PLIN2 and PLIN3, we observed remarkedly elevated colocalization between OGT and LDs following IAV infection, indicating that OGT was translocated to LDs (Supplementary Fig. 5c). Reconstitution of OGT-KO cells with WT OGT significantly reduced the number of LDs following IAV infection (Fig. 6f and Supplementary Fig. 5e). Therefore, OGT relocation to LDs correlates with an inhibitory role in LDs accumulation upon IAV infection. OGT-K908A mutant showed a comparable level of OGT-LDs colocalization and LDs inhibition as WT OGT, indicating no involvement of OGT catalytic activity in these processes. In contrast, deletion of OGT TPR4, but not TPR5, decreased OGT-LDs colocalization and showed no inhibitory effect on LDs accumulation compared to WT OGT. In sum, these results support that TPR4-dependent OGT-viral genomic RNA interaction promotes OGT relocation to LDs and negatively affects LDs accumulation.
We next employed both pharmacological and genetic approaches to examine if reduced LDs accumulation was responsible for TPR4-mediated antiviral function of OGT. Acetyl-CoA carboxylase (ACC) inhibitor (TOFA)62 or diacylglycerol O-acyltransferase (DGAT1) inhibitor (A922500) were used to inhibit fatty acid synthesis and LDs formation, respectively. Pretreatment of cells with either TOFA (50 μg/ml) or A922500 (20 μg/ml) reduced IAV NS1 and NP transcript levels and viral titers in cells with OGT ΔTPR4 to those in cells with WT OGT or OGT ΔTPR5 (Supplementary Fig. 6a, b). In addition, TOFA or A922500 treatment did not significantly affect ISG production compared to vehicle control (Supplementary Fig. 6c). We further genetically deleted PLIN2 in cells with various OGT genetic background (Fig. 6g) and found that PLIN2 deletion reduced IAV transcript levels (Fig. 6h) and viral titers (Fig. 6i) in cells with OGT ΔTPR4 to those in cells with WT OGT or OGT ΔTPR5 without affecting ISG production (Fig. 6j). Furthermore, the virus-restriction role of OGT-K908A compared to empty vector was completely abolished upon PLIN2 deletion (Fig. 6h, i). In sum, these results suggest TPR4-mediated OGT antiviral function depends on LDs inhibition, which supplements catalytic activity-dependent effect of OGT on MAVS signaling to antagonize viral replication.
OGT promotes PLIN2 degradation via K48-linked ubiquitination
It has been well documented that viral infection usually leads to enhanced fatty acid synthesis and LDs accumulation, which eventually benefits viral replication59,60,61,63. We next sought to determine the mechanism by which TPR4-dependent OGT LDs translocation suppresses LDs accumulation. Based on IAV-induced OGT interaction with PLIN2 and PLIN3 (Fig. 6d, e), we examined proteins levels of multiple perilipins by immunoblotting and found that only PLIN2 protein was remarkedly reduced by IAV infection in cells with WT OGT (Fig. 7a). Decreased PLIN2 protein was not due to lower gene transcription (Fig. 7b), indicating a viral-induced PLIN2 protein degradation. We further found that PLIN2 protein degradation was independent of catalytic activity of OGT, but instead required OGT TPR4, suggesting that TPR4-dependent OGT-PLIN2 interaction causes PLIN2 degradation (Fig. 7a).
a, b OGT-KO HT29 cells were reconstituted with empty vector (EV), GFP-tagged OGT (OGT-WT) or its indicated mutants, followed by the infection with PR8 virus at a MOI = 1 for 12 h. PLIN2-5 proteins expression (a) and PLIN2 mRNA (b) in cells were assessed by immunoblotting and RT-PCR respectively. c–e 293T cells were transfected with GFP-tagged PLIN2 and Myc-tagged OGT, in the presence of HA-tagged ubiquitin WT, K48-only, or K63-only mutants. After 48 h of transfection, cell lysates were immunoprecipitated with anti-GFP Agarose. Total (c), K48-only (d) or K63-only (e) ubiquitinated PLIN2 was assessed by immunoblotting with indicated antibodies. f, g 293T cells were transfected with GFP-tagged PLIN2 and Myc-tagged OGT, in the presence of HA-tagged ubiquitin WT and K48-only. After 30 h of transfection, cells were treated with MG132 (10 μΜ) for 6 h, and then the cell lysates were immunoprecipitated with anti-GFP Agarose. Total (f) and K48-only (g) ubiquitinated PLIN2 was assessed by immunoblotting with indicated antibodies. OGT-WT and its K908A mutant (h) or OGT-WT and its indicated mutants (i) HT29 cells were infected with PR8 virus at a MOI = 1 for 24 h, total PLIN2 was immunoprecipitated and followed by immunoblotting with anti-ubiquitin antibody. Data are representative of three independent experiments (b). Statistical significance was determined by two-way ANOVA followed by Tukey’s test (b), P values of statistical comparisons are shown in each graph. The error bars represent SEM from n = 3 independent biological replicates (b). Source data are provided as a Source Data file.
Previous studies have reported that OGT affects protein stability via a ubiquitin-proteasome system (UPS)-dependent mechanism64,65,66,67. We therefore examined whether OGT promotes PLIN2 protein degradation via ubiquitination. When 293T cells were transfected with plasmids expressing GFP-tagged PLIN2 and HA-tagged ubiquitin, total ubiquitination, K48-linked (K48-Ub) or K63-linked (K63-Ub) ubiquitination of PLIN2 were readily detected. Co-expression of OGT induced further enhancement in total and K48-Ub of PLIN2 but showed no effect on its K63-Ub (Fig. 7c and Supplementary Fig. 7a). We further assessed the effect of OGT on PLIN2 ubiquitination using either K48-only or K63-only ubiquitin mutants, which contain replacement of all lysine residues by alanine except lysine at position 48 and 63, respectively31. OGT increased PLIN2 ubiquitination in the presence of K48-only ubiquitin (Fig. 7d and Supplementary Fig. 7b), but not K63-only ubiquitin (Fig. 7e and Supplementary Fig. 7c), suggesting that OGT promotes K48-Ub of PLIN2. Treatment with proteosome inhibitor MG132 further elevated total and K48-Ub of PLIN2 (Fig. 7f, g). Moreover, IAV infection for 24 h induced a robust PLIN2 ubiquitination, which correlated with PLIN2 protein degradation, in the presence of WT OGT (OGT-WT, Fig. 7h). Deletion of OGT completely abolished PLIN2 ubiquitination and protein degradation, indicating a critical role of OGT in promoting PLIN2 protein degradation through K48-Ub. We further found that OGT-dependent PLIN2 ubiquitination and protein degradation did not involve its enzyme activity (OGT-K908A, Fig. 7i), but instead required TPR4 of OGT (OGT ΔTPR4, Fig. 7i). Together, these findings suggest that TPR4-dependent OGT-PLIN2 interaction correlates with PLIN2 ubiquitination and protein degradation, which is accompanied with LDs inhibition and viral replication (Supplementary Fig. 8).
Discussion
Since its initial discovery, OGT has been most recognized for its catalytic activity-dependent role in modifying proteins with O-GlcNAcylation68. Studying the catalytic activity-independent functions of OGT is crucial for enhancing our overall understanding of this key metabolic enzyme. To specifically focus on the catalytic activity-independent functions of OGT, we generated a conditional OGT catalytically impaired knock-in mouse and examined its phenotypes in an IAV infection model. Most surprisingly, OGT-K908A mutation decreased viral replication both in vitro and in vivo compared to OGT KO, although it was more permissive for viral replication than WT OGT. IAV infection activates RIG-I and subsequently induces IFN-I and ISGs in virus-infected cells2,13. Our previous study demonstrated that OGT catalytic activity-dependent antiviral effect requires MAVS- mediated IFN-I signaling31. In this study, we found that the inhibition of IAV replication by the catalytically impaired OGT K908A mutant involves a mechanism unrelated to MAVS signaling. These findings significantly expand the current knowledge of OGT by demonstrating that OGT has catalytic activity-independent antiviral function.
There are two evolutionarily conserved mechanisms for sensing viral pathogen-associated molecular patterns (PAMPs) that contribute to the innate immune response against viral infection11,12,13,14. One mechanism is mediated by RLRs and involves the induction of IFN-I and ISGs2,13. The other mechanism involves programmed cell death mediated by nucleotide-binding domain leucine-rich repeat (NLR) proteins69, such as NLRP170 and NLRP371 or ZBP17,9. A widely accepted concept is that activation of immune signaling in response to virus infection reprograms cellular metabolic activities, which not only provides the energy and substrates for efficient immune responses but also further conveys danger signals to other restorative stress responses31,63,72. Interestingly, one study reported that the glycolytic enzyme hexokinase unexpectedly acts as a primary pattern recognition receptor (PRR) that recognizes bacterial peptidoglycan and triggers activation of inflammasomes73. However, whether viral components can be detected directly by metabolic sensors as danger signals remains completely unknown. We sought to examine the mechanism by which OGT-K908A inhibits IAV replication through biochemical studies. Intriguingly, we discovered that OGT interacts with IAV genomic RNA in a manner independent of its catalytic activity. Thus, we propose that OGT, as a metabolic sensor, represents a branch in initiating the antiviral immune response by sensing IAV genomic RNA. It is worth noting that in some human cells, the catalytic activity of OGT may promote replication of viruses other than IAV such as herpes simplex virus and human cytomegalovirus based on the assays with the use of OGT inhibitor OSMI−174. Therefore, it seems that how OGT and O-GlcNAc levels impact viral infection are dependent on the virus and cell model used.
In this study, OgtK908A conditional knock-in model was achieved by crossing Ogt-K908Afl/fl with individual Cre-expressing deleter mouse strain. For Cre-loxP-mediated recombinant system, it is well known that individual Cre systems driven by different promoters function with different efficiencies. In our OgtK908A conditional knock-in models (cells and animals), it is unlikely that Cre-loxP-mediated recombinant will lead to 100% replacement of WT OGT by K908A. Similarly, OgtKO conditional knockout (by using Ogtfl/fl) will unlikely achieve an 100% Ogt gene deletion, as observed in most studies using Cre-loxP system. Regarding the effect of K908A mutation on the loss of catalytic activity, two studies reported a less than 10% activity of K908A mutant40,41. Therefore, incomplete knock-in efficacy and residual activity of K908A mutant are two major contributors to some residual OGT catalytic activity in K908A knock-in models in vitro and in vivo. Despite an incomplete loss-of-catalytic-activity of OGT in K908A knock-in models, we observed comparable levels of activation of antiviral immune signaling and ISG induction between OgtK908A and OgtKO cell and animals. These results support that impaired catalytical activity of OGT in OgtK908A cells and animal is sufficient to cause a similar defect phenotype in MAVS antiviral signaling compared to OgtKO models. Therefore, attenuated viral replication in OgtK908A models compared to OgtKO models may not be explained by residual activation of MAVS antiviral signaling. Indeed, when MAVS or IRF3 was removed from cells containing empty vector, OGT-WT or K908A mutant, different viral replication was abolished between cells containing OGT-WT or K908A, suggesting that OGT catalytic activity-mediated antiviral effect fully requires MAVS signaling. Meanwhile, OGT K908A still inhibits viral replication compared to cells without OGT, therefore highlighting OGT catalytic activity-independent antiviral effect.
OGT is composed of two separate domains: the N-terminal TPR repeats, which mediate the recognition of a broad range of target proteins and the C-terminal domain, which has glycosyltransferase activity45,46. Previous work has shown that IFIT1 and IFIT5 directly bind 5′ triphosphate RNA (5′ ppp-RNA) in a non-sequence-specific manner through their TPR motifs, representing a model of TPR-containing proteins that sense viral RNA47. The structural similarity between OGT and IFIT proteins suggests that viral RNA interaction may account for the antiviral ability of OGT-K908A mutant against IAV infection. Our current study showed that the N-terminal TPR4 motif of OGT is indeed required for the interaction between OGT and IAV genomic RNA. Deletion of TPR4 motif impaired the anti-IAV function of OGT, genetically verifying the hypothesis that the interaction with viral RNA is critical for OGT’s antiviral activity. This is similar to the antiviral mechanism of IFIT1/5 protein47. IAV PA and PB2 genomic RNAs, which are ligands for RIG-I75,76, were also sensed by OGT in this study. Whether OGT interacts with shorter subviral IAV gene segments, defective interfering (DI) RNA or dsRNA, or whether special RNA modifications are acquired for this interaction, remain to be elucidated. Moreover, our current results do not support that TPR4 directly interact with viral RNA. Instead, we reason that TPR4 may serve as a structural bridge between TPR3 and TPR5, facilitating the formation of a continuous TPR motif. This configuration may mediate RNA interacting through adjacent structural elements enriched in basic residues (e.g., R102, R113, R117, R172, and K182), which collectively contribute to the RNA-interacting interface of the OGT. Detailed interacting features between OGT and viral RNA requires structural biology techniques such as cryogenic electron microscopy in future studies.
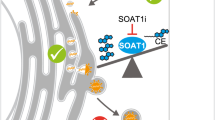
Here, we found that LDs accumulated significantly after IAV infection but were inhibited in the presence of OGT, indicating that viral infection reprograms lipid metabolism in cells and OGT negatively regulates this process. This finding is consistent with recent studies showing that viral infection enhances the activity of the fatty acid biosynthesis pathway59,60,61,62,77,78,79. Enhanced fatty acid and LDs biosynthesis is required for virus replication59. The perilipin family members, including PLINs 1-5, are major LD-associated proteins80,81. We identified the interaction between OGT and PLIN2 through unbiased LC-MS/MS analysis and found OGT induced PLIN2 degradation through its TPR4 motif. Importantly, PLIN2 deficiency leads to significantly decreased viral replication in OGT TPR4 deletion mutant cells, suggesting that PLIN2 is a key target of OGT on LDs. This finding aligns with recent studies showing that inhibition of the fatty acid biosynthesis pathway and LDs formation effectively restrict virus replication across a wide range of virus types61,62,82,83, including IAV84, thus supporting the promising therapeutic potential of targeting OGT in viral infectious diseases. It has been known for a long time that O-GlcNAc signaling significantly impacts lipid metabolism25,85,86. Here, our study showed that directly targeting PLIN2 by OGT resulted in a significant lipid metabolism reprogramming towards decreased LDs formation, demonstrating that the OGT-LDs axis is an antiviral route based on the crosstalk networks of sugar and lipid metabolism. In summary, our findings reveal OGT as a multifaceted metabolic sensor that integrates MAVS-dependent antiviral innate immune signaling and lipid metabolism. This expands our current understanding of the crosstalk between immune signaling pathways and glucose and lipid metabolism, highlighting the essential role of immunometabolism in viral infection-associated diseases.
Methods
Mice
For the generation of an inducible Ogt allele with the replacement of lysine 908 by alanine (Ogt-K908A), a targeting vector was generated in which the mutant exons 20–22 was cloned downstream of the wild type exons 20–22. The K908A (AAA to GCA) mutation was introduced to the mutant exon 20. This mutant module containing exons 20–22 was inserted downstream of a cassette containing an FRT-flanked neomycin-resistance gene and LoxP-flanked wild type exons 20–22 and transcriptional stop element (Supplementary Fig. 1a, b). The targeting vector was generated by Biocytogen and used to establish Ogt-K908Afl/fl mice by homologous recombination in BCGEN B61-6 (C57BL/6) embryonic stem cells. Ogt-K908Afl/fl mice were generated after successful germ-line transmission and backcrossed to C57BL/6J. C57BL/6J mice (000664) and R26-Cre-ERT2 (referred to as ER-Cre mice, 008463) mice were obtained from The Jackson Laboratory. Ogtfl/fl mice31 and Foxj1-Scgb1a1-Cre mice44 have been previously described. Ogtfl/fl and Ogt-K908Afl/fl mice were crossed with ER-Cre mice to generate tamoxifen-inducible Ogt gene-deletion (OgtKO) and Ogt catalytically impaired (OgtK908A) strains, respectively. OgtΔLEC and Ogt-K908AΔLEC mutant mice were generated by crossing Ogtfl/fl and Ogt-K908Afl/fl mice with Foxj1-Scgb1a1-Cre mice. Mice of both sexes were used in the study, gender-matched mice were randomly assigned to mice study. All mice were housed in standard rodent micro-isolator cages and acclimated to study conditions for at least 7 days before manipulation. Mice were kept in animal rooms maintained on 12-h light–dark cycle, temperature and humidity-controlled, between 68 and 74 ℉ and 30–70%, respectively. The animal work was approved by The Ohio State University and National Institute of Health Guide for the Care and Use of Laboratory Animals and the Institutional Animal Care and Use Committee (IACUC). All in vivo experiments were performed according to the guidelines established by The Ohio State University and National Institute of Health Guide for the Care and Use of Laboratory Animals and the Institutional Animal Care and Use Committee (protocol no. 2018A00000022-R1).
Reagents and antibodies
Tamoxifen (B5965) was from APExBIO. Diethyl pyrocarbonate (DEPC) (AC170250050) was from Acros Organics. X-tremeGENE HP DNA transfection reagent (06366546001) was from Roche. 10% neutral buffered formalin (5701) and AF488 goat anti-mouse secondary antibody (A−11001) were from Thermo Fisher Scientific. Bio-Safe Coomassie G-250 Stain (1610786) was from Bio-Rad Laboratories. TOFA (10005263) and DGAT−1 inhibitor A922500 (10012708) was from Cayman Chemical. Polybrene (H9268) was from Sigma. Flag peptide (RP21087) was from GenScript. Antibodies for immunoblotting included anti-OGT (5368), anti-MAVS (4983), anti-p-TBK1 (S172) (5483), anti-TBK1 (3504), anti-p-IRF3 (S396) (4947), anti-IRF3 (4962), anti-p-IKKα/β (S176/180) (2697), anti-p-IκBα (S32) (2859), anti-IκBα (9242S), anti-p-p65 (S536) (3033), anti-p65 (8242), and anti-ubiquitin (3933) from Cell Signaling Technology; anti-IAV (B65141G) from Meridian Life Science, Inc.; anti-IAV NA (11058-T62) from Sino biological; anti-MAVS (sc-365334), anti-GFP (sc-9996) and HRP-conjugated anti-β-actin (sc−1615) from Santa Cruz Biotechnology; HRP-conjugated anti-HA (11667475001), HRP-conjugated anti-Myc (11814150001) and HRP-conjugated anti-Flag (A8592) from Sigma; anti-IKKα (07−1107) from EMD Millipore; anti-PLIN3 (10694−1-AP), anti-PLIN4 (55404−1-AP) and anti-PLIN5 (26951−1-AP) from Proteintech; anti-O-GlcNAc (ab2739), anti-PLIN2 (ab78920) and anti-influenza A virus NP (ab20343) from Abcam. Antibody-conjugated agarose beads for immunoprecipitation included Anti-Flag M2 Affinity Gel (A2220, Sigma), Pierce Anti-Myc Agarose (20168, Thermo Fisher Scientific), GFP-Trap resin (gta-20, ChromoTek), and Protein A/G UltraLink Resin (53132, Thermo Fisher Scientific).
Cell culture
293T (CVCL_0063) and HT29 (CVCL_7297) cells were purchased from the American Type Culture Collection (ATCC). Madin-Darby Canine Kidney (MDCK) cells (NR-2628) were from BEI Resources. All these cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM; Gibco) supplemented with 10% fetal bovine serum (FBS). Mouse embryonic fibroblasts (MEFs) were generated from day−13.5 embryos of Ogtfl/fl, Ogt-K908Afl/fl, OgtKO and OgtK908A mice and cultured in DMEM supplemented with 10% FBS, as previously described87 (Supplementary Table 6).
Plasmids and molecular cloning
pCMV vector expressing human Myc-tagged full-length OGT, pWPXLd lentivector expressing OGT WT and K908A mutant have been previously described31,63. pCMV-myc-OGT and pWPXLd-OGT were used as templates to generate a series of OGT mutants (Supplementary Table 5). pcDNA3-HA-ubiquitin and Flag-tagged RIG-I has been previously described31,88. pBJG1 T7-8xHis-OGT (154271) was purchased from the Addgene and used as template to generate pBJG1 T7-8xHis-OGT ΔTPR4. All primers used for cloning and mutants constructing are listed in the Supplementary Table 2.
Cell transfection
293T cells were transfected with different combinations of expression plasmids as indicated in the Figure Legends for 48 h with X-tremeGENE HP DNA Transfection Reagent (Roche). For lentivector-based transduction, 293T cells were used to package the pWPXLd lentivirus expressing OGT WT or K908A mutant, harvesting supernatant from which and was further used for HT29 Cell transduction.
RT-PCR
Total RNA was extracted from cultured cells or lung homogenates using TRIzol (15596018, Thermo Fisher Scientific). cDNA was generated from total RNA by using Moloney murine leukemia virus reverse transcriptase (28025013, Thermo Fisher Scientific) at 38 °C for 60 min. RT-PCR was performed using SYBR Green PCR Master Mix in an Applied Biosystems StepOnePlus detection system. The fold difference of mRNA between different groups was determined by a standard delta-delta Ct method. Mouse β-actin and human GADPH were analyzed as an internal control. The primer sequences of individual genes are listed in Supplementary Table 1.
Immunoblotting and immunoprecipitation
For immunoblotting assay, electrophoresis of proteins was performed by using the NuPAGE system (Thermo Fisher Scientific) according to the manufacturer’s protocol. Briefly, cultured cells were collected and lysed with RIPA buffer. Proteins were separated on a NuPAGE gel and were transferred onto nitrocellulose membranes (Bio-Rad). Appropriate primary antibodies and HRP-conjugated secondary antibodies were used for immunoblotting and proteins were detected using the Enhanced Chemiluminescent (ECL) reagent (Thermo Fisher Scientific). The images were acquired with ChemiDoc MP System (Bio-Rad). For immunoprecipitation assay, cells were lysed with RIPA buffer supplemented with cOmpleteTM Protease Inhibitor Cocktail (Sigma). Cell lysates were incubated with Anti-Flag M2 Affinity Gel, Pierce Anti-Myc Agarose, or GFP-Trap resin overnight at 4 °C. The mixture was washed for 5 times with cold RIPA buffer and boiled at 95 °C for 5 min, followed by immunoblotting.
ELISA
IFN-β in lung tissue homogenates from mice study was quantified using the ELISA, which was performed using rat monoclonal anti-mouse IFN-β (7891, Cosmo Bio USA) as capture antibody and rabbit polyclonal anti-mouse IFN-β (32400−1, R&D Systems) as detection antibody, as previously described31.
Influenza A virus infection in vivo
Sex- and age-matched Ogtfl/fl, OgtΔLEC and Ogt-K908AΔLEC mice (7-week-old) were intranasally inoculated with influenza A/Puerto Rico/8/1934 (PR8; H1N1) strain at a 50% tissue culture infectious dose (TCID50) of 10 in 50 μl of PBS per mouse. Animal survival and body weight were monitored for 20 days after PR8 infection. Lung tissues were collected at indicated timepoints after infection for virus titration, immunological and histological analyses. Lung tissues were removed from mice at 2 days after IAV infection and fixed with 10% neutral-buffered formaldehyde. Fixed tissues were embedded in paraffin wax, sectioned and stained with Hematoxylin-Eosin (H&E) for histopathology assessment.
Virus titration
MDCK cells were seeded in 96-well plates, infected with 10-fold serially diluted viruses, and incubated at 37 °C for 72 h. Cells were fixed with 4% neutral buffered formaldehyde at room temperature for 30 min and permeabilized in PBS containing 0.1% Triton X−100 for 10 min and blocked at 37 °C for 1 h using 1% bovine serum albumin in PBS. Cells were incubated with anti-IAV NP protein monoclonal antibody at a dilution of 1:3000 at room temperature for 2 h, followed by incubation with AF488 goat anti-mouse secondary antibody at a dilution of 1:5000. Virus-positive cells were visualized under a microscope. 50% tissue culture infectious dose (TCID50) values were calculated according to the Reed-Muench method43.
Flow cytometry
Ogtfl/fl, Ogt-K908Afl/fl, OgtKO and OgtK908A MEFs were infected with PR8-GFP at an MOI of 1 for 12 h and then were fixed with 4% paraformaldehyde for 10 min at room temperature. The percentage of GFP-positive cells was quantified by flow cytometry. OGT-KO HT29 cells reconstituted with empty vector, GFP-tagged WT OGT, or a series of OGT mutants were infected with IAV (MOI of 1) for 12 h. Cells were stained with HCS LipidTOX™ Deep Red Lipid Stain at room temperature for 30 mins for detecting lipid droplets. Flow cytometry analysis was performed by a BD FACS-Canto flow cytometer and FlowJo software was used to analyze the data.
Confocal microscopy
OGT-KO HT29 cells reconstituted with empty vector, GFP-tagged WT OGT, or a series of OGT mutants were seeded in 8-well chamber slides (154526, Thermo Fisher Scientific). 12 h after PR8 infection (MOI = 1), cells were fixed with 4% paraformaldehyde in PBS for 8 min at room temperature, and were permeabilized with 0.01% Triton X−100 for 10 min, followed by blocking with 2% BSA at room temperature for 30 min. The samples were incubated with HCS LipidTOX™ Deep Red Lipid Stain (H34477, Thermo Fisher Scientific) for 30 min, followed by the incubation with DAPI (H−1200, Vector Laboratories) to stain nuclei. Images were acquired using a point scanning confocal unit (FV3000 Spectral, Olympus Scientific) on an Olympus IX83 inverted microscope stand (Olympus Scientific) equipped with a 60x/1.42 NA Plan ApoN objective lens (Olympus Scientific) and immoil-F30CC type F immersion oil (Olympus Scientific). DAPI fluorescence was excited with 405 laser line and collected using GaAsp multi-alkali photomultiplier tubes (PMT) with the following detection wavelengths 420-470 nm. Alexa Fluor 488 fluorescence was excited with 488 laser line and collected using GaAsp multi-alkali PMT with the following detection wavelengths 500-550 nm. Alexa Fluor 647 fluorescence was excited with 647 laser line and collected using GaAsp PMT with the following detection wavelengths 660–740 nm. Images were acquired with a Galvano scanner sequentially by line scanning at 4.0 μs pixel dwell time and acquired at 1.5× scan zoom, 1024 × 1024 image size, 3× line averaging controlled with FV315-SW software (Olympus Scientific). The intracellular distribution of GFP-OGT was quantified using the following ratio: (green fluorescence intensity of the cytosol/cytosolic area)/(green fluorescence intensity of the nucleus/nuclear area). The green fluorescence intensity of the cytosol was determined by subtracting the nuclear green fluorescence intensity from the total cellular green fluorescence intensity. Fluorescence intensity and area measurements for each cellular compartment were automatically obtained using the “Measure” function in ImageJ. To analyze OGT-lipid droplet colocalization, Pearson’s correlation coefficients were automatically calculated for each cell using the Coloc 2 algorithm in Fiji ImageJ 1.52p. For each biological replicate, ~10 cells per field were quantified across five randomly selected fields of view per group. A total of three biological replicates were performed.
Preparation of nuclear and cytoplasmic extracts from Cells
Nuclear and cytoplasmic extracts were prepared from HT29 cells using the Nuclear Extract Kit (40010, Active Motif) according to the manufacturer’s instructions. Briefly, HT29 cells grown in a 100 mm dish (~9 × 106 cells) were washed with ice-cold PBS containing phosphatase inhibitors. Next, 3 mL of ice-cold PBS with phosphatase inhibitors was added to each dish. Cells were scraped and collected by centrifugation at 400 × g for 5 min at 4 °C. The cell pellet was gently resuspended in 500 µL of 1X hypotonic buffer and incubated on ice for 15 min. Subsequently, 25 µL of detergent was added, and the suspension was vortexed at the highest setting for 10 s. The lysate was centrifuged at 14,000 × g for 30 s at 4 °C. The supernatant (cytoplasmic fraction) was collected and stored for immunoblotting analysis. The remaining pellet was resuspended in 50 µL of complete lysis buffer and incubated on ice for 30 min on a rocking platform set at 150 rpm. After incubation, the suspension was vortexed for 30 s at the highest setting and centrifuged at 14,000 × g for 10 min at 4 °C. The supernatant (nuclear fraction) was collected for immunoblotting analysis.
In vitro RNA transcription
Templates for T7 RNA transcription reaction were generated by PCR reaction from plasmid encoding PB2 segment of IAV PR8 virus using forward primer AGCRAAAGCAGGTCAATTATATTCA and reverse primer GCTAATACGACTCACTATAGGGAGTAGAAACAAGG. Plasmids encoding individual RNA segments of IAV PR8 virus have been previously described89. T7 transcription reactions were carried out with MEGAscript™ T7 Transcription Kit according to the manufacturer’s instructions (AM1333, Thermo Fisher Scientific). DNA was digested with 0.05 U/ml of DNase I (EN0525, Thermo Fisher Scientific) and incubated at 37 °C for 1 h followed by lithium chloride precipitation. Final concentrations of IAV PB2 genomic RNA products were measured by NanoDrop Spectrophotometer (Thermo Fisher Scientific) and further analyzed on denaturing agarose gels for correct size.
OGT protein generation and purification
For eukaryotic expression of human OGT protein, 293T cells were transfected with Flag-tagged OGT WT or mutants. Flag-tagged RIG-I was included as a positive control. After 48 h of transfection, cells were lysed with RIPA buffer supplemented with complete protease inhibitor cocktail (Sigma). Cell lysates were incubated with anti-Flag M2 affinity gel (Sigma) overnight at 4 °C. The bead mixture was washed 5 times with cold RIPA buffer and then eluted with Flag peptide (500 ng/ml, RP21087, GenScript) at 4 °C for 6 h. The supernatant was analyzed on SDS-PAGE gel with Coomassie blue staining and subjected to electrophoretic mobility shift assay. For prokaryotic expression of human OGT, pBJG1 T7-8xHis-OGT (154271, Addgene) and pBJG1 T7-8xHis-OGT ΔTPR4 were transformed in BL21 (DE3) cells and grown at 37 °C to an OD = 0.9−1.0. The culture was cooled to 16 °C and induced with 0.2 mM IPTG. After 20 h, cells were collected and resuspended in the binding buffer (250 mM NaCl, 20 mM Tris, 40 mM imidazole, pH = 7.7) and lysed with ultrasonication. Cell lysates were clarified by centrifugation at 17,000 × g, then loaded onto Ni2+-charged His Bind RESIN (69670, Sigma). The column was washed with three column volumes of 50 mM imidazole, pH = 7.5, 250 mM NaCl, and the protein was eluted with 250 mM imidazole, pH = 7.5, 250 mM NaCl. Elute fraction was dialyzed against 150 mM NaCl, 20 mM Tris, pH = 7.5 and stored at −80 °C.
OGT-IAV genomic RNA interaction
Two reciprocal immunoprecipitation assays were performed to investigate OGT interaction with IAV genomic RNA. First, 293 T cells were transfected with Flag-tagged OGT WT or a series of indicated mutants. Flag-tagged RIG-I was included as a positive control, as previously described88. At 12 h after transfection, cells were infected with IAV (MOI of 1). Cell lysates were harvested at 48 h after transfection and was incubated with Anti-Flag M2 Affinity Gel (Sigma) at 4 °C for 6 h. After washes of 3 times with lysis buffer, total RNA was extracted from beads mixture using TRIzol reagent. cDNA of viral PA and PB2 genomic RNA was generated using Uni−12 primer, which were further quantified by RT-PCR using the primers listed in the Supplementary Table 1 and the RT-PCR products were sequenced (Supplementary Table 4). Second, PB2 genomic RNA of IAV generated via in vitro transcription was biotinylated with Pierce RNA 3′ End Biotinylation Kit (20160, Thermo Fisher Scientific) according to the manufacturer’s instructions. Biotinylated RNA was incubated with MyOne Streptavidin C1 beads (Thermo Fisher Scientific) at 4 °C for 4 h with rotation in the presence of RNase inhibitor. RNA and beads mixture were washed three times and incubated with 1 ml of cell lysate containing overexpressed OGT at 4 °C for 6 h with rotation. The mixture was then washed three times and analyzed by immunoblotting. The mixture of cell lysate containing overexpressed OGT and MyOne Streptavidin C1 beads in the absence of PB2 genomic RNA was included as a negative control.
Electrophoretic mobility shift assay
PB2 genomic RNA of IAV generated via in vitro transcription (600 ng) was mixed with Flag-tagged OGT WT or mutants or Flag-tagged RIG-I protein at indicated concentrations in PBS buffer with additional 5 mM MgCl2 for 30 min at room temperature. Samples were then separated by non-denaturing 1% agarose gel electrophoresis in 0.5 × TB buffer (44.5 mM Tris, 44.5 mM boric acid) at 130 V for 1 h. For RNA signal, the gel was prestained with ethidium bromide and was imaged on ChemiDoc Touch Imaging System. The gel was stained with Bio-Safe Coomassie G-250 Stain and imaged on ChemiDoc Touch Imaging System for protein signal.
Isothermal titration calorimetry assay
Isothermal titration calorimetry (ITC) assay was carried out using a Nano ITC (TA waters), as previously described90. Briefly, OGT-WT or OGT ∆TPR4 protein (200 μM) in the ITC buffer (20 mM Tris buffer, pH = 7.5 ± 0.1, 150 mM NaCl) was loaded to the injection syringe. The reference cell was loaded with PB2 RNA (2 μM) in 300 µL of degassed ITC buffer. Titrations were performed at 298 K as a series of 2 µL injections with a 300 s delay between succeeding injections at a stirring rate of 300 rpm. The heat of dilution measured by titrating the buffer solution with protein solution of corresponding concentration was considered during fitting of the binding isotherm as the background heat. The data was analyzed and fitted with Nano Analyze software 2.5.0.
Co-immunoprecipitation of RNA-interacting OGT from IAV-infected cells
HEK 293 T cells (3 × 106 cells per 100 mm dish, two dishes per sample) were transfected with constructs encoding Flag-OGT or Flag-empty vector (10 μg DNA per dish) using polyethylenimine. 24 h after transfection, cells were infected with either PR8 (MOI of 2) or were left uninfected (mock). Cells were harvested 16 h after infection and lysed in RIPA buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 2 mM EDTA, 1% (v/v) NP-40 and 0.1% (v/v) SDS) supplemented with protease inhibitor for 30 min at 4 °C. Cell lysates were clarified by centrifugation at 17,000 × g for 20 min at 4 °C and supernatants were harvested. Aliquots of lysates were saved for total RNA input control. Lysates were then incubated with anti-Flag M2 Affinity Gel (A2220, Sigma) overnight with rotation at 4 °C. Precipitates were washed five times with RIPA buffer followed by treatment with 4 units proteinase K (EO0491, Thermo Fisher Scientific) at 55 °C for 30 min. The input and immunoprecipitated RNAs were extracted using TRIzol (Thermo Fisher Scientific) according to the manufacturer’s instructions.
RNA-seq analysis
RNA purified from Flag-OGT or Flag-empty vector precipitates from mock-or PR8-infected HEK 293T cells, as well as total RNA input samples, were subjected to RNA quality control test using Qubit Flex Fluorometer and Agilent 2100 Bioanalyzer. 100 ng total RNA was used for RNA-Seq Library preparation with QIAseq® FastSelect™ RNA Library Kit. Briefly, ribosomal RNA (rRNA) depletion was carried out using the QIAseq FastSelect rRNA HMR Kit (334387, Qiagen), following the manufacturer’s instructions. cDNA synthesis was performed using the QIAseq Low Input RNA Library Kit (334205, Qiagen), employing both the N6-T RT Primer (random hexamer) and the ODT-T RT Primer (oligo-dT primer). Library amplification/indexing were carried out with QIAseq UX Index kit UDI-A (331815, Qiagen), which incorporates unique 10-base dual indices (UDIs) into each library. The final library quality control test was again conducted using the Qubit Flex Fluorometer and the Agilent 2100 Bioanalyzer. Libraries were sequenced on the Illumina NovaSeq X Plus platform. Raw sequencing reads were quality-controlled, trimmed, and filtered using fastp (v0.23.2)91, followed by alignment to IAV PR8 strain reference genome (NCBI accession number: GCF_000865725.1) and human genome (GRCh38.99) using Hisat292, respectively. Human and IAV counts were normalized using Counts Per Million (CPM), adjusting for library size differences across samples (eq#1): \({\rm{CP}}{{\rm{M}}}_{{\rm{ij}}}=\frac{{x}_{{ij}}}{{\sum }_{k}{x}_{{kj}}}\times {10}^{6}\), where \({x}_{{ij}}\) is the count for gene \({i}\) in sample \(j\), and \({\sum }_{k}{x}_{{kj}}\) is the total number of counts in sample \(j\). Next, Flag-OGT and Flag empty vector precipitate counts were normalized to their respective input RNAs. OGT-specific transcript enrichment was calculated by dividing the normalized Flag-OGT precipitate counts through the normalized Flag-precipitate counts using R scripts. The data were multiplied and visualized in R using the ggplot2 package.
MS assay of OGT interactome
High resolution/accurate mass (HR/AM)-based quantitative proteomics strategy was employed to identify OGT-interacting partners. Briefly, OGT-KO HT29 cells reconstituted with empty vector or GFP-tagged WT OGT were infected with IAV (MOI of 1) for 24 h, followed by immunoprecipitation with GFP-Trap agarose. The protein complexes were boiled with SDS buffer and processed for proteomics analysis following the E3filter protocol described previously93. Three biological replicates of the IP assay were performed. The digests were desalted using C18 StageTips (CDS Analytical), dried in a SpeedVac and stored in −80 °C until further analysis.
For LC-MS/MS analysis, an Orbitrap Eclipse MS (Thermo Fisher Scientific) coupled with an Ultimate 3000 nanoLC system and a nanospray Flex ion source and a FAIMS Pro Interface (Thermo Fisher Scientific) was used. Peptides were first loaded onto a trap column (PepMap C18; 2 cm, length, 100 mm I.D.) and then separated by an analytical column (PepMap C18, 3.0 mm; 20 cm × 75 mm I.D.) using a binary buffer system (buffer A, 0.1% formic acid in water; buffer B, 0.1% formic acid in acetonitrile) with a 165-min gradient (1–25% buffer B over 95 min; 25–32% buffer B in 10 min, 32–95% buffer B over 5 min; back to 1% B in 5 min for equilibration after staying on 80% B for 15 min). MS data were acquired in a data-dependent top−12 method with a maximum injection time of 20 ms, a scan range of 350–1500 Da, and an automatic gain control target of 4 × 105. MS/MS was performed via higher energy collisional dissociation fragmentation with a target value of 5 × 104 and maximum injection time of 22 ms. Full MS and MS/MS scans were acquired by Orbitrap at resolutions of 60,000 and 17,500, respectively. Dynamic exclusion was set to 20 s. For FAIMS, a 3-CV combination (−40|−55|−75) was applied. MS data were searched against UniProt human protein database (20,387 sequences; Reviewed only; August 2021 release) using Sequest HT algorithm and Proteome Discoverer software (version 2.5, Thermo Scientific). Detailed parameters include: trypsin as an enzyme with maximally two missed cleavage sites; protein N-terminal acetylation and methionine oxidation as variable modifications; cysteine carbamidomethylation as a fixed modification; peptide length must be at least seven amino acids. False discovery rate was set at 1% for both proteins. We have also reorganized the raw data and provided the updated login details (MSV000097062).
Volcano plot visualization
Differential protein level analysis was performed using the DESeq2 package (Version 1.42.1) in RStudio and DESeqDataSet was constructed from raw MS assay count data94. A volcano plot was generated to visualize the relationship between fold change and statistical significance. The plot was created using the ggplot2 package, with log2 fold change on the x-axis and -log10 of the adjusted p-value on the y-axis. Proteins that met predefined thresholds for fold change (|log2 fold change| > 1) and adjusted p < 0.01 were highlighted to indicate statistically significant upregulation or downregulation (up or down). The subcellular localization of proteins was determined using data from the Human Protein Atlas database95 (Human Protein Atlas proteinatlas.org).
Venn diagram
The Venn diagram was generated using the online version of Jvenn96. The OGT interactome 157 and OGT interactome 256 were obtained from previously published studies. The OGT interactome presented in this study was derived from raw MS assay count data comparing IAV-treated and nontreated OGT WT.
CRISPR/Cas9 gene knockout
For human MAVS, IRF3 and PLIN2 knockout, Alt-R CRISPR-Cas9 crRNAs targeting MAVS (5′- GTAGATACAACTGACCCTGTGGG), IRF3 (5′- GAGGTGACAGCCTTCTACCGGGG), or PLIN2 (5′- TCATACGTGGAGCTCACCAAGGG) were used for ribonucleoprotein packaging in the presence of Cas9 protein (A36498, Thermo Fisher Scientific), Alt-R CRISPR-Cas9 tracrRNA ATTO 550 (1075927, IDT) and Alt-R Cas9 Electroporation Enhancer (1075915, IDT). Ribonucleoprotein complexes were electroporated into cells with Neon Transfection System (MPK5000, Thermo Fisher Scientific) according to the manufacturer’s instructions. Alt-R CRISPR-Cas9 negative control crRNA (1072544, IDT) was included as non-targeting control. After 24 h, cells with fluorescence were sorted with FACS for further use.
Statistics
Statistical analysis was carried out with GraphPad Prism 10.2.0 for macOS. All data are shown as SEM. The mean values for biochemical data from each group were compared by two-tailed Tukey’s test. Comparisons between multiple time points were analyzed by repeated-measurements analysis of variance (ANOVA) with Bonferroni post-tests. In all tests, P values of less than 0.05 were considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The RNA-seq data generated in this study have been deposited in the Gene Expression Omnibus (GEO) under accession number GSE297354. The MS data generated in this study have been deposited in the MassIVE server under accession number MSV000097062 (https://massive.ucsd.edu/ProteoSAFe/dataset.jsp?task=dffa2d6a49a04a69a79cf1df8218c2c4). Source data are provided with this paper.
References
Ablasser, A. & Hur, S. Regulation of cGAS- and RLR-mediated immunity to nucleic acids. Nat. Immunol. 21, 17–29 (2020).
Brubaker, S. W., Bonham, K. S., Zanoni, I. & Kagan, J. C. Innate immune pattern recognition: a cell biological perspective. Annu. Rev. Immunol. 33, 257–290 (2015).
Rehwinkel, J. & Gack, M. U. RIG-I-like receptors: their regulation and roles in RNA sensing. Nat. Rev. Immunol. 20, 537–551 (2020).
Seth, R. B., Sun, L., Ea, C. K. & Chen, Z. J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 122, 669–682 (2005).
Kenney, A. D. et al. Human genetic determinants of viral diseases. Annu. Rev. Genet. 51, 241–263 (2017).
Koehler, H. et al. Vaccinia virus E3 prevents sensing of Z-RNA to block ZBP1-dependent necroptosis. Cell Host Microbe 29, 1266–1276.e1265 (2021).
Thapa, R. J. et al. DAI senses influenza A virus genomic RNA and activates RIPK3-dependent cell death. Cell Host Microbe 20, 674–681 (2016).
Maelfait, J. et al. Sensing of viral and endogenous RNA by ZBP1/DAI induces necroptosis. EMBO J. 36, 2529–2543 (2017).
Zhang, T. et al. Influenza virus Z-RNAs induce ZBP1-mediated necroptosis. Cell 180, 1115–1129.e1113 (2020).
Zheng, M., Karki, R., Vogel, P. & Kanneganti, T. D. Caspase-6 is a key regulator of innate immunity, inflammasome activation, and host defense. Cell 181, 674–687.e613 (2020).
Fitzgerald, K. A. & Kagan, J. C. Toll-like receptors and the control of immunity. Cell 180, 1044–1066 (2020).
Cadena, C. & Hur, S. Filament-like assemblies of intracellular nucleic acid sensors: commonalities and differences. Mol. Cell 76, 243–254 (2019).
Roers, A., Hiller, B. & Hornung, V. Recognition of endogenous nucleic acids by the innate immune system. Immunity 44, 739–754 (2016).
Wu, J. & Chen, Z. J. Innate immune sensing and signaling of cytosolic nucleic acids. Annu. Rev. Immunol. 32, 461–488 (2014).
Buck, M. D., Sowell, R. T., Kaech, S. M. & Pearce, E. L. Metabolic instruction of immunity. Cell 169, 570–586 (2017).
O’Neill, L. A., Kishton, R. J. & Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 16, 553–565 (2016).
Everts, B. et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKvarepsilon supports the anabolic demands of dendritic cell activation. Nat. Immunol. 15, 323–332 (2014).
Lachmandas, E. et al. Microbial stimulation of different Toll-like receptor signalling pathways induces diverse metabolic programmes in human monocytes. Nat. Microbiol. 2, 16246 (2016).
O’Carroll, S. M., Henkel, F. D. R. & O’Neill, L. A. J. Metabolic regulation of type I interferon production. Immunol. Rev. 323, 276–287 (2024).
Wells, L., Vosseller, K. & Hart, G. W. Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science 291, 2376–2378 (2001).
Love, D. C., Krause, M. W. & Hanover, J. A. O-GlcNAc cycling: emerging roles in development and epigenetics. Semin Cell Dev. Biol. 21, 646–654 (2010).
Hart, G. W., Housley, M. P. & Slawson, C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 446, 1017–1022 (2007).
Torres, C. R. & Hart, G. W. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J. Biol. Chem. 259, 3308–3317 (1984).
Kreppel, L. K., Blomberg, M. A. & Hart, G. W. Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J. Biol. Chem. 272, 9308–9315 (1997).
Yang, X. & Qian, K. Protein O-GlcNAcylation: emerging mechanisms and functions. Nat. Rev. Mol. Cell Biol. 18, 452–465 (2017).
Hardiville, S. & Hart, G. W. Nutrient regulation of signaling, transcription, and cell physiology by O-GlcNAcylation. Cell Metab. 20, 208–213 (2014).
Hart, G. W., Slawson, C., Ramirez-Correa, G. & Lagerlof, O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu Rev. Biochem 80, 825–858 (2011).
Levine, Z. G. & Walker, S. The biochemistry of O-GlcNAc transferase: which functions make it essential in mammalian cells?. Annu. Rev. Biochem. 85, 631–657 (2016).
Bond, M. R. & Hanover, J. A. O-GlcNAc cycling: a link between metabolism and chronic disease. Annu Rev. Nutr. 33, 205–229 (2013).
Hart, G. W. Nutrient regulation of signaling and transcription. J. Biol. Chem. 294, 2211–2231 (2019).
Li, T. et al. O-GlcNAc transferase links glucose metabolism to MAVS-mediated antiviral innate immunity. Cell Host Microbe 24, 791–803.e796 (2018).
Song, N. et al. MAVS O-GlcNAcylation is essential for host antiviral immunity against lethal RNA viruses. Cell Rep. 28, 2386–2396.e2385 (2019).
Herzog, K. et al. Functional microRNA screen uncovers O-linked N-acetylglucosamine transferase as a host factor modulating hepatitis C virus morphogenesis and infectivity. Gut 69, 380–392 (2020).
Wang, Q. et al. O-GlcNAc transferase promotes influenza A virus-induced cytokine storm by targeting interferon regulatory factor-5. Sci. Adv. 6, eaaz7086 (2020).
Wang, X. et al. O-GlcNAcylation modulates HBV replication through regulating cellular autophagy at multiple levels. FASEB J. 34, 14473–14489 (2020).
Hu, J. et al. Hexosamine biosynthetic pathway promotes the antiviral activity of SAMHD1 by enhancing O-GlcNAc transferase-mediated protein O-GlcNAcylation. Theranostics 11, 805–823 (2021).
Mears, H. V. & Sweeney, T. R. Better together: the role of IFIT protein-protein interactions in the antiviral response. J. Gen. Virol. 99, 1463–1477 (2018).
Levine, Z. G. et al. Mammalian cell proliferation requires noncatalytic functions of O-GlcNAc transferase. Proc. Natl. Acad. Sci. USA 118. https://doi.org/10.1073/pnas.2016778118 (2021).
Authier, F., Ondruskova, N., Ferenbach, A. T., McNeilly, A. D., & van Aalten, D. M. F. Neurodevelopmental defects in a mouse model of O-GlcNAc transferase intellectual disability. Dis. Model. Mech. 17. https://doi.org/10.1242/dmm.050671 (2024).
Clarke, A. J. et al. Structural insights into mechanism and specificity of O-GlcNAc transferase. EMBO J. 27, 2780–2788 (2008).
Martinez-Fleites, C. et al. Structure of an O-GlcNAc transferase homolog provides insight into intracellular glycosylation. Nat. Struct. Mol. Biol. 15, 764–765 (2008).
Lazarus, M. B., Nam, Y., Jiang, J., Sliz, P. & Walker, S. Structure of human O-GlcNAc transferase and its complex with a peptide substrate. Nature 469, 564–567 (2011).
Kenney, A. D. et al. IFITM3 protects the heart during influenza virus infection. Proc. Natl. Acad. Sci. USA 116, 18607–18612 (2019).
Zhang, Y. et al. Respiratory enterovirus (like parainfluenza virus) can cause chronic lung disease if protection by airway epithelial STAT1 is lost. J. Immunol. 202, 2332–2347 (2019).
Iyer, S. P. & Hart, G. W. Roles of the tetratricopeptide repeat domain in O-GlcNAc transferase targeting and protein substrate specificity. J. Biol. Chem. 278, 24608–24616 (2003).
Jinek, M. et al. The superhelical TPR-repeat domain of O-linked GlcNAc transferase exhibits structural similarities to importin alpha. Nat. Struct. Mol. Biol. 11, 1001–1007 (2004).
Abbas, Y. M., Pichlmair, A., Gorna, M. W., Superti-Furga, G. & Nagar, B. Structural basis for viral 5’-PPP-RNA recognition by human IFIT proteins. Nature 494, 60–64 (2013).
Hoffmann, E., Stech, J., Guan, Y., Webster, R. G. & Perez, D. R. Universal primer set for the full-length amplification of all influenza A viruses. Arch. Virol. 146, 2275–2289 (2001).
Jiang, Z. et al. IFI16 directly senses viral RNA and enhances RIG-I transcription and activation to restrict influenza virus infection. Nat. Microbiol 6, 932–945 (2021).
Pichlmair, A. et al. IFIT1 is an antiviral protein that recognizes 5’-triphosphate RNA. Nat. Immunol. 12, 624–630 (2011).
Keane, S. C. et al. RNA structure. Structure of the HIV-1 RNA packaging signal. Science 348, 917–921 (2015).
Chiang, J. J. et al. Viral unmasking of cellular 5S rRNA pseudogene transcripts induces RIG-I-mediated immunity. Nat. Immunol. 19, 53–62 (2018).
Whelan, S. A., Lane, M. D. & Hart, G. W. Regulation of the O-linked beta-N-acetylglucosamine transferase by insulin signaling. J. Biol. Chem. 283, 21411–21417 (2008).
Bullen, J. W. et al. Cross-talk between two essential nutrient-sensitive enzymes: O-GlcNAc transferase (OGT) and AMP-activated protein kinase (AMPK). J. Biol. Chem. 289, 10592–10606 (2014).
Seo, H. G. et al. Identification of the nuclear localisation signal of O-GlcNAc transferase and its nuclear import regulation. Sci. Rep. 6, 34614 (2016).
Gao, J. et al. Proteomic analysis of the OGT interactome: novel links to epithelial-mesenchymal transition and metastasis of cervical cancer. Carcinogenesis 39, 1222–1234 (2018).
Martinez, M. et al. Quantitative proteomics reveals that the OGT interactome is remodeled in response to oxidative stress. Mol. Cell Proteom. 20, 100069 (2021).
Saka, H. A. & Valdivia, R. Emerging roles for lipid droplets in immunity and host-pathogen interactions. Annu. Rev. Cell Dev. Biol. 28, 411–437 (2012).
Laufman, O., Perrino, J. & Andino, R. Viral generated inter-organelle contacts redirect lipid flux for genome replication. Cell 178, 275–289.e216 (2019).
Herker, E. et al. Efficient hepatitis C virus particle formation requires diacylglycerol acyltransferase-1. Nat. Med. 16, 1295–1298 (2010).
Miyanari, Y. et al. The lipid droplet is an important organelle for hepatitis C virus production. Nat. Cell Biol. 9, 1089–1097 (2007).
Munger, J. et al. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat. Biotechnol. 26, 1179–1186 (2008).
Li, X. et al. O-GlcNAc transferase suppresses inflammation and necroptosis by targeting receptor-interacting serine/threonine-protein kinase 3. Immunity 50, 576–590.e576 (2019).
Zhang, F. et al. O-GlcNAc modification is an endogenous inhibitor of the proteasome. Cell 115, 715–725 (2003).
Yang, W. H. et al. Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat. Cell Biol. 8, 1074–1083 (2006).
Yan, W. et al. Cancer-cell-secreted miR-122 suppresses O-GlcNAcylation to promote skeletal muscle proteolysis. Nat. Cell Biol. 24, 793–804 (2022).
Song, T. et al. DOT1L O-GlcNAcylation promotes its protein stability and MLL-fusion leukemia cell proliferation. Cell Rep. 36, 109739 (2021).
Dong, H., Liu, Z. & Wen, H. Protein O-GlcNAcylation Regulates Innate Immune Cell Function. Front. Immunol. 13, 805018 (2022).
Ting, J. P. et al. The NLR gene family: a standard nomenclature. Immunity 28, 285–287 (2008).
Bauernfried, S., Scherr, M. J., Pichlmair, A., Duderstadt, K. E. & Hornung, V. Human NLRP1 is a sensor for double-stranded RNA. Science 371. https://doi.org/10.1126/science.abd0811 (2021).
Allen, I. C. et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 30, 556–565 (2009).
Zhang, W. et al. Lactate is a natural suppressor of RLR signaling by targeting MAVS. Cell 178, 176–189.e115 (2019).
Wolf, A. J. et al. Hexokinase is an innate immune receptor for the detection of bacterial peptidoglycan. Cell. https://doi.org/10.1016/j.cell.2016.05.076 (2016).
Angelova, M., Ortiz-Meoz, R. F., Walker, S. & Knipe, D. M. Inhibition of O-linked N-acetylglucosamine transferase reduces replication of herpes simplex virus and human cytomegalovirus. J. Virol. 89, 8474–8483 (2015).
Baum, A., Sachidanandam, R. & Garcia-Sastre, A. Preference of RIG-I for short viral RNA molecules in infected cells revealed by next-generation sequencing. Proc. Natl. Acad. Sci. USA 107, 16303–16308 (2010).
Liu, G., Park, H. S., Pyo, H. M., Liu, Q. & Zhou, Y. Influenza A virus panhandle structure is directly involved in RIG-I activation and interferon induction. J. Virol. 89, 6067–6079 (2015).
Li, Q., Pene, V., Krishnamurthy, S., Cha, H. & Liang, T. J. Hepatitis C virus infection activates an innate pathway involving IKK-alpha in lipogenesis and viral assembly. Nat. Med. 19, 722–729 (2013).
Yan, B. et al. Characterization of the lipidomic profile of human coronavirus-infected cells: implications for lipid metabolism remodeling upon coronavirus replication. Viruses 11. https://doi.org/10.3390/v11010073 (2019).
Dias, S. S. G. et al. Lipid droplets fuel SARS-CoV-2 replication and production of inflammatory mediators. PLoS Pathog. 16, e1009127 (2020).
Kimmel, A. R., Brasaemle, D. L., McAndrews-Hill, M., Sztalryd, C. & Londos, C. Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J. Lipid Res. 51, 468–471 (2010).
Kimmel, A. R. & Sztalryd, C. The perilipins: major cytosolic lipid droplet-associated proteins and their roles in cellular lipid storage, mobilization, and systemic homeostasis. Annu. Rev. Nutr. 36, 471–509 (2016).
Chu, J. et al. Pharmacological inhibition of fatty acid synthesis blocks SARS-CoV-2 replication. Nat. Metab. https://doi.org/10.1038/s42255-021-00479-4 (2021).
Yuan, S. et al. SREBP-dependent lipidomic reprogramming as a broad-spectrum antiviral target. Nat. Commun. 10, 120 (2019).
Episcopio, D. et al. Atorvastatin restricts the ability of influenza virus to generate lipid droplets and severely suppresses the replication of the virus. FASEB J. 33, 9516–9525 (2019).
Sodi, V. L. et al. Nutrient sensor O-GlcNAc transferase controls cancer lipid metabolism via SREBP-1 regulation. Oncogene 37, 924–934 (2018).
Baldini, S. F. & Lefebvre, T. O-GlcNAcylation and the metabolic shift in high-proliferating cells: all the evidence suggests that sugars dictate the flux of lipid biogenesis in tumor processes. Front Oncol. 6, 6 (2016).
Lei, Y. et al. The mitochondrial proteins NLRX1 and TUFM form a complex that regulates type I interferon and autophagy. Immunity 36, 933–946 (2012).
Lu, M. et al. N(6)-methyladenosine modification enables viral RNA to escape recognition by RNA sensor RIG-I. Nat. Microbiol 5, 584–598 (2020).
Guo, X. J. et al. Lung gammadelta T cells mediate protective responses during neonatal influenza infection that are associated with type 2 immunity. Immunity 49, 531–544.e536 (2018).
Kumar, N. et al. Dendritic molecular baskets for selective binding of toxic methotrexate. Angew. Chem. Int Ed. Engl. 64, e202420574 (2025).
Chen, S., Zhou, Y., Chen, Y. & Gu, J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890 (2018).
Kim, D., Paggi, J. M., Park, C., Bennett, C. & Salzberg, S. L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 37, 907–915 (2019).
Zhao, B. et al. SUSD2 suppresses CD8(+) T cell antitumor immunity by targeting IL-2 receptor signaling. Nat. Immunol. 23, 1588–1599 (2022).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Thul, P. J. et al. A subcellular map of the human proteome. Science 356. https://doi.org/10.1126/science.aal3321 (2017).
Bardou, P., Mariette, J., Escudie, F., Djemiel, C. & Klopp, C. Jvenn: an interactive Venn diagram viewer. BMC Bioinform. 15, 293 (2014).
Acknowledgements
We thank Dr. Xiaoyong Yang for Ogtfl/fl mice, Dr. Jing Wang for CRISPR/Cas9 gene deletion. This work was supported by NIH grants to H.W. (R01GM152205 and R01AI135234), X.C. (R01GM141394), J.L. (R01AI090060), J.S.Y. (R01AI130110), and S.L.L. (R01AI150473). Z. Liu was supported by Cure Cystic Fibrosis Columbus Research & Development Program (C3RDP) Trainee Grant. Y. Sun was supported by Pelotonia Postdoc Fellowship. The Ohio State University Comprehensive Cancer Center Genomics Shared Resource was supported by National Cancer Institute grant (P30-CA016058).
Author information
Authors and Affiliations
Contributions
H.D., C.L., and H.W. designed the experiments; H.D., C.L., Z.H.L., Y.S., and Z.W.L. performed experiments and provided intellectual input; Y.Y. performed key mass spectrometry experiment; J.Z., W.W., N.K., J.D.B., A.M., Q.M., X.C., and J.L. contributed to key protein-RNA interaction experiments and provided intellectual input; Y.Z., M.J.H., K.M.G., P.G.T., J.S.Y., and S.L.L. provided critical reagents and intellectual input. H.W. supervised the study, interpreted the data and wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dong, H., Liang, C., Zhang, J. et al. O-GlcNAc transferase plays dual antiviral roles by integrating innate immunity and lipid metabolism. Nat Commun 16, 7721 (2025). https://doi.org/10.1038/s41467-025-63085-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-63085-y